Abstract
Acute liver failure (ALF) due to Wilson disease (WD) is invariably fatal without emergency liver transplantation. Therefore, rapid diagnosis of WD should aid prompt transplant listing. To identify the best method for diagnosis of ALF due to WD (ALF-WD), data and serum were collected from 140 ALF patients (16 with WD), 29 with other chronic liver diseases and 17 with treated chronic WD. Ceruloplasmin (Cp) was measured by both oxidase activity and nephelometry and serum copper levels by atomic absorption spectroscopy. In patients with ALF, a serum Cp <20 mg/dL by the oxidase method provided a diagnostic sensitivity of 21% and specificity of 84% while, by nephelometry, a sensitivity of 56% and specificity of 63%. Serum copper levels exceeded 200 g/dL in all ALF-WD patients measured (13/16), but were also elevated in non-WD ALF. An alkaline phosphatase (AP) to total bilirubin (TB) ratio <4 yielded a sensitivity of 94%, specificity of 96%, and a likelihood ratio of 23 for diagnosing fulminant WD. In addition, an AST:ALT ratio > 2.2 yielded a sensitivity of 94%, a specificity of 86%, and a likelihood ratio of 7 for diagnosing fulminant WD. Combining the tests provided a diagnostic sensitivity and specificity of 100%. In conclusion, conventional WD testing utilizing serum ceruloplasmin and/or serum copper levels are less sensitive and specific in identifying patients with ALF-WD than other available tests. More readily available laboratory tests including alkaline phosphatase, bilirubin and serum aminotransferases by contrast provides the most rapid and accurate method for diagnosis of ALF due to WD.
Keywords: copper, ceruloplasmin
Introduction
Wilson disease (WD) is an infrequent cause of chronic liver disease with an estimated incidence of 1 per 30,000 in the general US population. Furthermore, WD is the identified etiology in ~5% of acute liver failure (ALF) patients worldwide.1 Prior series demonstrate that virtually all patients with WD presenting with ALF will die rapidly without urgent transplantation.2 Therefore, establishing the diagnosis quickly and unequivocally is critical for patient management and for family screening as well. Classically, reduced serum ceruloplasmin (Cp) levels have been associated with WD but are believed to be less reliable in the fulminant setting.3 Additional specific laboratory findings associated with fulminant WD include Coombs’ negative hemolytic anemia, low serum uric acid levels, low serum alkaline phosphatase activity and increased aspartate aminotransferase:alanine aminotransferase (AST:ALT) ratios.4-9 Prior studies have yielded conflicting results with respect to the ability of any of these serum biochemical markers to accurately differentiate WD from other causes of ALF.10-13 The predictive value of any of the currently available serum markers for the diagnosis of Wilson disease [i.e. a reduced Cp level, a reduced hemoglobin, elevated serum copper (Cu), ratios of alkaline phosphatase to bilirubin and AST to ALT] in the setting of ALF remains uncertain and is further hampered by the small numbers of well-characterized ALF cases available for study. In addition, serum Cp levels can be measured by two analytical methods (oxidase and immunonephelometry). However, direct comparison of the two methods in ALF patients has not been previously reported. The aim of the present study was to determine the sensitivity and specificity of each of the proposed serum markers for the diagnosis of WD in a group of well-characterized patients with ALF including 16 with ALF-WD derived from the United States Acute Liver Failure Study Group (ALFSG). In addition, we set out to compare the diagnostic utility of these tests in patients with fulminant WD compared to patients with other chronic liver diseases and in treated patients with stable chronic WD.
Patients and Methods
Overall Patient Group
More than 1,050 ALF patients have been enrolled at 23 sites since 1998 comprise the Acute Liver Failure Study Group registry. Inclusion criteria were any degree of altered mentation plus evidence of moderately severe coagulopathy [international normalized ratio (INR) ≥1.5] and a presumed hepatic illness of less than 26 weeks.1 Detailed clinical, laboratory, histological and outcome data were prospectively collected from all ALF patients. A consecutive series of 124 ALF patients was used in the present study, each of which had a day 1 serum sample available for analysis (>80% of patients have daily sera collected through 7 days). Because of the requirement of encephalopathy to enter the ALFSG registry, written informed consent was obtained from all patients’ surrogates before enrollment according to the regulations of local institutional review boards.
Wilson Disease Patient Cohort
Patients from the registry with a diagnosis of fulminant WD from 1998 to 2004 were included. All of these subjects had newly-diagnosed WD except for two who had been diagnosed more than 6 months prior to presentation but had developed typical ALF either due to non-adherence to treatment or treatment failure. Day 1 sera were available on all 16 in the ALF-WD study cohort (Table 1). While serum and liver Cu values were available in all 16, urine Cu measurements were available in 12/16 patients, slit lamp ophthalmologic exams in 5 patients, and Cu staining of biopsies in 7 patients.
Table 1.
Characteristics of Patients with WD and Acute Liver Failure.
| Case | Age | Sex | Cp by Oxidase |
Cp by Neph |
Serum Cu | Hepatic Cu (dry weight) |
Hepatic Cu (stain) |
Urine Cu | K-F Rings | Hgb | ALT | AST | AP:TB Ratio |
OLT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 53 | F | 48.4 | 35.8 | 428 | 1,328 | ND | ND | ND | 9.1 | 23 | 146 | 0.32 | No |
| 2 | 26 | F | 7.7 | 8.4 | 222 | 520 | Neg | 19,543 | Neg | 5.4 | 11 | 163 | 1.18 | No |
| 3 | 17 | F | 28.8 | 18.5 | 350 | 359 | ND | ND | ND | 7.6 | 23 | 251 | 0.64 | Yes |
| 4 | 14 | M | 17.1 | 11.1 | 105 | 228 | ND | 592 | ND | 9.6 | 64 | 142 | 3.72 | Yes |
| 5 | 14 | F | 21.5 | 13.4 | 200 | 712 | ND | 154 | ND | 6.6 | 10 | 124 | 0.32 | Yes |
| 6 | 52 | M | 21.6 | 21.3 | 1063 | 584 | ND | 630,672 | ND | 6 | 1463 | 4481 | 3.71 | No |
| 7 | 19 | F | 23.6 | 78.7 | 379 | 1,583 | Pos | 18,459 | Neg | 7 | 22 | 181 | 0.17 | Yes |
| 8 | 26 | F | 20.3 | 12.8 | 230 | 1,290 | Pos | 7,843 | Pos | 10.8 | 28 | 189 | 0.19 | Yes |
| 9 | 26 | M | 21.8 | 31.4 | 306 | 2,189 | Pos | 23,451 | ND | 6.8 | 26 | 234 | 0.84 | Yes |
| 10 | 15 | F | 17.7 | 11.7 | 312 | 1,815 | ND | ND | ND | 4.7 | 18 | 194 | 0.37 | Yes |
| 11 | 30 | F | 39.1 | 32.9 | 285 | 867 | Pos | 3,644 | ND | 8.5 | 13 | 236 | 0.49 | Yes |
| 12 | 27 | F | 23.6 | 25.9 | 397 | 472 | ND | ND | ND | 9.5 | 64 | 290 | 0.93 | Yes |
| 13 | 24 | F | 28.8 | 18.9 | 222 | 1,241 | Pos | 2,547 | ND | 7.9 | 7 | 128 | 0.31 | Yes |
| 14 | 26 | F | 27.3 | 17 | 133 | 508 | Pos | 564 | ND | 7 | 32 | 69 | 7.76 | Yes |
| 15 | 19 | F | ND | 13 | 259 | 690 | ND | 16,654 | Pos | 7.1 | 26 | 177 | 0.06 | Yes |
| 16 | 21 | F | ND | 20 | 126 | 833 | ND | 11,825 | Pos | 6.3 | 230 | 670 | 0.51 | Yes |
Abbreviations: Cp= ceruloplasmin (mg/dL); Neph= nephelometry; Cu= copper; K-F = Kayser-Fleischer rings; Hgb= hemoglobin (g/dL); AP:TB= alkaline phosphatase to total bilirubin ratio; OLT= orthotopic liver transplant; ND= not done; NA= not applicable; Neg= negative; Pos= positive. Hepatic copper was measured as stainable copper with rhodanine or by dry weight in μg/g, serum copper was measured in μg/dL, and urine copper was measured over 24 hours in mg.
Each case was reviewed in detail and met current standard diagnostic criteria including some, but not necessarily all, of the following features: biopsy Cu (>250 μg/g dry weight) elevated in 15/16; 24 hour urinary Cu (>100 μg/day) elevated in 12/12; serum Cu (>200 μg/dL) elevated in 13/16, Kayser Fleischer (KF) rings present in 3/5; and positive rhodanine histochemistry on liver biopsy present in 6/7.2 In this group of 16 patients, 5 patients met 2 criteria, 5 met 3 criteria, 5 met 4 criteria, and 1 met all 5 criteria for the diagnosis of WD. The 3 patients with low serum Cu levels each met 3 other criteria.
Other Disease Groups
For comparison and as controls, sera from 29 patients with other identifiable chronic liver diseases (9 with chronic hepatitis C, 5 with autoimmune hepatitis, 1 with chronic hepatitis B, 1 with sarcoidosis, 2 with alcoholic liver disease, 1 with hereditary hemochromatosis, 2 with nonalcoholic fatty liver disease, and 8 with cryptogenic liver disease) and from 17 with treated stable WD seen in outpatient clinics were also studied.
Chemical and Statistical Analyses
Serum Cu was measured using graphite furnace atomic absorption spectroscopy (normal range 70-130 g/dL). Concentration of serum Cp (normal range 20-40 mg/dL) was determined by immunonephelometry and by oxidase activity. Nephelometric measurement was performed using the Behring BN II nephelometer with standard reagents (Behring, Teaneck NJ). By oxidase, serum samples were thawed a single time after storage at −80C and used for determination of Cp oxidase activity as described previously.14 Immunonephelometry measures Cu-containing Cp as well as apoceruloplasmin which does not contain Cu. The oxidase assay measures Cu-containing Cp only. Specimens of liver were either dried or extracted from paraffin blocks and then dried and digested in nitric acid for determination of Cu content using a Hitachi Z8270 atomic absorption spectrophotometer with background subtraction as described previously.15 Biochemical data and blood counts were obtained from the ALF Study Group database and from patient records. Results were analyzed using SPSS software version 12. Analysis was performed using the two-sample Student t-test, Wilcoxon rank-sum test or Mann-Whitney U-test as appropriate for continuous variables and the Pearson chi-squared test for categorical variables. Spearman’s rank –order correlation coefficient was used to assess correlations.
Results
Clinical Features of the Patient Groups
The 124 ALF patients without WD included 92 women (74%) whose median age was 39 years (range=14-78) and 36 men (26%) whose median age was 28 years (range=14-76). Among the 16 patients with ALF WD, the median age was 25 years (range=14-53), and 13 were women (81%). Etiologies of ALF in the 124 non-WD ALF patients included acetaminophen hepatotoxicity (n=52; 37%), indeterminate etiology (n=21; 15%), idiosyncratic drug toxicity (n=18; 13%), viral hepatitis B (n=9; 6%), autoimmune hepatitis (n=7; 5%), viral hepatitis A (n=6; 4%), shock (n=6; 4%), Budd-Chiari or the HELLP (Hemolysis, Elevated Liver enzymes, Low Platelets) syndrome (2 each) and liver failure due to neoplasm (one patient). Median MELD scores were calculated for the WD patients and for all others: the median MELD score for WD patients was 40 while the median value for the non-WD patients was 34 (p < 0.01, Student t test).16 Thirteen WD patients were transplanted and three died.
Copper Metabolism Parameters
Serum Cp varied widely in patients with ALF, from below normal to marked elevation, regardless of etiology (Tables 1 and 2). In patients with ALF-WD, the median was 22.7 mg/dL determined by oxidase method, with 4 had values ≤20 mg/dl, and 2 were not measured. The range was 7.7-48.4 mg/dL. In the ALF patients with other etiologies, the median serum Cp value was 30.9 mg/dL (n=113, range=9.9-108.8 mg/dL, p=0.02) with 26 of 127 having a serum Cp ≤ 20 mg/dl. By immunonephelometry, the serum Cp in patients with ALF-WD had a median value of 18.7 mg/dL and 10 demonstrating values ≤20 mg/dl (range=8.4-78.7 mg/dL). However this reduction in Cp was not significantly different from the remainder of the ALF patients without WD whose median value was 22.9 mg/dL (n=116, range=1.8-103.0 mg/dL, p=0.2). A serum Cp ≤20 mg/dL in the setting of ALF (including those with WD) as measured by oxidase method provided a diagnostic sensitivity of 21%, specificity of 84%, and a likelihood ratio of a positive test of 1 for the diagnosis of Wilson disease (Table 3). Similarly, serum Cp ≤20 mg/dL as measured by nephelometry was found in 59 of 132 patients (including those with WD) yielding only a slightly better sensitivity of 56%, a specificity of 63%, and a likelihood ratio of 2 for the diagnosis of WD. Serum Cp was ≤20 mg/dL in the patients with other chronic liver diseases using the oxidase method in 13 of 46, yielding a sensitivity of 71%, a specificity of 97%, and a likelihood ratio of 20.5; whereas by nephelometry Cp was ≤20 in 18 of 46, yielding a sensitivity of 71%, a specificity of 79%, and a likelihood ratio of 3.4.
Table 2.
Serum Tests in Acute Liver Failure: Wilson Disease versus All Other Etiologies. All comparisons have a p <0.001, except for ceruloplasmin by oxidase p=0.02 and ceruloplasmin by nephelometry p=0.2.
| Serum Test |
Wilson Disease Median (range) N = 16 |
All Other Etiologies Median (range) N = 124 |
|---|---|---|
| ALT (IU/L) | 24.5 (7.0-1463.0) | 1830.0 (57.0-17670.0) |
| AST (IU/L) | 185.0 (69.0-4481.0) | 1337.0 (76.0-28870.0) |
| Total Bilirubin (mg/dL) | 42.1 (7.6-72.4) | 7.3 (0.7-49.8) |
| Alkaline Phosphatase (IU/L) | 20.5 (4.0-129.0) | 146.5 (56.0-524.0) |
| Hemoglobin (g/dL) | 7.0 (4.7-10.8) | 11.6 (4.3-21.0) |
| Ceruloplasmin-oxidase (mg/dL) | 22.7 (7.7-48.4) | 30.9 (9.3-108.8)† |
| Ceruloplasmin-nephelometry (mg/dL) |
18.7 (8.4-78.7) | 22.9 (1.8-103.0)‡ |
| Copper (μg/dL) | 272.0 (105.2-1063.0) | 91.5 (32.2-317.8) |
| Alk Phos:TB ratio | 0.5 (0.1-7.8) | 20.1 (2.8-194.3) |
| AST:ALT ratio | 7.5 (2.1-18.3) | 1.0 (0.1-5.7) |
Table 3.
Comparison of Screening Tests for WD in ALF: 1Ceruloplasmin; 2Alkaline Phosphatase:Total Bilirubin; 3Not applicable; 4Serum Copper. In the chronic liver disease group, zero subjects had an AP:TB ratio < 4, three subjects had Hg <10, one had Cu >200, and the AST:ALT ratio was an ineffective marker of WD.
| Group | Screening Test | Sensitivity % | Specificity % | Likelihood Ratio |
|---|---|---|---|---|
| Acute: | Cp1 (mg/dL)<20 by oxidase |
21 | 84 | 1 |
| Cp (mg/dL) <20 by nephelometry |
56 | 63 | 2 | |
| Hemoglobin (g/dL) <10 | 94 | 74 | 4 | |
| AP:TB2 ratio <4 | 94 | 96 | 23 | |
| AP:TB ratio <4 + AST:ALT ratio >2.2 |
100 | 100 | NA3 | |
| Cu4 (μg/dL) >200 | 75 | 96 | 17 | |
| AST:ALT ratio >2.2 | 94 | 86 | 7 | |
| Chronic: | Cp (mg/dL) <20 by oxidase |
71 | 97 | 21 |
| Cp (mg/dL) <20 by nephelometry |
71 | 79 | 3 |
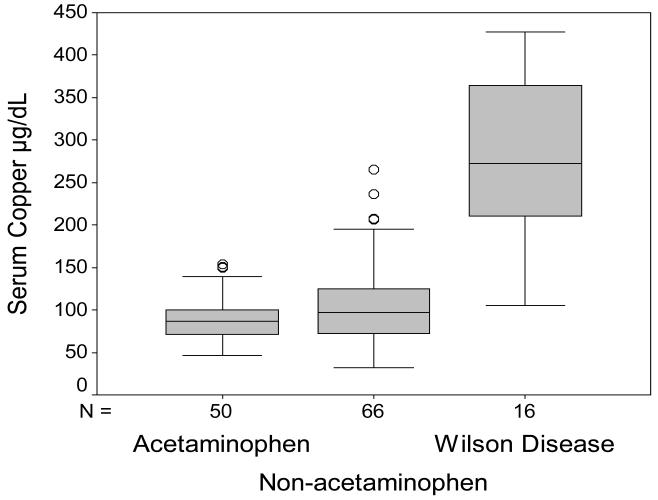
Serum Cu was >200 g/dL in only 4 patients in the ALF group without WD. Using this as a cutoff yielded a sensitivity of 75%, specificity of 96%, and a likelihood ratio of 17. Ranges of serum Cu for the separate patient groups with ALF (divided into those with acetaminophen injury, those without acetaminophen, and those with Wilson disease) are demonstrated in Figure 1. None of the subjects in the chronic liver disease group with or without WD had serum Cu levels that exceeded the 200 g/dL level (data not shown).
Figure 1.
Serum Copper in ALF. Results are shown for patients with ALF due to acetaminophen injury, non-acetaminophen injury and for patients with WD.
Standard Laboratory Tests
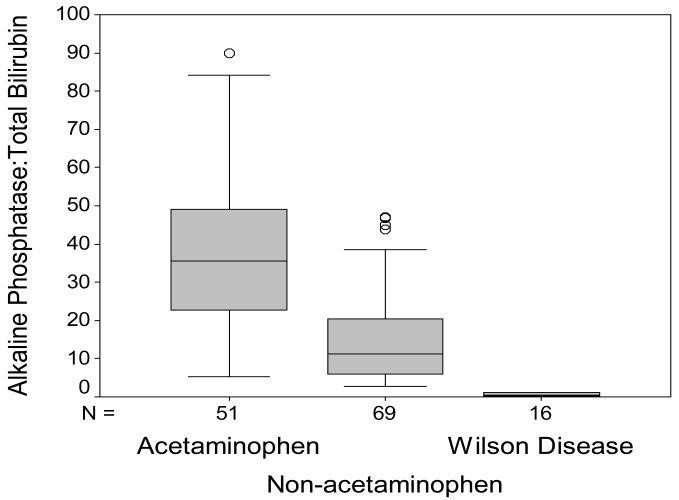
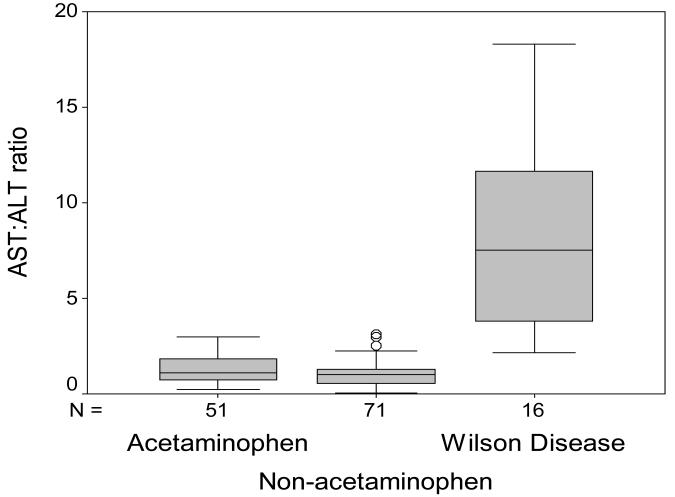
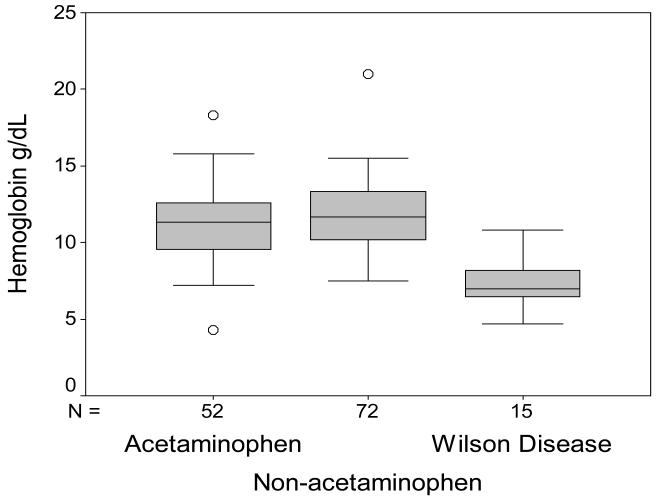
Routine biochemistry testing was recorded for all study patients. The serum alkaline phosphatase to total bilirubin ratio was significantly lower (p<0.001) in the group with ALF-WD than in all other groups as demonstrated in Figure 2. In the 16 patients with ALF-WD, the median ratio was 0.5 (range=0.1-7.8) and in all other etiologies the median ratio was 20.1 (n=120, range=2.8-194.3). This low ratio in WD reflects a higher median total bilirubin in WD (n=16, median=42.1 mg/dL, range=7.6-72.4 mg/dL) versus other etiologies (n=124, median=7.3 mg/dL, range = 0.7-49.8 mg/dL, p<0.001) as well as lower alkaline phosphatase levels (WD: n=16, median=20.5 IU/L, range=4.0-129.0 IU/L versus other ALF: n=120, median=146.5 IU/L, range= 56.0-524.0 IU/L, p<0.001). The serum AST to ALT ratio was another measure by which to distinguish WD from non-WD ALF patients (Figure 3). In WD, the AST:ALT ratio median was 7.5 (n=16, range=2.1-18.3) and in non-WD ALF, the ratio median was 1.0 (n=122, range=0.6-5.7, p<0.001). The mean hemoglobin concentration in the WD group was 7.08 g/dL (n=15/139, range=4.7-10.8 g/dL) and in the non-WD group it was 11.6 g/dL (n=124/139, range=4.3-21.0 g/dL, p<0.001, Figure 4).
Figure 2.
Alkaline Phosphatase to Total Bilirubin Ratio in ALF. Results are shown for patients with ALF due to acetaminophen injury, non-acetaminophen injury and for patients with WD.
Figure 3.
AST:ALT Ratio in ALF. Results are shown for patients with ALF due to acetaminophen injury, non-acetaminophen injury and for patients with WD.
Figure 4.
Hemoglobin in ALF. Results are shown for patients with ALF due to acetaminophen injury, non-acetaminophen injury and for patients with WD.
Screening for Wilson Disease in Acute Liver Failure
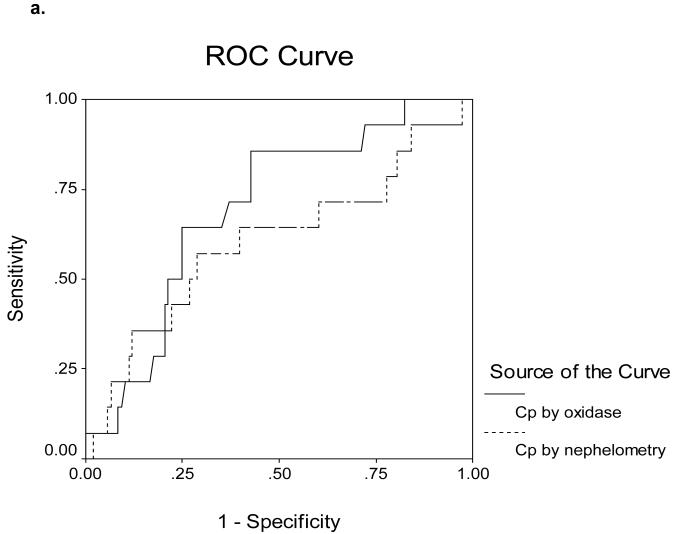
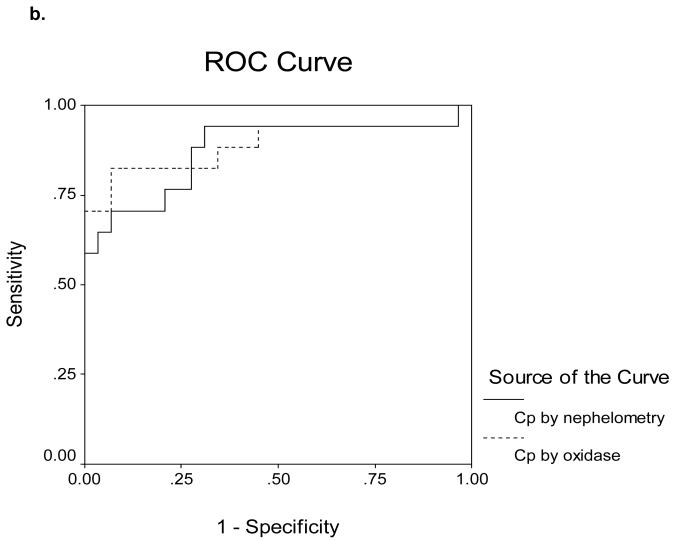
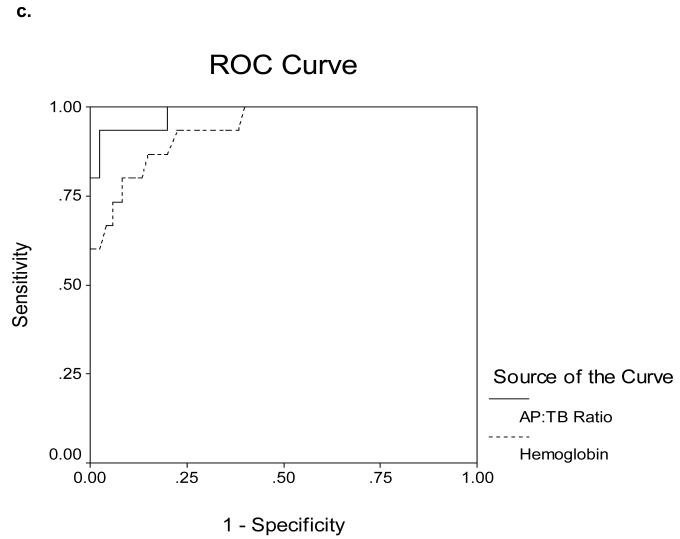
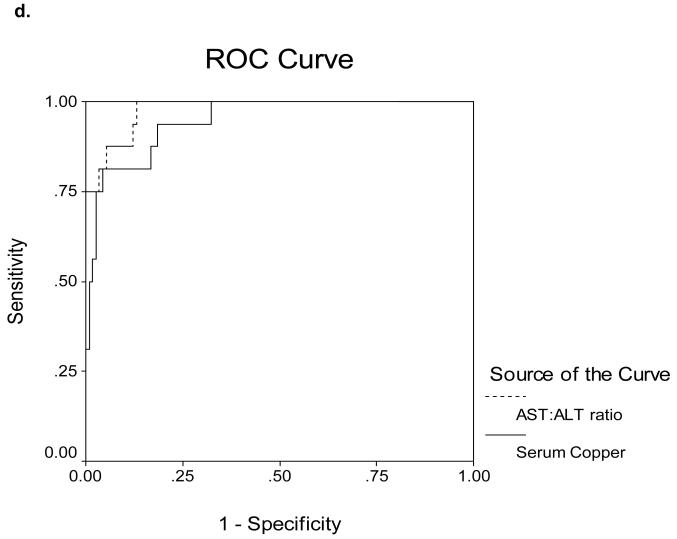
Receiver Operating Characteristics (ROC) curves for determining cut-off values are shown in Figures 5a-d. The area under the ROC curves (AUROC) for serum Cp as measured by nephelometry and oxidase in the 16 ALF-WD patients was 0.60 and 0.70, respectively (Figure 5a). In contrast, the AUROC for serum Cp as measured by nephelometry and oxidase in the 17 WD patients with chronic stable disease was 0.87 and 0.89, respectively (Figure 5b). In the ALF group, an alkaline phosphatase (AP) to total bilirubin (TB) ratio less than 4 yielded a sensitivity of 94%, specificity of 96%, a likelihood ratio of 23 and an AUROC of 0.98 (Table 3, Figure 5c). An AST:ALT ratio >2.2 yielded a sensitivity of 94%, a specificity of 86%, a likelihood ratio of 7 and an AUROC of 0.98 (Table 3, Figure 5d). By first screening patients for WD with the AP:TB ratio <4 and then sequentially screening with an AST:ALT ratio >2.2, the sensitivity and sensitivity increased to 100%. A hemoglobin <10 g/dL yielded a 94% sensitivity and a 74% specificity with a likelihood ratio of 4 and an AUROC of 0.94 (Table 3, Figure 5c). Serum Cu was also quite effective with a sensitivity and specificity of 74 and 94% and an AUROC of 0.95 (Table 3, Figure 5d). In the non-acute group with WD, no subjects had an AP:TB ratio < 4 and the AST:ALT ratio > 2.2 was not an effective marker of WD.
Figure 5.
a. Receiver Operating Characteristics for Ceruloplasmin Measured by Nephelometry and by Oxidase Methods in 140 patients with ALF. The area under the curve is 0.60 and 0.70, respectively.
b. Receiver Operating Characteristics for Ceruloplasmin Measured by Nephelometry and by Oxidase in 17 chronic Wilson Disease patients. The area under the curve is 0.87 and 0.89, respectively.
c. Receiver Operating Characteristics for Alkaline Phosphatase to Total Bilirubin Ratio and Hemoglobin (g/dL) and the diagnosis of Wilson Disease in ALF (ALF-WD). The area under the curve is 0.98 and 0.94, respectively.
d. Receiver Operating Characteristics for AST to ALT Ratio and Serum Copper (μg/dL) and the diagnosis of Wilson Disease in ALF. The area under the curve is 0.98 and 0.95, respectively.
Discussion
The diagnosis of WD in the setting of ALF can be difficult to ascertain but is vitally important due to its poor prognosis and implications for family members. The findings that most clinicians consider useful when evaluating a patient with suspected WD are those associated with the diagnosis in patients who present with chronic liver disease or neurological manifestations including: low serum ceruloplasmin, high 24 hour urine copper, elevated hepatic copper or special stains for copper, high serum copper and the presence of KF rings.2,17 However, in the setting of ALF, KF rings are only detectable in up to 50% of WD patients and obtaining a slit lamp exam in critically ill patients may be difficult.7,11-13,18 Furthermore, rapid testing for Cu in urine, serum or liver is rarely achieved due to the delay in obtaining Cu results from reference laboratories. This limits the value of these diagnostic tests in the ALF setting where rapid turnaround is essential to management.
In the initial phases of the ALFSG, urine samples were not routinely collected and so we could not evaluate the utility of urine Cu testing for the diagnosis of WD. Although urinary Cu excretion is a useful diagnostic marker in the non-acute setting, the presence of impaired renal function in up to 40% of ALF patients and the associated reduction in urine output would limit its potential utility19. In addition, since urinary Cu excretion is known to increase in diseases associated with extensive hepatocellular necrosis, there is a high likelihood of overlap between ALF due to Wilson’s disease and other etiologies.11
Liver biopsy to determine quantitative hepatic Cu levels, a useful test for diagnosis in stable outpatients with WD, is not usually practical in the patient with ALF due to concerns of bleeding. Hepatic Cu quantitation was available in all 16 WD-ALF patients but most of these were explant or autopsy samples. In one patient of the 7 for whom histologic evaluation was available, the histochemical stain for Cu was reportedly negative. This reinforces the poor negative predictive value of this test for WD. In all of the patients, there was histologic evidence of bridging fibrosis (1) or cirrhosis (15) as would be expected in fulminant WD.
This study represents a relatively large cohort of well-characterized ALF-WD patients. It confirms other previous series demonstrating that serum Cp was neither sensitive nor specific for the diagnosis of WD in ALF (Table 3, Figure 5).6,7,11 This study also suggests that there is little utility of Cp measurement in this setting and that routine liver biochemistries may be of greater value. The interesting differences in sensitivities and specificities and correlations for these assays for Cp in patients with ALF-WD and those who had been diagnosed at the time of non-acute presentations suggest that Cp is still useful in the non-ALF setting. In both the ALF and chronic groups the oxidase method for Cp determination seemed superior to nephelometry with respect to specificity for a diagnosis of WD, however sensitivities were poor overall (Table 3). For unexplained reasons, sensitivity was lowest using the oxidase method in the ALF setting (Table 3). Thus, patients with ALF showed more individual variability in the levels of serum Cp obtained by either the immunological or oxidase assays as compared to the correlation of the results in chronically treated patients with WD. Whether this was due to interfering substances in the assay in the setting of ALF is uncertain at this time.
We had intended to use determination of non-ceruloplasmin bound Cu (total Cu minus Cpx3, by the oxidase method) as a discriminant test (as opposed to the total Cu).17 However, as noted above, there was greater variability in the Cp value in the setting of ALF and the non-ceruloplasmin bound Cu likewise did not correlate well with presence of WD in this setting (data not shown). Since Cp was more variable in ALF, the serum copper alone proved a better predictor (see Figure 1)
Unlike the Cp assays in ALF-WD, we confirmed that the use of an AP:TB ratio <4 as an initial screening tool was highly sensitive and specific for this diagnosis (Table 3, Figures 2 and 5). In addition, by combining the AP:TB ratio with determining that the AST:ALT ratio was >2.2, we found 100% sensitivity and specificity in our ALF-WD patients. An earlier study by Berman et al12 used a AP:TB ratio cut-off of 2.0, (compared to our value of <4), in combination with a lower cut-off for AST:ALT of 4.0 (rather than our value of 2.2). We have no ready explanation for this difference. A proposed explanation for the high AST:ALT ratio in WD patients is presumably due to the known increase in AST levels in patients with cirrhosis. Since nearly all subjects with ALF-WD have evidence of advanced fibrosis on biopsy, this may account for the tendency towards a high AST:ALT ratio. Elevation in serum copper was highly sensitive for WD in our series, but was not very specific as many other ALF patients demonstrated elevated levels (Figure 1, 5b). Acute increases in serum copper are well reported in patients with any form of acute liver injury due to impaired biliary excretion of copper.
Non-immune mediated hemolytic anemia remains relatively unique clinical feature amongst WD patients presenting with ALF. However, ALF patients due to HELLP and cancer frequently have a component of intravascular hemolysis with markedly elevated serum bilirubin and DIC parameters at presentation as well. In our study group, there was a clear difference in hemoglobin levels in patients presenting with WD versus those with other causes of ALF (Table 2, Figure 4). However given the clear overlaps in the ranges for hemoglobin in patients with ALF of all etiologies, other testing must be utilized to establish a clear diagnosis of WD.
The importance of available and rapid testing to confirm the diagnosis of WD cannot be underestimated. Rapid identification of ALF-WD patients affects treatment decisions and provides accurate information in order to screen pre-symptomatic family members. Interestingly, the two patients with Wilson disease that developed acute liver failure due to non-adherence to their medications or failure of their medical therapy to control their disease appeared to be indistinguishable biochemically from those with ALF-WD as their initial presentation. This suggests that all patients with ALF-WD have an acute injury superimposed on underlying chronic liver injury; biopsy findings in our patients and in other studies support this idea.20,21
ALF-WD has been shown to portend 100% mortality without emergency liver transplant and this was observed in our patients; therefore, early identification of WD as the etiology of the liver failure affects the management of these patients.22 The MELD scores calculated for the WD group exceeded all other patients, again emphasizing the need for quick, accurate diagnosis and early referral. Recent interventions including exchange transfusion, plasmapheresis, molecular adsorbent recirculating system, and albumin dialysis aimed at rapid reduction of serum copper to help break the cycle of hemolytic anemia and perpetuation of liver damage appear promising but to date have not altered the overall outcome of ALF-WD patients.23-25 Despite these advances, early diagnosis and listing for transplantation seems the best course in this setting. To that end, our findings have important implications: conventional measures of serum ceruloplasmin and copper levels are of less value in fulminant WD patients compared to chronic WD patients and are more difficult to obtain than standard laboratory measures. In this study we could not assess the utility of urine testing and liver copper for WD in patients with ALF and liver tissue was available only in a limited number of patients. In those with fulminant WD, urine copper and liver copper were clearly elevated (Table 1), and similar to serum copper, would likely be useful in suggesting WD. However their predictive value for WD in ALF remains to be proven.
Limitations of the current study include the relatively small number of ALF-WD patients enrolled. However, this cohort of 16 patients represents the largest group of ALF-WD patients ever reported. In addition, consecutive ALF patients from multiple centers over several years were included suggesting that our findings may be generalizable. Unfortunately, not all of the diagnostic tests were applied to every patient but all 16 patients had available liver tissue and were confirmed to be bona fide cases using several other criteria. Furthermore, competing identifiable causes of ALF had been eliminated in all cases. A final limitation of our study is the lack of a validation population in which to confirm our findings of the diagnostic utility of routine biochemical parameters in identifying ALF-WD patients but prior studies have noted similar findings.11-13
In summary, screening for a diagnosis of WD in the setting of ALF using ceruloplasmin measurements is unreliable. To accurately distinguish ALF-WD patients from others with ALF, use of alkaline phosphatase to total bilirubin and AST to ALT ratios as well as hemoglobin levels at presentation provides an acceptable and rapidly available alternative. Serum and urine copper and screening for Kayser Fleischer rings should still be performed when the diagnosis of WD is suspected since these are non-invasive and confirmatory tests. However, our data suggest that the diagnosis of ALF-WD can be established with certainty even before these results are available. By using routinely available tests, patients with fulminant WD can be identified in a prompt manner, enabling rapid listing of transplantation for this rare but virtually uniformly fatal disorder.
Acknowledgments
Financial Support: Supported by NIH R-01-DK 56389. Additional support was provided by the Jeanne Roberts and the Rollin and Mary Ella King Funds of the Southwestern Medical Foundation, Dallas, Texas.
Abbreviations
- ALFSG
Acute Liver Failure Study Group
- Cp
Ceruloplasmin
- WD
Wilson Disease
- ALF
Acute Liver Failure
- AST
Aspartate Aminotransferase
- ALT
Alanine Aminotransferase
- AP
Alkaline Phosphatase
- TB
Total Bilirubin
- Cu
Copper
- INR
International Normalized Ratio
- KF
Kayser Fleischer
Footnotes
The Acute Liver Failure Study Group 1998-2004 was comprised of the following investigators and coordinators who worked tirelessly in support of this study: William M. Lee (PI), Julie Polson, Carla Pezzia, Ezmina Lalani, Linda S. Hynan, Joan S. Reisch, University of Texas Southwestern Medical Center, Dallas TX;
Anne M. Larson, Hao Do, University of Washington, Seattle WA
Jeffrey S. Crippin, Laura Gerstle, Washington University School of Medicine, St. Louis MO;
Timothy J. Davern, Katherine Partovi, University of California at San Francisco CA;
Sukru Emre, Mt Sinai Medical Center, New York NY;
Timothy M. McCashland, Tamara Bernard, University of Nebraska, Omaha NE;
J. Eileen Hay, Cindy Groettum, Mayo Clinic, Rochester MN;
Natalie Murray, Sonnya Coultrup, Baylor University Medical Center, Dallas TX
A. Obaid Shakil, Diane Morton, University of Pittsburgh Medical Center, Pittsburgh PA;
Andres T. Blei, Jeanne Gottstein, Northwestern University Medical School, Chicago IL;
Atif Zaman, Jonathan Schwartz, Ken Ingram, Oregon Health Sciences University, Portland OR;
Steven Han, Val Peacock, University of California at Los Angeles, Los Angeles CA;
Robert J. Fontana, Suzanne Welch, University of Michigan Medical Center, Ann Arbor Brendan McGuire, Linda Avant, University of Alabama, Birmingham AL;
Raymond Chung, Deborah Casson, Massachusetts General Hospital, Boston MA.
Robert Brown Jr. and Michael Schilsky, Laren Senkbeil, Columbia-Presbyterian Medical Center/Cornell-New York Hospital, New York NY
M. Edwyn Harrison, Rebecca Rush, Mayo Clinic, Scottsdale, Scottsdale AZ
Adrian Reuben, Nancy Huntley, Medical University of South Carolina, Charleston SC
Santiago Munoz, Chandra Misra, Albert Einstein Medical Center, Philadelphia PA
Todd Stravitz, Jennifer Salvatori, Virginia Commonwealth University, Richmond VA
Lorenzo Rossaro, Colette Prosser, University of California, Davis; Sacramento CA
Raj Satyanarayana, Wendy Taylor, Mayo Clinic, Jacksonville, Jacksonville FL
Raj Reddy, Mical Campbell, University of Pennsylvania, Philadelphia, PA
Tarek Hassenein, Fatma Barakat, University of California at San Diego
Alistair Smith, Duke University, Durham, NC
References
- 1.Ostapowicz G, Fontana RJ, Schiodt FV, Larson A, Davern TJ, Han SH, McCashland TM, Shakil AO, Hay JE, Hynan L, Crippin JS, Blei AT, Samuel G, Reisch J, Lee WM, U.S. Acute Liver Failure Study Group Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–54. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 2.Roberts E, Schilsky ML. A practice guideline on Wilson disease. Hepatology. 2003;37:1475–1492. doi: 10.1053/jhep.2003.50252. [DOI] [PubMed] [Google Scholar]
- 3.Schilsky ML, Sternlieb I. Overcoming obstacles to the diagnosis of Wilson’s disease. Gastroenterology. 1997;113:350–353. [PubMed] [Google Scholar]
- 4.Willson RA, Clayson KJ, Leon S. Unmeasurable serum alkaline phosphatase activity in Wilson’s disease associated with fulminant hepatic failure and hemolysis. Hepatology. 1987;7:613–615. doi: 10.1002/hep.1840070347. [DOI] [PubMed] [Google Scholar]
- 5.Willson RA, Hartleb M. Fulminant wilsonian hepatitis. Am J Gastroenterol. 1988;83:1309–1310. [PubMed] [Google Scholar]
- 6.Steindl P, Ferenci P, Dienes HP, et al. Wilson’s disease in patients presenting with liver disease: A diagnostic challenge. Gastroenterology. 1997;113:212–218. doi: 10.1016/s0016-5085(97)70097-0. [DOI] [PubMed] [Google Scholar]
- 7.Shaver WA, Bhatt H, Combes B. Low serum alkaline phosphatase activity in Wilson’s disease. Hepatology. 1986;6:859–863. doi: 10.1002/hep.1840060509. [DOI] [PubMed] [Google Scholar]
- 8.Lee JJ, Kim HJ, Chung IJ, et al. Acute hemolytic crisis with fulminant hepatic failure as the first manifestation of Wilson’s disease: A case report. J Korean Med Sci. 1998;13:548–550. doi: 10.3346/jkms.1998.13.5.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoshino T, Kumasaka K, Kawano K, et al. Low serum alkaline phosphatase activity associated with severe Wilson’s disease. is the breakdown of alkaline phosphatase molecules caused by reactive oxygen species? Clin Chim Acta. 1995;238:91–100. doi: 10.1016/0009-8981(95)06073-m. [DOI] [PubMed] [Google Scholar]
- 10.Schilsky ML. Diagnosis and treatment of Wilson’s disease. Pediatr Transplant. 2002;6:15–19. doi: 10.1034/j.1399-3046.2002.1r069.x. [DOI] [PubMed] [Google Scholar]
- 11.McCullough AJ, Fleming CR, Thistle JL, et al. Diagnosis of Wilson’s disease presenting as fulminant hepatic failure. Gastroenterology. 1983;84(1):161–167. [PubMed] [Google Scholar]
- 12.Berman DH, Leventhal RI, Gavaler JS, Cadoff EM, Van Thiel DH. Clinical differentiation of fulminant wilsonian hepatitis from other causes of hepatic failure. Gastroenterology. 1991;100:1129–1134. doi: 10.1016/0016-5085(91)90294-u. [DOI] [PubMed] [Google Scholar]
- 13.Sallie R, Katsiyiannakis L, Baldwin D, et al. Failure of simple biochemical indexes to reliably differentiate fulminant Wilson’s disease from other causes of fulminant liver failure. Hepatology. 1992;16:1206–1211. [PubMed] [Google Scholar]
- 14.Morell AG, Windsor J, Sternlieb I, Scheinberg IH. Measurement of the concentration of ceruloplasmin by dermination of its oxidase activity. In: Sunderman SW, Sunderman SW Jr., editors. Laboratory Diagnosis of Liver Diseases. Warren H. Green; St.Louis: 1968. pp. 103–195. [Google Scholar]
- 15.Schilsky ML, Quintana N, Volenberg I, Kabishcher V, Sternlieb I. Spontaneous cholangiofibrosis in Long–Evans Cinnamon rats: A rodent model for Wilson’s disease. Lab Anim Sci. 1998;48:156–161. [PubMed] [Google Scholar]
- 16.Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D’Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–70. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 17.Schilsky ML, Tavill AS. Wilson Disease. In: Schiff ER, Sorrell MF, Maddrey WC, editors. Schiff’s Diseases of the Liver. Tenth ed Lippincott-Williams and Wilkins; Philadelphia: 2007. pp. 1023–1106. [Google Scholar]
- 18.Gow PJ, Smallwood RA, Angus PW, Smith AL, Wall AJ, Sewell RB. Diagnosis of Wilson’s disease: An experience over three decades. Gut. 2000;46:415–419. doi: 10.1136/gut.46.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ring-Larsen H, Pallazo U. Renal failure in fulminant hepatic failure and terminal cirrhosis: a comparison between incidence, types, and prognosis. Gut. 1981;22:585–91. doi: 10.1136/gut.22.7.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schilsky ML, Scheinberg IH, Sternlieb I. Hepatic transplantation for Wilson’s disease: Indication and outcome. Hepatology. 1994;19:583–587. doi: 10.1002/hep.1840190307. [DOI] [PubMed] [Google Scholar]
- 21.Emre S, Atillasoy EO, Ozdemir S, Schilsky ML, Rathna Varma CVR, Thung SN, Sternlieb I, Guy SR, Sheiner PA, Schwartz ME, Miller CM. Orthotopic liver transplantation for Wilson’s disease: a single center experience. Transplantation. 2001;72:1232–1236. doi: 10.1097/00007890-200110150-00008. [DOI] [PubMed] [Google Scholar]
- 22.Sokol RJ, Frances PD, Gold SH, Ford DM, Lum GM, Ambruso DR. Orthotopic liver transplantation for acute fulminant Wilson disease. J Pediatr. 1985;107:549–552. doi: 10.1016/s0022-3476(85)80016-0. [DOI] [PubMed] [Google Scholar]
- 23.Auth MK, Kim HS, Beste M, Bonzel KE, Baumann U, et al. Removal of metabolites, cytokines and hepatic growth factors by extracorporeal liver support in children. J Pediatr Gastroenterol Nutr. 2005;40:54–59. doi: 10.1097/00005176-200501000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Kreymann B, Seige M, Schweigart U, Kopp KF, Classen M. Albumin dialysis: effective removal of copper in a patient with fulminant Wilson disease and successful bridging to liver transplantation: a new possibility for the elimination of protein-bound toxins. J Hepatol. 1999;31:1080–1085. doi: 10.1016/s0168-8278(99)80322-5. [DOI] [PubMed] [Google Scholar]
- 25.Rakela J, Kurtz SB, McCarthy JT, Ludwig J, Ascher NL, Bloomer JR, Claus PL. Fulminant Wilson’s disease treated with postdilution hemofiltration and orthotopic liver transplantation. Gastroenterology. 1986;90:2004–2007. doi: 10.1016/0016-5085(86)90274-x. [DOI] [PubMed] [Google Scholar]