Abstract
Background and Purpose
Mechanisms of skill learning are paramount components for stroke recovery. Recent noninvasive brain stimulation studies demonstrated that decreasing activity in the contralesional motor cortex might be beneficial, providing transient functional improvements after stroke. The more crucial question, however, is whether this intervention can also enhance the acquisition of complex motor tasks, yielding longer-lasting functional improvements. In the present study, we tested the capacity of cathodal transcranial direct current stimulation (tDCS) applied over the contralesional motor cortex during training to enhance the acquisition and retention of complex sequential finger movements of the paretic hand.
Method
Twelve well-recovered chronic patients with subcortical stroke attended 2 training sessions during which either cathodal tDCS or a sham intervention were applied to the contralesional motor cortex in a double-blind, crossover design. Two different motor sequences, matched for their degree of complexity, were tested in a counterbalanced order during as well as 90 minutes and 24 hours after the intervention. Potential underlying mechanisms were evaluated with transcranial magnetic stimulation.
Results
tDCS facilitated the acquisition of a new motor skill compared with sham stimulation (P=0.04) yielding better task retention results. A significant correlation was observed between the tDCS-induced improvement during training and the tDCS-induced changes of intracortical inhibition (R2=0.63).
Conclusions
These results indicate that tDCS is a promising tool to improve not only motor behavior, but also procedural learning. They further underline the potential of noninvasive brain stimulation as an adjuvant treatment for long-term recovery, at least in patients with mild functional impairment after stroke.
Keywords: motor learning, rehabilitation, stroke recovery, tDCS
Motor recovery after a stroke is fundamentally driven by the process of reacquiring motor skills that were lost due to the brain lesion. Thus, to a significant degree, the success of neurorehabilitation depends on the amount and effectiveness of rehabilitative training.1,2
The advent of noninvasive techniques to stimulate the brain (NIBS) has allowed to influence processes of neuronal synaptic plasticity and reorganization and may prove to be of great value for stroke rehabilitation.3–6 Among NIBS techniques, transcranial direct current stimulation (tDCS) is a safe, well-tolerated method, which has been shown to induce prolonged excitability changes in humans’ cortical regions, resulting in LTP/LTD-like synaptic modifications, a cellular correlate of learning and memory.6,7 To date, the mechanisms mediating these effects are not completely understood but animal and pharmacological evidence suggests that changes in γ-aminobutyric acidergic neurotrans-mission and N-methyl-D-aspartate-receptor activity might play a relevant role.8,9
Recently, tDCS was used to test whether cortical stimulation can improve hand and arm functions in the subacute and chronic phases after stroke. Based on an “interhemispheric competition model” in which motor deficits are suggested to be at least in part due to reduced output from the damaged hemisphere and increased inhibitory influence from the intact toward the stroke hemisphere,5,10 2 different therapeutic approaches have been tested. One was based on enhancing the output of the lesioned motor cortex (M1), the other was based on reducing the inhibitory influence of the contralesional M1 (cM1) toward the ipsilesional M1.11,12 Recent work suggested however also that the nonbeneficial role of contralesional cortical structures in the process of stroke recovery may not apply to all patients and to all time points after stroke.13,14 Up to date, the majority of studies were designed to evaluate the effects of stimulation on motor performance.11,12,15 However, based on recent animal and human studies, synchronous application of brain stimulation and motor learning might share synergistic impacts on synaptic plasticity and network reorganization, resulting in larger behavioral effects.8,16,17 Based on this notion, it is reasonable to assume that coupling the effects of tDCS and training might potentiate the acquisition and subsequent retention of a motor task to a level unattained by training alone.
In the present double-blind, sham-controlled study, we investigated the behavioral consequences of downregulating the excitability of the cM1 during a finger sequence training task in a sample of well-recovered patients with chronic stroke with subcortical lesions. We hypothesized enhanced skill acquisition and greater retention in the follow-up periods when training is combined with cathodal tDCS to cM1.
Materials and Methods
Patients
Twelve patients with a history of first-ever unilateral, subcortical ischemic stroke (6 women; mean age, 58.3 years; range, 31–73 years) who met the inclusion criteria for tDCS and transcranial magnetic stimulation (TMS) participated in the study (Table). All patients had demonstrated severe hand deficit at stroke onset (Medical Research Council grade <2) and subsequently recovered to the point of being able to perform the motor task used in this study. All subjects were free from neglect, aphasia, hemianopsia, serious cognitive impairment (Mini-Mental State Examination >23 of 30), and were not taking any central nervous system-affecting drugs. None of the patients was professional pianist or trained as a typist. The study was approved by the local institutional ethics committee and a written informed consent was obtained from all patients according to the declaration of Helsinki (www.wma.net/en/30publications).
Table.
Patient Data
| Patient No. | Age, y | Sex | Time After Stroke, mo | Lesion Site | Handedness (EDS) | MMSE | MRC | FMS | ASS |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 73 | F | 45 | R lenticular | R | 29/30 | 4.8 | 62/66 | 2 |
| 2 | 61 | M | 24 | R corona radiata | R | 27/30 | 5 | 64/66 | 0.5 |
| 3 | 45 | F | 36 | L globus pallidus | R | 29/30 | 4.8 | 64/66 | 0 |
| 4 | 39 | F | 42 | L thalamocapsular | R | 30/30 | 4.9 | 65/66 | 0 |
| 5 | 64 | F | 12 | R caudatolenticular | R | 28/30 | 4.5 | 61/66 | 0 |
| 6 | 68 | F | 64 | R corona radiata | R | 29/30 | 5 | 64/66 | 0 |
| 7 | 31 | F | 24 | L thalamocapsular | R | 30/30 | 5 | 65/66 | 0 |
| 8 | 48 | M | 22 | R lenticulocapsular | R | 28/30 | 4.8 | 64/66 | 0.5 |
| 9 | 67 | M | 18 | R frontal subcortical | R | 30/30 | 4.8 | 64/66 | 0 |
| 10 | 67 | M | 54 | L striatocapsular | R | 30/30 | 4.5 | 63/66 | 0.5 |
| 11 | 65 | M | 16 | L corona radiata | R | 29/30 | 5 | 65/66 | 0 |
| 12 | 72 | M | 44 | R lenticular–capsular | R | 30/30 | 5 | 65/66 | 0 |
| Mean | 58.3 | M | 30 | R=7, L=5 | . . . | 29/30 | 4.8 | 64/66 | 0.2 |
EDS indicates Edinburgh Handedness Scale; MMSE, Mini-Mental State Examination; MRC, Medical Research Council score of the upper extremity; FMS, Fugl-Meyer Scale of the upper extremity; ASS, Ashworth Spasticity Scale of the upper extremity; F, female; M, male; R, right; L, left.
Motor Task and Experimental Design
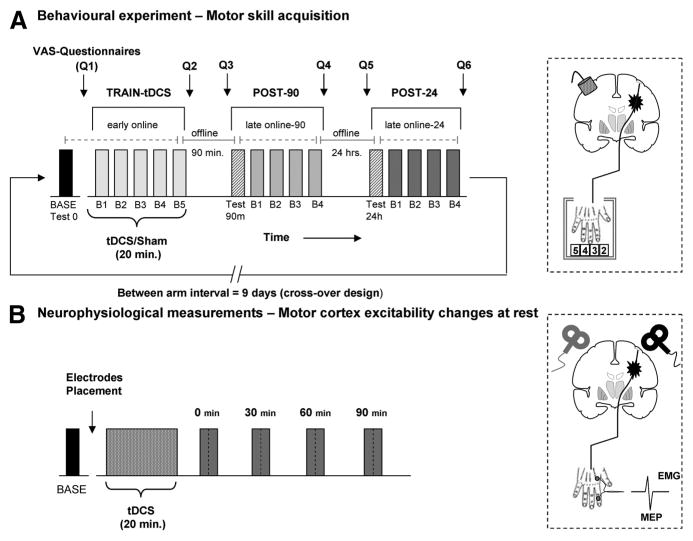
The task used in the current study is a well-established explicit finger movement task that engages activity in a distributed motor network.18,19 It consists of sequential pressing of a 5-element sequence on a 4-button electronic keyboard with the paretic hand as quickly and accurately as possible. Each of the participants (n=12) took part in 2 different arms separated by an interval of (9.2±2.2 days); each arm was divided into 3 different sessions: TRAIN-tDCS, 90 minutes after (POST-90), and 24 hours after (POST-24; see Figure 1A). During each arm, patients practiced a different motor sequence with a similar degree of complexity, length and number of repetitions confirmed by the Kolmogorov index successfully used in previous studies.20,21 The sequences were presented in a counterbalanced order across arms (eg, Sequence A: 3-5-2-4-2, Sequence B: 5-2-4-3-4). During the study, patients were seated in an armchair in front of a 20-inch screen monitor; the distance between the chair and keyboard was adjusted for subject comfort and kept constant during the whole study. Within each study arm, first, patients were familiarized with the task and then tested for their baseline performance. After baseline, they underwent a first training session (TRAIN-tDCS) composed of 5 blocks of 3 minutes each with 2 minutes breaks in between. Given that our main purpose was to evaluate whether the TRAIN-tDCS session could influence the performance in consecutive follow-up periods, 2 further retrain sessions were completed after 90 minutes and 24 hours (POST-90 and POST-24) organized in 4 blocks. Before each session, a retest block of 3 minutes was recorded to determine behavioral retention (Test-90 and Test-24) as our main outcome. As classically defined, retention has been used here to test performance of the motor task after an interval of practice to assess learning as the result of offline and online gains.22,23 A subgroup of 5 patients was additionally evaluated 90 days after training for long-term retention. The primary outcome measure was the number of correct sequences performed during each 3-minute block. Additionally, the total number of performed sequences in each block was analyzed as a secondary outcome measure (for a detailed description, see online-only Data Supplement Methods).
Figure 1.
Experimental design. A, After baseline (BASE), patients attended a training composed of 5 blocks (B1–B5) combined with tDCS or sham (TRAIN-tDCS) 90 minutes and 24 hours after the effects were re-evaluated in a testing block followed by 4 blocks of practice (POST-90 and POST-24). VAS questionnaires were recorded before and after each session. Online and offline effects were analyzed (see “Methods”). The diagram illustrates the position of the tDCS electrodes with the cathode placed over the projection of cM1. B, In a different experiment, MEP and SICI were measured before and after tDCS in both hemispheres in a counterbalanced order. tDCS indicates transcranial direct current stimulation; VAS, visual analog scale; cM1, contralesional motor cortex; MEP, motor-evoked potential; SICI, short interval intracortical inhibition.
Notably, the present design allowed to assess the temporal components of motor skill acquisition within and between each session as follows. Initially, fast learning with considerable within-session improvement can be expected for a novel and challenging motor task (“early online effects”).16,24 Further improvements can be expected after consecutive sessions of practice (“late online effects”).25 During the intermediate periods, between successive sessions, different phenomena occur with an either increased maintenance or deterioration of motor performance defined as “offline effects.”26
Additionally, we examined factors that might potentially influence motor learning such as attention level, perception of fatigue, and discomfort/pain due to the stimulation; they were assessed for both interventions in each session using visual analog scale questionnaires. After the completion of TRAIN-tDCS, patients were asked to identify whether they had received “real” or “placebo” stimulation.
Transcranial Direct Current Stimulation
The initial TRAIN-tDCS session was performed at the time of receiving cathodal tDCS or sham stimulation (Figure 1). tDCS was delivered through 2 sponge electrodes embedded in a saline-soaked solution; the surface of each electrode was 25 cm2 (Eldith, DC-stimulator; Neuroconn). The cathode was positioned on the projection of the hand knob area of the cM1 on the subject’s scalp, whereas the anode was placed on the contralateral supraorbital region.11 The hand knob area of the motor cortex was identified in each patient by single-pulse TMS (70-mm figure-8 coil; Magstim, Dyfed, UK) by standardized procedures.27 tDCS was applied for 20 minutes; the current was initially increased in a ramp-like fashion over several seconds (8 seconds) until reaching 1 mA (current density of 0.04 mA/cm2) as described in previous studies.10,11 During sham, just like during real tDCS, stimulation was started in a ramp-like fashion but faded out slowly after 30 seconds, a procedure demonstrated to warrant successful blinding.10 In this crossover design, each subject performed the experiment with each stimulation type in a pseudorandomized order. The participants and the examiner were blind for the type of stimulation.
Motor Cortical Excitability Determined by TMS
In a separate experiment, changes in corticospinal excitability and short interval intracortical inhibition (SICI) after cathodal tDCS application to the cM1 were evaluated in both hemispheres during rest with well-established single and paired-pulse TMS protocols.28 TMS was performed as follows: before tDCS (baseline); immediately; 30 minutes; 60 minutes and 90 minutes after offset of tDCS. The rationale of the present design was to determine the neurophysiological effects of cathodal tDCS in patients with stroke over both contralesional and ipsilesional M1. Seven of the 12 patients completed this experiment (for a detailed description, see the online-only Data Supplement Methods).
Data Acquisition and Analysis
Patients’ motor performance was recorded with an ergonomic 4-button electronic keyboard connected to a computer using Presentation software (Version 0.61; Neurobehavioral System, Albany, CA). For further analysis, we used a custom-made software routine using Matlab (Version 7.1.0.246; The MathWorks, Natick, MA) to automatically record the number of overall and correct sequences in each block. Normal distribution of the data was assessed by Kolmogorov–Smirnov tests. Repeated-measure analyses of variance were used to evaluate: (1) the level of attention and perception of fatigue toward the task with the factors INTERVENTION and VAS-TIME; (2) the effects of INTERVENTION on behavioral measurement during TRAIN-tDCS; and (3) the effects of INTERVENTION on Re-TEST. Online gains were calculated between the last and first block of each session (eg, Train-tDCSB5/Train-tDCSBase) and offline effects were assessed by contrasting the first block of the following and the last block of the previous session (eg, Test-90B1/Train-tDCSB5).16 Because of the small sample size, the effects of tDCS on TMS-TIME were assessed separately in each hemisphere by nonparametric Friedman analysis of variance and Wilcoxon signed-rank test for post hoc comparisons. All calculations with repeated-measure analysis of variance were Greenhouse-Geisser-corrected; post hoc testing was corrected for multiple comparisons if necessary. All statistical analyses were conducted with SPSS 15.0 (SPSS for Windows 15.0; SPSS, Chicago, IL). The level of significance was set at P<0.05.
Results
All patients completed the study; none of them reported any adverse effects with tDCS or TMS. All data were normally distributed as evaluated by Kolmogorov-Smirnov goodness-of-fit tests. There was no difference in pain or discomfort perception between sham and tDCS stimulation (t[11]=0.84, P=0.41); neither did the type of stimulation or session influence attention, fatigue, or hand tiredness (for statistics, see online-only Data Supplement Table I). Moreover, none of the patients were able to distinguish between tDCS and sham stimulation.
Carryover Effect Between Arms
Baseline comparisons revealed comparable performance for the 2 experimental stimulation conditions for correct sequences (BasetDCS=43.4±7.6, BaseSham=45.7±7.9; t[11]=1.2, P=0.26) and overall sequences (BasetDCS=60.2±8.6, BaseSham=63.4±8.9; t[11]=1.2, P=0.22). Additionally, the order of the sequence (Sequence A versus Sequence B) did not have any impact on the baseline performance in correct sequences (Base1st=43.1±7.5, Base2nd=46.3±8.2; t[11]=1.4, P=0.17) nor in overall sequences (Base1st=60.2±8.1, Base2nd=64.2±9.5; t[11]=1.7, P=0.11), consistent with the absence of relevant carryover effects.
Effects of Simulation on Retention
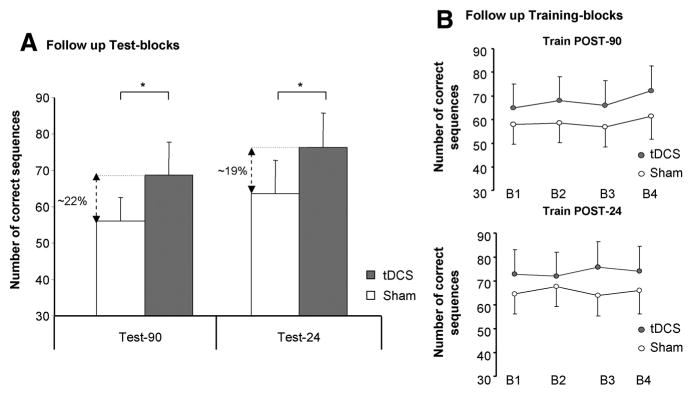
The analysis of the retest blocks at follow-up sessions revealed a significant effect of INTERVENTION (F[1,11]=5.1, P=0.04) and TEST (F[2,22]=29.9, P<0.01) in the number of correct sequences; furthermore, there was a significant INTERVENTION by TEST interaction (F[2,22]=4.2, P=0.02). Post hoc Scheffé test demonstrated that Test-90 and Test-24 were significantly different between tDCS and sham at 5% level (tDCSTest-90=68.8±9.1 and shamTest-90=56.1±6.4; tDCSTest-24=76.4±9.2, shamTest-24=63.5±9.1). Temporal components of skill acquisition (online and offline effects) in the follow-up periods demonstrated no differences in late online learning for both conditions in POST-90 (t[11]=0.26, P=nonsignificant) nor in POST-24 (t[11]=1.3, P=nonsignificant; Figure 2) in correct sequences. Furthermore, offline changes did not differ between the interventions for POST-90 (t[11]=−0.3, P=nonsignificant) and for POST-24 (t[11]=2.3, P=nonsignificant). Nonparametric Wilcoxon test revealed no significant difference between the correct sequences performed within INTERVENTION at the 3-month follow-up (tDCSTest-3m=49.1±9.2, shamTest-3m=47.4±9.3, Z=0.9, P=0.3).
Figure 2.
Follow-up sessions. A, The bar graph shows the number of correct sequences evaluated at Test-90 and Test-24; the percentages of improvement for tDCS compared with sham stimulation are included in the graph for both test blocks (*Scheffé post hoc P<0.05). B, Practice sessions performed at Post-90 minutes and Post-24 (late online learning) hours did not show further behavioral improvement (error bars=SEM). tDCS indicates transcranial direct current stimulation.
Effects of Stimulation on the TRAIN-tDCS Session
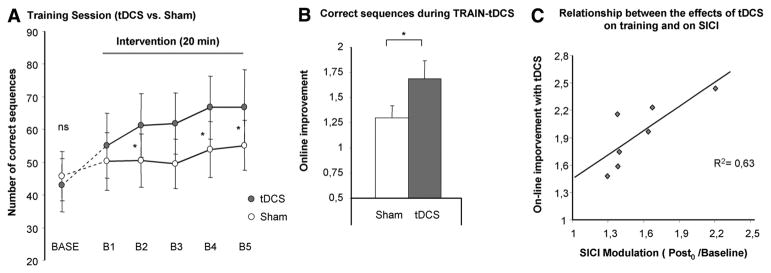
Repeated-measure analysis of variance revealed a significant improvement in the number of correct sequences with cathodal tDCS compared with sham stimulation demonstrated by the factor INTERVENTION (F[1,11]=4.7, P=0.04); there was also a significant effect on BLOCKS (F[5,55]=10.9, P<0.01); and more importantly, there was a significant INTERVENTION by BLOCKS (F[5,55]=3.9, P=0.01) interaction. Post hoc analysis demonstrated that, relative to sham treatment, tDCS facilitated the training effect to a larger extent at the second, fourth, and fifth blocks of training. A significant difference between the 2 stimulation conditions was apparent for the correct sequence (tDCSonline=1.7±0.2, shamonline=1.3±0.1, t[11]=2.2, P=0.04) as well as the overall sequences (tDCSonline=1.5±0.1, shamonline=1.2±0.1, t[11]=3.4, P<0.01) in the early online learning period (B5/Base). The increase in correct sequences with tDCS could potentially be driven by a change in the speed–accuracy relationship toward reduced speed and enhanced accuracy. To evaluate this question we conducted the same analysis for the overall sequences; here, too, we found a trend toward an increase in overall sequences for the factor INTERVENTION (F[1,11]=3.9, P=0.06) with a significant effect on BLOCKS (F[5,55]=15.2, P<0.01) and an INTERVENTION by BLOCKS (F[5,55]=3.6, P=0.03) interaction. Furthermore, the success rate did not decrease significantly with tDCS making a relevant change in the speed–accuracy–relation unlikely.
Effects of tDCS on Cortical Excitability
Motor-Evoked Potential
The resting motor threshold was 58.4%±6.4% in the cM1 and 64.6%±7.2% in the stroke M1 (P=0.09). Mean motor-evoked potential (MEP) amplitudes were 1.18±0.1 mV and 0.91±0.2 mV respectively for the baseline condition (Z=−1.6, P=0.11) and the power intensities were 66.2%±2.6% in the cM1 and 77.8%±3.8% in the lesioned M1 (P=0.03). After cathodal tDCS, a significant reduction in the MEPs over the unaffected motor cortex was demonstrated using a nonparametric Friedman analysis of variance test (χ2[4]=10.61, P=0.03). Post hoc analysis revealed a trend for MEP reduction at Post30 (from 1.18±0.1 to 0.89±0.1 mV, P=0.06) and a significant effect at Post0 (from 1.18±0.1 to 0.84±0.1 mV, P=0.02) and Post60 (from 1.18±0.1 to 0.77±0.1 mV, P<0.01) with a normalization of the values at Post90. On the other hand, there was no modification of the MEPs in the lesioned hemisphere (χ2[4]=1.1, P=0.8).
Short Interval Intracortical Inhibition
No significant differences were observed between SICI in both M1 during baseline (Z=−1.6, P=0.1). tDCS resulted in an increase (more inhibition) of SICI in the cM1 (χ2[4]=11.08, P=0.02). In contrast, a reduction of SICI (less inhibition) was demonstrated in the stroke hemisphere (χ2[4]= 9.37, P=0.05). Post hoc testing showed that disinhibition in the lesioned M1 was restricted to Post0 at the 0.0125 level of significance (from 43.8%±5.9% at baseline to 65.7%±7.3% of unconditioned MEP). All other comparisons were not significant (for more details, see the online-only Data Supplement).
Relationship Between Skill Acquisition and Cortical Excitability Changes
A significant correlation was observed between the tDCS-induced improvement during TRAIN-tDCS and the tDCS-induced modulation of SICI (reduced inhibition=disinhibition) in the stroke M1 (y=0.84x+0.54, R2=0.63, P=0.03; Figure 3C). Behavioral improvement was not correlated with the tDCS-induced changes of excitability within the cM1 determined by MEP amplitudes (r2=0.08, P=0.5) or SICI (r2=0.13, P=0.8).
Figure 3.
Early training session (TRAIN-tDCS). A, The number of correct sequences achieved in each block is displayed for cathodal tDCS and sham; the baselines blocks were not different between conditions (BASE). B, A significant improvement with tDCS compared with sham stimulation during the early training session was observed (early online learning). C, Relationship between tDCS-induced behavioral improvement and cortical excitability changes; the abscissa displays the online effects; the ordinate displays tDCS-induced decrease of SICI (Post0/baseline). tDCS indicates transcranial direct current stimulation; SICI, short interval intracortical inhibition.
Discussion
The main findings of the present study were that (1) the application of inhibitory tDCS to the cM1 concurrent with training can facilitate the effects of training yielding to subsequent improvement of the early online learning period; (2) this improvement translated into better performance for at least 24 hours; and (3) revealed an association between an intervention-induced decrease of SICI (less inhibition) within M1 of the lesioned hemisphere and a tDCS-induced enhancement of skill acquisition. Taken together, these findings support the beneficial effects of NIBS techniques in patients with stroke with mild motor impairment not only to enhance motor functions, as suggested previously,3,5,11,15 but also to boost the acquisition and potentially retention of complex motor functions with the paretic hand, a concept that might provide a basis for the improvement of longer-lasting functional recovery processes after stroke.
Clearly, acquisition of motor skills is essential to almost every daily life activity we perform from writing to driving a car or using a cell phone. Reacquisition of skills resulting in improved or more accurate motor performance is paramount to recovery of function after a brain lesion. Despite the structural changes and the abnormal patterns of neural activation during the execution of motor tasks in patients with stroke, robust evidence has been provided that acquisition of motor skills is not abolished.29 In this context, NIBS techniques have the ability to enhance lasting changes in neuroplasticity with the opportunity to modify behavior and interact beneficially with learning processes.6,8,11,30
Previous imaging and dual-pulse TMS studies demonstrated a deleterious contribution of the contralesional hemisphere in recovery of motor function after stroke.31,32 This abnormal and imbalanced interhemispheric interaction is 1 favorite model that underlies the experimental therapeutic strategies based on NIBS.33 Previous studies have shown the positive effect of NIBS targeting the healthy cM1 to transiently facilitate performance of activities of daily living (eg, Jebsen Taylor Hand function test),11,34 peak pinch acceleration,35 grasping,36 or simple and choice reaction times.37 However, in most of these studies, NIBS techniques were applied after patients reached stable levels of the task (eg, Jebsen Taylor Hand function test), likely reflecting a tDCS-induced performance improvement. It is not known if application of NIBS during an early acquisition period, before performance reaches an asymptotic level, could potentiate or even speed up the learning process of a novel procedural task with consecutive better retention.
Recently it was demonstrated that consecutive sessions of anodal tDCS over M1 can help healthy subjects to learn an isometric pinch task through an effect of consolidation.16 In the present design, the combined approach of tDCS with a skill acquisition task revealed a consistent improvement during the first training period, in line with a previous study performed with high-frequency repetitive TMS over the lesioned M1.38 Strikingly, in the present study, a longer-lasting improvement, beyond the pure effect of stimulation, could be demonstrated. Even after successive sessions of practice, the performance levels achieved with tDCS could not be reached after sham stimulation. How long will the effects of tDCS on retention last? This important question could not be sufficiently answered within the present study design. However, a 3-month follow-up session no longer revealed group differences between the 2 conditions probably due to the small subsample of 5 patients.
In the past decade, considerable evidence has accumulated regarding the plasticity of the human M1 as a function of motor learning. M1 is not only crucial to the execution, but also to acquisition and consolidation of novel motor skills. Motor practice is associated with an increase in excitability of the sensorimotor cortex, promoting plastic changes even in the chronic stage after a stroke.39 During acquisition of a novel motor skill, rewiring processes in M1 occur, consistent with rapid formation and stabilization of dendritic spines.40 These processes are most likely based on unmasking of pre-existing connections within the cortex, allowing rapid changes in sensorimotor representations by reducing the activity of existing inhibitory connections.41 Recent studies in humans using MR spectroscopy demonstrated that 30 minutes of motor learning generated a short-term and rapid reduction of the mean γ-aminobutyric acid concentration within the M1.42 Further pharmacological evidence supporting the notion that plasticity of the human sensorimotor cortex is modulated by changes in local γ-aminobutyric acid concentration comes from the observation that lorazepam, a γ-aminobutyric acid-A receptor agonist, might suppress use-dependent plasticity in healthy subjects.43 In this context, the decrease of SICI, a physiological measure hypothesized to reflect intracortical γ-aminobutyric acid-A neuronal activity,9,44 after tDCS, and its positive correlation with the tDCS-induced behavioral improvement during training might, at least in part, explain the behavioral benefits by modulation of intracortical inhibition. Thus, one possible conjecture is that the ongoing state of the cortex at the time of stimulation can reinforce the long-term effects induced by motor practice. If so, tDCS influences the ability of the affected M1 to undergo plastic modifications by preparing the “cortical ground” for successful plastic changes due to motor training. Currently this notion is still speculative in nature; however, it is consistent with recent studies conducted with animals and healthy humans.8,16,45
Limitations of the Study
In the present study, tDCS was applied to the contralesional M1; however, due to the size of the direct current electrodes (25 cm2), the spatial resolution is rather low. Thus, it is conceivable that the stimulation effects are not entirely restricted to M1, but also might have spread to adjacent structures like the premotor cortex.46 As recently discussed,47 the notion that the unaffected hemisphere has a nonbeneficial effect on the lesioned hemisphere and consecutive behavior does not apply to all groups of patients. This fact might relevantly depend on lesion location, time after stroke, and size and integrity of the corticospinal pathway among other factors, hypotheses, which are addressed in upcoming studies.13,14 Finally, it is important to keep in mind that the present results might only apply to this homogenic and selected subgroup of patients with subcortical lesions and mild impairment. Indeed, it is not yet known whether this intervention will have comparable beneficial effects in patients with more severe deficits who have experienced extensive cortical and subcortical lesions. We might even speculate that such an intervention might not prove to be beneficial for this subgroup.47 Upcoming studies designed to address these aspects are definitely needed to provide the basis for more patient-individualized therapeutic interventions.
In summary, noninvasive transcranial brain stimulation combined with motor training did enhance the acquisition of a novel skill with the paretic hand and led to a persistent enhancement of function in well-recovered patients with chronic stroke. The intervention dominantly influenced the early phase of online learning leading to behavioral improvements still apparent during the retention 24 hours after intervention. Thus, the present findings further support the potential of noninvasive brain stimulation in the treatment of functional deficits to enhance skill reacquisition and long-term functional recovery after brain lesions.
Supplementary Material
Acknowledgments
Sources of Funding
This research was supported by a grant from the German Academic Exchange Service to M.Z. (A/07/95990), the Alexander von Humboldt Foundation (Feodor-Lynen) to F.C.H., the Forschungsförder-ungsfonds Medizin of the University of Hamburg to F.C.H. (NWF-04/07) and to M.Z. (NWF-11/09), the Kompetenznetz Schlaganfall to C.G., and the German Research Foundation (SFB 936-C4) to F.C.H.
Footnotes
The online-only Data Supplement is available with this article at http://stroke.ahajournals.org/lookup/suppl/doi:10.1161/STROKEAHA.111.645382/-/DC1.
Disclosures
None.
References
- 1.Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. Lancet. 2011;377:1693–1702. doi: 10.1016/S0140-6736(11)60325-5. [DOI] [PubMed] [Google Scholar]
- 2.Hendricks HT, van Limbeek J, Geurts AC, Zwarts MJ. Motor recovery after stroke: a systematic review of the literature. Arch Phys Med Rehabil. 2002;83:1629–1637. doi: 10.1053/apmr.2002.35473. [DOI] [PubMed] [Google Scholar]
- 3.Nowak DA, Bosl K, Podubecka J, Carey JR. Noninvasive brain stimulation and motor recovery after stroke. Restor Neurol Neurosci. 2010;28:531–544. doi: 10.3233/RNN-2010-0552. [DOI] [PubMed] [Google Scholar]
- 4.Fregni F, Pascual-Leone A. Hand motor recovery after stroke: tuning the orchestra to improve hand motor function. Cogn Behav Neurol. 2006;19:21–33. doi: 10.1097/00146965-200603000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Schlaug G, Renga V, Nair D. Transcranial direct current stimulation in stroke recovery. Arch Neurol. 2008;65:1571–1576. doi: 10.1001/archneur.65.12.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rothwell JC. Plasticity in the human motor system. Folia Phoniatr Logop. 2010;62:153–157. doi: 10.1159/000314030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nitsche MA, Cohen L, Wassermann E, Priori A, Lang N, Antal A, et al. Transcranial direct current stimulation: state of the art 2008. Brain Stimulation. 2008;1:206–223. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Fritsch B, Reis J, Martinowich K, Schambra HM, Ji Y, Cohen LG, et al. Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron. 2010;66:198–204. doi: 10.1016/j.neuron.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ziemann U, Rothwell JC, Ridding MC. Interaction between intracortical inhibition and facilitation in human motor cortex. J Physiol (Lond) 1996;496:873–881. doi: 10.1113/jphysiol.1996.sp021734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gandiga PC, Hummel FC, Cohen LG. Transcranial dc stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol. 2006;117:845–850. doi: 10.1016/j.clinph.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Hummel F, Celnik P, Giraux P, Floel A, Wu WH, Gerloff C, et al. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain. 2005;128:490–499. doi: 10.1093/brain/awh369. [DOI] [PubMed] [Google Scholar]
- 12.Fregni F, Boggio PS, Mansur CG, Wagner T, Ferreira MJ, Lima MC, et al. Transcranial direct current stimulation of the unaffected hemisphere in stroke patients. Neuroreport. 2005;16:1551–1555. doi: 10.1097/01.wnr.0000177010.44602.5e. [DOI] [PubMed] [Google Scholar]
- 13.Bradnam LV, Stinear CM, Barber PA, Byblow WD. Contralesional hemisphere control of the proximal paretic upper limb following stroke. Cereb Cortex. doi: 10.1093/cercor/bhr344. Published online ahead of print December 1, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lotze M, Markert J, Sauseng P, Hoppe J, Plewnia C, Gerloff C. The role of multiple contralesional motor areas for complex hand movements after internal capsular lesion. J Neurosci. 2006;26:6096–6102. doi: 10.1523/JNEUROSCI.4564-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khedr EM, Ahmed MA, Fathy N, Rothwell JC. Therapeutic trial of repetitive transcranial magnetic stimulation after acute ischemic stroke. Neurology. 2005;65:466–468. doi: 10.1212/01.wnl.0000173067.84247.36. [DOI] [PubMed] [Google Scholar]
- 16.Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, Zarahn E, et al. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci U S A. 2009;106:1590–1595. doi: 10.1073/pnas.0805413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bolognini N, Pascual-Leone A, Fregni F. Using non-invasive brain stimulation to augment motor training-induced plasticity. J Neuroeng Rehabil. 2009;6:8. doi: 10.1186/1743-0003-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walker MP, Brakefield T, Hobson JA, Stickgold R. Dissociable stages of human memory consolidation and reconsolidation. Nature. 2003;425:616–620. doi: 10.1038/nature01930. [DOI] [PubMed] [Google Scholar]
- 19.Witt ST, Laird AR, Meyerand ME. Functional neuroimaging correlates of finger-tapping task variations: an ale meta-analysis. Neuroimage. 2008;42:343–356. doi: 10.1016/j.neuroimage.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sauseng P, Hoppe J, Klimesch W, Gerloff C, Hummel FC. Dissociation of sustained attention from central executive functions: local activity and interregional connectivity in the theta range. Eur J Neurosci. 2007;25:587–593. doi: 10.1111/j.1460-9568.2006.05286.x. [DOI] [PubMed] [Google Scholar]
- 21.Lempel A, Ziv J. On the complexity of finite sequences. IEEE Trans Inform Theory. 1976;22:75–81. [Google Scholar]
- 22.Dayan E, Cohen LG. Neuroplasticity subserving motor skill learning. Neuron. 2011;72:443–454. doi: 10.1016/j.neuron.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmidt RA, Lee TD. Motor control and learning: A behavioral emphasis. 5. Champaign, IL: Human Kinetics; 2011. Retention and Transfer; pp. 461–488. [Google Scholar]
- 24.Ungerleider LG, Doyon J, Karni A. Imaging brain plasticity during motor skill learning. Neurobiol Learn Mem. 2002;78:553–564. doi: 10.1006/nlme.2002.4091. [DOI] [PubMed] [Google Scholar]
- 25.Karni A, Meyer G, Rey-Hipolito C, Jezzard P, Adams MM, Turner R, et al. The acquisition of skilled motor performance: fast and slow experience-driven changes in primary motor cortex. Proc Natl Acad Sci U S A. 1998;95:861–868. doi: 10.1073/pnas.95.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krakauer JW, Shadmehr R. Consolidation of motor memory. Trends Neurosci. 2006;29:58–64. doi: 10.1016/j.tins.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siebner HR, Rothwell J. Transcranial magnetic stimulation: new insights into representational cortical plasticity. Exp Brain Res. 2003;148:1–16. doi: 10.1007/s00221-002-1234-2. [DOI] [PubMed] [Google Scholar]
- 28.Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, et al. Corticocortical inhibition in human motor cortex. J Physiol (Lond) 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krakauer JW. Motor learning: its relevance to stroke recovery and neurorehabilitation. Curr Opin Neurol. 2006;19:84–90. doi: 10.1097/01.wco.0000200544.29915.cc. [DOI] [PubMed] [Google Scholar]
- 30.Reis J, Robertson E, Krakauer JW, Rothwell J, Marshall L, Gerloff C, et al. Consensus: ‘Can tDCS and TMS enhance motor learning and memory formation?’. Brain Stimulat. 2008;1:363–369. doi: 10.1016/j.brs.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ward NS, Brown MM, Thompson AJ, Frackowiak RS. Neural correlates of motor recovery after stroke: a longitudinal fMRI study. Brain. 2003;126:2476–2496. doi: 10.1093/brain/awg245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004;55:400–409. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- 33.Nowak DA, Grefkes C, Ameli M, Fink GR. Interhemispheric competition after stroke: brain stimulation to enhance recovery of function of the affected hand. Neurorehabil Neural Repair. 2009;23:641–656. doi: 10.1177/1545968309336661. [DOI] [PubMed] [Google Scholar]
- 34.Boggio PS, Nunes A, Rigonatti SP, Nitsche MA, Pascual-Leone A, Fregni F. Repeated sessions of noninvasive brain DC stimulation is associated with motor function improvement in stroke patients. Restor Neurol Neurosci. 2007;25:123–129. [PubMed] [Google Scholar]
- 35.Takeuchi N, Chuma T, Matsuo Y, Watanabe I, Ikoma K. Repetitive transcranial magnetic stimulation of contralesional primary motor cortex improves hand function after stroke. Stroke. 2005;36:2681–2686. doi: 10.1161/01.STR.0000189658.51972.34. [DOI] [PubMed] [Google Scholar]
- 36.Nowak DA, Grefkes C, Dafotakis M, Eickhoff S, Kust J, Karbe H, et al. Effects of low-frequency repetitive transcranial magnetic stimulation of the contralesional primary motor cortex on movement kinematics and neural activity in subcortical stroke. Arch Neurol. 2008;65:741–747. doi: 10.1001/archneur.65.6.741. [DOI] [PubMed] [Google Scholar]
- 37.Mansur CG, Fregni F, Boggio PS, Riberto M, Gallucci-Neto J, Santos CM, et al. A sham stimulation-controlled trial of rTMS of the unaffected hemisphere in stroke patients. Neurology. 2005;64:1802–1804. doi: 10.1212/01.WNL.0000161839.38079.92. [DOI] [PubMed] [Google Scholar]
- 38.Kim YH, You SH, Ko MH, Park JW, Lee KH, Jang SH, et al. Repetitive transcranial magnetic stimulation-induced corticomotor excitability and associated motor skill acquisition in chronic stroke. Stroke. 2006;37:1471–1476. doi: 10.1161/01.STR.0000221233.55497.51. [DOI] [PubMed] [Google Scholar]
- 39.Liepert J. Motor cortex excitability in stroke before and after constraint-induced movement therapy. Cogn Behav Neurol. 2006;19:41–47. doi: 10.1097/00146965-200603000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Xu T, Yu X, Perlik AJ, Tobin WF, Zweig JA, Tennant K, et al. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature. 2009;462:915–919. doi: 10.1038/nature08389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rioult-Pedotti MS, Friedman D, Hess G, Donoghue JP. Strengthening of horizontal cortical connections following skill learning. Nat Neurosci. 1998;1:230–234. doi: 10.1038/678. [DOI] [PubMed] [Google Scholar]
- 42.Floyer-Lea A, Wylezinska M, Kincses T, Matthews PM. Rapid modulation of GABA concentration in human sensorimotor cortex during motor learning. J Neurophysiol. 2006;95:1639–1644. doi: 10.1152/jn.00346.2005. [DOI] [PubMed] [Google Scholar]
- 43.Butefisch CM, Davis BC, Wise SP, Sawaki L, Kopylev L, Classen J, et al. Mechanisms of use-dependent plasticity in the human motor cortex. Proc Natl Acad Sci U S A. 2000;97:3661–3665. doi: 10.1073/pnas.050350297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Di Lazzaro V, Oliviero A, Meglio M, Cioni B, Tamburrini G, Tonali P, et al. Direct demonstration of the effect of lorazepam on the excitability of the human motor cortex. Clin Neurophysiol. 2000;111:794–799. doi: 10.1016/s1388-2457(99)00314-4. [DOI] [PubMed] [Google Scholar]
- 45.Galea JM, Celnik P. Brain polarization enhances the formation and retention of motor memories. J Neurophysiol. 2009;102:294–301. doi: 10.1152/jn.00184.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wagner T, Fregni F, Fecteau S, Grodzinsky A, Zahn M, Pascual-Leone A. Transcranial direct current stimulation: a computer-based human model study. Neuroimage. 2007;35:1113–1124. doi: 10.1016/j.neuroimage.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 47.Hummel FC, Celnik P, Pascual-Leone A, Fregni F, Byblow WD, Buetefisch CM, et al. Controversy: noninvasive and invasive cortical stimulation show efficacy in treating stroke patients. Brain Stimul. 2008;1:370–382. doi: 10.1016/j.brs.2008.09.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.