Abstract
Aims
To determine if the association between electrocardiographic left ventricular hypertrophy (ECG-LVH) and ischaemic stroke is partially explained by the concomitant presence of QT prolongation.
Methods and results
A total of 24 948 (mean age = 65 ± 9.4 years; 40% black; 55% women) participants from the REasons for Geographic And Racial Differences in Stroke (REGARDS) study were included in this analysis. Electrocardiographic left ventricular hypertrophy was defined by the Sokolow–Lyon criteria. Heart rate-adjusted QT (QTa) was computed using a linear regression model. Adjudicated ischaemic stroke events were the outcome of interest. Cox regression was used to compute hazard ratios (HRs) and 95% confidence intervals (CIs) for associations between ECG-LVH and prolonged QTa, in isolation and combined, with ischaemic stroke. There were 2422 (9.7%) participants with ECG-LVH, 820 (3.3%) with prolonged QTa, and 161 (0.6%) with both. Over a median follow-up of 7.6 years, 714 (2.9%) ischaemic stroke events occurred. After adjustment for stroke risk factors and potential confounders, an increased risk of ischaemic stroke was observed among participants with ECG-LVH and prolonged QTa (HR = 1.85, 95% CI = 1.04–3.30), isolated ECG-LVH (HR = 1.40, 95% CI = 1.13–1.75), and isolated prolonged QTa (HR = 1.45, 95% CI = 1.04–2.03) compared with participants without either condition. When ECG-LVH and prolonged QTa were examined as separate variables, the risk of ischaemic stroke for each condition remained statistically significant.
Conclusion
The combination of ECG-LVH and prolonged QT is associated with a higher risk of ischaemic stroke compared with either condition in isolation, and the stroke risk for each condition does not depend on the presence of the other.
Keywords: Left ventricular hypertrophy, QT interval, Stroke
What's new?
It is unclear if QT prolongation in the presence of ECG-LVH should be considered an innocent finding that is commonly found with ECG-LVH, and whether the risk of stroke with ECG-LVH is partially explained by the concomitant presence of QT prolongation.
Using data from the REGARDS study, we have shown that ECG-LVH and prolonged QT commonly coexist and the combination of both is associated with a higher risk of ischaemic stroke compared with either condition in isolation. Additionally, the ischaemic stroke risk for each electrocardiographic finding did not depend on the presence of the other.
ECG-LVH and prolonged QT are distinct clinical entities associated with separate risk profiles for ischaemic stroke.
Potentially, persons with ECG-LVH and prolonged QT will benefit from preventive measures to reduce the risk of future cerebrovascular events.
Introduction
Experimental studies have demonstrated that electrocardiographic left ventricular hypertrophy (ECG-LVH) alters ventricular conduction and repolarization, possibly resulting in prolongation of the QT interval.1–4 This is supported by results from observational studies showing that ECG-LVH and prolonged QT commonly coexist.5–9
Electrocardiographic left ventricular hypertrophy is an established risk factor for stroke and is one of the components of the Framingham Stroke Risk Score.10–12 Similarly, prolongation of the QT interval is an independent predictor of stroke.13–15 However, it is unclear if QT prolongation in the presence of ECG-LVH should be considered an innocent finding that is commonly found with ECG-LVH and whether the risk of stroke with ECG-LVH is partially explained by the concomitant presence of QT prolongation. Therefore, the purpose of this study was to examine the interrelationship between ECG-LVH and prolonged QT and the risk of ischaemic stroke in the REasons for Geographic And Racial Differences in Stroke (REGARDS) study.
Methods
Study population and design
Details of REGARDS have been published previously.16 Briefly, REGARDS was designed to identify causes of regional and racial disparities in stroke mortality. Between January 2003 and October 2007, the study over sampled blacks and residents of the stroke belt (North Carolina, South Carolina, Georgia, Alabama, Mississippi, Tennessee, Arkansas, and Louisiana). A total of 30 239 participants were recruited from a commercially available list of residents using postal mailings and telephone data. Demographic information and medical histories were obtained using a computer-assisted telephone interview (CATI) system that was conducted by trained interviewers. Additionally, a brief in-home physical examination was performed 3–4 weeks after the telephone interview. During the in-home visit, trained staff obtained information on medications taken within the past 2 weeks, blood and urine samples, and a resting electrocardiogram. For the purpose of this analysis, participants were excluded with data anomalies (n = 56), baseline stroke (n = 1930), missing follow-up data (n = 456), and missing baseline characteristics (n = 2849).
Left ventricular hypertrophy and prolonged QTa
Electrocardiographic left ventricular hypertrophy was defined by the Sokolow–Lyon criteria using baseline electrocardiogram data.17 Guided by current recommendations, heart rate-adjusted QT (QTa) was computed using a linear regression model.18 Specifically, we used a linear regression model with the QT interval as the dependent variable and heart rate as the independent variable. Based on the β-coefficient associated with heart rate, the following formula was derived to adjust for heart rate: QTa = QT + 2.03 × (heart rate –60).19 Prolonged QTa was defined as QTa ≥460 ms for women and ≥450 ms for men.18
Ischaemic stroke events
The adjudication process for stroke events in REGARDS has been previously reported.20 Briefly, during follow-up, reports of possible strokes, transient ischaemic attacks, deaths, hospitalizations or emergency department visits for stroke symptoms, or unknown reasons generated a request for medical record review. Possible stroke events were centrally adjudicated by a team of physicians. For deaths without medical records, death certificates and/or proxy interviews were used to adjudicate events. Strokes were defined using the World Health Organization (WHO) definition.21 Events that did not meet the WHO definition but with symptoms lasting >24 h and with imaging consistent with acute ischaemia or haemorrhage were classified as ‘clinical strokes’. When adjudicators agreed that the event was likely a stroke but information was insufficient to meet other classifications, the event was classified as probable stroke. This analysis included WHO, clinical, and probable ischaemic stroke events.
Covariates
Age, sex, race, and smoking status were self-reported. Smoking was defined as current tobacco use. Fasting blood samples were obtained and assayed for serum glucose. Diabetes was defined as a fasting glucose level ≥126 mg/dL (or a non-fasting glucose ≥200 mg/dL among those failing to fast) or self-reported diabetes medication use. The current use of aspirin and antihypertensive medications was self-reported. The use of statins, warfarin, and QT prolonging medications was ascertained during the in-home visit by pill bottle review. After the participant rested for 5 min in a seated position, blood pressure was measured using a sphygmomanometer. Two values were obtained following a standardized protocol and averaged. Hypertension was defined as blood pressure ≥140/90 or by the self-reported use of antihypertensive medications. Body mass index was defined as the weight in kilograms divided by the height in meters squared. Atrial fibrillation was identified from the baseline electrocardiogram and also from self-reported history of a physician diagnosis during the CATI survey.22 Coronary heart disease was ascertained by self-reported history of myocardial infarction, coronary artery bypass grafting, coronary angioplasty or stenting, or if evidence of prior myocardial infarction was present on the baseline electrocardiogram.
Statistics
Categorical variables were reported as frequency and percentage while continuous variables were reported as mean ± standard deviation. Statistical significance for categorical variables was tested using the χ2 method and the Kruskal–Wallis procedure for continuous variables. Participants' characteristics were compared across categories stratified by ECG-LVH and prolonged QTa. Incidence rates per 1000 person-years were calculated for ischaemic stroke using the following categories: ECG-LVH and prolonged QTa, isolated prolonged QTa, isolated ECG-LVH, no ECG-LVH or prolonged QTa (reference group). Kaplan–Meier estimates were used to compute the cumulative incidence of ischaemic stroke for each category, and the differences in estimates were compared using the log-rank procedure. Follow-up time was defined as the time between the baseline electrocardiogram measurement until a diagnosis of ischaemic stroke, death, loss to follow-up, or end of follow-up (31 March 2014). The influence of ECG-LVH and prolonged QTa on each condition in terms of their association with ischaemic stroke was assessed using an approach similar to what has been used to describe the interrelationship between ECG-LVH and prolonged QT as predictors of all-cause mortality.19 Specifically, we examined the interrelationship between ECG-LVH and prolonged QTa as predictors of ischaemic stroke using two approaches. First, we used Cox regression to compute hazard ratios (HRs) and 95% confidence intervals (CIs) for the associations of ECG-LVH and prolonged QTa, in isolation and combination, with ischaemic stroke. In these models, different combinations of ECG-LVH and prolonged QTa were used as one categorical variable with the above four levels. This approach aimed to examine whether there was an additive risk of stroke when ECG-LVH and prolonged QTa coexist compared with each condition in isolation. Secondly, we examined the risk of stroke associated with ECG-LVH and prolonged QTa when entered in two separate models and with both entered in the same model as two separate variables (e.g. adjusting for each other). This method examined the attenuation of the magnitude of risk observed when the association between ECG-LVH and ischaemic stroke was adjusted for prolonged QTa, and vice versa. This approach determined how much the observed risk of ischaemic stroke associated with ECG-LVH was explained by prolonged QTa. In both approaches, the following models were constructed: Model 1, adjusted for age, sex, race, and age × race; Model 2, adjusted for Model 1 covariates plus systolic blood pressure, antihypertensive medications, current smoking, diabetes, body mass index, atrial fibrillation, and prevalent coronary heart disease; Model 3, adjusted for Model 2 covariates plus QT prolonging medications, statin use, warfarin use, and aspirin use. Additionally, we examined if the association between ECG-LVH, prolonged QTa, and ischaemic stroke varied by age (<65 years vs. ≥65 years), sex, race (black vs. white), hypertension, coronary heart disease, and obesity using a stratification technique and comparing models with and without interaction terms (see Supplementary material online). Statistical significance for all comparisons including interactions was defined as P < 0.05. All tests of significance were two-sided. SAS® Version 9.3 (Cary, NC, USA) was used for all analyses.
Results
A total of 24 948 (mean age = 65 ± 9.4 years; 40% black; 55% women) participants were included in this analysis. There were 2422 (9.7%) participants with ECG-LVH and 820 (3.3%) with prolonged QTa. Participants with ECG-LVH were more likely to have concomitant prolonged QTa (n = 161, 6.7%) than those without ECG-LVH (n = 659, 2.9%) (P < 0.0001). Baseline characteristics for study participants by ECG-LVH and prolonged QTa are shown in Table 1.
Table 1.
Baseline characteristics by ECG-LVH and prolonged QTa (n = 24 948)
| Characteristic | No ECG-LVH (n = 22 526) |
ECG-LVH (n = 2422) |
P-valuea | ||
|---|---|---|---|---|---|
| No prolonged QTa (n = 21 867) | Prolonged QTa (n = 659) | No prolonged QTa (n = 2261) | Prolonged QTa (n = 161) | ||
| Age, mean (SD), years | 64 (9.3) | 69 (9.6) | 66 (9.1) | 72 (9.6) | <0.0001 |
| Male (%) | 9754 (45) | 420 (64) | 941 (42) | 94 (58) | <0.0001 |
| Black (%) | 8320 (38) | 213 (32) | 1372 (61) | 83 (52) | <0.0001 |
| Current smoker (%) | 3189 (15) | 80 (12) | 201 (8.9) | 17 (11) | <0.0001 |
| Diabetes (%) | 4335 (20) | 169 (26) | 629 (28) | 59 (37) | <0.0001 |
| Systolic blood pressure, mean (SD) (mmHg) | 126 (16) | 130 (17) | 134 (18) | 135 (19) | <0.0001 |
| Body mass index, mean (SD) (kg/m2) | 29.1 (6.1) | 28.9 (5.9) | 31.2 (6.1) | 30.4 (6.4) | <0.0001 |
| Aspirin (%) | 9199 (42) | 365 (55) | 983 (43) | 96 (60) | <0.0001 |
| Warfarin (%) | 595 (2.7) | 76 (12) | 70 (3.1) | 12 (7.5) | <0.0001 |
| Statin (%) | 6618 (30) | 258 (39) | 739 (33) | 71 (44) | <0.0001 |
| Antihypertensive medications (%) | 10 837 (50) | 441 (67) | 1532 (68) | 128 (80) | <0.0001 |
| Atrial fibrillation (%) | 1658 (7.6) | 141 (21) | 187 (8.3) | 22 (14) | <0.0001 |
| Coronary heart disease (%) | 3363 (15) | 257 (39) | 482 (21) | 76 (47) | <0.0001 |
| QT prolonging medications (%) | 5330 (24) | 212 (32) | 510 (23) | 50 (31) | <0.0001 |
ECG-LVH, electrocardiographic left ventricular hypertrophy; QTa, heart rate-adjusted QT interval; SD, standard deviation.
aStatistical significance for categorical variables was tested using the χ2 method and for continuous variables the Kruskal–Wallis procedure was used.
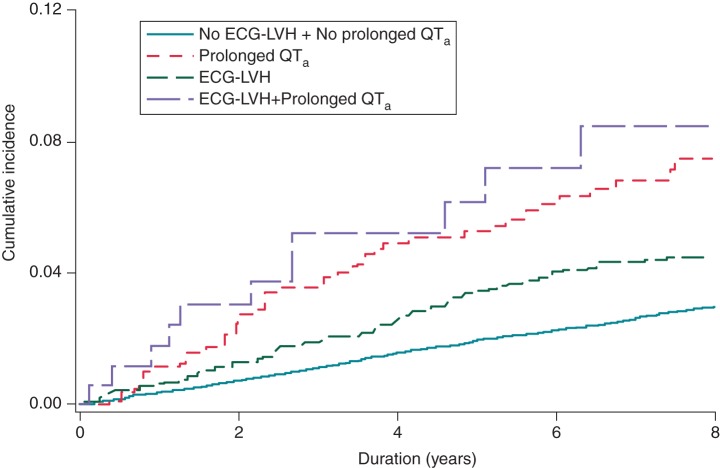
Over a median follow-up of 7.6 years, a total of 714 (2.9%) ischaemic stroke events occurred. A higher incidence of ischaemic stroke was observed among participants with ECG-LVH and prolonged QTa compared with those with isolated ECG-LVH, isolated prolonged QTa and those without either condition (Table 2). Cumulative incidence curves for ischaemic stroke events by each category are shown in Figure 1 and are statistically different (log-rank P < 0.0001).
Table 2.
Risk of ischaemic stroke associated with ECG-LVH and prolonged QTa
| ECG-LVH | Prolonged QTa | Incidence rate per 1000 person-years | Model 1a HR (95% CI) | Model 2b HR (95% CI) | Model 3c HR (95% CI) |
|---|---|---|---|---|---|
| Absent | Absent | 3.7 (3.4–4.0) | Referent | Referent | Referent |
| Present | Absent | 6.2 (5.1–7.5) | 1.49 (1.20–1.85) | 1.42 (1.14–1.77) | 1.41 (1.13–1.76) |
| Absent | Present | 9.3 (6.8–12.8) | 1.76 (1.26–2.45) | 1.46 (1.05–2.05) | 1.43 (1.02–2.00) |
| Present | Present | 13.8 (7.8–24.2) | 2.26 (1.27–4.01) | 1.88 (1.06–3.35) | 1.86 (1.04–3.31) |
CI, confidence interval; ECG-LVH, electrocardiographic left ventricular hypertrophy; HR, hazard ratio; QTa, heart rate-adjusted QT interval.
aModel 1, adjusted for age, sex, race, and age × race.
bModel 2, adjusted for Model 1 covariates plus systolic blood pressure, antihypertensive medications, current smoking, diabetes, body mass index, atrial fibrillation, and prevalent coronary heart disease.
cModel 3, adjusted for Model 2 covariates plus QT prolonging medications, statin use, warfarin use, and aspirin use.
Figure 1.
Unadjusted Kaplan–Meier survival curves stratified by ECG-LVH and prolonged QTa [cumulative incidence curves are statistically different (log-rank P < 0.0001)]. ECG-LVH, electrocardiographic left ventricular hypertrophy; QTa, heart rate-adjusted QT interval.
Electrocardiographic left ventricular hypertrophy and prolonged QTa, isolated ECG-LVH, and isolated prolonged QTa were associated with an increased risk of stroke compared with no ECG-LVH or prolonged QTa (Table 2). The results were consistent in subgroups stratified by age, sex, race, hypertension, coronary heart disease, and obesity (see Supplementary material online). When ECG-LVH and prolonged QTa were included separately in the model, both conditions remained significantly associated with ischaemic stroke (Table 3). Notably, the strength of the association was not materially altered when both conditions were included in the same model.
Table 3.
Ischaemic stroke risk associated with ECG-LVH and prolonged QTa with and without adjustment for each condition
| Model 1a HR (95% CI) | Model 2b HR (95% CI) | Model 3c HR (95% CI) | |
|---|---|---|---|
| ECG-LVH | 1.51 (1.23–1.85) | 1.42 (1.15–1.75) | 1.42 (1.15–1.75) |
| ECG-LVH with adjustment for prolonged QTa | 1.47 (1.19–1.81) | 1.40 (1.14–1.73) | 1.40 (1.13–1.72) |
| Prolonged QTa | 1.77 (1.32–2.37) | 1.47 (1.10–1.98) | 1.45 (1.08–1.94) |
| Prolonged QTa with adjustment for ECG-LVH | 1.70 (1.27–2.28) | 1.43 (1.06–1.92) | 1.40 (1.04–1.89) |
CI, confidence interval; ECG-LVH, electrocardiographic left ventricular hypertrophy; HR, hazard ratio; QTa, heart rate-adjusted QT interval.
aModel 1, adjusted for age, sex, race, and age × race.
bModel 2, adjusted for Model 1 covariates plus systolic blood pressure, antihypertensive medications, current smoking, diabetes, body mass index, atrial fibrillation, and prevalent coronary heart disease.
cModel 3, adjusted for Model 2 covariates plus QT prolonging medications, statin use, warfarin use, and aspirin use.
Discussion
In this analysis from REGARDS, we have shown that ECG-LVH and prolonged QT commonly coexist and the combination of both is associated with a higher risk of ischaemic stroke compared with either condition in isolation. Additionally, the stroke risk associated with each condition was not substantively altered when both ECG-LVH and prolonged QT were included in the same model, suggesting that the ischaemic stroke risk for each electrocardiographic finding does not depend on the presence of the other.
Several studies have shown that ECG-LVH and prolonged QT are risk factors for stroke.10–15 Owing to the fact that both ECG-LVH and prolonged QT commonly coexist, it was unclear if ECG-LVH and prolonged QT were two distinct entities with separate stroke risk profiles. However, the results of this analysis suggest that ECG-LVH and prolonged QT are distinct abnormalities that yield separate risks for ischaemic stroke. To our knowledge, only one study from the general Japanese population has attempted to show that each condition confers a separate stroke risk.23 Our results are in agreement with this study and confirm that both ECG-LVH and prolonged QT have different profiles regarding ischaemic stroke risk in a population of blacks and whites in the USA. Furthermore, our analysis accounted for medications that artificially prolong the QT interval and we used a method to calculate prolonged QT that is less susceptible to error when heart rate is high, such as Bazett's traditional formula for QT correction.18 Therefore, despite hypotheses that prolongation of the QT interval is a result of ECG-LVH, our findings show that ECG-LVH and prolonged QT are associated with separate risks of ischaemic stroke and suggest that prolongation of the QT interval is not an innocent consequence of repolarization.
Electrocardiographic left ventricular hypertrophy and prolonged QT have been linked with stroke by several mechanisms. Hypertrophy of the left ventricle is a likely consequence of long-standing hypertension and reflects a poor cardiovascular profile associated with an increased stroke risk.24–26 This is further supported by data that have shown the risk of stroke decreases with regression of ECG-LVH among patients who are treated with agents that block the renin–angiotensin–aldosterone system and suggest that neurohormonal mechanisms link ECG-LVH with stroke.27–29 Similarly, those with prolonged QT have poor risk factor profiles that also increase one's risk for cardiovascular events, including stroke.14,30 Another possible explanation is mediation by atrial fibrillation since both ECG-LVH and prolonged QT are associated with the development of this arrhythmia.31,32 However, we were unable to explore this hypothesis as we did not have incident atrial fibrillation in our dataset. Although we offer several mechanisms for the observed association, it is likely that both conditions are markers for poor cardiovascular profiles which predispose to the development of future cerebrovascular events rather than distinct pathological processes which result in stroke.
Electrocardiographic left ventricular hypertrophy and prolonged QT are commonly found in the general USA population.19 Our findings confirm that both conditions commonly coexist and confer an increased risk of ischaemic stroke when in combination. Additionally, both electrocardiographic findings are associated with separate risk profiles for ischaemic stroke. Potentially, preventive measures that result in decreasing the prevalence of ECG-LVH and prolonged QT are associated with reductions in ischaemic stroke risk. This is supported by data that have shown reductions in stroke risk among those with ECG-LVH regression,27–29 and further reduction in stroke risk possibly occurs with the return to a normal QT interval. However, it is currently unknown if reducing the QT interval will alter stroke risk in those with ECG-LVH, and further studies are needed before recommendations regarding clinical practice are made. It is more likely that reductions in the presence of ECG-LVH and prolonged QT will decrease the cardiovascular risk factor profile associated with conditions that serve as mediators in the causal pathway between ECG-LVH, prolonged QT, and ischaemic stroke (e.g. atrial fibrillation). Nonetheless, we have identified a group more likely to experience ischaemic stroke that possibly will benefit from preventive measures and risk factor modification (e.g. hypertension treatment) to reduce the risk of future cerebrovascular events. This finding likely is of interest to preventive cardiologists with aims to identify persons who are at risk for ischaemic stroke.
Our results should be interpreted in the context of several limitations. We had to exclude many participants with missing baseline data. However, these data were assumed to be missing at random. Also, the percentages of participants with ECG-LVH (8.5%) and future stroke events (2.6%) with missing data were comparable with those who had available data (ECG-LVH, P = 0.06; stroke, P = 0.50). Electrocardiographic left ventricular hypertrophy was defined by the Sokolow–Lyon criteria and the results possibly vary with different criteria. However, this definition is the most sensitive traditional ECG-LVH marker with the best overall diagnostic performance when compared with other criteria.33 Electrocardiographic left ventricular hypertrophy and the QT interval also are dynamic measurements that vary between electrocardiogram tracings, and the results potentially differ with subsequent recordings. Several baseline characteristics were self-reported and subjected our analysis to recall bias. Additionally, although we adjusted for several factors that are known to influence the development of ischaemic stroke, we acknowledge that residual confounding remains a possibility similar to other epidemiologic studies. We also were unable to adjust for conditions (e.g. incident atrial fibrillation) that potentially mediate the association between ECG-LVH, prolonged QT, and ischaemic stroke.
In conclusion, we have shown that ECG-LVH and prolonged QT are distinct clinical entities with separate risk profiles for ischaemic stroke. Further research is needed to confirm our findings and to explore the possible benefit of pharmacologic interventions that reduce the stroke burden in those with ECG-LVH and prolonged QT.
Supplementary material
Funding
This research project is supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, and Department of Health and Human Service. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health.
Supplementary Material
Acknowledgements
The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org.
Conflict of interest: none declared.
References
- 1. Winterton SJ, Turner MA, O'Gorman DJ, Flores NA, Sheridan DJ. Hypertrophy causes delayed conduction in human and guinea pig myocardium: accentuation during ischaemic perfusion. Cardiovasc Res 1994;28:47–54. [DOI] [PubMed] [Google Scholar]
- 2. McIntyre H, Fry CH. Abnormal action potential conduction in isolated human hypertrophied left ventricular myocardium. J Cardiovasc Electrophysiol 1997;8:887–94. [DOI] [PubMed] [Google Scholar]
- 3. Cooklin M, Wallis WR, Sheridan DJ, Fry CH. Changes in cell-to-cell electrical coupling associated with left ventricular hypertrophy. Circ Res 1997;80:765–71. [DOI] [PubMed] [Google Scholar]
- 4. Aronson RS. Characteristics of action potentials of hypertrophied myocardium from rats with renal hypertension. Circ Res 1980;47:443–54. [DOI] [PubMed] [Google Scholar]
- 5. Oikarinen L, Nieminen MS, Viitasalo M, Toivonen L, Wachtell K, Papademetriou V et al. Relation of QT interval and QT dispersion to echocardiographic left ventricular hypertrophy and geometric pattern in hypertensive patients. The LIFE study. The Losartan Intervention For Endpoint Reduction. J Hypertens 2001;19:1883–91. [DOI] [PubMed] [Google Scholar]
- 6. Johnson JN, Grifoni C, Bos JM, Saber-Ayad M, Ommen SR, Nistri S et al. Prevalence and clinical correlates of QT prolongation in patients with hypertrophic cardiomyopathy. Eur Heart J 2011;32:1114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wong KY, Lim PO, Wong SY, MacWalter RS, Struthers AD, MacDonald TM. Does a prolonged QT peak identify left ventricular hypertrophy in hypertension? Int J Cardiol 2003;89:179–86. [DOI] [PubMed] [Google Scholar]
- 8. Salles G, Leocadio S, Bloch K, Nogueira AR, Muxfeldt E. Combined QT interval and voltage criteria improve left ventricular hypertrophy detection in resistant hypertension. Hypertension 2005;46:1207–12. [DOI] [PubMed] [Google Scholar]
- 9. Opadijo OG, Omotoso AB, Araoye MA. Ventricular arrhythmias: Q-T prolongation and left ventricular hypertrophy in adult Nigerians with hypertensive heart disease. Niger Postgrad Med J 2003;10:76–8. [PubMed] [Google Scholar]
- 10. Wolf PA, D'Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke 1991;22:312–8. [DOI] [PubMed] [Google Scholar]
- 11. Verdecchia P, Porcellati C, Reboldi G, Gattobigio R, Borgioni C, Pearson TA et al. Left ventricular hypertrophy as an independent predictor of acute cerebrovascular events in essential hypertension. Circulation 2001;104:2039–44. [DOI] [PubMed] [Google Scholar]
- 12. Bots ML, Nikitin Y, Salonen JT, Elwood PC, Malyutina S, Freire de Concalves A et al. Left ventricular hypertrophy and risk of fatal and non-fatal stroke. EUROSTROKE: a collaborative study among research centres in Europe. J Epidemiol Community Health 2002;561(Suppl):i8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cardoso CR, Salles GF, Deccache W. QTc interval prolongation is a predictor of future strokes in patients with type 2 diabetes mellitus. Stroke 2003;34:2187–94. [DOI] [PubMed] [Google Scholar]
- 14. Soliman EZ, Howard G, Cushman M, Kissela B, Kleindorfer D, Le A et al. Prolongation of QTc and risk of stroke: the REGARDS (REasons for Geographic and Racial Differences in Stroke) study. J Am Coll Cardiol 2012;59:1460–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maebuchi D, Arima H, Doi Y, Ninomiya T, Yonemoto K, Tanizaki Y et al. QT interval prolongation and the risks of stroke and coronary heart disease in a general Japanese population: the Hisayama study. Hypertens Res 2010;33:916–21. [DOI] [PubMed] [Google Scholar]
- 16. Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ et al. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology 2005;25:135–43. [DOI] [PubMed] [Google Scholar]
- 17. Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J 1949;37:161–86. [DOI] [PubMed] [Google Scholar]
- 18. Rautaharju PM, Surawicz B, Gettes LS, Bailey JJ, Childers R, Deal BJ et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part IV: the ST segment, T and U waves, and the QT interval: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol 2009;53:982–91. [DOI] [PubMed] [Google Scholar]
- 19. Soliman EZ, Shah AJ, Boerkircher A, Li Y, Rautaharju PM. Inter-Relationship between electrocardiographic left ventricular hypertrophy and QT prolongation as predictors of increased risk of mortality in the general population. Circ Arrhythm Electrophysiol 2014;7:400–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Howard VJ, Kleindorfer DO, Judd SE, McClure LA, Safford MM, Rhodes JD et al. Disparities in stroke incidence contributing to disparities in stroke mortality. Ann Neurol 2011;69:619–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stroke–1989. Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO task force on stroke and other cerebrovascular disorders. Stroke 1989;20:1407–31. [DOI] [PubMed] [Google Scholar]
- 22. Soliman EZ, Howard G, Meschia JF, Cushman M, Muntner P, Pullicino PM et al. Self-reported atrial fibrillation and risk of stroke in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. Stroke 2011;42:2950–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ishikawa J, Ishikawa S, Kario K. Prolonged corrected QT interval is predictive of future stroke events even in subjects without ECG-diagnosed left ventricular hypertrophy. Hypertension 2014;65:554–60. [DOI] [PubMed] [Google Scholar]
- 24. Messerli FH, Aepfelbacher FC. Hypertension and left-ventricular hypertrophy. Cardiol Clin 1995;13:549–57. [PubMed] [Google Scholar]
- 25. Ohira T, Shahar E, Chambless LE, Rosamond WD, Mosley TH Jr, Folsom AR. Risk factors for ischemic stroke subtypes: the Atherosclerosis Risk in Communities study. Stroke 2006;37:2493–8. [DOI] [PubMed] [Google Scholar]
- 26. Kannel WB. Left ventricular hypertrophy as a risk factor: the Framingham experience. J Hypertens Suppl 1991;9:S3–8; discussion S-9. [DOI] [PubMed] [Google Scholar]
- 27. Larstorp AC, Okin PM, Devereux RB, Olsen MH, Ibsen H, Dahlof B et al. Changes in electrocardiographic left ventricular hypertrophy and risk of major cardiovascular events in isolated systolic hypertension: the LIFE study. J Hum Hypertens 2011;25:178–85. [DOI] [PubMed] [Google Scholar]
- 28. Okin PM, Devereux RB, Jern S, Kjeldsen SE, Julius S, Nieminen MS et al. Regression of electrocardiographic left ventricular hypertrophy during antihypertensive treatment and the prediction of major cardiovascular events. JAMA 2004;292:2343–9. [DOI] [PubMed] [Google Scholar]
- 29. Mathew J, Sleight P, Lonn E, Johnstone D, Pogue J, Yi Q et al. Reduction of cardiovascular risk by regression of electrocardiographic markers of left ventricular hypertrophy by the angiotensin-converting enzyme inhibitor ramipril. Circulation 2001;104:1615–21. [DOI] [PubMed] [Google Scholar]
- 30. Festa A, D'Agostino R Jr, Rautaharju P, O'Leary DH, Rewers M, Mykkanen L et al. Is QT interval a marker of subclinical atherosclerosis in nondiabetic subjects? The Insulin Resistance Atherosclerosis Study (IRAS). Stroke 1999;30:1566–71. [DOI] [PubMed] [Google Scholar]
- 31. Mandyam MC, Soliman EZ, Alonso A, Dewland TA, Heckbert SR, Vittinghoff E et al. The QT interval and risk of incident atrial fibrillation. Heart Rhythm 2013;10:1562–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA 1994;271:840–4. [PubMed] [Google Scholar]
- 33. Jain A, Tandri H, Dalal D, Chahal H, Soliman EZ, Prineas RJ et al. Diagnostic and prognostic utility of electrocardiography for left ventricular hypertrophy defined by magnetic resonance imaging in relationship to ethnicity: the Multi-Ethnic Study of Atherosclerosis (MESA). Am Heart J 2010;159:652–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.