Preface
Endothelial cells lining blood vessel capillaries are not just passive conduits for delivering blood. Tissue-specific endothelium establish specialized vascular niches that deploy specific sets of growth factors, known as angiocrine factors, which actively participate in inducing, specifying, patterning, and guiding organ regeneration and maintaining homeostasis and metabolism. Angiocrine factors upregulated in response to injury orchestrates self-renewal and differentiation of tissue-specific repopulating resident stem and progenitor cells into functional organs. Uncovering the precise mechanisms whereby physiological-levels of angiocrine factors are spatially and temporally produced, and distributed by organotypic endothelium to repopulating cells, will lay the foundation for driving organ repair without scarring.
Introduction
The microvascular circulation comprises a vast network of capillary endothelial cells (ECs) that connects the arteries to veins. These vascular beds, which are distinct from lymphatic vessels, were perceived as passive conduits with a responsibility for delivering oxygen and nutrients, modulating the coagulation of blood, regulating the transportation of inflammatory cells and serving as gatekeepers of cellular metabolism1, 2. However, these cells also perform other necessary physiological tasks: sustaining the homeostasis of resident stem cells and guiding the regeneration and repair of adult organs without provoking fibrosis.
This new paradigm emerged from microanatomical findings that epithelial, hematopoietic, mesenchymal and neuronal cells, along with their corresponding repopulating stem and progenitor cells, reside in close physical proximity to capillary ECs. Genetic and biochemical studies have shown that ECs serve as a fertile, instructive niche that plays key roles in sustaining homeostasis, metabolism and directing organ regeneration in a "perfusion-independent" manner. Tissue-specific ECs mastermind these complex tasks by supplying the repopulating cells with stimulatory and inhibitory growth factors, morphogens, extracellular matrix and chemokines. These EC-derived paracrine factors are collectively defined as angiocrine factors3,4 (Box 1).
Box 1. Physiology of the angiocrine factors.
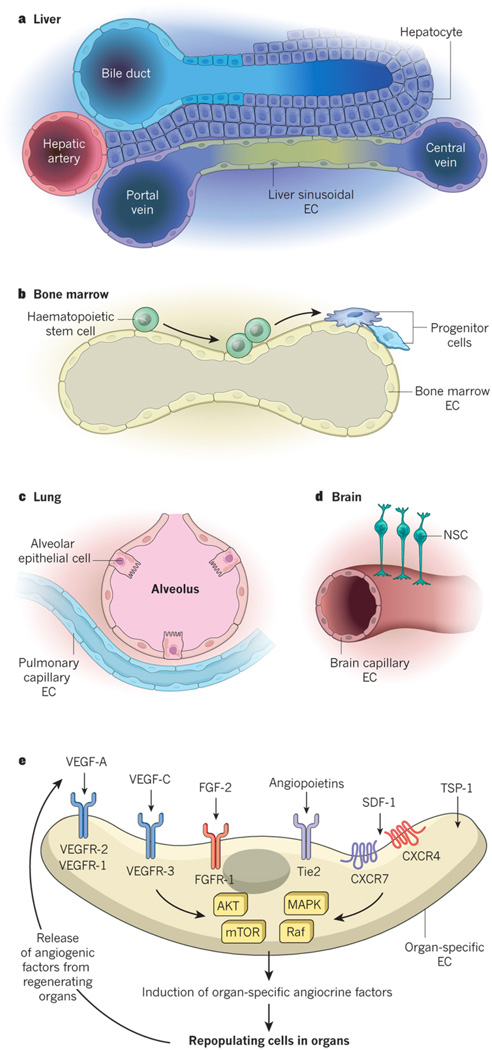
The paracrine factors produced by ECs that maintain organ homeostasis, balance the self-renewal and differentiation of stem cells and orchestrate organ regeneration and tumour growth are known as angiocrine factors. The term 'angiocrine' was created to emphasize the biological significance of the instructive factors produced by the ECs that influence the homeostasis of healthy and malignant tissues3. Angiocrine factors comprise secreted and membrane-bound inhibitory and stimulatory growth factors, trophogens, chemokines, cytokines, extracellular matrix components, exosomes and other cellular products that are supplied by tissue-specific ECs to help regulate homeostatic and regenerative processes in a paracrine or juxtacrine manner. These factors also play a part in adaptive healing and fibrotic remodelling. Subsets of angiocrine factors can act as morphogens to determine the shape, architecture, size and patterning of regenerating organs. The angiocrine profile of each tissue-specific bed of ECs is different and reflects the diversity of cell types found adjacent to ECs in organs (Fig. 1a–e). Although subsets of angiocrine factors are produced constitutively, some angiogenic factors can modulate the production of other tissue-specific angiocrine factors. For example, VEGF-A induces the expression of defined angiocrine factors through interaction with VEGFR-1 and VEGFR-2 (Fig. 1e). Similarly, FGF-2 (through the activation of FGFR-1) and the angiopoietins (through their interaction with the receptor Tie2) drive the expression of unique clusters of angiocrine factors. TSP-1 functions in a complex manner and can act as an inhibitory angiogenic factor as well as directly influence the differentiation of stem and progenitor cells. The molecular programmes that govern the production of context-dependent angiocrine factors from organ-specific ECs remain undefined.
The tissue-specific instructive functions of ECs have been demonstrated in studies showing that the deletion of angiocrine factors in adult ECs disrupts stem-cell homeostasis and impairs organ repair without compromising blood supply. Notably, intravenous transplantation and engraftment of tissue-specific ECs following injury augment organ reconstitution and function without instigating maladaptive fibrosis. On the basis of these observations, organotypic capillary ECs are now recognized as specialized niche cells that, through balanced physiological expression of angiocrine factors, maintain stem cells' capacity for quiescence and self-renewal. Spatially and temporally coordinated production of angiocrine factors after organ injury initiates and completes organ regeneration. This transformative model has opened a fresh chapter in translational vascular medicine. It has also raised the possibility that the inherent pro-regenerative potential of tissue-specific endothelium could be used therapeutically to orchestrate fibrosis-free healing and to restore homeostasis in tissues.
Although the angiocrine signals that guide the formation of the liver5 and pancreas6 in the fetus have been defined, the contribution of angiocrine signalling to the modulation of homeostasis and regeneration in adult organs has not been well studied until now. In this Review, we describe the instructive and inductive contributions of adult tissue-specific ECs to the homeostatic and regenerative functions of repopulating stem and progenitor cells.
Instructive interactions of capillary ECs
The adult human body contains 10 trillion–60 trillion ECs that cover a vast surface area7. Tightly intertwined monolayers of ECs form the lumen of the blood circulatory system, which consists of large arteries, veins and extensively branched capillaries. Lymphatic vessels run parallel to capillaries and exist as an independent and open circulatory system. The surface area of capillaries represents more than 95% of the total circulatory surface area and arborizes into almost every cellular component of organs.
Tissue-specific stem and progenitor cells are strategically positioned in close proximity to homotypic capillary ECs (Fig. 1a–d). This intimate cellular interaction facilitates the delivery of membrane-bound and soluble angiocrine factors from specialized ECs to the recipient cells, which are located on the basolateral surface of blood vessels. Moreover, the luminal surface of ECs can serve as a signalling platform for stem and immune cells that navigate through the circulation. Tissue-resident parenchymal and stem cells regulate the activation state and response of ECs to regenerative stimuli through the production of angiogenic factors such as vascular endothelial growth factor (VEGF)-A, fibroblast growth factor (FGF)-2, stromal-cell-derived factor (SDF-1; also known as CXCL12), angiopoietins and thrombospondin-1 (TSP-1) (Fig. 1e). Thus, the capillary network — without the influence of pericytes and mesenchymal cells — provides an adaptive platform that has the functional plasticity to integrate and relay these intravascular and extravascular cues to both resting and regenerating organs.
Figure 1. Cross-talk of specialized capillary ECs with organ-specific parenchymal cells and their corresponding stem cells modulate homeostatic and regenerative processes.
Each organ is arborized by an extensive network of specialized capillaries (A). Within each organ the capillaries assume unique structural, phenotypic, functional and angiocrine attributes. In the liver, hepatocytes are juxtaposed to fenestrated liver sinusoidal endothelial cells (LSECs) marked by the unique phenotype CD34-VEGFR1+VEGFR2+VEGFR3+VEcad+CXCR7+CD31low/-FactorVIII+ (B). In the hematopoietic organs such as bone marrow, the stem and progenitor cells are in direct cellular contact with arterial and fenestrated specialized sinusoidal vessels demarcated by VEGFR3+VEGFR2+VEcad+CD31+ ECs (C). In the lungs, the alveolar epithelial cells and their progenitors reside in the vicinity of continuous nonfenestrated pulmonary capillary endothelial cells (PCECs) defined by the signature VEGFR2+FGFR1+VEcad+CD31+ CD45 ECs (D). In the brain the majority of capillaries compose of tightly connected vessels with a common core phenotype of VEGFR2+VEcad+CD133+ thrombomodulin−/low ECs (E). At steady state conditions or during an angiogenic state upregulation of angiogenic factors, including VEGF-A through activation of its cognate tyrosine kinase receptors VEGFR2 and VEGFR1, FGF2 through FGFR1, angiopoietins through Tie2 not only modulate angiogenic processes, but also trigger or resolve the expression of tissue-specific angiocrine factors. Thrombospondins not only temper angiogenic response but also directly influence the proliferation and differentiation of the pancreatic islet and lung epithelial cells. Recruitment of pAkt-mTOR and MAPK/Raf signaling most likely play a role in choreographing the expression of the organotypic angiocrine factors (E).
Angiocrine-mediated self-renewal and differentiation
The formation of new blood vessels through angiogenesis is crucial to meet the metabolic demands of organs1, 2. Accumulating evidence indicates that ECs regulate organ homeostasis and repair through the production of angiocrine factors in an angiogenesis-independent manner (Box 1). The Greek philosopher and scientist Aristotle, who is widely considered to be the founder of classical biology, proposed that blood vessels direct the configuration of organs8. On pathophysiological stress (exposure to ionizing radiation, chemical injury or hypoxic conditions, for example) or loss of tissue mass, defined angiocrine factors emanate from activated ECs (Table 1). The activated ECs relay inflammatory and injury-induced angiocrine signals to quiescent tissue-specific stem cells, which drives regeneration and enforces developmental set points to re-establish homeostatic conditions. Microvascular ECs therefore fulfil the criteria for professional niche cells that choreograph tissue regeneration by cradling and nurturing stem cells with physiological levels and proper stoichiometry of angiocrine factors. The contribution of the endothelial niche to mediating stem-cell homeostasis and function has been studied in depth in neural stem cells (NSCs), spermatogonial stem cells and haematopoietic stem and progenitor cells (HSPCs).
Table 1.
Heterogeneity of the angiocrine factors at steady state and during regeneration
| EC origin | Repopulating cells | Regenerative Model EC Status |
Angiocrine Factor(s) | EC-specific gene knock out Emergent Phenotype |
|---|---|---|---|---|
| Bone Marrow sinusoidal ECs |
Hematopoietic stem and progenitor cells (HSPCs) |
Steady State Chemotherapy, Irradiation Angiogenic ECs |
Kit-ligand, SDF1, IGFBP2 DKK1, BMP2, BMP4, Dhh AKT-activated Kit-ligand, Jagged1, Jagged2, Dhh, FGF2, Angiopoietin2, EGF, Pleiotrophin AKT-MAPK activated IL6, G-CSF, GM-CSF, Ang2, Jagged1 and Jagged2 |
Kit-ligand61; SDF162: Stem cell depletion Jagged159: Impaired hematopoietic recovery and stem cell depletion VEGFR247: Angiocrine dysregulation, Hematopoietic failure VE-cadherin inhibition57: Hematopoietic recovery impairment |
| Testicular capillaries |
GPR125+ germline stem cells |
Chemical-induced sterilization |
GDNF, Fgf2 | |
| Brain Capillaries | Neural stem cells (NSCs) Transient amplifying cells Neuroblasts |
Steady state Regeneration Angiogenesis |
Jagged1, EphrinB2, NT-3 PEDF, Betacellulin, VEGF-C Nitric Oxide, BDNF |
Jagged119, Ephrinb219: Exhaustion of neural stem cells NT-320: Decrease in NSC cycling |
| Liver sinusoids Liver central vein ECs |
Hepatocytes and stellate cells Axin2+Tbx3+ liver stem cells |
70% partial hepatectomy Bile duct ligation Acute and chronic CCl4 Homeostatic conditions |
Id1-Wnt2, Id1-HGF, Angiopoietin-2 Anti-Fibrosis: Apelin, Follistatin- like-1, Noggin Pro-fibrosis: TGF-β, BMP4 Wnt2 and Wnt9b |
Tie2, Angiopoietin-285: Shift in liver recovery set-point Id181: Impaired liver regeneration Cxcr486: Decrease in liver fibrotic healing Cxcr786: Increase in pro-fibrotic healing Wntless (Wls)82: Defective liver regeneration and self-renewal |
| Pulmonary capillaries |
Lung alveolar epithelial cells Type 2 AECs Epithelial progenitors cells |
Left lung Pneumonectomy Bleomycin |
MMP14-mediated EGF-Receptor ligand release BMP4-NFAT/c- Thrombospondin1 |
MMP1490: Abrogated neo-alveologenesis but intact capillary vasculature Vegfr288, Fgfr188 Impaired alveolar regeneration Thrombospondin193: Impaired epithelial regeneration |
| Pancreatic capillaries |
Islet cells Pdx1+ islet progenitors |
Streptozocin Pluripotent stem cell derivatives |
BMP4, BMP2, Thrombospondin1 EGFL7 |
EGFL7100: Delayed Islet cell differentiation |
| White fat vessels | Adipose stem cells | Steady state | IGF1, IGFBP, TNF, IL1 | |
| Striated muscle microvessels |
Pax7+Myf5+ Satellite cells |
Steady state and post-myotoxin |
IGF, HGF. FGF, PDGF-BB | |
| Cardiac myocytes capillaries |
Myocytes | Coronary artery ligation | Neuregulin-1, Endothelin-1, Nitric oxide |
Neuregulin-1106: Impaired myocyte regeneration, Loss of cardiac protection |
| Osteogenesis Type-H and L vessels |
Osteoblasts | Steady state | Noggin, BMPs, Jagged-1 |
Noggin74: Impaired osteogenesis |
Neural stem cells
The adult brain contains two regions in which NSCs undergo neurogenesis: the ventricular subventricular zone (V-SVZ) and the subgranular zone (SGZ). In the V-SVZ, type B1 quiescent and activated NSCs give rise to type C transit amplifying cells and type A mature neuroblastic cell progenies, which are positioned in the proximity of capillary ECs9, 10, 11, 12 (Fig. 2a). Similarly, in the SGZ, which is located in the dentate gyrus of the hippocampus, NSCs and their progenies reside near capillaries13. Brain capillaries are lined with ECs that are positive for VEGF receptor (VEGFR)-2 and vascular endothelial (VE)-cadherin, positive or negative for the CD133 antigen, negative for or express only low levels of thrombomodulin, and that display zones of variable permeability4, 10, 12 (Figs 1d, 2a). Subsets of V-SVZ and SGZ blood vessels have a specialized planar morphology in which NSCs extend their endfeet to contact ECs. This close proximity supports the possibility that angiocrine factors regulate neurogenesis.
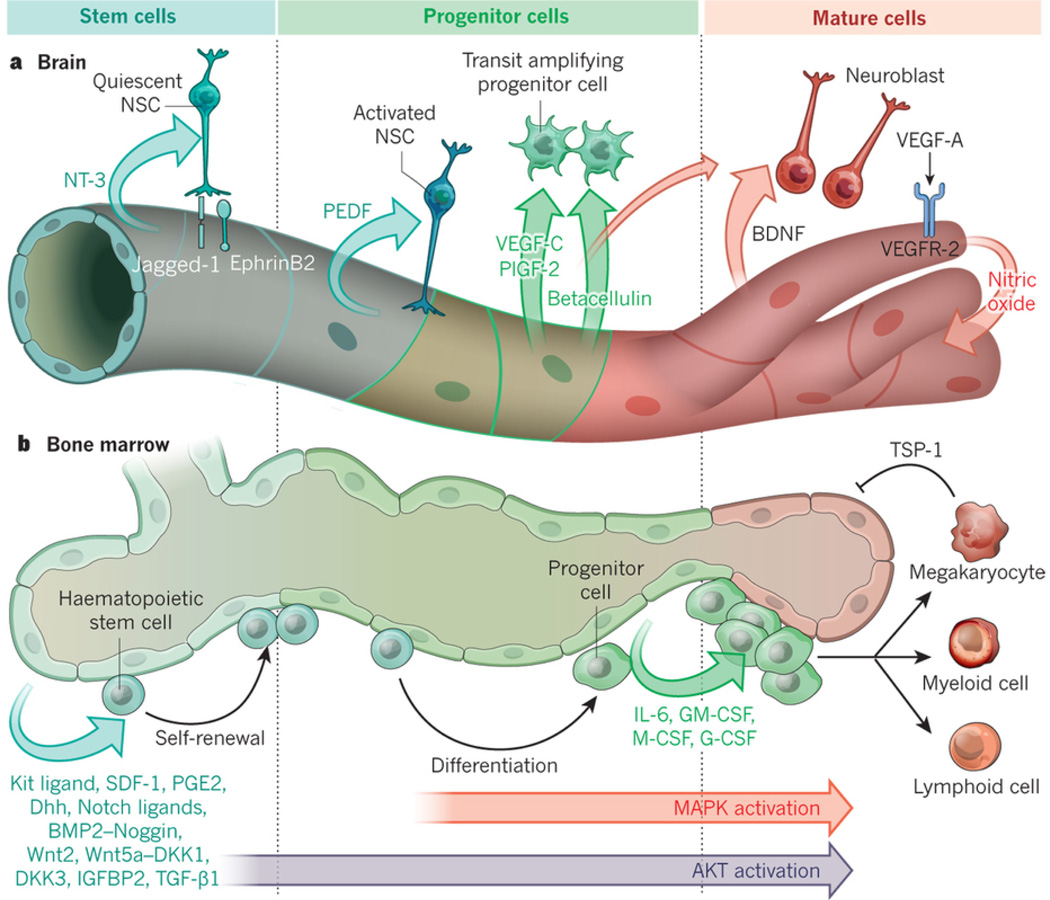
Figure 2. Tissue-specific ECs by supplying membrane-bound and secreted angiocrine factors orchestrate self-renewal and regeneration of stem and progenitors cells.
(A) Brain capillary ECs are strategically localized to the neural stem and progenitors cells. These ECs elaborate specific angiocrine factors, including membrane-bound Jagged-1 and EphrinB2 and NT-3 that establish the quiescence and survival of the Type-B1 quiescent NSCs (qNSCs) within the V-SVZ. ECs also deploy angiocrine factors, including PEDF, VEGF-C (mostly in SGZ) and PLGF2 that foster proliferation of Type-B1 activated NSCs (aNSCs). Angiocrine secretion of Betacellulin stimulates the amplification of Type-C progenitors (TAC). During angiogenesis, upregulation of VEGF-A through activation of VEGFR2 and induction of NO upregulates BDNF, which in conjunction with Betacellulin encourage the differentiation into neuroblast and mature neurons, leading to completion of neurogenesis.
(B) Bone marrow arterial and sinusoidal ECs produce angiocrine factors that support the maintenance and regeneration of hematopoietic stem and progenitor cells (HSPCs) following myeloablative insult. At steady state conditions low levels of AKT activation in ECs stimulate production of Kit-ligand, Cxcl12, Notch-ligands, IGFBP2, Wnts, BMP2, Dhh and BMP4, which maintain and promote the self-renewal of hematopoietic stem cells. After myeloablative stress, inflicted by chemotherapy or irradiation, co-activation of AKT and MAPK initiates expression of progenitor active angiocrine factors, including IL6, GM-CSF, G-CSF, M-CSF, Metalloproteinases, chemokines and other factors forcing balanced differentiation of stem cells into lineage-committed progenitors. EC-derived Notch-ligands, (i.e. Jagged-1) prevent exhaustion of HSPCs. Induction of thrombospondin1 (TSP1) expression by maturing megakaryocytes puts a brake on ongoing hemangiogenesis finalizing the regeneration process.
In vitro studies of neuronal cells that were co-cultured with heterotypic-derived ECs support a model in which ECs regulate NSC homeostasis and differentiation. Primary human umbilical vein ECs have been shown to produce brain-derived nerve growth factor (BDNF), which fosters the expansion of neuroblasts14. Bovine pulmonary artery ECs and polyoma-middle-T-immortalized mouse brain capillary ECs, but not smooth-muscle cells, trigger Notch signalling by secreting soluble factors that increase the self-renewal of NSCs and drive neurogenesis9. Follow-up studies showed that pigment epithelium-derived factor (PEDF) was one of the secreted angiocrine factors that stimulates Notch-dependent self-renewing symmetric divisions of NSCs15.
Subsequent in vivo experiments demonstrated that angiocrine factors derived from brain ECs regulate the homeostasis and regeneration of NSCs both through direct cellular contact and in a paracrine manner13, 16, 17, 18. Under steady-state conditions, angiocrine expression of the membrane-bound proteins EphrinB2 and Jagged-1 (refs 19, 20) sustains the dormancy of quiescent NSCs. Direct contact of EC-derived EphrinB2 and Jagged-1 with the endfeet of these cells suppresses their entry into the cell cycle and keeps them in an undifferentiated state. Moreover, neurotrophin-3 (NT-3), which is selectively produced by ECs in the brain and choroid plexus, maintains NSC quiescence, in part, through the induction of endothelial nitric-oxide synthase and the production of nitric oxide21, 22. Although NSCs could also supply endothelial nitric-oxide synthase, the angiocrine release of NT-3 in the V-SVZ and cerebrospinal fluid dictates nitric oxide production that sustains stem-cell quiescence. Conditional deletion of NT-3 in adult mouse brain ECs depletes NT-3 in both cerebrospinal fluid and the V-SVZ, which leads to an increase in dividing activated NSCs that express glial fibrillary acidic protein (GFAP) and accelerates the exhaustion of the NSC pool. Thus, angiocrine factors actively enforce the quiescence that is crucial for the long-term maintenance of the NSC population.
During regenerative processes, irrigation of the V-SVZ by soluble angiocrine factors such as BDNF14, PEDF23, betacellulin24 and placental growth factor-2 (PlGF-2)25, and of the SGZ by VEGF-C26, 27, orchestrates proliferation and differentiation of both quiescent and activated NSCs into transit amplifying cells and neuroblasts. Notably, graded angiocrine deposition of SDF-1 (ref. 28) and BDNF29 by blood vessels that run along the rostral migratory stream in the mouse brain guides the proliferation of transit amplifying cells and their migration to the olfactory bulb30. Therefore, brain capillary ECs not only supply the V-SVZ and SGZ with region-specific regenerative and path-finding cues, but also secrete angiocrine factors into cerebrospinal fluid to potentially modulate neuronal homeostasis throughout the brain.
Crosstalk between neuronal cells and angiogenic ECs allows the endothelial niche to adapt to regenerative neurogenesis (Fig. 1e). During vascular sprouting, cross-activation of ECs by neuronal-derived angiogenic factors regulates the differential production of angiocrine factors (Fig. 2a). After hypoxic injury, upregulation of VEGF-A through the activation of VEGFR-2 enhances the production of nitric oxide, which induces BDNF in brain capillary ECs to drive the expansion and maturation of transit amplifying cells16. Growth differentiation factor (GDF)-11 also enhances neurogenesis by remodelling the blood vessels31. Thus, endothelial niche cells in the brain possess a remarkable angiocrine plasticity that can adapt to the physiological demands of NSCs to initiate, execute and finalize neurogenic programmes.
Spermatogonial stem cells
Undifferentiated type A spermatogonial stem cells from mice reside in the vicinity of interstitial capillaries within the seminiferous tubules of the testes32. After perturbation of the testicular microenvironment, transplanted donor-derived spermatogonial stem cells localize to zones that are enriched in capillaries. In vitro studies have shown that spermatogonial stem cells that express the G-protein-coupled receptor GPR125 can directly convert to multipotent progenitor cells. Incubation of such spermatogonial stem cells with vascular-like stromal cells that carry the CD34 antigen is essential for the conversion of spermatogonial stem cells to pluripotent stem cells33, 34, 35, and indicates that angiocrine factors play an important part in regulating the maintenance and self-renewal of spermatogonial stem cells. Indeed, transcriptional analysis of testicular endothelium suggests that ECs could be a rich source of glial-cell-line-derived neurotrophic factor (GDNF)4. Further analysis of the phenotypic and functional properties of testicular ECs is necessary to determine the degree to which ECs influence spermatogonial stem-cell homeostasis by deploying angiocrine factors and depositing peritubular extracellular matrix components.
Hematopoietic stem cells
The first evidence that ECs establish an instructive niche for haematopoietic cells (Fig. 2b) was the demonstration that homotypic human bone-marrow-derived ECs expand human umbilical cord blood-derived CD34+ cells ex vivo36, 37. Furthermore, heterotypic primary ECs isolated from brain, heart and fetal tissues have since been shown to promote the proliferation of mouse38, 39, human40, 41 and non-human primate HSPCs42. However, these co-culture studies were performed in media supplemented by serum that contained supraphysiological doses of growth factors and under ambient oxygen tension, which masked the full potential of ECs to regulate the function of the cells.
The development of techniques for serum-free and xenobiotic-free culture of primary human or mouse homotypic EC monolayers (Box 2) has facilitated the identification of angiocrine factors that support the self-renewal and differentiation of HSPCs in such co-culture studies43, 44. Co-culture studies have also been used to demonstrate that bone-marrow sinusoidal ECs that are positive for VEGFR-3, VEGFR-2, VE-cadherin and CD31 stimulate the self-renewal of HSPCs by expressing soluble and membrane-bound angiocrine factors45, 46, 47, including bone morphogenic protein (BMP)2 and BMP4, insulin growth factor binding protein (IGFBP)2, SDF-1, Desert hedgehog (Dhh) protein, Notch ligands, Wingless-type MMTV integration site (Wnt)5a, and Kit ligand (Fig. 2b). Bone-marrow sinusoidal ECs also drive the lineage-specific differentiation of HSPCs by producing granulocyte macrophage colony-stimulating factor (GM-CSF), interleukin (IL)-6, IL-8, granulocyte colony-stimulating factor (G-CSF), IL-1, tumour necrosis factor (TNF), chemokines and metalloproteinases45. Notably, ECs that are transitioning through various activation states also produce inhibitory factors, such as transforming growth factor (TGF)-β1 (ref. 48), dickkopf-related protein (DKK)1 and DKK3, which block WNT signalling, and Noggin, which interferes with BMP signalling45 (Fig. 2b, Table 1). Thus, ECs express inhibitory and stimulatory angiocrine factors that regulate the quiescence and proliferation of HSPCs.
Box 2. In vitro endothelial niche platform.
To propagate pure populations of organ-specific capillary ECs and to avoid their contamination with lymphatic ECs, monoclonal antibodies to EC-specific surface markers are infused intravenously into mice. The animals are killed after 10 minutes to avoid leakage of antibodies into the lymphatic vessels. Next, the intravitally labelled ECs are isolated through enzymatic digestion of tissues and subsequent flow sorting to ensure the removal of pericytes and smooth-muscle cells4, 43. Purified ECs are then plated with angiogenic factors in the absence of pituitary extracts and serum to avoid the loss of tissue-specific signatures and to block the transition from endothelial to mesenchymal cells. However, these conditions can support the expansion of angiocrine-competent ECs only in the short term. To remove this hurdle, purified ECs can be transduced with lentiviral vectors that express myristoylated AKT or the non-oncogenic adenoviral gene E4ORF1 (ref. 43). E4ORF1 sustains the survival of ECs through specific activation of the AKT–mTOR pathway. ECs that express E4ORF1 are not transformed and behave like primary ECs that ultimately undergo replicative senescence. This approach enables their long-term cultivation, with high viability in serum-free and xenobiotic-free conditions, which establishes an ex vivo platform for endothelial niches that recapitulates organotypic niches4. Monolayers of E4ORF1+ ECs sustain their organotypic pro-stem-cell functions, making them superior to ECs that have been immortalized by simian virus 40 large T antigen or polyomavirus middle T antigen, which fail to expand stem cells45. Co-culture studies using E4ORF1+ ECs have helped to identify angiocrine pathways that foster the expansion and proliferation of parenchymal and stem cells. So far, E4ORF1+ ECs have not demonstrated any malignant potential and could be adapted to comply with regulatory clinical guidelines. Thus, these engineered ECs provide an ideal platform for interrogating the angiocrine function of ECs in models of organ regeneration and for translating the pro-regenerative therapeutic potential of these cells to the clinical setting.
ECs cultured under serum-free conditions were shown to supply angiocrine factors at physiological levels that increase the self-renewal of repopulating authentic mouse haematopoietic stem cells by 150-fold46 and of human cord blood severe combined immunodeficiency repopulating cells by 8-fold49. Direct contact between haematopoietic cells and ECs is essential for the self-renewal and differentiation of HSPCs45, 46, 47. Compared with mesenchymal cells, ECs are more efficient at expanding umbilical cord blood-derived HSPCs50. Other angiocrine factors, such as prostaglandin E2 (PGE2) (refs 51, 52), pleiotrophin53 and epidermal growth factor (EGF)54, drive haematopoietic reconstitution, which establishes ECs as a physiological repository of HSPC-supportive factors.
The first in vivo evidence to support the role of the endothelial niche in haematopoiesis came from a study of mice that are unable to produce soluble Kit ligand, an essential regulator of haematopoietic stem-cell biology55. It demonstrated that compartmentalized — yet interactive — stromal and endothelial niche cells regulate the regeneration of HSPCs. In response to physiological stress, the activation of matrix metalloproteinase (MMP)-9 leads to the release of soluble Kit ligand from cells in the niche, which stimulates the regeneration and proper transportation of HSPCs. Follow-up studies showed that phenotypically marked stem cells reside in close proximity to the endothelial niche56. Further evidence indicated that haematopoietic regeneration and thrombopoiesis after chemotherapy or irradiation is impaired by the conditional deletion of VEGFR-2 in ECs of adult mice47 and by the targeting of VE-cadherin to disrupt reconstitution of the endothelial niche57, 58. Therefore, the endothelial niche is essential not only for sustaining the self-renewal of haematopoietic stem cells, but also for multi-lineage reconstitution (Fig. 2b, Table 1).
Haematopoietic regeneration is orchestrated by the differential production of angiocrine factors that are induced by signalling pathways activated within ECs45(Fig. 2b). After myeloablative stress, angiogenic factors such as VEGF-A, VEGF-C, FGF-2 and the angiopoietins upregulate other angiocrine factors, including Jagged-1, through activation of AKT (also known as protein kinase B). Conditional deletion of Jagged-1 in ECs impairs haematopoietic recovery59, which suggests that Notch activation prevents the exhaustion of HSPCs. During the angiogenic phase of regeneration, AKT phosphorylation is accompanied by the activation of p42/p44 mitogen-activated protein kinase (MAPK). This triggers the secretion of G-CSF, macrophage colony-stimulating factor (M-CSF), GM-CSF and IL-6 to expand populations of myeloid, megakaryocytic and lymphoid progenitor cells and aid haematopoietic reconstitution45 (Fig. 2b). In turn, maturing haematopoietic cells produce inhibitory angiogenic factors that prevent excessive sprouting of regenerating sinusoidal vessels. For example, mature megakaryocytes produce TSP-1, which decelerates angiogenesis and shuts off the production of activating angiocrine factors to restore homeostasis4, 60. Notably, AKT-activated bone marrow ECs, which emulate some of the functions of in vivo angiogenic ECs, expand long-term repopulating haematopoietic stem cells under serum-free culture conditions, whereas bone-marrow-derived stromal cells direct stem-cell attrition44. Moreover, protection of the haematopoietic microenvironment through transplantation of AKT-activated bone marrow ECs, but not mesenchymal ones, accelerates haematopoietic recovery after lethal irradiation44. Therefore, the equilibrium between AKT and MAPK activation regulates multi-lineage haematopoietic recovery.
The contribution of the endothelial niche to steady-state haematopoiesis was unravelled by studies in which selective deletion in ECs of SDF-1, Kit ligand or Jagged-1 impaired the maintenance of HSPCs44, 61, 62, 63, 64. Several studies have also scrutinized the relative contribution of bone marrow perivascular cells to the homeostasis of HSPCs65, 66, 67. Because the functions and structural stability of endothelial and non-vascular cells is mutually dependent, the deletion of factors in one niche has the potential to perturb the constituents of the neighbouring one. Therefore, genetic manipulations within the intimately associated endothelial niche and accompanying perivascular cells could have off-target effects, which must be taken into consideration. Nonetheless, the findings of these in vivo and reductionist in vitro studies suggest that, irrespective of the localization of HSPCs, angiocrine factors that are presented by either arteriolar or sinusoidal endothelial niches have executive functions and serve as 'rheostats' that choreograph haematopoietic stem-cell self-renewal and differentiation during homeostasis and recovery after haematopoietic suppression. Furthermore, these studies demonstrate that some, but not all, heterotypic ECs can support HSPC expansion, which confirms that each organotypic vascular bed is endowed with unique angiocrine attributes that are suitable for stem-cell homeostasis and reconstitution4, 44, 46, 47.
During fetal development, inductive signals from ECs68 specify the development of haemogenic ECs69, 70. Thus, an endothelial niche could induce the direct conversion of all ECs into haemogenic ones, which give rise to definitive haematopoietic stem cells. Notably, endothelial niche-derived angiocrine signals are essential for the direct conversion of adult ECs into haematopoietic cells. In this approach, adult ECs were transduced with the transcription factors FosB, Gfi1, Runx1 and Spi1 (collectively termed FGRS). However, FGRS-transduced ECs failed to convert to engraftable haematopoietic cells unless they were co-cultured in direct contact with ECs71. Moreover, co-culture of haematopoietic cells that were derived from mouse and non-human primate pluripotent stem cells and an endothelial niche enhanced the engraftment of putative haematopoietic cells, in part through the deployment of Notch ligands72, 73. Thus, angiocrine signals from ECs participate in the specification, development, homeostasis, self-renewal and differentiation of haematopoietic stem cells.
Angiogenesis and osteogenesis
The skeletal system is constantly being remodelled, yet the ratio of skeletal mass to haematopoietic volume remains constant. Although penetrating bone marrow arteries and sinusoidal vessels provide instructive signals for maintenance of the haematopoietic mass, specialized type H and type L bone capillaries modulate osteogenesis without compromising haematopoiesis74, 75, 76. Type H vessels that are positive for CD31 and Endomucin regulate osteoblasts and the proliferation of chondrocytes74, 75. Type L vessels are an extension of type H vessels, and form sinusoidal vessels within the haematopoietic bone cavity. The angiocrine production of Noggin modulates skeletal patterning and ossification (Table 1). Notably, the expression of Noggin is downregulated by Notch signalling, which suggests that crosstalk between osteogenesis and haematopoiesis is conferred through the angiocrine expression of Jagged-1 (ref. 59). Hence, the balanced production of stimulatory and inhibitory angiocrine factors negotiates the allocation of space to bone and haematopoietic compartments.
Regeneration and fibrosis in the liver
Liver sinusoidal ECs distribute the intrahepatic blood flow between the hepatic artery, the hepatic vein and the portal vein77. The tightly regulated hepatic blood flow requires these cells to perform as an adaptable plexus network. Notably, non-lymphatic liver sinusoidal ECs form fenestrated ECs that lack a basement membrane and are negative for CD34, positive for VEGFR-1, VEGFR-2, VEGFR-3, VE-cadherin and the chemokine receptor CXCR7, have low levels or a lack of CD31 and express factor VIII. They also express stabilin-2, CD32b, CD209L and lymphatic vessel endothelial hyaluronic acid receptor 1 (Lyve1) (refs 78–81). A combination of these markers has been used to isolate, characterize and cultivate non-lymphatic liver sinusoidal ECs81.
Under homeostatic conditions, angiocrine signals modulate the expansion of hepatocytes by enabling the proliferation of diploid Axin2- and T-box transcription factor 3 (Tbx3)-positive cells that repopulate the liver82. These cells are located next to ECs in the central vein of the liver. Angiocrine production of Wnt2 and Wnt9b from these specialized ECs maintains Axin2 and Tbx3 double-positive hepatocytes that ultimately give rise to distal non-pericentral hepatocytes (Fig. 3a, Table 1). Deletion in ECs from adult mice of the gene Wntless, which encodes a specific transporter for Wnt-ligand secretion, depletes hepatic repopulating cells. Furthermore, specific angiocrine expression of the Wnt agonist Rspondin3 by ECs of the central vein of the liver — but not portal-vein ECs — establishes a β-catenin-dependent metabolic zonation83. Therefore, the repopulating potential and metabolic zonation of hepatocytes are established by extrinsic angiocrine signals that are derived from an 'inductive' central-vein-of-liver endothelial niche.
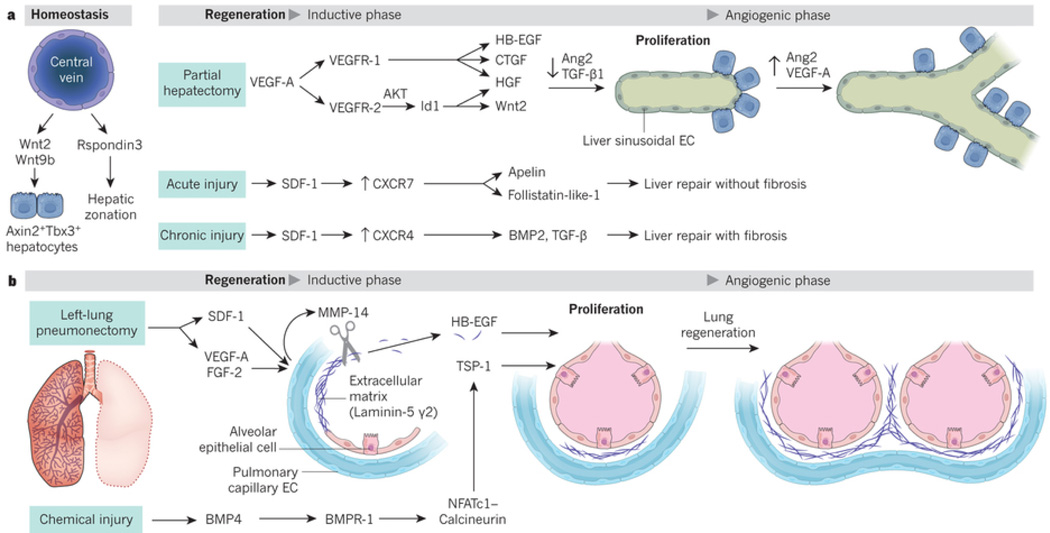
Figure 3. Tissue-specific ECs by supplying membrane-bound and secreted angiocrine factors support regeneration of alveolar epithelial cells and hepatocytes.
(A) At steady state Wnt2 and Wnt9b produced by liver central vein ECs sustains liver mass by replenishing the Axin2+Tbx3+ hepatic stem cell pools. After 70% partial hepatectomy (PH), VEGF-A via AKT activation induces Id 1 in LSECs upregulating HGF and Wnt2. VEGF-A through activation of VEGFR1 upregulates HGF, HB-EGF and CTGF. These angiocrine factors stimulate hepatocyte proliferation without provoking angiogenesis, (inductive phase). Four days after PH, increase in liver initiates proliferative angiogenesis. Upon PH downregulation of Ang2 in LSECs and TGF-β accelerates hepatic recovery. During resolution phase of liver regeneration (days 4–8 post-PH), VEGF-A and restoration of Ang2 stimulates angiogenesis and finalize hepatic reconstitution. Activation of CXCR7 on LSECs triggers pro-regenerative and anti-fibrotic angiocrine factors, including Apelin, follistatin-1-like that facilitates fibrosis-free healing. Chronic injury by persistent CXCR4 activation and CXCR7 suppression stimulates TGF-β and BMP4 leading to fibrosis. Balance of CXCR4 and CXCR7 in LSECs negotiates liver regeneration and fibrosis.
(B) After removal of mice left lung (pneumonectomy, PNX), the PCECs in right lung express membrane-bound MMP14, which unmasks the cryptic EGF-receptor ligands from HB-EGF and Laminin5 γ-2. This inductive phase orchestrates the angiogenesis-independent compensatory alveolar epithelial regeneration. After chemical injury (i.e. bleomycin), BMP4 through engagement of its receptor Bmpr1 sets up NFATc1/Calcineurin-dependent transcription of TSP1. TSP1 facilitates differentiation of lung epithelial progenitors into functional epithelial cells. After PNX, upregulation of the VEGF-A, FGF2 and deposition of platelets on PCECs by production of SDF1 and CXCR4 activation induces MMP14 initiating alveolar regeneration. Increase in lung mass triggers angiogenic phase of lung regeneration.
The angiocrine contribution of liver sinusoidal ECs to liver regeneration can be studied through the surgical resection of up to 70% of the liver's mass, known as a partial hepatectomy, which induces regeneration throughout the remaining liver. During the initial angiogenesis-independent inductive phase, which occurs 1–4 days after partial hepatectomy, VEGFR-2–AKT-dependent upregulation of transcription factor Id1 in non-proliferating liver sinusoidal ECs stimulates the expression of Wnt2 and hepatocyte growth factor (HGF) (Fig. 3a). Activation of VEGFR-1 on non-angiogenic liver sinusoidal ECs also induces the production of HGF and other pro-hepatic factors, such as heparin-binding EGF (HB-EGF), TGF-α and connective tissue growth factor (CTGF), to drive liver regeneration79. On days 4–12 after partial hepatectomy, liver sinusoidal ECs promote proliferative angiogenesis to meet the metabolic demands of the enlarging liver (Fig. 3a). Putative bone-marrow-derived ECs that co-express CD133, CD45 and CD31 and have the capacity to produce HGF can also engraft into populations of regenerating liver sinusoidal ECs, which helps to boost regeneration of the liver84.
Liver regeneration has a programmed set point to ensure restoration of the original hepatic mass that is modulated by liver sinusoidal ECs in an angiocrine-dependent manner85. During the inductive phase of liver regeneration, Angiopoietin-2 is downregulated in liver sinusoidal ECs, which diminishes the expression of the inhibitory factor TGF-β1. In the angiogenic phase of liver regeneration (4–8 days after partial hepatectomy), Angiopoietin-2 expression returns to normal levels to facilitate VEGFR-2-dependent angiogenesis (Fig. 3a). Liver regeneration is delayed in adult mice in which Angiopoietin-2–Tie2 signalling is disrupted, which underscores the angiocrine function of Angiopoietin-2 in hepatic regeneration (Table 1).
Liver sinusoidal ECs are capable of either guiding the regeneration of the liver or engendering its pathological recovery by fibrosis, depending on differentially activated signalling pathways. After an acute insult to the liver, upregulation and activation of the SDF-1 receptor CXCR7 induces the angiocrine factors apelin and follistatin-like-1, which promote fibrosis-free repair86 (Fig. 3a, Table 1). By contrast, chronic injury to the liver by repeated injection of carbon tetrachloride or bile-duct ligation leads to the upregulation of another SDF-1 receptor, CXCR4, and the suppression of CXCR7, which shifts the balance to angiocrine secretion of pro-fibrotic TGF-β1 and BMP2, and leads to scarring. Thus, liver sinusoidal ECs are a cellular node that governs the regeneration, homeostasis and pathology of the liver.
Regeneration of the lung epithelium
The pulmonary circulation has an expansive capillary surface area that is covered by a thin layer of ECs. A delicate alveolar–capillary membrane is formed by the juxtaposition of these cells and alveolar epithelial cells to mediate gas exchange87. This close association facilitates cellular crosstalk to modulate diverse pulmonary physiological processes. Using purification methods to eliminate lymphatic ECs (Box 2), pulmonary capillary ECs are identified by their expression of CD31, CD34, FGF receptor (FGFR)-1, VEGFR-1, and VEGFR-2, and their lack of CD45 (ref. 88). The contribution of pulmonary capillary ECs to lung regeneration can be demonstrated by removal of the left lung in mammals. Known as a pneumonectomy, the procedure leads to a compensatory growth of lung mass that is driven by the expansion of alveolar epithelial stem and progenitor cells, which include alveolar type (AT)2 epithelial cells89. After the pneumonectomy, the propagation of AT2 cells is elicited by MMP-14, which is induced in pulmonary capillary ECs88. MMP-14 activates the EGF receptor (EGFR) on alveolar epithelial progenitor cells through its unmasking of the cryptic EGF-like motif in HB-EGF and the γ2 chain of Laminin-5, and the proliferation of these cells achieves neo-alveologenesis (Fig. 3b, Table 1). Selective conditional deletion of FGFR-1 or VEGFR-2 from adult ECs impairs regeneration of the lung88 (Table 1). Following the pneumonectomy, recruitment of platelets and delivery of SDF-1 to its receptors on pulmonary capillary ECs could also lead to the upregulation of MMP-14 in ECs90. And selective conditional deletion of MMP-14 in adult ECs abrogates regeneration of the alveolar epithelium with negligible impact on vascular perfusion (Table 1). The progenitors of airway basal cells91 could also proliferate in response to angiocrine MMP-14 induction. FGF-2 and FGF-5 derived from cultured human basal cells stimulate the FGFR-1-dependent production of MMP-14 by pulmonary capillary ECs, which in turn supports the expansion and differentiation of basal cells92. Therefore, selective angiocrine upregulation of MMP-14 in pulmonary capillary ECs ignites the propagation of alveolar epithelial cells and basal cell progenitors.
Angiocrine signals also modulate the fate of other types of lung progenitor cell. BMP4 binds the BMP receptor BMPR-1 on ECs, which leads to NFATc1 transcriptional activation of TSP-1 and the differentiation of lung epithelial stem and progenitor cells93 (Fig. 3b, Table 1). In 3D-homotypic co-cultures, angiocrine-derived TSP-1 stimulates the differentiation of lung epithelial progenitor cells, and in Tsp1-deficient mice, the differentiation of progenitor cells into AT1 and AT2 cells is impaired. Collectively, these studies demonstrate that the activation of VEGFR-2, FGFR-1 and BMPR-1 on pulmonary capillary ECs instructs neo-alveologenesis to restore gas-exchange function in the regenerating lungs, which sets the stage for the treatment of emphysema-like disorders with EC transplantation or angiocrine factors94.
The modulation of metabolism
Molecular interactions between ECs and islet cells regulate pancreatic function. During the recuperation of islet cells from chemical injury, angiocrine signals drive regeneration of the pancreas and the resolution of diabetes95, 96. An angiocrine supply of BMP2 and BMP4 is crucial for islet-cell homeostasis96. Notably, the deposition of Laminin-α4 chain by ECs stimulates insulin production by islet cells97. Co-culture of islet cells with pancreatic ECs improves the survival of transplanted islet cells98. Angiocrine delivery of TSP-1, through the modulation of TGF-β1, promotes islet-cell proliferation99. Angiocrine signals from ECs also guide the stage-specific differentiation of pluripotent stem cells into islet cells. For example, EC-derived EGF-like 7 (EGFL7) specifies the fate and proliferation of pancreas and duodenum homeobox (Pdx)1-positive islet-cell progenitors100. Therefore, identification of the phenotypic and functional attributes of islet-specific ECs could unravel the angiocrine heterogeneity within pancreatic tissues and lead to new therapeutic strategies for islet-cell production.
Angiogenesis and transdifferentiation of ECs to adipogenic cells modulate thermogenesis and the remodelling of adipose tissue2, 101. Remarkably, ECs can also adjust metabolism in an angiogenesis-independent manner by deploying certain angiocrine factors. Adipose tissue regulates its mass by monitoring the differentiation of adipocyte stem cells into mature adipocytes. For example, in peroxisome proliferator-activated receptor-γ reporter mice, adipose stem cells were shown to embrace the white-fat vasculature, but not the vasculature of other tissues102. During adipogenesis, angiocrine signals such as TNF, IL-6, insulin growth factor (IGF)1 and IGFBPs modulate the expansion and differentiation of adipocyte stem cells. In turn, adipocytes produce angiogenic factors that fine-tune the induction of angiocrine factors. Deciphering the molecular details involved in the crosstalk between ECs and adipocyte stem cells has potential for the treatment of obesity, diabetes and metabolic syndromes.
Myogenic homeostasis
The idea that an endothelial niche modulates myogenesis stems from studies in which ECs were shown to facilitate the formation of striated muscle that was derived from pluripotent cells103. Moreover, satellite striated-muscle precursor cells that express Pax7 and Myf5 are positioned in close proximity to ECs104. Co-culture studies showed that ECs in heterotypic cultures support cycling of these satellite cells through the angiocrine production of IGF1, HGF, FGF-2, homodimers of platelet-derived growth factor B-chains (PDGF-BB) and VEGF-A (Table 1). Thus, homeostasis and regeneration of striated-muscle cells could also be coordinated by angiocrine signals.
Likewise, cardiac myocytes are surrounded by the unique capillary and specialized ECs that comprise the endocardium. Cardiac contractility and survival are controlled by EC-derived angiocrine factors, which include Neuregulin-1, endothelin-1 and nitric oxide105. Neuregulin-1 is induced and released in response to hypoxic stress by the microvascular endocardial and subendocardial coronary arteries106 (Table 1). Notably, deletion in adult ECs of the gene that encodes Neuregulin-1 decreases angiocrine-mediated protection of the ischaemic myocardium. Thus, after ischaemic injury, angiocrine factors execute regenerative programs that evoke cardiac repair and sustain myogenic-cell contractility.
Angiocrine functions as morphogens
Certain angiocrine factors, produced mainly by angiogenic ECs, consist of morphogens and chemokines that provide signals for proper tissue patterning and direct repopulating cells to their predetermined anatomical destinations107. During lung development, sprouting capillary ECs guide the patterning of alveolar epithelial cells108. Similarly, after hepatotoxin-induced liver injury, the remaining liver sinusoidal ECs provide the cellular roadmap that steers the proper assembly of the hepatic architecture109.
The size and activation state of the endothelial niche could also dictate the stem-cell mass and the extent to which an organ achieves its final volume and shape. Injury to ECs can decrease the surface area of the endothelial niche and deplete the stem-cell pool. This raises the possibility that an obligatory expansion in the mass of stem cells demands an equivalent increase in the surface area of the endothelial niche. Indeed, overexpression of VEGF-A in the V-SVZ of the brain increases the size of the endothelial niche, which augmentes neurogenesis17, 110. Whether the size of the endothelial niche in other organs is the main rheostat by which the mass of stem cells is set is an intriguing question that deserves further scrutiny.
Molecular determinants of angiocrine heterogeneity
In response to as yet unknown signals, organotypic ECs acquire unique structural features, such as fenestrations, and are induced to release specific sets of angiocrine factors4, 111. Notably, their response to various systemic endocrine factors, such as progesterone, is restricted to specific vascular boundaries — namely the uterine vascular bed112. The mechanisms through which ECs acquire and maintain tissue-specific attributes could be regulated by extrinsic biophysical cues as well as by the expression of intrinsic cues that include certain transcription factors.
Specific groups of transcription factors, such as those that are encoded by the homeobox, or Hox, genes, are expressed in organotypic ECs4. The differential expression of Hox genes provides positional identities to vascular zones at defined anatomic sites. In mice, Hoxa3 and Hoxc11, which are associated with anterior and posterior domains, respectively, are differentially expressed in ECs along the body axis113. Hox gene products might also contribute to organotypic angiocrine expression. For example, Hoxa9 triggers the expression of MMP-14 and EphrinB4, whereas Hoxb5, Hoxb3 and Hoxd3 control the positional expression of EphrinA1, VEGFR-2 and type I collagen114. It is plausible that extravascular cues, such as the biophysical forces that are imparted by shear stress and matrix elasticity, as well as crosstalk between parenchymal cells and ECs, enforce the expression of tissue-specific transcription factors in microvascular ECs and determine angiocrine heterogeneity.
Future directions
The emerging idea that organ homeostasis and regeneration are directed by tissue-specific microvascular ECs that function as instructive endothelial niches could be groundbreaking. This is because it focuses future work on what was conceived to be only a passive 'plumbing' system. These observations set the stage for capitalizing on the potential of specialized homotypic ECs as therapeutic drivers that can shepherd the regeneration of functional organs. However, the angiocrine profile of different organ-specific ECs is widely divergent, and a specific angiocrine profile must be established to elicit the desired effect on the local stem-cell population. Notably, the angiogenic and inflammatory states of ECs also play an important part in directing the proper production of angiocrine factors. Moreover, activation of signalling pathways other than AKT and MAPK — specifically, the Jak–Stat pathway — could switch on the expression of unique angiocrine factors. Therefore, to translate the potential of homotypic ECs to the clinical setting, the mechanism by which generic heterotypic ECs adopt tissue-specific angiocrine functions needs to be elucidated. The development of serum-free and xenobiotic-free co-culture platforms, such as E4ORF1+ ECs (Box 2), enables researchers to molecularly eavesdrop on the crosstalk between non-lymphatic homotypic or heterotypic ECs and tissue-repopulating stem cells. This could uncover the pathways that regulate the expression of tissue-specific angiocrine factors that are transcriptionally and biophysically induced.
In each organ, ECs perform the role of professional niche cells that elaborate a panoply of distinct sets of angiocrine factors that are distributed and delivered with precision to stem cells. Notably, ECs prevent stem cells from becoming exhausted or undergoing excessive self-renewal that could provoke the emergence of detrimental mutations. Our understanding of how stem cells integrate and interpret these myriad endothelial-derived signals to perform their organotypic functions is poor. Nonetheless, published data indicate that the coordinated presentation of a mixture of these angiocrine signals constitutes the long-sought holy grail of stem-cell self-renewal. Notably, the oscillatory release of inhibitory and stimulatory angiocrine factors might be crucial for the safe physiological self-renewal of engraftable stem cells, a process that is unlikely to be replicated by current approaches, in which stem cells are randomly pulsed with a cocktail of growth factors or small molecules. Thus, it is possible to translate the therapeutic potential of ECs for reconstituting pools of stem cells by recreating the appropriate endothelial niche, possibly through the transplantation of organotypic ECs.
The feasibility of this approach has been demonstrated by the intrajugular transplantation of pulmonary capillary ECs into the regenerating alveoli of mice. By supplying the proper doses of angiocrine factors, these engrafted cells drive repopulation of epithelial cells and improve respiratory function88, 90. Infusion of human cord blood-derived ECs into neonatal mice with hyperoxia-induced alveolar injury also enhances the repair of alveoli115. The intravascular transplantation of liver sinusoidal ECs rescues fibrosis-free regeneration of the liver in Id1-deficient mice, which are refractory to liver regeneration81. In mice, transplantation of ECs also restores liver function after chemical injury77, 84 and it corrects the phenotype of haemophilia A mice116. Administration of mouse brain capillary ECs117, 118, 119, lung ECs119, bone marrow ECs44 or other heterotypic ECs117, 118, 119, 120 enhances haematopoietic recovery after irradiation. Remarkably, senescent pancreatic islet cells regain their function121 when transplanted into young diabetic mice, which suggests that the infusion of young ECs could rejuvenate islet-cell function. Engineering clinical-grade tissue-specific or generic ECs43, 122, 123 that are capable of engrafting and adapting to an injured microenvironment, as well as producing the proper stoichiometry of angiocrine factors, will augment tissue regeneration and provide guidance for morphogenesis without instigating fibrosis.
It is plausible that the homeostasis and regenerative potential of almost every organ in mammals are regulated by angiocrine factors. Indeed, angiocrine signals might regulate the regenerative potential of stem cells in the hair-follicle bulge, Lgr5+ gastrointestinal repopulating cells, ovarian follicular cells, uterine syncytial cells, chondrocytes and tenocytes, as well as repopulating cells in endocrine organs and epithelial cells in the thymus and urogenital tissues. The development of technologies for isolating non-lymphatic capillary ECs (Box 2) from such sites will enable the angiocrine potential of ECs within these organs to be identified.
Although most of the studies described in this Review focused on angiocrine-derived growth factors and chemokines, the release of EC-derived exosomes and deposition of specific components of the extracellular matrix into the EC basal lamina, as well as the expression of adhesion molecules, such as E-selectin124, could also modulate homeostatic and reparative processes. Two notable examples are the release of Laminin-α4 by pancreatic ECs, which induces the production of insulin by islet cells125, and MMP-14 generated by lung ECs through unmasking of the cryptic EGF-like motif in HB-EGF that is embedded within the Laminin-5 γ2 chain88. Similarly, extracellular matrix components that are laid down by organotypic ECs could also modulate tissue homeostasis by refining the affinity of adhesion molecules or by exposing growth factors that are embedded in the extracellular matrix.
Aberrant production of angiocrine factors could constitute the underlying pathogenesis of various conditions, such as cardiovascular or cerebrovascular diseases and the ageing process. Indeed, it is not known whether senescent ECs develop dysfunction in angiocrine production. An important topic not addressed in this Review is the mechanism by which inflammatory and immune cells could alter the normal angiocrine profile of ECs and thereby influence organ repair and scarring. The isolation and molecular profiling of ECs from the tissues of people with various disorders, such as individuals who are experiencing the complications of age-related and inflammatory diseases, could reveal the contribution of perturbed angiocrine signalling to these disorders. Restoration of the angiocrine function of maladapted or senescent vasculature, possibly through the transplantation of engineered ECs, could set the stage for treatments and help to diminish the economic burden of such disorders.
Supplementary Material
Acknowledgments
We would like to acknowledge Drs. Ralph Nachman, David Hajjar, Eric Jaffe, Babette Wexler as well as the late Dr. Aaron Marcus and the late Dr. Judah Folkman whose pioneering work in vascular biology inspired us to uncover the remarkable instructive functions of endothelial cells. We would also like to thank esteemed colleagues and the members of our laboratory, including Drs William Schachterle, Michael Poulos, Koji Shido, Sina Rabbany, Pouneh Kermani, Raphael Lis, Marco Seandel, Arash Rafii, Daylon James, Joseph Scandura, Michael Ginsberg, Daniel Nolan, Jeffery Port, Geoff Davis, Fernando Martinez and Augustine Choi for critical review, scientific input and providing suggestions. We are grateful to Dr. Zhongwei Cao for providing outstanding expertise in designing artwork and figures.
This was supported by S.R.: Ansary Stem Cell Institute, the Empire State Stem Cell Board and New York State Department of Health grants (C026878, C028117, C029156), Howard Hughes medical Institute, National Heart, Lung, and Blood Institute R01HL115128, R01HL119872, and R01HL128158, National Cancer Institute U54CA163167 and the National Institute of Diabetes and Digestive and Kidney Diseases R01DK095039, Qatar National Priorities Research Program NPRP 6-131-3-268 and Tri-Institutional Stem Cell Initiative awards. J.M.B: Ansary Stem Cell Institute, Tri-Institutional Stem Cell Initiative, American Society of Hematology Scholar Award, and American Federation of Aging Research Award, Leukemia Lymphoma Society Quest for Cures Award. B.-S.D was supported by Ansary Stem Cell Institute and National Scientist Development Grant (12SDG1213004) from the American Heart Association.
We apologize to scientists whose work could not be highlighted because of space limitation.
Glossary of angiocrine factors
- Ang
Angiopoietin
- BDNF
Brain derived nerve growth factor
- BMP2, BMP4
Bone morphogenic proteins
- BTC
Betacellulin
- CTGF
Connective tissue growth factor
- DKK1, DKK3
Dickkopf WNT Signaling Pathway Inhibitor 1 and 3
- Dhh
Desert hedgehog
- EGFL7
Epidermal growth factor like-7
- EFNB2
EphrinB2
- FGF2
Fibroblast growth factor-2
- GDF11
Growth differentiation factor-11
- GDNF
Glial cell line-derived neurotrophic factor
- HB-EGF
Heparin binding-epidermal growth factor
- HGF
Hepatocyte growth factor
- IGFBP
Insulin growth factor binding protein
- Jag1
Jagged-1
- IL1
Interleukin-1
- IL6
Interleukin-6
- KL
Kit-ligand
- MMP14
Metalloproteinase 14
- NRG
Neuregulin
- NO
Nitric oxide
- NT-3
Neurotrophin-3
- PEDF
Pigment epithelium-derived factor
- PGE2
Prostaglandin-E2
- PlGF1
PlGF2, Placental growth factor-1 or 2
- SDF1
Stromal-derived factor-1 (Cxcl12)
- TSP1
Thrombospondin-1
- TNF
Tumor necrosis factor
- VEGF
Vascular endothelial growth factor
- VEGFR1
Vascular endothelial growth factor receptor-1 (Flt1)
- VEGFR2
Vascular endothelial growth factor receptor-2 (KDR, Flk1)
- Wls
Wntless
- Wnt2, Wnt9B
Wingless-type MMTV integration site family
References
- 1.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghesquiere B, Wong BW, Kuchnio A, Carmeliet P. Metabolism of stromal and immune cells in health and disease. Nature. 2014;511:167–176. doi: 10.1038/nature13312. [DOI] [PubMed] [Google Scholar]
- 3.Butler JM, Kobayashi H, Rafii S. Instructive role of the vascular niche in promoting tumour growth and tissue repair by angiocrine factors. Nat Rev Cancer. 2010;10:138–146. doi: 10.1038/nrc2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nolan DJ, et al. Molecular signatures of tissue-specific microvascular endothelial cell heterogeneity in organ maintenance and regeneration. Dev Cell. 2013;26:204–219. doi: 10.1016/j.devcel.2013.06.017. In this article the authors developed an intra-vital labeling approach to purify non-lymphatic mouse organ-specific ECs and by employing molecular profiling demonstrated the remarkable angiocrine heterogeneity in ECs among various tissues
- 5.Matsumoto K, Yoshitomi H, Rossant J, Zaret KS. Liver organogenesis promoted by endothelial cells prior to vascular function. Science. 2001;294:559–563. doi: 10.1126/science.1063889. [DOI] [PubMed] [Google Scholar]
- 6.Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science. 2001;294:564–567. doi: 10.1126/science.1064344. [DOI] [PubMed] [Google Scholar]
- 7.Augustin HG, Kozian DH, Johnson RC. Differentiation of endothelial cells: analysis of the constitutive and activated endothelial cell phenotypes. Bioessays. 1994;16:901–906. doi: 10.1002/bies.950161208. [DOI] [PubMed] [Google Scholar]
- 8.Crivellato E, Nico B, Ribatti D. Contribution of endothelial cells to organogenesis: a modern reappraisal of an old Aristotelian concept. J Anat. 2007;211:415–427. doi: 10.1111/j.1469-7580.2007.00790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shen Q, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. In this paper the authors demonstrated that heterotypic endothelial cells, but not stromal cells by producing soluble angiocrine factors supported the expansion and differentiation of co-cultured neural stem cells
- 10.Shen Q, et al. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mirzadeh Z, Merkle FT, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3:265–278. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tavazoie M, et al. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 14.Leventhal C, Rafii S, Rafii D, Shahar A, Goldman SA. Endothelial trophic support of neuronal production and recruitment from the adult mammalian subependyma. Mol Cell Neurosci. 1999;13:450–464. doi: 10.1006/mcne.1999.0762. [DOI] [PubMed] [Google Scholar]
- 15.Ramirez-Castillejo C, et al. Pigment epithelium-derived factor is a niche signal for neural stem cell renewal. Nat Neurosci. 2006;9:331–339. doi: 10.1038/nn1657. [DOI] [PubMed] [Google Scholar]
- 16. Goldman SA, Chen Z. Perivascular instruction of cell genesis and fate in the adult brain. Nat Neurosci. 2011;14:1382–1389. doi: 10.1038/nn.2963. This comprehensive review summarizes the studies elucidating how angiocrine factors produced by endothelial cells within subventricular and subgranular niches coordinate self-renewal of the neural stem cells and their differentiation into transient amplifying cells and neuroblasts.
- 17.Licht T, Keshet E. The vascular niche in adult neurogenesis. Mech Dev. 2015 doi: 10.1016/j.mod.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Silva-Vargas V, Crouch EE, Doetsch F. Adult neural stem cells and their niche: a dynamic duo during homeostasis, regeneration, and aging. Curr Opin Neurobiol. 2013;23:935–942. doi: 10.1016/j.conb.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Ottone C, et al. Direct cell-cell contact with the vascular niche maintains quiescent neural stem cells. Nat Cell Biol. 2014;16:1045–1056. doi: 10.1038/ncb3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ottone C, Parrinello S. Multifaceted control of adult SVZ neurogenesis by the vascular niche. Cell Cycle. 2015;14:2222–2225. doi: 10.1080/15384101.2015.1049785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delgado AC, et al. Endothelial NT-3 delivered by vasculature and CSF promotes quiescence of subependymal neural stem cells through nitric oxide induction. Neuron. 2014;83:572–585. doi: 10.1016/j.neuron.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 22.Silva-Vargas V, Doetsch F. A new twist for neurotrophins: endothelial-derived NT-3 mediates adult neural stem cell quiescence. Neuron. 2014;83:507–509. doi: 10.1016/j.neuron.2014.07.029. [DOI] [PubMed] [Google Scholar]
- 23.Andreu-Agullo C, Morante-Redolat JM, Delgado AC, Farinas I. Vascular niche factor PEDF modulates Notch-dependent stemness in the adult subependymal zone. Nat Neurosci. 2009;12:1514–1523. doi: 10.1038/nn.2437. [DOI] [PubMed] [Google Scholar]
- 24.Gomez-Gaviro MV, et al. Betacellulin promotes cell proliferation in the neural stem cell niche and stimulates neurogenesis. Proc Natl Acad Sci U S A. 2012;109:1317–1322. doi: 10.1073/pnas.1016199109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crouch EE, Liu C, Silva-Vargas V, Doetsch F. Regional and stage-specific effects of prospectively purified vascular cells on the adult V-SVZ neural stem cell lineage. J Neurosci. 2015;35:4528–4539. doi: 10.1523/JNEUROSCI.1188-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han J, et al. Vascular endothelial growth factor receptor 3 controls neural stem cell activation in mice and humans. Cell reports. 2015;10:1158–1172. doi: 10.1016/j.celrep.2015.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le Bras B, et al. VEGF-C is a trophic factor for neural progenitors in the vertebrate embryonic brain. Nat Neurosci. 2006;9:340–348. doi: 10.1038/nn1646. [DOI] [PubMed] [Google Scholar]
- 28.Kokovay E, et al. Adult SVZ lineage cells home to and leave the vascular niche via differential responses to SDF1/CXCR4 signaling. Cell Stem Cell. 2010;7:163–173. doi: 10.1016/j.stem.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Snapyan M, et al. Vasculature guides migrating neuronal precursors in the adult mammalian forebrain via brain-derived neurotrophic factor signaling. J Neurosci. 2009;29:4172–4188. doi: 10.1523/JNEUROSCI.4956-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whitman MC, Fan W, Rela L, Rodriguez-Gil DJ, Greer CA. Blood vessels form a migratory scaffold in the rostral migratory stream. J Comp Neurol. 2009;516:94–104. doi: 10.1002/cne.22093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katsimpardi L, et al. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science. 2014;344:630–634. doi: 10.1126/science.1251141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshida S, Sukeno M, Nabeshima Y. A vasculature-associated niche for undifferentiated spermatogonia in the mouse testis. Science. 2007;317:1722–1726. doi: 10.1126/science.1144885. [DOI] [PubMed] [Google Scholar]
- 33. Seandel M, et al. Generation of functional multipotent adult stem cells from GPR125+ germline progenitors. Nature. 2007;449:346–350. doi: 10.1038/nature06129. In this paper the authors report development of a serum-free and xenobiotic-free human vascular-like niche platform that supports the expansion of repopulating stem and progenitor cells.
- 34.Seandel M, et al. Niche players: spermatogonial progenitors marked by GPR125. Cell Cycle. 2008;7:135–140. doi: 10.4161/cc.7.2.5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim J, Seandel M, Falciatori I, Wen D, Rafii S. CD34+ testicular stromal cells support long-term expansion of embryonic and adult stem and progenitor cells. Stem Cells. 2008;26:2516–2522. doi: 10.1634/stemcells.2008-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rafii S, et al. Isolation and characterization of human bone marrow microvascular endothelial cells: hematopoietic progenitor cell adhesion. Blood. 1994;84:10–19. [PubMed] [Google Scholar]
- 37. Rafii S, et al. Human bone marrow microvascular endothelial cells support long-term proliferation and differentiation of myeloid and megakaryocytic progenitors. Blood. 1995;86:3353–3363. This is the first report of homotypic co-culture of human bone marrow ECs with human hematopoietic cells, underscoring the capacity of endothelial niche that by producing angiocrine factors support expansion of hematopoietic cells in the absence of exogenous growth factors.
- 38.Li W, et al. Primary endothelial cells isolated from the yolk sac and para-aortic splanchnopleura support the expansion of adult marrow stem cells in vitro. Blood. 2003;102:4345–4353. doi: 10.1182/blood-2003-03-0729. [DOI] [PubMed] [Google Scholar]
- 39.Li W, Johnson SA, Shelley WC, Yoder MC. Hematopoietic stem cell repopulating ability can be maintained in vitro by some primary endothelial cells. Exp Hematol. 2004;32:1226–1237. doi: 10.1016/j.exphem.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 40.Chute JP, Saini AA, Kampen RL, Wells MR, Davis TA. A comparative study of the cell cycle status and primitive cell adhesion molecule profile of human CD34+ cells cultured in stroma-free versus porcine microvascular endothelial cell cultures. Exp Hematol. 1999;27:370–379. doi: 10.1016/s0301-472x(98)00004-6. [DOI] [PubMed] [Google Scholar]
- 41.Chute JP, et al. Ex vivo culture with human brain endothelial cells increases the SCID-repopulating capacity of adult human bone marrow. Blood. 2002;100:4433–4439. doi: 10.1182/blood-2002-04-1238. [DOI] [PubMed] [Google Scholar]
- 42.Brandt JE, et al. Ex vivo expansion of autologous bone marrow CD34(+) cells with porcine microvascular endothelial cells results in a graft capable of rescuing lethally irradiated baboons. Blood. 1999;94:106–113. [PubMed] [Google Scholar]
- 43. Seandel M, et al. Generation of a functional and durable vascular niche by the adenoviral E4ORF1 gene. Proc Natl Acad Sci U S A. 2008;105:19288–19293. doi: 10.1073/pnas.0805980105. In this method report, it is demonstrated that transduction of a non-oncogenic adenoviral E4ORF1 gene enables serum-free and xenobiotic-free expansion of primary human ECs. E4ORF1+ ECs sustain their vascular properties and produce the proper stoichiometry of angiocrine factors, establishing an ideal endothelial niche for stem cell expansion and therapeutic organ regeneration
- 44.Poulos MG, et al. Vascular Platform to Define Hematopoietic Stem Cell Factors and Enhance Regenerative Hematopoiesis. Stem cell reports. 2015 doi: 10.1016/j.stemcr.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kobayashi H, et al. Angiocrine factors from Akt-activated endothelial cells balance self-renewal and differentiation of haematopoietic stem cells. Nature cell biology. 2010;12:1046–1056. doi: 10.1038/ncb2108. In this study, the authors show that differentially activated ECs by producing defined soluble and membrane-bound angiocrine factors balance self-renewal and differentiation of the hematopoietic stem cells. AKT-activated ECs foster expansion of stem cells, while co-activation with MAPKinase induce differentiation into large numbers of hematopoietic progrenitors
- 46. Butler JM, et al. Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell. 2010;6:251–264. doi: 10.1016/j.stem.2010.02.001. This article employs a serum-free endothelial niche platform to demonstrate that ECs foster the ex vivo expansion and self-renewal of Notch-dependent, authentic long-term mouse repopulating hematopoietic stem ells. Regeneration of the endothelial niche was essential for reconstitution of hematopoiesis.
- 47.Hooper AT, et al. Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell Stem Cell. 2009;4:263–274. doi: 10.1016/j.stem.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brenet F, Kermani P, Spektor R, Rafii S, Scandura JM. TGFbeta restores hematopoietic homeostasis after myelosuppressive chemotherapy. J Exp Med. 2013;210:623–639. doi: 10.1084/jem.20121610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Butler JM, et al. Development of a vascular niche platform for expansion of repopulating human cord blood stem and progenitor cells. Blood. 2012;120:1344–1347. doi: 10.1182/blood-2011-12-398115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raynaud CM, et al. Endothelial cells provide a niche for placental hematopoietic stem/progenitor cell expansion through broad transcriptomic modification. Stem cell research. 2013;11:1074–1090. doi: 10.1016/j.scr.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 51.North TE, et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447:1007–1011. doi: 10.1038/nature05883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoggatt J, et al. Differential stem- and progenitor-cell trafficking by prostaglandin E2. Nature. 2013;495:365–369. doi: 10.1038/nature11929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Himburg HA, et al. Pleiotrophin regulates the retention and self-renewal of hematopoietic stem cells in the bone marrow vascular niche. Cell reports. 2012;2:964–975. doi: 10.1016/j.celrep.2012.09.002. In this paper the authors provide evdience that Pleiotrophin primarily produced by endothelial cells support the reconstitution of hematopoiesis after myeloablative injury
- 54.Doan PL, et al. Epidermal growth factor regulates hematopoietic regeneration after radiation injury. Nat Med. 2013;19:295–304. doi: 10.1038/nm.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Heissig B, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires mmp-9 mediated release of kit-ligand. Cell. 2002;109:625–637. doi: 10.1016/s0092-8674(02)00754-7. This paper is the first report setting forth the proposition that endothelial and non-vascular niches cooperate by establishing a dynamic intertwined niches that by MMP-9 mediated increase in the bioavailibity of soluble Kit-ligand facilitate reconstitution of hematopoiesis and stem cell trafficking
- 56.Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C, Morrison SJ. SLAM Family Receptors Distinguish Hematopoietic Stem and Progenitor Cells and Reveal Endothelial Niches for Stem Cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 57.Avecilla ST, et al. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat Med. 2004;10:64–71. doi: 10.1038/nm973. [DOI] [PubMed] [Google Scholar]
- 58.Hamada T, et al. Transendothelial migration of megakaryocytes in response to stromal cell-derived factor 1 (SDF-1) enhances platelet formation. J Exp Med. 1998;188:539–548. doi: 10.1084/jem.188.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Poulos MG, et al. Endothelial jagged-1 is necessary for homeostatic and regenerative hematopoiesis. Cell reports. 2013;4:1022–1034. doi: 10.1016/j.celrep.2013.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kopp HG, et al. Thrombospondins deployed by thrombopoietic cells determine angiogenic switch and extent of revascularization. J Clin Invest. 2006;116:3277–3291. doi: 10.1172/JCI29314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457–462. doi: 10.1038/nature10783. The authors of this article show that angiocrine supply of kit-ligand by the endothelial cells is essential for hematopoietic maintenance, as conditional deletion of Kit-ligand selectively in the ECs results in impaired hematopoiesis
- 62.Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013 doi: 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Inra CN, et al. A perisinusoidal niche for extramedullary haematopoiesis in the spleen. Nature. 2015 doi: 10.1038/nature15530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kimura Y, et al. c-Kit-Mediated Functional Positioning of Stem Cells to Their Niches Is Essential for Maintenance and Regeneration of Adult Hematopoiesis. PLoS One. 2011;6:e26918. doi: 10.1371/journal.pone.0026918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kunisaki Y, et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013;502:637–643. doi: 10.1038/nature12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505:327–334. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Acar M, et al. Deep imaging of bone marrow shows non-dividing stem cells are mainly perisinusoidal. Nature. 2015;526:126–130. doi: 10.1038/nature15250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nguyen PD, et al. Haematopoietic stem cell induction by somite-derived endothelial cells controlled by meox1. Nature. 2014;512:314–318. doi: 10.1038/nature13678. [DOI] [PubMed] [Google Scholar]
- 69.Medvinsky A, Dzierzak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell. 1996;86:897–906. doi: 10.1016/s0092-8674(00)80165-8. [DOI] [PubMed] [Google Scholar]
- 70.Chen MJ, Yokomizo T, Zeigler BM, Dzierzak E, Speck NA. Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature. 2009;457:887–891. doi: 10.1038/nature07619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sandler VM, et al. Reprogramming human endothelial cells to haematopoietic cells requires vascular induction. Nature. 2014;511:312–318. doi: 10.1038/nature13547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gori JL, et al. Vascular niche promotes hematopoietic multipotent progenitor formation from pluripotent stem cells. J Clin Invest. 2015;125:1243–1254. doi: 10.1172/JCI79328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hadland BK, et al. Endothelium and NOTCH specify and amplify aorta-gonad-mesonephros-derived hematopoietic stem cells. J Clin Invest. 2015;125:2032–2045. doi: 10.1172/JCI80137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ramasamy SK, Kusumbe AP, Wang L, Adams RH. Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature. 2014;507:376–380. doi: 10.1038/nature13146. In this report, the authors describe the coupling of angiogenesis, angiocrine signals and osteogenesis. They demonstrate that angiocrine supply of noggin is essential for all aspects of osteogenesis
- 75.Ramasamy SK, Kusumbe AP, Adams RH. Regulation of tissue morphogenesis by endothelial cell-derived signals. Trends Cell Biol. 2015;25:148–157. doi: 10.1016/j.tcb.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kusumbe AP, Ramasamy SK, Adams RH. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 2014;507:323–328. doi: 10.1038/nature13145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.DeLeve LD. Liver sinusoidal endothelial cells and liver regeneration. J Clin Invest. 2013;123:1861–1866. doi: 10.1172/JCI66025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Falkowski M, Schledzewski K, Hansen B, Goerdt S. Expression of stabilin-2, a novel fasciclin-like hyaluronan receptor protein, in murine sinusoidal endothelia, avascular tissues, and at solid/liquid interfaces. Histochem Cell Biol. 2003;120:361–369. doi: 10.1007/s00418-003-0585-5. [DOI] [PubMed] [Google Scholar]
- 79.LeCouter J, et al. Angiogenesis-independent endothelial protection of liver: role of VEGFR-1. Science. 2003;299:890–893. doi: 10.1126/science.1079562. [DOI] [PubMed] [Google Scholar]
- 80.DeLeve LD. Liver sinusoidal endothelial cells in hepatic fibrosis. Hepatology. 2015;61:1740–1746. doi: 10.1002/hep.27376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ding BS, et al. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature. 2010;468:310–315. doi: 10.1038/nature09493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wang B, Zhao L, Fish M, Logan CY, Nusse R. Self-renewing diploid Axin2 cells fuel homeostatic renewal of the liver. Nature. 2015 doi: 10.1038/nature14863. This paper shows that at steady state conditons, angiocrine deployment of the Wnt2 and Wnt9B by the liver central vein ECs enables generation of dipoid repopulating hepatocytes maintaining the hepatic self-renewal and liver mass
- 83.Michalopoulos GK, Grompe M, Theise ND. Assessing the potential of induced liver regeneration. Nat Med. 2013;19:1096–1097. doi: 10.1038/nm.3325. [DOI] [PubMed] [Google Scholar]
- 84.Wang L, Wang X, Xie G, Hill CK, DeLeve LD. Liver sinusoidal endothelial cell progenitor cells promote liver regeneration in rats. J Clin Invest. 2012;122:1567–1573. doi: 10.1172/JCI58789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hu J, et al. Endothelial cell-derived angiopoietin-2 controls liver regeneration as a spatiotemporal rheostat. Science. 2014;343:416–419. doi: 10.1126/science.1244880. This report provides genetic evidence for the angiocrine-derived angiopoietin-2 that by activating its cognate Tie2 receptor and regulating TGF-β intiates and completes liver regeneration
- 86.Ding BS, et al. Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis. Nature. 2014;505:97–102. doi: 10.1038/nature12681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Morrisey EE, Hogan BL. Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev Cell. 2010;18:8–23. doi: 10.1016/j.devcel.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ding BS, et al. Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization. Cell. 2011;147:539–553. doi: 10.1016/j.cell.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hogan BL, et al. Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell. 2014;15:123–138. doi: 10.1016/j.stem.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rafii S, et al. Platelet-derived SDF-1 primes the pulmonary capillary vascular niche to drive lung alveolar regeneration. Nat Cell Biol. 2015;17:123–136. doi: 10.1038/ncb3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rock JR, et al. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci U S A. 2009;106:12771–12775. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ding BS, Gomi K, Rafii S, Crystal RG, Walters MS. Endothelial MMP14 is required for endothelial dependent growth support of human airway basal cells. J Cell Sci. 2015 doi: 10.1242/jcs.168179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Lee JH, et al. Lung stem cell differentiation in mice directed by endothelial cells via a BMP4-NFATc1-thrombospondin-1 axis. Cell. 2014;156:440–455. doi: 10.1016/j.cell.2013.12.039. This report describes a novel angiocrine pathway in which BMP4 through activation of Bmpr1-NFATc1 switches on the trancription of thrombospondin-1 thereby promoting lung epithelial regeneration after chemical injury
- 94.Petrache I, et al. Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nat Med. 2005;11:491–498. doi: 10.1038/nm1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rivas-Carrillo SD, et al. Endothelial cells promote pancreatic stem cell activation during islet regeneration in mice. Transplant Proc. 2011;43:3209–3211. doi: 10.1016/j.transproceed.2011.09.082. [DOI] [PubMed] [Google Scholar]
- 96.Talavera-Adame D, Dafoe DC. Endothelium-derived essential signals involved in pancreas organogenesis. World J Exp Med. 2015;5:40–49. doi: 10.5493/wjem.v5.i2.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nikolova G, et al. The vascular basement membrane: a niche for insulin gene expression and Beta cell proliferation. Dev Cell. 2006;10:397–405. doi: 10.1016/j.devcel.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 98.Pan X, et al. Islet graft survival and function: concomitant culture and transplantation with vascular endothelial cells in diabetic rats. Transplantation. 2011;92:1208–1214. doi: 10.1097/TP.0b013e3182356ca7. [DOI] [PubMed] [Google Scholar]
- 99.Olerud J, et al. Thrombospondin-1: an islet endothelial cell signal of importance for beta-cell function. Diabetes. 2011;60:1946–1954. doi: 10.2337/db10-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kao DI, et al. Endothelial cells control pancreatic cell fate at defined stages through EGFL7 signaling. Stem cell reports. 2015;4:181–189. doi: 10.1016/j.stemcr.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cao Y. Angiogenesis and vascular functions in modulation of obesity, adipose metabolism, and insulin sensitivity. Cell Metab. 2013;18:478–489. doi: 10.1016/j.cmet.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 102.Tang W, et al. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322:583–586. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shmelkov SV, et al. Cytokine preconditioning promotes codifferentiation of human fetal liver CD133+ stem cells into angiomyogenic tissue. Circulation. 2005;111:1175–1183. doi: 10.1161/01.CIR.0000157155.44008.0F. [DOI] [PubMed] [Google Scholar]
- 104.Christov C, et al. Muscle satellite cells and endothelial cells: close neighbors and privileged partners. Mol Biol Cell. 2007;18:1397–1409. doi: 10.1091/mbc.E06-08-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Noireaud J, Andriantsitohaina R. Recent insights in the paracrine modulation of cardiomyocyte contractility by cardiac endothelial cells. Biomed Res Int. 2014;2014:923805. doi: 10.1155/2014/923805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Hedhli N, et al. Endothelium-derived neuregulin protects the heart against ischemic injury. Circulation. 2011;123:2254–2262. doi: 10.1161/CIRCULATIONAHA.110.991125. The authors of this paper provide genetic evidence in which selective deletion of Neuregulin-1 in the adult mouse ECs impairs myogenic repair, implicating that angiocrine supply of Neuregulin-1 is essential for cardiac myocyte protection and regeneration
- 107.Cleaver O, Melton DA. Endothelial signaling during development. Nat Med. 2003;9:661–668. doi: 10.1038/nm0603-661. [DOI] [PubMed] [Google Scholar]
- 108.Lazarus A, et al. A perfusion-independent role of blood vessels in determining branching stereotypy of lung airways. Development. 2011;138:2359–2368. doi: 10.1242/dev.060723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hoehme S, et al. Prediction and validation of cell alignment along microvessels as order principle to restore tissue architecture in liver regeneration. Proc Natl Acad Sci U S A. 2010;107:10371–10376. doi: 10.1073/pnas.0909374107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Licht T, et al. Reversible modulations of neuronal plasticity by VEGF. Proc Natl Acad Sci U S A. 2011;108:5081–5086. doi: 10.1073/pnas.1007640108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Aird WC. Endothelial cell heterogeneity. Cold Spring Harb Perspect Med. 2012;2:a006429. doi: 10.1101/cshperspect.a006429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Goddard LM, et al. Progesterone receptor in the vascular endothelium triggers physiological uterine permeability preimplantation. Cell. 2014;156:549–562. doi: 10.1016/j.cell.2013.12.025. This reports highlights the remakable heterogeneity of the organotypic ECs demonstrating that selective expression of progresterone receptor in uterine endothelium modulate the unique physiological property such as permeablity and other functions to facilitate implantation
- 113.Pruett ND, et al. Evidence for Hox-specified positional identities in adult vasculature. BMC Dev Biol. 2008;8:93. doi: 10.1186/1471-213X-8-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Douville JM, Wigle JT. Regulation and function of homeodomain proteins in the embryonic and adult vascular systems. Can J Physiol Pharmacol. 2007;85:55–65. doi: 10.1139/y06-091. [DOI] [PubMed] [Google Scholar]
- 115.Alphonse RS, et al. Existence, functional impairment, and lung repair potential of endothelial colony-forming cells in oxygen-induced arrested alveolar growth. Circulation. 2014;129:2144–2157. doi: 10.1161/CIRCULATIONAHA.114.009124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Follenzi A, et al. Transplanted endothelial cells repopulate the liver endothelium and correct the phenotype of hemophilia A mice. J Clin Invest. 2008;118:935–945. doi: 10.1172/JCI32748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Salter AB, et al. Endothelial progenitor cell infusion induces hematopoietic stem cell reconstitution in vivo. Blood. 2009;113:2104–2107. doi: 10.1182/blood-2008-06-162941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chute JP, et al. Transplantation of vascular endothelial cells mediates the hematopoietic recovery and survival of lethally irradiated mice. Blood. 2007;109:2365–2372. doi: 10.1182/blood-2006-05-022640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li B, et al. Endothelial cells mediate the regeneration of hematopoietic stem cells. Stem cell research. 2010;4:17–24. doi: 10.1016/j.scr.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zachman DK, et al. Endothelial cells mitigate DNA damage and promote the regeneration of hematopoietic stem cells after radiation injury. Stem cell research. 2013;11:1013–1021. doi: 10.1016/j.scr.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Almaca J, et al. Young capillary vessels rejuvenate aged pancreatic islets. Proc Natl Acad Sci U S A. 2014;111:17612–17617. doi: 10.1073/pnas.1414053111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Ginsberg M, et al. Efficient Direct Reprogramming of Mature Amniotic Cells into Endothelial Cells by ETS Factors and TGFbeta Suppression. Cell. 2012;151:559–575. doi: 10.1016/j.cell.2012.09.032. This article describes an efficient method to convert human fetal mid-gestation amniotic cells into abundant functional ECs, without transitioning through destabilizing pluripotent state. Here, transient expression of the transcription factors ETV2 for 2 weeks and Fli1, Erg1 along with TGF-β inhbition resulted in generation of stable, engraftable and clinical scalable ECs
- 123.James D, et al. Expansion and maintenance of human embryonic stem cell-derived endothelial cells by TGFbeta inhibition is Id1 dependent. Nat Biotechnol. 2010;28:161–166. doi: 10.1038/nbt.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rafii S, et al. Human ESC-derived hemogenic endothelial cells undergo distinct waves of endothelial to hematopoietic transition. Blood. 2013;121:770–780. doi: 10.1182/blood-2012-07-444208. [DOI] [PubMed] [Google Scholar]
- 125.Nikolova G, Strilic B, Lammert E. The vascular niche and its basement membrane. Trends Cell Biol. 2007;17:19–25. doi: 10.1016/j.tcb.2006.11.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.