Abstract
Treatment for leishmaniasis, which is caused by Leishmania protozoan parasites, currently relies on a reduced arsenal of drugs. However, the significant increase in the incidence of drug therapeutic failure and the growing resistance to first-line drugs like antimonials in some areas of Northern India and Nepal limit the control of this parasitic disease. Understanding the molecular mechanisms of resistance in Leishmania is now a matter of urgency to optimize drugs used and to identify novel drug targets to block or reverse resistant mechanisms. Some members of the family of ATP-binding cassette (ABC) transporters in Leishmania have been associated with drug resistance. In this study, we have focused our interest to characterize LABCG2's involvement in drug resistance in Leishmania. Leishmania major parasites overexpressing the ABC protein transporter LABCG2 were generated in order to assess how LABCG2 is involved in drug resistance. Assays of susceptibility to different leishmanicidal agents were carried out. Analysis of the drug resistance profile revealed that Leishmania parasites overexpressing LABCG2 were resistant to antimony, as they demonstrated a reduced accumulation of SbIII due to an increase in drug efflux. Additionally, LABCG2 was able to transport thiols in the presence of SbIII. Biotinylation assays using parasites expressing LABCG2 fused with an N-terminal green fluorescent protein tag revealed that LABCG2 is partially localized in the plasma membrane; this supports data from previous studies which suggested that LABCG2 is localized in intracellular vesicles that fuse with the plasma membrane during exocytosis. In conclusion, Leishmania LABCG2 probably confers antimony resistance by sequestering metal-thiol conjugates within vesicles and through further exocytosis by means of the parasite's flagellar pocket.
INTRODUCTION
Leishmaniasis is a neglected tropical disease caused by Leishmania protozoan parasites and is spread by the bite of infected phlebotomine sand flies. Currently, 1.3 million new cases of leishmaniasis and 20,000 to 30,000 deaths occur annually through a variety of clinical presentations (1).
Although chemotherapy is the only current treatment option for leishmaniasis, its efficacy is increasingly limited by growing resistance to first-line drugs, especially antimonials; the frequent side effects associated with their use; and the high cost of treatment. There is a limited number of drugs available for treatment, including amphotericin B, especially as a liposomal formulation; paromomycin; miltefosine; or pentavalent antimonials. The World Health Organization recently recommended the use of a combination of leishmanicidal drugs in order to decrease the concentration and toxicity of the dosages required as well as to delay the development of resistance. Even so, emerging drug resistance constitutes one of the main problems facing current leishmaniasis chemotherapies. In India, 60% of patients suffering from visceral leishmaniasis do not respond to treatment with antimonials due to the parasite's increased resistance to these drugs (2).
One of the most characteristic mechanisms of antimony resistance in Leishmania is drug efflux mediated by ABC (ATP-binding cassette) transporters such as MRPA (formerly PGPA)/ABCC3 (3, 4) or ABCI4 (5), which results in a reduced degree of antimony accumulation in parasites. ABC transporters are one of the largest protein families known; they are highly evolutionarily conserved from bacteria to humans and are involved in the transport of different compounds through biological membranes. Leishmania has 42 ABC genes distributed across nine subfamilies (ABCA to ABCI), yet to date, only some transporters found in the ABCA, ABCB, ABCC, ABCG, and ABCI subfamilies have been characterized.
Overexpression of MRPA and ABCI4 in Leishmania confers SbIII resistance to the promastigote forms and SbIII or SbV resistance to the intracellular amastigote forms (5, 6). Leishmaniasis is treated with antimonials by using pentavalent antimony-based drugs. SbV can be taken up by amastigotes and reduced to SbIII inside macrophages so that it may become active against Leishmania parasites. This mechanism has not been fully elucidated, and there is apparently more than one SbV-to-SbIII conversion route. Reduced glutathione (GSH) has been observed to promote the reduction of SbV to SbIII in the phagolysosomes of macrophages (7). Alternatively, parasite-specific thiol-dependent reductase 1 (TDR1) and arsenate reductase (ACR2) found in Leishmania are also able to reduce SbV to SbIII (8, 9). As described previously for MRPA, the resulting SbIII can combine with thiols to form conjugates inside intracellular organelles, which are then effluxed from the parasite (3).
The involvement of the LABCG2 transporter in the phosphatidylserine (PS) externalization required for host macrophage infection was reported previously (10). Although PS synthesis in Leishmania has been a matter of intense debate, it was concluded that parasites in the late logarithmic phase contain PS (11, 12).
In Leishmania, LABCG4 and LABCG6 transporters have been involved in phosphatidylcholine transport and also confer resistance to different drugs, including miltefosine (13, 14). Considering that some ABC transporters present pleiotropic activity in response to therapeutic xenobiotics, we focused on the role of the Leishmania LABCG2 transporter in drug resistance.
MATERIALS AND METHODS
Chemical compounds.
Trivalent antimony (SbIII) (potassium antimony tartrate), trivalent arsenite (AsIII) (sodium meta-arsenite), amphotericin B, pentamidine, chloroquine, quinine, mefloquine, primaquine, vinblastine, G-418 (Geneticin), buthionine-(S,R)-sulfoximine (BSO), 4′,6-diamidino-2-phenylindole dilactate (DAPI), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), n-dodecyl-β-d-maltopyranoside (DDM), CdCl2, CoCl2, CuSO4, and GSH were purchased from Sigma-Aldrich. Miltefosine and perifosine were purchased from ÆternaZentaris. Pentavalent antimony (SbV) (sodium stibogluconate), tafenoquine, and sitamaquine dihydrochloride were provided by GlaxoSmithKline. Daunomycin was purchased from Pfizer.
Leishmania culture conditions.
Promastigotes of Leishmania major (MHOM/JL/80/Friedlin) and derivative lines used in this study were cultured at 28°C in RPMI 1640-modified medium (Invitrogen) supplemented with 20% heat-inactivated fetal bovine serum (hiFBS; Invitrogen).
Gene expression.
Total RNA from different L. major lines was extracted by using the High Pure RNA isolation kit (Roche Diagnostics GmbH). RNA was transcribed into cDNA by employing the qScript cDNA synthesis kit (Quanta Biosciences, Inc.) according to the manufacturer's instructions. The cDNA obtained was diluted (1:10 and 1:50), amplified with sense (5′-CCTACAGAGGACACCTACA) and antisense (5′-GAAGGGATTCTGGCAAG) primers for LABCG2 and with sense (5′-GAAGTACACGGTGGAGGCTG) and antisense (5′-CGCTGATCACGACCTTCTTC) primers for GAPDH (glyceraldehyde-3-phosphate dehydrogenase) (as an internal control), and electrophoresed on a 4% agarose gel.
Cell transfection and susceptibility analysis.
Promastigotes of L. major were transfected with the previously described pUCNEO (empty vector), pUCNEO-LABCG2, and pXG-GFP::LABCG2 constructs (10) and selected for G-418 resistance, as described previously (15). The susceptibilities of the respective pUCNEO (control), LABCG2, and GFP-LABCG2 promastigote lines to different compounds were determined by using an MTT colorimetric assay, as described previously (16). To analyze the relationship between thiol levels and susceptibility to SbIII, parasites were previously grown in M199 culture medium supplemented with 10% hiFBS plus 3 mM BSO (a γ-glutamylcysteine synthetase inhibitor) for 48 h at 28°C. For assays of susceptibility of intracellular Leishmania amastigotes to SbIII and SbV, stationary-phase promastigotes were used to infect macrophage-differentiated THP-1 cells at a macrophage/parasite ratio of 1:10, as described previously (17). After overnight infection at 35°C with 5% CO2 in RPMI 1640 medium plus 5% hiFBS, extracellular parasites were removed by washing with serum-free medium. Infected macrophages were incubated at 37°C with different concentrations of SbIII and SbV with 5% CO2 in RPMI 1640 medium plus 10% hiFBS for 72 and 120 h, respectively. Following incubation, the cultures were fixed and analyzed as described previously (17).
Cell surface biotinylation.
Parasite (1 × 108 promastigotes) surfaces were labeled as described previously (18) but by using 3% DDM instead of 1% Nonidet P-40 to cause parasite lysis and with a 60-min incubation instead of a 30-min incubation in lysis buffer coupled with a protease inhibitor cocktail (Sigma-Aldrich). Protein samples were fractionated by SDS-PAGE under standard conditions and electrotransferred onto Immobilon-P membranes (Millipore). Immunodetection was performed by using a 1:5,000 dilution of polyclonal anti-green fluorescent protein (GFP) (Rockland Immunochemicals) or a 1:3,000 dilution of polyclonal anti-plasma membrane (PM) protein-LRos3 (18) in phosphate-buffered saline (PBS) plus 0.01% Tween 20 and 0.1% bovine serum albumin (BSA). Control over PM integrity was determined by immunodetection using a monoclonal anti-cytosolic tryparedoxin peroxidase antibody at a 1:6,000 dilution (a gift from Ana M. Tomás, IBMC, Porto, Portugal). After washing, membranes were incubated with horseradish peroxidase-conjugated secondary goat anti-rabbit (1:5,000) immunoglobulin G (Dako) for GFP, LRos3, and tryparedoxin peroxidase. Signals were detected by using the ECL chemiluminescent substrate (Pierce).
Antimony accumulation and efflux.
Promastigotes (108/ml) were incubated at 28°C with 100 μM SbIII in RPMI 1640 culture medium at 28°C for different times. The parasites were centrifuged and pelleted to measure antimony accumulation after each time period (19). Antimony efflux was determined by incubating the different promastigote lines with compensated SbIII concentrations (100 μM for the pUCNEO line and 200 μM for the LABCG2 line) for 1 h at 28°C in culture medium in order to attain similar labeling in Leishmania lines. The parasites were then washed with PBS and resuspended in culture medium at 28°C, and the pellet was collected after different time points. The samples for uptake and efflux determination were measured by using inductively coupled plasma mass spectrometry (ICP-MS; PerkinElmer) as described previously (5).
Determination of nonprotein thiol levels.
Log-phase parasites (107 parasites/ml) were grown in M199 medium plus 10% hiFBS in order to measure thiol levels. They were then washed with PBS and incubated at 37°C with 2 μM CellTracker for 15 min. After incubation, the parasites were again washed with PBS and analyzed by flow cytometry using a FACScan flow cytometer (Becton-Dickinson). Fluorescence emission was quantified at between 515 and 545 nm by using Cell Quest software. The efflux of nonprotein thiols to the culture medium was determined by using the ThioStar thiol fluorescent detection reagent (Luminos) as described previously (5).
RESULTS AND DISCUSSION
Overexpression of LABCG2 confers resistance to antimony and other compounds.
The role of ABC transporters in resistance to different compounds has been studied previously (5, 19–21). As mentioned above, the Leishmania LABCG2 transporter is involved in the PS externalization required for macrophage infection (10). We have determined that the overexpression of LABCG2 in Leishmania parasites did not show differences in PS exposition (10) or cell growth.
LABCG2 belongs to the same subfamily as mammalian ABCG2, a well-characterized PS transporter (22) that also pumps drugs conferring a multidrug-resistant (MDR) phenotype in cancer cells (23, 24). Other Leishmania ABCG proteins, such as LABCG4 and LABCG6, have been described to be involved in phospholipid transport (phosphatidylcholine analogues) and drug resistance (alkyl-phospholipids) (13, 14). However, the role of the LABCG2 transporter in drug resistance has not yet been elucidated. Modulation of gene expression through gene amplification and gene deletion by homologous recombination is a common mechanism of drug resistance in Leishmania strains derived from both the laboratory and the field thanks to the plasticity of the Leishmania genome (25–27). We are therefore interested in determining whether the overexpression of LABCG2 in a L. major line (data not shown) could confer drug resistance. We analyzed the profile of resistance to different leishmanicidal drugs, including SbIII, amphotericin B, miltefosine, pentamidine, and sitamaquine, and other compounds such as AsIII, tafenoquine, primaquine, chloroquine, daunomycin, mefloquine, quinine, perifosine, and vinblastine (Table 1). As described previously, many of them are probably transported by other Leishmania ABCs (5, 13, 14, 28, 29). The results showed that promastigotes overexpressing LABCG2 were ∼6- and 7-fold more resistant to AsIII and SbIII, respectively, than the control line (pUCNEO) (Table 1), suggesting that these metal ions could be substrates for the LABCG2 transporter, as previously described for MRPA and ABCI4 (3, 5). We also observed that overexpression of LABCG2 did not affect susceptibility to other metal ions such as CdII, CoII, and CuII (Table 2). Contrary to other members of the Leishmania ABCG subfamily such as LABCG4 and LABCG6, LABCG2 does not confer resistance to the alkyl-phospholipids miltefosine and perifosine or the aminoquinolines sitamaquine and chloroquine (13, 14). Furthermore, the LABCG2 line “cured” for plasmid pUCNEO-LABCG2 (LABCG2rev) by maintaining the parasites in culture without drug selection for 3 months (LABCG2rev 90D) showed a susceptibility phenotype similar to that of the control line (Table 1).
TABLE 1.
Drug resistance profile in promastigote L. major linesa
| Drug | Mean EC50 (μM) ± SD (RI)b |
||
|---|---|---|---|
| pUCNEO | LABCG2 | LABCG2rev 90D | |
| SbIII | 16.02 ± 2.63 | 118.84 ± 11.50 (7.4)* | 11.53 ± 0.69 (0.7) |
| AsIII | 0.99 ± 0.30 | 6.02 ± 1.50 (6.0)* | 1.32 ± 0.02 (1.3) |
| Amphotericin B | 2.27 ± 0.73 | 2.33 ± 0.45 (1.0) | — |
| Miltefosine | 18.25 ± 0.22 | 17.64 ± 1.70 (0.9) | — |
| Pentamidine | 0.66 ± 0.11 | 1.03 ± 0.11 (1.6)* | 0.83 ± 0.03 (1.3) |
| Tafenoquine | 12.87 ± 3.16 | 15.92 ± 7.50 (1.2) | — |
| Sitamaquine | 21.88 ± 5.43 | 18.02 ± 0.82 (0.8) | — |
| Primaquine | 5.43 ± 0.32 | 6.11 ± 0.05 (1.1) | — |
| Chloroquine | 10.99 ± 0.53 | 9.37 ± 1.67 (0.8) | — |
| Daunomycin | 0.56 ± 0.09 | 1.06 ± 0.16 (1.9)* | 0.67 ± 0.06 (1.2) |
| Mefloquine | 2.02 ± 0.19 | 3.04 ± 0.34 (1.5) | — |
| Quinine | 23.62 ± 2.84 | 29.69 ± 0.26 (1.3) | — |
| Perifosine | 20.99 ± 1.95 | 20.96 ± 1.27 (1.0) | — |
| Vinblastine | 10.31 ± 2.16 | 13.25 ± 2.65 (1.3) | — |
Promastigotes of Leishmania lines were grown for 72 h at 28°C in the presence of increasing concentrations of drugs. Cell viability was determined by using an MTT-based assay as described in Materials and Methods. Bold font represents significant resistance.
Resistance indexes (RI) were calculated by dividing the EC50 for the Leishmania line overexpressing LABCG2 and LABCG2rev 90D by that for the Leishmania control line (pUCNEO). Data are the means ± standard deviations of results from three independent experiments. Significant differences were determined by using the Student t test (*, P < 0.01). —, not determined.
TABLE 2.
Heavy metal resistance profiles of L. major promastigote linesa
| Metal | Mean EC50 (μM) ± SD (RI)b |
|||
|---|---|---|---|---|
| pUCNEO | LABCG2 | 2-LABCG2 | GFP-LABCG2 | |
| SbIII | 16.02 ± 2.63 | 118.84 ± 11.50 (7.4)* | 42.92 ± 7.75 (2.7)* | 34.93 ± 1.00 (2.2)* |
| AsIII | 0.99 ± 0.30 | 6.02 ± 1.50 (6.0)* | 2.72 ± 0.78 (2.7)* | 3.20 ± 0.18 (3.2)* |
| CdII | 58.77 ± 0.82 | 52.79 ± 4.21 (0.9) | — | — |
| CoII | 28.46 ± 4.05 | 36.57 ± 5.27 (1.3) | — | — |
| CuII | 43.52 ± 7.02 | 60.68 ± 4.99 (1.4) | — | — |
Promastigotes of Leishmania lines were grown for 72 h at 28°C in the presence of increasing concentrations of metals. Cell viability was determined by using an MTT-based assay as described in Materials and Methods. Bold font represents significant resistance.
Resistance indexes (RI) were calculated by dividing the EC50 for Leishmania line overexpressing LABCG2, LABCG2rev at 90 days (LABCG2rev 90D), second-event LABCG2 (2-LABCG2), and GFP-LABCG2 by that for Leishmania control line (pUCNEO). Data are the means ± standard deviations of results from three independent experiments. Significant differences were determined by using the Student t test (*, P < 0.01). —, not determined.
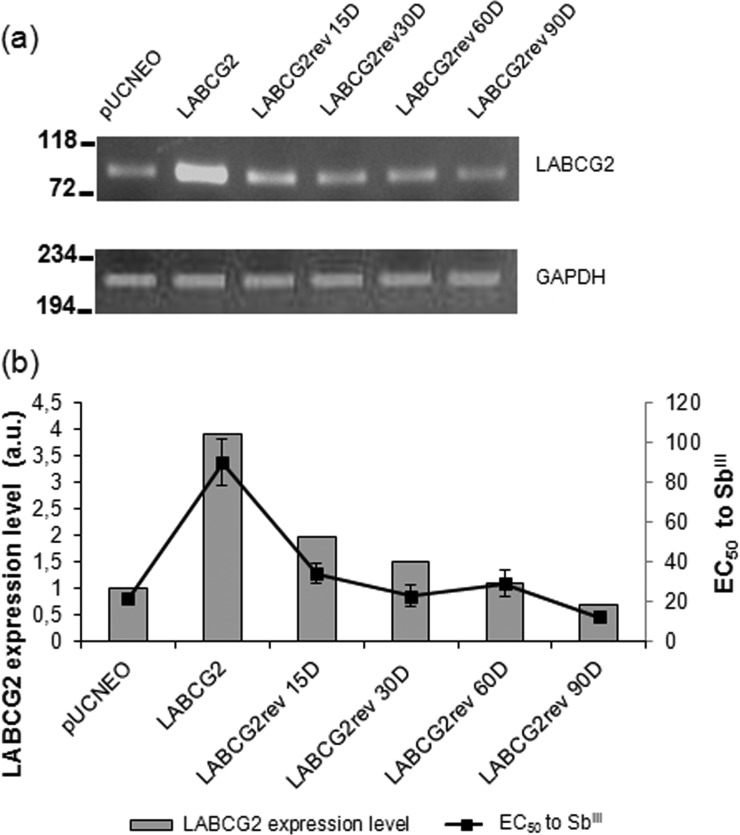
To analyze whether the expression levels of LABCG2 were correlated with susceptibility to SbIII, the LABCG2 lines were cultured in the absence of G-418 for 15, 30, 60, and 90 days to reduce plasmid copy numbers and LABCG2 expression levels (Fig. 1a). The results showed a direct relationship between LABCG2 expression levels and SbIII resistance (Fig. 1b). Consequently, a greater degree of LABCG2 expression generates a higher level of resistance to SbIII.
FIG 1.
RNA expression analysis of LABCG2 in L. major lines. (a, top) LABCG2 gene expression determined by reverse transcription-PCR, as indicated by the amplified 82-bp ABCG2 fragment. (Bottom) GAPDH gene expression as the internal loading control showing the amplified 227-bp GAPDH fragment. Total RNA was extracted from the pUCNEO line (control), LABCG2 lines, and LABCG2 parasites grown for 15, 30, 60, and 90 days in the absence of G-418 pressure (LABCG2rev 15D, LABCG2rev 30D, LABCG2rev 60D, and LABCG2rev 90D lines, respectively) and then reverse transcribed to single-stranded cDNA by specific priming as described in Materials and Methods. PCR products were electrophoresed on a 4% agarose gel, stained with ethidium bromide, and viewed under a UV illuminator, and the relative intensity was measured against that of GAPDH by using a densitometer. The positions of molecular markers (base pairs) are indicated on the left. (b) Relationship between LABCG2 expression (arbitrary units [a.u.]) and SbIII susceptibility (EC50 values ± standard deviations from three independent experiments) in L. major promastigote lines. Data from reverse transcription-PCR assays, representative of at least three independent experiments, are shown.
Further validation analysis showed that a second transfection event that facilitates LABCG2 overexpression in Leishmania (2-LABCG2) also conferred significant resistance to SbIII and AsIII (Table 2). The above-described experiments help to discard the possibility that the resistant phenotype observed was due to an intrinsic characteristic of the clone rather than a phenotypic characteristic of the overexpression of this transporter (Table 2). The differences observed in susceptibility to SbIII and AsIII between the two transfection events involving LABCG2 were due to variations in the parasites' degree of LABCG2 expression (data not shown). Furthermore, parasites overexpressing LABCG2 fused to GFP presented a susceptibility pattern similar to that of 2-LABCG2 parasites (Table 2), demonstrating that an N-terminal fusion of the GFP tag does not interfere with the functionality of the LABCG2 transporter.
The resistance to SbIII observed in the promastigote forms of L. major was retained in the intracellular amastigotes obtained after THP-1 cell infection (Table 3). Intracellular amastigotes overexpressing LABCG2 also presented significant resistance to sodium stibogluconate, a leishmanicidal drug containing SbV, which is reduced to SbIII inside macrophages (Table 3). LABCG2 has not been described as an antimony resistance marker in previous studies based on omics techniques, similarly as described previously for Leishmania ABCI4 (5), considering that not every change in the expression levels of proteins involved in antimony resistance is detected by these techniques. Our findings lend weight to the idea that the overexpression of LABCG2 confers antimony resistance to Leishmania parasites.
TABLE 3.
Susceptibility to antimony in intracellular amastigotes of L. major linesa
| Metal | Mean EC50 (μM) ± SD (RI)b |
||
|---|---|---|---|
| pUCNEO | LABCG2 | LABCG2rev 90D | |
| SbIII | 6.16 ± 0.07 | 21.30 ± 0.81 (3.4)* | 5.90 ± 0.11 (0.9) |
| SbV | 76.14 ± 2.88 | >200 (>2.6)* | 87.73 ± 9.71 (1.1) |
Macrophage-differentiated THP-1 cells infected with L. major lines using a macrophage/parasite ratio of 1:10 were incubated for 3 days in the presence of SbIII or for 5 days in the presence of SbV at different concentrations, as described in Materials and Methods. Antimony susceptibility was determined from the percentage of infected cells and the number of intracellular amastigotes per cell in antimony-treated cultures versus nontreated cultures. Infection was determined by DAPI staining of 300 macrophages/well.
Resistance indexes (RI) were calculated by dividing the EC50 for the Leishmania line overexpressing LABCG2 and LABCG2rev 90D by that for the Leishmania control line (pUCNEO). Data are the means ± standard deviations of results from two independent experiments. Significant differences were determined by using the Student t test (*, P < 0.01).
Reduction in accumulation of SbIII due to increased efflux in L. major lines overexpressing LABCG2.
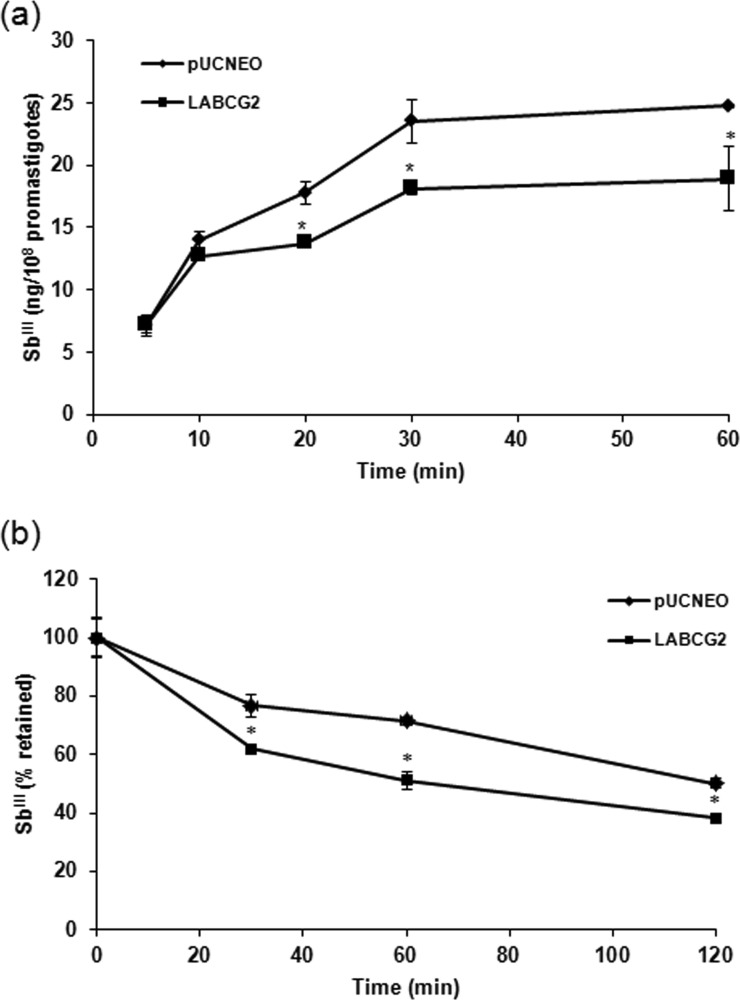
In order to uphold the suggestion that SbIII is a potential substrate for LABCG2, the intracellular accumulation of antimony metal ions in L. major lines was measured after different durations (10, 20, 30, and 60 min) by ICP-MS (Fig. 2a).
FIG 2.
Time-dependent accumulation and efflux of SbIII in Leishmania lines. (a) L. major lines (1 × 108 promastigotes/ml) carrying pUCNEO (control) and overexpressing LABCG2 were incubated with 100 μM SbIII, and samples were taken after different time points. Antimony accumulation was measured by ICP-MS. (b) An efflux assay was performed after incubation of Leishmania lines with compensated concentrations of SbIII for 1 h to ensure similar labeling in the different lines. The parasites were then washed and resuspended in PBS without SbIII and pelleted at different time points. The data are the means ± standard deviations of results from three independent experiments performed in duplicate. Significant differences versus the control line were determined by using the Student t test (*, P < 0.01).
Sixty minutes after incubation with SbIII (Fig. 2a), parasites overexpressing LABCG2 accumulated 76% of the total amount of SbIII accumulated by the control parasites. To assess whether the lower level of accumulation of this metal ion compound was due to increased efflux to the extracellular medium, Leishmania lines were loaded under conditions that generated similar amounts of intracellular SbIII. The amount of metal ion retained inside the parasites was then measured after different periods (Fig. 2b), and so we determined that SbIII efflux is faster in parasites overexpressing LABCG2 (Fig. 2b), leading to the conclusion that this transporter mediates SbIII elimination. As reported previously, the primary mechanism of resistance consists of decreasing the amount of active drug within the cell by a variety of routes (30). Parasites can decrease the uptake of, increase the efflux of, or inactivate the drug by sequestration, among other possible mechanisms. The LmAQP1 aquaglyceroporin is the only protein known to transport antimony inside L. major (31, 32), and its downregulation subsequently leads to increased drug resistance (33). Concerning efflux, members of the eukaryotic ABCC subfamily are involved in SbIII and AsIII resistance by exporting these metal ions outside the cells or by sequestering them within intracellular vesicles (34). MRPA from Leishmania is one of the best-known ABC transporters implicated in antimony resistance through the sequestration of SbIII-thiol complexes within an intracellular organelle near the flagellar pocket and then expulsion from the parasite by exocytosis (3). The levels of antimony efflux obtained for LABCG2 are slightly higher than the ones observed for MRPA (35) but lower than those for other MDR pumps (36, 37), suggesting similar mechanisms of action for MRPA and LABCG2. To the best of our knowledge, there have been no reports to suggest that the ABCG transporter is involved in SbIII or AsIII tolerance. Regarding other heavy metals, some plant full-size ABCG (PDR) transporters confer cadmium and lead tolerance (38–40). Therefore, this work presents the first description of the role of an ABCG transporter in the resistance of Leishmania to SbIII and AsIII.
Role of thiols in Leishmania ABCG2-mediated antimony resistance.
As described above, thiols conjugate with heavy metals and export them to the extracellular medium, which represents an antimony resistance mechanism in Leishmania (41). We therefore analyzed nonprotein thiol efflux in parasites overexpressing LABCG2 using ThioStar (Fig. 3). In the presence of SbIII (Fig. 3), the LABCG2 line showed significantly higher thiol efflux than did control parasites. This implies that the LABCG2 transporter confers resistance to SbIII by the efflux of SbIII-thiol complexes; however, we cannot discard the possibility of a cotransport activity of antimony and thiols. In the absence of SbIII (Fig. 3), we also observed a significant increase in thiol efflux for parasites overexpressing the LABCG2 transporter in comparison with the control line, although the increase was not as pronounced as that in the presence of SbIII. These results suggest that the LABCG2 transporter could export thiols without being conjugated to antimony.
FIG 3.
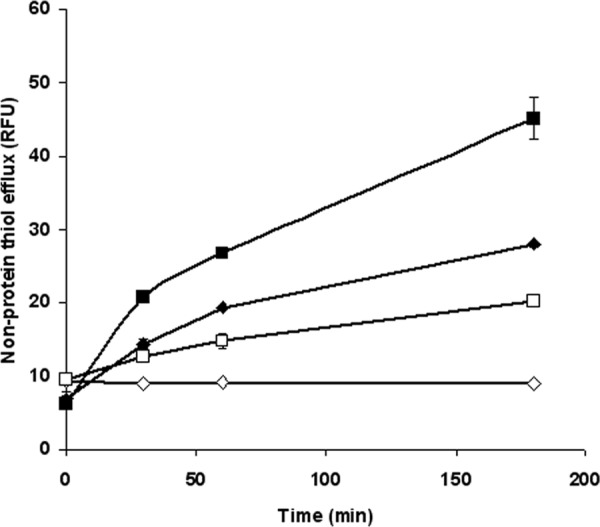
Nonprotein thiol efflux in Leishmania lines. L. major lines (1 × 108 promastigotes/ml) carrying pUCNEO (control) (diamonds) and overexpressing LABCG2 (squares) were incubated for 1 h either with (closed symbols) or without (open symbols) 100 μM SbIII for 1 h. The promastigotes were then washed with PBS, and the supernatants were processed after different periods. Sample fluorescence (excitation, 380 nm; emission, 510 nm) was determined by using an Infinite F200 luminescence system (Tecan Austria GmbH) and expressed in relative fluorescence units (RFU). The data are the means ± standard deviations of results from three independent experiments.
Since conjugation of thiol adducts of SbIII seems to be required for resistance to antimony, we determined the drug resistance profiles of L. major lines overexpressing LABCG2 after treatment with BSO, a γ-glutamylcysteine synthetase inhibitor. For these experiments, parasites were previously maintained in M199 culture medium supplemented with 10% hiFBS for 48 h at 28°C, a medium with lower levels of serum and thiols than those of RPMI 1640 medium with 20% hiFBS. We observed a significant decrease in SbIII 50% effective concentrations (EC50s) for parasites overexpressing LABCG2 after incubation with BSO than for those incubated without BSO (Table 4). The different EC50s of SbIII observed for the pUCNEO and LABCG2 lines without BSO (Table 4) with respect to values shown in Table 1 were due to differences in the contents of serum and thiols in the media used in both experiments. Hence, the SbIII resistance of L. major lines overexpressing LABCG2 is linked to thiol levels inside the parasites, probably due to the ability of LABCG2 to export thiol-conjugated adducts, as described previously for ABCI4 (5) and MRPA (3) in Leishmania. The implication of ABC transporters in detoxification by the efflux of metal-thiol complexes was described previously for other organisms, such as the vacuolar transporter YCF1 in Saccharomyces cerevisiae, which detoxifies bis-glutathione-cadmium complexes (42), or HTM1, an ABC transporter localized in fission yeast vacuolar vesicles that confers tolerance to cadmium by taking up glutathione-derived phytochelatin conjugated to CdII (43).
TABLE 4.
Susceptibility of L. major lines to antimony in the presence of BSOa
| Compound | Mean EC50 (μM) ± SD (RI [EC50 decrease]) |
|
|---|---|---|
| pUCNEO | LABCG2 | |
| SbIII | 6.50 ± 1.01 | 37.45 ± 1.50 (5.8)† |
| SbIII + BSO | 4.96 ± 1.23 (1.3) | 8.23 ± 0.91 (1.7 [4.5])* |
Parasites were grown in M199 culture medium supplemented with 10% hiFBS for 72 h at 28°C in the presence of increasing concentrations of SbIII. Cell viability was determined by using an MTT-based assay as described in Materials and Methods. Resistance indexes (RI), indicated in parentheses, were calculated by dividing the EC50 for the Leishmania line overexpressing LABCG2 by that for the Leishmania control line (pUCNEO) with the same treatment. The EC50 decrease, indicated in square brackets, was calculated by dividing the EC50 after SbIII treatment by that for treatment with SbIII plus BSO (a γ-glutamylcysteine synthetase inhibitor) in each Leishmania line. A total of 3 mM BSO was added to the culture medium 48 h before the susceptibility experiment was performed. The data are the means ± standard deviations of results from three independent experiments. Significant differences were determined by the Student t test (†, P < 0.01 for pUCNEO versus the LABCG2 line; *, P < 0.01 for the LABCG2 line treated with versus without BSO).
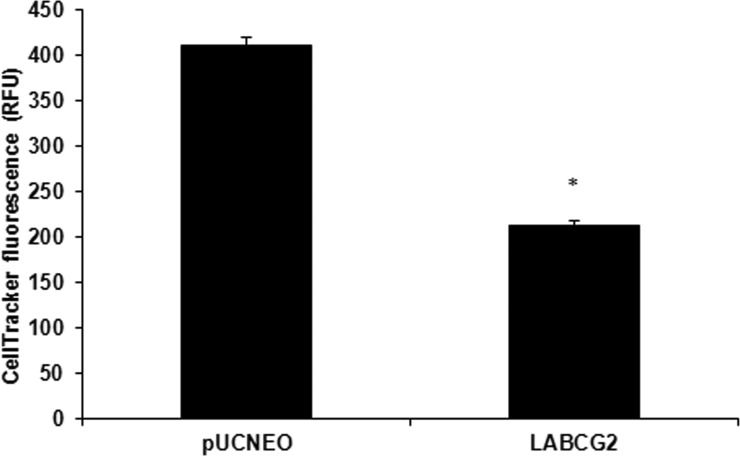
Considering that the LABCG2 transporter revealed an apparent capacity to export thiols in the absence of SbIII, we measured internal thiol levels in L. major lines by flow cytometry analysis using CellTracker. LABCG2 parasites presented lower thiols level than those of the control parasites (Fig. 4). Consequently, these results support the hypothesis that LABCG2 exports thiols to the extracellular medium without the need for conjugation to antimony. Decreased levels of GSH have been observed in MDCKII cells overexpressing human ABCG2/BCRP (44). Additionally, BCRP overexpression in HN4 cells was observed to increase extracellular GSH levels (45). However, measurement of GSH transport in membrane vesicles indicated that BCRP does not catalyze any significant GSH transport (46). In contrast, ABCC1/MRP1 mediated active GSH transport in cancer cells (47). Future studies using membrane reconstitution of purified LABCG2 in proteoliposomes will further our understanding of LABCG2-mediated thiol transport in the absence of SbIII.
FIG 4.
Determination of thiol levels in Leishmania lines. Promastigotes (107/ml) of L. major lines carrying pUCNEO (control) and overexpressing LABCG2 were incubated for 15 min at 37°C with 2 μM CellTracker and quantified by flow cytometry. The data are the means ± standard deviations of results from three independent experiments. Significant differences versus the control line were determined by using the Student t test (*, P < 0.01).
Determination of plasma membrane localization of LABCG2 in Leishmania parasites using a biotinylation assay.
We have previously used fluorescence microscopy assays to determine that LABCG2 partially colocalizes with the endosomal marker FM4-64 in L. major, suggesting that LABCG2 is located in the intracellular vesicles of the endocytic pathway of Leishmania parasites (10). However, we have not determined whether LABCG2 could be localized in the parasites' plasma membrane, where the transporter could be involved in the mechanism of drug efflux. We have previously described how Leishmania ABCI4 overexpression is localized in mitochondria, where it decreases the toxicity and accumulation of antimony, probably through efflux of the metal ion to the cytosol (5). Furthermore, ABCI4 that is localized in the parasitic plasma membrane may help to protect cells against the toxic effects of antimony and other compounds by effluxing them as conjugated thiol complexes (5).
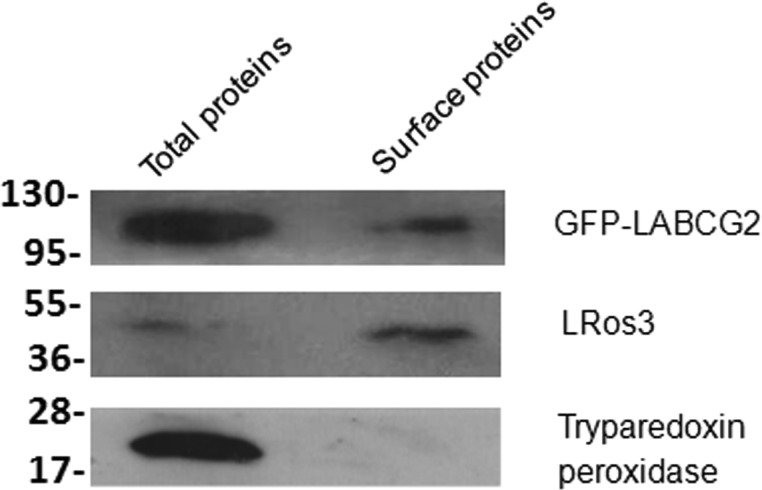
To corroborate the possible localization of LABCG2 in the parasitic plasma membrane, we performed biotinylation assays using parasites expressing LABCG2 fused with an N-terminal GFP tag. Expression of the GFP-LABCG2 protein was determined by Western blotting of whole-parasite lysates. As expected, a band corresponding to GFP-LABCG2 was detected at ∼100 kDa (Fig. 5). We also observed part of the protein localized in the PM extract, supporting the hypothesis that the transporter would be localized in intracellular vesicles, which would in turn fuse with the PM to release their content to the extracellular medium. The MRPA transporter has a similar subcellular localization, since it is found in intracellular membranes believed to correspond to vesicles that could be exocytosed via the flagellar pocket (3). Besides, we did not observe cytosolic tryparedoxin peroxidase among the biotinylated surface protein fractions, confirming that the labeling reagent did not penetrate the PM and consequently validating the specificity of the biotinylation procedure.
FIG 5.
LABCG2 expression levels in the plasma membrane of L. major parasites. Biotinylated proteins from the surface of promastigotes of Leishmania lines expressing GFP-LABCG2 were analyzed by immunoblotting with anti-GFP, anti-LRos3 (positive control), and anti-cytosolic tryparedoxin peroxidase (negative control) antibodies. Data from Western blot assays, representative of at least three independent experiments, are shown. The positions of molecular markers (kilodaltons) are indicated on the left.
In conclusion, overexpression of LABCG2 in the plasma membrane of Leishmania may help to protect cells against the toxic effects of antimony and other compounds by effluxing them as conjugated thiol complexes. Future work should endeavor to obtain null mutants for LABCG2 that could potentially be used to understand the role of LABCG2 in Leishmania as a thiol-X pump and to validate it as a marker of clinical antimony resistance.
ACKNOWLEDGMENTS
A.P. was a student of the biochemistry and molecular biology Ph.D. program of the University of Granada (Spain). We thank Stephen M. Beverley (Washington University School of Medicine, USA) for providing the pXG-GFP+2′ vectors used throughout this research work and Ana Maria Tomás (Institute for Molecular and Cell Biology, Porto, Portugal) for providing the monoclonal anti-cytosolic tryparedoxin peroxidase antibody.
A.P. was supported by a fellowship for predoctoral contracts for Ph.D. training from the Ministerio de Economia y Competitividad (in charge of project SAF2012-34267).
REFERENCES
- 1.World Health Organization. 2015. Leishmaniasis. World Health Organization, Geneva, Switzerland: http://www.who.int/mediacentre/factsheets/fs375/en/. [Google Scholar]
- 2.Sundar S. 2001. Drug resistance in Indian visceral leishmaniasis. Trop Med Int Health 6:849–854. doi: 10.1046/j.1365-3156.2001.00778.x. [DOI] [PubMed] [Google Scholar]
- 3.Legare D, Richard D, Mukhopadhyay R, Stierhof YD, Rosen BP, Haimeur A, Papadopoulou B, Ouellette M. 2001. The Leishmania ATP-binding cassette protein PGPA is an intracellular metal-thiol transporter ATPase. J Biol Chem 276:26301–26307. doi: 10.1074/jbc.M102351200. [DOI] [PubMed] [Google Scholar]
- 4.Moreira DS, Monte Neto RL, Andrade JM, Santi AM, Reis PG, Frézard F, Murta SM. 2013. Molecular characterization of the MRPA transporter and antimony uptake in four New World Leishmania spp. susceptible and resistant to antimony. Int J Parasitol Drugs Drug Resist 3:143–153. doi: 10.1016/j.ijpddr.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manzano JI, García-Hernandez R, Castanys S, Gamarro F. 2013. A new ABC half-transporter in Leishmania major is involved in resistance to antimony. Antimicrob Agents Chemother 57:3719–3730. doi: 10.1128/AAC.00211-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leprohon P, Legare D, Ouellette M. 2009. Intracellular localization of the ABCC proteins of Leishmania and their role in resistance to antimonials. Antimicrob Agents Chemother 53:2646–2649. doi: 10.1128/AAC.01474-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frezard F, Demicheli C, Ferreira CS, Costa MA. 2001. Glutathione-induced conversion of pentavalent antimony to trivalent antimony in meglumine antimoniate. Antimicrob Agents Chemother 45:913–916. doi: 10.1128/AAC.45.3.913-916.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denton H, McGregor JC, Coombs GH. 2004. Reduction of anti-leishmanial pentavalent antimonial drugs by a parasite-specific thiol-dependent reductase, TDR1. Biochem J 381:405–412. doi: 10.1042/BJ20040283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou Y, Messier N, Ouellette M, Rosen BP, Mukhopadhyay R. 2004. Leishmania major LmACR2 is a pentavalent antimony reductase that confers sensitivity to the drug pentostam. J Biol Chem 279:37445–37451. doi: 10.1074/jbc.M404383200. [DOI] [PubMed] [Google Scholar]
- 10.Campos-Salinas J, León-Guerrero D, González-Rey E, Delgado M, Castanys S, Pérez-Victoria JM, Gamarro F. 2013. LABCG2, a new ABC transporter implicated in phosphatidylserine exposure, is involved in the infectivity and pathogenicity of Leishmania. PLoS Negl Trop Dis 7:e2179. doi: 10.1371/journal.pntd.0002179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imbert L, Ramos RG, Libong D, Abreu S, Loiseau PM, Chaminade P. 2012. Identification of phospholipid species affected by miltefosine action in Leishmania donovani cultures using LC-ELSD, LC-ESI/MS, and multivariate data analysis. Anal Bioanal Chem 402:1169–1182. doi: 10.1007/s00216-011-5520-3. [DOI] [PubMed] [Google Scholar]
- 12.Weingärtner A, Kemmer G, Müller FD, Zampieri RA, Gonzaga dos Santos M, Schiller J, Pomorski TG. 2012. Leishmania promastigotes lack phosphatidylserine but bind annexin V upon permeabilization or miltefosine treatment. PLoS One 7:e42070. doi: 10.1371/journal.pone.0042070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castanys-Muñoz E, Pérez-Victoria JM, Gamarro F, Castanys S. 2008. Characterization of an ABCG-like transporter from the protozoan parasite Leishmania with a role in drug resistance and transbilayer lipid movement. Antimicrob Agents Chemother 52:3573–3579. doi: 10.1128/AAC.00587-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castanys-Muñoz E, Alder-Baerens N, Pomorski T, Gamarro F, Castanys S. 2007. A novel ATP-binding cassette transporter from Leishmania is involved in transport of phosphatidylcholine analogues and resistance to alkyl-phospholipids. Mol Microbiol 64:1141–1153. doi: 10.1111/j.1365-2958.2007.05653.x. [DOI] [PubMed] [Google Scholar]
- 15.Pérez-Victoria FJ, Gamarro F, Ouellette M, Castanys S. 2003. Functional cloning of the miltefosine transporter. A novel P-type phospholipid translocase from Leishmania involved in drug resistance. J Biol Chem 278:49965–49971. [DOI] [PubMed] [Google Scholar]
- 16.Kennedy ML, Cortes-Selva F, Pérez-Victoria JM, Jiménez IA, González AG, Muñoz OM, Gamarro F, Castanys S, Ravelo AG. 2001. Chemosensitization of a multidrug-resistant Leishmania tropica line by new sesquiterpenes from Maytenus magellanica and Maytenus chubutensis. J Med Chem 44:4668–4676. doi: 10.1021/jm010970c. [DOI] [PubMed] [Google Scholar]
- 17.Manzano JI, Lecerf-Schmidt F, Lespinasse MA, Di Pietro A, Castanys S, Boumendjel A, Gamarro F. 2014. Identification of specific reversal agents for Leishmania ABCI4-mediated antimony resistance by flavonoid and trolox derivative screening. J Antimicrob Chemother 69:664–672. doi: 10.1093/jac/dkt407. [DOI] [PubMed] [Google Scholar]
- 18.Sánchez-Cañete MP, Carvalho L, Pérez-Victoria FJ, Gamarro F, Castanys S. 2009. Low plasma membrane expression of the miltefosine transport complex renders Leishmania braziliensis refractory to the drug. Antimicrob Agents Chemother 53:1305–1313. doi: 10.1128/AAC.01694-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brochu C, Wang J, Roy G, Messier N, Wang XY, Saravia NG, Ouellette M. 2003. Antimony uptake systems in the protozoan parasite Leishmania and accumulation differences in antimony-resistant parasites. Antimicrob Agents Chemother 47:3073–3079. doi: 10.1128/AAC.47.10.3073-3079.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhandari V, Sundar S, Dujardin JC, Salotra P. 2014. Elucidation of cellular mechanisms involved in experimental paromomycin resistance in Leishmania donovani. Antimicrob Agents Chemother 58:2580–2585. doi: 10.1128/AAC.01574-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sauvage V, Aubert D, Escotte-Binet S, Villena I. 2009. The role of ATP-binding cassette (ABC) proteins in protozoan parasites. Mol Biochem Parasitol 167:81–94. doi: 10.1016/j.molbiopara.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Woehlecke H, Pohl A, Alder-Baerens N, Lage H, Herrmann A. 2003. Enhanced exposure of phosphatidylserine in human gastric carcinoma cells overexpressing the half-size ABC transporter BCRP (ABCG2). Biochem J 376:489–495. doi: 10.1042/bj20030886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Polgar O, Robey RW, Bates SE. 2008. ABCG2: structure, function and role in drug response. Expert Opin Drug Metab Toxicol 4:1–15. doi: 10.1517/17425255.4.1.1. [DOI] [PubMed] [Google Scholar]
- 24.Leslie EM, Deeley RG, Cole SP. 2005. Multidrug resistance proteins: role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol Appl Pharmacol 204:216–237. doi: 10.1016/j.taap.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 25.Ubeda JM, Legare D, Raymond F, Ouameur AA, Boisvert S, Rigault P, Corbeil J, Tremblay MJ, Olivier M, Papadopoulou B, Ouellette M. 2008. Modulation of gene expression in drug resistant Leishmania is associated with gene amplification, gene deletion and chromosome aneuploidy. Genome Biol 9:R115. doi: 10.1186/gb-2008-9-7-r115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leprohon P, Legare D, Raymond F, Madore E, Hardiman G, Corbeil J, Ouellette M. 2009. Gene expression modulation is associated with gene amplification, supernumerary chromosomes and chromosome loss in antimony-resistant Leishmania infantum. Nucleic Acids Res 37:1387–1399. doi: 10.1093/nar/gkn1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leprohon P, Fernandez-Prada C, Gazanion E, Monte-Neto R, Ouellette M. 2015. Drug resistance analysis by next generation sequencing in Leishmania. Int J Parasitol Drugs Drug Resist 5:26–35. doi: 10.1016/j.ijpddr.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ouellette M, Drummelsmith J, Papadopoulou B. 2004. Leishmaniasis: drugs in the clinic, resistance and new developments. Drug Resist Updat 7:257–266. doi: 10.1016/j.drup.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Coelho AC, Beverly SM, Cotrim PC. 2003. Functional genetic identification of PRP1, an ABC transporter superfamily member conferring pentamidine resistance in Leishmania major. Mol Biochem Parasitol 130:83–90. doi: 10.1016/S0166-6851(03)00162-2. [DOI] [PubMed] [Google Scholar]
- 30.Ashutosh S, Sundar S, Goyal N. 2007. Molecular mechanisms of antimony resistance in Leishmania. J Med Microbiol 56:143–153. doi: 10.1099/jmm.0.46841-0. [DOI] [PubMed] [Google Scholar]
- 31.Uzcategui NL, Zhou Y, Figarella K, Ye J, Mukhopadhyay R, Bhattacharjee H. 2008. Alteration in glycerol and metalloid permeability by a single mutation in the extracellular C-loop of Leishmania major aquaglyceroporin LmAQP1. Mol Microbiol 70:1477–1486. doi: 10.1111/j.1365-2958.2008.06494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gourbal B, Sonuc N, Bhattacharjee H, Legare D, Sundar S, Ouellette M, Rosen BP, Mukhopadhyay R. 2004. Drug uptake and modulation of drug resistance in Leishmania by an aquaglyceroporin. J Biol Chem 279:31010–31017. doi: 10.1074/jbc.M403959200. [DOI] [PubMed] [Google Scholar]
- 33.Marquis N, Gourbal B, Rosen BP, Mukhopadhyay R, Ouellette M. 2005. Modulation in aquaglyceroporin AQP1 gene transcript levels in drug-resistant Leishmania. Mol Microbiol 57:1690–1699. doi: 10.1111/j.1365-2958.2005.04782.x. [DOI] [PubMed] [Google Scholar]
- 34.Maciaszczyk-Dziubinska E, Wawrzycka D, Wysocki R. 2012. Arsenic and antimony transporters in eukaryotes. Int J Mol Sci 13:3527–3548. doi: 10.3390/ijms13033527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Callahan HL, Roberts WL, Rainey PM, Beverley SM. 1994. The PGPA gene of Leishmania major mediates antimony (SbIII) resistance by decreasing influx and not by increasing efflux. Mol Biochem Parasitol 68:145–149. doi: 10.1016/0166-6851(94)00154-5. [DOI] [PubMed] [Google Scholar]
- 36.Messaritakis I, Christodoulou V, Mazeris A, Koutala E, Vlahou A, Papadogiorgaki S, Antoniou M. 2013. Drug resistance in natural isolates of Leishmania donovani s.l. promastigotes is dependent of Pgp170 expression. PLoS One 8:e65467. doi: 10.1371/journal.pone.0065467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rai S, Bhaskar, Goel SK, Nath Dwivedi U, Sundar S, Goyal N. 2013. Role of efflux pumps and intracellular thiols in natural antimony resistant isolates of Leishmania donovani. PLoS One 8:e74862. doi: 10.1371/journal.pone.0074862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim DY, Bovet L, Maeshima M, Martinoia E, Lee Y. 2007. The ABC transporter AtPDR8 is a cadmium extrusion pump conferring heavy metal resistance. Plant J 50:207–218. doi: 10.1111/j.1365-313X.2007.03044.x. [DOI] [PubMed] [Google Scholar]
- 39.Nuruzzaman M, Zhang R, Cao HZ, Luo ZY. 2014. Plant pleiotropic drug resistance transporters: transport mechanism, gene expression, and function. J Integr Plant Biol 56:729–740. doi: 10.1111/jipb.12196. [DOI] [PubMed] [Google Scholar]
- 40.Oda K, Otani M, Uraguchi S, Akihiro T, Fujiwara T. 2011. Rice ABCG43 is Cd inducible and confers Cd tolerance on yeast. Biosci Biotechnol Biochem 75:1211–1213. doi: 10.1271/bbb.110193. [DOI] [PubMed] [Google Scholar]
- 41.Mukhopadhyay R, Dey S, Xu N, Gage D, Lightbody J, Ouellette M, Rosen BP. 1996. Trypanothione overproduction and resistance to antimonials and arsenicals in Leishmania. Proc Natl Acad Sci U S A 93:10383–10387. doi: 10.1073/pnas.93.19.10383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li ZS, Lu YP, Zhen RG, Szczypka M, Thiele DJ, Rea PA. 1997. A new pathway for vacuolar cadmium sequestration in Saccharomyces cerevisiae: YCF1-catalyzed transport of bis(glutathionato)cadmium. Proc Natl Acad Sci U S A 94:42–47. doi: 10.1073/pnas.94.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ortiz DF, Ruscitti T, McCue KF, Ow DW. 1995. Transport of metal-binding peptides by HMT1, a fission yeast ABC-type vacuolar membrane protein. J Biol Chem 270:4721–4728. doi: 10.1074/jbc.270.9.4721. [DOI] [PubMed] [Google Scholar]
- 44.Krzyzanowski D, Bartosz G, Grzelak A. 2014. Collateral sensitivity: ABCG2-overexpressing cells are more vulnerable to oxidative stress. Free Radic Biol Med 76:47–52. doi: 10.1016/j.freeradbiomed.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 45.Brechbuhl HM, Gould N, Kachadourian R, Riekhof WR, Voelker DR, Day BJ. 2010. Glutathione transport is a unique function of the ATP-binding cassette protein ABCG2. J Biol Chem 285:16582–16587. doi: 10.1074/jbc.M109.090506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gauthier C, Ozvegy-Laczka C, Szakacs G, Sarkadi B, Di Pietro A. 2013. ABCG2 is not able to catalyze glutathione efflux and does not contribute to GSH-dependent collateral sensitivity. Front Pharmacol 4:138. doi: 10.3389/fphar.2013.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trompier D, Chang XB, Barattin R, du Moulinet D'Hardemare A, Di Pietro A, Baubichon-Cortay H. 2004. Verapamil and its derivative trigger apoptosis through glutathione extrusion by multidrug resistance protein MRP1. Cancer Res 64:4950–4956. doi: 10.1158/0008-5472.CAN-04-0143. [DOI] [PubMed] [Google Scholar]