Abstract
Background
Aedes aegypti is the key vector of both the Yellow Fever and Dengue Fever viruses throughout many parts of the world. Low and variable transgene expression levels due to position effect and position effect variegation are problematic to efforts to create transgenic laboratory strains refractory to these viruses. Transformation efficiencies are also less than optimal, likely due to failure to detect expression from all integrated transgenes and potentially due to limited expression of the transposase required for transgene integration.
Results
Expression plasmids utilizing three heterologous promoters and three heterologous enhancers, in all possible combinations, were tested. The Hr3/IE1 enhancer-transactivator in combination with each of the constitutive heterologous promoters tested increased reporter gene expression significantly in transiently transfected Aedes albopictus C7-10 cells.
Conclusions
The addition of the Hr3 enhancer to expression cassettes and concomitant expression of the IE1 transactivator gene product is a potential method for increasing the level of transgene expression in insect systems. This mechanism could also potentially be used to increase the level of transiently-expressed transposase in order to increase the number of integration events in transposon-mediated transformation experiments.
Background
Through the efforts of many individuals in the past few years, it has become possible to genetically transform a wide variety of non-drosophilid insects of medical and agricultural importance [1]. The ability to genetically transform mosquito species allows researchers to better understand mechanisms of vector competence, design novel methods to disrupt vector-pathogen relationships and develop new insect control strategies [2-5]. New molecular methods could potentially augment continued traditional efforts to control malaria and other re-emerging arthropod-borne diseases. Similar approaches may also be used to stem the devastating infestation of economically important crops by insecticide-resistant pest strains.
Mosquitoes transmit to humans some of the most debilitating and deadly diseases known. According to the World Health Organization, malaria alone is responsible for one million deaths annually [6]. Additionally, the transmission of yellow fever, dengue fever, West Nile virus and a variety of other encephalitis viruses permanently disrupt or end untold numbers of lives. Both anopheline [7-10] and culicine [11-17] mosquito species have been successfully transformed. In all cases, the process is labor-intensive with a few successful experiments yielding transformation efficiencies ranging from 0.5% to 13%. These transformation efficiencies are low compared to the nearly 50% previously reported in Drosophila with vectors up to 8 kb in size [18]. Additionally, transgene expression in the yellow fever mosquito varies considerably both between and within families [11,12,19], likely due to differences in the transcriptional environments of specific insertion sites within the genome, such as the proximity of the transgene to enhancers or heterochromatic stretches of DNA. This phenomenon is of particular concern in the Ae. aegypti genome given its large size (~780 Mb) and its apparent pattern of short-period interspersion where single copy genes (1 to 2 kb) alternate with short (200–600 bp) or medium (1–4 kb) length repetitive sequences [20]. The problem is complex, however transposition has been shown to be dependent upon the amount of transiently available transposase to catalyze vector integration [21,22]. Also, the effective use of genetically-altered mosquitoes to augment current disease vector control requires the ability to create and maintain transgenic lines with consistent, predictable and high-level expression patterns of effector transgenes.
Looking to maximize the transcription of rare transgenes that do land in favorable environments and to potentially increase the levels of transiently available transposase, we tested the ability of three different enhancer elements; SV40 [23], copia ULR (Drosophila) [24] and Hr3 (Bombyx mori nuclear polyhedrosis virus or NPV) [25], to increase the levels of transcription from each of three heterologous promoters from the following genes: actin5C [26] and polyubiquitin (Ubi-p63E – hereafter referred to as pUb) [27] from Drosophila and the intermediate early gene (IE1) [28] from the Autographa californica multicapsid nuclear polyhedrosis virus (MNPV). Additionally, we tested the ability of the B. mori baculovirus IE1 gene product [29], which binds to repetitive sequences within the baculovirus homologous regions (Hrs) [30,31] and has previously been shown to function as a powerful transactivator in transfected lepidopteran cells [29], to yet further increase gene expression in mosquito cells.
Results
The Hr3 enhancer and the IE1 transactivator increase reporter gene activity in transiently transfected C7-10 Aedes albopictus cells
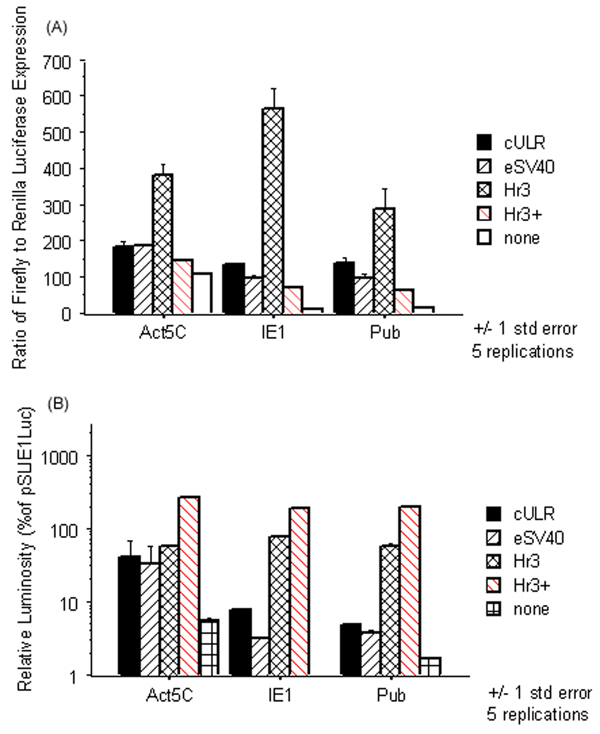
In transiently transfected C7-10 cells, the Act5C promoter resulted in the highest luciferase reporter activity in comparison with the remaining promoters alone (Fig. 1A). The level of measured activity directly corresponds to the amount of luciferase protein expressed by the transfected cells and thus presumably the level of transcription. Among the enhancers, Hr3 improved luciferase expression by 4-fold, 47-fold and 22-fold over the basal level expression from the Act5C, IE1 and pUb promoters respectively; cULR improved luciferase expression over basal level from the Act5C, IE1 and pUb promoters 2-fold, 11-fold and 10-fold respectively; and eSV40 resulted in 2-fold, 8-fold and 7-fold increases respectively in luciferase expression from the Act5C, IE1 and pUb promoters (Fig. 1A). Addition of the IE1 transactivator unexpectedly resulted in large increases in expression from the Renilla control plasmid, as well as from the Hr3-containing reporter plasmids. This is seen in the apparent drop of expression indicated by the red-shaded bars in Fig. 1A. This was confirmed in several independent experiments and was seen even with decreased concentrations of the Renilla plasmid (see additional file 1). In order to see the relative effect of the IE1 transactivator on expression from the Hr3/promoter constructs, the raw firefly luciferase values were converted to a % of average pSLIE1Luc expression and plotted on a log scale (Fig. 1B). Firefly luciferase expression increased 50–200-fold over the basal level expression of all of the promoters with the addition of the IE1 transactivator.
Figure 1.
Firefly luciferase expression from various promoter/enhancer plasmids in Aedes albopictus C7-10 cells. Cells were assayed for luciferase expression 24 hrs. post-transfection. The averages of five replications are reported and error is reported as +/- 1 standard error. (A) To normalize for differences in transfection efficiency and cell cycle state within the experiment, the firefly luciferase luminescence values for each construct were divided by the corresponding Renilla luciferase luminescence values measured in a dual luciferase assay. Bars in red indicate the presence of the IE1 transactivator. The Hr3 enhancer clearly outperforms both the cULR and the eSV40 enhancers in combination with each of the promoters. (B) Raw firefly luciferase values are reported as a % of pSLIE1Luc expression on a log scale. The bars in red show levels of firefly luciferase expression in the presence of the IE1 transactivator. Addition of the IE1 transactivating protein (Hr3+) increased firefly luciferase expression 2.5–4-fold over all Hr3-promoter combinations alone and 50–200-fold over basal promoter expression.
IE1 transactivator interacts with promoter sequences in addition to the Hr3 enhancer
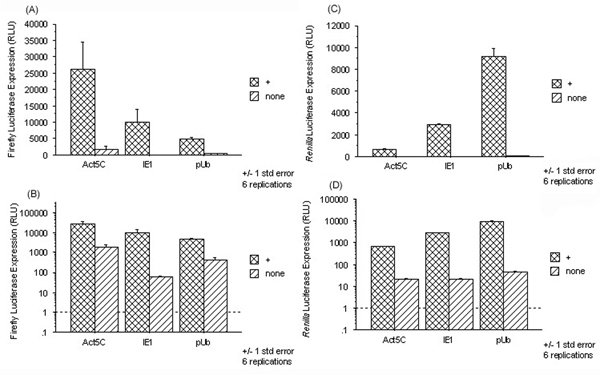
Analysis of multiple experiments (Fig. 2, Table 1, and data not shown) revealed an interesting trend regarding the effect of the IE1 transactivating protein upon the promoters themselves. This effect was different for each promoter when co-transfected with an identical plasmid expressing the Renilla luciferase control. Addition of the IE1 transactivator resulted in a 17-fold increase in expression of firefly luciferase from the Act5C promoter over its basal level expression and a concomitant 30-fold increase from the Renilla luciferase reporter under the control of the hsp82 promoter from Drosophila pseudobscura [32]. When the IE1 promoter was used to drive expression of firefly luciferase, expression increased 169-fold over the basal level expression, while Renilla luciferase expression from the hsp82 promoter increased 138-fold. Finally, firefly luciferase expression increased 11-fold relative to basal level expression from the pUb promoter with a corresponding 202-fold increase in Renilla luciferase expression from the hsp82 promoter.
Figure 2.
Differential effect of the IE1 transactivator on the transcription levels from various promoters. Each promoter-Luc construct was co-transfected with phsp82RenillaLuc, both in the absence and presence of the IE1 transactivator, and assayed for both firefly and Renilla luciferase expression, 24 hrs. post-transfection. One experiment with six replicates was performed with the same batch of cells, DNA/liposome complexes and luciferase reagents. Error is reported as +/- 1 standard error. Each set of data is plotted both on a linear and a log scale. (A) Addition of the transactivator (+) caused a 16-fold increase in firefly luciferase expression from the Act5C promoter, a 169-fold increase in expression from the IE1 promoter and an 11-fold increase in expression from the pUb promoter. (B) The same data as shown in (A) but plotted on a log scale. (C) Addition of the transactivator resulted in different levels of expression from the phsp82RenillaLuc construct depending upon which promoter was used to drive expression of the firefly luciferase construct. (D) The same data as shown in (C) but plotted on a log scale.
Table 1.
Change in Basal Luciferase Expression from Promoters with Addition of the IE1 Transactivator
| Promoter | Firefly Luciferase | Renilla Luciferase | Ratio |
| Act5C | ↑ 17 x | ↑ 30 x | ↓ 1.9 x |
| IE1 | ↑ 169 x | ↑ 138 x | ↑ 1.2 x |
| pUb | ↑ 11 x | ↑ 202 x | ↓ 18.3 x |
This summary of the data presented in Fig. 2 shows the fold change of firefly luciferase expression from each basal promoter following addition of the IE1 transactivator, the fold-increase in expression from the control Renilla luciferase plasmid under control of the hsp82 promoter and the overall change in ratio.
Discussion
Unexpectedly, the internal control for transfection and protein recovery, Renilla luciferase, could not reliably be used as such in the presence of the transactivator. The data presented reveal a differential effect of the IE1 transactivator (Fig. 2 and Table 1) that profoundly affects expression levels from the two luciferase plasmids in an enhancer/promoter-dependent manner. This compromises the ability to compare expression values both within an experiment where IE1 is present in some samples but not in others and between experiments where different batches of cells and assay reagents are employed. The results of a single experiment involving the transactivator are reported here, however additional experiments show similar results. The ratio of firefly to Renilla luciferase is reported for all promoter/enhancer combinations to allow accurate comparison of the three promoters alone and in combination with each of the three enhancers. It should be noted that the addition of the transactivator does significantly increase firefly luciferase expression from all three promoters with the Hr3 enhancer sequence, though this is masked by the simultaneous increase in expression from the Renilla luciferase control plasmid.
The IE1 transactivator is clearly interacting with the promoters in trans, even in the absence of the Hr3 enhancer element (Fig. 2, Table 1 and data not shown). This observation agrees with previously published data that the cytoplasmic A3 actin gene promoter of B. mori was upregulated as much as one hundred-fold by the co-transfection of a plasmid encoding the B. mori IE1 gene product (BmIE1) [29]. When the Hr3 enhancer is present, there is a cooperative effect, and luciferase expression increases as much as 200-fold (Fig. 1) over that of the promoter alone. This cooperativity is consistent with results obtained with Hr3-enhanced CAT expression cassettes driven by the B. mori cytoplasmic actin gene promoter co-expressed with the BmIE1 protein in lepidopteran cell lines Bm5 and Sf21 (an increase of up to three orders of magnitude) [29].
The significant differences seen in expression from each of the promoters tested (Fig. 2 and Table 1) reveal that not all promoters are affected in the same manner, nor is the co-transfected plasmid. The presence of the Hr3 enhancer region upstream of the promoter driving expression of the IE1 transactivator protein, results in high levels of IE1 protein from a relatively low amount of plasmid DNA. Despite this abundance of IE1 protein, it appears that transcription from the pUb promoter, in the absence of the Hr3 enhancer, increases only 11-fold (Fig. 2A and Table 1), while transcription from the hsp82 promoter driving Renilla luciferase expression is exceptionally high (Fig. 2B and Table 1). The simplest explanation is that the IE1 protein has different affinities for binding sites on the various promoters and/or the IE1 protein is sequestering necessary basal transcription factors. It has also been observed that some viral promoters, IE-0, IE-2 and PE-38, are inhibited by IE1 expression [33-35]. Clearly, the actions of the IE1 transactivator in this study are consistent with its ability to bind Hrs [36-38]. In addition, the protein has two independent functional acidic activation domains and two potential positively-charged inhibitory domains [39,40], consistent with its observed ability to both enhance and inhibit expression from different promoters. Also, lower concentrations of the plasmid bearing the IE1 gene sequence in these transient assays result in greater increases in luciferase expression (see additional file 2). This observation is consistent with the mechanism of negative regulation by the IE1 protein previously proposed [35] where the cooperative binding of the Hrs occurs at a lower concentration than that required for binding to the half sequence regions (Hs) present in negatively regulated promoters. It is also consistent with the presence of the Hr3 enhancer sequence on the plasmid producing the IE1 transactivating protein, which results in up-regulation of IE1 transcription, consequently reducing the number of plasmid copies needed to produce optimal protein levels. When this experiment was repeated using pUb to drive Renilla luciferase expression (data not shown and additional file 3), significant differences between promoters were also observed, though not the same differences described above with the hsp82-Renilla expression plasmid. Finally, it should be noted that each of the enhancers alone also differentially affected the expression from each promoter. These data collectively highlight the value of evaluating the effects of new promoter/enhancer/transactivator combinations on the expression of a reporter gene within a related cell line, prior to investing significant time and effort in the creation of transgenic lines. Though cell lines do not completely mimic the cellular and nuclear environment of an entire organism, they can yield significant insight into both the potential interaction between regulatory elements driving transgene expression and the potential impact of unknown endogenous transacting factors.
Conclusions
Clearly, we have shown that the baculovirus homologous region, Hr3, along with the IE1 transactivating protein, significantly increases transgene expression from each of the three heterologous, constitutive promoters tested in mosquito cells. Some concern does exist that endogenous promoters might be down-regulated by the presence of the IE1 protein, and that available host cell transcription factors might be sequestered by complexes stabilized by the IE1 transactivator, however a lower concentration of IE1 transactivator would likely mitigate these effects. Preliminary transposition assays confirm the ability of the Hr3 enhancer/IE1 transactivator combination to function in syncytial preblastoderm mosquito embryos and to significantly increase observed transposition frequencies when used to drive transposase expression (Coates, et al., unpublished data). Use of tissue-specific promoters/enhancers and/or inducible expression may effectively reduce any potential fitness load imposed by interactions of the ie1 protein with endogenous regulatory elements. Perhaps the most promising application is the use of HR/IE1 in helper plasmids transiently expressing transposase in an attempt to increase the number of stable transgene integration events by increasing the amount of available transposase, particularly if germline-specific promoters were used to express the transactivator protein. The HR/IE1 strategy is a promising tool for high-level transgene expression and/or increased transposition frequency in culicine mosquitoes and possibly other insect species as well.
Methods
Construction of the luciferase expression plasmids
A 2.7-kb HindIII-SalI fragment from pGL2-Basic (Promega), containing the firefly luciferase coding region and the SV40 poly-Adenylation signal, was inserted into the corresponding sites of pBCKS+ (Stratagene) to create pBCLuc. A 2.7-kb SmaI-SalI fragment from pBCLuc was inserted into the SmaI-SalI sites of pSLfa1180fa ([41] to create pSLLuc. The Drosophila Actin5C promoter was excised from pHermesA5CEGFP [13] by PstI and BamHI digestion and inserted into the corresponding sites of PSLLuc to create PSLAct5CLuc. The SacII site was removed from pIE1-3 (Novagen) and then the 657-bp EcoRI-BamHI fragment containing the AcMNPV IE1 promoter was inserted into the corresponding sites of pSLLuc to create pSLIE1Luc. A 2-kb KpnI-BamHI fragment from pB [pUB-nls-EGFP] [42] containing the Drosophila polyubiquitin promoter was inserted into the corresponding sites of pSLLuc to create pSLpUbLuc. The copia ULR was amplified by polymerase chain reaction (PCR) from copia LTR-ULR-CAT [43] using the primers 5'-AAGCTTGGGCCCAGTCCATGCCTA-3' and 5'-CCGCGGATTACGTTTAGCCTTGTC-3', cleaved by digestion with HindIII and SacII and inserted into the corresponding sites of pBCKS+ to create pBCcULR. The HindIII-SacII fragment from this plasmid was then inserted into the corresponding sites of pSLAct5CLuc and pSLIE1Luc to create pSLcULRAct5CLuc and pSLcULRIE1Luc. pBCcULR was digested with HindIII and the site filled with the Klenow fragment of DNA polymerase I (Promega), then digested with SacII and ligated into pSLpUbLuc which had been cut with NotI and the site filled with Klenow fragment, then cut with SacII to create pSLcULRpUbLuc. The SV40 enhancer region from pRL-SV40 (Promega) was PCR-amplified using the primers 5'-AAGCTTCTGAGGCGGAAAGAACCA-3' and 5'-CCGCGGAAAATTAGCCAGCCATGG-3', digested with HindIII and SacII and inserted into the corresponding sites of pBCKS+ to make pBCeSV40. The HindIII-SacII fragment of pBCeSV40 was then inserted into the corresponding sites of pSLAct5CLuc and pSLIE1Luc to create pSLeSV40Act5CLuc and pSLeSV40IE1Luc. pBCeSV40 was digested with HindIII, the site filled with Klenow fragment, then digested with SacII and ligated to pSLpUbLuc digested with NotI, the site filled with Klenow fragment and subsequently digested with SacII to produce pSLeSV40pUbLuc. A 1.2-kb PstI-BamHI fragment containing the B. mori NPV Hr3 enhancer from p153 [25] was inserted into the corresponding sites of pBCKS+ to create pBCHr3. The PstI-BamHI fragment of pBCHr3 was inserted into the corresponding sites of pSLAct5CLuc to create pSLHr3Act5CLuc. The HindIII-SacII fragment of pBCHr3 was inserted into the corresponding sites of pSLIE1Luc to create pSLHr3IE1Luc. The EcoRV-SacII fragment of pBCHr3 was ligated to pSLpUbLuc digested with NotI, the site filled with Klenow fragment, and then digested with SacII to create pSLHr3pUbLuc. phsp82RenillaLuc was created by inserting a 1-kb KpnI-BamHI fragment from pKhsp82 [44] into the corresponding sites of pBCKS+ and then inserting the KpnI-PstI fragment from this plasmid into the corresponding sites of pRL-SV40. ppUbRenillaLuc was created by first digesting pSLpUbLuc with NotI, filling in the site with Klenow fragment, then digesting with PstI to produce a2-kb fragment which was ligated to pRL-SV40 prepared by digestion with BglII, the site filled with Klenow fragment and then digested with PstI.
Cell cultures and transfections
Aedes albopictus C7-10 cells were maintained at 25°C with 5% CO2 in Eagle's media plus 5% fetal calf serum with the following additions per liter: 10 mL 10% (wt/vol) D(+)glucose, 10 mL 200 mM L-glutamine, 10 mL MEM vitamin solution, 20 mL MEM non-essential amino acids, 10 mL Penicillin/Streptomycin (10,000 U/mL), 29.3 mL sodium bicarbonate (7.5% w/v) [45]. 400 μL of cells at a density of 2 × 10-6 cells/mL were seeded into 24-well microtiter plates and incubated at 25°C for 24 hrs. Cells were transfected with 0.4 μg total DNA and 0.8 μL LipofectAMINE 2000 (Life Technologies) in 10 μL serum-free, antibiotic-free media. phsp82RenillaLuc and the firefly constructs were transfected at a 1:2 ratio. The IE1 transactivator plasmid [25] was present as 1/10 of the total DNA.
Luciferase assays
Transfected cells were assayed 24 hrs. post-transfection using a Turner Designs 20/20 luminometer and a Dual Luciferase Assay (Promega). The manufacturer's passive lysis protocol was followed. In addition, cell lysates were snap frozen in liquid nitrogen immediately after lysis to minimize luciferase protein degradation. All samples were diluted 20-fold in 1 × PLB (passive lysis buffer) in order to obtain a reading within the range of the luminometer.
Authors' contributions
CG carried out the studies described in this paper and drafted the manuscript. CC originated the study, participated in its design and planning and edited the manuscript. Both authors read and approved the final manuscript.
Supplementary Material
The IE1 transactivator significantly affects expression from the Renilla luciferase control plasmid. Cells were transfected as detailed in the methods with 0.27 μg Hr3Act5CLuc (firefly) plasmid, 0.04 μg IE1 transactivator plasmid and the indicated amount of Renilla luciferase control plasmid. Because this is a control plasmid with no Hr3 enhancer element present, one would expect the expression levels to parallel those shown in the absence of the IE1 transactivator (solid bars).
Lower concentrations of the IE1 transactivator result in greater expression from both the Hr3Actin5C (firefly luciferase) and the hsp82 (Renilla luciferase) promoters. Cells were transfected as detailed in the methods with 0.27 μg Hr3Act5CLuc (firefly) plasmid, 0.14 μg hsp82RenillaLuc plasmid and the indicated amount of IE1 transactivator plasmid.
The hsp82 versus the pUb promoter for Renilla luciferase expression. Cells were transfected as detailed in the methods with the only difference being the promoter used to drive expression of the Renilla luciferase control plasmid. The pUb promoter seems to be upregulated more than the hsp82 promoter in the presence of the transactivator. Interestingly, the corresponding expression from the Hr3Act5C firefly luciferase plasmid is less when the pUb Renilla plasmid is co-transfected. Other differences were seen when this experiment was repeated with Hr3IE1 and Hr3pUb firefly luciferase plasmids. Clearly, the IE1 transactivator binds sequences other than the Hr3 enhancer sequence in eukaryotic promoters and the effect is dependent upon the combination of promoters present.
Acknowledgments
Acknowledgements
We would like to thank Dr. Ann Fallon for her gift of the C7-10 cell line, Dr. Kostas Iatrou for his gift of the plasmids containing the IE1 transactivator and the Hr3 enhancer, and Yong Zhou and Jeremy Haag for their technical assistance. We would also like to thank the anonymous reviewers for their valuable comments and insights. This work was supported by NIH Grant #RO1 AI46432 to C.J.C.
Contributor Information
Christine E Gray, Email: c-gray@tamu.edu.
Craig J Coates, Email: c-coates@tamu.edu.
References
- Robinson Alan S., Franz Gerald, Atkinson Peter W. Insect transgenesis and its potential role in agriculture and human health. Insect Biochem Mol Biol. 2004;34:113–120. doi: 10.1016/j.ibmb.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Collins FH, James AA. Genetic modification of mosquitoes. Sci Med. 1996. pp. 52–61.
- Beerntsen BT, James AA, Christensen BM. Genetics of mosquito vector competence. Microbiol Mol Biol Rev. 2000;64:115–137. doi: 10.1128/MMBR.64.1.115-137.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair CD, Adelman ZN, Olson KE. Molecular strategies for interrupting arthropod-borne virus transmission by mosquitoes. Clin Microbiol Rev. 2000;13:651–661. doi: 10.1128/CMR.13.4.651-661.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alphey L, Andreasen M. Dominant lethality and insect population control. Mol Biochem Parasitol. 2002;121:173–178. doi: 10.1016/S0166-6851(02)00040-3. [DOI] [PubMed] [Google Scholar]
- WHO Malaria in Africa Infosheet http://www.rbm.who.int/cmc_upload/0/000/015/370/RBMInfosheet_3.htm
- Catteruccia F, Nolan T, Loukeris TG, Blass C, Savakis C, Kafatos FC, Crisanti A. Stable germline transformation of the malaria mosquito Anopheles stephensi. Nature. 2000;405:959–962. doi: 10.1038/35016096. [DOI] [PubMed] [Google Scholar]
- Grossman GL, Rafferty CS, Clayton JR, Stevens TK, Mukabayire O, Benedict MQ. Germline transformation of the malaria vector, Anopheles gambiae, with the piggyBac transposable element. Insect Mol Biol. 2001;10:597–604. doi: 10.1046/j.0962-1075.2001.00299.x. [DOI] [PubMed] [Google Scholar]
- Nolan Tony, Bower Tom M., Brown Anthony E., Crisanti Andrea, Catteruccia Flaminia. piggyBac-mediated germline transformation of the malaria mosquito Anopheles stephensi using the red fluorescent protein dsRED as a selectable marker. J Biol Chem. 2002;277:8759–8762. doi: 10.1074/jbc.C100766200. [DOI] [PubMed] [Google Scholar]
- Perera OP, Harrell RA, Handler AM. Germ-line transformation of the South American malaria vector, Anopheles albimanus, with a piggyBac/EGFP transposon vector is routine and highly efficient. Insect Mol Biol. 2002;11:291–297. doi: 10.1046/j.1365-2583.2002.00336.x. [DOI] [PubMed] [Google Scholar]
- Jasinskiene N, Coates CJ, Benedict MQ, A.J. Cornel, Salazar-Rafferty C, James AA, Collins FH. Stable, transposon-mediated transformation of the yellow fever mosquito, Aedes aegypti, using the Hermes element from the house fly. Proc Natl Acad Sci USA. 1998;95:3743–3747. doi: 10.1073/pnas.95.7.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates CJ, Jasinskiene N, James AA. Mariner transposition and transformation of the yellow fever mosquito, Aedes aegypti. Proc Natl Acad Sci USA. 1998;95:3748–3751. doi: 10.1073/pnas.95.7.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkerton AC, Michel K, O'Brochta DA, Atkinson PW. Green fluorescent protein as a genetic marker in transgenic Aedes aegypti. Insect Mol Biol. 2000;9:1–10. doi: 10.1046/j.1365-2583.2000.00133.x. [DOI] [PubMed] [Google Scholar]
- Kokoza V, Ahmed A, Wimmer EA, Raikhel AS. Efficient transformation of the yellow fever mosquito Aedes aegypti using the piggyBac transposable element vector pBac[3xP3-EGFP afm]. Insect Biochem Mol Biol. 2001;31:1137–1143. doi: 10.1016/S0965-1748(01)00120-5. [DOI] [PubMed] [Google Scholar]
- Allen Margaret L., O'Brochta David A., Atkinson Peter W., Levesque Cynthia S. Stable, Germ-Line Transformation of Culex quinquefasciatus (Diptera: Culicidae) J Med Entomol. 2001;38:701–710. doi: 10.1603/0022-2585-38.5.701. [DOI] [PubMed] [Google Scholar]
- Lobo NF, Hua-Van A, Li X, Nolen BM, Fraser Jr MJ. Germ line transformation of the yellow fever mosquito, Aedes aegypti, mediated by transpositional insertion of a piggyBac vector. Insect Mol Biol. 2002;11:133–139. doi: 10.1046/j.1365-2583.2002.00317.x. [DOI] [PubMed] [Google Scholar]
- Ito Junitsu, Ghosh Anil, Moreira Luciano A., Wimmer Ernst A., Jacobs-Lorena Marcelo. Transgenic anopheline mosquitoes impaired in transmission of a malaria parasite. Nature. 2002;417:452–455. doi: 10.1038/417452a. [DOI] [PubMed] [Google Scholar]
- Spradling AC. P element-mediated transformation. In: Roberts DB, editor. Drosophila: A Practical Approach. Oxford, IRL Press; 1986. pp. 175–197. [Google Scholar]
- Coates Craig J., Jasinskiene Nijole, Pott Gregory B., James Anthony A. Promoter-directed expression of recombinant fire-fly luciferase in the salivary glands of Hermes-transformed Aedes aegypti. Gene. 1999;226:317–325. doi: 10.1016/S0378-1119(98)00557-5. [DOI] [PubMed] [Google Scholar]
- Knudson DL, Zheng L, Gordon SW, Brown SE, Kafatos FC. Genome Organization of Vectors. In: Beatty BJ and Marquardt WC, editor. The Biology of Disease Vectors. Fort Collins, CO, University Press of Colorado; 1996. pp. 175–214. [Google Scholar]
- Kapetanaki Maria G., Loukeris Thanasis G., Livadaras Ioannis, Savakis Charalambos. High frequencies of Minos transposon mobilization are obtained in insects by using in vitro synthesized mRNA as a source of transposase. Nucl Acids Res. 2002;30:3333–3340. doi: 10.1093/nar/gkf455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson Ann E., Balciunas Darius, Mohn Deanna, Shaffer Jennifer, Hermanson Spencer, Sivasubbu Sridhar, Cliff M. Pat, Hackett Perry B., Ekker Stephen C. Efficient gene delivery and gene expression in zebrafish using the Sleeping Beauty transposon. Dev Biol. 2003;263:191–202. doi: 10.1016/j.ydbio.2003.07.013. [DOI] [PubMed] [Google Scholar]
- Moreau P, Hen R, Wasylyk B, Everett R, Gaub MP, Chambon P. The SV40 72 base pair repeat has a striking effect on gene expression both in SV40 and other chimeric recombinants. Nuc Acids Res. 1981;9:6047–6068. doi: 10.1093/nar/9.22.6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matyunina LV, Jordan IK, McDonald JF. Naturally occurring variation in copia expression is due to both element (cis) and host (trans) regulatory variation. Proc Natl Acad Sci USA. 1996;93:7097–7102. doi: 10.1073/pnas.93.14.7097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Farrell PJ, Johnson R, Iatrou K. A baculovirus (Bombyx mori nuclear polyhedrosis virus) repeat element functions as a powerful constitutive enhancer in transfected insect cells. J Biol Chem. 1997;272:30724–30728. doi: 10.1074/jbc.272.49.30724. [DOI] [PubMed] [Google Scholar]
- Thummel CS, Boulet AM, Lipshitz HD. Vectors for Drosophila P-element-mediated transformation and tissue culture transfection. Gene. 1988;74:445–456. doi: 10.1016/0378-1119(88)90177-1. [DOI] [PubMed] [Google Scholar]
- Lee HS, Simon JA, Lis JT. Structure and expression of ubiquitin genes of Drosophila melanogaster. Mol Cell Biol. 1988;8:4727–4735. doi: 10.1128/mcb.8.11.4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullen SS, Friesen PD. Early transcription of the IE-1 transregulator gene of Autographa californica nuclear polyhedrosis virus is regulated by DNA sequences within its 5' noncoding leader region. J Virol. 1995;69:156–165. doi: 10.1128/jvi.69.1.156-165.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Maolong, Johnson Russell R., Iatrou Kostas. Trans-activation of a cell housekeeping gene promoter by the ie1 gene product of baculoviruses. Virology. 1996;218:103–113. doi: 10.1006/viro.1996.0170. [DOI] [PubMed] [Google Scholar]
- Guarino LA, Summers MD. Interspersed homologous DNA of Autographa californica nuclear polyhedrosis virus enhances delayed-early gene expression. J Virol. 1986;60:215–223. doi: 10.1128/jvi.60.1.215-223.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer A, Knebel-Mordsdorf D. The early baculovirus he65 promoter: On the mechanism of transcriptional activation by IE1. Virology. 1998;249:336–351. doi: 10.1006/viro.1998.9288. [DOI] [PubMed] [Google Scholar]
- Blackman RK, Meselson M. Interspecific nucleotide sequence comparisons used to identify regulatory and structural features of the Drosophila hsp82 gene. J Mol Biol. 1986;188:499–515. doi: 10.1016/s0022-2836(86)80001-8. [DOI] [PubMed] [Google Scholar]
- Carson DD, Summers MD, Guarino LA. Transient expression of the Autographa californica nuclear polygefrosis virus intermediate-early gene, IE-N, is regulated by three viral elements. J Virol. 1991;65:945–951. doi: 10.1128/jvi.65.2.945-951.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs GR, Guarino LA, Summers MD. Novel regulatory properties of the ie1 and ie0 transactivators encoded by the baculovirus Autographa californica multicapsid nuclear polyhedrosis virus. J Virol. 1991;65:5281–5288. doi: 10.1128/jvi.65.10.5281-5288.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisy DJ, Rasmussen C, Owusu EO, Rohrmann GF. A mechanism for negative gene regulation in Autographa californica multinucleocapsid nuclear polyhedrosis virus. J Virol. 1997;71:5088–5094. doi: 10.1128/jvi.71.7.5088-5094.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarino LA, Dong W. Expression of an enhancer-binding protein in insect cells transfected with the Autographa californica nuclear polyhedrosis virus IE1 gene. J Virol. 1991;65 doi: 10.1128/jvi.65.7.3676-3680.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarino LA, Dong W. Functional dissection of the Autographa californica nuclear polyhedrosis enhancer element hr5. Virology. 1994;200:328–335. doi: 10.1006/viro.1994.1197. [DOI] [PubMed] [Google Scholar]
- Rodems SM, Friesen PD. Transcriptional enhancer activity of hr5 requires dual-palindrome half sites that mediate binding of a dimeric form of the baculovirus transregulator ie1. J Virol. 1995;69:5368–5375. doi: 10.1128/jvi.69.9.5368-5375.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Guarino LA. The baculovirus transactivator ie1 binds to viral enhancer elements in the absence of insect cell factors. J Virol. 1995;69:4548–4551. doi: 10.1128/jvi.69.7.4548-4551.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack JM, Blissard GW. Identification of two independent transcriptional activation domains in the Autographa californica multicapsid nuclear polyhedrosis virus ie1 protein. J Virol. 1997;71:9579–9587. doi: 10.1128/jvi.71.12.9579-9587.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn C, Wimmer EA. A versatile vector set for animal transgenesis. Dev Genes Evol. 2000;210:630–637. doi: 10.1007/s004270000110. [DOI] [PubMed] [Google Scholar]
- Handler AM, Harrell R. A. 2nd. Transformation of the Caribbean fruit fly, Anastrepha suspensa, with a piggyBac vector marked with polyubiquitin-regulated GFP. Insect Biochem Mol Biol. 2001;31:199–205. doi: 10.1016/S0965-1748(00)00119-3. [DOI] [PubMed] [Google Scholar]
- Wilson Susanne, Matyunina Lilya V., McDonald John F. An enhancer region within the copia untranslated leader contains binding sites for Drosophila regulatory proteins. Gene. 1998;209:239–246. doi: 10.1016/S0378-1119(98)00048-1. [DOI] [PubMed] [Google Scholar]
- Coates CJ, Howells AJ, O'Brochta DA, Atkinson PW. The 5' regulatory region from the Drosophila pseudobscura hsp82 gene results in a high level of reporter gene expression in Lucilia cuprina embryos. Gene. 1996;175:199–201. doi: 10.1016/0378-1119(96)00149-7. [DOI] [PubMed] [Google Scholar]
- Shih KM, Gerenday A, Fallon AM. Culture of mosquito cells in Eagle's medium. In Vitro Cell Dev Biol--Animal. 1998;34:629–630. doi: 10.1007/s11626-996-0010-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The IE1 transactivator significantly affects expression from the Renilla luciferase control plasmid. Cells were transfected as detailed in the methods with 0.27 μg Hr3Act5CLuc (firefly) plasmid, 0.04 μg IE1 transactivator plasmid and the indicated amount of Renilla luciferase control plasmid. Because this is a control plasmid with no Hr3 enhancer element present, one would expect the expression levels to parallel those shown in the absence of the IE1 transactivator (solid bars).
Lower concentrations of the IE1 transactivator result in greater expression from both the Hr3Actin5C (firefly luciferase) and the hsp82 (Renilla luciferase) promoters. Cells were transfected as detailed in the methods with 0.27 μg Hr3Act5CLuc (firefly) plasmid, 0.14 μg hsp82RenillaLuc plasmid and the indicated amount of IE1 transactivator plasmid.
The hsp82 versus the pUb promoter for Renilla luciferase expression. Cells were transfected as detailed in the methods with the only difference being the promoter used to drive expression of the Renilla luciferase control plasmid. The pUb promoter seems to be upregulated more than the hsp82 promoter in the presence of the transactivator. Interestingly, the corresponding expression from the Hr3Act5C firefly luciferase plasmid is less when the pUb Renilla plasmid is co-transfected. Other differences were seen when this experiment was repeated with Hr3IE1 and Hr3pUb firefly luciferase plasmids. Clearly, the IE1 transactivator binds sequences other than the Hr3 enhancer sequence in eukaryotic promoters and the effect is dependent upon the combination of promoters present.