Abstract
IκB kinase β (IKKβ), a central coordinator of inflammatory responses through activation of nuclear factor-κB (NF-κB), has been implicated as a critical molecular link between inflammation and metabolic disorders; however, the role of adipocyte IKKβ in obesity and related metabolic disorders remains elusive. Here we report an essential role of IKKβ in the regulation of adipose remodeling and adipocyte survival in diet-induced obesity. Targeted deletion of IKKβ in adipocytes does not affect body weight, food intake, and energy expenditure but results in an exaggerated diabetic phenotype when challenged with a high-fat diet (HFD). IKKβ-deficient mice have multiple histopathologies in visceral adipose tissue, including increased adipocyte death, amplified macrophage infiltration, and defective adaptive adipose remodeling. Deficiency of IKKβ also leads to increased adipose lipolysis, elevated plasma free fatty acid (FFA) levels, and impaired insulin signaling. Mechanistic studies demonstrated that IKKβ is a key adipocyte survival factor and that IKKβ protects murine and human adipocytes from HFD- or FFA-elicited cell death through NF-κB–dependent upregulation of antiapoptotic proteins and NF-κB–independent inactivation of proapoptotic BAD protein. Our findings establish IKKβ as critical for adipocyte survival and adaptive adipose remodeling in obesity.
Introduction
It is generally accepted that obesity is associated with a state of chronic low-grade inflammation that is a major contributor to insulin resistance and type 2 diabetes, yet the detailed molecular mechanisms remain elusive (1–3). IκB kinase β (IKKβ), a central coordinator of inflammatory responses through activation of nuclear factor-κB (NF-κB) (4–6), has been implicated as a key molecular link between obesity, inflammation, and metabolic disorders (7,8). It is known that overnutrition can activate IKKβ in vitro and in vivo (3,9,10) and that diet-induced insulin resistance has been associated with the activation of IKKβ/NF-κB in multiple tissues (9,11–15). Moreover, heterozygous deletion of IKKβ or inhibiting IKKβ with salicylates protected mice against insulin resistance triggered by high-fat (HF) diet (HFD) or obesity (11,16). Deletion of IKKβ in the liver improved diet-induced insulin resistance, and deficiency of IKKβ in myeloid cells rendered global insulin sensitivity upon HF feeding (12). By contrast, constitutive activation of IKKβ in the liver caused systemic insulin resistance (13).
Compared with other tissues and cell types, the role of adipose IKKβ signaling in obesity and metabolic disorders remains incompletely understood. Obesity is associated with elevated activity of IKKβ and another key stress/inflammatory kinase, c-Jun amino-terminal kinase (JNK), in adipose tissue (3,9,17). The contribution of JNK signaling to obesity-associated insulin resistance has been well-established (3,17). Although the role of IKKβ in inflammatory factor–mediated adipocyte insulin resistance has been confirmed by in vitro studies (9,18), in vivo studies using transgenic or knockout mice have generated inconsistent results. For example, overexpression of NF-κB subunit p65 or a constitutive active form of IKKβ in adipose tissue increased systemic and tissue inflammation but also resulted in improved insulin sensitivity (19,20). A very recent study showed that the adipocyte IKKβ also plays an important role in inflammation resolution by inducing interleukin 13 (IL-13) expression in adipose tissue (21). We previously demonstrated that IKKβ functions in adipocyte precursor cells to regulate adipogenesis and adipose tissue development (6). Deficiency of IKKβ in adipocyte precursor cells inhibited adipocyte differentiation and protected mice from diet-induced obesity and metabolic disorders (6). These findings suggest that the functions of IKKβ signaling in adipose tissue are complex and that further studies are needed to define the role of adipocyte IKKβ in obesity and insulin resistance.
The adipocyte is subject to constant turnover once formed, and obesity is associated with a higher adipocyte turnover due to an elevated rate of adipocyte death (22,23). In addition to mediating inflammatory responses, IKKβ is also a key cell survival factor (4,5) but the functions of IKKβ in adipocyte survival has not been thoroughly investigated under the condition of obesity. We demonstrate here that IKKβ plays an essential role in the regulation of adipocyte survival in diet-induced obesity and is required for adaptive adipose remodeling in response to nutrient changes.
Research Design and Methods
Animals
Adipocyte-specific IKKβ-knockout (IKKβΔAd) mice were generated by crossing IKKβF/F mice (6,24) with Adipoq-Cre transgenic mice (25). IKKβF/F mice were also mated with aP2-Cre mice to generate IKKβΔaP2 mice. IKKβ-deficient mice and IKKβF/F control littermates (4 weeks old) were fed a normal chow diet (ND) or a Western-type HFD (42% kcal from fat; Harlan Teklad) for 12 weeks until they were killed at 16 weeks of age (6). For the HFD-to-ND (HFD/ND) conversion diet experiment, mice were fed the HFD for 24 weeks then switched to the ND for 6 weeks. All animals were housed in an animal facility under a protocol approved by the University of Kentucky Institutional Animal Care and Use Committee.
Metabolic and Histological Analyses
Body weight was measured weekly, and body composition was measured by MRI (Echo MRI). Food intake, total activity, oxygen consumption, and carbon dioxide production were measured with a LabMaster system (6). Intraperitoneal glucose tolerance test and insulin tolerance test were performed as described previously (6). Ex vivo lipolysis was performed by using a Lipolysis Assay Kit (ZenBio) following the manufacturer’s protocol. Immunohistochemical staining of adipose tissue sections was performed following standard protocols (6). TUNEL staining for adipose tissue sections was performed using the In Situ Cell Death Detection Kit (Roche Applied Science).
MRI Analysis and Data Segmentation
HFD-fed IKKβΔAd and IKKβF/F littermates were imaged on a 7T CliniScan MRI (Bruker, Ettlingen, Germany). The fat and water images were generated from the in and out of phase images using Siemens Syngo software. FSL software was used for image analysis, and fat volume was calculated following standard protocols (26,27).
Adipose Stromal Vascular Cell Isolation and Analyses
Adipose stromal vascular (SV) cells and adipocytes were isolated as previously described (6). Flow cytometry analysis was performed using freshly isolated SV cells labeled with Alexa Fluor 488-F4/80 (Serotec) and PE-CD11b (BD Biosciences) antibodies. For the Cre-mediated IKKβ deletion assay, fully differentiated SV cells from IKKβF/F mice were infected with lentivirus expressing Cre or LacZ as the control. Cells were treated with a 0.5 mmol/L free fatty acid (FFA) mixture containing myristic, lauric, arachidonic, oleic, and linoleic acids (Sigma-Aldrich) or vehicle in the presence of 1% FFA-free BSA (9,10).
3T3-L1 Cell Differentiation and Short Interfering RNA Knockdown
3T3-L1 cells were first differentiated into mature adipocytes as previously described (6). Differentiated 3T3-L1 cells were transfected with control short interfering (si)RNA or siRNA targeting the mouse IKKβ or BAD gene sequences by electroporation using the Amaxa Cell Line Nucleofector Kit V (Lonza) (6). Cells were treated with 0.5 mmol/L FFA mixture or vehicle for Western blot analysis.
Quantitative PCR and Western Blot Analyses
Quantitative PCR and Western blot analyses were performed as described before (6,28,29). The primer sets used in this study are listed in Supplementary Table 1. Anti-phosphorylated (phospho)-BAD (Ser26) antibody was purchased from Abiocode, anti-actin antibody was purchased Sigma-Aldrich, and all of the other antibodies were purchased from Cell Signaling.
Human Subjects and Adult-Derived Human Adipose Stem Cell Isolation
Human adipocytes were derived from the differentiation of adult-derived human adipose stem cells (ADHASCs) (30,31). The adipose tissue was from a collagenase digestion of the lipoaspirate of young and healthy subjects undergoing liposuction of subcutaneous fat. Differentiation medium was used to induce differentiation, as previously described (30,31).
Statistical Analysis
Data are presented as the mean ± SEM. Individual pairwise comparisons were analyzed by two-sample, two-tailed Student t test in which P < 0.05 was regarded as significant.
Results
Adipocyte IKKβ Deficiency Does Not Affect Body Weight and Energy Expenditure but Results in an Exaggerated Diabetic Phenotype When Challenged With an HFD
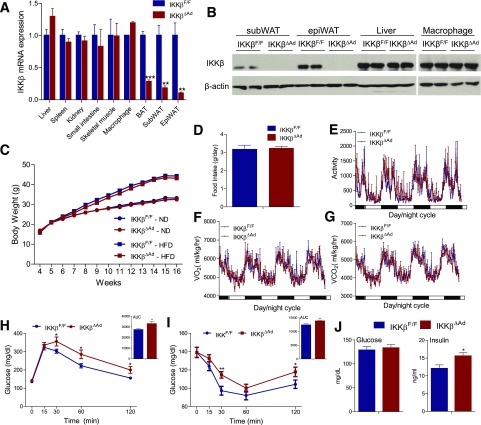
To investigate the functions of adipocyte IKKβ in vivo, we generated IKKβΔAd mice by crossing mice carrying loxP-flanked IKKβ alleles (IKKβF/F) (6,24) with adiponectin (Adipoq)-Cre transgenic mice (25,32). As expected, IKKβ mRNA levels were significantly reduced in white adipose tissue (WAT), including subcutaneous WAT (subWAT) and epididymal WAT (epiWAT), and in brown adipose tissue (BAT), but not in other major tissues or cell types such as liver and macrophages of IKKβΔAd mice (Fig. 1A). Western blot analysis also confirmed the deletion of IKKβ in WAT but not in liver or macrophages (Fig. 1B). Further, Adipoq-Cre was only present in mature adipocytes but not in adipose SV cells of IKKβΔAd mice, resulting in decreased IKKβ mRNA levels in adipocytes only (Supplementary Fig. 1A and B). Consistently, adipose SV cells of IKKβΔAd mice had similar adipogenic potential compared with those of IKKβF/F mice (Supplementary Fig. 1C). Taken together, these results validate the specific deletion of IKKβ in adipocytes of IKKβΔAd mice.
Figure 1.
Deficiency of adipocyte IKKβ does not affect diet-induced weigh gain but results in an exaggerated diabetic phenotype when challenged with an HFD. A: IKKβ mRNA expression levels in major organs of IKKβF/F and IKKβΔAd mice were analyzed by quantitative PCR (n = 3–7). B: Western blot analysis of IKKβ protein levels in subWAT, epiWAT, liver, and macrophages of IKKβF/F and IKKβΔAd mice. C: Growth curves of ND- or HFD-fed male IKKβF/F and IKKβΔAd mice (n = 19–28). Food intake (D), total activity (E), VO2 (F), and VCO2 (G) of 16-week-old male IKKβF/F and IKKβΔAd mice fed the HFD for 12 weeks (n = 5). Food intake was averaged from 4-day measurements. Glucose tolerance test (GTT) and the area under the curve (AUC) of GTT (H), insulin tolerance test (ITT), and AUC of ITT (I), and fasting glucose and insulin levels (J) in HFD-fed IKKβF/F and IKKβΔAd mice (n = 10–17). *P < 0.05; **P < 0.01; ***P < 0.001.
To assess the effect of deficiency of adipocyte IKKβ on obesity, 4-week-old male IKKβΔAd and IKKβF/F littermates were fed the ND or HFD. Body weight was significantly increased after HF feeding for both IKKβΔAd and IKKβF/F mice, but deficiency of adipocyte IKKβ did not affect the diet-induced weight gain (Fig. 1C). IKKβΔAd mice were also phenotypically normal in food intake, oxygen consumption, and carbon dioxide production compared with IKKβF/F mice (Fig. 1D–G). Unexpectedly, HFD-fed IKKβΔAd mice developed an exaggerated diabetic phenotype. Upon glucose and insulin tolerance testing, IKKβΔAd mice had worse glucose tolerance (Fig. 1H) and showed a decreased hypoglycemic response to the injected insulin (Fig. 1I). Further, deficiency of adipocyte IKKβ also elevated fasting serum insulin concentrations (Fig. 1J), suggesting worse insulin sensitivity.
Loss of Adipocyte IKKβ Causes Partial Lipodystrophy and Defects in Adaptive Adipose Remodeling in Response to Dietary Changes
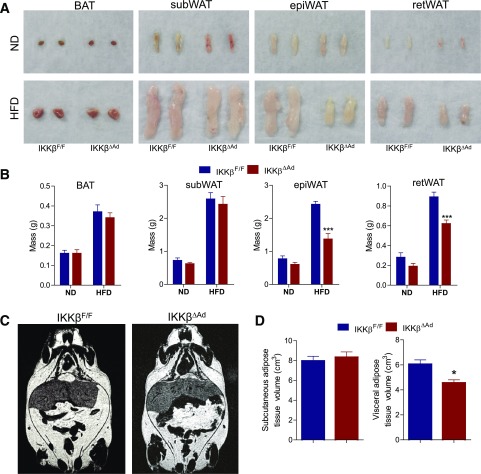
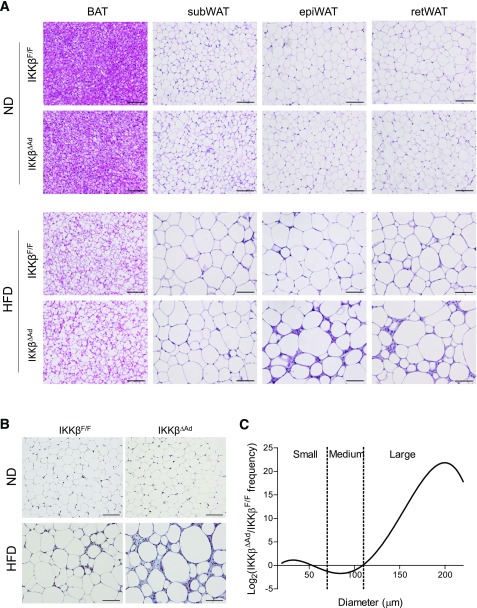
Intriguingly, we found that visceral (vis) adipose pads including epididymal (epi) and retroperitoneal fat pads of IKKβΔAd mice failed to expand properly in response to HF feeding (Fig. 2A and B). MRI analyses confirmed that IKKβΔAd mice had a significantly decreased volume of visWAT but a similar subWAT volume compared with IKKβF/F mice after HF feeding (Fig. 2C and D). Histological analyses showed that visWAT of HFD-fed IKKβΔAd mice exhibited a form of lipodystrophy with striking structure abnormalities and marked heterogeneity in adipocyte sizes (Fig. 3A). Massively increased collagen deposition in epiWAT of HFD-fed IKKβΔAd mice was confirmed by Masson’s trichrome stain (Fig. 3B). Quantification of adipocyte sizes demonstrated the aberrant adipocyte size distribution in IKKβ-deficient mice (Fig. 3C). These histopathological phenotypes suggest that visWAT of IKKβΔAd mice failed to execute the appropriate adipose remodeling program in response to HFD stress.
Figure 2.
Visceral adipose tissue of adipocyte IKKβ-deficient mice fails to expand properly in response to HF feeding. Representative photographs (A) and weight (B) of fat pads from ND- or HFD-fed male IKKβF/F and IKKβΔAd mice (n = 7–10). Representative coronal section MRIs (C) and visceral and subcutaneous adipose tissue volume (D) of HFD-fed male IKKβF/F and IKKβΔAd mice (n = 5–6). ret, retroperitoneal. *P < 0.05; ***P < 0.001.
Figure 3.
Deficiency of adipocyte IKKβ causes impaired visceral adipose tissue expansion when challenged with an HFD. A: Representative hematoxylin and eosin staining of BAT, subWAT, epiWAT, and retroperitoneal (ret)WAT of ND- or HFD-fed male IKKβF/F and IKKβΔAd mice. Scale bar, 100 μm. B: Trichrome staining for collagen deposition in epiWAT from ND- or HFD-fed IKKβF/F and IKKβΔAd mice. Scale bar, 100 μm. C: Quantitation of adipocyte cross-sectional sizes from HFD-fed mice, expressed as the ratio of the numbers of cells from IKKβΔAd and IKKβF/F mice defined as small (15–70 μm), medium (70–110 μm), and large (>110 μm).
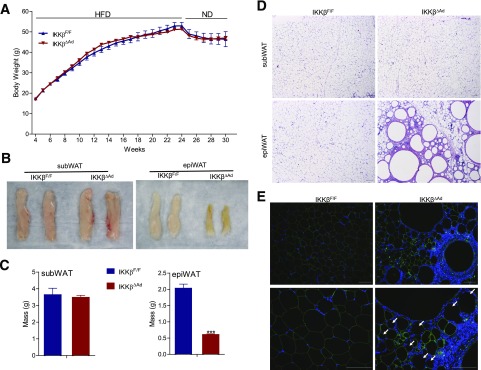
Because adipose tissue needs to expand and contract properly in response to nutrient status, we then tested whether adipocyte IKKβ plays a role in adipose tissue contraction capacity on withdrawal from the HFD. HFD-fed IKKβΔAd and control littermates were switched to the ND for 6 weeks. The control and IKKβΔAd mice both had similar decreased body weight in response to the HFD/ND change (Fig. 4A). Although subWAT was similar in IKKβΔAd and IKKβF/F mice, IKKβΔAd mice had much smaller and discolored epiWAT (Fig. 4B and C). Histological examination revealed a normal subWAT phenotype but severe degeneration of architecture and integrity in epiWAT of IKKβΔAd, indicative of defective adipose remodeling (Fig. 4D). Immunofluorescence staining confirmed the loss of perilipin, an adipocyte marker, for many cells in epiWAT of IKKβΔAd mice, suggesting increased adipocyte death (Fig. 4E). Further, unusual granuloma-like structures were also abundant in epiWAT of HFD/ND-fed IKKβΔAd mice (Fig. 4C and D), which were also observed in other mouse models of lipodystrophy (33). Taken together, the results from HFD-fed and HFD/ND-fed mice demonstrate the requirement of IKKβ in adaptive adipose tissue remodeling in response to dietary changes.
Figure 4.
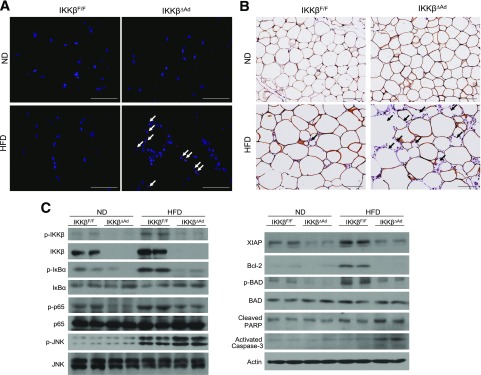
Loss of adipocyte IKKβ results in severe defects in adipose tissue remodeling in response to dietary changes. A: Growth curves of HFD-fed IKKβF/F and IKKβΔAd mice switched to the ND for 6 weeks (n = 6–10). Representative photographs (B), weight (C), and hematoxylin and eosin staining (D) of subWAT and epiWAT from HFD/ND-fed male IKKβF/F and IKKβΔAd mice (n = 3–7). ***P < 0.001. E: Representative immunofluorescence staining for perilipin (green) of epiWAT from IKKβF/F and IKKβΔAd mice fed HFD/ND. Bottom panels represent the magnification of areas in the top panels. The nuclei were stained with DAPI (blue), and the perilipin-negative adipocytes are indicated by arrows. Scale bar, 100 μm.
IKKβ Protects Adipocytes From HFD-Induced Cell Death
Obesity is associated with increased adipocyte death (23,34,35), and IKKβ is a well-established cell survival factor (4,5). Indeed, further analysis of WAT with TUNEL staining showed increased number of apoptotic (TUNEL-positive) cells in epiWAT of HFD-fed IKKβΔAd mice (Fig. 5A). Immunostaining of perilipin demonstrated that IKKβΔAd mice had many adipocytes that were devoid of perilipin labeling (Fig. 5B). Western blot analysis showed that HF feeding activated IKKβ/NF-κB signaling in epiWAT of IKKβF/F mice, as indicated by increased IKKβ expression and phosphorylation of the IKKβ substrates IκBα and p65 (Fig. 5C). In addition, JNK was also activated by HF feeding in epiWAT of IKKβF/F mice and further enhanced in that of IKKβΔAd mice (Fig. 5C). Although JNK signaling induces cell death, activation of NF-κB has been well known to inhibit JNK activation through upregulation of its target genes (36,37). Several NF-κB–regulated antiapoptotic proteins, including X-linked inhibitor of apoptosis (XIAP) and Bcl2, were increased in epiWAT of HFD-fed control mice but were repressed in that of IKKβΔAd mice (Fig. 5C). In addition to the NF-κB–dependent mechanism, IKKβ can also inhibit cell death in an NF-κB–independent manner by phosphorylating BAD, a BH3-only proapoptotic protein, to prime it for inactivation (38). Using a specific antibody against phospho-BAD (serine-26) (38), we also found that deficiency of IKKβ abolished HFD-elicited BAD phosphorylation without affecting total BAD protein levels (Fig. 5C). Consistently, the levels of activated caspase-3 and cleaved poly(ADP-ribose) polymerase (PARP) proteins, indicators of cell apoptosis, were markedly increased in epiWAT of HFD-fed IKKβΔAd mice (Fig. 5C). Collectively, these results suggest that IKKβ protects visceral adipocytes from HFD-induced cell death.
Figure 5.
IKKβ protects adipocytes from HFD-induced cell death. A: Representative TUNEL staining of epiWAT sections from ND- or HFD-fed IKKβF/F and IKKβΔAd mice. The nuclei were stained with DAPI (blue), and the TUNEL-positive cells were indicated by arrows. Scale bar, 50 μm. B: Representative immunohistochemistry for perilipin of epiWAT sections from ND- or HFD-fed IKKβF/F and IKKβΔAd mice. The nuclei were stained with hematoxylin (blue), and the perilipin-negative adipocytes were indicated by arrows. Scale bar, 100 μm. C: Western blot analysis with the indicated total and phosphorylated (p) antibodies in epiWAT from ND or HFD-fed IKKβF/F and IKKβΔAd mice.
IKKβ-Deficient Mice Have Increased Adipose Tissue Macrophage Infiltration and Elevated Lipolysis in Visceral Adipose Depots
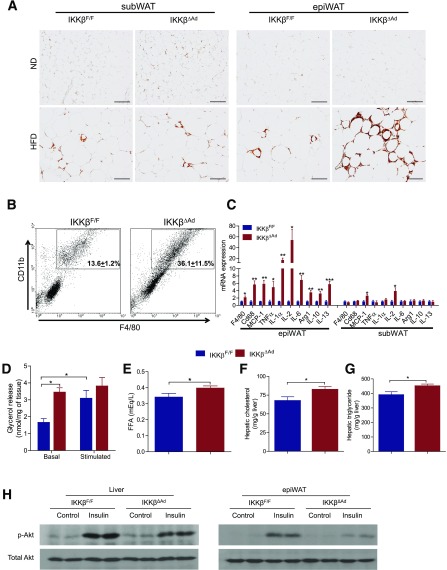
Adipocyte death has been implied as one of initial events that leads to adipose tissue macrophage (ATM) infiltration (33–35,39). Indeed, ATM infiltration was substantially increased in epiWAT of IKKβΔAd upon the HFD challenge (Fig. 6A). Flow cytometric analyses confirmed that the F4/80+CD11b+ macrophage population significantly increased in IKKβΔAd mice (Fig. 6B). Consistently, the mRNA levels of macrophage markers and several key proinflammatory cytokines, including tumor necrosis factor-α (TNF-α), MCP-1, and IL-2, generally considered M1 macrophage markers, were significantly elevated in epiWAT of IKKβΔAd mice (Fig. 6C). In addition, we also noticed the increased expression of IL-10 and Arg 1, markers for anti-inflammatory M2 macrophages, coincident with the increased adipocyte death. Interestingly, the expression levels of IL-13, another anti-inflammatory cytokine, were also significantly increased in epiWAT of IKKβΔAd mice (Fig. 6C). By contrast, deficiency of IKKβ did not affect the adipocyte apoptotic signaling in subWAT (Supplementary Fig. 2) or the ATM accumulation in subWAT (Fig. 6A). The expression levels of macrophage markers and most proinflammatory cytokines in subWAT were also comparable to that of IKKβF/F mice (Fig. 6C).
Figure 6.
Deficiency of adipocyte IKKβ increases macrophage infiltration and lipolysis in visceral adipose tissue and impairs insulin signaling in adipose tissue and liver. A: Representative immunohistochemistry for the macrophage marker F4/80 in subWAT and epiWAT from ND- or HFD-fed IKKβF/F and IKKβΔAd mice. Scale bar, 100 μm. B: Adipose SV cells isolated from epiWAT of HFD-fed mice were examined for expression of macrophage cell makers F4/80 and CD11b with flow cytometry. The percentages of F4/80+CD11b+ cells are as indicated in the flow profiles (n = 3–5). P < 0.05. C: Expression levels of macrophage markers and inflammatory genes in epiWAT and subWAT of HFD-fed mice were analyzed by quantitative PCR (n = 6). *P < 0.05; **P < 0.01; ***P < 0.001. D: Glycerol release in epiWAT from HFD-fed IKKβF/F and IKKβΔAd mice under basal conditions or stimulated with 1 μmol/L isoproterenol (n = 9–12). *P < 0.05. Plasma FFA concentration (E) and hepatic cholesterol (F) and triglyceride (G) levels in HFD-fed IKKβF/F and IKKβΔAd mice (n = 9–13). *P < 0.05. H: Western blot analysis of phospho(p)-Akt (ser473) and total Akt levels in liver and epiWAT of HFD-fed IKKβF/F and IKKβΔAd mice injected with saline or 0.35 units/kg body weight insulin.
Increased ATM number has been associated with elevated adipose lipolysis (40), and some proinflammatory cytokines, such as TNF-α, can also stimulate basal lipolysis in adipocytes (23,41). We found that epiWAT of IKKβΔAd mice also had significantly increased basal lipolysis but similar stimulated lipolysis compared with that of IKKβF/F mice (Fig. 6D). Consistently, IKKβΔAd mice had elevated plasma FFA levels as well as increased hepatic cholesterol and triglyceride concentrations (Fig. 6E–G). It is well established that increased plasma FFA levels and ectopic lipid accumulation in the liver impair insulin signaling and are highly responsible for insulin resistance. Next, HFD-fed mice IKKβF/F and IKKβΔAd mice were injected with insulin before being killed, and phosphorylation of Akt was analyzed in the liver and WAT. IKKβΔAd mice had decreased phosphorylation of Akt in response to insulin in WAT and in the liver (Fig. 6H), indicative of impaired insulin signaling in these mice.
aP2-Cre–Mediated IKKβ Deletion Also Results in Defects in Adipose Remodeling and Accentuated Inflammatory Responses After HF Feeding
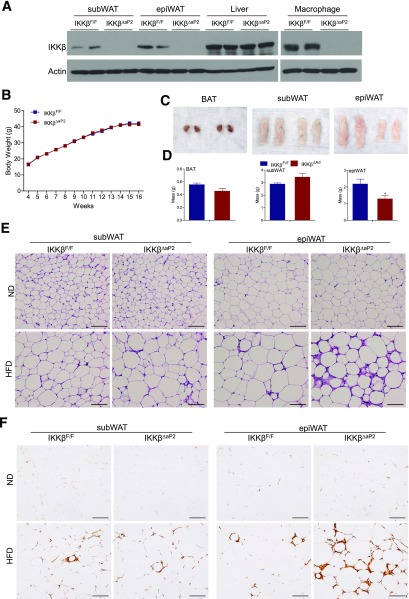
To further confirm the role of IKKβ in adipose tissue remodeling and adipocyte survival, we also used aP2-Cre transgenic mice to generate IKKβΔaP2 mice, another adipose IKKβ-deficient mouse model. As expected, the aP2-Cre line also led to effective deletion of IKKβ in WAT of IKKβΔaP2 mice (Fig. 7A). Consistent with previous reports demonstrating that the aP2 promoter is expressed by other cell types, including macrophages (42,43), IKKβ expression levels were also decreased in macrophages of IKKβΔaP2 mice (Fig. 7A). Similar to IKKβΔAd mice, IKKβΔaP2 mice displayed no significant phenotype under standard laboratory conditions and had similar body weight gain as littermate controls when challenged with the HFD (Fig. 7B). HFD-fed IKKβΔaP2 mice also had decreased size and weight of epiWAT but comparable BAT and subWAT compared with control mice (Fig. 7C and D). Although IKKβΔaP2 mice had IKKβ deficiency in adipocytes and macrophages, the epiWAT of IKKβΔaP2 mice exhibited the same histopathologies as that of IKKβΔAd mice, including aberrant adipose tissue expansion, increased heterogeneity in adipocyte sizes, and increased ATM accumulation (Fig. 7E and F). Therefore, these results confirmed that deficiency of IKKβ in adipocytes caused the defects in adipose remodeling and increased inflammatory responses after HFD feeding.
Figure 7.
aP2-Cre–mediated IKKβ deletion results in similar defects in adipose remodeling and accentuated inflammatory responses after HF feeding. A: Western blot analysis of IKKβ protein levels in subWAT, epiWAT, liver, and macrophages of IKKβF/F and IKKβΔaP2 mice. B: Growth curves of HFD-fed male IKKβF/F and IKKβΔaP2 mice (n = 24–38). Representative photographs (C) and weight of fat pads (D) of HFD-fed male IKKβF/F and IKKβΔaP2 mice (n = 5–7). *P < 0.05. E: Representative hematoxylin and eosin staining of subWAT and epiWAT of ND- or HFD-fed male IKKβF/F and IKKβΔaP2 mice. Scale bar, 100 μm. F: Representative immunohistochemistry for the macrophage marker F4/80 in subWAT and epiWAT from ND- or HFD-fed IKKβF/F and IKKβΔaP2 mice. Scale bar, 100 μm.
IKKβ-BAD Axis Is Required for Adipocyte Survival in Response to FFAs In Vitro
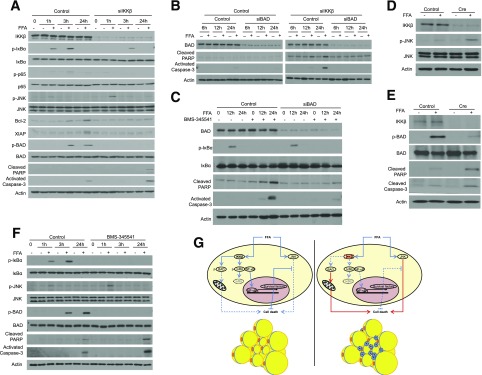
We next used cultured 3T3-L1 adipocytes as a model system to further investigate the role of IKKβ in adipocyte survival in response to overnutrition. Differentiated 3T3-L1 cells were introduced with control siRNA or siRNA against IKKβ (Fig. 8A) and then treated with a mixture of FFAs that activate IKKβ and induce adipocyte inflammation (9,10,18). FFA treatment activated IKKβ and increased phospho-IκBα and phospho-p65 levels in control but not in siIKKβ 3T3-L1 adipocytes (Fig. 8A). The levels of NF-κB–regulated antiapoptotic proteins, Bcl2 and XIAP, were suppressed by IKKβ knockdown, and FFA-elicited JNK activation was further enhanced in IKKβ-deficient cells (Fig. 8A). FFAs also induced IKKβ-mediated BAD phosphorylation in control but not in siIKKβ adipocytes (Fig. 8A). As a consequence, reduction of IKKβ expression increased FFA-triggered cell death as indicated by elevated levels of activated caspase-3 and cleaved PARP proteins (Fig. 8A) and increased TUNEL staining (Supplementary Fig. 3).
Figure 8.
IKKβ protects murine and human adipocytes from FFA-induced cell death. A: 3T3-L1 cells were differentiated into adipocytes and then introduced with control siRNA or siRNA against IKKβ. Western blot analysis with the indicated total and phosphorylated (p) antibodies in control or siIKKβ 3T3-L1 adipocytes treated with vehicle or FFAs for the indicated times. B: 3T3-L1 adipocytes were introduced with control siRNA or siRNA against IKKβ and/or BAD. Western blot analysis with the indicated antibodies in control, siBAD, siIKKβ, or siIKKβ/siBAD 3T3-L1 adipocytes treated with vehicle control or FFAs. C: 3T3-L1 adipocytes were introduced with control siRNA or siRNA against BAD. Western blot analysis with the indicated antibodies in control or siBAD 3T3-L1 adipocytes treated with vehicle control or FFAs in the absence or presence of 5 μmol/L IKKβ inhibitor, BMS-345541. Adipose SV cells isolated from IKKβF/F mice were differentiated into adipocytes and then infected with control lentivirus or lentivirus expressing Cre. Western blot analysis with the indicated antibodies in cells treated with vehicle control or FFAs for 3 h (D) or 24 h (E). F: ADHASCs were differentiated into adipocytes and then treated with FFAs in the presence or absence of 5 μmol/L IKKβ inhibitor, BMS-345541. Western blot analysis with the indicated antibodies in cells treated with control, FFAs and/or BMS-345541. G: Schematic representation of the mechanisms through which IKKβ protects adipocytes from cell death in diet-induced obesity. IKKβ inhibits FFA-induced adipocyte death through NF-κB–dependent upregulation of antiapoptotic proteins and NF-κB–independent inactivation of proapoptotic BAD protein.
To determine the importance of the NF-κB–independent mechanism in mediating adipocyte survival, we also used siRNA to knockdown BAD in control or siIKKβ 3T3-L1 cells (Fig. 8B). Knockdown of BAD did not affect control adipocytes that had intact IKKβ/NF-κB signaling but protected siIKKβ 3T3-L1 cells from FFA-induced cell death (Fig. 8B). In addition to genetic approaches, we also used a highly selective IKKβ inhibitor, BMS-345541 (6,44), and found that BMS-345541 treatment efficiently blocked IKKβ-mediated IκBα phosphorylation elicited by FFAs (Fig. 8C). BMS-345541–treated cells were more susceptible from FFA-induced cell death, as indicated by increased cleaved PARP and caspase-3 levels (Fig. 8C). However, knockdown of BAD protected BMS-345541–treated cells against FFA-induced cell death (Fig. 8C). Collectively, these results suggest that the newly identified IKKβ-BAD axis plays a critical role in protecting adipocytes from FFA-induced cell death.
IKKβ Protects Primary Murine and Human Adipocytes From FFA-Induced Cell Death
Lastly, we investigated the effect of IKKβ-deficiency on primary murine and human adipocyte survival. Adipose SV cells were isolated from IKKβF/F mice, differentiated into mature adipocytes, and then infected with lentivirus expressing Cre to acutely delete IKKβ (Fig. 8D). Deficiency of IKKβ enhanced FFA-elicited JNK activation (Fig. 8D) but abolished FFA-induced BAD phosphorylation (Fig. 8E). Accordingly, FFA treatment increased activated caspase-3 and cleaved PARP levels in IKKβ-deficient cells but not in control cells (Fig. 8E).
ADHASCs were isolated from healthy subjects (30,31). ADHASCs can efficiently differentiate into mature adipocytes in vitro (Supplementary Fig. 4). Differentiated mature adipocytes were treated with FFAs in the presence or absence of BMS-345541, the IKKβ inhibitor. Consistently, BMS-345541 inhibited FFA-elicited NF-κB activation and IKKβ-mediated BAD phosphorylation in human adipocytes (Fig. 8F). Control human adipocytes were protected from FFA-induced cell death but BMS-345541–treated cells had elevated cleaved PARP and caspase-3 levels upon FFA treatment (Fig. 8F). Taken together, these results demonstrated an essential role of IKKβ in protecting both murine and human adipocytes from FFA-induced cell death.
Discussion
In response to stresses linked to inflammation, cells are often forced to choose to die or survive. For example, in the immune system, TNF-α can signal both cell death and survival by activating JNK and IKKβ signaling, respectively (36,45). Previous studies have demonstrated that activation of NF-κB promotes cell survival by inducing expression of genes encoding inhibitor of JNK signaling (36,37). In addition to NF-κB–dependent functions, IKKβ can also inhibit TNF-α–induced apoptosis independently of NF-κB activation by phosphorylating and inactivating BAD, a key proapoptotic protein (38). Similar to TNF-α, FFAs can activate both JNK and IKKβ signaling in adipocytes (9,10). Although much attention has been focused on the role of IKKβ in linking inflammation to insulin resistance, the function of IKKβ in the regulation of adipocyte survival has not been thoroughly investigated. In the current study, we revealed an essential role of IKKβ in promoting adipocyte survival in diet-induced obesity. Under standard laboratory conditions, deficiency of adipocyte IKKβ did not affect adipose tissue development or metabolic function. However, when the mice were challenged with the HFD, IKKβ and JNK signaling were both activated in control mice, and ablation of IKKβ resulted in enhanced JNK activation and increased adipocyte death in IKKβΔAd mice. We also demonstrated that IKKβ can phosphorylate BAD in adipocytes and that knockdown of BAD protected IKKβ-deficient adipocytes from FFA-induced cell death, suggesting the importance of the IKKβ-BAD axis in mediating adipocyte survival. IKKβ is therefore a key adipocyte survival factor in obesity and can protect adipocytes from HFD- or FFA-elicited cell death through activation of the NF-κB–mediated survival factors and inactivation of the proapoptotic protein BAD (Fig. 8G).
Several groups have previously studied the role of adipose IKKβ/NF-κB signaling by overexpressing p65 or a constitutively active form of IKKβ in adipose tissue (19,20). Surprisingly, these transgenic mice had increased adipose tissue inflammation but were resistant to diet-induced obesity and insulin resistance, which may be partially explained by the increased energy expenditure (19,20). However, we found that deletion of endogenous IKKβ in adipocytes did not affect energy expenditure or diet-induced weight gain. While our work was in its final stages, Kwon et al. (21) also reported that deficiency of adipocyte IKKβ led to visceral adipose tissue inflammation without affecting body weight or energy expenditure under HF conditions. It is plausible that endogenous IKKβ in adipocytes does not affect energy metabolism at basal levels but that robustly constitutive activation of IKKβ/NF-κB signaling may cause increased energy expenditure. Future studies are required to determine the detailed mechanisms through which IKKβ/NF-κB signaling regulates energy homeostasis under basal and HF feeding conditions. Although the histopathologies in visWAT of our IKKβΔAd mice were consistent with those Kwon et al. reported (21), they attributed the increased inflammation phenotype to the decreased IL-13 expression of IKKβ-deficient mice (21). NF-κB signaling also plays an important role in inflammation resolution by regulating anti-inflammatory cytokines such as IL-10 and IL-13. However, IL-13 is one of many cytokines regulated by NF-κB signaling, and we found that the expression levels of both proinflammatory and anti-inflammatory cytokines, including MCP-1, TNF-α, IL-10, and IL-13, were all affected by IKKβ deficiency in the epiWAT of HFD-fed mice. Further, both IL-10 and IL-13 gene expression was significantly increased rather than decreased in the epiWAT of IKKβΔAd mice. Our mechanistic studies then suggest that the histopathologies in IKKβΔAd mice are likely due to the loss of protection against cell death in response to HFD stress. Thus, adipose IKKβ has other important functions beyond the scope of inflammation, and more studies are required to further investigate its role in obesity and insulin resistance.
The mechanisms triggering ATM recruitment remain incompletely understood (23,46,47), and adipocyte death has been proposed as a key mechanism that leads to macrophage infiltration into adipose tissue and metabolic disorders (33–35,39). For example, Alkhouri et al. (39) demonstrated that adipocyte death was a link between obesity and insulin resistance. They also showed that genetic inactivation of a proapoptotic molecule can reduce adipocyte death, prevent ATM infiltration, and protect mice against insulin resistance. Consistent with their observations, we found that the increased adipocyte death in visWAT of IKKβΔAd mice was associated with massive macrophage infiltration and elevated levels of proinflammatory cytokines. Increased ATM number has also been associated with elevated adipose lipolysis and circulating FFA levels (40). Indeed, deficiency of IKKβ increased adipose lipolysis and plasma FFA levels, leading to ectopic hepatic lipid accumulation and impaired insulin signaling. That ATM accumulation in IKKβΔAd mice is driven by increased lipolysis is also possible, and whether IKKβ can directly regulate adipocyte lipolysis would be an interesting study in the future.
It is intriguing that deficiency of adipocyte IKKβ promoted HFD-induced adipocyte death and ATM infiltration in visceral but not in subcutaneous fat. The regional differences between different fat depots have been well-recognized (48) and there is a major ontogenetic difference between visceral and subcutaneous fat because they have different developmental origins (49). HF feeding also rapidly and specifically activates adipogenesis in visceral but not in subcutaneous adipose tissue in mice (50). Consistently, adipocyte death rate was much higher in visWAT after long-term HF feeding (34). It is plausible that visceral adipocytes are more susceptible to HFD-induced cell death and that ablation of IKKβ therefore has detectable deleterious effects on visceral adipocytes compared with subcutaneous adipocytes. Future studies will be required to determine the detailed mechanisms that account for the different effects of IKKβ ablation on visceral versus subcutaneous fat.
In summary, we have revealed a pivotal role of IKKβ in adipocyte survival and adipose remodeling in obesity. IKKβ promotes adipocyte survival through NF-κB–dependent and NF-κB–independent mechanisms. Deficiency of IKKβ in adipocytes causes defects in adaptive adipose remodeling in response to dietary change, leading to an exaggerated diabetic phenotype coupled to partial lipodystrophy in visceral adipose tissue. IKKβ is therefore a key adipocyte survival factor and is required for adaptive adipose remodeling in obesity.
Article Information
Acknowledgments. The authors thank Dr. Michael Karin, University of California, San Diego, for IKKβF/F mice; Dr. Jason Kim, University of Massachusetts, for valuable discussions; Dr. Brandon Fornwalt, University of Kentucky, for assistance with MRI analysis; and Dr. Wendy Katz, University of Kentucky, for tissue sectioning and staining.
Funding. This work was supported in part by National Institutes of Health grants (R01-HL-123358, P20-GM-103527, R01-ES-023470, R21-ES-022745, R01-HL-131925, R01-DK-71349, U24-DK-093000, and UL1-TR-000117). Y.S. was supported by an American Heart Association postdoctoral fellowship (14POST18740064). R.N.H. was supported by a National Institutes of Health training grant (T32-DK-007778) and a Pharmaceutical Research and Manufacturers of America Foundation predoctoral fellowship.
Duality of Interest. P.A.K. received research funding from KinDex Pharmaceuticals. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. S.-H.P. and C.Z. researched data and contributed to the discussion and to writing, reviewing, and editing the manuscript. Z.L., Y.S., and R.N.H. researched data and contributed to the discussion and to reviewing and editing the manuscript. B.Z. and D.K.P. researched data. P.A.K. contributed to the discussion and to reviewing and editing the manuscript. C.Z. is the guarantor of this work and, as such, had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db15-1156/-/DC1.
References
- 1.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006;444:840–846 [DOI] [PubMed] [Google Scholar]
- 2.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature 2006;444:875–880 [DOI] [PubMed] [Google Scholar]
- 3.Hotamisligil GS, Erbay E. Nutrient sensing and inflammation in metabolic diseases. Nat Rev Immunol 2008;8:923–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell 2008;132:344–362 [DOI] [PubMed] [Google Scholar]
- 5.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature 2006;441:431–436 [DOI] [PubMed] [Google Scholar]
- 6.Sui Y, Park SH, Xu J, et al. IKKβ links vascular inflammation to obesity and atherosclerosis. J Exp Med 2014;211:869–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baker RG, Hayden MS, Ghosh S. NF-κB, inflammation, and metabolic disease. Cell Metab 2011;13:11–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Solinas G, Karin M. JNK1 and IKKbeta: molecular links between obesity and metabolic dysfunction. FASEB J 2010;24:2596–2611 [DOI] [PubMed] [Google Scholar]
- 9.Jiao P, Ma J, Feng B, et al. FFA-induced adipocyte inflammation and insulin resistance: involvement of ER stress and IKKβ pathways. Obesity (Silver Spring) 2011;19:483–491 [DOI] [PubMed] [Google Scholar]
- 10.Nguyen MT, Satoh H, Favelyukis S, et al. JNK and tumor necrosis factor-alpha mediate free fatty acid-induced insulin resistance in 3T3-L1 adipocytes. J Biol Chem 2005;280:35361–35371 [DOI] [PubMed] [Google Scholar]
- 11.Yuan M, Konstantopoulos N, Lee J, et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science 2001;293:1673–1677 [DOI] [PubMed] [Google Scholar]
- 12.Arkan MC, Hevener AL, Greten FR, et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med 2005;11:191–198 [DOI] [PubMed] [Google Scholar]
- 13.Cai D, Yuan M, Frantz DF, et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med 2005;11:183–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell 2008;135:61–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiang SH, Bazuine M, Lumeng CN, et al. The protein kinase IKKepsilon regulates energy balance in obese mice. Cell 2009;138:961–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim JK, Kim YJ, Fillmore JJ, et al. Prevention of fat-induced insulin resistance by salicylate. J Clin Invest 2001;108:437–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirosumi J, Tuncman G, Chang L, et al. A central role for JNK in obesity and insulin resistance. Nature 2002;420:333–336 [DOI] [PubMed] [Google Scholar]
- 18.Jiao P, Chen Q, Shah S, et al. Obesity-related upregulation of monocyte chemotactic factors in adipocytes: involvement of nuclear factor-kappaB and c-Jun NH2-terminal kinase pathways. Diabetes 2009;58:104–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang T, Zhang J, Yin J, et al. Uncoupling of inflammation and insulin resistance by NF-kappaB in transgenic mice through elevated energy expenditure. J Biol Chem 2010;285:4637–4644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiao P, Feng B, Ma J, et al. Constitutive activation of IKKβ in adipose tissue prevents diet-induced obesity in mice. Endocrinology 2012;153:154–165 [DOI] [PubMed] [Google Scholar]
- 21.Kwon H, Laurent S, Tang Y, Zong H, Vemulapalli P, Pessin JE. Adipocyte-specific IKKβ signaling suppresses adipose tissue inflammation through an IL-13-dependent paracrine feedback pathway. Cell Reports 2014;9:1574–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest 2011;121:2094–2101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell 2014;156:20–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li ZW, Omori SA, Labuda T, Karin M, Rickert RC. IKK beta is required for peripheral B cell survival and proliferation. J Immunol 2003;170:4630–4637 [DOI] [PubMed] [Google Scholar]
- 25.Eguchi J, Wang X, Yu S, et al. Transcriptional control of adipose lipid handling by IRF4. Cell Metab 2011;13:249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. Neuroimage 2012;62:782–790 [DOI] [PubMed] [Google Scholar]
- 27.Hu HH, Nayak KS, Goran MI. Assessment of abdominal adipose tissue and organ fat content by magnetic resonance imaging. Obesity Rev 2011;12:e504–e515 [DOI] [PMC free article] [PubMed]
- 28.Park SH, Sui Y, Gizard F, et al. Myeloid-specific IκB kinase β deficiency decreases atherosclerosis in low-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol 2012;32:2869–2876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sui Y, Helsley RN, Park SH, Song X, Liu Z, Zhou C. Intestinal pregnane X receptor links xenobiotic exposure and hypercholesterolemia. Mol Endocrinol 2015;29:765–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rasouli N, Yao-Borengasser A, Varma V, et al. Association of scavenger receptors in adipose tissue with insulin resistance in nondiabetic humans. Arterioscler Thromb Vasc Biol 2009;29:1328–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Finlin BS, Zhu B, Starnes CP, McGehee RE Jr, Peterson CA, Kern PA. Regulation of thrombospondin-1 expression in alternatively activated macrophages and adipocytes: role of cellular cross talk and omega-3 fatty acids. J Nutr Biochem 2013;24:1571–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee KY, Russell SJ, Ussar S, et al. Lessons on conditional gene targeting in mouse adipose tissue. Diabetes 2013;62:864–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pajvani UB, Trujillo ME, Combs TP, et al. Fat apoptosis through targeted activation of caspase 8: a new mouse model of inducible and reversible lipoatrophy. Nat Med 2005;11:797–803 [DOI] [PubMed] [Google Scholar]
- 34.Strissel KJ, Stancheva Z, Miyoshi H, et al. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes 2007;56:2910–2918 [DOI] [PubMed] [Google Scholar]
- 35.Cinti S, Mitchell G, Barbatelli G, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res 2005;46:2347–2355 [DOI] [PubMed] [Google Scholar]
- 36.Tang G, Minemoto Y, Dibling B, et al. Inhibition of JNK activation through NF-kappaB target genes. Nature 2001;414:313–317 [DOI] [PubMed] [Google Scholar]
- 37.De Smaele E, Zazzeroni F, Papa S, et al. Induction of gadd45beta by NF-kappaB downregulates pro-apoptotic JNK signalling. Nature 2001;414:308–313 [DOI] [PubMed] [Google Scholar]
- 38.Yan J, Xiang J, Lin Y, et al. Inactivation of BAD by IKK inhibits TNFα-induced apoptosis independently of NF-κB activation. Cell 2013;152:304–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alkhouri N, Gornicka A, Berk MP, et al. Adipocyte apoptosis, a link between obesity, insulin resistance, and hepatic steatosis. J Biol Chem 2010;285:3428–3438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kosteli A, Sugaru E, Haemmerle G, et al. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J Clin Invest 2010;120:3466–3479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang X, Zhang X, Heckmann BL, Lu X, Liu J. Relative contribution of adipose triglyceride lipase and hormone-sensitive lipase to tumor necrosis factor-α (TNF-α)-induced lipolysis in adipocytes. J Biol Chem 2011;286:40477–40485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee YH, Petkova AP, Granneman JG. Identification of an adipogenic niche for adipose tissue remodeling and restoration. Cell Metab 2013;18:355–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kang S, Kong X, Rosen ED. Adipocyte-specific transgenic and knockout models. Methods Enzymol 2014;537:1–16 [DOI] [PubMed] [Google Scholar]
- 44.Burke JR, Pattoli MA, Gregor KR, et al. BMS-345541 is a highly selective inhibitor of I kappa B kinase that binds at an allosteric site of the enzyme and blocks NF-kappa B-dependent transcription in mice. J Biol Chem 2003;278:1450–1456 [DOI] [PubMed] [Google Scholar]
- 45.Kyriakis JM. Life-or-death decisions. Nature 2001;414:265–266 [DOI] [PubMed] [Google Scholar]
- 46.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003;112:1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 2003;112:1821–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tchkonia T, Thomou T, Zhu Y, et al. Mechanisms and metabolic implications of regional differences among fat depots. Cell Metab 2013;17:644–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chau YY, Bandiera R, Serrels A, et al. Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source. Nat Cell Biol 2014;16:367–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jeffery E, Church CD, Holtrup B, Colman L, Rodeheffer MS. Rapid depot-specific activation of adipocyte precursor cells at the onset of obesity. Nat Cell Biol 2015;17:376–385 [DOI] [PMC free article] [PubMed] [Google Scholar]