Abstract
Introduction
There is an urgent need to identify biomarkers that can accurately detect and diagnose Alzheimer's disease (AD). Autoantibodies are abundant and ubiquitous in human sera and have been previously demonstrated as disease-specific biomarkers capable of accurately diagnosing mild-moderate stages of AD and Parkinson's disease.
Methods
Sera from 236 subjects, including 50 mild cognitive impairment (MCI) subjects with confirmed low CSF Aβ42 levels, were screened with human protein microarrays to identify potential biomarkers for MCI. Autoantibody biomarker performance was evaluated using Random Forest and Receiver Operating Characteristic curves.
Results
Autoantibody biomarkers can differentiate MCI patients from age-matched and gender-matched controls with an overall accuracy, sensitivity, and specificity of 100.0%. They were also capable of differentiating MCI patients from those with mild-moderate AD and other neurologic and non-neurologic controls with high accuracy.
Discussion
Autoantibodies can be used as noninvasive and effective blood-based biomarkers for early diagnosis and staging of AD.
Keywords: Mild Cognitive Impairment, Alzheimer's disease, Autoantibodies, Biomarkers, Blood biomarkers, Microarray, Autoantibody Biomarker, Antibody, Diagnostics
1. Introduction
AD is a devastating and progressive neurodegenerative disease affecting approximately 5.3 million people in the United States, including almost half of the population at 85 years and older [1], [2]. Microscopic hallmarks of the disease include neuritic plaques containing amyloid beta peptide (Aβ42) and neurofibrillary tangles composed of hyperphosphorylated tau [3]. Despite intensive research throughout the past two decades, a clear understanding of AD pathogenesis remains elusive and controversial. At best, current treatments only temporarily alleviate some symptoms and provide no relief for the pathology [4]. One point of agreement is that, in a high percentage of those afflicted, AD-related pathologic changes begin in the brain at least a decade before the emergence of telltale symptoms and clinical presentation [5], [6], [7], [8], [9], [10]. This makes it difficult to identify AD patients at early, pre-symptomatic disease stages, at a time when treatments are likely to be most beneficial. In view of this, intensive research is underway worldwide to discover and develop accurate, reliable, and cost-effective methods for early AD detection that can be widely implemented.
Much effort is being devoted to identification of soluble components in blood and cerebrospinal fluid (CSF) that can serve as useful and reliable AD biomarkers [11]. In CSF, the most established biomarkers include Aβ42, total tau, and phosphorylated tau (p-tau) and their relative ratios [12], [13], [14], [15], [16], [17]. Low CSF Aβ42 levels in individuals with mild cognitive impairment (MCI) are now considered to be strongly indicative of the presence of early ongoing AD pathology as well as predictive of the likelihood of rapid disease progression to AD [18], [19], [20], [21], [22], [23], [24]. A key limitation to the use of CSF in general is the means by which it is obtained, through a lumbar spinal puncture, which is considered invasive and not without risk [25], [26]. By contrast, procurement of blood is less invasive, and plasma proteins [27], [28], [29], lipids [30] as well as proteins and microRNAs enclosed within exosomes and lysosomal derivatives have all shown promise as biomarkers for early detection of AD pathology [31], [32]. Parallel advancements for early AD detection have been made in neuroimaging, such as magnetic resonance imaging (MRI) and positron emission tomography (PET) using tracers like Florbetapir (18F), Pittsburgh compound B (PiB), and fluorodeoxyglucose. The high cost of these procedures and inconsistencies in interpretation prohibits their use as initial disease screeners, and they may not be readily available to individuals in economically disadvantaged areas or remote geographical locations.
Using human protein microarrays as a platform, we have shown that humans possess thousands of autoantibodies in their blood, and that individual autoantibody profiles are influenced by age, gender, and the presence of disease [33]. Previous studies have suggested that autoantibodies may be useful as biomarkers for detection of neurodegenerative diseases, including AD and Parkinson's diseases (PD) [34], [35], [36], [37]. Ultimately, the practical utility of potential biomarkers depends on their capacity to accurately, specifically and reliably detect these diseases at early stages. Recently, we showed that a small panel of only four autoantibodies was sufficient to identify individuals with early-stage PD, as well as distinguish them from healthy individuals and others with mild-moderate PD [37]. In addition, these early PD biomarkers exhibited disease specificity by distinguishing subjects with early-stage PD from those with other neurodegenerative diseases, such as MCI, mild-moderate AD, multiple sclerosis, and early-stage breast cancer [37].
In the present study involving 236 subjects, our objective was to determine if autoantibodies can be used as biomarkers to accurately diagnose individuals with MCI that is driven by early stages of AD pathology. We obtained sera from MCI subjects exhibiting low CSF AΒ42 from the Alzheimer's Disease Neuroimaging Initiative (ADNI). Low CSF AΒ42 levels have been shown to be an independent surrogate biochemical biomarker indicative of the presence of ongoing early-stage AD pathology and a high likelihood of rapid progression to AD [38], [39], [40]. Our results show that a small panel of blood-borne autoantibody biomarkers can be used to distinguish subjects with AD-associated MCI from age-matched and gender-matched controls with an overall accuracy of 100%. In addition, MCI subjects were successfully differentiated from those with mild-moderate AD with similar overall accuracy, suggesting that this approach may also be useful for delineation of discrete disease stages along the MCI-to-AD continuum. Finally, the panel of AD-associated MCI biomarkers described here was highly specific for MCI in that they accurately distinguished AD-associated MCI subjects from those with other neurodegenerative and non-neurodegenerative diseases, including early and mild-moderate PD, multiple sclerosis, and early-stage breast cancer.
2. Methods
2.1. Study population
Clinical, biochemical, and imaging data on MCI subjects used here were obtained from the ADNI database at www.loni.ucla.edu/ADNI. Our study was focused on 50 ADNI participants who were diagnosed with amnestic MCI at baseline and had at least one follow-up visit (Table 1). All subjects enrolled in the ADNI were aged 55–91 years and had no evidence of cerebrovascular disease (Modified Hachinski Ischemia Score ≤ 4) [41], no evidence of depression (Geriatric Depression Scale <6 [42], stable medications, a study partner, no visual or hearing impairment, good general health, six grades of education or equivalent, English or Spanish fluency, and no medical contraindications to MRI. This included baseline data from individuals diagnosed with MCI with available neuropsychological test results, apolipoprotein E (APOE) status, CSF proteins, 18F-fluorodeoxyglucose PET, and structural MRI scans. In the ADNI samples, MCI was defined based on the following criteria: memory complaint verified by study partner; abnormal memory function based on education-adjusted cut-off on the Logical Memory II subscale from the Wechsler Memory Scale revised (Wechsler D. Wechsler Memory Scale-revised. San Antonio, TX: Psychological Corporation; 1987), MMSE [43] score of 24–30 (inclusive), Clinical Dementia Rating [44] score of 0.5, and cognitive and functional impairment not yet severe enough to meet criteria for AD or dementia.
Table 1.
Subject demographics
| Group | n | Age (y) | Range | Sex (% male) | Ethnicity (% Caucasian) | MMSE |
|---|---|---|---|---|---|---|
| Mild cognitive impairment | 50 | 73.0 ± 7.1 | 55–91 | 58 | 94 | 27.9 |
| Controls | 50 | 70.9 ± 5.1 | 62–87 | 56 | 78 | — |
| Mild-moderate Alzheimer's disease | 50 | 78.5 ± 8.8 | 61–97 | 42 | 88 | 16.5 |
| Mild-moderate Parkinson's disease | 25 | 73.9 ± 9.5 | 53–88 | 48 | 45 | — |
| Early-stage Parkinson's disease | 25 | 72.4 ± 2.9 | 67–79 | 56 | 96 | — |
| Multiple sclerosis | 25 | 53.8 ± 6.6 | 43–67 | 40 | 100 | — |
| Breast cancer | 11 | 52.5 ± 0.9 | 51–54 | 0 | 100 | — |
The number of individuals (n), age, range of age, gender, and ethnicity are listed for each disease group. For MCI and mild-moderate AD subjects, the mini-mental state examination (MMSE) score is included as a measure of cognitive impairment.
The ADNI was launched in 2003 by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, the Food and Drug Administration, private pharmaceutical companies, and non-profit organizations, as a $60 million, 5-year, public-private partnership. The primary goal of ADNI has been to test whether serial MRI, PET, and other biological markers, and clinical and neuropsychological assessments can be combined to measure the progression of MCI and early AD. Determination of sensitive and specific markers of very early AD progression is intended to aid researchers and clinicians to develop new treatments and monitor their effectiveness, as well as to lessen the time and cost of clinical trials.
The principal investigator of this initiative is Michael W. Weiner, VA Medical Center and University of California, San Francisco. ADNI is the result of the efforts of many co-investigators from a broad range of academic institutions and private corporations, and subjects have been recruited from more than 50 sites across the United States and Canada. The initial goal of ADNI was to recruit 800 adults, aged 55 to 90 years, to participate in the research approximately 200 cognitively normal older individuals to be followed for 3 years and 200 people with early AD to be followed for 2 years. For up-to-date information, see www.adni-info.org.
Participants with MCI were enrolled based on criteria outlined in the ADNI protocol (http://www.adni-info.org/Scientists/AboutADNI.aspx). Demographic data are presented in Table 1. The study was approved by the Rowan University Institutional Review Board.
Fifty MCI serum samples from subjects with confirmed low CSF Aβ42 levels were obtained. These came from subjects participating in the ADNI2 study, which is an ongoing longitudinal study with the goal of identifying individuals at risk for Alzheimer's disease, as well as the development of diagnostic and prognostic biomarkers of the disease [45]. Diagnosis of MCI was made based on a battery of tests, including MMSE scores, CDR, and other subjective memory assessments [45]. Twenty-five early-stage PD samples were obtained from the Parkinson's Study Group (Boston, MA). Twenty-five mild-moderate PD and 50 mild-moderate AD serum samples were obtained from Analytical Biological Systems, Inc. (Wilmington, DE). Eleven stage 0–2 breast cancer serum samples were obtained from Asterand, Inc. (Detroit, MI), and 25 multiple sclerosis (MS) patient serum samples were obtained from BioServe Biotechnologies Ltd (Beltsville, MD). Healthy age-matched and gender-matched control sera were obtained from several sources: 27 from BioServe Biotechnologies Ltd.; 11 from Asterand Inc.; 9 from The New Jersey Institute for Successful Aging at Rowan University (Stratford, NJ); and three from Analytical Biological Systems, Inc. All samples were handled using standard procedures and stored at −80°C until use. Stored samples were monitored using Sensaphone 1400 (Phonetics, Inc., Aston, PA). Demographic characteristics of the study population are listed in Table 1.
2.2. Human protein microarrays
To detect and identify autoantibodies in human sera, we used Invitrogen's ProtoArray v5.0 Human Protein Microarrays (Cat. No. PAH0525020, Invitrogen, Carlsbad, CA, USA), each containing 9486 unique human protein antigens (www.invitrogen.com/protoarray). All proteins were expressed as glutathione s-transferase (GST) fusion proteins in insect cells, purified under native conditions, and spotted in duplicate onto nitrocellulose-coated glass slides. Arrays were probed with serum, processed and scanned according to the manufacturer's instructions. Briefly, microarrays were blocked using Blocking Buffer (Cat. No. PA055, Invitrogen) and each was incubated with serum diluted to 1:500 in washing buffer. After washing, arrays were probed with antihuman IgG (H + L) conjugated to AlexaFluor 647 (Cat. No. A-21445, Invitrogen) diluted 1:2000 in washing buffer. Arrays were then washed, dried, and immediately scanned with a GenePix 4000B Fluorescence Scanner (Molecular Devices, Sunnyvale, CA, USA).
2.3. Microarray data analysis
Fluorescence data were acquired by aligning the Genepix Array List onto the microarray using the Genepix Pro analysis software. The resulting Genepix results files were imported into Invitrogen's Prospector 5.2 for analysis. The “group characterization” and “two-group comparison” features in the Immune Response Biomarker Profiling (IRBP) toolbox within Prospector then enabled M-statistical analysis of differential autoantibody expression between the two groups (Supplementary Fig. 1). Positive hits were determined by a Z-Factor >0.4, and a minimum signal intensity of 1500 RFU, which allows for stringent biomarker selection and minimizes the amount of false positives. Autoantibodies were sorted into descending order by difference of prevalence between MCI and control groups, and the top 50 most differentially expressed autoantibodies in the MCI group were chosen as potential diagnostic biomarkers. All data are MIAME compliant and raw data from the microarrays have been deposited in a MIAME compliant database (GEO) under accession number GSE74763.
Subjects were randomly split into Testing and Training Sets such that both sets included cases and controls matched by age and gender. The Training Set was used to rank candidate protein biomarkers by their predictive power and to establish the diagnostic logic. The initial Training Set for MCI consisted of 25 MCI and 25 control samples; the remaining samples were relegated to the independent Testing Set, thus also 25 MCI and 25 control subjects. We estimated the optimum number of autoantibody biomarkers, defined here as the minimum number of autoantibody biomarkers required to maintain maximum diagnostic accuracy for this population of MCI subjects. To accomplish this, we first compared the predictive capacity of the top 25 and bottom 25 biomarkers and then determined the efficacy of the top 10 alone using the original Training Set logic. In each case, the predictive classification accuracy of the selected biomarkers in the Training Set, Testing Set, and in both sets combined was tested with R's Random Forest (RF; v 4.6–10), using the default settings [46], [47]. Selected biomarkers were tested with the RF model algorithm, and classification accuracy is reported in a confusion matrix and misclassifications as an out-of-bag (OOB) error score. Receiver Operating Characteristic (ROC) curves, widely used to evaluate the utility of a diagnostic test, were generated using R (3.02) packages ROCR (v 1.0-5) and pROC (v 1.7.3) [48]. Based on the determined optimal number of biomarkers, a final model was constructed using these biomarkers and their associated Training Set logic and tested with the independent Testing Set.
Using the same Training and Testing Set strategy outlined above, we performed an additional round of biomarker discovery using only RF, instead of prevalence difference, to select potential biomarkers. After M-statistical analysis by Prospector, the data were analyzed using the “variable importance” function in RF, which is the prediction accuracy of the OOB error score reported for each tree, and also for each individual permutated biomarker. The difference between the two values was averaged over all trees and normalized by the standard error. The top 50 biomarkers based on the normalized variable importance score were chosen as potential diagnostic biomarkers and further analyzed for their diagnostic value as reported above.
3. Results
3.1. Selection of a panel of autoantibody biomarkers for AD-associated MCI diagnosis
We first sought to identify a panel of autoantibodies capable of detecting early-stage AD pathology using 50 ADNI MCI patient sera, all with low CSF AΒ42 levels consistent with the presence of ongoing AD-related pathology (from now on referred to as AD-associated MCI; Supplementary Fig. 2) and each with a clinical diagnosis of either early MCI (EMCI, n = 32) or late MCI (LMCI, n = 18), a distinction based on education-adjusted cut-off scores on the logical memory II subscale from the Wechsler Memory Scale revised, previously mentioned. These sera, along with those obtained from age-matched and gender-matched controls, were used to probe commercially available human protein microarrays containing 9486 proteins. First, samples were separated into Training and Testing Sets (Supplementary Fig. 1) each containing 25 ADNI MCI sera (16 EMCI + 9 LMCI) and 25 age-matched and gender-matched controls. The resulting individual autoantibody profiles for Training Set MCI subjects were compared with those of controls using Prospector analysis software. We identified 193 autoantibodies with a significantly (P < .05) higher prevalence in the MCI group compared to controls in the Training Set as potential diagnostic biomarkers. From this list, the top 50 most differentially expressed autoantibodies in the MCI group were chosen as a working diagnostic biomarker panel (Supplementary Table 1).
3.2. Verification of AD-associated MCI biomarkers via Training and Testing Set analysis
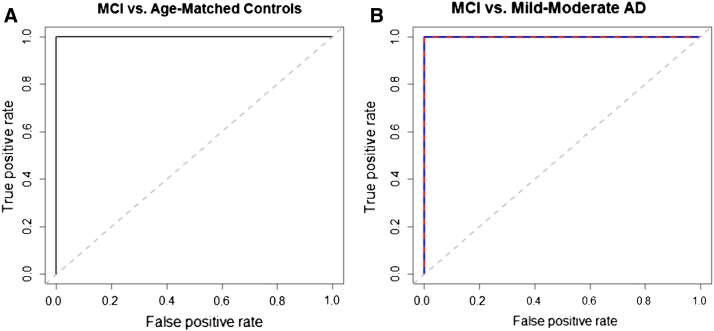
The top 50 MCI autoantibody biomarkers chosen from the Training Set were re-verified as significant predictors using Random Forest (RF). On RF evaluation of the Training Set samples (n = 50; 25 MCI, 25 controls) using the 50 selected biomarkers, MCI subjects were distinguished from age-matched and gender-matched controls with an average of 99.6% prediction accuracy based on five replicate runs. We then used these 50 biomarkers and the RF Training Set logic to classify MCI in Testing Set subjects, comprised of a completely independent group of samples that played no role in biomarker selection. RF was able to correctly classify 100% of MCI and controls among Testing Set subjects (n = 50; 25 MCI, 25 controls; Table 2). Combining both Training and Testing Set samples and using the Training Set logic, RF successfully distinguished MCI from controls with no error (Supplementary Fig. 1). The diagnostic utility of this panel of 50 MCI biomarkers was also evaluated using ROC curve analysis of Testing Set subjects (Fig. 1A). The ROC area under the curve (AUC) for this comparison was 1, indicating exceptional classification accuracy (Table 3). Diagnostic sensitivity, specificity, and positive- and negative-predictive values (PPV and NPV) for the 50 MCI biomarkers used to evaluate the Testing Set subjects are shown in Table 2, Table 3.
Table 2.
Diagnostic results using a panel of 50 AD-associated MCI biomarkers and RF
| MCI (n = 25) vs. |
MCI (n = 11) vs. |
|||||
|---|---|---|---|---|---|---|
| Age-matched controls | Mild-Moderate AD∗ | Early-stage PD | Mild-Moderate PD | Multiple sclerosis | Breast cancer | |
| n | 25 | 50 | 25 | 25 | 25 | 11 |
| Sensitivity, % | 100.0 | 100.0 | 100.0 | 96.0 | 100.0 | 100.0 |
| Specificity, % | 100.0 | 98.0 | 96.0 | 96.0 | 100.0 | 100.0 |
| PPV, % | 100.0 | 96.2 | 96.2 | 96.0 | 100.0 | 100.0 |
| NPV, % | 100.0 | 100.0 | 100.0 | 96.0 | 100.0 | 100.0 |
| Overall accuracy, % | 100.0 | 98.7 | 98.0 | 96.0 | 100.0 | 100.0 |
| Overall error, % | 0 | 1.3 | 2.0 | 4.0 | 0 | 0 |
Diagnostic performance was assessed using RF. Using Testing Set samples, RF successfully distinguished AD-associated MCI subjects (n = 25) from age-matched and gender-matched controls as well as those with mild-moderate AD, early-stage PD, mild-moderate PD, multiple sclerosis and breast cancer with high overall accuracies.
The average of 25 MCI samples versus two Testing Sets of 25 mild-moderate AD samples each.
Fig. 1.
(A) and (B) Biomarker analysis and Receiver Operating Characteristic (ROC) curve assessment of the utility of autoantibody biomarkers for detection of AD-associated MCI. (A) and (B) ROC assessment of autoantibody biomarkers for detection of MCI in Testing Set subjects and AD progression. (A) Comparison of MCI (n = 25) vs. age-matched controls (n = 25) using a panel of 50 MCI-specific biomarkers demonstrates that this biomarker panel can be used to detect MCI with relatively high overall accuracy. The dashed line represents the line of no discrimination. (B) Comparison of MCI Testing Set subjects (n = 25) vs. two groups of mild-moderate AD subjects (n = 50) (first group = blue line, second group = red line) using a panel of 50 MCI biomarkers shows that these biomarkers can be used to accurately distinguish these two different stages of AD progression. The ROC AUC, sensitivity, and specificity values for the 50 biomarkers are shown in Table 3.
Table 3.
ROC curve assessment of the diagnostic utility of the top 50 AD-associated MCI biomarkers
| Top 50 values | |||
|---|---|---|---|
| MCI (n = 25) vs. | Top 50 markers |
||
| AUC (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | |
| Age-matched controls (n = 25) | 1 | 1 | 1 |
| Early-stage PD (n = 25) | 1 | 1 | 1 |
| Mild-moderate PD (n = 25) | 1 | 1 | 1 |
| Mild-moderate AD∗ (n = 50) | 1 | 1 | 1 |
| Multiple sclerosis (n = 25) | 1 | 1 | 1 |
| Breast cancer (n = 11) | 1 | 1 | 1 |
ROC curve analyses (Testing Set subjects only) showing the diagnostic utility of the top 50 biomarkers for distinguishing AD-associated MCI subjects from age-matched controls and from the subject groups listed. Area under the curve (AUC) values at 95% confidence are listed along with values for sensitivity and specificity derived from ROC curve output data.
The average of 25 MCI samples versus two Testing Sets of 25 mild-moderate AD samples each.
3.3. Exchanging Training and Testing Sets yields similar biomarker panels with comparable diagnostic accuracy
As a further test of the utility of autoantibodies as biomarkers for detecting AD pathology in MCI patients, we also carried out a second round of biomarker discovery in which we exchanged the Training and Testing Sets and compared the resulting, second round biomarkers with those chosen in the first round. Using the panel of 50 second round biomarkers, RF was able to correctly classify 98% of MCI and controls using Testing Set subjects (sensitivity = 96.0%; specificity = 100.0%; PPV = 100.0%; NPV = 96.2%; ROC AUC = 1). Importantly, 26 of 50 (52%) second round biomarkers overlapped with those chosen in the first round.
3.4. Comparison of MCI biomarker selection strategies: RF vs. prevalence difference
We next carried out a completely different and unbiased MCI biomarker selection process using RF only. Here, instead of first ranking potential MCI biomarkers based on prevalence difference, we uploaded data from Prospector directly into R and asked RF to independently choose the top 50 MCI biomarkers as described in the methods. As shown in Supplementary Table 2, using the panel of 50 RF-selected biomarkers, RF was able to correctly classify MCI and controls in Testing Set subjects with an average of 100% overall accuracy in five replicate runs, thus comparable to both panels derived from prevalence difference described above. Importantly, 19 of 50 (38%) of the RF-selected biomarkers overlapped with those chosen in the first round. Because this is a small and preliminary “proof of concept” study, and there were no significant performance differences between the three different MCI biomarker panels described thus far, we opted to choose the first biomarker panel described in Supplementary Table 1 for subsequent studies and comparisons.
3.5. Fewer than 50 autoantibody biomarkers are sufficient for accurate detection of AD-associated MCI
The top 50 first round biomarkers sorted according to decreasing prevalence difference are shown in Supplementary Table 1. We next compared the relative diagnostic accuracy of the top and bottom 25 biomarkers within this selected panel for detecting AD-associated MCI in Testing Set subjects. Results showed an overall accuracy of 100% for the top 25 biomarkers (sensitivity = 100.0%; specificity = 100.0%; ROC AUC = 1) and 98.0% for the bottom 25 biomarkers (sensitivity = 100.0%; specificity = 96.0%; ROC AUC = 1) for distinguishing MCI subjects from corresponding age-matched and gender-matched controls (Table 4, Table 5). Next, the top 10 biomarkers were tested independently and showed an overall accuracy of 98.0% (sensitivity = 96.0%; specificity = 100.0%; ROC AUC = 1), suggesting that a small panel with roughly 10 biomarkers may be sufficient to achieve maximal overall accuracy (Table 4, Table 5).
Table 4.
Diagnostic accuracies of the top 10, top 25, and bottom 25 AD-associated MCI biomarkers
| MCI (n = 25) vs. |
MCI (n = 11) vs. |
||||||
|---|---|---|---|---|---|---|---|
| Age-matched controls | Mild-moderate AD∗ | Early-stage PD | Mild-moderate PD | Multiple sclerosis | Breast cancer | ||
| Top 10 MCI biomarkers | n | 25 | 50 | 25 | 25 | 25 | 11 |
| Sensitivity, % | 96.0 | 96.0 | 96.0 | 96.0 | 96.0 | 100.0 | |
| Specificity, % | 100.0 | 96.0 | 100.0 | 96.0 | 100.0 | 100.0 | |
| PPV, % | 100.0 | 98.0 | 100.0 | 96.0 | 100.0 | 100.0 | |
| NPV, % | 96.2 | 92.3 | 96.0 | 96.0 | 96.0 | 100.0 | |
| Overall accuracy, % | 98.0 | 96.0 | 98.0 | 96.0 | 98.0 | 100.0 | |
| Overall error, % | 2.0 | 4.0 | 2.0 | 4.0 | 2.0 | 0 | |
| Top 25 MCI biomarkers | n | 25 | 50 | 25 | 25 | 25 | 11 |
| Sensitivity, % | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | |
| Specificity, % | 100.0 | 98.0 | 96.0 | 96.0 | 100.0 | 100.0 | |
| PPV, % | 100.0 | 96.2 | 96.2 | 96.2 | 100.0 | 100.0 | |
| NPV, % | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | |
| Overall Accuracy, % | 100.0 | 98.7 | 98.0 | 98.0 | 100.0 | 100.0 | |
| Overall error, % | 0 | 1.3 | 2.0 | 2.0 | 0 | 0 | |
| Bottom 25 MCI biomarkers | n | 25 | 50 | 25 | 25 | 25 | 11 |
| Sensitivity, % | 100.0 | 100.0 | 100.0 | 100.0 | 96.0 | 100.0 | |
| Specificity, % | 96.0 | 94.0 | 92.0 | 96.0 | 92.0 | 81.8 | |
| PPV, % | 96.2 | 89.3 | 92.6 | 96.2 | 92.3 | 84.6 | |
| NPV, % | 100.0 | 100.0 | 100.0 | 100.0 | 95.8 | 100.0 | |
| Overall accuracy, % | 98.0 | 96.0 | 96.0 | 98.0 | 94.0 | 90.9 | |
| Overall error, % | 2.0 | 4.0 | 4.0 | 2.0 | 6.0 | 9.1 | |
Diagnostic results using different panels of AD-associated MCI biomarkers and RF. The performance of the top 10, top 25, and bottom 25 biomarkers were assessed using RF. Using Testing Set samples, RF successfully distinguished MCI (n = 25) from age-matched and gender-matched controls, mild-moderate AD, early-stage PD, mild-moderate PD, multiple sclerosis, and breast cancer with high overall accuracies.
indicates the average of 25 MCI samples vs. two Testing Sets of 25 mild-moderate AD samples each.
Table 5.
ROC curve assessment of diagnostic utility of top 10, top 25, and bottom 25 AD-associated MCI biomarkers
| MCI (n = 25) vs. | Top 10, top 25, and bottom 25 values |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Top 10 markers |
Top 25 markers |
Bottom 25 markers |
|||||||
| AUC (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | AUC (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | AUC (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | |
| Age-matched controls (n = 25) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Early-stage PD (n = 25) | 1 | 1 | 1 | 1 | 1 | 1 | 0.97 (0.92–1) | 0.92 (0.8–1) | 1 |
| Mild-moderate PD (n = 25) | 0.99 (0.99–1) | 1 | 0.96 (0.88–1) | 1 | 1 | 1 | 0.98 (0.96–1) | 0.96 (0.88–1) | 1 |
| Mild-moderate AD∗ (n = 50) | 1 | 1 | 1 | 1 | 1 | 1 | 0.99 (0.9892–1) | 1 | 0.96 (0.88–1) |
| Multiple sclerosis (n = 25) | 1 | 1 | 1 | 1 | 1 | 1 | 0.9 (0.92–1) | 0.92 (0.8–1) | 0.96 (0.88–1) |
| Breast cancer (n = 11) | 1 | 1 | 1 | 1 | 1 | 1 | 0.95 (0.88–1) | 1 | 0.81 (0.54–1) |
NOTE. ROC curve assessment (Testing Set subjects only) of the diagnostic utility of AD-associated MCI biomarkers. ROC curve analysis was used to assess the diagnostic utility of the top 10, top 25, and bottom 25 biomarkers for distinguishing MCI subjects from age-matched controls and from the other subject groups listed. Area under the curve (AUC) values at 95% confidence are listed along with values for sensitivity and specificity derived from ROC curve output data.
The average of 25 MCI samples versus two Testing Sets of 25 mild-moderate AD samples each.
3.6. Disease specificity of the selected biomarkers for AD-associated MCI
We next evaluated the disease specificity of the panels of the top 50 and 10 autoantibody biomarkers for the detection of AD-associated MCI, i.e., whether they can successfully differentiate ADNI MCI subjects from those with other neurological and non-neurological diseases. To eliminate the possibility that the selected biomarkers were simply detecting nonspecific CNS degeneration, the same 25 MCI serum samples from Testing Set subjects were compared to sera obtained from 25 subjects with early-stage PD, 25 subjects with mild-moderate PD, 25 subjects with MS, and 11 subjects with stage 0–2 breast cancer. Using the original panel of 50 biomarkers, MCI sera were readily distinguished from early-stage PD sera with an overall accuracy of 98.0% (sensitivity = 100.0%; specificity = 96.0%; ROC AUC = 1) and 96.0% for mild-moderate PD (sensitivity = 96.0%; specificity = 96.0%; ROC AUC = 1) (Table 2, Table 3). Similarly, this biomarker panel was able to distinguish ADNI MCI subjects from MS and stage 0–2 breast cancer subjects with comparable overall accuracy (Table 2, Table 3). Similar results were obtained using only the top 10 biomarkers (Table 4, Table 5).
Finally, to further assess the specificity of the selected biomarkers for MCI, we asked if these biomarkers could be used to differentiate the same neurologic and non-neurologic diseases mentioned previously from a group of normal control subjects. Using the panel of the top 50 MCI biomarkers along with their associated logic, RF was unable to successfully differentiate any of the disease groups from the control group, as indicated by the ROC curve analyses shown in Supplementary Fig. 3. These results clearly demonstrate the specificity of the top 50 biomarkers for AD-associated MCI, ruling out the possibility that they are nonspecific for CNS neurodegeneration or disease in general.
3.7. Staging of AD: autoantibody biomarkers can distinguish ADNI AD-associated MCI subjects from those with mild-moderate AD
We next asked if autoantibody biomarkers can be used to distinguish different stages of AD. To address this, we used our original panel of 50 biomarkers (Supplementary Table 1) and the RF logic derived from the Training Set to test whether 25 ADNI Testing Set MCI samples could be distinguished from 50 subjects with mild-moderate AD. The latter were randomly split into two groups of 25 each and compared to the same Testing Set of 25 MCI samples, and the average overall accuracy from both runs was 98.7% (Table 2). ROC curve analyses of all these comparisons are presented in Fig. 1B along with sensitivity, specificity, PPV, and NPV (Table 3). Taken together, these results confirm that, although AD-associated MCI and mild-moderate AD are different stages of the same disease and are expected to share biomarkers, the panel of 50 autoantibody biomarkers for MCI selected here along with its corresponding diagnostic logic was capable of differentiating AD-associated MCI from a more pathologically advanced stage of AD.
In addition to the 50 MCI-specific biomarkers, we also sought to determine if biomarkers specific to mild-moderate AD could distinguish between these two discreet stages of the disease. To test this, 50 mild-moderate AD samples were compared to the same group of 50 control samples used for discovery of MCI biomarkers, using the same Training/Testing Set strategy as before. The top 50 most differentially expressed autoantibody biomarkers in mild-moderate AD subjects compared to controls were selected and verified as significant using the methods described above. Using this set of 50 mild-moderate AD biomarkers, MCI was readily distinguished from mild-moderate AD with an overall accuracy, sensitivity, and specificity of 100.0% (data not shown). Comparison of the top 50 biomarkers for MCI and mild-moderate AD revealed an overlap of 5 biomarkers, or 10% of the total biomarkers (data not shown), thus confirming the expected presence of common biomarkers between these disease stages.
3.8. Sera from patients classified by ADNI as early and late MCI were indistinguishable
Among the 50 MCI sera obtained from the ADNI, 32 were classified as early MCI (EMCI) and 18 as late MCI (LMCI), a distinction based on assessments as part of the ADNI2 protocol. After the successful differentiation of MCI from mild-moderate AD using separate panels of both MCI-specific and mild-moderate AD-specific biomarkers, we then asked if we could use a similar strategy to generate biomarker panels that could distinguish between EMCI and LMCI. Thirty-two EMCI and 18 LMCI samples were each compared to the same group of 25 controls that were used to generate a panel of the top 50 most differentially expressed autoantibodies for each group. Among the top 50 biomarkers for EMCI and LMCI, 34 (68%) were found to overlap. Overlapping biomarkers were then removed, and the remaining 16 biomarkers unique to each stage were then tested in an attempt to differentiate EMCI subjects from those with LMCI. Each panel was separately tested using RF, which was unable to differentiate between the two MCI subgroups with <40% overall error in both instances (data not shown). The significant overlap of biomarkers in the EMCI and LMCI biomarker panels and our inability to correctly distinguish EMCI and LMCI suggest that there is little or no detectable pathologic difference between these two stages. This conclusion is further supported by the fact that both EMCI and LMCI subjects had comparable CSF Aβ42 levels (Supplementary Fig. 2), a biomarker known to be indicative of ongoing early AD pathology and strongly predictive of conversion from MCI to AD.
4. Discussion
The goals of this study were to determine if autoantibodies can be used effectively as blood-based biomarkers to identify individuals diagnosed with MCI due to ongoing early-stage AD pathology (AD-associated MCI) and to distinguish these MCI individuals from subjects with a more advanced stage of AD or other neurodegenerative and non-neurodegenerative diseases. This study resulted in five main findings. First, we demonstrate that a panel of autoantibodies can be used to distinguish individuals with MCI linked to early-stage AD pathology from healthy age-matched and gender-matched controls with high overall accuracy. The subjects used were diagnosed with MCI by ADNI investigators and were selected because they also exhibit low CSF Aβ42 levels. The latter has been established as a reliable surrogate biochemical biomarker indicative of ongoing early AD pathology and a high likelihood of imminent further progression and transition to full-blown AD. Second, implementation of independent biomarker discovery strategies was found to yield biomarker panels showing substantial overlap of selected autoantibody biomarkers and comparable diagnostic performance outcomes. Third, the selected panel of AD-associated MCI biomarkers and its accompanying diagnostic logic were disease stage specific in that it was possible to differentiate MCI subjects from individuals at a more advanced (mild-moderate) stage of AD. Fourth, these biomarkers were also disease-specific as they were capable of distinguishing MCI subjects from those with early or mild-moderate stage PD, MS, and early-stage breast cancer. Finally, these biomarkers were unsuccessful in distinguishing subjects with more subtle clinical designations that are not rooted in significant differences in the extent of pathology.
In a recent study, we used human protein microarrays to demonstrate that autoantibodies can serve as useful blood-based biomarkers to distinguish patients with early-stage PD from healthy age-matched and gender-matched control subjects [37]. Results from 398 subjects, including 103 early-stage PD subjects derived from the Deprenyl and Tocopherol Antioxidative Therapy of Parkinsonism (DATATOP) study, showed that a panel of as few as four autoantibody biomarkers can distinguish subjects with early-stage PD from matched controls with an overall accuracy of 87.9%. These biomarkers also differentiated patients with early-stage PD from those with more advanced, mild-moderate PD with an overall accuracy of 97.5% and could also distinguish subjects with early-stage PD from those with other neurological (e.g., Alzheimer's disease and multiple sclerosis) and non-neurological (e.g., breast cancer) diseases with comparable accuracies.
In the present study, we analyzed sera from 236 subjects, including 50 derived from patients diagnosed with MCI by ADNI investigators that also demonstrated low CSF Aβ42 levels, a feature consistent with the presence of ongoing AD-related pathology and a high likelihood of rapid transition to AD [49], [50]. In these MCI patients, a much larger volume of brain tissue is expected to be involved in the pathology compared to that in early-stage PD subjects, where the pathology is initially confined to the substantia nigra and surrounding regions. For this reason and in the context of the results obtained for early-stage PD described above, we expected to achieve the same or better overall accuracy for detection of AD-associated MCI. This expectation was realized using the MCI autoantibody biomarkers identified in the present study, which showed 100% overall accuracy in the Testing Set in distinguishing AD-associated MCI subjects from matched controls. This result is in agreement with our earlier study showing that autoantibodies can be used to distinguish individuals with mild-moderate AD from matched controls as well as the studies of Restrepo et al. and Reddy et al. [34], [51]. The latter group used the affinity of autoantibodies for peptoid microarrays and showed that specific autoantibody binding patterns could be used to diagnose AD. In fact, there now exists a rapidly growing literature spanning many fields reporting the identification of specific autoantibodies associated with a wide variety of diseases. In many cases, these autoantibodies are being pursued as diagnostic biomarkers for these diseases, and it is now becoming increasingly clear that these are not just isolated cases where autoantibodies are somehow related to the presence of disease. We have recently proposed that the presence of these autoantibodies are instead reflecting the immune system's natural response to disease as part of the body's attempt to clear debris generated by the disease from the blood [33], [52]. Additional studies are needed to test this possibility.
To fully test all 50 ADNI MCI subjects, we also exchanged Training and Testing Set subjects, carried out a second round of biomarker discovery and compared the resulting new biomarkers with those chosen in the first round. Using the panel of 50 second round biomarkers, RF was able to correctly classify 98% of MCI and matched controls in the new Testing Set subjects. Many of the newly selected biomarkers in this second round overlapped with first round biomarkers, thus reaffirming their selection and their utility for detection of early-stage AD pathology associated with MCI. In addition, as an independent and completely unbiased method of AD-associated biomarker selection, we recruited RF to choose the top 50 autoantibody biomarkers using the data uploaded directly from Prospector into R. In five replicate runs, the panel of 50 RF-selected biomarkers correctly classified MCI and control Testing Set subjects with an average of 100% overall accuracy, thus showing results comparable to both the first and second round autoantibody biomarker panels derived from prevalence difference. Also, it is noteworthy that 19 and 15 of the top 50 RF-selected biomarkers overlapped with those chosen via prevalence difference in the first and second rounds of biomarker discovery, respectively.
We also preliminarily addressed the question of how many autoantibody biomarkers are required for accurate detection of AD-associated MCI by sorting the top 50 biomarkers according to greatest prevalence difference and then comparing the diagnostic performance of the top 25 and bottom 25 biomarkers on this list. Results revealed only a slight reduction in overall diagnostic accuracy from 100% to 98% for the bottom 25 biomarkers, with the top 10 biomarkers showing an overall accuracy of 98%. These results suggest that most of the top 50 biomarkers are potentially useful as diagnostic indicators and that a smaller panel of 10–15 biomarkers may be sufficient for accurate detection and diagnosis of AD-associated MCI. Of course, final selection of the most useful biomarkers and a proper determination of the number necessary to achieve consistently accurate detection of AD-associated MCI will require a much larger verification study carried out on a broader subject population.
To investigate the possibility that the chosen AD-associated MCI biomarkers might have potential application for distinguishing different stages of disease progression, we tested their capacity to differentiate patients with MCI from those with more advanced (mild-moderate) AD. Results showed that this distinction was made with an overall accuracy of 98.5%, suggesting that this MCI biomarker panel along with its corresponding RF diagnostic logic is specific for early-stage AD associated with MCI. The ability to distinguish different stages or phases of disease progression is a highly desirable feature because it would offer physicians the possibility of monitoring the progress of their patients while under treatment. It would also be invaluable for early enrollment into clinical trials for new therapies where slowing of disease progression would be evidenced by a delayed transition from one phase to the next.
Of course, as with any disease-associated biomarkers, it is critical to demonstrate that the chosen biomarkers are disease specific. This is especially true for neurodegenerative diseases in which the cell types involved in the pathology closely resemble one another. Here, we show that the AD-associated MCI biomarkers were capable of distinguishing ADNI MCI subjects from those with other neurologic (e.g., PD and MS) and non-neurologic (e.g., breast cancer) diseases with exceptionally high accuracy. However, these biomarkers are not useful for distinguishing other neurologic and non-neurologic diseases from controls, thus further confirming their specificity for MCI (Supplementary Fig. 3). At disease stages where telltale symptoms are evident, this need for disease specificity may be less stringent. However, as the use of blood-based biomarkers, including autoantibodies, moves into prodromal disease phases that lack the crutch of “telltale” symptoms, the requirement for disease specificity will become particularly stringent. Additionally, when symptoms of different pathologic conditions resemble those of MCI, such as drug side-effects, poor vascular perfusion of the brain, and chronic depression, these biomarkers will be useful in accurately diagnosing those patients with MCI due only to ongoing AD-related pathology.
The autoantibody biomarker panel used here to distinguish patients with AD-associated MCI from matched controls is presented in Supplementary Table 1. The constituent biomarkers comprise many categories including kinases, potassium channel subunits, ribosomal and mitochondrial proteins, as well as a variety of receptor, adapter, and other accessory proteins, and cytoskeletal components, among others. Based on available information from database searches, proposed functions of some of the selected biomarkers include cell signaling, RNA modification, protein modification, regulation of apoptosis, hematopoiesis, neuron polarization, axon regeneration, synaptic vesicle trafficking, cell proliferation and migration, various inflammatory processes, and many more. As was also true for our previous study describing early-stage PD biomarkers, at first glance, there is a surprising lack of well-known or “usual-suspect” proteins in the list of top biomarkers for AD-associated MCI. Although such expectations represent a bias and may reflect a perception that we understand the pathogenic mechanisms of AD better than we do in reality, it is noteworthy to point out that we are working under the assumption that the autoantibodies being detected are produced by the immune system in response to the release of debris from the site(s) of pathology in the brain.
This study has a number of strengths. The first is that it describes a blood-based diagnostic approach that is independent of symptoms and, because all MCI subjects also possess low CSF Aβ42 levels, presumably linked directly to ongoing pathology. Given that AD has a long prodromal period that may extend for more than a decade during which the pathologic changes are well underway, the use of autoantibodies as biomarkers for AD may open the door to pre-symptomatic detection. Another strength is that this approach takes advantage of the body's own response to the presence of pathology. Unlike many blood proteins and lipids, antibodies are unusually stable in the blood, a fact which ensures that their production and detection will be largely independent of circadian as well as day to day production variations. Also, it is noteworthy to highlight that the biomarker selection process used here is unbiased and that multiple biomarker discovery strategies yielded comparable results and overlapping biomarker panels, providing additional confirmation of the utility of specific autoantibodies as diagnostic indicators of AD-associated MCI. We have taken advantage of recent progress in human protein microarray technology which has allowed us to use a very large number of potential protein targets (nearly 10,000 in this case) and cast as wide a net as possible in an effort to identify potentially useful autoantibody biomarkers. This has also helped us to gain a better appreciation for the complexity of individual autoantibody profiles that are typically present in human blood. Rather than selecting the biomarkers based on a highly restricted subset of likely favorites, we were able to compare the expression levels of thousands of autoantibodies among groups of diseased and nondiseased individuals in an unbiased fashion and identify those that are differentially expressed among the two groups and thus represent potentially useful diagnostic indicators. Additional strengths of this study are the demonstration of disease specificity, especially in the context of other neurodegenerative diseases, and the ability to distinguish different pathologic stages of the disease. For the latter, a much larger study using clinically well-characterized samples (imaging data, CSF, blood work, neuropsychological tests, and so forth) and appropriate matched controls will be necessary to validate this capability and determine the number of stages that can be properly delineated. Finally, in support of their direct link to disease pathology, our data suggest that autoantibodies cannot be effectively used to discriminate between disease stages that do not exhibit substantial differences in the site and/or extent of pathology, as was shown to be the case in our failed attempt to distinguish patients designated by ADNI as EMCI and LMCI.
This study has several weaknesses, the most obvious of which is that it is a very small study intended to be a “proof of concept” study that was focused on addressing the question of whether autoantibodies can potentially be used as biomarkers to detect early-stage AD pathology in patients diagnosed with MCI. As such, it is important to note that the data are limited to this group of ADNI MCI subjects, and it is acknowledged that much larger studies will be needed to determine the utility of the biomarkers chosen here or select additional biomarkers that will be needed for application to the general population. Another limitation was that this study was not longitudinal. A longitudinal study would allow identification of more subtle, individual changes in autoantibody profiles associated with successive stages of disease progression, and then a determination of which changes are common among individuals at the same disease stage. Another weakness is that the age-matched and gender-matched controls used here did not have measurements of CSF Aβ42 levels. Finally, we did not test the efficacy of the AD-associated MCI biomarker panel for use in distinguishing subjects with AD-associated MCI from patients with MCI due to other causes (e.g., cerebrovascular disease, drug side-effects, depression, excessive alcohol use, poor vascular perfusion of the brain, and neurodegeneration unrelated to AD).
In conclusion, we report here a method for early AD diagnosis at the MCI stage. Early diagnosis of dementia has many potential benefits for clinicians, patients, and family members alike. These benefits include, but are not limited to earlier treatment, which may delay symptom progression, enrollment into clinical trials, which could potentially facilitate the development of new therapies and drug targets, as well as the ability to make lifestyle arrangements and manage future medical care. In addition to MCI, this simple blood-based diagnostic method has also been verified in the detection and staging of early-stage PD, suggesting the potential for widespread application of this platform as a multi-disease diagnostic tool.
Research in context.
-
1.
Systematic review: The authors reviewed electronic literature through PubMed, as well as both meeting and conference abstracts and presentations. Publications describing efforts to establish diagnostic biomarkers for AD have been appropriately cited in the text.
-
2.
Interpretation: Our findings lead to the creation of a simple, inexpensive, and noninvasive blood test with the potential to diagnose and stage AD with high overall accuracy, sensitivity, and specificity. To the best of our knowledge, this is the first diagnostic blood test using autoantibody biomarkers capable of detecting early stages of AD.
-
3.
Future directions: This article proposes a “proof of concept” study describing the potential utility of a diagnostic panel of autoantibody biomarkers for the detection and staging of AD. Additional studies using larger, clinically characterized sample cohorts with longitudinal data are needed to further evaluate the utility, and/or modify the chosen biomarker panel for optimal application to the general population.
Acknowledgments
Data collection and sharing for this project were funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer's Association; Alzheimer's Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Author contributions: C.D., E.N., and R.N. wrote the first draft of the article. Microarrays were processed by C.D., N.K.A., G.G., A.S., E.G., M.H., and E.N. All authors contributed to manuscript writing/revision. Statistical analysis was performed by C.D., E.N., M.H., N.K.A., and U.T. MCI serum samples were provided by the ADNI. DATATOP serum samples from Parkinson's disease patients were provided by the Parkinson's Study Group.
This research was supported in part by the Osteopathic Heritage Foundation and the Michael J. Fox Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the article.
We would also like to acknowledge the use of Parkinson's disease samples provided by the Parkinson Study Group.
Footnotes
Data used in the preparation of this article were obtained from the ADNI database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in the analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc/wp-content/uploads/how_to_apply/ADNI_Acknowledgment_List.pdf.
The authors have the following competing interests: R.N. has received research funding from the Michael J. Fox Foundation, the Osteopathic Heritage Foundation, GlaxoSmithKline, the Foundation Venture Capital Group, and the Boye Foundation. These funders had no role in study design, data collection and analysis, decision to publish, or preparation of the article. R.N. is also co-founder of Durin Technologies, Inc., serves as its Chief Scientific Officer, and has received consulting fees. He may accrue revenue in the future based on patents submitted by Rowan University wherein he is a co-inventor. B.B. is also co-founder of Durin Technologies, Inc., serves as its Chief Executive Officer, and has received consulting fees. He may accrue revenue in the future based on patents submitted by Rowan University. A patent has been submitted for the MCI autoantibody biomarker panel. There are no marketed products to declare.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.dadm.2016.03.002.
Supplementary data
Biomarker selection and Training/Testing Set analysis strategy for AD-associated MCI. The total sample pool (n = 100) was randomly split into two groups: a Training Set and Testing Set. Prospector statistical analysis was performed on the Training Set to identify the top 50 most differentially expressed autoantibody classifiers in MCI samples compared to controls. The diagnostic accuracy of these selected biomarkers was tested using Random Forest to predict sample classification in the Training Set. This logic was then applied to Testing Set analysis and then to analysis of both sets combined. The values represent an average of five runs for each of the three steps of the biomarker discovery and validation.
CSF Aβ42 levels in MCI samples. CSF Aβ42 (pg/mL) for EMCI (blue) and LMCI (red) samples.
Specificity of the biomarker panel and diagnostic logic for AD-associated MCI. Comparison of several neurodegenerative controls including early-stage PD, mild-moderate PD, mild-moderate AD, multiple sclerosis, and one non-neurodegenerative control, breast cancer, to normal control samples using the selected panel of 50 biomarkers to assess the specificity of the panel for AD-associated MCI. The low sensitivity and specificity of the ROC curves clearly demonstrates the specificity of the top 50 biomarkers for AD-associated MCI, ruling out the possibility that the chosen biomarkers are nonspecific for CNS degeneration or disease in general. Dashed line represents the line of no discrimination.
References
- 1.Alzheimer's Association 2015 Alzheimer's disease facts and figures. Alzheimers Dement. 2015;11:332–384. doi: 10.1016/j.jalz.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Trojanowski J.Q., Hampel H. Neurodegenerative disease biomarkers: guideposts for disease prevention through early diagnosis and intervention. Prog Neurobiol. 2011;95:491–495. doi: 10.1016/j.pneurobio.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blennow K., de Leon M.J., Zetterberg H. Alzheimer's disease. Lancet. 2006;368:387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- 4.Casey D.A., Antimisiaris D., O'Brien J. Drugs for Alzheimer's disease: are they effective? P T. 2010;35:208–211. [PMC free article] [PubMed] [Google Scholar]
- 5.Braak H., Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging. 1997;18:351–357. doi: 10.1016/s0197-4580(97)00056-0. [DOI] [PubMed] [Google Scholar]
- 6.de Leon M.J., Convit A., Wolf O.T., Tarshish C.Y., DeSanti S., Rusinek H. Prediction of cognitive decline in normal elderly subjects with 2-[(18)F]fluoro-2-deoxy-D-glucose/poitron-emission tomography (FDG/PET) Proc Natl Acad Sci U S A. 2001;98:10966–10971. doi: 10.1073/pnas.191044198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hulette C.M., Welsh-Bohmer K.A., Murray M.G., Saunders A.M., Mash D.C., McIntyre L.M. Neuropathological and neuropsychological changes in “normal” aging: evidence for preclinical Alzheimer disease in cognitively normal individuals. J Neuropathol Exp Neurol. 1998;57:1168–1174. doi: 10.1097/00005072-199812000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Morris J.C., Storandt M., McKeel D.W., Jr., Rubin E.H., Price J.L., Grant E.A. Cerebral amyloid deposition and diffuse plaques in “normal” aging: Evidence for presymptomatic and very mild Alzheimer's disease. Neurology. 1996;46:707–719. doi: 10.1212/wnl.46.3.707. [DOI] [PubMed] [Google Scholar]
- 9.Price J.L., Morris J.C. Tangles and plaques in nondemented aging and “preclinical” Alzheimer's disease. Ann Neurol. 1999;45:358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 10.Petersen R.C. Early diagnosis of Alzheimer's disease: is MCI too late? Curr Alzheimer Res. 2009;6:324–330. doi: 10.2174/156720509788929237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blennow K., Dubois B., Fagan A.M., Lewczuk P., de Leon M.J., Hampel H. Clinical utility of cerebrospinal fluid biomarkers in the diagnosis of early Alzheimer's disease. Alzheimers Dement. 2015;11:58–69. doi: 10.1016/j.jalz.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Vlies A.E., Verwey N.A., Bouwman F.H., Blankenstein M.A., Klein M., Scheltens P. CSF biomarkers in relationship to cognitive profiles in Alzheimer disease. Neurology. 2009;72:1056–1061. doi: 10.1212/01.wnl.0000345014.48839.71. [DOI] [PubMed] [Google Scholar]
- 13.Petersen R.C., Smith G.E., Waring S.C., Ivnik R.J., Tangalos E.G., Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 14.Albert M.S., DeKosky S.T., Dickson D., Dubois B., Feldman H.H., Fox N.C. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henriksen K., O'Bryant S.E., Hampel H., Trojanowski J.Q., Montine T.J., Jeromin A. The future of blood-based biomarkers for Alzheimer's disease. Alzheimers Dement. 2014;10:115–131. doi: 10.1016/j.jalz.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cummings J.L. Biomarkers in Alzheimer's disease drug development. Alzheimers Dement. 2011;7:e13–e44. doi: 10.1016/j.jalz.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Blennow K., Hampel H., Weiner M., Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol. 2010;6:131–144. doi: 10.1038/nrneurol.2010.4. [DOI] [PubMed] [Google Scholar]
- 18.Hulstaert F., Blennow K., Ivanoiu A., Schoonderwaldt H.C., Riemenschneider M., De Deyn P.P. Improved discrimination of AD patients using beta-amyloid(1-42) and tau levels in CSF. Neurology. 1999;52:1555–1562. doi: 10.1212/wnl.52.8.1555. [DOI] [PubMed] [Google Scholar]
- 19.Riemenschneider M., Lautenschlager N., Wagenpfeil S., Diehl J., Drzezga A., Kurz A. Cerebrospinal fluid tau and beta-amyloid 42 proteins identify Alzheimer disease in subjects with mild cognitive impairment. Arch Neurol. 2002;59:1729–1734. doi: 10.1001/archneur.59.11.1729. [DOI] [PubMed] [Google Scholar]
- 20.Hansson O., Zetterberg H., Buchhave P., Londos E., Blennow K., Minthon L. Association between CSF biomarkers and incipient Alzheimer's disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol. 2006;5:228–234. doi: 10.1016/S1474-4422(06)70355-6. [DOI] [PubMed] [Google Scholar]
- 21.Mattsson N., Zetterberg H., Hansson O., Andreasen N., Parnetti L., Jonsson M. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA. 2009;302:385–393. doi: 10.1001/jama.2009.1064. [DOI] [PubMed] [Google Scholar]
- 22.Blennow K., Zetterberg H. Cerebrospinal fluid biomarkers for Alzheimer's disease. J Alzheimers Dis. 2009;18:413–417. doi: 10.3233/JAD-2009-1177. [DOI] [PubMed] [Google Scholar]
- 23.Hampel H., Burger K., Teipel S.J., Bokde A.L., Zetterberg H., Blennow K. Core candidate neurochemical and imaging biomarkers of Alzheimer's disease. Alzheimers Dement. 2008;4:38–48. doi: 10.1016/j.jalz.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Mattsson N., Blennow K., Zetterberg H. CSF biomarkers: pinpointing Alzheimer pathogenesis. Ann N Y Acad Sci. 2009;1180:28–35. doi: 10.1111/j.1749-6632.2009.04944.x. [DOI] [PubMed] [Google Scholar]
- 25.Kang J.H., Korecka M., Toledo J.B., Trojanowski J.Q., Shaw L.M. Clinical utility and analytical challenges in measurement of cerebrospinal fluid amyloid-beta(1-42) and tau proteins as Alzheimer disease biomarkers. Clin Chem. 2013;59:903–916. doi: 10.1373/clinchem.2013.202937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morris G.P., Clark I.A., Vissel B. Inconsistencies and controversies surrounding the amyloid hypothesis of Alzheimer's disease. Acta Neuropathol Commun. 2014;2:135. doi: 10.1186/s40478-014-0135-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Bryant S.E., Xiao G., Barber R., Huebinger R., Wilhelmsen K., Edwards M. A blood-based screening tool for Alzheimer's disease that spans serum and plasma: findings from TARC and ADNI. PLoS One. 2011;6:e28092. doi: 10.1371/journal.pone.0028092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Bryant S.E., Gupta V., Henriksen K., Edwards M., Jeromin A., Lista S. Guidelines for the standardization of preanalytic variables for blood-based biomarker studies in Alzheimer's disease research. Alzheimers Dement. 2015;11:549–560. doi: 10.1016/j.jalz.2014.08.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sattlecker M., Kiddle S.J., Newhouse S., Proitsi P., Nelson S., Williams S. Alzheimer's disease biomarker discovery using SOMAscan multiplexed protein technology. Alzheimers Dement. 2014;10:724–734. doi: 10.1016/j.jalz.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 30.Mapstone M., Cheema A.K., Fiandaca M.S., Zhong X., Mhyre T.R., MacArthur L.H. Plasma phospholipids identify antecedent memory impairment in older adults. Nat Med. 2014;20:415–418. doi: 10.1038/nm.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kiddle S.J., Sattlecker M., Proitsi P., Simmons A., Westman E., Bazenet C. Candidate blood proteome markers of Alzheimer's disease onset and progression: a systematic review and replication study. J Alzheimers Dis. 2014;38:515–531. doi: 10.3233/JAD-130380. [DOI] [PubMed] [Google Scholar]
- 32.Cheng L., Quek C.Y., Sun X., Bellingham S.A., Hill A.F. The detection of microRNA associated with Alzheimer's disease in biological fluids using next-generation sequencing technologies. Front Genet. 2013;4:150. doi: 10.3389/fgene.2013.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagele E.P., Han M., Acharya N.K., DeMarshall C., Kosciuk M.C., Nagele R.G. Natural IgG autoantibodies are abundant and ubiquitous in human sera, and their number is influenced by age, gender, and disease. PLoS One. 2013;8:e60726. doi: 10.1371/journal.pone.0060726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reddy M.M., Wilson R., Wilson J., Connell S., Gocke A., Hynan L. Identification of candidate IgG biomarkers for Alzheimer's disease via combinatorial library screening. Cell. 2011;144:132–142. doi: 10.1016/j.cell.2010.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagele E., Han M., Demarshall C., Belinka B., Nagele R. Diagnosis of Alzheimer's disease based on disease-specific autoantibody profiles in human sera. PLoS One. 2011;6:e23112. doi: 10.1371/journal.pone.0023112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han M., Nagele E., DeMarshall C., Acharya N., Nagele R. Diagnosis of Parkinson's disease based on disease-specific autoantibody profiles in human sera. PLoS One. 2012;7:e32383. doi: 10.1371/journal.pone.0032383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeMarshall C.A., Han M., Nagele E.P., Sarkar A., Acharya N.K., Godsey G. Potential utility of autoantibodies as blood-based biomarkers for early detection and diagnosis of Parkinson's disease. Immunol Lett. 2015;168:80–88. doi: 10.1016/j.imlet.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 38.Trojanowski J.Q., Vandeerstichele H., Korecka M., Clark C.M., Aisen P.S., Petersen R.C. Update on the biomarker core of the Alzheimer's Disease Neuroimaging Initiative subjects. Alzheimers Dement. 2010;6:230–238. doi: 10.1016/j.jalz.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shaw L.M., Vanderstichele H., Knapik-Czajka M., Clark C.M., Aisen P.S., Petersen R.C. Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duits F.H., Teunissen C.E., Bouwman F.H., Visser P.J., Mattsson N., Zetterberg H. The cerebrospinal fluid “Alzheimer profile”: easily said, but what does it mean? Alzheimers Dement. 2014;10:713–723.e2. doi: 10.1016/j.jalz.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 41.Rosen W.G., Terry R.D., Fuld P.A., Katzman R., Peck A. Pathological verification of ischemic score in differentiation of dementias. Ann Neurol. 1980;7:486–488. doi: 10.1002/ana.410070516. [DOI] [PubMed] [Google Scholar]
- 42.Sheikh J.I., Yesavage J.A. Geriatric Depression Scale (GDS) Recent evidence and development of a shorter version. In: Brink T.L., editor. Clinical Gerontology: A Guide to Assessment and Intervention. The Haworth Press, Inc; New York: 1986. pp. 165–173. [Google Scholar]
- 43.Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 44.Morris J.C. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 45.Weiner M.W., Veitch D.P., Aisen P.S., Beckett L.A., Cairns N.J., Green R.C. The Alzheimer's Disease Neuroimaging Initiative: a review of papers published since its inception. Alzheimers Dement. 2013;9:e111–e194. doi: 10.1016/j.jalz.2013.05.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O'Bryant S.E., Xiao G., Barber R., Cullum C.M., Weiner M., Hall J. Molecular neuropsychology: creation of test-specific blood biomarker algorithms. Dement Geriatr Cogn Disord. 2014;37:45–57. doi: 10.1159/000345605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Breiman L. Random Forests. Machine Learning. 2001;45:5–32. [Google Scholar]
- 48.O'Bryant S.E., Xiao G., Barber R., Reisch J., Hall J., Cullum C.M. A blood-based algorithm for the detection of Alzheimer's disease. Dement Geriatr Cogn Disord. 2011;32:55–62. doi: 10.1159/000330750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bazenet C., Lovestone S. Plasma biomarkers for Alzheimer's disease: much needed but tough to find. Biomark Med. 2012;6:441–454. doi: 10.2217/bmm.12.48. [DOI] [PubMed] [Google Scholar]
- 50.Rissman R.A., Trojanowski J.Q., Shaw L.M., Aisen P.S. Longitudinal plasma amyloid beta as a biomarker of Alzheimer's disease. J Neural Transm (Vienna) 2012;119:843–850. doi: 10.1007/s00702-012-0772-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Restrepo L., Stafford P., Magee D.M., Johnston S.A. Application of immunosignatures to the assessment of Alzheimer's disease. Ann Neurol. 2011;70:286–295. doi: 10.1002/ana.22405. [DOI] [PubMed] [Google Scholar]
- 52.DeMarshall C., Sarkar A., Nagele E.P., Goldwaser E., Godsey G., Acharya N.K. Utility of Autoantibodies as Biomarkers for Diagnosis and Staging of Neurodegenerative Diseases. Int Rev Neurobiol. 2015;122:1–51. doi: 10.1016/bs.irn.2015.05.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Biomarker selection and Training/Testing Set analysis strategy for AD-associated MCI. The total sample pool (n = 100) was randomly split into two groups: a Training Set and Testing Set. Prospector statistical analysis was performed on the Training Set to identify the top 50 most differentially expressed autoantibody classifiers in MCI samples compared to controls. The diagnostic accuracy of these selected biomarkers was tested using Random Forest to predict sample classification in the Training Set. This logic was then applied to Testing Set analysis and then to analysis of both sets combined. The values represent an average of five runs for each of the three steps of the biomarker discovery and validation.
CSF Aβ42 levels in MCI samples. CSF Aβ42 (pg/mL) for EMCI (blue) and LMCI (red) samples.
Specificity of the biomarker panel and diagnostic logic for AD-associated MCI. Comparison of several neurodegenerative controls including early-stage PD, mild-moderate PD, mild-moderate AD, multiple sclerosis, and one non-neurodegenerative control, breast cancer, to normal control samples using the selected panel of 50 biomarkers to assess the specificity of the panel for AD-associated MCI. The low sensitivity and specificity of the ROC curves clearly demonstrates the specificity of the top 50 biomarkers for AD-associated MCI, ruling out the possibility that the chosen biomarkers are nonspecific for CNS degeneration or disease in general. Dashed line represents the line of no discrimination.