Abstract
Acne is a multifactorial skin disorder frequently observed during adolescence with different grades of severity. Multiple factors centering on sebum secretion are implicated in acne pathogenesis. Despite the recognized role of sebum, its compositional complexity and limited analytical approaches have hampered investigation of alterations specifically associated with acne. To examine the profiles of lipid distribution in acne sebum, 61 adolescents (29 males and 32 females) were enrolled in this study. Seventeen subjects presented no apparent clinical signs of acne. The 44 affected individuals were clinically classified as mild (13 individuals), moderate (19 individuals), and severe (12 individuals) acne. Sebum was sampled from the forehead with SebutapeTM adhesive patches. Profiles of neutral lipids were acquired with rapid-resolution reversed-phase/HPLC-TOF/MS in positive ion mode. Univariate and multivariate statistical analyses led to the identification of lipid species with significantly different levels between healthy and acne sebum. The majority of differentiating lipid species were diacylglycerols (DGs), followed by fatty acyls, sterols, and prenols. Overall, the data indicated an association between the clinical grading of acne and sebaceous lipid fingerprints and highlighted DGs as more abundant in sebum from adolescents affected with acne.
Keywords: mass spectrometry, glycerides, skin lipidome, chemometrics
Acne is a complex and multifactorial skin disorder targeting the pilosebaceous unit, wherein the sebaceous gland produces and secretes a lipid-rich mixture known as sebum. Increased sebogenesis is pivotal in the pathogenesis of acne and predisposes skin to deregulated inflammatory responses (1–3). Quantitative and qualitative modifications of sebaceous lipids likely trigger inflammatory responses causative of acne lesions commonly called comedones (4–7). Juvenile acne, which affects approximately 80% of adolescents in industrialized countries, develops during puberty when androgens and other endocrine factors stimulate sebum secretion, thereby generating conditions for development of lesions (8). Studies aimed at the identification of lipid alterations involved in acne pathogenesis date back to the early 70s to late 80s (9–14); however, the complexity of the sebum composition has represented a major limitation to understanding lipid modifications involved with acne. The recent availability of methods with improved analytical performance and high-throughput opens new perspectives to understanding sebum alterations in acne.
GC-MS has been widely employed to investigate sebum lipids. GC-MS conditions are suitable for analysis of FFAs, squalene, and cholesterol and also allow for detection of wax esters and sterol oxidation products (15). The latest advances in GC-MS analysis have provided structural information and detailed fingerprints of intact sebaceous lipids (16, 17). Coupling the separation power of HPLC with high-resolution MS has also proved to meet the difficult task of analyzing intact structures of sebaceous mixture components (18). On the other hand, no separative spectroscopic methods based on NMR have been used for the quantitative analysis of sebum lipids independent of their fatty acid composition (19).
We have defined a method for analysis of intact lipids in sebum that requires minimal manipulation of the sample and is both efficient and accurate. The HPLC-MS method enabled detection of intact neutral lipids belonging to chemical classes known to enrich sebum, including esters of glycerol, cholesterol, and wax, as well as squalene and FFAs (20). For the purposes of the current study, this analytical method offered a suitable basis for semi-targeted analysis of characterized lipids in acne sebum and an untargeted approach to address the sebaceous lipidome in its entirety. The primary focus of the current study was to identify differences in the lipid composition of healthy and acne sebum in order to understand the role of sebum lipid alterations in this disease. Discovery of new disease markers and clues on the enzymatic pathways involved in acne pathogenesis are crucial to characterizing new targets for successful acne management in the near future.
MATERIALS AND METHODS
Chemicals and reagents
Methanol (MeOH), isopropyl alcohol (iPrOH), acetone (Me2O), and ethanol (EtOH) were of HPLC-MS grade and were purchased from Merck (Darmstadt, Germany), whereas HPLC-MS grade ammonium formate (HCO2NH4) was purchased in granular form from Fluka (Buchs, St. Gallen, Switzerland). Deuterated cholesterol-2,2,3,4,4,6-d6 (d6CH) and glyceryl-d5-trihexadecanoate (d5TG 48:0), selected as the internal standards (ISTDs), were purchased from CDN Isotopes Inc., Pointe-Claire, Quebec, Canada.
Study design and sample collection
The study was approved by the Institutional Ethics Committee and was conducted in agreement with the principles expressed in the Declaration of Helsinki. Female and male Caucasian adolescents were enrolled consecutively in the first 4 months of the year. Acne patients were recruited among subjects at their first access to the Acne Unit. The control group was enrolled among healthy subjects undergoing mole check in the same Institution. Informed consent was obtained from their parents or a legal representative. The acne severity scores and the clinical grading were assessed by dermatologists. Criteria of inclusions were absence of systemic diseases and of skin disorders other than acne. Oral or topic pharmacological treatments at the first visit or up to 2 weeks before the examination, smoking, and use of sun beds were exclusion criteria. Sixty-one subjects (32 females, age 14.1 ± 1.7 years; 29 males, age 14.4 ± 1.4 years) resulted as eligible for the study. None of the enrolled patients had a history of isotretinoin use. The intensity of clinical manifestations was evaluated on the face areas according to the Global European Acne (GEA) severity (GEAS) criteria (21). Clinical classification of the subjects was based on four grades scales adapted from the reported GEAS scores. Briefly, subjects were classified as non-affected (clear) when no or very few lesions were clinically observable (0 ≤ GEAS < 1). Acne patients were subdivided into mild, moderate, and severe groups. Patients with GEA scores between 1 and 2 (1 ≤ GEAS ≤ 2) were referred to as the mild group, whereas patients with GEAS 3 and 4–5 were classified as moderate and severe groups, respectively. Seventeen, 13, 19, and 12 subjects were included in the clear (10 female/7 male), mild (8 female/5 male), moderate (12 female/7 male), and severe (2 female/10 male) groups, respectively. Noninvasive sampling of sebum lipids was performed from central foreheads as previously described (20). Briefly, the sampling surface was cleansed with a gauze pad wetted with 70% EtOH solution beforehand. After allowing the surface to dry, two sebum-adsorbent tapes (SebutapesTM; CuDerm Corp., Dallas, TX), which had been preweighed beforehand, were held onto the skin for 30 min. The tapes were reweighted for the gravimetric assessment of sebum excretion rates (SERs, micrograms per square centimeter per minute). Supplementary Table 1 and supplementary Fig. 1 summarize demographics, distribution of subjects according to the GEAS, and average SER for the respective groups.
Sample preparation
Extraction of tapes was performed according to the previously defined procedure (20). Briefly, tapes were extracted with 10 ml of absolute EtOH containing 0.025% butylated hydroxytoluene. EtOH was evaporated almost to dryness under nitrogen. The concentrated EtOH solution was subjected to liquid-liquid extraction with ethyl acetate to abate matrix-derived material. The final lipid extract was dissolved in Me2O/MeOH/iPrOH (40/40/20) to obtain a solution of sebum at the final weight per volume concentration of 5 mg/ml. Before analysis, the concentrated solutions were diluted 1:5 in Me2O/MeOH/iPrOH (40/40/20). A mixture of ISTDs, namely d6CH and d5TG 48:0, were added at a final concentration of 50 and 10 μM, respectively, to control the analytical performance and to calculate the relative abundance of detected lipids.
HPLC
The chromatographic apparatus consisted of a 1200 series rapid-resolution (RR)-HPLC (Agilent Technologies, Santa Clara, CA), equipped with a degasser, a binary pump, an autosampler, and a thermostated column compartment from the same manufacturer. For the RR-reversed-phase (RP)-HPLC separation, two columns were connected in series. The first one was a Zorbax SB-C8 RR cartridge 2.1 × 30 mm 3.5 μm particle size (Agilent Technologies, Santa Clara, CA) and the second one was a Zorbax SB-C8 RR HT 2.1 × 100 mm 1.8 μm particle size with a maximal operational backpressure at 600 Bar (from the same manufacturer). Sebum samples and authentic standards were eluted with a binary gradient of (A) 5 mM ammonium formate in MilliQ water (18.2 Ω) and (B) MeOH/iPrOH 95/5. The mobile phases were filtered through 0.45 μm glass filters and continuously degassed under vacuum. The elution program was as follows: 0–1 min 70% B, 20 min 99% B, 20–32 min 99% B, 34 min 100% B, 34–44 min 100% B, 56 min 70% B. A post run of 4 min at 70% B was included. The flow rate was maintained at 0.25 ml/min during the entire HPLC run and post-run time (4 min). The column was thermostated at 60°C. The injection volume was 0.5 μl. The injector needle was washed with the mobile phase in the wash port during the HPLC runs. The eluent outlet was connected to two different MS analyzers for the detection and characterization.
MS
Accurate mass measurements were conducted with a G6220A series TOF-MS (Agilent Technologies, Santa Clara, CA) equipped with an ESI interface operating in the positive ion mode. Analytes eluted from the RR-RP-HPLC system were introduced into the TOF-MS apparatus at the operating chromatographic flow rate (see chromatographic conditions described in the HPLC section). Nitrogen was used as the nebulizing and desolvation gas. The temperature and the flow of the drying gas were 350°C and 10 l/min, respectively. The capillary and the cone voltage were 4,000 and 60 V, respectively. Scan mode TOF mass spectra were acquired in the positive and negative ion mode by using the TOF at 10,000 mass resolving power for scans over the range from m/z 100 to 1,000. To enhance accurate mass measurement for the ion species, a reference solution of two compounds with m/z 121.050873 and 922.009798, respectively, was vaporized in continuum in the spray chamber by means of a separate nebulizer.
Data acquisition and extraction
LC-MS-ESI TOF data were acquired and deconvoluted into individual chemical peaks using the Mass HunterTM acquisition software (B.01.03 version; Agilent Technologies). Untargeted and semi-targeted mining of the LC-MS-ESI TOF data were performed with the molecular feature extraction algorithm in the Mass Hunter software. Due to the nature of the sebum samples, quality controls could not be prepared from the study samples. Thus, instrumental drifts and responses were controlled with the help of lipid standard mixtures and the ISTDs. The features extracted were exported into a compound exchange format reporting the retention time, the accurate mass, and the absolute abundance for each entity to be processed in the subsequent chemometric analysis. Alignment of both m/z and retention time was performed against the two ISTDs, d6CH and d5TG, whereas normalization of peak intensities was performed against d6CH.
Data analysis
Mass Profiler Professional (MPP) (version 12.6.1; Agilent Technologies) followed by a statistical procedure developed in-house (22) were used to process the above data. Retention times were aligned by setting a retention time window of 0.6 min, whereas mass-to-charge ratio (m/z) binning was performed by setting windows at 100 ppm. Grouping of biological replicates was set according to the clinical classification of patients with acne. Absolute abundance of each entity was normalized by the absolute abundance of the d6CH ISTD. Data were filtered by frequency of detection, which reflects the number of samples that presented particular features. A frequency filter was applied to data extracted from MPP and only entities present in 75% of samples belonging to at least one of the investigated groups were retained for the statistical analysis. Fold changes of filtered entities were compared between subsets of the studied population by volcano plots in the MPP tools. Fold changes with P < 0.05 after the Bonferroni’s correction were considered as significant. Identification of entities within the MPP workflow was performed based on the METLIN Metabolomics Database (http://metlin.scripps.edu/).
Multivariate data analysis
Golpe procedure was used to perform partial least squares (PLS) algorithm (23). After a preliminary screening focused on enlightening the main differences in the expression of lipid species among the investigated grades of acne (see Data analysis and Results for further details), clear and moderate acne samples were taken as representative of non-affected and affected condition, respectively. Thus, PLS algorithm was performed assigning a categorical activity of −1 for clear and 1 for moderate samples and considering the log2 of the obtained peaks area. Pareto scaling (24) was used to pretreat the data. PLS weights plot has been analyzed in order to select the most important entities responsible for the discrimination between unaffected and moderate acne samples. The selected entities were then identified searching for a correspondence of exact mass in the LIPID MAPS (http://www.lipidmaps.org/) structure database (25).
Statistical analyses
Statistical significance was calculated by Student’s t-test or ANOVA following post hoc Tukey’s honestly significant difference (HSD) test using XLSTAT version 2015.6.01.24027 (Addinosoft, Paris, France). A statistical probability of P < 0.05 was considered significant.
Nomenclature and abbreviations used to describe lipid components
Two letter abbreviations for lipids were used, consistent with the literature (26, 27). Notation of fatty acids, monoacylglycerols, diacylglycerols (DGs), and TGs included the total number of carbon atoms arising from the fatty acyls and the total number of carbon-carbon double bonds in the fatty acyls, independent of their distribution.
RESULTS
Participants in the current study included adolescent subjects with juvenile acne, the most common type of the disease. Supplementary Table 1 shows the distribution of age, gender, and clinical grades (clear, mild, moderate, and severe ones) of participants. Acne severity groups were similarly distributed in terms of age and gender. Mild and severe acne were observed with higher frequency in females and males, respectively. To evaluate sebometry, a parameter associated with acne pathogenesis, the SER, was determined for each participant. Distribution of SER values in the clinical groups is provided in a scattergram in supplementary Fig. 1. The SER was not significantly different in mild and moderate acne compared with unaffected sebum. In contrast, severe acne was associated with a significantly higher SER in both genders. To minimize the effects of SER values on sebum chemometrics, all samples were analyzed with the same weight to volume concentration of sebum in order to compare lipid abundance profiles.
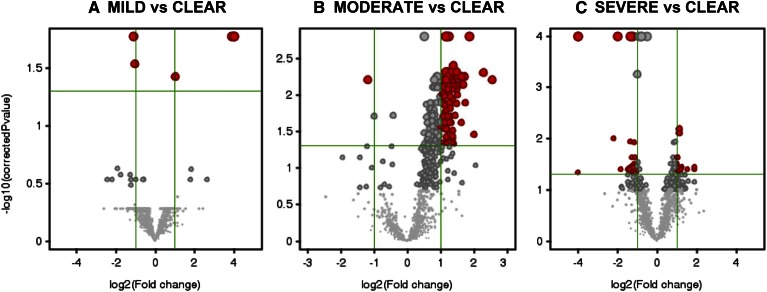
The applied analytical procedure allowed for the untargeted detection of lipid species expressed in the sebum samples. The minimal number of investigated lipid species was obtained by retaining those species that were detected in 75% of samples in at least one condition, which was coincident with the acne severity grade. The applied filtering retrieved 1,492 entities. Measurement of intensity ratios was used to establish relative abundance within a single sample. Intensity ratios were obtained by normalizing the peak area of detected species by the peak area of the d6CH ISTD. To show differences in the expression of untargeted lipid species in sebum from subjects with different acne grades, we plotted fold-changes in the relative abundance of multiple lipids versus sebum from unaffected donors. Thus, we generated volcano plots of normalized intensities of the 1,492 entities in pair-wise comparisons of the sebum lipidome in mild, moderate, and severe acne with that of unaffected subjects (i.e., clear) as a function of estimated effect size (in log2 fold-change) and the statistical significance (P < 0.05, Bonferroni correction; Fig. 1). Gray-colored entities on both sides indicate no significant change with respect to control sebum. Red-colored entities on the left and the right indicate that the normalized intensities of the corresponding entities were statistically lower and higher, respectively, in affected sebum. Changes in the relative abundance of a small number of lipid species were statistically different in sebum from mild grades of acne. In contrast, moderate acne showed significantly greater expression of several lipids compared with control sebum. In sebum from severe acne, we detected both significant decreases and increases in the relative abundance of numerous sebum lipids. The differences highlighted in the volcano plots were consistent with the extent of acne severity.
Fig. 1.
Volcano plots of entities found differently regulated in acne grades versus clear skin conditions. Comparison of relative abundance of entities between non-affected sebum and different acne grades was performed by applying univariate statistical analysis to 1,492 entities found in the 75% of samples belonging to at least one skin condition, namely clear, mild, moderate, and severe acne grades. The log2 of the fold-change values is plotted on the x axis, whereas the −log10 of the t-test P values is plotted on the y axis. The vertical and the horizontal green lines mark the threshold of the fold-change and of the P values with Bonferroni’s correction set at 2 and 0.05, respectively. Annotation of species found differently expressed is provided in the consequent assignment of putative identity. Volcano plots returned 6, 95, and 46 entities expressed at significantly different extent in mild (A), moderate (B), and severe (C) acne grades, respectively, when compared with unaffected sebum.
An Eulero-Venn diagram of entities significantly perturbed in acne sebum showed commonalities between the different acne grades (supplementary Fig. 2). In particular, among lipids with a fold-change ≥2 with respect to unaffected sebum, moderate acne shared one lipid in common with mild acne and nine with severe acne. Class assignment of these lipids was completed using an in-house algorithm designed to match the exact mass of each entity with entries of the LIPID MAPS structure database. Annotation of the shared entities is reported in supplementary Table 2. The epidemiology of moderate acne (28) combined with the outcomes of above comparisons were the basis for its selection as a representative of overall changes associated with average juvenile acne. Thus, the sebum lipidome in moderate acne was further investigated with multivariate approaches. PLS algorithm was performed in order to reinforce the separation between controls and moderate acne in terms of sebum lipid profile.
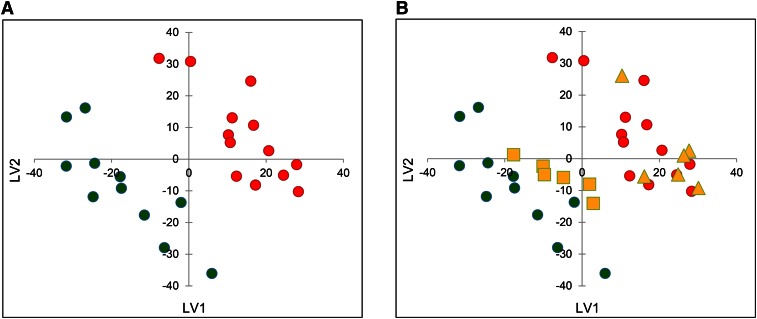
The PLS training set was built selecting samples from 11 clear and 13 moderate subjects. The remaining 12 samples, half from controls and half from the moderate acne group, were then used as a test set to assess the goodness-of-fit and predictive ability of the proposed model. The PLS score plot of the obtained model [PLS first latent variable (LV1) versus the PLS second latent variable (LV2)] is reported in Fig. 2A, which shows obvious separation between control and moderate acne sebum samples, suggestive of significant differences in the lipidome of the two groups. The correlation coefficient of recomputed versus experimental PLS activity reached 0.92 at the LV2. As an external validation, the test set was projected on the model and found to agree perfectly with the experimental results. Indeed, the six samples belonging to unaffected subjects were correctly projected among clear samples of the training set, while the six samples belonging to subjects with moderate acne were projected among the moderate samples (Fig. 2B).
Fig. 2.
PLS score plot (LV1 versus LV2) of training set model obtained from lipid species detected in unaffected (green) and moderate acne subjects (red) (A), and relative test set projection (B). Clear samples (yellow squares) and moderate samples (yellow triangles) correctly projected among green and red training set samples, respectively.
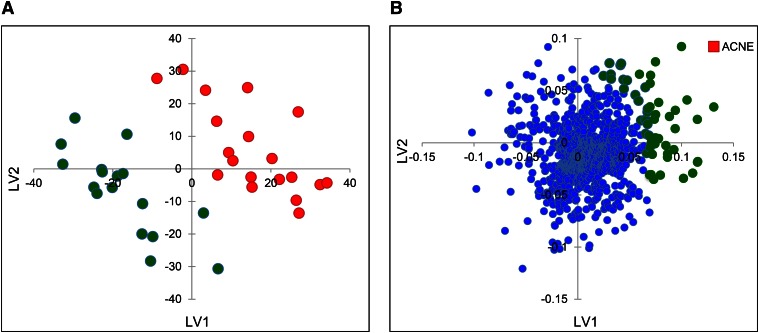
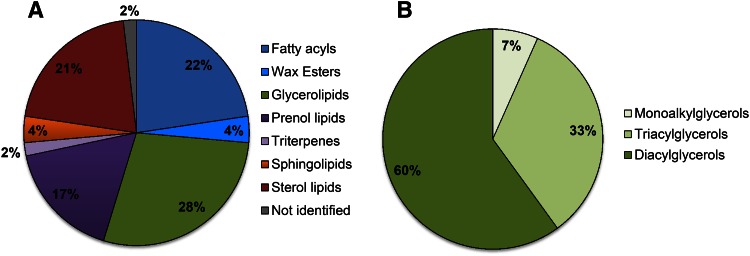
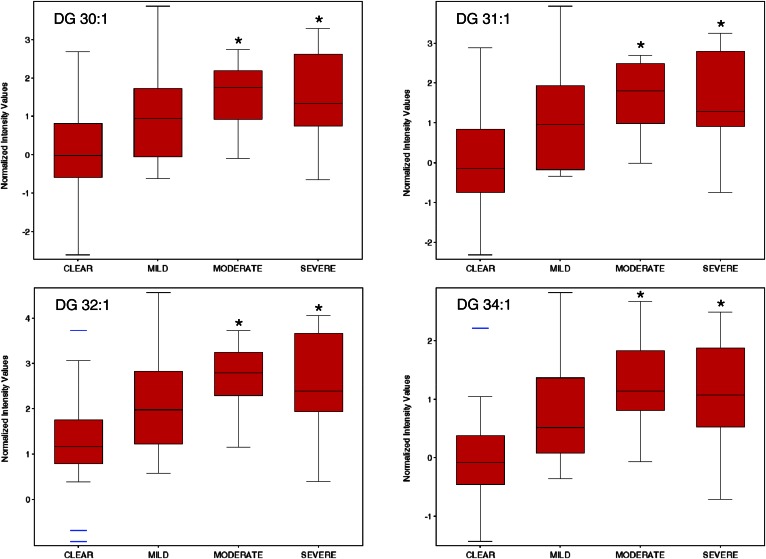
Having assessed both the goodness and predictive ability of the obtained PLS model, a new global model was built in that was comprised of both training and test sets in order to extract the desired sebum lipidome-activity relationship. For the obtained global model (Fig. 3A), a recalculated/experimental correlation coefficient of 0.88 was achieved. The PLS weights plot is reported in Fig. 3B. In general, PLS weights plot analysis allows a straightforward individuation of variables that are most correlated with the proposed activity. In our case, among the entities responsible for the differences in sebum lipidome profiles between the examined groups, the entities highlighted in green in Fig. 3B were those more positively correlated to the acne activity (red square). The pie charts in Fig. 4 illustrate the results of this first identification by showing the distribution of the assigned lipid class. The majority of differentiating lipid species were glycerolipids, followed by fatty acyls, sterols, and prenols. Wax esters and squalene-related compounds (triterpenes) accounted for a small percentage of the total discriminating lipids. The fatty acyl family also included FFAs that are normally present in sebum. Consistent with the literature, squalene and members of the FFA family were found expressed at higher levels in moderate acne (data not shown) (13, 29). Because glycerolipids were predominant among the differentiating lipids (Fig. 4A) and DGs were found to be the most significantly affected glycerolipids in acne (Fig. 4B), we focused our attention on this group of compounds. The identity of DGs found to be expressed at significantly different levels in moderate acne were tentatively assigned using the LIPID MAPS database based on mass and isotope pattern, and then confirmed according to diagnostic ions in the MS spectrum (20). The relative abundance of annotated DGs in clear and moderate acne groups are reported in Table 1. Sixteen out of the 17 annotated DGs were expressed at significantly higher levels in moderate acne (P < 0.05). On average, the levels of these DGs in moderate acne was double that in clear skin secretions (fold-change ≥2). DG assignments and expression in acne were further confirmed with the IDBrowser Identification search tool in the MPP package based on the METLIN Metabolomics database. Notably, consistent assignment of DG identities was achieved with METLIN and LIPID MAPS databases. To determine whether DG expression levels correlated with acne severity, we assessed the relative abundance of this lipid species with MPP statistical tools. Box plots reported in Fig. 5 illustrate how the relative abundance of DGs 30:1, 31:1, 32:1, and 34:1 varied across acne grades. These DGs were accounted among those lipids that were modified with a fold-change ≥2 in both moderate and severe acne (supplementary Fig. 2, supplementary Table 2). In particular, there was an apparent positive correlation between the relative abundance of these DGs species and the severity of acne. Moreover, we used box plots to show the variation in the relative abundance of these DG species within each grade of acne, with clear and mild grades associated with a large inter-individual variability. To further support the assignment of DG identities, respective MS spectra are reported in supplementary Fig. 3. The ESI-MS spectra showed the formation of [M+H]+, [M+NH4]+, and [M+H-H2O]+ ions, which are typically observed for DGs in the used analytical conditions.
Fig. 3.
A: PLS score plot (LV1 versus LV2) of the global model obtained from lipid species detected in the 17 unaffected (green) and 19 moderate acne subjects (red). B: PLS weights plot of the global model with acne (red square) most positively correlated entities highlighted in green.
Fig. 4.
Pie charts of the lipids identification results: distribution of identified lipid class (A) and distribution of identified lipids of the glycerolipids class (B).
TABLE 1.
Chemically identified DGs which show a significant difference in their relative abundance between sebum collected from clear and moderate acne subjects
| Retention Time ± SDa | m/z | Parts per Million ± SD | Ion Formula | Ion | Annotation | Clear ± SDb | Moderate ± SDb | FCc | d |
| 22.52 ± 0.09 | 530.4784 | 1.94 ± 1.1 | C31 H64 N O5 | [M+NH4]+ | DG 28:0 | 0.092 ± 0.099 | 0.212 ± 0.126 | 2.31 | 0.003 |
| 21.47 ± 0.08 | 528.4628 | 1.99 ± 0.99 | C31 H62 N O5 | [M+NH4]+ | DG 28:1 | 0.099 ± 0.112 | 0.226 ± 0.139 | 2.27 | 0.005 |
| 23.27 ± 0.07 | 509.4570 | 1.99 ± 1.6 | C32 H61 O4 | [M+H-H2O]+ | DG 29:0 | 0.434 ± 0.447 | 0.801 ± 0.368 | 1.85 | 0.011 |
| 22.31 ± 0.07 | 542.4784 | 1.92 ± 1.2 | C32 H64 N O5 | [M+NH4]+ | DG 29:1 | 0.248 ± 0.271 | 0.551 ± 0.287 | 2.22 | 0.003 |
| 24.07 ± 0.07 | 558.5097 | 1.97 ± 0.78 | C33 H68 N O5 | [M+NH4]+ | DG 30:0 | 0.711 ± 0.578 | 1.57 ± 0.771 | 2.20 | 0.001 |
| 23.14 ± 0.08 | 556.4941 | 1.96 ± 0.98 | C33 H66 N O5 | [M+NH4]+ | DG 30:1 | 0.928 ± 0.935 | 2.11 ± 1.06 | 2.28 | 0.001 |
| 24.71 ± 0.09 | 572.5254 | 1.87 ± 1.4 | C34 H70 N O5 | [M+NH4]+ | DG 31:0 | 0.651 ± 0.519 | 1.52 ± 0.778 | 2.33 | 0.000 |
| 23.90 ± 0.06 | 570.5097 | 1.99 ± 1.6 | C34 H68 N O5 | [M+NH4]+ | DG 31:1 | 1.45 ± 1.12 | 2.34±1.20 | 3.01 | 0.000 |
| 22.96 ± 0.06 | 568.4941 | 1.88 ± 1.6 | C34 H66 N O5 | [M+NH4]+ | DG 31:2 | 0.324 ± 0.341 | 0.643 ± 0.310 | 1.99 | 0.006 |
| 25.43 ± 0.08 | 586.5410 | 1.68 ± 1.2 | C35 H72 N O5 | [M+NH4]+ | DG 32:0 | 0.727 ± 0.477 | 1.51 ± 0.729 | 2.07 | 0.001 |
| 24.61 ± 0.07 | 584.5254 | 1.86 ± 1.6 | C35 H70 N O5 | [M+NH4]+ | DG 32:1 | 1.96 ± 1.77 | 4.42 ± 2.141 | 2.25 | 0.001 |
| 23.73 ± 0.07 | 582.5097 | 1.86 ± 1.8 | C35 H68 N O5 | [M+NH4]+ | DG 32:2 | 1.37 ± 1.32 | 2.56 ± 1.32 | 1.94 | 0.009 |
| 25.06 ± 0.08 | 598.5410 | 1.77 ± 1.9 | C36 H72 N O5 | [M+NH4]+ | DG 33:1 | 0.816 ± 0.703 | 1.71 ± 0.961 | 2.09 | 0.003 |
| 24.37 ± 0.07 | 596.5254 | 1.84 ± 2.1 | C36 H70 N O5 | [M+NH4]+ | DG 33:2 | 0.549 ± 0.584 | 1.16 ± 0.589 | 2.11 | 0.004 |
| 25.81 ± 0.06 | 612.5567 | 1.83 ± 1.2 | C37 H74 N O5 | [M+NH4]+ | DG 34:1 | 0.631 ± 0.506 | 1.58 ± 0.921 | 2.50 | 0.001 |
| 25.01 ± 0.08 | 610.5410 | 1.88 ± 0.99 | C37 H72 N O5 | [M+NH4]+ | DG 34:2 | 1.40 ± 1.28 | 2.20 ± 1.29 | 1.57 | 0.129 |
| 24.37 ± 0.09 | 608.5254 | 1.84 ± 0.99 | C37 H70 N O5 | [M+NH4]+ | DG 34:3 | 0.263 ± 0.262 | 0.566 ± 0.322 | 2.15 | 0.006 |
Retention times in minutes.
Average abundance relative to the ISTD.
Fold change (FC) of moderate acne versus clear skin conditions.
Calculated with impaired Student’s t-test.
Fig. 5.
Box plots of DGs differently regulated in acne sebum according to the severity grade. *P ≤ 0.05 indicates statistically significant differences of affected subjects toward clear participants calculated by one-way ANOVA followed by post hoc Tukey’s HSD test.
DISCUSSION
Acne is a multifaceted disorder, as demonstrated by the different types of lesions that develop and a fairly wide spectrum of severity. Multiple factors involved in acne pathogenesis include increased sebogenesis and occurrence of inflammatory processes. Indeed, incretion of proinflammatory mediators, namely interleukin-1, and sebaceous lipids are a common occurrence at the lesional skin surface (30). The acne grades of participants in the present study were classified as mild, moderate, and severe acne and were representative of the normally observed spectrum of acne manifestations. The level of perturbation in the sebum composition appeared to be consistent with the severity of acne as demonstrated by slight alterations found in mild grades versus more profound changes associated with severe manifestations. Due to its intermediate characteristics, moderate acne was found to be suitable for investigating sebum modifications associated with acne overall and was used to identify sebum lipids most affected in acne. This is the first study to investigate modifications of intact sebum lipids in healthy and acne subjects. Previous studies have centered on the arrangement of fatty acids in sebaceous lipids and the level of squalene expression, which is the most abundant in sebum. Many analytical approaches used to date have been used to assess fatty acids resulting from decomposition of esters of glycerol, cholesterol, and fatty alcohols (7). Thin-layer chromatography has been extensively used to assess the abundance of sebaceous lipids, such as fatty acids, TGs, waxes, cholesterol esters, and squalene, alone and in combination with other analytical processes (14, 31). Targeting the composition of fatty acids resulting from the transesterification of surface lipids has shown that the levels of exogenous linoleic acid are significantly lower in acne. In contrast, the endogenous sebaceous sebaleic acid was not found to be modified in acne (11). The available evidence supports an increased level of sebaceous fatty acids (i.e., sapienic acid) and squalene in acne sebum (29, 32). Nevertheless, derangement of fatty acid distribution has also been observed in the epidermal lipids of comedones (33). However, the available information related to sebum lipids is limited by small study sizes and laborious procedures required to investigate sebum clinically. Our approach, on the other hand, allowed for detection of the majority of lipid components in sebum.
Our results demonstrated that several classes of lipids are associated with acne development and severity. In particular, this is the first evidence that sebum sampled from acne patients has significantly higher levels of DGs.
Presence of DGs has been reported in human sebum (12, 13). To the best of our knowledge, association of DGs with acne has no previous evidence. In contrast, FFAs are recognized to be implicated in the acne symptoms. Indeed, it is generally assumed that the skin resident flora is involved in the elevation of the FFA portion due to the hydrolytic degradation of TGs (13). Upon hydrolysis, TGs release DGs along with FFAs. Based on this assumption, one can infer that DGs are by-products of TG degradation. Nevertheless, while Propionibacterium acnes colonization is accounted among the pathogenic factors of acne, occurrence of P. acnes is not necessarily associated with manifestation of acne. The reverse direction of the TG degradation is the TG biosynthesis catalyzed by DG acyltransferases that transfer one FFA moiety to the DG backbone. Thus, the hypothesis that DGs may also arise from incomplete TG synthesis can be explored in order to understand the role played by endogenous lipid biosynthetic pathways in the acne pathogenesis. The observation that the abundance of specific DG members is associated with acne severity indicates they are potential biomarkers of disease stage and prospectively of therapeutic response. Interestingly, outcomes of recent metabolomics studies have revealed DGs are associated with inflammatory processes and tissue insulin resistance, which are implicated in acne development (34–36). In conclusion, our findings present new perspectives in the search for lipid triggers of inflammation in acne.
Supplementary Material
Acknowledgments
The authors are grateful to Ms. Marisa Galante for the assistance in the collection and preparation of sebum samples, and to Dr. Alessia Mammone for the statistical description of group demographics.
Footnotes
Abbreviations:
- DG
- diacylglycerol
- d6CH
- deuterated cholesterol-2,2,3,4,4,6-d6
- d5TG 48:0
- glyceryl-d5-trihexadecanoate
- EtOH
- ethanol
- GEA
- Global European Acne
- GEAS
- Global European Acne severity
- HSD
- honestly significant difference
- iPrOH
- isopropyl alcohol
- ISTD
- internal standard
- LV1
- first latent variable
- LV2
- second latent variable
- Me2O
- acetone
- MeOH
- methanol
- MPP
- Mass Profiler Professional
- PLS
- partial least squares
- RP
- reversed-phase
- RR
- rapid-resolution
- SER
- sebum excretion rate
This study was supported by the Italian Ministry of Health Grant RF-2010-2316435.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Zouboulis C. C., Eady A., Philpott M., Goldsmith L. A., Orfanos C., Cunliffe W. C., and Rosenfield R.. 2005. What is the pathogenesis of acne? Exp. Dermatol. 14: 143–152. [DOI] [PubMed] [Google Scholar]
- 2.Zouboulis C. C., Jourdan E., and Picardo M.. 2014. Acne is an inflammatory disease and alterations of sebum composition initiate acne lesions. J. Eur. Acad. Dermatol. Venereol. 28: 527–532. [DOI] [PubMed] [Google Scholar]
- 3.Shi V. Y., Leo M., Hassoun L., Chahal D. S., Maibach H. I., and Sivamani R. K.. 2015. Role of sebaceous glands in inflammatory dermatoses. J. Am. Acad. Dermatol. 73: 856–863. [DOI] [PubMed] [Google Scholar]
- 4.Ottaviani M., Camera E., and Picardo M.. 2010. Lipid mediators in acne. Mediators Inflamm. 2010: 858176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Picardo M., Ottaviani M., Camera E., and Mastrofrancesco A.. 2009. Sebaceous gland lipids. Dermatoendocrinol. 1: 68–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jeremy A. H., Holland D. B., Roberts S. G., Thomson K. F., and Cunliffe W. J.. 2003. Inflammatory events are involved in acne lesion initiation. J. Invest. Dermatol. 121: 20–27. [DOI] [PubMed] [Google Scholar]
- 7.Akaza N., Akamatsu H., Numata S., Matsusue M., Mashima Y., Miyawaki M., Yamada S., Yagami A., Nakata S., and Matsunaga K.. 2014. Fatty acid compositions of triglycerides and free fatty acids in sebum depend on amount of triglycerides, and do not differ in presence or absence of acne vulgaris. J. Dermatol. 41: 1069–1076. [DOI] [PubMed] [Google Scholar]
- 8.Taylor M., Gonzalez M., and Porter R.. 2011. Pathways to inflammation: acne pathophysiology. Eur. J. Dermatol. 21: 323–333. [DOI] [PubMed] [Google Scholar]
- 9.Powell E. W., and Beveridge G. W.. 1970. Sebum excretion and sebum composition in adolescent men with and without acne vulgaris. Br. J. Dermatol. 82: 243–249. [DOI] [PubMed] [Google Scholar]
- 10.Cunliffe W. J. 1971. The relationship between surface lipid composition and acne vulgaris. Br. J. Dermatol. 85: 86–89. [DOI] [PubMed] [Google Scholar]
- 11.Morello A. M., Downing D. T., and Strauss J. S.. 1976. Octadecadienoic acids in the skin surface lipids of acne patients and normal subjects. J. Invest. Dermatol. 66: 319–323. [DOI] [PubMed] [Google Scholar]
- 12.Pochi P. E., Strauss J. S., and Downing D. T.. 1977. Skin surface lipid composition, acne, pubertal development, and urinary excretion of testosterone and 17-ketosteroids in children. J. Invest. Dermatol. 69: 485–489. [DOI] [PubMed] [Google Scholar]
- 13.Rebillo T., and Hawk J. L.. 1978. Skin surface glycerol levels in acne vulgaris. J. Invest. Dermatol. 70: 352–354. [DOI] [PubMed] [Google Scholar]
- 14.Nordstrom K. M., Labows J. N., McGinley K. J., and Leyden J. J.. 1986. Characterization of wax esters, triglycerides, and free fatty acids of follicular casts. J. Invest. Dermatol. 86: 700–705. [DOI] [PubMed] [Google Scholar]
- 15.Girod A., and Weyermann C.. 2014. Lipid composition of fingermark residue and donor classification using GC/MS. Forensic Sci. Int. 238: 68–82. [DOI] [PubMed] [Google Scholar]
- 16.Michael-Jubeli R., Bleton J., and Baillet-Guffroy A.. 2011. High-temperature gas chromatography-mass spectrometry for skin surface lipids profiling. J. Lipid Res. 52: 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michael-Jubeli R., Tfayli A., Bleton J., and Baillet-Guffroy A.. 2011. Chemometric approach for investigating the skin surface lipids (SSLs) composition: influence of geographical localization. Eur. J. Dermatol. 21: 63–71. [DOI] [PubMed] [Google Scholar]
- 18.Rolim A. E., Henrique-Araújo R., Ferraz E. G., de Araújo Alves Dultra F. K., and Fernandez L. G.. 2015. Lipidomics in the study of lipid metabolism: current perspectives in the omic sciences. Gene. 554: 131–139. [DOI] [PubMed] [Google Scholar]
- 19.Robosky L. C., Wade K., Woolson D., Baker J. D., Manning M. L., Gage D. A., and Reily M. D.. 2008. Quantitative evaluation of sebum lipid components with nuclear magnetic resonance. J. Lipid Res. 49: 686–692. [DOI] [PubMed] [Google Scholar]
- 20.Camera E., Ludovici M., Galante M., Sinagra J. L., and Picardo M.. 2010. Comprehensive analysis of the major lipid classes in sebum by rapid resolution high-performance liquid chromatography and electrospray mass spectrometry. J. Lipid Res. 51: 3377–3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dréno B., Poli F., Pawin H., Beylot C., Faure M., Chivot M., Auffret N., Moyse D., Ballanger F., and Revuz J.. 2011. Development and evaluation of a Global Acne Severity Scale (GEA Scale) suitable for France and Europe. J. Eur. Acad. Dermatol. Venereol. 25: 43–48. [DOI] [PubMed] [Google Scholar]
- 22.Baroni M., Costantino G., Cruciani G., Riganelli D., Valigi R., and Clementi S.. 1993. Generating optimal linear PLS estimations (GOLPE): an advanced chemometric tool for handling 3D-QSAR problems. Quantitative Structure-Activity Relationships. 12: 9–20. [Google Scholar]
- 23.Wold S., and Sjostrom M.. 2001. PLS-regression: a basic tool of chemometrics. Chemom. Intell. Lab. Syst. 58: 109–130. [Google Scholar]
- 24.Eriksson L., Johansson E., Kettaneh-Wold N., and Wold S.. 1999. Scaling. In Introduction to Multi- and Megavariate Data Analysis using Projection Methods (PCA & PLS). Umetrics, Sweden. 213–225. [Google Scholar]
- 25.Sud M., Fahy E., Cotter D., Brown A., Dennis E. A., Glass C. K., Merrill A. H. Jr., Murphy R. C., Raetz C. R., Russell D. W., et al. . 2007. LMSD: LIPID MAPS structure database. Nucleic Acids Res. 35: D527–D532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fahy E., Subramaniam S., Brown H. A., Glass C. K., Merrill A. H. Jr., Murphy R. C., Raetz C. R., Russell D. W., Seyama Y., Shaw W., et al. . 2005. A comprehensive classification system for lipids. J. Lipid Res. 46: 839–861. [DOI] [PubMed] [Google Scholar]
- 27.Fahy E., Subramaniam S., Murphy R. C., Nishijima M., Raetz C. R., Shimizu T., Spener F., van Meer G., Wakelam M. J., and Dennis E. A.. 2009. Update of the LIPID MAPS comprehensive classification system for lipids. J. Lipid Res. 50: S9–S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhate K., and Williams H. C.. 2013. Epidemiology of acne vulgaris. Br. J. Dermatol. 168: 474–485. [DOI] [PubMed] [Google Scholar]
- 29.Pappas A., Johnsen S., Liu J. C., and Eisinger M.. 2009. Sebum analysis of individuals with and without acne. Dermatoendocrinol. 1: 157–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dreno B., Gollnick H. P., Kang S., Thiboutot D., Bettoli V., Torres V., and Leyden J.; Global Alliance to Improve Outcomes in Acne. 2015. Understanding innate immunity and inflammation in acne: implications for management. J. Eur. Acad. Dermatol. Venereol. 29: 3–11. [DOI] [PubMed] [Google Scholar]
- 31.Warren R., Wertz P. W., Kirkbride T., Brunner M., and Gross M. C.. 2011. Comparative analysis of skin surface lipids of the labia majora, inner thigh, and forearm. Skin Pharmacol. Physiol. 24: 294–299. [DOI] [PubMed] [Google Scholar]
- 32.Smith R. N., Braue A., Varigos G. A., and Mann N. J.. 2008. The effect of a low glycemic load diet on acne vulgaris and the fatty acid composition of skin surface triglycerides. J. Dermatol. Sci. 50: 41–52. [DOI] [PubMed] [Google Scholar]
- 33.Perisho K., Wertz P. W., Madison K. C., Stewart M. E., and Downing D. T.. 1988. Fatty acids of acylceramides from comedones and from the skin surface of acne patients and control subjects. J. Invest. Dermatol. 90: 350–353. [DOI] [PubMed] [Google Scholar]
- 34.Erion D. M., and Shulman G. I.. 2010. Diacylglycerol-mediated insulin resistance. Nat. Med. 16: 400–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grkovich A., and Dennis E. A.. 2009. Phosphatidic acid phosphohydrolase in the regulation of inflammatory signaling. Adv. Enzyme Regul. 49: 114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Melnik B. C. 2015. Linking diet to acne metabolomics, inflammation, and comedogenesis: an update. Clin. Cosmet. Investig. Dermatol. 8: 371–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.