Summary
Immune checkpoints are crucial for the maintenance of self-tolerance and for the modulation of immune responses in order to minimize tissue damage. Tumor cells take advantage of these mechanisms to evade immune recognition. A significant proportion of tumors, including breast cancers, can express co-inhibitory molecules that are important formediating the escape from T cell-mediated immune surveillance. The interaction of inhibitory receptors with their ligands can be blocked by specific molecules. Monoclonal antibodies (mAbs) directed against the cytotoxic T lymphocyte-associated antigen-4 (CTLA4) and, more recently, against the programmed cell death protein 1 (PD1), have been approved for the therapy of melanoma (anti-CTLA4 and anti-PD1 mAbs) and non-small cell lung cancer (anti-PD1 mAbs). Moreover, inhibition of PD1 signaling has shown extremely promising signs of activity in breast cancer. An increasing number of molecules directed against other immune checkpoints are currently under clinical development. In this review, we summarize the evidence supporting the implementation of checkpoint inhibition in breast cancer by reviewing in detail data on PD-L1 expression and its regulation. In addition, opportunities to boost anti-tumor immunity in breast cancer with checkpoint inhibitor-based immunotherapies alone and in combination with other treatment options will be discussed.
Keywords: Antibody therapy, Biomarker, Breast cancer, Immune system, Immunomodulation, Immunotherapy, Tumor marker
Introduction
Due to the recent advances in technologies for high-throughput analyses, molecular mechanisms responsible for T cell-mediated cancer elimination have been elucidated [1,2,3]. For their proper activation, T cells require 2 signals regulating T cell survival, proliferation, and/or responsiveness to antigens. The first signal is initiated by the T cell receptor (TCR) through antigen recognition, while the second one is mediated by an interaction between receptors and ligands of co-stimulatory and/or co-inhibitory signals, also known as immune checkpoints, which include in particular members of the B7 family [4,5]. Under physiologic conditions, there exists a balance between co-inhibitory and co-stimulatory signals, which is crucial for the maintenance of self-tolerance and immune homeostasis, thereby protecting tissues from unnecessary damage when the immune system has efficiently cleared the pathogen [6]. In tumors, immune inhibitory molecules are expressed following oncogenic transformation resulting in the attenuation of excessive immune reactions and immune resistance. T cells are able to control diverse immune responses by integrating both adaptive and innate effector mechanisms. Therefore, agonists of co-stimulatory receptors or antagonists of inhibitory receptors might lead to an amplification of antigen-specific T cell response [7,8]. A list of immune checkpoints and co-stimulatory molecules is provided in supplemental table 1 (www.karger.com/?DOI=445335). Indeed, the blockade of immune checkpoints using respective monoclonal antibodies (mAbs) has been shown to trigger efficient anti-tumor responses not only in classical ‘immunogenic’ tumor types, such as melanoma and renal cell carcinoma [9,10,11,12], but also in many other solid tumors, including lung [13], colorectal [14], ovarian [15], esophageal [16], bladder [12], and more recently, breast cancer [17,18]. In this review, the features of the best characterized checkpoints (cytotoxic T lymphocyte-associated antigen-4 (CTLA4) and programmed cell death protein 1 (PD1)) will be described. Data regarding the expression and regulation of the PD1 ligand PD-L1 in breast cancer will be discussed in detail. Finally, the state of the art of breast cancer checkpoint inhibition approaches and opportunities to increase their efficacy will be summarized.
Characteristics of the Cytotoxic T Lymphocyte-Associated Antigen-4
CTLA4 has been identified as the first immune checkpoint receptor, which counteracts the T cell co-stimulatory receptor CD28 [19]. After antigen recognition, CD28 interaction with its ligands CD80 and CD86 amplifies TCR signaling to activate T cells, which is followed by an upregulation of CTLA4 [20]. CTLA4 has a higher affinity for CD80 and CD86, thereby dampening the T cell activation and out-competing CD28 [21]. Although CTLA4 is expressed by activated CD8+ effector T cells, it is in particular important for CD4+ T cells where it modulates the T helper cell activity and enhances regulatory T cell (Treg)-mediated immune suppression [22,23]. It is noteworthy that CTLA4 can also be expressed by cancer cells. In breast cancer, a recent study reported that the presence of cytoplasmic CTLA4 dots was associated with short survival [24]. Although the significance of this expression is unclear, studies in mouse models have shown that the soluble form of CTLA4 can exert a regulatory effect on T cells [25].
The anti-CTLA4 mAb ipilimumab has been approved by the Food and Drug Association (FDA) for the treatment of metastatic and high-risk resected melanoma (in 2011 and 2015, respectively). It is broadly accepted that the mechanism of action of ipilimumab relies in the enhancement of T cell response through the inhibition of CTLA4 signaling. However, it has also been shown that ipilimumab can mediate antibody-dependent cytotoxicity of CTLA4-positive melanoma cell lines [26].
Results from phase II trials in lung cancer, in which ipilimumab was tested in combination with chemotherapy, have been less exciting [27] but still intriguing, and phase III trials are currently ongoing.
Features of Programmed Cell Death Protein 1 and Its Ligand PD-L1
In addition to anti-CTLA4, mAbs directed against PD1 and PD-L1 are emerging as important therapeutic tools in the treatment of cancer patients. These drugs are characterized by a better safety profile and more pronounced anti-tumor activity. PD1 is an immune inhibitory receptor which is expressed on activated T cells, B cells, and monocytes, but also on Tregs. Following interaction with its ligands (i.e., PD-L1 and/or PD-L2), PD1 induces T cell anergy, therefore representing an important immune escape mechanism [28,29,30]. PD-L1 is the best characterized of the 2 known PD1 ligands. It can be expressed by tumor cells as well as by T and B cells, macrophages, and dendritic cells [23,31]. The anti-PD1 mAbs nivolumab and pembrolizumab have already been approved by the FDA for the treatment of metastatic melanoma (in 2014) and non-small cell lung cancer (in 2015), while anti-PD-L1 have demonstrated similar anti-tumor activities and are currently in an effervescent stage of development [32,33,34].
Regulation of PD-L1 Expression in Breast Cancers
The regulation of PD-L1 is extremely complex. PD-L1 is an interferon (IFN)-stimulated gene, and its modulation is tightly regulated by IFN-γ. This in part explains the observed strong correlation between the level of PD-L1 and the density of tumor-infiltrating lymphocytes (TILs) [35] which upon activation secrete large amounts of IFN-γ. In fact, in breast cancer, PD-L1 transcript expression linearly correlates with that of IFN-γ and other inflammatory genes [36]. The intrinsic genetic program of tumor cells is also involved in the modulation of PD-L1, albeit with varying strength in distinct diseases. In non-Hodgkin's lymphoma, for example, amplification of chromosome 9p24 represents a recurrent genomic alteration accompanied by high expression levels of PD-L1 and PD-L2 [37]. In breast cancer, the same chromosomal amplification, which is associated with higher expression of PD1 ligands, was observed in 12 of 41 triple-negative breast cancer (TNBC) cases, but not in the estrogen receptor (ER)-positive or human epidermal growth factor receptor 2 (HER2)-positive mammary carcinoma tissues [38]. Another study has reported a higher frequency of PD-L1 amplification or gain in basal-like tumors when compared to the other subtypes [39]. In general, in breast cancer, PD-L1 transcripts correlate significantly but not heavily with copy number aberrations [40].
In a pan-cancer analysis of TCGA (The Cancer Genome Atlas) data, amplification 9p24 was associated with the degree of cytolytic activity determined as average expression of granzyme A (GZMA) and perforin (PRF1), which in turn correlated with PD-L1 expression [41]. In the tumor subtype analysis, however, the association between 9p24 amplification and cytolytic activity was only significant in stomach, head and neck, cervical, colorectal, and lung squamous tumors, but not in the other cancers such as lung adenocarcinoma, glioma, melanoma, and breast, kidney, ovarian, liver, uterine, prostate, kidney, and bladder cancer [41].
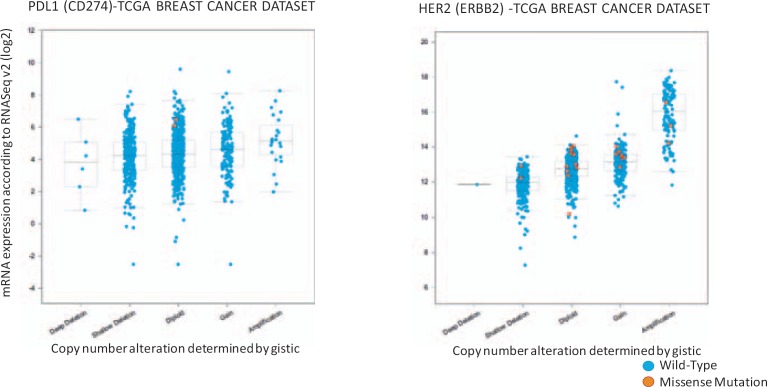
However, it should be mentioned that the interpretation of correlative results between copy number and PD-L1 is challenging. In fact, the absence of PD-L1 could make a tumor more permissive to T cell invasion. Then, IFN-γ secreted by activated lymphocytes (or natural killer cells) can induce tumor cells to express PD-L1 resulting in a lack of linearity between PD-L1 constitutional activation and ex vivo expression. Vice versa, a constitutive expression of PD-L1 (e.g., following amplification of the corresponding gene) can counteract T cell infiltration, with consequent decreased release of IFN-γ and less sustained PD-L1 expression. Therefore, even though copy number variations can influence the expression of PD-L1, their correlation with PD-L1 expression ex vivo will never be perfect. As an example, the different relationship between copy number variations and transcript levels for HER2 whose expression is largely controlled by DNA amplification, and for PD-L1 whose expression is significantly modified by micro-environmental variables, is shown in figure 1.
Fig. 1.
Copy number variation and transcript levels: ERBB2 (HER2) vs. CD274 (PD-L1). The relationship between copy number variations and transcript levels for ERB2 and CD274 in the TCGA (The Cancer Genome Atlas) breast cancer datasets is shown. Plots are generated with cBioportal (www.cbioportal.org/) [103].
Oncogenic pathways can also modulate the expression of PD-L1, adding complexity to the interpretation of correlative ex vivo studies. In lung cancer, oncogenic activation of the mTOR-AKT induces expression of PD-L1 [42]. In breast cancer, deletion of PTEN, a negative modulator of the PI3K pathway, triggers the expression of PD-L1, which is followed by diminished T cell proliferation and increased apoptosis [43].
Moreover, it has been shown that PD-L1 expression could be induced in breast cancer cells by chemotherapeutics such as paclitaxel, etoposide, and 5-fluorouracil. This promoted PD-L1-mediated T cell apoptosis, thereby demonstrating a potential link between chemotherapy and cancer immune resistance [44]. In contrast, doxorubicin treatment caused a downregulation of PD-L1 surface expression in vitro, which was also confirmed in a xenograft mouse model. Interestingly, the doxorubicin-mediated downregulation of PD-L1 surface expression was accompanied by an upregulation of PD-L1 in the nucleus. This cellular re-distribution of PD-L1 into the nuclei of breast cancer cells suggests a function of PD-L1 beyond inhibition of T cells [45].
PD-L1 Expression in Breast Cancer
PD-L1 expression has been shown in different cancers, such as kidney, lung, pancreas, esophagus, ovarian, colorectal, head and neck squamous cell carcinoma, melanoma, and glioma [35,46,47,48].
The first study on the prevalence of PD-L1 expression in breast cancer dates back to 2006 when Ghebeh et al. [49] reported that expression of this molecule, evaluated by immunohistochemistry (IHC) on either tumor cells or TILs, was present in 22/44 (50%) of the analyzed primary breast cancer samples.
Investigations into this matter have intensified in the last couple of years largely due to the enthusiasm generated by the results of PD1 blockade in multiple tumors. These studies are difficult to compare due to different cut-offs used for PD-L1 positivity (e.g., cut-off at 1 or 5%), staining evaluated on cancer cells or immune infiltrates, the kind of antibodies employed, as well as the use of different assays (IHC, gene expression profiling, or in situ RNA hybridization); however, some common findings have emerged from these reports. First, PD-L1 expression positively associates with the presence of immune-infiltrates [39,40,43,48,49,50,51,52,53,54]. Second, TNBC (or basal-like) tumors express PD-L1 more frequently than other subtypes [39,40,51,55]. The main finding of studies that assessed PD-L1 expression in breast cancer are summarized in supplemental table 2 (www.karger.com/?DOI=445335) [39,40,43,48,49,50,51,52,53,54,55,56,57].
The largest IHC evaluation assessing almost 4,000 breast cancer samples detected PD-L1 expression (cut-off at 1%) in 1.7 % of all tumors and in 19% of the 302 TNBC samples [40]. However, PD-L1 expression of TILs was present in 6% overall and in 39% of TNBCs. Similarly, Mittendorf et al. [43] reported a PD-L1 positivity rate of 19% by assessing 105 TNBCs (cut-off at 5% on tumor cell membranous staining).
In another study with 161 TNBCs, PD-L1 positivity using a 1% cut-off was even higher: 64% for tumor cell membranous staining, 80% for cytoplasmic staining, and 93% for stromal staining [52]. Even with a 5% cut-off, the proportion of PD-L1-positive tumors remains high: 60, 77, and 93%, for tumor cell membranous, cytoplasmic, and stromal staining, respectively [52]. It is possible that these discrepancies are at least in part influenced by the kind of antibody used, therefore highlighting the urgent need for harmonization procedures [58,59]. Luminal subtypes (e.g., luminal A and B) are the most prevalent breast cancer tumors. Although PD-L1 expression is not that frequent in luminal subtypes given their high prevalence, they still represent a considerable proportion of PD-L1-positive tumors (i.e., 44% of all PD-L1-positive tumors in the study by Ali et al. [40]). This subgroup of luminal PD-L1-positive patients might benefit from immunotherapy.
A transcriptomic meta-analysis of 5,454 breast cancer lesions demonstrated a highly variable frequency of PD-L1 mRNA expression [39]. Expression was most prominent in basal tumors, followed by HER2, and then luminal subtypes (supplemental table 2, www.karger.com/?DOI=445335). High PD-L1 expression levels were associated with negative prognostic features such as large tumor size, high grade, lack of ER, progesterone receptor and HER2, and high proliferative index [39]. Another study confirmed that high proliferative capacity in breast cancer cell lines is associated with higher PD-L1 expression [60]. It is noteworthy that the frequency of PD-L1 is higher in inflammatory breast cancer (IBC) when compared to non-IBC [54]. Quite interestingly, PD-L1 expression is higher in breast cancer cell lines bearing a basal-like phenotype as compared with luminal subtype [39]. In the study by Gatalica et al. [48], PD-L1expression in solid tumors was correlated with high mutational load of the tumor suppressor gene TP53, while no association between TP53 and PD-L1 expression was observed in breast cancer by Ali et al. [40].
Recently, PD-L1 expression was detected in metastatic tumor cells circulating in the blood of hormone receptor-positive, HER2-negative breast cancer patients [61]. Thus, PD-L1 expression of circulating tumors cells might be used for stratification and monitoring of tumor patients undergoing immune checkpoint blockade using liquid biopsy.
PD-L1 Expression in Breast Cancer and Clinical Relevance
A multitude of studies across solid tumors has shown that tumors displaying a T helper 1 polarization respond better to immunotherapy and are associated with a better prognosis [2,62,63,64,65]. These tumors are characterized by the activation of specific molecular pathways that are also found in other forms of immune-mediated rejection, such as allograft rejection or flares of autoimmunity [66,67]. We refer to them as the Immunologic Constant of Rejection (ICR) [2,68,69,70,71]. These pathways comprise the IFN-stimulated gene pathway (centered on IRF1 and STAT1), CXCR3 and CCR5 ligand pathways (e.g., CXCL9-11 and CCL3-5), and immune effector function genes (e.g., perforin, granulysin) [2,69]. As discussed in detail elsewhere [36], various predictive and prognostic immune-related signatures described in breast cancer are centered on the ICR pathways. In breast cancer, the activation of the ICR pathways has been associated with prolonged survival and response to neoadjuvant chemotherapy [72,73,74,75,76] or adjuvant anti-HER2 therapy [77]. However, the prognostic value of immune gene signatures is influenced by intrinsic molecular subtypes and proliferative capacity [36,78,79].
It is tempting to speculate that the prognostic role of the ICR pathways resides in their ability to describe an intra-tumor immune response that can slow down tumor growth or counteract metastatic processes [2]. Their predictive role in the context of immunotherapy could rely on the ability to capture a more permissive cancer phenotype in which immune manipulations might more easily trigger the development of an acute anti-tumor inflammatory process. As for the prediction of response to conventional therapy, it has been proposed that the presence of a subacute inflammatory status can facilitate tumor clearance following the induction of chemotherapy-mediated immunologic cell death [80] or, as for anti-HER2 therapy, the enhancement of an antibody-dependent mediated cytotoxicity [81].
However, the presence of such a molecular inflammatory status is accompanied by the activation of immune-regulatory mechanisms, and a strong correlation exists between pro-inflammatory (e.g., CXCL9-11, CCL5, IRF1, and STAT1) and regulatory (e.g., CTLA4, PD1, PDL1, FOXP3, and IDO1) transcripts [36,63,64,74].
In anti-PD1 trials of melanoma and lung cancer, PD-L1 expression [9,35,82,83] as well as a high mutational load [13,84] have been invariably associated with response to checkpoint inhibitors.
In primary basal-like breast cancers or TNBCs, most of the studies, and in particular the largest ones [39,40,53], have reported a positive correlation between PD-L1 expression and favorable prognosis. However, in overall populations, a reverse correlation between PD-L1 and prognosis has been noted by some investigators, including a large study in the Chinese population [55]. As expression of PD-L1 is associated with negative prognostic features, it is possible that the prognostic role of those unfavorable variables can prevail when the analysis is not stratified according to clinicopathologic groups. Nevertheless, one relatively large study showed a reverse association between PD-L1 expression and survival in all but the luminal A subtypes [51], and the reasons for this discrepancy are not immediately clear (supplemental table 2, www.karger.com/?DOI=445335). Prospective validations of these findings are lacking although it is unlikely that PD-L1 expression alone will retain significance as a prognostic factor when confronted with other variables.
The influence of confounding variables is less strong in the therapeutic setting where expression of PD-L1, which is in turn associated with the expression of ICR genes, is correlated with responsiveness to neoadjuvant breast cancer chemotherapy [54,74]. The predictive role of PD-L1 in the metastatic setting is completely unknown.
Checkpoint Inhibitors as Novel Strategies for Breast Cancer and Opportunities to Further Boost Anti-Tumor Immune Response
Breast cancer was considered non-immunogenic for a long time, and therefore patients had limited access to immunotherapies. In the metastatic setting, vaccination strategies have shown some signs of activity [85,86], but results have been overall disappointing with low objective response (OR) rates. Adoptive therapy with TILs is extremely active in melanoma patients [87,88]. However, this approach has not yet been implemented in breast cancer due to the difficulty to generate TIL cultures with specificity against the tumor from which they are generated [89]. A phase I/IIa study in metastatic breast cancer by Domschke et al. [90] and Stefanovic et al. [91] demonstrated encouraging results in terms of immunological response, disease control, and survival by using bone marrow-derived tumor-reactive memory T cells. The investigators obtained an intriguing median overall survival (OS) of 34 months, with 3 (20%) patients alive at last follow-up and more than 7 years after treatment. Interestingly, the survival rate correlates with the immunological response in the peripheral blood. The same group is now testing this approach in combination with cyclophosphamide to counteract the response to Tregs in a phase II study (Schuetz F., personal communication; Breast Cancer Immunotherapy Symposium, Doha, Qatar, 2015).
The first study employing checkpoint inhibitors tested the anti-CTLA4 mAb tremelimumab in combination with endocrine therapy (examestane) in metastatic ER-positive patients. Unfortunately, no OR was induced by treatment although 42% of patients achieved stable disease for more than 3 months [92].
The anti-CTLA4 mAb ipilimumab is now being tested in patients with lower tumor burden. In early breast cancer, addition of ipilimumab to preoperative cryotherapy was able to induce a stronger expansion of clonal TILs as compared with either approach alone [93]. Investigators are now planning to evaluate in a follow-up randomized trial whether this expansion correlates with clinical outcome.
Based on the predictive and/or prognostic role of TILs [94,95] and immune signatures [36] in breast cancers, and in view of the striking activity of PD1 blockade across multiple tumors, this strategy has been recently investigated in mammary carcinoma.
Because TNBCs have, in general, a higher density of TILs, and considering that the prognostic role of TILs is more prominent in TNBC than in other subtypes, the efficacy of PD1 inhibition has so far been evaluated in this setting. Results from 2 studies assessing the anti-PD1 mAb pembrolizumab and the anti-PDL1 atezolizumab were recently presented.
The pembrolizumab phase Ib KEYNOTE-012 trial recruited 32 metastatic TNBC patients, most of whom had previously received at least 3 lines of chemotherapy for metastatic disease [17]. Only patients with PD-L1 staining in the stroma or in ≥ 1 % of tumor cells (evaluated by IHC) in archived samples were eligible. An extremely promising OR rate of 19 % was detected, including 1 complete and 4 partial responders.
The atezolizumab phase Ia expansion trial enrolled 54 TNBC patients [18]. Even in this case, patients were heavily pretreated (85% had received 4 or more lines of chemotherapy). In the 21 PD-L1 patients in whom efficacy was evaluable, a similar OR rate of 24% was reported, including 3 partial and 2 complete responses. In this case, tumors were considered PD-L1-positive if PD-L1 was expressed in 5% or more of the infiltrating immune cells.
Although the OR rate in breast cancer was lower than the rates obtained in PD-L1-positive melanoma (55-60%) [9,96] or lung cancer (45%) [83], this is the first time that a single immunotherapeutic agent induced tumor shrinkage (and in some cases disappearance of the tumor) in a considerable proportion of breast cancer patients. Similarly to what was observed in other tumor types, responses tend to be long-lasting, with some ongoing at the time of this report [17,18].
Importantly, both molecules were extremely well tolerated, with toxicities similar to those in other disease settings. Phase III trials are currently ongoing testing pembrolizumab alone vs. chemotherapy or atezolizumab in combination with abraxane (a new generation taxane) (supplemental table 3, www.karger.com/?DOI=445335)[85].
The combination of the anti-PD1 mAb nivolumab and ipilimumab has been demonstrated to be more effective than either strategy alone [96] in melanoma and has been recently approved by the FDA in this setting. A flurry of early combinatorial trials has been initiated to assess the activity of these and other anti-PD1/PD-L1 mAbs in multiple tumors, including breast cancer. These trials include combinations with co-stimulatory molecules (e.g., anti-OX-40 and anti-CD-27), other checkpoint inhibitors (IDO inhibitors, anti-CTLA4), p53 vaccine, anti-HER2 mAb (trastuzumab and trastuzumab emtansine (TDM1)), histone deacetylase inhibitors (etinostat, vorinostat), eribulin (a novel microtubule synamic inhibitor), PLX3397 (a novel tyrosine kinase inhibitor), poly I:C (a toll-like receptor agonist), bevacizumab (an anti-angiogenic mAb), and radiotherapy, as summarized in supplemental table 3 (www.karger.com/?DOI=445335).
An emerging approach to increase the activity of checkpoint inhibition is represented by targeting oncogenic pathways associated with immune suppression and T cell exclusion [97]. It has been recently reported that suppression of TIL recruitment or retention in breast cancer is associated with genomic alterations of Ras/MAPK [98]. This was further confirmed by i) in vitro data demonstrating that MEK inhibition upregulated MHC class I surface antigens and reduced immunosuppressive markers, and ii) a combinatorial treatment of a syngeneic mouse model of breast cancer with MEK inhibitors and anti-PD1 antibodies demonstrating synergistic effects. Based on these results, a combination of MEK inhibition and anti-PD-L1 expression might be a promising novel therapeutic approach for the treatment of this disease. In addition, we described specific MAPK mutations associated with the absence of ICR pathway activation [99].
As PTEN deletion influences the expression of PD-L1, another intriguing approach could be represented by the combination of checkpoint inhibitors with PI3K inhibitors. Studies in melanoma animal models have recently demonstrated the therapeutic efficacy of this approach [43].
Although some clinical studies are dissecting the effects of combination therapies on breast cancer immunity and therapy response, experimental models mimicking the human disease are urgently required. This has recently become possible by the modification of the well characterized transgenic BALB/c WAP-T mouse model for breast cancer with strong similarities to the corresponding human disease by an additional transgene coding for an immune-dominant T cell epitope of the nucleoprotein (WAP-TNP), which allows the monitoring of T cell responses. Using the WAP-TNP model, it could be shown that the impaired T cell response was due to PD1 expression, which could be overcome by treatment with anti-PD1 antibodies, suggesting the WAP-TNP mice to be a suitable tool to analyze parameters to overcome the blockade of immune checkpoints in breast cancer patients including combination therapies [100].
Conclusion
Until now, immune checkpoint inhibitor agents have shown promising results in the treatment of solid tumors, including breast cancer. However, further investigations are required to determine whether these co-inhibitors could be used in combination with each other or may require the use of chemotherapy or radiation [101,102], and whether the outcome would be synergistic. Additionally, the safety profile of such combinations needs to be carefully assessed. Furthermore, other immune checkpoint blockade agents should be developed to optimize the anti-tumor qualities of the immune system to enhance the immune response and abrogate the immunosuppressive microenvironment in breast cancers.
The heterogeneity of PD-L1 expression in breast cancer subtypes postulates that anti-PD1/PD-L1 agents may only be part of the solution, suggesting that additional agents are required to complement tumor cell killing. The ability to distinguish between ‘super’-responders and non-responders using biomarkers might be the key to future combinatorial regimens. The selection criteria for enrolling breast cancer patients for immune checkpoint inhibitors would not only depend on the heterogeneity of the primary tumor and subtype regarding the expression of PD-L1, but also on the putative distinct immune signature of tumor-initiating stem cells, as well as on the individual profile of tumor-infiltrating and peripheral immune cells. Therefore, the molecular characterization of breast tumors may further identify other phenotypes that could benefit from immunotherapy in addition to TNBCs.
We believe that the intensification of genomic studies assessing the genetic determinants of spontaneous or treatment-induced anti-tumor immunity combined with the optimization of pre-clinical breast models and the implementation of combinatorial strategies will lead to a significant increase in the therapeutic efficacy of immune manipulations in the near future.
Online Supplemental Tables
Supplemental Table 1. Immune checkpoints and co-stimulatory molecules and their targeting agents
Supplemental Table 2. Expression of PD-L1 in breast cancer lesions
Supplemental Table 3. Immune checkpoint agents under clinical development in breast cancer
To access the online supplemental tables, please refer to www.karger.com/?DOI=445335.
Disclosure Statement
The authors have declared that no conflict of interest exists.
Supplementary Material
Supplementary data
Acknowledgment
We would like to thank Sylvi Magdeburg and Nicole Ott for excellent secretarial help. This work was supported by the Mildred Scheel grant (Deutsche Krebshilfe 34102510; BS) and by the Sidra Medical and Research Center (Research Branch).
References
- 1.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348:69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 2.Galon J, Angell Helen K, et al. The continuum of cancer immunosurveillance: prognostic, predictive, and mechanistic signatures. Immunity. 2013;39:11–26. doi: 10.1016/j.immuni.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 3.Wang E, Bedognetti D, Tomei S, Marincola FM. Common pathways to tumor rejection. Ann N Y Acad Sci. 2013;1284:75–79. doi: 10.1111/nyas.12063. [DOI] [PubMed] [Google Scholar]
- 4.Ceeraz S, Nowak EC, Noelle RJ. B7 family checkpoint regulators in immune regulation and disease. Trends Immunol. 2013;34:556–563. doi: 10.1016/j.it.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jung K, Choi I. Emerging co-signaling networks in T cell immune regulation. Immune Netw. 2013;13:184–193. doi: 10.4110/in.2013.13.5.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maj T, Wei S, Welling T, Zou W. T cells and costimulation in cancer. Cancer J. 2013;19:473–482. doi: 10.1097/PPO.0000000000000002. [DOI] [PubMed] [Google Scholar]
- 8.Sanmamed MF, Pastor F, Rodriguez A, et al. Agonists of co-stimulation in cancer immunotherapy directed against CD137, OX40, GITR, CD27, CD28, and ICOS. Semin Oncol. 2015;42:640–655. doi: 10.1053/j.seminoncol.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 9.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 10.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24:207–212. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bracarda S, Altavilla A, Hamzaj A, et al. Immunologic checkpoints blockade in renal cell, prostate, and urothelial malignancies. Semin Oncol. 2015;42:495–505. doi: 10.1053/j.seminoncol.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ojalvo LS, Nichols PE, Jelovac D, Emens LA. Emerging immunotherapies in ovarian cancer. Discov Med. 2015;20:97–109. [PubMed] [Google Scholar]
- 16.Shah MA. Update on metastatic gastric and esophageal cancers. J Clin Oncol. 2015;33:1760–1769. doi: 10.1200/JCO.2014.60.1799. [DOI] [PubMed] [Google Scholar]
- 17.Nanda R, Chow LQ, Dees EC, et al. A phase Ib study of pembrolizumab (MK-3475) in patients with advanced triple-negative breast cancer. Cancer Res. 2015;75:abstr S1–09. [Google Scholar]
- 18.Emens LA, Braiteh FB, Cassier P, et al. Inhibition of PD-L1 by MPDL3280A leads to clinical activity in patients with metastatic triple negative breast cancer. Cancer Res. 2015;75 abstr PD1-6. [Google Scholar]
- 19.Walunas TL, Lenschow DJ, Bakker CY, et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. [PubMed] [Google Scholar]
- 20.Krummel MF, Allison JP. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J Exp Med. 1996;183:2533–2540. doi: 10.1084/jem.183.6.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Egen JG, Allison JP. Cytotoxic T lymphocyte antigen-4 accumulation in the immunological synapse is regulated by TCR signal strength. Immunity. 2002;16:23–35. doi: 10.1016/s1074-7613(01)00259-x. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi T, Tagami T, Yamazaki S, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu H, Yang J, Jiao S, et al. Cytotoxic T lymphocyte antigen 4 expression in human breast cancer: implications for prognosis. Cancer Immunol Immunother. 2015;64:853–860. doi: 10.1007/s00262-015-1696-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ward FJ, Dahal LN, Wijesekera SK, et al. The soluble isoform of CTLA-4 as a regulator of T-cell responses. Eur J Immunol. 2013;43:1274–1285. doi: 10.1002/eji.201242529. [DOI] [PubMed] [Google Scholar]
- 26.Laurent S, Queirolo P, Boero S, et al. The engagement of CTLA-4 on primary melanoma cell lines induces antibody-dependent cellular cytotoxicity and TNF-alpha production. J Transl Med. 2013;11:108. doi: 10.1186/1479-5876-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lynch TJ, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol. 2012;30:2046–2054. doi: 10.1200/JCO.2011.38.4032. [DOI] [PubMed] [Google Scholar]
- 28.Fife BT, Pauken KE, Eagar TN, et al. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat Immunol. 2009;10:1185–1192. doi: 10.1038/ni.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Francisco LM, Salinas VH, Brown KE, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Latchman Y, Wood CR, Chernova T, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 31.Sponaas AM, Moharrami NN, Feyzi E, et al. PDL1 Expression on plasma and dendritic cells in myeloma bone marrow suggests benefit of targeted anti PD1-PDL1 therapy. PLoS One. 2015;10:e0139867. doi: 10.1371/journal.pone.0139867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anagnostou VK, Brahmer JR. Cancer immunotherapy: a future paradigm shift in the treatment of non-small cell lung cancer. Clin Cancer Res. 2015;21:976–984. doi: 10.1158/1078-0432.CCR-14-1187. [DOI] [PubMed] [Google Scholar]
- 33.Adachi K, Tamada K. Immune checkpoint blockade opens an avenue of cancer immunotherapy with a potent clinical efficacy. Cancer Sci. 2015;106:945–950. doi: 10.1111/cas.12695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melero I, Grimaldi AM, Perez-Gracia JL, Ascierto PA. Clinical development of immunostimulatory monoclonal antibodies and opportunities for combination. Clin Cancer Res. 2013;19:997–1008. doi: 10.1158/1078-0432.CCR-12-2214. [DOI] [PubMed] [Google Scholar]
- 35.Taube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20:5064–5074. doi: 10.1158/1078-0432.CCR-13-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bedognetti D, Hendrickx W, Marincola FM, Miller LD. Prognostic and predictive immune gene signatures in breast cancer. Curr Opin Oncol. 2015;27:433–444. doi: 10.1097/CCO.0000000000000234. [DOI] [PubMed] [Google Scholar]
- 37.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med. 2015;372:311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barrett MT, Anderson KS, Lenkiewicz E, et al. Genomic amplification of 9p24.1 targeting JAK2, PD-L1, and PD-L2 is enriched in high-risk triple negative breast cancer. Oncotarget. 2015;6:26483–26493. doi: 10.18632/oncotarget.4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sabatier R, Finetti P, Mamessier E, et al. Prognostic and predictive value of PDL1 expression in breast cancer. Oncotarget. 2015;6:5449–5464. doi: 10.18632/oncotarget.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ali HR, Glont SE, Blows FM, et al. PD-L1 protein expression in breast cancer is rare, enriched in basal-like tumours and associated with infiltrating lymphocytes. Ann Oncol. 2015;26:1488–1493. doi: 10.1093/annonc/mdv192. [DOI] [PubMed] [Google Scholar]
- 41.Rooney MS, Shukla SA, Wu CJ, et al. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. 2015;160:48–61. doi: 10.1016/j.cell.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lastwika KJ, Wilson W, 3rd, Li QK, et al. Control of PD-L1 expression by oncogenic activation of the AKT-mTOR pathway in non-small cell lung cancer. Cancer Res. 2016;76:227–238. doi: 10.1158/0008-5472.CAN-14-3362. [DOI] [PubMed] [Google Scholar]
- 43.Mittendorf EA, Philips AV, Meric-Bernstam F, et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014;2:361–370. doi: 10.1158/2326-6066.CIR-13-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang P, Su DM, Liang M, Fu J. Chemopreventive agents induce programmed death-1-ligand 1 (PD-L1) surface expression in breast cancer cells and promote PD-L1-mediated T cell apoptosis. Mol Immunol. 2008;45:1470–1476. doi: 10.1016/j.molimm.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 45.Ghebeh H, Lehe C, Barhoush E, et al. Doxorubicin downregulates cell surface B7-H1 expression and upregulates its nuclear expression in breast cancer cells: role of B7-H1 as an anti-apoptotic molecule. Breast Cancer Res. 2010;12:R48. doi: 10.1186/bcr2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Teng MW, Ngiow SF, Ribas A, Smyth MJ. Classifying cancers based on T-cell infiltration and PD-L1. Cancer Res. 2015;75:2139–2145. doi: 10.1158/0008-5472.CAN-15-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu P, Wu D, Li L, et al. PD-L1 and survival in solid tumors: a meta-analysis. PLoS One. 2015;10:e0131403. doi: 10.1371/journal.pone.0131403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gatalica Z, Snyder C, Maney T, et al. Programmed cell death 1 (PD-1) and its ligand (PD-L1) in common cancers and their correlation with molecular cancer type. Cancer Epidemiol Biomarkers Prev. 2014;23:2965–2970. doi: 10.1158/1055-9965.EPI-14-0654. [DOI] [PubMed] [Google Scholar]
- 49.Ghebeh H, Mohammed S, Al-Omair A, et al. The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia. 2006;8:190–198. doi: 10.1593/neo.05733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cimino-Mathews A, Thompson E, Taube JM, et al. PD-L1 (B7-H1) expression and the immune tumor microenvironment in primary and metastatic breast carcinomas. Hum Pathol. 2016;47:52–63. doi: 10.1016/j.humpath.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Muenst S, Schaerli AR, Gao F, et al. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2014;146:15–24. doi: 10.1007/s10549-014-2988-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beckers RK, Selinger CI, Vilain R, et al. PDL1 expression in triple-negative breast cancer is associated with tumour-infiltrating lymphocytes and improved outcome. Histopathology. 2015 doi: 10.1111/his.12904. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 53.Schalper KA, Velcheti V, Carvajal D, et al. In situ tumor PD-L1 mRNA expression is associated with increased TILs and better outcome in breast carcinomas. Clin Cancer Res. 2014;20:2773–2782. doi: 10.1158/1078-0432.CCR-13-2702. [DOI] [PubMed] [Google Scholar]
- 54.Bertucci F, Finetti P, Colpaert C, et al. PDL1 expression in inflammatory breast cancer is frequent and predicts for the pathological response to chemotherapy. Oncotarget. 2015;6:13506–13519. doi: 10.18632/oncotarget.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qin T, Zeng YD, Qin G, et al. High PD-L1 expression was associated with poor prognosis in 870 Chinese patients with breast cancer. Oncotarget. 2015;6:33972–33981. doi: 10.18632/oncotarget.5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baptista MZ, Sarian LO, Derchain SF, et al. Prognostic significance of PD-L1 and PD-L2 in breast cancer. Hum Pathol. 2016;47:78–84. doi: 10.1016/j.humpath.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 57.Park IH, Kong SY, Ro JY, et al. Prognostic implications of tumor-infiltrating lymphocytes in association with Programmed Death Ligand 1 Expression in early-stage breast cancer. Clin Breast Cancer. 2016;16:51–58. doi: 10.1016/j.clbc.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 58.Yuan J, Hegde PS, Clynes R, et al. Novel technologies and emerging biomarkers for personalized cancer immunotherapy. J Immunother Cancer. 2016;4:3. doi: 10.1186/s40425-016-0107-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bedognetti D, Balwit JM, Wang E, et al. SITC/iSBTc Cancer Immunotherapy Biomarkers Resource Document: online resources and useful tools - a compass in the land of biomarker discovery. J Transl Med. 2011;9:155. doi: 10.1186/1479-5876-9-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ghebeh H, Tulbah A, Mohammed S, et al. Expression of B7-H1 in breast cancer patients is strongly associated with high proliferative Ki-67-expressing tumor cells. Int J Cancer. 2007;121:751–758. doi: 10.1002/ijc.22703. [DOI] [PubMed] [Google Scholar]
- 61.Mazel M, Jacot W, Pantel K, et al. Frequent expression of PD-L1 on circulating breast cancer cells. Mol Oncol. 2015;9:1773–1782. doi: 10.1016/j.molonc.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bedognetti D, Spivey TL, Zhao Y, et al. CXCR3/CCR5 pathways in metastatic melanoma patients treated with adoptive therapy and interleukin-2. Br J Cancer. 2013;109:2412–2423. doi: 10.1038/bjc.2013.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ji RR, Chasalow SD, Wang L, et al. An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol Immunother. 2012;61:1019–1031. doi: 10.1007/s00262-011-1172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ulloa-Montoya F, Louahed J, Dizier B, et al. Predictive gene signature in MAGE-A3 antigen-specific cancer immunotherapy. J Clin Oncol. 2013;31:2388–2395. doi: 10.1200/JCO.2012.44.3762. [DOI] [PubMed] [Google Scholar]
- 66.Spivey TL, De Giorgi V, Zhao Y, et al. The stable traits of melanoma genetics: an alternate approach to target discovery. BMC Genomics. 2012;13:156. doi: 10.1186/1471-2164-13-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Spivey TL, Uccellini L, Ascierto ML, et al. Gene expression profiling in acute allograft rejection: challenging the immunologic constant of rejection hypothesis. J Transl Med. 2011;9:174. doi: 10.1186/1479-5876-9-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bedognetti D, Wang E, Sertoli MR, Marincola FM. Gene-expression profiling in vaccine therapy and immunotherapy for cancer. Expert Rev Vaccines. 2010;9:555–565. doi: 10.1586/erv.10.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang E, Bedognetti D, Marincola FM. Prediction of response to anticancer immunotherapy using gene signatures. J Clin Oncol. 2013;31:2369–2371. doi: 10.1200/JCO.2013.49.2157. [DOI] [PubMed] [Google Scholar]
- 70.Murtas D, Maric D, De Giorgi V, et al. IRF-1 responsiveness to IFN-gamma predicts different cancer immune phenotypes. Br J Cancer. 2013;109:76–82. doi: 10.1038/bjc.2013.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tomei S, Bedognetti D, De Giorgi V, et al. The immune-related role of BRAF in melanoma. Mol Oncol. 2015;9:93–104. doi: 10.1016/j.molonc.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stoll G, Enot D, Mlecnik B, et al. Immune-related gene signatures predict the outcome of neoadjuvant chemotherapy. Oncoimmunology. 2014;3:e27884. doi: 10.4161/onci.27884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bedognetti D, Wang E, Marincola FM. Meta-analysis and metagenes: CXCL-13-driven signature as a robust marker of intratumoral immune response and predictor of breast cancer chemotherapeutic outcome. Oncoimmunology. 2014;3:e28727. doi: 10.4161/onci.28727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Denkert C, von Minckwitz G, Brase JC, et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol. 2015;33:983–991. doi: 10.1200/JCO.2014.58.1967. [DOI] [PubMed] [Google Scholar]
- 75.Lee HJ, Lee JJ, Song IH, et al. Prognostic and predictive value of NanoString-based immune-related gene signatures in a neoadjuvant setting of triple-negative breast cancer: relationship to tumor-infiltrating lymphocytes. Breast Cancer Res Treat. 2015;151:619–627. doi: 10.1007/s10549-015-3438-8. [DOI] [PubMed] [Google Scholar]
- 76.Ignatiadis M, Singhal SK, Desmedt C, et al. Gene modules and response to neoadjuvant chemotherapy in breast cancer subtypes: a pooled analysis. J Clin Oncol. 2012;30:1996–2004. doi: 10.1200/JCO.2011.39.5624. [DOI] [PubMed] [Google Scholar]
- 77.Perez-Gracia JL, Labiano S, Rodriguez-Ruiz ME, et al. Orchestrating immune check-point blockade for cancer immunotherapy in combinations. Curr Opin Immunol. 2014;27:89–97. doi: 10.1016/j.coi.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 78.Miller LD, Chou JW, Black MA, et al. Immune gene signatures and tumor intrinsic markers delineate novel immunogenic subtypes of breast cancer. J Immunother Cancer. 2014;2(suppl 3):P256. [Google Scholar]
- 79.Nagalla S, Chou JW, Willingham MC, et al. Interactions between immunity, proliferation and molecular subtype in breast cancer prognosis. Genome Biol. 2013;14:R34. doi: 10.1186/gb-2013-14-4-r34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity. 2013;39:74–88. doi: 10.1016/j.immuni.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 81.Perez EA, Thompson EA, Ballman KV, et al. Genomic analysis reveals that immune function genes are strongly linked to clinical outcome in the North Central Cancer Treatment Group n9831 Adjuvant Trastuzumab Trial. J Clin Oncol. 2015;33:701–708. doi: 10.1200/JCO.2014.57.6298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bedognetti D, Tomei S, Hendrickx W, et al. Toward the identification of genetic determinants of responsiveness to cancer immunotherapy. In: Ascierto PA, Stroncek DF, Wang E, editors. Developments in T Cell Based Cancer Immunotherapies. New York, NY: Humana Press; 2015. [Google Scholar]
- 83.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 84.Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Emens LA, Tuohy VK, Stanton SE. Immunotherapy for breast cancer: is it feasible? Immunotherapy. 2015;7:1135–1143. doi: 10.2217/imt.15.83. [DOI] [PubMed] [Google Scholar]
- 86.Emens LA. Breast cancer immunobiology driving immunotherapy: vaccines and immune checkpoint blockade. Expert Rev Anticancer Ther. 2012;12:1597–1611. doi: 10.1586/era.12.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rosenberg SA, Yang JC, Sherry RM, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tomei S, Wang E, Delogu LG, et al. Non-BRAF-targeted therapy, immunotherapy, and combination therapy for melanoma. Expert Opin Biol Ther. 2014;14:663–686. doi: 10.1517/14712598.2014.890586. [DOI] [PubMed] [Google Scholar]
- 89.Hinrichs CS, Rosenberg SA. Exploiting the curative potential of adoptive T-cell therapy for cancer. Immunol Rev. 2014;257:56–71. doi: 10.1111/imr.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Domschke C, Ge Y, Bernhardt I, et al. Long-term survival after adoptive bone marrow T cell therapy of advanced metastasized breast cancer: follow-up analysis of a clinical pilot trial. Cancer Immunol Immunother. 2013;62:1053–1060. doi: 10.1007/s00262-013-1414-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stefanovic S, Schuetz F, Sohn C, et al. Adoptive immunotherapy of metastatic breast cancer: present and future. Cancer Metastasis Rev. 2014;33:309–320. doi: 10.1007/s10555-013-9452-6. [DOI] [PubMed] [Google Scholar]
- 92.Vonderheide RH, LoRusso PM, Khalil M, et al. Tremelimumab in combination with exemestane in patients with advanced breast cancer and treatment-associated modulation of inducible costimulator expression on patient T cells. Clin Cancer Res. 2010;16:3485–3494. doi: 10.1158/1078-0432.CCR-10-0505. [DOI] [PubMed] [Google Scholar]
- 93.Page DB, Diab A, Yuan J, et al. Pre-operative immunotherapy with tumor cryoablation (cryo) plus ipilimumab (ipi) induces potentially favorable systemic and intratumoral immune effects in early stage breast cancer (ESBC) patients. J Immunother Cancer. 2015;3:P1. [Google Scholar]
- 94.Savas P, Salgado R, Denkert C, et al. Clinical relevance of host immunity in breast cancer: from TILs to the clinic. Nat Rev Clin Oncol. 2015 doi: 10.1038/nrclinonc.2015.215. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 95.Salgado R, Denkert C, Demaria S, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26:259–271. doi: 10.1093/annonc/mdu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature. 2015;523:231–235. doi: 10.1038/nature14404. [DOI] [PubMed] [Google Scholar]
- 98.Loi S, Dushyanthen S, Beavis PA, et al. RAS/MAPK activation is associated with reduced tumor-infiltrating lymphocytes in triple-negative breast cancer: therapeutic cooperation between MEK and PD-1/PD-L1 immune checkpoint inhibitors. Clin Cancer Res. 2015 doi: 10.1158/1078-0432.CCR-15-1125. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Simeone I, Hendricks W, Miller L, et al. Toward the identification of genetic determinants of breast cancer immune responsiveness. Breast Cancer Immunotherapy Symposium (BRECIS): Sidra Symposia Series, April 13-14, Doha, Qatar. 2015 (JITC suppl in press) [Google Scholar]
- 100.Bruns M, Wanger J, Utermohlen O, Deppert W. An inducible transgenic mouse breast cancer model for the analysis of tumor antigen specific CD8+ T-cell responses. Oncotarget. 2015;6:38487–38503. doi: 10.18632/oncotarget.5750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst. 2013;105:256–265. doi: 10.1093/jnci/djs629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vanpouille-Box C, Diamond JM, Pilones KA, et al. TGFbeta is a master regulator of radiation therapy-induced antitumor immunity. Cancer Res. 2015;75:2232–2242. doi: 10.1158/0008-5472.CAN-14-3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data