Abstract
Sexual minorities in the United States are at elevated risk of bullying, discrimination, and violence victimization, all stressors that have been linked to psychological and behavioral stress responses including depressive and anxious symptoms and substance use. Acute and chronic stressors may also elicit physiologic stress responses, including changes in the regulation of the hypothalamic-pituitary-adrenocortical (HPA) axis. Few studies, however, have examined the relationship between minority sexual orientation and diurnal cortisol patterns. The present study included 1670 young adults ages 18–32 years (69% female, 31% male) from the Growing Up Today Study, a prospective cohort of U.S. youth. Participants provided five saliva samples over one day to estimate diurnal cortisol patterns. Sexual orientation groups included: completely heterosexual with no same-sex partners (referent), completely heterosexual with same-sex partners/mostly heterosexual, and gay/lesbian/bisexual. Covariates included perceived stress and stressful life events in the past month. Sex-stratified multilevel models of log-transformed cortisol values were used to model diurnal cortisol patterns, and generalized estimating equations were used to model area under the curve (AUC), both with respect to ground (AUCg) and increase (AUCi). Among females, sexual minorities reported significantly more stressful life events in the past month than their heterosexual counterparts. In adjusted multilevel models, sexual orientation was not significantly associated with diurnal cortisol patterns or with AUCg or AUCi in either females or males. There were no significant interactions between sexual orientation and stressful life events. Time-varying negative mood was significantly associated with higher cortisol levels across the day for both female and male participants, after adjusting for all covariates. This study from a large cohort of U.S. young adults did not detect a relationship between sexual orientation and diurnal cortisol patterns. Despite consistent evidence indicating that, compared to heterosexuals, sexual minorities experience elevated exposure to multiple forms of stressors and adversity across the life course, we did not find differences in diurnal cortisol rhythms by sexual orientation. One possible explanation is that sexual minority participants in the study exhibited physiologic resilience.
Keywords: Sexual orientation, Cortisol, Diurnal rhythm, HPA axis, Stressful life events, Young adults
1. Introduction
Health disparities related to sexual orientation are well-documented in the United States, with multiple studies finding sexual minorities (e.g., lesbian, gay, bisexual [LGB], and mostly heterosexual populations) reporting more depressive and anxious symptoms, post-traumatic stress disorder (PTSD), substance use, eating disorder symptoms, and other health risk indicators relative to heterosexual populations (Austin et al., 2013; Institute of Medicine, 2011; Ward et al., 2014). Sexual minority stress theory posits that health inequities may be explained by societal stigmatization of sexual minorities, resulting in differential exposures to acute and chronic stressors such as discrimination, abuse, and violence (Meyer, 2003; Rosario et al., 2002). In support of this theory, elevated rates of violence victimization (Austin et al., 2008; Berlan et al., 2010; Friedman et al., 2011; Katz-Wise and Hyde, 2012; Roberts et al., 2010a; Saewyc et al., 2006) and discrimination (Hatzenbuehler et al., 2015, 2010) are well-documented and have been linked to psychological and behavioral outcomes (Almeida et al., 2009b; Roberts et al., 2012, 2013a). Research on physiological markers of chronic stress will be essential to understanding the mechanisms underlying health disparities—and, more broadly, to understanding the ways that stigmatizing or hostile social environments may “get under the skin” (Hatzenbuehler et al., 2009). Relatively few studies, however, have examined markers of stress physiology across sexual orientation groups.
Chronic stressors can affect health via several pathways, including through psychological responses, coping-related risk behaviors, and neurobiological disruptions. One of the most widely studied physiological stress pathways involves the hypothalamic-pituitary-adrenocortical (HPA) axis, which governs the release of cortisol. Threats to physical and social self-preservation have been shown to influence HPA-axis regulation in numerous studies (Adam and Gunnar, 2001; Dickerson and Kemeny, 2004; Miller et al., 2007). Cortisol has a diurnal rhythm that typically peaks shortly after awakening in the morning (known as the cortisol awakening response [CAR]) and then gradually drops throughout the day. Dysregulation of diurnal cortisol rhythms may result from exposure to chronic stressors (Almeida et al., 2009a; Miller et al., 2007). For instance, experiences of adversity may be associated with hypocortisolism in adulthood, manifesting as low morning cortisol levels and shallow decreases across the day (Gunnar and Vazquez, 2001). Because cortisol is critical to regulation of other physiological systems, including metabolic and immune systems (Dickerson and Kemeny, 2004), cortisol dysregulation may have wide-ranging impacts on pathophysiology (Heim et al., 2000a; Pervanidou and Chrousos, 2012; Segerstrom and Miller, 2004). Specifically, the CAR has also been linked to psychological wellbeing and physical health status, although it not clear whether positive outcomes are associated with larger or smaller CARs (Clow et al., 2004). Flatter cortisol slopes over the course of the day have been associated with PTSD in adults (e.g., (Yehuda et al., 1996)), a history of internalizing disorders in older adolescents (e.g., (Doane et al., 2013)), and chronic conditions such as fibromyalgia and rheumatoid arthritis (Heim et al., 2000a).
Empirical data show clear disparities in victimization and discrimination adversely affecting sexual minorities (Austin et al., 2008; Berlan et al., 2010; Friedman et al., 2011; Hatzenbuehler et al., 2015, 2010; Katz-Wise and Hyde, 2012; Roberts et al., 2010a; Saewyc et al., 2006), and theoretical work offers plausible pathways through which minority sexual orientation might be linked to HPA-axis dysregulation (Meyer, 2003; Rosario et al., 2002). Given that hypocortisolism, characterized by low morning cortisol levels and a blunted decline across the day, is the most common pattern observed among people exposed to adversity (Gunnar and Vazquez, 2001; Heim et al., 2000a), such patterns might be expected among sexual minority populations; however, evidence from sexual minority samples is lacking. To date, only a handful of studies including sexual minorities have assessed cortisol reactivity in experimental settings or diurnal cortisol. One study of gay and bisexual adult men found that degree of “outness” (sexual orientation disclosure) at work was related to higher mean cortisol levels over the course of a work day (Huebner and Davis, 2005). A study of HPA-axis reactivity to a laboratory stressor found LGB young adults who grew up in states classified as highly stigmatizing to sexual minorities as adolescents had a reduced cortisol response to a stress task compared to those who grew up in states classified as having low levels of stigma (Hatzenbuehler and McLaughlin, 2014). This finding reflects prior research showing lower cortisol response following an acute stressor among individuals exposed to adversities in adolescence (Bosch et al., 2012) or in earlier life (Carpenter et al., 2007, 2011). However, much remains to be understood about what distinguishes adaptive from maladaptive cortisol responses and how these responses might be affected by other stressors or buffers relevant for sexual minority populations. For example, another study based on the same LGB sample as used in the Hatzenbuehler and McLaughlin (2014) study above found that participants with greater parental support—a potential buffer against stigma—had a reduced cortisol response relative to those with less parental support (Burton et al., 2014).
Notably, none of the above studies included a comparison group of heterosexuals. One study of LGB and heterosexual adults in Montreal found no significant differences in diurnal cortisol profiles by sexual orientation identity, although within the LGB group, sexual identity disclosure was associated with lower cortisol levels at 30 min after waking (Juster et al., 2013) A study of HPA axis reactivity in this same sample found differences by sexual orientation and gender, such that lesbian/bisexual women had higher cortisol reactivity following exposure to a laboratory stressor than heterosexual women while gay/bisexual men showed lower overall cortisol levels throughout the study than heterosexual men (Juster et al., 2015). In addition, one study examined associations between sexual orientation identity and other stress-related biomarkers in a nationally representative cohort of U.S. young adults (Everett et al., 2014; Hatzenbuehler et al., 2013). Biomarkers of inflammation and poorer immune function (C-reactive protein and Epstein-Barr virus antibodies) that may be consequences of HPA-axis activation were found to be at higher levels in sexual minority men than in both heterosexual men and sexual minority women (Everett et al., 2014). These studies underscore the importance of considering the intersectionality (Bauer, 2014) of both gender and sexual orientation when examining stress biomarkers.
New research is needed to build a more complete understanding of the relationships among sexual orientation, exposure to stressors, and HPA-axis regulation. In addition, studies are needed that include less well-understood subgroups of sexual minorities such as mostly heterosexuals and heterosexuals with same-sex sexual partners, both of which have been shown to have elevated risk of trauma compared to heterosexuals with no same-sex partners (Roberts et al., 2010a, 2012). The present study sought to address these gaps by examining diurnal patterns of salivary cortisol by gender and sexual orientation in a large epidemiologic cohort of U.S. young adults. Hypotheses of this study were:
Compared to heterosexual young adults, sexual minorities will exhibit lower morning levels of diurnal cortisol and shallower decreases across the day;
Higher rates of stressful life events among sexual minorities compared to heterosexuals will partly explain differences in diurnal cortisol dysregulation.
2. Methods
2.1. Study sample
Study participants were drawn from the Growing Up Today Study (GUTS), a national, prospective cohort of 27,324 youth. GUTS participants, who were ages 9–16 years at enrollment, are children of women in the Nurses’ Health Study 2, a prospective cohort of over 116,000 U.S. women. The first stage of the cohort (GUTS1) began in 1996, followed by a second stage (GUTS2) in 2004. Surveys have been administered annually or biennially since the inception of the cohort. The sample is primarily white (94%) and has a restricted socioeconomic range, as all participants’ mothers have a four-year nursing degree.
The current study is based on a subset of GUTS youth who participated in the 2011–2014 GUTS Saliva Substudy, designed to examine sexual orientation in relation to stress response physiology. Only those who had responded to the previous GUTS survey wave in 2010–2011 were invited to participate. Sample selection for the GUTS Saliva Substudy was designed to achieve sufficient subgroup sizes of sexual minorities. All sexual minority participants (i.e., participants who identified as mostly heterosexual, bisexual, mostly homosexual, gay, lesbian on the 2007 or 2010–2011 waves, or reported any history of same-sex sexual partners on the 2005, 2007 or 2010–2011 waves) were recruited. Participants were invited to the GUTS Saliva Substudy by email and then screened for eligibility. Youth were considered ineligible if pregnant currently or in previous six months or if they reported past month use of oral or inhaled steroids, any history of cancer treatment, or ever diagnosed with an endocrine disorder. In total, 6980 GUTS participants were invited, of whom 1966 (28%) took part. Of these, 1686 individuals returned at least one usable saliva sample. We excluded individuals from analysis who were missing information on wakeup time (n = 16), resulting in an analytic sample of 1670 participants. Among the 1966 who participated in the GUTS Saliva Substudy, compared to those included in analyses, those excluded because they did not return at least one usable saliva sample (n = 280) were more likely to be heterosexual, of white ethnicity, and older (P < 0.05) but did not differ by sex (P > 0.05) compared to those included.
2.2. Survey measures
2.2.1. Sexual orientation
Sexual orientation was assessed on the GUTS 2005, 2007, 2010–2011, and 2013 survey waves using two widely used measures. The first measure asked participants to report which of the following best describes them: Completely heterosexual (attracted to persons of the opposite sex); mostly heterosexual; bisexual (equally attracted to men and women); mostly homosexual; completely homosexual, gay, or lesbian (attracted to persons of the same sex); or unsure (Remafedi et al., 1992). The second asked participants to report the sex of any sexual partners they have had (female[s], male[s], female[s] and male[s], or no sexual contact). Due to sample size limitations, sexual orientations groups were aggregated for analysis into three groups: (1) completely heterosexual with no same-sex partners, (2) mostly heterosexual or completely heterosexual with same-sex partners, and (3) lesbian, gay, or bisexual. Prior research (Roberts et al., 2010b), including in GUTS (Roberts et al., 2012), indicates that completely heterosexuals with same-sex partners and mostly heterosexuals have elevated health risks compared to completely heterosexuals with no same-sex partners, so the former two groups were combined to make an additional category for analyses. Sexual orientation was carried forward from the most recent prior wave for participants missing sexual orientation on the 2013 wave.
2.2.2. Recent stressful life events
The GUTS Saliva Substudy included a daily diary and brief questionnaire that participants completed on the day of saliva collection. This questionnaire included a modified version of the Stressful Life Events Screening Questionnaire (Goodman et al., 1998) assessing self-reported lifetime exposure to 13 types of traumatic experiences (e.g., life-threatening events, death of a loved one). To capture a full breadth of stressful life experiences, the original measure was modified by adding four questions about stressful life events pertaining to financial hardship and divorce or separation. In addition, to ascertain recent exposures to stressors, the time frame was modified to focus on experiences in the past 30 days and past year. One binary variable was created indicating whether any of the stressful events had been experienced within the past 30 days (any vs. none) and a second variable indicated any experiences in the past year.
2.2.3. Daily diary measures and additional covariates
The saliva diary included measures accounting for factors that may impact diurnal rhythms (Adam and Kumari, 2009). These included measures of sleep duration and quality (time at falling asleep on night before data collection, time of awakening on day of data collection, previous night’s hours of sleep, and usual hours of sleep) and mood during each saliva collection (“Do you feel worried, anxious, or fearful right now?” and “Do you feel happy, excited, or content right now?” with response options from (1) Not at all to (4) Extremely). The “worried” and “happy” mood items were included as ordinal time-varying covariates in the model, with higher scores indicating greater levels of worry or happiness at each time point. The saliva diary also included the four-item Perceived Stress Scale (PSS-4) (Cohen et al., 1983), which asked participants to report feelings and thoughts in the previous month (e.g., “In the last month, how often have you felt you were unable to control the important things in your life?” with response options from (0) Never to (4) Very often) (Cohen et al., 1983; Cohen and Williamson, 1988). PSS-4 scores were obtained by reverse coding the two positive items and taking the mean of the four items, such that higher scores indicated higher perceived stress.
Participants were asked to report whether they had, on the day of saliva collection, smoked any cigarettes (yes/no), had any alcoholic beverages (yes/no), engaged in any vigorous physical activity (increasing heart rate and sweat; yes/no), and to list any drugs or medications taken. Women were asked if they were currently using or had used in the previous month any form of hormonal contraception (yes/no). Additional covariates included participant age (in years) and non-compliance with the wakeup sample (yes/no; non-compliance defined as the first saliva sample not being taken within 15 min of waking up).
2.3. Salivary cortisol collection
Participants received saliva collection tubes through the mail along with written instructions and the daily diary. Samples were collected from participants on a single weekday (Monday–Thursday). After completing the informed consent process, participants were instructed to provide five passive-drool saliva samples over the course of the day: at awakening, 45 min, 4, and 10 h after awakening, and at bedtime. Participants were instructed not to brush their teeth before the first (awakening) sample was collected and not to eat, drink, chew anything or do vigorous physical activity for at least 30 min before each subsequent sample. To assess adherence, participants recorded the time of collection for each sample and whether or not they had brushed teeth, eaten, drunk, or exercised in the previous 30 min. Filled tubes were stored in baggies in a refrigerator in the participant’s home until sampling was complete and then returned along with an ice pack via two-day delivery in a postage-paid mailer. All saliva samples were collected between August 2011 and February 2014. Participants received $25 upon return of saliva samples. This study was approved by the Brigham & Women’s Hospital Institutional Review Board.
2.4. Cortisol processing
Saliva samples were shipped to the Channing Division of Network Medicine (CDNM) biorepository, aliquoted, stored at −20 °Centigrade, and assayed by Rohleder Lab at Brandeis University along with quality control (QC) samples provided by the CDNM. The CDNM biorepository routinely stocks pooled QC samples. Three distinct QC pools of saliva were obtained. Aliquots of QC pools were distributed randomly among the participant samples and labeled with alias IDs for blinding. Each batch of samples analyzed contained two QC pool samples from at least two of the three available pools. Each pool was represented in at least one-third of the batches analyzed. At Rohleder Lab, cortisol was measured in duplicate using a competitive chemiluminescence immunoassay (CLIA; IBL-International, Toronto, ON, Canada). Inter- and intra-assay coefficients of all assays were below 10%. Rohleder Lab provided CDNM with final data from study samples and QC samples, which were un-blinded and QC results were compiled and analyzed across the study. Coefficients of variation for the study met the biorepository standard of 15% or below (observed range: 9.7%–13.3%). Batch adjustment was performed to adjust for any potential batch effects (Rosner et al., 2008).
2.5. Statistical analysis
Multilevel models of log-transformed cortisol values were used to examine diurnal cortisol patterns (interpreted in nmol/L). The models included individual-level, day-level, and sample-level covariates in addition to individual-level random intercept (awakening cortisol level) and slope terms (time since waking). All continuous covariates were centered around their mean. The primary predictor was sexual orientation, with completely heterosexual without same-sex partners as the referent group. In addition to sexual orientation, the models included a linear, quadratic, and cubic effect of time since waking and an indicator variable for non-compliance with the wake-up sample time (defined as >15 min from wake-up time). Models also included an estimate of cortisol awakening response (CAR) and an interaction between CAR and sexual orientation to test whether the CAR differed by sexual orientation. CAR is defined as the difference between the wake-up sample (Sample 1) and the sample collected 45 min later (Sample 2). Only those covariates that were either associated with cortisol or sexual orientation were included in final models. As models were of log-transformed values of cortisol, parameter estimates can be transformed (as 100*(exp(β) − 1) for interpretation as percentage increase or decrease in mean cortisol value (Adam et al., 2006). All analyses were sex-stratified so that models for females could account for hormonal contraception use.
In models examining the effect of stressful events on cortisol, a main effect of stressful events and an interaction of stressful events with sexual orientation were additionally included in the model to determine whether stressful events influenced cortisol level and whether this effect differed by sexual orientation. We also calculated cortisol area under the curve (AUC), both with respect to ground (AUCg) to assess overall cortisol output and with respect to increase (AUCi) to assess changes in cortisol output over time (Fekedulegn et al., 2007; Pruessner et al., 2003). Generalized estimating equations were used to estimate associations between sexual orientation and AUCg/AUCi. Models were adjusted for covariates that were either associated with AUCg/AUCi or sexual orientation. All analyses were conducted using SAS v9.3 (SAS, 2012)
3. Results
3.1. Descriptive statistics
The analytic sample included 1152 (68.9%) female participants and 518 (31.1%) male participants ages 18–32 years old. Approximately 8% of the sample identified as bisexual, gay, or lesbian (n = 130), 26% of the sample identified as mostly heterosexual or completely heterosexual with same-sex partners (n = 427; hereafter referred to as “mostly heterosexual”) and 67% of the sample identified as completely heterosexual and had no same-sex partners (n = 1113). At the bivariate level, sexual minority status was significantly associated with reported stressful life events in the past month for female participants. Male sexual minorities reported somewhat more stressful life events than heterosexuals, but this difference did not reach statistical significance (Table 1). Among females, 15% of completely heterosexual participants reported two or more stressful events in the past year, compared to 19% of mostly heterosexual and 31% of lesbian or bisexual participants (p = 0.001). Among males, 16% of completely heterosexual, 25% of mostly heterosexual, and 20% of bisexual or gay participants reported two or more stressful events in the past month (p = 0.13).
Table 1.
Participant characteristics for females and males in the Growing Up Today Study who completed saliva sampling in 2011–2014 by sexual orientation identity (n = 1670).
| FEMALES |
MALES |
|||||||
|---|---|---|---|---|---|---|---|---|
| Completely heterosexual (CH) (n = 758) |
Mostly heterosexual or CH w/SS partners (n = 324) |
Bisexual or lesbian (n = 70) |
p | Completely heterosexual (CH) (n = 362) |
Mostly heterosexual or CH w/SS partners (n = 95) |
Bisexual or gay (n = 61) |
p | |
| Age in years, M (SD) | 24.4 (3.5) | 25.1 (3.1) | 24.9 (3.6) | 0. 01 | 24.8 (3.5) | 25.1 (3.3) | 24.7 (3.1) | 0.63 |
| Sleep, M(SD) | ||||||||
| Hours of sleep in previous night | 7.8 (1.3) | 7.6 (1.3) | 7.7 (1.5) | 0.21 | 7.3 (1.4) | 7.4 (1.4) | 7.5 (1.3) | 0.70 |
| Hours of sleep on typical night | 7.7 (0.9) | 7.7 (1.0) | 7.8 (0.9) | 0.59 | 7.4 (1.0) | 7.5 (0.9) | 7.4 (0.8) | 0.67 |
| Waking time (HH:MM) | 7:57 AM (1:36) | 7:59 AM (1:37) | 8:13 AM (1:38) | 0.43 | 7:57 AM (1:46) | 8:03 AM (1:44) | 8:16 AM (2:12) | 0.43 |
| Health-related behaviors, n (%) | ||||||||
| Any alcohol use | 164 (21.9) | 104 (32.6) | 15 (21.4) | <0.001 | 94 (26.6) | 32 (34.4) | 22 (37.3) | 0.12 |
| Any cigarette smoking | 14 (1.9) | 17 (5.3) | 5 (7.1) | 0.002 | 17 (4.7) | 7 (7.4) | 5 (8.2) | 0.39 |
| Hormonal contraception use (female only), n (%) | 389 (51.6) | 172 (53.3) | 22 (31.4) | 0.003 | n/a | n/a | n/a | |
| Perceived stress scale, M (SD) | 2.2 (0.7) | 2.4 (0.7) | 2.5 (0.7) | >.001 | 2.2 (0.7) | 2.5 (0.8) | 2.5 (0.8) | <0.001 |
| Time-varying worry score, M (SD) | ||||||||
| Sample 1 (Wake up) | 1.5 (0.6) | 1.5 (0.6) | 1.5 (0.6) | 0.13 | 1.4 (0.5) | 1.5 (0.6) | 1.5 (0.6) | 0.02 |
| Sample 2 (45 min post-wake up) | 1.5 (0.6) | 1.5 (0.6) | 1.5 (0.6) | 0.69 | 1.4 (0.5) | 1.5 (0.6) | 1.5 (0.6) | 0.06 |
| Sample 3 (4 post-wake up) | 1.5 (0.6) | 1.6 (0.6) | 1.5 (0.6) | 0.05 | 1.4 (0.6) | 1.5 (0.6) | 1.5 (0.6) | 0.03 |
| Sample 4 (10 post-wake up) | 1.5 (0.6) | 1.6 (0.7) | 1.6 (0.7) | 0.04 | 1.4 (0.6) | 1.6 (0.7) | 1.6 (0.7) | 0.03 |
| Sample 5 (Before bed) | 1.5 (0.6) | 1.6 (0.7) | 1.7 (0.7) | 0.002 | 1.4 (0.6) | 1.6 (0.7) | 1.4 (0.7) | 0.01 |
| Time-varying happy score, M (SD) | ||||||||
| Sample 1 (Wake up) | 2.0 (0.7) | 1.8 (0.6) | 1.9 (0.5) | 0.001 | 1.9 (0.7) | 1.9 (0.7) | 1.8 (0.6) | 0.66 |
| Sample 2 (45 min post-wake up) | 2.1 (0.6) | 2.0 (0.6) | 2.1 (0.6) | 0.05 | 2.1 (0.6) | 2.0 (0.7) | 2.0 (0.5) | 0.38 |
| Sample 3 (4 post-wake up) | 2.3 (0.7) | 2.1 (0.6) | 2.1 (0.7) | <0.001 | 2.3 (0.7) | 2.1 (0.7) | 2.2 (0.6) | 0.02 |
| Sample 4 (10 post-wake up) | 2.4 (0.7) | 2.2 (0.7) | 2.2 (0.6) | 0.02 | 2.3 (0.7) | 2.1 (0.8) | 2.2 (0.7) | 0.11 |
| Sample 5 (Before bed) | 2.2 (0.7) | 2.1 (0.7) | 2.1 (0.6) | 0.18 | 2.3 (0.8) | 2.0 (0.7) | 2.1 (0.7) | 0.03 |
| Stressful events in past month, n (%) | 0.001 | 0.25 | ||||||
| None | 643 (84.8) | 261 (80.6) | 48 (68.6) | 301 (83.2) | 72 (75.8) | 49 (80.3) | ||
| 1+ | 115 (15.2) | 63 (19.4) | 22 (31.4) | 61 (16.9) | 23 (24.2) | 12 (19.7) | ||
| Compliant with wakeup sample, n (%) | 728 (96.7) | 306 (94.4) | 66 (94.3) | 0.18 | 352 (97.8) | 89 (98.9) | 60 (98.4) | 0.78 |
Notes: P-values based on Chi-square tests for categorical variables and ANOVA for continuous variables. Compliant with wake up sample = saliva sample taken within 15 min of waking. All numbers are rounded to nearest zero.
3.2. Diurnal cortisol by sexual orientation
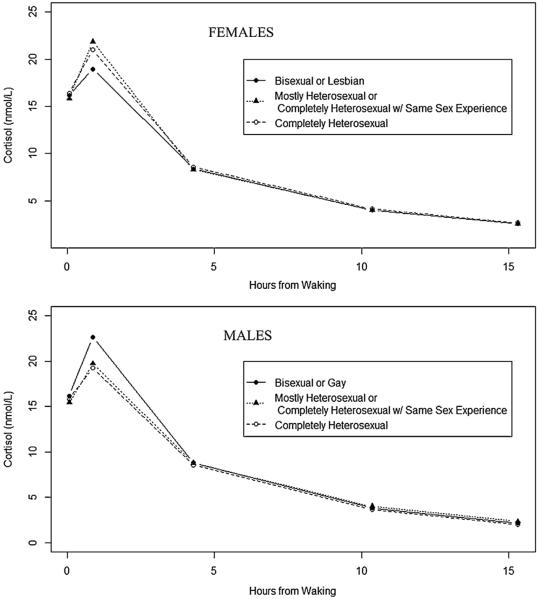
As depicted in Fig. 1, on average, salivary cortisol concentration followed the expected diurnal pattern, after accounting for key covariates. Participants exhibited high mean cortisol values upon awakening (Female (F): 15.8 nmol/L; Male (M): 16.0 nmol/L), an increase in concentration in the first 45 min after awakening (F: 48% increase; M: 39% increase), and decrease across the rest of the day (F: 15% lower and M: 14% lower per hour than at wakeup) (Table 2 for females and Table 3 for males).
Fig. 1.
Estimated model-based mean cortisol values as a function of time since awakening, by sexual orientation among female and male participants ages 18–32 years in the Growing Up Today Study (GUTS) Saliva Substudy. Notes: estimates based on model presented in Table 3. Saliva sample times: sample 1 = 0 from waking; sample 2 = 45 min from waking; sample 3 = 4 from waking; sample 4 = 10 from waking; sample 5 = before bed. No significant differences in cortisol were seen across sexual orientation groups.
Table 2.
Multilevel model of log(cortisol) including random effects for wakeup cortisol and time since waking, and fixed effects of cortisol awakening response and time since waking squared and cubed among females in GUTS Saliva Substudy (n = 1113).
| Variable | Estimate | SE | p-value | Interpretation (% increase/decrease per unit change of predictor)b | |
|---|---|---|---|---|---|
| Model for wakeup cortisol level | |||||
| Intercept | 2.763 | 0.021 | <0.0001 | 15.9 nmol/L mean wakeup cortisol a | |
| Age in years | 0.030 | 0.003 | <0.0001 | 3.0% higher for each year older | |
| Hours sleep, previous night | −0.031 | 0.008 | 0.0001 | 3.1% lower for each hour less sleep | |
| Hours sleep, typical night | −0.015 | 0.010 | 0.16 | −1.5% (n.s.) | |
| Waking time | 0.000 | 0.000 | <0.0001 | 0.001% lower per minute later of wakeup time | |
| Any cigarette smoking | 0.100 | 0.053 | 0.06 | 10.5% (n.s.) | |
| Any alcoholic drinks | 0.006 | 0.021 | 0.80 | 0.6% (n.s.) | |
| Perceived stress scale | 0.020 | 0.014 | 0.14 | 2.0% (n.s.) | |
| Hormonal contraception use | 0.064 | 0.018 | 0.0005 | 6.6% higher if on hormonal contraception | |
| Noncompliance (wakeup sample) | 0.091 | 0.053 | 0.09 | 9.6% (n.s.) | |
| Sexual orientation (Ref = CH) | |||||
| MH or CHwSS | −0.033 | 0.030 | 0.27 | −3.3% (n.s.) | |
| Bisexual or Lesbian | −0.012 | 0.056 | 0.82 | −1.2% (n.s.) | |
| Model for cortisol level | |||||
| Worry level (at each sample) | 0.025 | 0.013 | 0.06 | 2.5% (n.s.) | |
| Happiness level (at each sample) | −0.011 | 0.012 | 0.37 | −1.1% (n.s.) | |
| Model for time since waking effect | |||||
| Intercept | −0.168 | 0.005 | <0.0001 | 15.5% lower per hour since wakeup | |
| Sexual orientation (Ref = CH) | |||||
| MH or CHwSS | −0.000 | 0.004 | 0.99 | −0.0% (n.s.) | |
| Bisexual or Lesbian | −0.002 | 0.007 | 0.77 | −0.2% (n.s.) | |
| Model for time since waking squared effect | |||||
| Intercept | 0.003 | 0.000 | <0.0001 | 0.4% per hour squared since wakeup | |
| Model for time since waking cubed effect | |||||
| Intercept | −0.000 | 0.000 | <0.0001 | 0.0% per hour cubed since wakeup | |
| Model for cortisol awakening response effect | |||||
| Intercept | 0.389 | 0.023 | <0.0001 | 47.6% higher if CAR | |
| Noncompliance (wakeup sample) | −0.190 | 0.088 | 0.03 | 17.3% lower if non-compliant with wakeup sample | |
| Sexual orientation (Ref = CH) | |||||
| MH or CHwSS | 0.074 | 0.042 | 0.08 | 7.6% (n.s.) | |
| Bisexual or Lesbian | −0.087 | 0.078 | 0.26 | −8.3% (n.s.) | |
| Random Effects | Variance estimate | SE | p-value | ||
|
| |||||
| Awakening cortisol | 0.029 | 0.006 | <0.0001 | ||
| Time since waking | 0.001 | 0.000 | <0.0001 | ||
Notes: Variables centered around their means are age, hours sleep in previous night, hours sleep on a typical night, waking time, perceived stress score, level of worry during each saliva sample. Sexual orientation categories: (1) Bisexual or Lesbian (2) MH or CHwSS = Mostly Heterosexual or Completely Heterosexual with same-sex partners; (3) CH = Completely Heterosexual with no same-sex partners (Ref). All numbers are rounded to nearest zero.
Mean wakeup cortisol among CH, non-smoking, no alcohol, no hormonal contraception use, compliant participants with mean age sleep, waking time, stress, and worryscores.
In interpreting effect sizes, use of a logarithmic outcome allows coefficients to be interpreted as percentage change in the outcome per unit change in the independent variable, after applying the following transformation: B%change = [exp (Braw)] – 1. The “Interpretation” column of table includes the percentage change interpretation based on this transformation.
Table 3.
Multilevel model of log(cortisol) including random effects for wakeup cortisol and time since waking, and fixed effects of cortisol awakening response and time since waking squared and cubed among males in GUTS Saliva Substudy (n = 487).
| Variable | Estimate | SE | p-value | Interpretation (% increase/decrease per unit change of predictor)b | |
|---|---|---|---|---|---|
| Model for wakeup cortisol level | |||||
| Intercept | 2.773 | 0.032 | <0.0001 | 16.0 nmol/L mean wakeup cortisol a | |
| Age in years | 0.030 | 0.005 | <0.0001 | 3.0% higher for each year older | |
| Hours sleep, previous night | 0.000 | 0.013 | 0.99 | 0.0% (n.s.) | |
| Hours sleep, typical night | −0.025 | 0.017 | 0.15 | −2.5% (n.s.) | |
| Waking time | 0.000 | 0.000 | 0.12 | 0.0% (n.s.) | |
| Any cigarette smoking | −0.052 | 0.067 | 0.44 | −5.1% (n.s.) | |
| Any alcoholic drinks | −0.038 | 0.033 | 0.25 | −3.8% (n.s.) | |
| Perceived stress scale | −0.016 | 0.023 | 0.48 | −1.6% (n.s.) | |
| Noncompliance (wakeup sample) | 0.300 | 0.124 | 0.02 | 35.0% higher if non-compliant with wakeup sample | |
| Sexual orientation (Ref = CH) | |||||
| MH or CHwSS | −0.023 | 0.058 | 0.69 | −2.2% (n.s.) | |
| Bisexual or Gay | 0.023 | 0.069 | 0.74 | 2.3% (n.s.) | |
| Model for cortisol level | |||||
| Worry level (at each sample) | 0.055 | 0.024 | 0.02 | 5.7% higher with increased worry | |
| Happiness level (at each sample) | −0.022 | 0.020 | 0.27 | −2.2% (n.s.) | |
| Model for time since waking effect | |||||
| Intercept | −0.145 | 0.010 | <0.0001 | 13.5% lower per hr since wakeup | |
| Sexual orientation (Ref = CH) | |||||
| MH or CHwSS | 0.011 | 0.007 | 0.12 | 1.1% (n.s.) | |
| Bisexual or Gay | 0.002 | 0.008 | 0.85 | 0.2% (n.s.) | |
| Model for time since waking squared effect | |||||
| Intercept | 0.000 | 0.001 | 0.78 | 0.0% (n.s.) | |
| Model for time since waking cubed effect | |||||
| Intercept | 0.000 | 0.000 | 0.03 | 0.0% per hour cubed since wakeup | |
| Model for cortisol awakening response effect | |||||
| Intercept | 0.321 | 0.039 | <0.0001 | 37.8% higher if CAR | |
| Noncompliance (wakeup sample) | −0.219 | 0.207 | 0.29 | −19.7% (n.s.) | |
| Sexual orientation (Ref = CH) | |||||
| MH or CHwSS | 0.038 | 0.084 | 0.65 | 3.9% (n.s.) | |
| Bisexual or Gay | 0.139 | 0.101 | 0.17 | 14.9% (n.s.) | |
| Random Effects | Variance estimate | SE | p-value | ||
|
| |||||
| Awakening cortisol | 0.020 | 0.011 | 0.04 | ||
| Time since waking | 0.001 | 0.000 | <0.0001 | ||
Notes: Variables centered around their means are age, hours sleep in previous night, hours sleep on a typicalnight, waking time, perceived stress score, level of worry during each saliva sample. Sexual orientation categories: (1) Bisexual or Gay (2) MH or CHwSS = Mostly Heterosexual or Completely Heterosexual with same-sex partners; (3) CH = Completely Heterosexual with no same-sex partners (Ref). All numbers are rounded to nearest zero.
Mean wakeup cortisol among CH, non-smoking, no alcohol, compliant participants with mean age sleep, waking time, stress, and worry scores.
In interpreting effect sizes, use of a logarithmic outcome allows coefficients to be interpreted as percentage change in the outcome per unit change in the independent variable, after applying the following transformation: B%change = [exp (Braw)] – 1. The “Interpretation” column of table includes the percentage change interpretation based on this transformation.
Sexual orientation was not significantly associated with diurnal cortisol for females or males (see Tables 2 and 3). There was no significant interaction between sexual orientation and stressful life events in the past 30 days on diurnal cortisol for females or males (Table 4 for females and Table 5 for males), and results were similar for past-year stressful life events (data not shown). Time-varying negative mood (self-reported worry/anxiety at each saliva collection) was significantly associated with increased mean cortisol levels across the day for both female and male participants, after adjustment for recent stressful life events.
Table 4.
Multilevel model of log(cortisol) including any stressful life events in the previous month and random effects for wakeup cortisol and time since waking, and fixed effects of cortisol awakening response and time since waking squared and cubed among females in GUTS Saliva Substudy (n = 1113).
| Variable | Estimate | SE | p-value | Interpretation (% increase/decrease per unit change of predictor)b | |
|---|---|---|---|---|---|
| Model for wakeup cortisol level | |||||
| Intercept | 2.761 | 0.022 | <0.0001 | 15.8 nmol/L mean wakeup cortisol a | |
| Age in years | 0.030 | 0.003 | <0.0001 | 3.0% higher for each year older | |
| Hours sleep, previous night | −0.030 | 0.008 | 0.0002 | 3.0% lower for each hour less sleep | |
| Hours sleep, typical night | −0.016 | 0.010 | 0.13 | −1.6% (n.s.) | |
| Waking time | 0.000 | 0.000 | <0.0001 | 0.001% lower per minute later of wakeup time | |
| Any cigarette smoking | 0.098 | 0.053 | 0.06 | 10.3% (n.s.) | |
| Any alcoholic drinks | 0.006 | 0.021 | 0.77 | 0.6% (n.s.) | |
| Stress scale | 0.014 | 0.014 | 0.32 | 1.4% (n.s.) | |
| Hormonal contraception use | 0.0654 | 0.018 | 0.0004 | 6.7% higher if on hormonal contraception | |
| Noncompliance (wakeup sample) | 0.092 | 0.053 | 0.08 | 9.7% (n.s.) | |
| 1+ traumatic events, past month | 0.009 | 0.032 | 0.78 | 0.9% (n.s.) | |
| Sexual orientation (Ref = CH) | |||||
| MH or CHwSS | −0.045 | 0.031 | 0.15 | −4.4% (n.s.) | |
| Bisexual or Lesbian | −0.022 | 0.061 | 0.72 | −2.2% (n.s.) | |
| Sexual orientation by stressful events interaction (Ref = CH w/no past month stressful events) | |||||
| 1+ stressful events × MH/CHwSS | 0.067 | 0.054 | 0.21 | 6.9% (n.s.) | |
| 1+ stressful events × Bi/Lesbian | 0.032 | 0.085 | 0.70 | 3.3% (n.s.) | |
| Model for cortisol level | |||||
| Worry level (at each sample) | 0.025 | 0.013 | 0.06 | 2.5% higher with increased worry | |
| Happiness level (at each sample) | −0.011 | 0.012 | 0.35 | −1.1% (n.s.) | |
| Model for time since waking effect | |||||
| Intercept | −0.168 | 0.005 | <0.0001 | 15.5% lower per hour since wakeup | |
| Sexual orientation (Ref = CH) | |||||
| MH or CHwSS | −0.000 | 0.004 | 0.99 | −0.0% (n.s.) | |
| Bisexual or Lesbian | −0.002 | 0.007 | 0.76 | −0.2% (n.s.) | |
| Model for time since waking squared effect | |||||
| Intercept | 0.003 | 0.000 | <0.0001 | 0.4% per hour squared since wakeup | |
| Model for time since waking cubed effect | |||||
| Intercept | −0.00002 | 0.000004 | <0.0001 | 0.0% per hour cubed since wakeup | |
| Model for cortisol awakening response (CAR) effect | |||||
| Intercept | 0.389 | 0.023 | <0.0001 | 47.6% higher if CAR | |
| Noncompliance (wakeup sample) | −0.190 | 0.088 | 0.03 | 17.3% lower if non-compliant with wakeup sample | |
| Sexual orientation (Ref = CH) | |||||
| MH or CHwSS | 0.074 | 0.042 | 0.08 | 7.7% (n.s.) | |
| Bisexual or Lesbian | −0.087 | 0.078 | 0.26 | −8.3% (n.s.) | |
| Random Effects | Variance estimate | SE | p-value | ||
|
| |||||
| Awakening cortisol | 0.029 | 0.006 | <0.0001 | ||
| Time since waking | 0.001 | 0.000 | <0.0001 | ||
Notes: Variables centered around their means are age, hours sleep in previous night, hours sleep on a typical night, waking time, perceived stress, worry, happiness. Sexual orientation categories: (1) Bisexual or Lesbian; (2) MH, CHwSS = Mostly Heterosexual or Completely Heterosexual with same-sex partners; (3) CH = Completely Heterosexual with no same-sex partners (Ref). All numbers are rounded to nearest zero.
Mean wakeup cortisol among CH, non-smoking, no alcohol, compliant participants with no reported stressful events in the previous month and mean sleep, waking time, stress, worry, and happiness scores.
In interpreting effect sizes, use of a logarithmic outcome allows coefficients to be interpreted as percentage change in the outcome per unit change in the independent variable, after applying the following transformation: B%change = [exp (Braw)] – 1. The “Interpretation” column of table includes the percentage change interpretation based on this transformation.
Table 5.
Multilevel model of log(cortisol) including any stressful life events in the previous month and random effects for wakeup cortisol and time since waking, and fixed effects of cortisol awakening response and time since waking squared and cubed among males in GUTS Saliva Substudy (n = 487).
| Variable | Estimate | SE | p-value | Interpretation (% increase/decrease per unit change of predictor)b | |
|---|---|---|---|---|---|
| Model for wakeup cortisol level | |||||
| Intercept | 2.783 | 0.033 | <0.0001 | 16.2 nmol/L mean wakeup cortisol a | |
| Age in years | 0.030 | 0.005 | <0.0001 | 3.0% higher for each year older | |
| Hours sleep, previous night | −0.001 | 0.013 | 0.95 | −0.1% (n.s.) | |
| Hours sleep, typical night | −0.023 | 0.017 | 0.19 | −2.3% (n.s.) | |
| Waking time | 0.000 | 0.000 | 0.14 | 0.0% (n.s.) | |
| Any cigarette smoking | −0.063 | 0.067 | 0.35 | −6.1% (n.s.) | |
| Any alcoholic drinks | −0.039 | 0.033 | 0.25 | −3.8% (n.s.) | |
| Perceived stress | −0.010 | 0.024 | 0.70 | −1.0% (n.s.) | |
| Noncompliance (wakeup sample) | 0.305 | 0.124 | 0.01 | 35.6% higher if noncompliant with wakeup sample | |
| 1+ stressful events, past month | −0.050 | 0.050 | 0.31 | −4.9% (n.s.) | |
| Sexual orientation (Ref = CH) | |||||
| MH or CHwSS | −0.003 | 0.061 | 0.96 | −0.3% (n.s.) | |
| Bisexual or Gay | −0.009 | 0.073 | 0.90 | 0.0% (n.s.) | |
| Sexual orientation by stressful events interaction (Ref = CH w/no past month stressful events) | |||||
| 1+ stressful events × MH/CHwSS | −0.078 | 0.096 | 0.42 | −7.5% (n.s.) | |
| 1+ stressful events × Bi/Gay | 0.156 | 0.118 | 0.19 | 17.0% (n.s.) | |
| Model for cortisol level | |||||
| Worry level (at each sample) | 0.056 | 0.024 | 0.02 | 5.7% higher with increased worry | |
| Happiness level (at each sample) | −0.022 | 0.020 | 0.28 | −2.2% (n.s.) | |
| Model for time since waking effect | |||||
| Intercept | −0.145 | 0.010 | <0.0001 | 13.5% lower per hour since wakeup | |
| Sexual orientation (Ref = CH) | |||||
| MH or CHwSS | 0.011 | 0.007 | 0.12 | 1.1% (n.s.) | |
| Bisexual or Gay | 0.002 | 0.008 | 0.85 | 0.2% (n.s.) | |
| Model for time since waking squared effect | |||||
| Intercept | 0.000 | 0.001 | 0.79 | 0.0% (n.s.) | |
| Model for time since waking cubed effect | |||||
| Intercept | 0.000 | 0.000 | 0.03 | 0.0% per hour cubed since wakeup | |
| Model for cortisol awakening response effect | |||||
| Intercept | 0.321 | 0.039 | <0.0001 | 37.8% higher if CAR | |
| Noncompliance (wakeup sample) | −0.219 | 0.207 | 0.29 | −19.7% (n.s.) | |
| Sexual orientation (Ref = CH) | |||||
| MH or CHwSS | 0.038 | 0.084 | 0.65 | 3.9% (n.s.) | |
| Bisexual or Gay | 0.138 | 0.101 | 0.17 | 14.8% (n.s.) | |
| Random Effects | Variance estimate | SE | p-value | ||
|
| |||||
| Awakening cortisol | 0.020 | 0.011 | 0.04 | ||
| Time since waking | 0.001 | 0.000 | <0.0001 | ||
Notes: Variables centered around their means are age, hours sleep in previous night, hours sleep on a typical night, waking time, perceived stress, worry, happiness. Sexual orientation categories: (1) Bisexual or Gay; (2) MH, CHwSS = Mostly Heterosexual or Completely Heterosexual with same-sex partners; (3) CH = Completely Heterosexual with no same-sex partners (Ref). All numbers are rounded to nearest zero.
Mean wakeup cortisol among CH, nonsmoking, no alcohol, compliant participants with no reported stressful events in the previous month and mean sleep, waking time, stress, worry, and happiness scores.
In interpreting effect sizes, use of a logarithmic outcome allows coefficients to be interpreted as percentage change in the outcome per unit change in the independent variable, after applying the following transformation: B%change = [exp (Braw)] – 1. The “Interpretation” column of table includes the percentage change interpretation based on this transformation.
Table 6 presents results of generalized estimating equations examining sexual orientation as a predictor of overall cortisol output as measured by AUCg, adjusted for covariates. Sexual orientation was not significantly associated with AUCg; findings for AUCi were similar (data not shown).
Table 6.
Adjusted generalized estimating equations (GEE) models of cortisol output (AUCg) by sexual orientation among females and males in GUTS Saliva Substudy (n = 1502).
| FEMALES (n = 1047) |
MALES (n = 455) |
|||||
|---|---|---|---|---|---|---|
| Beta | SE | p-value | Beta | SE | p-value | |
| Intercept | 240.07 | 22.76 | <.0001 | 212.38 | 25.90 | <.0001 |
| Sexual orientation (Ref = CH) | 0.58 | 0.08 | ||||
| MH or CHwSS | 4.53 | 4.67 | 0.33 | 14.96 | 8.03 | 0.06 |
| Bisexual,Lesbian, or Gay | −1.90 | 6.22 | 0.76 | 12.00 | 8.10 | 0.14 |
| Hours sleep, previous night | −7.37 | 1.63 | <.0001 | −1.18 | 3.10 | 0.70 |
| Hours sleep, typical night | −3.18 | 2.22 | 0.15 | −4.94 | 2.64 | 0.06 |
| Wake time today | −0.0024 | 0.0003 | <.0001 | −0.0016 | 0.0005 | <0.01 |
| Cigarette | 37.54 | 15.40 | 0.01 | 1.89 | 12.31 | 0.88 |
| Alcohol | 3.60 | 3.64 | 0.32 | −4.86 | 5.20 | 0.35 |
| Perceived stress | 3.79 | 2.17 | 0.08 | 5.45 | 4.09 | 0.18 |
| Hormonal contraception use | −4.39 | 3.43 | 0.20 | |||
| Noncompliance (wakeup sample) | −6.19 | 7.84 | 0.43 | 4.84 | 30.07 | 0.87 |
Notes: Variables centered around their means are hours sleep in previous night, hours sleep on a typical night, waking time, perceived stress score. Sexual orientation categories: (1) Bisexual, Gay or Lesbian; (2) MH, CHwSS = Mostly Heterosexual or Completely Heterosexual with same-sex partners; (3) CH = Completely Heterosexual with no same-sex partners (Ref). All numbers are rounded to nearest zero.
Bold indicates statistical significance.
4. Discussion
To our knowledge, this is the largest study to date to examine associations between sexual orientation and any marker of HPA-axis functioning, and one of the larger studies of diurnal cortisol patterns in young adults in general. We did not find support for a hypothesized association between sexual orientation and diurnal cortisol rhythms. We observed a statistically significantly higher prevalence of stressful life events in sexual minority females compared to heterosexual females, as would be predicted by sexual minority stress theory, though only a suggestion of higher prevalence among sexual minority males compared to heterosexual males. We did not find evidence of an interaction between stressful life events and sexual orientation.
These findings represent an important contribution to the literature on social stressors in relation to stress physiology. Most prior research on sexual orientation and stress biomarkers has focused on within-group analyses of sexual minorities without a heterosexual comparison group (Burton et al., 2014; Hatzenbuehler et al., 2014; Huebner and Davis, 2005). The only other study to assess diurnal cortisol and sexual orientation with a heterosexual comparison group also found no significant associations between sexual orientation identity and cortisol profile in a sample of 87 LGB and heterosexual adults (Juster et al., 2013).
What might explain the lack of differences by sexual orientation in cortisol diurnal rhythms in young adults? One possible explanation could be that the similarity across sexual orientation groups may be the result of physiologic resilience in sexual minority participants in the study. Sexual minority youth and adults are exposed to both chronic and acute social stressors related to stigma and discrimination (Institute of Medicine, 2011). Indeed, sexual minority female participants in this study reported a higher prevalence of recent stressful life events relative to completely heterosexuals. Researchers have proposed that sexual minorities, particularly those who have access to supportive social contexts, may be able to develop adaptive coping strategies that heighten resilience (Hatzenbuehler et al., 2009). Following from this logic, it is possible that young sexual minority adults in the GUTS cohort have developed coping strategies that enhance their ability to manage stigma-related stressors and are reflected in HPA-axis responses that parallel those of their heterosexual peers. Prior research on stress reactivity finding low cortisol response to a laboratory stressor among gay/bisexual men in relation to heterosexual men was also hypothesized to be due to successful adaptation to sexual minority stigma (Juster et al., 2015). That said, the well-documented higher rates of depressive symptoms, PTSD, eating disorder symptoms, substance use, and other health risk indicators found in sexual minority compared to heterosexuals in the GUTS cohort (Austin et al., 2013; Corliss et al., 2010; Roberts et al., 2012, 2013b) raises the question as to whether coping strategies that mitigate dysregulation of the HPA axis or, more particularly, cortisol diurnal rhythms, may be different from strategies that mitigate mental health problems.
Another possible explanation may be that diurnal cortisol rhythms may be driven more by proximal emotions than by past stressful events. Most of the evidence for disruptions in cortisol regulation among adults with abuse histories or among LGB adults who grew up in high-stigma or low-family-support environments is based on reactivity to an acute stressor or challenge administered in a laboratory setting (Burton et al., 2014; Hatzenbuehler and McLaughlin, 2014; Heim et al., 2008, 2000b, 2002). In the present study, self-reported current level of worry/anxiety, which did not exhibit large variations by sexual orientation, was one of the few covariates that was significantly associated with diurnal cortisol levels, which is consistent with findings that feelings such as anger/tension predict higher evening cortisol levels (Adam et al., 2006) and that daily stressors, such as arguments at home, predict increased total cortisol output (Stawski et al., 2013)
The nature and timing of the stressor also play a critical role in diurnal cortisol patterns, according to a meta-analysis of studies on stress and HPA-axis functioning (Fekedulegn et al., 2007). For example, traumatic stress or stress due to physical threat, like combat, produces a high and flat diurnal cortisol pattern, while stress that threatens the social self, such as divorce, produces significantly higher cortisol secretion at certain times of day. In the present study, although we did not see associations between past-month stressful life events and cortisol patterns, if different types of life events produce conflicting diurnal cortisol profiles, then the analyses presented here would not be able to detect these relationships. Future research should investigate the role of specific types of stressors (e.g., physical threats such as overt violence versus social-evaluative threats such as covert employment discrimination) in the diurnal cortisol patterns of LGB and heterosexual populations. Finally, previous research has found associations between diurnal cortisol patterns and sexual identity milestones (e.g., sexual orientation disclosure) (Huebner and Davis, 2005; Juster et al., 2016, 2013). Although outside the scope of the present study, further research is needed to examine how such processes may or may not be protective against HPA-axis dysregulation.
These findings must be considered in light of several limitations. In spite of the large sample size, power limitations are a concern for this analysis, particularly given the relatively small sample of LGB individuals who provided saliva for the study, made even smaller when stratified by sex. Future studies with even larger samples are needed because, as indicated by prior research on stress-related biomarkers (Everett et al., 2014; Juster et al., 2015, 2013) and guided by intersectionality theory (Bauer, 2014), it is critically important to be able to examine the intersection of sexual orientation and gender to improve understanding of physiological stress response. Larger samples and oversampling of transgender participants would also allow for analyses of gendered patterns beyond those seen in cisgender populations. We did not monitor compliance with sampling protocols electronically, although we did capture self-reported sampling times and compliance with instructions (e.g., not eating or drinking 30 min prior), and models were adjusted for sampling-time noncompliance. Furthermore, the low response rate may have introduced bias in our findings. Finally, participants were members of an ongoing cohort of the children of U.S. nurses and are predominantly white and raised in largely middle-income households; thus, these findings may not be generalizable to other young adult populations.
5. Conclusion
Given the well-documented health inequities faced by U.S. sexual minority populations and mounting evidence that social stressors such as experiences of stigma and discrimination can influence both mental and physical health, this study offers a needed contribution to the research literature on the dynamics of stress biomarkers in relation to the health of socially marginalized populations. If future studies replicate our results, that will suggest that diurnal cortisol dysregulation, at least as currently conceived and measured, may not be on a pathway linking sexual minority stressors to the widely observed elevated risk of depressive and anxious symptoms, PTSD, eating disorders, and substance use among sexual minority adolescents and young adults. Future research on sexual orientation and the HPA axis must also examine specific types of social stressors, given the dependence of cortisol patterns on the specific type and timing of the stressor. Finally, given the rapidly shifting status of civil rights for sexual minority individuals in the United States, longitudinal research on the relationship between sexual orientation and stress biomarkers may aid in evaluating whether and how changes in the social environment may impact individual physiological stress processes.
Acknowledgments
The authors would like to thank the Channing Division of Network Medicine Biorepository and Growing Up Today Study (GUTS) team of investigators for their contributions to this paper and the thousands of young people across the country participating in the GUTS cohort.
Role of funding sources
This study was funded by grants HD057368 and HD066963 from the National Institutes of Health. S.B. Austin is supported by the Leadership Education in Adolescent Health project, Maternal and Child Health Bureau, Health Resources and Services Administration grants T71-MC00009 and T76-MC00001. The funders played no role in the study design, collection, analysis or interpretation of data, in writing of the manuscript, or in the decision to submit the manuscript for publication.
Footnotes
Conflict of interest
The authors have no conflicts of interest to declare.
Contributors
SB Austin, M Rosario were responsible for study conceptualization. SB Austin, M Rosario, KA McLaughlin, AL Roberts, S Missmer, and L Anatale-Tardiff were responsible for data collection. SB Austin, M Rosario, KA McLaughlin, AL Roberts, AR Gordon, V Sarda, S Missmer, L Anatale-Tardiff, and EA Scherer were responsible for data analysis, interpretation, and article preparation.
References
- Adam EK, Gunnar MR. Relationship functioning and home and work demands predict individual differences in diurnal cortisol patterns in women. Psychosom. Med. 2001;26:189–208. doi: 10.1016/s0306-4530(00)00045-7. [DOI] [PubMed] [Google Scholar]
- Adam EK, Kumari M. Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology. 2009;34:1423–1436. doi: 10.1016/j.psyneuen.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT. Day-to-day dynamics of experience–cortisol associations in a population-based sample of older adults. Proc. Natl. Acad. Sci. U. S. A. 2006;103:17058–17063. doi: 10.1073/pnas.0605053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida DM, McGonagle K, King H. Assessing daily stress processes in social surveys by combining stressor exposure and salivary cortisol. Biodemogr. Soc. Biol. 2009a;55:219–237. doi: 10.1080/19485560903382338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida J, Johnson RM, Corliss HL, Molnar BE, Azrael D. Emotional distress among LGBT youth: the influence of perceived discrimination based on sexual orientation. J. Youth Adolesc. 2009b;38:1001–1014. doi: 10.1007/s10964-009-9397-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin SB, Jun H-J, Jackson B, Spiegelman D, Rich-Edwards J, Corliss HL, Wright RJ. Disparities in child abuse victimization in lesbian, bisexual, and heterosexual women in the Nurses’ Health Study II. J. Women’s Health. 2008;17:597–606. doi: 10.1089/jwh.2007.0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin SB, Nelson LA, Birkett MA, Calzo JP, Everett B. Eating disorder symptoms and obesity at the intersections of gender ethnicity, and sexual orientation in US high school students. Am. J. Public Health. 2013;103:e16–22. doi: 10.2105/AJPH.2012.301150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer GR. Incorporating intersectionality theory into population health research methodology: challenges and the potential to advance health equity. Soc. Sci. Med. 2014;110:10–17. doi: 10.1016/j.socscimed.2014.03.022. [DOI] [PubMed] [Google Scholar]
- Berlan ED, Corliss HL, Field AE, Goodman E, Austin SB. Sexual orientation and bullying among adolescents in the Growing Up Today Study. J. Adolesc. Health. 2010;46:366–371. doi: 10.1016/j.jadohealth.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch NM, Riese H, Reijneveld SA, Bakker MP, Verhulst FC, Ormel J, Oldehinkel AJ. Timing matters: long term effects of adversities from prenatal period up to adolescence on adolescents’ cortisol stress response; The TRAILS study. Psychoneuroendocrinology. 2012;37:1439–1447. doi: 10.1016/j.psyneuen.2012.01.013. [DOI] [PubMed] [Google Scholar]
- Burton CL, Bonanno GA, Hatzenbuehler ML. Familial social support predicts a reduced cortisol response to stress in sexual minority young adults. Psychoneuroendocrinology. 2014;47:241–245. doi: 10.1016/j.psyneuen.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Carvalho JP, Tyrka AR, Wier LM, Mello AF, Mello MF, Anderson GM, Wilkinson CW, Price LH. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biol. Psychiatry. 2007;62:1080–1087. doi: 10.1016/j.biopsych.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Shattuck TT, Tyrka AR, Geracioti TD, Price LH. Effect of childhood physical abuse on cortisol stress response. Psychopharmacology (Berl.) 2011;214:367–375. doi: 10.1007/s00213-010-2007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clow A, Thorn L, Evans P, Hucklebridge F. The awakening cortisol response: methodological issues and significance. Stress. 2004;7:29–37. doi: 10.1080/10253890410001667205. [DOI] [PubMed] [Google Scholar]
- Cohen S, Williamson G. The Social Psychology of Health: The Claremont Symposium on Applied Social Psychology. Sage; Newbury Park, CA: 1988. [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J. Health Soc. Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- Corliss HL, Rosario M, Wypij D, Wylie SA, Frazier AL, Austin SB. Sexual orientation and drug use in a longitudinal cohort study of U.S. adolescents. Addict. Behav. 2010;35:517–521. doi: 10.1016/j.addbeh.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol. Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Doane LD, Mineka S, Zinbarg RE, Craske M, Griffith JW, Adam EK. Are flatter diurnal cortisol rhythms associated with major depression and anxiety disorders in late adolescence? The role of life stress and daily negative emotion. Dev. Psychopathol. 2013;25:629–642. doi: 10.1017/S0954579413000060. [DOI] [PubMed] [Google Scholar]
- Everett BG, Rosario M, McLaughlin KA, Austin SB. Sexual orientation and gender differences in markers of inflammation and immune functioning. Ann. Behav. Med. 2014;47:57–70. doi: 10.1007/s12160-013-9567-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekedulegn DB, Andrew ME, Burchfiel CM, Violanti JM, Hartley TA, Charles LE, Miller DB. Area under the curve and other summary indicators of repeated waking cortisol measurements. Psychosom. Med. 2007;69:651–659. doi: 10.1097/PSY.0b013e31814c405c. [DOI] [PubMed] [Google Scholar]
- Friedman MS, Marshal MP, Guadamuz TE, Wei C, Wong CF, Saewyc E, Stall R. A meta-analysis of disparities in childhood sexual abuse, parental physical abuse, and peer victimization among sexual minority and sexual nonminority individuals. Am. J. Public Health. 2011;101:1481–1494. doi: 10.2105/AJPH.2009.190009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman LA, Corcoran C, Turner K, Yuan N, Green BL. Assessing traumatic event exposure: general issues and preliminary findings for the Stressful Life Events Screening Questionnaire. J. Trauma. Stress. 1998;11:521–542. doi: 10.1023/A:1024456713321. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and flattening of expected daytime rhythm: potential indices of risk in human development. Dev. Psychopathol. 2001;13:515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Hatzenbuehler ML, McLaughlin KA. Structural stigma and hypothalamic-pituitary-adrenocortical axis reactivity in lesbian, gay, and bisexual young adults. Ann. Behav. Med. 2014;47:39–47. doi: 10.1007/s12160-013-9556-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzenbuehler ML, Nolen-Hoeksema S, Dovidio J. How does stigma get under the skin? The mediating role of emotion regulation. Psychol. Sci. 2009;20:1282–1289. doi: 10.1111/j.1467-9280.2009.02441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzenbuehler ML, McLaughlin KA, Keyes KM, Hasin DS. The impact of institutional discrimination on psychiatric disorders in lesbian, gay, and bisexual populations: a prospective study. Am. J. Public Health. 2010;100:452–459. doi: 10.2105/AJPH.2009.168815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzenbuehler ML, McLaughlin KA, Slopen N. Sexual orientation disparities in cardiovascular biomarkers among young adults. Am. J. Prev. Med. 2013;44:612–621. doi: 10.1016/j.amepre.2013.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzenbuehler ML, Jun H-J, Corliss HL, Austin SB. Structural stigma and sexual orientation disparities in adolescent drug use. Addict. Behav. 2015;46:14–18. doi: 10.1016/j.addbeh.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000a;25:1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Miller AH, Nemeroff CB. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000b;284:592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Wagner D, Wilcox MM, Miller AH, Nemeroff CB. The role of early adverse experience and adulthood stress in the prediction of neuroendocrine stress reactivity in women: a multiple regression analysis. Depress. Anxiety. 2002;15:117–125. doi: 10.1002/da.10015. [DOI] [PubMed] [Google Scholar]
- Heim C, Mletzko T, Purselle D, Musselman DL, Nemeroff CB. The dexamethasone/corticotropin-releasing factor test in men with major depression: role of childhood trauma. Biol. Psychiatry. 2008;63:398–405. doi: 10.1016/j.biopsych.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Huebner DM, Davis MC. Gay and bisexual men who disclose their sexual orientations in the workplace have higher workday levels of salivary cortisol and negative affect. Ann. Behav. Med. 2005;30:260–267. doi: 10.1207/s15324796abm3003_10. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine . The Health of Lesbian, Gay, Bisexual and Transgender People: Building a Foundation for Better Understanding. The National Academies Press; Washington, DC: 2011. [PubMed] [Google Scholar]
- Juster RP, Smith NG, Ouellet E, Sindi S, Lupien SJ. Sexual orientation and disclosure in relation to psychiatric symptoms diurnal cortisol, and allostatic load. Psychosom. Med. 2013;75:103–116. doi: 10.1097/PSY.0b013e3182826881. [DOI] [PubMed] [Google Scholar]
- Juster R-P, Hatzenbuehler ML, Mendrek A, Pfaus JG, Smith NG, Johnson PJ, Lefebvre-Louis J-P, Raymond C, Marin M-F, Sindi S, Lupien SJ, Pruessner JC. Sexual orientation modulates endocrine stress reactivity. Biol. Psychiatry. 2015;77:668–676. doi: 10.1016/j.biopsych.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juster R-P, É O, Lefebvre-Louis J-P, Sindi S, Johnson PJ, Smith NG, Lupien SJ. Retrospective coping strategies during sexual identity formation and current biopsychosocial stress. Anxiety Stress Coping. 2016;29:119–138. doi: 10.1080/10615806.2015.1004324. [DOI] [PubMed] [Google Scholar]
- Katz-Wise SL, Hyde JS. Victimization experiences of lesbian, gay, and bisexual individuals: a meta-analysis. J. Sex Res. 2012;49:142–167. doi: 10.1080/00224499.2011.637247. [DOI] [PubMed] [Google Scholar]
- Meyer IH. Prejudice, social stress, and mental health in lesbian gay, and bisexual populations: conceptual issues and research evidence. Psychol. Bull. 2003;129:674–697. doi: 10.1037/0033-2909.129.5.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Phychol. Bull. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Pervanidou P, Chrousos GP. Metabolic consequences of stress during childhood and adolescence. Metabolism. 2012;61:611–619. doi: 10.1016/j.metabol.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Remafedi G, Resnick M, Blum R, Harris L. Demography of sexual orientation in adolescents. Pediatrics. 1992;89:714–721. [PubMed] [Google Scholar]
- Roberts AL, Austin SB, Corliss HL, Morris A, Koenen K. Pervasive trauma exposure among U: S. sexual orientation minority adults linked to posttraumatic stress disorder risk. Am. J. Public Health. 2010a;100:2433–2441. doi: 10.2105/AJPH.2009.168971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AL, Austin SB, Corliss HL, Vandermorris AK, Koenen KC. Pervasive trauma exposure among US sexual orientation minority adults and risk of posttraumatic stress disorder. Am. J. Public Health. 2010b;100:2433–2441. doi: 10.2105/AJPH.2009.168971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AL, Rosario M, Corliss HL, Koenen K, Austin SB. Elevated risk of posttraumatic stress in sexual minority youths: mediation by childhood abuse and gender nonconformity. Am. J. Public Health. 2012;102:1587–1593. doi: 10.2105/AJPH.2011.300530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AL, Rosario M, Corliss HL, Wypij D, Lightdale JR, Austin SB. Sexual orientation and functional pain in U.S. young adults: the mediating role of childhood abuse and psychological symptoms. PLoS One. 2013a;e54702;8 doi: 10.1371/journal.pone.0054702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AL, Rosario M, Slopen N, Calzo JP, Austin SB. Childhood gender nonconformity, bullying victimization, and depressive symptoms across adolescence and early adulthood: an 11-year longitudinal study. J. Am. Acad. Child Adolesc. Psychiatry. 2013b;52:143–152. doi: 10.1016/j.jaac.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario M, Schrimshaw EW, Hunter J, Gwadz M. Gay-related stress and emotional distress among gay, lesbian, and bisexual youths: a longitudinal examination. J. Consult. Clin. Psychol. 2002;70:967–975. doi: 10.1037//0022-006x.70.4.967. [DOI] [PubMed] [Google Scholar]
- Rosner B, Cook N, Portman R, Daniels S, Falkner B. Determination of blood pressure percentiles in normal-weight children: some methodological issues. Am. J. Epidemiol. 2008;167:653–666. doi: 10.1093/aje/kwm348. [DOI] [PubMed] [Google Scholar]
- SAS, I. SAS 93 for Windows. SAS Institute; Cary, NC: 2012. [Google Scholar]
- Saewyc EM, Skay CL, Pettingell SL, Reis EA, Bearinger L, Resnick M, Murphy A, Combs L. Hazards of stigma: the sexual and physical abuse of gay, lesbian, and bisexual adolescents in the United States and Canada. Child Welfare. 2006;85:195–213. [PubMed] [Google Scholar]
- Segerstrom SC, Miller GE. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol. Bull. 2004;130:601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stawski RS, Cichy KE, Piazza JR, Almeida DM. Associations among daily stressors and salivary cortisol: findings from the National Study of Daily Experiences. Psychoneuroendocrinology. 2013;38:2654–2665. doi: 10.1016/j.psyneuen.2013.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BW, Dahlhamer JM, Galinsky AM, Joestl SS. Sexual orientation and health among U.S. adults: national health interview survey, 2013. Natl. Health Stat. Rep. 2014:1–10. [PubMed] [Google Scholar]
- Yehuda R, Teicher MH, Trestman RL, Levengood RA, Siever LJ. Cortisol regulation in posttraumatic stress disorder and major depression: a chronobiological analysis. Biol. Psychiatry. 1996;40:79–88. doi: 10.1016/0006-3223(95)00451-3. [DOI] [PubMed] [Google Scholar]