Abstract
Background and Purpose
Acute triggers for ischemic stroke, which may include infection, are understudied, as is whether background cardiovascular disease (CVD) risk modifies such triggering. We hypothesized that infection increases acute stroke risk, especially among those with low CVD risk.
Methods
Hospitalized strokes and infections were identified in the Atherosclerosis Risk in Communities (ARIC) cohort. A case-crossover design and conditional logistic regression were used to compare hospitalized infections among stroke patients (14, 30, 42, and 90 days before stroke) with corresponding control periods 1 year and 2 years prior. Background CVD risk was assessed at both visit 1 and the visit most proximal to stroke, with risk dichotomized at the median.
Results
A total of 1,008 adjudicated incident ischemic strokes were included. Compared to control periods, hospitalized infection was more common within 2 weeks before stroke (14 day odds ratio (OR) = 7.7 (2.1, 27.3)); the strength of association declined with increasing time in the exposure window before stroke (30 day OR = 5.7 (2.3, 14.3), 42 day OR = 4.5 (2.0, 10.2), and 90 day OR = 3.6 (2.1, 6.5)). Stroke risk was higher among those with low compared with high CVD risk, with this interaction reaching statistical significance for some exposure periods.
Conclusions
These results support the hypothesis that hospitalized infection is a trigger of ischemic stroke, and may explain some cryptogenic strokes. Infection control efforts may prevent strokes. CVD preventive therapies may prevent strokes if used in the peri-infection period but clinical trials are needed.
Keywords: stroke, infection, trigger, crossover design
Introduction
Population-based cohort studies have identified many long-term risk factors for incident ischemic stroke that are both modifiable, like high blood pressure, elevated serum cholesterol, and smoking, and non-modifiable, like male sex, non-white race, family history, and greater age.1–3 Short term risk factors – or triggers – of acute ischemic stroke include hypertensive crisis4, alcohol abuse5, and may include acute infection.5 Herein, we explore the relationship between hospitalization with infection and short-term stroke risk. Identifying and understanding stroke triggers offers potential strategies for stroke prevention during periods of vulnerability.
Several case-control studies have reported an association between various measures of infection and ischemic stroke.6–12 Two studies used a case cross-over design to identify an association between hospitalization with infection and short-term risk of ischemic stroke.13,14 Additionally, these two studies suggested that stroke risk after acute infection is greater in those with fewer vascular risk factors.13,14 The authors hypothesized that acute triggers, such as infection, were less relevant in people with more vascular risk factors since they are already at elevated stroke risk.
While recognition of infection as a trigger of ischemic stroke is growing, previous studies were often small, lacked prospective data, and failed to properly account for potential confounding factors. Further, previous studies have considered effect modification by individual vascular risk factors but have not yet examined how the infection-stroke triggering association varies by overall CVD risk level.
We used the data collected in the long-running, prospective cohort Atherosclerosis Risk in Communities (ARIC) Study to study the association further. Based on the previous literature, we hypothesized that there is an association between hospitalization with infection and acute ischemic stroke risk, and that infection is a stronger stroke trigger among patients with low background CVD risk.
Methods
Study Population
The ARIC Study is a prospective population-based cohort study comprising 15,792 adults aged 45–64 years at recruitment in 1987–1989.15 Subsequent exams took place 1990–1992 (visit 2), 1993–1995 (visit 3), 1996–1998 (visit 4), and 2011–2013 (visit 5). Cohort participants were selected from four U.S. communities: Forsyth County, North Carolina; Jackson, Mississippi; suburbs of Minneapolis, Minnesota; and Washington County, Maryland. A detailed discussion of ARIC study design and objectives is provided elsewhere.15
Study Design
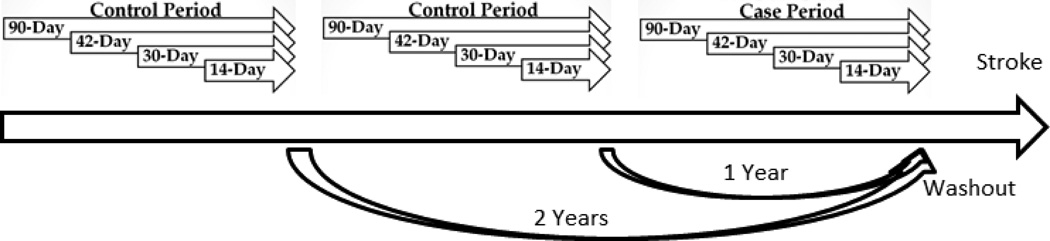
A case-crossover study design was used in which all ARIC participants who suffered an ischemic stroke during follow-up (n=1,062) served as their own control. The case-crossover design affords the ability to isolate exposures that vary over time within person and better control for potential confounding that might occur between people. The occurrence of hospitalization with infection at intervals of 14, 30, 42, and 90 days prior to the stroke date were compared with two preceding control periods (1 year and 2 years prior to stroke). A washout period between the stroke admission date and preceding hospitalization with infection discharge date was used to exclude infections that may have been diagnosed secondarily at a stroke hospitalization. Cases whose control periods occurred prior to ARIC study enrollment were excluded (n=54) leaving a study sample of n=1,008. The study design is summarized in Figure 1.
Figure 1.
Case-crossover study design employed to study stroke in relation to triggering by hospitalized infection, ARIC. Presence of hospitalized infection was assessed for each of the 12 time periods, and case period was compared to control periods.
Stroke ascertainment
Incident ischemic strokes were ascertained from hospitalizations reported by annual contact with participants, cardiovascular disease discharge lists provided by local hospitals, and death certificates. Incident ischemic strokes were identified and classified as thrombotic or cardioembolic by a computer algorithm and physician review based on signs, symptoms, neuroimaging (CT/MRI), and other diagnostic reports, according to criteria adapted from the National Survey of Stroke. Details on quality assurance for ascertainment and classification of ischemic stroke events are published elsewhere.3 All definite and probable incident ischemic strokes between study enrollment and year end 2011 were included in the analysis.
Main independent variable: hospitalization with infection
The exposure of interest was hospitalization with infection up to 90 days before ischemic stroke for the case period and equivalent 90-day control periods exactly 1 and 2 years before the ischemic stroke. The admission date for hospitalization with stroke was considered the stroke date, while the discharge date for hospitalization with infection was considered the infection date. Infection was assessed using abstracted hospital record discharge ICD-9-CM codes. The following ICD-9 codes for infection adapted from methodology used by Elkind et al.13 were included:
Respiratory - 460–466,480–487
Urinary tract - 599.0, 595, 590
Skin and subcutaneous tissue - 680–686
Bacteremia - 790.7
Osteomyelitis - 730.0–730.2
Other Infectious Diseases - 001–134
Codes in any position were counted. A washout period was used to exclude infections that may have been diagnosed secondarily at a stroke hospitalization. Washout periods of both 2 and 4 days between stroke admission date and preceding hospitalization with infection discharge date were used and compared.
Measurement of other covariates
Background CVD risk was measured using the ACC/AHA 10-year CVD risk score.16 The CVD risk score uses sex, age, race, total cholesterol, blood pressure, diabetes status, and smoking status to estimate 10-year CVD risk based on pooled cohort data. Data on risk factors were obtained at baseline (visit 1) and again during follow-up visits. We compared CVD risk score ascertained at baseline with that assessed at the participant’s study visit prior to the first control period to examine any differences in the magnitude of the associations of interest.
Statistical Analysis
Potential confounders that are stable within an individual are controlled in the case-crossover study design by having cases serve as their own controls, a type of individual matching. Confounding by overall health status related to age is possible since deteriorating health could be a common cause of both infection and stroke. As participants age and their health status decreases, their stroke risk and hospitalization with infection risk may increase suggesting potential positive confounding by health status.
To reduce potential confounding, only time periods proximal to stroke (1 and 2 years previous) were included. We further controlled for the total number of hospitalizations in the 9 months preceding the start of each of the three exposure periods (case period and two control periods) to account for potential decline in overall health status.
The prevalence of hospitalization with infection 14, 30, 42, and 90-days prior to ischemic stroke was compared to the corresponding time periods exactly 1 and 2 years prior to the stroke. Conditional logistic regression was used to estimate odds ratios (OR) of stroke and 95% confidence intervals (CIs) for each time period (14, 30, 42, and 90 days). Interactions between hospitalized infection and CVD 10-year risk score, modeled continuously, were tested by adding an interaction term to the conditional logistic regression models. ORs associated with hospitalized infection were also estimated after dichotomizing CVD risk score at the median (10.00% for visit 1 and 15.38% for most recent visit) for ease of interpretation. Finally, models stratified by ischemic stroke sub-type (thrombotic and cardioembolic) were considered to evaluate if the infection-stroke triggering effect differed by ischemic stroke sub-type. Ischemic stroke sub-type definitions previously published in the ARIC study literature were used.3 For all analyses, both crude models and models adjusted for the number of hospitalizations were evaluated and presented.
Results
Of the 15,792 ARIC participants, 4,964 (31.4%) had a least one hospitalization with infection during follow-up and 1,062 (6.7%) suffered an ischemic stroke. Of the 1,062 strokes identified during the follow-up period, 54 were excluded because their control periods occurred prior to ARIC study enrollment, leaving a total of 1,008 strokes for analysis. Baseline (ARIC visit 1) characteristics are provided in Table 1 for both stroke participants and non-stroke participants (for comparison). The majority of the stroke participants were white (60%), they were equally split between males and females, and had an average age of 56 years at baseline. Of the 1,008 stroke participants, 37 had a hospitalization with infection during the preceding 90 days. Models constructed using a 4-day washout period produced results similar to the models constructed using a 2-day washout and thus only models using a 2-day washout period are presented. As shown in Table 2, hospitalized infection was more common in all case periods compared to equivalent control periods but the ORs decrease with elapsed time: 14 day OR (95% CI) = 7.7 (2.1, 27.3), 30 day OR (95% CI) = 5.7 (2.3, 14.3), 42 day OR (95% CI) = 4.5 (2.0, 10.2), and 90 day OR (95% CI) = 3.6 (2.1, 6.5). Controlling for the number of hospitalizations slightly attenuated the association between hospitalization with infection and stroke.
Table 1.
Baseline (Visit 1) Characteristics of ARIC Participants by Future Stroke Status, 1987–89
| Baseline Characteristic | Stroke Participants (n=1,008) |
Non-Stroke Participants (n=14,730) |
|---|---|---|
| Age, years, mean ± SD Age at Stroke, years, mean ± SD |
56.1 ± 5.5 69.4 ± 7.6 |
54.0 ± 5.7 |
| Sex, Count (%) | ||
| Male | 506 (50.2) | 8,185 (55.6) |
| Female | 502 (49.8) | 6545 (44.4) |
| Race, Count (%) | ||
| White | 607 (60.2) | 10,844 (73.6) |
| Black | 400 (39.7) | 3,839 (26.1) |
| Asian | 1 (0.1) | 33 (0.2) |
| Current Smoker, Count (%) | ||
| No | 679 (67.4%) | 10,934 (74.3) |
| Yes | 328 (35.6) | 3,781 (25.7) |
| Diabetes | ||
| No | 739 (74.4) | 13,004 (89.1) |
| Yes | 254 (25.6) | 1,594 (10.9) |
| Treatment for Hypertension | ||
| No | 540 (53.6) | 10,386 (70.6) |
| Yes | 468 (46.4) | 4,4336 (29.5) |
| Systolic Blood Pressure, mm Hg mean ± SD |
129.8 ± 21.2 | 120.7 ± 18.6 |
| HDL cholesterol, mg/dL, mean ± SD | 48.4 ± 16.0 | 51.8 ± 17.2 |
| Total Cholesterol, mg/dL mean ± SD | 220.6 ± 45.4 | 214.5 ± 41.7 |
| CVD 10-year risk probability, mean ± SD |
0.12 ± 0.10 | 0.07 ± 0.07 |
Table 2.
Association of Recent Hospitalization with Infection and Risk of Ischemic Stroke
| Infection hospitalization by interval before stroke |
Case (n) | Control (n) | Unadjusted OR, 95% CI |
Adjusted* OR, 95%CI |
|---|---|---|---|---|
| 14 Days | ||||
| No | 996 | 2013 | 8.0 (2.3, 28) | 7.7 (2.1, 27) |
| Yes | 12 | 3 | ||
| 30 Days | ||||
| No | 988 | 2010 | 6.3 (2.5, 16) | 5.7 (2.3, 14) |
| Yes | 20 | 6 | ||
| 42 Days | ||||
| No | 987 | 2008 | 5.3 (2.3, 12) | 4.5 (2.1, 10) |
| Yes | 21 | 8 | ||
| 90 Days | ||||
| No | 971 | 1995 | 3.9 (2.2, 6.9) | 3.6 (2.1, 6.5) |
| Yes | 37 | 21 |
Adjusted for total number of hospitalizations
In all interaction models, the infection-stroke association was stronger in those with low background CVD risk compared to those with high background CVD risk (Table 3). These interactions were significant for CVD risk measured at visit 1 for the 30-day (OR High CVD Risk – 2.5 vs. OR Low CVD Risk – 28.0) and 42-day (OR High CVD Risk – 2.0 vs. OR Low CVD Risk – 14.0) periods, and borderline significant for CVD risk measured at the most recent visit for the 30-day (OR High CVD Risk – 3.5 vs. OR Low CVD Risk – 26.0) period.
Table 3.
Association of Recent Hospitalization with Infection and Risk of Ischemic Stroke by Background CVD Risk Score
| Crude OR, Stratified by CVD Risk Score at Visit 1 | |||
| High CVD Risk* (n = 489) OR (95% CI) |
Low CVD Risk† (n=490) OR (95% CI) |
Interaction P-Value‡ |
|
| 14-Day | 5.0 (1.0, 26) | 12.0 (1.4, 100) | 0.10 |
| 30-Day | 2.5 (0.7, 9.3) | 28.0 (3.7, 213) | 0.03 |
| 42-Day | 2.0 (0.6, 6.9) | 14.0 (3.2, 62) | 0.04 |
| 90-Day | 2.7 (1.1, 6.3) | 5.3 (2.3, 12) | 0.31 |
| Crude OR, Stratified by CVD Risk Score at Recent Visit | |||
| High CVD Risk§ (n=489) OR (95% CI) |
Low CVD Risk‖ (n=488) OR (95% CI) |
Interaction P-Value‡ |
|
| 14-Day | 7.0 (1.5, 34) | 10.0 (1.2, 86) | 0.39 |
| 30-Day | 3.5 (1.0, 12) | 26.0 (3.4, 199) | 0.07 |
| 42-Day | 3.2 (1.0, 9.8) | 13.0 (2.9, 58) | 0.13 |
| 90-Day | 3.3 (1.5, 7.6) | 5.4 (2.3, 13) | 0.20 |
| Adjusted# OR, Stratified by CVD Risk Score at Visit 1 | |||
| High CVD Risk* (n=489) Adjusted# OR (95% CI) |
Low CVD Risk† (n=490) Adjusted# OR (95% CI) |
Interaction P-Value‡ |
|
| 14-Day | 4.2 (0.8, 23) | 12.6 (1.5, 105) | 0.10 |
| 30-Day | 1.7 (0.4, 6.9) | 25.9 (3.4, 198) | 0.01 |
| 42-Day | 1.4 (0.4, 5.2) | 13.0 (2.9, 57) | 0.02 |
| 90-Day | 2.5 (1.0, 5.9) | 5.1 (2.2, 12) | 0.29 |
| Adjusted# OR, Stratified by CVD Risk Score at Recent Visit | |||
| High CVD Risk§ (n=489) Adjusted# OR (95% CI) |
Low CVD Risk‖ (n=488) Adjusted# OR (95% CI) |
Interaction P-Value‡ |
|
| 14-Day | 6.2 (1.3, 30) | 10.6 (1.2, 91) | 0.36 |
| 30-Day | 3.5 (1.0, 12) | 26.0 (3.4, 199) | 0.07 |
| 42-Day | 2.4 (0.8, 7.7) | 12.0 (2.7, 53) | 0.11 |
| 90-Day | 3.0 (1.3, 7.0) | 5.2 (2.2, 12.4) | 0.20 |
10-year CVD Risk ≥10.00%
10-year CVD Risk < 10.00%
P-value from model with continuous CVD risk score
10-year CVD Risk ≥15.38%
10-year CVD Risk < 15.38%
Adjusted for total number of hospitalizations
Results from the analysis stratified by ischemic stroke sub-type are presented in Table 4. The infection-stroke association was stronger among thrombotic strokes for the 14-day period (OR thrombotic – 9.6 vs OR cardioembolic - 8.0) but stronger for cardioembolic strokes for the 30-day (OR thrombotic 3.1 vs. OR cardioembolic – 16.3), 42-day (OR thrombotic – 2.4 vs. OR cardioembolic – 18.3), and 90-day (OR thrombotic – 2.4 vs. OR cardioembolic – 9.9) periods. The differences between thrombotic and cardioembolic strokes did not reach statistical significance for any time periods.
Table 4.
Association of Recent Hospitalization with Infection and Risk of Ischemic Stroke by Ischemic Stroke Sub-Type
| Crude OR Stratified by Ischemic Stroke Sub-Type | ||
| Thrombotic Stroke (n=752) OR (95% CI) |
Cardioembolic Stroke (n=256) OR, (95% CI) |
|
| 14 Day | 12.0 (1.4, 99.7) | 8.0 (0.9, 71.6) |
| 30 Day | 4.4 (1.5, 12.7) | 16.0 (2.0, 127.9) |
| 42 Day | 3.4 (1.4, 8.7) | 18.0 (2.3, 142.1) |
| 90 Day | 2.7 (1.4, 5.2) | 10.0 (2.9, 34.5) |
| Adjusted* OR Stratified by Ischemic Stroke Sub-Type | ||
| Thrombotic Stroke (n=752) OR (95% CI) |
Cardioembolic Stroke (n=256) OR, (95% CI) |
|
| 14 Day | 9.6 (1.1, 81.4) | 8.0 (2.0, 130.1) |
| 30 Day | 3.1 (1.0, 9.3) | 16.3 (2.0, 130.1) |
| 42 Day | 2.4 (0.9, 6.4) | 18.3 (2.3, 144.3) |
| 90 Day | 2.4 (1.2, 4.7) | 9.9 (2.8, 34.2) |
Adjusted for total number of hospitalizations
Discussion
This case-crossover study within a population-based cohort demonstrated that ischemic stroke risk is higher after hospitalization with infection. Patients with infection had higher odds of stroke up to 90 days after the hospitalization with infection compared to equivalent control periods 1 and 2 years previous. This supports our hypothesis that hospitalized infection is associated with higher ischemic stroke risk. The association between hospitalization with infection and stroke was graded, such that stroke risk was highest in the exposure periods most proximal to the stroke event and decreased as the time window before stroke increased. These results corroborate previous work that found that infection may function as a stroke trigger and that the stoke risk varies by time since infection.13 We found some evidence that stroke risk following hospitalization with infection appears to be more prominent in those with low background CVD risk. This pattern of interaction is consistent with results reported by Elkind et al.,13 but corroboration with larger studies is needed. Though the infection-stroke association may be stronger for cardioembolic strokes, it is difficult to determine if infection is a stronger trigger for a particular stroke sub-type given the imprecise estimates and wide confidence intervals due to small cell sizes.
Previous investigators have suggested possible mechanisms by which infection can trigger CVD events. Corrales-Medina et al. posited mechanisms by which infection may trigger coronary events, including via inflammation, prothrombosis, increased biomechanical stress on coronary arteries, variations in the coronary arterial tone, disturbed hemodynamic homoeostasis, and altered myocardial metabolic balance.17 Considering stroke, specifically, Elkind et al. suggest that infection may trigger stroke events through hypercoagulability, platelet activation, and impaired endothelial function.13 They further hypothesize that infection may contribute to dehydration and immobility which increase stroke risk.13 Additional research on the mechanisms linking infection and stroke including considering infection and stroke triggering by infection and stroke sub-type is warranted.
Our study has a number of strengths, including a large sample size from a community cohort, a rigorous methodology to adjudicate ischemic strokes, and a cross-over design to control for potential confounding. It also has limitations. Like all case crossover studies, our study may suffer from survival bias since we did not consider infections in participants who did not have a stroke. Confounding by age is possible because as participants age their risk of both stroke and hospitalization with infection increase. To reduce potential confounding, only time periods proximal to stroke (1 and 2 years previous) were examined and the total number of hospitalizations was included in the adjusted models. Other confounders such as medication use that may vary between the exposure and control periods were not measured. Relatively few infections prior to stroke yielded OR estimates that were imprecise, with wide confidence intervals. Since we used the hospital admission as the stroke date, stroke dates for patients who do not seek immediate medical attention may be inaccurate, but we think that this is rare since most patients immediately seek care. We certainly are under-ascertaining infections, especially minor ones, by including only hospitalizations with infections. This would most likely lead to non-differential misclassification of the exposure that would typically bias ORs towards the null.
Our study has a number of implications. Identification of hospitalization with infection as a stroke trigger may prompt more aggressive treatment with standard preventive strategies, including antiplatelet agents and statins, during and immediately following hospitalization with infection to reduce the increased risk of acute stroke. This time period immediately following infection is referred to as a “treatable moment”.13 While antibiotics have not been shown to prevent vascular events18–20, other infection control efforts, including evidence-based vaccination, may be considered because of their ability to not only reduce infection but stroke also.21 Further, considering past infections may help clinicians identify causes of so-called cryptogenic strokes.
Conclusion
Ischemic stroke patients have higher odds of a hospitalization with infection up to 90 days prior to their stroke compared to equivalent control periods 1 and 2 years previous. Stroke risk following hospitalization with infection appears to be higher in those with low background CVD risk. There may be a role from primary prevention with anti-platelet therapy in the immediate aftermath of an infection, though clinical trials and cost-benefit analysis should be considered.
Acknowledgments
We thank the staff and participants of the ARIC study for their important contributions.
Funding:
The ARIC Study was supported by National Heart, Lung, and Blood Institute (NHLBI) contracts HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C. L.T.C. is supported by an NHLBI training grant T32 HL007779. A.A. is supported by grant R01 HL122200 from the National Institutes of Health and 16EIA2641001 from the American Heart Association.
Footnotes
Disclosures:
None
References
- 1.Arnold AM, Psaty BM, Kuller LH, Burke GL, Manolio TA, Fried LP, et al. Incidence of cardiovascular disease in older americans: The cardiovascular health study. Journal of the American Geriatrics Society. 2005;53:211–218. doi: 10.1111/j.1532-5415.2005.53105.x. [DOI] [PubMed] [Google Scholar]
- 2.Petrea RE, Beiser AS, Seshadri S, Kelly-Hayes M, Kase CS, Wolf PA. Gender differences in stroke incidence and poststroke disability in the framingham heart study. Stroke; a journal of cerebral circulation. 2009;40:1032–1037. doi: 10.1161/STROKEAHA.108.542894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosamond WD, Folsom AR, Chambless LE, Wang CH, McGovern PG, Howard G, et al. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the atherosclerosis risk in communities (aric) cohort. Stroke; a journal of cerebral circulation. 1999;30:736–743. doi: 10.1161/01.str.30.4.736. [DOI] [PubMed] [Google Scholar]
- 4.Vilela-Martin JF, Vaz-de-Melo RO, Kuniyoshi CH, Abdo AN, Yugar-Toledo JC. Hypertensive crisis: Clinical-epidemiological profile. Hypertension research : official journal of the Japanese Society of Hypertension. 2011;34:367–371. doi: 10.1038/hr.2010.245. [DOI] [PubMed] [Google Scholar]
- 5.Guiraud V, Amor MB, Mas JL, Touze E. Triggers of ischemic stroke: A systematic review. Stroke; a journal of cerebral circulation. 2010;41:2669–2677. doi: 10.1161/STROKEAHA.110.597443. [DOI] [PubMed] [Google Scholar]
- 6.Syrjanen J, Valtonen VV, Iivanainen M, Kaste M, Huttunen JK. Preceding infection as an important risk factor for ischaemic brain infarction in young and middle aged patients. British medical journal. 1988;296:1156–1160. doi: 10.1136/bmj.296.6630.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grau AJ, Buggle F, Heindl S, Steichen-Wiehn C, Banerjee T, Maiwald M, et al. Recent infection as a risk factor for cerebrovascular ischemia. Stroke; a journal of cerebral circulation. 1995;26:373–379. doi: 10.1161/01.str.26.3.373. [DOI] [PubMed] [Google Scholar]
- 8.Bova IY, Bornstein NM, Korczyn AD. Acute infection as a risk factor for ischemic stroke. Stroke; a journal of cerebral circulation. 1996;27:2204–2206. doi: 10.1161/01.str.27.12.2204. [DOI] [PubMed] [Google Scholar]
- 9.Grau AJ, Buggle F, Becher H, Zimmermann E, Spiel M, Fent T, et al. Recent bacterial and viral infection is a risk factor for cerebrovascular ischemia: Clinical and biochemical studies. Neurology. 1998;50:196–203. doi: 10.1212/wnl.50.1.196. [DOI] [PubMed] [Google Scholar]
- 10.Macko RF, Ameriso SF, Gruber A, Griffin JH, Fernandez JA, Barndt R, et al. Impairments of the protein c system and fibrinolysis in infection-associated stroke. Stroke; a journal of cerebral circulation. 1996;27:2005–2011. doi: 10.1161/01.str.27.11.2005. [DOI] [PubMed] [Google Scholar]
- 11.Grau AJ, Preusch MR, Palm F, Lichy C, Becher H, Buggle F. Association of symptoms of chronic bronchitis and frequent flu-like illnesses with stroke. Stroke; a journal of cerebral circulation. 2009;40:3206–3210. doi: 10.1161/STROKEAHA.109.561019. [DOI] [PubMed] [Google Scholar]
- 12.Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. The New England journal of medicine. 2004;351:2611–2618. doi: 10.1056/NEJMoa041747. [DOI] [PubMed] [Google Scholar]
- 13.Elkind MS, Carty CL, O'Meara ES, Lumley T, Lefkowitz D, Kronmal RA, et al. Hospitalization for infection and risk of acute ischemic stroke: The cardiovascular health study. Stroke; a journal of cerebral circulation. 2011;42:1851–1856. doi: 10.1161/STROKEAHA.110.608588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levine DA, Langa KM, Rogers MA. Acute infection contributes to racial disparities in stroke mortality. Neurology. 2014;82:914–921. doi: 10.1212/WNL.0000000000000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The ARIC Investigators. The atherosclerosis risk in communities (aric) study: Design and objectives. American journal of epidemiology. 1989;129:687–702. [PubMed] [Google Scholar]
- 16.Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D'Agostino RB, Gibbons R, et al. 2013 acc/aha guideline on the assessment of cardiovascular risk: A report of the american college of cardiology/american heart association task force on practice guidelines. Circulation. 2014;129:S49–S73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 17.Corrales-Medina VF, Madjid M, Musher DM. Role of acute infection in triggering acute coronary syndromes. The Lancet. Infectious diseases. 2010;10:83–92. doi: 10.1016/S1473-3099(09)70331-7. [DOI] [PubMed] [Google Scholar]
- 18.Muhlestein JB, Anderson JL, Carlquist JF, Salunkhe K, Horne BD, Pearson RR, et al. Randomized secondary prevention trial of azithromycin in patients with coronary artery disease: Primary clinical results of the academic study. Circulation. 2000;102:1755–1760. doi: 10.1161/01.cir.102.15.1755. [DOI] [PubMed] [Google Scholar]
- 19.Vainas T, Stassen FR, Schurink GW, Tordoir JH, Welten RJ, van den Akker LH, et al. Secondary prevention of atherosclerosis through chlamydia pneumoniae eradication (space trial): A randomised clinical trial in patients with peripheral arterial disease. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. 2005;29:403–411. doi: 10.1016/j.ejvs.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Grayston JT, Kronmal RA, Jackson LA, Parisi AF, Muhlestein JB, Cohen JD, et al. Azithromycin for the secondary prevention of coronary events. The New England journal of medicine. 2005;352:1637–1645. doi: 10.1056/NEJMoa043526. [DOI] [PubMed] [Google Scholar]
- 21.Udell JA, Zawi R, Bhatt DL, Keshtkar-Jahromi M, Gaughran F, Phrommintikul A, et al. Association between influenza vaccination and cardiovascular outcomes in high-risk patients: A meta-analysis. Jama. 2013;310:1711–1720. doi: 10.1001/jama.2013.279206. [DOI] [PubMed] [Google Scholar]