Abstract
Chagas disease is an important public health problem in Latin America, and its treatment by chemotherapy with benznidazole (BZ) or nifurtimox remains unsatisfactory. In order to design new alternative strategies to improve the current etiological treatments, in the present work, we comprehensively evaluated the in vitro and in vivo anti-Trypanosoma cruzi effects of clomipramine (CMP) (a parasite-trypanothione reductase-specific inhibitor) combined with BZ. In vitro studies, carried out using a checkerboard technique on trypomastigotes (T. cruzi strain Tulahuen), revealed a combination index (CI) of 0.375, indicative of a synergistic effect of the drug combination. This result was correlated with the data obtained in infected BALB/c mice. We observed that during the acute phase (15 days postinfection [dpi]), BZ at 25 mg/kg of body weight/day alone decreased the levels of parasitemia compared with those of the control group, but when BZ was administered with CMP, the drug combination completely suppressed the parasitemia due to the observed synergistic effect. Furthermore, in the chronic phase (90 dpi), mice treated with both drugs showed less heart damage as assessed by the histopathological analysis, index of myocardial inflammation, and levels of heart injury biochemical markers than mice treated with BZ alone at the reference dose (100 mg/kg/day). Collectively, these data support the notion that CMP combined with low doses of BZ diminishes cardiac damage and inflammation during the chronic phase of cardiomyopathy. The synergistic activity of BZ-CMP clearly suggests a potential drug combination for Chagas disease treatment, which would allow a reduction of the effective dose of BZ and an increase in therapeutic safety.
INTRODUCTION
Chagas disease is a neglected disease caused by the protozoan parasite Trypanosoma cruzi, and it represents a significant public health burden in Latin America. It is estimated that >8 million people are infected worldwide and >300,000 new cases are diagnosed every year (1–4). This tropical disease has become a serious global public health problem due to migrations to countries of nonendemicity and the resurgence of the disease in areas of endemicity (5, 6). To date, an efficient and safe chemotherapy regimen for the treatment of Chagas disease is not available, and an effective prophylactic vaccine has yet to be developed.
The disease is a complex condition resulting from the successful establishment of T. cruzi in key host tissues. It develops from an initial acute phase that lasts 2 to 3 months and is characterized by circulating parasites that are detectable in the bloodstream (6–8). The infection then enters the chronic phase and, without successful treatment, becomes permanent. Several years or even decades after the initial infection, approximately 30% to 40% of all infected individuals develop a chronic inflammatory disease that primarily affects the heart tissue (6, 8, 9). Chagasic myocarditis is the most common form of nonischemic cardiomyopathy worldwide and the most expressive manifestation of the disease because of its frequency and severity (8).
The available drugs with proven in vitro and in vivo efficacy against T. cruzi are nifurtimox (NFX) (Lampit; Bayer) and benznidazole (BZ) (Rochagan and Radanil; Hoffman-La Roche), developed >50 years ago. BZ distribution was discontinued in 2011, and it is currently manufactured and distributed by Maprimed and ELEA Laboratories (Abarax) in Argentina (10–13). Both drugs have significant activity in the acute phase, with a 60% to 80% parasitological cure rate (13, 14). Despite their long history in the treatment of Chagas disease, both NFX and BZ have frequent side effects, especially in adults.
The main side effect of BZ is hepatic intolerance or hepatotoxicity, usually concomitant with hypersensitivity reactions at the beginning of treatment, and medullar toxicity and peripheral neuropathies at the end of treatment (15–19).
Since the 1980s, the WHO has included both BZ and NFX on the Model Lists of Essential Medicines (http://www.who.int/medicines/publications/essentialmedicines/en/). However, it is important to point out that neither drug has not been fully validated by evidence-based medicine. These drugs are used but not completely recommended by the U.S. Food and Drug Administration or the European Medicines Agency (6). Reports about the differential toxicities of the two drugs are controversial; nevertheless, it has been proposed that BZ is usually better tolerated than NFX (6, 18).
As a consequence, chemotherapy with trypanocidal agents remains unsatisfactory, due to the significant limitations of the currently available drugs and the multiple side effects associated with therapy, which frequently result in noncompliance with long-term treatment, particularly in the treatment of chronic patients (8, 9).
Considering the limitations of currently available etiological treatments, the development of novel strategies employing new formulations and/or combinations of existing drugs is an alternative to be considered (20).
In the last 2 decades, new attempts to improve chemotherapy treatments for Chagas disease have been made, specifically directed to different parasite targets, such as triazole derivatives, squalene synthase inhibitors, and cysteine protease inhibitors, among other compounds (12, 21). In particular, clomipramine (CMP), a tricyclic antidepressant, inhibits trypanothione reductase activity and has evidenced antitrypanocidal activity when used alone (22, 49) or in combination with BZ (23). Furthermore, considering the possible existence of resistant parasite strains and more than one strain in the same patient, combined treatment with two drugs bearing different mechanisms of action might be a strategy to improve the pharmacotherapy of Chagas disease (14, 23, 24).
Given the need for a new safe and effective pharmacotherapeutic treatment for Chagas disease, the aim of this work was to evaluate the in vitro trypanocidal activity of the combination of CMP and BZ by means of assessment in the form of a checkerboard study. We found that CMP exerted a parasiticidal synergistic effect with BZ. The in vitro studies allowed us to propose approximate doses for in vivo treatment of infected BALB/c mice. The results reveal that CMP in combination with lower doses of BZ diminishes cardiac damage and inflammation during chronic cardiomyopathy.
MATERIALS AND METHODS
Materials. (i) Drugs.
The drugs used were CMP (hydrochloride) (Parafarm; USP grade, Buenos Aires, Argentina) and BZ (Radanil; Hoffman-La Roche, Argentina). This 2-nitroimidazole was extracted and purified from commercial available tablets, according to the following details. One hundred tablets were crushed in a mortar. The powder obtained was transferred into a glass beaker and dispersed with 2 ml of ethanol per tablet (Anedra) under soft controlled heating conditions (40 to 45°C) and constant stirring for 30 min. After that, it was filtered, the remaining solid was discarded, and BZ was obtained from the leaked liquid by recrystallization upon addition of 200 ml of cold distilled water and under an ice bath. The solid obtained by filtration under a vacuum was dried in an oven at 45°C until it reached constant weight. The solid BZ was stored at room temperature in well-closed light-resistant glass containers, and samples were subjected to identification and purity analysis. The crystalline solid obtained had a purity of 99.2% ± 0.5% and yield of 87% ± 2%, as determined spectrophotometrically, and it showed a melting temperature of 190.2 to 191.2°C, which was evaluated by thermal analysis.
(ii) Animals.
Three-month-old male BALB/c mice weighing 24 ± 3 g were purchased from the Universidad Nacional de La Plata (Argentina) and housed in the animal facility of the Centro de Investigaciones en Bioquímica Clínica e Inmunología (CIBICI)-National Scientific and Technical Research Council (CONICET) (Office of Laboratory Animal Welfare [OLAW] assurance no. A5802-01). The animals had ad libitum access to water and feed in all experiments. All animal experiments and procedures were carried out according to the guidelines of the Committee for Animal Care and Use of the Facultad de Ciencias Químicas, Universidad Nacional de Córdoba, Argentina (ethics committee approval no. HCD 753/14), in strict accordance with the recommendations of the Guide to the Care and Use of Experimental Animals published by the Canadian Council on Animal Care (http://www.ccac.ca/en_/standards/guidelines#application).
In vitro assays. (i) Mammalian cell cultures and parasites.
Vero cell monolayers were infected with trypomastigote forms of T. cruzi strain Tulahuen and maintained in RPMI medium (Gibco Invitrogen Corporation) at 37°C in a 5% CO2 atmosphere.
The parasites were collected from the supernatant of infected cells and harvested by centrifugation at 4,400 rpm for 5 min.
The pellet of parasites was resuspended in Dulbecco's modified Eagle's medium (DMEM; Gibco Invitrogen Corporation) and counted by direct microscopic observation using a Neubauer chamber.
(ii) Checkerboard test.
Trypomastigotes (1 × 106 parasites/ml) were placed in DMEM supplemented with 10% fetal bovine serum (FBS; Gibco Invitrogen Corporation) (pH 7.4), 2 mM l-glutamine (Gibco; Life Technologies), and 50 mg/liter gentamicin in 96-well plates. Then, 20 μl of peripheral blood was added to each well in the presence or absence of increasing concentrations of BZ and CMP (range of concentrations, 0 to 64 μg/ml). The assay was screened in triplicate. The drugs were dissolved in dimethyl sulfoxide (DMSO) and DMEM. The final concentration of DMSO did not exceed 1%.
After 24 h at 37°C in humidified 5% CO2 atmosphere (25), the parasites were counted by direct microscopic observation using a Neubauer hemocytometer previously incubated with lysis buffer. Parasite viability was examined by observation of their mobility under an inverted microscope (Zeiss Axio Vert 25, D-07740; Jena, Germany).
The effect of combining BZ and CMP was analyzed by isobologram and the combination index (CI) proposed by Chou and Taladay (26) and reviewed by Zhao et al. (27). According to these authors, a synergistic effect is defined as an effect produced by a combination of drugs that is greater than the sum of the effects produced by each drug alone, represented by a CI of <1 (26). The graphical interpretation by isobolographic analysis was also used to evaluate the pharmacological interaction between BZ and CMP. A synergistic effect is evidenced when lower concentrations of two drugs in combination provide the same effect as higher concentrations of the drugs alone, and when they are plotted in an isobologram, the resulting curve is located below the line of additivity (27).
(iii) Antiamastigote activity.
To obtain bone marrow-derived macrophages (BMDM), intact femurs and tibias were aseptically dislocated from the hind legs of the mice. The marrow was flushed with 5 ml of phosphate-buffered saline (PBS) using a 25-gauge sterile needle. After filtration, bone marrow cells were centrifuged for 5 min at 2,300 rpm. Briefly, the cells were cultured in growth medium supplemented with 13% supernatant of the mouse L929 cell line (conditioned medium) for 7 days. For cell culture, 150,000 BMDM were infected with T. cruzi at a 1:5 host-to-parasite cell ratio for 24 h and then washed and subjected to treatment designated BZ 8 (8 μg/ml), BZ 2 (2 μg/ml), or BZ 2 plus CMP 2 (2 μg/ml each) or maintained in medium for 48 h. The number of amastigotes/100 BMDM was calculated by use of immunofluorescence assays (28).
In vivo assays in the acute phase of T. cruzi infection.
BALB/c mice were infected intraperitoneally with 103 bloodstream trypomastigotes of T. cruzi (Tulahuen strain). For this experimental model, the survival rate was 100%.
For all treatment schedules, the drugs were administered by oral gavage for 14 days, with the first dose 24 h after infection. To administer the solid drug, doses were dispersed into 30 μl of sterilized water just before oral administration. For acute-phase studies, the animals were analyzed at 15 days postinfection (dpi). The animals were divided into groups of 6, according to the different experimental designs. Noninfected animals (NI) and infected/nontreated mice (INT) were used as a control.
(i) First experimental design.
To carry out the first experimental design assay, the animals were divided in 10 groups: NI, INT, those infected and treated with BZ alone at 100, 50, 25, and 12.5 mg/kg of body weight per day (BZ, BZ 50, BZ 25, and BZ 12.5, respectively), and those infected and treated with BZ at 100, 50, 25, and 12.5 mg/kg of body weight per day in combination with CMP at a fixed dose of 7.5 mg/kg of body weight per day (BZ 100 + CMP 7.5, BZ 50 + CMP 7.5, BZ 25 + CMP 7.5, and BZ 12.5 + CMP 7.5, respectively).
(ii) Second experimental design.
The animals were divided into seven groups: NI, INT, and those infected and treated with CMP at 12.5, 10, 7.5, 5, and 2.5 mg/kg of body weight per day in combination with BZ at a fixed dose of 25 mg/kg of body weight per day (BZ 25 + CMP 12.5, BZ 25 + CMP 10, BZ 25 + CMP 7.5, BZ 25 + CMP 5, and BZ 25 + CMP 2.5, respectively).
In both designs, the animals were sacrificed at 15 dpi.
In vivo assay in T. cruzi chronic infection.
The animals were divided into the following groups: NI, those infected and nontreated (INT-CCD), those infected and treated with BZ alone at 100 mg/kg of body weight per day (BZ-CCD), and those treated with BZ at 25 mg/kg of body weight per day in combination with CMP at 7.5 mg/kg of body weight per day (BZ-CMP-CCD). The animals were infected, and the administration was made in the same way as detailed in “In vivo assays in the acute phase of T. cruzi infection,” above.
After 14 days of drug administration, the animals were kept and supervised in-house in the animal facility until 90 dpi.
Hepatotoxicity in vivo assay.
The animals were divided into the following groups: untreated/healthy animals (control), those treated with BZ alone at 100 mg/kg of body weight per day (BZ-H), and those treated with BZ at 25 mg/kg of body weight per day in combination with CMP at 7.5 mg/kg of body weight per day (BZ-CMP-H).
The animals were treated by 14 days of oral gavage administration of the drugs, which were dispersed into 30 μl of sterilized water just before oral administration.
Parasitemia and survival rate analysis.
The animals were anesthetized with isoflurane (Forane; Abbott, fractioned in Argentina), and peripheral blood was extracted by intracardiac puncture. After 10 min with lysis buffer, the parasites were counted by direct microscopic observation in a Neubauer chamber. The survival of the mice was monitored every day.
Recording of weight of relevant organs.
At the indicated time points, infected mice were perfused with cold PBS (Gibco, Invitrogen) and livers, hearts, and spleens were weighed.
Tissue injury biochemical markers.
Samples of blood were centrifuged at 2,500 rpm for 5 min, and the plasma was analyzed in order to determine the activities of glutamate oxaloacetate transaminase (GOT) and glutamate pyruvate transaminase (GPT) as liver injury biomarkers (16). On the other hand, creatine phosphokinase (total CK) and creatine phosphokinase myocardial band isoform MB (CK-MB) as a cardiac injury marker were tested (29). These trials were outsourced to Biocon Laboratory (Córdoba, Argentina).
Heart histology and inflammatory index.
Hearts were obtained from T. cruzi-infected, treated and nontreated, and noninfected mice, fixed in 10% buffered formalin, and embedded in paraffin. Five-micrometer-thick sections were stained with hematoxylin and eosin. Photographs were taken using a Nikon Eclipse TE2000-U inverted microscope using a 40× objective. Inflammation was evaluated semiquantitatively on low-power microscopic examination, according to the distribution and extent of inflammatory cells (focal, confluent, or diffuse) in epicardium and myocardium (1+ for a single inflammatory foci, 2+ multiple nonconfluent foci of inflammatory infiltrate, 3+ for confluent inflammation, and 4+ for diffuse inflammation extended throughout the section) (30, 31). Furthermore, it also assessed enlarged endothelial cells, perivascular edema, and disrupted and necrotic myocardial fibers. The numerical sum for each heart (n = 4) section represented an estimate of the inflammation index.
Parasite load.
For determination of tissue parasitism, genomic DNA was purified from hearts of infected mice at 90 dpi using TRIzol reagent, according to the manufacturer's instructions. Satellite DNA from T. cruzi (GenBank accession no. AY520036) was quantified by real-time PCR using specific custom TaqMan gene expression assay (Applied Biosystems) using the primer and probe sequences described by Piron et al. (50). A sample containing 2 μg of genomic DNA was amplified. An abundance of satellite DNA from T. cruzi was normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) abundance (TaqMan rodent GAPDH control reagent; Applied Biosystems) and expressed in arbitrary units.
Statistical analysis.
The statistical significance of the comparisons of mean values was assessed by a two-tailed Student t test and two-way analysis of variance (ANOVA), followed by Bonferroni's posttest using the GraphPad software. A P value of <0.05 was considered significant.
RESULTS
In vitro assay by checkerboard test.
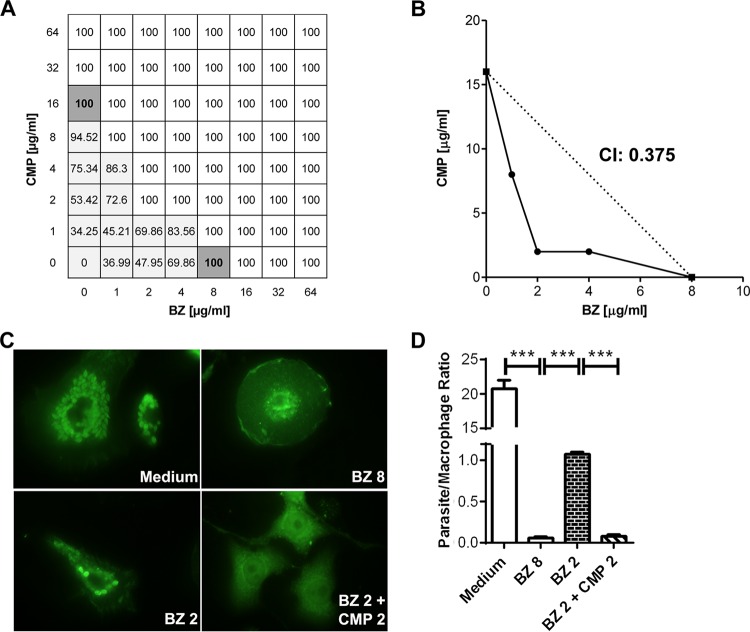
In order to determine whether the combination of CMP and BZ had a synergistic effect on the survival of the infective parasite form in vitro, we developed a checkerboard test according to the scheme described in Fig. 1A. The parasite survival rate was evaluated by counting the live trypomastigotes after 24 h of culture in a medium containing murine peripheral blood. The combinations of drug concentrations that achieved 100% parasite death were further plotted on an isobologram and were used to calculate the CI. The isobolographic analysis and the CI of 0.375 revealed that combination of BZ and CMP had a synergistic effect (Fig. 1B). Antiamastigote activity was evaluated in infected murine BMDM exposed to BZ 8, BZ 2, or BZ 2 + CMP 2 or maintained in medium alone. A significant inhibition of parasite growth was observed in BZ 2 + CMP 2 compared to that with BZ 2 (Fig. 1C and D).
FIG 1.
(A) Scheme of the checkerboard test employed. White wells show the combinations of drug concentrations that suppress parasitemia. Light gray wells show the combinations of drugs that reduced parasitemia levels, and dark gray wells show the minimal concentration of each drug that suppresses parasitemia by itself. (B) Isobologram of the combinations of CMP and BZ. The points below the dotted line indicate a synergistic effect. The calculated CI is also displayed. (C) Representative immunofluorescence images of infected BMDM treated with BZ at 8 µg/ml (BZ 8), BZ at 2 µg/ml (BZ 2), or BZ and CMP at 2 µg/ml each (BZ 2 + CMP 2). (D) Quantitative analysis of antiamastigote activity. The error bars show the standard error of the mean. ***, P < 0.001.
These results indicate that CMP might improve the effectiveness of BZ, and thus, the therapeutic combination of the two drugs might potentiate lower doses of BZ with a concomitant diminution of its collateral effects.
In vivo studies in the acute phase of T. cruzi infection.
Considering the effective-trypanocidal concentration range obtained from in vitro studies, we took into account the relative/oral bioavailability reported for BZ (>90%) (32) and CMP (24.8 to 29.7%) (33) in animal models, as well as the mouse total volume of circulating blood (55 to 80 ml/kg of body weight; average, 1.5 ml) to estimate the dose ranges of each drug to be orally administered in the in vivo experiments.
First experimental design.
To determine the in vivo effect of the BZ-CMP combination, different groups of mice received various doses of BZ, alone or in combination with fixed doses of CMP (7.5 mg/kg/day), daily by oral gavage for 14 days starting 24 h after infection. Infected and nontreated (INT) mice receiving drinking water by oral gavage were used as controls. At 15 days postinfection (dpi), we evaluated the parasitemia, spleen and heart weights, and the proportional activity of the creatine kinase MB isoenzyme (CK-MB)/total creatine kinase (total CK) in plasma, which is a highly sensitive and specific biochemical marker for acute heart injury (29). All groups showed 100% survival under the evaluated conditions. It was observed that the therapeutic dose of BZ currently used in the Chagas mouse model (100 mg/kg/day) (5, 23) and BZ at 50 mg/kg/day were able to override the parasitemia. As was expected, BZ at 25 mg/kg/day and 12.5 mg/kg/day only decreased the parasitemia levels (P < 0.001) relative to those of the INT group. Interestingly, it was observed that BZ at 25 mg/kg/day in combination with CMP at 7.5 mg/kg/day was able to override the parasitemia. In addition, BZ at 12.5 mg/kg/day in combination with CMP at 7.5 mg/kg/day was able to significantly decrease parasitemia levels (P < 0.001) in comparison with the same dose of BZ alone (Fig. 2).
FIG 2.
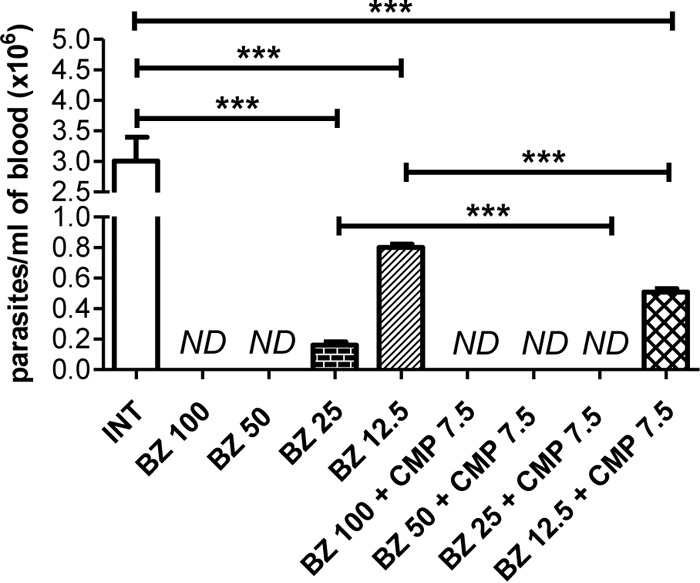
Effects of BZ alone and in combination with CMP, at a fixed dose, on parasitemia in infected mice. The values were obtained at 15 dpi. Asterisks indicate a significant difference between the indicated groups (***, P < 0.001). ND, parasites not detected.
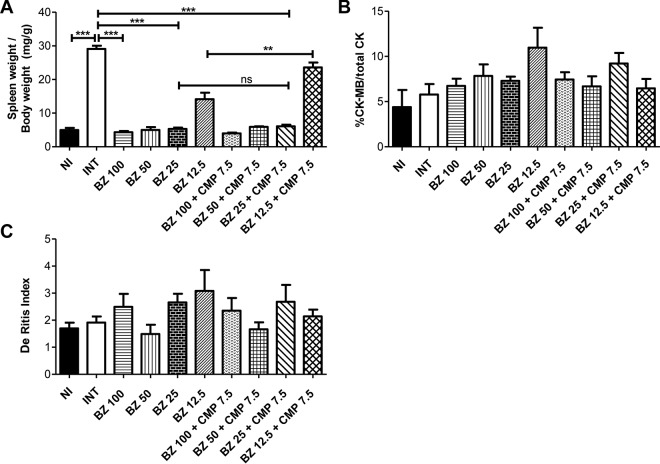
As expected, the relative spleen weight was higher in the INT mouse group than in the NI group (P < 0.001). Furthermore, treatment with BZ at 100 mg/kg/day, 50 mg/kg/day, and 25 mg/kg/day, alone and in combination with CMP (7.5 mg/kg/day), induced a decrease in relative spleen weight compared to that with the INT group (P < 0.001) (Fig. 3A).
FIG 3.
Effects of BZ alone and in combination with CMP at a fixed dose in infected mice at 15 dpi. (A) Relative spleen weight. ***, P < 0.001; **, P < 0.01; ns, no significant difference. (B) Relative percentage of CK-MB enzyme with respect to total CK in plasma. The differences between groups were not statistically significant. (C) De Ritis index. The differences between groups were not statistically significant. The error bars show the standard error of the mean.
There were no significant between-group differences in the ratio of plasma CK-MB to total CK enzyme activity (Fig. 3B). In the same way, the De Ritis indices (relationship between GOT and GPT) (34) were similar in all groups of animals (Fig. 3C).
Second experimental design.
In the second experimental design, we evaluated the same parameters in different groups of animals that received fixed doses of BZ (25 mg/kg/day) and various doses of CMP, according to the protocols described in the first experimental design.
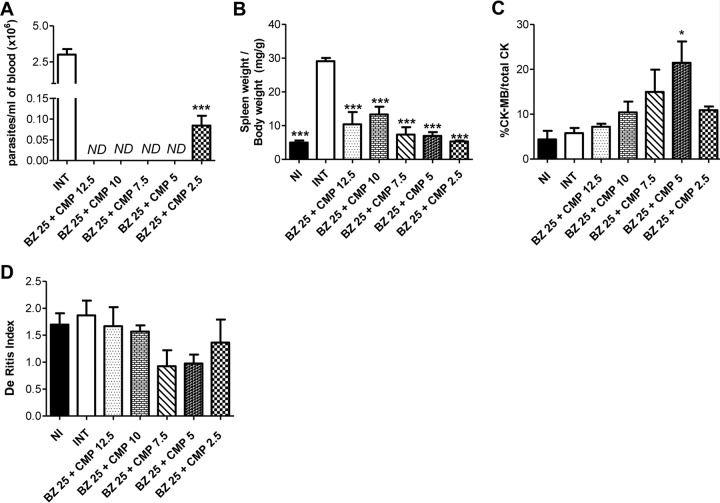
All groups of animals exhibited 100% survival. After 15 dpi, CMP at 12.5 mg/kg/day, 10 mg/kg/day, 7.5 mg/kg/day, or 5 mg/kg/day combined with BZ at 25 mg/kg/day was able to override the parasitemia. Furthermore, CMP at 2.5 mg/kg/day combined with the same doses of BZ was able to significantly decrease parasitemia levels (P < 0.001) relative to those of the INT group (Fig. 4A).
FIG 4.
Effects of BZ at a fixed dose in combination with CMP at different doses in infected mice at 15 dpi. (A) Parasitemia levels. Asterisks indicate a significant difference between the indicated group and the control (***, P < 0.001). ND, parasites not detected. (B) Relative spleen weight. Asterisks indicate the significant differences between the analyzed group and the control of infected/nontreated animals (INT) (***, P < 0.001). (C) Relative percentage of CK-MB enzyme in plasma. The asterisk indicates the significant differences between the tested group and INT (*, P < 0.05). (D) De Ritis index. The differences between groups were not statistically significant. The error bars show the standard error of the mean.
The INT group experienced a significant increase in relative spleen weight compared with that of the other groups, including the NI animals (P < 0.001) (Fig. 4B).
It is important to stress that the CK-MB-to-total CK ratios were similar in most groups, except in the BZ 25 mg/kg/day + CMP 5 mg/kg/day group, which showed a higher CKMB-to-total CK ratio than the INT group (P < 0.05) (Fig. 4C). On the other hand, the De Ritis indices were similar in all groups (Fig. 4D).
In vivo assay in the chronic phase of T. cruzi infection.
Considering the results obtained with the first and second experimental designs in the early stages of infection, we chose the combination of BZ at 25 mg/kg/day and CMP at 7.5 mg/kg/day (BZ-CMP-CCD) and compared it with BZ alone (100 mg/kg/day) (BZ-CCD) to determine the impact of the combination treatment in the chronic phase of the infection, according to the protocol described above. To this end, we analyzed the parasite load by real-time PCR, relative heart weight, heart histology and inflammatory index, and biochemical markers of heart injury at 90 dpi.
The relative heart weights in the BZ-CCD and BZ-CMP-CCD groups were not significantly different from those of the INT-CCD group. However, the relative heart weights of mice treated with BZ-CCD were significantly higher than those of BZ-CMP-CCD group of mice (P < 0.05) (Fig. 5A). In addition, it was observed that animals treated with BZ-CCD presented higher relative heart weights (P < 0.01) than either mice treated with the combination of drugs or NI mice (Fig. 5A). In accordance with these results, the relative percentage of CK-MB enzymatic activity was significantly higher in mice treated with BZ-CCD than that in the BZ-CMP-CCD, NI, and INT-CCD groups (P < 0.001, <0.01, and <0.05, respectively) (Fig. 5B).
FIG 5.
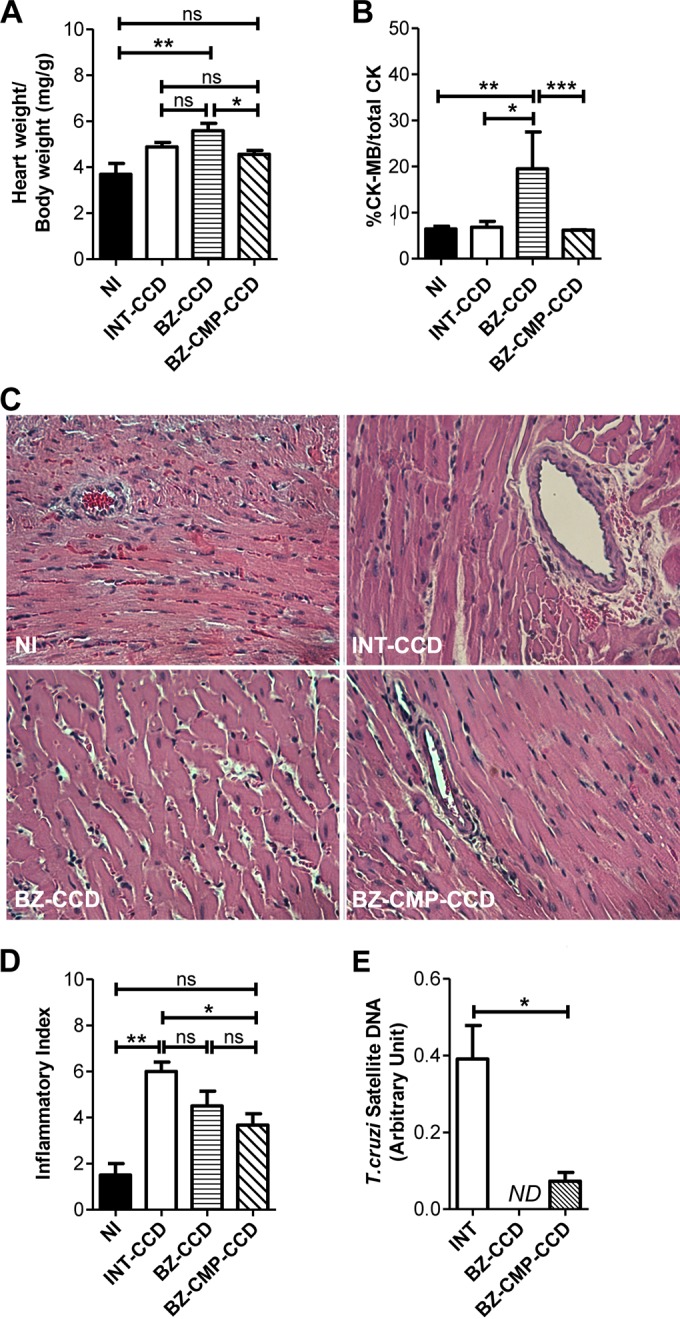
Effects of BZ alone or in combination with CMP in the experimental model of chronic Chagas disease (90 dpi). (A) Relative heart weight. (B) Relative percentage of CK-MB enzyme in plasma. (C) Representative histological sections of noninfected and infected heart tissue stained with hematoxylin and eosin (H&E) from mice treated with BZ alone or BZ-CMP (400× magnification). (D) Inflammatory index of myocardial tissue. The inflammatory index was calculated according to the details outlined in Materials and Methods. (E) Parasite DNA detected by real-time PCR in cardiac tissues. Asterisks indicate the significant differences between the indicated groups. ND, not detected. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, no significant difference.
Histopathological analysis of the heart sections showed that the severity of the inflammatory lesions observed (inflammatory foci in myocardium and epicardium, edema of blood vessels, and myocardial fiber disruption) was higher in INT sections than that observed in the treated groups (Fig. 5C). Hearts from mice treated with BZ alone or combined with CMP presented a small number of cardiac alterations, including isolated inflammatory infiltrates and edemas. There was no statistically significant difference between the INT-CCD group and the group of mice treated with BZ-CCD, and the INT-CCD group presented a higher inflammatory index than the NI group (P < 0.01). However, mice treated with BZ-CMP-CCD showed minor edema and less inflammatory infiltration than INT-CCD animals (P < 0.05), and there was no statistically significant difference between the BZ-CMP-CCD and NI groups (P > 0.05). In addition, there were no significant treatment-related changes in the inflammatory index (Fig. 5D).
The cardiac parasite burden was significantly higher in the INT-CCD group than in mice treated with the combination of BZ-CMP-CCD at 90 dpi (P < 0.05). In addition, mice treated with BZ-CCD showed no detectable levels of parasite DNA in heart tissue (Fig. 5E).
To summarize, the results show that a significant reduction in BZ concentration in combination with a low quantity of CMP might be a valid option for decreasing the parasite load, with fewer adverse effects.
In vivo hepatotoxicity assay.
In view of the results obtained with the first and second experimental designs in the acute phase, we evaluated the in vivo effect on the liver of BZ alone at 100 mg/kg/day (BZ-H) and at 25 mg/kg/day in combination with CMP at 7.5 mg/kg/day (BZ-CMP-H).
Drug treatments were administered by oral gavage to NI groups of mice for 14 days. The control group received water by oral gavage in place of drug treatment. We evaluated liver weight and plasma GPT levels, a specific liver injury biomarker (16).
There were no significant differences in relative liver weights between the BZ-H or BZ-CMP-H group and the control group (Fig. 6A).
FIG 6.
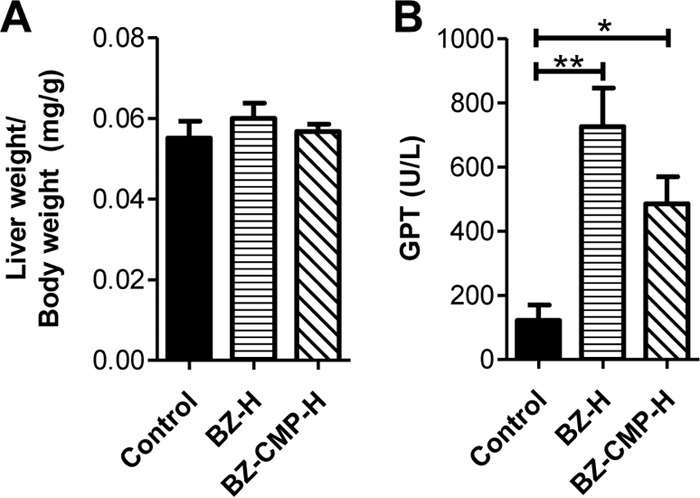
Effects of BZ alone and in combination with CMP in noninfected mice. (A) Relative liver weight. (B) Levels of GPT activity in plasma. *, P < 0.05; **, P < 0.01.
The mice in both treated groups presented higher GPT levels in plasma than the control group mice (P < 0.01 for BZ-H and P < 0.05 for BZ-CMP-H). Furthermore, BZ-H mice presented slightly higher levels of GPT than mice receiving BZ at a lower dose when combined with CMP (Fig. 6B).
DISCUSSION
In this study, we demonstrate that CMP synergizes BZ trypanocidal activity in vitro, and the combination of the two drugs at low doses ameliorates heart inflammation in experimental Chagas cardiomyopathy. In fact, treatment with BZ at 25 mg/kg/day in combination with CMP at 7.5 mg/kg/day for 14 days reduced the severity of infection and tissue lesions compared with those using the reference drug treatment (BZ at 100 mg/kg/day).
Chagas disease pharmacotherapy is based on two agents, NFX and BZ, which are recommended for all acute-stage, early chronic-stage, and reactivated cases (11–14). However, <1% of T. cruzi-infected persons have received any treatment, despite the current recommendations (35). Even when treatment with BZ is clinically recommended, the evidence-based medicine has not been fully validated, and its use in the chronic phase of the disease is still controversial (6). The effect of BZ treatment on patients with well-established Chagas cardiomyopathy was recently reported in a well-conducted BENEFIT trial (36). After 5 years of follow up, the results evidence that treatment with BZ is unlikely to have a major preventive effect on the progression of heart disease in patients with advanced Chagas infection. On the other hand, a high dropout rate has been shown for BZ treatment, especially during the chronic phase, due to the high incidence of side effects, which are usually more severe in adults than in children (14, 37). The development of new specific chemotherapeutic approaches to this disease has also been stalled for decades due to the controversy surrounding its pathogenesis. Although the role of T. cruzi in the pathology of the acute phase and the importance of etiological treatment at that stage have widely been accepted, several investigations have implicated autoimmune phenomena as a primary reason for persistent inflammation. As a consequence, the use of antiparasitic treatment in the chronic stage of Chagas disease was considered irrelevant (35, 38). Nowadays, there is a general consensus that parasite eradication must be a prerequisite for arresting the evolution of Chagas disease, and consequently, it should be treated primarily as an infectious, not an autoimmune, condition (8, 12, 39). For this reason, in the last 2 decades, new approaches focusing on parasite-specific targets to address this illness have been studied, and different strategies with the overall aim of finding a cure for Chagas disease are under investigation. In particular, we focused on trypanothione reductase, a specific enzyme of T. cruzi that performs vital functions in the parasite, and on CMP, which presents inhibitory effects on trypanothione reductase activity (40).
In our study, we employed the checkerboard technique to verify the potential synergistic effects of combination therapy using BZ and CMP on trypomastigote viability. The isobologram of this combination showed a synergic antitrypomastigote effect, evidenced by a CI of <1 (CI, 0.375). These data, together with the pharmacokinetic parameters of the drugs (32, 33) and mouse total volume of circulating blood, allow us to anticipate that BZ doses could be reduced to 25% of the clinically recommended concentration (100 mg/kg/day) when combined with 7.5 mg/kg/day of CMP. The checkerboard strategy was employed to test the capacities of other compounds to interact with BZ and whether these interactions could increase its trypanocidal activity (41, 42). The synergistic effect of combining BZ and CMP could be explained by considering the mechanism of action of each drug. Particularly, BZ produces free radicals and/or electrophilic metabolites and induces oxidative stress in the parasite (17). On the other hand, CMP inhibits trypanothione reductase (22, 40, 43), an essential component of the antioxidant defenses of T. cruzi. As a consequence, it is plausible to think that these mechanisms would work together against the parasite.
For the in vivo studies, we first selected different doses of BZ, alone or in combination with CMP at a fixed dose, to study efficacy and safety in the acute and chronic phases of experimental Chagas disease. The results obtained with the first experimental design allowed us to choose 25 mg/kg/day as an effective dose of BZ when combined with CMP at 7.5 mg/kg/day. This drug combination overrides parasitemia and induces a significant diminution of relative spleen weights in comparison with infected control mice (P < 0.001). Our results are in agreement with a previous work in which BZ at 50 mg/kg/day administered by the oral route was combined with the intraperitoneal administration of CMP at 5 mg/kg/day (23). It is important to highlight that the intraperitoneal route is rarely used for systemic drug administration in human medicine (44, 45).
In order to determine the minimal dose of CMP that can suppress parasitemia in combination with BZ at 25 mg/kg/day, we carried out the second experimental design, in which we assayed BZ at a fixed dose (25 mg/kg/day) in combination with different doses of CMP. The results obtained from this assay showed us that CMP at 5 mg/kg/day combined with BZ was able to completely suppress parasitemia. However, since this combination exhibited a higher CK-MB-to-total CK ratio, even higher than the corresponding ratio in the INT group, we selected the doses of 7.5 mg/kg/day of CMP and 25 mg/kg/day of BZ for subsequent assays (29).
Considering the side effects of BZ, in particular its hepatic intolerance and hepatotoxicity due to the inhibition of DNA and protein synthesis in hepatocytes (16, 42), we evaluated the toxicities of the two drugs at the selected doses. Even though the differences in the indicators measured (liver weight and plasma GPT levels) were not statistically significant, it is important to stress that both treatments showed a moderate increase in GPT levels compared with those in the control group receiving drinking water by gavage (INT). As expected, BZ alone produced liver alterations (16), while the presence of CMP seemed not to induce additional apparent injuries (23).
In order to determine the impact of the combined treatment in the chronic phase of infection (90 dpi), we administered the combination of drugs at the selected doses and compared the results with those of BZ alone (100 mg/kg/day), employing the same experimental model used for the acute-infection studies. Although the administration of combined drugs was effective at completely suppressing parasitemia, the results obtained by real-time PCR evidenced parasite DNA in the heart tissue of infected mice after CMP-BZ treatment. These results suggest that a longer treatment might be necessary to achieve the complete absence of parasites, as was observed in BZ-treated mice. Several therapeutic studies show PCR to be a useful tool to evaluate the early detection of treatment failure in either the acute or chronic phase of Chagas disease (46, 47). In this sense, our results emphasize the importance of evaluating the parasite load by real-time PCR as an accurate method to measure the impact of trypanocidal drugs throughout the treatment of Chagas disease.
Several studies have demonstrated that BZ chemotherapy produces a decrease in cardiac lesions and dysfunction during the chronic phase of T. cruzi infection (48). In this work, we demonstrate that treatment with CMP combined with lower doses of BZ diminishes cardiac inflammation and heart tissue damage in comparison with clinical doses of BZ.
In conclusion, the synergistic activity of BZ combined with CMP clearly evidences potential usefulness in the treatment of Chagas disease, which would allow the use of lower effective doses of BZ with increased safety. The therapeutic combination of existing drugs takes advantage of independent mechanisms of action that act in synergy in order to improve treatment.
ACKNOWLEDGMENTS
M. P. Aoki and A. F. Jimenez-Kairuz are staff researchers of CONICET.
M. C. García, L. M. Sanmarco, and N. E. Ponce thank CONICET for doctoral and postdoctoral fellowships. We thank the Coordinación Nacional de Control de Vectores and Ministerio de Salud de la Nación (filial Córdoba) for supplying the Radanil tablets used to do this work. We also thank Cinthia Stempin, David Rojas, and Ximena Volpini for assistance with the antiamastigote activity assay, and Pilar Crespo, Fabricio Navarro, Diego Lutti, Carolina Florit, Victoria Blanco, and Alejandra Romero for optimal technical assistance.
This work was supported by Agencia Nacional de Promoción Científica y Tecnológica – Fondo para la Investigación Científica y Tecnológica (grants PICT 2012-0173 and PICT 2013-2885), Consejo Nacional de Investigaciones Científicas y Técnicas de la República Argentina (grants PIP 11220120100620 and PIP 11220120100461), Secretaría de Ciencia y Tecnología, Universidad Nacional de Córdoba (grants 05/C642 and 05/C539), Secretaría de Ciencia y Tecnología, Ministerio de Ciencia y Tecnología de la Provincia de Córdoba (grant 1143/10).
Funding Statement
This work, including the efforts of Nicolás Eric Ponce, Liliana Maria Sanmarco, and Maria Pilar Aoki, was funded by Secretaría de Ciencia y Tecnología, Universidad Nacional de Córdoba (05/C642), Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT), Fondo para la Investigación Científica y Tecnológica (PICT 2013-2885), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) (PIP 11220120100620), and Ministerio de Ciencia y Tecnología, Gobierno de la Provincia de Córdoba, Secretaría de Ciencia y Tecnología (1143/10). This work, including the efforts of Mónica Cristina Garcia, Rubén Hilario Manzo, and Alvaro Federico Jimenez-Kairuz, was funded by Secretaría de Ciencia y Tecnología, Universidad Nacional de Córdoba (05/C539), Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT), Fondo para la Investigación Científica y Tecnológica (PICT 2012-0173), and Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) (PIP 11220120100461).
REFERENCES
- 1.World Health Organization. 2015. Chagas disease. World Health Organization, Geneva, Switzerland: http://www.who.int/tdr/research/ntd/chagas/en/. [Google Scholar]
- 2.Campi-Azevedo A, Gomes J, Teixeira-Carvalho A, Silveira-Lemos D, Vitelli-Avelar D, Sathler-Avelar R, Peruhype-Magalhães V, Béla S, Silvestre K, Batista M, Schachnik N, Correa-Oliveira R, Eloi-Santos S, Martins-Filho O. 2015. Etiological treatment of Chagas disease patients with benznidazole lead to a sustained pro-inflammatory profile counterbalanced by modulatory events. Immunobiology 220:564–574. doi: 10.1016/j.imbio.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Moncayo A, Silveira A. 2009. Current epidemiological trends for Chagas disease in Latin America and future challenges in epidemiology, surveillance and health policy. Mem Inst Oswaldo Cruz 104(Suppl 1):17–30. [DOI] [PubMed] [Google Scholar]
- 4.Schofield C, Jannin J, Salvatella R. 2006. The future of Chagas disease control. Trends Parasitol 22:583–588. doi: 10.1016/j.pt.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 5.Cencig S, Coltel N, Truyens C, Carlier Y. 2012. Evaluation of benznidazole treatment combined with nifurtimox, posaconazole or AmBisome in mice infected with Trypanosoma cruzi strains. Int J Antimicrob Agents 40:527–532. doi: 10.1016/j.ijantimicag.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Rassi AJ, Rassi A, Marin-Neto J. 2010. Chagas disease. Lancet 375:1388–1402. doi: 10.1016/S0140-6736(10)60061-X. [DOI] [PubMed] [Google Scholar]
- 7.Aoki M, Carrera-Silva E, Cuervo H, Fresno M, Gironès N, Gea S. 2012. Nonimmune cells contribute to crosstalk between immune cells and inflammatory mediators in the innate response to Trypanosoma cruzi infection. J Parasitol Res 2012:1–13. doi: 10.1155/2012/737324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coura J, Borges-Pereira J. 2012. Chagas disease. What is known and what should be improved: a systemic review. Rev Soc Bras Med Trop 45:286–296. [DOI] [PubMed] [Google Scholar]
- 9.Lo Presti M, Bazán P, Strauss M, Báez A, Rivarola H, Paglini-Oliva P. 2015. Trypanothione reductase inhibitors: overview of the action of thioridazine in different stages of Chagas disease. Acta Trop 145:79–87. doi: 10.1016/j.actatropica.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 10.Maya J, Orellana M, Ferreira J, Kemmerling U, López-Muñoz R, Morello A. 2010. Chagas disease: present status of pathogenic mechanisms and chemotherapy. Biol Res 43:323–331. [PubMed] [Google Scholar]
- 11.Rodrigues Coura J, de Castro SL. 2002. A critical review on Chagas disease chemotherapy. Mem Inst Oswaldo Cruz 97:3–24. [DOI] [PubMed] [Google Scholar]
- 12.Urbina J. 2010. Specific chemotherapy of Chagas disease: relevance, current limitations and new approaches. Acta Trop 115:55–68. doi: 10.1016/j.actatropica.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 13.Von A, Zaragoza E, Jones D, Rodriguez-Morales AJ, Franco-Paredes C. 2007. New insights into Chagas disease: a neglected disease in Latin America. J Infect Dev Ctries 1:99–111. [Google Scholar]
- 14.Urbina J, Docampo R. 2003. Specific chemotherapy of Chagas disease: controversies and advances. Trends Parasitol 19:495–501. doi: 10.1016/j.pt.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Castro JA, de Mecca MM, Bartel LC. 2006. Toxic side effects of drugs used to treat Chagas' disease (American trypanosomiasis). Hum Exp Toxicol 25:471–479. [DOI] [PubMed] [Google Scholar]
- 16.Davies C, Dey N, Negrette OS, Parada LA, Basombrio MA, Garg NJ. 2014. Hepatotoxicity in mice of a novel anti-parasite drug candidate hydroxymethylnitrofurazone: a comparison with benznidazole. PLoS Negl Trop Dis 8:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maya J, Cassels B, Iturriaga-Vásquez P, Ferreira J, Faúndez M, Galanti N, Ferreira A, Morello A. 2007. Mode of action of natural and synthetic drugs against Trypanosoma cruzi and their interaction with the mammalian host. Comp Biochem Physiol A Mol Integr Physiol 146:601–620. doi: 10.1016/j.cbpa.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Rojo G, Castillo C, Duaso J, Liempi A, Droguett D, Galanti N, Maya J, López-Muñoz R, Kemmerling U. 2014. Toxic and therapeutic effects of nifurtimox and benznidazol on Trypanosoma cruzi ex vivo infection of human placental chorionic villi explants. Acta Trop 132:112–118. doi: 10.1016/j.actatropica.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Castro J, Montalto de Mecca M, Díaz Gómez M, Castro G. 2015. Enfermedad de Chagas: contribuciones del Centro de Investigaciones Toxicológicas. Acta Bioquim Clin Latinoam 49:73–82. [Google Scholar]
- 20.Chatelain E, Ioset J. 2011. Drug discovery and development for neglected diseases: the DNDi model. Drug Des Devel Ther 5:175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bern C. 2011. Antitrypanosomal therapy for chronic Chagas' disease. N Engl J Med 364:2527–2534. doi: 10.1056/NEJMct1014204. [DOI] [PubMed] [Google Scholar]
- 22.Fauro R, Lo Presti S, Bazan C, Baez A, Strauss M, Triquell F, Cremonezzi D, Negrete OS, Willhuber G, Paglini-Oliva P, Rivarola HW. 2013. Use of clomipramine as chemotherapy of the chronic phase of Chagas disease. Parasitology 140:917–927. doi: 10.1017/S0031182013000103. [DOI] [PubMed] [Google Scholar]
- 23.Strauss M, Lo Presti M, Bazán P, Baez A, Fauro R, Esteves B, Sanchez Negrete O, Cremonezzi D, Paglini-Oliva P, Rivarola H. 2013. Clomipramine and benznidazole association for the treatment of acute experimental Trypanosoma cruzi infection. Parasitol Int 62:293–299. doi: 10.1016/j.parint.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Araújo M, Martins-Filho O, Pereira M, Brener Z. 2000. A combination of benznidazole and ketoconazole enhances efficacy of chemotherapy of experimental Chagas' disease. J Antimicrob Chemother 45:819–824. doi: 10.1093/jac/45.6.819. [DOI] [PubMed] [Google Scholar]
- 25.Andrews NW, Colli W. 1982. Adhesion and interiorization of Trypanosoma cruzi in mammalian cells. J Protozool 29:264–269. doi: 10.1111/j.1550-7408.1982.tb04024.x. [DOI] [PubMed] [Google Scholar]
- 26.Chou TC. 2010. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res 70:440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 27.Zhao L, Au JLS, Wientjes MG. 2010. Comparison of methods for evaluating drug-drug interaction. Front Biosci (Elite Ed) 2:241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stempin C, Giordanengo L, Gea S, Cerbán F. 2002. Alternative activation and increase of Trypanosoma cruzi survival in murine macrophages stimulated by cruzipain, a parasite antigen. J Leukoc Biol 72:727–734. [PubMed] [Google Scholar]
- 29.Kindermann I, Barth C, Mahfoud F, Ukena C, Lenski M, Yilmaz A, Klingel K, Kandolf R, Sechtem U, Cooper L, Böhm M. 2012. Update on myocarditis. J Am Coll Cardiol 59:779–792. doi: 10.1016/j.jacc.2011.09.074. [DOI] [PubMed] [Google Scholar]
- 30.Martin DL, Postan M, Lucas P, Gress R, Tarleton RL. 2007. TGF-beta regulates pathology but not tissue CD8+ T cell dysfunction during experimental Trypanosoma cruzi infection. Eur J Immunol 37:2764–2771. doi: 10.1002/eji.200737033. [DOI] [PubMed] [Google Scholar]
- 31.Postan M, Bailey JJ, Dvorak JA, McDaniel JP, Pottala EW. 1987. Studies of Trypanosoma cruzi clones in inbred mice. III. Histopathological and electrocardiographical responses to chronic infection. Am J Trop Med Hyg 37:541–559. [DOI] [PubMed] [Google Scholar]
- 32.Workman P, White RA, Walton MI, Owen LN, Twentyman PR. 1984. Preclinical pharmacokinetics of benznidazole. Br J Cancer 50:291. doi: 10.1038/bjc.1984.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoo SD, Yoon BM, Lee HS, Lee KC. 1999. Increased bioavailability of clomipramine after sublingual administration in rats. J Pharm Sci 88:1119–1121. doi: 10.1021/js990163p. [DOI] [PubMed] [Google Scholar]
- 34.Botros M, Sikaris KA. 2013. The De Ritis ratio: the test of time. Clin Biochem Rev 34:117–130. [PMC free article] [PubMed] [Google Scholar]
- 35.Ribeiro I, Sevcsik A-M, Alves F, Diap G, Don R, Harhay MO, Chang S, Pecoul B. 2009. New, improved treatments for Chagas disease: from the R&D pipeline to the patients. PLoS Negl Trop Dis 3:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morillo C, Marin-Neto J, Avezum A, Sosa-Estani S, Rassi A Jr, Rosas F, Villena E, Quiroz R, Bonilla R, Britto C, Guhl F, Velazquez E, Bonilla L, Meeks B, Rao-Melacini P, Pogue J, Mattos A, Lazdins J, Rassi A, Connolly S, Yusuf S, BENEFIT Investigators. 2015. Randomized trial of benznidazole for chronic Chagas' cardiomyopathy. N Engl J Med 373:1295–1306. doi: 10.1056/NEJMoa1507574. [DOI] [PubMed] [Google Scholar]
- 37.Pinazo M, Muñoz J, Posada E, López-Chejade P, Gállego M, Ayala E, del Cacho E, Soy D, Gascon J. 2010. Tolerance of benznidazole in treatment of Chagas' disease in adults. Antimicrob Agents Chemother 54:4896–4899. doi: 10.1128/AAC.00537-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Urbina JA. 2015. Recent clinical trials for the etiological treatment of chronic Chagas disease: advances, challenges and perspectives. J Eukaryot Microbiol 62:149–156. doi: 10.1111/jeu.12184. [DOI] [PubMed] [Google Scholar]
- 39.Tarleton RL, Gürtler RE, Urbina JA, Ramsey J, Viotti R. 2014. Chagas disease and the London Declaration on Neglected Tropical Diseases. PLoS Negl Trop Dis 8:e3219. doi: 10.1371/journal.pntd.0003219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gobbi P, Baez A, Lo Presti MS, Fernandez AR, Enders JE, Fretes R, Gea S, Paglini-Oliva PA, Rivarola HW. 2010. Association of clomipramine and allopurinol for the treatment of the experimental infection with Trypanosoma cruzi. Parasitol Res 107:1279–1283. doi: 10.1007/s00436-010-2002-z. [DOI] [PubMed] [Google Scholar]
- 41.Pelizzaro-Rocha KJ, Tiuman TS, Izumi E, Ueda-Nakamura T, Dias Filho BP, Nakamura CV. 2010. Synergistic effects of parthenolide and benznidazole on Trypanosoma cruzi. Phytomedicine 18:36–39. [DOI] [PubMed] [Google Scholar]
- 42.Valdez RH, Tonin LT, Ueda-Nakamura T, Silva SO, Dias Filho BP, Kaneshima EN, Yamada-Ogatta SF, Yamauchi LM, Sarragiotto MH, Nakamura CV. 2012. In vitro and in vivo trypanocidal synergistic activity of N-butyl-1-(4-dimethylamino)phenyl-1,2,3,4-tetrahydro-beta-carboline-3-carboxamide associated with benznidazole. Antimicrob Agents Chemother 56:507–512. doi: 10.1128/AAC.05575-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Benson TJ, McKie JH, Garforth J, Borges A, Fairlamb AH, Douglas KT. Rationally designed selective inhibitors of trypanothione reductase. Phenothiazines and related tricyclics as lead structures. Biochem J 286:9–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chaudhary K, Haddadin S, Nistala R, Papageorgio C. 2010. Intraperitoneal drug therapy: an advantage. Curr Clin Pharmacol 5:82–88. doi: 10.2174/157488410791110779. [DOI] [PubMed] [Google Scholar]
- 45.Quante M, Thate-Waschke I, Schofer M. 2012. What are the reasons for patient preference? A comparison between oral and subcutaneous administration. Z Orthop Unfall 150:397–403. (In German.) [DOI] [PubMed] [Google Scholar]
- 46.Caldas S, Caldas I, Diniz Lde F, Lima WG, Oliveira Rde P, Cecilio AB, Ribeiro I, Talvani A, Bahia MT. 2012. Real-time PCR strategy for parasite quantification in blood and tissue samples of experimental Trypanosoma cruzi infection. Acta Trop 123:170–177. doi: 10.1016/j.actatropica.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 47.Zulantay I, Honores P, Solari A, Apt W, Ortiz S, Osuna A, Rojas A, López B, Sánchez G. 2004. Use of polymerase chain reaction (PCR) and hybridization assays to detect Trypanosoma cruzi in chronic chagasic patients treated with itraconazole or allopurinol. Diagn Microbiol Infect Dis 48:253–257. doi: 10.1016/j.diagmicrobio.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 48.Viotti R, Vigliano C, Lococo B, Bertocchi G, Petti M, Alvarez M, Postan M, Armenti A. 2006. Long-term cardiac outcomes of treating chronic Chagas disease with benznidazole versus no treatment: a nonrandomized trial. Ann Intern Med 144:724–734. doi: 10.7326/0003-4819-144-10-200605160-00006. [DOI] [PubMed] [Google Scholar]
- 49.Rivarola HW, Bustamante JM, Lo Presti S, Fernández AR, Enders JE, Gea S, Fretes R, Paglini-Oliva P. 2005. Trypanosoma cruzi: chemotherapeutic effects of clomipramine in mice infected with an isolate obtained from an endemic area. Exp Parasitol 111:80–86. doi: 10.1016/j.exppara.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 50.Piron M, Fisa R, Casamitjana N, López-Chejade P, Puig L, Vergés M, Gascón J, Gómez i Prat J, Portús M, Sauleda S. 2007. Development of a real-time PCR assay for Trypanosoma cruzi detection in blood samples. Acta Trop 103:195–200. doi: 10.1016/j.actatropica.2007.05.019. [DOI] [PubMed] [Google Scholar]