ABSTRACT
Vascular grafts under 5 mm or less in diameter are not developed due to a problem caused by early thrombus formation, neointimal hyperplasia, etc. Bombyx mori silk fibroin (SF) which has biodegradability and tissue infiltration is focused as tube and coating material of vascular grafts. Coating is an important factor to maintain the strength of the anastomotic region of vascular grafts, and to prevent the blood leak from the vascular grafts after implantation. Therefore, in this research, we focused on the SF concentration of the coating solution, and tissue infiltration and remodeling were compared among each SF concentration. Silk poly (-ethylene) glycol diglycidyl ether (PGDE) coating with concentrations of 1.0%, 2.5%, 5.0%, and 7.5% SF were applied for the double-raschel knitted small-sized vessel with 1.5 mm diameter and 1cm in length. The grafts were implanted in the rat abdominal aorta and removed after 3 weeks or 3 months. Vascular grafts patency was monitored by ultrasound, and morphological evaluation was performed by histopathological examination. SF concentration had no significant effects on the patency rate. However, tissue infiltration was significantly higher in the sample of 2.5% SF in 3 weeks, and 1.0% and 2.5% SF in 3 months. Also, in comparison of length inside of the graft, stenosis were not found in 3 weeks, however, found with 5.0% and 7.5% in 3 months. From these results, it is clear that 2.5% SF coating is the most suitable concentration, based on the characteristics of less stenosis, early tissue infiltration, and less neointimal hyperplasia.
KEYWORDS: artificial vascular graft, endothelial cells, in vivo evaluation, silk fibroin, small diameter
INTRODUCTION
Small diameter artificial vascular grafts play an important role in cardiac and peripheral revascularization. Autologous arterial and venous grafts currently show favorable outcomes for small-diameter bypass grafts.1 However, autologous arterial and venous grafts have material weaknesses, such as vasospasm, limited length, poor quality, and prior use.2 Therefore, conventional vascular prostheses, such as expanded polytetrafluoroethylene (ePTFE) and polyethylene terephthalate (Dacron), are used for small-diameter bypass grafts. Unfortunately, satisfactory results with long-term patency have not been achieved, especially with grafts with an internal diameter of less than 6 mm.2-4 This problem appears to be due to the lack of endothelial cells, thrombus formation by foreign bodies, and intimal hyperplasia at the graft-vein anastomotic site due to compliance mismatch with the native vessels.3,5-8
Silk fibroin (SF) is a natural polymer that is biocompatible, biodegradable and has various processing abilities, which makes it an ideal material for small diameter artificial vascular grafts.9-13 Silk fibers are primarily composed of 2 types of proteins, sericin and SF. Sericin is an antigenic gum-like protein surrounding the fibers. SF is the core filaments of silk consisting of highly organized β-sheet crystal- and semicrystalline regions, which is responsible for silk's elasticity compared with fibers of similar tensile integrity.12
In the present study, SF was used to construct artificial vascular grafts. The biological responses to the SF fibers were comparable with the responses to most other commonly used biomaterials. In addition, SF is susceptible to proteolytic degradation in vivo and is slowly absorbed.14-17
In past research, SF vascular grafts showed one-year patency and the patency rate was higher than that of ePTFE grafts.18 Furthermore, formation of endothelial cells and smooth muscle cells were found in the early-stages. Although previous studies show that SF is useful for knitted tube material,19,20 numerous problems still exist. The knitted SF tube cannot maintain its anastomotic strength. In addition, the tube is highly permeable and significant amounts of blood could leak through the graft during/after implantation. To avoid these disadvantages, it is necessary to apply a coating to the tube material.21 We have used SF as the coating material which has shown its advantages. However, from in our experience the concentration of the SF coating has a great effect on handling specifications during the operation. At high SF concentrations there are difficulties regarding the suture technique. On the other hand, when SF concentrations are too low, the grafts cannot maintain their structural integrity immediately after implantation. The SF concentration affects all of the advantages of SF coating; biocompatibility and improved tissue infiltration. Although it has not been fully evaluated, it seems to have a strong effect on the results of graft implantation.
The purpose of this research was to evaluate the effect of SF concentrations in the coating materials on handling and remodeling of vascular grafts. This was done by conducting physical property tests and implantation studies in the rat abdominal aorta.
RESULTS
Scanning electron microscope images of the 4 types of grafts
Scanning electron microscope (SEM) images of the graft tubes are shown in Figure 1.
FIGURE 1.
Scanning electron microscope images of silk fibroin (SF) knitted graft tube. Inner surface (A) and outer surface (B) are shown.
The inner and outer part of the 4 types of vascular grafts are shown in Figure 2. Spaces were observed in the grafts before coating, and decreased as the coating concentrations increased.
FIGURE 2.
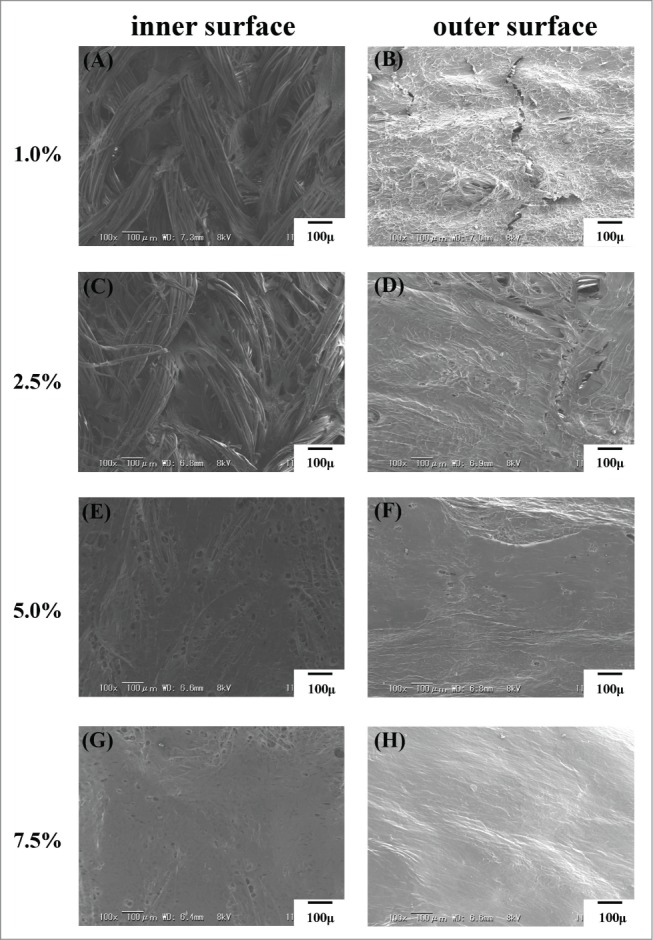
Scanning electron microscope images of coated vascular grafts. Inner and outer surface of the each coating concentration was shown respectively.
The amount of SF of the 4 types of grafts
The amount of SF in each coating is shown in Fig. 3A. The amount of SF was increased as the concentration of SF coating was increased.
FIGURE 3.
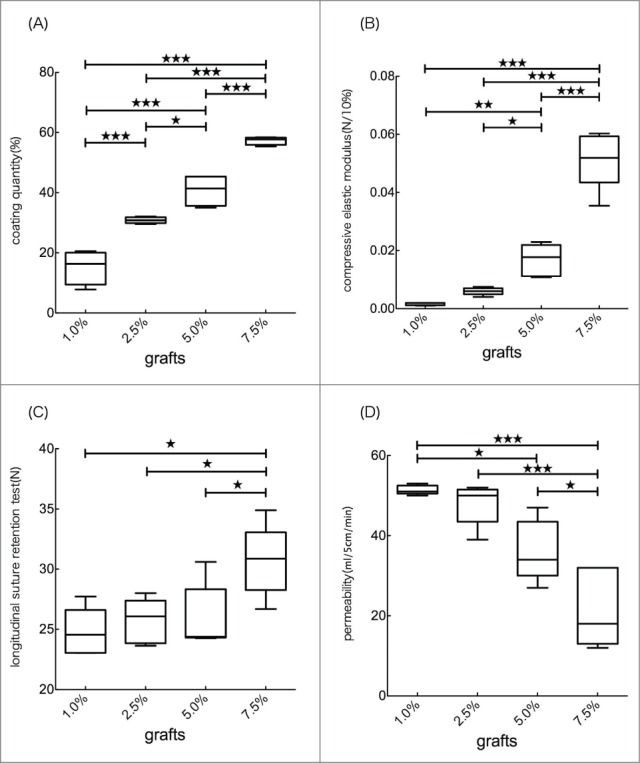
(A) Coating quantity of 4 types of grafts were shown in %. There was significant difference in each grafts. (B) Compression test of 4 types of grafts. The values was interpreted as the compressive elastic modulus (N/10%). There was no significant difference between 1.0% and 2.5%. Compressive elastic modulus in 5.0% and 7.5% was significant higher than that in 1.0% and 2.5%. (C) Longitudinal suture retention test of 4 types of grafts. The values was interpreted as the retention of strength at anastomotic site. There was significant difference between 7.5% and other grafts. (D) Permeability of 4 types of grafts. There was significant difference between 7.5% and other graft.
In vivo experiments
All 48 implantations were performed successfully with no complications such as uncontrolled bleeding. All rats survived until the scheduled date of explantation and were sacrificed either at 3 weeks or at 3 months. Patency of all implanted grafts was observed by an ultrasound Doppler flow study on day one and just before explantation. Patency rate on Day 1 after implantation was 100%. At three weeks, one 2.5% and one 7.5% SF-coated grafts were occluded. At three months, one graft with 5.0% SF coating was occluded (Table 1).
Table 1.
Patency of each graft at 3 weeks and 3 months after implantation
| 1.0% | 2.5% | 5.0% | 7.5% | |
|---|---|---|---|---|
| 3 weeks | 6/6 | 5/6 | 6/6 | 5/6 |
| 3 months | 6/6 | 6/6 | 5/6 | 6/6 |
Gross appearance
The abdominal aortic pulse was monitored just before explantation. The patent grafts removed from rats 3 months after implantation had a clean coated layer on the external surface (Fig. 4). The gross appearance of the inner lumen of the patent grafts showed no thrombus at 3 weeks or 3 months after implantation.
FIGURE 4.
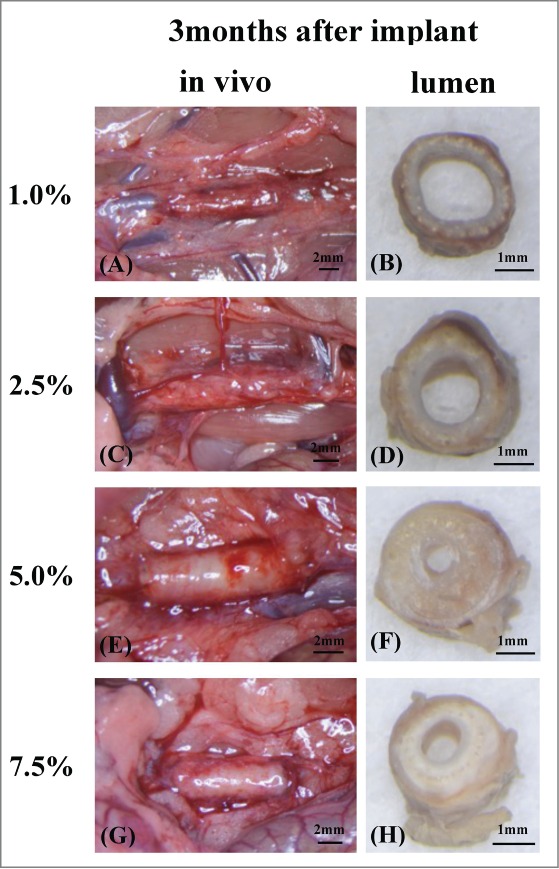
The grafts 3 months after implant just before removed from rat abdominal aortic (A, C, E, G). It was able to confirm much tissue around grafts so that coating density was low. Grafts lumen at the mid-portion were checked after removed from rat (B, D, F, H). There were many coating around grafts in high density. Also lumen became thick than low density grafts because of neointimal hyperplasia (F, H). On the other hand in low density grafts, there were no neointimal hyperplasia and thin inner layer were confirmed and most of coating were decomposed (B, D).
Physical property test
For in vitro graft evaluation, differences in physical properties were observed for all 4 types of vascular grafts. For compressive elastic modulus, 7.5% SF-coating grafts had the highest measurements and significant differences were seen between each of the groups except for between 1.0% and 2.5% SF-coated grafts (Fig. 3B). In the longitudinal suture retention test 7.5% SF-coated grafts had the highest scores, and significant differences were confirmed between the 7.5% and other grafts (Fig. 3C). Permeability tests showed that the 1.0% SF-coated grafts were most permeable (Fig. 3D).
Histopathological examination
The grafts before implantation, graft mid-portions thickness were checked and HE staining and MTC staining were confirmed (Fig. 5).
FIGURE 5.
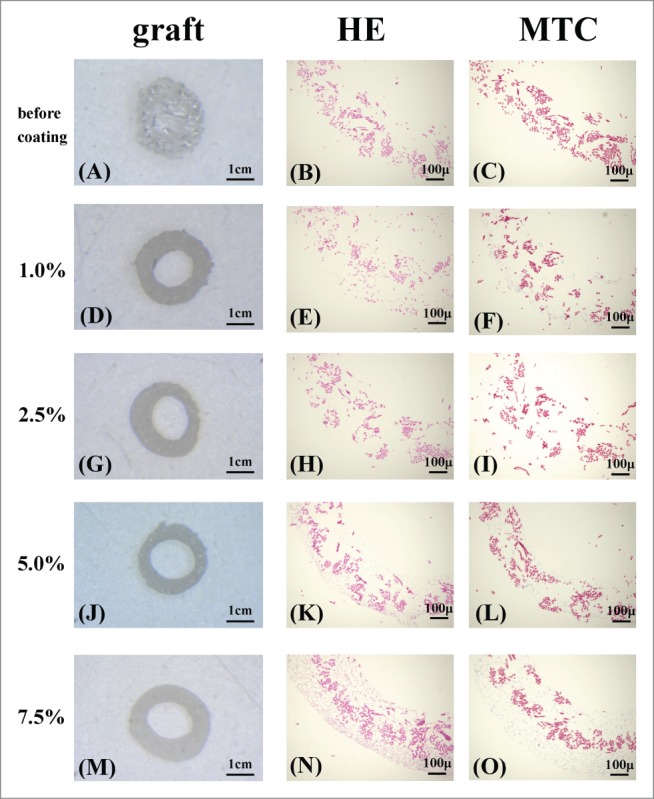
The grafts before implant [(A),(D),(G),(J),(M)]. The graft before coating has a lot of space (A). The space was covered by each concentration coating. Also there was no difference in the graft thickness (D, G, J, M). Histological micrographs of the grafts: Hematoxylin and eosin (HE) staining [before coating (B) 1.0% (E), 2.5% (H), 5.0% (K), 7.5% (N)], Masson trichrome (MTC) staining [before coating (C), 1.0% (F), 2.5% (I), 5.0% (L), 7.5% (O)] of grafts before implant.
Three weeks after implantation, HE staining confirmed inflammatory cells in all 4 types of vascular grafts (Fig. 6). In addition, MTC staining indicated collagen fiber infiltration inside 1.0% and 2.5% SF-coated grafts. On the other hand, collagen fibers were formed around the 5.0% and 7.5% SF-coated grafts.
FIGURE 6.
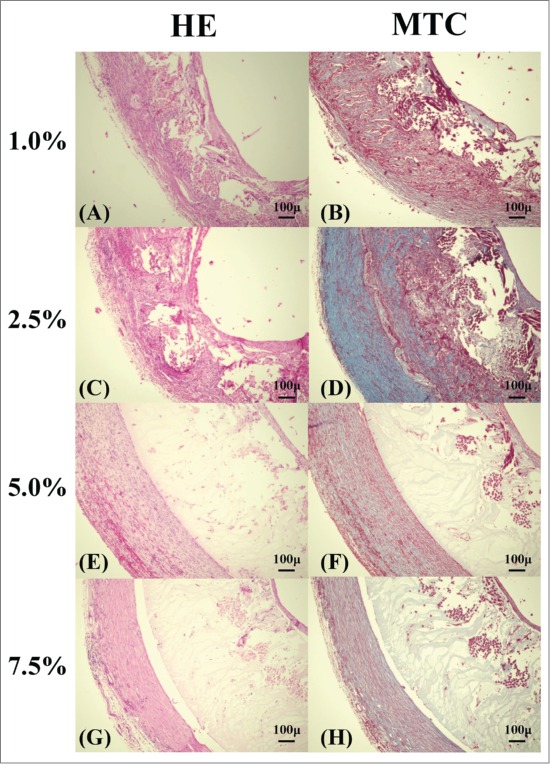
Histological micrographs of the removed grafts: Hematoxylin and eosin (HE) staining [1.0% (A), 2.5% (C), 5.0% (E), 7.5% (G)], Masson trichrome (MTC) staining [1.0% (B), 2.5% (D), 5.0% (F), 7.5% (H)] of grafts removed 3 weeks after implant.
After three months, most of the coating was degraded in 1.0% SF-coated grafts, and tissue infiltration was confirmed around the SF fibers of the grafts (Fig. 7). Collagen fibers decreased in comparison with 2.5% SF-coated grafts after 3 weeks and additional organized infiltration was observed. Furthermore, the collagen fibers around the grafts decreased in the 5.0% and 7.5% SF-coated grafts, with little tissue infiltration inside of these grafts.
FIGURE 7.
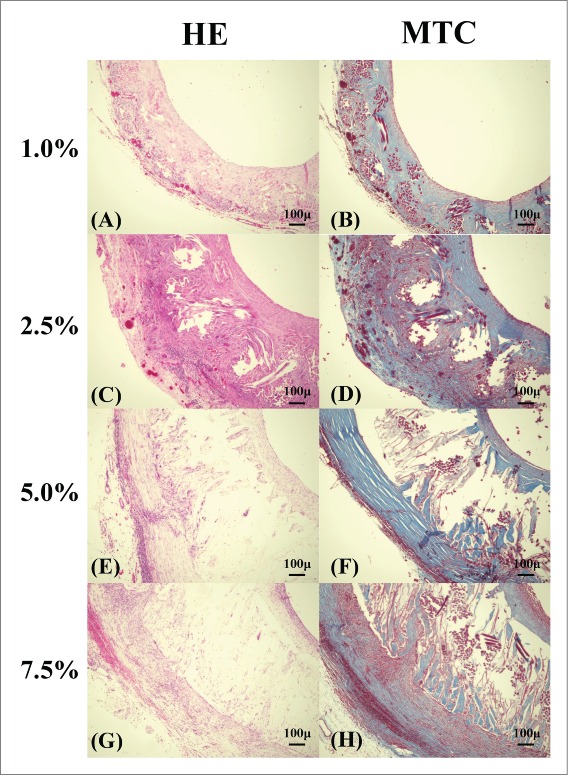
Histological micrographs of the removed grafts: Hematoxylin and eosin (HE) staining [1.0% (A), 2.5% (C), 5.0% (E), 7.5% (G)], Masson trichrome (MTC) staining [1.0% (B), 2.5% (D), 5.0% (F), 7.5% (H)] of grafts removed 3months after implant.
Immunostaining with CD31 was used as a marker of endothelial cells and α-smooth muscle actin was used as marker of smooth muscle cells. Figure 8 shows CD31 and α-smooth muscle actin of 4 types of grafts 3 months after implantation.
FIGURE 8.
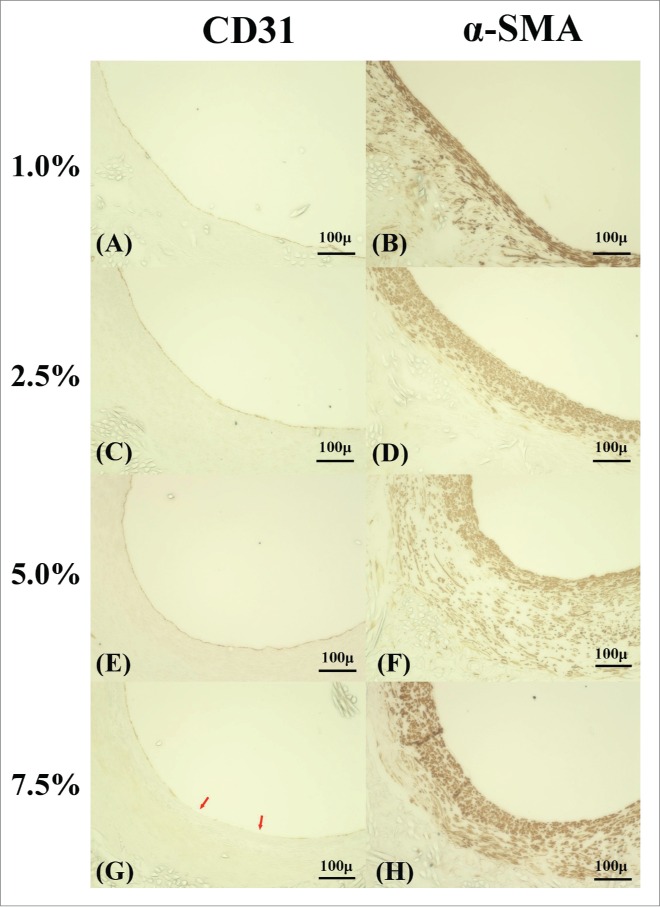
Endothelial cells and smooth muscle cells in 4 types of grafts 3 months after implant. Endothelial cells were stained by CD31 antibody (A, C, E, G), smooth muscle cells were stained by using α-SMA antibody (B, D, F, H). (A, C, E) Endothelial cells complete coverage the luminal surface in 1.0%, 2.5% and 5.0%. (G) Some part of luminal surface in 7.5% wasn't covered by endothelial cells. The red arrow indicates the lack of endothelial cells. (B, D) Thinner smooth muscle cells were formed in 1.0% and 2.5%. (F, H) Thick smooth muscular layer was formed, and a lumen became stenosis.
Endothelial cells were observed on the almost of the luminal surface except for 7.5%. Smooth muscle cells, which were formed on the media of the blood vessel were formed thinly in 1.0% and 2.5%. On the other hand, a thick layer of smooth muscle cells was formed thick in 5.0% and 7.5%. Because the excessive outbreak of the smooth muscle cells caused neointimal hyperplasia, it was thought to be the cause of stenosis of the grafts.
Imaging analysis
Three weeks after implantation, the 2.5% SF-coated grafts had significantly higher tissue infiltration rates compared to the others (Fig. 9A), and 1.0% and 2.5% SF-coated grafts showed significantly higher tissue infiltration compared to 5.0% and 7.5% SF-coated grafts (Fig. 9B). There were no significant differences in internal diameter of the grafts at 3 weeks (Fig. 9C), however, after 3 months, 5.0% and 7.5% SF-coated grafts showed significantly decreased internal diameter compared to the others (Fig. 9D). A single-layer of tissue including smooth muscle cells was found inside the grafts for all grafts types, but it was thin in 1.0% and 2.5% and thick in 5.0% and 7.5% SF-coated grafts.
FIGURE 9.
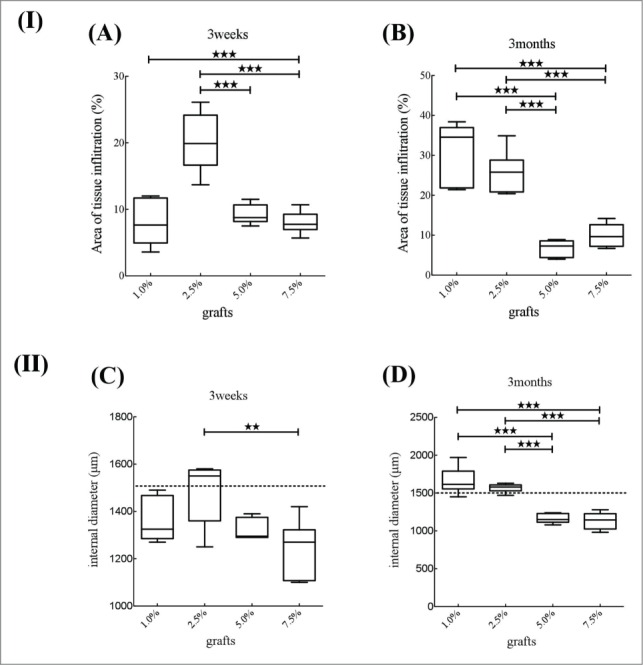
The results of analysis of histopathological examination of the 4 types of grafts. Area of tissue infiltration were calculated in %. (A) Three weeks after implantation, graft with 2.5% SF coating shows significantly higher tissue infiltration rate than other grafts. (B) Three months after implantation, graft with 1.0% and 2.5% SF coating shows significantly higher tissue infiltration compared to that with 5.0% and 7.5% SF coating. Internal diameter were shown in millimeter. (C) Three weeks after implantation, there was significant difference between 2.5% and 7.5%. (D) Three months after implantation, 1.0% and 2.5% had significantly longer compared to 5.0% and 7.5%.
DISCUSSION
Regardless of their size, implanted vascular grafts are subject to suffer from both thrombus formation in early stages of implantation, and neointimal hyperplasia formation in middle or long-term implantation. They are serious problems especially for small diameter artificial vascular grafts.22-26
To improve graft patency, thrombus formation and neointimal hyperplasia should be controlled. Early stage endothelialisation offers a potential solution. In a previous study, it was shown that tissue infiltration in large diameter vascular grafts facilitates endothelialisation.27 SF is known to have a histotropic character and stimulates cell migration.18,28 This character could contribute to endothelialisation in the early stages following implantation.
In this study, tissue infiltration in 1.0% and 2.5% SF-coated grafts was significantly higher than in 5.0% and 7.5% SF-coated grafts 3 months after implantation. Most of the coating material had disappeared after 3 months in 1.0% and 2.5% SF-coated grafts. Therefore, we hypothesized that the coating decomposed as tissue infiltration progressed in the earlier stages and coating materials were completely replaced by the host tissue in 3 months.
Surprisingly, tissue infiltration in 2.5% SF-coated grafts was higher than that of 1% SF-coated grafts 3 weeks after implantation. Generally, 1.0% SF coating is more susceptible to decomposition and induces more tissue infiltration compared to 2.5%, however, 2.5% SF coating showed more tissue infiltration 3 weeks after implantation. Histopathological examinations indicated that the decomposition rates of 1.0% and 2.5% SF coatings were similar and that the characteristic of SF to accelerate the migration of tissue likely induces higher tissue infiltration.18,28 So far, coating materials are considered a negative factor because they act as a physical barrier. However, this study proved that using SF as a coating material could improve tissue infiltration and endothelialisation if used appropriately. Although SF effectively stimulates cell migration, excessive amounts can disturb tissue infiltration. This study shows that 2.5% SF coating is the optimal concentration to accelerate tissue infiltration.
Neointimal hyperplasia is also a serious problem for small diameter artificial vascular grafts, affecting the mid- to long-term results. Even if endothelialisation is complete, neointimal hyperplasia can cause mid- to long-term stenosis or occlusion. Neointimal hyperplasia is caused by the difference in compliance between native vessels and artificial grafts.3,5-8 Decomposition of SF coating, tissue infiltration, and fragmentation of the SF fibers were important factors for normalizing the compliance of implanted grafts. In particular, decomposition of SF coating and tissue infiltration are greatly influenced by coating materials. In this study, excessive vascular intima by smooth muscle cells was observed in 5.0% and 7.5% SF coating and was responsible for stenosis after 3 months, whereas no neointimal hyperplasia was observed in 1.0% and 2.5% SF coating. As the grafts we used in this research are 1cm in length, stenosis observed in 5.0% and 7.5% are due to neo-intimal hyperplasia. Overexpression of smooth muscle cells can cause neo-intimal hyperplasia.5,6,29 Lack of tissue infiltration in 5.0% and 7.5% caused the compliance mismatch. Compliance mismatch can cause and responsible for the turbulent blood flow and also cause overexpression of smooth muscle cells.
After implant the graft endothelial cells is important to prevent thrombus. In this study 3 months after implantation the inner surface was covered withe endothelial cells exclusive of 7.5% grafts. The past study revealed that the tissue infiltration into the grafts will encourage the settlement of endothelial cells.30 Additionally, the stretched capillary vessels from the tissue around the implanted grafts are thought to have effect to the endothelialization.31 In the present research, we could not reveal the cause for the lack of endothelial cells in some graft in 7.5%, however tissue infiltration of 7.5% was significantly low compared to that of 1.0% and 2.5%. Also, 3 month after the implantation, in 7.5%, there are inessential tissue proliferation around the graft, which were thought to inhibit the tissue infiltration like a barrier.
The physical properties of the coating materials are also crucial. The inside diameter of grafts with 1.0% SF coating increased after implantation. Physical property observations further showed that the circumferential tensile strength test and compressive elastic moduli were low in 1.0% SF coating. This indicates that SF tubes cannot retain their form without tissue infiltration and appropriate concentrations of SF. The retention of the suture strength at anastomotic sites also showed the contribution of the SF coating. This strength increased as the coating concentration increased. However, 2.5% SF coating offered sufficient practical strength and a higher concentration was unnecessary.
CONCLUSIONS
To fulfill the demand of small diameter artificial vascular grafts, better tissue infiltration, an unexpandable nature and anastomotic strength are essential factors which can be seriously impacted by the characteristics of coating materials. To optimise the coating material, the composition and concentrations have to be considered.
MATERIALS AND METHODS
Vascular graft tube
The tubes with 1.5 mm inner diameters were made of Bombyx mori SF fiber, using a computer-controlled double-raschel knitting machine (Fukui Tateami Kogyo Co., Ltd., Japan).12,32 Double-raschel knitted tubes were shown to have strength and elasticity.19,33 Scanning electron microscopy (SEM; VE-7800 Keyence, Japan) was used to check the morphology of the SF grafts.19
Coating process applied to graft tubes
The SF tube was coated with SF sponge, which was prepared from an aqueous solution of SF and poly (-ethylene) glycol diglycidyl ether (PGDE) as porogen.19,21,33
PGDE coating has several material characteristics; it prevents leakage of blood from the graft in the implantation stage, adds softness to the graft, holds tissue, and prevents water infiltrations. Furthermore, after implantation PGDE decomposes rapidly.34
The poly (vinylchloride) rod was inserted into the double raschel knitted SF tube and soaked in 1.0%, 2.5%, 5% and 7.5% SF solutions(concentrations that of the SF alone) containing PGDE (SF:PGDE ratio of 1 : 1) for approximately 30 min. The SF graft was then placed under 0.01 MPa reduced pressure until no bubbles were visible from the tube. The tube containing the surrounding the SF/PGDE solution was frozen at -20°C overnight and immersed in distilled water for 3 d to remove the PGDE. The SF graft was sterilized in an autoclave at 120°C for 20 min and kept it in distilled water until the animal implantation experiment.
Weight measurements of coating materials
The weight of coating materials attached to each vascular graft was measured as described by Fuhua et al.35 First, the weight of the graft tube (cut into 10 mm sections) was measured and recorded as the weight before coating. After coating, graft tubes were allowed to air-dry under ambient conditions, and the weight of each graft tube was measured again. This weight was considered as the weight after coating, and the increase in weight after coating [(the weight of the graft tube after coating - the weight before coating)/ the weight of the graft tube after coating] was calculated.
Animals
Female Sprague-Dawley (SD) rats (Charles River Laboratories, Japan) weighing 200–300 g were used for the in vivo study. All rats were kept in micro-isolator cages with a 12-h light/dark cycle. All experimental procedures and protocols were approved by Tokyo University of Agriculture and Technology (Approval number: 26–76), and rats were managed and cared for in accordance with the standards established by Tokyo University of Agriculture and Technology (TUAT) and described in its "Guide for the Care and Use of Laboratory Animals."
Surgical procedure
Four types of vascular grafts (10 mm in length × 1.5 mm inner diameter) were implanted in the rat abdominal aorta. Each type of graft was implanted in 12 rats respectively, 6 of which were removed at 3 weeks and the remaining 6, 3 months after implantation. Thus, 48 rats were used. For graft implantation, rats were anesthetised by intraperitoneal injection of pentobarbital (50 mg/kg body weight). The abdominal aorta was exposed carefully and the aortic branches in this segment were ligated. After intravenous injection of heparin (100 IU/kg), the proximal and distal portions of the infrarenal aorta were clamped with non-crushing vascular clamps. A 10 mm segment of aorta was removed and replaced with a vascular graft by end-to-end anastomosis using interrupted 9–0 monofilament nylon sutures (BEAR, Japan), starting with 2 stay sutures at 180° to each other, then suturing the front wall, followed by the back wall. Each anastomosis required 8–10 stitches. The distal, then the proximal vascular clamps were slowly removed, and flow was restored through the grafts. Total clamp time was 45–60 min. Graft patency was confirmed visually. No anticoagulant or antiplatelet agent was administered postoperatively. The graft patency was monitored by color Doppler imaging (e-flow imaging), and pulse waves were recorded with a 7.5-MHz linear probe and echo-imaging apparatus (Prosound α-10, Hitachi-Aloka, Japan) at one week and again just before explantation, under anesthesia with pentobarbital. Graft diameter, together with any sign of thrombosis or aneurysm formation, were carefully checked by B-mode imaging, and blood flow velocity was measured using a pulsed Doppler flow meter. Before euthanasia, rats underwent a general physical examination to evaluate their condition. At death, the rats were perfused with 0.9% saline solution through the left ventricle. The grafts were carefully removed together with the surrounding tissue.
Physical property observations
A longitudinal suture retention test was performed to evaluate handling, and a compression test was performed to evaluate usefulness as previously described [21, 25].
When suturing vascular grafts, fraying of the grafts is a major problem. We measured the retention strength of the anastomotic part using EZ graph (Shimadzu, Japan) and evaluated whether it is suitable for operation. We cut the grafts into 20 mm sections and passed the sutures 2 mm from the end, pulled by the clamp 3 mm/min by a 100 N operator cell until break point and analysis.
It is important for graft compliance to be able to adjust to the operability and pulsatile environments after transplantation. Therefore, the universal testing machine, EZ graph (Shimadzu, Japan) was used with specific evaluation software to evaluate circumferential compressive moduli of specimens.34
The circumferential compressive elastic modulus of the silk fibroin graft tube was also tested. The test machine which is the same as that used for tensile strength was used. Ring-shaped specimens with an axial length of 10 mm were also prepared for each tube and the outer diameters were measured. Specimens were hydrated in a saline solution for 1 h before testing. The load cell was 5 N and the rate of compression was 2 mm/min. The compressive strength was measured when the specimen was compressed by 10% of the value of the inner diameter. For each test, a stress–strain curve was drawn using the image analysis software installed on the computer as previously described.
The water reservoir was set to apply a hydrostatic pressure to the graft of approximately 120 mmHg. The water permeating through the graft wall was collected and calculated in ml/min/cm2. Ten specimens were tested for each double-raschel tube.
Histopathological examinations
The grafts were cut transversely into 3 equal pieces and graft mid-portions (~4 mm from the end of the graft) were fixed in 2% glutaraldehyde for histological analyses. Fixed samples (6 μm in thickness) were embedded in paraffin and processed for hematoxylin and eosin (HE) staining and Masson's trichrome (MTC) staining. The sections for immunohistochemical staining were incubated with primary antibodies, including α-smooth muscle actin (clone 1A4; Nichirei Biosciences Inc., Japan) or CD31 mouse anti-rat monoclonal antibody (Lifespan biosciences, United States of America). This was followed by incubation with biotinylated anti-mouse immunoglobulin G secondary antibody (Nichirei Biosciences Inc.), and subsequent color development was done using diaminobenzidine solution (Nichirei Biosciences Inc.). Nuclei were counterstained with hematoxylin.
Imaging analysis
Biological reactions of the stained specimens were visualised with the All-in-One fluorescence microscope (Keyence BZ-9000, Keyence): surrounding fibrosis, tissue infiltration into the graft wall, and the length inside of the graft were examined.
The area of the vascular graft and the area of collagenous fibers inside the graft were also measured, and the percentage of tissue infiltration (area of collagenous fibers inside the graft/ area of the vascular graft) was calculated and considered as the degree of biocompatibility (analysis of tissue infiltration).22,35,36
The inside length of the graft was divided by π to measure the diameters of the 4 graft types at 3 weeks and 3 months after implantation. This value was regarded as the degree of intimal hyperplasia.37,38
Statistical analysis
Data are presented as mean ± standard error (SD). Comparison of means was performed by one-way analysis of variance, followed by the Bonferroni post-hoc test. Data analyses were performed using the commercial statistics software package GraphPad Prism (Version 5.0a, GraphPad, San Diego, CA, USA). Statistical significance was defined as P < 0.05.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
ACKNOWLEDGMENT
The authors wish to thank Fukui Tateami Kogyo Co. Ltd. for providing us the tubes of the SF vascular grafts with 1.5 mm diameter.
Funding
This study was funded by a grant from the Ministry of Agriculture, Forestry and Fisheries of Japan (Agri-Health Translational Research Project (2010–2015).
REFERENCE
- 1.Tomizawa Y. Vascular prostheses for aortocoronary bypass grafting: a review. Artif Organs 1995; 19:39-45; PMID:7741637; http://dx.doi.org/ 10.1111/j.1525-1594.1995.tb02242.x [DOI] [PubMed] [Google Scholar]
- 2.Teebken OE, Haverich A. Tissue engineering of small diameter vascular grafts. Eur J Vasc Endovasc Surg 2002; 23:475-85; PMID:12093061; http://dx.doi.org/ 10.1053/ejvs.2002.1654 [DOI] [PubMed] [Google Scholar]
- 3.Pawlowski KJ, Rittgers SE, Schmidt SP, Bowlin GL. Endothelial cell seeding of polymeric vascular grafts. Front Biosci 2004; 9:1412-21; PMID:14977556; http://dx.doi.org/ 10.2741/1302 [DOI] [PubMed] [Google Scholar]
- 4.Faries PL, Logerfo FW, Arora S, Hook S, Pulling MC, Akbari CM, Campbell DR, Pomposelli FB Jr. A comparative study of alternative conduits for lower extremity revascularization: all-autogenous conduit versus prosthetic grafts. J Vasc Surg 2000; 32:1080-90; PMID:11107079; http://dx.doi.org/ 10.1067/mva.2000.111279 [DOI] [PubMed] [Google Scholar]
- 5.Ballyk PD, Walsh C, Butany J, Ojha M. Compliance mismatch may promote graft-artery intimal hyperplasia by altering suture-line stresses. J Biomech 1998; 31:229-37; PMID:9645537; http://dx.doi.org/ 10.1016/S0197-3975(97)00111-5 [DOI] [PubMed] [Google Scholar]
- 6.Haruguchi H, Teraoka S. Intimal hyperplasia and hemodynamic factors in arterial bypass and arteriovenous grafts: a review. J Artif Organs 2003; 6:227-35; PMID:14691664; http://dx.doi.org/ 10.1007/s10047-003-0232-x [DOI] [PubMed] [Google Scholar]
- 7.Kuwabara F, Narita Y, Yamawaki-Ogata A, Satake M, Kaneko H, Oshima H, Usui A, Ueda Y. Long-term results of tissue-engineered small-caliber vascular grafts in a rat carotid arterial replacement model. J Artif Organs 2012; 15:399-405; PMID:22806242; http://dx.doi.org/ 10.1007/s10047-012-0652-6 [DOI] [PubMed] [Google Scholar]
- 8.Pektok E, Nottelet B, Tille JC, Gurny R, Kalangos A, Moeller M, Walpoth BH. Degradation and healing characteristics of small-diameter poly(epsilon-caprolactone) vascular grafts in the rat systemic arterial circulation. Circulation 2008; 118:2563-70; PMID:19029464; http://dx.doi.org/ 10.1161/CIRCULATIONAHA.108.795732 [DOI] [PubMed] [Google Scholar]
- 9.Zhang X, Baughman CB, Kaplan DL. In vitro evaluation of electrospun silk fibroin scaffolds for vascular cell growth. Biomaterials 2008; 29:2217-27; PMID:18279952; http://dx.doi.org/ 10.1016/j.biomaterials.2008.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X, Zhang X, Castellot J, Herman I, Iafrati M, Kaplan DL. Controlled release from multilayer silk biomaterial coatings to modulate vascular cell responses. Biomaterials 2008; 29:894-903; PMID:18048096; http://dx.doi.org/ 10.1016/j.biomaterials.2007.10.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X, Kluge JA, Leisk GG, Kaplan DL. Sonication-induced gelation of silk fibroin for cell encapsulation. Biomaterials 2008; 29:1054-64; PMID:18031805; http://dx.doi.org/ 10.1016/j.biomaterials.2007.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen J, Lu H, Richmond J, Kaplan DL. Silk-based biomaterials. Biomaterials 2003; 24:401-16; PMID:12423595; http://dx.doi.org/ 10.1016/S0142-9612(02)00353-8 [DOI] [PubMed] [Google Scholar]
- 13.Zhang X, Wang X, Keshav V, Wang X, Johanas JT, Leisk GG, Kaplan DL. Dynamic culture conditions to generate silk-based tissue-engineered vascular grafts. Biomaterials 2009; 30:3213-23; PMID:19232717; http://dx.doi.org/ 10.1016/j.biomaterials.2009.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lam KH, Nijenhuis AJ, Bartels H, Postema AR, Jonkman MF, Pennings AJ, Nieuwenhuis P. Reinforced poly(L-lactic acid) fibres as suture material. J Appl Biomater 1995; 6:191-7; PMID:7492810; http://dx.doi.org/ 10.1002/jab.770060308 [DOI] [PubMed] [Google Scholar]
- 15.Rossitch E Jr., Bullard DE, Oakes WJ. Delayed foreign-body reaction to silk sutures in pediatric neurosurgical patients. Child's Nerv Syst 1987; 3:375-8; PMID:3329961; http://dx.doi.org/ 10.1007/BF00270712 [DOI] [PubMed] [Google Scholar]
- 16.Salthouse TN, Matlaga BF, Wykoff MH. Comparative tissue response to six suture materials in rabbit cornea, sclera, and ocular muscle. Am J Ophthalmol 1977; 84:224-33; PMID:888893; http://dx.doi.org/ 10.1016/0002-9394(77)90856-X [DOI] [PubMed] [Google Scholar]
- 17.Soong HK, Kenyon KR. Adverse reactions to virgin silk sutures in cataract surgery. Ophthalmology 1984; 91:479-83; PMID:6377167; http://dx.doi.org/ 10.1016/S0161-6420(84)34273-7 [DOI] [PubMed] [Google Scholar]
- 18.Enomoto S, Sumi M, Kajimoto K, Nakazawa Y, Takahashi R, Takabayashi C, Asakura T, Sata M. Long-term patency of small-diameter vascular graft made from fibroin, a silk-based biodegradable material. J Vasc Surg 2010; 51:155-64; PMID:19954921; http://dx.doi.org/ 10.1016/j.jvs.2009.09.005 [DOI] [PubMed] [Google Scholar]
- 19.Aytemiz D, Sakiyama W, Suzuki Y, Nakaizumi N, Tanaka R, Ogawa Y, Takagi Y, Nakazawa Y, Asakura T. Small-diameter silk vascular grafts (3 mm diameter) with a double-raschel knitted silk tube coated with silk fibroin sponge. Adv Healthc Mater 2013; 2:361-8; PMID:23184438; http://dx.doi.org/ 10.1002/adhm.201200227 [DOI] [PubMed] [Google Scholar]
- 20.Fukayama T, Takagi K, Tanaka R, Hatakeyama Y, Aytemiz D, Suzuki Y, Asakura T. Biological reaction to small-diameter vascular grafts made of silk fibroin implanted in the abdominal aortae of rats. Ann Vasc Surg 2015; 29:341-52; PMID:25449988; http://dx.doi.org/ 10.1016/j.avsg.2014.10.008 [DOI] [PubMed] [Google Scholar]
- 21.Min S, Gao X, Liu L, Tian L, Zhu L, Zhang H, Yao J. Fabrication and characterization of porous tubular silk fibroin scaffolds. J Biomater Sci Polym Ed 2009; 20:1961-74; PMID:19793450; http://dx.doi.org/ 10.1163/156856208X396056 [DOI] [PubMed] [Google Scholar]
- 22.Soldani G, Losi P, Bernabei M, Burchielli S, Chiappino D, Kull S, Briganti E, Spiller D. Long term performance of small-diameter vascular grafts made of a poly(ether)urethane-polydimethylsiloxane semi-interpenetrating polymeric network. Biomaterials 2010; 31:2592-605; PMID:20035992; http://dx.doi.org/ 10.1016/j.biomaterials.2009.12.017 [DOI] [PubMed] [Google Scholar]
- 23.Baguneid M, de Mel A, Yildirimer L, Fuller BJ, Hamilton G, Seifalian AM. In vivo study of a model tissue-engineered small-diameter vascular bypass graft. Biotechnol Appl Biochem 2011; 58:14-24; PMID:21446955; http://dx.doi.org/ 10.1002/bab.8 [DOI] [PubMed] [Google Scholar]
- 24.Shin YM, Lee YB, Kim SJ, Kang JK, Park JC, Jang W, Shin H. Mussel-inspired immobilization of vascular endothelial growth factor (VEGF) for enhanced endothelialization of vascular grafts. Biomacromolecules 2012; 13:2020-8; PMID:22617001; http://dx.doi.org/ 10.1021/bm300194b [DOI] [PubMed] [Google Scholar]
- 25.McClure MJ, Simpson DG, Bowlin GL. Tri-layered vascular grafts composed of polycaprolactone, elastin, collagen, and silk: Optimization of graft properties. J Mech Behav Biomed Mater 2012; 10:48-61; PMID:22520418; http://dx.doi.org/ 10.1016/j.jmbbm.2012.02.026 [DOI] [PubMed] [Google Scholar]
- 26.Janairo RR, Henry JJ, Lee BL, Hashi CK, Derugin N, Lee R, Li S. Heparin-modified small-diameter nanofibrous vascular grafts. IEEE Trans Nanobioscience 2012; 11:22-7; PMID:22434651; http://dx.doi.org/ 10.1109/TNB.2012.2188926 [DOI] [PubMed] [Google Scholar]
- 27.Noishiki Y, Tomizawa Y, Yamane Y, Matsumoto A. Autocrine angiogenic vascular prosthesis with bone marrow transplantation. Nat Med 1996; 2:90-3; PMID:8564850; http://dx.doi.org/ 10.1038/nm0196-90 [DOI] [PubMed] [Google Scholar]
- 28.Nakazawa Y, Sato M, Takahashi R, Aytemiz D, Takabayashi C, Tamura T, Enomoto S, Sata M, Asakura T. Development of Small-Diameter Vascular Grafts Based on Silk Fibroin Fibers from Bombyx mori for Vascular Regeneration. J Biomater Sci Polym Ed 2010; 22(1-3):195-206 [DOI] [PubMed] [Google Scholar]
- 29.Klinkert P, Post PN, Breslau PJ, van Bockel JH. Saphenous vein versus PTFE for above-knee femoropopliteal bypass. A review of the literature. Eur J Vasc Endovasc Surg 2004; 27:357-62; PMID:15015183; http://dx.doi.org/ 10.1016/j.ejvs.2003.12.027 [DOI] [PubMed] [Google Scholar]
- 30.Narayan D, Venkatraman SS. Effect of pore size and interpore distance on endothelial cell growth on polymers. J Biomed Mater Res A 2008; 87:710-8; PMID:18200559; http://dx.doi.org/ 10.1002/jbm.a.31749 [DOI] [PubMed] [Google Scholar]
- 31.Noishiki Y, Yamane Y, Tomizawa Y, Matsumoto A. Transplantation of autologous tissue fragments into an e-PTFE graft with long fibrils. Artif Organs 1995; 19:17-26; PMID:7741633; http://dx.doi.org/ 10.1111/j.1525-1594.1995.tb02239.x [DOI] [PubMed] [Google Scholar]
- 32.Makaya K, Terada S, Ohgo K, Asakura T. Comparative study of silk fibroin porous scaffolds derived from salt/water and sucrose/hexafluoroisopropanol in cartilage formation. J Biosci Bioeng 2009; 108:68-75; PMID:19577196; http://dx.doi.org/ 10.1016/j.jbiosc.2009.02.015 [DOI] [PubMed] [Google Scholar]
- 33.Yagi T, Sato M, Nakazawa Y, Tanaka K, Sata M, Itoh K, Takagi Y, Asakura T. Preparation of double-raschel knitted silk vascular grafts and evaluation of short-term function in a rat abdominal aorta. J Artif Organs 2011; 14:89-99; PMID:21344164; http://dx.doi.org/ 10.1007/s10047-011-0554-z [DOI] [PubMed] [Google Scholar]
- 34.Asakura T, Saotome T, Aytemiz D, Shimokawatoko H, Yagi T, Fukayama T, et al.. Characterization of silk sponge in the wet state using13C solid state NMR for development of a porous silk vascular graft with small diameter. RSC Adv 2014; 4:4427-34; http://dx.doi.org/ 10.1039/C3RA45190A [DOI] [Google Scholar]
- 35.Huang F, Sun L, Zheng J. In vitro and in vivo characterization of a silk fibroin-coated polyester vascular prosthesis. Artif Organs 2008; 32:932-41; PMID:19133021; http://dx.doi.org/ 10.1111/j.1525-1594.2008.00655.x [DOI] [PubMed] [Google Scholar]
- 36.Wu HC, Wang TW, Kang PL, Tsuang YH, Sun JS, Lin FH. Coculture of endothelial and smooth muscle cells on a collagen membrane in the development of a small-diameter vascular graft. Biomaterials 2007; 28:1385-92; PMID:17141865; http://dx.doi.org/ 10.1016/j.biomaterials.2006.11.012 [DOI] [PubMed] [Google Scholar]
- 37.Ao PY, Hawthorne WJ, Vicaretti M, Fletcher JP. Development of intimal hyperplasia in six different vascular prostheses. Eur J Vasc Endovasc Surg 2000; 20:241-9; PMID:10986022; http://dx.doi.org/ 10.1053/ejvs.2000.1177 [DOI] [PubMed] [Google Scholar]
- 38.Isenberg BC, Williams C, Tranquillo RT. Small-diameter artificial arteries engineered in vitro. Circ Res 2006; 98:25-35; PMID:16397155 [DOI] [PubMed] [Google Scholar]


