Abstract
The international prognostic score (IPS-7) is the most commonly used risk stratification tool for advanced Hodgkin lymphoma (HL), however recent studies suggest the IPS-7 is less discriminating due to improved outcomes with contemporary therapy. We evaluated the seven variables for IPS-7 recorded at study entry for 854 patients enrolled on E2496. Univariate and multivariate Cox models were used to assess their prognostic ability for freedom from progression (FFP) and overall survival (OS). The IPS-7 remained prognostic however its prognostic range has narrowed. On multivariate analysis 2 factors (age, stage) remained significant for FFP and 3 factors (age, stage, hemoglobin) for OS. An alternative prognostic index, the IPS-3, was constructed using age, stage, and hemoglobin, which provided 4 distinct risk groups [FFP (p=0.0001) and OS (p<0.0001)]. IPS-3 outperformed the IPS-7 on risk prediction for both FFP and OS by model fit and discrimination criteria. Using reclassification calibration 18% of IPS-7 low risk patients were re-classified as intermediate risk and 13% of IPS-7 intermediate risk patients as low risk. For patients with advanced HL, the IPS-3 may provide a simpler and more accurate framework for risk assessment in the modern era. Validation of these findings in other large data sets is planned.
Keywords: Hodgkin lymphoma, IPS, prognostic score, ABVD, Stanford V
Introduction
Hodgkin lymphoma (HL) is the most common lymphoid neoplasm in young patients, with a median age at diagnosis of 38 years, and approximately 40% of patients under age 35 Cancer facts and figures 2013. Atlanta GA: American Cancer Society, 2013.. In the current era, more than 75% of patients are cured with contemporary frontline therapy (Engert, et al 2012, Federico, et al 2009b, Gordon, et al 2013, Viviani, et al 2011). Despite this success, patients with primary refractory disease or those who relapse after salvage strategies continue to have poor outcomes (Arai, et al 2013). The most widely utilized clinical index to assign upfront risk in HL is the International Prognostic Score (IPS), a retrospectively developed clinical model with a primary endpoint of freedom from progression (FFP) (Hasenclever and Diehl 1998). The IPS was constructed in 1998 based on outcomes from approximately 4,600 patients treated on protocols for advanced stage HL prior to 1992. Complete data were available on 1,600 of these patients, and were used to fit the final Cox model. While the majority of patients had advanced stage (45% stage III, 43% stage IV), approximately 13% of patients were classified as stage I or II, and 22% had bulky mediastinal presentation. Therapy was variable and while the majority of patients (75%) were treated with at least 4 cycles of doxorubicin containing chemotherapy, 20% received mechlorethamine, oncovin, procarbazine, and prednisone (MOPP) or a similar regimen, which have been proved to be inferior to ABVD or other doxorubicin-containing regimens. Seven clinical parameters determined to be significant on multivariate analysis were independently associated with adverse clinical outcome; male sex, age >45 years, stage IV disease, hemoglobin <10.5g/dl, white blood count (wbc) ≥ 15 × 109/L, lymphocyte count < 0.6 × 109/L or <8% of total WBC, and albumin < 40g/L. On the basis of the number of factors present at diagnosis the IPS identified 6 subgroups of patients with 5 year FFP ranging from 42% to 84%, and overall survival (OS) of 56%-89% (Hasenclever and Diehl 1998).
Since the development of the IPS, there have been considerable improvements in therapy and supportive care in both the front line and relapsed setting, resulting in significant improvement in outcome (Eich, et al 2010, Engert, et al 2010, Straus, et al 2004, Younes, et al 2012). Additionally newer imaging modalities i.e. PET/CT may allow for more precise staging and response assessment during treatment (Barrington, et al 2014, Biggi, et al 2013, Cheson, et al 2014, Gallamini, et al 2007, Hutchings, et al 2005).
Although the IPS continues to be widely used, its utility for patients treated with contemporary regimens has been challenged. A retrospective analysis from British Columbia Cancer Agency (BCCA) in patients treated between 1980 and 2010 with ABVD, or an equivalent regimen reported an improvement in outcome and a diminished prognostic range of the IPS-7 with FFP ranging from 62% to 88% and OS ranging from 67% to 98% (Moccia, et al 2012). To assess the utility of the individual IPS-7 factors in the contemporary era we analyzed data from a prospective phase III randomized trial ECOG 2496, a study that evaluated ABVD versus Stanford V in advanced HL (Gordon, et al 2013).
Patients and Methods
Patient Population
Between 1996 and 2006, 854 patients were enrolled on the North American Intergroup trial E2496, a Randomized Phase III Trial of ABVD versus Stanford V in Locally Extensive and Advanced Stage Hodgkin Lymphoma (Gordon, et al 2013). As IPS was one of the stratification factors used in the trial, all 7 IPS variables were recorded at the time of study entry.
Statistical Analysis
FFP was defined as the time from study entry to disease progression or relapse; deaths that occurred during remission that were not preceded by disease progression/relapse were censored. OS was defined as the time from study entry to death from any cause. The Kaplan-Meier method and Cox proportional regression model were used to estimate failure rates, hazard ratios (HRs), and 95% CIs. Log-rank test was used to compare the survival distributions between groups Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association 53: 457-481, 1958., Cox, D. R.; Oakes, D. (1984). Analysis of Survival Data. New York: Chapman & Hall. ISBN 041224490X.
The prognostic ability of individual IPS factors was evaluated for FFP and OS in both univariate and multivariable Cox regression models. A new 3-factor prognostic score (IPS-3) was constructed utilizing factors that were significant in multivariate Cox models. Possible alternative cut-off points for the 3 selected factors were evaluated using Chi-square statistics. FFP and OS curves were used to classify patients into 3 risk groups low, intermediate, and high based on number of risk factors: 0-2, 3-4 and ≥5 factors for IPS-7; 0, 1-2 and 3 factors for IPS-3, respectively. The prognostic performance of IPS-7 and IPS-3 was evaluated using the global model fit criteria Akaike information criterion (AIC), concordance probability estimate (CPE), and time-dependent AUC (area under ROC curve) for both FFP and OS Akaike, H. (1974) A new look at the statistical model identification. IEEE Transactions on Automatic Control, 19, 716–723, Gonen, M. & Heller, G. (2005) Concordance probability and discriminative power of proportional hazards regression. Biometrika, 92, 965–970. Lower AIC, higher CPE, and/or higher AUC indicated better concordance. The reclassification calibration method was used to cross-tabulate all risk groups by IPS-7 and IPS-3 (Cook and Ridker 2009, Heagerty and Zheng 2005). Specifically, for cases that were re-classified to different risk group by IPS-3, the observed FFP and OS estimates were compared to survival rates predicted by IPS-7 and IPS-3 respectively. The primary analysis includes all randomized patients. An additional subset analysis was performed on patients with advanced stage III-IV. The analysis was performed using SAS 9.3 and R 3.0.3.
Results
Patient Characteristics
Patient characteristics comparing E2496, original IPS report and the BCCA cohort are shown in Table S1. In E2496 most patients (62%) had stage III or IV disease and the remainder (37%) bulky stage I or II disease.
Evaluation of Outcome for E2496 Patients by IPS-7
As previously reported, there were no significant differences between the two arms (ABVD versus Stanford V) for failure free survival (FFS) and OS at a median follow-up of 6.4 years (Gordon, et al 2013).
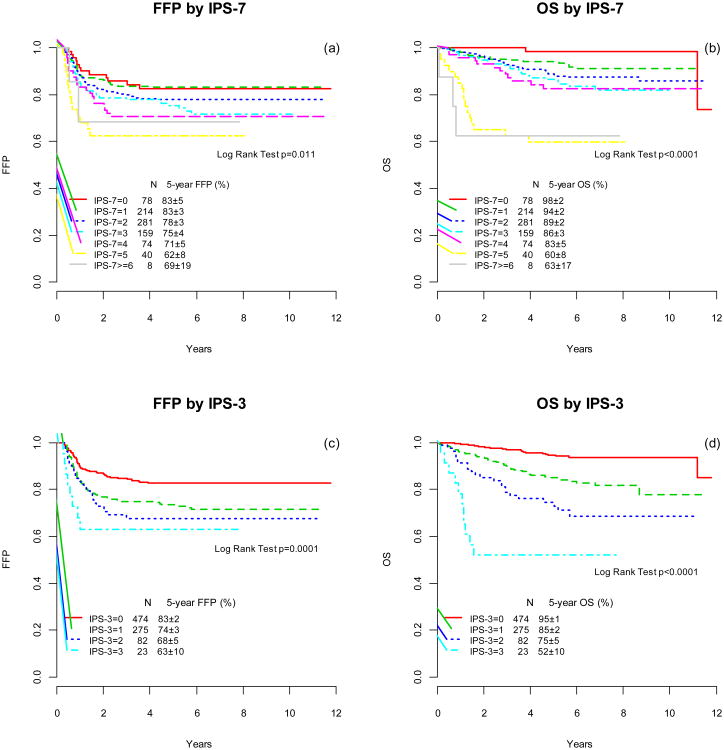
The IPS-7 remained prognostic for FFP (p=0.012) and OS (p<.0001) as shown in Figure 1a and 1b, however, the separation among IPS-7 groups has narrowed. For patients in both the highest and lowest risk groups, Kaplan-Meier curves of FFP and OS largely overlap. Risk prediction by IPS-7 in the E2496 cohort is similar to the BCC report, but differs from the original report by Hasenclever (Table S2).
Figure 1.
Freedom from progression (FFP) and overall survival (OS) according to the International Prognostic Score (IPS) factors (a, b) and the simpler prognostic index IPS-3 (c,d)
Significance of Individual Seven Factors Used in IPS-7
Table 1 summarizes the results of univariate and multivariate Cox regression assessing the prognostic ability of the 7 individual IPS factors for both FFP and OS. On multivariate analysis, two factors (hemoglobin level and stage) were significant in predicting FFP and three factors (age, hemoglobin level, and stage) were significant for OS. Lymphocyte count was marginally significant for FFP prediction in univariate analysis (p=0.08), but lost its prognostic effect (p=0.29) when put together in the model adjusting for hemoglobin level and stage. A similar observation was seen for albumin in predicting OS (p=0.04 in univariate analysis and 0.31 in multivariate analysis).
Table 1. Individual IPS factors with FFP and OS by Univariate and Multivariate Analysis.
| FFP | OS | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | |||||
| IPS factor | HR(95% CI) | p-value | HR(95% CI) | p-value | HR(95% CI) | p-value | HR(95% CI) | p-value |
| Age≥45 years | 1.3 (0.9-1.8) | 0.15 | 1.2 (0.9-1.8) | 0.27 | 4.0 (2.7-5.9) | <0.0001 | 3.7 (2.5-5.5) | <0.0001 |
| Albumin<4g/dl | 1.0 (0.7-1.4) | 0.92 | 0.8 (0.6-1.1) | 0.21 | 1.6 (1.0-2.5) | 0.04 | 1.3 (0.8-2.1) | 0.31 |
| WBC≥15,000 mm3 | 1.3 (0.9-2.0) | 0.20 | 1.3 (0.8-1.9) | 0.28 | 1.1 (0.7-2.0) | 0.64 | 1.2 (0.7-2.1) | 0.52 |
| Hgb<10.5g/dl | 1.8 (1.3-2.6) | 0.0003 | 1.7 (1.2-2.5) | 0.004 | 2.8 (1.9-4.2) | <0.0001 | 2.1 (1.3-3.3) | 0.002 |
| Lymph count <600 mm3or <8% of WBC | 1.3 (1.0-1.9) | 0.08 | 1.2 (0.8-1.7) | 0.29 | 1.2 (0.7-1.9) | 0.48 | 1.0 (0.6-1.6) | 0.91 |
| Male | 1.1 (0.8-1.5) | 0.55 | 1.1 (0.8-1.5) | 0.55 | 1.1 (0.8-1.6) | 0.57 | 1.1 (0.7-1.6) | 0.68 |
| Stage IV | 1.7 (1.3-2.4) | 0.0005 | 1.5 (1.1-2.1) | 0.02 | 2.3 (1.6-3.4) | <0.0001 | 1.7 (1.1-2.5) | 0.02 |
Factors and numbers in bold indicate significant p-values in the analysis.
Evaluation of a Simpler 3 Factor Prognostic Score (IPS-3)
An alternative prognostic score (IPS-3) was constructed utilizing the variables that remained significant for predicting FFP or OS on multivariate analysis (Table 1): age≥45 years, hemoglobin<10.5g/dl, and stage IV. More than half (56%) of the patients had none of these risk factors, 32% of patients had 1 risk factor, and 10% 2 risk factors. Only 3% (23 patients) had all 3 risk factors. The IPS-3 was significant for both FFP (p=0.0001) and OS (p<.0001) and separated patients into 4 distinct risk groups (Figures 1c and 1d). The five-year FFP by IPS-3 was 83%, 74%, 68%, and 63% and OS 95%, 85%, 75%, and 52% for patients with 0, 1, 2, and 3 risk factors, respectively.
We also explored different cut-off values of age, stage, and hemoglobin: age 50 versus 55 versus 60, stage III/IV versus stage I/II, and hemoglobin of < 10 g/dl versus <9.5 g/dl. Chi-square statistics and hazard ratio associated with different cut-off values are displayed in Table S2. The largest Chi-square statistics selected to refine the IPS-3 were age>55, stage III/IV, and hemoglobin <10.5 g/dl. The results comparing the two models (original versus refined IPS-3) did not show meaningful difference to support the alternative cut-offs. There were very little differences in CPE between the two models (0.58 versus 0.60 for FFP, and 0.65 versus 0.65 for OS). Therefore for further analysis the original cut-off points were used in IPS-3.
Subset Analysis in Patients with Advanced Stage Diseases
To assess the utility of the IPS-7 and the IPS-3 in patients restricted to advanced disease (stage III-IV) we performed an additional subset analysis. Among the 529 (62%) patients in this category, IPS-7 was not significant for FFP [p=0.15, Figure S1 (a)], but remained highly prognostic for OS [p<=0.0001; Figure S1 (b)]. For IPS-3 there is separation for FFP curves [Figure S1, (c)], however the difference is not statistically significant (p=0.11). IPS-3 also remained highly prognostic for OS [p<=0.0001; Figure S1 (d)].
Comparison of IPS-3 to IPS-7
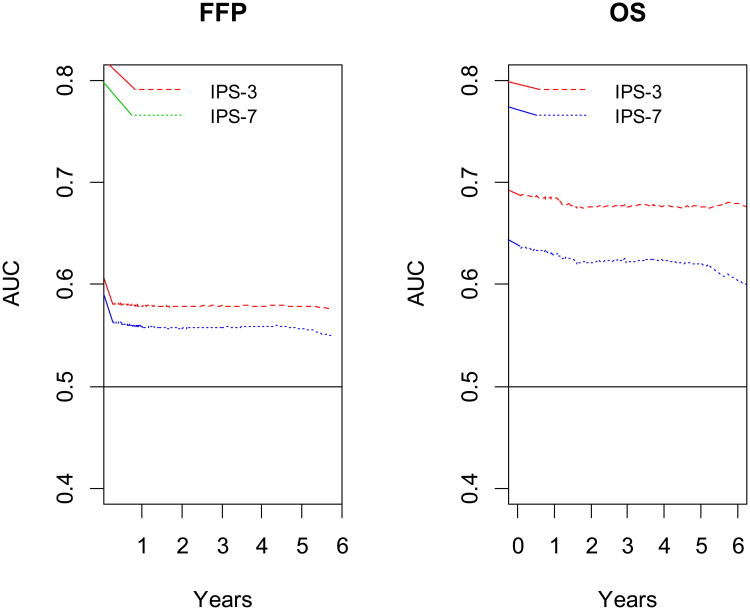
The time-dependent AUC ranked IPS-3 higher than IPS-7 at all times for both FFP and OS (Figure 2a and 2b). IPS-3 was also ranked higher than IPS-7 using the global fit discriminatory measure (CPE), with 0.58 versus 0.55 for FFP, and 0.65 versus 0.59 for OS. These results suggest a better concordance between the observed data and IPS-3. Lower AIC was seen for IPS-3 than for IPS-7 (2375 versus 2381 for FFP, and 1313 versus 1336 for OS) again indicating better model fit with IPS-3 for both FFP and OS.
Figure 2.
Time weighted area under the receiver operator characteristics curves (AUC) for freedom-from progression (A) and overall survival (B) according to the International Prognostic Score factors IPS-7 and simpler prognostic index IPS-3.
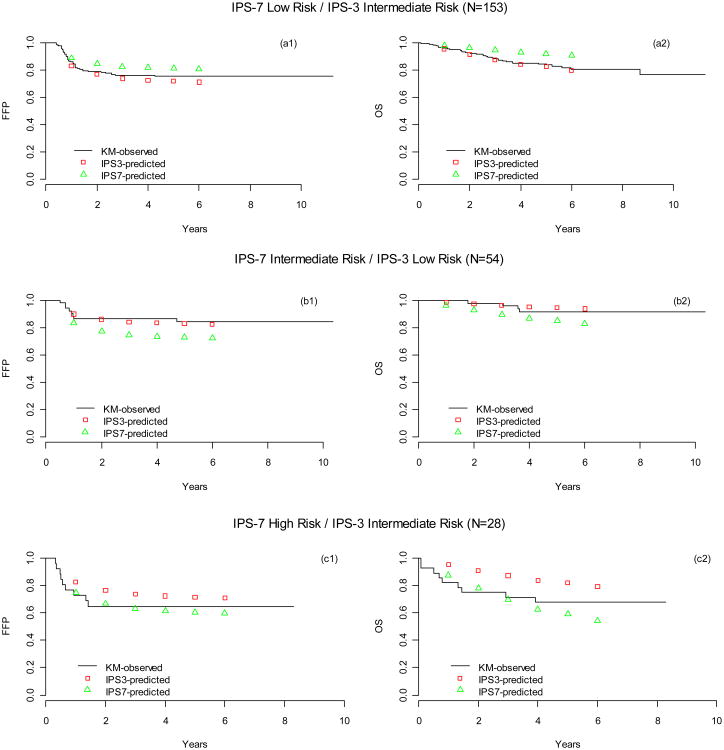
We also compared IPS-3 to IPS-7 using reclassification calibration methods. A total of 238 (28%) patients were reclassified to different risk categories by IPS-3 vs IPS-7 (Table 2). Among the IPS-7 low risk patients, 153 (18%) were reclassified as intermediate risk by IPS-3. Both FFP and OS were closer to the predicted survival rates made by IPS-3 than by IPS-7, suggesting that IPS-3 was more accurate for the reclassified 153 patients [Figure 3 (a1, a2)]. Similarly the IPS-3 reclassified 54 (13%) of IPS -7 intermediate risk patients as low risk [Figure 3 (b1, b2)], with FFS and OS curves closer to the predicted survival rates made by IPS-3 than by IPS-7. A very small number (n=28, 3%) of patients were reclassified from IPS-7 high risk to IPS-3 intermediate risk. For this latter group IPS-7 was a better predictor of FFP than IPS-3, however there was no substantial difference in predicting OS, [Figure 3 (c1,c2)].
Table 2. Reclassification: Number of Patients in Each Risk Category by IPS-7 and IPS-3.
| IPS-7 | |||||
|---|---|---|---|---|---|
| Low Risk (0-2) | Intermediate Risk (3-4) | High Risk (5-7) | Total | ||
| IPS-3 | Low Risk (0) | 420 | 54 | 0 | 474 (56%) |
| Intermediate Risk (1-2) | 153 | 176 | 28 | 357 (42%) | |
| High Risk (3) | 0 | 3 | 20 | 23 (3%) | |
| Total | 573 (67%) | 233 (27%) | 48 (6%) | 854 | |
Numbers in bold indicate patients who were reclassified.
Figure 3. Kaplan-Meier (KM) analysis of observed and predicted (by IPS-7 and IPS-3) FFP and OS for patients whose risk categories were reclassified by IPS-3.
N=153 patients low risk by IPS-7 but intermediate risk by IPS-3 (a1, a2); n=54 patients intermediate risk by IPS-7 but low risk by IPS-3 (b1, b2); n=28 patients high risk by IPS-7 but intermediate risk by IPS-3 (c1,c2). The predicted survival rates were produced by univariate Cox models using IPS-3 and IPS-7 as covariates.
Discussion
Our analysis of data from E2496 shows that the IPS-7 remains prognostic in the contemporary era, however the magnitude of the differences for both FFP and OS for the lowest and highest risk patients have narrowed, and are consistent with the findings from the BCCA (Moccia, et al 2012). In our dataset, only 2 of the original 7 factors used in the IPS-7 remain significant for FFP (age and stage), and 3 factors for OS (age, stage, and hemoglobin). Based on this, a simpler prognostic score the IPS-3 was constructed that identified 4 distinct risk groups based on 0,1,2, or 3 risk factors, which provided risk-stratification for both FFP (p=0.0001) and OS (p<0.0001). As compared to IPS-7, better global prognostic performance of IPS-3 was suggested by model fit and discrimination criteria. In addition, IPS-3 reclassified 18% of IPS-7 low risk patients as intermediate risk, and 13% of IPS-7 intermediate risk patients as low risk, with observed FFP and OS rate estimates among reclassified patients closer to those predicted by the corresponding IPS-3 category. In our data set, the number of patients in the highest risk group were too few to draw any valid conclusions.
Our study population differs from original IPS-7 report with respect to stage at diagnosis. In the latter study patients with early stage high-risk disease (stages I, II) comprised 13% of the patient population, compared to 37% high-risk stage I and II patients in E2496. Thus to evaluate whether this discrepancy skewed outcomes towards more favorable outcomes with IPS-3 we performed an additional subset analysis restricted to the patients with advanced stage (III-IV) disease. While 4 distinct risk groups were noted the differences were not statistically significant for FFP (p=0.11) and remained highly significant for OS (p<=0.0001). This was also the case for IPS-7 in our study where differences in FFP were not significant (p=0.15). This suggests that patients with a high IPS-3 or IPS-7 at diagnosis may have a worse outcome at relapse than patients with low risk scores.
Therapeutic advances such as the replacement of MOPP chemotherapy with ABVD, use of autologous stem cell transplant at relapse, growth factors to support dose intensity, improved imaging modalities which allow for accurate staging, and increased diagnostic accuracy are some of the key reasons which have contributed to the improvement in patient outcomes in the contemporary era. Yet despite these advances, some patients continue to have extremely poor outcomes. Clinical prognostic factors incorporated within the IPS-7 reflect the interaction of the biologic heterogeneity of HL with treatment. In the original IPS-7 analyses all risk factors were equally weighted. In contrast in the contemporary era, only 3 of these risk factors remained significant and were used to construct IPS-3. Advanced age is well recognized in several studies to be associated with inferior outcome (Engert, et al 2005, Evens, et al 2013, Landgren, et al 2003). Elderly patients are not well represented in the original IPS-7, as only 9% of patients were older than 55 years, with none older than 65 years (Hasenclever and Diehl 1998). By comparison, patients up to age 83 were included in E2496, and 157 (18%) of patients were older than 45 years. Assessing alternative age cutoffs did not appear to improve the prognostic power of the IPS-3, as only a minority (n=12, 1%) of patients were included in the highest risk groups; however using an age cutoff of 50 rather than 45 did appear to potentially discriminate better Additionally age did not appear to affect the total chemotherapy dose received(Evens, et al 2013). It is less clear why anemia conferred a higher risk factor for poor OS than other lab parameters such as low albumin, leukopenia, and lymphocytosis. The anemia may have correlated with poor overall fitness and/or poor marrow reserve. It is plausible that anemia may be associated with adverse tumor biology, increased inflammatory cytokine production, and/or macrophage infiltration. Clearly this warrants further investigation.
A potential limitation of our study is that the study patients in E2496 were treated with one of two chemotherapy regimens (ABVD or Stanford V), thus the question of whether the IPS-3 would be applicable to other current dose intense regimens such as escalated BEACOPP, or to novel therapies such as brentuximab vedotin remains to be studied. Another limitation is that despite the overall large sample size of our study, there were very few patients (n=23) who had all 3 IPS-3 risk factors. Finally for the very small number of patients (n=28) of patients who were reclassified from IPS-7 high risk to IPS-3 intermediate risk, the IPS-7 was a better predictor of FFP than IPS-3, however there was no substantial difference in predicting OS. Therefore validation of the IPS-3 in other data sets is definitely required, and modification of the IPS-3 to further refine risk assessment is ongoing. In a largely curable disease it is important to individualize therapy by accurately selecting patients based on risk factors at diagnosis. With the multiplicity of therapeutic options now available for HL, accurate assessment of risk based on outcomes expected with contemporary therapy is more important now than ever before. The distinction of 4 groups by IPS-3 has clinical significance as new therapeutic advances are required for high risk patients, while minimizing therapy to alter the spectrum of long-term therapy related toxicity becomes more important for the low risk patients. As therapeutic strategies evolve these alternative indices are increasingly necessary for patient identification and risk stratification for clinical trials that utilize risk-adapted treatment approaches. There is precedent to refine prognostic scores in other lymphomas as clinical outcomes have evolved with improved therapies as reflected by the revised IPI (R-IPI) in DLBCL, which redistributed the 5 IPI elements into 3 prognostic groups with 4 year OS ranging from 55% to 94% (Sehn, et al 2007), the elderly IPI (E-IPI) which used an age cut off of 70 years rather than the 60 years used in the IPI, and provided additional prognostic discrimination for elderly patients of low and low-intermediate risk (Advani, et al 2010), the NCCN IPI in DLBCL(Zhou, et al 2014), and the Follicular Lymphoma International Prognostic Index-2 (FLIPI-2) which stratified patients into 3 quartiles based on low risk (0), intermediate risk (1-2), and high risk (Federico, et al 2009a).
Advances in of HL biology suggest the significance of the interaction of the HRS cell with its tumor microenvironment for clinical outcomes. Expression of CD68+ and CD163 by tumor-associated macrophages (TAMS) is correlated with inferior PFS and OS (Kamper, et al 2011, Steidl, et al 2012, Steidl, et al 2010, Tan, et al 2012). More recently, NanoString digital expression profiling described a 23-gene signature which identified a high risk subset of advanced stage HL patients (Scott, et al 2013). Other novel prognostic indicators such as EBV expression and lymphocyte/monocyte ratio have also shown to have prognostic significance (Kanakry, et al 2013). Moving forward, a truly comprehensive risk assesment platform must take these biologic features as well as clinical factors into account. Efforts to incorporate these advances into the IPS-3 are ongoing.
Supplementary Material
Key Points.
Analysis of 854 advanced HL patients treated on E2496 confirms that the IPS-7 has decreased prognostic discrimination in the contemporary era.
A simpler prognostic score, the IPS-3, outperformed the IPS-7 and may provide a more accurate framework for risk prediction.
Acknowledgments
Supported in part by research funding from the American Cancer Society (MRSG-14-052-01-LIB) CD
This study was coordinated by the ECOG-ACRIN Cancer Research Group (Robert L. Comis, MD and Mitchell D. Schnall, MD, PhD, Group Co-Chairs) and supported in part by Public Health Service CA21115, CA180820, CA180794, CA23318, CA66636, CA17145, CA13650, CA180790, CA21076, CA180799, CA180816, CA11083, CA27525, CA32102, CA46282, CA77440, CA77597, CA77658, CA31946, CA33601, CA77202 and from the National Cancer Institute, National Institutes of Health and the Department of Health and Human Services. Its content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Footnotes
Presented in part: 9th International Hodgkin Lymphoma Symposium, Cologne 2013; American Society of Hematology Annual Meeting, 2013.
Authorship Contributions: CD, HL, FH, RA designed the research.
CD, HL, FH, RA performed the research.
LG, RF, NB, MC, RG, HW, PS, BC, DS, BK, JF, KB, TH, JT, RH, SH, RA collected data.
CD, HL, FH, RA analyzed and interpreted data
HL, FH performed statistical analysis
CD, HL, FH, RA wrote the manuscript.
All authors reviewed and approved the manuscript.
Disclosures of Conflicts of Interest: CD: no conflicts of interest to disclose
HL: no conflicts of interest to disclose
FH: no conflicts of interest to disclose
LG: no conflicts of interest to disclose
RF: no conflicts of interest to disclose
NB: no conflicts of interest to disclose
MC: no conflicts of interest to disclose
RD: no conflicts of interest to disclose
HW: no conflicts of interest to disclose
PS: no conflicts of interest to disclose
BC: no conflicts of interest to disclose
DS: no conflicts of interest to disclose
BK: no conflicts of interest to disclose
JF: no conflicts of interest to disclose
KB: no conflicts of interest to disclose
TH: no conflicts of interest to disclose
JT: no conflicts of interest to disclose
RH: no conflicts of interest to disclose
SH: no conflicts of interest to disclose
RA: no conflicts of interest to disclose
References
- Advani RH, Chen H, Habermann TM, Morrison VA, Weller EA, Fisher RI, Peterson BA, Gascoyne RD, Horning SJ Eastern Cooperative Oncology, G.,Cancer, Leukemia Group, B. & Southwest Oncology, G. Comparison of conventional prognostic indices in patients older than 60 years with diffuse large B-cell lymphoma treated with R-CHOP in the US Intergroup Study (ECOG 4494, CALGB 9793): consideration of age greater than 70 years in an elderly prognostic index (E-IPI) Br J Haematol. 2010;151:143–151. doi: 10.1111/j.1365-2141.2010.08331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai S, Fanale M, DeVos S, Engert A, Illidge T, Borchmann P, Younes A, Morschhauser F, McMillan A, Horning SJ. Defining a Hodgkin lymphoma population for novel therapeutics after relapse from autologous hematopoietic cell transplant. Leuk Lymphoma. 2013;54:2531–2533. doi: 10.3109/10428194.2013.798868. [DOI] [PubMed] [Google Scholar]
- Barrington SF, Mikhaeel NG, Kostakoglu L, Meignan M, Hutchings M, Mueller SP, Schwartz LH, Zucca E, Fisher RI, Trotman J, Hoekstra OS, Hicks RJ, O'Doherty MJ, Hustinx R, Biggi A, Cheson BD. Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol. 2014;32:3048–3058. doi: 10.1200/JCO.2013.53.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggi A, Gallamini A, Chauvie S, Hutchings M, Kostakoglu L, Gregianin M, Meignan M, Malkowski B, Hofman MS, Barrington SF. International validation study for interim PET in ABVD-treated, advanced-stage hodgkin lymphoma: interpretation criteria and concordance rate among reviewers. J Nucl Med. 2013;54:683–690. doi: 10.2967/jnumed.112.110890. [DOI] [PubMed] [Google Scholar]
- Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, Lister TA, Alliance AL, Lymphoma G Eastern Cooperative Oncology, G., European Mantle Cell Lymphoma, C., Italian Lymphoma, F., European Organisation for, R., Treatment of Cancer/Dutch Hemato-Oncology, G., Grupo Espanol de Medula, O., German High-Grade Lymphoma Study, G., German Hodgkin's Study, G., Japanese Lymphorra Study, G., Lymphoma Study, A., Group, N.C.T.,Nordic Lymphoma Study, G., Southwest Oncology, G. & United Kingdom National Cancer Research, I. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–3068. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook NR, Ridker PM. Advances in measuring the effect of individual predictors of cardiovascular risk: the role of reclassification measures. Ann Intern Med. 2009;150:795–802. doi: 10.7326/0003-4819-150-11-200906020-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eich HT, Diehl V, Gorgen H, Pabst T, Markova J, Debus J, Ho A, Dorken B, Rank A, Grosu AL, Wiegel T, Karstens JH, Greil R, Willich N, Schmidberger H, Dohner H, Borchmann P, Muller-Hermelink HK, Muller RP, Engert A. Intensified chemotherapy and dose-reduced involved-field radiotherapy in patients with early unfavorable Hodgkin's lymphoma: final analysis of the German Hodgkin Study Group HD11 trial. J Clin Oncol. 2010;28:4199–4206. doi: 10.1200/JCO.2010.29.8018. [DOI] [PubMed] [Google Scholar]
- Engert A, Ballova V, Haverkamp H, Pfistner B, Josting A, Duhmke E, Muller-Hermelink K, Diehl V German Hodgkin's Study, G. Hodgkin's lymphoma in elderly patients: a comprehensive retrospective analysis from the German Hodgkin's Study Group. J Clin Oncol. 2005;23:5052–5060. doi: 10.1200/JCO.2005.11.080. [DOI] [PubMed] [Google Scholar]
- Engert A, Haverkamp H, Kobe C, Markova J, Renner C, Ho A, Zijlstra J, Kral Z, Fuchs M, Hallek M, Kanz L, Dohner H, Dorken B, Engel N, Topp M, Klutmann S, Amthauer H, Bockisch A, Kluge R, Kratochwil C, Schober O, Greil R, Andreesen R, Kneba M, Pfreundschuh M, Stein H, Eich HT, Muller RP, Dietlein M, Borchmann P, Diehl V German Hodgkin Study, G., Swiss Group for Clinical Cancer, R. & Arbeitsgemeinschaft Medikamentose, T. Reduced-intensity chemotherapy and PET-guided radiotherapy in patients with advanced stage Hodgkin's lymphoma (HD15 trial): a randomised, open-label, phase 3 non-inferiority trial. Lancet. 2012;379:1791–1799. doi: 10.1016/S0140-6736(11)61940-5. [DOI] [PubMed] [Google Scholar]
- Engert A, Plutschow A, Eich HT, Lohri A, Dorken B, Borchmann P, Berger B, Greil R, Willborn KC, Wilhelm M, Debus J, Eble MJ, Sokler M, Ho A, Rank A, Ganser A, Trumper L, Bokemeyer C, Kirchner H, Schubert J, Kral Z, Fuchs M, Muller-Hermelink HK, Muller RP, Diehl V. Reduced treatment intensity in patients with early-stage Hodgkin's lymphoma. N Engl J Med. 2010;363:640–652. doi: 10.1056/NEJMoa1000067. [DOI] [PubMed] [Google Scholar]
- Evens AM, Hong F, Gordon LI, Fisher RI, Bartlett NL, Connors JM, Gascoyne RD, Wagner H, Gospodarowicz M, Cheson BD, Stiff PJ, Advani R, Miller TP, Hoppe RT, Kahl BS, Horning SJ. The efficacy and tolerability of adriamycin, bleomycin, vinblastine, dacarbazine and Stanford V in older Hodgkin lymphoma patients: a comprehensive analysis from the North American intergroup trial E2496. Br J Haematol. 2013;161:76–86. doi: 10.1111/bjh.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federico M, Bellei M, Marcheselli L, Luminari S, Lopez-Guillermo A, Vitolo U, Pro B, Pileri S, Pulsoni A, Soubeyran P, Cortelazzo S, Martinelli G, Martelli M, Rigacci L, Arcaini L, Di Raimondo F, Merli F, Sabattini E, McLaughlin P, Solal-Celigny P. Follicular lymphoma international prognostic index 2: a new prognostic index for follicular lymphoma developed by the international follicular lymphoma prognostic factor project. J Clin Oncol. 2009a;27:4555–4562. doi: 10.1200/JCO.2008.21.3991. [DOI] [PubMed] [Google Scholar]
- Federico M, Luminari S, Iannitto E, Polimeno G, Marcheselli L, Montanini A, La Sala A, Merli F, Stelitano C, Pozzi S, Scalone R, Di Renzo N, Musto P, Baldini L, Cervetti G, Angrilli F, Mazza P, Brugiatelli M, Gobbi PG Trial, H.D.G.I.p.l.S.d.L. ABVD compared with BEACOPP compared with CEC for the initial treatment of patients with advanced Hodgkin's lymphoma: results from the HD2000 Gruppo Italiano per lo Studio dei Linfomi Trial. J Clin Oncol. 2009b;27:805–811. doi: 10.1200/JCO.2008.17.0910. [DOI] [PubMed] [Google Scholar]
- Gallamini A, Hutchings M, Rigacci L, Specht L, Merli F, Hansen M, Patti C, Loft A, Di Raimondo F, D'Amore F, Biggi A, Vitolo U, Stelitano C, Sancetta R, Trentin L, Luminari S, Iannitto E, Viviani S, Pierri I, Levis A. Early interim 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography is prognostically superior to international prognostic score in advanced-stage Hodgkin's lymphoma: a report from a joint Italian-Danish study. J Clin Oncol. 2007;25:3746–3752. doi: 10.1200/JCO.2007.11.6525. [DOI] [PubMed] [Google Scholar]
- Gordon LI, Hong F, Fisher RI, Bartlett NL, Connors JM, Gascoyne RD, Wagner H, Stiff PJ, Cheson BD, Gospodarowicz M, Advani R, Kahl BS, Friedberg JW, Blum KA, Habermann TM, Tuscano JM, Hoppe RT, Horning SJ. Randomized phase III trial of ABVD versus Stanford V with or without radiation therapy in locally extensive and advanced-stage Hodgkin lymphoma: an intergroup study coordinated by the Eastern Cooperative Oncology Group (E2496) J Clin Oncol. 2013;31:684–691. doi: 10.1200/JCO.2012.43.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin's disease. International Prognostic Factors Project on Advanced Hodgkin's Disease. N Engl J Med. 1998;339:1506–1514. doi: 10.1056/NEJM199811193392104. [DOI] [PubMed] [Google Scholar]
- Heagerty PJ, Zheng Y. Survival model predictive accuracy and ROC curves. Biometrics. 2005;61:92–105. doi: 10.1111/j.0006-341X.2005.030814.x. [DOI] [PubMed] [Google Scholar]
- Hutchings M, Mikhaeel NG, Fields PA, Nunan T, Timothy AR. Prognostic value of interim FDG-PET after two or three cycles of chemotherapy in Hodgkin lymphoma. Ann Oncol. 2005;16:1160–1168. doi: 10.1093/annonc/mdi200. [DOI] [PubMed] [Google Scholar]
- Kamper P, Bendix K, Hamilton-Dutoit S, Honore B, Nyengaard JR, d'Amore F. Tumor-infiltrating macrophages correlate with adverse prognosis and Epstein-Barr virus status in classical Hodgkin's lymphoma. Haematologica. 2011;96:269–276. doi: 10.3324/haematol.2010.031542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanakry JA, Li H, Gellert LL, Lemas MV, Hsieh WS, Hong F, Tan KL, Gascoyne RD, Gordon LI, Fisher RI, Bartlett NL, Stiff P, Cheson BD, Advani R, Miller TP, Kahl BS, Horning SJ, Ambinder RF. Plasma Epstein-Barr virus DNA predicts outcome in advanced Hodgkin lymphoma: correlative analysis from a large North American cooperative group trial. Blood. 2013;121:3547–3553. doi: 10.1182/blood-2012-09-454694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgren O, Algernon C, Axdorph U, Nilsson B, Wedelin C, Porwit-MacDonald A, Grimfors G, Bjorkholm M. Hodgkin's lymphoma in the elderly with special reference to type and intensity of chemotherapy in relation to prognosis. Haematologica. 2003;88:438–444. [PubMed] [Google Scholar]
- Moccia AA, Donaldson J, Chhanabhai M, Hoskins PJ, Klasa RJ, Savage KJ, Shenkier TN, Slack GW, Skinnider B, Gascoyne RD, Connors JM, Sehn LH. International Prognostic Score in advanced-stage Hodgkin's lymphoma: altered utility in the modern era. J Clin Oncol. 2012;30:3383–3388. doi: 10.1200/JCO.2011.41.0910. [DOI] [PubMed] [Google Scholar]
- Scott DW, Chan FC, Hong F, Rogic S, Tan KL, Meissner B, Ben-Neriah S, Boyle M, Kridel R, Telenius A, Woolcock BW, Farinha P, Fisher RI, Rimsza LM, Bartlett NL, Cheson BD, Shepherd LE, Advani RH, Connors JM, Kahl BS, Gordon LI, Horning SJ, Steidl C, Gascoyne RD. Gene expression-based model using formalin-fixed paraffin-embedded biopsies predicts overall survival in advanced-stage classical Hodgkin lymphoma. J Clin Oncol. 2013;31:692–700. doi: 10.1200/JCO.2012.43.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehn LH, Berry B, Chhanabhai M, Fitzgerald C, Gill K, Hoskins P, Klasa R, Savage KJ, Shenkier T, Sutherland J, Gascoyne RD, Connors JM. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood. 2007;109:1857–1861. doi: 10.1182/blood-2006-08-038257. [DOI] [PubMed] [Google Scholar]
- Steidl C, Diepstra A, Lee T, Chan FC, Farinha P, Tan K, Telenius A, Barclay L, Shah SP, Connors JM, van den Berg A, Gascoyne RD. Gene expression profiling of microdissected Hodgkin Reed-Sternberg cells correlates with treatment outcome in classical Hodgkin lymphoma. Blood. 2012;120:3530–3540. doi: 10.1182/blood-2012-06-439570. [DOI] [PubMed] [Google Scholar]
- Steidl C, Lee T, Shah SP, Farinha P, Han G, Nayar T, Delaney A, Jones SJ, Iqbal J, Weisenburger DD, Bast MA, Rosenwald A, Muller-Hermelink HK, Rimsza LM, Campo E, Delabie J, Braziel RM, Cook JR, Tubbs RR, Jaffe ES, Lenz G, Connors JM, Staudt LM, Chan WC, Gascoyne RD. Tumor-associated macrophages and survival in classic Hodgkin's lymphoma. The New England journal of medicine. 2010;362:875–885. doi: 10.1056/NEJMoa0905680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus DJ, Portlock CS, Qin J, Myers J, Zelenetz AD, Moskowitz C, Noy A, Goy A, Yahalom J. Results of a prospective randomized clinical trial of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) followed by radiation therapy (RT) versus ABVD alone for stages I, II, and IIIA nonbulky Hodgkin disease. Blood. 2004;104:3483–3489. doi: 10.1182/blood-2004-04-1311. [DOI] [PubMed] [Google Scholar]
- Tan KL, Scott DW, Hong F, Kahl BS, Fisher RI, Bartlett NL, Advani RH, Buckstein R, Rimsza LM, Connors JM, Steidl C, Gordon LI, Horning SJ, Gascoyne RD. Tumor-associated macrophages predict inferior outcomes in classic Hodgkin lymphoma: a correlative study from the E2496 Intergroup trial. Blood. 2012;120:3280–3287. doi: 10.1182/blood-2012-04-421057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viviani S, Zinzani PL, Rambaldi A, Brusamolino E, Levis A, Bonfante V, Vitolo U, Pulsoni A, Liberati AM, Specchia G, Valagussa P, Rossi A, Zaja F, Pogliani EM, Pregno P, Gotti M, Gallamini A, Rota Scalabrini D, Bonadonna G, Gianni AM, Michelangelo F Gruppo Italiano di Terapie Innovative nei, L. & Intergruppo Italiano, L. ABVD versus BEACOPP for Hodgkin's lymphoma when high-dose salvage is planned. N Engl J Med. 2011;365:203–212. doi: 10.1056/NEJMoa1100340. [DOI] [PubMed] [Google Scholar]
- Younes A, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, Ramchandren R, Bartlett NL, Cheson BD, de Vos S, Forero-Torres A, Moskowitz CH, Connors JM, Engert A, Larsen EK, Kennedy DA, Sievers EL, Chen R. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin's lymphoma. J Clin Oncol. 2012;30:2183–2189. doi: 10.1200/JCO.2011.38.0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Sehn LH, Rademaker AW, Gordon LI, Lacasce AS, Crosby-Thompson A, Vanderplas A, Zelenetz AD, Abel GA, Rodriguez MA, Nademanee A, Kaminski MS, Czuczman MS, Millenson M, Niland J, Gascoyne RD, Connors JM, Friedberg JW, Winter JN. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood. 2014;123:837–842. doi: 10.1182/blood-2013-09-524108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.