Abstract
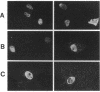
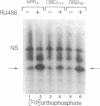
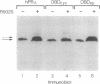
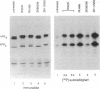
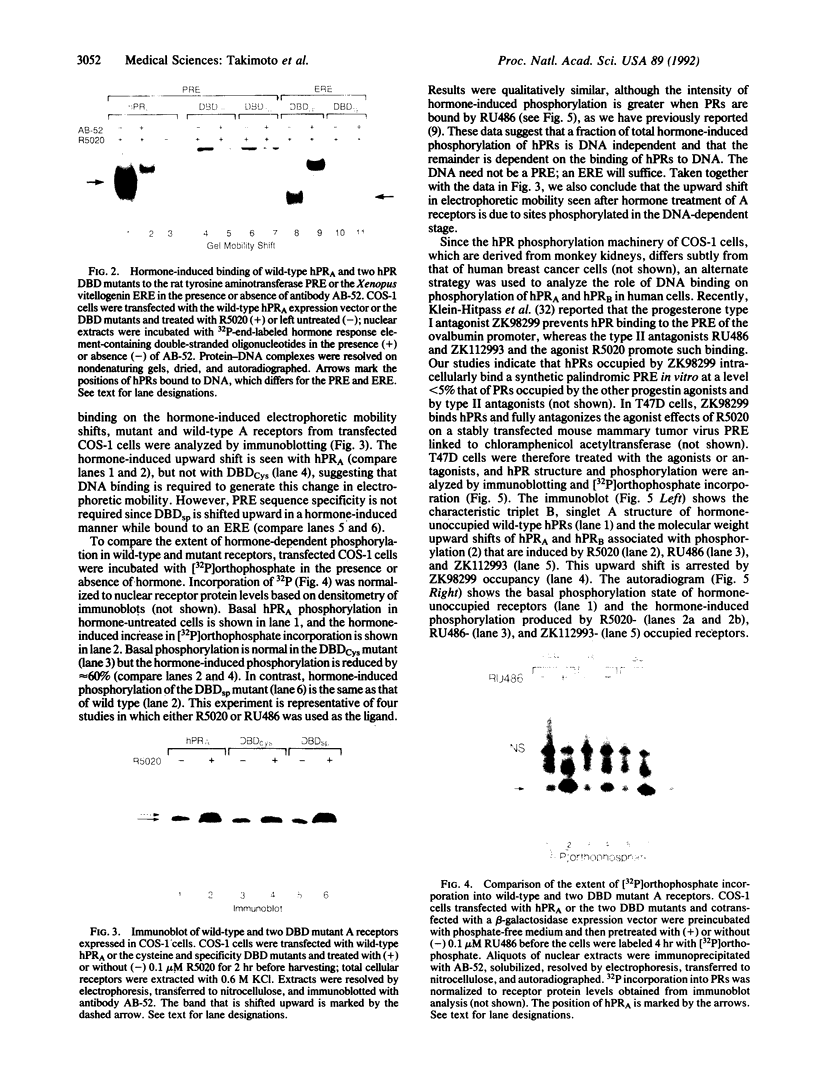
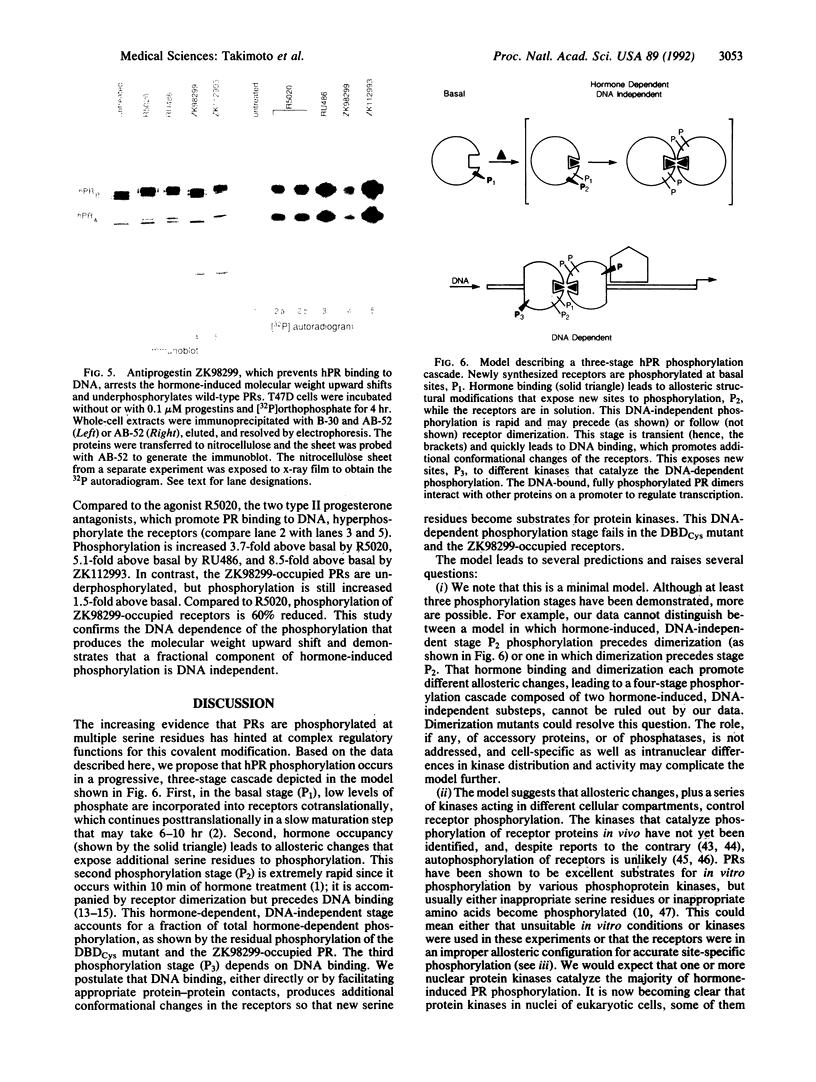
Human progesterone receptors (hPRs) are phosphorylated at multiple serine residues, first in a basal step and then in a hormone-induced step. To determine whether hormone-induced phosphorylation precedes or follows the interaction of hPRs with DNA two strategies were used. (i) DNA binding was prevented or altered with site-specific mutants of the A form of hPR; (ii) DNA binding of wild-type hPR forms A and B was prevented with the progesterone antagonist ZK98299. Two hPRA mutants were constructed: DBDCys, which lacks a critical cysteine residue in the first zinc finger, and DBDsp, which is mutated at three discriminatory amino acids to change its DNA binding specificity from a progesterone response element to an estrogen response element. Receptors were transiently expressed in PR-negative cells and were intranuclear. DBDCys did not bind DNA in vitro and DBDsp bound only the estrogen response element. Transiently expressed hPRA and DBDsp showed the upward shift in electrophoretic mobility characteristic of hormone-induced phosphorylation; it was absent with DBDCys. Hormone-induced [32P] orthophosphate incorporation into transiently expressed DBDCys was reduced 60% compared to hPRA and DBDsp but was not eliminated. ZK98299 binds hPRs but prevents their interaction with DNA. Compared to R5020, the antagonist reduced phosphorylation of hPRB and hPRA in T47D breast cancer cells by 60% and totally prevented the mobility shift. We conclude that the hormone-induced phosphorylation of hPR includes DNA-independent and DNA-dependent stages and that only DNA-dependent sites contribute to the mobility shift.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ankenbauer W., Strähle U., Schütz G. Synergistic action of glucocorticoid and estradiol responsive elements. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7526–7530. doi: 10.1073/pnas.85.20.7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auwerx J., Sassone-Corsi P. IP-1: a dominant inhibitor of Fos/Jun whose activity is modulated by phosphorylation. Cell. 1991 Mar 8;64(5):983–993. doi: 10.1016/0092-8674(91)90322-p. [DOI] [PubMed] [Google Scholar]
- Bailly A., Le Page C., Rauch M., Milgrom E. Sequence-specific DNA binding of the progesterone receptor to the uteroglobin gene: effects of hormone, antihormone and receptor phosphorylation. EMBO J. 1986 Dec 1;5(12):3235–3241. doi: 10.1002/j.1460-2075.1986.tb04634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerji J., Rusconi S., Schaffner W. Expression of a beta-globin gene is enhanced by remote SV40 DNA sequences. Cell. 1981 Dec;27(2 Pt 1):299–308. doi: 10.1016/0092-8674(81)90413-x. [DOI] [PubMed] [Google Scholar]
- Binétruy B., Smeal T., Karin M. Ha-Ras augments c-Jun activity and stimulates phosphorylation of its activation domain. Nature. 1991 May 9;351(6322):122–127. doi: 10.1038/351122a0. [DOI] [PubMed] [Google Scholar]
- Bodwell J. E., Ortí E., Coull J. M., Pappin D. J., Smith L. I., Swift F. Identification of phosphorylated sites in the mouse glucocorticoid receptor. J Biol Chem. 1991 Apr 25;266(12):7549–7555. [PubMed] [Google Scholar]
- Carter T., Vancurová I., Sun I., Lou W., DeLeon S. A DNA-activated protein kinase from HeLa cell nuclei. Mol Cell Biol. 1990 Dec;10(12):6460–6471. doi: 10.1128/mcb.10.12.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsen M., Hinck L., Ringold G. M. Two amino acids within the knuckle of the first zinc finger specify DNA response element activation by the glucocorticoid receptor. Cell. 1989 Jun 30;57(7):1131–1138. doi: 10.1016/0092-8674(89)90050-0. [DOI] [PubMed] [Google Scholar]
- Denner L. A., Schrader W. T., O'Malley B. W., Weigel N. L. Hormonal regulation and identification of chicken progesterone receptor phosphorylation sites. J Biol Chem. 1990 Sep 25;265(27):16548–16555. [PubMed] [Google Scholar]
- Denner L. A., Weigel N. L., Maxwell B. L., Schrader W. T., O'Malley B. W. Regulation of progesterone receptor-mediated transcription by phosphorylation. Science. 1990 Dec 21;250(4988):1740–1743. doi: 10.1126/science.2176746. [DOI] [PubMed] [Google Scholar]
- Denner L. A., Weigel N. L., Schrader W. T., O'Malley B. W. Hormone-dependent regulation of chicken progesterone receptor deoxyribonucleic acid binding and phosphorylation. Endocrinology. 1989 Dec;125(6):3051–3058. doi: 10.1210/endo-125-6-3051. [DOI] [PubMed] [Google Scholar]
- Diamond M. I., Miner J. N., Yoshinaga S. K., Yamamoto K. R. Transcription factor interactions: selectors of positive or negative regulation from a single DNA element. Science. 1990 Sep 14;249(4974):1266–1272. doi: 10.1126/science.2119054. [DOI] [PubMed] [Google Scholar]
- Estes P. A., Suba E. J., Lawler-Heavner J., Elashry-Stowers D., Wei L. L., Toft D. O., Sullivan W. P., Horwitz K. B., Edwards D. P. Immunologic analysis of human breast cancer progesterone receptors. 1. Immunoaffinity purification of transformed receptors and production of monoclonal antibodies. Biochemistry. 1987 Sep 22;26(19):6250–6262. doi: 10.1021/bi00393a045. [DOI] [PubMed] [Google Scholar]
- Eul J., Meyer M. E., Tora L., Bocquel M. T., Quirin-Stricker C., Chambon P., Gronemeyer H. Expression of active hormone and DNA-binding domains of the chicken progesterone receptor in E. coli. EMBO J. 1989 Jan;8(1):83–90. doi: 10.1002/j.1460-2075.1989.tb03351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia T., Jung-Testas I., Baulieu E. E. Tightly bound nuclear progesterone receptor is not phosphorylated in primary chick oviduct cultures. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7573–7577. doi: 10.1073/pnas.83.20.7573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia T., Tuohimaa P., Mester J., Buchou T., Renoir J. M., Baulieu E. E. Protein kinase activity of purified components of the chicken oviduct progesterone receptor. Biochem Biophys Res Commun. 1983 Jun 29;113(3):960–966. doi: 10.1016/0006-291x(83)91092-6. [DOI] [PubMed] [Google Scholar]
- Gonzalez G. A., Montminy M. R. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989 Nov 17;59(4):675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- Gordon M. S., Notides A. C. Computer modeling of estradiol interactions with the estrogen receptor. J Steroid Biochem. 1986 Aug;25(2):177–181. doi: 10.1016/0022-4731(86)90414-0. [DOI] [PubMed] [Google Scholar]
- Graham M. L., 2nd, Dalquist K. E., Horwitz K. B. Simultaneous measurement of progesterone receptors and DNA indices by flow cytometry: analysis of breast cancer cell mixtures and genetic instability of the T47D line. Cancer Res. 1989 Jul 15;49(14):3943–3949. [PubMed] [Google Scholar]
- Guiochon-Mantel A., Loosfelt H., Lescop P., Sar S., Atger M., Perrot-Applanat M., Milgrom E. Mechanisms of nuclear localization of the progesterone receptor: evidence for interaction between monomers. Cell. 1989 Jun 30;57(7):1147–1154. doi: 10.1016/0092-8674(89)90052-4. [DOI] [PubMed] [Google Scholar]
- Horwitz K. B., Francis M. D., Wei L. L. Hormone-dependent covalent modification and processing of human progesterone receptors in the nucleus. DNA. 1985 Dec;4(6):451–460. doi: 10.1089/dna.1985.4.451. [DOI] [PubMed] [Google Scholar]
- Jackson S. P., MacDonald J. J., Lees-Miller S., Tjian R. GC box binding induces phosphorylation of Sp1 by a DNA-dependent protein kinase. Cell. 1990 Oct 5;63(1):155–165. doi: 10.1016/0092-8674(90)90296-q. [DOI] [PubMed] [Google Scholar]
- Kastner P., Krust A., Turcotte B., Stropp U., Tora L., Gronemeyer H., Chambon P. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J. 1990 May;9(5):1603–1614. doi: 10.1002/j.1460-2075.1990.tb08280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein-Hitpass L., Cato A. C., Henderson D., Ryffel G. U. Two types of antiprogestins identified by their differential action in transcriptionally active extracts from T47D cells. Nucleic Acids Res. 1991 Mar 25;19(6):1227–1234. doi: 10.1093/nar/19.6.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein-Hitpass L., Schorpp M., Wagner U., Ryffel G. U. An estrogen-responsive element derived from the 5' flanking region of the Xenopus vitellogenin A2 gene functions in transfected human cells. Cell. 1986 Sep 26;46(7):1053–1061. doi: 10.1016/0092-8674(86)90705-1. [DOI] [PubMed] [Google Scholar]
- Klein-Hitpass L., Tsai S. Y., Weigel N. L., Allan G. F., Riley D., Rodriguez R., Schrader W. T., Tsai M. J., O'Malley B. W. The progesterone receptor stimulates cell-free transcription by enhancing the formation of a stable preinitiation complex. Cell. 1990 Jan 26;60(2):247–257. doi: 10.1016/0092-8674(90)90740-6. [DOI] [PubMed] [Google Scholar]
- Kumar V., Chambon P. The estrogen receptor binds tightly to its responsive element as a ligand-induced homodimer. Cell. 1988 Oct 7;55(1):145–156. doi: 10.1016/0092-8674(88)90017-7. [DOI] [PubMed] [Google Scholar]
- Kurl R. N., Jacob S. T. Phosphorylation of purified glucocorticoid receptor from rat liver by an endogenous protein kinase. Biochem Biophys Res Commun. 1984 Mar 15;119(2):700–705. doi: 10.1016/s0006-291x(84)80307-1. [DOI] [PubMed] [Google Scholar]
- Logeat F., Le Cunff M., Pamphile R., Milgrom E. The nuclear-bound form of the progesterone receptor is generated through a hormone-dependent phosphorylation. Biochem Biophys Res Commun. 1985 Aug 30;131(1):421–427. doi: 10.1016/0006-291x(85)91819-4. [DOI] [PubMed] [Google Scholar]
- Logeat F., Le Cunff M., Rauch M., Brailly S., Milgrom E. Characterization of a casein kinase which interacts with the rabbit progesterone receptor. Differences with the in vivo hormone-dependent phosphorylation. Eur J Biochem. 1987 Dec 30;170(1-2):51–57. doi: 10.1111/j.1432-1033.1987.tb13666.x. [DOI] [PubMed] [Google Scholar]
- Meyer M. E., Gronemeyer H., Turcotte B., Bocquel M. T., Tasset D., Chambon P. Steroid hormone receptors compete for factors that mediate their enhancer function. Cell. 1989 May 5;57(3):433–442. doi: 10.1016/0092-8674(89)90918-5. [DOI] [PubMed] [Google Scholar]
- Meyer M. E., Pornon A., Ji J. W., Bocquel M. T., Chambon P., Gronemeyer H. Agonistic and antagonistic activities of RU486 on the functions of the human progesterone receptor. EMBO J. 1990 Dec;9(12):3923–3932. doi: 10.1002/j.1460-2075.1990.tb07613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen C. J., Sando J. J., Pratt W. B. Evidence that dephosphorylation inactivates glucocorticoid receptors. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1398–1402. doi: 10.1073/pnas.74.4.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power R. F., Lydon J. P., Conneely O. M., O'Malley B. W. Dopamine activation of an orphan of the steroid receptor superfamily. Science. 1991 Jun 14;252(5012):1546–1548. doi: 10.1126/science.2047861. [DOI] [PubMed] [Google Scholar]
- Puri R. K., Toft D. O. Peptide mapping analysis of the avian progesterone receptor. J Biol Chem. 1986 Apr 25;261(12):5651–5657. [PubMed] [Google Scholar]
- Sanchez E. R., Pratt W. B. Phosphorylation of L-cell glucocorticoid receptors in immune complexes: evidence that the receptor is not a protein kinase. Biochemistry. 1986 Mar 25;25(6):1378–1382. doi: 10.1021/bi00354a028. [DOI] [PubMed] [Google Scholar]
- Severne Y., Wieland S., Schaffner W., Rusconi S. Metal binding 'finger' structures in the glucocorticoid receptor defined by site-directed mutagenesis. EMBO J. 1988 Aug;7(8):2503–2508. doi: 10.1002/j.1460-2075.1988.tb03097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan P. L., Evans R. M., Horwitz K. B. Phosphotryptic peptide analysis of human progesterone receptor. New phosphorylated sites formed in nuclei after hormone treatment. J Biol Chem. 1989 Apr 15;264(11):6520–6528. [PubMed] [Google Scholar]
- Sheridan P. L., Francis M. D., Horwitz K. B. Synthesis of human progesterone receptors in T47D cells. Nascent A- and B-receptors are active without a phosphorylation-dependent post-translational maturation step. J Biol Chem. 1989 Apr 25;264(12):7054–7058. [PubMed] [Google Scholar]
- Sheridan P. L., Krett N. L., Gordon J. A., Horwitz K. B. Human progesterone receptor transformation and nuclear down-regulation are independent of phosphorylation. Mol Endocrinol. 1988 Dec;2(12):1329–1342. doi: 10.1210/mend-2-12-1329. [DOI] [PubMed] [Google Scholar]
- Smith D. F., Faber L. E., Toft D. O. Purification of unactivated progesterone receptor and identification of novel receptor-associated proteins. J Biol Chem. 1990 Mar 5;265(7):3996–4003. [PubMed] [Google Scholar]
- Strähle U., Klock G., Schütz G. A DNA sequence of 15 base pairs is sufficient to mediate both glucocorticoid and progesterone induction of gene expression. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7871–7875. doi: 10.1073/pnas.84.22.7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strähle U., Schmid W., Schütz G. Synergistic action of the glucocorticoid receptor with transcription factors. EMBO J. 1988 Nov;7(11):3389–3395. doi: 10.1002/j.1460-2075.1988.tb03212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan W. P., Madden B. J., McCormick D. J., Toft D. O. Hormone-dependent phosphorylation of the avian progesterone receptor. J Biol Chem. 1988 Oct 15;263(29):14717–14723. [PubMed] [Google Scholar]
- Takimoto G. S., Tasset D. M., Miller L. A., Horwitz K. B. Epitope mapping of the anti-human progesterone receptor monoclonal antibody, AB-52. J Steroid Biochem Mol Biol. 1991 Nov;39(5A):687–692. doi: 10.1016/0960-0760(91)90368-f. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Herr W. Differential transcriptional activation by Oct-1 and Oct-2: interdependent activation domains induce Oct-2 phosphorylation. Cell. 1990 Feb 9;60(3):375–386. doi: 10.1016/0092-8674(90)90589-7. [DOI] [PubMed] [Google Scholar]
- Tsai S. Y., Carlstedt-Duke J., Weigel N. L., Dahlman K., Gustafsson J. A., Tsai M. J., O'Malley B. W. Molecular interactions of steroid hormone receptor with its enhancer element: evidence for receptor dimer formation. Cell. 1988 Oct 21;55(2):361–369. doi: 10.1016/0092-8674(88)90059-1. [DOI] [PubMed] [Google Scholar]
- Tsai S. Y., Tsai M. J., O'Malley B. W. Cooperative binding of steroid hormone receptors contributes to transcriptional synergism at target enhancer elements. Cell. 1989 May 5;57(3):443–448. doi: 10.1016/0092-8674(89)90919-7. [DOI] [PubMed] [Google Scholar]
- Umesono K., Evans R. M. Determinants of target gene specificity for steroid/thyroid hormone receptors. Cell. 1989 Jun 30;57(7):1139–1146. doi: 10.1016/0092-8674(89)90051-2. [DOI] [PubMed] [Google Scholar]
- Wei L. L., Sheridan P. L., Krett N. L., Francis M. D., Toft D. O., Edwards D. P., Horwitz K. B. Immunologic analysis of human breast cancer progesterone receptors. 2. Structure, phosphorylation, and processing. Biochemistry. 1987 Sep 22;26(19):6262–6272. doi: 10.1021/bi00393a046. [DOI] [PubMed] [Google Scholar]
- Woo D. D., Fay S. P., Griest R., Coty W., Goldfine I., Fox C. F. Differential phosphorylation of the progesterone receptor by insulin, epidermal growth factor, and platelet-derived growth factor receptor tyrosine protein kinases. J Biol Chem. 1986 Jan 5;261(1):460–467. [PubMed] [Google Scholar]