Abstract
Background
The efficacy of short-term antiarrhythmic drugs (AADs) use compared with no-AADs prescription after catheter ablation of atrial fibrillation (AF) in preventing atrial arrhythmia recurrence is uncertain.
Methods
We searched PubMed, Embase, and the Cochrane Library through December 2015 to identify randomized controlled trials (RCTs) which evaluated the efficacy of short-term AADs use compared with no-AADs prescription after AF ablation in preventing atrial arrhythmia recurrence. The primary outcome was labeled as early atrial arrhythmia recurrence within 3 months after ablation. Secondary outcome was defined as late recurrence after 3 months of ablation. Random-effects model or fixed-effects model was used to estimate relative risks (RRs) with 95% confidence intervals (CIs).
Results
Six RCTs with 2,667 patients were included into this meta-analysis. Compared with no-AADs administration after AF ablation, short-term AADs use was associated with significant reduction of early atrial arrhythmia recurrence (RR, 0.68; 95% CI, 0.52–0.87; p = 0.003). Trial sequential analysis (TSA) showed that the cumulative Z-curve crossed the trial sequential monitoring boundary for benefit, establishing sufficient and conclusive evidence. However, compared with no-AADs prescription, short-term AADs use after AF ablation didn’t significantly reduce the risk of late atrial arrhythmia recurrence (RR, 0.92; 95% CI, 0.83–1.03; p = 0.15). TSA supported this result; meanwhile the estimated required information size (1,486 patients) was also met.
Conclusion
Short-term use of AADs after AF ablation can significantly decrease the risk of early atrial arrhythmia recurrence but not lead to corresponding reduction in risk of late atrial arrhythmia recurrence.
Introduction
Arial fibrillation (AF), predisposing patients to an increased risk of stroke, heart failure and death, is the most common arrhythmia encountered in clinical practice[1,2]. A key therapeutic goal of AF is to restore and maintain sinus rhythm. Catheter ablation, as a guideline-supported treatment option to restore sinus rhythm for patients with drug refractory AF, is increasingly performed in routine clinical practice[1].
However, the recurrences of AF or other atrial arrhythmia following catheter ablation are very common, estimated up to 50% after a single intervention[3,4,5,6,7]. Referred to early recurrence of atrial arrhythmias, one of the most prominent causes is the transient post-ablation inflammation following the histopathologic tissue damage that resulted from RF energy, which contributes to the heterogeneity of action potential durations in the myocardium of the atrial and/or pulmonary veins by inflammatory cytokines and ultimately results in the creation of an arrhythmogenic substrate that may be related to the initiation of AF[8,9,10,11]. It is known that antiarrhythmic drugs (AADs), especially the class I and III AADs, could lead to the prolongation of the effective refractory period and the action potential durations of atrial myocytes by blocking the sodium and potassium channels. Therefore, theoretically, the use of AADs after catheter ablation of AF may prevent the early recurrence of atrial arrhythmias through improving the heterogeneity of action potential durations of the atrial myocardium. Moreover, early recurrence of atrial arrhythmias, although not a predictor of treatment failure in AF patients who received catheter ablation[1,12], has been shown to be a strong indicator of late recurrence[13,14,15]. Thus, pharmacologic rhythm control approach with short-term use of AADs within 3 months after ablation has been proposed by the guidelines and expert consensus[1,12]. Short-term use of AADs is also often prescribed by experience in clinical practice.
However, the efficacy of AADs short-term use after catheter ablation of AF was just investigated in several small size randomized controlled trials (RCTs) [13,16,17], each of which enrolled only 50–200 patients until the results of a large-scale multicentre study with 2038 patients were published on European Heart Journal in October 2015[14].
Evidence from the above RCTs reported inconsistent results. And a published small-size meta-analysis conducted by Xu et al[18] was underpowered to reach determinate conclusions because of the limitations that need to be addressed: first, the included patients in the meta-analysis had obvious clinical heterogeneity, as the included trial by Brignole et al[19] involved AF patients received atrioventricular junction ablation but not PVI-based catheter ablation, and in another included RCT by Jun et al[20] the control group received either class I or III AADs but not placebo while the extensive AADs therapy group received both class I and III AADs. Second, apart from these two above mentioned RCTs with clinical heterogeneity, only 4 RCTs with 555 patients had the appropriate clinical homogeneity for meta-analysis. Thus, we conducted a meta-analysis of the latest and most convincing evidence to evaluate the efficacy of short-term AADs use compared with no-AADs after catheter ablation of AF in preventing atrial arrhythmia recurrence; and we further applied trial sequential analysis (TSA) to determine whether the currently available evidence was sufficient and conclusive.
Methods
This meta-analysis was performed according to the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions[21] and was reported in compliance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement) guidelines[22]. There was no registered protocol for this meta-analysis.
Literature search strategy
We searched the published literature in PubMed, Embase and the Cochrane Library from inception through December 27, 2015. The systematic electronic searches were conducted using exploded Medical Subject Headings (MeSH) terms and the corresponding keywords in Title/abstract. The search terms used in this meta-analysis were (MeSH exp ‘Atrial Fibrillation’, and keywords ‘atrial’, ‘atrium’, ‘auricular’, ‘fibrillation’, ‘fibrillations’,), (MeSH exp ‘Anti-Arrhythmia Agents’, and keywords ‘anti-arrhythm*’, ‘antiarrhythm*’, ‘antifibri*’), and (MeSH exp ‘Catheter Ablation’ and keywords ‘ablation’,). No language restriction was applied. To ensure literature saturation, we re-ran the searches on December 31, 2015. We also searched ClinicalTrials.gov registry (https://clinicaltrials.gov/) and manually checked the reference lists of previous published reviews and included trials to identify other potentially eligible trials. Two reviewers (W.C. and H.L.) independently conducted the initial search, deleted duplicate records, screened the titles and abstracts for relevance, and identified records as included, excluded or uncertain. In case of uncertainty, full-text article was acquired to identify eligibility. Doubts and disagreements were solved by discussion and consensus.
Selection criteria
Published RCTs meeting the following criteria were included: (1) Population: AF patients undergoing catheter ablation with pulmonary vein isolation (PVI)-based strategy; (2) Intervention: short-term administration of AADs within 3 months after ablation of AF; (3) Comparison: no-AADs prescription after ablation procedure; and (4) Outcome: early recurrence of atrial arrhythmia lasting more than 30 s within the first 3 months after ablation, late recurrence of atrial arrhythmia lasting more than 30 s post the 3 months’ “blank period” after ablation.
Data extraction
Data extraction was conducted by Weijie Chen, and independently confirmed by other authors (Z.L. and Y.Y.). The following information was obtained using a standardized Excel (Microsoft Corporation) data extraction form: first author, year of publication, country, study population (AF type, AF duration), number of patients, demographics of patients, echocardiographic parameters (left ventricular ejection fraction, left atrial dimension), comorbidities of patients, ablation procedural data (ablation strategy, ablation procedural endpoints) and outcomes data (follow-up duration, study endpoints, AADs in antiarrhythmic group, therapeutic strategy in control group, anti-arrhythmia duration after ablation, early recurrent events, early cardioversion events, and late recurrent events). Additionally, we also reviewed supplementary appendices of included RCTs and contacted the corresponding authors to verify extracted data and request the unreachable data, if necessary. Discrepancies during data extraction were resolved by discussion with co-authors. Predefined primary outcome was early recurrence of atrial arrhythmia within the first 3 months after ablation and the secondary outcome was late recurrence of atrial arrhythmia.
Risk of Bias Assessment
Risk of bias was independently assessed by two reviewers (W.C. and Y.X.) using the Cochrane risk-of-bias tool[23]. According to the tool, each included trial was reviewed and scored as ‘high’, ‘low’, or ‘unclear’ risk with the following criteria: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other bias. Trials with high risk of bias for any domain were considered as at high risk of bias; while trials with low risk of bias for all key domains were considered as at low risk of bias; otherwise they were considered as at unclear risk of bias.
Grading Quality of Evidence
The quality of evidence for primary and secondary outcomes of this meta-analysis was independently evaluated by two reviewers (W.C. and J.F.) according to the GRADE methodology for risk of bias, inconsistency, indirectness, imprecision, and publication bias; and classified as very low, low, moderate, or high. Summary tables were constructed using the GRADE Profiler (GRADEpro, version 3.6.1)[24].
Statistical Analysis
Statistical analyses were performed using Stata 13.0 (StataCorp LP, College Station, Texas) and Review Manager 5.3. (The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). Relative risks (RRs) with 95% CIs were calculated for dichotomous outcomes. Heterogeneity across studies was quantified using the I2 statistic[25]. Studies with an I2 statistic of 25% to 50% were considered to have low heterogeneity, those with an I2 statistic of 50% to 75% were considered to have moderate heterogeneity, and those > 75% were considered to have a high degree of heterogeneity[25]. An I2 value greater than 50% indicates significant heterogeneity, and the Mantel-Haenszel method with random effects model was used to calculate pooled RRs and 95% CIs; otherwise, the fixed effects model was used. For the meta-analyses with significant heterogeneity, sensitivity analysis was performed to evaluate the influence of single studies on the summary estimates and the consistency of the outcome. Because graphical evaluation with funnel plot can be subjective, we performed both Harbord[26] and Peters[27] tests, as formal statistical tests for publication bias. P value < 0.05 was considered statistically significant, except where otherwise specified such as p < 0.1 indicated statistically significant for heterogeneity test and publication bias evaluation with Harbord and Peters tests.
In meta-analysis, random errors because of sparse data and repetitive testing of accumulating data increase the risk of type I error[28,29]. To avoid this and correct for the increased risk of random errors, trial sequential analysis (TSA) was recommended to be used, which can determine whether the evidence in a meta-analysis is reliable and conclusive[28,30]. TSA is a methodology that combines an estimated required information size calculation for a meta-analysis with the adaptation of monitoring boundaries to evaluate the accumulated evidence. Similar to a sample size calculation for a single trial, the estimated required information size calculation involves a methodology that includes type I error, type II error, the control event proportion, and the effect size, allowing a quantification of the reliability of cumulative data in meta-analyses. Based on the theory of TSA, when the cumulative Z-curve crosses the trial sequential monitoring boundary or enters the futility area, a sufficient level of evidence for the anticipated intervention effect may have been reached and no further trials are needed. If the Z-curve does not cross any of the boundaries and the required information size has not been reached, evidence to reach a conclusion is insufficient. For our TSAs in this review, the required information size was estimated using α = 0.05 (two sided), β = 0.20 (power 80%), the control event proportions calculated from the control group of this meta-analysis, and an estimated relative risk reduction of 25% in early recurrence of atrial arrhythmia and 20% in late recurrence of atrial arrhythmia. All these trial sequential analyses were performed using software TSA version 0.9 beta (http://www.ctu.dk/tsa, Copenhagen Trial Unit, 2011)[31].
Results
Literature search
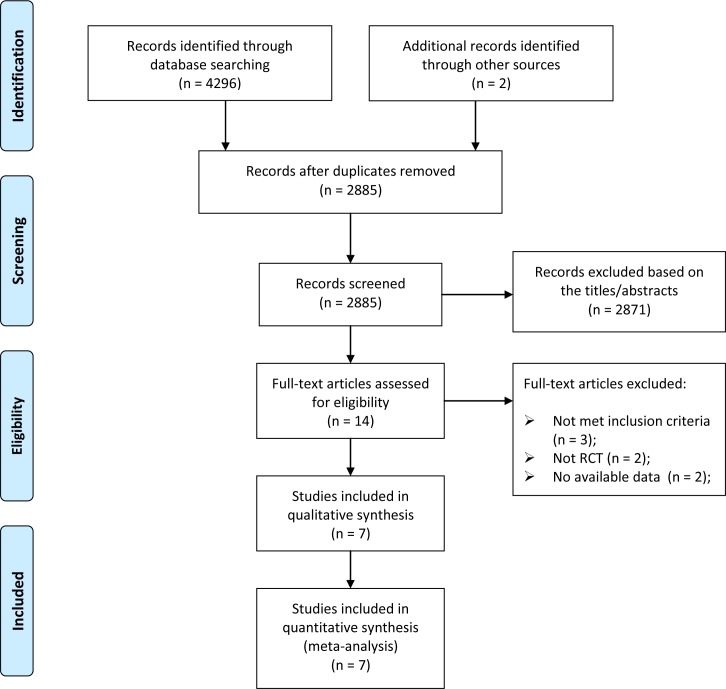
The results of literature search and selection are shown in the PRISMA flowchart (Fig 1). Our initial search yielded 4298 records. After removing duplicates and screening the titles and abstracts, 14 articles were thought to be potentially eligible for inclusion. After full-text review, 7 published reports[13,14,16,17,32,33,34] with the results from 6 RCTs were finally included in this meta-analysis. Two of the published reports were results of the first 6 weeks and 6 months related to the same study.
Fig 1. Selection of randomized controlled trials for this meta-analysis.
RCT = randomized controlled trials.
Trials Characteristics
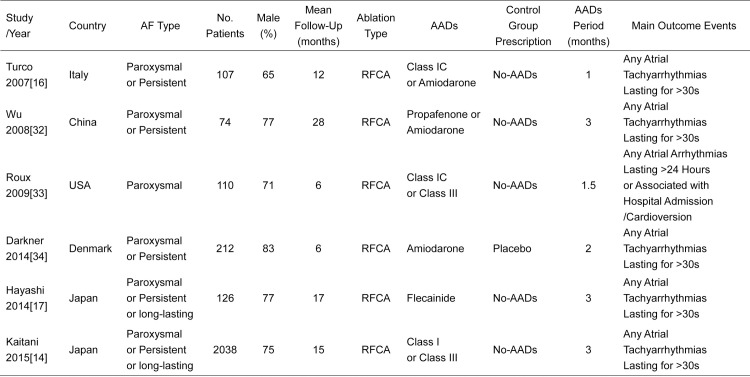
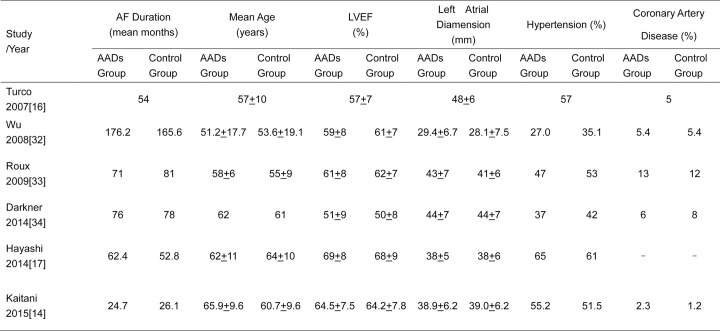
The main characteristics of 6 included RCTs with 2667 patients are shown in Figs 2 and 3. The population sizes of trials ranged from 74 to 2038. A total of 1329 patients in AADs group received short-term AADs therapy and 1338 patients in control group received no-AADs after ablation. Most of the RCTs included patients with paroxysmal and persistent AF except the trial by Roux et al[33] which only enrolled patients with paroxysmal AF. The mean AF duration of these RCTs ranged from 24 to 81 months except the trial by Wu et al[32] which included patients with more than 10 years of AF history. The mean age ranged from 51 to 65 years, and the proportion of men ranged from 65% to 83%. Most of the included patients had normal left ventricular ejection fraction (LVEF) with the baseline LVEF ranging from 50% to 69%. At baseline, mean left atrial diameter varied from 28 to 48 mm and did not differ in those who received short-term AADs therapy versus those who didn’t. Enrolled Patients had variable underlying heart diseases, while the most common comorbidity was hypertension. All enrolled patients underwent catheter ablation with PVI-based strategy. Patients in AADs group received class I or III AADs therapy for 1 to 3 months, while patients in control group received no-AADs treatment after AF ablation. And mean follow-up duration of RCTs ranged from 6 to 28 months.
Fig 2. PICO characteristics of the 6 Included Randomized Controlled Trials in This Meta-Analysis.
AF = atrial fibrillation. AADs = antiarrhythmic drugs. RFCA = radiofrequency catheter ablation.
Fig 3. Detailed patient information of the 6 Included Randomized Controlled Trials in This Meta-Analysis.
AF = atrial fibrillation. AADs = antiarrhythmic drugs. LVEF = left ventricular ejection fraction.
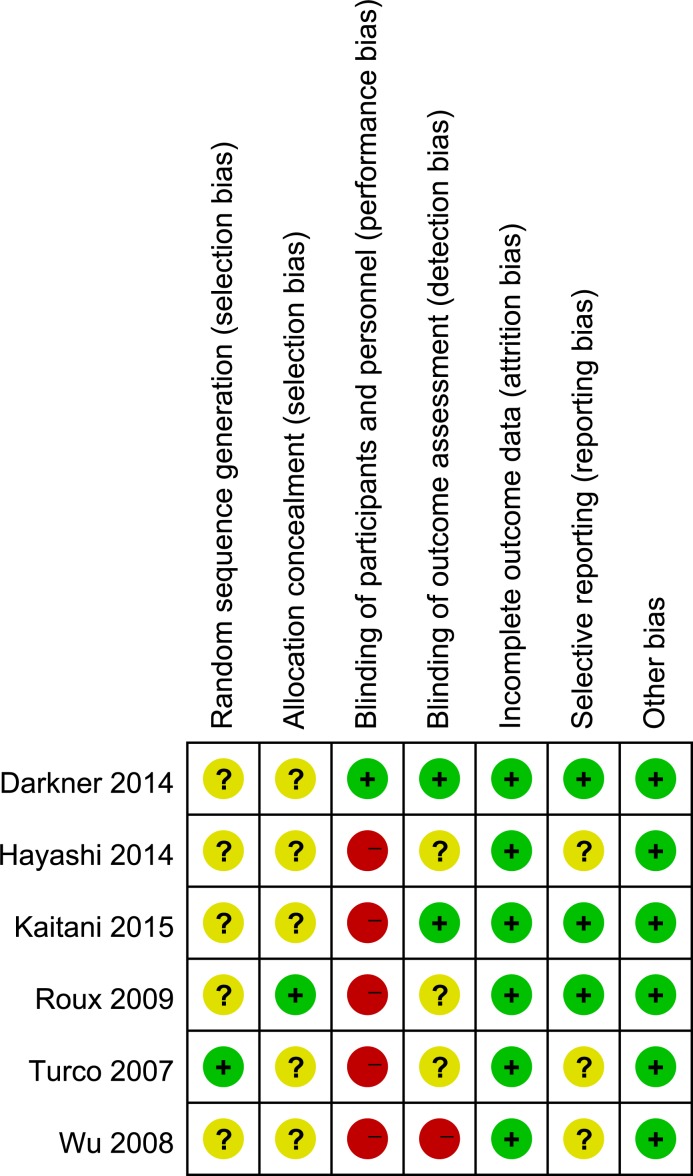
Risk of Bias Assessment
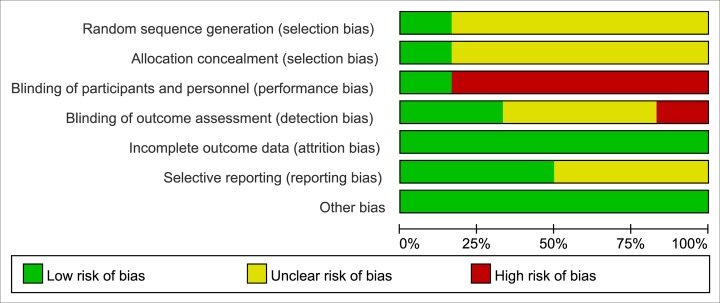
Details of risk of bias are summarized in Figs 4 and 5. According to the Cochrane risk-of-bias tool, 5 of the included RCTs are open-label studies[14,16,17,32,33] without blinding of participants and personnel, resulting in high risk of performance bias. All of the included RCTs didn’t report the information of random sequence generation except the trial by Turco et al[16]; five RCTs[14,16,17,32,34] didn’t report adequate information about allocation concealment. These problems resulted in the unclear risk of selection bias. For blinding of outcome assessment, one trial[32] was openly assessed by the outcome assessor and 3 RCTs[16,17,33] didn’t report the related information. Overall, based on the above information, one trial[34] was categorized as at unclear risk of bias, and five[14,16,17,32,33] as at high risk of bias. Therefore, the evidence level for primary and secondary outcome of this meta-analysis was partly downgraded.
Fig 4. Risk of bias for this meta-analysis: judgements about each risk of bias item presented as percentages across all included randomized controlled trials.
Fig 5. Risk of bias summary of the included randomized controlled trials: details about each risk of bias item for each included trials.
Green = low risk of bias, Yellow = unclear risk of bias, Red = high risk of bias.
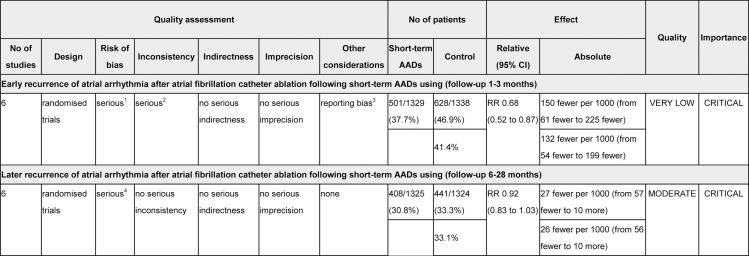
Primary Outcome
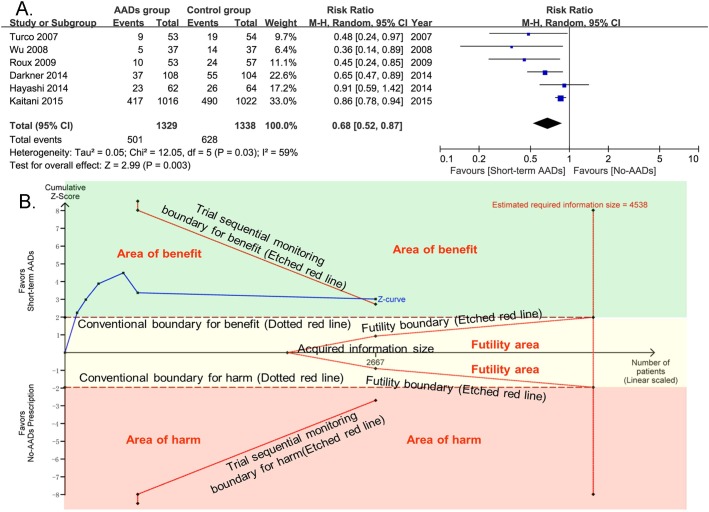
For the primary outcome of early recurrence of atrial arrhythmia, 6 included RCTs with 2667 patients provided the related information. Compared with no-AADs prescription in control group, short-term use of AADs after AF ablation significantly reduced the risk of early recurrence of atrial arrhythmia (RR, 0.68; 95% CI, 0.52–0.87; p = 0.003; Fig 6A), with moderate heterogeneity (I2 = 59%; phet = 0.03). In consideration of the moderate heterogeneity, to evaluate the influence of single studies on the pooled estimate and the consistency of primary outcome, sensitivity analysis with consecutively excluding one single trial each time was performed. The meta-analyses after excluding every trial one at a time had no significant effect on the pooled estimate and 95% confidence interval (Fig 7). Moreover, potential publication bias was found by Harbord (p = 0.048) and Peters tests (p = 0.05). Despite the estimating required information size was not met (4538 patients), TSA results showed that the cumulative Z-curve crossed both the conventional boundary for benefit and the trial sequential monitoring boundary for benefit and entered the area of benefit, which established sufficient and conclusive evidence for a 25% reduction in the relative risk of early recurrence of atrial fibrillation with short-term AADs use after ablation. Based on the above TSA results, further trials aimed to evaluate the effect of short-term AADs use on prevention of early recurrence of atrial arrhythmia after AF ablation, are not required and are unlikely to alter the present conclusions (Fig 6B).
Fig 6. Effect of short-term AADs use versus no-AADs prescription after AF ablation on early recurrence of atrial arrhythmias.
AADs = antiarrhythmic drugs; CI = confidence intervals. TSA results showed that the cumulative Z-curve crossed both the conventional boundary for benefit and the trial sequential monitoring boundary for benefit and entered the area of benefit, which established sufficient and conclusive evidence. X-axis: the number of patients included; Y-axis: the cumulative Z-Score; The red dotted lines: conventional boundaries (Z-score = 1.96, two-sided P value = 0.05); The red etched lines: trial sequential monitoring boundaries; Blue full line: the cumulative Z-curve; Vertical red etched line: the estimated required information size; The estimated required information size of 4538 patients was calculated using α = 0.05 (two sided), β = 0.20 (power 80%), an anticipated relative risk reduction of 25%, and an event proportion of 46.9% in the control group. Upper green square area: area of benefit; Middle faint yellow square area: futility area; Lower red square area: area of harm.
Fig 7. Sensitivity analysis of early recurrence of atrial arrhythmia.
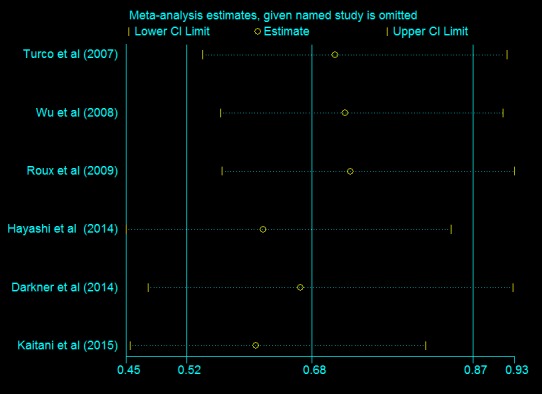
Single trial was excluded each time, however, pooled estimate and 95% confidence interval had no significant changes.
Secondary Outcomes
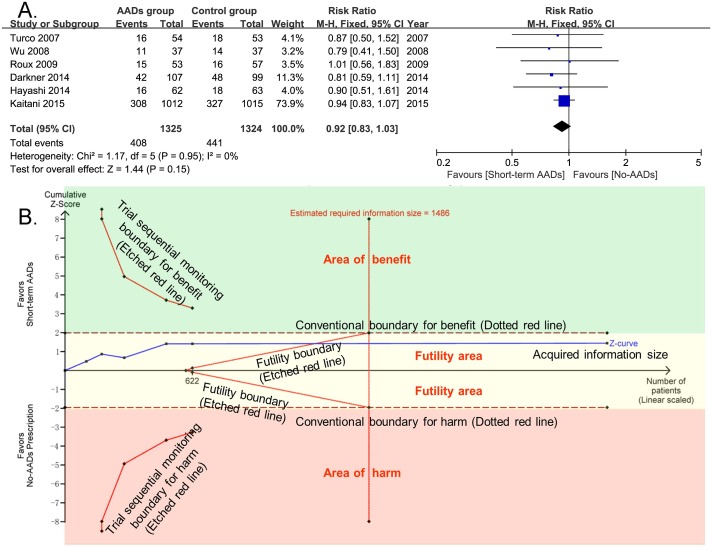
For late recurrence of atrial arrhythmia, 6 included RCTs totaling 2649 patients provided the related information. Compared with no-AADs prescription in control group, short-term use of AADs after AF ablation didn’t significantly reduce the risk of late recurrence of atrial arrhythmia (RR, 0.92; 95% CI, 0.83–1.03; p = 0.15; Fig 8A), without heterogeneity (I2 = 0%; phet = 0.95). For publication bias, both Harbord (p = 0.27) and Peters tests (p = 0.27) were not significant. TSA results showed that the estimating required information size was met with the present enrolled RCTs(1486 patients). And the cumulative Z-curve crossed the futility boundary and entered the futility area, indicating sufficient and conclusive evidence for a 20% reduction in the relative risk of late recurrence of atrial arrhythmia with short-term AADs use after ablation. The results also suggested that further clinical trials, aimed to evaluate the effect of short-term AADs use on prevention of late recurrence of atrial arrhythmia after AF ablation, are not required and are unlikely to alter the present negative conclusions (Fig 8B).
Fig 8. Effect of short-term AADs use versus no-AADs prescription after AF ablation on late recurrence of atrial arrhythmias.
AADs = antiarrhythmic drugs; CI = confidence intervals. TSA results showed that the cumulative Z-curve crossed the futility boundary and entered the futility area, indicating the negative result was also sufficient and conclusive. X-axis: the number of patients included; Y-axis: the cumulative Z-Score; The red dotted lines: conventional boundaries (Z-score = 1.96, two-sided P value = 0.05); The red etched lines: trial sequential monitoring boundaries; Blue full line: the cumulative Z-curve; Vertical red etched line: the estimated required information size; The reached estimating required information size of 622 patients was calculated using α = 0.05 (two sided), β = 0.20 (power 80%), an anticipated relative risk reduction of 20%, and an event proportion of 33.3% in the control group. Upper green square area: area of benefit; Middle faint yellow square area: futility area; Lower red square area: area of harm.
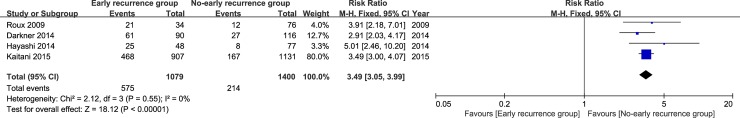
Additionally, the data related to late atrial arrhythmia recurrence events between patients with and without evidence of early atrial arrhythmia recurrence were extracted from 4 included RCTs with 2479 patients. The pooled RR was calculated with fixed effects Mantel-Hænzel model. The result showed that the risk of late atrial arrhythmia recurrence was 2.49-fold higher in patients with early recurrence of atrial arrhythmia than in patients without early recurrence (RR, 3.49; 95% CI, 3.05–3.99; p < 0.001; Fig 9), without heterogeneity (I2 = 0%; phet = 0.55).
Fig 9. Early atrial arrhythmia recurrence after atrial fibrillation ablation is a risk factor of late recurrence of atrial arrhythmia.
The pooled RR was calculated with fixed effects Mantel-Hænzel model.
GRADE Profile Evidence
The GRADE evidence profiles for the primary and secondary outcomes are shown in Fig 10. The GRADE Working Group grades level of evidence is very low for early recurrence of atrial arrhythmia (short-term AADs use versus no-AADs prescription after ablation), and moderate for late recurrence of atrial arrhythmia (short-term AADs use versus no-AADs prescription after ablation).
Fig 10. The GRADE Evidence Profile for the Primary and Secondary outcome of This Meta-Analysis.
GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. 1 Although most of included RCTs were judged as high risk of performance bias because of without blinding of participants and personnel, the predefined objective outcome was just partly influenced. 2 Heterogeneity (I2 = 59%) was found. 3 As shown by Harbord and Peters tests, publication bias may exist. 4 Although most of included RCTs were judged as high risk of performance bias because of without blinding of participants and personnel, the predefined objective outcome was partly influenced.
Discussion
Main Findings
The question addressed by this meta-analysis was whether short-term AADs use after catheter ablation of AF, compared with no-AADs prescription, could prevent the recurrence of atrial arrhythmia. Our meta-analysis comprehensively and systematically reviewed the current available literature, included 2667 patients from 6 RCTs, and found that (1) Short-term use of AADs compared with no-AADs prescription after AF ablation significantly reduced the risk of early recurrence of atrial arrhythmia. The evidence of this benefit was confirmed by TSA, which demonstrated this conclusion was reliable and conclusive. (2) Short-term use of AADs after AF ablation had no significant benefit on the prevention of late recurrence of atrial arrhythmia compared with no-AADs prescription in control group, which was also confirmed by TSA and the estimating required information size had been met with the present data. (3) Early recurrence of atrial arrhythmia was definitely a risk factor of late recurrence of atrial arrhythmia.
Comparison with Published Meta-analysis
Preceding this review, one meta-analysis on this topic conducted by Xu et al[18] was published on Cardiovascular Therapeutics in August 2015. Although the main finding of our meta-analysis was consistent with the meta-analysis conducted by Xu et al[18], differences between our respective results should be noted. First, the previous published meta-analysis included 6 RCTs with a total of 814 patients. In comparison, our present meta-analysis included 6 RCTs totaling 2667 patients. Secondly, as mentioned, the included patients in the meta-analysis published by Xu et al had obvious clinical heterogeneity, as in one of the included trials by Brignole et al[19], the enrolled AF patients received atrioventricular junction ablation treatment but not PVI-based catheter ablation and in another RCT by Jun et al[20] the control group received either class I or III AADs but not placebo while the extensive AADs therapy group received both class I and III AADs. Apart from these two mentioned RCTs with clinical heterogeneity, only 4 RCTs with 555 patients in previous published meta-analysis had the appropriate clinical homogeneity for meta-analysis. In contrast, all the enrolled AF patients in our meta-analysis received PVI-based catheter ablation and the patients in control group received no-AADs prescription after ablation procedure. Third, we further applied TSA to provide more conservative estimate. The results of TSA showed that our present meta-analysis established sufficient and conclusive evidence. Last but not the least, we reported our meta-analysis in strict compliance with the PRISMA guidelines and also evaluated the quality of evidence for outcomes using GRADE to help healthcare professionals to make clinical decisions.
The relationship between early recurrence and late recurrence
Our meta-analysis showed that although short-term AADs use after AF ablation significantly reduced the risk of early recurrence of atrial arrhythmia, it did not lead to the corresponding reduction in the risk of late recurrence of atrial arrhythmia. However, it has been shown that early recurrence of atrial arrhythmias after ablation was strongly associated with late atrial arrhythmia recurrence[14,15]. And our results also showed that early recurrence of atrial arrhythmia was definitely a risk factor of late atrial arrhythmia recurrence. Overall, the reasons to why early recurrence of atrial arrhythmia, as a risk factor for late atrial arrhythmia recurrence, can be decreased by short-term AADs use but not results in corresponding reduction of late recurrence of atrial arrhythmia, have not been intensively investigated.
The proposed mechanisms of early recurrence after AF ablation include mechanical and thermal injury provoking an inflammatory response[9,35,36], and the modification of autonomic nervous system[37,38]. Previous studies have demonstrated that the levels of inflammatory cytokines were significantly elevated after catheter ablation[35,39]. Among these cytokines, tumor necrosis factor (TNF) can directly alter Ca2+ handling in cardiomyocytes, which is crucial for the initiation of AF and atrial electrical remodeling[10,40,41]; platelet-derived growth factor (PDGF) can reduce the duration of action potentials and Ca2+ transients of cardiomyocytes[42]; and also C-reactive Protein is associated with an increased number of identified nonpulmonary vein ectopies and high-frequency sites in the left atrium[43,44]. Additionally, inflammation also increases the heterogeneity of conduction and AF duration[44,45]. Thus, elevated inflammatory cytokines can irritate the left atrium by influencing the electrophysiological characteristics of atrial myocardium and increase vulnerability to AF, which may be part of the reason for high rate of early atrial arrhythmia recurrences after AF ablation. It is known that AADs, especially the class I and III AADs, could lead to the prolongation of the effective refractory period and the action potential durations of atrial myocytes by blocking the sodium and potassium channels. Therefore, as we theoretically hypothesized, short-term use of AADs may prevent the above alteration of atrial electrophysiological characteristics through blocking ion channels and ultimately leads to reduction of early atrial arrhythmia recurrences.
The mechanisms for early recurrence and late recurrence of atrial arrhythmias are different. Previous studies indicated that late recurrences of atrial arrhythmias were mainly ascribed to incomplete pulmonary vein isolation, recovery of electrical conduction between the pulmonary veins and left atrium, or that of other block lines created in the previous procedure[1,46,47]. Moreover, the experiences of centers for catheter ablation procedure of AF were also an important factor that influenced the incidence of late atrial arrhythmia recurrences. However, all these factors can not be effectively resolved by short-term use of AADs. A recent report by Zhang et al[48] has shown that a second ablation procedure was more effective in maintaining sinus rhythm than AADs in patients with late atrial arrhythmia recurrence after catheter ablation of AF. As we above discussed, the main causes for early recurrence of atrial arrhythmias included post-ablation inflammations, temporary autonomic imbalance, and also the delay of atrial radiofrequency lesion formation[49]. Moreover, recovery of pulmonary veins-left atrium conduction was also proposed as a potential cause for early atrial arrhythmia recurrences[50,51]. However, the referred to effect of post-ablation inflammatory cytokines on early atrial arrhythmia recurrences were elevated in the days immediately following PVI but returned to baseline within 30 days[52], while significant autonomic imbalance was observed at one week following PVI and spontaneous recovered within one month[37]. It has been proposed that these transient factors would not be expected to lead to late atrial arrhythmia recurrences[50]. Previous studies have demonstrated that early atrial arrhythmia recurrences within one month following PVI, which may be promoted by these transient factors, were less predictive for late recurrences compared with that initiated in the second and third months post-ablation which may mainly be induced by pulmonary veins-left atrium electrical reconnection[53,54]. Therefore, as this meta-analysis shown, short-term use of AADs after AF ablation, through the mechanism of preventing the alteration of atrial electrophysiological characteristics induced by the above transient factors, although significantly reduced the risk of early atrial arrhythmia recurrences, but not resulted in the corresponding reduction of late atrial arrhythmia recurrences.
Implications for Clinical Practice
Based on our meta-analysis results, in clinical practice, short-term use of AADs after AF ablation just reduces early recurrence of atrial arrhythmia within 3 months but not affect the risk of late atrial arrhythmia recurrences. For late recurrence of atrial arrhythmia, it has been suggested by RCT[48] and guidelines[1] that repeat ablation may be a preferable option. However, the reduction of early atrial arrhythmia recurrence may lead to better medical compliance of patients. Thus, pharmacologic rhythm control approach with short-term use of antiarrhythmic drugs (AADs) but not repeat ablation within 3 months after AF ablation has been proposed by the guidelines[1] and expert consensus[12].
Limitations
Our meta-analysis also had limitations. First, this is a meta-analysis at the study level and a variety of differences exist in ablation procedures, follow-up periods, physician experience, and antiarrhythmic drugs used among enrolled RCTs. Second, most of the included trials were not blinded, which may result in performance and detection bias and influence the evidence level of outcomes.
Conclusions
Short-term use of AADs after AF ablation can significantly decrease the risk of early recurrence of atrial arrhythmia, which is a risk factor of late atrial arrhythmia recurrence, but not lead to the corresponding reduction in the risk of late atrial arrhythmia recurrence.
Supporting Information
(DOC)
(DOCX)
Acknowledgments
The authors wish to acknowledge professor Meiso Hayashi for his help in verifying the details of outcome data from his research work.
The authors do not have any possible conflicts of interest.
Abbreviations
- AADs
antiarrhythmic drugs
- AF
atrial fibrillation
- CI
confidence intervals
- CRP
C-reactive protein
- GRADE
Grading of Recommendations Assessment, Development, and Evaluation
- LVEF
left ventricular ejection fraction
- MeSH
Medical Subject Headings
- PDGF
platelet-derived growth factor
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement
- PVI
pulmonary vein isolation
- RCT
randomized controlled trial
- RR
relative risk
- TNF
tumor necrosis factor
- TSA
trial sequential analysis
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014; 64: e1–76. 10.1016/j.jacc.2014.03.022 [DOI] [PubMed] [Google Scholar]
- 2.Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014; 129: 837–847. 10.1161/CIRCULATIONAHA.113.005119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oral H, Pappone C, Chugh A, Good E, Bogun F, Pelosi F Jr, et al. Circumferential pulmonary-vein ablation for chronic atrial fibrillation. N Engl J Med. 2006; 354: 934–941. 10.1056/NEJMoa050955 [DOI] [PubMed] [Google Scholar]
- 4.Cosedis NJ, Mortensen LS, Hansen PS. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. N Engl J Med. 2013; 368: 478–479. 10.1056/NEJMc1214193 [DOI] [PubMed] [Google Scholar]
- 5.Morillo CA, Verma A, Connolly SJ, Kuck KH, Nair GM, Champagne J, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of paroxysmal atrial fibrillation (RAAFT-2): a randomized trial. JAMA. 2014; 311: 692–700. 10.1001/jama.2014.467 [DOI] [PubMed] [Google Scholar]
- 6.Aytemir K, Oto A, Canpolat U, Sunman H, Yorgun H, Şahiner, et al. Immediate and medium-term outcomes of cryoballoon-based pulmonary vein isolation in patients with paroxysmal and persistent atrial fibrillation: single-centre experience. J Interv Card Electrophysiol. 2013; 38: 187–195. 10.1007/s10840-013-9834-2 [DOI] [PubMed] [Google Scholar]
- 7.Canpolat U, Aytemir K, Yorgun H, Şahiner, Kaya EB, Oto A. A proposal for a new scoring system in the prediction of catheter ablation outcomes: promising results from the Turkish Cryoablation Registry. Int J Cardiol. 2013; 169: 201–206. 10.1016/j.ijcard.2013.08.097 [DOI] [PubMed] [Google Scholar]
- 8.Fujiki A, Sakamoto T, Nishida K, Mizumaki K, Inoue H. Relation of interleukin-6 and C-reactive protein levels to sinus maintenance after pharmacological cardioversion in persistent atrial fibrillation. J Cardiovasc Pharmacol. 2007; 50: 264–266. 10.1097/FJC.0b013e318074f952 [DOI] [PubMed] [Google Scholar]
- 9.Koyama T, Tada H, Sekiguchi Y, Arimoto T, Yamasaki H, Kuroki K, et al. Prevention of atrial fibrillation recurrence with corticosteroids after radiofrequency catheter ablation: a randomized controlled trial. J Am Coll Cardiol. 2010; 56: 1463–1472. 10.1016/j.jacc.2010.04.057 [DOI] [PubMed] [Google Scholar]
- 10.Lee SH, Chen YC, Chen YJ, Chang SL, Tai CT, Wongcharoen W, et al. Tumor necrosis factor-alpha alters calcium handling and increases arrhythmogenesis of pulmonary vein cardiomyocytes. Life Sci. 2007; 80: 1806–1815. 10.1016/j.lfs.2007.02.029 [DOI] [PubMed] [Google Scholar]
- 11.Rizos I, Tsiodras S, Rigopoulos AG, Dragomanovits S, Kalogeropoulos AS, Papathanasiou S, et al. Interleukin-2 serum levels variations in recent onset atrial fibrillation are related with cardioversion outcome. Cytokine. 2007; 40: 157–164. 10.1016/j.cyto.2007.08.013 [DOI] [PubMed] [Google Scholar]
- 12.Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm. 2012; 9: 632–696.e21. 10.1016/j.hrthm.2011.12.016 [DOI] [PubMed] [Google Scholar]
- 13.Leong-Sit P, Roux JF, Zado E, Callans DJ, Garcia F, Lin D, et al. Antiarrhythmics after ablation of atrial fibrillation (5A Study): six-month follow-up study. Circ Arrhythm Electrophysiol. 2011; 4: 11–14. 10.1161/CIRCEP.110.955393 [DOI] [PubMed] [Google Scholar]
- 14.Kaitani K, Inoue K, Kobori A, Nakazawa Y, Ozawa T, Kurotobi T, et al. Efficacy of Antiarrhythmic Drugs Short-Term Use After Catheter Ablation for Atrial Fibrillation (EAST-AF) trial. Eur Heart J. 2015. 10.1093/eurheartj/ehv501 [DOI] [PubMed] [Google Scholar]
- 15.Oral H, Knight BP, Ozaydin M, Tada H, Chugh A, Hassan S, et al. Clinical significance of early recurrences of atrial fibrillation after pulmonary vein isolation. J Am Coll Cardiol. 2002; 40: 100–104. [DOI] [PubMed] [Google Scholar]
- 16.Turco P, De Simone A, La Rocca V, Iuliano A, Capuano V, Astarita C, et al. Antiarrhythmic drug therapy after radiofrequency catheter ablation in patients with atrial fibrillation. Pacing Clin Electrophysiol. 2007; 30 Suppl 1: S112–115. 10.1111/j.1540-8159.2007.00618.x [DOI] [PubMed] [Google Scholar]
- 17.Hayashi M, Miyauchi Y, Iwasaki YK, Yodogawa K, Tsuboi I, Uetake S, et al. Three-month lower-dose flecainide after catheter ablation of atrial fibrillation. Europace. 2014; 16: 1160–1167. 10.1093/europace/euu041 [DOI] [PubMed] [Google Scholar]
- 18.Xu X, Alida CT, Yu B. Administration of antiarrhythmic drugs to maintain sinus rhythm after catheter ablation for atrial fibrillation: a meta-analysis. Cardiovasc Ther. 2015; 33: 242–246. 10.1111/1755-5922.12133 [DOI] [PubMed] [Google Scholar]
- 19.Brignole M, Menozzi C, Gasparini M, Bongiorni MG, Botto GL, Ometto R, et al. An evaluation of the strategy of maintenance of sinus rhythm by antiarrhythmic drug therapy after ablation and pacing therapy in patients with paroxysmal atrial fibrillation. Eur Heart J. 2002; 23: 892–900. 10.1053/euhj.2001.2971 [DOI] [PubMed] [Google Scholar]
- 20.Gu J, Liu X, Tan H, Zhou L, Gu J, Jiang W, et al. Extensive antiarrhythmic drugs after catheter ablation of persistent atrial fibrillation. Acta Cardiol. 2012; 67: 407–414. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0 The Cochrane Collaboration, 2011. Available from: http://www.cochrane.org/handbook [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009; 339: b2535 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011; 343: d5928 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008; 336: 924–926. 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327: 557–560. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med. 2006; 25: 3443–3457. 10.1002/sim.2380 [DOI] [PubMed] [Google Scholar]
- 27.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006; 295: 676–680. 10.1001/jama.295.6.676 [DOI] [PubMed] [Google Scholar]
- 28.Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol. 2008; 61: 64–75. 10.1016/j.jclinepi.2007.03.013 [DOI] [PubMed] [Google Scholar]
- 29.Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta-analyses may be inconclusive—Trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta-analyses. Int J Epidemiol. 2009; 38: 287–298. 10.1093/ije/dyn188 [DOI] [PubMed] [Google Scholar]
- 30.Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta-analyses. J Clin Epidemiol. 2008; 61: 763–769. 10.1016/j.jclinepi.2007.10.007 [DOI] [PubMed] [Google Scholar]
- 31.Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manualfor trial sequential analysis (TSA) Copenhagen Trial Unit, Centre for Clinical Intervention Research, 588 Copenhagen, Denmark: 2011. p.1–115. Available from www.ctu.dk/tsa [Google Scholar]
- 32.Wu G, Jiang H, Huang CX, Yang B, Huang H, Wang YL, et al. [Effects of antiarrhythmic drug use on atrial fibrillation recurrence in atrial fibrillation patients post circumferential pulmonary vein ablation]. Zhonghua Xin Xue Guan Bing Za Zhi. 2008; 36: 623–626. [PubMed] [Google Scholar]
- 33.Roux JF, Zado E, Callans DJ, Garcia F, Lin D, Marchlinski FE, et al. Antiarrhythmics After Ablation of Atrial Fibrillation (5A Study). Circulation. 2009; 120: 1036–1040. 10.1161/CIRCULATIONAHA.108.839639 [DOI] [PubMed] [Google Scholar]
- 34.Darkner S, Chen X, Hansen J, Pehrson S, Johannessen A, Nielsen JB, et al. Recurrence of arrhythmia following short-term oral AMIOdarone after CATheter ablation for atrial fibrillation: a double-blind, randomized, placebo-controlled study (AMIO-CAT trial). Eur Heart J. 2014; 35: 3356–3364. 10.1093/eurheartj/ehu354 [DOI] [PubMed] [Google Scholar]
- 35.McCabe JM, Smith LM, Tseng ZH, Badhwar N, Lee BK, Lee RJ, et al. Protracted CRP elevation after atrial fibrillation ablation. Pacing Clin Electrophysiol. 2008; 31: 1146–1151. 10.1111/j.1540-8159.2008.01155.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yokokawa M, Tada H, Koyama K, Ino T, Naito S, Oshima S, et al. Thickening of the left atrial wall shortly after radiofrequency ablation predicts early recurrence of atrial fibrillation. Circ J. 2010; 74: 1538–1546. [DOI] [PubMed] [Google Scholar]
- 37.Hsieh MH, Chiou CW, Wen ZC, Wu CH, Tai CT, Tsai CF, et al. Alterations of heart rate variability after radiofrequency catheter ablation of focal atrial fibrillation originating from pulmonary veins. Circulation. 1999; 100: 2237–2243. [DOI] [PubMed] [Google Scholar]
- 38.Pappone C, Santinelli V, Manguso F, Vicedomini G, Gugliotta F, Augello G, et al. Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation. Circulation. 2004; 109: 327–334. 10.1161/01.CIR.0000112641.16340.C7 [DOI] [PubMed] [Google Scholar]
- 39.Stein A, Wessling G, Deisenhofer I, Busch G, Steppich B, Estner H, et al. Systemic inflammatory changes after pulmonary vein radiofrequency ablation do not alter stem cell mobilization. Europace. 2008; 10: 444–449. 10.1093/europace/eun041 [DOI] [PubMed] [Google Scholar]
- 40.Saba S, Janczewski AM, Baker LC, Shusterman V, Gursoy EC, Feldman AM, et al. Atrial contractile dysfunction, fibrosis, and arrhythmias in a mouse model of cardiomyopathy secondary to cardiac-specific overexpression of tumor necrosis factor-{alpha}. Am J Physiol Heart Circ Physiol. 2005; 289: H1456–1467. 10.1152/ajpheart.00733.2004 [DOI] [PubMed] [Google Scholar]
- 41.Sawaya SE, Rajawat YS, Rami TG, Szalai G, Price RL, Sivasubramanian N, et al. Downregulation of connexin40 and increased prevalence of atrial arrhythmias in transgenic mice with cardiac-restricted overexpression of tumor necrosis factor. Am J Physiol Heart Circ Physiol. 2007; 292: H1561–1567. 10.1152/ajpheart.00285.2006 [DOI] [PubMed] [Google Scholar]
- 42.Musa H, Kaur K, O'Connell R, Klos M, Guerrero-Serna G, Avula UM, et al. Inhibition of platelet-derived growth factor-AB signaling prevents electromechanical remodeling of adult atrial myocytes that contact myofibroblasts. Heart Rhythm. 2013; 10: 1044–1051. 10.1016/j.hrthm.2013.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin YJ, Tsao HM, Chang SL, Lo LW, Tuan TC, Hu YF, et al. Prognostic implications of the high-sensitive C-reactive protein in the catheter ablation of atrial fibrillation. Am J Cardiol. 2010; 105: 495–501. 10.1016/j.amjcard.2009.10.019 [DOI] [PubMed] [Google Scholar]
- 44.Hu YF, Chen YJ, Lin YJ, Chen SA. Inflammation and the pathogenesis of atrial fibrillation. Nat Rev Cardiol. 2015; 12: 230–243. 10.1038/nrcardio.2015.2 [DOI] [PubMed] [Google Scholar]
- 45.Ishii Y, Schuessler RB, Gaynor SL, Yamada K, Fu AS, Boineau JP, et al. Inflammation of atrium after cardiac surgery is associated with inhomogeneity of atrial conduction and atrial fibrillation. Circulation. 2005; 111: 2881–2888. 10.1161/CIRCULATIONAHA.104.475194 [DOI] [PubMed] [Google Scholar]
- 46.Sotomi Y, Inoue K, Ito N, Kimura R, Toyoshima Y, Masuda M, et al. Cause of very late recurrence of atrial fibrillation or flutter after catheter ablation for atrial fibrillation. Am J Cardiol. 2013; 111: 552–556. 10.1016/j.amjcard.2012.10.040 [DOI] [PubMed] [Google Scholar]
- 47.Lee SH, Tai CT, Hsieh MH, Tsai CF, Lin YK, Tsao HM, et al. Predictors of early and late recurrence of atrial fibrillation after catheter ablation of paroxysmal atrial fibrillation. J Interv Card Electrophysiol. 2004; 10: 221–226. 10.1023/B:JICE.0000026915.02503.92 [DOI] [PubMed] [Google Scholar]
- 48.Zhang XD, Gu J, Jiang WF, Zhao L, Zhou L, Wang YL, et al. Optimal rhythm-control strategy for recurrent atrial tachycardia after catheter ablation of persistent atrial fibrillation: a randomized clinical trial. Eur Heart J. 2014; 35: 1327–1334. 10.1093/eurheartj/ehu017 [DOI] [PubMed] [Google Scholar]
- 49.Fenelon G, Brugada P. Delayed effects of radiofrequency energy: mechanisms and clinical implications. Pacing Clin Electrophysiol. 1996; 19: 484–489. [DOI] [PubMed] [Google Scholar]
- 50.Das M, Wynn GJ, Morgan M, Lodge B, Waktare JE, Todd DM, et al. Recurrence of atrial tachyarrhythmia during the second month of the blanking period is associated with more extensive pulmonary vein reconnection at repeat electrophysiology study. Circ Arrhythm Electrophysiol. 2015; 8: 846–852. 10.1161/CIRCEP.115.003095 [DOI] [PubMed] [Google Scholar]
- 51.Ouyang F, Antz M, Ernst S, Hachiya H, Mavrakis H, Deger FT, et al. Recovered pulmonary vein conduction as a dominant factor for recurrent atrial tachyarrhythmias after complete circular isolation of the pulmonary veins: lessons from double Lasso technique. Circulation. 2005; 111: 127–135. 10.1161/01.CIR.0000151289.73085.36 [DOI] [PubMed] [Google Scholar]
- 52.Lim HS, Schultz C, Dang J, Alasady M, Lau DH, Brooks AG, et al. Time course of inflammation, myocardial injury, and prothrombotic response after radiofrequency catheter ablation for atrial fibrillation. Circ Arrhythm Electrophysiol. 2014; 7: 83–89. 10.1161/CIRCEP.113.000876 [DOI] [PubMed] [Google Scholar]
- 53.Bertaglia E, Stabile G, Senatore G, Zoppo F, Turco P, Amellone C, et al. Predictive value of early atrial tachyarrhythmias recurrence after circumferential anatomical pulmonary vein ablation. Pacing Clin Electrophysiol. 2005; 28: 366–371. 10.1111/j.1540-8159.2005.09516.x [DOI] [PubMed] [Google Scholar]
- 54.Themistoclakis S, Schweikert RA, Saliba WI, Bonso A, Rossillo A, Bader G, et al. Clinical predictors and relationship between early and late atrial tachyarrhythmias after pulmonary vein antrum isolation. Heart Rhythm. 2008; 5: 679–685. 10.1016/j.hrthm.2008.01.031 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.