Abstract
In this study, we aim to identify environmental molecules that can inhibit cholesterol biosynthesis, potentially leading to the same biochemical defects as observed in cholesterol biosynthesis disorders, which are often characterized by congenital malformations and developmental delay. Using the Distributed Structure-Searchable Toxicity (DSSTox) Database Network developed by EPA, we first carried out in silico screening of environmental molecules that display structures similar to AY9944, a known potent inhibitor of 3β-hydroxysterol-Δ7-reductase (DHCR7)—the last step of cholesterol biosynthesis. Molecules that display high similarity to AY9944 were subjected to test in mouse and human neuroblastoma cells for their effectiveness in inhibiting cholesterol biosynthesis by analyzing cholesterol and its precursor using gas chromatography-mass spectrometry. We found that a common disinfectant mixture, benzalkonium chlorides (BACs), exhibits high potency in inhibiting DHCR7, as suggested by greatly elevated levels of the cholesterol precursor, 7-dehydrocholesterol (7-DHC). Subsequent structure-activity studies suggested that the potency of BACs as Dhcr7 inhibitors decrease with the length of their hydrocarbon chain: C10 > C12 ≫ C14 > C16. Real-time qPCR analysis revealed upregulation of the genes related to cholesterol biosynthesis and downregulation of the genes related to cholesterol efflux, suggesting a feedback response to the inhibition. Furthermore, an oxidative metabolite of 7-DHC that was previously identified as a biomarker in vivo was also found in cells exposed to BACs by liquid chromatography-mass spectrometry. Our findings suggest that certain environmental molecules could potently inhibit cholesterol biosynthesis, which could be a new link between environment and developmental disorders.
Keywords: benzalkonium chlorides, cholesterol biosynthesis inhibition, DHCR7, 7-dehydrocholesterol, quaternary ammonium compounds.
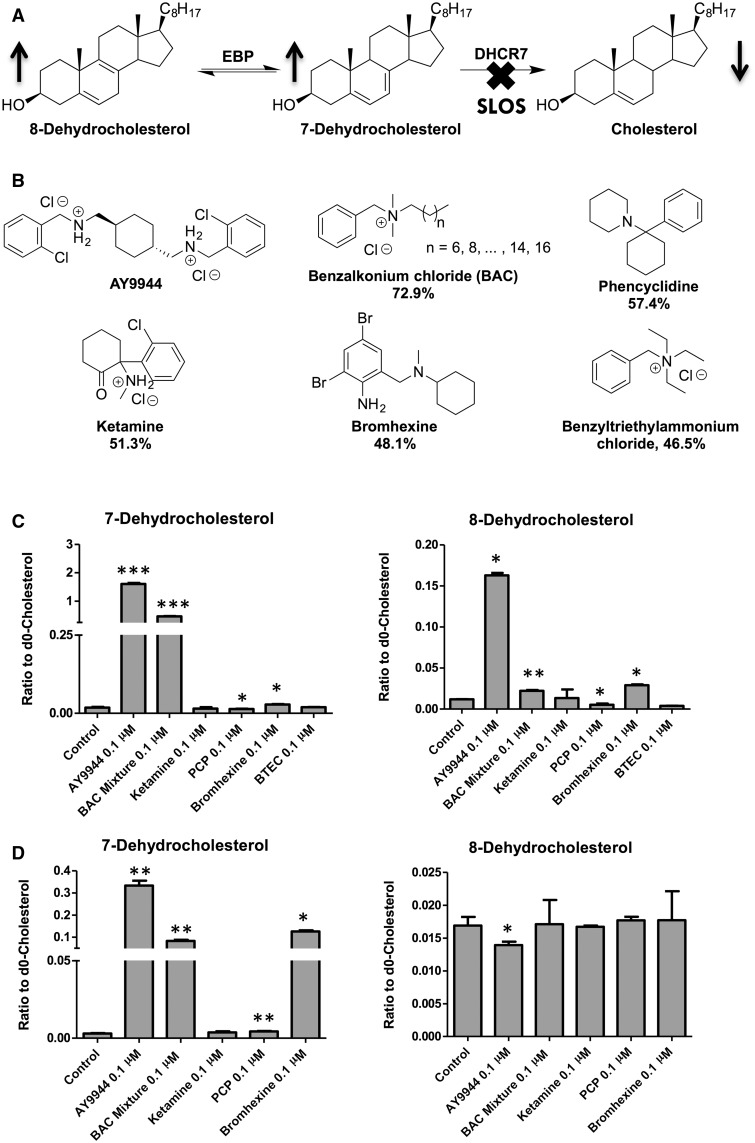
Cholesterol plays important roles in embryonic development (Porter et al., 1996), synapse formation and function (Koudinov and Koudinova 2001; Mauch et al., 2001), and myelination (Saher et al., 2005). Almost all cholesterol in the brain is synthesized locally and independently (Bjorkhem and Meaney, 2004). We have been interested in the cholesterol biosynthesis disorder, Smith-Lemli-Opitz syndrome (SLOS), which is caused by defective 3β-hydroxysterol-Δ7-reductase (DHCR7; EC 1.3.1.21) (Fitzky et al., 1998; Wassif et al., 1998; Waterham et al., 1998). This defect leads to greatly elevated levels of cholesterol precursors, including 7-dehydrocholesterol (7-DHC) and 8-dehydrocholesterol (8-DHC), and decreased levels of cholesterol (Tint et al., 1994, 1995) (Figure 1A). The formation of 8-DHC is due to the activity of 3β-hydroxysterol-Δ8,Δ7-isomerase (EBP; EC 5.3.3.5). The SLOS phenotype includes multiple congenital malformations, developmental delay, cognitive impairment, and behavior problems (Bukelis et al., 2007; Nowaczyk and Irons 2012; Porter and Herman 2011; Sikora et al., 2006). We found that 7-DHC and 8-DHC are exceptionally prone to peroxidation and their oxidation products, i.e. oxysterols, contribute to the pathophysiology of SLOS (Korade et al., 2010, 2013, 2014; Xu et al., 2012, 2013).
FIG. 1.
(A) The enzymatic step, DHCR7, affected in Smith–Lemli–Opitz syndrome; (B) Structures of the known cholesterol biosynthesis inhibitor and similar environmental agents (similarity score to AY9944 obtained from DSSTox are shown underneath each structure); (C) Effect of the compounds similar to AY9944 on cholesterol biosynthesis in Neuro2a cells and (D) in human SK-N-SH cells after incubation in lipid-free medium for 48 hrs, shown as the ratios of the cholesterol precursors to cholesterol. *, P < .05; **, P < .005; ***, P < .0005; n = 3; all statistical analyses are relative to Control using Student’s t-test.
Interestingly, some small molecules, such as AY9944 (a known teratogenic agent) and BM15.766, can potently inhibit DHCR7, leading to the same biochemical defect as seen in SLOS, i.e. increased levels of 7-DHC and decreased levels of cholesterol. Because of this, AY9944 and BM15.766 have been used to create a pharmacological animal model of SLOS (Fliesler et al., 2004; Kolf-Clauw et al., 1996, 1997; Xu et al., 1995). Many other small molecules have been reported to affect different steps of cholesterol biosynthesis. For example, antipsychotic drugs such as haloperidol, aiprasidone, risperidone, aripiprazole, and trazodone were found to inhibit DHCR7 (Canfran-Duque 2013; Hall 2013); breast cancer drugs tamoxifen and raloxifene, an infertility drug clomifene, fungicides tridemorph and fenpropimorph, and additional antipsychotic drugs fluphenazine and perphenazine, were found to inhibit EBP (Korade et al., 2016; Moebius, 1998a).
Much work has been done by the US EPA to screen environmental molecules for their biological activities, such as their effect on key transcription regulators (Martin et al., 2010), various receptor signaling pathways (Sipes et al., 2013), and zebrafish development (Padilla et al., 2012). However, none of the previous reports addressed the effect of environmental agents on cholesterol biosynthesis, which is important to development as discussed above. Thus, we aim to identify environmental molecules and drugs that display similar structures to DHCR7 inhibitors and that can inhibit cholesterol biosynthesis, potentially leading to the same biochemical defect as observed in SLOS. In this study, we rely on the Distributed Structure-Searchable Toxicity (DSSTox) Database Network (Kavlock and Dix 2010; Richard and Williams 2002) to carry out an initial in silico screening to identify structures similar to AY9944, one of the most potent inhibitors of DHCR7 known to date. The reason to choose AY9944 over BM15.766 as a model inhibitor here is because AY9944 displays almost 100 times higher potency than BM15.766 (Moebius et al., 1998b). The DSSTox was built by EPA’s National Center for Computational Toxicology in order to facilitate structure-activity studies and enable predictive toxicology. One feature of DSSTox is that it can calculate the similarity score (Tanimoto similarity coefficient) (Schneider 2000) of the molecules in the database (over 10 000 so far) to a given chemical structure and rank these structures based on their similarity.
Here we report (1) identification of benzalkonium chlorides (BACs) as a class of compounds that are highly similar structurally to AY9944 and can inhibit the Dhcr7 in both mouse and human neuroblastoma cell lines; (2) structure–activity studies suggest that the potency of BACs as Dhcr7 inhibitors decrease with the length of the hydrocarbon chain: C10 > C12 ≫ C14 > C16; 3) cholesterol biosynthesis-related genes were upregulated and a cholesterol efflux gene was downregulated in the cells upon exposure to BACs; and 4) 7-DHC-derived oxysterols were observed in cells exposed to BACs.
MATERIALS AND METHODS
Materials. HPLC grade solvents (hexanes, 2-propanol, methanol and water) were purchased from Thermo Fisher Scientific Inc. [25,26,26,26,27,27,27-d7]-7-DHC and d7-DHCEO were prepared as reported previously (Xu et al., 2011a,b). Non-deuterated BAC mixture, individual BACs (C10, C12, C14, and C16), ketamine, phencyclidine, bromhexine, benzyltriethylammonium chloride, dimethylalkyl amines with different lengths of alkyl chains and d7-benzyl chloride were purchased from Sigma-Aldrich Co. d7-BACs were synthesized as described below. All sterol standards were purchased from Avanti Polar Lipids unless otherwise noted.
Cell cultures and treatment with different compounds. Mouse Neuro2a and human SK-N-SH neuroblastoma cell lines were purchased from the American Type Culture Collection (Rockville, MD). Both cell lines were maintained in DMEM supplemented with L-glutamine, 10% fetal bovine serum (FBS; Thermo Scientific HyClone, Logan, Utah), and penicillin/streptomycin at 37ºC and 5% CO2. For treatment of Neuro2a cells with different chemicals, the cells were plated in 100 mm plates at the density of 1.0 × 106 cells/plate and left to adhere overnight. The following day, the media was replaced with DMEM high glucose media without serum, but with the addition of N2-supplement, L-glutamine and penicillin/streptomycin, and with or without the chemicals at the concentrations specified in the main text (stock solutions of the chemicals were made in DMSO at 1000x concentrations). 0.1% DMSO was used as the vehicle control and AY9944, a known Dhcr7 inhibitor, was used as the positive control.
The SK-N-SH cells were maintained and treated as described for the Neuro2a cells.
Lipid extraction, analysis of sterols by GC-MS, and analysis of oxysterol by HPLC-MS/MS. Cell pellets were lysed using 150 µl of lysis buffer (120 mM NaCl, 50 mM Hepes, 1% IGEPAL, 1% saturated PMSF) and the protein weight measured with the BioRad-DC protein Assay Kit. Before lipid extraction, internal standards, d7-cholesterol and 13C3-lanosterol (1 μg of each) were added to each sample. To extract the lipids, 1 ml of NaCl aqueous solution (0.9%) and 4ml of Folch solution (chloroform:methanol = 2:1, containing 1 mM BHT and 1 mM PPh3) was added to the cell lysates. The mixture was briefly vortexed and centrifuged for 5 min. The lower organic phase was taken and dried under nitrogen. The samples were re-dissolved in 300 µl of methylene chloride and 150 µl of the sample was transferred to a GC vial and then blown dry by nitrogen or argon. About 40 µl of acetonitrile and 20 µl of BSTFA were added to each sample before being analyzed by GC-MS.
GC-MS analysis was performed on a Shimadzu QP2010 instrument with a DB-5 column, electron ionization and a single-quadrupole mass analyzer. The injector temperature is 280 °C. The column temperature program starts at 220 °C, then increases to 275 °C at 15 °C/min, then to 280 °C at 1 °C/min and maintain for 2 min, then increases to 315 °C at 15 °C/min and maintain for 2.5 min. The interface temperature is 270 °C and the MS operates at 70 eV. The m/z values for monitoring cholesterol, d7-cholesterol, lanosterol, 13C3-lanosterol (Korade et al., 2016), 7-dehydrocholesterol, 8-dehydrocholesterol, 7-dehydrodesmosterol, desmosterol, lathosterol, zymostenol, and zymosterol are 329, 336, 393, 396, 325, 325, 349, 343, 458, 458, and 456, respectively. The levels of cholesterol and lanosterol were calculated based on their isotope-labeled internal standards. The levels of other sterols were calculated based on their relative response to the internal standard d7-cholesterol. A typical chromatogram for the analysis of the sterol standards by this method is included in the supporting information (Supplementary Figure S6).
Oxysterols were analyzed by normal phase HPLC-MS/MS as described previously (Xu et al., 2011a, 2013). Endogenous DHCEO (m/z 399 → m/z 381) were quantified by comparing its relative response to the d7-DHCEO (m/z 406 → m/z 388) internal standard. HPLC column and conditions: Phenomenex Luna 4.6 × 150 mm Si column; 3 μm particle size; 1.0 ml/min; elution solvent: 10% 2-propanol in hexanes.
Analysis of BACs by LC-MS/MS. Prior to extraction, a known amount of deuterated internal standards (d7-BAC-C10, d7-BAC-C12, d7-BAC-C14, and d7-BAC-C16) were added to each cell lysate sample. The extraction was performed the same as described above. The dried extracts were re-dissolved in Water (0.1% formic acid)/[Acetonitrile/2-propanol (0.1% formic acid) (50/50)] (30/70). The samples were stored at −80ºC until analysis using HPLC- Electrospray Ionization (ESI)-MS/MS. LC separations were performed on a Waters Acquity UPLC system equipped with autosampler (Waters, Milford, Massachusetts). HPLC conditions: Phenomenex Kinetex C18, 100A (100 × 2.1 mm) column; 1.7 μm particle size; mobile phase solvent: Water (0.1% formic acid)/[Acetonitrile/2-propanol (0.1% formic acid) (50/50)] (30/70); isocratic solvent at 0.200 ml/min flow rate; 10 μl injection volume. MS detections were done using a ThermoFinnigan TSQ tandem mass spectrometer (ThermoFisher, Waltham, Massachusetts) and data was acquired using Finnigan Xcalibur software package. MS condition: spray voltage, 3200 V; sheath gas pressure, 8 psi; sweep gas pressure, 0 psi; aux gas pressure, 3 psi; capillary temperature, 205.68 °C; tube lens, 103.61 V; skimmer offset, 14 V; collision pressure, 1.5 mTorr; collision energy, 14 V.
Cell viability and membrane integrity assay. To determine the toxicity of each component of BAC, cell viability studies were performed on Neuro2A and SK-N-SH cells at two different time points (24 and 48 h). Neuro2a cells were plated in 96-well plates at the density of 5000 cells/well and left in the incubator to adhere overnight. The following day, the media was replaced with 200 µl of DMEM high glucose media without serum with the addition of N2-supplement, l-glutamine, and penicillin/streptomycin and BAC at various concentrations in DMSO. DMSO levels were kept below 0.1% and the final concentration levels of BAC were: 10 µM, 5µ M, 1 µM, 100 nM, and 10 nM. 0.1% DMSO was used as a vehicle control. The Neuro2a cells were kept in culture for 24 and 48 h in the presence of BAC. The CellTiter-Glo Luminescent Cell Viability Assay (Promega, cat No.G7570) was performed to determine the cell viability as described previously (Xu et al., 2010). In parallel, CytoTox-ONE™ Homogeneous Membrane Integrity Assay (Promega, cat No. G7890) based on the releasing of lactate dehydrogenase (LDH) was performed to examine the cell membrane integrity. Statistical significance was determined via Student’s t-test (Excel).
Gene expression studies. Neuro2a cells were plated in 6 well plates at the density of 0.1 × 106 cells/well and left in the incubator to adhere to the plate overnight. The following day, the media was replaced with DMEM high glucose media without serum and the addition of N2-supplement, L-glutamine, and penicillin/streptomycin with or without chemicals. Total RNA was isolated from the cells using the RNeasy Mini Kit (Qiagen, catalog No.74104). The concentration of total RNA was measured on a Nanodrop instrument. RNA (200 ng) from each sample was reverse transcribed to cDNA using a reverse transcriptase PCR kit (Life Technologies, catalog No.4368814). Quantitative PCR (qPCR) was performed with an Applied Biosystems 7300 Machine. A 2X mastermix was prepared (Rox dye, 2× SYBR green, 100 µM dNTPs, 25 mM MgCl2, and 10× buffer) and each reaction contained the 2X mastermix, gene-specific primers, Taq polymerase, and cDNA (from 2ng of RNA). The primers were designed using the primer software on <http://frodo.wi.mit.edu/>. The primers were designed to yield 90-110 bp PCR amplicons and were 20-22 bp long. Primers for Srebf2 and Fasn were as reported (Yang et al., 2001). The primers used are shown in Table 1. All samples were run in quadruplicate. Significance was determined by Student’s t-test (Microsoft Excel).
TABLE 1.
Primers for qPCR Studies in Neuro2a Cells
| Gene name | Protein name | Sequence | |
|---|---|---|---|
| mHmgcr | 3-Hydroxy-3-Methylglutaryl-CoA Reductase | Forward | AACTATTGCACCGACAAGAAGC |
| Reverse | CACCTCTCTCACCACCTTGG | ||
| mDhcr7 | 7-dehydrocholesterol reductase | Forward | CCAAGAAGGTGCCATTACTCC |
| Reverse | TTCACAAACCAGAGGATGTGG | ||
| mSrebp-2 | Sterol Regulatory Element Binding Protein-2 | Forward | GCGTTCTGGAGACCATGGA |
| Reverse | ACAAAGTTGCTCTGAAAACAAATCA | ||
| mFasn | fatty acid synthase | Forward | GCTGCGGAAACTTCAGGAAAT |
| Reverse | AGAGACGTGTCACTCCTGGACTT | ||
| mEbp | 3β-hydroxysterol-Δ8,Δ7-isomerase | Forward | TCACGTGGCTGTTGTCTAGC |
| Reverse | CCCTCGATCACAAGGTGAAT | ||
| mAbca1 | ATP-binding cassette, sub-family A-1 | Forward | AGGTGATGTTTCTGACCAACG |
| Reverse | GTTGAGGGACTTGATCTTCAGG |
Synthesis. Reactions were monitored by TLC and the plates were visualized by UV and stained with phosphomolybdic acid or Ninhydrin. A general procedure for the synthesis of d7-BACs using dimethylalkylamine and d7-benzyl chloride is described below:
Basic Dowex was washed with CH2Cl2, acetone, and ethanol. The Dowex (0.90 g, 1g/mmol of amine) was added to a solution of N,N-dimethylhexadecylamine (0.20 ml, 0.84 mmol) and d7-benzyl chloride (0.10 ml, 0.87 mmol) in ethanol (4 ml), then the reaction mixture was heated to reflux. After 2 h, the reaction mixture was cooled and filtered. The filtrate was concentrated, then redissolved in CH2Cl2 and dried over MgSO4. The solution was filtered, concentrated, and the product dried under high vacuum. The product (0.30 g, 100%) was isolated as a colorless oil. All BACs were synthesized following the same procedure and their NMR data follows.
d7-C10-BAC: colorless oil; 1H NMR (300 MHz, CDCl3) δ 3.35-3.29 (m, 2H), 3.03 (s, 6H), 1.56 (br s, 2H), 1.07 and 0.99 (br s, 14H), 0.63 (t, 3H, J = 6.4 Hz); 13C NMR (75 MHz, CDCl3) δ 132.9 (m), 128.3 (m), 127.1, 63.5, 49.2, 31.6, 29.1, 29.0, 28.9, 26.1, 22.7, 22.4, 13.9; HRMS (ESI) calculated 283.3125 (M - Cl), observed 283.3133.
d7-C12-BAC: colorless oil; 1H NMR (300 MHz, CDCl3) δ 3.40-3.35 (m, 2H), 3.10 (s, 6H), 1.61 (br s, 2H), 1.12 and 1.05 (br s, 18H), 0.69 (t, 3H, J = 6.9 Hz); 13C NMR (75 MHz, CDCl3) δ 132.6 (m), 128.3 (m), 127.2, 63.5, 49.3, 31.7, 29.4, 29.3, 29.2, 29.12, 29.08, 26.2, 22.7, 22.5, 13.9; HRMS (ESI) calculated 311.3438 (M - Cl), observed 311.3444.
d7-C14-BAC: colorless oil; 1H NMR (300 MHz, CDCl3) δ 3.38-3.36 (m, 2H), 3.10 (s, 6H), 1.61 (br s, 2H), 1.11 and 1.06 (br s, 22H), 0.68 (t, 3H, J = 7.0 Hz); 13C NMR (75 MHz, CDCl3) δ 132.6 (m), 128.3 (m), 127.2, 63.5, 49.3, 31.7, 29.5, 29.44, 29.39, 29.3, 29.21, 29.15, 29.1, 26.2, 22.8, 22.5, 13.9; HRMS (ESI) calculated 339.3751 (M - Cl), observed 339.3744.
d7-C16-BAC: white powder; 1H NMR (300 MHz, CDCl3) δ 3.45-3.39 (m, 2H), 3.18 (s, 6H), 1.67 (br s, 2H), 1.18 and 1.13 (br s, 26H), 0.75 (t, 3H, J = 7.0 Hz); 13C NMR (75 MHz, CDCl3) δ 132.6 (m), 128.3 (m), 127.2, 63.5, 49.4, 31.8, 29.6, 29.52, 29.45, 29.32, 29.26, 29.2, 29.1, 26.2, 22.8, 22.6, 14.0; HRMS (ESI) calculated 367.4064 (M - Cl), observed 367.4063.
RESULTS
In silico screening of environmental and pharmaceutical small molecules for potential DHCR7 inhibitors using DSSTox and identification of BACs as Dhcr7 inhibitors in cells
Using the DSSTox Database Network as described above, we found that there are six classes of molecules that show > 45% of structural similarity to AY9944 (Figure 1B), including the antiseptic benzalkonium chloride compounds (BACs), the recreational drug phencyclidine (PCP), the anesthetic drug ketamine, mucus expectorant bromhexine, and the industrial chemical benzyltriethylammonium chloride (BTEC). Among them, BAC displays the highest similarity to AY9944 at 72.9%.
Neuro2a cells were chosen to test the effect of each compound or compound mixture (BAC) on cholesterol biosynthesis because these cells express high levels of Dhcr7 and Dhcr7-knockdown Neuro2a cells have been demonstrated to be a useful model for studying SLOS (Korade et al., 2009 and 2010; Xu et al., 2011a). Briefly, Neuro2a cells were exposed to each compound at 100 nM, lipids were extracted, and sterols were analyzed by GC-MS. We found that BAC is indeed a potent inhibitor of Dhcr7, leading to significant accumulation of 7-DHC and 7-dehydrodesmosterol (7-DHD) at 100 nM (Figure 1C and Supplementary Figure S1A). Importantly, BAC as a mixture is only slightly less potent than the positive control, AY9944. We note that in control Neuro2a cells, the baseline levels of desmosterol is high, possibly due to intrinsic low expression or activity of 3β-hydroxysterol-Δ24-reductase (Dhcr24). As a result of this, the level of 7-DHD also increased when Dhcr7 was inhibited (Supplementary Figure S1A). In a subsequent experiment, we found that BAC mix can inhibit Dhcr7 even at 1 nM, as indicated by the formation of 7-dehydrodesmosterol (Supplementary Figure S1B).
We then carried out similar experiments using a human neuroblastoma cell line, SK-N-SH. We found that the BAC mixture can also inhibit DHCR7 effectively at 100 nM (Figure 1D). Interestingly, bromhexine displayed high potency in inhibiting DHCR7, a drastic increase from its potency in Neuro2a cells, which suggests that there may be some structural differences in the human DHCR7 enzyme from its mouse equivalent. We also note that BAC mixture did not affect the level 8-DHC as much as AY9944 did.
Structure-activity studies of individual BACs as inhibitors of Dhcr7
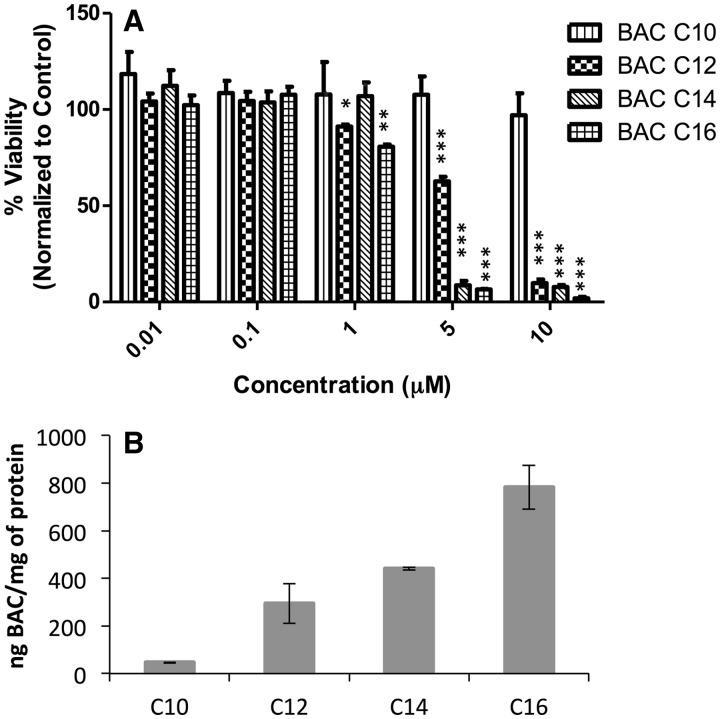
BACs are a class of quaternary ammonium compounds (QACs) that differ by the length of the alkyl side-chain. In this study, we chose the most common components of BACs, i.e. those with an alkyl chain of C10, C12, C14, or C16. We first examined the cytotoxicity of individual BACs on the Neuro2a cells using MTS assay after 24 or 48 h of treatment. Cell viability results after 48 hrs are shown in Figure 2A, which suggests that BAC-C16 is the most toxic component, followed by C14, C12, and C10, with IC50 values of 2.5, 2.5, 5, and >10 μM, respectively. A similar trend in cytotoxicity was observed after 24 hr of treatment as shown in Supplementary Figure S2. The SK-N-SH cells displayed similar vulnerability to BAC using the same cytotoxicity test (Supplementary Figure S3).
FIG. 2.
(A) Cytotoxicity of individual BACs on Neuro2a cells after 48 h of treatment. (B) Incorporation levels of individual BACs in Neuro2a cells after 48 h of treatment of each compound at 100 nM. BACs were analyzed by UPLC-MS/MS as described in Materials and Methods. *, P < .05; **, P < .005; ***, P < .0005; n = 3; all statistical analyses are relative to Control using Student’s t-test.
As the main mechanism of action of BACs against microbes is through disruption of the lipid membrane (Gilbert and Moore 2005; McDonnell and Russell 1999), quantification of the incorporation of individual BACs to the cells would reflect such action. Thus, we developed a fast and sensitive analytical method using isotope-dilution UPLC-MS/MS. Deuterated (d7)-standards of BAC-C10, C12, C14, and C16 were synthesized following known chemistry (see Materials and Methods). After testing two different UPLC columns (reverse phase C8 and C18), we established an UPLC-MS/MS method that allows complete analysis of all four BACs within three minutes (Supplementary Figure S4). After incubating the Neuro2a cells in the presence of individual BAC, BAC was extracted by Folch solution in the presence of the deuterated standards and was analyzed by UPLC-MS/MS (Figure 2b). As shown in the Figure, the incorporation levels increase with the chain length of the BAC (C10 ≪ C12 < C14 < C16), which appears to reflect their cLogP values of 2.1, 3.2, 4.2, and 5.3, respectively. Accordingly, the levels of individual BACs in the corresponding media decrease with the chain length (Supplementary Figure S5). Thus, the cytotoxicity of each BAC is consistent with its level of incorporation into the cells. To examine whether membrane-breakage contributed to the cytotoxicity, we carried out a cell permeability test using a membrane integrity assay based on the release of LDH from the cells and found that no BAC led to cell leakage at 1 μM or lower (Supplementary Figure S7), which suggests that at 100 nM used in this study, the effect of BAC was not caused by the breakage of the cell membrane.
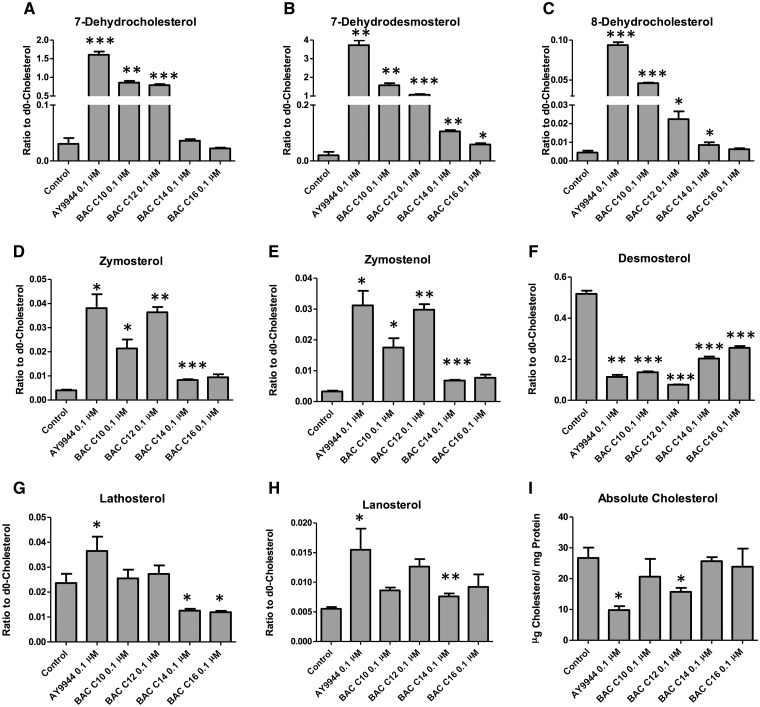
To understand the structural features of BACs that are responsible for their potency as Dhcr7 inhibitor, Neuro2a cells were exposed to individual BACs at the non-cytotoxic concentration (100 nM), and the sterol profiles were analyzed by GC-MS (Figure 3). We found that both BAC-C10 and BAC-C12 inhibit Dhcr7 potently, leading to much elevated levels of 7-DHC and 7-DHD, while C14 and C16 are not very good inhibitors, suggesting that the proper length of the side chain plays a critical role for binding to the active site of Dhcr7. This result, combined with the results in Figure 2B, suggests that BAC-C10 is a much more potent inhibitor of Dhcr7 than it appears to be as it was incorporated at a much lower level than other BACs. We also observed that BAC-C10 and C12 are also inhibitors of EBP as indicated by the formation of zymosterol and zymostenol, although at much lower potency. Interestingly, although BAC-C14 and C16 did not inhibit any steps of the cholesterol biosynthesis examined here, they both led to significantly lower levels of desmosterol while they did not affect the total levels of cholesterol (Figure 3I).
FIG. 3.
Structure-activity studies on the effect of individual BACs on the cholesterol biosynthesis. The Neuro2a cells were exposed to individual BACs at 100 nM in lipid-free medium for 48 hrs and the levels of cholesterol and its precursors were analyzed by GC-MS as described in the Materials and Methods. *P < .05; **P < .005; ***P < .0005; n = 3; all statistical analyses are relative to Control using Student’s t-test.
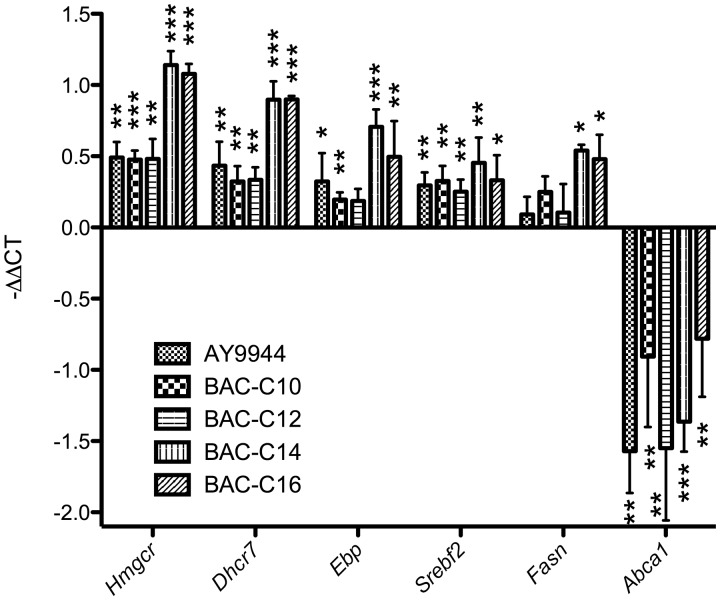
To confirm the effect of BACs on the total cholesterol homeostasis, gene expression analysis was carried out on related genes by qPCR, including Srebf2, Hmgcr, Ebp, and Dhcr7. We found that all BACs led to significant upregulation of the genes that are related to cholesterol biosynthesis (Figure 4) and downregulation of Abca1, the gene that is responsible for cholesterol efflux (Figure 4). All these observed gene expression changes suggest feedback response to the inhibition of the cholesterol biosynthesis by BAC. It is worth noting that although BAC-C14 and C16 did not show high potency in inhibiting cholesterol biosynthesis, they behaved similarly to C10 and C12 on the regulation of the cholesterol homeostasis-related genes. This observation suggests that BAC-C14 and C16 could interact with other proteins that regulate cholesterol biosynthesis and remain to be identified. The decreased levels of desmosterol in response to BAC-C14 and C16 treatment (without any precursors being accumulated) also provided support to this notion. Interestingly, BAC-C14 and C16 also led to upregulation of fatty acid synthase (Fasn), suggesting that they may target additional lipid metabolic pathways.
FIG. 4.
Effect of Individual Component of BAC on expression of the genes related to cholesterol biosynthesis and cholesterol efflux as analyzed by qPCR in Neuro2a cells after treatment of each compound at 100 nM for 20 h. The expression level of each gene was normalized to Actin. *P < .05; **P < .005; ***P < .0005; four biological replicates for each group and four technical replicates for each sample were performed; all statistical analyses are relative to Control using Student’s t-test.
7-DHC-derived oxysterols are formed in BAC-treated cells
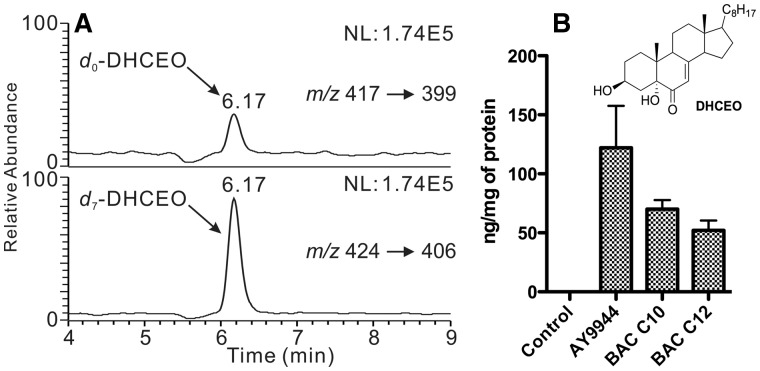
Because of the high reactivity of 7-DHC toward free radical peroxidation (Xu et al., 2009, 2010), one would expect the formation of 7-DHC-derived oxysterols in BAC-treated cells. Thus, lipids were extracted from the cells that were treated with individual BAC for two days, and the 7-DHC oxysterol biomarker, 3β,5α-dihydroxycholest-7-en-6-one (DHCEO) (Xu et al., 2011a), was analyzed by normal phase HPLC-MS/MS (Xu et al., 2011a, 2011b, 2013). We found that DHCEO was indeed formed in cells that were treated with AY9944, BAC-C10, or BAC-C12, but not with BAC-C14 and BAC-C16, which is consistent with the profile of the cholesterol precursors under these conditions (Figure 5A). The levels of DHCEO are proportional to the levels of 7-DHC as shown in Figure 5B.
FIG. 5.
Normal phase HPLC-MS/MS analysis (A) and quantitation (B) of DHCEO using d7-DHCEO as the internal standard in Neuro2a cells treated with different compounds at 100 nM for 2 days.
DISCUSSION
Environmental factors have been implicated in neurodevelopmental and neurodegenerative diseases, such as autism, Parkinson’s disease, and Alzheimer’s disease (Hallmayer et al., 2011; Kanthasamy et al., 2012; Landrigan et al., 2005). Cholesterol biosynthesis disorders are characterized by defects in embryonic and neural development (Porter and Herman, 2011). Thus, it seems to be plausible that inhibition of cholesterol biosynthesis by non-genetic factors, such as small molecules in the environment or pharmaceuticals, could also contribute to such developmental disorders.
In this study, the DSSTox Database Network allowed us to carry out initial structural screening of environmental molecules and drugs based on their similarity to AY9944, which led to the identification of BACs as a class of molecules that inhibit Dhcr7, the enzyme that was affected in the cholesterol biosynthesis disorder, SLOS. Structure–activity relationship (SAR) studies allowed us to identify BAC-C10 and C12 to be the active components that are responsible for the inhibition of Dhcr7 while also serving as a less potent inhibitor of Ebp. On the other hand, although BAC-C14 and C16 did not lead to accumulation of sterol precursors, they both lowered the levels of desmosterol, a major sterol in Neuro2a cells (∼50% of the levels of cholesterol in control Neuro2a cells), suggesting that they likely affect the cholesterol biosynthesis at an earlier step, likely before the formation of the first sterol. More broad scale lipidomic analysis could reveal the particular enzymatic steps that were affected by BAC-C14 and C16. The observed upregulation of cholesterol biosynthesis-related genes suggest that BACs act as direct inhibitors of the enzymes involved in the biosynthesis. This SAR study suggests that the benzyl group, and proper length of the side chain are critical for binding to the active site of Dhcr7. Interestingly, a recently reported potent inhibitor of Dhcr7 also displays the benzyl dialkyl amine moiety (Horling et al., 2012), which seems to be consistent with our findings. We note that this new inhibitor is only about two times more potent than AY9944 based on their comparison with BM15.766 (no direct comparison with AY9944 was done in this study) (Horling et al., 2012; Moebius et al., 1998b). Similarity between BAC/AY9944 and BM15.766 appears to be much smaller.
Significantly, even at non-cytotoxic concentrations, BACs can potently inhibit Dhcr7. This suggests that exposure of BACs could have severe consequences even when there is no apparent acute toxicity. It has been reported that orally administered BAC can cross the blood-brain barrier in rats (a 1.2 μg/g concentration in serum leads to 0.2 μg/g in brain) (Xue et al., 2002, 2004), which suggests that BAC could pose threat to the central nervous system. Additionally, vaginal treatment of pregnant rats with BAC led to fetal death, reduced litter size, and birth defects (Buttar, 1985). A recent study by Hrubec, Hunt, and coworkers demonstrated that chronic consumption of QAC-containing food (including BAC) led to significantly decreased fertility and fecundity in mice, as well increased mortality of the dam (Melin et al., 2014). The latter report suggests that BAC may penetrate the blood–placenta barrier. The inhibition of cholesterol biosynthesis by BACs could contribute to some of the deleterious effects on embryonic development.
The observation of a 7-DHC-derived oxysterol, DHCEO, in BAC-C10 and C12-treated cells further confirmed that these cells resemble the biochemical features of SLOS cells and tissues. DHCEO was established as a biomarker of the oxidation of 7-DHC and was observed at the highest level (3.5 μM) among all oxysterols in the brain of a Dhcr7-KO mouse (Xu et al., 2011a). Importantly, DHCEO exerts a variety of biological activities, such as affecting expression of the genes related to cholesterol biosynthesis (Korade et al., 2010) and cell growth and inducing differentiation and arborization of neuronal cells (Xu et al., 2012). Thus, 7-DHC-derivedy oxysterols could be a major contributor to the biological consequences of exposure to BACs.
Quaternary ammonium compounds (QAC), including BACs, have been used as antimicrobials for more than 50 years (Gilbert and Moore, 2005), exerting their effect on microbes mainly through the disruption of the negatively charged lipid membrane mediated by its positively charged head group (Gilbert and Moore 2005; McDonnell and Russell 1999). QACs are widely used in cleaning products (such as Lysol and Clorox solutions, wipes, and hand sanitizers), medical products (such as eye and nasal drops), and the food processing industries (Gilbert and Moore 2005; Holah et al., 2002; McDonnell and Russell 1999; Ratani et al., 2012). High levels of QAC have been found in grapefruit seed extracts (Takeoka et al., 2005), food additives (Kröckel et al., 2003), fruits (The Federal Institute for Risk Assessment (BfR) of Germany, 2012a,b), and processed food such as milk and other dairy products (The Federal Institute for Risk Assessment (BfR) of Germany, 2012a,b). Thus, the sources of exposure of BACs to human beings are diverse and the levels are potentially high. These reports, combined with the high potency of BACs as cholesterol inhibitor (nM in Neuro2a cells and SK-N-SH cells), suggest that BACs could be a legitimate threat to human health.
A number of deleterious effects of the usage of QAC have been reported. For example, long-term use of BAC-preserved eye and nasal drops can cause ocular and corneal surface diseases (Baudouin et al., 2010; De Saint Jean et al., 1999; Leung et al., 2008; Pisella et al., 2002), and aggravate rhinitis medicamentosa (Graf, 2001), respectively. Evidence also suggests that BAC exposure can lead to occupational asthma (Burge and Richardson 1994; Purohit et al., 2000). More importantly, BAC was found to be toxic to peripheral neurons, such as enteric and ganglion neuronal cells, at μM concentrations (Herman and Bass 1989; Sarkar et al., 2012). Significantly, BACs (Roccal in the study) stopped the development of over 50% of clam eggs and completely killed larvae at only 0.2 ppm (equivalent to approximately 500 nM when calculating using the molecular weight of BAC-C16) (Davis and Hidu, 1969). The inhibition effect of BACs on cholesterol biosynthesis could provide an alternative or additional mechanism for these observed toxicities.
In summary, a combination of in silico screening and in vitro tests led to the discovery of a class of common antiseptic compounds, BACs, as potent inhibitors of the last step of cholesterol biosynthesis, Dhcr7. This finding suggests that exposure to these compounds at critical developmental stages could contribute to the pathogenesis of developmental disorders. An epidemiological study may be warranted in order to systematically assess the risk of exposure to BACs.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
ACKNOWLEDGMENTS
We thank Dr. Karoly Mirnics for the use of the tissue culture facility and qPCR instrument at Vanderbilt University and for helpful discussion on the project. We also would like to thank Dr. Zeljka Korade for sharing the design of the mEbp primers.
FUNDING
National Institutes of Health grants (R00 HD073270) and a pilot grant of NIH (P30 ES000267) to L.X. and the startup funds to LX from the Department of Medicinal Chemistry, School of Pharmacy of the University of Washington.
References
- Baudouin C., Labbe A., Liang H., Pauly A., Brignole-Baudouin F. (2010). Preservatives in eyedrops: The good, the bad and the ugly. Prog. Retin. Eye Res. 29, 312–334. [DOI] [PubMed] [Google Scholar]
- Bjorkhem I., Meaney S. (2004). Brain cholesterol: Long secret life behind a barrier. Arterioscler. Thromb. Vasc. Biol. 24, 806–815. [DOI] [PubMed] [Google Scholar]
- Bukelis I., Porter F. D., Zimmerman A. W., Tierney E. (2007). Smith-Lemli-Opitz syndrome and autism spectrum disorder. Am. J. Psychiatry 164, 1655–1661. [DOI] [PubMed] [Google Scholar]
- Burge P. S., Richardson M. N. (1994). Occupational asthma due to indirect exposure to lauryl dimethyl benzyl ammonium chloride used in a floor cleaner. Thorax 49, 842–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttar H. S. (1985). Embryotoxicity of benzalkonium chloride in vaginally treated rats. J. Appl. Toxicol. 5, 398–401. [DOI] [PubMed] [Google Scholar]
- Canfran-Duque A. et al. (2013). Atypical antipsychotics alter cholesterol and fatty acid metabolism in vitro. J. Lipid Res. 54, 310–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis H. C., Hidu H. (1969). Effects of pesticides on embryonic development of clams and oysters and on survival and growth of the larvae. Fish Bull. 67, 393–404. [Google Scholar]
- De Saint Jean M., Brignole F., Bringuier A. F., Bauchet A., Feldmann G., Baudouin C. (1999). Effects of benzalkonium chloride on growth and survival of Chang conjunctival cells. Invest. Ophthalmol. Vis. Sci. 40, 619–630. [PubMed] [Google Scholar]
- Fitzky B. U., Witsch-Baumgartner M., Erdel M., Lee J. N., Paik Y. K., Glossmann H., Utermann G, Moebius F. F. (1998). Mutations in the Delta 7-sterol reductase gene in patients with the Smith-Lemli-Opitz syndrome. Proc. Natl. Acad. Sci. USA 95, 8181–8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliesler S. J., Peachey N. S., Richards M. J., Nagel B. A, Vaughan D. K. (2004). Retinal degeneration in a rodent model of Smith-Lemli-Opitz syndrome: Electrophysiologic, biochemical, and morphologic features. Arch. Ophthalmol. 122, 1190–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert P., Moore L. E. (2005). Cationic antiseptics: Diversity of action under a common epithet. J. Appl. Microbiol. 99, 703–715. [DOI] [PubMed] [Google Scholar]
- Graf P. (2001). Benzalkonium chloride as a preservative in nasal solutions: Re-examining the data. Respir. Med. 95, 728–733. [DOI] [PubMed] [Google Scholar]
- Hall P. et al. (2013). Aripiprazole and trazodone cause elevations of 7-dehydrocholesterol in the absence of Smith-Lemli-Opitz Syndrome. Mol. Genet. Metab. 110, 176–178. [DOI] [PubMed] [Google Scholar]
- Hallmayer J., Cleveland S., Torres A., Phillips J., Cohen B., Torigoe T., Miller J., Fedele A., Collins J., Smith K. et al. (2011). Genetic heritability and shared environmental factors among twin pairs with autism. Arch. Gen. Psychiatry 68, 1095–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman J. R., Bass P. (1989). Enteric neuronal ablation: Structure–activity relationship in a series of alkyldimethylbenzylammonium chlorides. Fundam. Appl. Toxicol. 13, 576–584. [DOI] [PubMed] [Google Scholar]
- Holah J. T., Taylor J. H., Dawson D. J, Hall K. E. (2002). Biocide use in the food industry and the disinfectant resistance of persistent strains of Listeria monocytogenes and Escherichia coli. Symp. Ser Soc. Appl. Microbiol. 111S–120S. [PubMed] [Google Scholar]
- Horling A., Muller C., Barthel R., Bracher F., Imming P. (2012). A new class of selective and potent 7-dehydrocholesterol reductase inhibitors. J. Med. Chem. 55, 7614–7622. [DOI] [PubMed] [Google Scholar]
- Kanthasamy A., Jin H., Anantharam V., Sondarva G., Rangasamy V., Rana A. (2012). Emerging neurotoxic mechanisms in environmental factors-induced neurodegeneration. Neurotoxicology 33, 833–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavlock R., Dix D. (2010). Computational toxicology as implemented by the U.S. EPA: Providing high throughput decision support tools for screening and assessing chemical exposure, hazard and risk. J. Toxicol. Environ. Health B Crit. Rev. 13, 197–217. [DOI] [PubMed] [Google Scholar]
- Kolf-Clauw M., Chevy F., Siliart B., Wolf C., Mulliez N., Roux C. (1997). Cholesterol biosynthesis inhibited by BM15.766 induces holoprosencephaly in the rat. Teratology 56, 188–200. [DOI] [PubMed] [Google Scholar]
- Kolf-Clauw M., Chevy F., Wolf C., Siliart B., Citadelle D., Roux C. (1996). Inhibition of 7-dehydrocholesterol reductase by the teratogen AY9944: A rat model for Smith-Lemli-Opitz syndrome. Teratology 54, 115–125. [DOI] [PubMed] [Google Scholar]
- Korade Z., Kenworthy A. K., Mirnics K. (2009). Molecular consequences of altered neuronal cholesterol biosynthesis. J. Neurosci. Res. 87, 866–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korade Z., Kim H. Y., Tallman K. A., Liu W., Koczok K., Balogh I., Xu L., Mirnics K, Porter N. A. (2016). The effect of small molecules on sterol homeostasis: Measuring 7-dehydrocholesterol in Dhcr7-deficient neuro2a cells and human fibroblasts. J. Med. Chem. 59, 1102–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korade Z., Xu L., Harrison F. E., Ahsen R., Hart S. E., Folkes O. M., Mirnics K, Porter N. A. (2014). Antioxidant supplementation ameliorates molecular deficits in Smith-Lemli-Opitz syndrome. Biol. Psychiatry 75, 215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korade Z., Xu L., Mirnics K, Porter N. A. (2013). Lipid biomarkers of oxidative stress in a genetic mouse model of Smith-Lemli-Opitz syndrome. J. Inherit. Metab. Dis. 36, 113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korade Z., Xu L., Shelton R, Porter N. A. (2010). Biological activities of 7-dehydrocholesterol-derived oxysterols: Implications for Smith-Lemli-Opitz syndrome. J. Lipid Res. 51, 3259–3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koudinov A. R., Koudinova N. V. (2001). Essential role for cholesterol in synaptic plasticity and neuronal degeneration. FASEB J. 15, 1858–1860. [DOI] [PubMed] [Google Scholar]
- Kröckel L., Jira W., Wild D. (2003). Identification of benzalkonium chloride in food additives and its inefficacy against bacteria in minced meat and raw sausage batters. Eur. Food Res. Technol. 216, 402–406. [Google Scholar]
- Landrigan P. J., Sonawane B., Butler R. N., Trasande L., Callan R., Droller D. (2005). Early environmental origins of neurodegenerative disease in later life. Environ. Health Perspect. 113, 1230–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung E. W., Medeiros F. A, Weinreb R. N. (2008). Prevalence of ocular surface disease in glaucoma patients. J. Glaucoma 17, 350–355. [DOI] [PubMed] [Google Scholar]
- Martin M. T., Dix D. J., Judson R. S., Kavlock R. J., Reif D. M., Richard A. M., Rotroff D. M., Romanov S., Medvedev A., Poltoratskaya N. et al. (2010). Impact of environmental chemicals on key transcription regulators and correlation to toxicity end points within EPA's ToxCast program. Chem. Res. Toxicol. 23, 578–590. [DOI] [PubMed] [Google Scholar]
- Mauch D. H., Nagler K., Schumacher S., Goritz C., Muller E. C., Otto A, Pfrieger F. W. (2001). CNS synaptogenesis promoted by glia-derived cholesterol. Science 294, 1354–1357. [DOI] [PubMed] [Google Scholar]
- McDonnell G., Russell A. D. (1999). Antiseptics and disinfectants: activity, action, and resistance. Clin. Microbiol. Rev. 12, 147–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melin V. E., Potineni H., Hunt P., Griswold J., Siems B., Werre S. R, Hrubec T. C. (2014). Exposure to common quaternary ammonium disinfectants decreases fertility in mice. Repro. Toxicol. 50, 163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moebius F. F. et al. (1998a). Pharmacological analysis of sterol delta8-delta7 isomerase proteins with [3H]ifenprodil. Mol. Pharmacol. 54, 591–598. [DOI] [PubMed] [Google Scholar]
- Moebius F. F., Fitzky B. U., Lee J. N., Paik Y. K., Glossmann H. (1998b). Molecular cloning and expression of the human delta7-sterol reductase. Proc. Natl. Acad. Sci. USA 95, 1899–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowaczyk M. J., Irons M. B. (2012). Smith-Lemli-Opitz syndrome: phenotype, natural history, and epidemiology. Am. J. Med. Genet. C Semin Med. Genet. 160C, 250–262. [DOI] [PubMed] [Google Scholar]
- Padilla S., Corum D., Padnos B., Hunter D. L., Beam A., Houck K. A., Sipes N., Kleinstreuer N., Knudsen T., Dix D. J. et al. (2012). Zebrafish developmental screening of the ToxCast Phase I chemical library. Reprod. Toxicol. 33, 174–187. [DOI] [PubMed] [Google Scholar]
- Pisella P. J., Pouliquen P., Baudouin C. (2002). Prevalence of ocular symptoms and signs with preserved and preservative free glaucoma medication. Br. J. Ophthalmol. 86, 418–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter F. D., Herman G. E. (2011). Malformation syndromes caused by disorders of cholesterol synthesis. J. Lipid Res. 52, 6–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter J. A., Young K. E, Beachy P. A. (1996). Cholesterol modification of hedgehog signaling proteins in animal development. Science 274, 255–259. [DOI] [PubMed] [Google Scholar]
- Purohit A., Kopferschmitt-Kubler M. C., Moreau C., Popin E., Blaumeiser M., Pauli G. (2000). Quaternary ammonium compounds and occupational asthma. Int. Arch. Occup. Environ. Health 73, 423–427. [DOI] [PubMed] [Google Scholar]
- Ratani S. S., Siletzky R. M., Dutta V., Yildirim S., Osborne J. A., Lin W., Hitchins A. D., Ward T. J., Kathariou S. (2012). Heavy metal and disinfectant resistance of Listeria monocytogenes from foods and food processing plants. Appl. Environ. Microbiol. 78, 6938–6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard A. M., Williams C. R. (2002). Distributed structure-searchable toxicity (DSSTox) public database network: A proposal. Mutat. Res. 499, 27–52. [DOI] [PubMed] [Google Scholar]
- Saher G., Brugger B., Lappe-Siefke C., Mobius W., Tozawa R., Wehr M. C., Wieland F., Ishibashi S, Nave K. A. (2005). High cholesterol level is essential for myelin membrane growth. Nat. Neurosci. 8, 468–475. [DOI] [PubMed] [Google Scholar]
- Sarkar J., Chaudhary S., Namavari A., Ozturk O., Chang J. H., Yco L., Sonawane S., Khanolkar V., Hallak J., Jain S. (2012). Corneal neurotoxicity due to topical benzalkonium chloride. Invest. Ophthalmol. Vis. Sci. 53, 1792–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider G. 2000-2013. Analysis of Chemical Space In Madame Curie Bioscience Database [Internet]. Landes Bioscience, Austin (TX: ). Available at: http://www.ncbi.nlm.nih.gov/books/NBK6062/. Last accessed on March 02, 2016. [Google Scholar]
- Sikora D., Pettit-Kekel K., Penfield J., Merkens L., Steiner R. (2006). The near universal presence of autism spectrum disorders in children with Smith-Lemli-Opitz syndrome. Am. J. Med. Genet. 140A, 1511–1518. [DOI] [PubMed] [Google Scholar]
- Sipes N. S., Martin M. T., Kothiya P., Reif D. M., Judson R. S., Richard A. M., Houck K. A., Dix D. J., Kavlock R. J, Knudsen T. B. (2013). Profiling 976 ToxCast chemicals across 331 enzymatic and receptor signaling assays. Chem. Res. Toxicol. 26, 878–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeoka G. R., Dao L. T., Wong R. Y, Harden L. A. (2005). Identification of benzalkonium chloride in commercial grapefruit seed extracts. J. Agric. Food Chem. 53, 7630–7636. [DOI] [PubMed] [Google Scholar]
- The Federal Institute for Risk Assessment (BfR) of Germany. 2012a. Health assessment of benzalkonium chloride residues in food. BfR opinion No 032/2012 Available at: http://www.bfr.bund.de/cm/349/health-assessment-of-benzalkonium-chloride-residues-in-food.pdf. Last accessed on March 02, 2016.
- The Federal Institute for Risk Assessment (BfR) of Germany. 2012b. Health assessment of didecyldimethylammonium chloride (DDAC) residues in food. BfR opinion No 027/2012. Available at: http://www.bfr.bund.de/cm/349/health-assessment-of-didecyldimethylammonium-chloride-ddac-residues-in-food.pdf. Last accessed on March 02, 2016.
- Tint G. S., Irons M., Elias E. R., Batta A. K., Frieden R., Chen T. S., Salen G. (1994). Defective cholesterol biosynthesis associated with the Smith-Lemli-Opitz syndrome. N. Engl. J. Med. 330, 107–113. [DOI] [PubMed] [Google Scholar]
- Tint G. S., Seller M., Hughes-Benzie R., Batta A. K., Shefer S., Genest D., Irons M., Elias E., Salen G. (1995). Markedly increased tissue concentrations of 7-dehydrocholesterol combined with low levels of cholesterol are characteristic of the Smith-Lemli-Opitz syndrome. J. Lipid Res. 36, 89–95. [PubMed] [Google Scholar]
- Wassif C. A., Maslen C., Kachilele-Linjewile S., Lin D., Linck L. M., Connor W. E., Steiner R. D, Porter F. D. (1998). Mutations in the human sterol Delta(7)-reductase gene at 11q12-13 cause Smith-Lemli-Opitz syndrome. Am. J. Hum. Genet. 63, 55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterham H. R., Wijburg F. A., Hennekam R. C., Vreken P., Poll-The B. T., Dorland L., Duran M., Jira P. E., Smeitink J. A., Wevers R. A. et al. (1998). Smith-Lemli-Opitz syndrome is caused by mutations in the 7-dehydrocholesterol reductase gene. Am. J. Hum. Genet. 63, 329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G., Salen G., Shefer S., Ness G. C., Chen T. S., Zhao Z., Salen L, Tint G. S. (1995). Treatment of the cholesterol biosynthetic defect in Smith-Lemli-Opitz syndrome reproduced in rats by BM 15.766. Gastroenterology 109, 1301–1307. [DOI] [PubMed] [Google Scholar]
- Xu L., Davis T. A, Porter N. A. (2009). Rate constants for peroxidation of polyunsaturated fatty acids and sterols in solution and in liposomes. J. Am. Chem. Soc. 131, 13037–13044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Korade Z, Porter N. A. (2010). Oxysterols from free radical chain oxidation of 7-dehydrocholesterol: Product and mechanistic studies. J. Am. Chem. Soc. 132, 2222–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Korade Z., Rosado D. A,, Jr., Mirnics K, Porter N. A. (2013). Metabolism of oxysterols derived from nonenzymatic oxidation of 7-dehydrocholesterol in cells. J. Lipid Res. 54, 1135–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Korade Z., Rosado D. A., Liu W., Lamberson C. R, Porter N. A. (2011a). An oxysterol biomarker for 7-dehydrocholesterol oxidation in cell/mouse models for Smith-Lemli-Opitz syndrome. J. Lipid Res. 52, 1222–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Liu W., Sheflin L. G., Fliesler S. J, Porter N. A. (2011b). Novel oxysterols observed in tissues and fluids of AY9944-treated rats — a model for Smith-Lemli-Opitz Syndrome. J. Lipid Res. 52, 1810–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Mirnics K., Bowman A. B., Liu W., Da J., Porter N. A., Korade Z. (2012). DHCEO accumulation is a critical mediator of pathophysiology in a Smith-Lemli-Opitz syndrome model. Neurobiol. Dis. 45, 923–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y., Hieda Y., Kimura K., Nishiyama T., Adachi T. (2002). Sensitive determination of benzalkonium chloride in blood and tissues using high-performance liquid chromatography with solid-phase extraction. Leg Med. (Tokyo) 4, 232–238. [DOI] [PubMed] [Google Scholar]
- Xue Y., Hieda Y., Saito Y., Nomura T., Fujihara J., Takayama K., Kimura K., Takeshita H. (2004). Distribution and disposition of benzalkonium chloride following various routes of administration in rats. Toxicol. Lett. 148, 113–123. [DOI] [PubMed] [Google Scholar]
- Yang J., Goldstein J. L., Hammer R. E., Moon Y. A., Brown M. S., Horton J. D. (2001). Decreased lipid synthesis in livers of mice with disrupted Site-1 protease gene. Proc. Natl. Acad. Sci. USA 98, 13607–13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.