Abstract
Vesicle exocytosis releases content to mediate many biological events, including synaptic transmission essential for brain functions. Following exocytosis, endocytosis is initiated to retrieve exocytosed vesicles within seconds to minutes. Decades of studies in secretory cells reveal three exocytosis modes coupled to three endocytosis modes: (a) full-collapse fusion, in which vesicles collapse into the plasma membrane, followed by classical endocytosis involving membrane invagination and vesicle reformation; (b) kiss-and-run, in which the fusion pore opens and closes; and (c) compound exocytosis, which involves exocytosis of giant vesicles formed via vesicle-vesicle fusion, followed by bulk endocytosis that retrieves giant vesicles. Here we review these exo- and endocytosis modes and their roles in regulating quantal size and synaptic strength, generating synaptic plasticity, maintaining exocytosis, and clearing release sites for vesicle replenishment. Furthermore, we highlight recent progress in understanding how vesicle endocytosis is initiated and is thus coupled to exocytosis. The emerging model is that calcium influx via voltage-dependent calcium channels at the calcium microdomain triggers endocytosis and controls endocytosis rate; calmodulin and synaptotagmin are the calcium sensors; and the exocytosis machinery, including SNARE proteins (synaptobrevin, SNAP25, and syntaxin), is needed to coinitiate endocytosis, likely to control the amount of endocytosis.
Keywords: kiss-and-run, compound exocytosis, bulk endocytosis, synaptic plasticity, calmodulin, calcium channels
INTRODUCTION
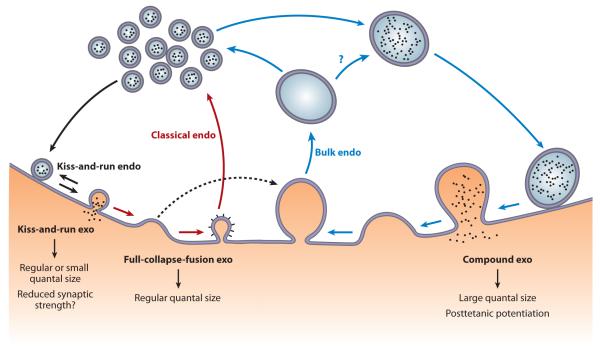
Vesicle exocytosis and endocytosis are fundamental biological events (1, 2). Vesicle exocytosis, the fusion of a vesicle with the plasma membrane, releases vesicular contents to exert various functions. For example, the secretion of transmitters from neurons mediates synaptic transmission essential for brain functions, the neuronal secretion of peptides (e.g., neuropeptide Y) and hormones (e.g., vasopressin, oxytocin) regulates labor and mental state, the secretion of insulin from pancreatic cells regulates blood glucose level (critical in diabetes), the secretion of catecholamine and peptides from adrenal chromaffin cells is involved in the stress response, and blood cell exocytosis is involved in immune responses (1, 3, 4). Following exocytosis, vesicular membrane and proteins are retrieved from the plasma membrane through endocytosis, which recycles vesicles, maintains exocytosis, and prevents secretory cells from swelling or shrinking (2, 5). Secretory cells employ three exocytosis modes—full-collapse fusion, kiss-and-run, and compound exocytosis—to control the rate and amount of vesicular content release and to thus control exocytosis strength and mediate plasticity of exocytosis, including synaptic plasticity (Figure 1). These three exocytosis modes are followed by three endocytosis modes—classical endocytosis, kiss-and-run, and bulk endocytosis, respectively—that ensure sufficient vesicle recycling during different activities (Figure 1). Here we review the supporting evidence for each exo- and endocytosis mode and their underlying functions observed in various cell types over the past four decades, but with a focus on neuronal terminals, where exo- and endocytosis have been studied intensely. Furthermore, we highlight recent progress in understanding how endocytosis is initiated and coupled to exocytosis, a puzzle since the discovery of secretory vesicle endocytosis in the 1970s (6, 7). We present an emerging exo-endocytosis coupling model in which a calcium signaling pathway and the exocytosis machinery are involved in controlling the endocytosis rate and amount.
Figure 1.
Schematic drawing of modes of exocytosis, endocytosis, and their coupling. Three modes of exocytosis (exo)—full-collapse fusion, kiss-and-run, and compound exocytosis—are coupled to classical endocytosis (endo), kiss-and-run, and bulk endocytosis, respectively. Full-collapse fusion may also be coupled to bulk endocytosis (dotted arrow), although this hypothesis remains to be tested. The functions of each exocytosis mode in regulating quantal size and generating synaptic plasticity are also listed. Question marks indicate an unclear function or transition.
THREE EXOCYTOSIS MODES: SUPPORTING EVIDENCE
Full-Collapse Fusion
Full-collapse fusion has been suggested mainly on the basis of freeze-fracture electron microscopic (EM) observations that vesicle-like structures (with diameters of ~60–120 nm) larger than a regular vesicle (with diameters of ~30 nm) are more abundant after stimulation at the neuromuscular junction (Figure 2ai) (8). Other lines of evidence indirectly support this fusion mode. For example, cell-attached recordings of secretory cells, including nerve terminals, show that the membrane capacitance up step caused by single-vesicle fusion is accompanied by a fusion pore conductance increase reflecting pore expansion (Figure 2aii,iii) (9–11). The pore diameter increases to a value beyond the detection limit (~4–6 nm) and is not accompanied by a down step within a few seconds, suggesting rapid pore expansion (Figure 2aii,iii). Amperometric measurements of release from catecholamine-containing vesicles (Figure 2aii) and imaging of fluorescently tagged vesicle proteins and lipids reveal rapid release kinetics, suggesting a larger fusion pore or pore expansion (9, 10, 12–15). Quantum dots with an ~15-nm diameter that are preloaded in synaptic vesicles can be released (Figure 2aiv), suggesting a fusion pore larger than 15 nm or vesicle collapse (16). Although the above evidence led to the acceptance of full-collapse fusion, direct observation of this morphological change in live cells is still needed, which seems feasible, given the recent development of superresolution imaging techniques.
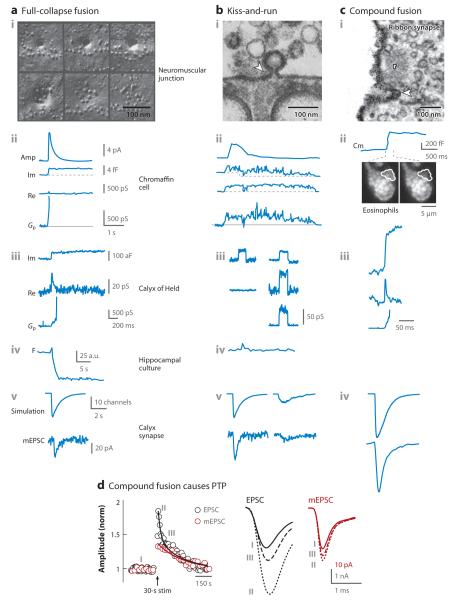
Figure 2.
Modes of exocytosis: supporting evidence and functions. (a) Full-collapse fusion. (i) Electron microscopic (EM) images showing the hypothesized sequence of full-collapse fusion (from upper left to upper right and then from lower left to lower right). Images were taken from neuromuscular junctions that were frozen 3.7 ms, 5.2 ms, 5.2 ms (top montage from left to right), 5.2 ms, 20 ms, and 50 ms (bottom montage from left to right) after stimulation. (ii) Simultaneous cell-attached capacitance and amperometric (Amp) recordings of a chromaffin cell. The fusion pore conductance (Gp) is calculated from recordings of the imaginary (Im, reflecting capacitance) and the real (Re) component of the admittance. The rapid and large Amp spike, the absence of a down step after the up step, and the rapid Gp increase suggest full-collapse fusion. (iii) Full-collapse fusion revealed by cell-attached capacitance recordings at the release face of the calyx of Held. (iv) Release of a quantum dot (F denotes fluorescence) during exocytosis of a vesicle preloaded with a quantum dot (15-nm diameter) reveals full-collapse fusion at cultured hippocampal synapses. (v) (Upper) Simulated miniature excitatory postsynaptic currents (mEPSCs) from full-collapse fusion. (Lower) Representative mEPSC recorded from the calyx of Held synapse. The scale bars shown in panel a apply to all the panels in the same row, except when a scale bar is otherwise provided. Panels i, ii, and iv are adapted with permission from References 8, 10, and 16, respectively. Panels iii and v are adapted from Reference 11.
(b) Kiss-and-run. (i) EM image of an Ω profile (arrowhead), a candidate for kiss-and-run fusion, in a frog neuromuscular junction that was stimulated for 2 h in the presence of horseradish peroxidase. (ii) Similar arrangements as in panel aii, but showing a capacitance flicker caused by kiss-and-run. (iii) Similar arrangements as in panel aiii, but showing capacitance flickers reflecting kiss-and-run. One flicker is not accompanied by detectable Re changes (left), because the fusion pore is too fast and/or too large to resolve. (iv) Similar arrangements as in panel aiv, but showing transient fluorescence increase and decrease (the quantum dot is pH sensitive) caused by kiss-and-run. (v) (Upper) Simulated mEPSCs for kiss-and-run with large (left) and small (right) fusion pore conductance. (Lower) Representative mEPSCs that are recorded from the calyx of Held synapse and that are similar to the corresponding simulated mEPSCs. Panels i, ii, and iv are adapted with permission from References 173 (copyright © 1972, Rockefeller University Press), 9, and 16, respectively. Panels iii and v are adapted from Reference 11. (c) Compound exocytosis. (i) An EM image of a large fusion-like structure (arrowhead), a candidate for compound exocytosis, near the ribbon (r) at a goldfish retinal bipolar nerve terminal fixed immediately after stimulation in the presence of cationized ferritin. (ii) Compound exocytosis from eosinophils stained with LysoTracker®. A whole-cell capacitance (Cm) up step much larger than a single vesicle’s membrane capacitance is accompanied by sequential loss of fluorescence from six granules (here showing the final loss, marked by white outlines). (iii) Similar arrangements as in panel aiii, but showing a giant capacitance up step. (iv) Similar arrangements as in panel av, but showing a simulated mEPSC caused by compound exocytosis (upper) and a giant mEPSC recorded during intense stimulation (lower). Panels i and ii are adapted with permission from References 43 and 36, respectively. Panels iii and iv are adapted from Reference 44. (d) Compound exocytosis mediates a large portion of posttetanic potentiation (PTP) at the calyx of Held synapse. A 30-s train of presynaptic action potentials at 100 Hz (arrow) potentiates both the EPSC (black) and the mEPSC (red) [normalized to baseline (norm)]. Sampled EPSCs and mEPSCs collected at the times indicated (I, II, III) are also shown. Panel d is adapted from Reference 44.
Kiss-and-Run
Kiss-and-run was recently reviewed in the Annual Review of Physiology (17). Here we briefly summarize its supporting evidence. Kiss-and-run was originally proposed on the basis of EM observations of an Ω profile at the neuromuscular junction (Figure 2bi) (7, 18, 19), although whether the observed Ω profile is an intermediate structure en route to collapse or pore closure is uncertain. The first convincing evidence comes from the observation of capacitance flickers, during which a measurable pore conductance corresponding to a narrow pore of ~0.5–3-nm diameter is sometimes detected in secretory cells, including nerve terminals (Figure 2bii,iii) (9–11, 20). Cell-attached capacitance recordings and amperometric recordings show that the capacitance flicker is sometimes accompanied by a stand-alone foot of the amperometric signal in chromaffin cells and PC12 cells, suggesting that kiss-and-run may partially release transmitter (10, 21, 22). Fluorescently tagged vesicle membrane or lumen proteins (e.g., phogrin, tissue plasminogen activator) in chromaffin, PC12, and insulin-containing cells may stay clustered upon fusion and are retrieved as a cluster within seconds to minutes, which reflects a slow mode of kiss-and-run termed cavicapture (13, 14, 23). At small synapses like cultured hippocampal synapses (16, 24–28) and lamprey reticulospinal synapses (29), kiss-and-run is suggested on the basis of imaging results, such as partial release of FM dyes, rapid synaptopHluorin increase and decrease that may reflect transient fusion pore opening and closure, and in particular the recent observation that quantum dot–containing vesicles do not release the ~15-nm quantum dot during exo- and endocytosis (Figure 2biv) (16, 28). However, other imaging studies are not in agreement with this suggestion (30–33) (see also References 17 and 34 for reviews). Whether kiss-and-run represents a significant fraction of exo- and endocytosis at synapses remains unsettled.
Compound Exocytosis
EM observations of multivesicular structures in pancreatic acinar cells (35) and many other cells suggest that vesicles may fuse with each other (36, 37). Capacitance recordings show that up steps can be much larger than a regular vesicle’s membrane capacitance in mast cells and eosinophils, suggesting fusion of very large vesicles preformed via vesicle-vesicle fusion (38, 39). In eosinophils in which a regular vesicle is ~1.7 μm, a capacitance up step equivalent to several vesicles’ membrane capacitances is accompanied by the release of multiple fluorescently labeled vesicles (Figure 2cii) (36). In pancreatic acinar cells, vesicle fusion on the already fused, but not collapsed, vesicle membrane, termed sequential compound fusion, has been observed by fluorescence imaging (40).
Although compound exocytosis is well established at nonneuronal cells containing large vesicles (~300–2,000 nm), it has been suggested only recently at synapses containing small vesicles (~20–50 nm). Synaptic vesicle-vesicle fusion is mechanistically possible, because both vesicular and membrane-targeted SNARE proteins essential for exocytosis are present at vesicles (41), and vesicles isolated from synaptosomes may fuse with each other in vitro (42). At a ribbon-type synapse, large vesicular structures are found immediately after stimulation (Figure 2ci). Like regular vesicles, these large vesicular structures are attached to ribbons via filaments, suggesting that large vesicles may be formed by vesicle-vesicle fusion (43). At calyx of Held synapses, cell-attached capacitance measurements at the release face reveal capacitance up steps ~10 times larger than a regular vesicle’s capacitance (Figure 2ciii) (44). These large up steps are not due to simultaneous or sequential multivesicle fusion for the following reasons: (a) They rise instantaneously at 0.6-ms time resolution, (b) they occur not only during stimulation but also after stimulation during which there is no depolarization to evoke synchronous release, and (c) they are sometimes accompanied by a fusion pore conductance increase reflecting pore expansion of a fusing vesicle (Figure 2ciii) (44). Large capacitance up steps are accompanied by EM observation of giant vesicles and by giant miniature excitatory postsynaptic currents (mEPSCs) recorded from postsynaptic neurons (Figure 2civ), suggesting compound exocytosis (44). Removing extracellular calcium or synaptotagmin 2, the calcium sensor for synchronized exocytosis at calyces, abolishes giant capacitance up steps, giant vesicles, and giant mEPSCs, suggesting that, like regular fusion, calcium binding with synaptotagmin triggers compound exocytosis (44).
THREE EXOCYTOSIS MODES: DIFFERENTIAL ROLES
Full-Collapse Fusion and Kiss-and-Run
Full-collapse fusion releases vesicular contents completely and rapidly via a rapidly expanding pore (Figure 2aii,iii,v) (9–11, 20). In contrast, kiss-and-run is often considered to have a narrow pore and may thus release contents slowly and/or partially for the following reasons. (a) During capacitance flickers, small fusion pore conductance is sometimes detectable (Figure 2bii,iii), which predicts a slow transmitter release and thus a smaller quantal size at synapses (Figure 2bv). (b) Amperometric recording shows a stand-alone foot that may be due to rapid fusion pore closure. (c) Imaging shows retention of large vesicular lumen proteins during cavicapture of large dense-core vesicles (9–11, 13, 20, 21). At synapses, kiss-and-run is considered to be a mechanism to reduce quantal size (Figure 2bv), to reduce synaptic strength, and to mediate synaptic plasticity, although these functions have not been directly demonstrated (11, 17, 20, 45, 46). Kiss-and-run may also open a large pore, which would ensure rapid and complete release (47). In fact, fusion pore size is too large to resolve in most capacitance flickers at the calyx nerve terminal (Figure 2biii, left), suggesting that most kiss-and-run events release transmitter as rapidly and completely as full-collapse fusion (Figure 2bv) (11). Thus, kiss-and-run should not be viewed as a mode capable of generating only slower and smaller quantal responses.
Because kiss-and-run bypasses vesicle collapse, it may save energy spent on vesicle collapse and the subsequent vesicle reformation and recollection of vesicle proteins from the plasma membrane. It may also provide a mechanism for rapid clearance of vesicle membrane and proteins from active zones after exocytosis, which, as discussed below, may facilitate vesicle replenishment to the release sites and may help recover short-term synaptic depression (48–50). The key questions in understanding the importance of kiss-and-run are what promotes kiss-and-run and determines its pore size and why kiss-and-run is needed in physiological conditions. These questions remain largely unsettled (17, 34), although a variety of conditions in which kiss-and-run may occur have been implicated (11, 16, 20, 22, 24–29, 47, 51).
Compound Exocytosis
Compound exocytosis increases quantal size (Figure 2civ) and mediates a large part of posttetanic potentiation after repetitive firing at calyces (Figure 2d) (44, 52, 53). Compound exocytosis is likely a common mechanism to enhance synaptic strength, because (a) posttetanic potentiation is observed at many synapses during physiological stimulation (54, 55) and (b) large organelles (6, 56–59) accompanied by giant mEPSCs at the same synapse, which may be caused by compound exocytosis, are widely observed (60–63). Compound exocytosis may explain the increases in vesicle size, miniature potential, and synaptic strength observed after high natural crawling activities in Drosophila (64). Compound exocytosis also provides an alternative mechanism to explain the multivesicular release observed at many synapses (65–68).
ENDOCYTOSIS MODES: SUPPORTING EVIDENCE
We discuss here supporting evidence for classical endocytosis and bulk endocytosis. The supporting evidence for kiss-and-run is described above in the discussion of kiss-and-run exocytosis.
Classical Endocytosis
Classical endocytosis was first suggested on the basis of EM observations of endocytic-like intermediates, including shallow and deep membrane invaginations and coated and uncoated pits, in stimulated frog neuromuscular junctions (Figure 3ai) (6, 69). These intermediates are accumulated at nerve terminals lacking proteins involved in clathrin-dependent endocytosis, such as amphiphysin, endophilin, AP180, auxilin, and dynamin (reviewed in Reference 2). Furthermore, endocytosis is prolonged by knockdown of clathrin, AP2, and stonin 2; by pharmacological block of proteins involved in clathrin-dependent endocytosis; and by knockout of endophilin and auxilin (Figure 3aii) (33, 49, 50, 70–77). At pituitary nerve terminals and the calyx release face, capacitance down steps reflecting single-vesicle fission are not preceded within a few seconds by equal-sized up steps, suggesting classical, but not kiss-and-run, endocytosis (Figure 3aiii) (11, 20, 44). These results led to the widespread acceptance of clathrin-dependent classical endocytosis at secretory cells (2, 5, 17). Visualization of the dynamic structural changes underlying classical endocytosis in live cells would provide the key missing evidence.
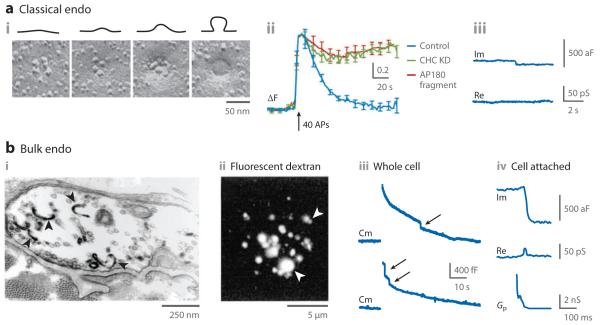
Figure 3.
Modes of endocytosis: supporting evidence. (a) Classical endocytosis. (i) Freeze-fracture electron microscopic (EM) images of frog neuromuscular junctions arranged in this order to illustrate the hypothetical endocytic process starting from a shallow pit to a vesicle-like Ω profile (see schematic drawings above the images). (ii) In cultured hippocampal neurons transfected with pHluorin-tagged synaptophysin, knockdown of clathrin heavy chain (CHC, green) or overexpression of the C-terminal fragment of AP180 (red) blocks endocytosis (control, blue). Here the pHluorin fluorescence increase and decrease indicate exocytosis and endocytosis, respectively. (iii) Cell-attached capacitance recordings (Im, imaginary; Re, real) from the release face of the calyx showing a capacitance down step. This down step may reflect classical endocytosis because the absence of a preceding up step indicates that it is not kiss-and-run and that its size is too small to be caused by bulk endocytosis. Panels i, ii, and iii are adapted with permission from References 69, 33, and 44, respectively. (b) Bulk endocytosis. (i) An EM image of a frog neuromuscular junction fixed after tetanic stimulation (1 min, 30 Hz) in the presence of dye (FM1-43). Large cisternae containing photoconverted dye (dark structures, black arrowheads) may be generated by bulk endocytosis. (ii) A fluorescence image of a goldfish retinal bipolar nerve terminal that has taken up tetramethylrhodamine-labeled 40-kDa dextran after intense stimulation. Large fluorescent spots (arrowheads) may be caused by bulk endocytosis. (iii) Whole-cell capacitance (Cm) changes at calyces induced by ten 20-ms depolarizing pulses at 10 Hz (applied right before the Cm jump). The Cm increase reflects exocytosis, whereas the Cm decrease indicates endocytosis. The downward Cm shifts (arrows), which are much larger than an averaged vesicle’s membrane capacitance (~0.07 fF), reflect bulk endocytosis. Two capacitance traces are shown to illustrate that bulk endocytosis may take place within a few seconds (lower) to 20 s (upper) after stimulation. (iv) Cell-attached capacitance recordings [Im, Re, fusion pore conductance (Gp)] of a large down step reflecting fission pore closure during bulk endocytosis at the release face of a calyx. Panels i, ii, iii, and iv are adapted with permission from References 56, 80, 87, and 44, respectively.
Bulk Endocytosis
EM examination after intense nerve stimulation reveals endosome-like structures or cisternae much larger than regular vesicles (Figure 3bi) (6, 44, 56–58, 78, 79). Some of these structures take up extracellular horseradish peroxidase or FM dyes, suggesting their endocytic origin (Figure 3bi). Imaging reveals large fluorescent spots that may correspond to endosome-like structures taking up extracellular dyes (Figure 3bii) (79–83). Endosome-like structures may be generated by bulk endocytosis or by vesicle-vesicle fusion. The first possibility is supported by EM observations that some endosome-like structures are deep invaginations of the plasma membrane, particularly in conditions that inhibit endocytosis (69, 78, 84–86), although the possibility that deep membrane invagination is a platform for fission of regular vesicles has not been excluded. The second possibility, vesicle-vesicle fusion, was proposed in the 1970s (6) and was recently proposed again on the basis of the observation that in vitro, endocytosed vesicles isolated from synaptosomes may fuse with each other or with endosomes isolated from PC12 cells (42). To determine whether bulk endocytosis occurs, large-vesicle pinch off needs to be resolved in live cells. Capacitance measurement offers an approach for detecting large-vesicle pinch off.
At calyces, whole-cell capacitance recordings at a millisecond time resolution reveal capacitance down steps of more than 20 fF (Figure 3biii) (87), much larger than a regular vesicle’s capacitance (~0.07 fF) (Figure 3aiii) (11, 88). Large down steps are accompanied by a fission pore conductance decrease reflecting a pore that closes from a diameter of ~3–19 nm at a rate of ~4 nm/100 ms (87). Thus, large down steps reflect bulk endocytosis (87). These large whole-cell down steps are also observed in pituitary cells containing dense-core vesicles and in nonneuronal cells (89, 90). One drawback of whole-cell recordings is that they can resolve only giant down steps corresponding to ~0.8–4-μm-diameter vesicles, which are much larger than 20–50-nm synaptic vesicles (87). This drawback can be overcome by cell-attached recordings, which reveal down steps of ~0.02–3 fF at the calyx release face, corresponding to ~20–330-nm vesicles (Figure 3biv). Some of these large cell-attached down steps are accompanied by a fission pore conductance decrease, reflecting rapid pore closure during bulk endocytosis (Figure 3biv) (44). Together, whole-cell and cell-attached capacitance measurements (44, 87) cover the size range of endosome-like structures (6, 56–58, 78, 80) and indicate the existence of bulk endocytosis, particularly during intense stimulation.
ROLES OF ENDOCYTOSIS
Maintenance of Exocytosis
The function of endocytosis is best demonstrated in the Drosophila mutant shibirets (where ts denotes temperature sensitive), in which inactivation of the GTPase dynamin at nonpermissive temperatures (e.g., 32°C) inhibits endocytosis, depletes vesicles, abolishes synaptic transmission (Figure 4a), and paralyzes the animal (78, 84, 91). Clearly, endocytosis maintains exocytosis via recycling vesicles to prevent vesicle exhaustion. Endocytosis may help recover synaptic transmission by maintaining the size of nerve terminals (92).
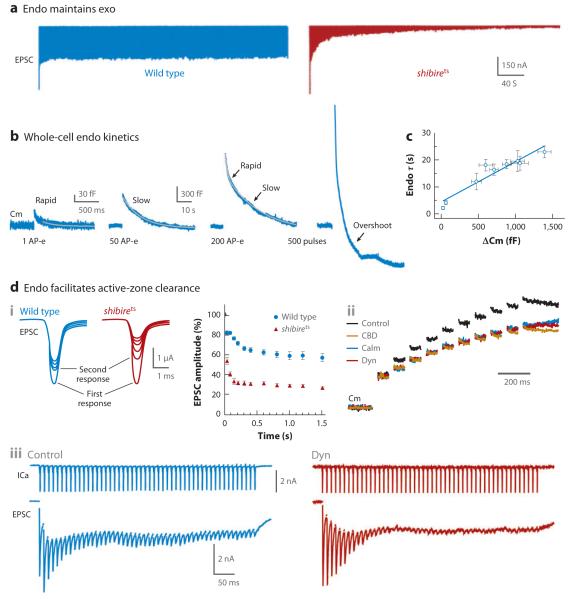
Figure 4.
Roles of endocytosis. (a) Maintenance of exocytosis. Synaptic currents induced by repetitive 10-Hz stimulation at Drosophila neuromuscular junctions at a nonpermissive temperature (32°C) in wild type (left) and in a shibire temperature-sensitive mutant (shibirets) (right). Block of endocytosis in the shibirets mutant causes gradual decline of synaptic currents toward 0. EPSC denotes excitatory postsynaptic current. Adapted with permission from Reference 91. (b) Activity-dependent changes of endocytosis kinetics observed with whole-cell capacitance (Cm) recordings at calyces. A single action potential–equivalent stimulus (AP-e) (1-ms depolarization) causes rapid endocytosis; 50 AP-e at 100 Hz cause slow endocytosis; 200 AP-e at 100 Hz cause both rapid (τ1 1.8 s) and slow (τ2 = 24.0 s) endocytosis; and 500 depolarizing pulses (0.5-ms depolarization) at 200 Hz cause endocytosis overshoot. = Different endocytosis kinetics may be due to different modes of endocytosis (see text for discussion). Adapted from References 98 and 49. (c) Endocytosis time constant versus endocytosis amplitude at calyces after stimuli ranging from mild to intense (see Reference 98 for details about the stimuli). The data were fit with a linear regression line with a slope of 1.4 s/100 fF. When endocytosis is biexponential, as induced by more intense stimuli, only the slow component, and not the rapid component, of endocytosis is included in the plot. Adapted from Reference 98. (d) Endocytosis facilitates active-zone clearance and thus vesicle replenishment to the readily releasable pool (RRP). (i) Sample (left; lines indicate first and second responses) and mean (right) synaptic current changes during 50-Hz stimulation in wild-type and shibirets mutant Drosophila neuromuscular junctions at a nonpermissive temperature (33°C). More severe short-term depression is observed within 20–40 ms of stimulation in shibirets mutants. (ii) Blocking endocytosis inhibits rapid vesicle replenishment of the RRP at calyces. RRP replenishment is monitored as capacitance jumps induced by ten 20-ms depolarizing pulses at 10 Hz; each pulse depletes the RRP. Compared with control, endocytosis blockers, including CBD (calmodulin binding-domain peptide, a calmodulin inhibitor), Calm (calmidazolium, a calmodulin inhibitor), and Dyn (dynasore, a dynamin inhibitor), reduce capacitance increases and thus RRP replenishment. The capacitance traces are normalized to the jump induced by the first 20-ms depolarization. (iii) Short-term depression of EPSCs (lower) during a train of action potential–like stimuli (1.5-ms depolarization, 50 stimuli at 100 Hz) is enhanced by Dyn at the calyx. Dyn does not affect calcium current (ICa) (upper). Panels i, ii, and iii are adapted with permission from References 48, 49, and 50, respectively.
Contribution of Each Endocytosis Mode to Whole-Cell Endocytosis
Endocytosis is often evaluated as the averaged behavior of the entire cell or many boutons. The amplitude and time course of this behavior may depend on modes of endocytosis. We first introduce various averaged endocytosis behaviors, termed whole-cell endocytosis, and then discuss how different modes of endocytosis contribute to these behaviors.
Whole-cell endocytosis: rapid, slow, and overshoot
The kinetics of whole-cell endocytosis is highly plastic and depends on the stimulation intensity (Figure 4b) (49, 66, 70, 88, 93–99). The calyx of Held shows a typical example. At the calyx (Figure 4b), a mild stimulus, such as an action potential–like (APlike) stimulus, induces a low level of exocytosis and rapid endocytosis with a time constant (τ) of ~1–3 s (88, 98). An intermediate stimulus intensity, such as a short APlike train or a 20-ms depolarization, induces endocytosis with a τ of ~10–30 s and bulk endocytosis (49, 66, 88, 98, 100). An intense stimulus, such as a prolonged, high-frequency APlike train or ten pulses of 20-ms depolarization at 10 Hz, reactivates rapid endocytosis together with slow endocytosis and bulk endocytosis (49, 98). Finally, a very intense stimulation, such as a prolonged APlike train at very high frequency or ten pulses of 50-ms depolarization at 10 Hz, induces endocytosis overshoot that retrieves more vesicles than the exocytosed amount, together with rapid, slow, and bulk endocytosis (49, 100, 101).
Analogous to changes from rapid to slow endocytosis as the stimulus intensity changes from mild to intermediate at calyces (Figure 4b, left two traces; Figure 4c), increasing the stimulation intensity increases exocytosis and prolongs the endocytosis τ at frog neuromuscular junctions, hippocampal synapses, goldfish retinal bipolar cells, and chromaffin cells (70, 93, 96, 97, 102). Such a rule does not apply to intense stimuli that trigger the reappearance of rapid endocytosis (Figure 4b, right two traces). However, if only the slow component of endocytosis induced by intense stimuli is included for analysis, with endocytosis induced by mild and intermediate stimuli, the endocytosis τ increases linearly with the exocytosis amount (Figure 4c) (98). This phenomenon is attributed to saturation of the endocytic capacity (97, 99) or to the inhibition of endocytosis by a global calcium increase (103).
Similar to the reappearance of rapid endocytosis after intense stimulation at calyces, rapid endocytosis in hair cells is more often observed at higher calcium concentration (>15 μM) increases induced by calcium uncaging (104). Akin to the overshoot observed during a very intense stimulus at calyces, prolonged depolarizing pulses or high calcium increases induce endocytosis overshoot in chromaffin cells (95) and in pituitary cells (105).
Different whole-cell behaviors due to different endocytosis modes
Classical endocytosis may mediate whole-cell slow endocytosis because slow endocytosis is inhibited by perturbation of proteins involved in clathrin-dependent endocytosis (Figure 3aii) (33, 49, 50, 70–77). Cavicapture, a slow form of kiss-and-run, contributes to slow endocytosis to a minor extent at chromaffin cells (14). Bulk endocytosis, which takes a few to tens of seconds, may also contribute to slow endocytosis (Figure 3biii) (87). In brief, all three endocytosis modes may contribute to slow endocytosis, but classical endocytosis is likely the dominant mode, given that inhibition of clathrin-dependent endocytosis largely inhibits slow endocytosis (Figure 3aii) (33, 70–75). Because slow endocytosis is observed during most stimulation conditions at nearly all secretory cells examined (Figure 4b) (5, 70, 72, 106, 107), classical endocytosis is generally considered to be the most important endocytosis mode.
Rapid kiss-and-run is an obvious candidate for mediating whole-cell rapid endocytosis (51). However, bulk endocytosis is also a candidate because large capacitance down steps reflecting bulk endocytosis may occur within a few seconds after stimulation (Figure 3biii) (87). Furthermore, strong stimulation, which induces a larger component of rapid endocytosis, also induces more capacitance down steps reflecting bulk endocytosis (49, 87) and generates large organelles and large fluorescent spots at neuromuscular junctions and central synapses within seconds of stimulation (69, 81, 82, 108). Despite classical endocytosis being slower, individual vesicle endocytosis is stochastic and can be fast in a fraction of vesicles, raising the possibility that classical endocytosis contributes to rapid endocytosis (31). Consistent with this possibility, a retrievable vesicle pool at hippocampal synapses and calyces (101, 109–111), which likely correspond to preformed endocytic structures at the plasma membrane, speeds up endocytosis, likely by bypassing membrane invagination (101). Although the relative contribution of each mode to rapid endocytosis remains to be determined, classical endocytosis is unlikely to be the main mechanism, because rapid endocytosis induced by strong stimulation (e.g., Figure 4b) is much larger than predicted from a stochastic process (49, 98) and inhibition of clathrin-dependent endocytosis does not block rapid endocytosis (70, 71). Rapid endocytosis is therefore likely mediated largely by kiss-and-run and/or by bulk endocytosis.
Endocytosis overshoot may retrieve fused vesicles stranded at the plasma membrane (49), which may correspond to a retrievable vesicle pool or to preformed endocytic structures (109–111). Like rapid and slow endocytosis, inhibition of calcium influx, calmodulin, calcineurin, and dynamin blocks endocytosis overshoot at calyces, suggesting a similar underlying mechanism between endocytosis overshoot and compensatory endocytosis (101). Which mode of endocytosis contributes most to endocytosis overshoot remains to be determined.
In summary, the studies discussed above lead to a working model in which (a) classical endocytosis is a major endocytosis mode for slow endocytosis in most conditions; (b) bulk endocytosis, as activated during intermediate to strong stimulation intensity, may contribute to rapid and slow endocytosis to enhance endocytosis capacity; and (c) kiss-and-run may contribute to rapid endocytosis. Although the quantitative contribution of each mode to each whole-cell form of endocytosis remains largely unclear, it can be determined with two approaches in the future. The first is to record an individual vesicle’s endocytosis mode, to reconstruct overall endocytosis from individual vesicle endocytosis, and to compare reconstructed endocytosis with simultaneously recorded whole-cell endocytosis. The second is to understand the molecular mechanisms of each mode, to develop specific tools to block each mode, and to determine the outcome of the block.
Roles for High-Speed Endocytosis
Kiss-and-run may be dominant during the first few action potentials at low-frequency firing and may recycle vesicles within the readily releasable pool (RRP) in seconds to tens of seconds at hippocampal synapses (16, 17). However, other studies argue against a major contribution of kiss-and-run to overall endocytosis (30–32, 34). A recent study at calyces shows that both whole-cell rapid endocytosis and slow endocytosis, which include kiss-and-run if it contributes to whole-cell endocytosis, do not recycle vesicles within the RRP (92).
If not for recycling within the RRP, then why do cells need rapid endocytosis? There are at least two reasons. First, rapid endocytosis, as activated by intense stimulation, recycles vesicles in a recycling pool beyond the RRP to prevent vesicle exhaustion (92). Second, rapid endocytosis may rapidly restore the structure of release sites during intense stimulation that adds many vesicle membranes and proteins to release sites (92). As described below, clearance of the release site by endocytosis facilitates vesicle replenishment to the RRP.
Endocytosis Clears Active Zones to Facilitate Readily Releasable Pool Replenishment and Recovery of Synaptic Depression
In addition to recycling vesicles, endocytosis may facilitate vesicle replenishment to the RRP, likely by clearance of exocytotic materials from active zones. This new role may lead to reinterpretation of the mechanisms underlying RRP replenishment, which is thought to involve only exocytic mechanisms in counteracting short-term depression during repetitive firing.
Supporting evidence for active-zone clearance
Dynamin defect or clathrin knockdown in Drosophila neuromuscular junctions increases short-term synaptic depression during the first 20–40 ms of repetitive stimuli at 50 Hz (Figure 4di) (48, 112). This event seems to occur too quickly to be caused by an effect on vesicle recycling (48). Accordingly, investigators proposed that endocytosis facilitates vesicle replenishment to the RRP (48). However, alternative explanations, such as blocking kiss-and-run that recycles vesicles within 20–40 ms, a reduced RRP size that causes more depletion of the RRP (113, 114), and calcium current inactivation (115) that causes more short-term depression, have not been excluded. These possibilities can be distinguished at the calyx. At this large nerve terminal, replenishment of the RRP after a 20-ms depolarization that depletes the RRP is biexponential, with τ of ~0.3 and ~5–10 s, respectively (92, 113, 114). In contrast, endocytosis τ is ~10–20 s after a 20-ms depolarization (66, 88, 98, 100), which is clearly slower and thus not responsible for RRP replenishment. Furthermore, activation of rapid endocytosis does not speed up RRP replenishment, and block of both rapid and slow endocytosis by GTPγS does not affect RRP replenishment (92). Thus, RRP replenishment is not due to recycling directly from rapidly or slowly endocytosed vesicles (92). However, RRP replenishment is inhibited by calmodulin, dynamin, and AP2 blockers, all of which inhibit endocytosis without affecting the RRP size or the calcium current (Figure 4dii,iii) (49, 50). These results suggest that endocytosis facilitates RRP replenishment through clearance of the active zone, by removing exocytosed vesicle membranes and proteins that may prevent vesicle docking and priming (49, 50).
Active-zone clearance must take place before fission because RRP replenishment is faster than vesicle retrieval. This fast action may explain why GTPγS, which blocks endocytosis by affecting the final GTP-dependent fission process, does not affect RRP replenishment (92), whereas other dynamin inhibitors affect RRP replenishment, likely because dynamin is involved in active-zone clearance in addition to the final fission step (49, 50).
Reinterpreting calmodulin-dependent RRP replenishment
Active-zone clearance offers a new explanation for calcium/calmodulin-mediated facilitation of RRP replenishment (114, 116–118). Similar to the case of dynamin and AP2 inhibitors, blocking calcium influx or calmodulin inhibits endocytosis (for details, see Figures 5–7 below) and RRP replenishment (Figure 4dii) (49). Given that calcium-dependent RRP replenishment is critical in recovering from short-term depression, a widely observed form of synaptic plasticity involved in neuronal circuit functions (119), endocytosis may play an important role in short-term depression and circuit information processing (Figure 4d).
Figure 5.
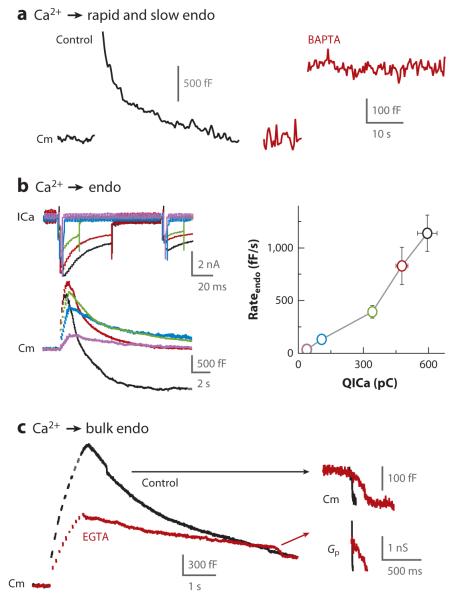
Calcium influx triggers endocytosis and controls the endocytosis rate. (a) Compared with control (left), the fast calcium buffer BAPTA (10 mM in the pipette) (right) abolishes endocytosis, as recorded with capacitance measurements from calyces. The stimulus is twenty 20-ms depolarizing pulses at 10 Hz. (b) Sample (left) and summary (right) data showing the steep dependence of the endocytosis rate (Cm decay rate) on calcium current charge (QICa) induced by 2-ms (purple), 5-ms (blue), 20-ms (green), and 50-ms (red) depolarizing pulses (10 times) at 10 Hz in 2 mM extracellular calcium and ten 50-ms depolarizing pulses at 10 Hz in 5.5 mM calcium (black). For sample ICa (left), only the response to the first depolarization and a fraction of the second response (out of ten) are shown. (c) Sample Cm in control (black) and in the presence of a slow calcium buffer, EGTA (70 mM in the pipette) (red). EGTA significantly slows down fission of bulk endocytosis (arrows; large downward capacitance shifts shown in larger scale with accompanying Gp changes). The stimulus is ten 50-ms depolarization pulses at 10 Hz in 5.5 mM extracellular calcium. Panels a, b, and c are adapted from Reference 49.
Figure 7.
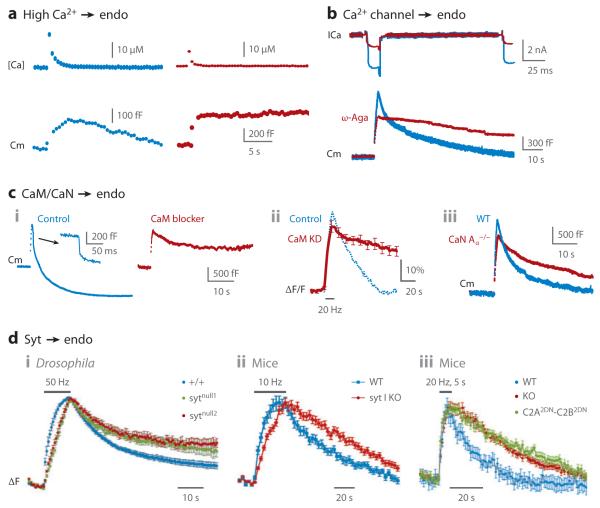
Large calcium influx via voltage-gated calcium channels triggers endocytosis by activating calmodulin (CaM), calcineurin (CaN), and synaptotagmin (syt). (a) A high calcium increase is needed to initiate endocytosis. Endocytosis (Cm decay; lower) is observed after high (left, blue) but not low (right, red) calcium concentration increase induced by uncaging of a caged calcium compound in the calyx (upper). (b) P/Q-type calcium channels are involved in triggering endocytosis. Sampled ICa and Cm induced by ten 10-ms depolarizing pulses at 10 Hz in control (blue) and in the presence of the P/Q-type calcium channel blocker ω-agatoxin-IVA (ω-Aga; red) (200 nM), which reduces ICa and blocks endocytosis. For ICa (upper), only the response to the first depolarization and a fraction of the second response (out of 10) are shown. Panel a is adapted with permission from Reference 50, and panel b is from Reference 129. (c) CaM and CaN in endocytosis. (i) Cm traces from calyces show inhibition of rapid, slow, and bulk endocytosis and endocytosis overshoot by calmidazolium (red), a CaM blocker. The control (blue), stimulated to the same extent (ten 50-ms depolarizing pulses at 10 Hz with 5.5 mM extracellular calcium), shows rapid, slow, and bulk endocytosis (arrow, enlarged trace) and endocytosis overshoot. (ii) SynaptopHluorin fluorescence (ΔF/F) imaging shows inhibition of slow endocytosis by CaM knockdown (KD) (red) at cultured hippocampal synapses (control in blue). The stimulus is a 10-s action potential train at 20 Hz. (iii) CaN Aα knockout inhibits both rapid endocytosis and slow endocytosis as recorded with Cm in calyces (red). The stimulus is ten 20-ms depolarizing pulses at 10 Hz, which induce both rapid endocytosis and slow endocytosis in wild-type mouse (WT) (blue). Panel i is adapted from Reference 49; panels ii and iii, from Reference 130. (d) Syt in endocytosis. Endocytosis is prolonged by deletion of syt in Drosophila neuromuscular junctions (i), by deletion of syt 1 (KO) at cultured cortical synapses (ii,iii), and by mutation of the C2A and C2B domains (calcium-binding domains) of syt 1 at cultured hippocampal synapses (iii). Panels i, ii, and iii are adapted with permission from References 154, 153, and 155, respectively.
SNARE involvement in RRP replenishment
A recent study shows that cleavage of two SNARE proteins critical for exocytosis, SNAP25 and syntaxin, inhibits endocytosis (see Figure 8 below) and RRP replenishment at the calyx, suggesting the involvement of SNARE proteins in both endocytosis and RRP replenishment (120). Given the role of endocytosis in active-zone clearance, SNAP25 and syntaxin may facilitate RRP replenishment via active-zone clearance in addition to the well-characterized interaction between vesicular and membrane-targeted SNAREs (120).
Figure 8.
Exocytosis and SNARE proteins are involved in initiating endocytosis. (a) Exocytosis is needed for endocytosis initiation. ICa and Cm of a calyx at 2 min (blue) and 14 min (red) after break-in with a pipette containing botulinum neurotoxin C (BoNT/C). The stimulus is ten 20-ms depolarizing pulses applied at 10 Hz. The red traces denote no exo- or endocytosis. Adapted from Reference 98. (b) SNAREs (synaptobrevin, SNAP25, and syntaxin) in endocytosis. (i) Cm traces at calyces in control (blue) and in the presence of tetanus toxin (TeTx) (red), showing inhibition of slow endocytosis by TeTx that cleaves synaptobrevin. The stimulus is twenty 1-ms depolarizing pulses at 100 Hz. (ii) Cm traces from calyces in control (blue) and in the presence of TeTx, BoNT/E (cleaves SNAP25), or BoNT/C (cleaves syntaxin), showing inhibition of rapid and slow endocytosis by toxins (red). The stimulus (ten 20-ms depolarizing pulses at 10 Hz) induces rapid and slow endocytosis in control (blue). (iii) Synaptophysin-pHluorin fluorescence changes (ΔF, normalized) in control (blue) and in synaptobrevin (Syb) or SNAP25 knockdown (KD) (red), showing a slowdown of endocytosis due to knockdown in cultured hippocampal synapses. The stimulus is a 10-s train at 20 Hz. Panels i, ii, and iii are adapted with permission from References 50, 120, and 160, respectively.
COUPLING BETWEEN EXO- AND ENDOCYTOSIS
Mode Coupling: A Vesicle Size Match
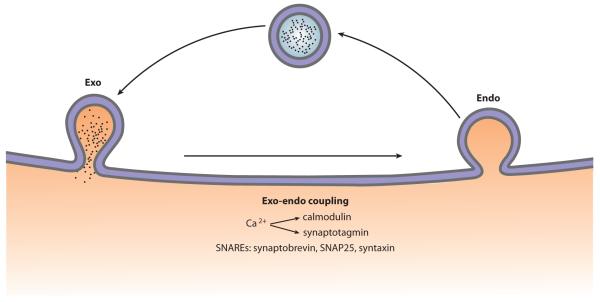
Full-collapse fusion is generally thought to be followed by classical endocytosis that forms a vesicle with a size similar to that of the exocytosed one. This hypothesis is supported by the observations of similar-sized endocytosed and exocytosed vesicles (6, 8, 69) and similar capacitance up and down steps (Figures 2aiii and 3aiii) (11, 20). Kiss-and-run retrieves the same exocytosed vesicle. Compound exocytosis is suggested to couple to bulk endocytosis because (a) large capacitance up steps reflecting compound exocytosis are followed by large down steps reflecting bulk endocytosis (44) and (b) both compound exocytosis and bulk endocytosis are more frequently observed during intense stimulation (6, 39, 43, 44, 56–58, 78). However, the possibility that bulk endocytosis also follows full-collapse fusion of regular-sized vesicles has not been excluded. In summary, there are at least three size-matched exo-endocytosis couplings: (a) full-collapse fusion and classical endocytosis, (b) kiss-and-run, and (c) compound exocytosis and bulk endocytosis (Figure 1).
Tight Exo-Endocytosis Coupling at the Whole-Cell Level
At the whole-cell level, exocytosis is followed by endocytosis, which retrieves a similar amount of exocytosed vesicle membrane and protein within a few seconds to a few minutes (Figures 3aii and 4b) (5, 95). Such a tight exo-endocytosis coupling in amount and timing, often referred to as compensatory endocytosis (95, 121), maintains exocytosis and prevents cells from swelling or shrinking. One apparent exception is endocytosis overshoot. However, endocytosis overshoot may retrieve fused vesicles stranded at the plasma membrane (49) and may thus reflect coupling between early exocytosis and endocytosis.
What mediates exo-endocytosis coupling, controls endocytosis timing, and matches the amount of exo-endocytosis? Presynaptic scaffold proteins have been speculated to be involved (121). Recent studies, as discussed in the following two sections, suggest that calcium influx may activate calmodulin and synaptotagmin to initiate endocytosis and control endocytosis timing, whereas exocytosis, particularly the SNARE proteins that mediate exocytosis, may be involved in matching the exo-endocytosis amount.
CALCIUM INFLUX VIA VOLTAGE-GATED CHANNELS TRIGGERS ALL FORMS OF ENDOCYTOSIS BY ACTIVATING CALMODULIN AND SYNAPTOTAGMIN
Early Studies on Calcium and Endocytosis
Early studies at frog and Drosophila neuromuscular junctions found that after calcium-independent release induced by the neurotoxin black widow spider venom, horseradish peroxidase or FM dye can be taken up into vesicles, and this dye uptake is reduced when the extracellular calcium is removed (122–124). Because voltage-dependent calcium channels (VDCCs) are not activated, this result suggests that extracellular calcium regulates endocytosis (122–124). However, dye uptake was measured after calcium-independent exocytosis. It is unclear whether this result applies to endocytosis that follows physiological calcium-dependent exocytosis. In synaptosomes preloaded with FM dye in the presence of extracellular barium, FM dye release is less than that in synaptosomes preloaded with FM dye in the presence of extracellular calcium, implying that extracellular calcium may regulate a step in vesicle cycling, such as endocytosis, vesicle mobilization to the RRP, or vesicle release probability (125, 126). To determine whether intracellular calcium regulates endocytosis, 0.5–1 μM calcium was dialyzed into goldfish retinal nerve terminals, which inhibited endocytosis (93). However, subsequent studies show that calcium influx facilitates endocytosis. At hippocampal synapses, increasing the extracellular calcium and thus calcium influx during action potentials accelerates slow endocytosis induced by action potentials (99, 127). At retinal nerve terminals and calyces, dialysis of the calcium buffer EGTA slows down rapid endocytosis (94, 98). In hair cells, rapid endocytosis is more often observed at higher calcium concentration increases induced by calcium uncaging (104). At chromaffin cells, replacing extracellular calcium with barium blocks rapid endocytosis (70).
The above studies raise two questions. First, does calcium influx regulate or trigger endocytosis? Second, is calcium influx the universal trigger of all forms of endocytosis? The difference between regulation and trigger is best demonstrated in studies of exocytosis: Calcium influx triggers exocytosis by increasing the exocytosis rate by several orders of magnitude during action potentials, whereas residual calcium after action potentials regulates exocytosis during subsequent action potentials by increasing the exocytosis rate severalfold (128). Although results from most studies suggest that calcium regulates endocytosis rate by only ~1–3 fold, a triggering role cannot be excluded, because these studies did not reduce calcium influx by a large extent while maintaining sufficient exocytosis for reliable endocytosis measurements. Furthermore, EM or FM dye imaging techniques used in early studies do not provide sufficiently quantitative endocytosis rates. These experimental difficulties can be overcome at calyces, where (a) up to ~20,000 vesicles can be released within 1 s, allowing for reliable quantification of subsequent endocytosis at millisecond resolution with whole-cell capacitance measurements (66, 98), and (b) a wide range of calcium currents can be induced and quantified while sufficient exocytosis is maintained for subsequent endocytosis measurement (49, 129).
Calcium Influx Triggers All Forms of Endocytosis at Secretory Cells
Five sets of evidence suggest that calcium influx triggers all forms of endocytosis detected with whole-cell recordings at calyces, including slow, rapid, and bulk endocytosis and endocytosis overshoot. First, decreasing the extracellular calcium from 5 to 0.25 mM decreases the calcium current and the slow endocytosis rate by ~50 fold (49). Second, 10 mM BAPTA, a fast calcium buffer, re duces the endocytosis rate by ~1,000–1,500 fold (Figure 5a) (49), and 1 mM BAPTA blocks slow endocytosis induced by APlike trains (50). Third, as the calcium current charge increases, the initial endocytosis rate increases by several orders of magnitude. For example, at near resting condition with 0.5–0.75 μM calcium in calyces, the endocytosis τ is ~600 s (49); ten 2-ms depolarization pulses at 10 Hz induce endocytosis with a τ of ~15 s; and ten 50-ms depolarization pulses at 10 Hz induce a larger calcium current charge and an endocytosis τ of ~1 s (Figure 5b) (49). Thus, endocytosis τ may decrease by ~600 fold, depending on the calcium influx. These three sets of evidence suggest that calcium influx triggers rapid and slow endocytosis. Fourth, strong stimulation increases the frequency of large capacitance down steps reflecting bulk endocytosis by more than 10 fold (87). The frequency increase depends on the calcium current charge. EGTA (70 mM) nearly abolishes this increase and slows down the rate of fission pore closure from ~100 nm/s to ~ 10 nm/s, an estimate from fission pore conductance measurements (Figure 5c) (49). These results suggest that calcium influx triggers bulk endocytosis (49, 87). Fifth, increasing the extracellular calcium from 1.3 to 10 mM increases the calcium current charge and the frequency of endocytosis overshoot induced by a strong stimulus from 0 to 100%, whereas 10–70 mM EGTA abolishes endocytosis overshoot, suggesting that calcium influx triggers endocytosis overshoot (49, 101).
Reducing or buffering calcium influx abolishes endocytosis and also reduces exocytosis (e.g., Figure 5a). Would the reduced exocytosis abolish endocytosis? The answer is no, as several studies show (49, 98, 129). Three sets of supporting evidence are described here.
First, except for the rapid component of endocytosis induced by intense stimuli, endocytosis τ increases as the exocytosis amount increases in control conditions (Figure 4c) (70, 93, 96–98, 102). Thus, a reduced exocytosis amount (e.g., Figure 5a) may shorten endocytosis τ but not prolong or block endocytosis.
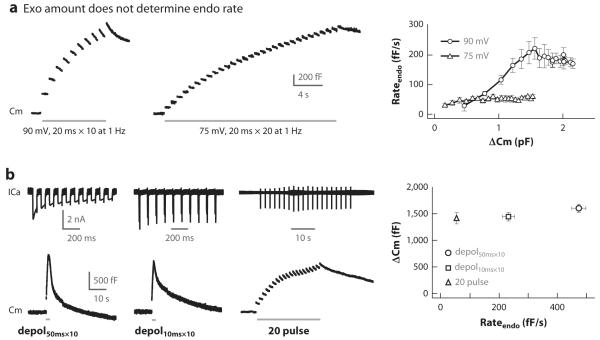
Second, the appearance of rapid endocytosis upon stronger calcium influx is independent of the exocytosis amount. For example, the initial rate of endocytosis induced by a 20-ms depolarization from −80 mV to +10 mV increases as the pulse is repeated at 1 Hz to activate rapid endocytosis (Figure 6a) (98). However, the increase in endocytosis rate reaches a plateau as depolarization is repeated five to six times while the capacitance that reflects exocytosis continues to increase (Figure 6a). In contrast, when a 20-ms depolarization from −80 mV to −5 mV, which induces a much smaller calcium current than the depolarization to +10 mV, is repeated at 1 Hz, the initial endocytosis rate barely increases as exocytosis increases to a similar amount as for repeated depolarization to +10 mV (Figure 6a) (98). Thus, increasing exocytosis does not increase endocytosis rate. Rather, manipulation of calcium current charge through varying depolarization affects endocytosis rate (Figure 6a) (98). Similarly, three different stimuli induce similar capacitance increases but different initial endocytosis rates (by ~10 times) at calyces, owing to the differences in calcium current charge, amplitude, and/or frequency of stimulation (Figure 6b) (129).
Figure 6.
Increasing the exocytosis amount does not speed up endocytosis. (a) (Left and middle) Sampled Cm induced by either ten 20-ms depolarizing pulses from −80 to +10 mV (left, 90-mV depolarization) or thirty pulses to −5 mV (middle, 75-mV depolarization, inducing smaller calcium currents) at 1 Hz. (Right) The initial capacitance decay rate that reflects endocytosis rate (Rateendo) after each 20-ms depolarization during the two stimuli mentioned above (n = 4–7 calyces). (b) (Left three panels) Sampled calcium currents (ICa) and Cm induced by 10 pulses of 50-ms depolarization to +10 mV at 10 Hz (left, depol50ms × 10, large ICa), 10 pulses of 10-ms depolarization to +10 mV at 10 Hz (middle, depol10ms × 10, large ICa but smaller ICa charge), and 20 pulses of 20-ms depolarization to −5 mV at 0.5 Hz (right, 20 pulse, small ICa and low frequency). (Right) The ΔCm (mean ± SE, n = 12–18 calyces) is plotted versus the endocytosis rate after depol50ms × 10 (circle), depol10ms × 10 (square), and the 20-pulse train (triangle). Panels a and b are adapted from References 98 and 129, respectively.
Third, reducing calcium currents or application of 70 mM EGTA reduces the rate of bulk endocytosis, even when the reduction of exocytosis is taken into account (49). EGTA at 10 mM abolishes endocytosis overshoot in conditions of comparable capacitance jump (49). Taken together, the findings indicate that decreasing the exocytosis amount alone does not slow down endocytosis.
As the calcium current charge increases, first slow endocytosis is observed, followed by bulk endocytosis, rapid endocytosis, and finally endocytosis overshoot, suggesting that the threshold calcium concentration for triggering endocytosis ranges from low to high for slow, bulk, and rapid endocytosis and endocytosis overshoot (49, 87). One exception to this rule is that very mild stimulation also triggers rapid endocytosis (Figure 4b) (93, 96, 98). Saturation of the endocytic capacity or inhibition of endocytosis by residual calcium increase may explain why endocytosis slows down as the stimulus intensity increases from mild to intermediate (93, 97, 103).
The finding that calcium influx triggers all forms of whole-cell endocytosis at calyces is consistent with the observation that calcium influx triggers rapid endocytosis at chromaffin cells (70) and slow endocytosis at hippocampal synapses (130). This finding may account for the previous observation that calcium upregulates rapid and slow endocytosis at hippocampal synapses (99, 127), retinal nerve terminals (94), the calyx of Held (98), and hair cells (104). It explains why more intense stimulation, which generates higher calcium influx, induces more endosome-like structures that may be caused by bulk endocytosis at various synapses (6, 44, 56–58, 78, 79, 131) and induces endocytosis overshoot at chromaffin cells and pituitary cells (95, 105). Accordingly, calcium influx is suggested as the universal trigger for all forms of vesicle endocytosis in secretory cells in which calcium triggers exocytosis (49).
Micro/Nanodomain Calcium Coupling of Exo- and Endocytosis
Three sets of evidence suggest that more than a few micromolars of calcium at the calcium micro/nanodomain are needed to trigger slow endocytosis. First, endocytosis at 0.5–0.75 μM calcium is extremely slow, with a τ of ~600 s (49). Second, EGTA slows down endocytosis (Figure 5c), whereas BAPTA, a faster calcium buffer, abolishes endocytosis (Figure 5a) (49, 50, 132). This result suggests that, analogous to the sensitivity of exocytosis to BAPTA and EGTA (133), calcium influx triggers not only exocytosis but also endocytosis at the microdomain near calcium channels (49, 50, 132), whose calcium concentration is more than 10 μM (134, 135). As the calyx matures, the coupling between calcium and exocytosis and that between calcium and endocytosis change from the microdomain to the nanodomain (132, 136). Third, photolysis of a caged calcium induces endocytosis when the calcium concentration is more than ~10 μM (Figure 7a) (50). These results suggest that the endocytosis trigger takes place at or near the release site, challenging a seemingly established principle that endocytosis takes place at the periactive zone (6, 69). Consistent with this suggestion, FM dye uptake at snake neuromuscular junctions is near the active zone (86), a retrievable vesicle pool at the plasma membrane of hippocampal boutons was implied to be within ~100–300 nm of the active zone (111), clathrin may be located at Drosophila active zones (112), and endocytic sites are within 500 nm of exocytic sites in PC12 cells (137).
Transient, But Not Prolonged, Calcium Increase Triggers Endocytosis
Increasing the duration of APlike trains or depolarization trains prolongs endocytosis at goldfish retinal bipolar nerve terminals (93), frog neuromuscular junctions (102), hippocampal synapses (97, 99), calyces (88, 98, 100, 138), and chromaffin cells (51). For three reasons, these observations are not in conflict with the finding that calcium influx at the micro/nanodomain triggers endocytosis. First, calcium from the micro/nanodomain dissipates within milliseconds after action potentials, whereas action potentials are often repeated at an interval of ~50 ms or longer. Thus, repetitive action potentials at low to intermediate frequencies may not substantially increase the transient calcium concentration at the micro/nanodomain (128). Second, repeated stimulation causes more exocytosis, which may saturate the endocytic capacity, resulting in apparently slower endocytosis (97, 99). Third, repeated stimulation increases global calcium concentration to the submicromolar range for a prolonged period of time (102, 113). Prolonged dialysis of calcium at 1 μM or more in goldfish retinal nerve terminals and chromaffin cells inhibits endocytosis (51, 93). Thus, transient, but not prolonged, calcium increase triggers endocytosis.
Voltage-Dependent Calcium Channels at the Plasma Membrane Mediate Calcium Influx to Trigger Endocytosis
What type of calcium channel mediates calcium influx to trigger endocytosis? In a recent study at Drosophila synapses, researchers proposed that the calcium channel is Flower, a vesicular membrane protein conserved from Drosophila to humans (139). Upon vesicle fusion, Flower is thought to transfer from vesicles to the plasma membrane to mediate calcium influx (139). An earlier study of sea urchin eggs suggests that upon exocytosis, VDCCs are translocated from the vesicular membrane to the plasma membrane to mediate the calcium influx needed for endocytosis (140). These studies led to the hypothesis that vesicular calcium channels conserved from sea urchins to nerve terminals mediate exo-endocytosis coupling (141). However, Flower insertion increases calcium to the submicromolar range at Drosophila nerve terminals after prolonged tetanic stimulation (139), which may inhibit endocytosis (93) but is insufficient to trigger endocytosis (49, 50, 142). Calcium increase via Flower channels takes ~10–60 min (139), which is too slow to trigger endocytosis that typically lasts for tens of seconds in secretory cells (Figure 4b), including Drosophila synapses (5, 106, 143). Compared with the complete block of endocytosis by BAPTA (Figure 5a) (49, 50, 132), loss of Flower impairs endocytosis by only ~40% (139). Thus, Flower may play a regulatory role but not a triggering role in endocytosis.
At calyces at which both VDCCs and Flower are present, the fusion of thousands of vesicles at the plasma membrane does not generate any calcium current or influx, indicating that vesicles do not contain VDCCs and that Flower does not generate calcium influx to trigger endocytosis (129). The rate of endocytosis depends on calcium influx via VDCCs (Figure 5b) (49), including P/Q-type VDCCs (Figure 7b), at the plasma membrane, but not on the amount of exocytosed vesicles (Figure 6b) (129). These results suggest that calcium influx via VDCCs, including P/Q-type VDCCs, at the plasma membrane triggers endocytosis (49, 129). Consistent with this suggestion, blocking P/Q- and L-type calcium channels inhibits endocytosis in chromaffin cells (144). Given that calcium influx may trigger all forms of endocytosis at secretory cells in which calcium influx through VDCCs triggers exocytosis, we suggest that VDCCs at the plasma membrane trigger endocytosis at neuronal nerve terminals and in nonneuronal secretory cells.
Calcium Sensors for Endocytosis
Recent studies show that calmodulin, a calcium-binding protein, is involved in rapid, slow, and bulk endocytosis and in endocytosis overshoot, whereas synaptotagmin, another calcium-binding protein, is involved in slow endocytosis. Thus, calmodulin and synaptotagmin may act as calcium sensors for endocytosis.
Calmodulin
Various calmodulin inhibitors block rapid, slow, and bulk endocytosis and endocytosis overshoot at calyces (Figure 7ci) (49). They also block rapid endocytosis in chromaffin cells (107). Knockdown of calmodulin inhibits slow endocytosis at cultured hippocampal synapses (Figure 7cii) (130). These results suggest that calmodulin is the calcium sensor for all forms of endocytosis (49).
Calcineurin
One of the downstream targets of calmodulin is calcineurin, which may dephosphorylate many endocytic proteins (145). Calcineurin is composed of a catalytic A subunit and a regulatory B subunit. Among the three isoforms of the A subunit, calcineurin Aα and Aβ are expressed in the brain. Knockout of calcineurin Aα inhibits rapid and slow endocytosis at calyces (Figure 7ciii), whereas knockout of calcineurin Aβ inhibits slow endocytosis at hippocampal synapses (130). These results suggest the involvement of calcineurin in both rapid and slow endocytosis (130). The downstream target for calcineurin may include dynamin 1 because dynamin 1 dephosphorylation by calcineurin may be involved in bulk endocytosis (146, 147).
On the basis of knockout studies (130), it is tempting to propose that calcineurin-mediated dephosphorylation of dynamin (146) is involved in triggering endocytosis. However, a large number of studies using blockers of calcineurin do not reach a consensus. At chromaffin cells, calcineurin blockers such as cyclosporine A and FK506 were reported to have either no effect or an inhibitory effect on endocytosis (107, 148, 149). In synaptosomes, calcineurin blockers were implied to inhibit endocytosis in adult, but not in juvenile, animals, leading to the proposal that the calcium sensor switches from an unknown one to calcineurin as synapses mature (150). In contrast, calcineurin blockers inhibit endocytosis at immature calyces (130), but not at mature ones, leading to the proposal that the endocytic calcium sensor switches from calcineurin to an as-yet-unidentified one (132). At Drosophila neuromuscular junctions and cerebellar synapses, calcineurin blockers do not block endocytosis (150, 151). It is unclear whether such controversy is due to the specificity of synapses or pharmacological blockers. Resolving this issue would be critical in understanding how calcium influx triggers endocytosis.
Synaptotagmin
Synaptotagmin isoforms 1 and 2 are the calcium sensors for synchronized exocytosis (152). Deletion of synaptotagmin 1 prolongs the τ of slow endocytosis by approximately 0.5–1 fold in Drosophila neuromuscular junctions and at cortical and hippocampal synapses (Figure 7di–iii) (153–155). Deletion or mutation of both the C2A and C2B domains, the calcium-binding domains of synaptotagmin 1, prolongs the τ of slow endocytosis by ~30–50% (Figure 7diii) (155). These results suggest a regulatory role for synaptotagmin in slow endocytosis. At the calyx, where synaptotagmin 2 is the calcium sensor for synchronized exocytosis, deletion of synaptotagmin 2 has a negligible effect on endocytosis (49). At chromaffin cells, deletion of synaptotagmin 1 prolongs the duration of the fission pore closure but does not abolish endocytosis. Taken together, these findings suggest that synaptotagmin may regulate endocytosis but is not essential for endocytosis.
Comparison between calmodulin and synaptotagmin
Because the impairment of endocytosis in synaptotagmin 1 or 2 knockout synapses is not as severe as block of calmodulin (Figure 7c,d), the calmodulin signaling pathway is likely the dominant pathway in mediating calcium-triggered endocytosis. Furthermore, calmodulin is involved in all whole-cell forms of endocytosis, whereas synaptotagmin is involved only in slow endocytosis. How calmodulin and synaptotagmin trigger endocytosis is unclear. Calcineurin is a potential downstream target of calmodulin, although this issue remains controversial. Even if calcineurin is involved in endocytosis, how calcineurin-mediated dephosphorylation of endocytic proteins, including dynamin 1, speeds up endocytosis is unclear. Adaptor proteins AP2 and stonin are potential targets of synaptotagmin because both bind to synaptotagmin (156–158).
EXOCYTOSIS AND SNARE PROTEINS IN EXO-ENDOCYTOSIS COUPLING
Exocytosis Is Needed for Endocytosis Initiation
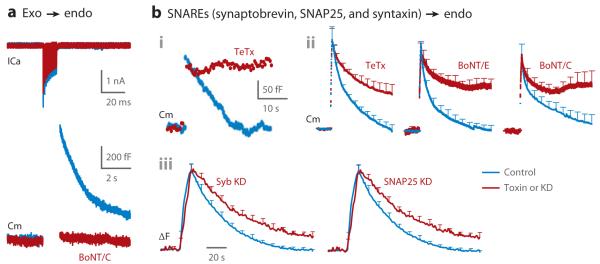
Although the exocytosis amount does not control the endocytosis rate (Figure 6), capacitance increase or vesicular protein-tagged pHluorin signal increase is usually followed by a decay to the baseline (e.g., Figures 3aii, 4b), indicating a match between exocytosis amount and endocytosis amount. When exocytosis is abolished by botulinum neurotoxins that cleave SNARE proteins essential for exocytosis, no endocytosis takes place, despite strong calcium influx induced by depolarization (Figure 8a) (98, 138). Thus, in addition to calcium influx, exocytosis is needed for endocytosis initiation, likely in controlling the endocytosis amount. An apparent exception is endocytosis overshoot. However, it may also require exocytosis because endocytosis overshoot retrieves vesicles previously fused but stranded at the plasma membrane (49, 101).
SNARE Proteins in Endocytosis
The requirement of exocytosis raises the possibility that proteins mediating exocytosis and/or vesicular membrane proteins exocytosed to the plasma membrane may be the substrates for endocytosis initiation. An early study implicates the involvement of synaptobrevin, a vesicular SNARE critical in mediating exocytosis, in rapid endocytosis at hippocampal synapses (159), although the technique used to detect rapid endocytosis is indirect and controversial (16, 32, 34). A recent study shows that tetanus toxin, which cleaves synaptobrevin, blocks slow endocytosis at calyces, suggesting the involvement of synaptobrevin in slow endocytosis (Figure 8bi) (50). A very recent study reveals that tetanus toxin blocks not only slow endocytosis but also rapid endocytosis at calyces (Figure 8bii) and that botulinum neurotoxins that cleave SNAP25 or syntaxin, each a membrane-targeted SNARE critical for exocytosis, also inhibit both rapid and slow endocytosis at calyces (Figure 8bii) (120). Furthermore, knockdown of synaptobrevin or SNAP25 inhibits slow endocytosis at cultured hippocampal synapses (Figure 8biii) (160). In conclusion, all three SNAREs that catalyze exocytosis—synaptobrevin, SNAP25, and syntaxin—are involved in both rapid and slow endocytosis at the calyx of Held and hippocampal synapses. Given that SNARE proteins mediate exocytosis at all nerve terminals and in nonneuronal secretory cells, the dual role of SNARE proteins in exo- and endocytosis is likely a general principle.
How SNARE proteins are involved in endocytosis remains unclear. Binding studies provide clues. For example, synaptobrevin binds to the ANTH domain of endocytic adaptors AP180 and clathrin assembly lymphoid myeloid leukemia protein (161, 162). SNAP25 binds to the endocytic protein intersectin (163). Syntaxin binds to dynamin (164).
Calcium Influx and SNAREs Comediate Exo-Endocytosis Coupling: A Working Model
We describe above the dual conditions required to initiate endocytosis: (a) calcium influx that activates calmodulin and synaptotagmin and (b) exocytosis machinery including the three SNARE proteins (Figure 9). A dual requirement is more advantageous than a requirement for only one of them. For example, if calcium influx is the only requirement, when an action potential–induced calcium influx fails to evoke exocytosis, which occurs at many low-release-probability synapses (165), calcium influx still triggers endocytosis, resulting in the shrinkage of the nerve terminal. Such futile endocytosis and terminal shrinkage are prevented by the dual requirement of calcium and exocytosis. If the same SNARE protein molecules involved in exocytosis also mediate the subsequent endocytosis, these molecules may carry the information about the exocytosis amount and may thus provide a mechanism for mediating exo-endocytosis coupling with regard to the amount.
Figure 9.
A schematic drawing showing that two pathways mediate exo-endocytosis coupling: (a) calcium influx that activates calmodulin and/or synaptotagmin and (b) SNARE proteins (synaptobrevin, SNAP25, and syntaxin).
The requirement of calcium provides a mechanism by which to regulate the rate of endocytosis and by which secretory cells can cope with different physiological conditions. For example, during high-frequency firing, large calcium influx triggers rapid endocytosis, bulk endocytosis, and even endocytosis overshoot (49, 82, 98). Activation of these different forms of endocytosis increases the endocytic rate and capacity, which may prevent exhaustion of synaptic vesicles and thus maintain synaptic transmission during high-frequency firing.
BROADER IMPACT
The principles described here are likely to have a broader impact on protein and vesicle trafficking in a variety of cell types. For example, analogous to calcium-triggered endocytosis observed at mammalian secretory cells, calcium influx via VDCCs is required for a form of endocytosis that takes place after cortical granule exocytosis and lasts for as long as 15 min (140). Calcium influx is also required for a rapid form of endocytosis in baby hamster kidney cells and cardiac myocytes (90) and for exo- and endocytosis in Chinese hamster ovary cells and 3T3 fibroblasts (166). At postsynaptic dendritic spines, SNARE-mediated AMPA receptor exocytosis is involved in generating long-term synaptic potentiation (167), whereas calcium and calcineurin may activate AMPA receptor endocytosis (168, 169), which may mediate long-term synaptic depression (170). These observations are similar to those observed at nerve terminals in which SNAREs mediate exocytosis and calcium, calmodulin, and calcineurin may be involved in triggering endocytosis. These similarities suggest that some or all of the principles described here, including exo-endocytosis modes, functions, and coupling mechanisms, are likely to apply well beyond secretory vesicles in the nerve terminals and endocrine cells described here, such as in the contexts of muscle, adipose tissues, immune cells, and neuronal dendrites (167, 171, 172). The general observation that most molecules involved in mediating exo- and endocytosis are evolutionally conserved in different systems and organisms (1, 2) further supports this possibility.
SUMMARY POINTS.
Three modes of exocytosis—full-collapse fusion, kiss-and-run, and compound exocytosis—control quantal size and thus the strength of exocytosis, by which synaptic plasticity, including posttetanic potentiation, can be generated.
Three modes of endocytosis—classical clathrin-dependent endocytosis, kiss-and-run, and bulk endocytosis—control the rate and capacity of endocytosis and thus maintain exocytosis under various stimulation conditions.
Endocytosis not only recycles vesicles but also mediates calcium-dependent facilitation of vesicle replenishment to the readily releasable pool via active-zone clearance, which helps recover short-term synaptic depression caused by repetitive firing.
There are three size-matched exo-endocytosis couplings: full-collapse fusion and classical endocytosis, kiss-and-run, and compound exocytosis and bulk endocytosis.
Calcium influx via voltage-dependent calcium channels at the plasma membrane at the micro/nanodomain triggers all forms of endocytosis at the whole-cell level. Calmodulin is the main calcium sensor, although synaptotagmin may also contribute to this process.
Exocytosis and the SNARE proteins synaptobrevin, SNAP25, and syntaxin are needed for endocytosis initiation.
Calcium signaling pathways and exocytosis machinery may mediate exo-endocytosis coupling with respect to timing and amount.
The principles described here for vesicle exo- and endocytosis in secretory cells may have a broader impact on protein and vesicle trafficking.
ACKNOWLEDGMENTS
We sincerely thank Drs. Justin Taraska, Zhen Zhang, Jiansong Sheng, and Fujun Luo for helpful comments on the review. This work was supported by the National Institute of Neurological Disorders and Stroke Intramural Research Program.
Glossary
- Full-collapse fusion
upon vesicle fusion with the plasma membrane, a fusion pore opens and dilates until the vesicle fully collapses
- Kiss-and-run
during vesicle fusion with the plasma membrane, a fusion pore opens and closes without vesicle collapse
- Compound exocytosis
fusion of a giant vesicle preformed by vesicle-vesicle fusion or by fusion of vesicles on the already fused, but not collapsed, vesicle
- Classical endocytosis
generation of a vesicle via membrane invagination, formation of an Ω profile, and fission of the Ω profile
- Bulk endocytosis
endocytosis that retrieves a large endosome-like structure
- Membrane capacitance
an electrophysiologically recorded value linearly proportional to the membrane area; an increase reflects exocytosis, and a decrease indicates endocytosis
- Capacitance flicker
capacitance up and down steps of equal size within a few seconds
- SNARE
soluble NSF (N-ethylmaleimide-sensitive factor) attachment protein receptor
- Calyx of Held
a large glutamatergic nerve terminal in the medial nucleus of the trapezoid body in the auditory brain stem; this structure is accessible to electrophysiological recordings
- Exo-endocytosis coupling
endocytosis follows exocytosis within seconds to minutes to retrieve a similar amount of exocytosed vesicle membrane and protein
Footnotes
This is a work of the US Government and is not subject to copyright protection in the United States.
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Südhof TC. The synaptic vesicle cycle. Annu. Rev. Neurosci. 2004;27:509–47. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- 2.Saheki Y, De Camilli P. Synaptic vesicle endocytosis. Cold Spring Harb. Perspect. Biol. 2012;4:a005645. doi: 10.1101/cshperspect.a005645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kasai H, Takahashi N, Tokumaru H. Distinct initial SNARE configurations underlying the diversity of exocytosis. Physiol. Rev. 2012;92:1915–64. doi: 10.1152/physrev.00007.2012. [DOI] [PubMed] [Google Scholar]
- 4.Rorsman P, Braun M. Regulation of insulin secretion in human pancreatic islets. Annu. Rev. Physiol. 2013;75:155–79. doi: 10.1146/annurev-physiol-030212-183754. [DOI] [PubMed] [Google Scholar]
- 5.Wu LG, Ryan TA, Lagnado L. Modes of vesicle retrieval at ribbon synapses, calyx-type synapses, and small central synapses. J. Neurosci. 2007;27:11793–802. doi: 10.1523/JNEUROSCI.3471-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heuser JE, Reese TS. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J. Cell Biol. 1973;57:315–44. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ceccarelli B, Hurlbut WP, Mauro A. Turnover of transmitter and synaptic vesicles at the frog neuromuscular junction. J. Cell Biol. 1973;57:499–524. doi: 10.1083/jcb.57.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heuser JE, Reese TS. Structural changes after transmitter release at the frog neuromuscular junction. J. Cell Biol. 1981;88:564–80. doi: 10.1083/jcb.88.3.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alvarez de Toledo G, Fernandez-Chacon R, Fernandez JM. Release of secretory products during transient vesicle fusion. Nature. 1993;363:554–58. doi: 10.1038/363554a0. [DOI] [PubMed] [Google Scholar]
- 10.Albillos A, Dernick G, Horstmann H, Almers W, Alvarez de Toledo G, Lindau M. The exocytotic event in chromaffin cells revealed by patch amperometry. Nature. 1997;389:509–12. doi: 10.1038/39081. [DOI] [PubMed] [Google Scholar]
- 11.He L, Wu XS, Mohan R, Wu LG. Two modes of fusion pore opening revealed by cell-attached recordings at a synapse. Nature. 2006;444:102–5. doi: 10.1038/nature05250. [DOI] [PubMed] [Google Scholar]
- 12.Chow RH, von Ruden L, Neher E. Delay in vesicle fusion revealed by electrochemical monitoring of single secretory events in adrenal chromaffin cells. Nature. 1992;356:60–63. doi: 10.1038/356060a0. [DOI] [PubMed] [Google Scholar]
- 13.Taraska JW, Perrais D, Ohara-Imaizumi M, Nagamatsu S, Almers W. Secretory granules are recaptured largely intact after stimulated exocytosis in cultured endocrine cells. Proc. Natl. Acad. Sci. USA. 2003;100:2070–75. doi: 10.1073/pnas.0337526100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perrais D, Kleppe IC, Taraska JW, Almers W. Recapture after exocytosis causes differential retention of protein in granules of bovine chromaffin cells. J. Physiol. 2004;560:413–28. doi: 10.1113/jphysiol.2004.064410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zenisek D, Steyer JA, Feldman ME, Almers W. A membrane marker leaves synaptic vesicles in milliseconds after exocytosis in retinal bipolar cells. Neuron. 2002;35:1085–97. doi: 10.1016/s0896-6273(02)00896-6. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Q, Li Y, Tsien RW. The dynamic control of kiss-and-run and vesicular reuse probed with single nanoparticles. Science. 2009;323:1448–53. doi: 10.1126/science.1167373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alabi AA, Tsien RW. Perspectives on kiss-and-run: role in exocytosis, endocytosis, and neuro-transmission. Annu. Rev. Physiol. 2013;75:393–422. doi: 10.1146/annurev-physiol-020911-153305. [DOI] [PubMed] [Google Scholar]
- 18.Torri-Tarelli F, Grohovaz F, Fesce R, Ceccarelli B. Temporal coincidence between synaptic vesicle fusion and quantal secretion of acetylcholine. J. Cell Biol. 1985;101:1386–99. doi: 10.1083/jcb.101.4.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fesce R, Grohovaz F, Valtorta F, Meldolesi J. Neurotransmitter release: fusion or “kiss and run”? Trends Cell Biol. 1994;4:1–4. doi: 10.1016/0962-8924(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 20.Klyachko VA, Jackson MB. Capacitance steps and fusion pores of small and large-dense-core vesicles in nerve terminals. Nature. 2002;418:89–92. doi: 10.1038/nature00852. [DOI] [PubMed] [Google Scholar]
- 21.Wang CT, Lu JC, Bai J, Chang PY, Martin TF, et al. Different domains of synaptotagmin control the choice between kiss-and-run and full fusion. Nature. 2003;424:943–47. doi: 10.1038/nature01857. [DOI] [PubMed] [Google Scholar]
- 22.Fulop T, Smith C. Physiological stimulation regulates the exocytic mode through calcium activation of protein kinase C in mouse chromaffin cells. Biochem. J. 2006;399:111–19. doi: 10.1042/BJ20060654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsuboi T, McMahon HT, Rutter GA. Mechanisms of dense core vesicle recapture following “kiss and run” (“cavicapture”) exocytosis in insulin-secreting cells. J. Biol. Chem. 2004;279:47115–24. doi: 10.1074/jbc.M408179200. [DOI] [PubMed] [Google Scholar]
- 24.Aravanis AM, Pyle JL, Tsien RW. Single synaptic vesicles fusing transiently and successively without loss of identity. Nature. 2003;423:643–47. doi: 10.1038/nature01686. [DOI] [PubMed] [Google Scholar]
- 25.Gandhi SP, Stevens CF. Three modes of synaptic vesicular recycling revealed by single-vesicle imaging. Nature. 2003;423:607–13. doi: 10.1038/nature01677. [DOI] [PubMed] [Google Scholar]
- 26.Harata NC, Choi S, Pyle JL, Aravanis AM, Tsien RW. Frequency-dependent kinetics and prevalence of kiss-and-run and reuse at hippocampal synapses studied with novel quenching methods. Neuron. 2006;49:243–56. doi: 10.1016/j.neuron.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 27.Richards DA, Bai J, Chapman ER. Two modes of exocytosis at hippocampal synapses revealed by rate of FM1-43 efflux from individual vesicles. J. Cell Biol. 2005;168:929–39. doi: 10.1083/jcb.200407148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park H, Li Y, Tsien RW. Influence of synaptic vesicle position on release probability and exocytotic fusion mode. Science. 2012;335:1362–66. doi: 10.1126/science.1216937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Photowala H, Blackmer T, Schwartz E, Hamm HE, Alford S. G protein βγ-subunits activated by serotonin mediate presynaptic inhibition by regulating vesicle fusion properties. Proc. Natl. Acad. Sci. USA. 2006;103:4281–86. doi: 10.1073/pnas.0600509103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fernandez-Alfonso T, Ryan TA. The kinetics of synaptic vesicle pool depletion at CNS synaptic terminals. Neuron. 2004;41:943–53. doi: 10.1016/s0896-6273(04)00113-8. [DOI] [PubMed] [Google Scholar]
- 31.Balaji J, Ryan TA. Single-vesicle imaging reveals that synaptic vesicle exocytosis and endocytosis are coupled by a single stochastic mode. Proc. Natl. Acad. Sci. USA. 2007;104:20576–81. doi: 10.1073/pnas.0707574105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Granseth B, Odermatt B, Royle SJ, Lagnado L. Comment on “The dynamic control of kiss-and-run and vesicular reuse probed with single nanoparticles. Science. 2009;325:1499. doi: 10.1126/science.1175790. [DOI] [PubMed] [Google Scholar]
- 33.Granseth B, Odermatt B, Royle SJ, Lagnado L. Clathrin-mediated endocytosis is the dominant mechanism of vesicle retrieval at hippocampal synapses. Neuron. 2006;51:773–86. doi: 10.1016/j.neuron.2006.08.029. [DOI] [PubMed] [Google Scholar]
- 34.He L, Wu LG. The debate on the kiss-and-run fusion at synapses. Trends Neurosci. 2007;30:447–55. doi: 10.1016/j.tins.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 35.Ichikawa A. Fine structural changes in response to hormonal stimulation of the perfused canine pancreas. J. Cell Biol. 1965;24:369–85. doi: 10.1083/jcb.24.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hafez I, Stolpe A, Lindau M. Compound exocytosis and cumulative fusion in eosinophils. J. Biol. Chem. 2003;278:44921–28. doi: 10.1074/jbc.M306013200. [DOI] [PubMed] [Google Scholar]
- 37.Pickett JA, Edwardson JM. Compound exocytosis: mechanisms and functional significance. Traffic. 2006;7:109–16. doi: 10.1111/j.1600-0854.2005.00372.x. [DOI] [PubMed] [Google Scholar]
- 38.Alvarez de Toledo G, Fernandez JM. Compound versus multigranular exocytosis in peritoneal mast cells. J. Gen. Physiol. 1990;95:397–409. doi: 10.1085/jgp.95.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scepek S, Lindau M. Focal exocytosis by eosinophils—compound exocytosis and cumulative fusion. EMBO J. 1993;12:1811–17. doi: 10.1002/j.1460-2075.1993.tb05829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nemoto T, Kimura R, Ito K, Tachikawa A, Miyashita Y, et al. Sequential-replenishment mechanism of exocytosis in pancreatic acini. Nat. Cell Biol. 2001;3:253–58. doi: 10.1038/35060042. [DOI] [PubMed] [Google Scholar]
- 41.Takamori S, Holt M, Stenius K, Lemke EA, Gronborg M, et al. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–46. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 42.Rizzoli SO, Bethani I, Zwilling D, Wenzel D, Siddiqui TJ, et al. Evidence for early endosome-like fusion of recently endocytosed synaptic vesicles. Traffic. 2006;7:1163–76. doi: 10.1111/j.1600-0854.2006.00466.x. [DOI] [PubMed] [Google Scholar]
- 43.Matthews G, Sterling P. Evidence that vesicles undergo compound fusion on the synaptic ribbon. J. Neurosci. 2008;28:5403–11. doi: 10.1523/JNEUROSCI.0935-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.He L, Xue L, Xu J, McNeil BD, Bai L, et al. Compound vesicle fusion increases quantal size and potentiates synaptic transmission. Nature. 2009;459:93–97. doi: 10.1038/nature07860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Richards DA. Vesicular release mode shapes the postsynaptic response at hippocampal synapses. J. Physiol. 2009;587:5073–80. doi: 10.1113/jphysiol.2009.175315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choi S, Klingauf J, Tsien RW. Fusion pore modulation as a presynaptic mechanism contributing to expression of long-term potentiation. Philos. Trans. R. Soc. Lond. Ser. B. 2003;358:695–705. doi: 10.1098/rstb.2002.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ales E, Tabares L, Poyato JM, Valero V, Lindau M, Alvarez de Toledo G. High calcium concentrations shift the mode of exocytosis to the kiss-and-run mechanism. Nat. Cell Biol. 1999;1:40–44. doi: 10.1038/9012. [DOI] [PubMed] [Google Scholar]
- 48.Kawasaki F, Hazen M, Ordway RW. Fast synaptic fatigue in shibire mutants reveals a rapid requirement for dynamin in synaptic vesicle membrane trafficking. Nat. Neurosci. 2000;3:859–60. doi: 10.1038/78753. [DOI] [PubMed] [Google Scholar]
- 49.Wu XS, McNeil BD, Xu J, Fan J, Xue L, et al. Ca2+ and calmodulin initiate all forms of endocytosis during depolarization at a nerve terminal. Nat. Neurosci. 2009;12:1003–10. doi: 10.1038/nn.2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hosoi N, Holt M, Sakaba T. Calcium dependence of exo- and endocytotic coupling at a glutamatergic synapse. Neuron. 2009;63:216–29. doi: 10.1016/j.neuron.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 51.Elhamdani A, Azizi F, Artalejo CR. Double patch clamp reveals that transient fusion (kiss-and-run) is a major mechanism of secretion in calf adrenal chromaffin cells: High calcium shifts the mechanism from kiss-and-run to complete fusion. J. Neurosci. 2006;26:3030–36. doi: 10.1523/JNEUROSCI.5275-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xue L, Wu LG. Post-tetanic potentiation is caused by two signaling mechanisms affecting quantal size and quantal content. J. Physiol. 2010;588:4987–94. doi: 10.1113/jphysiol.2010.196964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fioravante D, Chu Y, Myoga MH, Leitges M, Regehr WG. Calcium-dependent isoforms of protein kinase C mediate posttetanic potentiation at the calyx of Held. Neuron. 2011;70:1005–19. doi: 10.1016/j.neuron.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu. Rev. Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
- 55.Borst JGG, Soria van Hoeve J. The calyx of Held synapse: from model synapse to auditory relay. Annu. Rev. Physiol. 2012;74:199–224. doi: 10.1146/annurev-physiol-020911-153236. [DOI] [PubMed] [Google Scholar]
- 56.Richards DA, Guatimosim C, Betz WJ. Two endocytic recycling routes selectively fill two vesicle pools in frog motor nerve terminals. Neuron. 2000;27:551–59. doi: 10.1016/s0896-6273(00)00065-9. [DOI] [PubMed] [Google Scholar]
- 57.de Lange RPJ, de Roos ADG, Borst JGG. Two modes of vesicle recycling in the rat calyx of Held. J. Neurosci. 2003;23:10164–73. doi: 10.1523/JNEUROSCI.23-31-10164.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paillart C, Li J, Matthews G, Sterling P. Endocytosis and vesicle recycling at a ribbon synapse. J. Neurosci. 2003;23:4092–99. doi: 10.1523/JNEUROSCI.23-10-04092.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Coggins MR, Grabner CP, Almers W, Zenisek D. Stimulated exocytosis of endosomes in goldfish retinal bipolar neurons. J. Physiol. 2007;584:853–65. doi: 10.1113/jphysiol.2007.140848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heuser JE. Proceedings: A possible origin of the “giant” spontaneous potentials that occur after prolonged transmitter release at frog neuromuscular junctions. J. Physiol. 1974;239:P106–8. doi: 10.1113/jphysiol.1974.sp010593. [DOI] [PubMed] [Google Scholar]
- 61.Henze DA, McMahon DB, Harris KM, Barrionuevo G. Giant miniature EPSCs at the hippocampal mossy fiber to CA3 pyramidal cell synapse are monoquantal. J. Neurophysiol. 2002;87:15–29. doi: 10.1152/jn.00394.2001. [DOI] [PubMed] [Google Scholar]
- 62.Llano I, Gonzalez J, Caputo C, Lai FA, Blayney LM, et al. Presynaptic calcium stores underlie large-amplitude miniature IPSCs and spontaneous calcium transients. Nat. Neurosci. 2000;3:1256–65. doi: 10.1038/81781. [DOI] [PubMed] [Google Scholar]
- 63.Wall MJ, Usowicz MM. Development of the quantal properties of evoked and spontaneous synaptic currents at a brain synapse. Nat. Neurosci. 1998;1:675–82. doi: 10.1038/3677. [DOI] [PubMed] [Google Scholar]
- 64.Steinert JR, Kuromi H, Hellwig A, Knirr M, Wyatt AW, et al. Experience-dependent formation and recruitment of large vesicles from reserve pool. Neuron. 2006;50:723–33. doi: 10.1016/j.neuron.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 65.Wadiche JI, Jahr CE. Multivesicular release at climbing fiber–Purkinje cell synapses. Neuron. 2001;32:301–13. doi: 10.1016/s0896-6273(01)00488-3. [DOI] [PubMed] [Google Scholar]
- 66.Sun JY, Wu LG. Fast kinetics of exocytosis revealed by simultaneous measurements of postsynaptic capacitance and postsynaptic currents at a central synapse. Neuron. 2001;30:171–82. doi: 10.1016/s0896-6273(01)00271-9. [DOI] [PubMed] [Google Scholar]
- 67.Singer JH, Lassova L, Vardi N, Diamond JS. Coordinated multivesicular release at a mammalian ribbon synapse. Nat. Neurosci. 2004;7:826–33. doi: 10.1038/nn1280. [DOI] [PubMed] [Google Scholar]
- 68.Li GL, Keen E, Andor-Ardo D, Hudspeth AJ, Von Gersdorff H. The unitary event underlying multiquantal EPSCs at a hair cell’s ribbon synapse. J. Neurosci. 2009;29:7558–68. doi: 10.1523/JNEUROSCI.0514-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miller TM, Heuser JE. Endocytosis of synaptic vesicle membrane at the frog neuromuscular junction. J. Cell Biol. 1984;98:685–98. doi: 10.1083/jcb.98.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Artalejo CR, Henley JR, McNiven MA, Palfrey HC. Rapid endocytosis coupled to exocytosis in adrenal chromaffin cells involves Ca2+, GTP, and dynamin but not clathrin. Proc. Natl. Acad. Sci. USA. 1995;92:8328–32. doi: 10.1073/pnas.92.18.8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jockusch WJ, Praefcke GJ, McMahon HT, Lagnado L. Clathrin-dependent and clathrin-independent retrieval of synaptic vesicles in retinal bipolar cells. Neuron. 2005;46:869–78. doi: 10.1016/j.neuron.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 72.He Z, Fan J, Kang L, Lu J, Xue Y, et al. Ca2+ triggers a novel clathrin-independent but actin-dependent fast endocytosis in pancreatic beta cells. Traffic. 2008;9:910–23. doi: 10.1111/j.1600-0854.2008.00730.x. [DOI] [PubMed] [Google Scholar]
- 73.Kim SH, Ryan TA. Synaptic vesicle recycling at CNS synapses without AP-2. J. Neurosci. 2009;29:3865–74. doi: 10.1523/JNEUROSCI.5639-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yim YI, Sun T, Wu LG, Raimondi A, De Camilli P, et al. Endocytosis and clathrin-uncoating defects at synapses of auxilin knockout mice. Proc. Natl. Acad. Sci. USA. 2010;107:4412–17. doi: 10.1073/pnas.1000738107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.von Kleist L, Stahlschmidt W, Bulut H, Gromova K, Puchkov D, et al. Role of the clathrin terminal domain in regulating coated pit dynamics revealed by small molecule inhibition. Cell. 2011;146:471–84. doi: 10.1016/j.cell.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 76.Willox AK, Royle SJ. Stonin 2 is a major adaptor protein for clathrin-mediated synaptic vesicle retrieval. Curr. Biol. 2012;22:1435–39. doi: 10.1016/j.cub.2012.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Milosevic I, Giovedi S, Lou X, Raimondi A, Collesi C, et al. Recruitment of endophilin to clathrin-coated pit necks is required for efficient vesicle uncoating after fission. Neuron. 2011;72:587–601. doi: 10.1016/j.neuron.2011.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Koenig JH, Ikeda K. Synaptic vesicles have two distinct recycling pathways. J. Cell Biol. 1996;135:797–808. doi: 10.1083/jcb.135.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wenzel EM, Morton A, Ebert K, Welzel O, Kornhuber J, et al. Key physiological parameters dictate triggering of activity-dependent bulk endocytosis in hippocampal synapses. PLoS ONE. 2012;7:e38188. doi: 10.1371/journal.pone.0038188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Holt M, Cooke A, Wu MM, Lagnado L. Bulk membrane retrieval in the synaptic terminal of retinal bipolar cells. J. Neurosci. 2003;23:1329–39. doi: 10.1523/JNEUROSCI.23-04-01329.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Teng H, Lin MY, Wilkinson RS. Macroendocytosis and endosome processing in snake motor boutons. J. Physiol. 2007;582:243–62. doi: 10.1113/jphysiol.2007.130989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Clayton EL, Evans GJ, Cousin MA. Bulk synaptic vesicle endocytosis is rapidly triggered during strong stimulation. J. Neurosci. 2008;28:6627–32. doi: 10.1523/JNEUROSCI.1445-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gaffield MA, Romberg CF, Betz WJ. Live imaging of bulk endocytosis in frog motor nerve terminals using FM dyes. J. Neurophysiol. 2011;106:599–607. doi: 10.1152/jn.00123.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Koenig JH, Ikeda K. Disappearance and reformation of synaptic vesicle membrane upon transmitter release observed under reversible blockage of membrane retrieval. J. Neurosci. 1989;9:3844–60. doi: 10.1523/JNEUROSCI.09-11-03844.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Takei K, Mundigl O, Daniell L, De Camilli P. The synaptic vesicle cycle: a single vesicle budding step involving clathrin and dynamin. J. Cell Biol. 1996;133:1237–50. doi: 10.1083/jcb.133.6.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Teng H, Wilkinson RS. Clathrin-mediated endocytosis near active zones in snake motor boutons. J. Neurosci. 2000;20:7986–93. doi: 10.1523/JNEUROSCI.20-21-07986.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu W, Wu LG. Rapid bulk endocytosis and its kinetics of fission pore closure at a central synapse. Proc. Natl. Acad. Sci. USA. 2007;104:10234–39. doi: 10.1073/pnas.0611512104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sun JY, Wu XS, Wu LG. Single and multiple vesicle fusion induce different rates of endocytosis at a central synapse. Nature. 2002;417:555–59. doi: 10.1038/417555a. [DOI] [PubMed] [Google Scholar]
- 89.Rosenboom H, Lindau M. Exo-endocytosis and closing of the fission pore during endocytosis in single pituitary nerve terminals internally perfused with high calcium concentrations. Proc. Natl. Acad. Sci. USA. 1994;91:5267–71. doi: 10.1073/pnas.91.12.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lariccia V, Fine M, Magi S, Lin MJ, Yaradanakul A, et al. Massive calcium-activated endocytosis without involvement of classical endocytic proteins. J. Gen. Physiol. 2011;137:111–32. doi: 10.1085/jgp.201010468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Delgado R, Maureira C, Oliva C, Kidokoro Y, Labarca P. Size of vesicle pools, rates of mobilization, and recycling at neuromuscular synapses of a Drosophila mutant, shibire. Neuron. 2000;28:941–53. doi: 10.1016/s0896-6273(00)00165-3. [DOI] [PubMed] [Google Scholar]
- 92.Wu XS, Wu LG. Rapid endocytosis does not recycle vesicles within the readily releasable pool. J. Neurosci. 2009;29:11038–42. doi: 10.1523/JNEUROSCI.2367-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Von Gersdorff H, Matthews G. Inhibition of endocytosis by elevated internal calcium in a synaptic terminal. Nature. 1994;370:652–55. doi: 10.1038/370652a0. [DOI] [PubMed] [Google Scholar]
- 94.Neves G, Gomis A, Lagnado L. Calcium influx selects the fast mode of endocytosis in the synaptic terminal of retinal bipolar cells. Proc. Natl. Acad. Sci. USA. 2001;98:15282–87. doi: 10.1073/pnas.261311698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Smith C, Neher E. Multiple forms of endocytosis in bovine adrenal chromaffin cells. J. Cell Biol. 1997;139:885–94. doi: 10.1083/jcb.139.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chan SA, Smith C. Physiological stimuli evoke two forms of endocytosis in bovine chromaffin cells. J. Physiol. 2001;537:871–85. doi: 10.1111/j.1469-7793.2001.00871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sankaranarayanan S, Ryan TA. Real-time measurements of vesicle-SNARE recycling in synapses of the central nervous system. Nat. Cell Biol. 2000;2:197–204. doi: 10.1038/35008615. [DOI] [PubMed] [Google Scholar]
- 98.Wu W, Xu J, Wu XS, Wu LG. Activity-dependent acceleration of endocytosis at a central synapse. J. Neurosci. 2005;25:11676–83. doi: 10.1523/JNEUROSCI.2972-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Balaji J, Armbruster M, Ryan TA. Calcium control of endocytic capacity at a CNS synapse. J. Neurosci. 2008;28:6742–49. doi: 10.1523/JNEUROSCI.1082-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Renden R, Von Gersdorff H. Synaptic vesicle endocytosis at a CNS nerve terminal: faster kinetics at physiological temperatures and increased endocytotic capacity during maturation. J. Neurophysiol. 2007;98:3349–59. doi: 10.1152/jn.00898.2007. [DOI] [PubMed] [Google Scholar]
- 101.Xue L, McNeil BD, Wu XS, Luo F, He L, Wu LG. A membrane pool retrieved via endocytosis overshoot at nerve terminals: a study of its retrieval mechanism and role. J. Neurosci. 2012;32:3398–404. doi: 10.1523/JNEUROSCI.5943-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wu LG, Betz WJ. Nerve activity but not intracellular calcium determines the time course of endocytosis at the frog neuromuscular junction. Neuron. 1996;17:769–79. doi: 10.1016/s0896-6273(00)80208-1. [DOI] [PubMed] [Google Scholar]
- 103.Leitz J, Kavalali ET. Ca2+ influx slows single synaptic vesicle endocytosis. J. Neurosci. 2011;31:16318–26. doi: 10.1523/JNEUROSCI.3358-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Moser T, Beutner D. Kinetics of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse of the mouse. Proc. Natl. Acad. Sci. USA. 2000;97:883–88. doi: 10.1073/pnas.97.2.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Thomas P, Lee AK, Wong JG, Almers W. A triggered mechanism retrieves membrane in seconds after Ca2+-stimulated exocytosis in single pituitary cells. J. Cell Biol. 1994;124:667–75. doi: 10.1083/jcb.124.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Royle SJ, Lagnado L. Endocytosis at the synaptic terminal. J. Physiol. 2003;553:345–55. doi: 10.1113/jphysiol.2003.049221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Artalejo CR, Elhamdani A, Palfrey HC. Calmodulin is the divalent cation receptor for rapid endocytosis, but not exocytosis, in adrenal chromaffin cells. Neuron. 1996;16:195–205. doi: 10.1016/s0896-6273(00)80036-7. [DOI] [PubMed] [Google Scholar]
- 108.Llobet A, Beaumont V, Lagnado L. Real-time measurement of exocytosis and endocytosis using interference of light. Neuron. 2003;40:1075–86. doi: 10.1016/s0896-6273(03)00765-7. [DOI] [PubMed] [Google Scholar]
- 109.Fernandez-Alfonso T, Kwan R, Ryan TA. Synaptic vesicles interchange their membrane proteins with a large surface reservoir during recycling. Neuron. 2006;51:179–86. doi: 10.1016/j.neuron.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 110.Wienisch M, Klingauf J. Vesicular proteins exocytosed and subsequently retrieved by compensatory endocytosis are nonidentical. Nat. Neurosci. 2006;9:1019–27. doi: 10.1038/nn1739. [DOI] [PubMed] [Google Scholar]
- 111.Hua Y, Sinha R, Thiel CS, Schmidt R, Huve J, et al. A readily retrievable pool of synaptic vesicles. Nat. Neurosci. 2011;14:833–39. doi: 10.1038/nn.2838. [DOI] [PubMed] [Google Scholar]
- 112.Kawasaki F, Iyer J, Posey LL, Sun CE, Mammen SE, et al. The DISABLED protein functions in CLATHRIN-mediated synaptic vesicle endocytosis and exoendocytic coupling at the active zone. Proc. Natl. Acad. Sci. USA. 2011;108:E222–29. doi: 10.1073/pnas.1102231108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wu LG, Borst JGG. The reduced release probability of releasable vesicles during recovery from short-term synaptic depression. Neuron. 1999;23:821–32. doi: 10.1016/s0896-6273(01)80039-8. [DOI] [PubMed] [Google Scholar]
- 114.Sakaba T, Neher E. Calmodulin mediates rapid recruitment of fast-releasing synaptic vesicles at a calyx-type synapse. Neuron. 2001;32:1119–31. doi: 10.1016/s0896-6273(01)00543-8. [DOI] [PubMed] [Google Scholar]
- 115.Xu J, Wu LG. The decrease in the presynaptic calcium current is a major cause of short-term depression at a calyx-type synapse. Neuron. 2005;46:633–45. doi: 10.1016/j.neuron.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 116.Stevens CF, Wesseling JF. Activity-dependent modulation of the rate at which synaptic vesicles become available to undergo exocytosis. Neuron. 1998;21:415–24. doi: 10.1016/s0896-6273(00)80550-4. [DOI] [PubMed] [Google Scholar]
- 117.Dittman JS, Regehr WG. Calcium dependence and recovery kinetics of presynaptic depression at the climbing fiber to Purkinje cell synapse. J. Neurosci. 1998;18:6147–62. doi: 10.1523/JNEUROSCI.18-16-06147.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang L-Y, Kaczmarek LK. High-frequency firing helps replenish the readily releasable pool of synaptic vesicles. Nature. 1998;394:384–38. doi: 10.1038/28645. [DOI] [PubMed] [Google Scholar]
- 119.Abbott LF, Regehr WG. Synaptic computation. Nature. 2004;431:796–803. doi: 10.1038/nature03010. [DOI] [PubMed] [Google Scholar]
- 120.Xu J, Luo F, Zhang Z, Xue L, Wu XS, et al. SNARE proteins synaptobrevin, SNAP-25 and syntaxin are involved in rapid and slow endocytosis at synapses. Cell Rep. 2013;3:1414–21. doi: 10.1016/j.celrep.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Haucke V, Neher E, Sigrist SJ. Protein scaffolds in the coupling of synaptic exocytosis and endocytosis. Nat. Rev. Neurosci. 2011;12:127–38. doi: 10.1038/nrn2948. [DOI] [PubMed] [Google Scholar]
- 122.Ceccarelli B, Hurlbut WP. Ca2+-dependent recycling of synaptic vesicles at the frog neuromuscular junction. J. Cell Biol. 1980;87:297–303. doi: 10.1083/jcb.87.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ramaswami M, Krishnan KS, Kelly RB. Intermediates in synaptic vesicle recycling revealed by optical imaging of Drosophila neuromuscular junctions. Neuron. 1994;13:363–75. doi: 10.1016/0896-6273(94)90353-0. [DOI] [PubMed] [Google Scholar]
- 124.Henkel AW, Betz WJ. Monitoring of black widow spider venom (BWSV) induced exo- and endocytosis in living frog motor nerve terminals with FM1-43. Neuropharmacology. 1995;34:1397–406. doi: 10.1016/0028-3908(95)00126-q. [DOI] [PubMed] [Google Scholar]
- 125.Marks B, McMahon HT. Calcium triggers calcineurin-dependent synaptic vesicle recycling in mammalian nerve terminals. Curr. Biol. 1998;8:740–49. doi: 10.1016/s0960-9822(98)70297-0. [DOI] [PubMed] [Google Scholar]
- 126.Cousin MA, Robinson PJ. Ba2+ does not support synaptic vesicle retrieval in rat cerebrocortical synaptosomes. Neurosci. Lett. 1998;253:1–4. doi: 10.1016/s0304-3940(98)00610-7. [DOI] [PubMed] [Google Scholar]
- 127.Sankaranarayanan S, Ryan TA. Calcium accelerates endocytosis of vSNAREs at hippocampal synapses. Nat. Neurosci. 2001;4:129–36. doi: 10.1038/83949. [DOI] [PubMed] [Google Scholar]
- 128.Neher E, Sakaba T. Multiple roles of calcium ions in the regulation of neurotransmitter release. Neuron. 2008;59:861–72. doi: 10.1016/j.neuron.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 129.Xue L, Zhang Z, McNeil BD, Luo F, Wu XS, et al. Voltage-dependent calcium channels at the plasma membrane, but not vesicular channels, couple exocytosis to endocytosis. Cell Rep. 2012;1:632–38. doi: 10.1016/j.celrep.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sun T, Wu XS, Xu J, McNeil BD, Pang ZP, et al. The role of calcium/calmodulin-activated calcineurin in rapid and slow endocytosis at central synapses. J. Neurosci. 2010;30:11838–47. doi: 10.1523/JNEUROSCI.1481-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Clayton EL, Cousin MA. The molecular physiology of activity-dependent bulk endocytosis of synaptic vesicles. J. Neurochem. 2009;111:901–14. doi: 10.1111/j.1471-4159.2009.06384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yamashita T, Eguchi K, Saitoh N, Von Gersdorff H, Takahashi T. Developmental shift to a mechanism of synaptic vesicle endocytosis requiring nanodomain Ca2+ Nat. Neurosci. 2010;13:838–44. doi: 10.1038/nn.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Borst JGG, Sakmann B. Calcium influx and transmitter release in a fast CNS synapse. Nature. 1996;383:431–34. doi: 10.1038/383431a0. [DOI] [PubMed] [Google Scholar]
- 134.Bollmann JH, Sakmann B, Borst JGG. Calcium sensitivity of glutamate release in a calyx-type terminal. Science. 2000;289:953–57. doi: 10.1126/science.289.5481.953. [DOI] [PubMed] [Google Scholar]
- 135.Schneggenburger R, Neher E. Intracellular calcium dependence of transmitter release rates at a fast central synapse. Nature. 2000;406:889–93. doi: 10.1038/35022702. [DOI] [PubMed] [Google Scholar]
- 136.Wang LY, Neher E, Taschenberger H. Synaptic vesicles in mature calyx of Held synapses sense higher nanodomain calcium concentrations during action potential–evoked glutamate release. J. Neurosci. 2008;28:14450–58. doi: 10.1523/JNEUROSCI.4245-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sochacki KA, Larson BT, Sengupta DC, Daniels MP, Shtengel G, et al. Imaging the post-fusion release and capture of a vesicle membrane protein. Nat. Commun. 2012;3:1154. doi: 10.1038/ncomms2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yamashita T, Hige T, Takahashi T. Vesicle endocytosis requires dynamin-dependent GTP hydrolysis at a fast CNS synapse. Science. 2005;307:124–27. doi: 10.1126/science.1103631. [DOI] [PubMed] [Google Scholar]
- 139.Yao CK, Lin YQ, Ly CV, Ohyama T, Haueter CM, et al. A synaptic vesicle–associated Ca2+ channel promotes endocytosis and couples exocytosis to endocytosis. Cell. 2009;138:947–60. doi: 10.1016/j.cell.2009.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Smith RM, Baibakov B, Ikebuchi Y, White BH, Lambert NA, et al. Exocytotic insertion of calcium channels constrains compensatory endocytosis to sites of exocytosis. J. Cell Biol. 2000;148:755–67. doi: 10.1083/jcb.148.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Vogel SS. Channeling calcium: a shared mechanism for exocytosis-endocytosis coupling. Sci. Signal. 2009;2:e80. doi: 10.1126/scisignal.2102pe80. [DOI] [PubMed] [Google Scholar]
- 142.Beutner D, Voets T, Neher E, Moser T. Calcium dependence of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse. Neuron. 2001;29:681–90. doi: 10.1016/s0896-6273(01)00243-4. [DOI] [PubMed] [Google Scholar]
- 143.Poskanzer KE, Marek KW, Sweeney ST, Davis GW. Synaptotagmin I is necessary for compensatory synaptic vesicle endocytosis in vivo. Nature. 2003;426:559–63. doi: 10.1038/nature02184. [DOI] [PubMed] [Google Scholar]
- 144.Rosa JM, Torregrosa-Hetland CJ, Colmena I, Gutierrez LM, Garcia AG, Gandia L. Calcium entry through slow-inactivating L-type calcium channels preferentially triggers endocytosis rather than exocytosis in bovine chromaffin cells. Am. J. Physiol. Cell Physiol. 2011;301:C86–98. doi: 10.1152/ajpcell.00440.2010. [DOI] [PubMed] [Google Scholar]
- 145.Cousin MA, Robinson PJ. The dephosphins: Dephosphorylation by calcineurin triggers synaptic vesicle endocytosis. Trends Neurosci. 2001;24:659–65. doi: 10.1016/s0166-2236(00)01930-5. [DOI] [PubMed] [Google Scholar]
- 146.Clayton EL, Anggono V, Smillie KJ, Chau N, Robinson PJ, Cousin MA. The phospho-dependent dynamin-syndapin interaction triggers activity-dependent bulk endocytosis of synaptic vesicles. J. Neurosci. 2009;29:7706–17. doi: 10.1523/JNEUROSCI.1976-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Xue J, Graham ME, Novelle AE, Sue N, Gray N, et al. Calcineurin selectively docks with the dynamin Ixb splice variant to regulate activity-dependent bulk endocytosis. J. Biol. Chem. 2011;286:30295–303. doi: 10.1074/jbc.M111.273110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Engisch KL, Nowycky MC. Compensatory and excess retrieval: two types of endocytosis following single step depolarizations in bovine adrenal chromaffin cells. J. Physiol. 1998;506(Pt. 3):591–608. doi: 10.1111/j.1469-7793.1998.591bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chan SA, Chow R, Smith C. Calcium dependence of action potential–induced endocytosis in chromaffin cells. Pflüg. Arch. 2003;445:540–46. doi: 10.1007/s00424-002-0966-y. [DOI] [PubMed] [Google Scholar]
- 150.Smillie KJ, Evans GJ, Cousin MA. Developmental change in the calcium sensor for synaptic vesicle endocytosis in central nerve terminals. J. Neurochem. 2005;94:452–58. doi: 10.1111/j.1471-4159.2005.03213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kuromi H, Yoshihara M, Kidokoro Y. An inhibitory role of calcineurin in endocytosis of synaptic vesicles at nerve terminals of Drosophila larvae. Neurosci. Res. 1997;27:101–13. doi: 10.1016/s0168-0102(96)01132-7. [DOI] [PubMed] [Google Scholar]
- 152.Xu J, Mashimo T, Südhof TC. Synaptotagmin-1, -2, and -9: Ca2+ sensors for fast release that specify distinct presynaptic properties in subsets of neurons. Neuron. 2007;54:567–81. doi: 10.1016/j.neuron.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 153.Nicholson-Tomishima K, Ryan TA. Kinetic efficiency of endocytosis at mammalian CNS synapses requires synaptotagmin I. Proc. Natl. Acad. Sci. USA. 2004;101:16648–52. doi: 10.1073/pnas.0406968101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Poskanzer KE, Fetter RD, Davis GW. Discrete residues in the C2B domain of synaptotagmin I independently specify endocytic rate and synaptic vesicle size. Neuron. 2006;50:49–62. doi: 10.1016/j.neuron.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 155.Yao J, Kwon SE, Gaffaney JD, Dunning FM, Chapman ER. Uncoupling the roles of synaptotagmin I during endo- and exocytosis of synaptic vesicles. Nat. Neurosci. 2011;15:243–49. doi: 10.1038/nn.3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhang JZ, Davletov BA, Südhof TC, Anderson RG. Synaptotagmin I is a high affinity receptor for clathrin AP-2: implications for membrane recycling. Cell. 1994;78:751–60. doi: 10.1016/s0092-8674(94)90442-1. [DOI] [PubMed] [Google Scholar]
- 157.Haucke V, Wenk MR, Chapman ER, Farsad K, De Camilli P. Dual interaction of synaptotagmin with μ2- and α-adaptin facilitates clathrin-coated pit nucleation. EMBO J. 2000;19:6011–19. doi: 10.1093/emboj/19.22.6011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Maritzen T, Podufall J, Haucke V. Stonins—specialized adaptors for synaptic vesicle recycling and beyond? Traffic. 2010;11:8–15. doi: 10.1111/j.1600-0854.2009.00971.x. [DOI] [PubMed] [Google Scholar]
- 159.Deak F, Schoch S, Liu X, Südhof TC, Kavalali ET. Synaptobrevin is essential for fast synaptic-vesicle endocytosis. Nat. Cell Biol. 2004;6:1102–8. doi: 10.1038/ncb1185. [DOI] [PubMed] [Google Scholar]
- 160.Zhang Z, Wang D, Sun T, Xu J, Chiang HC, et al. The SNARE proteins SNAP25 and synaptobrevin are involved in endocytosis at hippocampal synapses. J. Neurosci. 2013;33:9169–75. doi: 10.1523/JNEUROSCI.0301-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Koo SJ, Markovic S, Puchkov D, Mahrenholz CC, Beceren-Braun F, et al. SNARE motif–mediated sorting of synaptobrevin by the endocytic adaptors clathrin assembly lymphoid myeloid leukemia (CALM) and AP180 at synapses. Proc. Natl. Acad. Sci. USA. 2011;108:13540–45. doi: 10.1073/pnas.1107067108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Miller SE, Sahlender DA, Graham SC, Honing S, Robinson MS, et al. The molecular basis for the endocytosis of small R-SNAREs by the clathrin adaptor CALM. Cell. 2011;147:1118–31. doi: 10.1016/j.cell.2011.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Okamoto M, Schoch S, Südhof TC. EHSH1/intersectin, a protein that contains EH and SH3 domains and binds to dynamin and SNAP-25. A protein connection between exocytosis and endocytosis? J. Biol. Chem. 1999;274:18446–54. doi: 10.1074/jbc.274.26.18446. [DOI] [PubMed] [Google Scholar]
- 164.Galas MC, Chasserot-Golaz S, Dirrig-Grosch S, Bader MF. Presence of dynamin–syntaxin complexes associated with secretory granules in adrenal chromaffin cells. J. Neurochem. 2000;75:1511–19. doi: 10.1046/j.1471-4159.2000.0751511.x. [DOI] [PubMed] [Google Scholar]
- 165.Sheng J, He L, Zheng H, Xue L, Luo F, et al. Calcium-channel number critically influences synaptic strength and plasticity at the active zone. Nat. Neurosci. 2012;15:998–1006. doi: 10.1038/nn.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Coorssen JR, Schmitt H, Almers W. Ca2+ triggers massive exocytosis in Chinese hamster ovary cells. EMBO J. 1996;15:3787–91. [PMC free article] [PubMed] [Google Scholar]
- 167.Kennedy MJ, Ehlers MD. Mechanisms and function of dendritic exocytosis. Neuron. 2011;69:856–75. doi: 10.1016/j.neuron.2011.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ehlers MD. Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron. 2000;28:511–25. doi: 10.1016/s0896-6273(00)00129-x. [DOI] [PubMed] [Google Scholar]
- 169.Beattie EC, Carroll RC, Yu X, Morishita W, Yasuda H, et al. Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nat. Neurosci. 2000;3:1291–300. doi: 10.1038/81823. [DOI] [PubMed] [Google Scholar]
- 170.Newpher TM, Ehlers MD. Glutamate receptor dynamics in dendritic microdomains. Neuron. 2008;58:472–97. doi: 10.1016/j.neuron.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Foley K, Boguslavsky S, Klip A. Endocytosis, recycling, and regulated exocytosis of glucose transporter 4. Biochemistry. 2011;50:3048–61. doi: 10.1021/bi2000356. [DOI] [PubMed] [Google Scholar]
- 172.Almena M, Merida I. Shaping up the membrane: Diacylglycerol coordinates spatial orientation of signaling. Trends Biochem. Sci. 2011;36:593–603. doi: 10.1016/j.tibs.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 173.Ceccarelli B, Hurlbut WP, Mauro A. Depletion of vesicles from frog neuromuscular junctions by prolonged tetanic stimulation. J. Cell Biol. 1972;54:30–38. doi: 10.1083/jcb.54.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]