Abstract
Background and Objectives
Photodynamic therapy (PDT) has shown potentially beneficial results in treating port-wine stain, but its benefit–risk profile remains undefined. This study aimed to evaluate the efficacy and safety of PDT conducted with hemoporfin and a 532 nm continuous wave laser to treat port-wine stain clinically.
Patients and Methods
This randomized clinical trial was conducted in eight hospitals in China. Participants were adolescent and adult patients (age range: 14–65 years old) with port-wine stain. During stage 1 (day 1 to week 8) all patients were randomized at a 3:1 ratio to treatment (532 nm laser irradiation (96–120 J/cm2) with hemoporfin (5mg/kg; PDT-hemoporfin, n = 330)) or placebo groups (irradiation with placebo (PDT-placebo, n = 110)); during stage 2 (week 8 to 16) patients in both groups were offered treatment. Clinician-evaluators, who were blind to the study, classified each case on the following four-level scale according to assessment of before and after standardized pictures of the lesion area: no improvement: <20%; some improvement: 20–59%; great improvement: 60–89%; or nearly completely resolved: ≥90%. The primary efficacy endpoint was proportion of patients achieving at least some improvement at week 8. The secondary efficacy endpoints were proportion of patients achieving nearly completely resolved or at least great improvement at week 8, proportion of patients achieving early completely resolved, at least great improvement, or at least some improvement at week 16, and the corresponding satisfaction of the investigators and the patients (designated as ‘excellent’, ‘good’, ‘moderate’, or ‘ineffective’) at weeks 8 and 16.
Results
Compared to the PDT-placebo group, the PDT-hemoporfin group showed a significantly higher proportion of patients that achieved at least some improvement (89.7% [n = 295; 95% CI, 85.9%-92.5%] vs. 24.5% [n = 27; 95% CI, 17.4%-33.3%]) at week 8 (P < 0.0001) and higher improvements for all secondary efficacy endpoints. Treatment reactions occurred in 99.5% (n = 731; 95% CI, 98.7%-99.8%) of the PDT-hemoporfin treatments (n = 735). Hyperpigmentation occurred in 22.9 per 100 patient-treatments (n = 168; 95% CI, 20.0–26.0) in the PDT-hemoporfin treated patients.
Conclusions
Hemoporfin-mediated PDT is an effective and safe treatment option for adolescent and adult patients with port-wine stain.
Trial Registration
Chinese Clinical Trial Registry ChiCTR-TRC-08000213
Introduction
Port-wine stain (PWS) is the most common congenital vascular malformation reported in 0.3% of infants born worldwide [1]. The visible manifestation of this disorder is often considered a disfigurement and the accompanying social stigma often causes psychological problems for the affected individuals [2]. While no cure for PWS has yet been found, many treatment options have been developed and put into clinical practice; these approaches range from moderately risky (such as covering the PWS with tattoos) to substantially risky (such as radiation), yet cosmetically acceptable results are rarely achieved [3,4]. Even with the current preferred clinical treatment of pulsed-dye laser (PDL), 19–27% of patients achieved ≥75% clearance in randomized clinical trials [5,6], and recurrence or redarkening of the treated PWS occur frequently [7,8]. Therefore, the need for effective and safe modalities to treat PWS remains unfulfilled.
One potential treatment modality is photodynamic therapy (PDT), which uses photosensitizer, light, and oxygen to induce a photochemical reaction that generates highly-reactive singlet oxygen molecules, which are able to cause cell death via apoptosis, necrosis or autophagy [9]. PDT has already been shown to be a successful management tool for treating neoplastic and non-malignant diseases [9,10]. Studies of PDT as a vascular-targeted approach to treat PWS have provided potentially beneficial results [11,12].
PDT using the porphyrin-related photosensitizer hematoporphyrin monomethyl ether (HMME), combined with application of alternative light sources (such as copper vapour laser), appears to represent an effective approach for treating PWS [13,14]. Preliminary studies for PDT using a new product of HMME, hemoporfin, have identified the optimal wavelength as 532 nm [15]. However, the effectiveness and safety of this comprehensive modality (PDT + hemoporfin + 532 nm wavelength) remains to be established by a prospective study of a large population. Therefore, this study was designed as a randomized, double-blind, placebo-controlled phase 3 clinical trial to test the hypothesis that treatment with PDT using an optimized protocol (5mg/kg hemoporfin and 532 nm continuous wave lasers with fluence of 96–120 J/cm2) would be effective and safe for patients with PWS.
Materials and Methods
Patients
Adolescent and adult patients (age range: 14 to 65 years-old) with clinical diagnosis of PWS were recruited to the study from eight research centers in China (one each located in Beijing, Nanjing, Guangzhou, Xian, Wuhan, and Changsha, and two in Shanghai), all of which are affiliated with large general teaching hospitals. For study enrollment, each patient was required to have adequate renal (serum creatinine and blood urea nitrogen ≤1.5 upper limit of normal [ULN]) and hepatic (alanine aminotransferase and aspartate transaminase ≤1ULN, and total bilirubin ≤1.5 ULN) functions and no history of treatment with isotope, laser or PDT, or systemic treatment for PWS during the past 4 weeks, or topical treatment during the past 2 weeks. Patients were considered ineligible if any one or more of the following conditions were present: other vascular malformations, vessel-related syndromes, or other conditions that might interfere with the study; allergy to porphyrins and analogues; photosensitivity; porphyria; allergic constitution; scar diathesis; immunocompromised conditions; electrocardiographic abnormalities or organic heart diseases; coagulation disorders; psychiatric diseases; severe endocrinopathies; current or one-month previous history of medications that might cause photosensitivity; women who were currently pregnant or lactating.
The study protocols and amendments were approved by the Ethics Committee of Peking University First Hospital. Written informed consent was obtained from all study participants prior to enrollment; for patients younger than 18-years-old, the informed consent was provided by a parent or legal guardian. This study was registered in the Chinese Clinical Trial Registry (Registration number: ChiCTR-TRC-08000213, URL: http://www.chictr.org.cn/showprojen.aspx?proj=9313).
Interventions
The injectable formulation of hemoporfin (sterile, lyophilized powder) was manufactured in accordance with the national Good Manufacturing Processes (GMP) standard of China, and supplied by Shanghai Fudan-Zhangjiang Bio-Pharmaceutical Co., Ltd. (China). At each center involved in this study, a dedicated manager was assigned for the hemoporfin storage and dispensing.
Before treatment, a treatment-target epidermal site (≤7 cm diameter) was chosen in the PWS area, and the surrounding skin was carefully covered. A fresh working solution of hemoporfin (5 mg/kg, the dose was determined based on our previous studies [16,17]) was prepared by dissolving in normal saline for immediate transfusion (constant speed over 20 min). A group of patients were transfused with normal saline alone and served as the placebo control group. The infusion apparatus was prepared by a nurse and completely covered to avoid potential photodecomposition. At 10 minutes after the transfusion had been initiated, the 532 nm continuous wave laser (see S1 Table for features) was applied to the target site with a power density of 80–100 mW/cm2 for a total of 20 minutes; these parameters were used according to pharmacokinetic parameters and the results of a phase IIa study of hemoporfin [16]. Therefore, the fluence was 96–120 J/cm2. No allergy testing against hemoporfin was performed prior to the treatment. No anesthesia was required before or after the treatment. Patients were not sedated and wore protective eye goggles throughout the treatment. After the treatment, patients were instructed to avoid strong light exposure and to wear sunglasses, a hat, and long-sleeved clothing if any outdoor activities were required for two weeks, in order to prevent effects of photosensitivity.
Study design
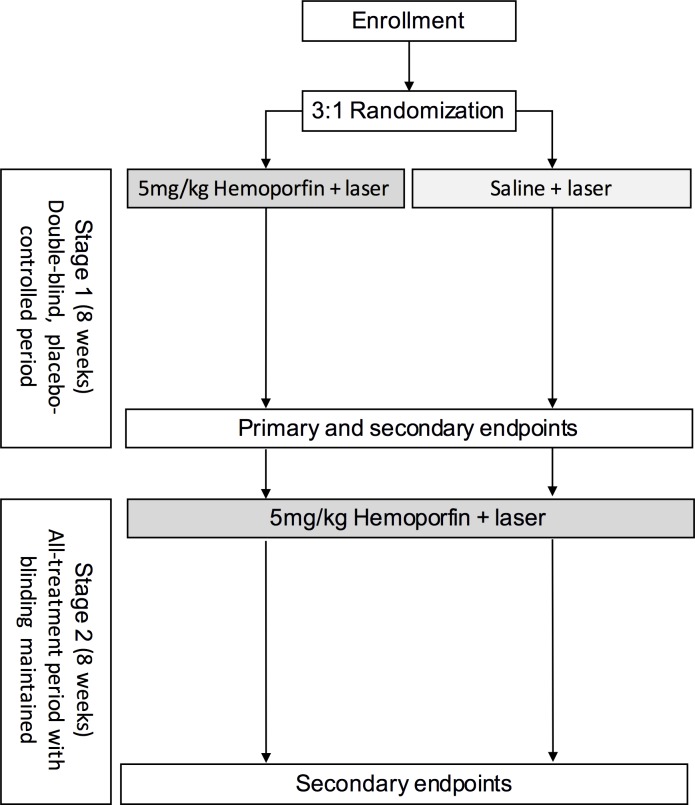
This study was designed to be conducted in two stages over a total 16-week period. The initial 8-week stage represented the double-blind, placebo-controlled treatment period (stage 1: day 1 to week 8) and aimed to establish efficacy. The subsequent 8-week stage represented the all-treatment period (stage 2: week 8 to week 16) and aimed to assess the overall efficacy and safety profiles.
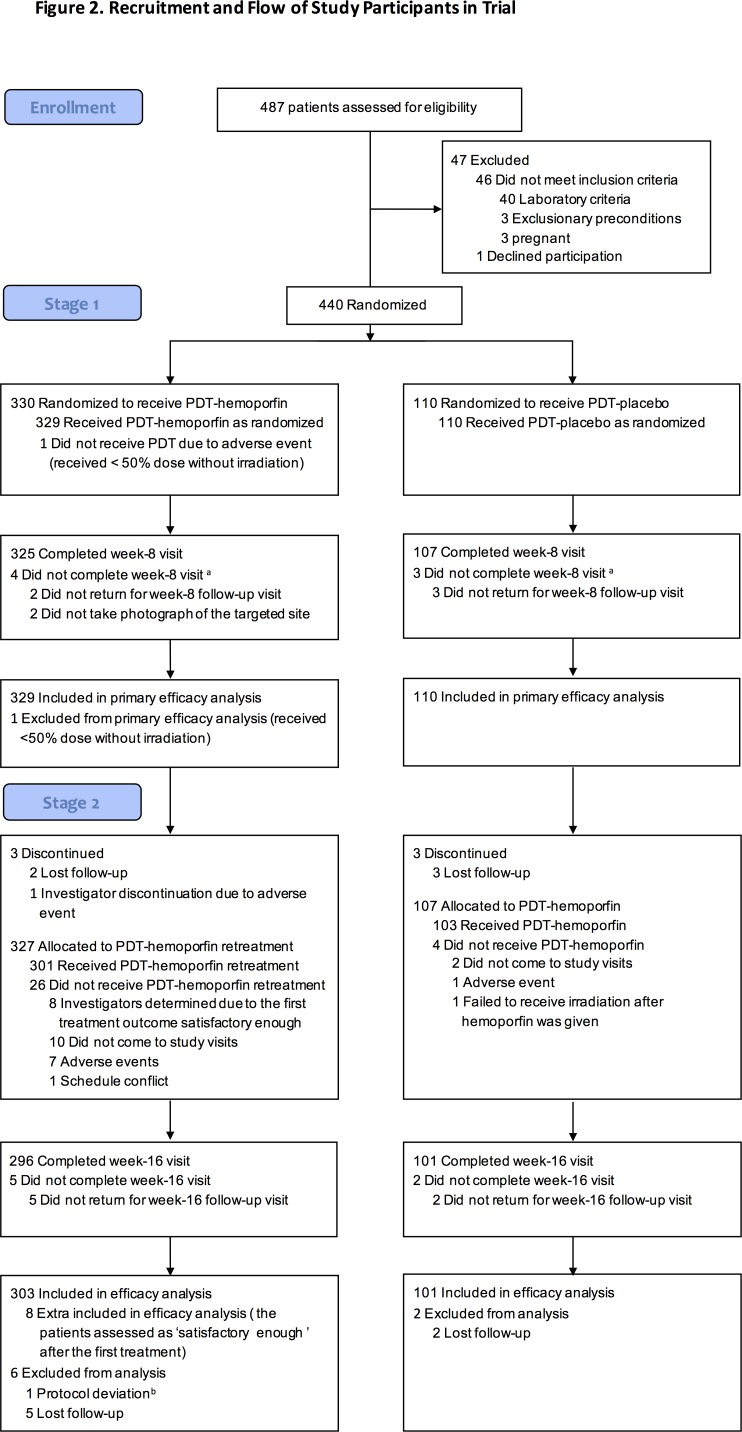
Upon enrollment, patients were randomized at a 3:1 ratio to receive hemoporfin or placebo, respectively, by using a block randomization scheme (block size = 4, 110 blocks) stratified according to investigational site. At day 1 (Stage 1), the patients received laser irradiation with hemoporfin (designated as the PDT-hemoporfin group) or irradiation with placebo (designated as the PDT-placebo group), with both the physician and the patients unaware of the assignment. To ensure all patients received appropriate treatment for their PWS, including those in the placebo group, the study was designed so that at week 8 (stage 2), all the patients received hemoporfin-PDT. In addition to the days of treatment (day 1 and week 8) visits, follow-up visits occurred on post-treatment day 4, 4 days after the week 8 visit, and week 16. If an adequately satisfactory treatment response had been achieved at week 8, the patient could opt to not receive the second treatment. The profile of patients throughout the study is summarized in Fig 1, and the trial design is shown in Fig 2.
Fig 1. Flowchart of patient enrollment, treatment group allotment, and progression through the study period.
Abbreviations: PDT-hemoporfin, hemoporfin-mediated PDT; PDT-placebo, laser irradiation plus placebo. Denotations: aData from participants were included in the primary efficacy analysis, missing data were imputed as no improvement. bA different area of the targeted lesion was treated.
Fig 2. Trial design.
Efficacy analyses
Standardized digital photos of the targeted sites were taken from three different angles (at 90° and at 45° to the left and right of the treated surface) before and after each treatment, and at each visit (standard operating procedure described in the S3 File). Prior to photographing, each PWS lesion was labeled with a marker of red coloration that was used as a reference marker and as a quality control marker in the photographs for subsequent judgment of treatment efficacy. Three blinded evaluators (one dermatologist and two plastic surgeons, who were otherwise not involved in the study) independently reviewed the photos from both stages of the study and graded the extent of PWS fading (improvement) according to color blanching from the baseline in the treated area and using the following four-level scale: no improvement (NI): <20%; some improvement (SI): 20–59%; great improvement (GI): 60–89%; or nearly completely resolved (CR): ≥90%. The results were deemed valid when two or more evaluators agreed; otherwise, the response was re-evaluated until a consensus of two or more was achieved. Initial disagreement in the evaluation occurred for only 4.62% of the patients in the primary efficacy analysis. The primary efficacy endpoint was proportion of patients achieving at least SI at week 8. The secondary efficacy endpoints were proportion of patients achieving CR and at least GI at week 8, proportion of patients achieving CR, at least GI or at least SI at week 16, and corresponding satisfaction of the investigators and the patients themselves, which was designated as ‘excellent’, ‘good’, ‘moderate’, or ‘ineffective’ at weeks 8 and 16.
For post hoc objective efficacy assessment, the digital photos were analyzed as previously described [18,19]. Briefly, after opening in the ImageJ software [20], each photo was converted into an erythema index (EI, representing the intensity of redness) image file. A region of interest (ROI) was selected and its EI was automatically measured. For each image, triplicate ROIs within the PWS lesion (ls) and within adjacent normal skin (ns) were given EI measurements for comparative analysis of the averaged values, respectively. To maintain operational accuracy, we created a macro of the ImageJ software which was then used to process the images.
The EI difference (ΔEI) was calculated as: EIls−EIns. Hence, the ΔEI value represented the difference in the degree of erythema between the PWS lesion and the adjacent normal skin.
Safety analyses
Clinical assessments by the physicians and all local or systemic events reported by the patients were recorded in detail. The following symptoms were recorded as treatment reactions: local burning sensation, pain, pruritus, numbness, edema, purpura, blistering, and crusting at the treatment site. Laboratory examinations (including routine blood and urine tests, liver and renal function tests, as well as electrocardiograms) were also performed (at baseline, day 4, week 8, and 4 days after week 8) to monitor adverse events. All adverse events and treatment reactions recorded during the 16-week trial period were followed-up until they had completely resolved. The adverse events were coded according to the World Health Organization Adverse Reaction Terminology (WHO-ART, version 2000) [21] and the extent of each adverse event was defined as follows: mild, awareness of a sign or symptom that is otherwise easily tolerable; moderate, discomfort that is sufficient to cause interference with normal activities; severe, incapacitating and inhibiting the ability to perform normal activities. Causality between the study drug and an adverse event was defined using the WHO-UMC causality assessment system [22]. A serious adverse event was defined as any adverse events occurring at any doses that might result in death, threaten the life of the patient, require hospitalization or a prolonged stay in the hospital, cause long-term or significant disability, or cause congenital malformations.
Statistical analyses
With chi-square test, we calculated that the sample size of 88 patients (66 in the treatment group and 22 in the placebo group) would provide 90% power to detect a 40% difference in the proportion of patients achieving at least SI (improvement ≥20%) at week 8, on the basis of a two-sided significance level of 0.05 and assuming that 75% of the patients in the treatment group would achieve at least SI according to the prior phase II studies [16]. However, considering the minimum sample size of 300 cases that is demanded by official regulatory for safety observation for new drug registration in China and assuming a 10% drop-out rate, we planned to include 440 patients (330 in treatment group and 110 in the placebo group).
Analysis of the primary outcome was conducted with chi-square test in the intent-to-treat (ITT) population (all randomized patients who had received at least one dose of treatment in each group); the seven patients (1.59%; four in the PDT-hemoporfin group and three in the placebo group) with missing post-baseline data were imputed as ‘no improvement’, according to the non-responder imputation approach. Univariate and stepwise multivariate logistic regression analyses were performed to assess the significance of differences in the treatment group and to identify potential confounding factors. Further subgroup analyses were made by the chi-square tests or Fisher’s exact tests (S2 and S3 Tables according to sex, age group (adolescent: 14–18 years old, young adult:19–30 years old, and older adult: 31–65 years old), PWS type (pink, purple, and hypertrophic), and location (centrofacial, non-centrofacial, and neck).
Analysis of the secondary outcomes, including proportion of patients achieving CR or at least GI at week 8, proportion of patients achieving CR, at least GI, or at least SI at week 16, and the satisfaction of the investigators and the patients at weeks 8 and 16, were made using chi-square test or Fisher’s exact test. An additional analysis was performed using t-test to compare the changes of ΔEI between two groups at week 8 and 16. Missing data were not imputed for some secondary outcomes. Eight of the patients who had achieved an adequately satisfactory response at week 8 after the initial treatment and opted to not receive a second treatment were imputed in the efficacy analysis at week 16; data from these patients were analyzed according to their original treatment group (all were in the PDT-hemoporfin group).
The safety analysis was based on event incidence rates adjusted for exposure (one treatment of a patient was defined as a patient-treatment). The evaluable-for-safety population consisted of those patients who had received study medication and who had at least one post-baseline safety evaluation. The treatment reactions and adverse events were analyzed separately using chi-square tests or Fisher’s exact test.
All statistical analyses were carried out by the SAS statistical software package (version 9.1.3). All statistical tests were two-sided with a significance (a) level of 0.05.
Results
Study patients
The study was conducted from 2008 to 2010. All 440 study participants had skin type III-IV on the Fitzpatrick phototype scale. The PDT-hemoporfin group (n = 329) had slightly more males, greater height and weight, and less pink-type PWS lesions than the PDT-placebo group (n = 110) (Table 1). 5.0% of the total study population had received prior treatment for PWS with laser, medication, or other therapeutic procedures.
Table 1. Patients’ demographics and disease history†.
| Feature | PDT-hemoporfin | PDT-placebo | |
|---|---|---|---|
| n = 329 | n = 110 | ||
| Age (years) | 24.95±7.80 | 24.17±6.84 | |
| Age group (years, n[%]) | |||
| 14–18 | 48(14.6) | 13(11.8) | |
| 19–30 | 220(66.9) | 82(74.5) | |
| 31–65 | 61(18.5) | 15(13.6) | |
| Males, n(%) | 138(41.9) | 33(30.0) | |
| Females, n(%) | 191(58.1) | 77(70.0) | |
| Chinese ethnicity, n(%) | |||
| Han | 323(98.2) | 110(100.0) | |
| Non-Han | 6(1.8) | 0(0.0) | |
| Occupation, n(%) | |||
| PL | 36(10.9) | 15(13.6) | |
| Non-PL | 293(89.1) | 95(86.4) | |
| Height (cm) | 165.09±7.95 | 163.39±7.31 | |
| Weight (kg) | 57.35±9.97 | 54.67±8.33 | |
| Systolic blood pressure (mmHg) | 113.01±13.09 | 112.85±12.07 | |
| Diastolic blood pressure (mmHg) | 72.85±9.00 | 73.31±8.69 | |
| Heart rate (bpm) | 80.38±10.59 | 80.09±10.82 | |
| Previous therapy, n(%) | 19(5.8) | 3(2.7) | |
| Location of PWS, n(%) | |||
| Non-CF | 105(31.9) | 36(32.7) | |
| CF | 162(49.2) | 64(58.2) | |
| Neck | 60(18.2) | 9(8.2) | |
| Other | 2(0.6) | 1(0.9) | |
| Type of PWS‡, n(%) | |||
| Pink | 111(33.7) | 49(44.5) | |
| Purple | 189(57.4) | 56(50.9) | |
| Hypertrophic | 29(8.8) | 5(4.5) | |
| Area of targeted site (cm2) | 34.38±15.81 | 31.64±16.00 | |
| Power density (mW/cm2) | 86.51±3.27 | 86.63±3.18 | |
| ΔEI at baseline (95% CI) | 39.94(38.04 to 41.84) | 39.90(36.92 to 42.88) | |
Abbreviations: PL, physical laborers; CF, centrofacial
Denotations
† Data are presented as means ± SD, unless otherwise specified.
‡ Pink type: flat PWS lesion with color of light pink to red, which fades completely upon pressure; Purple type: flat PWS lesion with color of purple to dark purple, which fades completely or incompletely upon pressure; Hypertrophic type: thickened PWS lesion with a nodular or raised surface (above the around normal skin plane), having a dark purple color, which incompletely fades or shows no fade upon pressure.
Efficacy
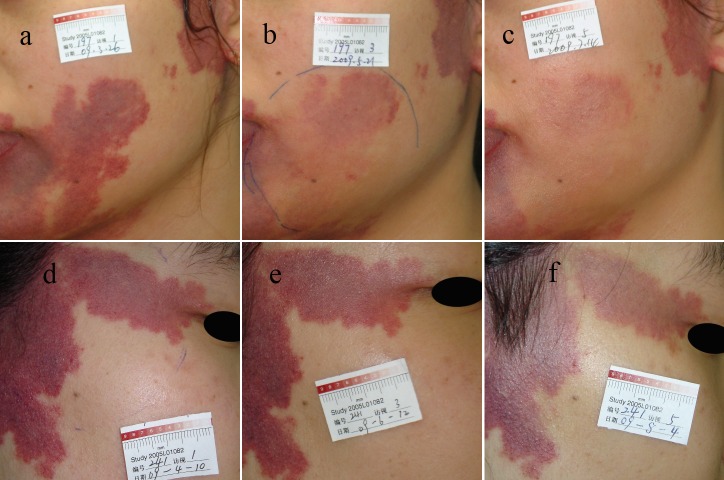
In total, 439 patients were eligible for primary analysis. (Fig 3A–3F) shows representative images of PWS patients during the 16-week study period. In general, the PDT-hemoporfin group had significantly higher proportions of patients in all response categories at post-treatment week 8 (vs. PDT-placebo: at least SI, 89.7% (n = 295; 95% CI, 85.9%-92.5%) vs. 24.5% (n = 27; 95% CI, 17.4%-33.3%); at least GI, 43.5% (n = 143; 95% CI, 38.2%-48.9%) vs. 0.9% (n = 1; 95% CI, 0.2%-5.0%); CR, 11.2% (n = 37; 95% CI, 8.3%-15.1%) vs. 0.0% (n = 0; 95% CI, 0.0%-3.4%); P<0.0005) (Table 2).
Fig 3. Efficacy of PDT-hemoporfin for treating PWS during the 16-week study period.
Panels a, b and c represent a patient from the PDT-hemoporfin group and d, e and f represent a patient from the placebo group; Panels a and d: baseline; b: after 1 PDT-hemoporfin treatment at week 8; c: after 2 PDT-hemoporfin treatments at week 16; e: after PDT-placebo at week 8; f: after 1 PDT-hemoporfin treatment at week 16; Panels c, b, f and e show PWS fading by ≥90%, 60–89%, 20–59% and <20%, respectively.
Table 2. Efficacy assessment at week 8 and 16.
| Efficacy assessment | Week 8 | Week 16 | ||||
|---|---|---|---|---|---|---|
| PDT-hemoporfin | PDT-placebo | P value | HH | PH | P value | |
| Grading and response rates of PWS fading, n(% [95% CI]) | ||||||
| N | 329 # | 110 ¶ | - | 303※ | 101 | - |
| At least SI (≥20%) | 295 (89.7 [85.9 to 92.5]) | 27 (24.5 [17.4 to 33.3]) | <0.0001a | 295 (97.4 [94.9 to 98.7]) | 91 (90.1 [82.7 to 94.5]) | 0.0022 |
| At least GI (≥60%) | 143 (43.5 [38.2 to 48.9]) | 1 (0.9 [0.2 to 5.0]) | <0.0001 | 194 (64.0 [58.5 to 69.2]) | 43 (42.6 [33.4 to 52.3]) | 0.0001 |
| CR (≥90%) | 37 (11.2 [8.3 to 15.1]) | 0 (0.0 [0.0 to 3.4]) | 0.0002 | 85 (28.1 [23.3 to 33.4]) | 8 (7.9 [4.1 to 14.9]) | <0.0001 |
| GI (60–89%) | 106 (32.2 [27.4 to 37.4]) | 1 (0.9 [0.2 to 5.0]) | - | 109 (36.0 [30.8 to 41.5]) | 35 (34.7 [26.1 to 44.3]) | - |
| SI (20–59%) | 152 (46.2 [40.9 to 51.6]) | 26 (23.6 [16.7 to 32.4]) | - | 101 (33.3 [28.3 to 38.8]) | 48 (47.5 [38.1 to 57.2]) | - |
| NI (<20%) | 34 (10.3 [7.5 to 14.1]) | 83 (75.5 [66.6 to 82.5]) | - | 8 (2.6 [1.3 to 5.1]) | 10 (9.9 [5.5 to 17.3]) | - |
| ΔEI, mean(95% CI) | ||||||
| N | 329 # | 110 ¶ | - | 301(2) § | 101 | - |
| ΔEIbefore | 39.94 (38.04 to 41.84) | 39.90 (36.92 to 42.88) | 0.9840 | - | - | - |
| ΔEIafter | 29.58 (27.92 to 31.24) | 38.42 (34.78 to 42.05) | <0.0001 | 25.11 (23.35 to 26.88) | 31.16 (27.90 to 34.41) | 0.0010 |
| Subjective assessment of efficacy by the physicians, n(% [95% CI]) | ||||||
| n(missing) | 327(2) § | 107(3) § | - | 303 | 101 | - |
| Good to Excellent | 231 (70.7 [65.5 to 75.4]) | 2 (1.9 [0.5 to 6.6]) | <0.0001 | 252 (83.1 [78.5 to 86.9]) | 61 (60.4 [50.6 to 69.4]) | <0.0001 |
| Excellent | 82 (25.1) | 0 (0.0) | - | 121 (39.9) | 20 (19.8) | - |
| Good | 149 (45.6) | 2 (1.9) | - | 131 (43.2) | 41 (40.6) | - |
| Moderate | 80 (24.5) | 3 (2.8) | - | 45 (14.9) | 28 (27.7) | - |
| Ineffective | 16 (4.9) | 102 (95.3) | - | 6 (2.0) | 12 (11.9) | - |
| Subjective assessment of efficacy by the patients, n(% [95% CI]) | ||||||
| n(missing) | 327(2) § | 108(2) § | - | 303 | 101 | - |
| Good to Excellent | 221 (67.6 [62.3 to 72.4]) | 3 (2.8 [1.0 to 7.9]) | <0.0001 | 245 (80.9 [76.1 to 84.9]) | 61 (60.4 [50.6 to 69.4]) | <0.0001 |
| Excellent | 96 (29.4) | 0 (0.0) | - | 102 (33.7) | 17 (16.8) | - |
| Good | 125 (38.2) | 3 (2.8) | - | 143 (47.2) | 44 (43.6) | - |
| Moderate | 80 (24.5) | 8 (7.4) | - | 48 (15.8) | 30 (29.7) | - |
| Ineffective | 26 (8.0) | 97 (89.8) | - | 10 (3.3) | 10 (9.9) | - |
Abbreviations: HH, patients who received a second PDT-hemoporfin treatment; PH, patients who received the first PDT-hemoporfin treatment at week 8 (stage 2); NI, no improvement; SI, some improvement; GI, great improvement; CR, nearly completely resolved.
Denotations
# Four missing data included and imputed as”no improvement”
¶ Three missing data included and imputed as”no improvement”
§ Missing data not included in the n
※ Eight of the patients achieved ‘adequately satisfactory’ at week 8 and did not receive the second treatment, but were imputed in the efficacy analyses at week 16; among these eight patients, three, four and one patient were graded as having achieved PWS fading of >90%, 60–89%, and 20–59%, respectively
a primary efficacy end point; ΔEI was analyzed using t-test and all other comparisons were conducted using chi-square or Fisher’s exact test.
The multivariate logistic regression analysis confirmed that PDT-hemoporfin treatment was significantly related to a higher likelihood of achieving at least SI (OR 29.324, 95% CI 16.490–54.244, P <0.001), with location and type of PWS as influential factors (a lower proportion of the patients having hypertrophic type and a higher proportion of those having lesions on the neck achieved at least SI, Table 3). A significantly greater proportion of the PDT-hemoporfin group was evaluated as ‘good to excellent’ by the investigators (70.7% [n = 231; 95% CI, 65.5%-75.4%] vs. 1.9% [n = 2; 95% CI, 0.5%-6.6%]; P<0.0001). Similarly, a significantly greater proportion of the PDT-hemoporfin patients assessed their own response as ‘good to excellent’ (67.6% [n = 221; 95% CI, 62.3%-72.4%] vs. 2.8% [n = 3; 95% CI, 1.0%-7.9%]; P<0.0001).
Table 3. The logistic regression analysis to identify factors relevant to achieving at least SI at week 8*.
| Variables | Estimated Odds Ratio (95% CI) | P value |
|---|---|---|
| PDT-hemoporfin vs. PDT-placebo | 29.324(16.490,54.244) | <0.001 |
| Location of PWS (vs. Centrofacial) | ||
| Non-centrofacial | 1.107(0.603,2.052) | 0.743 |
| Neck | 4.030(1.475,12.629) | 0.010 |
| Other | 1.059(0.053,39.141) | 0.973 |
| Type of PWS (vs. Pink type) | ||
| Purple type | 1.140(0.617,2.092) | 0.673 |
| Hypertrophic type | 0.354(0.137,0.951) | 0.035 |
Abbreviations: SI, some improvement; CI, confidence interval.
Denotations
* Candidate continuous variables considered were age, systolic blood pressure (mmHg), diastolic blood pressure (mmHg), heart rate (bpm), body-mass index, area of targeted site (cm2), ΔEI at baseline and power density of laser irradiation (mW/cm2). Candidate categorical variables considered were intervention group (PDT-hemoporfin vs. PDT-placebo), study site (other sites vs. site 1), sex (male vs. female), occupation (physical laborers vs. non-physical laborers), location of PWS (other locations vs. centrofacial) and type of PWS (other types vs. pink type). The variables with significance level of P < 0.2 in univariate analyses were included in the stepwise multivariate logistic regression; ethnicity and previous therapy was not included in the analyses because of the small number of cases in the subgroups. The logistic regression model fit was tested with the likelihood ratio test (P < 0.001).
Patients in the PDT-hemoporfin group who received a second treatment (n = 303) showed significantly higher proportions of response (in all response categories) at week 16 (vs. PDT-placebo group after one treatment with PDT-hemoporfin (n = 101): at least SI, 97.4% (n = 295; 95% CI, 94.9%-98.7%) vs. 90.1% (n = 91; 95% CI, 82.7%-94.5%); at least GI, 64.0% (n = 194; 95% CI, 58.5%-69.2%) vs. 42.6% (n = 43; 95% CI, 33.4%-52.3%); CR, 28.1% (n = 85; 95% CI, 23.3%-33.4%) vs. 7.9% (n = 8; 95% CI, 4.1%-14.9%); P<0.005). At week 16, compared with the PDT-placebo patients after one treatment with PDT-hemoporfin, a significantly greater proportion of the PDT-hemoporfin patients who received a second treatment were evaluated as having ‘good to excellent’ response by the investigators (83.1% [n = 252; 95% CI, 78.5%-86.9%] vs. 60.4% [n = 61; 95% CI, 50.6%-69.4%], P<0.0001), and so were by the patients (80.9% [n = 245; 95%CI, 76.1%-84.9%] vs. 60.4% [n = 61; 95%CI, 50.6%-69.4%], P<0.0001).
In post hoc analyses, the ΔEI was found to not be significantly different between the PDT-hemoporfin group (n = 329) and the PDT-placebo group (n = 110) at baseline (39.94 [95% CI, 38.04–41.84] vs. 39.90 [95%CI, 36.92–42.88]; P = 0.984). However, at week 8, the ΔEI of the PDT-hemoporfin group was significantly lower (vs. PDT-placebo group: 29.58 [95% CI, 27.92–31.24] vs. 38.42 [95% CI, 34.78–42.05]; P<0.0001). At week 16, the PDT-hemoporfin group who received a second treatment (n = 301) had significantly lower ΔEI than the patients in the PDT-placebo group who had received only one PDT-hemoporfin treatment (n = 101) (25.11 [95% CI, 23.35–26.88] vs. 31.16 [95% CI, 27.90–34.41]; P = 0.0010) (S1 Fig and Table 2).
Safety
The exposure-adjusted rates of treatment reactions and adverse events are presented in Table 4. Treatment reactions (at stage 1 and/or stage 2) occurred in 99.5% (n = 731, 95% CI, 98.7%-99.8%) of the PDT-hemoporfin treatments (n = 735 in total), compared to only 39.1% (n = 43, 95% CI, 30.5%-48.4%) of the PDT-placebo treatments (n = 110) and the difference was statistically significant (P<0.0001). The median number of treatment reactions per patient-treatment was also significantly higher in the PDT-hemoporfin treatments than in the PDT-placebo treatments (4 [range: 1–7] vs. 1 [range: 1–4]; P<0.0001, Mann-Whitney test). All the treatment reactions reported in the PDT-placebo treatments were mild to moderate. In contrast, the patients who received PDT-hemoporfin experienced the appreciable amounts of severe treatment reactions, including: pain (19.9% [n = 142; 95% CI, 17.1%-23.0%]), burning sensation (12.1% [n = 71; 95% CI, 9.7%-15.0%]), pruritus (4.6% [n = 18; 95% CI, 2.9%-7.2%]), edema (25.6% [n = 179; 95% CI, 22.5%-29.0%]), crusting (4.8% [n = 24; 95% CI, 3.2%-7.1%]), purpura (0.0% [n = 0; 95% CI, 0.0%-5.2%]), and vesicle rash (2.0% [n = 1; 95% CI, 0.4%-10.4%]). No significant difference was seen for most of the treatment reactions that were experienced in stage 1 and stage 2 by the patients who received the PDT-hemoporfin treatments, with the notable exceptions of a lower percentage of crusting (73.3% [n = 242; 95% CI, 68.3%-77.8%] vs. 58.8% [n = 177; 95% CI, 53.2%-64.2%]; P = 0.0002) and a higher percentage of edema (93.0% [n = 307; 95% CI, 89.7%-95.3%] vs. 96.7% [n = 291; 95% CI, 94.0%-98.2%]; P = 0.0130).
Table 4. Treatment reactions and adverse events possibly related to the treatment.
| Event | PDT-hemoporfin, 735 PT of 434 patients | PDT-placebo, 110 PT of 110 patients | P value | |||||
|---|---|---|---|---|---|---|---|---|
| n | Event rate† | Days to resolve‡ | n | Event rate† | Days to resolve‡ | |||
| Treatment reactions | ||||||||
| Pain | 713 | 97.0 (95.5 to 98.0) | 0–19 | 7 | 6.4 (3.1 to 12.6) | 0–2 | <0.0001 | |
| Burning sensation | 589 | 80.1 (77.1 to 82.9) | 0–10 | 32 | 29.1 (21.4 to 38.2) | 0–4 | <0.0001 | |
| Pruritus | 388 | 52.8 (49.2 to 56.4) | 0–47 | 12 | 10.9 (6.4 to 18.1) | 0–9 | <0.0001 | |
| Numbness | 1 | 0.1 (0.0 to 0.8) | 0 | 0 | 0.0 (0.0 to 3.4) | - | >0.999 | |
| Edema | 698 | 95.0 (93.1 to 96.3) | 0–21 | 4 | 3.6 (1.4 to 9.0) | 0–4 | <0.0001 | |
| Crusting | 496 | 67.5 (64.0 to 70.8) | 0–46 | 0 | 0.0 (0.0 to 3.4) | - | <0.0001 | |
| Purpura | 70 | 9.5 (7.6 to 11.9) | 2–15 | 0 | 0.0 (0.0 to 3.4) | - | 0.0007 | |
| Blistering | 51 | 6.9 (5.3 to 9.0) | 1–24 | 1 | 0.9 (0.2 to 5.0) | 3 | 0.0139 | |
| Adverse events | ||||||||
| Total adverse events | 241 | 32.8 (29.5 to 36.3) | - | 8 | 7.3 (3.7 to 13.7) | - | <0.001 | |
| Light-exposure related reactions | 10 | 1.4 (0.7 to 2.5) | - | 2 | 1.8 (0.5 to 6.4) | - | 0.955 | |
| Dyspnea, rash and photophobia | 1 | 0.1 (0.0 to 0.8) | 0 | 0 | 0.0 (0.0 to 3.4) | - | - | |
| Urticaria | 1 | 0.1 (0.0 to 0.8) | 0 | 0 | 0.0 (0.0 to 3.4) | - | - | |
| Photosensitive cheilitis | 1 | 0.1 (0.0 to 0.8) | 8 | 0 | 0.0 (0.0 to 3.4) | - | - | |
| Photosensitive dermatitis | 0 | 0.0 (0.0 to 0.5) | - | 1 | 0.9 (0.2 to 5.0) | 9 | - | |
| Dizziness and photophobia | 5 | 0.7 (0.3 to 1.6) | 0–21 | 0 | 0.0 (0.0 to 3.4) | - | - | |
| Photophobia | 2 | 0.3 (0.1 to 1.0) | 4–18 | 1 | 0.9 (0.2 to 5.0) | 5 | - | |
| Local adverse events | ||||||||
| Hyperpigmentation | 168 | 22.9 (20.0 to 26.0) | 16–379a | 0 | 0.0 (0.0 to 3.4) | - | <0.001 | |
| Hypopigmentation | 14 | 1.9 (1.1 to 3.2) | 27–268b | 0 | 0.0 (0.0 to 3.4) | - | 0.288 | |
| Temporary skin lesions | 8 | 1.1 (0.6 to 2.1) | 0 | 0.0 (0.0 to 3.4) | - | 0.566 | ||
| Rash maculo-papular | 3 | 0.4 (0.1 to 1.2) | 0–3 | 0 | 0.0 (0.0 to 3.4) | - | - | |
| Exudation | 1 | 0.1 (0.0 to 0.8) | 2 | 0 | 0.0 (0.0 to 3.4) | - | - | |
| Eczema | 1 | 0.1 (0.0 to 0.8) | 14 | 0 | 0.0 (0.0 to 3.4) | - | - | |
| Scaling | 1 | 0.1 (0.0 to 0.8) | 9 | 0 | 0.0 (0.0 to 3.4) | - | - | |
| Erythema | 1 | 0.1 (0.0 to 0.8) | 10 | 0 | 0.0 (0.0 to 3.4) | - | - | |
| Textural change | 1 | 0.1 (0.0 to 0.8) | 105 | 0 | 0.0 (0.0 to 3.4) | - | - | |
| Wound infection | 8 | 1.1 (0.6 to 2.1) | 2–12c | 0 | 0.0 (0.0 to 3.4) | - | 0.566 | |
| Atrophic scar | 4 | 0.5 (0.2 to 1.4) | 48–208d | 0 | 0.0 (0.0 to 3.4) | - | >0.999 | |
| Systemic adverse events | ||||||||
| Liver and biliary system disorders e | 8 | 1.1 (0.6 to 2.1) | 2 | 1.8 (0.5 to 6.4) | 0.854 | |||
| ALT, AST and TBil increase | 1 | 0.1 (0.0 to 0.8) | 52 | 1 | 0.9 (0.2 to 5.0) | - | ||
| ALT and AST increase | 3 | 0.4 (0.1 to 1.2) | 28–119 | 0 | 0.0 (0.0 to 3.4) | - | - | |
| ALT increase | 1 | 0.1 (0.0 to 0.8) | 3 | 1 | 0.9 (0.2 to 5.0) | - | ||
| AST increase | 2 | 0.3 (0.1 to 1.0) | 6–7 | 0 | 0.0 (0.0 to 3.4) | - | - | |
| TBil increase | 1 | 0.1 (0.0 to 0.8) | 54 | 0 | 0.0 (0.0 to 3.4) | - | - | |
| Gastro-intestinal system disorders | 6 | 0.8 (0.4 to 1.8) | 0–1 | 0 | 0.0 (0.0 to 3.4) | - | >0.999 | |
| Nausea | 5 | 0.7 (0.3 to 1.6) | 0–1 | 0 | 0.0 (0.0 to 3.4) | - | - | |
| Vomiting | 1 | 0.1 (0.0 to 0.8) | 0 | 0 | 0.0 (0.0 to 3.4) | - | - | |
| Cardiac disorders | 4 | 0.5 (0.2 to 1.4) | 47–154 | 1 | 0.9 (0.2 to 5.0) | 51 | 0.504 | |
| Ventricular arrhythmia | 1 | 0.1 (0.0 to 0.8) | 154 | 0 | 0.0 (0.0 to 3.4) | - | - | |
| Conduction disorder | 1 | 0.1 (0.0 to 0.8) | 47 | 1 | 0.9 (0.2 to 5.0) | 51 | - | |
| Non-specific ECG abnormal | 2 | 0.3 (0.1 to 1.0) | 47–50 | 0 | 0.0 (0.0 to 3.4) | - | - | |
| Respiratory system disorders | 3 | 0.4 (0.1 to 1.2) | 0–2 | 0 | 0.0 (0.0 to 3.4) | - | >0.999 | |
| Dyspnea | 3 | 0.4 (0.1 to 1.2) | 0–2 | 0 | 0.0 (0.0 to 3.4) | - | - | |
| General symptoms | 3 | 0.4 (0.1 to 1.2) | 2–36 | 0 | 0.0 (0.0 to 3.4) | - | >0.999 | |
| Asthenia | 2 | 0.3 (0.1 to 1.0) | 2–30 | 0 | 0.0 (0.0 to 3.4) | - | - | |
| Sweating increased | 1 | 0.1 (0.0 to 0.8) | 36 | 0 | 0.0 (0.0 to 3.4) | - | - | |
| Eye abnormality | 2 | 0.3 (0.1 to 1.0) | 4–16 | 0 | 0.0 (0.0 to 3.4) | - | >0.999 | |
| Conjunctivitis | 1 | 0.1 (0.0 to 0.8) | 16 | 0 | 0.0 (0.0 to 3.4) | - | - | |
| Xerophthalmia | 1 | 0.1 (0.0 to 0.8) | 4 | 0 | 0.0 (0.0 to 3.4) | - | - | |
| White cell and RES disorders | 1 | 0.1 (0.0 to 0.8) | 2 | 0 | 0.0 (0.0 to 3.4) | - | >0.999 | |
| Leukocytosis | 1 | 0.1 (0.0 to 0.8) | 2 | 0 | 0.0 (0.0 to 3.4) | - | - | |
| Nervous system disorders | 1 | 0.1 (0.0 to 0.8) | 7 | 1 | 0.9 (0.2 to 5.0) | 2 | 0.244 | |
| Dizziness | 0 | 0.0 (0.0 to 0.5) | - | 1 | 0.9 (0.2 to 5.0) | 2 | - | |
| Vertigo | 1 | 0.1 (0.0 to 0.8) | 7 | 0 | 0.0 (0.0 to 3.4) | - | - | |
| Tooth disorder | 1 | 0.1 (0.0 to 0.8) | 0 | 0 | 0.0 (0.0 to 3.4) | - | >0.999 | |
| Tooth infection | 1 | 0.1 (0.0 to 0.8) | 0 | 0 | 0.0 (0.0 to 3.4) | - | - | |
| Urinary system disorders | 0 | 0.0 (0.0 to 0.5) | - | 2 | 1.8 (0.5 to 6.4) | 52–54 | 0.017 | |
| Hematuria | 0 | 0.0 (0.0 to 0.5) | - | 1 | 0.9 (0.2 to 5.0) | 54 | - | |
| Proteinuria | 0 | 0.0 (0.0 to 0.5) | - | 1 | 0.9 (0.2 to 5.0) | 52 | - | |
Abbreviations: PT, patient-treatments; AST, aspartate transaminase; ALT, alanine aminotransferase; TBil, total bilirubin; ECG, electrocardiogram; RES, reticuloendothelial system.
Denotations
† Exposure adjusted event rate per 100 patient-treatments, Mean (95% CI)
‡ range, where 0 indicates resolution in less than one day
a Nine patients lost to follow-up
b Two patients lost to follow-up
c Healed with application of topical antibiotics
d One patient lost to follow-up
e An increase was defined as ALT or AST exceeding the upper limit of normal (ULN), or TBil exceeding 1.5ULN; All statistical comparisons were conducted using chi-square or Fisher’s exact test.
None of the study participants experienced any treatment related serious adverse events. Only two types of the adverse events reported differed significantly between the patients who received PDT-hemoporfin treatments and the PDT-placebo treatments; in particular, the PDT-hemoporfin treatments were associated with a significantly higher proportion of hyperpigmentation (22.9 [95% CI, 20.0–26.0] vs. 0.0 [95% CI, 0.0–3.4] per 100 patient-treatment; P<0.001) and a significantly lower rate of urinary system disorder (0.0 [95% CI, 0.0–0.5] vs. 1.8 [95%CI, 0.5–6.4] per 100 patient-treatments; P = 0.017). All the adverse events were mild to moderate, except for 2.4% (95% CI, 1.5%-3.8%) of the hyperpigmentation that were severe. All the adverse events resolved without sequelae during follow-up; however, the final outcome of 12 adverse events remains unknown since those patients were lost of follow-up before the event had been fully resolved. All rates of adverse events in the PDT-hemoporfin group were similar between stage 1 and stage 2.
Discussion
Ideally, PWS should be treated in a manner that provides the best cosmetic outcome. To achieve this goal, innovative approaches in PDT need to be developed, possibly at the levels of drug as well as and the treatment protocol. Hemoporfin is a new product of the porphyrin-related photosensitizer HMME, and has been shown to elicit a stronger photodynamic effect, lower toxicity, and a shorter-term skin phototoxicity than the most commonly used photosensitizer, photofrin [16,23,24]. The protocol used in the current study, which was designed according to the results from our exploratory phase IIa [16] and IIb studies to identify an optimized irradiation procedure and drug dosage, is novel. However, the effectiveness and safety of this protocol remains to be established by a prospective study of a large population. To our knowledge, this is the first randomized clinical trial of a large sample population to assess the efficacy and safety of PDT-hemoporfin for treating PWS in adolescents and adults. The stage 1 was designed as randomized double-blind, placebo-controlled, to establish efficacy; and in stage 2, all patients in both group were included for treatment, for a better safety evaluation and ethical practice.
Efficacy profile of PDT-hemoporfin for treating PWS
Although it is possible to quantify PWS lesion response to treatment by colorimetry or reflectance spectrophotometry [25], the clinical utility of these techniques is limited by a lack of repeatability [18,26]. However, use of a defined scoring system based on comparative analysis of pre- and post-treatment images has been shown to be a valid method for assessing treatment response of PWS, and has been successfully applied to other clinical studies, including randomized clinical trials [27–29]. This method was applied in the current study, and indicated that at post-treatment week 8 a statistically significant and clinically meaningful reduction in disease severity of PWS had occurred in patients who underwent PDT-hemoporfin treatment, as opposed to the placebo-control group who underwent laser irradiation without hemoporfin.
Previous studies have suggested that efficacy of PDT might be affected by sex and age, as well as PWS location and type (the pink, purple and hypertrophic type is relevant to lesion severity of PWS), presumably due to variations in the lesion-involved skin and vessel properties [30,31]. The current study also found that patients who were female and having cervical and pink type PWS responded better to the PDT-hemoporfin treatment (Table 3, S2 and S3 Tables).
The higher proportions of physician- and patient-rated satisfaction with treatment response in the PDT-hemoporfin group, and after the second treatment application, suggest that multiple treatment sessions may be preferable for PWS. EI analysis has been previously used to quantitatively evaluate the intensity of erythema [18] and the efficacy of treatment for PWS [19]. Indeed, in post hoc analyses, the significantly lower ΔEI that was achieved after a single PDT-hemoporfin treatment (vs. the PDT-placebo treatment) was even further reduced after a second application of the procedure.
Alternative treatments exist for PWS, and many have been systematically studied as well. For example, in a comparative analysis of PDT and pulsed-dye laser (PDL) in a small series of PWS patients, PDT was shown to be at least as effective as PDL, and in some cases to be superior [14,32]. It has been suggested that PDT might be effective in treating PDL-resistant PWS lesions [33], or that a combination PDT plus PDL treatment strategy may provide better results for PWS patients [34]; however, these possibilities must be assessed in future studies.
Safety profile of PDT-hemoporfin for treating PWS
Treatment reactions were observed in almost all of the patient-treatments of the current study. While the rates of treatment reactions in the PDT-placebo treatments were lower and less severe than those in the PDT-hemoporfin treatments, the fact that these reactions occurred indicates the potential of the laser irradiation component of the procedure causing some of the treatment reactions. Assessment of the most frequently experienced treatment reactions provided insights into the possible management of these undesirable side effects. For example, in the case of pain, the symptom usually began around 5–10 minutes after the initiation of laser exposure and blowing cold air on the area during the treatment process might help to ease the discomfort [35]. The patients who received a second PDT-hemoporfin treatment had different incidences of some treatment reactions, namely crusting and edema. PDT-induced microstructural changes in the treated skin may play a role in this phenomenon [36,37], but further study is needed to determine the underlying mechanism.
Our treatment showed similar rates of treatment reaction, but some different rates of long term side effects compared to a retrospective study. The differences might be owing to study design, treatment protocol or methods of observation for AEs [38]. Transient hyperpigmentation was the principal adverse event associated with the PDT-hemoporfin treatment in this study. PDT is limited by its induction of prolonged systemic photosensitivity to visible light, which can last for 1~2 months following application of porphyrin derivatives, such as photocarcinorin [11,39]. However, in the current study, when simple and convenient protection practices were used over the 2 week period following the treatment procedure, the incidence of light-exposure related reactions were not significantly increased in the PDT-hemoporfin treated patients. Thus, the patient might be allowed to cautiously resume normal daily activities shortly after treatment.
Although fine tuning of drug dose or exposure time according to selected observation parameters (e.g. age, sex, PWS type and location, or changes of the skin during irradiation) might be helpful to improve the efficacy of the treatment and reduce the adverse effects, it would hard for practice and might be risky without known the risk/benefit profile defined in the population. Our study has settled the basis of this possibility by define the risk/benefit profile of an optimized protocol. In our study, PWS patients treated with PDT using our protocol achieved satisfactory efficacy at the risk of only short-term treatment reactions and partly temporary hyperpigmentation, no significant scar or systemic side effects. Thus the risk/benefit profile of the treatment was remarkable. Retrospective or small scale studies have been reported to compare the efficacy and safety profiles of PDT with those of PDL [14,32,40]. The rate of excellent response in PDT group was significantly higher than that in PDL group (23.5–37.5% vs 3.1–16.1%), and incidences of pigmentation and scar formation in PDT group were significantly lower than PDL group (8.3–10.2% vs 21.1–24.7%) [14, 40]. A significantly greater blanching effect of PWS has been shown after a single-session PDL treatment compared with a single-session PDT treatment [32]. However, the real risk/benefit difference between PDT and PDL is necessary to be studied in large scale prospective studies.
Limitations
This study had several limitations. Firstly, the current study did not attempt to compare the effects of PDT with PDL. Because large multicenter studies to compare the efficacies of these two treatments have, unfortunately, been unfeasible at our settings due to the limitations of PDL being more operator-dependent than PDT and other practical reasons. Secondly, in practice, the efficacy of treatment for PWS will be stable following resolution of acute responses; thus, the efficacy at week 16 likely represents the response over the period of upcoming years [30]. However, as the alternative PWS treatment of PDL is associated with significant recurrence or redarkening of the treated lesion during long-term follow-up [7,8], the long-term efficacy of PDT-hemoporfin should also be evaluated in further studies. Lastly, the half-life of intravenous-administered hemoporfin is short (1.31 ± 0.33h [16]); therefore, we assume that the observation period of 8 weeks used in each stage of this study was likely sufficient for monitoring adverse events related to the treatment. However, longer-term (>16 weeks) monitoring of PDT-hemoporfin-treated patients is necessary to help identify very rare adverse events. Further randomized controlled trials to evaluate the effect in comparisons with PDL and the long term efficacy and safety are in planning.
Conclusions
Hemoporfin-mediated PDT using 5mg/kg hemoporfin and 532 nm continuous wave lasers with fluence of 96–120 J/cm2 is effective and safe for adolescent and adult patients with port-wine stain.
Supporting Information
(PDF)
(DOCX)
(PDF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We thank all of the patients and investigators involved with this study. This manuscript has been edited and proofread by Medjaden Bioscience Limited.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was co-sponsored by Shanghai Fudan-Zhangjiang Bio-Pharmaceutical Co., Ltd. and the Chinese PLA General Hospital, and was funded by the former. The sponsors had no role in the collection, management, or analysis of the data, or interpretation of the data, preparation of the manuscript, or decision to submit the manuscript for publication. In collaboration with the academic coauthors, both cosponsors were involved in the design of the study. The funder was involved in the conduct of the study and reviewed the manuscript prior to submission, and provided support in the form of salaries for authors [JN Tao], but did not have any additional role in the data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Jacobs AH, Walton RG. The incidence of birthmarks in the neonate. Pediatrics. 1976;58: 218–22. [PubMed] [Google Scholar]
- 2.Malm M, Carlberg M. Port-wine stain—a surgical and psychological problem. Ann Plast Surg. 1988;20: 512–6. [DOI] [PubMed] [Google Scholar]
- 3.Dixon JA, Huether S, Rotering R. Hypertrophic scarring in argon laser treatment of port-wine stains. Plast Reconstr Surg. 1984;73: 771–9. [DOI] [PubMed] [Google Scholar]
- 4.Kelly KM, Choi B, McFarlane S, Motosue A, Jung B, Khan MH, et al. Description and analysis of treatments for port-wine stain birthmarks. Arch Facial Plast Surg. 2005;7: 287–94. 7/5/287 [pii] 10.1001/archfaci.7.5.287 [DOI] [PubMed] [Google Scholar]
- 5.Greve B, Raulin C. Prospective study of port wine stain treatment with dye laser: comparison of two wavelengths (585 nm vs. 595 nm) and two pulse durations (0.5 milliseconds vs. 20 milliseconds). Lasers Surg Med. 2004;34: 168–73. 10.1002/lsm.20003 [DOI] [PubMed] [Google Scholar]
- 6.Tomson N, Lim SP, Abdullah A, Lanigan SW. The treatment of port-wine stains with the pulsed-dye laser at 2-week and 6-week intervals: a comparative study. Br J Dermatol. 2006;154: 676–9. BJD7113 [pii] 10.1111/j.1365-2133.2005.07113.x [DOI] [PubMed] [Google Scholar]
- 7.Orten SS, Waner M, Flock S, Roberson PK, Kincannon J. Port-wine stains. An assessment of 5 years of treatment. Arch Otolaryngol Head Neck Surg. 1996;122: 1174–9. [DOI] [PubMed] [Google Scholar]
- 8.Huikeshoven M, Koster PH, de Borgie CA, Beek JF, van Gemert MJ, van der Horst CM. Redarkening of port-wine stains 10 years after pulsed-dye-laser treatment. N Engl J Med. 2007;356: 1235–40. 356/12/1235 [pii] 10.1056/NEJMoa064329 [DOI] [PubMed] [Google Scholar]
- 9.Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, et al. Photodynamic therapy of cancer: an update. CA Cancer J Clin. 2011;61: 250–81. caac.20114 [pii] 10.3322/caac.20114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mayor S. NICE recommends photodynamic therapy for certain patients with macular degeneration. BMJ. 2003;327: 698 10.1136/bmj.327.7417.698-b 327/7417/698-b [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gu Y, Li J, Shan H. Clinical Application of Copper Vapor Laser in PDT for Fifty Cases of PWS. Chin J Laser Med Surg. 1994;3: 215–217. [Google Scholar]
- 12.Orenstein A, Nelson JS, Liaw LH, Kaplan R, Kimel S, Berns MW. Photochemotherapy of hypervascular dermal lesions: a possible alternative to photothermal therapy? Lasers Surg Med. 1990;10: 334–43. [DOI] [PubMed] [Google Scholar]
- 13.Gu Y, Huang NY, Liang J, Pan YM, Liu FG. Clinical study of 1949 cases of port wine stains treated with vascular photodynamic therapy (Gu’s PDT). Ann Dermatol Venereol. 2007;134: 241–4. MDOI-AD-03-2007-134-3-0151-9638-101019-200520004 [pii] [DOI] [PubMed] [Google Scholar]
- 14.Yuan KH, Li Q, Yu WL, Zeng D, Zhang C, Huang Z. Comparison of photodynamic therapy and pulsed dye laser in patients with port wine stain birthmarks: a retrospective analysis. Photodiagnosis Photodyn Ther. 2008;5: 50–7. S1572-1000(07)00127-5 [pii] 10.1016/j.pdpdt.2007.12.001 [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Gu Y, Zuo Z, Huang N. Choosing optimal wavelength for photodynamic therapy of port wine stains by mathematic simulation. J Biomed Opt. 2011;16: 098001 10.1117/1.3616127 [DOI] [PubMed] [Google Scholar]
- 16.Zhao Y, Zhou Z, Zhou G, Tu P, Zheng Q, Tao J, et al. Efficacy and safety of hemoporfin in photodynamic therapy for port-wine stain: a multicenter and open-labeled phase IIa study. Photodermatol Photoimmunol Photomed. 2011;27: 17–23. 10.1111/j.1600-0781.2010.00555.x [DOI] [PubMed] [Google Scholar]
- 17.Sun PH, Zhao X, Zhou Y, Liang Y, Zhang HL, Cui YM, et al. Tolerance and pharmacokinetics of single-dose intravenous hemoporfin in healthy volunteers. Acta Pharmacol Sin. 2011;32: 1549–54. aps2011132 [pii] 10.1038/aps.2011.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamamoto T, Takiwaki H, Arase S, Ohshima H. Derivation and clinical application of special imaging by means of digital cameras and Image J freeware for quantification of erythema and pigmentation. Skin Res Technol. 2008;14: 26–34. SRT256 [pii] 10.1111/j.1600-0846.2007.00256.x [DOI] [PubMed] [Google Scholar]
- 19.Zhao Y, Tao J, Tu P. Quantitative evaluation of efficacy of photodynamic therapy for port-wine stains using erythema index image analysis. Photodiagnosis Photodyn Ther. 2013;10: 96–102. S1572-1000(12)00104-4 [pii] 10.1016/j.pdpdt.2012.10.001 [DOI] [PubMed] [Google Scholar]
- 20.Rasband W. ImageJ—image processing and analysis in Java. [Internet]. Available: http://rsb.info.nih.gov/ij/
- 21.Uppsala Monitoring Center. The WHO-ART Adverse Reaction Terminology 2000 (Chinese version). [Internet]. 2000. Available: http://www.umc-products.com/graphics/3149.pdf
- 22.World Health Organisation. Uppsala Monitoring Centre system for standardised case causality assessment. [Internet]. Available: http://www.who-umc.org
- 23.Song K, Kong B, Qu X, Li L, Yang Q. Phototoxicity of Hemoporfin to ovarian cancer. Biochem Biophys Res Commun. 2005;337: 127–32. S0006-291X(05)02006-1 [pii] 10.1016/j.bbrc.2005.09.021 [DOI] [PubMed] [Google Scholar]
- 24.Wei Y, Kong B, Song K, Qu X, Jin Q, Yang Q. Involvement of mitochondria-caspase pathway in Hemoporfin-mediated cell death. Photochem Photobiol. 2007;83: 1319–24. PHP160 [pii] 10.1111/j.1751-1097.2007.00160.x [DOI] [PubMed] [Google Scholar]
- 25.Le KV, Shahidullah H, Frieden IJ. Review of modern techniques in detecting port-wine stain response to laser therapy. Dermatol Surg. 1999;25: 127–32. [DOI] [PubMed] [Google Scholar]
- 26.Jung B, Kim CS, Choi B, Kelly KM, Nelson JS. Use of erythema index imaging for systematic analysis of port wine stain skin response to laser therapy. Lasers Surg Med. 2005;37: 186–91. 10.1002/lsm.20218 [DOI] [PubMed] [Google Scholar]
- 27.Currie CL, Monk BE. Can the response of port-wine stains to laser treatment be reliably assessed using subjective methods? Br J Dermatol. 2000;143: 360–4. bjd3663 [pii] [DOI] [PubMed] [Google Scholar]
- 28.Faurschou A, Togsverd-Bo K, Zachariae C, Haedersdal M. Pulsed dye laser vs. intense pulsed light for port-wine stains: a randomized side-by-side trial with blinded response evaluation. Br J Dermatol. 2009;160: 359–64. BJD8993 [pii] 10.1111/j.1365-2133.2008.08993.x [DOI] [PubMed] [Google Scholar]
- 29.Yung A, Sheehan-Dare R. A comparative study of a 595-nm with a 585-nm pulsed dye laser in refractory port wine stains. Br J Dermatol. 2005;153: 601–6. BJD6707 [pii] 10.1111/j.1365-2133.2005.06707.x [DOI] [PubMed] [Google Scholar]
- 30.Xiao Q, Li Q, Yuan KH, Cheng B. Photodynamic therapy of port-wine stains: long-term efficacy and complication in Chinese patients. J Dermatol. 2011;38: 1146–52. 10.1111/j.1346-8138.2011.01292.x [DOI] [PubMed] [Google Scholar]
- 31.Klapman MH, Yao JF. Thickening and nodules in port-wine stains. J Am Acad Dermatol. 2001;44: 300–2. S0190-9622(01)07090-6 [pii] 10.1067/mjd.2001.111353 [DOI] [PubMed] [Google Scholar]
- 32.Gao K, Huang Z, Yuan KH, Zhang B, Hu ZQ. Side-by-side comparison of photodynamic therapy (PDT) and pulsed dye laser (PDL) treatment of port-wine stain (PWS) birthmarks. Br J Dermatol. 2012; 10.1111/bjd.12130 [DOI] [PubMed] [Google Scholar]
- 33.Yuan KH, Li Q, Yu WL, Huang Z. Photodynamic therapy in treatment of port wine stain birthmarks—recent progress. Photodiagnosis Photodyn Ther. 2009;6: 189–94. S1572-1000(09)00128-8 [pii] 10.1016/j.pdpdt.2009.08.001 [DOI] [PubMed] [Google Scholar]
- 34.Tournas JA, Lai J, Truitt A, Huang YC, Osann KE, Choi B, et al. Combined benzoporphyrin derivative monoacid ring photodynamic therapy and pulsed dye laser for port wine stain birthmarks. Photodiagnosis Photodyn Ther. 2009;6: 195–9. S1572-1000(09)00135-5 [pii] 10.1016/j.pdpdt.2009.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stangeland KZ, Kroon S. Cold air analgesia as pain reduction during photodynamic therapy of actinic keratoses. J Eur Acad Dermatol Venereol. 2012;26: 849–54. 10.1111/j.1468-3083.2011.04167.x [DOI] [PubMed] [Google Scholar]
- 36.Jiang L, Gu Y, Li X, Zhao X, Li J, Wang K, et al. Changes of skin perfusion after photodynamic therapy for port wine stain. Chin Med J Engl. 1998;111: 136–8. [PubMed] [Google Scholar]
- 37.Huang N, Cheng G, Wang Y, Zeng J, Qiu H, Gu Y. Influence of laser wavelength on the damage of comb’s vasculature by photodynamic therapy—simulation and validation of mathematical models. Lasers Med Sci. 2011;26: 665–72. 10.1007/s10103-011-0890-5 [DOI] [PubMed] [Google Scholar]
- 38.Yuan K-H, Gao J-H, Huang Z. Adverse effects associated with photodynamic therapy (PDT) of port-wine stain (PWS) birthmarks. Photodiagnosis Photodyn Ther. 2012;9: 332–336. 10.1016/j.pdpdt.2012.03.007 [DOI] [PubMed] [Google Scholar]
- 39.Qin Z-P, Li K-L, Ren L, Liu X-J. Photodynamic therapy of port wine stains-a report of 238 cases. Photodiagnosis Photodyn Ther. 2007; 53–9. 10.1016/j.pdpdt.2007.01.001 [DOI] [PubMed] [Google Scholar]
- 40.Zhang B, Zhang T-H, Huang Z, Li Q, Yuan K-H, Hu Z-Q. Comparison of pulsed dye laser (PDL) and photodynamic therapy (PDT) for treatment of facial port-wine stain (PWS) birthmarks in pediatric patients. Photodiagnosis Photodyn Ther. 2014;11: 491–497. 10.1016/j.pdpdt.2014.06.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(DOCX)
(PDF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.