Abstract
Vilazodone is a novel antidepressant having a selective serotonin (5-HT) reuptake inhibitor and 5-HT1A receptor partial agonist profile, so it has been regarded as a serotonin partial agonist-reuptake inhibitor (SPARI). We aimed to provide Vilazodone's clinical implications mainly by reviewing published clinical trials. Vilazodone has been speculated to have three potential benefits including faster onset of action, greater efficacy, and better tolerability owning to its SPARI properties. However, no studies conducted so far have directly proven the above speculations. Five initial phase II trials failed to distinguish vilazodone from placebo in the treatment of MDD, but 4 randomized clinical trials (RCT), 3 post-hoc or pooled analysis, 1 long-term open label study, and a meta-analysis showed vilazodone's superior efficacy over placebo. The studies also showed vilazodone is generally safe and tolerable. However, diarrhea, nausea, headache, dizziness, dry mouth, and insomnia warrant close attention in clinical practice because they have been constantly noted throughout the clinical studies. 2 RCTs recently documented the efficacy and safety of vilazodone in patients with generalized anxiety disorder, which could be a start of broadening vilazodone's usage or FDA approval in diverse anxiety disorders.
Keywords: Antidepressive agents, Anxiety disorders, 5-HT1 receptor agonists, Serotonin uptake inhibitors, Vilazodone hydrochloride
INTRODUCTION
Major depressive disorder (MDD), which has a lifetime prevalence of 15-20%, is a debilitating illness affecting patients' general lives by causing severe functional impairment.1 Despite numerous antidepressants being available, more than 30% of patients with MDD do not achieve adequate relief.2 Other important obstacles with conventional antidepressants such as selective serotonin reuptake inhibitors (SSRI) and selective serotonin and norepinephrine reuptake inhibitors (SNRI) include the therapeutic lag (approximately 2-4 weeks) between administration and onset of clinical improvement and safety and tolerability issues such as sexual dysfunction.3,4,5 Therefore, additional novel antidepressants are needed to overcome the above obstacles of the conventional antidepressant.
The monoamine hypothesis of depression has been the subject of controversy for more than a decade.6 In order to provide a mechanisms beyond monoamines, diverse potential agents targeting glutamate receptors, promoting neuroplasticity, modulating cholinergic transmission, and enhancing neuroinflammation and gamma-butyric acid (GABA) transmission were tested.6,7,8 However, none of these agents have yet successfully developed as an antidepressant, so almost all antidepressants available are based on monoamine theory. Thus, the action mechanism of antidepressants has inevitably moved from multiple monoamine transporters/receptors (Monoamine inhibitors and Tricyclic antidepressants) first to selective (SSRI/SNRI) and then recently to selectively multiple. In this respect, the introduction of vilazodone, an antidepressant which received FDA approval for the treatment of major depressive disorder (MDD) in 2011,9 is timely because it is one of the first antidepressants targeting selectively multiple monoamine transporters or receptors. Vilazodone is known to contain a selective serotonin (5-HT) reuptake inhibitor with 5-HT1A receptor partial agonist profile.10
The purpose of this article is to provide review of clinical studies evaluating vilazodone's role as an antidepressant. We aimed to provide its clinical implications mainly by reviewing published clinical trials. In order to provide unbiased information, we have also included 5 unpublished phase II trials.
GENERAL INFORMATION
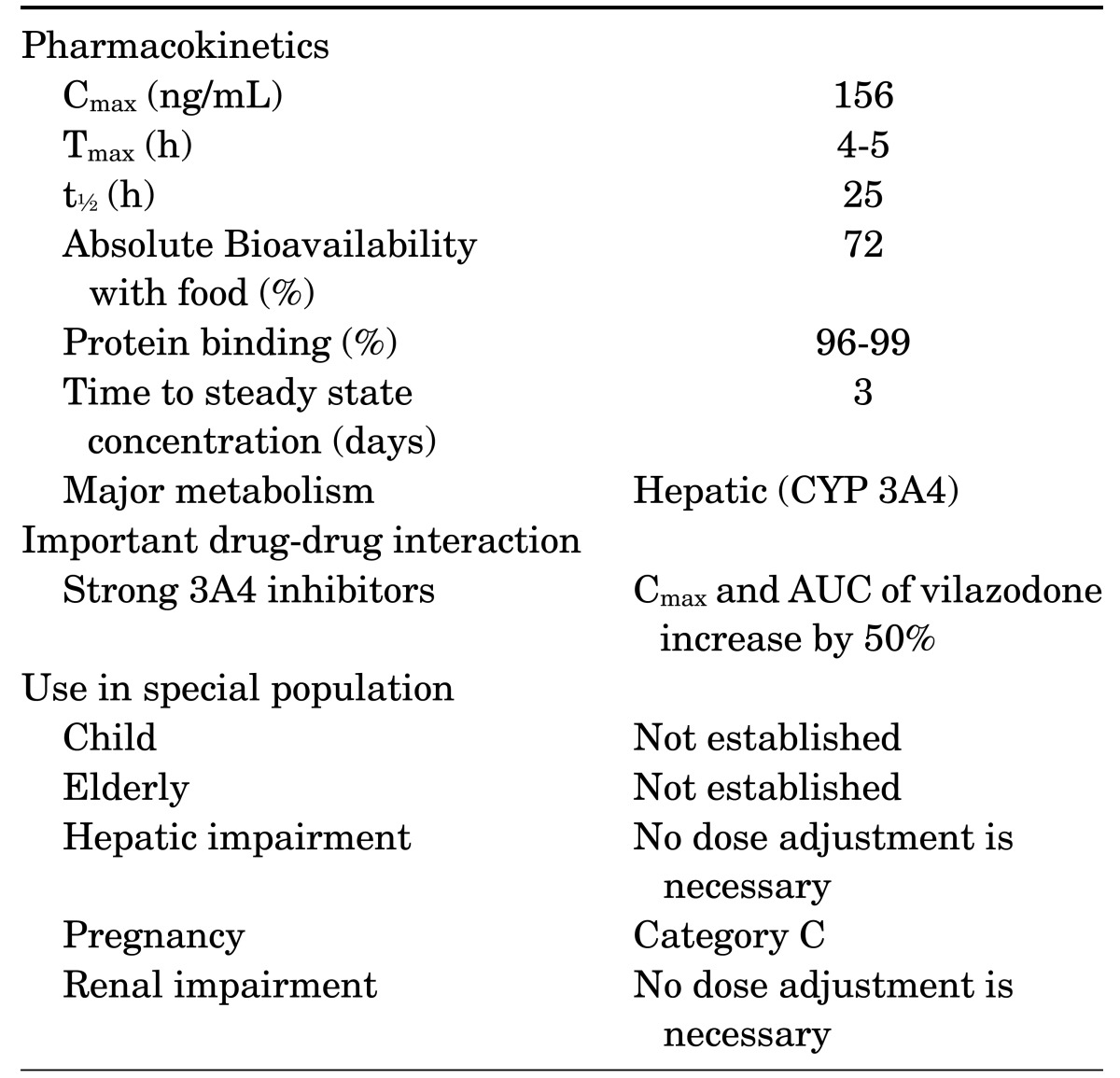
Vilazodone's activity is primarily related to its parent drug, and its pharmacokinetics doses are proportional from 5 to 80 mg.9 Vilazodone is primarily metabolized via hepatic (CYP3A4) system, so its dosage should be reduced to 20 mg/d when used in combination with a strong CYP3A4 inhibitor such as ketoconazole. Pharmacokinetics of vilazodone were similar in participants with mild, moderate, or severe hepatic impairment and healthy controls following a single, 20-mg oral dose of vilazodone.11 Thus, dose adjustment is not needed for patients with major depressive disorder who have mild, moderate, or severe hepatic impairment. Likewise, another study suggested that no dose adjustment was required for patients having mild to moderate renal dysfunction.12 Vilazodone's initial recommended daily dosage is 10 mg/d. The dose should be increased gradually to 40 mg/d over 3 weeks in order to prevent gastrointestinal discomfort. There has been no reported data on the use of vilazodone in children, adolescents, or elderly. Vilazodone's general information is summarized in Table 1.
TABLE 1. General information of vilazodonea.
AUC: area under the curve, Cmax: maximum plasma vilazodone concentration, t½: terminal elimination half-life, Tmax: time to Cmax.
aTable adapted from Wang et al.25
MECHANISM OF ACTION
The affinity of vilazodone is much higher in the 5-HT reuptake site (Ki=0.1 nM) than in norepinephrine (Ki=56 nM) and dopamine (Ki=37 nM) sites.13 Therefore, it is known to have SSRI activity and a partial agonist at the 5-HT1A receptor and has been characterized as a serotonin partial agonist-reuptake inhibitor (SPARI).14
In order to understand its suggested mechanism of action, it is crucial to first review the fact that the pre- and postsynaptic 5HT1A receptors have opposite roles in serotonin turnover;15,16,17
- Pre-synaptic 5HT1A receptors located in raphe nuclei (autoreceptors) → activation cause decrement in serotonin secretion → can be desensitized with chronic stimulation
- Post-synaptic 5HT1A receptors located in the hippocampus → activation causes serotonin secretion → does not desensitize with chronic stimulation
- In order for an antidepressant to show an effect → extra-neuronal serotonin must first be increased → resulting desensitization of pre-synaptic autoreceptors+activation of post-synaptic 5HT1A receptors → lead to increase release of serotonin
When a conventional antidepressant, such as an SSRI, is administered acutely, serotonin transporters will be blocked causing extra-neuronal serotonin to be increased. However, this increase of 5-HT will be counteracted due to the increased 5-HT which will stimulate the pre-synaptic 5-HT1A autoreceptors initiating neuronal negative feedback mechanisms.18 This negative feedback mechanism is known to cause therapeutic lag because a minimum of 2-4 weeks is required to overcome the autoreceptor-mediated serotonin inhibition and 5-HT1A autoreceptor down-regulation with SSRI.19
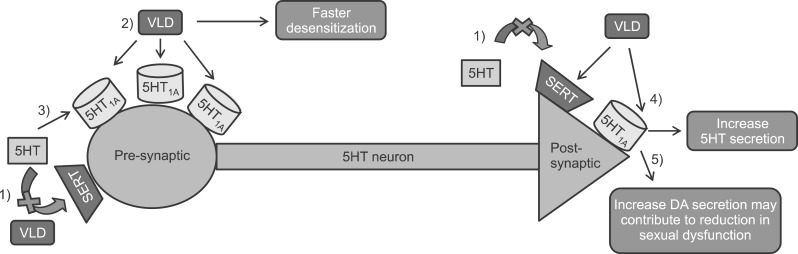
In this respect, Stahl speculated that vilazodone has three potential benefits owning to its SPARI property including faster onset of action, greater efficacy, and better tolerability, in particular lower sexual side effects (Fig. 1).14 Vilazodone intake may result in quicker and greater desensitization of 5-HT1A autoreceptors without resulting in excess activation of 5-HT1A autoreceptor- mediated serotonin inhibition by acting only as a partial agonist at these receptors,20,21 In addition, vilazodone binds to 5-HT1A with a higher affinity in a longer time than serotonin, which also contributes to faster desensitization of 5-HT1A autoreceptors.22,23,24 The quicker and greater desensitization of 5-HT1A autoreceptors, of course, could lead to faster onset of action.25 Synergistic with its SSRI properties and activating 5-HT1A postsynaptic receptors, vilazodone could yield even more serotonin release leading to greater efficacy.26 Lastly, activation of 5-HT1A postsynaptic receptors increases in dopamine release, which might contribute to a reduction in sexual dysfunction.
FIG. 1. Vilazodone's propsed mechanism of action.13,25 1) Vilazodone can increase extra-neuronal serotonin by blocking SERT. 2) By acting only as a partial agonist at 5-HT1A autoreceptors, vilazodone may more rapidly desensitize 5-HT1A autoreceptors without causing excess activation of 5-HT1A autoreceptor-mediated serotonin inhibition. 3) Vilazodone binds to 5-HT1A With a higher affinity in a longer time than serotonin contributing to faster desensitization of 5-HT1A autoreceptors. 4) Vilazodone will enhance 5-HT by activating 5-HT1A postsynaptic receptors, and synergistic with its SSRI properties, vilazodone would yield even more serotonin release leading to greater efficacy. 5) Activating 5-HT1Apostsynaptic receptors increase dopamine release, which contribute to reduction in sexual dysfunction. 5HT: Serotonin, SERT: Serotonin transporter, SSRI: Selective serotonin reuptake inhibitor. Figure adapted from Wang et al.25.
One could argue that similar actions could be obtained by combining SSRI with atypical antipsychotic or a 5-HT1A partial agonist, buspirone. One must realize that administration of vilazodone is not identical to administration of SSRI+atypical antipsychotics because atypical antipsychotics have additional desirable pharmacologic actions and undesirable adverse reactions. In terms of SSRI+buspirone, vilazodone is known to be a higher partial 5-HT1A agonist and occupy more 5-HT1A for a longer time than buspirone.14 However, no head-to-head clinical trials have confirmed that vilazodone has a faster treatment onset, greater efficacy, and better tolerability (lower sexual side effects) than other antidepressants (i.e.: SSRIs and SNRIs).
CLINICAL EVIDENCE
1. Unpublished phase II studies
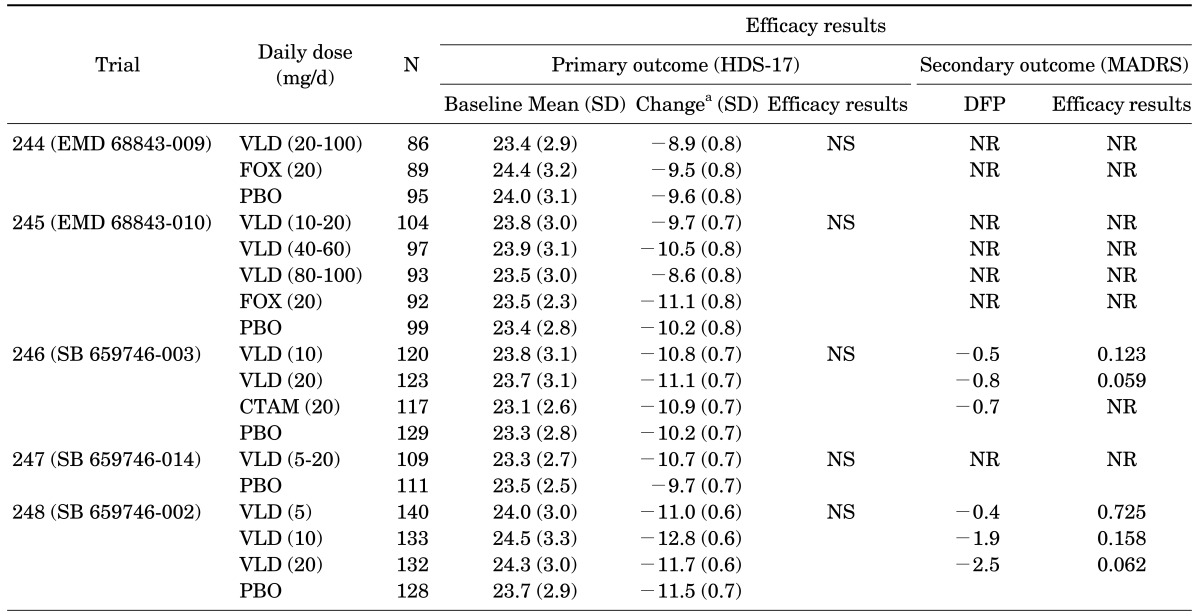
Five unpublished studies having almost identical designs (8-week, double-blind, randomized, and placebo-controlled) using vilazodone doses from 5 to 100 mg/d were carried out as part of the phase II program (Table 2).13,27 None of these trials were able to prove statistically significant treatment effects of vilazodone on its primary endpoint, the Hamilton Depression Rating Scale (HDS). However, all active comparators also failed to show superior efficacy over placebo in the three trials with active comparator (trials 244-246). Thus, two studies without an active comparator could be regarded as "negative" studies (trial 247-248), but the three trials with active comparator (trials 244-246) could be recognized as "failed" studies. More importantly, the two fixed-dose trials (trials 246 and 248) showed a possible superior efficacy of vilazodone (20 mg/day) over placebo on the Montgomery Asberg Depression Rating Scale (MADRS), which was the studies' secondary outcome measure. The results also suggested possible dose response relationships; depression symptoms tended to decrease as dose of vilazodone increased from 10 mg/d to 20 mg/d (trial 246, p=.12 and .06 for vilazodone 10 mg and 20 mg respectively; trial 248, p=.73, .12, and .06 for vilazodone 5 mg, 10 mg and 20 mg respectively). These results led the industry to conduct phase III studies using higher, fixed daily doses of vilazodone.
TABLE 2. Summary of phase II (unpublished) clinical trials of vilazodone in patients with major depressive disorder13,27.
aLeast-square mean change from baseline to end-point (week 8).
CTAM: citalopram, FOX: fluoxetine, HDS-17: 17 item-Hamilton depression rating scale, MADRS: Montgomery-Asberg depression rating scale, NR: not reported, SD: standard deviation, SE: standard error, DFP: difference from placebo, PBO: placebo, VLD: vilazodone.
2. Published randomized clinical studies
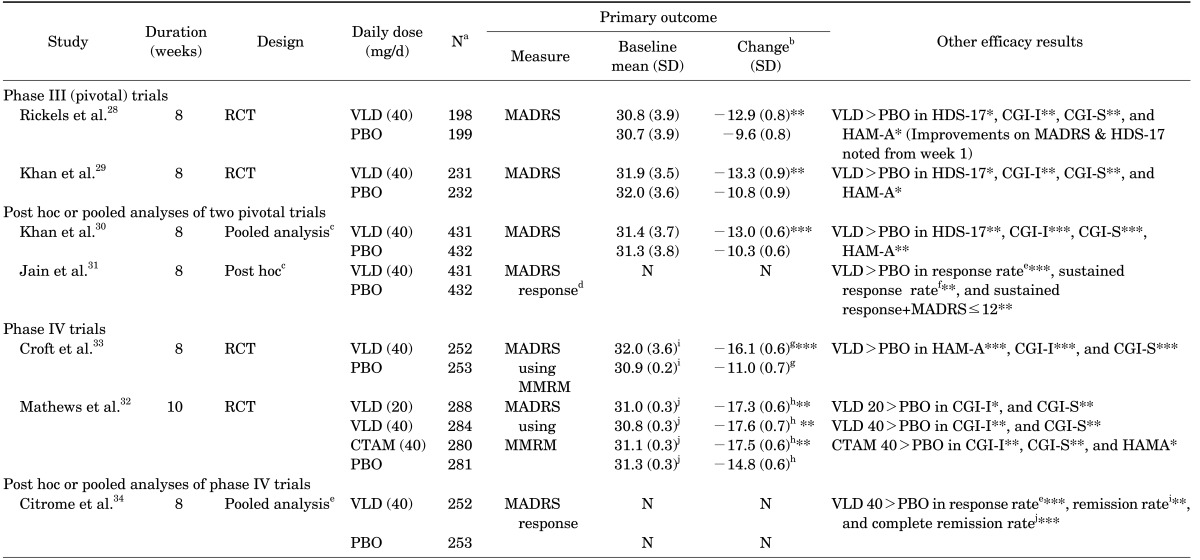
Two pivotal 8-week, randomized, double-blind, placebo-controlled phase III trials enabled approval of vilazodone in treatment of MDD.28,29 Key inclusion criteria were patients having current MDD episodes of ≥4 weeks and <2 years with HDS-17 score ≥22. In the first study by Rickel et al, vilazodone 40 mg/d showed significant, superior efficacy over a placebo in mean changes on the MADRS, HDS, Clinical global impressions-improvement scale (CGI-I), and the Clinical global impressions-severity of illness scale (CGI-S). In addition, significant differences on the MADRS and HDS were observed at week 1.28 The second study by Khan et al also showed asuperior outcome for vilazodone over the placebo in mean changes in the MADRS, HDS, CGI-I, CGI-S, and Hamilton rating scale for anxiety (HAM-A) total scores.29
Two pooled studies from the pivotal trials were conducted subsequently.30,31 The first pooled analysis aimed to assess the efficacy of vilazodone across a range of symptoms and severities of depression.30 The study, by including 431 vilazodone treated and 432 placebo treated patients (n=intention to treat population for both groups), compared the efficacy of vilazodone and a placebo. Vilazodone showed significantly greater improvement in depressive symptoms at week 8 (mean change of MADRS) than the placebo (p<0.0001). In addition, the statistically significant differences were apparent from week 1 (p<0.01, all weeks). Similar results were also noted for HDS, CGI-S, and CGI-I scores, except for HAM-A which showed significant differences at week 6. A second study retrospectively examined the timing of depressive symptom improvement of vilazodone. 31 This post hoc analysis, which contained pooled data from the two pivotal phase III trials, showed vilazodone's superior efficacy over the placebo in response rates, sustained response rates, and the rate of sustained response+MADRS <12. The significant superiority of vilazodone in cumulative response rates was evident from week 1 (p<0.05), and the time to cumulative response was quicker in the vilazodone group than the placebo group (p<0.0001).
Two phase IV clinical trials repeated and extended the efficacy and safety of vilazodone in patients with MDD. Mathews et al.32 conducted a phase IV study to fulfill a post-marketing commitment with the FDA to identify the minimum effective dose of vilazodone. In order to so, the study investigated the efficacy, safety, and tolerability of vilazodone 20 and 40 mg/d in patients with MDD while including citalopram as an active control for assay sensitivity. A total of 1,133 patients with MDD were randomized for vilazodone 20, 40 mg/d, citalopram 40 mg/d, or placebo in a 1:1:1:1 ratio. The results showed that MADRS (Primary efficacy) and CGI-S (Secondary efficacy) changes from baseline to week 10 were significantly greater for vilazodone 20 mg/d, vilazodone 40 mg/d, and citalopram than for the placebo. Croft et al.33 conducted an 8-week Phase IV RCT to further characterize the efficacy, safety, and tolerability of vilazodone 40 mg/d versus placebo. Mean difference changes from baseline to week 8 was statistically significant for vilazodone versus placebo on MADRS and CGI-S (for both p<.00001). Moreover, the statistical significant change versus the placebo occurred at week 2 and persisted for the study duration. The MADRS sustained response rate was also higher in vilazodone then in the placebo (p<.01).
Citrome et al. conducted post-hoc analyses of the phase IV trial by Croft et al. to provide additional information about the effects of vilazodone.34 The primary efficacy endpoint was a mean change in the MADRS total score. The results showed that significant outcomes were obtained with vilazodone versus the placebo for the MADRS response, remission, and complete remission. In addition, patients meeting remission criteria for HAM-A and CGI-S were also greater for the vilazodone group than for the placebo group. The study suggested that vilazodone at 40 mg/d may help patients with MDD to achieve remission of depression and/or anxiety symptoms. Please see Table 3 for summary of published clinical trials of vilazodone in the treatment of MDD.
TABLE 3. Summary of published clinical trials of investigating efficacy of vilazodone in patients with major depressive disorder.
aNumber of total intent-to-treat patients, bLeast-square mean change from baseline to end-point (week 8), cStudies included Khan et al and Rickels et al.28,29, d≥50% reduction in baseline score at Week 8, eRate of >50% reduction in baseline MADRS total score at Week 8, fRate of ≥50% reduction in baseline MADRS total score at last two study visits, gStandard error mean, hStandard error, iTotal score of MADRS≤10 at week 8, jTotal score of MADRS≤5 at week 8.
*p<0.05, **p<0.01, ***p<0.001.
CGI-I: clinical global impressions-improvement scale, CGI-S: clinical global impressions-severity of illness scale, HAM-A: Hamilton rating scale for anxiety, HDS-17: 17-item Hamilton rating scale for depression, MADRS: Montgomery-Asberg depression rating scale, N: not applicable, RCT: randomized double-blind placebo controlled trials, PBO: placebo, SD: standard deviation, SE: standard error, VLD: vilazodone.
3. Other published studies
Long-term efficacy of vilazodone for MDD were investigated in a 1-year, open-label, multicenter trial containing 599 MDD safety population with a HDS-17 score ≥18.35 254 (41.2%) patients completed the entire study. The mean MADRS total scores continuously lowered from 29.9 at baseline to 11.4 at week 8. The MADRS total scores were further improved to 7.1 by week 52, but statistical significance analysis was not provided.
A claims database study compared the use of healthcare resources and healthcare costs among vilazodone treated MDD patients with that other SSRIs treated MDD patients. The study cohort included 49,861 patients (vilazodone=3,527, citalopram=12,187, escitalopram=8,275, fluoxetine=10,142, paroxetine=3,146, and sertraline=12,584). The results showed that all-cause inpatient hospital visits, duration of hospital stays, and frequency of emergency department visits were significantly lower for patients taking vilazodone than for patients taking other SSRIs including citalopram, escitalopram, fluoxetine, paroxetine, and sertraline. In addition, all-cause medical service costs were significantly lower for vilazodone ($758) than for all other SSRI cohorts ($1,165) (p<0.05).
A meta-analysis evaluating the efficacy and safety of vilazodone in MDD was published very recently.36 The meta-analysis, by including 4 RCTs with MDD28,29,32,33 and 1 with GAD,37 contained a total of 2393 patients with MDD (Vilazodone=1,200, Placebo=1,193). The authors chose to use a fixed-effect model because no significant heterogeneity was detected across the 5 studies (p>0.05 for all). The results showed that the pooled estimates for the standardized mean difference were −3.58, −0.33, −0.54, and −1.64 (95% CI −4.59 to −2.56, −0.41 to −0.25, −0.62 to −0.46, and −2.07 to −1.22, respectively; p<0.00001 for all) for MADRS, CGI-S, CGI-I, and HAM-A respectively. Thus, the study suggested that compared with placebo, vilazodone showed dramatically greater reductions in MADRS, CGI-S, CGI-I, and HAM-A scores.
SAFETY AND TOLERABILITY
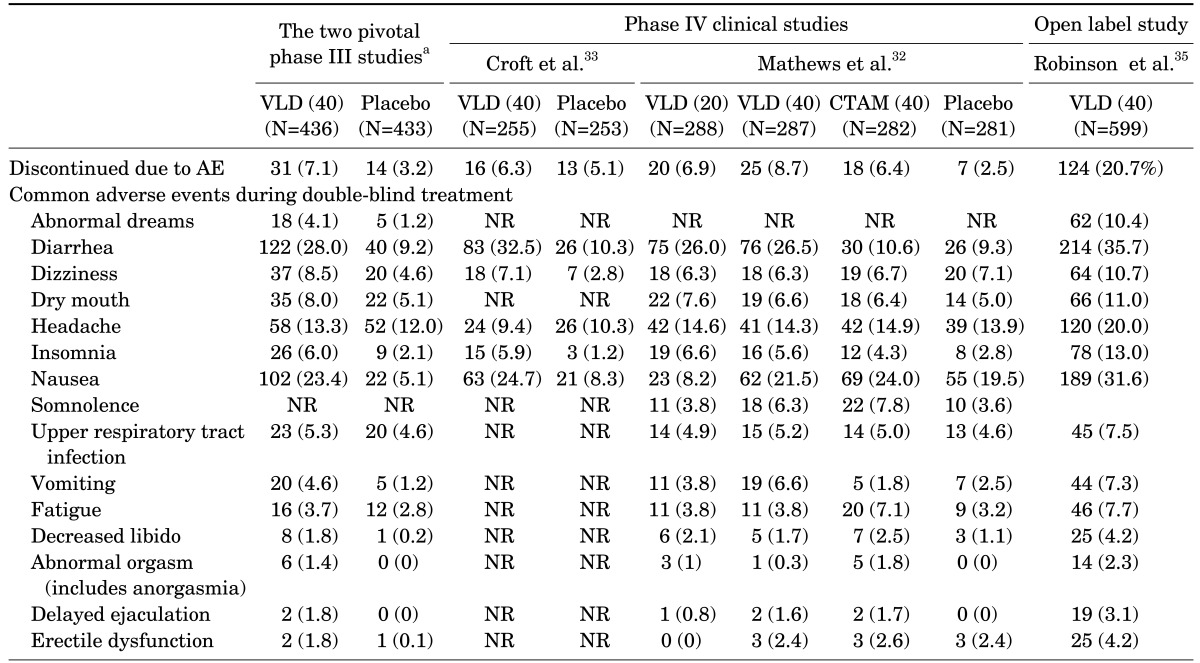
The most frequent adverse events (AE), although varied from study to study, included diarrhea, nausea, vomiting, headache, and insomnia. After pooling the two pivotal 8-week RCTS together,28,29 the discontinuation rates due to AEs were shown to be 7.1% and 3.2%, respectively.38 the number needed to treat (NNT) values vs. placebo for response and remission were 8 (95% CI 6-16) and 14 (95% CI 8-55) respectively. Number needed hard (NNH) values vs. placebo for frequently observed AEs such as diarrhea, nausea, vomiting and insomnia were 6 (95% Confidence interval, CI 5-8), 6 (95% CI 5-8), 30 (95% CI 18-82) and 26 (95% CI 16-78), respectively. However, the majority of AEs were mild to moderate in their intensities indicating that vilazodone is generally safe and well tolerated (Table 4). In addition, an open-label, multicenter study documented that vilazodone 40 mg/day for 1 year was safe and well tolerated in MDD patients. Only one published RCT compared the safety and tolerability of vilazodone with that of another SSRI.32 The results showed generally comparable rates of discontinuation for vilazodone with citalopram: 6.4% for citalopram, 6.9% for vilazodone 20 mg/d, 8.7% for vilazodone 40 mg/d, and 2.5% for placebo (no statistical analysis was presented).
TABLE 4. Summary of treatment-emergent adverse events of two randomized controlled studies in patients with MDD.
aData adopted from reference L. Citrome.38
NR: nor reported, VLD: vilazodone.
A similar trend was found in a meta-analysis which included 4 RCTs with MDD and 1 RCT with GAD.36 The study showed that incidences of diarrhea (OR 3.54, 95% CI 2.84-4.45; p<0.00001) and nausea (OR 3.85, 95% CI 3.00-4.96; p<0.00001), were higher in the vilazodone group than in the placebo group. In addition, discontinuation due to AEs was significantly higher with vilazodone then the placebo (OR 2.71, 95% CI 1.81-4.05; p<0.00001). The study added that vilazodone-related diarrhea and nausea were mild or moderate in intensity, and only a very few led to premature discontinuations. The incidence of abnormal laboratory or vital sign changes was low in the vilazodone treated group which was similar to that of the placebo treated group. Thus, the study concluded that treatment with vilazodone was overall well tolerated.
Vilazodone's postsynaptic 5-HT1A receptor actions were speculated to lower the risk of causing sexual side effects and improve depressive symptoms caused from the sexual dysfunctions.38,39,40,41 Whether or not vilazodone has an advantage over other SSRIs in terms of sexual dysfunction has long been a center of debate. Clayton et al.,42 investigated vilazodone's effects on sexual function in depressed patients by summoning data from the two pivotal phase III RCTS and one open label long-term study.28,29,35 Arizona Sexual Experience Scale (ASEX) or Changes in Sexual Functioning Questionnaire (CSFQ) were used to assess sexual dysfunction. Although changes from baseline to end of treatment for ASEX and CSFQ did not statistically differ between vilazodone and placebo treated groups, significantly more patients on vilazodone (8.0%) reported at least one sexual-function-related TAEs the placebo (0.9%) group (p<0.001). Mathews et al reported that vilazodone (20 and 40 mg/day) groups had greater improvement on the CSFQ relative to citalopram 40 mg/day and the placebo, but the differences were not statistically significant.32
Insomnia, another important frequently observed AE of vilazodone, was investigated by analyzing electroencephalogram (EEG) patterns in a randomized crossover study.43 The study included 10 healthy young men who received either a single dose of vilazodone 20 mg or a placebo. The vilazodone group exhibited significantly less total sleep time which was significant compared to the placebo group (p<0.01). Rapid eye movement almost totally disappeared in the vilazodone group. These findings suggest that sleep disturbances could happen even at a lower than therapeutic dosage of vilazodone.
BEYOND DEPRESSION: VILAZODONE'S EVIDENCE IN ANXIETY DISORDER
1. Clinical efficacy
Both 5-HT1A partial agonist, buspirone, and SSRI have proven efficacy for treating anxiety disorders, especially generalized anxiety disorder (GAD).44,45 Due to vilazodone's dual mechanisms of action, both of which have been found useful in treating GAD, it has been long been speculated that vilazodone could be effective in patients with GAD.25
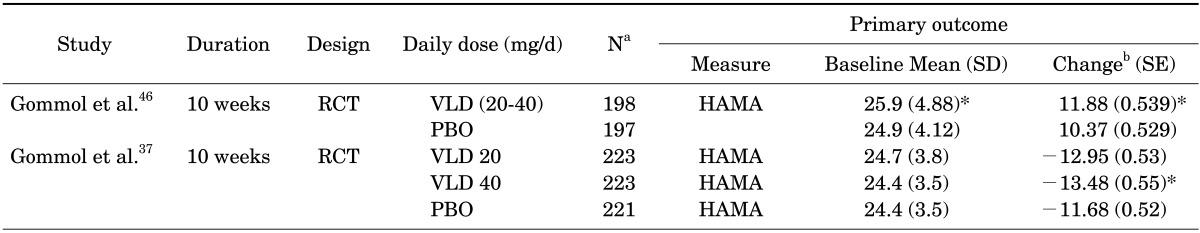
Two studies were recently conducted to verify this important clinical speculation. The first study conducted by Gommoll et al., investigated the efficacy, safety, and tolerability of vilazodone for generalized anxiety disorder in 395 patients [intent-to-treat analysis (ITT), vilazodone n=198 and placebo n=197].46 Participants (18-70 years) meeting the Diagnostic and Statistical Manual of Mental Disorders, 4th ed., text revision, criteria for GAD were equally randomized to placebo or flexible-dose vilazodone (20-40 mg/day) groups. The total duration of the study was 10 weeks, which consisted of a 1-week screening, an 8-week double-blind treatment period, and a 1-week double-blind down-taper period. Vilazodone showed significant superior efficacy over the placebo in mean changes on HAM-A from baseline to the end of double-blind treatment (week 8).
The second study comprised of 667 patients with GAD [(ITT), n=223 for vilazodone 20 mg/day, n=223 for vilazodone 40 mg/day, n=221 for placebo].37 The results showed that vilazodone 40 mg/d group, but not 20 mg/d group, had significant superior efficacy over placebo group in mean changes on HAM-A from baseline to the end of double-blind treatment (week 8). The outcome summary of these clinical studies is presented in Table 5.
TABLE 5. Summary of published (Phase III) clinical trials investigating efficacy of vilazodone in patient with generalized anxiety disorder.
aNumber of total intent-to-treat patients, bBased on mean change from baseline to end-of-double blind phase (Week 8) using mixed-effects model for repeated measures.
*p<0.05.
HAM-A: Hamilton rating scale for anxiety, ITT: intent to treat, RCT: randomized double-blind placebo controlled trials, PBO: placebo, SD: standard deviation, SE: standard error, VLD: vilazodone.
2. Safety and tolerability
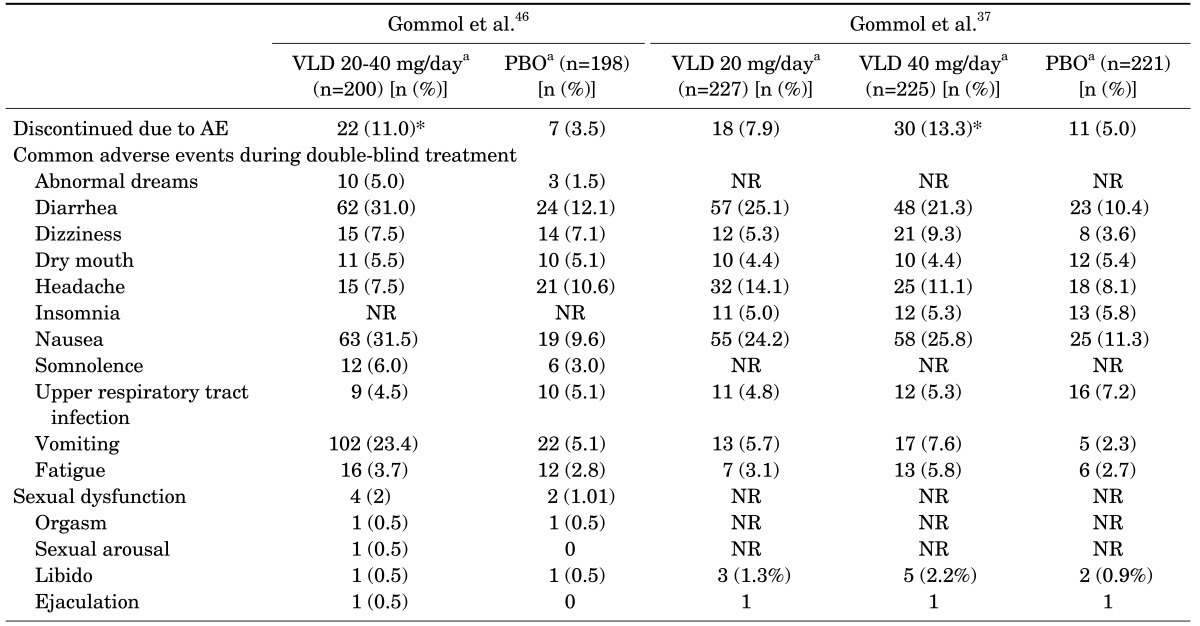
In accordance with studies conducted in MDD patients, the most frequent overall AEs in GAD were diarrhea, nausea, vomiting, and headache. The majority of the AEs were mild to moderate in their intensities (Table 6). In the first study, more vilazodone-treated patients (83%) than placebo-treated patients (60%) reported treatment-emergent AEs (TEAEs), and the incidence of discontinuation due to AEs was also significantly higher for the vilazodone group than for the placebo group.46 TEAS associated with sexual function were reported by 4 patients in the vilazodone group and 2 patients in placebo group. All sexual function-related TEAEs were mild or moderate in intensities, and none discontinued treatment due to sexual dysfunction. A similar trend was observed in the second study.37 The frequency of discontinuation due to AEs was higher for vilazodone 40 mg/d than for the placebo (p<.05). Sexual function related AEs were also more common in vilazodone- than placebo-treated patients, but discontinuation due to sexual dysfunction were observed equally throughout the three groups (n=1 for vilazodone 20 mg/day, 40 mg/day, placebo). Most sexual function AEs were considered mild or moderate.
TABLE 6. Summary of treatment-emergent adverse events of two randomized controlled studies in patients with generalized anxiety disorder.
aSafey population total intent-to-treat patients.
*p<0.05.
PBO: placebo, NR: nor reported, VLD: vilazodone.
EXPERT OPINION
Vilazodone, a novel antidepressant having dual mechanism action on 5-HT reuptake inhibition and 5-HT1A partial agonist, has extended treatment options for MDD. Although all of the phase II trials did not distinguish vilazodone from the placebo in treatment of MDD, its efficacy in depression was later well documented in 4 RCTs (2 Phase III and Phase IV RCTs), 3 post-hoc or pooled analysis, and 1 long-term open label study. Moreover, a meta-analysis indicated vilazodone's superior efficacy over placebo in MADRS, CGI-S, CGI-I, and HAM-A scores in patients with MDD. Likewise, 4 RCTs (2 Phase III and Phase IV RCTs), 1 long-term open label study, and 1 meta-analysis demonstrated vilazodone's safety and tolerability. However, AEs such as diarrhea, nausea, headache, dizziness, dry mouth, and insomnia have been consistently noted throughout the clinical studies rendering attention in clinical practice. the superiority of vilazodone over other antidepressants has yet to be shown through head-to-head studies. Yet, vilazodone has been speculated to have three potential benefits owning to its SPARI properties, which includes faster onset of action, greater efficacy, and better tolerability, and in particular lower sexual side effects. Two pivotal trials, a pooled analysis, and a post-hoc study indeed suggested depressive symptoms could be improved within 1 week after vilazodone administration. In addition, two phase IV trials have also shown that statistically significant improvement versus placebo occurred early in the trial, starting at week 2. However, no direct head-to-head studies have yet confirmed this putative benefit. Augmenting non-antidepressants having 5-HT1A partial agonist (i.e.: atypical antipsychotics and buspirone) have long been one of the treatment options in patients showing partial or inadequate response to current antidepressants.3,47,48 In this respect, vilazodone could be expected to have greater efficacy in patients with treatment-refractory depression (TRD) without increasing the AE burden due to polypharmacy.41 Studies comparing the efficacy and tolerability of vilazodone with that of other conventional antidepressants (SSRI and SNRI) should be conducted to shed light on this important issue. Nevertheless, the fact that all-cause inpatient hospital visits, duration of hospital stays, and frequency of emergency department visits were significantly lower for patients taking vilazodone than for patients taking other SSRIs in a claims database study might suggest its greater efficacy over other conventional antidepressants. It is still unclear whether vilazodone has lower risk of causing sexual side effects than conventional antidepressants. Antidepressant-induced sexual dysfunctions are known to increase with prolonged antidepressant use.49,50 Likewise, the rate of sexual dysfunction was higher in the 1-year open label study (around 16%) than other short-term RCTs (3-7%). Thus, head-to-head to trials comparing rate of sexual dysfunction between vilazdone with other conventional antidepressants having longer follow-up periods (>1 year) could be more appropriate.
Two phase III RCTs documented the efficacy and safety of vilazodone in patients with generalized anxiety disorder. The results could be the start of broadening vilazodone's usage or FDA approval in diverse anxiety disorders. In this perspective, vilazodone may be effective in patients with other anxiety disorders, and this speculation should also be established with future studies.
In conclusion, vilazodone is an effective and safe treatment option having novel mechanisms of action in patients with MDD. Relapse prevention effects and long-term efficacy of vilazodone should be established in adequately powered, large RCTs in the near future. More importantly, vilazodone's efficacy and tolerability should be compared with SSRI+buspirone in well controlled clinical trials.
Footnotes
CONFLICT OF INTEREST STATEMENT: This study was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI12C0003).
References
- 1.Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen HU. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21:169–184. doi: 10.1002/mpr.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kulkarni SK, Dhir A. Current investigational drugs for major depression. Expert Opin Investig Drugs. 2009;18:767–788. doi: 10.1517/13543780902880850. [DOI] [PubMed] [Google Scholar]
- 3.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 4.Serretti A, Chiesa A. Treatment-emergent sexual dysfunction related to antidepressants: a meta-analysis. J Clin Psychopharmacol. 2009;29:259–266. doi: 10.1097/JCP.0b013e3181a5233f. [DOI] [PubMed] [Google Scholar]
- 5.Kim JM, Hong JP, Kim SD, Kang HJ, Lee YS. Development of a Korean Version of the Perceived Deficits Questionnaire-Depression for Patients with Major Depressive Disorder. Clin Psychopharmacol Neurosci. 2016;14:26–32. doi: 10.9758/cpn.2016.14.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Massart R, Mongeau R, Lanfumey L. Beyond the monoaminergic hypothesis: neuroplasticity and epigenetic changes in a transgenic mouse model of depression. Philos Trans R Soc Lond B Biol Sci. 2012;367:2485–2494. doi: 10.1098/rstb.2012.0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Artigas F. Serotonin receptors involved in antidepressant effects. Pharmacol Ther. 2013;137:119–131. doi: 10.1016/j.pharmthera.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Wang SM, Han C, Lee SJ, Patkar AA, Masand PS, Pae CU. Five potential therapeutic agents as antidepressants: a brief review and future directions. Expert Rev Neurother. 2015;15:1015–1029. doi: 10.1586/14737175.2015.1071192. [DOI] [PubMed] [Google Scholar]
- 9.Forest Pharmaceuticals I. Viibryd (Vilazodone) Prescribing Information. [updated April 201120 July 2012]. Available from: http://www.frx.com/pi/viibryd_pi.pdf.
- 10.Hughes ZA, Starr KR, Langmead CJ, Hill M, Bartoszyk GD, Hagan JJ, et al. Neurochemical evaluation of the novel 5-HT1A receptor partial agonist/serotonin reuptake inhibitor, vilazodone. Eur J Pharmacol. 2005;510:49–57. doi: 10.1016/j.ejphar.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 11.Boinpally R, Henry D, Gupta S, Edwards J, Longstreth J, Periclou A. Pharmacokinetics and Safety of Vilazodone in Hepatic Impairment. Am J Ther. 2015;22:269–277. doi: 10.1097/MJT.0000000000000173. [DOI] [PubMed] [Google Scholar]
- 12.Boinpally R, Alcorn H, Adams MH, Longstreth J, Edwards J. Pharmacokinetics of vilazodone in patients with mild or moderate renal impairment. Clin Drug Investig. 2013;33:199–206. doi: 10.1007/s40261-013-0061-5. [DOI] [PubMed] [Google Scholar]
- 13.Administration. UFaD. Vilazodone Drug Approval Package. [updated 1 March 2011; cited 2012 20 July]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/022567Orig1s000TOC.cfm.
- 14.Stahl SM. Mechanism of action of the SPARI vilazodone: serotonin 1A partial agonist and reuptake inhibitor. CNS Spectr. 2014;19:105–109. doi: 10.1017/S1092852914000169. [DOI] [PubMed] [Google Scholar]
- 15.Sprouse JS, Aghajanian GK. Electrophysiological responses of serotoninergic dorsal raphe neurons to 5-HT1A and 5-HT1B agonists. Synapse. 1987;1:3–9. doi: 10.1002/syn.890010103. [DOI] [PubMed] [Google Scholar]
- 16.Casanovas JM, Lésourd M, Artigas F. The effect of the selective 5-HT1A agonists alnespirone (S-20499) and 8-OH-DPAT on extracellular 5-hydroxytryptamine in different regions of rat brain. Br J Pharmacol. 1997;122:733–741. doi: 10.1038/sj.bjp.0701420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lanfumey L, Hamon M. Central 5-HT(1A) receptors: regional distribution and functional characteristics. Nucl Med Biol. 2000;27:429–435. doi: 10.1016/s0969-8051(00)00107-4. [DOI] [PubMed] [Google Scholar]
- 18.Blier P, Ward NM. Is there a role for 5-HT1A agonists in the treatment of depression? Biol Psychiatry. 2003;53:193–203. doi: 10.1016/s0006-3223(02)01643-8. [DOI] [PubMed] [Google Scholar]
- 19.Celada P, Puig M, Amargós-Bosch M, Adell A, Artigas F. The therapeutic role of 5-HT1A and 5-HT2A receptors in depression. J Psychiatry Neurosci. 2004;29:252–265. [PMC free article] [PubMed] [Google Scholar]
- 20.Starr KR, Price GW, Watson JM, Atkinson PJ, Arban R, Melotto S, et al. SB-649915-B, a novel 5-HT1A/B autoreceptor antagonist and serotonin reuptake inhibitor, is anxiolytic and displays fast onset activity in the rat high light social interaction test. Neuropsychopharmacology. 2007;32:2163–2172. doi: 10.1038/sj.npp.1301341. [DOI] [PubMed] [Google Scholar]
- 21.Hughes ZA, Starr KR, Scott CM, Newson MJ, Sharp T, Watson JM, et al. Simultaneous blockade of 5-HT1A/B receptors and 5-HT transporters results in acute increases in extracellular 5-HT in both rats and guinea pigs: in vivo characterization of the novel 5-HT1A/B receptor antagonist/5-HT transport inhibitor SB-649-915-B. Psychopharmacology (Berl) 2007;192:121–133. doi: 10.1007/s00213-006-0691-x. [DOI] [PubMed] [Google Scholar]
- 22.Duxon MS, Starr KR, Upton N. Latency to paroxetine-induced anxiolysis in the rat is reduced by co-administration of the 5-HT(1A) receptor antagonist WAY100635. Br J Pharmacol. 2000;130:1713–1719. doi: 10.1038/sj.bjp.0703496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hogg S, Dalvi A. Acceleration of onset of action in schedule-induced polydipsia: combinations of SSRI and 5-HT1A and 5-HT1B receptor antagonists. Pharmacol Biochem Behav. 2004;77:69–75. doi: 10.1016/j.pbb.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 24.Page ME, Cryan JF, Sullivan A, Dalvi A, Saucy B, Manning DR, et al. Behavioral and neurochemical effects of 5-(4-[4-(5-Cyano-3-indolyl)-butyl)-butyl]-1-piperazinyl)-benzofuran-2-carboxamide (EMD 68843): a combined selective inhibitor of serotonin reuptake and 5-hydroxytryptamine(1A) receptor partial agonist. J Pharmacol Exp Ther. 2002;302:1220–1227. doi: 10.1124/jpet.102.034280. [DOI] [PubMed] [Google Scholar]
- 25.Wang SM, Han C, Lee SJ, Patkar AA, Masand PS, Pae CU. Vilazodone for the treatment of major depressive disorder: focusing on its clinical studies and mechanism of action. Psychiatry Investig. 2015;12:155–163. doi: 10.4306/pi.2015.12.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hellerstein DJ, Flaxer J. Vilazodone for the treatment of major depressive disorder: an evidence-based review of its place in therapy. Core Evid. 2015;10:49–62. doi: 10.2147/CE.S54075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laughren TP, Gobburu J, Temple RJ, Unger EF, Bhattaram A, Dinh PV, et al. Vilazodone: clinical basis for the US Food and Drug Administration's approval of a new antidepressant. J Clin Psychiatry. 2011;729:1166–1173. doi: 10.4088/JCP.11r06984. [DOI] [PubMed] [Google Scholar]
- 28.Rickels K, Athanasiou M, Robinson DS, Gibertini M, Whalen H, Reed CR. Evidence for efficacy and tolerability of vilazodone in the treatment of major depressive disorder: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2009;70:326–333. doi: 10.4088/jcp.08m04637. [DOI] [PubMed] [Google Scholar]
- 29.Khan A, Cutler AJ, Kajdasz DK, Gallipoli S, Athanasiou M, Robinson DS, et al. A randomized, double-blind, placebo-controlled, 8-week study of vilazodone, a serotonergic agent for the treatment of major depressive disorder. J Clin Psychiatry. 2011;72:441–447. doi: 10.4088/JCP.10m06596. [DOI] [PubMed] [Google Scholar]
- 30.Khan A, Sambunaris A, Edwards J, Ruth A, Robinson DS. Vilazodone in the treatment of major depressive disorder: efficacy across symptoms and severity of depression. Int Clin Psychopharmacol. 2014;29:86–92. doi: 10.1097/YIC.0000000000000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jain R, Chen D, Edwards J, Mathews M. Early and sustained improvement with vilazodone in adult patients with major depressive disorder: post hoc analyses of two phase III trials. Curr Med Res Opin. 2014;30:263–270. doi: 10.1185/03007995.2013.855188. [DOI] [PubMed] [Google Scholar]
- 32.Mathews M, Gommoll C, Chen D, Nunez R, Khan A. Efficacy and safety of vilazodone 20 and 40 mg in major depressive disorder: a randomized, double-blind, placebo-controlled trial. Int Clin Psychopharmacol. 2015;30:67–74. doi: 10.1097/YIC.0000000000000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Croft HA, Pomara N, Gommoll C, Chen D, Nunez R, Mathews M. Efficacy and safety of vilazodone in major depressive disorder: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2014;75:e1291–e1298. doi: 10.4088/JCP.14m08992. [DOI] [PubMed] [Google Scholar]
- 34.Citrome L, Gommoll CP, Tang X, Nunez R, Mathews M. Evaluating the efficacy of vilazodone in achieving remission in patients with major depressive disorder: post-hoc analyses of a phase IV trial. Int Clin Psychopharmacol. 2015;30:75–81. doi: 10.1097/YIC.0000000000000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robinson DS, Kajdasz DK, Gallipoli S, Whalen H, Wamil A, Reed CR. A 1-year, open-label study assessing the safety and tolerability of vilazodone in patients with major depressive disorder. J Clin Psychopharmacol. 2011;31:643–646. doi: 10.1097/JCP.0b013e31822c6741. [DOI] [PubMed] [Google Scholar]
- 36.Zhang XF, Wu L, Wan DJ, Liu RZ, Dong Z, Chen M, et al. Evaluation of the efficacy and safety of vilazodone for treating major depressive disorder. Neuropsychiatr Dis Treat. 2015;11:1957–1965. doi: 10.2147/NDT.S87968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gommoll C, Durgam S, Mathews M, Forero G, Nunez R, Tang X, et al. A double-blind, randomized, placebo-controlled, fixed-dose phase III study of vilazodone in patients with generalized anxiety disorder. Depress Anxiety. 2015;32:451–459. doi: 10.1002/da.22365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Citrome L. Vilazodone for major depressive disorder: a systematic review of the efficacy and safety profile for this newly approved antidepressant - what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Pract. 2012;66:356–368. doi: 10.1111/j.1742-1241.2011.02885.x. [DOI] [PubMed] [Google Scholar]
- 39.Le Poul E, Laaris N, Doucet E, Laporte AM, Hamon M, Lanfumey L. Early desensitization of somato-dendritic 5-HT1A autoreceptors in rats treated with fluoxetine or paroxetine. Naunyn Schmiedebergs Arch Pharmacol. 1995;352:141–148. doi: 10.1007/BF00176767. [DOI] [PubMed] [Google Scholar]
- 40.Li Q, Muma NA, Battaglia G, Van de Kar LD. A desensitization of hypothalamic 5-HT1A receptors by repeated injections of paroxetine: reduction in the levels of G(i) and G(o) proteins and neuroendocrine responses, but not in the density of 5-HT1A receptors. J Pharmacol Exp Ther. 1997;282:1581–1590. [PubMed] [Google Scholar]
- 41.Wang SM, Han C, Lee SJ, Patkar AA, Masand PS, Pae CU. A review of current evidence for vilazodone in major depressive disorder. Int J Psychiatry Clin Pract. 2013;17:160–169. doi: 10.3109/13651501.2013.794245. [DOI] [PubMed] [Google Scholar]
- 42.Clayton AH, Kennedy SH, Edwards JB, Gallipoli S, Reed CR. The effect of vilazodone on sexual function during the treatment of major depressive disorder. J Sex Med. 2013;10:2465–2476. doi: 10.1111/jsm.12004. [DOI] [PubMed] [Google Scholar]
- 43.Murck H, Frieboes RM, Antonijevic IA, Steiger A. Distinct temporal pattern of the effects of the combined serotonin-reuptake inhibitor and 5-HT1A agonist EMD 68843 on the sleep EEG in healthy men. Psychopharmacology (Berl) 2001;155:187–192. doi: 10.1007/s002130100703. [DOI] [PubMed] [Google Scholar]
- 44.Loane C, Politis M. Buspirone: what is it all about? Brain Res. 2012;1461:111–118. doi: 10.1016/j.brainres.2012.04.032. [DOI] [PubMed] [Google Scholar]
- 45.Hoge EA, Ivkovic A, Fricchione GL. Generalized anxiety disorder: diagnosis and treatment. BMJ. 2012;345:e7500. doi: 10.1136/bmj.e7500. [DOI] [PubMed] [Google Scholar]
- 46.Gommoll C, Forero G, Mathews M, Nunez R, Tang X, Durgam S, et al. Vilazodone in patients with generalized anxiety disorder: a double-blind, randomized, placebo-controlled, flexible-dose study. Int Clin Psychopharmacol. 2015;30:297–306. doi: 10.1097/YIC.0000000000000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rush AJ, Warden D, Wisniewski SR, Fava M, Trivedi MH, Gaynes BN, et al. STAR*D: revising conventional wisdom. CNS Drugs. 2009;23:627–647. doi: 10.2165/00023210-200923080-00001. [DOI] [PubMed] [Google Scholar]
- 48.Bouwer C, Stein DJ. Buspirone is an effective augmenting agent of serotonin selective re-uptake inhibitors in severe treatment-refractory depression. S Afr Med J. 1997;87(4 Suppl):534–537. 540. [PubMed] [Google Scholar]
- 49.Segraves RT. Sexual dysfunction associated with antidepressant therapy. Urol Clin North Am. 2007;34:575–579. doi: 10.1016/j.ucl.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 50.Segraves RT, Balon R. Antidepressant-induced sexual dysfunction in men. Pharmacol Biochem Behav. 2014;121:132–137. doi: 10.1016/j.pbb.2013.11.003. [DOI] [PubMed] [Google Scholar]