Abstract
Objective
To longitudinally assess the effect of native tissue vaginal apical prolapse repair with anti-incontinence surgery on quality of life, sexual function, and body image between uterosacral and sacrospinous suspensions.
Methods
A planned secondary analysis was performed on 374 women enrolled in a randomized trial of the two types of native tissue repair for apical prolapse. Condition-specific and generic quality of life, sexual function, overall and de novo dyspareunia, and body image were assessed using validated instruments at baseline, 6, 12, and 24 months postoperatively, and changes from baseline were assessed and compared between surgical groups. General linear mixed models were used for comparisons and clinically significant differences were assessed using minimum important differences.
Results
Of the women randomized, 82% had outcomes available at 2 years. Overall, clinically and statistically significant improvements in generic and condition-specific quality of life and sexual function were observed after surgery. Dyspareunia rates decreased from 25% to 16% by 24 months, with only 3% of all women undergoing treatment. De novo dyspareunia occurred in 5% and 10% by 12 and 24 months, respectively. Body image scores also significantly improved from baseline. There were no clinically meaningful or statistically significant differences between groups for any of these outcomes (all p>0.05).
Conclusion
Native tissue vaginal prolapse surgery results in statistically and clinically significant improvements in quality of life, sexual function, and body image at 24 months with no significant differences between uterosacral and sacrospinous suspensions. One in 10 women experience de novo dyspareunia but few requested treatment.
Clinical Trial Registration
ClinicalTrials.gov, www.clinicaltrials.gov, NCT00597935.
Introduction
Pelvic organ prolapse (POP) is a common condition affecting millions of women with significant negative impact on quality of life, sexual function, and body image.1–4 Treatment options include expectant, conservative and surgical management, with up to 13% of women undergoing surgery in their lifetime.5 While a variety of surgical approaches exist to correct apical POP, the majority of repairs are still approached vaginally.6 Transvaginal uterosacral ligament suspension and sacrospinous ligament fixation are the two most common native tissue approaches to apical POP, but they have distinct anatomic variation. Traditionally, sacrospinous ligament fixation is performed with a unilateral deviation of the vaginal apex to the right sacrospinous ligament, while uterosacral suspension replaces the apex in the midline to the hollow of the sacrum.7,8 Theoretically, the location of apical support could impact sexual function and dyspareunia rates. While others have shown improvement in quality of life and sexual function after native tissue prolapse repairs, data comparing these two procedures is limited.9–12
The primary outcomes from the Operations and Pelvic Muscle Training in the Management of Apical Support Loss (OPTIMAL) trial demonstrated similar surgical success rates of approximately 63–65% with no difference in subjective or anatomic failures between the two surgical approaches, and low retreatment rates of approximately 5% at 2 years.13 The addition of perioperative behavioral therapy with pelvic floor muscle training had no significant impact on overall prolapse or urinary incontinence rates compared to usual care. The goals of this analysis were to report on the secondary outcomes of quality of life, sexual function, and body image longitudinally over the 2-year follow up period of the original study.
Materials and Methods
The OPTIMAL trial was conducted by the Pelvic Floor Disorders Network at 9 clinical sites and was IRB approved at each site as a, multicenter, randomized, blinded, 2×2 factorial designed trial comparing two native tissue vaginal prolapse apical suspensions (uterosacral ligament suspension vs. sacrospinous ligament fixation) with midurethral sling surgery in women with stage 2 or greater uterine or vaginal prolapse and stress urinary incontinence and perioperative behavioral therapy with pelvic floor muscle training vs. usual perioperative care. Details of this study have been previously published.13,14 Briefly, the OPTIMAL trial had 2 primary aims: 1) to compare surgical outcomes of sacrospinous ligament fixation to uterosacral ligament suspension 24 months after vaginal surgery for apical or uterine prolapse and stress incontinence and 2) to evaluate the impact of a perioperative behavioral-pelvic muscle therapy program on urinary and prolapse outcomes. Each enrolled patient first underwent a 1:1 perioperative behavioral and pelvic muscle therapy vs. usual care randomization followed by a second 1:1 surgical randomization. Patients were sequentially randomized using a permuted block design and stratified by clinical site. Surgical randomization was also stratified by surgeon and concomitant hysterectomy. This was a planned secondary analysis of the longitudinal quality of life outcomes between the two surgical methods, which was IRB-approved by the 9 clinical sites.
Participants enrolled in the OPTIMAL trial were masked to surgical treatment and underwent standardized evaluations by masked assessors at baseline, 6, 12 and 24 months including administration of validated patient-reported outcome measures to assess generic and condition-specific health related quality of life, sexual function, and body image. The SF-36, with its mental and physical components, was used to assess generic quality of life.15 The Pelvic Floor Distress Inventory (PFDI), was used to assess pelvic floor symptoms and associated bother and has 3 subscales: the pelvic organ prolapse distress inventory (POPDI); the colorectal-anal distress inventory (CRADI); and the urinary distress inventory (UDI).16 The Pelvic Floor Impact Questionnaire (PFIQ) also has 3 subscales: the pelvic organ prolapse impact questionnaire (POPIQ); the colorectal-anal impact questionnaire (CRAIQ); and the urinary impact questionnaire (UIQ) and was used to assess condition specific quality of life.16
Sexual activity was assessed in all participants at baseline and post operatively with the question “In the past 3 months, have you engaged in sexual activities with a partner? Yes or No.” Sexual function in sexually active and inactive women was assessed with questions from the Prolapse-Incontinence Sexual Function Questionnaire short form (PISQ-12). For sexually active women, total scores were calculated. For inactive women, only select questions were used to ascertain dyspareunia as defined below. Overall sexual activity and dyspareunia rates were evaluated at each time point for both active and inactive women: In sexually active women, dyspareunia was defined as a response of “usually or always” to PISQ question #B5: “Do you feel pain during sexual intercourse?” or any postoperative treatment for dyspareunia. For those women who were not sexually active, dyspareunia was defined as a response of “usually or always” to question #C4: “Does fear of pain during sexual intercourse restrict your activity?” or any post operative treatment for dyspareunia. De novo dyspareunia was assessed at each time point in women who were sexually active at baseline without pain, who then either a) were still sexually active but reported dyspareunia (“usually or always” as above) or b) were not active due to fear of pain (“usually or always”) or received any post operative treatment for dyspareunia. Treatment for dyspareunia was assessed at each time point with the question: “Have you undergone any treatment for painful or difficult intercourse because your vagina was too narrow or too tight?” Additionally, any of the following outcomes including surgical intervention and use of dilators, physical therapy prescribed outside of the protocol or vaginal estrogen was considered treatment. Sexual activity limitations due to urinary incontinence and prolapse symptoms were defined, in both sexually active and inactive women, as responses of “usually or always” to the questions “Does fear of incontinence (either urine or stool) during sexual intercourse restrict your sexual activity?” and “Do you avoid sexual intercourse because of bulging in the vagina (either the bladder, rectum, or vagina falling out)?” Body image was evaluated using a modification of the body image scale described by Hopwood et al. Five questions were asked at baseline for a maximum score of 15 and nine questions were asked at follow up for a maximum score of 27.17,18 Given the discrepancy in scoring for baseline and follow up, these scores were normalized to a 100 point scale for purposes of analyzing change from baseline and statistical modeling as recommended by Hopwood et al.18
Longitudinal changes over time within and between surgical groups for PFDI, PFIQ, SF-36, PISQ-12, and body image scores were assessed using longitudinal general linear mixed models. Changes from baseline at 6-, 12-, and 24-month time points were estimated and compared between groups. As in the primary analysis, models included effects for surgical and behavioral treatments, hysterectomy status, time, and interactions between surgical and behavioral therapy treatment effects and time. Surgeon was included as a random effect in the model because of the large number of surgeons in the trial. In addition, for these analyses, age was included in the models as a fixed effect due to the known impact of age on sexual activity and function. Similar models were fit for dichotomous outcomes of sexual activity and de novo dyspareunia rates; longitudinal generalized linear mixed models including the same effects described above were used to evaluate changes over time and compare surgical groups. Data from baseline, 6-, 12-, and 24-month time points were used in the analyses.
Missing patient-reported outcomes were defined based on the scoring instructions for the respective instrument and were excluded from analysis. Participants were included in the analysis models at time points where their outcomes were non-missing. For each scale, models were constructed to predict missing data status based on treatment assignment to determine whether the missing data were associated with surgical treatment group. Statistical significance was determined using a p value of < 0.05 and clinical significance was defined as exceeding published minimum important differences of 2 to 3 for SF-36, 11 for UDI, 16 for UIQ, 11 for CRADI, 18 for CRAQI and 6 for PISQ-12.19–22 As there are no published minimum important differences for the POPDI, POPIQ, or body image scale, clinically significant changes in these scores were assessed using ½ the baseline standard deviation as a conservative estimate of minimum important difference (34.3 for POPDI, 48.4 for POPIQ, and 13.0 for body image).23
Sample size calculations were based on the primary OPTIMAL study, which determined that 170 patients per group would be sufficient to detect an effect size of 0.30 with 80% power (or 90% power to detect an effect size of 0.36) based on a two-sided t test and the assumption of normally distributed differences.14 Assuming a standard deviation for the scales of the PFDI of 68, this would provide 80% power to detect a difference of 21 points or higher between groups at a significance level of 0.05.16 Under the same assumptions, 150 women per group provides 80% power to detect a difference of 22 points or higher between groups. SAS statistical software was used for analysis (version 9.4, SAS Institute Inc., Cary, NC), and the MIXED and GLIMMIX procedures were used to model continuous and categorical outcomes, respectively.
Results
The baseline characteristics of the 374 women randomized to surgery (188 uterosacral ligament suspension vs.186 sacrospinous ligament fixation) and to behavioral therapy with pelvic floor muscle training (186 behavioral therapy with pelvic floor muscle training vs.188 usual care) have been previously reported.13 The follow-up rate at 2 years was 82% for quality of life measures and surgical treatment group was not predictive of missing outcome data for any of the quality of life outcomes (data not shown). There were no significant differences in demographic variables between participants with vs. without missing outcomes at 6 months (data not shown). The only significant difference between participants with vs. without missing outcomes at 24 months was in current smoking (20% vs. 6% respectively, p<0.001). Tables 1 and 2 display the number of participants who provided outcomes at various study time points. In the 2 surgical groups (uterosacral ligament suspension, sacrospinous ligament fixation, respectively), the mean age (± standard deviation) was 57.2 years (57.3±10.8, 57.2±11.0), and mean body mass index was 28.8 (28.7±5.2, 29.0±5.7). The median number of vaginal deliveries was 3 (3, 2), Overall, 57% of women had stage 3 pelvic organ prolapse (59%, 55%). The majority of women were Caucasian 84% (84%, 84%) and postmenopausal 66% (65%, 66%). Systemic estrogen replacement therapy was used by 12% (11%, 14%) and vaginal estrogen was used by 24% (26%, 21%). Prior hysterectomy was reported in 27% (26%, 28%) of participants, 4% (4%, 3%) had prior surgery for stress incontinence, and 7% (5%, 9%) prior surgery for pelvic organ prolapse.
Table 1.
Changes in symptom distress and quality of life scores after vaginal prolapse surgery
| Variable | BASELINE | Change from Baseline to 6 MONTHS | Change from Baseline to 12 MONTHS | Change from Baseline to 24 MONTHS | Time (P-value)* | ULS vs. SSLF (P-value)* |
|---|---|---|---|---|---|---|
|
| ||||||
|
PFDI
| ||||||
| UDI | <.01 | 0.81 | ||||
| N | 354 | 328 | 312 | 292 | 6 vs. 12MON: 0.76 | |
| Mean ± SE | 126.5 ± 3.2 | −100.0 ± 4.5† | −100.6 ± 4.5† | −89.5 ± 4.5† | 6 vs. 24MON: <0.01 | |
| Min, Max | 9.6, 292.3 | −276.5, 95.1 | −276.5, 73.1 | −274.6, 107.5 | 12 vs. 24MON: <0.01 | |
|
| ||||||
| POPDI | <.01 | 0.51 | ||||
| N | 354 | 328 | 312 | 292 | 6 vs. 12MON: 0.43 | |
| Mean ± SE | 123.8 ± 3.6 | −89.5 ± 4.9† | −91.4 ± 4.9† | −78.8 ± 4.9† | 6 vs. 24MON: <0.01 | |
| Min, Max | 0.0, 300.0 | −278.0, 121.4 | −273.2, 130.4 | −288.7, 131.5 | 12 vs. 24MON: <0.01 | |
|
| ||||||
| CRADI | <.01 | 6MON: 0.42 | ||||
| N | 354 | 328 | 312 | 292 | Time × Surgery interaction: <.01 | 12MON: 0.70 |
| 24MON: 0.18 | ||||||
| Mean ± SE | 110.6 ± 4.5 | −73.5 ± 4.9† | −74.0 ± 4.9† | −59.0 ± 5.0† | ||
| Min, Max | 0.0, 380.4 | −295.7, 89.5 | −363.3, 126.2 | −363.3, 206.2 | ||
| CRADI – ULS | 6 vs. 12MON: 0.18 | |||||
| N | 175 | 163 | 153 | 144 | 6 vs. 24MON: 0.16 | |
| Mean ± SE | 107.7 ± 6.5 | −70.3 ± 6.4† | −75.6 ± 6.5† | −64.6 ± 6.5† | 12 vs. 24MON: <.01 | |
| Min, Max | 0.0, 380.4 | −283.6, 84.5 | −363.3, 126.2 | −363.3, 96.7 | ||
| CRADI – SSLF | 6 vs. 12MON: 0.28 | |||||
| N | 179 | 165 | 159 | 148 | 6 vs. 24MON: <.01 | |
| Mean ± SE | 113.4 ± 6.1 | −76.7 ± 6.3† | −72.5 ± 6.3† | −53.5 ± 6.4† | 12 vs. 24MON: <.01 | |
| Min, Max | 0.0, 357.1 | −295.7, 89.5 | −295.7, 115.1 | −283.6, 206.2 | ||
|
| ||||||
|
PFIQ
| ||||||
| UIQ | <.01 | Surgery × BPMT interaction | ||||
| N | 354 | 328 | 312 | 292 | 6 vs. 12MON: 0.41 | Effect of surgery: |
| Mean ± SE | 137.1 ± 5.5 | −102.5 ± 7.0† | −104.8 ± 7.0† | −90.4 ± 7.0† | 6 vs. 24MON: <0.01 | within BPMT: 0.40 |
| Min, Max | 0.0, 400.0 | −383.8, 280.3 | −383.8, 277.5 | −383.8, 298.1 | 12 vs. 24MON: <0.01 | within usual care: 0.046 ‡ |
|
| ||||||
| POPIQ | <.01 | Surgery × BPMT interaction | ||||
| N | 354 | 328 | 312 | 295 | 6 vs. 12MON: 0.65 | Effect of surgery: |
| Mean ± SE | 73.0 ± 5.1 | −60.3 ± 6.6† | −58.9 ± 6.7† | −39.5 ± 6.7† | 6 vs. 24MON: <0.01 | within BPMT: 0.07 |
| Min, Max | 0.0, 392.2 | −386.8, 160.5 | −392.2, 321.7 | −386.8, 370.3 | 12 vs. 24MON: <0.01 | within usual care: 0.15 |
|
| ||||||
| CRAIQ | <.01 | Surgery × BPMT interaction | ||||
| N | 354 | 328 | 312 | 295 | 6 vs. 12MON: 0.50 | Effect of surgery: |
| Mean ± SE | 99.3 ± 5.8 | −83.3 ± 8.6† | −85.3 ± 8.7† | −69.6 ± 8.7† | 6 vs. 24MON: <0.01 | within BPMT: 0.07 |
| Min, Max | 0.0, 400.0 | −397.0, 268.6 | −391.4, 316.2 | −−386.8, 341.6 | 12 vs. 24MON: <0.01 | within usual care:0.06 |
|
| ||||||
|
SF36
| ||||||
| Mental Component | 0.21 | 0.66 | ||||
| N | 357 | 327 | 313 | 292 | ||
| Mean ± SE | 47.6 ± 0.6 | 2.8 ± 0.7† | 2.9 ± 0.7† | 2.0 ± 0.7† | ||
| Min, Max | −3.4, 68.0 | −35.8, 32.0 | −35.7, 47.5 | −36.8, 39.5 | ||
|
| ||||||
| Physical Component | 0.66 | 0.75 | ||||
| N | 357 | 327 | 313 | 292 | ||
| Mean ± SE | 43.9 ± 0.5 | 5.8 ± 0.7† | 6.1 ± 0.7† | 5.7 ± 0.7† | ||
| Min, Max | 13.7, 63.4 | −22.1, 35.2 | −37.9, 45.4 | −25.0, 44.9 | ||
ULS=uterosacral ligament suspension. SSLF=sacrospinous ligament fixation. PFDI=Pelvic Floor Distress Inventory. UDI= Urinary Distress Inventory. POPDI=Pelvic Organ Prolapse Distress Inventory, CRADI=Colorectal-anal Distress Inventory. PFIQ=Pelvic Floor Impact Questionnaire. UIQ=Urinary Impact Questionnaire. POPIQ=Pelvic Organ Prolapse Impact Questionnaire. CRAIQ=Colorectal-anal Impact Questionnaire. SE=standard error. MON=Month. BPMT=behavioral therapy-pelvic muscle training.
P-values for Time and for ULS vs. SSLF are from longitudinal general linear mixed models of changes from baseline.
Values at 6-month, 12-month, and 24-month time points are adjusted mean changes from baseline and standard errors from the longitudinal general linear mixed models. Mean changes from baseline are significantly different from zero at the p<0.01 level.
Surgical treatment effect within the usual care group is significant with P-value=0.046. Adjusted mean ± SE at each time point for ULS = 150.3 ± 11.6, −117.9 ± 11.3, −123.5 ± 11.3, −104.0 ± 11.4. SSLF = 121.1 ± 10.7, −88.1 ± 11.3, −93.7 ± 11.4, −79.9 ± 11.4. P-values for comparisons between ULS and SSLF at 6-, 12-, and 24-month time points within the usual care group are: 0.04, 0.04, and 0.10, respectively.
Table 2.
Sexual function and body image after vaginal prolapse surgery
| Variable | BASELINE | 6 MONTHS | 12 MONTHS | 24 MONTHS | Time (P-value)* | ULS vs. SSLF (P-value)* |
|---|---|---|---|---|---|---|
|
| ||||||
|
Sexually Active
| ||||||
| Overall | 184/346 (53.2%) | 195/331 (58.9%) | 186/317 (58.7%) | 169/305 (55.4%) | 0.65 | 0.16 |
| De novo | N/A | 27/145 (18.6%) | 28/134 (20.9%) | 37/136 (27.2%) | 0.17 | 0.19 |
| Stopped | N/A | 7/169 (4.1%) | 10/161 (6.2%) | 26/150 (17.3%) | <0.01 | 0.02 |
| ULS – Stopped | N/A | 1/84 (1.2%) | 2/80 (2.5%) | 12/76 (15.8%) | 6 vs. 12MON: 0.52 | 6MON: 0.054 |
| SSLF – Stopped | N/A | 6/85 (7.1%) | 8/81 (9.9%) | 14/74 (18.9%) | 6 vs. 24MON: <0.01 12 vs. 24MON: <0.01 |
12MON: 0.01 24MON: 0.44 |
|
| ||||||
|
Not
Active/Restricted
| ||||||
| Due to Pain | 77/312 (24.7%) | 57/283 (20.1%) | 56/281 (19.9%) | 62/270 (23.0%) | 0.63 | 0.97 |
| Due to Bulge | 106/316 (33.5%) | 59/284 (20.8%)† | 57/283 (20.1%)† | 57/278 (20.5%)† | 0.98 | 0.91 |
| Due to Incontinence | 55/313 (17.6%) | 30/286 (10.5%)† | 28/284 (9.9%)† | 32/272 (11.8%)‡ | 0.78 | >0.99 |
|
| ||||||
|
Dyspareunia§
| ||||||
| Overall Dyspareunia = PISQ-12 Questions B5 or C4 or Treatment | 77/312 (24.7%) | 31/301 (10.3%) † | 26/296 (8.8%) † | 45/275 (16.4%) † | 0.01 6 vs. 12MON: 0.64 6 vs. 24MON: 0.02 12 vs. 24MON: <0.01 |
0.14 |
| De novo Dyspareunia | N/A | 7/123 (5.7%) | 6/122 (4.9%) | 11/112 (9.8%) | 0.41 | 0.63 |
| B5: Pain during intercourse | 48/182 (26.4%) | 21/194 (10.8%) † | 14/186 (7.5%) † | 24/165 (14.5%) † | 0.12 | 0.92 |
| C4: Fear of pain | 29/130 (22.3%) | 9/107 (8.4%) † | 8/109 (7.3%) † | 15/110 (13.6%) | 0.22 | 0.20 |
| Treatment for Dyspareunia‖ | N/A | 1/352 (0.3%) | 4/341 (1.2%) | 10/319 (3.1%) | N/A | 0.21 |
|
| ||||||
|
PISQ-12
| ||||||
| N | 183 | 161 | 150 | 124 | 0.02 | 0.65 |
| Mean ± SE | 30.7 ± 0.6 | 7.1 ± 0.7¶ | 7.2 ± 0.7¶ | 5.9 ± 0.7¶ | 6 vs. 12MON: 0.87 | |
| Minimum, Maximum | 6.5, 46.0 | −11.0, 25.5 | −10.0, 29.0 | −13.6, 25.0 | 6 vs. 24MON: 0.01 12 vs. 24MON: 0.01 |
|
|
| ||||||
|
Normalized Body Image
Score
| ||||||
| N | 354 | 340 | 325 | 300 | <0.01 | 0.54 |
| Mean ± SE | 25.3 ± 0.2 | −20.7 ± 1.7¶ | −20.8 ± 1.7¶ | −17.6 ± 1.7¶ | 6 vs. 12MON: 0.85 | |
| Minimum, Maximum | 0.0, 100 | −100, 66.7 | −100, 92.6 | −100, 96.3 | 6 vs. 24MON: <0.01 12 vs. 24MON: <0.01 |
|
ULS=uterosacral ligament suspension. SSLF=sacrospinous ligament fixation. MON=Month. PISQ-12=Pelvic Organ Prolapse-Urinary Incontinence Sexual Questionnaire. NA = Not Applicable. SE=Standard error.
P-values for Time and for ULS vs. SSLF are from longitudinal generalized linear mixed models of dichotomous outcomes (sexually active, not active or restricted, and dyspareunia) or longitudinal general linear mixed models of changes from baseline for continuous outcomes (PISQ-12 and normalized body image score).
Proportion of women with the outcome is significantly different from baseline at the p<0.01 level based on the longitudinal generalized linear mixed model.
Proportion of women with the outcome is significantly different from baseline at the p<0.05 level based on the longitudinal generalized linear mixed model.
Dyspareunia was defined by a response of either “Always” or “Usually” PISQ-12 question B5 “Do you feel pain during intercourse” (for women who were sexually active), or a response of either “Always” or “Usually” to PISQ-12 question C4 “Does fear of pain during sexual intercourse restrict your activity” (for women who were not sexually active), or any postoperative treatment for dyspareunia. De novo dyspareunia was assessed in the subset of women who were sexually active without pain at baseline.
Due to the cumulative nature of treatment for dyspareunia, testing for differences between surgical groups is only performed on treatment by 24 months and time is not included in the model.
Values at 6-month, 12-month, and 24-month time points are adjusted mean changes from baseline and standard errors from the longitudinal general linear mixed models. Mean changes from baseline are significantly different from zero at the p<0.01 level.
For all women undergoing vaginal prolapse repair, the adjusted mean baseline and change in scores of PFDI, PFIQ, and SF-36 along with statistical comparisons over time and between surgical groups are noted in Figure 1 and Table 1. At each post-operative time point, clinically and statistically significant improvements from baseline occurred in both subscales of the generic quality of life, and in all the condition-specific quality of life measures (PFDI and PFIQ subscales), and no clinically meaningful differences were noted between surgical groups. There was a clinically and statistically significant deterioration in urinary symptoms between 12 and 24 months, however scores were still substantially better than before surgery. There were no significant differences in UDI scores between uterosacral ligament suspension and sacrospinous ligament fixation. There was a statistically significant interaction for CRADI scores between the effects of time and surgery, indicating that changes over time postoperatively differed between the surgical groups. Specifically, there was a deterioration in bowel symptoms between 12 and 24 months in both surgical groups with the uterosacral ligament suspension difference just reaching clinical significance (minimum important difference of 11) and the sacrospinous ligament fixation difference exceeding it; however, there were no statistically significant differences in bowel symptom scores between the two surgical groups and significant improvement from baseline was maintained at each time point. There was a statistically significant deterioration in the POPDI postoperatively between 12 and 24 months; however, this change is likely not clinically important using 34.3 as an estimate of the minimum important difference.
Figure 1.
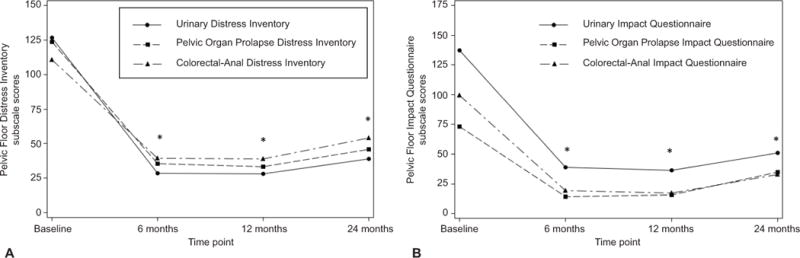
Changes in Pelvic Floor Distress Inventory (A) and Pelvic Floor Impact Questionnaire (B) subscale scores following prolapse surgery. *P<.01 from baseline.
Similarly, there were clinically and statistically significant improvements from baseline in PFIQ scores for all women after surgery. There were statistically significant interactions between surgical and behavioral therapy with pelvic muscle therapy groups for all PFIQ subscales. Among women in the usual care group, those assigned to uterosacral ligament suspension had a greater reduction from baseline in the urinary-specific UIQ scores at 6 and 12 months compared to those randomized to sacrospinous ligament fixation (Table 1 footnote). These differences between surgical groups were clinically significant based on a UIQ minimum important difference of 16; the difference at 24 months was clinically but not statistically significant (p=0.10). For the bowel-specific measure CRAIQ, differences between the surgical groups within both the behavioral therapy with pelvic muscle therapy and usual care groups were not significantly different at the p<0.05 level (p=0.07 and 0.06 respectively). There were no clinically significant differences (based on an estimated minimum important difference of 48.4) in prolapse-specific POPIQ scores between treatment groups or over time postoperatively (Table 1). Generic quality of life measures (SF-36 mental and physical component summary scales) improved clinically and statistically between baseline and 6 months, but did not significantly change after 6 months and showed no difference between surgical groups (Table 1).
Overall changes in sexual function, activity, and dyspareunia are reported in Table 2 along with comparisons over time and between surgical groups. Clinically and statistically significant improvement in PISQ-12 scores were seen after surgery in sexually active women, which exceed the minimum important difference of 6 points at 6 and 12 months and nearly reached minimum important difference at 24 months. There were no significant differences between surgical groups. Overall sexual activity rates remained between 50% and 60%, across all time points. By 24 months postoperatively, up to 27% of women who were not sexually active at baseline reported new onset of activity while 17% of women who had been sexually active at baseline reported discontinuation. There were no differences between surgical groups with respect to overall sexual activity or new onset activity. More women in the sacrospinous ligament fixation group stopped sexual activity by 12 months; however, by 24 months, discontinuation of activity was similar for both surgical groups.
Overall, dyspareunia was reported by 25% of all women at baseline; this decreased to 10% at 6 months postoperatively (p<0.01) with an increase between 6 and 24-months to 16% (p=0.02) (Table 2). Of those sexually active women without dyspareunia at baseline (n=134), 6%, 5% and 10% reported de novo dyspareunia at 6, 12, and 24 months, respectively. There were no significant differences in dyspareunia or de novo dyspareunia between surgical groups. Of all women (with and without baseline dyspareunia), a total of 67 reported or were treated for dyspareunia at one or more of the post-operative time points (Table 2). A total of 4 received treatment in the first 12 months and 10 had received treatment by 24 months. Among women without baseline dyspareunia, a total of 19 women reported de novo dyspareunia at one or more time points: 7 at 6 mo, 6 at 12 mo and 11 at 24 months. None of those with denovo dyspareunia were treated at 6 or 12 months; 3 received treatment by 24 months. Prior to surgery, sexual inactivity due to bulge was 34% and due to incontinence was 18%, with these rates decreasing overall to 21% and 12% respectively by 24 months after surgery. There were no differences in sexual inactivity due to incontinence or bulge between surgical groups.
Normalized mean body image scores at baseline and changes from baseline at 6, 12 and 24 months are noted in Table 2 and reflect a statistically (p<0.01) and clinically significant (based on an estimated minimum important difference of 13.0) improvement from baseline to 6 months with statistically but not clinically significant deterioration in body image between 12 and 24 months. There were no significant differences between surgical groups.
Discussion
In women with apical prolapse and stress urinary incontinence, generic and condition specific quality of life, sexual function, sexual activity, dyspareunia and body image all improved after native tissue vaginal prolapse surgery with midurethral sling with no clinically significant differences between the uterosacral ligament suspension and sacrospinous ligament fixation. Although slight deteriorations in condition-specific quality of life, sexual function and body image were observed post operatively over time, significant improvement over baseline persisted at 24 months. Overall, structural dyspareunia rates after native tissue vaginal repair improved from 25% to 16%, which included women with denovo dyspareunia by 24 months. Additionally, de novo dyspareunia was infrequently reported, rarely required intervention, and did not require surgery in any participant; and there was no difference between surgical groups. Any anatomic differences in the vaginal axis following sacrospinous ligament fixation do not appear to be associated with adverse sexual outcomes. Surgeons should feel comfortable offering either approach for correction of prolapse without fear of worsening quality of life or sexual function.
Our findings are consistent with a recent study comparing sacrospinous hysteropexy to hysterectomy with uterosacral vault suspension, which demonstrated no significant differences in overall sexual activity rates after native tissue repair and no difference between surgical approaches.12 In contrast to our findings, their study failed to demonstrate statistically or clinically significant improvement in sexual function or differences in median dyspareunia scores. Unfortunately, they did not report on overall rates of dyspareunia. Our reduction in rates of dyspareunia after native tissue repair suggests that some women may experience pre-operative dyspareunia that is prolapse-related. These findings warrant further study.
Individual decisions regarding sexual activity are complex and multi-factorial. The trend toward increasing rates of dyspareunia in our population over time may reflect age-related or menopausal changes and also deserves further exploration.24 Additionally, the effect of prolapse and urinary incontinence and body image on sexual activity adds a health dimension that is poorly understood and likely to be a multi-dimensional effect. The high proportion of previously inactive women who resumed sexual activity after surgery suggests that improvement in body image as well as the anatomic aspects of prolapse combined with improvements in bladder control is a benefit of native tissue prolapse repair with anti-incontinence surgery.
Much of the evidence that vaginal repair of POP improves quality of life includes studies with short follow up and small sample sizes.25 Similar to this study, several recent publications offer stronger evidence for improvement in quality of life: Larson et al reported high satisfaction rates eight years after sacrospinous ligament suspension, even in those women with pelvic floor defects at the hymenal ring after surgery.26 Ulrich and colleagues reported that improvements in vaginal and sexual function depended on the type of repair, with patients undergoing levator plication reporting less benefit compared to women undergoing posterior prolapse repairs without levator plication27. Our study adds a large sample of highly characterized patients reporting improvement using standardized validated questionnaires. Additional randomization to behavioral therapy with behavioral and physical therapy also did not seem to affect quality of life in our study, similar to other recent studies.28
This analysis benefits from the robust randomized trial design. The similarity of overall outcomes between these two native tissue vaginal repairs and lack of clinically relevant effects from peri-operative behavioral and pelvic muscle therapy allow us to consider this group of research participants as a larger cohort. Limitations of this study revolve around the patient population, which included only women undergoing transvaginal native tissue repair with concomitant midurethral sling. These eligibility criteria reduce our ability to comment on the effect of de novo stress urinary incontinence following sacrospinous ligament fixation or uterosacral ligament suspension on quality of life or sexual function. We also acknowledge that although an 82% follow up rate is high, we could be insufficiently powered to identify statistically significant differences in quality of life measures and dyspareunia rates between groups. However, we are reassured by the fact that we were unable to identify clinically meaningful differences between groups. Additionally, the sexual function questionnaire was only designed for use in sexually active women with pelvic organ prolapse and urinary incontinence.
Our findings suggest that native tissue vaginal prolapse surgery results in statistically and clinically significant improvements in generic and condition-specific quality of life, sexual function, and body image at 24 months; with low rates of de novo dyspareunia and no significant differences between uterosacral and sacrospinous suspensions. One in 10 women experience de novo dyspareunia but few require treatment.
Supplementary Material
Acknowledgments
Supported by grants U01 HD041249, U10 HD041250, U10 HD041261, U10 HD041267, U10 HD054136, U10 HD054214, U10 HD054215, U01 HD069031, and U10 HD054241 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the NIH Office of Research on Women’s Health.
Financial Disclosure: Emily S. Lukacz has received research grants support from Boston Scientific, Uroplasty, and Pfizer; she has been a consultant to AMS (Astora), Axonics, and Renew Medical; and she has received royalties from UpToDate. Holly E. Richter has received research grants from Pelvalon, has been a consultant for Pelvalon and Kimberly Clark, and she has received royalties from UpToDate. Linda Brubaker has received editor fees from UpToDate. Matthew D. Barber has received a research grant from the Foundation for Female Health Awareness.
Footnotes
The other authors did not report any potential conflicts of interest.
For a list of members of the Pelvic Floor Disorders Network who participated in this study, see Appendix 1 online at http://links.lww.com/xxx.
Presented at the 2015 annual International Urogynecologic Association (IUGA) meeting, Nice, France, June 10–13, 2015, and the 2015 annual American Urogynecologic Society (AUGS) meeting, Seattle, Washington, October 14–17, 2015.
Contributor Information
Emily S. Lukacz, Department of Reproductive Medicine, UC San Diego Health Systems, San Diego, CA.
Lauren Klein Warren, Social, Statistical & Environmental Sciences, RTI International, Research Triangle Park, NC.
Holly E. Richter, Department of Obstetrics and Gynecology, University of Alabama at Birmingham, Birmingham, AL.
Linda Brubaker, Department of Obstetrics and Gynecology and Urology, Loyola University Chicago Stritch School of Medicine, Chicago, IL.
Matthew D. Barber, Obstetrics, Gynecology and Women’s Health Institute, Cleveland Clinic, Cleveland, OH.
Peggy Norton, Department of Obstetrics and Gynecology, University of Utah Medical Center, Salt Lake City, UT.
Alison C. Weidner, Department of Obstetrics and Gynecology, Duke Medical Center, Durham, NC.
John N. Nguyen, Department of Obstetrics and Gynecology, Southern California Kaiser Permanente, Downey, CA.
Marie G. Gantz, Social, Statistical & Environmental Sciences, RTI International, Research Triangle Park, NC.
References
- 1.Lawrence JM, Lukacz ES, Nager CW, Hsu JW, Luber KM. Prevalence and co-occurrence of pelvic floor disorders in community-dwelling women. Obstet Gynecol. 2008;111(3):678–85. doi: 10.1097/AOG.0b013e3181660c1b. [DOI] [PubMed] [Google Scholar]
- 2.Nygaard I, Barber MD, Burgio KL, Kenton K, Meikle S, Schaffer J, et al. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008;300(11):1311–6. doi: 10.1001/jama.300.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Athanasiou S, Grigoriadis T, Chalabalaki A, Protopapas A, Antsaklis A. Pelvic organ prolapse contributes to sexual dysfunction: a cross-sectional study. Acta Obstet Gynecol Scand. 2012;91(6):704–9. doi: 10.1111/j.1600-0412.2012.01396.x. [DOI] [PubMed] [Google Scholar]
- 4.Lowenstein L, Gamble T, Sanses TV, van Raalte H, Carberry C, Jakus S, et al. Sexual function is related to body image perception in women with pelvic organ prolapse. J Sex Med. 2009;6(8):2286–91. doi: 10.1111/j.1743-6109.2009.01329.x. [DOI] [PubMed] [Google Scholar]
- 5.Wu JM, Matthews CA, Conover MM, Pate V, Jonsson Funk M. Lifetime risk of stress urinary incontinence or pelvic organ prolapse surgery. Obstet Gynecol. 2014;123(6):1201–6. doi: 10.1097/AOG.0000000000000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montoya TI, Grande KB, Rahn DD. Apical vaginal prolapse surgery: practice patterns and factors guiding route of repair. Female Pelvic Med Reconstr Surg. 2012;18(6):315–20. doi: 10.1097/SPV.0b013e3182713ccc. [DOI] [PubMed] [Google Scholar]
- 7.Kearney R, DeLancey JO. Selecting suspension points and excising the vagina during Michigan four-wall sacrospinous suspension. Obstet Gynecol. 2003;101(2):325–30. doi: 10.1016/s0029-7844(02)02464-x. [DOI] [PubMed] [Google Scholar]
- 8.Shull BL, Bachofen C, Coates KW, Kuehl TJ. A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse with uterosacral ligaments. Am J Obstet Gynecol. 2000;183(6):1365–73. doi: 10.1067/mob.2000.110910. discussion 73–4. [DOI] [PubMed] [Google Scholar]
- 9.Silva WA, Pauls RN, Segal JL, Rooney CM, Kleeman SD, Karram MM. Uterosacral ligament vault suspension: five-year outcomes. Obstet Gynecol. 2006;108(2):255–63. doi: 10.1097/01.AOG.0000224610.83158.23. [DOI] [PubMed] [Google Scholar]
- 10.Glavind K, Larsen T, Lindquist AS. Sexual function in women before and after surgery for pelvic organ prolapse. Acta Obstet Gynecol Scand. 2015;94(1):80–5. doi: 10.1111/aogs.12524. [DOI] [PubMed] [Google Scholar]
- 11.Pauls RN. Impact of gynecological surgery on female sexual function. Int J Impot Res. 2010;22(2):105–14. doi: 10.1038/ijir.2009.63. [DOI] [PubMed] [Google Scholar]
- 12.Detollenaere RJ, Kreuwel IA, Dijkstra JR, Kluivers KB, van Eijndhoven HW. The Impact of Sacrospinous Hysteropexy and Vaginal Hysterectomy With Suspension of the Uterosacral Ligaments on Sexual Function in Women With Uterine Prolapse: A Secondary Analysis of a Randomized Comparative Study. J Sex Med. 2016 doi: 10.1016/j.jsxm.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Barber MD, Brubaker L, Burgio KL, Richter HE, Nygaard I, Weidner AC, et al. Comparison of 2 transvaginal surgical approaches and perioperative behavioral therapy for apical vaginal prolapse: the OPTIMAL randomized trial. JAMA: the journal of the American Medical Association. 2014;311(10):1023–34. doi: 10.1001/jama.2014.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barber MD, Brubaker L, Menefee S, Norton P, Borello-France D, Varner E, et al. Operations and pelvic muscle training in the management of apical support loss (OPTIMAL) trial: design and methods. Contemporary clinical trials. 2009;30(2):178–89. doi: 10.1016/j.cct.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ware JE., Jr SF-36 health survey update. Spine. 2000;25(24):3130–9. doi: 10.1097/00007632-200012150-00008. [DOI] [PubMed] [Google Scholar]
- 16.Barber MD, Kuchibhatla MN, Pieper CF, Bump RC. Psychometric evaluation of 2 comprehensive condition-specific quality of life instruments for women with pelvic floor disorders. Am J Obstet Gynecol. 2001;185(6):1388–95. doi: 10.1067/mob.2001.118659. [DOI] [PubMed] [Google Scholar]
- 17.Jelovsek JE, Barber MD. Women seeking treatment for advanced pelvic organ prolapse have decreased body image and quality of life. Am J Obstet Gynecol. 2006;194(5):1455–61. doi: 10.1016/j.ajog.2006.01.060. [DOI] [PubMed] [Google Scholar]
- 18.Hopwood P, Fletcher I, Lee A, Al Ghazal S. A body image scale for use with cancer patients. European journal of cancer. 2001;37(2):189–97. doi: 10.1016/s0959-8049(00)00353-1. [DOI] [PubMed] [Google Scholar]
- 19.Barber MD, Spino C, Janz NK, Brubaker L, Nygaard I, Nager CW, et al. The minimum important differences for the urinary scales of the Pelvic Floor Distress Inventory and Pelvic Floor Impact Questionnaire. Am J Obstet Gynecol. 2009;200(5):580 e1–7. doi: 10.1016/j.ajog.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ware JM. User’s Manual for the SF-36v2 Health Survey. QualityMetric Inc.; 2007. Determining Important Differences in Scores; pp. 125–33. [Google Scholar]
- 21.Jelovsek JE, Chen Z, Markland AD, Brubaker L, Dyer KY, Meikle S, et al. Minimum important differences for scales assessing symptom severity and quality of life in patients with fecal incontinence. Female Pelvic Med Reconstr Surg. 2014;20(6):342–8. doi: 10.1097/SPV.0000000000000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mamik MM, Rogers RG, Qualls CR, Morrow JD. The minimum important difference for the Pelvic Organ Prolapse-Urinary Incontinence Sexual Function Questionnaire. Int Urogynecol J. 2014;25(10):1321–6. doi: 10.1007/s00192-014-2342-9. [DOI] [PubMed] [Google Scholar]
- 23.Sloan JA, Cella D, Hays RD. Clinical significance of patient-reported questionnaire data: another step toward consensus. J Clin Epidemiol. 2005;58(12):1217–9. doi: 10.1016/j.jclinepi.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 24.Dennerstein L, Dudley E, Burger H. Are changes in sexual functioning during midlife due to aging or menopause? Fertil Steril. 2001;76(3):456–60. doi: 10.1016/s0015-0282(01)01978-1. [DOI] [PubMed] [Google Scholar]
- 25.Maher C, Feiner B, Baessler K, Schmid C. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2013;4 doi: 10.1002/14651858.CD004014.pub5. [DOI] [PubMed] [Google Scholar]
- 26.Larson KA, Smith T, Berger MB, Abernethy M, Mead S, Fenner DE, et al. Long-term patient satisfaction with michigan four-wall sacrospinous ligament suspension for prolapse. Obstet Gynecol. 2013;122(5):967–75. doi: 10.1097/AOG.0b013e3182a7f0d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ulrich D, Dwyer P, Rosamilia A, Lim Y, Lee J. The effect of vaginal pelvic organ prolapse surgery on sexual function. Neurourol Urodyn. 2015;34(4):316–21. doi: 10.1002/nau.22569. [DOI] [PubMed] [Google Scholar]
- 28.Pauls RN, Crisp CC, Novicki K, Fellner AN, Kleeman SD. Impact of physical therapy on quality of life and function after vaginal reconstructive surgery. Female Pelvic Med Reconstr Surg. 2013;19(5):271–7. doi: 10.1097/SPV.0b013e31829c64ea. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


