Abstract
Orexin-1 receptors (Ox1Rs) have been implicated in the motivation for drugs of abuse. Here, we utilized a within-session behavioral-economic threshold procedure to screen for individual differences in economic demand for the ultra-short acting opioid remifentanil and to test whether antagonism of Ox1Rs reduces remifentanil demand. The behavioral-economic procedure revealed robust individual differences in free consumption of remifentanil (Q0 parameter; hedonic set point). Rats with low baseline Q0 (low takers) displayed high demand elasticity (α parameter; reduced responding as drug price increased indicating low motivation for drug), whereas subjects with a higher Q0 (high takers) exhibit low demand elasticity (low α) by continuing to self-administer remifentanil despite increased cost (reflecting higher motivation for drug). In a punished responding paradigm utilizing footshock, subjects that were classified as high takers at baseline withstood twice as much shock as low takers to continue self-administering remifentanil. Interestingly, Ox1R antagonism with SB-334867 reduced Q0 and increased α in low takers but not in high takers. Similarly, the Ox1R antagonist attenuated cue-, but not drug-, induced reinstatement of remifentanil seeking in low takers, but had no significant effect on reinstatement of drug seeking in high takers. Together, these data reveal a novel role of orexins in demand for remifentanil: Ox1Rs modulate demand in low takers but not in individuals that exhibit addictive-like behaviors (high takers). Finally, the behavioral assays in this study can serve as a novel laboratory model for studying individual differences in opioid use disorders.
Keywords: addiction, behavioral economics, drug abuse, orexin, remifentanil, self-administration
Introduction
Abuse of prescription opioids as well as heroin has drastically risen (Compton & Volkow, 2006). A plethora of drug abuse research has focused on psychostimulants; however, a growing number of studies demonstrate that opioid abuse differs from psychostimulant abuse in a number of important ways (Badiani et al., 2011). Indeed, the behavioral profile of opioid self-administration as well as the biological mechanisms of action of opioids are different from those of psychostimulants (Ettenberg & Geist, 1993; Caprioli et al., 2008; Caprioli et al., 2009; Celentano et al., 2009).
Orexins, also known as hypocretins, are neuropeptides that regulate sleep and arousal (Siegel, 2004; Ohno & Sakurai, 2008) as well as modulate motivation for drugs of abuse (Aston-Jones et al., 2010; España, 2012; Mahler et al., 2014a; Baimel et al., 2015). For example, signaling at orexin-1 receptors (Ox1Rs) facilitates high effort responding for cocaine (such as on progressive-ratio or economic demand schedules of reinforcement) but not low effort cocaine seeking (such as fixed-ratio 1 schedules (FR1) of reinforcement) (Borgland et al., 2009; España et al., 2010; Bentzley & Aston-Jones, 2015). In contrast, blockade of Ox1Rs reduces responding for heroin under FR1 and progressive-ratio schedules of reinforcement (Smith & Aston-Jones, 2012).
Behavioral-economic demand curve (BE) methods for quantifying motivation for drugs of abuse are of growing popularity, because they capture individual differences in motivation for drugs, facilitate comparisons across different drug classes, and provide a number of unique parameters of motivation. In BE procedures consumption is graphed as a function of price, generating demand curves, and an exponential demand equation (Hursh & Silberberg, 2008) can then be applied to these curves to generate parameters such as Q0 and α. Q0 is a theoretical measure of how much drug one would consume when drug is completely free (free consumption, also a measure of hedonic set point) and is quantified by where the demand curve intercepts the ordinate (Bentzley et al., 2013; Bentzley et al., 2014). The slope of the demand curve, α, captures how consumption changes in relation to price independently of Q0 (Hursh & Silberberg, 2008; Bentzley et al., 2013; Bentzley et al., 2014). Within-session BE threshold procedures are particularly useful because they generate a demand curve for each individual session and allow for within-session manipulations of drug demand (España et al., 2010; Oleson et al., 2011; Bentzley et al., 2013; Bentzley et al., 2014). Utilizing these procedures, signaling at OX1Rs has been shown to modulate economic demand for cocaine. Peripheral as well as intra-ventral tegmental area (VTA) administration of an Ox1R antagonist has been shown to reduce demand for the psychostimulant cocaine (España et al., 2010; Bentzley & Aston-Jones, 2015). However, the role of Ox1Rs in mediating economic demand for opioids remains unexplored.
In the present study, we tested the hypothesis that Ox1Rs modulate economic demand for opioids using a within-session BE threshold-demand curve procedure. Ox1Rs have previously been shown to be important in simple fixed-ratio and progressive-ratio responding for heroin (Smith & Aston-Jones, 2012); however, the long duration of action and sedative properties of heroin reduces the number of trials subjects can complete in a session, making it sub-optimal for testing multiple drug doses within one session. A short-acting drug is critical for testing multiple doses within a session, as occurs in the within-session BE threshold procedure. Therefore, we utilized the potent, ultra-short-acting μ-opioid receptor agonist remifentanil as a reinforcer. Remifentanil has a much shorter duration of action than traditional opioids (Glass et al., 1999), making it ideally suited for the within-session BE tests. Indeed, the elimination half-life of remifentanil from the rat's blood is ~0.3 min and from the nucleus accumbens (NAc) ~10 min (Crespo et al., 2005). Remifentanil can be as reinforcing as cocaine (Wade-Galuska et al., 2007; Freeman & Woolverton, 2011; Koffarnus et al., 2012) and has the potential to be abused (Levine & Bryson, 2010). Laboratory rats will readily self-administer remifentanil, and on a progressive-ratio test rats achieve similar breakpoints for remifentanil as for heroin (Panlilio & Schindler, 2000), demonstrating that remifentanil is a highly reinforcing opioid. Additionally, during morphine withdrawal monkeys and rats will self-administer significantly more remifentanil than subjects not experiencing opioid withdrawal (Cooper et al., 2008; Wade-Galuska et al., 2011).
Using the within-session BE task, we discovered individual differences in the behavioral profiles of drug demand as well as previously unknown relationships between free consumption (Q0) and demand elasticity (α) for remifentanil. Individual differences in demand measured in our BE task predict how much punishment rats will later endure to take drug. Additionally, using a within-subjects design, we reveal surprising individual differences in the effectiveness of Ox1Rs to modulate opioid free consumption and demand elasticity. Finally, individual differences are also found in the role of Ox1Rs in mediating cue-induced reinstatement of drug seeking, a model of drug relapse.
Materials and Methods
Subjects
Twenty-four male Sprague Dawley rats (250-275g upon arrival) obtained from Charles River Laboratories (Raleigh, NC) were used as subjects in this study. Rats were single-housed and maintained on a reverse light/dark cycle (lights off at 6:00; on at 18:00), with all behavioral testing occurring during the dark cycle. Rat chow and water were available ad libitum in the home cages. All experiments were approved by the Medical University of South Carolina Institutional Animal Care and Use Committee and conducted in accordance with their guidelines.
Surgery
Animals were anesthetized with an intraperitoneal (i.p.) injection of ketamine (56.5 mg/kg) and xylazine (11.3 mg/kg), and the non-opioid analgesic carprofen (5 mg/kg) was administered subcutaneously. Rats were implanted with chronic indwelling jugular vein catheters as previously described (Smith et al., 2009). Beginning 3 days after surgery, catheters were flushed daily with 0.1 mL cefazolin (100mg/mL) and 0.1 mL heparin. Subjects were given a minimum of 7 days to recover from surgery before behavioral training began.
Drugs
Remifentanil (Ultiva; obtained from the Medical University of South Carolina hospital pharmacy) was dissolved in 0.9% sterile saline for self-administration. SB-334867 is a highly selective antagonist for the Ox1R, compared to, e.g., the Ox2R. SB-334867 (generously donated by the National Institute of Drug Abuse) was suspended in 10% 2-hydroxypropyl-b-cyclodextrin in sterile water and 2% dimethyl sulfoxide (Sigma-Aldrich, St. Louis, MO); this vehicle solution was also used for control injections. SB-334867 and vehicle were injected i.p. at a volume of 4.0 mL/kg.
Self-Administration Training
All self-administration experiments were conducted in operant chambers obtained from MED Associates (St. Albans, VT). Each operant chamber was equipped with two levers, a cue light above each lever, a tone generator, a house light, an infusion pump, and a floor grid capable of delivering electrical shocks.
A timeline of experimental training and tests is found in Supplemental Figure 1. After at least 7 days for recovery from surgery, subjects were trained to self-administer remifentanil intravenously on an FR1 schedule of reinforcement. At the beginning of each FR1 session, two levers extended into the chamber. A response on the active lever resulted in a 1 μg infusion of remifentanil delivered over 4 sec. This dose of remifentanil was chosen because it corresponds to ~3.2 μg/kg (based on the average weights of subjects when the experiment began), which is a dose that is readily self-administered by rats (Panlilio & Schindler, 2000; Panlilio et al., 2003) and facilitates Pavlovian conditioned approach (Yager et al., 2015). Each infusion was accompanied by the illumination of the cue light above the active lever and an auditory tone; cues were presented for the duration of each infusion. After each infusion a 20-sec time out was imposed whereby no infusions or cues could be earned. Throughout the session responses on the inactive lever resulted in no infusions or cue presentations. Each session ended after 2 hrs or when 80 infusions were earned, whichever first occurred. Subjects were trained on the FR1 procedure for a minimum of 6 sessions.
After stable responding was observed (defined as < 20% variation in number of infusions earned for at least 3 consecutive sessions), the effects of an Ox1R antagonist (SB-334867; SB) on FR1 self-administration of remifentanil were tested. On test days, 30 mg/kg SB or vehicle was injected i.p. 30 min prior the beginning of an FR1 session (n=16). A within-subjects design was used whereby each rat received both SB and vehicle; order was counter-balanced across subjects.
Behavioral Economics Threshold Procedure
Economic demand training for remifentanil was conducted consistently with previous cocaine BE studies to facilitate comparisons between the two drugs (Bentzley et al., 2013; Bentzley et al., 2014; Bentzley & Aston-Jones, 2015). To advance from FR1 training to the BE threshold procedure, subjects were required to earn a minimum of 25 infusions within 2 hrs for at least 3 consecutive sessions. As previously described (Oleson et al., 2011; Bentzley et al., 2013), the BE procedure varies the cost of drug by changing the length of infusion while maintaining an FR1 schedule of reinforcement. The dose of remifentanil resulting from a response on the active lever decreased every 10 min; therefore, each 110-min session tested 11 doses of remifentanil (2, 1, 0.6, 0.3, 0.2, 0.1 0.06, 0.03, 0.02, 0.01, 0.006 μg/infusion). Similarly to FR1 training, each infusion resulting from a response on the active lever was accompanied by presentation of a light-tone compound cue; responses on the inactive lever had no consequence. However, unlike the FR1 training procedure, there were no limits on how much drug subjects could take in a session nor any time out periods in which drug was unavailable (except additional presses during drug infusions did not result in additional drug); eliminating these restrictions on drug taking allowed for individual differences in drug taking patterns to be more readily observed. Subjects were trained daily on the BE procedure until stable responding, defined as ≤ 20% variation in α was observed for at least 3 consecutive days.
To test the effects of Ox1R signaling on economic demand for remifentanil, subjects received 0 (vehicle), 10, or 30 mg/kg SB 30 min prior to a BE session. Each subject received each dose, and dose order was counter-balanced across subjects. The day following a test session, subjects resumed training on the BE procedure for a minimum of 3 days and until behavior re-stabilized before undergoing the next test. On average, subjects underwent one test per week.
Intermittent behavioral economics procedure
To control for the potential confounds of drug satiation and session length in the BE procedure, a subset of subjects (n = 6) experienced an intermittent version of the task whereby the following doses of remifentanil were presented in ascending or descending order (within subjects procedure) across 10-minute intervals on different days: 2, 0.6, 0.2, 0.06, 0.02 μg/infusion. Additionally, a 20-min time out was imposed between each 10-min drug dose interval. Similarly to the threshold BE procedure, subjects were trained on the intermittent procedure until behavior stabilized, defined as ≤ 20% variation in α for at least 3 consecutive days. The order of training with ascending or descending remifentanil doses was counterbalanced across subjects. Two economic parameters, the slope of the demand curve (α) and free consumption (Q0), were compared when doses of remifentanil were presented in ascending versus descending order.
Using the intermittent threshold procedure, if the normally observed decreases in drug consumption as cost increased were merely an effect of satiation or exhaustion from lever pressing, different α and Q0 values would be expected when remifentanil doses were experienced in ascending versus descending order. However, if the economic parameters obtained from the BE procedures are valid measures of motivation for drug under varying costs, then similar values would be expected to be obtained regardless of the order in which drug prices were presented.
Punished responding
To examine motivation to continue taking drug despite adverse consequences, subjects (n = 16) were tested on a punished responding procedure as previously described (Bentzley et al., 2014). In contrast to the threshold BE procedure in which drug dose was varied, the infusion dose remained constant throughout the session (1 μg remifentanil/infusion), and cost was assessed by increasing intensities of footshock accompanying infusions, in 5-min intervals throughout the session. As in the BE procedure, drug was continually available with no time out periods.
Subjects first completed 2 shock-free sessions (55 min each) during which they could self-administer remifentanil (1 μg/infusion) without concomitant footshock. This established baseline levels of drug intake shock-free. After a stable baseline was observed, the shock test occurred the following day. During the first 5 min of the shock test, no shocks were given; after this period, a 0.5 sec shock accompanied each infusion. The intensity of shock increased every 5 min [0.10, 0.13, 0.16, 0.20, 0.25, 0.32, 0.40, 0.50, 0.63, 0.79 milliamps (mA)]. All shocks were contingent upon pressing the active lever to receive an infusion of remifentanil.
Extinction and Reinstatement Tests
After self-administration and BE tests were completed, subjects underwent extinction training. During each 2-hr extinction session, remifentanil and drug-associated cues (cue light and tone) were unavailable; lever presses on either lever had no consequence. Consistent with our previous studies using cocaine as the reinforcer (Smith et al., 2009; Smith & Aston-Jones, 2012; Bentzley et al., 2014; Mahler et al., 2014b), subjects underwent extinction training until they had ≤ 25 presses on the previously active lever for at least 2 consecutive sessions and a total of at least 7 extinction sessions.
To test the role of Ox1R signaling on cue-induced and remifentanil-induced reinstatement of drug seeking, subjects received 30 mg/kg SB or a control infusion of vehicle 30 min before each reinstatement test. A within-subjects design was used whereby rats received up to 4 reinstatement test sessions (vehicle or 30 mg/kg SB for separate cue- and drug-induced reinstatement tests); drug dose and type of reinstatement test were counter-balanced across subjects. On cue-induced reinstatement tests, presses on the active lever resulted in presentation of the drug-associated cues (tone and cue light); however, no remifentanil was delivered. On drug-induced reinstatement tests, rats received one intravenous priming infusion of remifentanil (2 μg delivered over 8 sec). Immediately after the infusion, levers became available but responses on the levers neither yielded any further infusions nor presentation of the cues. Although previous studies of cocaine- or heroin-induced reinstatement delivered the drugs i.p., remifentanil is not suitable for this route of administration due to its short half-life (Crespo et al., 2005). Therefore, the remifentanil prime was delivered intravenously – the same route of administration utilized throughout the study.
Locomotor Testing
To measure possible general locomotor effects of SB, 22 subjects were screened in VersaMax (Columbus, OH) open field chambers on a day after completion of the aforementioned behavioral experiments. Subjects were given one session to habituate to the chambers. In two subsequent sessions, rats were pretreated with 30 mg/kg SB or vehicle 30 min prior to locomotor testing. A within-subjects design was utilized, and drug order was counterbalanced across rats. Locomotion was monitored for 2 hrs, the same length of time as the self-administration experiments. Horizontal activity as well as the total number of beam breaks was used to quantify locomotor activity.
Data Analysis
Economic demand curves were generated for each threshold BE session as previously described (Bentzley et al., 2013). An exponential demand equation (Hursh & Silberberg, 2008) was applied to these curves to generate Q0 and α parameters. The slope of the demand curve, α, is the measure of demand elasticity capturing how consumption changes in relation to price. Larger α values indicates greater demand elasticity and are characterized by a greater reduction in responding as drug price increases. Smaller α values indicate less demand elasticity and are symptomatic of continued responding for drug despite increases in the costs to obtain drug. Q0, the theoretical measure of free consumption (amount consumed at zero price), is the point at which the demand curve intercepted the ordinate. The curve fitting was performed similarly to our previous cocaine BE experiments (Bentzley et al., 2013; Bentzley et al., 2014; Bentzley & Aston-Jones, 2015), whereby all data points up until two bins past the point at which maximal responding was observed (Omax) were included in the generation of demand curves. Although our previous cocaine studies excluded the first data bin because rats self-administering cocaine “load up” to achieve their preferred brain levels of cocaine and then maintain these levels (Bentzley et al., 2013), excluding the first bin was unnecessary here because subjects were not observed to “load up” on remifentanil. This is consistent with previous studies (Panlilio et al., 2003; Crespo et al., 2005).
To facilitate examination of individual differences, rats were categorized as low or high takers based upon a median split of average Q0 values across training. Specifically, average Q0 values were calculated across the 3 stable baseline periods prior to the SB tests in the BE procedure.
Parametric statistical analyses were performed in GraphPad Prism 5 (La Jolla, CA) and IBM SPSS Statistics Version 22 (Armonk, NY). Planned a priori contrasts were used to analyze the effects of SB on economic demand. Acquisition of self-administration, individual differences in economic demand parameters, and the effects of SB on FR1 responding and reinstatement were analyzed using ANOVAs and t-tests. Corrections (Bonferroni or Dunnett's) were applied to post-hoc tests to reduce the risk of type 1 errors. Two-way ANOVAs were used to examine the effects of increasing shock intensity on drug taking in low and high takers. Linear regression was used to correlate individual Q0 and α values, establish the relationship between Q0 and α on total charge self-administered in the punished responding test, and to test whether the order in which SB doses were administered affected the BE results.
Results
Antagonism of Ox1Rs has minimal effects on FR1 responding for remifentanil
Rats quickly acquired self-administration, with the majority of rats earning the maximum 80 infusions per session within a few sessions (Fig. 1A). Additionally, rats performed significantly more lever presses on the active lever versus the inactive lever (main effect, F(1,138) = 283.69, p < 0.0001) beginning on the second session (Bonferroni post hoc test, p < 0.05) and throughout the remainder of the training sessions (p < 0.001).
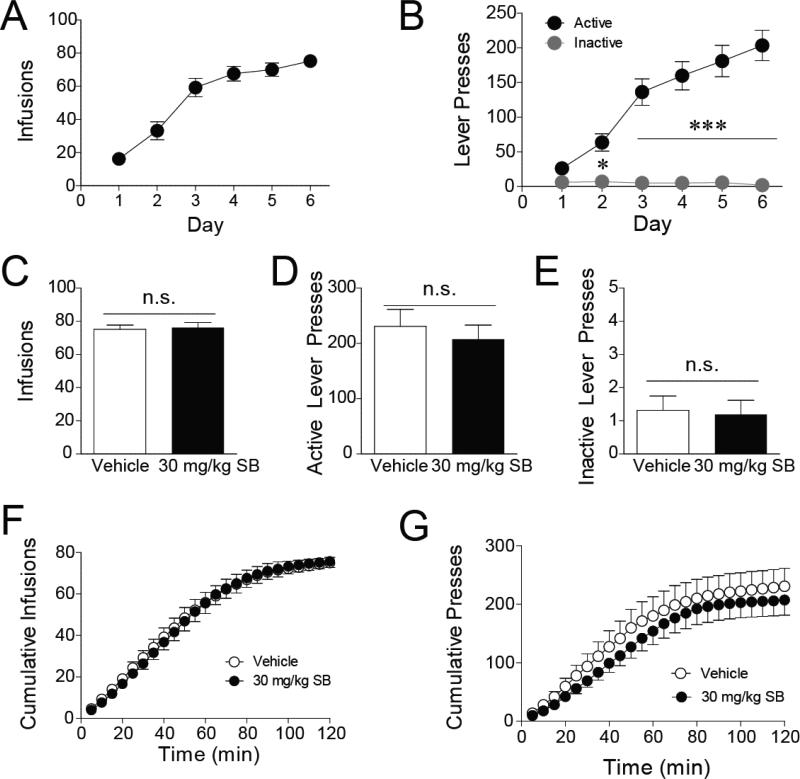
Figure 1. Blockade of Ox1Rs does not significantly alter FR1 responding for remifentanil.
A) Acquisition of remifentanil self-administration (n = 24). B) Rats quickly learned to discriminate between the lever that delivers drug (the active lever) and the inactive lever. C-G) After stabilizing on the FR1 self-administration procedure, subjects were pretreated with 30 mg/kg of the OxR1 antagonist SB-334867 or vehicle prior to an FR1 session (n = 16, within-subjects design). SB did not affect the number of infusions earned (C) or the number of active (D) or inactive (E) lever presses. Time course analyses revealed no effect of SB on the cumulative number of infusions throughout the session (F); however, there was a modest effect of SB on cumulative active lever presses (G). n.s. = not statistically different; *p < 0.05; p < 0.001.
To test the effect of Ox1R antagonism on self-administration of remifentanil, 30 mg/kg of SB or vehicle was administered on sessions after FR1 behavior had stabilized. Blockade of Ox1Rs did not significantly change the total number of infusions earned (Fig. 1C; t(15) = 0.531, p = 0.603) or the total number of active (Fig. 1D; t(15) = 1.304, p = 0.212) or inactive (Fig. 1E; t(15) = 0.229, p = 0.822) lever presses. Examination of the time course of infusions revealed no effect of SB on cumulative infusions throughout the session (Fig. 1F; F(1, 360) = 1.751, p = 0.187). In contrast, there was a modest but statistically significant main effect of SB on cumulative active lever presses (Fig. 1G; F(1, 360) = 34.74, p < 0.001); however, none of the specific time bins reached statistical significance in the post-hoc analyses with Bonferroni corrections.
Individual differences in demand for remifentanil
After rats acquired remifentanil self-administration, they were trained on a BE threshold procedure during which demand for drug under varying costs was evaluated in a within-session design. The exponential demand equation was fit to each subject's data for each daily session to generate demand curves and to calculate α and Q0 (example demand curve presented in Fig. 2A). Animals were trained daily on this task until behavior stabilized (as described in Methods).
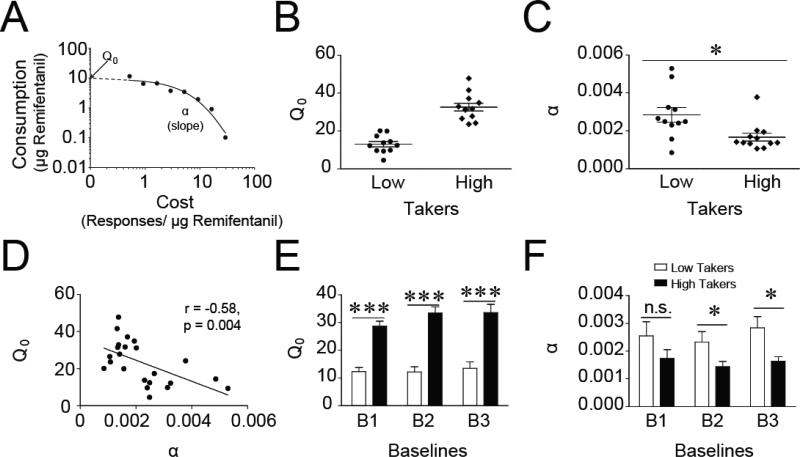
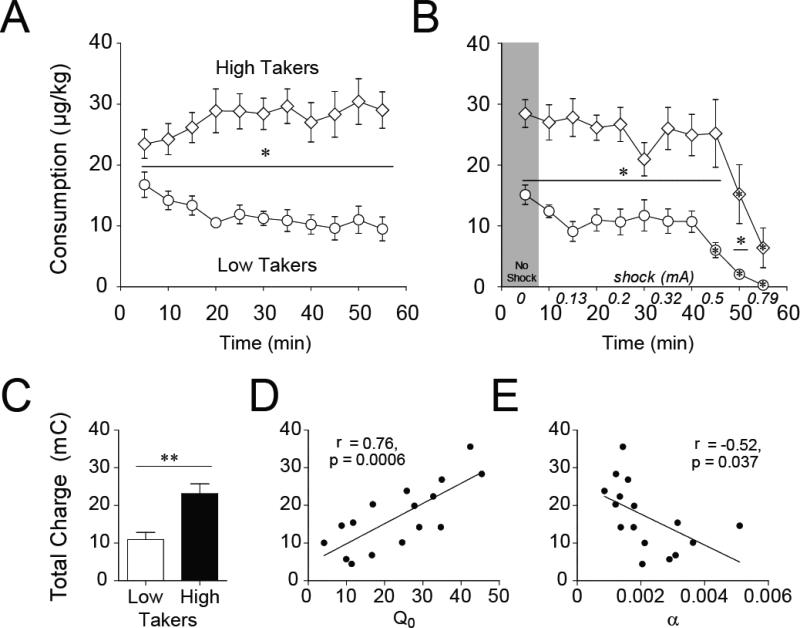
Figure 2. Individual differences in motivation for remifentanil during a within-session behavioral-economic procedure.
A) A representative example of a demand curve for remifentanil of a single animal during a single session. B) To examine individual differences in demand for remifentanil, rats were divided into low (n = 12) and high (n = 12) takers based on Q0. C) High takers exhibited more inelastic responding than low takers as price increased. D) Preferred consumption of remifentanil at null cost (Q0) and demand elasticity (α) correlated with each other with high takers displaying enhanced motivation for remifentanil. E) Individual differences in Q0 were evident consistently throughout training based upon the average stable baseline sessions prior to each SB test. F) Individual differences in α between low and high takers emerged over BE training. *p < 0.05; ***p < 0.001; n.s. = not statistically different.
Interestingly, large individual differences were observed in the amount of drug subjects would consume at null cost, i.e. Q0. As described in the methods, subjects were divided into two groups for analysis based upon their average Q0 values. For simplicity, rats with a low Q0 value were classified as “low takers” and those with a higher Q0 were termed “high takers” (Fig. 2B; t(21) = 7.676, p < 0.001). High takers had significantly lower α values (lower demand elasticity) compared to low takers (Fig. 2C), indicating that high takers exhibited more persistent drug taking as the cost for remifentanil increased (i.e., higher motivation for the drug) than low takers. Consistent with this, there was a significant correlation between Q0 and α (Fig. 2D; r = −0.576, p = 0.004), such that high takers displayed significantly higher motivation for remifentanil than low takers. Together, these data demonstrate that individuals who exhibit high free consumption of remifentanil (high Q0) also exhibit higher motivation for drug (more inelastic behavior, lower α) as the price increases.
To test whether these individual differences emerged over extended training, time course analyses were conducted examining average Q0 and α values from the beginning, middle, and end of BE training, defined as the stable averages prior to each of the three SB/vehicle test sessions. Although high takers had significantly greater Q0 values than low takers during all three time points (Fig.2E; main effect of group, F(1,19) = 249.931, p < 0.001; post hoc tests with Bonferroni corrections, p < 0.001 at every time point), high takers had statistically lower α values (i.e. less elasticity; higher motivation) than low takers during the second and third, but not the first, time points (Fig.2F; main effect of group, F(1,19) = 5.215, p = 0.034; post hoc tests with Bonferroni corrections: time 1, p = 0.169; time 2, p = 0.038; time 3, p = 0.006). These effects occurred independently of whether rats received SB on the first, second or third test. Therefore, the observed individual differences in set point (Q0) are consistent throughout BE training; however, the differences in demand elasticity emerged over the weeks of drug experience.
Acquisition of remifentanil FR1 self-administration (Fig. 1A-B) was reanalyzed based upon whether subjects were subsequently classified as low takers or high takers in the behavioral economic threshold procedure. Overall, high takers more quickly acquired remifentanil self-administration (Supplemental Fig. 1A; main effect of takers, F(1, 21) = 7.319, p = 0.013). Posthoc tests with Bonferroni correction revealed that the differences occurred in sessions 3-5 (p < 0.05); the majority of both low and high takers earned the maximum number of infusion by session 6. Additionally, high takers pressed the active lever significantly more than low takers (Supplemental Fig. 1B; main effect of takers, F(1, 21) = 7.107, p = 0.015; ). High and low takers similarly had low levels of responding on the inactive lever (Supplemental Fig. 1C; main effect of takers, F(1, 21) = 0.356, p = 0.557).
Intermittent BE procedure validates measures of economic demand
To ensure that decreased consumption as cost increased was not confounded by session length or satiation, a subset of the same rats completed an intermittent version of the threshold procedure (Bentzley et al., 2014), whereby the dose per infusion was delivered in ascending or descending order during separate sessions (within subjects), as described in the Methods. Additionally, a 20-min time out was imposed between each dose.
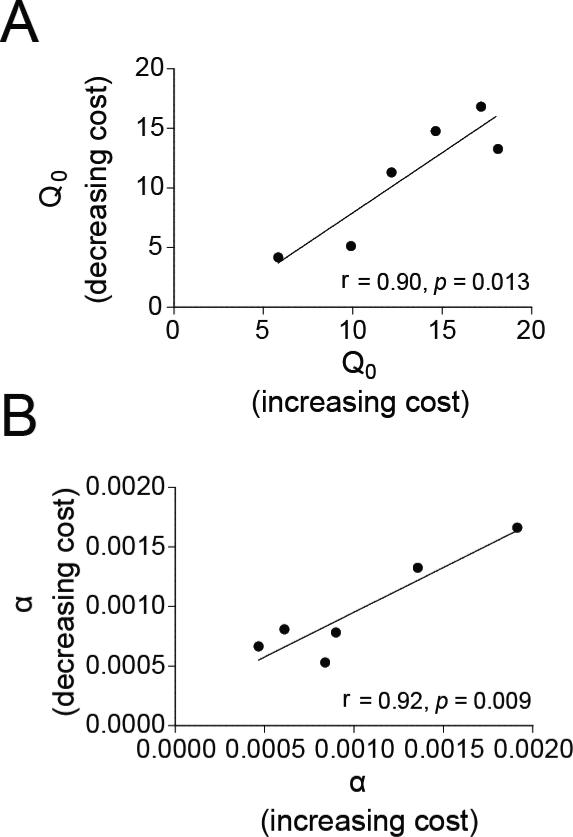
Individual rats’ Q0 values were highly correlated between the ascending and descending versions of the intermittent paradigm (Fig. 3A; r = 0.904, p = 0.013). The slopes of individuals’ demand curves were also correlated for the two orders in which prices were tested (Fig. 3B; r = 0.920, p = 0.009). This experiment demonstrates that the economic parameters (Q0 and α) reflect motivation for remifentanil independent of time in the session or satiation.
Figure 3. Remifentanil demand is consistent regardless of the order of price, demonstrating that time or satiation do not confound the results.
A-B) Economic demand for remifentanil when it is free (A; Q0) and demand elasticity (B; α) were consistent regardless of whether remifentanil prices were presented in ascending or descending order in the intermittent version of the BE task (n = 6).
Blockade of Ox1Rs reduces economic demand for remifentanil in low takers but not in high takers
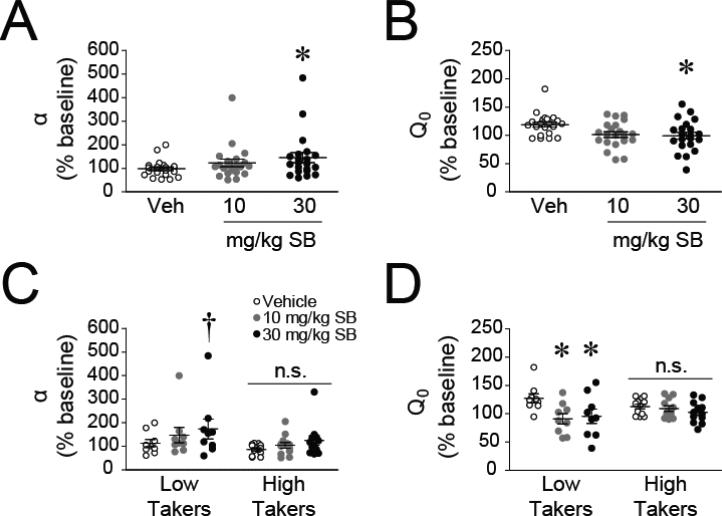
To test the hypothesis that signaling at Ox1Rs modulates opioid motivation, rats were treated with the Ox1R antagonist SB or vehicle prior to BE sessions. As predicted, blockade of Ox1Rs dose-dependently increased demand elasticity (decreased motivation for remifentanil; Fig. 4A; 30 mg/kg SB vs vehicle, F(1,19) = 6.295, p = 0.021; 10 mg/kg SB vs vehicle, F(1,19) = 2.240, p = 0.151). Additionally, both doses of SB reduced remifentanil free consumption (Q0) compared to vehicle (Fig. 4B; 10 mg/kg SB, F(1,19) = 8.226, p = 0.010; 30 mg/kg SB, F(1,19) = 9.763, p = 0.006). The order in which subjects received each dose of SB did not account for the SB-induced reduction in free consumption (β = −0.081, t = −0.665, p = 0.509) or increase in demand elasticity (β = −0.080, t = −0.662, p = 0.510).
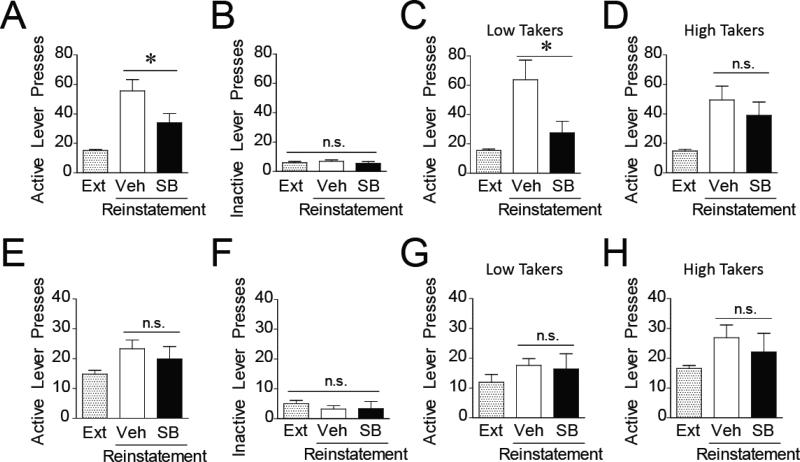
Figure 4. Individual differences in the effectiveness of Ox1R antagonism on reducing free consumption and demand for remifentanil.
A-B) SB overall increases demand elasticity (α; A; n = 21) and reduces consumption at null cost (Q0; B). C-D) SB modulated α (C) and Q0 (D) in low takers (n = 9) but was largely ineffective in high takers (n = 12). *p < 0.05 compared to vehicle; †p = 0.056 compared to vehicle; n.s. = not statistically different.
Previous work revealed greater effects of SB in rats with a high demand for cocaine (Bentzley & Aston-Jones, 2015); therefore, individual differences in the effectiveness of SB at altering α and Q0 were examined. Surprisingly, in contrast to cocaine reward, SB had greater effects on demand for remifentanil in low takers than in high takers. SB dose-dependently increased demand elasticity in low takers (Fig. 4C; 10 mg/kg dose, p = 0.207; 30 mg/kg dose, p = 0.056) but not in high takers (Fig 4C; 10 mg/kg dose, p = 0.447; 10 mg/kg dose, p = 0.155). Additionally, both doses of SB significantly reduced Q0 in low takers (Fig. 4D; 10 mg/kg, p = 0.003; 30 mg/kg, p = 0.006) but neither significantly affected Q0 in high takers (10 mg/kg, p = 0.259; 30 mg/kg, p = 0.685).
The effects of SB on FR1 responding (Fig. 1) were reanalyzed based upon whether subjects were subsequently classified as low or high takers from their performance on the behavioral economic threshold procedure (Fig. 2). Antagonism of Ox1Rs was similarly ineffective at reducing total number of infusions on the FR1 task in low and high takers (Supplemental Fig. 2A; main effect of SB versus vehicle pretreatment, F(1, 14) = 0.248, p = 0.626). SB similarly did not alter total active (Supplemental Fig. 2B; F(1, 14) = 1.812, p = 0.120) or inactive (Supplemental Fig. 2C; F(1, 14) = 0.013, p = 0.911) lever presses in low or high takers.
High takers endure more aversive punishment than low takers to continue taking remifentanil
A prominent characteristic in substance use disorders is continued drug use despite adverse consequences (Deroche-Gamonet et al., 2004; APA, 2013). We hypothesized that high takers would exhibit a more prominent addiction-like phenotype; therefore, we tested whether the high takers would sustain more footshock to receive remifentanil than low takers. After establishing a stable baseline of drug FR1 self-administration without footshock, subsequent sessions paired escalating intensity of footshocks with infusions (as described in the Methods).
During baseline (shock-free) sessions, high takers consumed significantly more remifentanil than low takers, also when accounting for body weight (Fig. 5A, F(1, 14) = 35.23, p < 0.001; p ≤ 0.05 all time bins). During punished responding (shock) sessions, high takers continued consuming significantly more drug than low takers despite the footshocks (Fig. 5B, F(1, 14) = 22.77, p < 0.001; bins 1-10, p < 0.05; bin 11, p = 0.08). Furthermore, high takers reduced footshock-paired drug consumption (relative to the shock-free baseline; Fig. 5A) at a higher shock intensity than low takers (Fig. 5B; low takers p < 0.05 beginning at 0.5 mA bin; high takers p < 0.05 beginning at 0.63 mA bin). Overall, high takers endured twice as much electrical charge as low takers during the shock test (Fig.5C, t(14) = 3.814, p = 0.002), demonstrating a persistent motivation for drug despite adverse consequences.
Figure 5. High takers withstand greater footshock to continue self-administering remifentanil.
A) During shock-free baseline sessions, high takers (n = 8) consistently self-administer more remifentanil than low takers (n = 8). B) During the shock test when each infusion is paired with a foot shock of escalating intensity, high takers continue to self-administer more drug than low takers. (Asterisks in symbols compared to shock-free baseline in A; asterisks over line compare high and low takers). C) High takers self-administer twice as much electrical charge to take remifentanil. D) Preferred levels of remifentanil consumption when drug is free (Q0) during the threshold BE procedure robustly predicts the amount of shock rats will subsequently withstand to continue self-administering remifentanil during the shock test. E) Demand elasticity (α) also predicts the total charge subjects will withstand to take drug. *p < 0.05; **p < 0.01.
Furthermore, drug consumption at null cost (Q0) during the BE threshold procedure predicted the amount of electrical shock sustained during the shock test (Fig. 5D, r = 0.763, p = 0.0006). There was also a significant relationship between demand elasticity (α) during the BE threshold procedure and the amount of electrical charge subjects endured to continue taking drug (Fig. 5E, r = −0.524, p = 0.037).
Ox1Rs mediate cue-induced, but not remifentanil-induced, reinstatement of drug seeking in low takers
After self-administration experiments concluded, subjects underwent a conventional extinction protocol, during which lever presses were neither reinforced by drug delivery nor cues. Low takers and high takers did not significantly differ in rates of extinction (data not shown). Once rates of responding were consistently less than 25 presses per session, animals were pretreated with vehicle or 30 mg/kg SB before cue-induced or remifentanil-induced reinstatement sessions to test whether blockade of Ox1Rs decreases reinstatement of drug seeking behavior.
Presentation of remifentanil-associated cues with active lever presses prompted a significant reinstatement of drug seeking measured by increased responding on the active lever (Fig. 6A; main effect, F(2,26) = 13.47, p < 0.0001; Dunnett's post hoc test, vehicle significantly greater than extinction, p < 0.001). As hypothesized, antagonism of Ox1Rs attenuated the reinstatement of cue-induced drug seeking (Fig. 6A; SB significantly lower than vehicle, p < 0.05). There was no change in responding on the inactive lever (Fig. 6B; F(2,26) = 0.39, p = 0.683), demonstrating that the increased responding on the active lever was not a general locomotor effect but rather targeted drug seeking behavior. Interestingly, individual differences were observed in the effect of SB on cue-induced reinstatement. Consistent with the individual differences in SB's effects on demand elasticity and set point, SB significantly attenuated cue-induced reinstatement in low takers (Fig. 6C; p < 0.05) but not high takers (Fig. 6D; p > 0.05).
Figure 6. Cue- and remifentanil-induced reinstatement of drug seeking.
A) Antagonism of Ox1Rs via pretreatment with 30 mg/kg SB attenuates cue-induced reinstatement (n = 14). B) There are no significant effects of SB or vehicle on inactive lever presses. C-D) The attenuation of cue-induced reinstatement of drug seeking by the OxR1 antagonist specifically occurs in low takers (C; n = 6) but not high takers (D; n = 8). E-H) Blockade of OxR1s does not affect remifentanil-induced reinstatement of drug seeking (E; n = 13) and also has no effect on inactive lever presses (F). Drug-induced reinstatement is similarly unaffected by SB in both low takers (G; n = 5) and high takers (H; n = 8). *p < 0.05; n.s. = not statistically different.
An i.v. infusion of 2 μg of remifentanil resulted in a modest reinstatement in drug seeking behavior (Fig. 6E; main effect, F(2,24) = 3.31, p = 0.05; Dunnett's post hoc test, vehicle significantly higher than extinction, p < 0.05) with no effect on inactive lever responding (Fig. 6F; main effect, F(2,24) = 1.09, p = 0.35). SB had no significant effects on this drug-induced reinstatement behavior (Fig. 6E; vehicle compared to SB, p > 0.05), in contrast to cue-induced reinstatement. The lack of effect of SB on drug-induced reinstatement was consistent in both low takers (Fig. 6G; p > 0.05) and high takers (Fig. 6H; p > 0.05).
Locomotor effects of SB
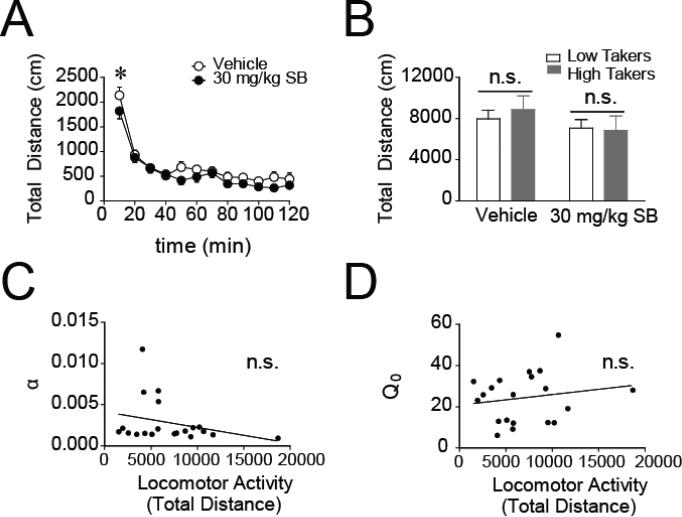
Consistent with previous studies (Smith et al., 2009), SB (30 mg/kg, i.p.) caused a modest, but statistically significant, reduction in general locomotor activity (Fig. 7A; main effect of treatment, F(1,252) = 18.17, p < 0.001). Post hoc analyses with Bonferroni corrections revealed that SB significantly decreased locomotor activity during the first time bin. SB did not differentially affect locomotor activity in high takers versus low takers (Fig. 7B; main effect of group, F(1,20) = 0.05; p = 0.83; interaction, F(1,20) = 0.98; p = 0.33). Furthermore, the locomotor effects of SB did not correlate with the effects of SB on α (Fig. 7C; r = −0.288, p = 0.22) or Q0 (Fig. 7D; r = 0.171, p = 0.47) during the threshold BE procedure, indicating that general locomotor effects of the drug are unlikely to be responsible for SB's modulation of economic demand for remifentanil.
Figure 7. SB has similar locomotor effects in low and high takers, which are not responsible for the drug's modulation of demand elasticity or free consumption of remifentanil.
A) SB modestly decreases spontaneous locomotor activity (n=22). B) The locomotor effects of vehicle and SB are similar in high and low takers. C-D) The locomotor effects of SB do not correspond to the drug's effects on demand elasticity (α; C) or consumption at null cost (Q0; D). *p < 0.05; n.s. = not statistically significant.
Discussion
Using a within-session BE procedure, this study reveals previously unknown individual differences in motivation for remifentanil and the role of Ox1Rs in mediating motivation for an opioid reward. Specifically, antagonism of Ox1Rs via SB increases demand elasticity (α), reduces remifentanil consumption when drug is free (Q0), and attenuates cue-induced reinstatement of drug seeking in low takers (low Q0 subjects), but not high takers (high Q0 rats). Together, these results indicate that Ox1Rs modulate opioid set point and demand in individuals with low baseline remifentanil demand and free consumption; however, the ineffectiveness of SB in altering motivation in high takers indicates that in addiction-prone individuals, a nonorexin mechanism may exert more control over opioid-seeking.
Individual differences in motivation for remifentanil
Although many people use addictive drugs, only a subset of users develops a substance use disorder. Therefore, examining individual differences is critical to modeling addiction in the laboratory. The within-session BE threshold procedure revealed a wide range of individual differences in remifentanil demand. Subjects were divided into low and high takers based upon average Q0 values, a measure of free consumption, or “hedonic set point” (Bentzley et al., 2013; Bentzley et al., 2014; Bentzley & Aston-Jones, 2015). The opponent process theory of addiction purports that higher hedonic set points are characteristic of a need to take more drug to achieve preferred drug levels and compensate for the allostatic state that occurs in the transition to addiction (Ahmed & Koob, 1998; Koob & Le Moal, 2001; Koob, 2015); therefore, individuals with a high set point in the BE threshold task are hypothesized to exhibit at least one component of addictive-like behaviors. Indeed, individuals with high set points (high Q0) also had more inelastic demand curves; in other words, high takers exhibited more persistent drug taking despite increases in drug price (Fig. 2), demonstrating that high takers were more motivated to acquire remifentanil than low takers. Time course analyses revealed that the difference in α between low and high takers emerged over weeks of remifentanil self-administration (Fig. 2F); thus, extended drug exposure may have supported a transition to addiction in susceptible individuals.
Additionally, when given unlimited access to low-cost remifentanil for an hour, high takers self-administered significantly more drug than low takers (Fig. 5A), and high takers withstood twice as much aversive shock to continue taking the drug (Fig. 5B-C). Individual differences in shock sensitivity are unlikely to account for the changes in drug self-administration. In the present study high takers self-administered twice as much charge as low takers (Fig. 5C) throughout the session; however, the first shock intensity at which high takers significantly reduce drug-taking is only one bin higher than low takers (Fig. 5B; 0.63 mA for high takers vs 0.50 mA for low takers). If subjects had markedly different shock sensitivities, the point at which the consumption curves significantly decrease from baseline would be very different between the two groups, which it is not (Fig. 5). Furthermore, human studies have shown that opiate addicts have a lower pain tolerance than controls (Compton et al., 2001) and there is evidence that having a history of opioid use can increase pain sensitivity in rats (Larcher et al., 1998). Therefore, if there were differences in pain sensitivity between high and low takers, we would hypothesize that the high takers would have greater pain sensitivity and therefore footshock would be more effective in reducing drug seeking. However, high takers maintained their consistent level of remifentanil self-administration on footshock compared to baseline shock-free sessions (Fig. 5A-B) which does not indicate a difference in pain sensitivity compared to low takers. Finally, previous experiments have found that footshock did not reduce cocaine-seeking in rats with extensive cocaine and sucrose self-administration histories (they were resistant to the punishing effects of footshock on cocaine-seeking similar to our high takers); however, footshock robustly attenuated sucrose consumption, demonstrating that the continued drug-seeking despite adverse consequences is not an effect of insensitivity to footshock but a high motivation for drug (Pelloux et al., 2007).
In summary, high takers consume more remifentanil at low cost, exert greater effort to consume drug, and withstand greater punishment to take drug. All of these factors are consistent with the conclusion that high takers represent more of an addiction phenotype than low takers. Moreover, these factors parallel our clinical observation that patients who require higher maintenance doses of buprenorphine have higher rates of relapse to illicit opioid use (Bentzley et al., 2015). Therefore, the within-session BE and shock procedures used here are useful for studying individual differences in susceptibility to opioid use disorders.
Ox1R antagonism reduces remifentanil motivation in low takers but not high takers
Previously, our lab has examined individual differences in the effectiveness of Ox1R blockade to alter motivation for other drugs of abuse. SB was shown to be more effective in attenuating cue-induced reinstatement in rats with high demand for cocaine (Bentzley & Aston-Jones, 2015). Similarly, SB caused greater reductions in ethanol consumption in high alcohol-preferring rats than in low-preferring rats (Moorman & Aston-Jones, 2009).
In contrast to these previous studies using non-opioid reinforcers, here we found that SB was more effective in reducing motivation for remifentanil in low takers than in high-takers. Specifically, SB reduced free consumption (Q0), increased demand elasticity (alpha), and attenuated cue-induced reinstatement in low takers but not in high takers. These differences highlight the importance of investigating the neurobiology of numerous classes of drugs of abuse considering different neurobiological mechanisms may mediate motivation for different drugs.
A potential explanation for the difference between the results of the current study and our previous studies examining the effects of Ox1R antagonism on motivation for other drugs of abuse is that chronic opioid exposure may spur adaptations in the orexin system of individuals with high motivation for remifentanil (high takers) whereby orexins no longer modulate opioid-seeking behaviors. Indeed, orexin neurons contain μ-opioid receptors (Georgescu et al., 2003), providing a direct mechanism for opioids to modulate orexin neurons. Specifically, morphine decreases the firing rate of orexin neurons, and chronic (65 hr) morphine exposure attenuates the morphine-induced decrease in neuronal activity while increasing the sensitivity of orexin cells to naloxone and decreasing their sensitivity to met-enkephalin (Li & van den Pol, 2008). If morphine exposure over a few days can significantly change the responsivity of orexin neurons to μ-opioid receptor agonists and antagonists (Li & van den Pol, 2008), it is plausible that the many of weeks of remifentanil self-administration experienced by our rats, particularly the high takers, could cause adaptations in orexin neurons to alter their activity and opioid responsiveness. Indeed, individuals with opioid addiction have been shown to have lower orexin levels than healthy participants (Zhang et al., 2013), supporting the conclusion that chronic opioid use causes adaptations in the orexin systems of those susceptible to addiction (high takers). The precise neurobiological mechanisms whereby this change occurs resulting in Ox1R blockade effectively reducing remifentanil-seeking in low takers not high takers remain to be elucidated.
Role of Ox1Rs in economic demand and motivation for opioids versus psychostimulants
Previous work has shown that SB reduces cocaine intake on high-effort, but not low-effort, schedules of reinforcement (Borgland et al., 2009). Similarly, systemic and intra-VTA site-specific blockade of Ox1Rs increases demand elasticity (α) for cocaine but does not affect free consumption (Q0) (España et al., 2010; Bentzley & Aston-Jones, 2015). In contrast, SB has been shown to reduce heroin intake in both low (FR1) and high ratio (progressive-ratio) schedules of reinforcement (Smith & Aston-Jones, 2012).
In the present study, similarly to cocaine, the number of remifentanil infusions earned on an FR1 schedule was not significantly reduced by SB; however, the cumulative number of active lever presses was modestly reduced (Fig. 1). Using the identical BE threshold procedure, we found that SB increased demand elasticity (increased α; decreased motivation) for both remifentanil (Fig. 4A) and cocaine; however, SB decreased free consumption (set point; Q0) for remifentanil (Fig. 4B), but not for cocaine (Bentzley & Aston-Jones, 2015). Although there was a significant correlation between α and Q0 in remifentanil-seeking (Fig. 2D), this relationship was not observed when cocaine was used as the reinforcer (Bentzley et al., 2014), revealing an important difference between the behavioral profiles for opioid and psychostimulant demand.
Consistent with previous reinstatement studies using self-administration of cocaine or heroin (Smith et al., 2009; Smith & Aston-Jones, 2012), SB attenuated cue- but not drug-induced reinstatement of remifentanil seeking. However, this effect depended on individual differences in set point: the SB-induced attenuation of cue-induced reinstatement occurred primarily in low takers, but not high takers, which contrasts with what our lab observed for cue-induced reinstatement of cocaine-seeking where baseline alpha, but not Q0 values, predicted effects of SB on reinstatement behavior (Bentzley & Aston-Jones, 2015). Although the lack of effect of SB on remifentanil-induced reinstatement behavior is consistent with the literature on orexin's role in drug-induced reinstatement, this finding may not be conclusive given the comparatively low levels of reinstatement observed. In view of prior reports for cocaine self-administration, the results of this study indicate that Ox1Rs modulate motivation for remifentanil differently than for cocaine.
The mechanisms underlying the reinforcing properties of cocaine and remifentanil differ with important implications for the neurobiology of opioid versus psychostimulant addiction (Zernig et al., 2007). Psychostimulants such as cocaine (Paulson & Robinson, 1995; Aragona et al., 2008; Porter-Stransky et al., 2011) as well as opioids (Di Chiara & Imperato, 1988; Vander Weele et al., 2014) including remifentanil (Crespo et al., 2005) increase dopamine transmission in the nucleus accumbens (NAc). Rewarding properties of opioids are traditionally thought to occur through activation of μ-opioid receptors in the midbrain that inhibit GABA neurons causing disinhibition of dopamine neurons, whereas cocaine primarily acts at dopamine transporters in terminal regions such as NAc (Luscher & Ungless, 2006). Orexins can facilitate motivation for rewards via actions in ventral tegmental area (VTA), among other targets such as the paraventricular nucleus of the thalamus (Matzeu et al., 2014) and the hypothalamic paraventricular nucleus (Schneider et al., 2007). Indeed, SB attenuates cocaine-induced increases in tonic and phasic patterns of dopamine transmission in NAc (España et al., 2010; Prince et al., 2015). Although much evidence implicates dopamine in facilitating the rewarding properties of psychostimulants, dopamine is not always necessary for opioid reward (Ettenberg et al., 1982; Pettit et al., 1984; Dworkin et al., 1988; Gerrits & Van Ree, 1996; Hnasko et al., 2005; Badiani et al., 2011). Additionally, whereas cocaine and amphetamine increase neuronal spine density and dendritic branching in NAc shell and mPFC, morphine has been shown to cause the opposite effects in these brain regions (Robinson & Kolb, 2004), further suggesting that psychostimulants and opioids have different effects within motivational circuitry. Together, these studies demonstrate that psychostimulants and opioids have distinct mechanisms of action, and these differences may be responsible for the differential role of Ox1Rs in modulating economic demand, free consumption, and cue-induced reinstatement for opioids (present study) versus psychostimulants (Bentzley & Aston-Jones, 2015).
Furthermore, dynorphin and orexin can be co-released, and these two neuropeptides have opposing effects on VTA dopamine neurons and behavior (Muschamp et al., 2014); the interaction of these peptides poses a possible mechanism whereby Ox1R antagonism may differentially affect motivation for cocaine versus remifentanil. Supporting this possibility, Fischer rats experiencing cocaine withdrawal exhibited increases in orexin and preprodynorphin mRNA in the lateral hypothalamus (Zhou et al., 2008), whereas subjects experiencing morphine withdrawal had increased orexin but not preprodynorphin mRNA (Zhou et al., 2006). Although the effects of changes in orexin and dynorphin mRNA on peptide release are unclear, these studies pose the intriguing possibility that opioids could differentially affect the balance of orexin and dynorphin release compared to psychostimulants and therefore differentially modulate remifentanil- versus cocaine-seeking.
Conclusion
This series of experiments reveals that individual ‘trait’ differences exist in free consumption and motivation for the opioid remifentanil, and these differences correspond to the role of Ox1Rs in modulating opioid demand. High takers, defined by their preference for higher levels of remifentanil when it is free (Q0), consumed significantly more drug in low effort sessions when drug availability was unlimited, exhibited more effortful responding for remifentanil as cost increased, and withstood more footshock to take drug, compared to low takers. Antagonism of Ox1Rs modulated three unique measures of motivation for remifentanil in low takers: SB reduced demand for drug at null cost (Q0), increased demand elasticity (α), and attenuated cue-induced reinstatement of drug seeking. Surprisingly, SB had none of these effects in high takers. These results were in marked contrast to the pattern of results in experiments examining cocaine demand, thereby revealing substantial differences in the role of Ox1Rs in modulating motivation for opioids versus psychostimulants. One possibility is that some individuals (high takers) develop an orexin-independent adaptation to chronic opioid exposure that is associated with an opioid addiction phenotype.
Supplementary Material
Acknowledgments
This work was supported by U.S. Public Health Service grant awards R37/R01-DA006214, T32 GM008716, and F30-DA035065. We would like to thank Dr. Ben Zimmer for helpful discussions about this project.
Footnotes
Author Contribution
GAJ & KAPS designed the study. KAPS collected the data. BSB formulated the macros necessary to analyze the economic demand curves, and KAPS analyzed the data. KAPS drafted the manuscript with critical revisions provided by GAJ and BSB.
References
- Ahmed S, Koob G. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- APA . Diagnostic and statistical manual of mental disorders. 5th ed. American Psychiatric Publishing; Arlington: 2013. [Google Scholar]
- Aragona BJ, Cleaveland NA, Stuber GD, Day JJ, Carelli RM, Wightman RM. Preferential enhancement of dopamine transmission within the nucleus accumbens shell by cocaine is attributable to a direct increase in phasic dopamine release events. J Neurosci. 2008;28:8821–8831. doi: 10.1523/JNEUROSCI.2225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Smith RJ, Sartor GC, Moorman DE, Massi L, Tahsili-Fahadan P, Richardson KA. Lateral hypothalamic orexin/hypocretin neurons: a role in reward-seeking and addiction. Brain research. 2010;1314:74–90. doi: 10.1016/j.brainres.2009.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badiani A, Belin D, Epstein D, Calu D, Shaham Y. Opiate versus psychostimulant addiction: the differences do matter. Nature Reviews Neuroscience. 2011;12:685–700. doi: 10.1038/nrn3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baimel C, Bartlett SE, Chiou LC, Lawrence AJ, Muschamp JW, Patkar O, Tung LW, Borgland SL. Orexin/hypocretin role in reward: implications for opioid and other addictions. Br. J. Pharmacol. 2015;172:334–348. doi: 10.1111/bph.12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzley BS, Aston-Jones G. Orexin-1 receptor signaling increases motivation for cocaine-associated cues. European Journal of Neuroscience. 2015;41:1149–1156. doi: 10.1111/ejn.12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzley BS, Barth KS, Back SE, Book SW. Discontinuation of buprenorphine maintenance therapy: Perspectives and outcomes. Journal of substance abuse treatment. 2015;52:48–57. doi: 10.1016/j.jsat.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzley BS, Fender KM, Aston-Jones G. The behavioral economics of drug self-administration: A review and new analytical approach for within-session procedures. Psychopharmacology. 2013;226:113–125. doi: 10.1007/s00213-012-2899-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzley BS, Jhou TC, Aston-Jones G. Economic demand predicts addiction-like behavior and therapeutic efficacy of oxytocin in the rat. Proceedings of the National Academy of Sciences. 2014;111:11822–11827. doi: 10.1073/pnas.1406324111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Chang S-J, Bowers MS, Thompson JL, Vittoz N, Floresco SB, Chou J, Chen BT, Bonci A. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. The Journal of neuroscience. 2009;29:11215–11225. doi: 10.1523/JNEUROSCI.6096-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprioli D, Celentano M, Dubla A, Lucantonio F, Nencini P, Badiani A. Ambience and drug choice: cocaine-and heroin-taking as a function of environmental context in humans and rats. Biological psychiatry. 2009;65:893–899. doi: 10.1016/j.biopsych.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Caprioli D, Celentano M, Paolone G, Lucantonio F, Bari A, Nencini P, Badiani A. Opposite environmental regulation of heroin and amphetamine self-administration in the rat. Psychopharmacology. 2008;198:395–404. doi: 10.1007/s00213-008-1154-3. [DOI] [PubMed] [Google Scholar]
- Celentano M, Caprioli D, Di Pasquale P, Cardillo V, Nencini P, Gaetani S, Badiani A. Drug context differently regulates cocaine versus heroin self-administration and cocaine-versus heroin-induced Fos mRNA expression in the rat. Psychopharmacology. 2009;204:349–360. doi: 10.1007/s00213-009-1467-x. [DOI] [PubMed] [Google Scholar]
- Compton P, Charuvastra V, Ling W. Pain intolerance in opioid-maintained former opiate addicts: effect of long-acting maintenance agent. Drug and alcohol dependence. 2001;63:139–146. doi: 10.1016/s0376-8716(00)00200-3. [DOI] [PubMed] [Google Scholar]
- Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug and alcohol dependence. 2006;81:103–107. doi: 10.1016/j.drugalcdep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Cooper ZD, Truong YNT, Shi YG, Woods JH. Morphine deprivation increases self-administration of the fast- and short-acting mu-opioid receptor agonist remifentanil in the rat. Journal of Pharmacology and Experimental Therapeutics. 2008;326:920–929. doi: 10.1124/jpet.108.139196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo JA, Sturm K, Saria A, Zernig G. Simultaneous intra-accumbens remifentanil and dopamine kinetics suggest that neither determines within-session operant responding. Psychopharmacology. 2005;183:201–209. doi: 10.1007/s00213-005-0180-7. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin SI, Guerin GF, Co C, Goeders NE, Smith JE. Lack of an effect of 6-hydroxydopamine lesions of the nucleus accumbens on intravenous morphine self-administration. Pharmacology Biochemistry and Behavior. 1988;30:1051–1057. doi: 10.1016/0091-3057(88)90138-4. [DOI] [PubMed] [Google Scholar]
- España RA. Hypocretin/Orexin Involvement in Reward and Reinforcement. Vitamins and hormones. 2012;89:185–208. doi: 10.1016/B978-0-12-394623-2.00010-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- España RA, Oleson EB, Locke JL, Brookshire BR, Roberts D, Jones SR. The hypocretin–orexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system. European Journal of Neuroscience. 2010;31:336–348. doi: 10.1111/j.1460-9568.2009.07065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenberg A, Geist TD. Qualitative and quantitative differences in the operant runway behavior of rats working for cocaine and heroin reinforcement. Pharmacology Biochemistry and Behavior. 1993;44:191–198. doi: 10.1016/0091-3057(93)90298-8. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Pettit HO, Bloom FE, Koob GF. Heroin and cocaine intravenous self-administration in rats: mediation by separate neural systems. Psychopharmacology. 1982;78:204–209. doi: 10.1007/BF00428151. [DOI] [PubMed] [Google Scholar]
- Freeman KB, Woolverton WL. Self-administration of cocaine and remifentanil by monkeys: choice between single drugs and mixtures. Psychopharmacology. 2011;215:281–290. doi: 10.1007/s00213-010-2131-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgescu D, Zachariou V, Barrot M, Mieda M, Willie JT, Eisch AJ, Yanagisawa M, Nestler EJ, DiLeone RJ. Involvement of the lateral hypothalamic peptide orexin in morphine dependence and withdrawal. The Journal of neuroscience. 2003;23:3106–3111. doi: 10.1523/JNEUROSCI.23-08-03106.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrits MA, Van Ree JM. Effect of nucleus accumbens dopamine depletion on motivational aspects involved in initiation of cocaine and heroin self-administration in rats. Brain research. 1996;713:114–124. doi: 10.1016/0006-8993(95)01491-8. [DOI] [PubMed] [Google Scholar]
- Glass PS, Gan TJ, Howell S. A review of the pharmacokinetics and pharmacodynamics of remifentanil. Anesthesia & Analgesia. 1999;89:7. doi: 10.1097/00000539-199910001-00003. [DOI] [PubMed] [Google Scholar]
- Hnasko TS, Sotak BN, Palmiter RD. Morphine reward in dopamine-deficient mice. Nature. 2005;438:854–857. doi: 10.1038/nature04172. [DOI] [PubMed] [Google Scholar]
- Hursh SR, Silberberg A. Economic demand and essential value. Psychological review. 2008;115:186. doi: 10.1037/0033-295X.115.1.186. [DOI] [PubMed] [Google Scholar]
- Koffarnus MN, Hall A, Winger G. Individual differences in rhesus monkeys’ demand for drugs of abuse. Addiction biology. 2012;17:887–896. doi: 10.1111/j.1369-1600.2011.00335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. The dark side of emotion: The addiction perspective. European journal of pharmacology. 2015 doi: 10.1016/j.ejphar.2014.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Larcher A, Laulin J, Celerier E, Le Moal M, Simonnet G. Acute tolerance associated with a single opiate administration: involvement of N-methyl-D-aspartate-dependent pain facilitatory systems. Neuroscience. 1998;84:583–589. doi: 10.1016/s0306-4522(97)00556-3. [DOI] [PubMed] [Google Scholar]
- Levine AI, Bryson EO. Intranasal self-administration of remifentanil as the foray into opioid abuse by an anesthesia resident. Anesthesia & Analgesia. 2010;110:524–525. doi: 10.1213/ANE.0b013e3181c5f069. [DOI] [PubMed] [Google Scholar]
- Li Y, van den Pol AN. μ-Opioid receptor-mediated depression of the hypothalamic hypocretin/orexin arousal system. The Journal of neuroscience. 2008;28:2814–2819. doi: 10.1523/JNEUROSCI.5447-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Ungless MA. The mechanistic classification of addictive drugs. PLoS Med. 2006;3:e437. doi: 10.1371/journal.pmed.0030437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Moorman DE, Smith RJ, James MH, Aston-Jones G. Motivational activation: a unifying hypothesis of orexin/hypocretin function. Nature neuroscience. 2014a;17:1298–1303. doi: 10.1038/nn.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Vazey EM, Beckley JT, Keistler CR, McGlinchey EM, Kaufling J, Wilson SP, Deisseroth K, Woodward JJ, Aston-Jones G. Designer receptors show role for ventral pallidum input to ventral tegmental area in cocaine seeking. Nature neuroscience. 2014b;17:577–585. doi: 10.1038/nn.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzeu A, Zamora-Martinez ER, Martin-Fardon R. The paraventricular nucleus of the thalamus is recruited by both natural rewards and drugs of abuse: recent evidence of a pivotal role for orexin/hypocretin signaling in this thalamic nucleus in drug-seeking behavior. Front. Behav. Neurosci. 2014;8 doi: 10.3389/fnbeh.2014.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman DE, Aston-Jones G. Orexin-1 receptor antagonism decreases ethanol consumption and preference selectively in high-ethanol–preferring Sprague–Dawley rats. Alcohol. 2009;43:379–386. doi: 10.1016/j.alcohol.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschamp JW, Hollander JA, Thompson JL, Voren G, Hassinger LC, Onvani S, Kamenecka TM, Borgland SL, Kenny PJ, Carlezon WA. Hypocretin (orexin) facilitates reward by attenuating the antireward effects of its cotransmitter dynorphin in ventral tegmental area. Proceedings of the National Academy of Sciences. 2014;111:E1648–E1655. doi: 10.1073/pnas.1315542111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno K, Sakurai T. Orexin neuronal circuitry: Role in the regulation of sleep and wakefulness. Front. Neuroendocrinol. 2008;29:70–87. doi: 10.1016/j.yfrne.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Oleson EB, Richardson JM, Roberts DC. A novel IV cocaine self-administration procedure in rats: differential effects of dopamine, serotonin, and GABA drug pre-treatments on cocaine consumption and maximal price paid. Psychopharmacology. 2011;214:567–577. doi: 10.1007/s00213-010-2058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panlilio LV, Katz JL, Pickens RW, Schindler CW. Variability of drug self-administration in rats. Psychopharmacology. 2003;167:9–19. doi: 10.1007/s00213-002-1366-x. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Schindler CW. Self-administration of remifentanil, an ultra-short acting opioid, under continuous and progressive-ratio schedules of reinforcement in rats. Psychopharmacology. 2000;150:61–66. doi: 10.1007/s002130000415. [DOI] [PubMed] [Google Scholar]
- Paulson PE, Robinson TE. Amphetamine-Induced time-dependent sensitization of dopamine neurotransmission in the dorsal and ventral striatum: A microdialysis study in behaving rats. Synapse. 1995;19:56–65. doi: 10.1002/syn.890190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelloux Y, Everitt BJ, Dickinson A. Compulsive drug seeking by rats under punishment: effects of drug taking history. Psychopharmacology. 2007;194:127–137. doi: 10.1007/s00213-007-0805-0. [DOI] [PubMed] [Google Scholar]
- Pettit HO, Ettenberg A, Bloom FE, Koob GF. Destruction of dopamine in the nucleus accumbens selectively attenuates cocaine but not heroin self-administration in rats. Psychopharmacology. 1984;84:167–173. doi: 10.1007/BF00427441. [DOI] [PubMed] [Google Scholar]
- Porter-Stransky KA, Wescott SA, Hershman M, Badrinarayan A, Vander Weele CM, Lovic V, Aragona BJ. Cocaine must enter the brain to evoke unconditioned dopamine release within the nucleus accumbens shell. Neurosci Lett. 2011;504:13–17. doi: 10.1016/j.neulet.2011.08.028. [DOI] [PubMed] [Google Scholar]
- Prince CD, Rau AR, Yorgason JT, España RA. Hypocretin/Orexin Regulation of Dopamine Signaling and Cocaine Self-Administration Is Mediated Predominantly by Hypocretin Receptor 1. ACS chemical neuroscience. 2015;6:138–146. doi: 10.1021/cn500246j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47:33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Schneider ER, Rada P, Darby RD, Leibowitz SF, Hoebel BG. Orexigenic peptides and alcohol intake: Differential effects of orexin, galanin, and ghrelin. Alcoholism. 2007;31:1858–1865. doi: 10.1111/j.1530-0277.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- Siegel JM. Hypocretin (OREXIN): Role in Normal Behavior and Neuropathology*. Annu. Rev. Psychol. 2004;55:125–148. doi: 10.1146/annurev.psych.55.090902.141545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Aston-Jones G. Orexin/hypocretin 1 receptor antagonist reduces heroin self-administration and cue-induced heroin seeking. European Journal of Neuroscience. 2012;35:798–804. doi: 10.1111/j.1460-9568.2012.08013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, See RE, Aston-Jones G. Orexin/hypocretin signaling at the orexin 1 receptor regulates cue-elicited cocaine-seeking. European Journal of Neuroscience. 2009;30:493–503. doi: 10.1111/j.1460-9568.2009.06844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Weele CM, Porter-Stransky KA, Mabrouk OS, Lovic V, Singer BF, Kennedy RT, Aragona BJ. Rapid dopamine transmission within the nucleus accumbens: Dramatic difference between morphine and oxycodone delivery. European Journal of Neuroscience. 2014;40:3041–3054. doi: 10.1111/ejn.12709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade-Galuska T, Galuska CM, Winger G. Effects of daily morphine administration and deprivation on choice and demand for remifentanil and cocaine in rhesus monkeys. Journal of the experimental analysis of behavior. 2011;95:75–89. doi: 10.1901/jeab.2011.95-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade-Galuska T, Winger G, Woods JH. A behavioral economic analysis of cocaine and remifentanil self-administration in rhesus monkeys. Psychopharmacology. 2007;194:563–572. doi: 10.1007/s00213-007-0858-0. [DOI] [PubMed] [Google Scholar]
- Yager LM, Pitchers KK, Flagel SB, Robinson TE. Individual Variation in the Motivational and Neurobiological Effects of an Opioid Cue. Neuropsychopharmacology. 2015;40:1269–1277. doi: 10.1038/npp.2014.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zernig G, Ahmed SH, Cardinal RN, Morgan D, Acquas E, Foltin RW, Vezina P, Negus SS, Crespo JA, Stöckl P, Grubinger P, Madlung E, Haring C, Kurz M, Saria A. Explaining the escalation of drug use in substance dependence: models and appropriate animal laboratory tests. Pharmacology. 2007;80:65–119. doi: 10.1159/000103923. [DOI] [PubMed] [Google Scholar]
- Zhang HR, Lian Z, Yan SY, Bao YP, Liu ZM. Different Levels in Orexin Concentrations and Risk Factors Associated with Higher Orexin Levels: Comparison between Detoxified Opiate and Methamphetamine Addicts in 5 Chinese Cities. Biomed Res. Int. 2013 doi: 10.1155/2013/282641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Bendor J, Hofmann L, Randesi M, Ho A, Kreek MJ. Mu opioid receptor and orexin/hypocretin mRNA levels in the lateral hypothalamus and striatum are enhanced by morphine withdrawal. Journal of endocrinology. 2006;191:137–145. doi: 10.1677/joe.1.06960. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Cui C-L, Schlussman SD, Choi JC, Ho A, Han J-S, Kreek MJ. Effects of cocaine place conditioning, chronic escalating-dose “binge” pattern cocaine administration and acute withdrawal on orexin/hypocretin and preprodynorphin gene expressions in lateral hypothalamus of Fischer and Sprague–Dawley rats. Neuroscience. 2008;153:1225–1234. doi: 10.1016/j.neuroscience.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.