Abstract
The EEG recorded from epileptic patients presents with interictal discharges that are not associated with detectable clinical symptoms but are valuable for diagnostic purposes. Experimental studies have shown that interictal discharges and ictal events (i.e., seizures) are characterized intracellularly by similar (but for duration) neuronal depolarizations leading to sustained action potential firing, thus indicating that they may share similar cellular and pharmacological mechanisms. It has also been proposed that interictal discharges may herald the onset of electrographic seizures, but other studies have demonstrated that interictal events interfere with the occurrence of ictal activity. The relationship between interictal and ictal activity thus remains ambiguous. Here we will review this issue in animal models of limbic seizures that are electrographically close to those seen in TLE patients. In particular we will: (i) focus on the electrophysiological and pharmacological characteristics of, at least, two types of interictal discharge; (ii) propose that they play opposite roles in leading to ictogenesis; and (iii) discuss the possibility that mimicking one of these two types of interictal activity by low frequency repetitive stimulation can control ictogenesis. Finally, we will also review evidence indicating that specific types of interictal discharge may play a role in epileptogenesis.
Keywords: GABA, Extracellular potassium, Interictal spikes, Seizures, Temporal lobe epilepsy
1. Introduction
The electroencephalogram (EEG) of patients with partial epileptic disorders presents, between seizures, with interictal discharges that include spikes (<50 ms duration) and sharp waves (50–200 ms duration) (Chatrian et al., 1974; de Curtis and Avanzini, 2001), as well as high frequency oscillations (not specifically addressed in the present review, but see Bragin et al., 1999, 2004; Jefferys et al., 2012). Interictal discharges are not associated with detectable clinical symptoms and are valuable both for diagnosing the epileptic condition and for localizing the epileptogenic area (Jacobs et al., 2010; de Curtis and Avanzini, 2001).
Experimental work performed in both in vivo and in vitro animal models of seizures and epilepsy have demonstrated that interictal spikes/sharp waves and ictal discharges are characterized intracellularly by similar (but for duration) neuronal depolarizations that lead to sustained action potential firing (Ayala et al., 1973; Sherwin, 1984; Prince, 1993; Lopantsev and Avoli, 1998). Therefore, interictal and ictal discharges may share similar cellular and pharmacological mechanisms.
It has also been proposed that interictal events may herald the onset of electrographic seizures. For instance, experiments performed in the early 1970s led to the hypothesis that the fading of post-interictal spike hyperpolarizations could play a role in the transition from interictal to ictal discharge (see Ayala et al., 1973). However, clinical studies have later shown that the rate of occurrence of interictal discharges does not change before seizure onset in temporal lobe epilepsy (TLE) (Lange et al., 1983; Gotman and Marciani, 1985; Gotman, 1991). Moreover, evidence obtained in both in vivo (Engel and Ackermann, 1980) and in vitro animal models (Swartzwelder et al., 1987; Bragdon et al., 1992; Barbarosie and Avoli, 1997; Librizzi and de Curtis, 2003; see for review Avoli and de Curtis, 2011) indicates that interictal spiking may interfere with the occurrence of ictal discharges. The relationship between interictal and ictal activity thus remains ambiguous.
Here we will review this issue in animal models of limbic seizures that are electrographically close to those seen in TLE patients. In particular we will: (i) focus on the electrophysiological and pharmacological characteristics of, at least, two types of interictal discharge that are recorded in brain slices maintained in vitro; (ii) propose that they play opposite roles in leading to ictogenesis; and (iii) discuss the possibility that mimicking one of these two types of interictal activity by low frequency repetitive stimulation can control ictogenesis. In addition, we will briefly review experimental and clinical evidence indicating that specific types of interictal discharge may play a role in epileptogenesis.
2. Two interictal identities
Focal application of GABAA receptor antagonists to the cortex in intact preparations or superfusion of brain slices maintained in vitro with medium containing these drugs have represented the core of basic epilepsy research for more than half a century. These studies, however, rarely addressed the relation between interictal and ictal discharges, since the latter seldom occur under these experimental conditions. This problem has been solved by using manipulations that do not fully block GABAA receptor inhibition and even enhance it. Accordingly, it has been shown that prolonged epileptiform events, which may represent the equivalent of ictal phenomena, along with interictal activity are induced by application of the K+ channel blocker 4-aminopyridine (4AP; see for review Avoli and de Curtis, 2011), the cholinergic agonist pilocarpine (Nagao et al., 1996), or medium containing either high [K+] (Jensen and Yaari, 1988) or nominally zero Mg2+ (Dreier and Heinemann, 1991; Kohling et al., 2000). Since TLE patients present with seizure discharges in several limbic structures, most of these studies have been carried out on rodent brain slices that include interconnected portions of hippocampal and parahippocampal areas.
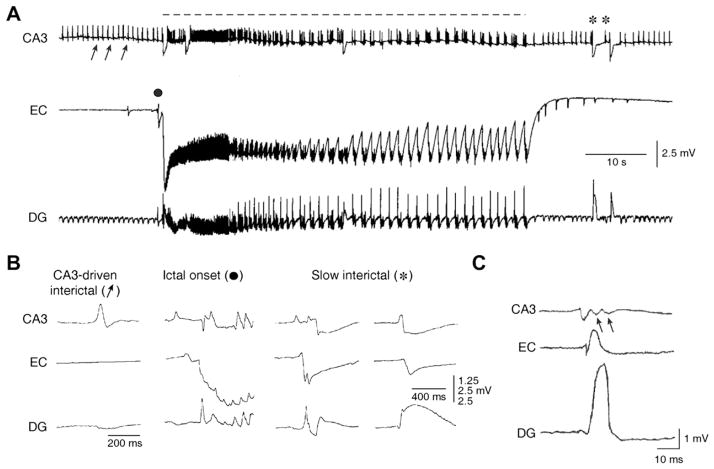
As illustrated in Fig. 1A, these “extended” brain slices – when treated, for instance, with 4AP – generate epileptiform activity resembling both interictal and ictal events. Ictal discharges (dotted line in Fig. 1A): (i) depend on the activation of both NMDA and non-NMDA glutamatergic and GABAA receptors and (ii) in the adult brain slice they appear to initiate outside the hippocampus proper. In fact, depending upon how brain slices are cut (and thus which parahippocampal structures are included and how they are reciprocally interconnected), ictal activity can originate from the entorhinal cortex (EC) (Fig. 1A and B, ictal onset panel), the perirhinal cortex (PC), the amygdala, as well as the insular or cingulate cortices (Avoli and de Curtis, 2011). Clinically these are all brain structures that are prone to generate seizures in patients with complex partial epilepsies (Rutecki et al., 1989; Gloor, 1992; Spencer and Spencer, 1994; Isnard et al., 2000; Bartolomei et al., 2005; Vaugier et al., 2009).
Fig. 1.
Epileptiform patterns induced by 4AP in rodent hippocampus-entorhinal cortex slices. (A) Field potential recordings performed simultaneously in the rat CA3 subfield, EC and dentate gyrus demonstrate the occurrence of three different types of activity; the first (discontinuous line) is recorded synchronously in all areas and consists of a sustained ictal-like epileptiform discharge; the second type (arrows) consists of continuous fast interictal-like events, and it is seen in the CA3 and dentate areas only; the third type (asterisk) is recorded in all areas and is characterized by a slow field potential. (B) Expanded traces of the field potential recordings illustrated in A show the modalities of onset and spread of CA3-drive interictal events, ictal discharge onset, and slow interictal discharge; note that the ictal discharge initiates in the EC while different sites of origin characterize the two examples of slow interictal events. (C) Expanded traces of a CA3-driven interictal discharge recorded from a mouse hippocampus-EC slice; note that this interictal discharge initiates in the CA3 region and propagates to the EC and DG; arrows point at the “late” components of the CA3-driven interictal discharge recorded in CA3, presumably representing the reentry of synchronous activity from the DG. In this and following figures abbreviations are: entorhinal cortex (EC); dentate gyrus (DG).
Close inspection of interictal activity recorded during 4AP application also reveals two distinct types of discharge: one type has a relatively low rate of occurrence (intervals can last up to 50 s), appears in all limbic areas, and has variable site of initiation (Fig. 1A and B, asterisks); the other type (Fig. 1A and B, arrows) occurs at short intervals (approx. every 1–4 s), originates in the CA3 area and can propagate to the EC/PC via the subiculum and then re-enter the hippocampus proper through the dentate gyrus (Fig. 1C). Moreover, both the dynamics and the velocity of spread of the first type of interictal discharge are slower than those characterizing CA3-driven interictal events (Perreault and Avoli, 1991, 1992); hence, these two types of discharges will be thereafter referred to as slow and fast interictal discharges, respectively.
Pharmacological analysis (reviewed in Avoli and de Curtis, 2011) has shown that the slow interictal events continue to occur and to propagate during blockade of NMDA and non-NMDA glutamatergic receptors (albeit at a lower pace), but are sensitive to GABAA receptor antagonists or μ-opioid receptor agonists, a pharmacological procedure that abolishes the presynaptic GABA release (Capogna et al., 1993). Hence, these pharmacological findings strongly suggest that the slow interictal discharges largely reflect the postsynaptic response of principal, glutamatergic neurons to GABA released by interneurons.
Similar hypersynchronous, glutamate receptor-independent, GABAergic potentials are recorded from several areas of the isolated in vitro guinea pig brain preparation during application of 4AP and ionotropic glutamatergic receptor antagonists (Uva et al., 2009; Carriero et al., 2010). As in the in vitro slice preparation, these synchronous events, which are independent on glutamatergic transmission, appear to be capable of propagating to distant limbic structures, even to across hemispheres, in the in vitro guinea pig brain (Uva et al., 2009). At variance, CA3-driven interictal activity recorded in brain slices is not influenced by NMDA receptor antagonists, can be reinforced by pharmacological procedures that interfere with GABAA receptor signalling, and is readily blocked by non-NMDA glutamatergic receptor antagonists (see Avoli and de Curtis, 2011).
2.1. Slow GABAergic interictal discharges lead to ictogenesis
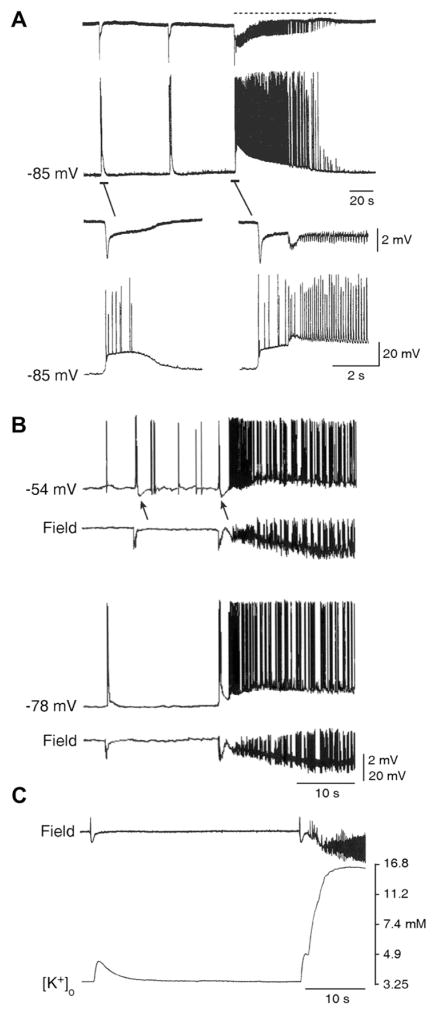
As mentioned above, pharmacological procedures that cause epileptiform synchronization by fully blocking GABAA receptor signaling do not easily induce ictal discharges while either a reduction or a reinforcement of GABAergic inhibition can lead to ictogenesis. Indeed, electrophysiological and pharmacological findings obtained in the in vitro 4AP model indicate that ictal discharge onset is characterized by an event that shares similar extracellular and intracellular features with the slow interictal discharge (which, as already mentioned, largely reflects GABAA receptor-mediated conductances; see Fig. 2A). In keeping with the presumptive inhibitory nature of the process leading to ictogenesis, the initial component of the ictal discharge is hyperpolarizing at lower membrane potentials (Fig. 2B; Avoli and de Curtis, 2011).
Fig. 2.
Relationship between slow interictal spikes and ictal discharges recorded from the EC during 4AP application. (A) Simultaneous extracellular (Field) and “sharp” intracellular (−85 mV) recordings obtained from the rat EC during 4AP application reveal slow interictal spikes and an ictal discharge. Note in the expanded traces that the ictal discharge appears to be initiated by an event similar to that associated to the interictal spike. (B) Field and intracellular characteristics of slow interictal events and ictal discharge onset in an EC neuron that was recorded with K-acetate-filled microelectrode during 4AP application both at resting membrane potential (−78 mV) and during intracellular injection of steady positive current (−54 mV). Note that this procedure causes a decrease of the ictal depolarization that is preceded by a hyperpolarizing event similar to what seen during the slow interictal event (arrows). (C) Simultaneous field and [K+]o recordings obtained from the rat EC show that the slow interictal spike is associated with a transient increases in [K+]o, while a sustained elevation is seen during the tonic phase of the ictal discharge; note that the ictal discharge onset is characterized by a “transient-like” increase in [K+]o that is larger than what observed during the isolated slow interictal discharges.
Experiments employing K+ selective microelectrodes have demonstrated that the slow interictal discharges occurring in isolation as well as those that immediately precede the ictal discharge are accompanied by transient elevations in [K+]o. In addition, the [K+]o increase associated with ictal discharge onset is larger than that recorded in association with the isolated slow spikes occurring during the interictal period (Fig. 2C). For instance, we have reported in the EC that [K+]o elevations associated with the slow interictal discharges reach peak values of 4.4 ± 0.3 mM, while those preceding ictal discharges had values of 6.0 ± 0.2 mM (Avoli et al., 1996).
The transient increases in [K+]o associated with the slow interictal spikes attain similar values both under control conditions (i.e., during application of 4AP only) and following blockade of ionotropic glutamatergic receptors, thus suggesting that GABA per se is the main factor for the [K+]o increases (Avoli et al., 1996). This conclusion is supported by the elegant work of Barolet and Morris (1991) who demonstrated that [K+]o elevations occur in the hippocampus during application of exogenous GABA or GABAA (but not GABAB) receptor agonists in the presence of the voltage-gated Na+ channel blocker tetrodotoxin; these results, thus, rule out a meaningful contribution of action potential firing to the elevations in [K+]o recorded during the GABAA receptor-mediated interictal-like events. In addition both slow interictal events and their associated [K+]o increases recorded during blockade of excitatory glutamatergic receptors are readily decreased and eventually abolished by applying the GABAA receptor antagonist bicuculline methiodide (Fig. 3A). Similar results can also be observed during pharmacological activation of μ-opioid receptors, a procedure that, as already mentioned blocks the release of GABA from interneurons. Pre-ictal GABA-mediated activities have also been observed in the neocortex; there, however, they appear to sub-serve a different role, namely imposing a restraint to activity propagation (Trevelyan et al., 2006; Trevelyan, 2009).
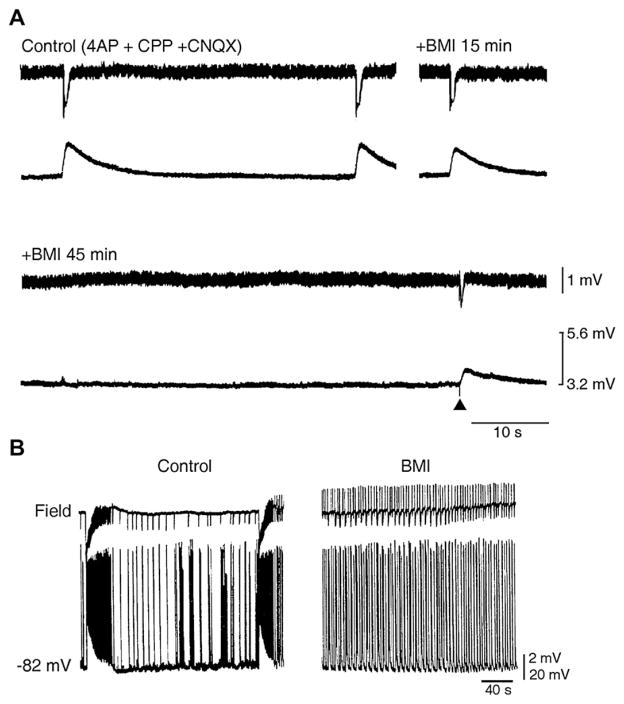
Fig. 3.
GABAA receptors contribute to the [K+]o increases associated with slow interictal discharges and to ictogenesis. (A) Simultaneous field and [K+]o recordings performed in the rat EC during application of 4AP and glutamatergic receptor antagonists (Control) and at different times after addition of the GABAA receptor antagonist bicuculline methiodide (BMI). Note that after 45 min of BMI application, no spontaneous events can be observed as well as that the response induced by an electrical stimulus delivered close to the recording site induces reduced field and [K+]o signals. (B) Application of BMI abolishes slow interictal and ictal discharges recorded from an EC slice with simultaneous extracellular (Field trace) and intracellular microelectrodes (−78 mV trace) while disclosing rhythmic short-lasting epileptiform events.
Overall, these data have led us to hypothesize that the increase in [K+]o caused by GABAA receptor activation during the slow spikes can lead to hypersynchronization of neuronal networks and thus to ictal activity. This hypothesis is supported by experiments in which drugs that interfere with GABAA receptor transmission were shown to abolish both the slow interictal and the ictal discharges that occur in the presence of 4AP (Fig. 3B). A similar slow interictal-ictal discharge relationship has been found in isolated rat hippocampal slices that were obtained from young rats (Avoli et al., 1996). Moreover, slow interictal spikes have been reported to occur just ahead of seizures in the hippocampus and the EC in the guinea pig isolated brain in vitro following arterial perfusion of 4AP (Uva et al., 2009; Carriero et al., 2010). It has also been shown in the guinea pig isolated brain preparation that a brief arterial application of bicuculline (which only reduces GABAergic inhibition by approx. 40%) causes electrographic seizures in the EC that are shortly preceded by large amplitude interictal spikes, which correspond to GABAA receptor-mediated hyperpolarizations in principal neurons and correlate to a burst discharge in putative interneurons (Gnatkovsky et al., 2008). These presumptive GABAergic spikes entrain interneuronal networks to generate fast oscillatory activity that is typically observed at seizure onset (Gnatkovsky et al., 2008). Finally, Kohling et al. (2000) have reported in the Mg2+-free model that the onset of ictal discharges generated by CA1 neurons is preceded by GABAA receptor-mediated gamma oscillations and that this ictal activity is abolished by GABAA receptor antagonists. Hence, recruitment of inhibitory mechanisms during slow interictal events is crucial for seizure activity initiation and occurrence.
However, a different picture was recently described in brain slices of the human subiculum that was obtained from post-surgery tissue of patients with TLE resistant to antiepileptic drugs. In these experiments, interictal spikes are sustained by GABAergic neurotransmission (Cohen et al., 2002) but transition to seizure involves an emergent glutamatergic population activity (Huberfeld et al., 2011). In addition, Zhang et al. (2012) have recently reported that interictal and early pre-ictal states recorded in the CA3 region of the intact mouse hippocampus superfused with low-Mg2+/high-K+ medium, are dominated by GABAergic activity while seizure onset is heralded by exhaustion of presynaptic release of GABA, and an unopposed increase in glutamatergic mechanisms. It should also be emphasized that GABAergic mechanisms have been reported to participate to acute seizure-like discharges that are induced in hippocampal slices maintained in vitro (Ziburkus et al., 2006; Fujiwara-Tsukamoto et al., 2007).
2.2. Fast interictal activity controls ictogenesis
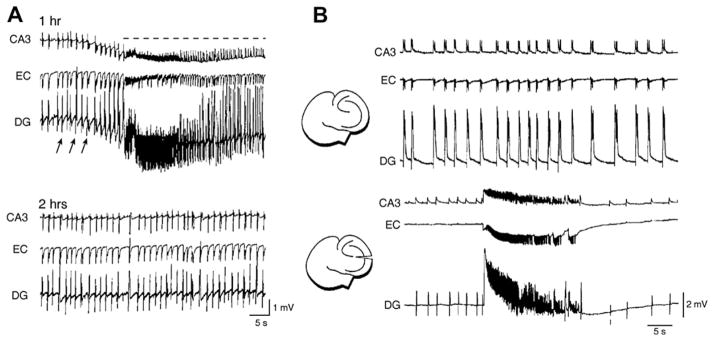
Fast CA3-driven interictal discharges can surprisingly control the propensity of parahippocampal structures to ictogenesis. This mechanism was originally proposed by Swartzwelder (Swartzwelder et al., 1987), who discovered that ictal discharges in the Mg2+-free model are inversely correlated in frequency when interictal activity is reduced by applying the GABAB receptor agonist baclofen. This process was later confirmed by Barbarosie and Avoli (1997) in mouse combined (and functionally interconnected) hippocampus-EC slices, in which interictal and ictal events are generated by distinct limbic networks (see above). As illustrated in Fig. 4A, ictal discharges – which are consistently initiated by the EC – disappear in these experiments after 1–2 h of bath application of 4AP or Mg2+-free medium, while fast CA3-driven interictal activity continues to occur for several hours throughout the experiment. Moreover, a subsequent Schaffer collateral cut, which abolishes the spread of the CA3-driven interictal discharges to the EC, restores ictal activity generation thus suggesting that CA3-driven activity exerts an unexpected “inhibitory” effect on the expression of ictal discharges (Fig. 4B).
Fig. 4.
(A) Epileptiform activity recorded 1 and 2 h after initiation of continuous bath application of 4AP from a combined mouse hippocampus-EC slice. Note that ictal (dotted line) and fast-developing interictal (arrows) activity is recorded at 1 h; at 2 h of 4AP application, ictal discharges disappear. (B) Spontaneous epileptiform activity induced by super fusing a combined mouse hippocampus-EC slice with Mg2+-free medium before and after Schaffer collateral cut. Note that before the lesion (top panel), interictal discharges are recorded from all limbic structures, while after sectioning the Schaffer collaterals (bottom panel) interictal discharges disappear in the EC and ictal activity, which is recorded in the three areas, is disclosed.
This mechanism of ictogenesis control has later been confirmed in rat brain slices in which structures such as the EC and PC or the amygdala were interconnected; these results have been recently reviewed by Avoli & de Curtis (2011). In this context, studies of the Holmes’ group (Zhou et al., 2007) point into a similar direction. While this study did not directly address the interplay between interictal spikes and ictal discharges, it nevertheless reported a reduction of hippocampal cell firing up to 2 s after the occurrence of interictal spikes in two different models of epilepsy in vivo. Taken together, these studies strongly suggest that CA3-driven interictal discharges likely control rather than sustain limbic seizures.
3. Activity-dependent changes in network excitability and the K+ link
Several mechanisms, which include activity-dependent changes in synaptic and non-synaptic excitability as well as pH changes and gap-junction coupling (de Curtis et al., 1998), may contribute to the ability of CA3-driven interictal discharges to prevent the occurrence of ictal discharges in parahippocampal structures. However, evidence that was mainly obtained from the 4AP model suggest that a main player in controlling ictogenesis rests on the ability of the CA3-driven interictal activity to hamper transient elevations in [K+]o associated with the slow interictal events, which are indeed instrumental for ictal discharge onset. In keeping with this view, the slow interictal discharges recorded after Schaffer collateral cut in the EC are accompanied by larger [K+]o elevations than those seen during the pre-cut control period, and can attain peak values that are sufficient for triggering ictal discharges (Barbarosie et al., 2002). Thus, according with this view, CA3-driven interictal events serve to insure that GABA release during the slow interictal discharges is down-regulated, thus keeping at bay the associated [K+]o elevations.
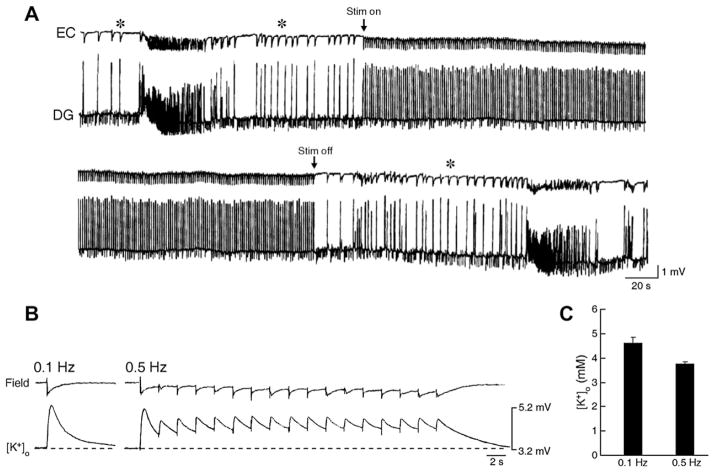
This hypothesis is further supported by experiments in which parahippocampal structures such as the EC, the amygdala or the insular cortex are stimulated electrically at frequencies that are similar to those of the CA3-driven interictal activity (i.e., at 0.5–1.0 Hz). As illustrated in Fig. 5A, these studies have revealed that following Schaffer collateral cut, ictal discharge generation is depressed throughout such stimulation period. Of relevance for the mechanisms underlying the ability of low frequency stimulation to abate in vitro ictal activity, recent experiments in our laboratories have shown that the elevations in [K+]o induced by electrical stimuli delivered at 0.5 Hz during ionotropic glutamate receptor blockade attain values that are significantly lower that those associated with responses elicited by stimuli delivered at frequency of, or lower than 0.1 Hz (Fig. 5B and C).
Fig. 5.
(A) Effect induced by stimulation at 1 Hz delivered in the CA1 on the 4AP-induced epileptiform activity recorded after Schaffer collateral cut from a combined mouse hippocampus-EC slice. Note that ictal discharge is abolished during the stimulation period and re-appears on termination of the stimulation; note also the persistence of interictal discharges (asterisks), presumably of EC origin, before and after the ictal activity. (B) Simultaneous field and [K+]o recordings obtained from the rat EC during application of 4AP and glutamatergic receptor antagonists. Electrical stimuli were delivered in the EC (subicular side) at 0.1 and 0.5 Hz. Note that the peak elevations in [K+]o, were smaller during the latter protocol. (C) Histogram of the peak elevations in [K+]o induced by 0.1 and 0.5 Hz stimulating protocols during 6 trials in two experiments; note that the peak value is smaller during repetitive stimuli delivered at 0.5 Hz. Values are mean ± SEM.
Therefore, it can be concluded that either hippocampal output activity in the form of CA3-driven interictal discharges or low-frequency electrical stimuli may control the ictogenic propensity of parahippocampal networks by hampering excessive levels of synchronization that are, at least in this in vitro model, facilitated by GABAA receptor-mediated increases in [K+]o. It should also be mentioned that during the last decade clinical studies have shown that low frequency stimulation – delivered through transcranial magnetic or deep-brain electrical procedures –can reduce seizures in patients presenting with epileptic disorders refractory to conventional antiepileptic therapy, including TLE (Tergau et al., 1999; Theodore and Fisher, 2004; Vonck et al.,2002; Yamamoto et al.,2006).
4. Interictal activity and epileptogenesis
The data discussed in the previous section underscore the role of hippocampal output activity in controlling ictogenesis. This aspect may be indeed relevant for the pathophysiology of TLE, a condition that is known to be associated with a rather specific pattern of seizure-induced cell damage in both humans and animal models such as reproduced in rodents following an initial pilocarpine-induced status epilepticus (Gloor, 1997). Accordingly, CA3-driven interictal discharges have been reported to be less frequent in slices obtained from pilocarpine-treated epileptic mice or rats as compared to non-epileptic controls (D’Antuono et al., 2002; Panuccio et al., 2010). Thus, in epileptic animals (and perhaps in TLE patients) a decrease in hippocampal output activity secondary to cell damage, could let the EC and other parahippocampal structures generate ictal discharges. In addition, it has been proposed that such decrease in hippocampal output activity leads to the functional disclosure of a direct temporo-ammonic path that excites both CA1 and subicular neurons (D’Antuono et al., 2002).
The potential role played by different types of interictal events in epileptogenesis is also supported by data obtained by performing chronic EEG recordings during the latent period up to and including the chronic epileptic state in rats that had initially experienced a pilocarpine-induced, 120 min long, status epilepticus (Bortel et al., 2010). In this study, the frequency of interictal discharges recorded from the EC was significantly lower in the latent than in the chronic period of recurring seizures while interictal spikes in the CA3 area diminished in duration following seizure appearance. Finally, interictal spikes recorded from the amygdala, as in the case on the EC, occurred at rates that were significantly higher in the chronic than in the latent period. Hence, these in vivo experimental data suggest that interictal discharges undergo structure-specific modifications once chronic seizures begin to recur in this animal model of TLE.
Evidence supporting the view that interictal activity plays a role in epileptogenesis (and the gravity of the epileptic condition) also stems from a clinical study, in which it was shown that TLE patients with no or few interictal epileptic discharges in scalp EEG recordings have a higher age at seizure onset, less frequent and less severe seizures, and a lower incidence of hippocampal atrophy than those with frequent interictal abnormalities (Rosati et al., 2003). Depth electrode recordings were not used in this study to establish the presence of interictal discharges in mesial temporal structures; nonetheless, an additional finding reported by Rosati et al. (Rosati et al., 2003) was that these so-called “oligospikers” had an excellent surgical outcome. This finding has been later confirmed by Krendl et al. (Krendl et al., 2008).
5. Conclusions
We have presented here evidence that indicates that interictal spikes can have both anti- and pro-seizure actions. Indeed, pro-seizure interictal spikes in in vitro experiments appear to be sustained by GABAergic network activation. Specifically, these findings suggest a scenario in which interneuron discharge associated to these interictal events causes large transient increases in [K+]o that exceed those induced by non-GABAergic interictal spikes. It is well known that elevated [K+]o can depolarize neurons and cause seizure activity both in vivo (Zuckermann and Glaser, 1968) and in vitro (Jensen and Yaari, 1988). It should also be emphasized that this evidence points at GABAA receptor-mediated signaling as a major active player in synchronizing neuronal networks in seizure-prone brain structures such as those of the limbic system.
Historically, the existence of prognostically “bad” and “good” interictal spike dates back to the electrocorticographic studies performed during epilepsy surgery by W. Penfield and T. Rasmussen in the 1950s and 1960s who termed these interictal spikes “red” and “green”, respectively (W. Feindel, personal communication). It should be, however, emphasized that although the data presented here support such a concept, differentiation between good and bad spikes in the clinical contest remains difficult since their exact cellular correlate cannot be determined from the EEG recordings obtained from epileptic patients.
The mechanisms by which some types of interictal discharges can exert an anti-ictogenic action remain unclear. However, as reviewed in Section 3, they may induce desynchronizing effects similar to those observed during repetitive, low frequency stimulation. According with this view, both hippocampal output activity in the form of CA3-driven interictal discharges and low-frequency electrical stimuli may control seizure occurrence by hampering excessive levels of synchronization that are presumably linked to GABAA receptor-mediated increases in [K+]o.
In conclusion, interictal discharges can either facilitate or prevent ictal activity depending on their underlying mechanisms and, perhaps, the brain structure in which they originate. Moreover, a similar conflicting role of interictal activity may exist during epileptogenesis (Bortel et al., 2010), although more studies are required to identify specific roles. Indeed, by establishing them, we can reasonably anticipate new, more efficacious, and safer antiepileptic strategies.
Acknowledgments
The original studies reviewed in this paper were supported by grants from the Canadian Institutes of Health Research, the Savoy Foundation, CURE, the Piergiorgio and Luisa Mariani Foundation, The Wellcome Trust and the Italian Health Ministry.
References
- Avoli M, de Curtis M. GABAergic synchronization in the limbic system and its role in the generation of epileptiform activity. Prog Neurobiol. 2011;95:104–132. doi: 10.1016/j.pneurobio.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avoli M, Louvel J, Kurcewicz I, Pumain R, Barbarosie M. Extracellular free potassium and calcium during synchronous activity induced by 4-aminopyridine in the juvenile rat hippocampus. J Physiol. 1996;493:707–717. doi: 10.1113/jphysiol.1996.sp021416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala GF, Dichter M, Gumnit RJ, Matsumoto H, Spencer WA. Genesis of epileptic interictal spikes. New knowledge of cortical feedback systems suggests a neurophysiological explanation of brief paroxysms. Brain Res. 1973;1973(52):1–17. doi: 10.1016/0006-8993(73)90647-1. [DOI] [PubMed] [Google Scholar]
- Barbarosie M, Avoli M. CA3-driven hippocampal-entorhinal loop controls rather than sustains in vitro limbic seizures. J Neurosci. 1997;17:9308–9314. doi: 10.1523/JNEUROSCI.17-23-09308.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbarosie M, Louvel J, D’Antuono M, Kurcewicz I, Avoli M. Masking synchronous GABA-mediated potentials controls limbic seizures. Epilepsia. 2002;43:1469–1479. doi: 10.1046/j.1528-1157.2002.17402.x. [DOI] [PubMed] [Google Scholar]
- Barolet AW, Morris ME. Changes in extracellular K+ evoked by GABA, THIP and baclofen in the guinea-pig hippocampal slice. Exp Brain Res. 1991;84:591–598. doi: 10.1007/BF00230971. [DOI] [PubMed] [Google Scholar]
- Bartolomei F, Khalil M, Wendling F, Sontheimer A, Regis J, Ranjeva JP, Guye M, Chauvel P. Entorhinal cortex involvement in human mesial temporal lobe epilepsy: an electrophysiologic and volumetric study. Epilepsia. 2005;46:677–687. doi: 10.1111/j.1528-1167.2005.43804.x. [DOI] [PubMed] [Google Scholar]
- Bortel A, Levesque M, Biagini G, Gotman J, Avoli M. Convulsive status epilepticus duration as determinant for epileptogenesis and interictal discharge generation in the rat limbic system. Neurobiol Dis. 2010;40:478–489. doi: 10.1016/j.nbd.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragdon AC, Kojima H, Wilson WA. Suppression of interictal bursting in hippocampus unleashes seizures in entorhinal cortex: a proepileptic effect of lowering [K+]o and raising [Ca2+]o. Brain Res. 1992;590:128–135. doi: 10.1016/0006-8993(92)91088-v. [DOI] [PubMed] [Google Scholar]
- Bragin A, Engel J, Jr, Wilson CL, Fried I, Buzsaki G. High-frequency oscillations in human brain. Hippocampus. 1999;9:137–142. doi: 10.1002/(SICI)1098-1063(1999)9:2<137::AID-HIPO5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Bragin A, Wilson CL, Almajano J, Mody I, Engel J., Jr High-frequency oscillations after status epilepticus: epileptogenesis and seizure genesis. Epilepsia. 2004;45:1017–1023. doi: 10.1111/j.0013-9580.2004.17004.x. [DOI] [PubMed] [Google Scholar]
- Capogna M, Gahwiler BH, Thompson SM. Mechanism of mu-opioid receptor-mediated presynaptic inhibition in the rat hippocampus in vitro. J Physiol. 1993;470:539–558. doi: 10.1113/jphysiol.1993.sp019874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carriero G, Uva L, Gnatkovsky V, Avoli M, de Curtis M. Independent epileptiform discharge patterns in the olfactory and limbic areas of the in vitro isolated Guinea pig brain during 4-aminopyridine treatment. J Neurophysiol. 2010;103:2728–2736. doi: 10.1152/jn.00862.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatrian GE, Bergamini L, Dondey M, Klass DW, Lennox-Buchthal M, Petersen I. A glossary of terms most commonly used by clinical electroencephalographers. Electroenceph Clin Neurophysiol. 1974;37:538–548. doi: 10.1016/0013-4694(74)90099-6. [DOI] [PubMed] [Google Scholar]
- Cohen I, Navarro V, Clemenceau S, Baulac M, Miles R. On the origin of interictal activity in human temporal lobe epilepsy in vitro. Science. 2002;298:1418–1421. doi: 10.1126/science.1076510. [DOI] [PubMed] [Google Scholar]
- D’Antuono M, Benini R, Biagini G, D’Arcangelo G, Barbarosie M, Tancredi V, Avoli M. Limbic network interactions leading to hyperexcitability in a model of temporal lobe epilepsy. J Neurophysiol. 2002;87:634–639. doi: 10.1152/jn.00351.2001. [DOI] [PubMed] [Google Scholar]
- de Curtis M, Avanzini G. Interictal spikes in focal epileptogenesis. Progr Neurobiol. 2001;63:541–567. doi: 10.1016/s0301-0082(00)00026-5. [DOI] [PubMed] [Google Scholar]
- de Curtis M, Manfridi A, Biella G. Activity-dependent pH changes and periodicity of spontaneous interictal spikes. J Neurosci. 1998;15:7543–7551. doi: 10.1523/JNEUROSCI.18-18-07543.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreier JP, Heinemann U. Regional and time dependent variations of low Mg2+ induced epileptiform activity in rat temporal cortex slices. Exp Brain Res. 1991;87:581–596. doi: 10.1007/BF00227083. [DOI] [PubMed] [Google Scholar]
- Engel J, Ackermann R. Interictal EEG spikes correlate with decreased, rather than increased, epileptogenicity in amygdaloid kindled rats. Brain Res. 1980;190:543–548. doi: 10.1016/0006-8993(80)90296-6. [DOI] [PubMed] [Google Scholar]
- Fujiwara-Tsukamoto Y, Isomura Y, Imanishi M, Fukai T, Takada M. Distinct types of ionic modulation of GABA actions in pyramidal cells and interneurons during electrical induction of hippocampal seizurelike network activity. Eur J Neurosci. 2007;25:2713–2725. doi: 10.1111/j.1460-9568.2007.05543.x. [DOI] [PubMed] [Google Scholar]
- Gloor P. Role of the amygdala in temporal lobe epilepsy. In: Aggleton J, editor. The Amygdala: Neurobiological Aspects of Emotion, Memory and Mental Dysfunction. Wiley-Liss; New York: 1992. pp. 505–538. [Google Scholar]
- Gloor P. The Temporal Lobe and Limbic System. Oxford University Press; New York: 1997. [Google Scholar]
- Gnatkovsky V, Librizzi L, Trombin F, de Curtis M. Fast activity at seizure onset is mediated by inhibitory circuits in the entorhinal cortex in vitro. Ann Neurol. 2008;64:674–686. doi: 10.1002/ana.21519. [DOI] [PubMed] [Google Scholar]
- Gotman J, Marciani M. Electroencephalographic spiking activity, drug levels, and seizure occurrence in epileptic patients. Ann Neurol. 1985;17:597–603. doi: 10.1002/ana.410170612. [DOI] [PubMed] [Google Scholar]
- Gotman J. Relationships between interictal spiking and seizures: human and experimental evidence. Can J Neurol Sci. 1991;18:573–576. doi: 10.1017/s031716710003273x. [DOI] [PubMed] [Google Scholar]
- Huberfeld G, Menendez de la Prida L, Pallud J, Cohen I, Le Van Quyen M, Adam C, Clemenceau S, Baulac M, Miles R. Glutamatergic pre-ictal discharges emerge at the transition to seizure in human epilepsy. Nat Neurosci. 2011;14:627–634. doi: 10.1038/nn.2790. [DOI] [PubMed] [Google Scholar]
- Isnard J, Guenot M, Ostrowsky K, Sindou M, Mauguiere F. The role of the insular cortex in temporal lobe epilepsy. Ann Neurol. 2000;48:614–623. [PubMed] [Google Scholar]
- Jacobs J, Kobayashi K, Gotman J. High-frequency changes during interictal spikes detected by time-frequency analysis. Clin Neurophysiol. 2010 doi: 10.1016/j.clinph.2010.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferys JGR, Menendez de la Prida L, Wendling F, Bragin A, Avoli M, Timofeev I, Lopes da Silva FH. Mechanisms of physiological and epileptic HFO generation. Prog Neurobiol. 2012 Mar 7; doi: 10.1016/j.pneurobio.2012.02.005. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MS, Yaari Y. The relationship between interictal and ictal paroxysms in an in vitro model of focal hippocampal epilepsy. Ann Neurol. 1988;24:591–598. doi: 10.1002/ana.410240502. [DOI] [PubMed] [Google Scholar]
- Kohling R, Vreugdenhil M, Bracci E, Jefferys JG. Ictal epileptiform activity is facilitated by hippocampal GABAA receptor-mediated oscillations. J Neurosci. 2000;20:6820–6829. doi: 10.1523/JNEUROSCI.20-18-06820.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krendl R, Lurger S, Baumgartner C. Absolute spike frequency predicts surgical outcome in TLE with unilateral hippocampal atrophy. Neurology. 2008;71:413–418. doi: 10.1212/01.wnl.0000310775.87331.90. [DOI] [PubMed] [Google Scholar]
- Lange HH, Lieb JP, Engel J, Jr, Crandall PH. Temporo-spatial patterns of pre-ictal spike activity in human temporal lobe epilepsy. EEG Clin Neurophysiol. 1983;56:543–555. doi: 10.1016/0013-4694(83)90022-6. [DOI] [PubMed] [Google Scholar]
- Librizzi L, de Curtis M. Epileptiform ictal discharges are prevented by periodic interictal spiking in the olfactory cortex. Ann Neurol. 2003;53:382–389. doi: 10.1002/ana.10471. [DOI] [PubMed] [Google Scholar]
- Lopantsev V, Avoli M. Participation of GABAA-mediated inhibition in ictallike discharges in the rat entorhinal cortex. J Neurophysiol. 1998;79:352–360. doi: 10.1152/jn.1998.79.1.352. [DOI] [PubMed] [Google Scholar]
- Nagao T, Alonso A, Avoli M. Epileptiform activity induced by pilocarpine in the rat hippocampal-entorhinal slice preparation. Neuroscience. 1996;72:399–408. doi: 10.1016/0306-4522(95)00534-x. [DOI] [PubMed] [Google Scholar]
- Panuccio G, D’Antuono M, de Guzman P, De Lannoy L, Biagini G, Avoli M. In vitro ictogenesis and parahippocampal networks in a rodent model of temporal lobe epilepsy. Neurobiol Dis. 2010;39:372–780. doi: 10.1016/j.nbd.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault P, Avoli M. Physiology and pharmacology of epileptiform activity induced by 4-aminopyridine in rat hippocampal slices. J Neurophysiol. 1991;65:771–785. doi: 10.1152/jn.1991.65.4.771. [DOI] [PubMed] [Google Scholar]
- Perreault P, Avoli M. 4-aminopyridine-induced epileptiform activity and a GABA-mediated long-lasting depolarization in the rat hippocampus. J Neurosci. 1992;12:104–115. doi: 10.1523/JNEUROSCI.12-01-00104.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince DA. In: Basic Mechanisms of Focal Epileptogenesis. Avanzini G, Fariello R, Heinemann U, Mutani R, editors. Libbey; London: 1993. pp. 17–27. [Google Scholar]
- Rosati A, Aghakhani Y, Bernasconi A, Olivier A, Andermann F, Gotman J, Dubeau F. Intractable temporal lobe epilepsy with rare spikes is less severe than with frequent spikes. Neurology. 2003;60:1290–1295. doi: 10.1212/01.wnl.0000058761.12715.0e. [DOI] [PubMed] [Google Scholar]
- Rutecki PA, Grossman RG, Armstrong D, Irish-Loewen S. Electrophysiological connections between the hippocampus and entorhinal cortex in patients with complex partial seizures. J Neurosurg. 1989;70:667–675. doi: 10.3171/jns.1989.70.5.0667. [DOI] [PubMed] [Google Scholar]
- Sherwin I. Ictal-interictal unit firing pattern differences in penicillin-induced primary and secondary epileptogenic foci. Exp Neurol. 1984;84:463–477. doi: 10.1016/0014-4886(84)90242-5. [DOI] [PubMed] [Google Scholar]
- Spencer DD, Spencer SS. Hippocampal resections and the use of human tissue in defining temporal lobe epilepsy syndromes. Hippocampus. 1994;4:243–249. doi: 10.1002/hipo.450040303. [DOI] [PubMed] [Google Scholar]
- Swartzwelder HS, Lewis DV, Anderson WW, Wilson WA. Seizure-like events in brain slices: suppression by interictal activity. Brain Res. 1987;410:362–366. doi: 10.1016/0006-8993(87)90339-8. [DOI] [PubMed] [Google Scholar]
- Tergau F, Naumann U, Paulus W, Steinhoff B. Low-frequency repetitive transcranial magnetic stimulation improves intractable epilepsy. Lancet. 1999;353:2209–2215. doi: 10.1016/S0140-6736(99)01301-X. [DOI] [PubMed] [Google Scholar]
- Theodore WH, Fisher RS. Brain stimulation for epilepsy. Lancet Neurol. 2004;3:111–118. doi: 10.1016/s1474-4422(03)00664-1. [DOI] [PubMed] [Google Scholar]
- Trevelyan AJ, Sussillo D, Watson BO, Yuste R. Modular propagation of epileptiform activity: evidence for an inhibitory veto in neocortex. J Neurosci. 2006;26:12447–12455. doi: 10.1523/JNEUROSCI.2787-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevelyan AJ. The direct relationship between inhibitory currents and local field potentials. J Neurosci. 2009;29:15299–15307. doi: 10.1523/JNEUROSCI.2019-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uva L, Avoli M, de Curtis M. Synchronous GABA-receptor-dependent potentials in limbic areas of the in-vitro isolated adult guinea pig brain. Eur J Neurosci. 2009;29:911–920. doi: 10.1111/j.1460-9568.2009.06672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaugier L, Aubert S, McGonigal A, Trebuchon A, Guye M, Gavaret M, Regis J, Chauvel P, Wendling F, Bartolomei F. Neural networks underlying hyperkinetic seizures of “temporal lobe” origin. Epilepsy Res. 2009;86:200–208. doi: 10.1016/j.eplepsyres.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Vonck K, Boon P, Achten E, De Reuck J, Caemaert J. Long-term amygdalo-hippocampal stimulation for refractory temporal lobe epilepsy. Ann Neurol. 2002;52:556–565. doi: 10.1002/ana.10323. [DOI] [PubMed] [Google Scholar]
- Yamamoto J, Ikeda A, Kinoshita M, Matsumoto R, Satow T, Takeshita K, Matsuhashi M, Mikuni N, Miyamoto S, Hashimoto N, Shibasaki H. Low-frequency electric cortical stimulation decreases interictal and ictal activity in human epilepsy. Seizure. 2006;15:520–527. doi: 10.1016/j.seizure.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Zhang ZJ, Koifman J, Shin DS, Ye H, Florez CM, Zhang L, Valiante TA, Carlen PL. Transition to seizure: ictal discharge is preceded by exhausted presynaptic GABA release in the hippocampal CA3 region. J Neurosci. 2012;32:2499–2512. doi: 10.1523/JNEUROSCI.4247-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou JL, Lenck-Santini PP, Zhao Q, Holmes GL. Effect of interictal spikes on single-cell firing patterns in the hippocampus. Epilepsia. 2007;48:720–731. doi: 10.1111/j.1528-1167.2006.00972.x. [DOI] [PubMed] [Google Scholar]
- Ziburkus J, Cressman JR, Barreto E, Schiff SJ. Interneuron and pyramidal cell interplay during in vitro seizure-like events. J Neurophysiol. 2006;95:3948–3954. doi: 10.1152/jn.01378.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckermann EC, Glaser GH. Hippocampal epileptic activity induced by localized ventricular perfusion with high-potassium cerebrospinal fluid. Exp Neurol. 1968;20:87–110. doi: 10.1016/0014-4886(68)90126-x. [DOI] [PubMed] [Google Scholar]