Abstract
Dystonia has been defined as a syndrome of involuntary, sustained muscle contractions affecting one or more sites of the body, frequently causing twisting and repetitive movements or abnormal postures. Dystonia is also a clinical sign that can be the presenting or prominent manifestation of many neurodegenerative and neuro-metabolic disorders. Etiological categories include primary dystonia, secondary dystonia, heredodegenerative diseases with dystonia, and dystonia plus. Primary dystonia includes syndromes in which dystonia is the sole phenotypic manifestation with the exception that tremor can be present as well. Most primary dystonia begins in adults, and approximately 10% of probands report one or more affected family members. Many cases of childhood- and adolescent-onset dystonia are due to mutations in TOR1A and THAP1. Mutations in THAP1 and CIZ1 have been associated with sporadic and familial adult-onset dystonia. Although significant recent progress had been made in defining the genetic basis for most of the dystonia-plus and heredodegenerative diseases with dystonia, a major gap remains in understanding the genetic etiologies for most cases of adult-onset primary dystonia. Common themes in the cellular biology of dystonia include G1/S cell cycle control, monoaminergic neurotransmission, mitochondrial dysfunction, and the neuronal stress response.
I. INTRODUCTION
Although the term “dystonia” was coined in 1911 by Hermann Oppenheim, a leading German neurologist of his time, numerous earlier descriptions of dystonia had appeared in the medical literature under various names during the previous century (Fahn, 2011; Goetz et al., 2001). Oppenheim believed that dystonia was an abnormality of tone with both hypertonic and hypotonic components. In 1836, J.H. Kopp eloquently described the clinical features of writer’s cramp, a form of hand–forearm dystonia, in a German medical monograph. In his manual of diseases of the nervous system published in 1893, Sir William Richard Gowers used the term “tetanoid chorea” to describe what was apparently dystonia. In 1908, Schwalbe employed the term “tonic cramps” and described hereditary contributions to dystonia.
Under the heavy influence of Freud, dystonia was predominantly viewed as a psychiatric disorder for several decades in the early nineteenth century. This is not surprising given that truly psychogenic dystonia is relatively common and the often bizarre anatomical patterns, task specificity and gestes antagonistes characteristic of organic dystonia. In 1944, dystonia was resurrected as a true neurological disorder by Herz who used frame-by-frame cinematography to typify dystonic movements as slow, sustained, and forceful contortions of the trunk and limbs. Herz dropped “hypotonia” from the definition of dystonia, as initially applied by Oppenheim.
More recently (1984), dystonia was defined by an ad hoc committee of the Dystonia Medical Research Foundation (André Barbeau, Donald B. Calne, Stanley Fahn, C. David Marsden, John Menkes, and G. Frederick Wooten) as “a syndrome of sustained muscle contractions, frequently causing twisting and repetitive movements or abnormal postures.” Although the clinical definition of dystonia has remained static over nearly 30 years, the classification of dystonia has evolved (Fahn, 2011). In 1976, Fahn and Eldridge provided an etiological classification of dystonia: primary (hereditary or sporadic), secondary (associated with heredodegenerative disease or environmental insults), or psychological. In the same year, David Marsden and colleagues (1976) published an anatomical classification of dystonia: focal, segmental, or generalized. In general, dystonia can be classified by etiology (primary or secondary), age of onset (<20 or >20 years), and anatomical distribution (focal, segmental, multifocal, hemidystonia, or generalized) (Fahn, 1987, 1988; Fahn et al., 1998). More recent iterations and refinements of this basic classification scheme have been largely driven by developments in the genetics of dystonia and related neurological disorders (Fahn, 2011).
In some sense, genetics has biased the study of dystonia. Primary generalized dystonias usually affect children and a subset of cases is due to a ΔGAG mutation in Exon 5 of TOR1A (DYT1). TOR1A was the first gene to be causally associated with primary dystonia. In recent years, many patients with early-onset primary generalized DYT1 dystonia have responded dramatically to deep brain stimulation. Other “genetic” forms of dystonia such as DYT5, DYT11, and DYT12 also exhibit striking phenotypes and/or very positive responses to treatment. In reality, however, the vast majority of dystonia (>90%) seen by subspecialists in neurology clinics is late onset and focal or segmental in distribution (Xiao et al., 2010). The genetics underpinnings of late-onset primary dystonia are only beginning to be unraveled (Xiao et al., 2009, 2010, 2011, 2012).
II. CLINICAL FEATURES
A. Phenomenology and Demographics
Primary dystonia includes syndromes in which dystonia is the sole phenotypic manifestation with the exception that tremor can be present as well. Tremor is included in this definition since rhythmic activation of contiguous muscle groups can be seen in a significant fraction of patients with focal dystonias, particularly those with cervical or hand–forearm dystonia. Dystonia is characterized by (1) “abnormal” co-contraction of agonist and antagonist muscle groups, (2) “abnormal” prolongation of EMG bursts in muscles normally required for a specific motor act, (3) “impaired” volitional control of a group of somatotopically contiguous muscles, and (4) “impaired” inhibition of spinal and brainstem reflexes beyond the somatotopic extent of clinical involvement. “Abnormal” and “impaired” are not binary terms, which make it difficult to differentiate primary dystonia from psychogenic dystonia and other movement disorders based strictly on neurophysiological criteria. Most commonly, however, dystonia remains a purely clinical diagnosis made by experienced neurologists who know how to differentiate dystonia from other movement disorders. There are no definitive laboratory tests for primary dystonia—psychogenic dystonia has been reported in carriers of the DYT1 TOR1A ΔGAG mutation.
Overall, dystonia is more common in females (LeDoux, 2012a; Xiao et al., 2010). For late-onset primary dystonia affecting the craniocervical musculature (e.g., blepharospasm, laryngeal dystonia, and cervical dystonia), the M:F ratio is 1:1.5–2. In contrast, the M:F ratio may be closer to 1:1 for hand–forearm dystonia including writer’s cramp and other task-specific appendicular dystonias. For some genetically defined forms of dystonia such as DYT5 due to mutations in GCH1, penetrance is higher in females.
Dystonia, in general, and certain genetic etiologies are more common in certain ethnic or racial groups. For example, the TOR1A ΔGAG mutation is more common in Ashkenazi Jews than in other populations. In the United States, dystonia may be less common in African-Americans (Marras et al., 2007), although this may reflect ascertainment bias (Puschmann et al., 2011).
Up to 10% of patients with adult-onset primary dystonia may experience remissions, which are more common early in the disease course but may occasionally occur in long-standing dystonia (LeDoux, 2012a). Remissions may be permanent, but, more commonly, only last months or a few years, and have been reported in patients with a genetic diagnosis (e.g., THAP1 dystonia). Sustained relief of motor symptoms has also been reported after cessation of GPi DBS. These findings suggest that dystonia may be the consequence of aberrant neural networks becoming trapped in local minima rather than irreversible cellular pathology (LeDoux et al., 2012a).
Dystonia is typically mobile and abates with sleep. Dystonia is often precipitated by action or worsens with movement. A small percentage of patients with dystonia have “fixed dystonia,” a term which has been the subject of controversy for many years. Fixed dystonia is often associated with peripheral trauma, complex regional pain syndrome, and psychiatric comorbidities. However, fixed dystonia can be the terminal consequence of long-standing, untreated primary “mobile” dystonia. For instance, in the years prior to botulinum toxin injections and deep brain stimulation, many patients with cervical dystonia progresses to fixed abnormal head postures.
Tremor is common in patients with dystonia and may be dystonic and/or non-dystonic. Appendicular tremors, usually non-dystonic, are common in patients with craniocervical dystonia. Familial essential tremor is often associated with dystonia and may represent a distinct subtype of essential tremor (Hedera et al., 2010).
Task specificity and sensory tricks (gestes antagonistes) are relatively unique features of the dystonias. Initially, task-specific hand–forearm dystonia may be extreme and only present when writing certain numbers or letters (Shamim et al., 2011). Over time, task specificity may wane. Classic examples of task-specific dystonia include writer’s cramp and embouchure dystonia. However, task-specific dystonia is not limited to the hand and face. For illustration, task-specific leg dystonia may only manifest when walking down steps, whereas walking on a level surface, up steps, and down steps backward are normal. Task-specific dystonias have been reported in golfers, typists, pianists, violinists, and croupiers. Sensory tricks are reported by more than 50% of patients with focal dystonia. Tricks are associated with transient improvement or resolution of dystonia. Classic examples include touching the chin, cheek, or occiput in cervical dystonia; and singing, touching the lateral brow, and looking downward in blepharospasm. In some patients, simply thinking about the trick (interoceptive stimulus) may help to alleviate their dystonia.
In general, the relationship between anatomical site of dystonia onset and age of onset follows a caudal-to-rostral gradient: blepharospasm (58 years), oromandibular dystonia (53 years), spasmodic dysphonia (46 years), cervical dystonia (45 years), hand–forearm dystonia (35 years), and distal leg dystonia (<20 years). Most commonly, distal leg dystonia begins in childhood with inversion and plantar flexion at the ankle and spreads rostrally. However, leg dystonia may occasionally appear in adults without foot inversion (Van Gerpen et al., 2010). Site of onset may be gene specific. For example, DYT6/THAP1 dystonia usually begins in an arm or the neck, whereas DYT1 dystonia often begins in a leg.
Dystonia may spread from an initial site of onset (Weiss et al., 2006). Risk for rostral spread is high in early-onset DYT1 dystonia that begins in a leg. In contrast, among the late-onset dystonias, risk of spread is highest for blepharospasm. Blepharospasm often spreads to the lower face and masticatory muscles and, in a smaller subset of patients, to the cervical musculature (LeDoux, 2009; Waln and LeDoux, 2011). Only rarely does blepharospasm spread to become generalized.
Patients with dystonia may experience pain and suffer from psychiatric comorbidities. Pain is perhaps most common in subjects with cervical dystonia but is also reported by individuals with blepharospasm, masticatory dystonia, and limb dystonia. Pain intensity may correlate poorly with the apparent severity of dystonic contractures. In some clinical series, subjects with primary focal dystonia are reported to have more obsessive-compulsive tendencies and a higher frequency of depressive disorders than matched control groups.
B. Classification
Dystonia can be classified by age of onset, distribution, and etiology (Table 2.1) (Fahn, 1987, 1988, 2011; Fahn et al., 1998). In contrast to the presentation in Table 2.1 (Fahn, 1987, 1988; Fahn et al., 1998), some of the more recently published classification schemes used 26 years of age as the dividing line between early- and late- or adult-onset dystonia (Fahn, 2011). However, based on normal patterns of human development, 20 years is a biologically rational separation point (Kuczmarski et al., 2000). Application of 26 years arose from recommendations for DYT1 ΔGAG diagnostic testing (Bressman et al., 2000) and is not applicable to non-DYT1 primary dystonia. In brief, DYT1 ΔGAG diagnostic testing was recommended for all individuals with primary dystonia with onset before age 26. Testing after age 26 may be considered for subjects having an affected relative with early onset. It should be emphasized that these guidelines only apply to DYT1 dystonia, which comprises less than 1% of all primary dystonia (Xiao et al., 2009).
Table 2.1.
Classification of dystonia
| Age of onset |
| Early onset (<20 years) |
| Late onset (>20 years) |
| Distribution |
| Focal: single body region (e.g., cervical dystonia, blepharospasm, oromandibular dystonia, spasmodic dysphonia, and task-specific dystonias) |
| Segmental: contiguous regions (e.g., cranial + cervical) |
| Multifocal: non-contiguous regions (e.g., cervical + leg) |
| Generalized: leg + trunk + one other body part |
| Hemidystonia: ipsilateral arm + leg |
| Etiology |
| Primary dystonia: syndromes in which dystonia is the sole phenotypic manifestation with the exception that tremor can be present as well |
| Secondary dystonia: due to structural lesions, neural insults, or medications (e.g., stroke, trauma, encephalitis, etc.) |
| Dystonia plus: dystonia plus another movement disorder without overt evidence of neurodegeneration (dopa-responsive dystonia [DRD/DYT5a and DYT5b], myoclonus-dystonia syndrome [MDS/DYT11], rapid-onset dystonia–parkinsonism [RDP/DYT12], and early-onset dystonia with parkinsonism [DYT16]) |
| Heredodegenerative diseases with dystonia: dystonia may be a prominent feature (e.g., X-linked dystonia–parkinsonism [DYT3], progressive supranuclear palsy [PSP], Parkinson’s disease, multiple system atrophy, corticobasal ganglionic degeneration, spinocerebellar ataxia type 3 [SCA3]) |
| Paroxysmal dyskinesias: sudden episodes of involuntary movement, dystonia is often a major clinical feature |
| Psychogenic dystonia: dystonia is primarily due to psychological factors |
| Pseudodystonia: dystonia mimics associated with abnormal postures |
Age of onset is usually easy to establish and guides the clinician to underlying etiologies. For instance, DYT1 (Table 2.2) typically presents around 10 years of age with distal lower extremity dystonia. In contrast, the mean age of onset for primary focal dystonias of the head and neck is approximately 50 years (Xiao et al., 2009, 2010). Among the more common focal dystonias are cervical dystonia (i.e., spasmodic torticollis), blepharospasm (abnormal contractions of the orbicularis oculi musculature), and task-specific hand–forearm dystonia (e.g., writer’s cramp). The dystonia-plus category is superficially distinct from both the primary dystonias and here-dodegenerative diseases with dystonia given that the definition of neurodegeneration is fuzzy and postmortem tissue for rigorous pathological analysis at the structural and ultrastructural levels has been limited. More problematic perhaps is the absence of parkinsonism in most cases of dopa-responsive dystonia (DRD) due to autosomal dominant mutations in GTP cyclohydrolase I, uncertain presence of parkinsonism in DYT16, and not infrequent absence of dystonia in individuals with mutations in SGCE.
Table 2.2.
Hereditary dystonias with Mendelian inheritance patterns
| HUGO/OMIM | Common name | Locus/gene | Mode | Mutant protein |
|---|---|---|---|---|
| DYT1 (MIM 128100) | Oppenheim’s dystonia | 9q34.11/TOR1A | AD | TorsinA (Ozelius et al., 1997) |
| DYT2 (MIM 224500) | Autosomal recessive dystonia | Unknown | AR | Unknown |
| DYT3 (MIM 314250) | Lubag (X-linked dystonia–parkinsonism) | Xq13.1/TAF1 | XLR | Reduced TAF1 expression (Makino et al., 2007; Nolte et al., 2003) |
| DYT4 (MIM 128101) | Australian whispering dysphonia family | Unknown | AD | Unknown |
| DYT5a (MIM 128230) | Dopa-responsive dystonia | 14q22.2/GCH1 | AD | GTP cyclohydrolase I (Ichinose et al., 1994) |
| DYT5b | Dopa-responsive dystonia | 11p.15.5/TH | AR | Tyrosine hydroxylase (Lüdecke et al., 1995) |
| DYT5b | Dopa-responsive dystonia | 2q13.2/SPR | AR | Sepiapterin reductase (Bonafé et al., 2001) |
| DYT6 (MIM 602629) | Mixed-type dystonia | 8p11.21 | AD | THAP1 (Fuchs et al., 2009) |
| DYT7 (MIM 602124) | Familial torticollis | 18p | AD | Unknown |
| DYT8 (MIM 118800) | Paroxysmal nonkinesigenic dyskinesia (PNKD) | 2q35/PNKD | AD | Paroxysmal nonkinesigenic protein (Rainier et al., 2004) |
| DYT9 (MIM 601042) | Paroxysmal choreoathetosis/spasticity | 1p34.2/SLC2A1 | AD | Glucose transporter 1 (GLUT1) (Weber et al., 2011) |
| DYT10 (MIM 128200) | Paroxysmal kinesigenic dyskinesia (PKD) | 16p11.2/PRRT2 | AD | Proline-rich transmembrane protein 2 (Chen et al., 2011) |
| DYT11 (MIM 159900) | Myoclonus-dystonia syndrome | 7q21.3/SGCE | AD | ε-sarcoglycan (Zimprich et al., 2001) |
| DYT12 (MIM 128235) | Rapid-onset dystonia–parkinsonism | 19q13.2/ATP1A3 | AD | Na+/K+–ATPase α-3 subunit (de Carvalho Aguiar et al., 2004) |
| DYT13 (MIM 607671) | Italian family-primary torsion dystonia | 1p36.32-p36.13 | AD | Unknown |
| DYT15 (MIM 607488) | Myoclonus dystonia, Canadian family | 18p11 | AD | Unknown |
| DYT16 (MIM 612067) | Young-onset dystonia–parkinsonism | 2q31.2/PRKRA | AR | Stress-response protein PRKRA (Camargos et al., 2008) |
| DYT17 (MIM 612406) | Generalized dystonia with dysarthria and dysphonia | 20p11.2-q13.12 | AR | Unknown |
| DYT18 (MIM 61216) | Paroxysmal exertional dyskinesia associated with hemolytic anemia | 1p34.2/SLC2A1 | AR | Glucose transporter 1 (GLUT1) (Weber et al., 2008) |
| DYT19 (MIM 611031) | Paroxysmal kinesigenic dyskinesia (PKD) | 16q13-q22.1 | AD | Unknown |
| DYT20 (MIM 611147) | Paroxysmal nonkinesigenic dyskinesia 2 (PNKD2) | 2q31 | AD | Unknown |
| DYT21 | Adult-onset mixed dystonia | 2q14.3-q21.3 | AD | Unknown |
AD, autosomal dominant; AR, autosomal recessive.
GTP cyclohydrolase I is the rate-limiting enzyme in the synthesis of tetrahydrobiopterin. Tetrahydrobiopterin is a cofactor for tyrosine hydroxylase, tryptophan hydroxylase, and phenylalanine hydroxylase. Patients with DRD characteristically respond dramatically to very small doses of levodopa, a clinical finding that distinguishes DYT5 from the other hereditary dystonias. Secondary dystonias are due to acquired neural insults such as head trauma or stroke and can be distinguished from primary dystonias by the presence of additional abnormalities on neurological examination, magnetic resonance imaging (MRI) or computed tomographic imaging abnormalities, and their distinctive temporal profiles. Tardive dystonia is also classified as a secondary dystonia.
In many neurodegenerative diseases, dystonia may be either a prominent or presenting feature. Either cervical or appendicular dystonia may be present in up to one-third of patients with parkinsonian syndromes such as multiple system atrophy and progressive supranuclear palsy (PSP). However, in these patients, characteristic neurological and neuroimaging findings readily permit an accurate diagnosis. In particular, the presence of dementia, autonomic dysfunction, and/or oculomotor abnormalities in the hereditary and neurodegenerative diseases with dystonia sets these disorders apart from the primary dystonias. Family history, response to levodopa, and rate of disease progression provide additional, often useful, diagnostic clues.
C. Genetic Designations
Analysis of the individual DYT syndromes provides important insight into possible genetic and molecular mechanisms underlying the development of the late-onset primary focal/segmental dystonias, although the “DYT” nomenclature includes many syndromes that are not considered to be primary dystonias. In particular, DYT5, DYT11, and DYT14 are dystonia-plus syndromes, and patients with DYT8, DYT9, and DYT10 mutations frequently exhibit additional motor manifestations such as ataxia or chorea. Review of Table 2.2 reveals that inheritance may occur in dominant, recessive (e.g., DYT2), or X-linked (DYT3) Mendelian patterns. The DYT2 and DYT17 loci may be responsible for sporadic cases of DYT1-negative childhood-onset dystonia. Autosomal recessive inheritance also suggests that haploinsufficiency may contribute to some adult-onset primary dystonias.
III. PRIMARY DYSTONIA
A. TOR1A (DYT1)
1. Genotypes and phenotypes
TOR1A (12 kb) is a five exon gene located on the reverse strand of Chr 9q34.11. The classic c.904_906delGAG (ΔGAG) mutation in Exon 5 of TOR1A typically manifests as early-onset generalized dystonia with onset in the distal lower extremities (Ozelius et al., 1997). However, DYT1 dystonia is genetically and phenotypically heterogeneous. Although relatively uncommon, the DYT1 ΔGAG mutation has also been associated with late-onset focal, segmental, and multifocal dystonia (Gambarin et al., 2006; Gasser et al., 1998; Grundmann et al., 2003; Kabakci et al., 2004; O’Riordan et al., 2002; Valente et al., 1998). The carrier frequency of the classic DYT1 ΔGAG mutation is estimated at 1:1000–1:3000 in Ashkenazi Jews (Risch et al., 1995) and less than 1:30,000 in non-Jews (Frédéric et al., 2007). The penetrance of the DYT1 ΔGAG mutation is 30–40%. A p.Asp216His sequence variant may alter penetrance. Risch et al. (2007) found that the frequency of the 216His allele to be increased in ΔGAG mutation carriers without dystonia. Haplotype analysis demonstrated a protective effect of the His allele in trans with the ΔGAG mutation. Moreover, the Asp216 allele in cis may be required for penetrance of the ΔGAG mutation. The work of Risch et al. (2007) was not replicated in the French population (Frédéric et al., 2009).
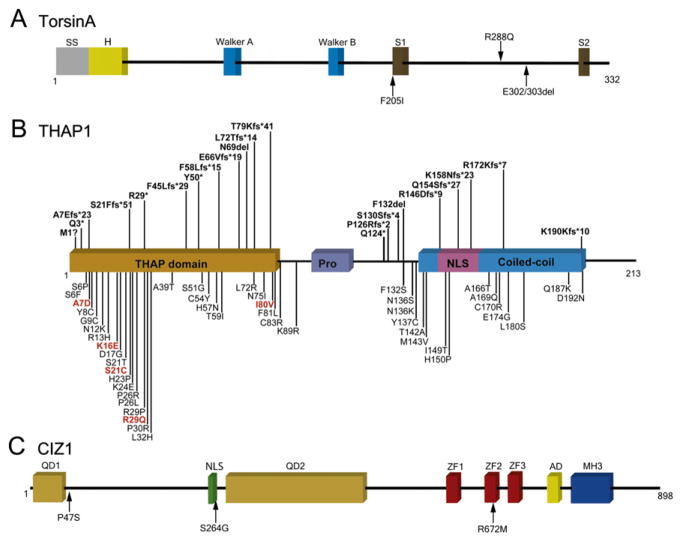
Another sequence variant in Exon 5 of TOR1A (c.863G>A, Fig. 2.1A) has been described in one female patient with severe childhood-onset generalized dystonia (Zirn et al., 2008a). The G>A transition results in exchange of an arginine for a glutamine. Two additional sequence variants have been described in Exon 5. Leung et al. (2001) reported a subject with early-onset dystonia and myoclonus who harbored an 18-bp deletion in Exon 5 (p.Phe323_Tyr328del), which eliminates a putative phosphorylation site. The causality of the 18-bp deletion is doubtful since the same subject was subsequently found to have a mutation in SGCE, the gene associated with myoclonus-dystonia syndrome (Klein et al., 2002). In another study, a 4-bp deletion (c.934_937delAGAG) found in a putatively healthy blood donor should result in a premature stop at position 325 in the carboxy terminus of torsinA (Kabakci et al., 2004).
Figure 2.1.
TorsinA, THAP1, and CIZ1. (A) Functional domains of torsinA and the location of coding variants that have been associated with dystonia. Walker A and B motifs are involved in ATP binding and hydrolysis. SS, signal sequence; H, hydrophobic domain; SI, sensor 1; and S2, sensor 2. (B) Functional domains of THAP1 and the location of coding variants that have been associated with dystonia. THAP, thanatos-associated protein domain; Pro, low-complexity proline-rich region; and NLS, nuclear localization signal. M1?, c.2delT or c.1A>G. (C) Functional domains of CIZ1 and the location of variants that have been associated with cervical dystonia. QD1, glutamine-rich domain 1; NLS, putative nuclear localization sequence; QD2, glutamine-rich domain 2; ZF, zinc finger domains; AD, acidic domain; and MH3, matrin (MATR3)-homologous domain 3. For color version of this figure, the reader is referred to the online version of this book.
A novel sequence variant (c.613T>A, p.F205I) in a patient with late-onset, masticatory and facial dystonia was reported by Calakos et al. (2010). The variant is located in a conserved AAA-ATPase domain of torsinA. Expression assays revealed that expression of p.F205I torsinA produced frequent intracellular inclusions. Despite functional data, the causality of this variant must be questioned given that the affected subject had a long history of psychiatric disease with neuroleptic exposure.
Xiao et al. (2009) sought to identify TOR1A Exon 5 mutations in a large cohort of subjects (>1000) with mainly non-generalized primary dystonia. High resolution melting (HRM) was used to examine the entire TOR1A Exon 5 coding sequence. HRM of Exon 5 showed high (100%) diagnostic sensitivity and specificity, reliably differentiating the ΔGAG and c.863G>A mutations. Melting curves were normal in 250/250 controls and 1012/1014 subjects with primary dystonia. The two subjects with shifted melting curves were found to harbor the classic ΔGAG deletion: (1) a non-Jewish Caucasian female with childhood-onset multifocal dystonia and (2) an Ashkenazi Jewish female with adolescent-onset spasmodic dysphonia. Although Exon 5 mutations in TOR1A are rarely associated with late-onset non-generalized primary dystonia, the role of sequence variants in Exons 1–4 and non-coding regions of TOR1A remains largely unknown. Clearly, the classic DYT1 ΔGAG mutation is uncommon in non-generalized primary dystonia and quite rare in late-onset primary dystonia.
Growing databases of sequence variants such as dbSNP, Exome Variant Server, and 1000 Genomes point out the richness of genetic variation and potential complexity of genotype–phenotype correlations. For instance, a substantial number of TOR1A variants have been reported in putatively normal controls (coding: p.Asp185His, p.Asp216His, p.Asp228His, and p.Asp259His; splice site; 5′UTR; 3′UTR; intronic; and promoter region). Unfortunately, however, most of the variants have not been validated with Sanger sequencing and many are probably next-gen read errors.
The characteristic DYT1 phenotype is characterized by early-onset in a limb, most commonly a leg, with spread to other limbs and the trunk over several years. Hand–forearm dystonia initially manifest as writer’s cramp or more extensive upper limb dystonia without task specificity is the most common presentation of adolescent- or late-onset primary dystonia due to the DYT1 ΔGAG mutation, and most of these subjects will have a positive family history of dystonia (Gambarin et al., 2006). However, DYT1 dystonia is phenotypically heterogeneous with oftentimes striking intrafamilial and interfamilial variability (Edwards et al., 2003; Gajos et al., 2007; Leube et al., 1999; Opal et al., 2002). Atypical presentations include (1) childhood-onset cervical dystonia with much later development of laryngeal dystonia and writer’s cramp and (2) isolated laryngeal involvement for over 40 years (Xiao et al., 2009). Other remarkable phenotypes described in the literature include onset of focal dystonia at 64 years, status dystonicus, and late-onset dystonia precipitated by exposure to a neuroleptic (Edwards et al., 2003; Opal et al., 2002).
2. TorsinA
TorsinA is a member of the ATPases associated with a variety of cellular activities (AAA+) superfamily of proteins that includes torsinB and two related gene products (TOR2A and TOR3A) in mammals, and OOC-5 in Caenorhabditis elegans. AAA+ proteins function as molecular chaperones for protein quality control (protein complex assembly, operation, disassembly, protein folding, unfolding, and degradation), membrane fusion and vesicular transport, and cytoskeletal regulation (Neuwald et al., 1999; Ogura and Wilkinson, 2001; Vale, 2000). TorsinA harbors an N-terminal signal sequence, a single AAA+ module that includes Walker A and Walker B nucleotide-binding motifs, sensor 1 and sensor 2 regions, and two biochemically confirmed glycosylation sites (Callan et al., 2007; Kamm et al., 2004; Neuwald et al., 1999; Ozelius et al., 1997, 1999). TorsinA is an endoplasmic reticulum (ER) luminal monotopic membrane protein (Vander Heyden et al., 2011). The hydrophobic N-terminal domain of torsinA directs static retention of torsinA within the ER by excluding it from ER exit sites. TorsinA functions as a homohexamer. TorsinA is enriched at the nuclear envelope when overexpressed in vitro (Goodchild and Dauer, 2004; Naismith et al., 2004).
TorsinA is present in neuron perikarya and extends to the distal tips of dendrites and axons (Augood et al., 2003; Kamm et al., 2004; Konakova and Pulst, 2001; Konakova et al., 2001). TorsinA may function as a chaperone for unfolded or degraded proteins and may facilitate movement of polytopic proteins to the cell surface (Torres et al., 2004). TorsinA has been localized to Lewy bodies in Parkinson’s disease brain and inclusion bodies in trinucleotide repeat diseases (Sharma et al., 2001; Shashidharan et al., 2000; Walker et al., 2003). Overexpression of torsinA suppresses aggregation of α-synuclein in human neuroglioma cells (McLean et al., 2002) and polyglutamine-induced protein aggregation in C. elegans (Caldwell et al., 2003). TorsinA facilitates clearance of another dystonia-related protein, ε-sarcoglycan, by the ubiquitin proteosome system (Esapa et al., 2007).
TorsinA appears to protect PC12 cells against cellular insults, such as serum deprivation and oxidative stress (Esapa et al., 2007; Kuner et al., 2003; Shashidharan et al., 2004), and dopaminergic neurons from oxidative stress in mice (Kuner et al., 2004) and C. elegans (Cao et al., 2005). The chaperone functions of torsinA may be essential during developmental processes, which seemingly involve interaction with cytoskeletal elements (Ferrari-Toninelli et al., 2004; Hewett et al., 2006; Kamm et al., 2004). The expression of torsinA shows prolonged increases after insults to the central nervous system (CNS) and peripheral nervous system (Zhao et al., 2008). Moreover, expression of torsinA in reactive astrocytes in the CNS and satellite cells in the peripheral nervous system indicates that glial cells may contribute to the pathobiology of DYT1 dystonia (Zhao et al., 2008).
The expression of torsinA is developmentally regulated with the highest levels of transcript and protein seen during the prenatal and early postnatal periods (Xiao et al., 2004). The expression of torsinA is intense in cerebellar cortex and striatal cholinergic interneurons at Postnatal Day 14, a period of intense dendritogenesis in these areas (Vasudevan et al., 2006; Xiao et al., 2004).
The torsinA homolog present in C. elegans (OOC-5) contributes to PAR protein localization. Mutations of ooc-5 result in polarity defects in C. elegans embryos (Basham and Rose, 2001). Attenuated torsinA expression promotes neurite outgrowth in SH-SY5Y human neuroblastoma cells (Ferrari-Toninelli et al., 2004), whereas overexpression of mutant torsinA interferes with neurite extension (Hewett et al., 2006). TorsinA may play direct or indirect roles in neuritogenesis and/or associated nuclear rotation.
Cytoskeletal interactions at the nuclear envelope and ER may be an important aspect of torsinA biology. TorsinA knockout and homozygous ΔGAG knockin mice show ultrastructural morphological abnormalities of the nuclear envelope (Goodchild et al., 2005). TorsinA has been shown to interact with LAP1 in the nuclear envelope and LULL1 in the ER (Goodchild and Dauer, 2005). In a yeast two-hybrid study, torsinA was found to interact with kinesin light chain (Kamm et al., 2004). TorsinA co-immunoprecipitates with a multimolecular complex that includes vimentin, tubulin, actin, kinesin light chain, LAP1, LULL1, and nesprin (Hewett et al., 2006; Nery et al., 2008) and related in vitro studies showed that mutant torsinA interferes with cytoskeletal events that involve vimentin (Hewett et al., 2006). Vimentin, a member of the intermediate filament family of proteins, is expressed in developing brain (Hutchins and Casagrande, 1989; Sancho-Tello et al., 1995) and reactive glia (Braun et al., 1998; Kindy et al., 1992).
B. DYT2
Initially described in Spanish gypsies (Gimenez-Roldan et al., 1988; Santangelo, 1934), autosomal recessive generalized dystonia (DYT2; MIM 224500) has also been reported in Iranian (Khan et al., 2003b) and Arab-American (Moretti et al., 2005) families. In theory, DYT2 loci may be responsible for sporadic cases of DYT1- and DYT6-negative early-onset dystonia. Autosomal recessive inheritance also suggests that haploin-sufficiency may contribute to some late-onset primary dystonias.
C. DYT4
The Australian “whispering dysphonia” kindred was first described by Parker (1985). Although the “whispering” descriptor suggests the presence of the abductor subtype, most affected individuals have reportedly presented with the adductor subtype of spasmodic dysphonia. DYT4 patients often progress to craniofacial and cervical dystonia. Some of the more severe cases progress to generalized dystonia. DYT4 appears to obey an autosomal dominant inheritance pattern. Studies of the Northern Queensland kindred have been confounded by the presence of Wilson’s disease in several family members. However, ATP7B mutations do not segregate with dystonia (Wilcox et al., 2011). Somewhat similar to DYT11, alcohol improves symptoms especially early in the disease course (Wilcox et al., 2011). There is no linkage to the DYT1, DYT6, DYT7, DYT11, or DYT13 loci and no identified mutations in THAP1 (DYT6) or PRKRA (DYT16).
D. THAP1 (DYT6)
1. Genotype and phenotypes
THAP1 (THAP domain containing, apoptosis associated protein 1) was the second gene to be associated with primary dystonia (Fuchs et al., 2009). The seminal THAP1 mutation identified in Amish-Mennonite families was a heterozygous 5-bp (GGGTT) insertion followed by a 3-bp deletion (AAC) in Exon 2 (Fuchs et al., 2009). At the protein level, this mutation causes a frameshift and premature stop codon (F45Lfs*29).
THAP1 is located on the reverse strand of Chr 8. THAP1 consists of three exons and is alternatively spliced into three (2189 bp) and two (1993 bp) exon variants. THAP1 mutations are an important cause of both early-and late-onset primary dystonia. In contrast to TOR1A, mutations in THAP1 show great diversity with missense mutations broadly distributed across its three exons (Bressman et al., 2009; LeDoux et al., 2012b; Xiao et al., 2010). In addition, frameshift, non-coding, and homozygous mutations in THAP1 have also been associated with dystonia (Houlden et al., 2010; Schneider et al., 2011; Xiao et al., 2010). Although initially described in Amish-Mennonites living in the Eastern United States, THAP1 dystonia has now been reported in individuals of Caucasians in most European countries and Chinese.
A promoter variant, 5′ to the THAP1 coding sequence, was identified in African-Americans subjects with dystonia and African-American controls (Puschmann et al., 2011). Highlighting the importance of matched control groups in candidate gene studies of dystonia, this variant (g.42698477C>T/A) was not found in Caucasians. Similarly, an initial report suggested that a nearby 5′UTR dinucleotide GA>TT variant was associated with increased risk for dystonia (Djarmati et al., 2009), whereas a subsequent large, well-matched case–control study failed to confirm this association (Xiao et al., 2011). However, the TT variant may be pathological in the homozygous state since all three published carriers had manifest dystonia (Djarmati et al., 2009; Xiao et al., 2011).
Initial reports suggested that c.71+126T>C (Houlden et al., 2010) and c.71+9C>A (Xiao et al., 2010) variants may increase risk for primary dystonia, and follow-up studies have been underpowered to substantiate or refute the pathogenicity of these variants (Djarmati et al., 2009; Groen et al., 2010; Lohmann et al., 2012b). Although beyond the core splice site, the c.71+9C>A variant may possibly alter splicing efficiency and the ratio of the two THAP1 isoforms. Similarly, the c.71+126T>C variant could exert cryptic effects on splicing or reduce overall gene expression.
At least 75 dystonia-associated coding variants have been published to date. Among this collection are 45 missense variants, 8 silent variants, and 22 indels or nonsense variants (Fig. 2.1B). All reported THAP1 indels and nonsense mutations are predicted to elicit nonsense-mediated decay or generate truncated proteins. None of the mutations shown in Fig. 2.1B are predicted to generate an elongated THAP1. Missense variants are concentrated within the THAP domain. Other missense variants are present within the coiled-coil domain and nuclear localization signal. There are no missense variants within the proline-rich region. All but two missense variants (p.L32H and p.N136S) were heterozygous (Houlden et al., 2010; Schneider et al., 2011). The subject harboring p.N136S had no family history of dystonia, whereas three siblings exhibited early-onset generalized dystonia in the kindred identified with the p.L32H variant (Schneider et al., 2011). Heterozygous carriers of the p.L32H variant were reportedly asymptomatic.
All reported silent variants are associated with sporadic dystonia. Silent mutations have the potential to be pathogenic if they activate cryptic splice sites, leading to exon skipping or the inclusion of intronic sequences into mature transcripts. For example, the c.267G>A (p.K89K) silent variant is located at the Exon 2 to Intron 2 boundary and was shown to reduce the expression of THAP1 RNA in lymphocytes (Cheng et al., 2011).
Age of onset for THAP1 dystonia ranges from 3 to over 60 years with a mean of 16.8 years (LeDoux et al., 2012b). Common sites of onset are the arm (42.6%) and neck (24.6%), and 43.1% of affected individuals progress to generalized dystonia (LeDoux et al., 2012b). Ultimately, the cranium, mainly the lower face, jaw, tongue, or pharynx, is affected in approximately half of patients with THAP1 dystonia. Mutations near the N-terminus of THAP1 are associated with an earlier age of onset and tended to be associated with more extensive anatomical involvement (LeDoux et al., 2012b). Mutations within the THAP domain are associated with an earlier age of onset than non-THAP domain mutations (LeDoux et al., 2012b). THAP domain mutations are also associated with more extensive anatomical distributions but have no significant effect on site of onset.
Each THAP1 sequence variant probably exhibits a unique penetrance value that is modified by environmental factors and overall genetic background. The penetrance of the Amish-Mennonite indel is 50–60% (Fuchs et al., 2009), whereas the penetrance for many of the published missense variants is probably much lower. Proposing specific values for penetrance is compromised by intrafamilial variability in age of onset and assigning affection status to subtle phenotypes.
2. THAP1
THAP1 and related family members are defined by their zinc-binding THAP (Thanatos [Greek god of death]-associated protein) domain that is found in a number of proteins involved in various aspects of transcriptional regulation, apoptosis, and cell cycle control. The human genome contains 12 THAP family members. The THAP domain contains a C2-CH zinc finger that is similar to the DNA-binding domain of the Drosophila P-element transposase (Roussigne et al., 2003). The THAP domain (1-81aa) of THAP1 recognizes an 11 nucleotide target sequence (AGTACGGGCAA) (Clouaire et al., 2005). THAP1 also contains a low-complexity proline-rich region and bipartite nuclear localization signal. THAP1 probably functions as a homodimer within a larger multimeric DNA-binding complex. Amino acid residues 154 to 166 within the coiled-coil domain are critical for dimerization of THAP1 (Sengel et al., 2011). THAP1 is widely expressed in the brain and extra-neural tissues, including whole blood, liver, kidney, skeletal muscle, thyroid gland, and prostate.
E. DYT7
Leube et al. (1996) performed linkage analysis on a large German family (Family K) with seven definitely affected individuals (6/7 with cervical dystonia and 1/7 with spasmodic dysphonia) and six possibly affected individuals. Of the affected subjects, one also had blepharospasm and another had hand–forearm dystonia. The distribution of affected subjects was most compatible with autosomal dominant inheritance. A maximal LOD score of 3.17 was assigned to the region telomeric to marker D18S1153 on Chr 18p. In follow-up work, Leube et al. (1997) suggested that many sporadic cases of dystonia from Northwest Germany inherited the same mutation as Family K from a common ancestor and narrowed the causal mutation to a 6-cm region close to marker D18S1098.
F. DYT13
Dystonia has been linked to Chr 1p36.13–36.32 in a large Italian kindred with 11 definitely affected members (Bentivoglio et al., 2004; Valente et al., 2001). Linkage analysis using an autosomal dominant model generated a maximum LOD score of 3.44 between the disease and marker D1S2667. Age at onset ranged from 5 to 43 years, and similar to DYT6 dystonia, site of onset occurred either in the craniocervical region or arms. Dystonia generalized in only two cases and was relatively mild compared to DYT1 generalized dystonia. Penetrance was incomplete (~58%).
G. DYT17
Using homozygosity mapping with 382 microsatellite markers, DYT17 was mapped to a 20.5-Mb interval on Chr 20 in a large consanguineous Lebanese family with three affected individuals (Chouery et al., 2008). Of 270 genes in this interval, causal variants were excluded in 27 candidate genes. Dystonia became manifest with cervical involvement between 14 and 19 years of age in the three affected individuals. Dystonia became generalized in one subject.
H. DYT21
Holmgren et al. (1995) reported a large Swedish family with 10 affected members with age of onset ranging from 18 to 50 years. Dystonia was quite variable with focal and generalized distributions. Initial presentations included blepharospasm, arm dystonia, and dysarthria.
In this Swedish kindred, dystonia was inherited in an autosomal dominant manner with a penetrance of approximately 90%. Using SNP-based linkage analysis, the dystonia locus was mapped to Chr 2q14.3-q21.3. Microsatellite confirmation generated a maximum LOD score of 5.59 for marker D2S1260. No causal mutations were identified in 22 candidate genes within the disease interval on Chr 2p (Norgren et al., 2011).
I. CIZ1
In very recent work (Xiao et al., 2012), solution-based whole-exome capture and massively parallel sequencing in combination with micro-satellite-based linkage analysis was used to identify the causal sequence variant in a large Caucasian pedigree with adult-onset primary cervical dystonia first described by Uitti and Maraganore (1993). Cervical dystonia showed strongest linkage to microsatellite marker D9S159 located on Chr 9q34.11, and whole-exome sequencing identified an exonic splicing enhancer mutation in Exon 7 of CIZ1 (c.790A>G, p.S264G), a gene within the candidate region. CIZ1 encodes a p21Cip1/Waf1-interacting zinc finger protein (CIZ1) that is involved in DNA synthesis and G1/S cell cycle control. After confirmation of co-segregation with dystonia, high-throughput screening identified two additional CIZ1 missense mutations (p.P47S and p.R672M) among a population of 308 Caucasians with familial or sporadic adult-onset cervical dystonia (Fig. 2.1C).
J. Other Adult-Onset Primary Dystonia
Although at least 10-fold more common than early-onset (<20 years) primary dystonia and far more prevalent than well-known neurological disorders such as Huntington disease, amyotrophic lateral sclerosis, and Duchenne muscular dystrophy, very little is known about the biological underpinnings of the primary adult-onset focal dystonias. Moreover, we do not know if focal dystonias such as cervical dystonia, spasmodic dysphonia, blepharospasm, and hand–forearm dystonia (e.g., writer’s cramp) are (1) distinct and separate entities or (2) localized manifestations of primary generalized dystonia. Clinical information suggests that age, gender, and environment are major contributors to the anatomical distribution of dystonia. Alternatively, the primary adult-onset focal dystonias are caused by shared genetic variant(s) transmitted in autosomal dominant fashion with variable expressivity. Phenotypic concordance–discordance is approximately 50%/50% for adult-onset primary dystonia. An example of phenotypic discordance would be blepharospasm in a proband and cervical dystonia in one of the proband’s siblings.
Current thinking suggests that sporadic primary dystonia, like many other adult-onset diseases, is due to complex interactions between the genome and environment. In this regard, peripheral trauma (e.g., ocular surface irritation) or intense sensorimotor training (e.g., writing) may trigger dystonia in genetically predisposed individuals. Clearly, genetic factors play a major role in adult-onset primary dystonia since 10–20% of patients with primary adult-onset dystonia have one or more family members affected with dystonia (Defazio et al., 2011; Xiao et al., 2010) and several of the early-onset primary dystonias inherited in Mendelian fashion begin focally, show incomplete penetrance, and exhibit variable anatomical expressivity. It is likely that rare sequence variants of low to moderate penetrance in THAP1, TOR1A, CIZ1, PRKRA, and within the DYT2, DYT4, DYT7, DYT13, DYT16, DYT17, and DYT21 loci contribute to risk for largely sporadic adult-onset primary dystonia.
IV. DYSTONIA PLUS
A. DYT5/DRD
First described by Segawa et al. (1976), DRD is something of a misnomer since several other neurogenetic disorders may exhibit dystonia that responds to levodopa. On the other hand, classic DRD due to mutations in GCH1 is imminently treatable with low dosages of levodopa and, diagnostically, should not be missed by clinicians.
Classic DRD (DYT5a) is due to deficiency of GTP cyclohydrolase I (GCH1), the rate-limiting enzyme for synthesis of the cofactor tetrahy-drobiopterin (BH4) (Werner et al., 2011). BH4 is an essential co-factor for the aromatic amino acid hydroxylases tyrosine hydroxylase (TH), tryptophan hydroxylase (TPH2), and phenylalanine hydroxylase (PAH) (Thony and Blau, 2006). BH4 also regulates nitric oxide synthase. TH is the rate-limiting enzyme for synthesis of dopamine, norepinephrine, and epinephrine. Tryptophan hydroxylase is the rate-limiting enzyme involved in synthesis of serotonin. The synthesis of BH4 requires the serial action of GTP cyclohydrolase I (GCH1), 6-pyruvoyl-tetrahydropterin synthase (PTS), and sepiapterin reductase (SPR). Autosomal recessive mutations in PTS are associated with hyperphenylalaninemia and can cause a wide range of neurological manifestations including microcephaly, oculogyric crises, sialorrhea, developmental delay, parkinsonism, seizures, and dystonia (Dudesek et al., 2001).
Cofactor regeneration requires pterin-4a-carbinolamine dehydratase (PCBD1) and quinoid dihydropteridine reductase (QDPR) (Werner et al., 2011). Recessive mutations in QDPR are associated with hyperphenylalaninemia, development delay, intracerebral calcifications, seizures, and, occasionally, dystonia (Larnaout et al., 1998). In contrast, recessive mutations in PCBD1 cause hyperphenylalaninemia but only mild, transient neurological features.
1. DYT5a (GTP cyclohydrolase I)
GCH1 contains six exons and is located on Chr 14q22.2. Grotzsch et al. (2002) mapped another locus for DRD (DYT14) to Chr 14q13. However, Wider et al. (2008) showed that this Swiss family with DRD had a heterozygous deletion of GCH1 Exons 3 to 6. The vast majority of dominant mutations in GCH1 are associated with the classic phenotype of childhood-and limb-onset dystonia that is completely responsive to low-dose levodopa (<400 mg/day). Transient levodopa-induced dyskinesias may be seen in some patients. Mean age of onset is approximately 6 years. The penetrance is notably higher in females.
Over time, the phenotype of GTP cyclohydrolase I–deficient DRD has expanded to include adult-onset parkinsonism, adult-onset focal dystonias including cervical dystonia, spastic diplegia, or cerebral palsy like syndrome, and a variety of co-morbidities including depression, anxiety, and sleep disorders. Upon careful examination, many untreated patients with childhood-onset DRD show evidence of mild parkinsonism with rigidity, bradykinesia, and rapid fatiguing with repetitive arm movements. Most patients report diurnal fluctuation of symptoms with worsening in the late afternoon hours.
The majority of GCH1 mutations can be detected with Sanger sequencing. However, a significant minority of affected families harbor large deletions or duplications or mutations in noncoding regions (Sharma et al., 2011; Zirn et al., 2008b). Therefore, most commercial laborites employ the quantitative polymerase chain reaction or multiplex ligation-dependent probe amplification if routine sequencing is unrevealing.
Rarely, GTP cyclohydrolase I deficiency is recessive. These cases are commonly associated with hyperphenylalaninemia and severe neurological dysfunction including seizures, developmental delay, and involuntary movements. These severe cases demand treatment with BH4, levodopa, and 5-hydroxytryptophan. Milder variants without hyperphenylalaninemia have also been reported and may respond to levodopa alone (Horvath et al., 2008; Opladen et al., 2011).
2. DYT5b (tyrosine hydroxylase)
TH deficiency shows a much broader phenotypic spectrum than GTP cyclohydrolase I deficiency (Willemsen et al., 2010). At one end of the spectrum is mild dystonia that responds to low-dose levodopa for decades. In contrast, more severe cases are associated with infantile parkinsonism with onset prior to 6 months of age, developmental delay, levodopa-induced dyskinesias, ptosis, gastroparesis, and oculogyric crises. Although the vast majority of cases are autosomal recessive, heterozygotes may manifest subtle exertion-induced dystonia or rigidity or restless legs syndrome (Swoboda et al., 2006). Most reported variants have been missense mutations, either homozygous or heterozygous. Frameshift and promoter region mutations have also been described (Verbeek et al., 2007). The promoter mutations have been localized to a cAMP response element.
3. DYT5b (sepiapterin reductase)
In general, the lack of hyperphenylalaninemia distinguishes sepiapterin reductase deficiency from other autosomal recessive disorders of BH4 synthesis (Bonafé et al., 2001). On average, clinical manifestations are more severe than those associated with GTP cyclohydrolase I deficiency. The disease spectrum includes cognitive delay, oculogyric crises, microcephaly, hyperactivity, hypersomnolence, parkinsonism, dystonia, tremor, seizures, and, if untreated, mental retardation. Onset may occur in infancy. Several novel homozygous and compound heterozygous missense, splice site, nonsense, and frameshift mutations in SPR have been reported since 2001 (Lohmann et al., 2012a). In rare cases, sepiapterin reductase deficiency may be autosomal dominant (Steinberger et al., 2004).
B. DYT11/MDS
Myoclonus-dystonia syndrome is due to mutations in SGCE, which encodes ε-sarcoglycan (Zimprich et al., 2001). SGCE is maternally imprinted and covers 71 kb on the reverse strand of Chr 7q21.3. Virtually, all affected individuals have myoclonus, which is concentrated in the upper extremities, neck, and trunk with infrequent involvement of the legs. Approximately 50–65% of patients have dystonia, usually affecting the neck or arms. Very rarely, dystonia may be the only disease manifestation. Onset is usually in childhood or early adolescence but has been reported in the fourth decade of life. Many affected individuals reported a dramatic reduction in myoclonus after consumption of alcohol. Psychiatric disorders including depression and anxiety can be prominent non-motor features of the disorder.
Given that SGCE is maternally imprinted, penetrance is significantly higher when the mutant allele is inherited from the father. DYT11 may be caused by a variety of mutations in SGCE (nonsense, missense, insertions, and deletions). Testing for exonic deletions should be considered in individuals with a classic phenotype in whom Sanger sequencing is unrevealing (Asmus et al., 2005; Grunewald et al., 2008).
In muscle, the sarcoglycans are part of the large transmembrane dystrophin–glycoprotein complex required for the stability of striated muscle membranes. Muscle expression of ε-sarcoglycan is highest in the early postnatal period with only minimal expression in adult muscle (Xiao and LeDoux, 2003). In brain, ε-sarcoglycan is expressed at high levels in cerebellar cortex (Xiao and LeDoux, 2003), and a major brain-specific isoform shows relatively high expression in Purkinje cells and the dentate nucleus (Ritz et al., 2011). In neurons, the dystrophin–glycoprotein complex may be involved in the clustering and stabilization of GABAergic synapses (Waite et al., 2009).
C. DYT12/RDP
Although denoted “rapid-onset dystonia–parkinsonism (RDP),” relatively few patients have all four cardinal features of Parkinson’s disease (resting tremor, bradykinesia, postural instability, and rigidity). In fact, the term “rapid-onset dystonia” would perhaps be more appropriate if it was not for the fact that many patients with largely sporadic adult-onset dystonia wake up with their disorder or report onset over a couple of days. Despite these caveats, RDP is fairly distinct with abrupt onset of dystonia with varying degrees of bradykinesia. The dystonia obeys a rostral-caudal gradient (face → arm → trunk) with minimal leg involvement and often prominent bulbar involvement with dysphagia and dysarthria. Patients do not respond to levodopa. Typically, RDP first manifests in teenagers or young adults, although age of onset ranges from 4 to 55 years of age.
RDP is an autosomal-dominant disorder due to mutations in ATP1A3, which encodes ATPase, Na+/K+ transporting, and α-3 polypeptide. The α-3 subunit of Na+/K+–ATPase is an integral membrane protein and a member of the P-type cation transport ATPases. Members of this family help to maintain electrochemical gradients across the plasma membrane. The α subunit of Na+/K+–ATPase has three isoforms (α-1, α-2, and α-3) encoded by distinct genes rather than alternative splicing of a single gene.
ATP1A3 contains 23 exons and covers 27.6 kb of genomic DNA. Mutations described to date have been concentrated in Exons 8, 14, 15, 17, 20, and 23 (Brashear et al., 2007). Missense mutations, a 3-bp deletion and a 3-bp insertion, have been reported, mainly in Caucasians (Brashear et al., 2007; de Carvalho Aguiar et al., 2004; Tarsy et al., 2010). An RDP p.D923N mutation in ATP1A3 shows approximately 200-fold reduction of Na+ affinity for activation of phosphorylation from ATP (Einholm et al., 2010). In general, RDP mutant forms of Na+/K+ ATPase show reduced affinity for cytoplasmic Na+ (Rodacker et al., 2006).
RDP is genetically heterogeneous. ATP1A3 mutations have not been identified in all subjects with an RDP phenotype (Brashear et al., 2007). In particular, Kabakci et al. (2005) reported a large RDP kindred that did not harbor an ATP1A3 mutation or show linkage to Chr 19.
D. DYT16
High-density autozygosity mapping (Illumina HumanHap550) was used to identify a disease-segregating region of homozygosity in affected family members from two families with early onset, apparently autosomal recessive dystonia in southeast-central Brazil (Camargos et al., 2008). The relationship between these two families remains unclear. Age of onset ranged from 2 to 18 years. Lower limb onset was apparent in 4/7 subjects. Dystonia became generalized with neck, trunk, laryngeal, and oromandibular involvement. Some patients were mildly bradykinetic and possibly parkinsonian. Moreover, 3/7 subjects had evidence of upper motor neuron dysfunction. Sanger sequencing of all genes within a 1.2-Mb interval on Chr 2q31 revealed a single disease-segregating mutation, c.665C>T (P222L), in PRKRA, which encodes protein kinase, interferon-inducible double-stranded RNA-dependent activator. In a single follow-up study, Seibler et al. (2008) reported a heterozygous frameshift mutation in a patient with early-onset dystonia first manifest in a leg. At last examination, this subject had generalized dystonia that spared the cranial and facial muscles. The pathogenicity of this variant must be questioned given that PRKRA-null mice do not show evidence of dystonia or significant neurological disease (Peters et al., 2009). Furthermore, in contrast to the Brazilian patients, dystonia spared the cranial muscles in the subject from Germany.
V. HEREDODEGENERATIVE DYSTONIA
A. X-Linked Dystonia–Parkinsonism (XDP/DYT3)
Also known as Lubag, X-linked dystonia–parkinsonism (XDP) is limited to Filipinos with origin from the Island of Panay. Parkinsonism is usually the presenting manifestation in the fourth decade of life. Craniocervical dystonia typically begins later with involvement of the masticatory, cervical, and upper facial muscles in many patients. Appendicular dystonia is less common. Parkinsonism may partially respond to levodopa and dopamine agonists. Males are affected more severely than females and moderate phenotypic variability is noted with isolated parkinsonism or focal dystonia in some subjects.
Subjects with XDP harbor five distinct single nucleotide sequence variants in addition to a 48-bp deletion and an SVA (short interspersed nuclear element, variable number of tandem repeats, and Alu composite; SINE/VNTR/Alu) element (Makino et al., 2007; Nolte et al., 2003). The SVA is located in Intron 32 of TATA-binding protein-associated factor 1 gene (TAF1) and may alter expression of a neuron-specific isoform of TAF1 (N-TAF1). In rat brain, N-TAF1 is expressed in medium spiny neurons, preferentially in the striosome compartment of the striatum (Sako et al., 2011). This finding is consistent with postmortem neuropathology in human subjects, which is characterized by a mosaic pattern of striatal gliosis with more prominent neuronal loss in striosomes than in the matrix (Evidente et al., 2002; Goto et al., 2005).
B. Parkinson’s Disease
Dystonia is very common in sporadic and familial Parkinson’s disease (Tolosa and Compta, 2006). Dystonia is a frequent “side-effect” of pharmacological and surgical treatment of Parkinson’s disease. Well-recognized manifestations include AM off-dystonia in the lower extremities, blepharospasm with apraxia of eyelid opening, cervical dystonia, peak-dose dystonia, and diphasic dystonia.
Dystonia is often a presenting sign in early-onset Parkinson’s disease. Classically, this manifests as action dystonia in a distal leg, occasionally only precipitated by exercise (Khan et al., 2003a). Over 30% of individuals with recessive mutations in PARK2, which encodes parkin, present with leg dystonia. Distal leg dystonia is also a common presentation of recessive mutations in PARK6 (PINK1) and PARK7 (DJ1) mutations (Schneider and Bhatia, 2010a).
C. Tauopathies
Largely based on postmortem pathological identification of tau protein aggregation, a group of neurodegenerative disorders including PSP, corticobasal ganglionic degeneration (CBGD), frontotemporal dementia and parkinsonism linked to Chr 17 (FTDP-17), argyrophilic grain disease, and Pick’s disease have been commonly referred to as tauopathies. Dystonia is a common clinical manifestation in PSP (Barclay and Lang, 1997) and CBGD (Reich and Grill, 2009). Tau is encoded by MAPT, which covers 134 kb on Chr 17q21.31. Tau is mainly expressed in neurons and contributes to the assembly and stabilization of microtubules. Alternative splicing of Exon 10 determines the number of microtubule-binding repeats (3R or 4R). In PSP and CBGD, accumulation of 4R-tau predominates, whereas 3R-tau is deposited in Pick’s disease.
Case–control studies have demonstrated significant associations between the H1 haplotype, an inversion polymorphism of MAPT and risk for PSP and CGBD (Baker et al., 1999; Houlden et al., 2001). Missense mutations in MAPT have also been associated with sporadic and familial PSP (e.g., p.R5L and p.G303V) (Choumert et al., 2011; Poorkaj et al., 2002). There are no reported relationships among MAPT haplotypes and sporadic primary dystonia. Rare cases of CGBD have been associated with mutations in PGRN, which encodes progranulin (Spina et al., 2007). PGRN mutations are more commonly associated with a clinical–pathological phenotype sometimes described as behavior-predominant frontotemporal dementia.
D. Neurodegeneration with Brain Iron Accumulation
Dystonia may be a presenting or prominent clinical feature of the neuro-degeneration with brain iron accumulations (NBIAs), which are characterized by iron deposition in the brain, particularly the basal ganglia (Schneider and Bhatia, 2010b). With the advent of MRI and increased use of T2★ and T2 fast-spin echo sequences, these disorders are being recognized with increasing frequency (McNeill et al., 2008). It should be emphasized, however, that iron deposition in the basal ganglia is a part of normal aging, and increased iron deposition has been associated with numerous acquired and hereditary disorders that affect the CNS (Aquino et al., 2009).
The classic disorder in this family is NBIA1 (PANK2, pantothenate kinase 2), previously called Hallervorden–Spatz disease. Other NBIAs and NBIA-like disorders include NBIA2 (PLA2G6; calcium-dependent cytosolic phospholipase A2, group VI) also known as PARK4 or infantile neuroaxonal dystrophy (INAD); NBIA3 (FTL, ferritin light chain) or neuroferritinopathy; NBIA4 (C19orf12, an orphan mitochondrial protein); aceruloplasminemia (CP, ceruloplasmin); FAHN (fatty acid hydroxylase neurodegeneration) disease or SPG35 (FA2H, fatty acid 2-hydroxylase); and Kufor–Rakeb syndrome (ATP13A2, lysosomal type 5 ATPase), also known as PARK9. With the exception of neuroferritinopathy, all of these disorders are autosomal recessive.
VI. DYSTONIA IN ASSOCIATION WITH OTHER NEUROGENETIC DISORDERS
A. Paroxysmal Dyskinesias
This group of neurological disorders is characterized by the sudden onset of involuntary movements that may include one or more of the following: dystonia, chorea, athetosis, and ballism (Bhatia, 2011). The paroxysmal dyskinesias are divided into four major types: paroxysmal kinesigenic dyskinesia (PKD), paroxysmal nonkinesigenic dyskinesia (PNKD), paroxysmal exertion-induced dyskinesia (PED), and paroxysmal hypnogenic dyskinesia. Precipitating factors are included within the name of each type: kinesigenic (sudden movements), nonkinesigenic (may occur spontaneously but often triggered by stressors such as anxiety, temperature extremes, or caffeine), exertion induced (running or prolonged walking), and hypnogenic (non-rapid eye movement sleep). The paroxysmal dyskinesias may be familial (hereditary), sporadic, or secondary (or symptomatic) to other neurological or metabolic disorders (e.g., head trauma, multiple sclerosis, hypoparathyroidism). Overall, genetic etiologies have been identified for a modest number affected families and small number of sporadic/idiopathic cases.
1. DYT8 (PNKD)
Many cases of primary, familial PNKD are due to missense mutations (p.A7V, p.A9V, and p.A33P) in the N-terminal mitochondrial targeting sequence of myofibrillogenesis regulator-1 or PNKD (MR-1 or PNKD; Bruno et al., 2007; Ghezzi et al., 2009; Lee et al., 2004; Rainier et al., 2004). Patients with PNKD due to mutations in PNKD have attacks that last from 10 min to 1 h, often precipitated by caffeine, alcohol, or emotional stress. Onset occurs in infancy or early childhood and typically includes a combination of chorea and dystonia with involvement of the face, trunk, and limbs, often bilaterally.
PNKD shows homology to glyoxalase II and is probably involved in the cellular stress response. Although the primary substrate of PNKD is not known, glyoxalase II catalyzes the second step in the glutathione-dependent glyoxylase pathway, which converts methylglyoxal to D-lactic acid. Lower glutathione levels have been noted in the brains of PNKD knockout mice (Shen et al., 2011).
2. DYT9/DYT18 (PED)
Recent work has shown that DYT9 (paroxysmal choreoathetosis/spasticity) and DYT18 (paroxysmal exercise-induced dyskinesia) are allelic disorders due to mutations in SLC2A1, which encodes the glucose transporter type 1 (GLUT1) (Weber and Lerche, 2009; Weber et al., 2011).
Auburger et al. (1996) described a large pedigree with autosomal dominant “paroxysmal choreoathetosis/spasticity.” The pedigree included 18 affected family members. Age of onset ranged from 2 to 15 years with episodes of involuntary movements lasting less than 20 min and manifest mainly as dystonia. The frequency of episodes was highly variable and ranged from daily to twice yearly. Episodes were triggered by a broad range of triggers including emotional stress, exercise, lack of sleep, and alcohol. Some family members demonstrated interictal spasticity in the lower extremities. The causal gene was mapped to Chr 1p.
Weber and co-workers (Schneider et al., 2009; Weber et al., 2008) showed that paroxysmal-exertion (or exercise)-induced dyskinesias (PED) were due to mutations in SLC2A1. They described a large three-generation family with PED, epilepsy, developmental delay, and hemolytic anemia. Cerebrospinal glucose levels were also reduced. Mutations in SLC2A1 have also been identified in sporadic PED (Schneider et al., 2009). In subsequent work, the kindred initially described by Auburger et al. (1996) and an Australian monozygotic twin pair were found to have causal mutations in SLC2A1 with decreased glucose uptake in functional assays.
The classical GLUT1 deficiency phenotype is characterized by early-onset cognitive and motor delay, intractable epilepsy, microcephaly, involuntary movements (dystonia, chorea, ataxia, and choreoathetosis), spasticity, and microcephaly (Verrotti et al., 2012). Most patients with GLUT1 syndromes have abnormally low levels of cerebrospinal fluid glucose. The most effective treatment for these disorders is a ketogenic diet (Verrotti et al., 2012).
GLUT1 is expressed in the brain microvasculature, astrocytes, and red blood cells. Most SLC2A1 mutations reported to date appear to manifest as haploinsufficiency. It is possible that paroxysmal movement disorders are caused by astrocyte failure in critical regions of motor system such as the cerebellum (Schneider et al., 2009).
3. DYT10/DYT19 (PKD)
Using linkage and haplotype analysis, Valente et al. (2000) identified a 15.8-cm candidate region for PKD in a large Indian family. The candidate region is flanked by markers D16S685 and D16S503 on Chr 16q13-q22.1 with a maximum LOD score of 3.66 at D16S419. This candidate region is telomeric to the locus identified in Japanese families with PKD (Tomita et al., 1999) but showed overlap with a region identified in an African-American family with PKD (Bennett et al., 2000). The candidate region for infantile familial convulsions and paroxysmal choreoathetosis (ICCA) was also mapped to the pericentromeric region of Chr 16 in both French (Szepetowski et al., 1997) and Chinese (Lee et al., 1998) families. PKD is genetically heterogeneous, however, and, in at least one British pedigree, does not map to Chr 16 (Spacey et al., 2002).
The family described by Valente et al. (2000) included 13 subjects with PKD. In addition, four deceased family members had probably been affected based on historical accounts. Age at onset ranged from 7 to 13 years. Consistent with PKD, attacks were precipitated by sudden movements and lasted less than 2 min. Attacks included choreiform and dystonic movements and occurred up to 20 times daily. Two family members with PKD and three unaffected family members had generalized tonic–clonic seizures as teenagers. However, epilepsy did not co-segregate with the PKD haplotype. Penetrance was 75%, and over half of the affected family members had spontaneous remissions in their early twenties (Spacey et al., 2002).
Just recently, several distinct loss-of-function frameshift mutations leading to protein truncation or nonsense-mediated decay in proline-rich transmembrane protein 2 (PRRT2) have been associated with PKD in numerous Han Chinese families (Chen et al., 2011; Li et al., 2012; Liu et al., 2012; Wang et al., 2011). Missense mutations (c.796C>T, p.R266W; c.913G>A, p.G305R) have been identified in a single family and one sporadic case of PKD (Liu et al., 2012; Wang et al., 2011). PRRT2 contains two predicted transmembrane domains and is highly expressed in the developing nervous system, particularly the cerebellum (Chen et al., 2011). PRRT2 is located on Chr 16p.11.2, within the ICCA, Japanese PKD, and African-American candidate regions but outside the Indian PKD candidate regions. In addition to classic carbamazepine-responsive PKD, the phenotypic spectrum of PRRT2 mutations appears to include ICCA, a “PNKD-like” syndrome and PED (Liu et al., 2012).
4. DYT20 (PNKD2)
PNKD is genetically heterogeneous (Bruno et al., 2007). For instance, Spacey et al. (2006) described a Canadian family with PNKD and without a mutation in PNKD. In this kindred, the PNKD2 locus maps to Chr 2p31.
B. Ataxias
Dystonia can be a prominent clinical feature or presenting sign in many of the dominant (van Gaalen et al., 2011) and recessive (Perlman, 2011) ataxias. For example, leg or hand–forearm dystonia can be presenting signs in spinocerebellar ataxia type 6 (SCA6) and SCA17 (Hagenah et al., 2004; Sethi and Jankovic, 2002). Similarly, cervical dystonia has been reported as a presenting sign in SCA1 (Wu et al., 2004) and SCA6 (Arpa et al., 1999). Among the SCAs, dystonia is most common in SCA17, SCA3, and SCA2 (van Gaalen et al., 2011).
Although relatively infrequent, dystonia, appendicular and cervical, has been well documented in Friedrich ataxia (FA) (Hou and Jankovic, 2003). FA is perhaps the most common and intensively studied of the hereditary ataxias. FA is caused by reduced expression of the mitochondrial protein frataxin due to a GAA trinucleotide expansion within Intron 1 of FXN. Frataxin plays a critical role in mitochondrial iron metabolism (Shan and Cortopassi, 2011), and FA can be considered a mitochondrial disease caused by a mutation in nuclear DNA (nDNA). In some patients, point mutations are present in heterozygosity. Dystonia may also manifest in several other recessive and X-linked ataxias (Table 2.3).
Table 2.3.
Dystonia associated with other neurogenetic disorders
| Group | Disorder | MIM | Gene | Inheritance | Protein | Phenotype |
|---|---|---|---|---|---|---|
| Dominant ataxias | SCA1 | 164400 | ATXN1 | AD | Ataxin-1 | Ataxia, slow saccades, dysarthria, corticospinal signs, dystonia |
| SCA2 | 183090 | ATXN2 | AD | Ataxin-2 | Ataxia, slow saccades, dysarthria, hyporeflexia, dystonia | |
| SCA3 | 109150 | ATXN3 | AD | Ataxin-3 | Ataxia, ophthalmoplegia, parkinsonism, dystonia, spasticity, neuropathy | |
| SCA6 | 183086 | CACNA1A | AD | α-1A subunit P/Q voltage-gated Ca2+ channel | Ataxia, nystagmus, dystonia | |
| SCA7 | 164500 | ATXN7 | AD | Ataxin-7 | Ataxia, macular degeneration, dystonia, spasticity | |
| SCA8 | 608768 | ATXN8 | AD | Ataxin-8 | Ataxia, nystagmus, spasticity, dystonia, sensory neuropathy | |
| SCA12 | 604326 | PPP2R2B | AD | Serine/threonine-protein phosphatase 2A 55-kDa regulatory subunit B, β isoform | Ataxia, facial myokymia, action tremor, dystonia, parkinsonism, dementia | |
| SCA14 | 605361 | PRKCG | AD | Protein kinase C, gamma | Ataxia, myoclonus, slow saccades, focal dystonia | |
| SCA17 | 607136 | TBP | AD | TATA box-binding protein | Ataxia, spasticity, seizures, dystonia, dementia | |
| Dentatorubral- pallidoluysian atrophy (DRPLA) | 125370 | ATN1 | AD | Atrophin 1 | Ataxia, myoclonus, seizures, dystonia | |
| Recessive ataxias | Friedreich ataxia | 606829 | FXN | AR | Frataxin | Ataxia, nystagmus, optic atrophy, cardiomyopathy, sensory neuropathy, spasticity, dystonia, diabetes, scoliosis, pes cavus |
| Ataxia telangiectasia | 208900 | AT | AR | Serine-protein kinase ATM | Ataxia, telangiectasis, oculomotor apraxia, immune deficiency, seizures, dystonia | |
| Ataxia with oculomotor apraxia type 1 | 208920 | APTX | AR | Aprataxin | Ataxia, oculomotor apraxia, scoliosis, pes cavus, dystonia, choreoathetosis, neuropathy | |
| Ataxia with oculomotor apraxia type 2 | 606002 | SETX | AR | Senataxin | Ataxia, oculomotor apraxia, nystagmus, amyotrophy, dystonia, chorea, neuropathy | |
| Ataxia with selective vitamin E deficiency | 277460 | TTPA | AR | α-Tocopherol transfer protein | Ataxia, areflexia, loss of proprioception, dystonia, xanthelasma, tendon xanthomas | |
| X-linked ataxias | Mental retardation, X-linked syndromic, Christianson type | 300243 | SLC9A6 | X-linked dominant | Sodium/hydrogen exchanger 6 | Ataxia, spasticity, mental retardation, dysmorphic features, microcephaly, dystonia, autistic |
| Dopamine metabolism | AADC deficiency | 608643 | AADC | AR | Aromatic L-amino acid decarboxylase | Psychomotor delay, ptosis, oculogyric crises, dystonia, truncal hypotonia, temperature instability, hypotension |
| Infantile parkinsonism–dystonia | 613135 | SLC6A3 | AR | Presynaptic dopamine transporter | Parkinsonism, dystonia, developmental delay, upper motor neuron signs | |
| Deafness | Mohr–Tranebjaerg syndrome | 300356 | TIMM8A | X-linked | Translocase of inner mitochondrial membrane 8 | Sensorineural deafness at an early age followed by varying degrees of dystonia along with visual and cognitive disability |
| Woodhouse–Sakati syndrome | 241080 | DCAF17 | AR | Nucleolar protein | Sensorineural deafness, seizures, sensory neuropathy, mental retardation, alopecia, hypogonadism, diabetes mellitus | |
| Spasticity | Spastic paraplegia 35 (SPG35) | 612319 | FA2H | AR | Fatty acid 2-hydroxylase | Spasticity, cognitive decline, dystonia, optic atrophy, brain iron accumulation, leukodystrophy |
| SPG2, Pelizaeus– Merzbacher disease | 312920, 312080 | PLP1 | X-linked | Proteolipid protein-1 | Spasticity, ataxia, dystonia, developmental delay, hypomyelinative leukodystrophy | |
| SPG15 | ZFYVE26 | KI | AR | Spastizin | Spasticity, amyotrophy, ataxia, mental retardation, dystonia | |
| Metabolic and storage | Wilson disease | 277900 | ATP7B | AR | ATPase, Cu2+ transporting β polypeptide | Hepatitis, liver failure, Kayser–Fleischer rings, tremor, dystonia, personality changes |
| Lesch–Nyhan syndrome | 300322 | HPRT1 | X-linked recessive | Hypoxanthine guanine phosphoribosyl-transferase I | Hyperuricemia, gout, nephrolithiasis, self-injurious behavior, dystonia, mental retardation, athetosis | |
| Glutaric academia I | 231670 | GCDH | AR | Glutaryl-CoA dehydrogenase | Macrocephaly, hepatomegaly, dystonia, choreoathetosis, infantile encephalopathy, striatal necrosis | |
| Pyruvate dehydrogenase deficiency | 312170 | PDHA1 | X-linked dominant | Pyruvate dehydrogenase E1-α deficiency | Low birth weight, microcephaly, dysmorphic features, psychomotor retardation, seizures, choreoathetosis, dystonia, ataxia, lactic acidosis | |
| Homocystinuria | 236200 | CBS | AR | Cystathionine β-synthase deficiency | Tall stature, ectopic lentis, skeletal abnormalities, seizures, mental retardation, dystonia, thromboembolism | |
| Biotin-responsive basal ganglia disease | 607483 | AR | Subacute encephalopathy, dysphagia, dysarthria, dystonia, nystagmus, seizures | |||
| Niemann–Pick type C1 | 257220 | NPC1 | AR | Neimann-Pick C1 protein | Hepatosplenomegaly, supranuclear gaze palsy, dementia, dysarthria, dystonia, ataxia | |
| Niemann–Pick type C2 | 607625 | NPC2 | AR | Epididymal secretory protein E1 | Hepatosplenomegaly, respiratory failure, supranuclear gaze play, dementia, dysarthria, dystonia, ataxia | |
| GM1 gangliosidosis | 230500 | GLB1 | AR | β-Galactosidase | Dysmorphic face/neck, dwarfism, macular cherry-red spot, hepatosplenomegaly, mental retardation, dystonia | |
| GM2 gangliosidosis | 272750 | GM2A | AR | Ganglioside GM2 activator | Macular cherry-red spot, blindness, psychomotor delay, dementia, seizures, dystonia | |
| Tay–Sachs disease | 272800 | HEXA | AR | β-Hexosaminidase, subunit α | Macular cherry-red spot, blindness, psychomotor deterioration, seizures, dementia, dystonia | |
| Neuronal ceroid-lipofuscinosisa | 204200 | CLN3 | AR | Battenin | Retinitis pigmentosa with progressive vision loss, psychomotor degeneration with dementia, parkinsonism, ataxia, dystonia, seizures | |
| Metachromatic leukodystrophy | 250100 | ARSA | AR | Arylsulfatase A | Optic atrophy, dementia, ataxia, chorea, dystonia, seizures, demyelinating polyneuropathy | |
| Mitochondrial | Leber hereditary optic atrophy (LHON) | 53500 | MTND1b | M | NADH dehydrogenase I | Optic atrophy, action tremor, dystonia, peripheral neuropathy |
| Leigh syndromec | 25600 | MTND2 | AR, M, X-linked | NAD dehydrogenase subunit 2 | Ophthalmoplegia, pigmentary retinopathy, psychomotor retardation, ataxia, dystonia, spasticity, seizures, lactic acidosis | |
| Other neuro- degenerative disorders | Rett syndrome | 312750 | MECP2 | X-linked dominant | MECP2 | Mental retardation, stereotypies, parkinsonism, dystonia |
| Huntington disease (HD) | 143100 | HTT | AD | Huntington | Chorea, personality changes, dementia, dystonia | |
| Huntington disease-like 2 (HDL2) | 606438 | JPH3 | AD | Junctophilin 3 | Dementia, akinetic-rigid syndrome, mild chorea, mild dystonia | |
| Choreoacanthocytosis | 200150 | VPS13A | AR | Chorein | Orofacial dyskinesias, dysphagia, choreoathetosis, dystonia, seizures, parkinsonism, acanthocytosis | |
| Creutzfeldt–Jakob disease, familial | 123400 | PRNP | AD | Prion protein | Dementia, delirium, myoclonus, akinetic-rigid syndrome, dystonia, ataxia |
AD, autosomal dominant; AR, autosomal recessive; M, mitochondrial.
Neuronal ceroid lipofuscinoses are a group of genetically heterogeneous disorders characterized by intracellular accumulation of autofluorescent lipopigment and include dementia, movement disorders, vision loss, and seizures as phenotypic features (Getty and Pearce, 2011).
LHON has been associated with variants in ND1, ND2, ND4, ND4L, ND5, ND6, MTCYB, MTCO1, and MTAP6 (Toñska et al., 2010).
Leigh syndrome is associated with marked genetic hetereogeneity and can be caused by mutations in either mtDNA or nDNA (Finsterer, 2008).
Le Ber et al. (2006) reported eight families characterized by the combination of dystonia and MRI evidence of cerebellar atrophy. Age of dystonia onset ranged from 9 to 42 years. Most individuals had clinical evidence of laryngeal or arm dystonia at disease onset. Dystonia generalized in 5/12 individuals. Cerebellar ataxia was relatively mild and slowly progressive. Inheritance was either recessive or X-linked. Similar families have been described by other groups (Hagenah et al., 2007).
C. Other
Dystonia may be a clinical sign in a diverse array of neurogenetic disorders including those associated with autism and mental retardation, mitochondrial dysfunction, deafness, spasticity, dopamine neurotransmission, and metabolic storage defects (Table 2.3). In many of these conditions, the functional impact of dystonia on quality of life is relatively minor compared to the effects of the causal mutation on cognition, hearing, fine and gross motor skills, or extra-neural systems. There are several exceptions, however, and dystonia can occasionally be a presenting feature or major source of disability.
Dystonia-deafness syndrome (Mohr–Tranebjaerg syndrome) is an X-linked recessive disorder caused by mutations in DDP1, which encodes deafness-dystonia peptide 1 or translocase of inner mitochondrial membrane 8. Males develop childhood-onset deafness, spasticity, blindness, and dystonia. Dystonia can generalize and affect the craniocervical musculature (Kim et al., 2007). Cervical dystonia and hand–forearm dystonia (e.g., writer’s cramp) have been reported in female carriers (Swerdlow and Wooten, 2001). The combination of deafness and dystonia is also seen in the Woodhouse–Sakati syndrome (Schneider and Bhatia, 2008), which is due to mutations in DCAF17 that encodes a nucleolar protein (Alazami et al., 2008).
Spasticity and dystonia may coexist as “spastic dystonia” or “spastic ataxia” syndromes or in a subset of patients with spastic paraplegia 35 (SPG35 or FAHN disease) and the allelic disorders, SGP2 and Pelizaeus–Merzbacher disease (Table 2.3). Gilbert et al. (2009) have mapped a novel spastic dystonia locus to Chr 2q24-31. Their Ohio kindred manifests spasticity and dystonia without ataxia or dementia. One subject showed marked improvement of dystonia with deep brain stimulation. Portneuf spastic ataxia with eukoencephalopathy is an autosomal recessive disorder mapped to 2q33-34 and associated with spasticity, ataxia, mild cognitive impairment, dystonia, nystagmus, and scoliosis (Thiffault et al., 2006).
Screening ceruloplasmin and uric acid levels should be obtained in all children and young adults presenting with dystonia to exclude Wilson and Lesch–Nyhan diseases, respectively. Wilson disease is due to recessive mutations in ATP7B, which encodes an ATPase, Cu2+ transporting β polypeptide. In developed countries, Wilson disease is often diagnosed prior to the onset of significant neurological dysfunction. Virtually all patients with neurological disease have Kayser–Fleischer rings due to copper deposition in Descemet membrane. Dystonia is often among the protean neuropsychiatric manifestations of more advanced Wilson disease. Severe generalized dystonia, mental retardation, gout, and self-injurious behavior are seen is the classic form of Lesch–Nyhan disease. Attenuated variants of this disorder may present with isolated focal or segmental dystonia ( Jinnah et al., 2010). Nonsense and deletion mutations are usually associated with classic Lesch–Nyhan disease ( Jinnah et al., 2010).
Dystonia can be a manifestation of disorders linked to mitochondrial dysfunction due to mutations in either nDNA or mtDNA. Examples of dystonia-associated nuclear genes include TIMM8A in X-linked dystonia-deafness syndrome and TACO1 in Leigh syndrome (Weraarpachai et al., 2009). An enormous array of mtDNA mutations have been tied to Leber hereditary optic neuropathy (Tonska et al., 2010) and Leigh syndrome (Finsterer, 2008). Mitochondrial dysfunction may also be a feature of more common forms of dystonia. For example, Schapira et al. (1997) found a 22% decrease in the activity of complex I, but not complex II/III or complex IV, in 20 patients with idiopathic focal dystonia compared to 22 controls. Similarly, Benecke et al. (1992) found a 37% reduction in complex I activity in patients with focal dystonia and a 67% decrease in complex I activity in eight patients with segmental or generalized dystonia. These data suggest that sequence variants in mtDNA may contribute to risk for largely sporadic adult-onset primary dystonia.
Dystonia is an important clinical feature in several of the hereditary and neurodegenerative choreas, particularly Huntington disease (HTT) and choreoacanthocystosis (VPS13A). The combination of chorea and dystonia may also been seen in familial or idiopathic basal ganglia calcifications (IBGC). In a large family with autosomal dominant IBGC, the disease locus was mapped to 14q (Geschwind et al., 1999). Many other families with IBGC do not show linkage to 14q (Oliveira et al., 2004).
VII. CONCLUSIONS
Major recent discoveries have appeared in the field of dystonia genetics. First, mutations in the transcription factor THAP1 and DNA replication factor CIZ1 have been linked to adult-onset primary dystonia (Xiao et al., 2010, 2012). Second, proline-rich transmembrane protein 2 has been tied to paroxysmal kinesigenic dyskinesia (Chen et al., 2011). Third, there has been consolidation of DYT syndromes—DYT9 (paroxysmal choreoathetosis/spasticity) and DYT18 (paroxysmal exertional dyskinesia) are now known to be treatable allelic disorders, and DYT14 has been redefined as DYT5 due to a deletion mutation in GCH1 (Wider et al., 2008).
Recent advances in high-throughput sequencing and bioinformatics should enable additional discoveries. A major focus of future efforts should be the genetics of adult-onset primary dystonia. Unfortunately, this work will be compromised by the paucity of large kindreds with highly penetrant disease. Although there is no good evidence that adult-onset primary dystonia is a complex disease, well-designed genome-wide association studies of specific forms of focal dystonia may point out loci that contribute to disease penetrance. Work to date has implicated several cellular pathways in the pathophysiology of dystonia. These include G1/S cell cycle control in primary dystonia; and monoamine neurotransmission, the neuronal stress response, and mitochondrial dysfunction in non-primary dystonia. Hopefully, future work can serve to unify, in whole or part, the cellular and systems biology of dystonia.
Acknowledgments
My work on dystonia has been supported by the Neuroscience Institute at the University of Tennessee Health Science Center, Dystonia Medical Research Foundation, NIH grants R01NS048458 and R01NS069936, NIH U54 Dystonia Coalition (1U54NS065701) Pilot Projects Program, and the Parkinson’s & Movement Disorder Foundation.
References
- Alazami AM, Al-Saif A, Al-Semari A, Bohlega S, Zlitni S, Alzahrani F, Bavi P, Kaya N, Colak D, Khalak H, et al. Mutations in C2orf37, encoding a nucleolar protein, cause hypogonadism, alopecia, diabetes mellitus, mental retardation, and extrapyramidal syndrome. Am J Hum Genet. 2008;83:684–691. doi: 10.1016/j.ajhg.2008.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aquino D, Bizzi A, Grisoli M, Garavaglia B, Bruzzone MG, Nardocci N, Savoiardo M, Chiapparini L. Age-related iron deposition in the basal ganglia: quantitative analysis in healthy subjects. Radiology. 2009;252:165–172. doi: 10.1148/radiol.2522081399. [DOI] [PubMed] [Google Scholar]
- Arpa J, Cuesta A, Cruz-Martinez A, Santiago S, Sarria J, Palau F. Clinical features and genetic analysis of a Spanish family with spinocerebellar ataxia 6. Acta Neurol Scand. 1999;99:43–47. doi: 10.1111/j.1600-0404.1999.tb00656.x. [DOI] [PubMed] [Google Scholar]
- Asmus F, Salih F, Hjermind LE, Ostergaard K, Munz M, Kuhn AA, Dupont E, Kupsch A, Gasser T. Myoclonus-dystonia due to genomic deletions in the epsilon-sarcoglycan gene. Ann Neurol. 2005;58:792–797. doi: 10.1002/ana.20661. [DOI] [PubMed] [Google Scholar]
- Auburger G, Ratzlaff T, Lunkes A, Nelles HW, Leube B, Binkofski F, Kugel H, Heindel W, Seitz R, Benecke R, et al. A gene for autosomal dominant paroxysmal choreoathetosis/spasticity (CSE) maps to the vicinity of a potassium channel gene cluster on chromosome 1p, probably within 2 cM between D1S443 and D1S197. Genomics. 1996;31:90–94. doi: 10.1006/geno.1996.0013. [DOI] [PubMed] [Google Scholar]
- Augood SJ, Keller-McGandy CE, Siriani A, Hewett J, Ramesh V, Sapp E, DiFiglia M, Breakefield XO, Standaert DG. Distribution and ultrastructural localization of torsinA immunoreactivity in the human brain. Brain Res. 2003;986:12–21. doi: 10.1016/s0006-8993(03)03164-0. [DOI] [PubMed] [Google Scholar]
- Baker M, Litvan I, Houlden H, Adamson J, Dickson D, Perez-Tur J, Hardy J, Lynch T, Bigio E, Hutton M. Association of an extended haplotype in the tau gene with progressive supranuclear palsy. Hum Mol Genet. 1999;8:711–715. doi: 10.1093/hmg/8.4.711. [DOI] [PubMed] [Google Scholar]
- Barclay CL, Lang AE. Dystonia in progressive supranuclear palsy. J Neurol Neurosurg Psychiatry. 1997;62:352–356. doi: 10.1136/jnnp.62.4.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basham SE, Rose LS. The Caenorhabditis elegans polarity gene ooc-5 encodes a Torsin-related protein of the AAA ATPase superfamily. Development. 2001;128:4645–4656. doi: 10.1242/dev.128.22.4645. [DOI] [PubMed] [Google Scholar]
- Benecke R, Strumper P, Weiss H. Electron transfer complex I defect in idiopathic dystonia. Ann Neurol. 1992;32:683–686. doi: 10.1002/ana.410320512. [DOI] [PubMed] [Google Scholar]
- Bennett LB, Roach ES, Bowcock AM. A locus for paroxysmal kinesigenic dyskinesia maps to human chromosome 16. Neurology. 2000;54:125–130. doi: 10.1212/wnl.54.1.125. [DOI] [PubMed] [Google Scholar]
- Bentivoglio AR, Ialongo T, Contarino MF, Valente EM, Albanese A. Phenotypic characterization of DYT13 primary torsion dystonia. Mov Disord. 2004;19:200–206. doi: 10.1002/mds.10634. [DOI] [PubMed] [Google Scholar]
- Bhatia KP. Paroxysmal dyskinesias. Mov Disord. 2011;26:1157–1165. doi: 10.1002/mds.23765. [DOI] [PubMed] [Google Scholar]
- Bonafé L, Thöny B, Penzien JM, Czarnecki B, Blau N. Mutations in the sepiapterin reductase gene cause a novel tetrahydrobiopterin-dependent mono-amine-neurotransmitter deficiency without hyperphenylalaninemia. Am J Hum Genet. 2001;69:269–277. doi: 10.1086/321970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brashear A, Dobyns WB, de Carvalho Aguiar P, Borg M, Frijns CJ, Gollamudi S, Green A, Guimaraes J, Haake BC, Klein C, et al. The phenotypic spectrum of rapid-onset dystonia-parkinsonism (RDP) and mutations in the ATP1A3 gene. Brain. 2007;130:828–835. doi: 10.1093/brain/awl340. [DOI] [PubMed] [Google Scholar]
- Braun N, Zhu Y, Krieglstein J, Culmsee C, Zimmermann H. Upregulation of the enzyme chain hydrolyzing extracellular ATP after transient forebrain ischemia in the rat. J Neurosci. 1998;18:4891–4900. doi: 10.1523/JNEUROSCI.18-13-04891.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressman SB, Raymond D, Fuchs T, Heiman GA, Ozelius LJ, Saunders-Pullman R. Mutations in THAP1 (DYT6) in early-onset dystonia: a genetic screening study. Lancet Neurol. 2009;8:441–446. doi: 10.1016/S1474-4422(09)70081-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressman SB, Sabatti C, Raymond D, de Leon D, Klein C, Kramer PL, Brin MF, Fahn S, Breakefield X, Ozelius LJ, et al. The DYT1 phenotype and guidelines for diagnostic testing. Neurology. 2000;54:1746–1752. doi: 10.1212/wnl.54.9.1746. [DOI] [PubMed] [Google Scholar]
- Bruno MK, Lee HY, Auburger GW, Friedman A, Nielsen JE, Lang AE, Bertini E, Van Bogaert P, Averyanov Y, Hallett M, et al. Genotype-phenotype correlation of paroxysmal nonkinesigenic dyskinesia. Neurology. 2007;68:1782–1789. doi: 10.1212/01.wnl.0000262029.91552.e0. [DOI] [PubMed] [Google Scholar]
- Calakos N, Patel VD, Gottron M, Wang G, Tran-Viet KN, Brewington D, Beyer JL, Steffens DC, Krishnan RR, Zuchner S. Functional evidence implicating a novel TOR1A mutation in idiopathic, late-onset focal dystonia. J Med Genetic. 2010;47:646–650. doi: 10.1136/jmg.2009.072082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell GA, Cao S, Sexton EG, Gelwix CC, Bevel JP, Caldwell KA. Suppression of polyglutamine-induced protein aggregation in Caenorhabditis elegans by torsin proteins. Hum Mol Genet. 2003;12:307–319. doi: 10.1093/hmg/ddg027. [DOI] [PubMed] [Google Scholar]
- Callan AC, Bunning S, Jones OT, High S, Swanton E. Biosynthesis of the dystonia-associated AAA+ ATPase torsinA at the endoplasmic reticulum. Biochem J. 2007;401:607–612. doi: 10.1042/BJ20061313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargos S, Scholz S, Simon-Sanchez J, Paisan-Ruiz C, Lewis P, Hernandez D, Ding J, Gibbs JR, Cookson MR, Bras J, et al. DYT16, a novel young-onset dystonia-parkinsonism disorder: identification of a segregating mutation in the stress-response protein PRKRA. Lancet Neurol. 2008;7:207–215. doi: 10.1016/S1474-4422(08)70022-X. [DOI] [PubMed] [Google Scholar]
- Cao S, Gelwix CC, Caldwell KA, Caldwell GA. Torsin-mediated protection from cellular stress in the dopaminergic neurons of Caenorhabditis elegans. J Neurosci. 2005;25:3801–3812. doi: 10.1523/JNEUROSCI.5157-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WJ, Lin Y, Xiong ZQ, Wei W, Ni W, Tan GH, Guo SL, He J, Chen YF, Zhang QJ, et al. Exome sequencing identifies truncating mutations in PRRT2 that cause paroxysmal kinesigenic dyskinesia. Nat Genet. 2011;43:1252–1255. doi: 10.1038/ng.1008. [DOI] [PubMed] [Google Scholar]
- Cheng FB, Ozelius LJ, Wan XH, Feng JC, Ma LY, Yang YM, Wang L. THAP1/DYT6 sequence variants in non-DYT1 early-onset primary dystonia in China and their effects on RNA expression. J Neurol. 2011;259:342–347. doi: 10.1007/s00415-011-6196-5. [DOI] [PubMed] [Google Scholar]
- Chouery E, Kfoury J, Delague V, Jalkh N, Bejjani P, Serre JL, Megarbane A. A novel locus for autosomal recessive primary torsion dystonia (DYT17) maps to 20p11.22-q13.12. Neurogenetics. 2008;9:287–293. doi: 10.1007/s10048-008-0142-4. [DOI] [PubMed] [Google Scholar]
- Choumert A, Poisson A, Honnorat J, Le Ber I, Camuzat A, Broussolle E, Thobois S. G303V tau mutation presenting with progressive supranuclear palsy-like features. Mov Disord. 2011 doi: 10.1002/mds.24060. http://dx.doi.org/10.1002/mds.24060. [DOI] [PubMed]
- Clouaire T, Roussigne M, Ecochard V, Mathe C, Amalric F, Girard JP. The THAP domain of THAP1 is a large C2CH module with zinc-dependent sequence-specific DNA-binding activity. Proc Natl Acad Sci U S A. 2005;102:6907–6912. doi: 10.1073/pnas.0406882102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carvalho Aguiar P, Sweadner KJ, Penniston JT, Zaremba J, Liu L, Caton M, Linazasoro G, Borg M, Tijssen MA, Bressman SB, et al. Mutations in the Na+/K+-ATPase alpha3 gene ATP1A3 are associated with rapid-onset dystonia parkinsonism. Neuron. 2004;43:169–175. doi: 10.1016/j.neuron.2004.06.028. [DOI] [PubMed] [Google Scholar]
- Defazio G, Abbruzzese G, Aniello MS, Bloise M, Crisci C, Eleopra R, Fabbrini G, Girlanda P, Liguori R, Macerollo A, et al. Environmental risk factors and clinical phenotype in familial and sporadic primary blepharospasm. Neurology. 2011;77:631–637. doi: 10.1212/WNL.0b013e3182299e13. [DOI] [PubMed] [Google Scholar]
- Djarmati A, Schneider SA, Lohmann K, Winkler S, Pawlack H, Hagenah J, Bruggemann N, Zittel S, Fuchs T, Rakovic A, et al. Mutations in THAP1 (DYT6) and generalised dystonia with prominent spasmodic dysphonia: a genetic screening study. Lancet Neurol. 2009;8:447–452. doi: 10.1016/S1474-4422(09)70083-3. [DOI] [PubMed] [Google Scholar]
- Dudesek A, Roschinger W, Muntau AC, Seidel J, Leupold D, Thöny B, Blau N. Molecular analysis and long-term follow-up of patients with different forms of 6-pyruvoyl-tetrahydropterin synthase deficiency. Eur J Pediatr. 2001;160:267–276. doi: 10.1007/s004310000722. [DOI] [PubMed] [Google Scholar]
- Edwards M, Wood N, Bhatia K. Unusual phenotypes in DYT1 dystonia: a report of five cases and a review of the literature. Mov Disord. 2003;18:706–711. doi: 10.1002/mds.10411. [DOI] [PubMed] [Google Scholar]
- Einholm AP, Toustrup-Jensen MS, Holm R, Andersen JP, Vilsen B. The rapid-onset dystonia parkinsonism mutation D923N of the Na+, K+-ATPase alpha3 isoform disrupts Na+ interaction at the third Na+ site. J Biol Chem. 2010;285:26245–26254. doi: 10.1074/jbc.M110.123976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esapa CT, Waite A, Locke M, Benson MA, Kraus M, McIlhinney RA, Sillitoe RV, Beesley PW, Blake DJ. SGCE missense mutations that cause myoclonus-dystonia syndrome impair epsilon-sarcoglycan trafficking to the plasma membrane: modulation by ubiquitination and torsinA. Hum Mol Genet. 2007;16:327–342. doi: 10.1093/hmg/ddl472. [DOI] [PubMed] [Google Scholar]
- Evidente VG, Advincula J, Esteban R, Pasco P, Alfon JA, Natividad FF, Cuanang J, Luis AS, Gwinn-Hardy K, Hardy J, et al. Phenomenology of “Lubag” or X-linked dystonia-parkinsonism. Mov Disord. 2002;17:1271–1277. doi: 10.1002/mds.10271. [DOI] [PubMed] [Google Scholar]
- Fahn S. Systemic therapy of dystonia. Can J Neurol Sci. 1987;14:528–532. doi: 10.1017/s0317167100038051. [DOI] [PubMed] [Google Scholar]
- Fahn S. Concept and classification of dystonia. Adv Neurol. 1988;50:1–8. [PubMed] [Google Scholar]
- Fahn S. Classification of movement disorders. Mov Disord. 2011;26:947–957. doi: 10.1002/mds.23759. [DOI] [PubMed] [Google Scholar]
- Fahn S, Bressman SB, Marsden CD. Classification of dystonia. Adv Neurol. 1998;78:1–10. [PubMed] [Google Scholar]
- Ferrari-Toninelli G, Paccioretti S, Francisconi S, Uberti D, Memo M. TorsinA negatively controls neurite outgrowth of SH-SY5Y human neuronal cell line. Brain Res. 2004;1012:75–81. doi: 10.1016/j.brainres.2004.02.080. [DOI] [PubMed] [Google Scholar]
- Finsterer J. Leigh and Leigh-like syndrome in children and adults. Pediatr Neurol. 2008;39:223–235. doi: 10.1016/j.pediatrneurol.2008.07.013. [DOI] [PubMed] [Google Scholar]
- Frédéric M, Lucarz E, Monino C, Saquet C, Thorel D, Claustres M, Tuffery-Giraud S, Collod-Beroud G. First determination of the incidence of the unique TOR1A gene mutation, c. 907delGAG, in a Mediterranean population. Mov Disord. 2007;22:884–888. doi: 10.1002/mds.21391. [DOI] [PubMed] [Google Scholar]
- Frédéric MY, Clot F, Blanchard A, Dhaenens CM, Lesca G, Cif L, Durr A, Vidailhet M, Sablonniere B, Calender A, et al. The p. Asp216His TOR1A allele effect is not found in the French population. Mov Disord. 2009;24:919–921. doi: 10.1002/mds.22407. [DOI] [PubMed] [Google Scholar]
- Fuchs T, Gavarini S, Saunders-Pullman R, Raymond D, Ehrlich ME, Bressman SB, Ozelius LJ. Mutations in the THAP1 gene are responsible for DYT6 primary torsion dystonia. Nat Genet. 2009;41:286–288. doi: 10.1038/ng.304. [DOI] [PubMed] [Google Scholar]
- Gajos A, Piaskowski S, Slawek J, Ochudlo S, Opala G, Lobinska A, Honczarenko K, Budrewicz S, Koszewicz M, Pelszynska B, et al. Phenotype of the DYT1 mutation in the TOR1A gene in a Polish population of patients with dystonia. A preliminary report. Neurol Neurochir Pol. 2007;41:487–494. [PubMed] [Google Scholar]
- Gambarin M, Valente EM, Liberini P, Barrano G, Bonizzato A, Padovani A, Moretto G, Fiorio M, Dallapiccola B, Smania N, et al. Atypical phenotypes and clinical variability in a large Italian family with DYT1-primary torsion dystonia. Mov Disord. 2006;21:1782–1784. doi: 10.1002/mds.21056. [DOI] [PubMed] [Google Scholar]
- Gasser T, Windgassen K, Bereznai B, Kabus C, Ludolph AC. Phenotypic expression of the DYT1 mutation: a family with writer’s cramp of juvenile onset. Ann Neurol. 1998;44:126–128. doi: 10.1002/ana.410440119. [DOI] [PubMed] [Google Scholar]
- Geschwind DH, Loginov M, Stern JM. Identification of a locus on chromosome 14q for idiopathic basal ganglia calcification (Fahr disease) Am J Hum Genet. 1999;65:764–772. doi: 10.1086/302558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getty AL, Pearce DA. Interactions of the proteins of neuronal ceroid lipofuscinosis: clues to function. Cell Mol Life Sci. 2011;68:453–474. doi: 10.1007/s00018-010-0468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghezzi D, Viscomi C, Ferlini A, Gualandi F, Mereghetti P, DeGrandis D, Zeviani M. Paroxysmal non-kinesigenic dyskinesia is caused by mutations of the MR-1 mitochondrial targeting sequence. Hum Mol Genet. 2009;18:1058–1064. doi: 10.1093/hmg/ddn441. [DOI] [PubMed] [Google Scholar]
- Gilbert DL, Leslie EJ, Keddache M, Leslie ND. A novel hereditary spastic paraplegia with dystonia linked to chromosome 2q24–2q31. Mov Disord. 2009;24:364–370. doi: 10.1002/mds.22363. [DOI] [PubMed] [Google Scholar]
- Gimenez-Roldan S, Delgado G, Marin M, Villanueva JA, Mateo D. Hereditary torsion dystonia in gypsies. Adv Neurol. 1988;50:73–81. [PubMed] [Google Scholar]
- Goetz CG, Chmura TA, Lanska DJ. History of dystonia: part 4 of the MDS-sponsored history of movement disorders exhibit, Barcelona, June, 2000. Mov Disord. 2001;16:339–345. doi: 10.1002/mds.1067. [DOI] [PubMed] [Google Scholar]
- Goodchild RE, Dauer WT. Mislocalization to the nuclear envelope: an effect of the dystonia-causing torsinA mutation. Proc Natl Acad Sci U S A. 2004;101:847–852. doi: 10.1073/pnas.0304375101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodchild RE, Dauer WT. The AAA+ protein torsinA interacts with a conserved domain present in LAP1 and a novel ER protein. J Cell Biol. 2005;168:855–862. doi: 10.1083/jcb.200411026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodchild RE, Kim CE, Dauer WT. Loss of the dystonia-associated protein torsinA selectively disrupts the neuronal nuclear envelope. Neuron. 2005;48:923–932. doi: 10.1016/j.neuron.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Goto S, Lee LV, Munoz EL, Tooyama I, Tamiya G, Makino S, Ando S, Dantes MB, Yamada K, Matsumoto S, et al. Functional anatomy of the basal ganglia in X-linked recessive dystonia-parkinsonism. Ann Neurol. 2005;58:7–17. doi: 10.1002/ana.20513. [DOI] [PubMed] [Google Scholar]
- Groen JL, Ritz K, Contarino MF, van de Warrenburg BP, Aramideh M, Foncke EM, van Hilten JJ, Schuurman PR, Speelman JD, Koelman JH, et al. DYT6 dystonia: mutation screening, phenotype, and response to deep brain stimulation. Mov Disord. 2010;25:2420–2427. doi: 10.1002/mds.23285. [DOI] [PubMed] [Google Scholar]
- Grotzsch H, Pizzolato GP, Ghika J, Schorderet D, Vingerhoets FJ, Landis T, Burkhard PR. Neuropathology of a case of dopa-responsive dystonia associated with a new genetic locus, DYT14. Neurology. 2002;58:1839–1842. doi: 10.1212/wnl.58.12.1839. [DOI] [PubMed] [Google Scholar]
- Grundmann K, Laubis-Herrmann U, Bauer I, Dressler D, Vollmer-Haase J, Bauer P, Stuhrmann M, Schulte T, Schols L, Topka H, et al. Frequency and phenotypic variability of the GAG deletion of the DYT1 gene in an unselected group of patients with dystonia. Arch Neurol. 2003;60:1266–1270. doi: 10.1001/archneur.60.9.1266. [DOI] [PubMed] [Google Scholar]
- Grunewald A, Djarmati A, Lohmann-Hedrich K, Farrell K, Zeller JA, Allert N, Papengut F, Petersen B, Fung V, Sue CM, et al. Myoclonus-dystonia: significance of large SGCE deletions. Hum Mutat. 2008;29:331–332. doi: 10.1002/humu.9521. [DOI] [PubMed] [Google Scholar]
- Hagenah J, Reetz K, Zuhlke C, Rolfs A, Binkofski F, Klein C. Predominant dystonia with marked cerebellar atrophy: a rare phenotype in familial dystonia. Neurology. 2007;68:2157. doi: 10.1212/01.wnl.0000269478.69285.7e. [DOI] [PubMed] [Google Scholar]
- Hagenah JM, Zuhlke C, Hellenbroich Y, Heide W, Klein C. Focal dystonia as a presenting sign of spinocerebellar ataxia 17. Mov Disord. 2004;19:217–220. doi: 10.1002/mds.10600. [DOI] [PubMed] [Google Scholar]
- Hedera P, Phibbs FT, Fang JY, Cooper MK, Charles PD, Davis TL. Clustering of dystonia in some pedigrees with autosomal dominant essential tremor suggests the existence of a distinct subtype of essential tremor. BMC Neurol. 2010;10:66. doi: 10.1186/1471-2377-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewett JW, Zeng J, Niland BP, Bragg DC, Breakefield XO. Dystonia-causing mutant torsinA inhibits cell adhesion and neurite extension through interference with cytoskeletal dynamics. Neurobiol Dis. 2006;22:98–111. doi: 10.1016/j.nbd.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Holmgren G, Ozelius L, Forsgren L, Almay BG, Holmberg M, Kramer P, Fahn S, Breakefield XO. Adult onset idiopathic torsion dystonia is excluded from the DYT 1 region (9q34) in a Swedish family. J Neurol Neurosurg Psychiatry. 1995;59:178–181. doi: 10.1136/jnnp.59.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath GA, Stockler-Ipsiroglu SG, Salvarinova-Zivkovic R, Lillquist YP, Connolly M, Hyland K, Blau N, Rupar T, Waters PJ. Autosomal recessive GTP cyclohydrolase I deficiency without hyperphenylalaninemia: evidence of a phenotypic continuum between dominant and recessive forms. Mol Genet Metab. 2008;94:127–131. doi: 10.1016/j.ymgme.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Hou JG, Jankovic J. Movement disorders in Friedreich’s ataxia. J Neurol Sci. 2003;206:59–64. doi: 10.1016/s0022-510x(02)00321-0. [DOI] [PubMed] [Google Scholar]
- Houlden H, Baker M, Morris HR, MacDonald N, Pickering-Brown S, Adamson J, Lees AJ, Rossor MN, Quinn NP, Kertesz A, et al. Corticobasal degeneration and progressive supranuclear palsy share a common tau haplotype. Neurology. 2001;56:1702–1706. doi: 10.1212/wnl.56.12.1702. [DOI] [PubMed] [Google Scholar]
- Houlden H, Schneider SA, Paudel R, Melchers A, Schwingenschuh P, Edwards M, Hardy J, Bhatia KP. THAP1 mutations (DYT6) are an additional cause of early-onset dystonia. Neurology. 2010;74:846–850. doi: 10.1212/WNL.0b013e3181d5276d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchins JB, Casagrande VA. Vimentin: changes in distribution during brain development. Glia. 1989;2:55–66. doi: 10.1002/glia.440020107. [DOI] [PubMed] [Google Scholar]
- Ichinose H, Ohye T, Takahashi E, Seki N, Hori T, Segawa M, Nomura Y, Endo K, Tanaka H, Tsuji S, et al. Hereditary progressive dystonia with marked diurnal fluctuation caused by mutations in the GTP cyclohydrolase I gene. Nat Genet. 1994;8:236–242. doi: 10.1038/ng1194-236. [DOI] [PubMed] [Google Scholar]
- Jinnah HA, Ceballos-Picot I, Torres RJ, Visser JE, Schretlen DJ, Verdu A, Larovere LE, Chen CJ, Cossu A, Wu CH, et al. Attenuated variants of Lesch-Nyhan disease. Brain. 2010;133:671–689. doi: 10.1093/brain/awq013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabakci K, Hedrich K, Leung JC, Mitterer M, Vieregge P, Lencer R, Hagenah J, Garrels J, Witt K, Klostermann F, et al. Mutations in DYT1: extension of the phenotypic and mutational spectrum. Neurology. 2004;62:395–400. doi: 10.1212/01.wnl.0000113024.84178.f7. [DOI] [PubMed] [Google Scholar]
- Kabakci K, Isbruch K, Schilling K, Hedrich K, de Carvalho Aguiar P, Ozelius LJ, Kramer PL, Schwarz MH, Klein C. Genetic heterogeneity in rapid onset dystonia-parkinsonism: description of a new family. J Neurol Neurosurg Psychiatry. 2005;76:860–862. doi: 10.1136/jnnp.2004.046730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamm C, Boston H, Hewett J, Wilbur J, Corey DP, Hanson PI, Ramesh V, Breakefield XO. The early onset dystonia protein torsinA interacts with kinesin light chain 1. J Biol Chem. 2004;279:19882–19892. doi: 10.1074/jbc.M401332200. [DOI] [PubMed] [Google Scholar]
- Khan NL, Graham E, Critchley P, Schrag AE, Wood NW, Lees AJ, Bhatia KP, Quinn N. Parkin disease: a phenotypic study of a large case series. Brain. 2003a;126:1279–1292. doi: 10.1093/brain/awg142. [DOI] [PubMed] [Google Scholar]
- Khan NL, Wood NW, Bhatia KP. Autosomal recessive, DYT2-like primary torsion dystonia: a new family. Neurology. 2003b;61:1801–1803. doi: 10.1212/01.wnl.0000099076.17187.9a. [DOI] [PubMed] [Google Scholar]
- Kim HT, Edwards MJ, Tyson J, Quinn NP, Bitner-Glindzicz M, Bhatia KP. Blepharospasm and limb dystonia caused by Mohr-Tranebjaerg syndrome with a novel splice-site mutation in the deafness/dystonia peptide gene. Mov Disord. 2007;22:1328–1331. doi: 10.1002/mds.21351. [DOI] [PubMed] [Google Scholar]
- Kindy MS, Bhat AN, Bhat NR. Transient ischemia stimulates glial fibrillary acid protein and vimentin gene expression in the gerbil neocortex, striatum and hippocampus. Mol Brain Res. 1992;13:199–206. doi: 10.1016/0169-328x(92)90027-9. [DOI] [PubMed] [Google Scholar]
- Klein C, Liu L, Doheny D, Kock N, Muller B, de Carvalho Aguiar P, Leung J, de Leon D, Bressman SB, Silverman J, et al. Epsilon-sarcoglycan mutations found in combination with other dystonia gene mutations. Ann Neurol. 2002;52:675–679. doi: 10.1002/ana.10358. [DOI] [PubMed] [Google Scholar]
- Konakova M, Huynh DP, Yong W, Pulst SM. Cellular distribution of torsin A and torsin B in normal human brain. Arch Neurol. 2001;58:921–927. doi: 10.1001/archneur.58.6.921. [DOI] [PubMed] [Google Scholar]
- Konakova M, Pulst SM. Immunocytochemical characterization of torsin proteins in mouse brain. Brain Res. 2001;922:1–8. doi: 10.1016/s0006-8993(01)03014-1. [DOI] [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL. CDC growth charts: United States. Adv Data. 2000;314:1–27. [PubMed] [Google Scholar]
- Kuner R, Teismann P, Trutzel A, Naim J, Richter A, Schmidt N, Bach A, Ferger B, Schneider A. TorsinA, the gene linked to early-onset dystonia, is upregulated by the dopaminergic toxin MPTP in mice. Neurosci Lett. 2004;355:126–130. doi: 10.1016/j.neulet.2003.10.069. [DOI] [PubMed] [Google Scholar]
- Kuner R, Teismann P, Trutzel A, Naim J, Richter A, Schmidt N, von Ahsen O, Bach A, Ferger B, Schneider A. TorsinA protects against oxidative stress in COS-1 and PC12 cells. Neurosci Lett. 2003;350:153–156. doi: 10.1016/s0304-3940(03)00904-2. [DOI] [PubMed] [Google Scholar]
- Larnaout A, Belal S, Miladi N, Kaabachi N, Mebazza A, Dhondt JL, Hentati F. Juvenile form of dihydropteridine reductase deficiency in 2 Tunisian patients. Neuropediatrics. 1998;29:322–323. doi: 10.1055/s-2007-973586. [DOI] [PubMed] [Google Scholar]
- Le Ber I, Clot F, Vercueil L, Camuzat A, Viemont M, Benamar N, De Liege P, Ouvrard-Hernandez AM, Pollak P, Stevanin G, et al. Predominant dystonia with marked cerebellar atrophy: a rare phenotype in familial dystonia. Neurology. 2006;67:1769–1773. doi: 10.1212/01.wnl.0000244484.60489.50. [DOI] [PubMed] [Google Scholar]
- LeDoux MS. Meige syndrome: what’s in a name? Parkinsonism Relat Disord. 2009;15:483–489. doi: 10.1016/j.parkreldis.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux MS. Dystonia: phenomenology. Parkinsonism Relat Disord. 2012a;18(Supp 1):S162–164. doi: 10.1016/S1353-8020(11)70050-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux MS, Xiao J, Rudzińska M, Bastian RW, Wszolek ZK, Van Gerpen JA, Puschmann A, Momčilović D, Vemula S, Zhao Y. Genotype-phenotype correlations in THAP1 dystonia: molecular foundations and description of new cases. Parkinsonism Relat Disord. 2012b;18:414–425. doi: 10.1016/j.parkreldis.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HY, Xu Y, Huang Y, Ahn AH, Auburger GW, Pandolfo M, Kwiecinski H, Grimes DA, Lang AE, Nielsen JE, et al. The gene for paroxysmal non-kinesigenic dyskinesia encodes an enzyme in a stress response pathway. Hum Mol Genet. 2004;13:3161–3170. doi: 10.1093/hmg/ddh330. [DOI] [PubMed] [Google Scholar]
- Lee WL, Tay A, Ong HT, Goh LM, Monaco AP, Szepetowski P. Association of infantile convulsions with paroxysmal dyskinesias (ICCA syndrome): confirmation of linkage to human chromosome 16p12-q12 in a Chinese family. Hum Genet. 1998;103:608–612. doi: 10.1007/s004390050876. [DOI] [PubMed] [Google Scholar]
- Leube B, Hendgen T, Kessler KR, Knapp M, Benecke R, Auburger G. Sporadic focal dystonia in northwest Germany: molecular basis on chromosome 18p. Ann Neurol. 1997;42:111–114. doi: 10.1002/ana.410420117. [DOI] [PubMed] [Google Scholar]
- Leube B, Kessler KR, Ferbert A, Ebke M, Schwendemann G, Erbguth F, Benecke R, Auburger G. Phenotypic variability of the DYT1 mutation in German dystonia patients. Acta Neurol Scand. 1999;99:248–251. doi: 10.1111/j.1600-0404.1999.tb07356.x. [DOI] [PubMed] [Google Scholar]
- Leube B, Rudnicki D, Ratzlaff T, Kessler KR, Benecke R, Auburger G. Idiopathic torsion dystonia: assignment of a gene to chromosome 18p in a German family with adult onset, autosomal dominant inheritance and purely focal distribution. Hum Mol Genet. 1996;5:1673–1677. doi: 10.1093/hmg/5.10.1673. [DOI] [PubMed] [Google Scholar]
- Leung JC, Klein C, Friedman J, Vieregge P, Jacobs H, Doheny D, Kamm C, DeLeon D, Pramstaller PP, Penney JB, et al. Novel mutation in the TOR1A (DYT1) gene in atypical early onset dystonia and polymorphisms in dystonia and early onset parkinsonism. Neurogenetics. 2001;3:133–143. doi: 10.1007/s100480100111. [DOI] [PubMed] [Google Scholar]
- Li J, Zhu X, Wang X, Sun W, Feng B, Du T, Sun B, Niu F, Wei H, Wu X, et al. Targeted genomic sequencing identifies PRRT2 mutations as a cause of paroxysmal kinesigenic choreoathetosis. J Med Genet. 2012;49:76–78. doi: 10.1136/jmedgenet-2011-100635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Qi Z, Wan XH, Li JY, Shi L, Lu Q, Zhou XQ, Qiao L, Wu LW, Liu XQ, et al. Mutations in PRRT2 result in paroxysmal dyskinesias with marked variability in clinical expression. J Med Genet. 2012;49:79–82. doi: 10.1136/jmedgenet-2011-100653. [DOI] [PubMed] [Google Scholar]
- Lohmann E, Koroglu C, Hanagasi HA, Dursun B, Tasan E, Tolun A. A homozygous frameshift mutation of sepiapterin reductase gene causing parkinsonism with onset in childhood. Parkinsonism Relat Disord. 2012a;18:191–193. doi: 10.1016/j.parkreldis.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Lohmann K, Uflacker N, Erogullari A, Lohnau T, Winkler S, Dendorfer A, Schneider SA, Osmanovic A, Svetel M, Ferbert A, et al. Identification and functional analysis of novel THAP1 mutations. Eur J Hum Genet. 2012b;20:171–175. doi: 10.1038/ejhg.2011.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüdecke B, Dworniczak B, Bartholomé K. A point mutation in the tyrosine hydroxylase gene associated with Segawa’s syndrome. Hum Genet. 1995;95:123–125. doi: 10.1007/BF00225091. [DOI] [PubMed] [Google Scholar]
- Makino S, Kaji R, Ando S, Tomizawa M, Yasuno K, Goto S, Matsumoto S, Tabuena MD, Maranon E, Dantes M, et al. Reduced neuron-specific expression of the TAF1 gene is associated with X-linked dystonia-parkinsonism. Am J Hum Genet. 2007;80:393–406. doi: 10.1086/512129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marras C, Van den Eeden SK, Fross RD, Benedict-Albers KS, Klingman J, Leimpeter AD, Nelson LM, Risch N, Karter AJ, Bernstein AL, et al. Minimum incidence of primary cervical dystonia in a multiethnic health care population. Neurology. 2007;69:676–680. doi: 10.1212/01.wnl.0000267425.51598.c9. [DOI] [PubMed] [Google Scholar]
- McLean PJ, Kawamata H, Shariff S, Hewett J, Sharma N, Ueda K, Breakefield XO, Hyman BT. TorsinA and heat shock proteins act as molecular chaperones: suppression of alpha-synuclein aggregation. J Neurochem. 2002;83:846–854. doi: 10.1046/j.1471-4159.2002.01190.x. [DOI] [PubMed] [Google Scholar]
- McNeill A, Birchall D, Hayflick SJ, Gregory A, Schenk JF, Zimmerman EA, Shang H, Miyajima H, Chinnery PF. T2* and FSE MRI distinguishes four subtypes of neurodegeneration with brain iron accumulation. Neurology. 2008;70:1614–1619. doi: 10.1212/01.wnl.0000310985.40011.d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti P, Hedera P, Wald J, Fink J. Autosomal recessive primary generalized dystonia in two siblings from a consanguineous family. Mov Disord. 2005;20:245–247. doi: 10.1002/mds.20228. [DOI] [PubMed] [Google Scholar]
- Naismith TV, Heuser JE, Breakefield XO, Hanson PI. TorsinA in the nuclear envelope. Proc Natl Acad Sci U S A. 2004;101:7612–7617. doi: 10.1073/pnas.0308760101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nery FC, Zeng J, Niland BP, Hewett J, Farley J, Irimia D, Li Y, Wiche G, Sonnenberg A, Breakefield XO. TorsinA binds the KASH domain of nesprins and participates in linkage between nuclear envelope and cytoskeleton. J Cell Sci. 2008;121:3476–3486. doi: 10.1242/jcs.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuwald AF, Aravind L, Spouge JL, Koonin EV. AAA+: a class of chaperone-like ATPases associated with the assembly, operation, and disassembly of protein complexes. Genome Res. 1999;9:27–43. [PubMed] [Google Scholar]
- Nolte D, Niemann S, Müller U. Specific sequence changes in multiple transcript system DYT3 are associated with X-linked dystonia parkinsonism. Proc Natl Acad Sci U S A. 2003;100:10347–10352. doi: 10.1073/pnas.1831949100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgren N, Mattson E, Forsgren L, Holmberg M. A high-penetrance form of late-onset torsion dystonia maps to a novel locus (DYT21) on chromosome 2q14.3-q21.3. Neurogenetics. 2011;12:137–143. doi: 10.1007/s10048-011-0274-9. [DOI] [PubMed] [Google Scholar]
- Ogura T, Wilkinson AJ. AAA+ superfamily ATPases: common structure–diverse function. Genes Cells. 2001;6:575–597. doi: 10.1046/j.1365-2443.2001.00447.x. [DOI] [PubMed] [Google Scholar]
- Oliveira JR, Spiteri E, Sobrido MJ, Hopfer S, Klepper J, Voit T, Gilbert J, Wszolek ZK, Calne DB, Stoessl AJ, et al. Genetic heterogeneity in familial idiopathic basal ganglia calcification (Fahr disease) Neurology. 2004;63:2165–2167. doi: 10.1212/01.wnl.0000145601.88274.88. [DOI] [PubMed] [Google Scholar]
- Opal P, Tintner R, Jankovic J, Leung J, Breakefield XO, Friedman J, Ozelius L. Intrafamilial phenotypic variability of the DYT1 dystonia: from asymptomatic TOR1A gene carrier status to dystonic storm. Mov Disord. 2002;17:339–345. doi: 10.1002/mds.10096. [DOI] [PubMed] [Google Scholar]
- Opladen T, Hoffmann G, Horster F, Hinz AB, Neidhardt K, Klein C, Wolf N. Clinical and biochemical characterization of patients with early infantile onset of autosomal recessive GTP cyclohydrolase I deficiency without hyperphenylalaninemia. Mov Disord. 2011;26:157–161. doi: 10.1002/mds.23329. [DOI] [PubMed] [Google Scholar]
- O’Riordan S, Cockburn D, Barton D, Lynch T, Hutchinson M. Primary torsion dystonia due to the Tor1A GAG deletion in an Irish family. Ir J Med Sci. 2002;171:31–32. doi: 10.1007/BF03168938. [DOI] [PubMed] [Google Scholar]
- Ozelius LJ, Hewett JW, Page CE, Bressman SB, Kramer PL, Shalish C, de Leon D, Brin MF, Raymond D, Corey DP, et al. The early-onset torsion dystonia gene (DYT1) encodes an ATP-binding protein. Nat Genet. 1997;17:40–48. doi: 10.1038/ng0997-40. [DOI] [PubMed] [Google Scholar]
- Ozelius LJ, Page CE, Klein C, Hewett JW, Mineta M, Leung J, Shalish C, Bressman SB, de Leon D, Brin MF, et al. The TOR1A (DYT1) gene family and its role in early onset torsion dystonia. Genomics. 1999;62:377–384. doi: 10.1006/geno.1999.6039. [DOI] [PubMed] [Google Scholar]
- Parker N. Hereditary whispering dysphonia. J Neurol Neurosurg Psychiatry. 1985;48:218–224. doi: 10.1136/jnnp.48.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman SL. Spinocerebellar degenerations. Handb Clin Neurol. 2011;100:113–140. doi: 10.1016/B978-0-444-52014-2.00006-9. [DOI] [PubMed] [Google Scholar]
- Peters GA, Seachrist DD, Keri RA, Sen GC. The double-stranded RNA-binding protein, PACT, is required for postnatal anterior pituitary proliferation. Proc Natl Acad Sci U S A. 2009;106:10696–10701. doi: 10.1073/pnas.0900735106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poorkaj P, Muma NA, Zhukareva V, Cochran EJ, Shannon KM, Hurtig H, Koller WC, Bird TD, Trojanowski JQ, Lee VM, et al. An R5L tau mutation in a subject with a progressive supranuclear palsy phenotype. Ann Neurol. 2002;52:511–516. doi: 10.1002/ana.10340. [DOI] [PubMed] [Google Scholar]
- Puschmann A, Xiao J, Bastian RW, Searcy JA, LeDoux MS, Wszolek ZK. An African-American family with dystonia. Parkinsonism Relat Disord. 2011;17:547–550. doi: 10.1016/j.parkreldis.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainier S, Thomas D, Tokarz D, Ming L, Bui M, Plein E, Zhao X, Lemons R, Albin R, Delaney C, et al. Myofibrillogenesis regulator 1 gene mutations cause paroxysmal dystonic choreoathetosis. Arch Neurol. 2004;61:1025–1029. doi: 10.1001/archneur.61.7.1025. [DOI] [PubMed] [Google Scholar]
- Reich SG, Grill SE. Corticobasal degeneration. Curr Treat Options Neurol. 2009;11:179–185. doi: 10.1007/s11940-009-0021-9. [DOI] [PubMed] [Google Scholar]
- Risch N, de Leon D, Ozelius L, Kramer P, Almasy L, Singer B, Fahn S, Breakefield X, Bressman S. Genetic analysis of idiopathic torsion dystonia in Ashkenazi Jews and their recent descent from a small founder population. Nat Genet. 1995;9:152–159. doi: 10.1038/ng0295-152. [DOI] [PubMed] [Google Scholar]
- Risch NJ, Bressman SB, Senthil G, Ozelius LJ. Intragenic Cis and Trans modification of genetic susceptibility in DYT1 torsion dystonia. Am J Hum Genet. 2007;80:1188–1193. doi: 10.1086/518427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz K, van Schaik BD, Jakobs ME, van Kampen AH, Aronica E, Tijssen MA, Baas F. SGCE isoform characterization and expression in human brain: implications for myoclonus-dystonia pathogenesis? Eur J Hum Genet. 2011;19:438–444. doi: 10.1038/ejhg.2010.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodacker V, Toustrup-Jensen M, Vilsen B. Mutations Phe785Leu and Thr618Met in Na+, K+-ATPase, associated with familial rapid-onset dystonia parkinsonism, interfere with Na+ interaction by distinct mechanisms. J Biol Chem. 2006;281:18539–18548. doi: 10.1074/jbc.M601780200. [DOI] [PubMed] [Google Scholar]
- Roussigne M, Cayrol C, Clouaire T, Amalric F, Girard JP. THAP1 is a nuclear proapoptotic factor that links prostate-apoptosis-response-4 (Par-4) to PML nuclear bodies. Oncogene. 2003;22:2432–2442. doi: 10.1038/sj.onc.1206271. [DOI] [PubMed] [Google Scholar]
- Sako W, Morigaki R, Kaji R, Tooyama I, Okita S, Kitazato K, Nagahiro S, Graybiel AM, Goto S. Identification and localization of a neuron-specific isoform of TAF1 in rat brain: implications for neuropathology of DYT3 dystonia. Neuroscience. 2011;189:100–107. doi: 10.1016/j.neuroscience.2011.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho-Tello M, Valles S, Montoliu C, Renau-Piqueras J, Guerri C. Developmental pattern of GFAP and vimentin gene expression in rat brain and in radial glial cultures. Glia. 1995;15:157–166. doi: 10.1002/glia.440150208. [DOI] [PubMed] [Google Scholar]
- Santangelo G. Contributo clinico alla conoscenza delle forme familiari della dysbasia lordotica progressiva (spasmo di torsione) Psychiat Neuropat. 1934;62:52–77. [Google Scholar]
- Schapira AH, Warner T, Gash MT, Cleeter MW, Marinho CF, Cooper JM. Complex I function in familial and sporadic dystonia. Ann Neurol. 1997;41:556–559. doi: 10.1002/ana.410410421. [DOI] [PubMed] [Google Scholar]
- Schneider SA, Bhatia KP. Dystonia in the Woodhouse Sakati syndrome: a new family and literature review. Mov Disord. 2008;23:592–596. doi: 10.1002/mds.21886. [DOI] [PubMed] [Google Scholar]
- Schneider SA, Bhatia KP. Rare causes of dystonia parkinsonism. Curr Neurol Neurosci Rep. 2010a;10:431–439. doi: 10.1007/s11910-010-0136-0. [DOI] [PubMed] [Google Scholar]
- Schneider SA, Bhatia KP. Secondary dystonia–clinical clues and syndromic associations. Eur J Neurol. 2010b;17(Suppl 1):52–57. doi: 10.1111/j.1468-1331.2010.03051.x. [DOI] [PubMed] [Google Scholar]
- Schneider SA, Paisan-Ruiz C, Garcia-Gorostiaga I, Quinn NP, Weber YG, Lerche H, Hardy J, Bhatia KP. GLUT1 gene mutations cause sporadic paroxysmal exercise-induced dyskinesias. Mov Disord. 2009;24:1684–1688. doi: 10.1002/mds.22507. [DOI] [PubMed] [Google Scholar]
- Schneider SA, Ramirez A, Shafiee K, Kaiser FJ, Erogullari A, Bruggemann N, Winkler S, Bahman I, Osmanovic A, Shafa MA, et al. Homozygous THAP1 mutations as cause of early-onset generalized dystonia. Mov Disord. 2011;26:858–861. doi: 10.1002/mds.23561. [DOI] [PubMed] [Google Scholar]
- Segawa M, Hosaka A, Miyagawa F, Nomura Y, Imai H. Hereditary progressive dystonia with marked diurnal fluctuation. Adv Neurol. 1976;14:215–233. [PubMed] [Google Scholar]
- Seibler P, Djarmati A, Langpap B, Hagenah J, Schmidt A, Bruggemann N, Siebner H, Jabusch HC, Altenmuller E, Munchau A, et al. A heterozygous frameshift mutation in PRKRA (DYT16) associated with generalised dystonia in a German patient. Lancet Neurol. 2008;7:380–381. doi: 10.1016/S1474-4422(08)70075-9. [DOI] [PubMed] [Google Scholar]
- Sengel C, Gavarini S, Sharma N, Ozelius LJ, Bragg DC. Dimerization of the DYT6 dystonia protein, THAP1, requires residues within the coiled-coil domain. J Neurochem. 2011;118:1087–1100. doi: 10.1111/j.1471-4159.2011.07386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi KD, Jankovic J. Dystonia in spinocerebellar ataxia type 6. Mov Disord. 2002;17:150–153. doi: 10.1002/mds.1252. [DOI] [PubMed] [Google Scholar]
- Shamim EA, Chu J, Scheider LH, Savitt J, Jinnah HA, Hallett M. Extreme task specificity in writer’s cramp. Mov Disord. 2011;26:2107–2109. doi: 10.1002/mds.23827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan Y, Cortopassi G. HSC20 interacts with frataxin and is involved in iron-sulfur cluster biogenesis and iron homeostasis. Hum Mol Genet. 2011 doi: 10.1093/hmg/ddr582. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N, Armata IA, Multhaupt-Buell TJ, Ozelius LJ, Xin W, Sims KB. Mutation in 5′ upstream region of GCHI gene causes familial dopa-responsive dystonia. Mov Disord. 2011;26:2140–2141. doi: 10.1002/mds.23786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N, Hewett J, Ozelius LJ, Ramesh V, McLean PJ, Breakefield XO, Hyman BT. A close association of torsinA and alpha-synuclein in Lewy bodies: a fluorescence resonance energy transfer study. Am J Path. 2001;159:339–344. doi: 10.1016/s0002-9440(10)61700-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shashidharan P, Good PF, Hsu A, Perl DP, Brin MF, Olanow CW. TorsinA accumulation in Lewy bodies in sporadic Parkinson’s disease. Brain Res. 2000;877:379–381. doi: 10.1016/s0006-8993(00)02702-5. [DOI] [PubMed] [Google Scholar]
- Shashidharan P, Paris N, Sandu D, Karthikeyan L, McNaught KS, Walker RH, Olanow CW. Overexpression of torsinA in PC12 cells protects against toxicity. J Neurochem. 2004;88:1019–1025. doi: 10.1046/j.1471-4159.2003.02233.x. [DOI] [PubMed] [Google Scholar]
- Shen Y, Lee HY, Rawson J, Ojha S, Babbitt P, Fu YH, Ptácek LJ. Mutations in PNKD causing paroxysmal dyskinesia alters protein cleavage and stability. Hum Mol Genet. 2011;20:2322–2332. doi: 10.1093/hmg/ddr125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spacey SD, Adams PJ, Lam PC, Materek LA, Stoessl AJ, Snutch TP, Hsiung GY. Genetic heterogeneity in paroxysmal nonkinesigenic dyskinesia. Neurology. 2006;66:1588–1590. doi: 10.1212/01.wnl.0000217332.51740.7c. [DOI] [PubMed] [Google Scholar]
- Spacey SD, Valente EM, Wali GM, Warner TT, Jarman PR, Schapira AH, Dixon PH, Davis MB, Bhatia KP, Wood NW. Genetic and clinical heterogeneity in paroxysmal kinesigenic dyskinesia: evidence for a third EKD gene. Mov Disord. 2002;17:717–725. doi: 10.1002/mds.10126. [DOI] [PubMed] [Google Scholar]
- Spina S, Murrell JR, Yoshida H, Ghetti B, Bermingham N, Sweeney B, Dlouhy SR, Crowther RA, Goedert M, Keohane C. The novel Tau mutation G335S: clinical, neuropathological and molecular characterization. Acta Neuropathol. 2007;113:461–470. doi: 10.1007/s00401-006-0182-5. [DOI] [PubMed] [Google Scholar]
- Steinberger D, Blau N, Goriuonov D, Bitsch J, Zuker M, Hummel S, Müller U. Heterozygous mutation in 5′-untranslated region of sepiapterin reductase gene (SPR) in a patient with dopa-responsive dystonia. Neurogenetics. 2004;5:187–190. doi: 10.1007/s10048-004-0182-3. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH, Wooten GF. A novel deafness/dystonia peptide gene mutation that causes dystonia in female carriers of Mohr-Tranebjaerg syndrome. Ann Neurol. 2001;50:537–540. doi: 10.1002/ana.1160. [DOI] [PubMed] [Google Scholar]
- Swoboda KJ. Disorders of amine biosynthesis. Future Neurol. 2006;1:605–614. [Google Scholar]
- Szepetowski P, Rochette J, Berquin P, Piussan C, Lathrop GM, Monaco AP. Familial infantile convulsions and paroxysmal choreoathetosis: a new neurological syndrome linked to the pericentromeric region of human chromosome 16. Am J Hum Genet. 1997;61:889–898. doi: 10.1086/514877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarsy D, Sweadner KJ, Song PC. Case records of the Massachusetts General Hospital. Case 17-2010-a 29-year-old woman with flexion of the left hand and foot and difficulty speaking. New Engl J Med. 2010;362:2213–2219. doi: 10.1056/NEJMcpc1002112. [DOI] [PubMed] [Google Scholar]
- Thiffault I, Rioux MF, Tetreault M, Jarry J, Loiselle L, Poirier J, Gros-Louis F, Mathieu J, Vanasse M, Rouleau GA, et al. A new autosomal recessive spastic ataxia associated with frequent white matter changes maps to 2q33-34. Brain. 2006;129:2332–2340. doi: 10.1093/brain/awl110. [DOI] [PubMed] [Google Scholar]
- Thöny B, Blau N. Mutations in the BH4-metabolizing genes GTP cyclohydrolase I, 6-pyruvoyl-tetrahydropterin synthase, sepiapterin reductase, carbinolamine-4a-dehydratase, and dihydropteridine reductase. Hum Mutat. 2006;27:870–878. doi: 10.1002/humu.20366. [DOI] [PubMed] [Google Scholar]
- Tolosa E, Compta Y. Dystonia in Parkinson’s disease. J Neurol. 2006;253(Suppl 7):VII7–VII13. doi: 10.1007/s00415-006-7003-6. [DOI] [PubMed] [Google Scholar]
- Tomita H, Nagamitsu S, Wakui K, Fukushima Y, Yamada K, Sadamatsu M, Masui A, Konishi T, Matsuishi T, Aihara M, et al. Paroxysmal kinesigenic choreoathetosis locus maps to chromosome 16p11.2-q12.1. Am J Hum Genet. 1999;65:1688–1697. doi: 10.1086/302682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tońska K, Kodron A, Bartnik E. Genotype-phenotype correlations in Leber hereditary optic neuropathy. Biochim Biophys Acta. 2010;1797:1119–1123. doi: 10.1016/j.bbabio.2010.02.032. [DOI] [PubMed] [Google Scholar]
- Torres GE, Sweeney AL, Beaulieu JM, Shashidharan P, Caron MG. Effect of torsinA on membrane proteins reveals a loss of function and a dominant-negative phenotype of the dystonia-associated DeltaE-torsinA mutant. Proc Natl Acad Sci U S A. 2004;101:15650–15655. doi: 10.1073/pnas.0308088101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uitti RJ, Maraganore DM. Adult onset familial cervical dystonia: report of a family including monozygotic twins. Mov Disord. 1993;8:489–494. doi: 10.1002/mds.870080413. [DOI] [PubMed] [Google Scholar]
- Vale RD. AAA proteins. Lords of the ring. J Cell Biol. 2000;150:F13–19. doi: 10.1083/jcb.150.1.f13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente EM, Bentivoglio AR, Cassetta E, Dixon PH, Davis MB, Ferraris A, Ialongo T, Frontali M, Wood NW, Albanese A. DYT13, a novel primary torsion dystonia locus, maps to chromosome 1p36.13–36.32 in an Italian family with cranial-cervical or upper limb onset. Ann Neurol. 2001;49:362–366. [PubMed] [Google Scholar]
- Valente EM, Spacey SD, Wali GM, Bhatia KP, Dixon PH, Wood NW, Davis MB. A second paroxysmal kinesigenic choreoathetosis locus (EKD2) mapping on 16q13-q22.1 indicates a family of genes which give rise to paroxysmal disorders on human chromosome 16. Brain. 2000;123:2040–2045. doi: 10.1093/brain/123.10.2040. [DOI] [PubMed] [Google Scholar]
- Valente EM, Warner TT, Jarman PR, Mathen D, Fletcher NA, Marsden CD, Bhatia KP, Wood NW. The role of DYT1 in primary torsion dystonia in Europe. Brain. 1998;121:2335–2339. doi: 10.1093/brain/121.12.2335. [DOI] [PubMed] [Google Scholar]
- van Gaalen J, Giunti P, van de Warrenburg BP. Movement disorders in spinocerebellar ataxias. Mov Disord. 2011;26:792–800. doi: 10.1002/mds.23584. [DOI] [PubMed] [Google Scholar]
- Van Gerpen JA, LeDoux MS, Wszolek ZK. Adult-onset leg dystonia due to a missense mutation in THAP1. Mov Disord. 2010;25:1306–1307. doi: 10.1002/mds.23086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heyden AB, Naismith TV, Snapp EL, Hanson PI. Static retention of the lumenal monotopic membrane protein torsinA in the endoplasmic reticulum. EMBO J. 2011;30:3217–3231. doi: 10.1038/emboj.2011.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan A, Breakefield XO, Bhide PG. Developmental patterns of torsinA and torsinB expression. Brain Res. 2006;1073–1074:139–145. doi: 10.1016/j.brainres.2005.12.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeek MM, Steenbergen-Spanjers GC, Willemsen MA, Hol FA, Smeitink J, Seeger J, Grattan-Smith P, Ryan MM, Hoffmann GF, Donati MA, et al. Mutations in the cyclic adenosine monophosphate response element of the tyrosine hydroxylase gene. Ann Neurol. 2007;62:422–426. doi: 10.1002/ana.21199. [DOI] [PubMed] [Google Scholar]
- Verrotti A, D’Egidio C, Agostinelli S, Gobbi G. Glut1 deficiency: when to suspect and how to diagnose? Eur J Paediatr Neurol. 2012;16:3–9. doi: 10.1016/j.ejpn.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Waite A, Tinsley CL, Locke M, Blake DJ. The neurobiology of the dystrophin-associated glycoprotein complex. Ann Med. 2009;41:344–359. doi: 10.1080/07853890802668522. [DOI] [PubMed] [Google Scholar]
- Walker RH, Good PF, Shashidharan P. TorsinA immunoreactivity in inclusion bodies in trinucleotide repeat diseases. Mov Disord. 2003;18:1041–1044. doi: 10.1002/mds.10487. [DOI] [PubMed] [Google Scholar]
- Waln O, LeDoux MS. Blepharospasm plus cervical dystonia with predominant anterocollis: a distinctive subphenotype of segmental craniocervical dystonia? Tremor Other Hyperkinet Mov (NY) 2011;1 doi: 10.7916/D8SQ8Z4T. pii: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JL, Cao L, Li XH, Hu ZM, Li JD, Zhang JG, Liang Y, San A, Li N, Chen SQ, et al. Identification of PRRT2 as the causative gene of paroxysmal kinesigenic dyskinesias. Brain. 2011;134:3493–3501. doi: 10.1093/brain/awr289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber YG, Kamm C, Suls A, Kempfle J, Kotschet K, Schule R, Wuttke TV, Maljevic S, Liebrich J, Gasser T, et al. Paroxysmal choreoathetosis/spasticity (DYT9) is caused by a GLUT1 defect. Neurology. 2011;77:959–964. doi: 10.1212/WNL.0b013e31822e0479. [DOI] [PubMed] [Google Scholar]
- Weber YG, Lerche H. Genetics of paroxysmal dyskinesias. Curr Neurol Neurosci Rep. 2009;9:206–211. doi: 10.1007/s11910-009-0031-8. [DOI] [PubMed] [Google Scholar]
- Weber YG, Storch A, Wuttke TV, Brockmann K, Kempfle J, Maljevic S, Margari L, Kamm C, Schneider SA, Huber SM, et al. GLUT1 mutations are a cause of paroxysmal exertion-induced dyskinesias and induce hemolytic anemia by a cation leak. J Clin Invest. 2008;118:2157–2168. doi: 10.1172/JCI34438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss EM, Hershey T, Karimi M, Racette B, Tabbal SD, Mink JW, Paniello RC, Perlmutter JS. Relative risk of spread of symptoms among the focal onset primary dystonias. Mov Disord. 2006;21:1175–1181. doi: 10.1002/mds.20919. [DOI] [PubMed] [Google Scholar]
- Weraarpachai W, Antonicka H, Sasarman F, Seeger J, Schrank B, Kolesar JE, Lochmuller H, Chevrette M, Kaufman BA, Horvath R, et al. Mutation in TACO1, encoding a translational activator of COX I, results in cytochrome c oxidase deficiency and late-onset Leigh syndrome. Nat Genet. 2009;41:833–837. doi: 10.1038/ng.390. [DOI] [PubMed] [Google Scholar]
- Werner ER, Blau N, Thöny B. Tetrahydrobiopterin: biochemistry and pathophysiology. Biochem J. 2011;438:397–414. doi: 10.1042/BJ20110293. [DOI] [PubMed] [Google Scholar]
- Wider C, Melquist S, Hauf M, Solida A, Cobb SA, Kachergus JM, Gass J, Coon KD, Baker M, Cannon A, et al. Study of a Swiss dopa-responsive dystonia family with a deletion in GCH1: redefining DYT14 as DYT5. Neurology. 2008;70:1377–1383. doi: 10.1212/01.wnl.0000275527.35752.c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox RA, Winkler S, Lohmann K, Klein C. Whispering dysphonia in an Australian family (DYT4): a clinical and genetic reappraisal. Mov Disord. 2011;26:2404–2408. doi: 10.1002/mds.23866. [DOI] [PubMed] [Google Scholar]
- Willemsen MA, Verbeek MM, Kamsteeg EJ, de Rijk-van Andel JF, Aeby A, Blau N, Burlina A, Donati MA, Geurtz B, Grattan-Smith PJ, et al. Tyrosine hydroxylase deficiency: a treatable disorder of brain catecholamine biosynthesis. Brain. 2010;133:1810–1822. doi: 10.1093/brain/awq087. [DOI] [PubMed] [Google Scholar]
- Wu YR, Lee-Chen GJ, Lang AE, Chen CM, Lin HY, Chen ST. Dystonia as a presenting sign of spinocerebellar ataxia type 1. Mov Disord. 2004;19:586–587. doi: 10.1002/mds.10708. [DOI] [PubMed] [Google Scholar]
- Xiao J, Bastian RW, Perlmutter JS, Racette BA, Tabbal SD, Karimi M, Paniello RC, Blitzer A, Batish SD, Wszolek ZK, et al. High-throughput mutational analysis of TOR1A in primary dystonia. BMC Med Genet. 2009;10:24. doi: 10.1186/1471-2350-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Gong S, Zhao Y, LeDoux MS. Developmental expression of rat torsinA transcript and protein. Brain Res Dev Brain Res. 2004;152:47–60. doi: 10.1016/j.devbrainres.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Xiao J, LeDoux MS. Cloning, developmental regulation and neural localization of rat epsilon-sarcoglycan. Mol Brain Res. 2003;119:132–143. doi: 10.1016/j.molbrainres.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Xiao J, Uitti RJ, Zhao Y, Vemula SR, Perlmutter JS, Wszolek ZK, Maraganore DM, Auburger G, Leube B, Lehnhoff K, et al. Mutations in CIZ1 cause adult-onset primary cervical dystonia. Ann Neurol. 2012;71:458–469. doi: 10.1002/ana.23547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Zhao Y, Bastian RW, Perlmutter JS, Racette BA, Tabbal SD, Karimi M, Paniello RC, Wszolek ZK, Uitti RJ, et al. Novel THAP1 sequence variants in primary dystonia. Neurology. 2010;74:229–238. doi: 10.1212/WNL.0b013e3181ca00ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Zhao Y, Bastian RW, Perlmutter JS, Racette BA, Tabbal SD, Karimi M, Paniello RC, Wszolek ZK, Uitti RJ, et al. The c.-237_236GA>TT THAP1 sequence variant does not increase risk for primary dystonia. Mov Disord. 2011;26:549–552. doi: 10.1002/mds.23551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Xiao J, Ueda M, Wang Y, Hines M, Nowak TS, Jr, LeDoux MS. Glial elements contribute to stress-induced torsinA expression in the CNS and peripheral nervous system. Neuroscience. 2008;155:439–453. doi: 10.1016/j.neuroscience.2008.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimprich A, Grabowski M, Asmus F, Naumann M, Berg D, Bertram M, Scheidtmann K, Kern P, Winkelmann J, Muller-Myhsok B, et al. Mutations in the gene encoding epsilon-sarcoglycan cause myoclonus-dystonia syndrome. Nat Genet. 2001;29:66–69. doi: 10.1038/ng709. [DOI] [PubMed] [Google Scholar]
- Zirn B, Grundmann K, Huppke P, Puthenparampil J, Wolburg H, Riess O, Müller U. Novel TOR1A mutation p. Arg288Gln in early-onset dystonia (DYT1) J Neurol Neurosurg Psychiatry. 2008a;79:1327–1330. doi: 10.1136/jnnp.2008.148270. [DOI] [PubMed] [Google Scholar]
- Zirn B, Steinberger D, Troidl C, Brockmann K, von der Hagen M, Feiner C, Henke L, Müller U. Frequency of GCH1 deletions in dopa-responsive dystonia. J Neurol Neurosurg Psychiatry. 2008b;79:183–186. doi: 10.1136/jnnp.2007.128413. [DOI] [PubMed] [Google Scholar]