Abstract
Molecular evolution is an established technique for inferring gene homology but regulatory DNA turns over so rapidly that inference of ancestral networks is often impossible. In silico evolution is used to compute the most parsimonious path in regulatory space for anterior-posterior patterning linking two Dipterian species. The expression pattern of gap genes has evolved between Drosophila (fly) and Anopheles (mosquito), yet one of their targets, eve, has remained invariant. Our model predicts that stripe 5 in fly disappears and a new posterior stripe is created in mosquito, thus eve stripe modules 3+7 and 4+6 in fly are homologous to 3+6 and 4+5 in mosquito. We can place Clogmia on this evolutionary pathway and it shares the mosquito homologies. To account for the evolution of the other pair-rule genes in the posterior we have to assume that the ancestral Dipterian utilized a dynamic method to phase those genes in relation to eve.
Author Summary
The last common ancestor of the fruit fly (Drosophila) and mosquito (Anopheles) lived more than 200 Million years ago. Can we use available data on insects alive today to infer what their ancestor looked like? In this manuscript, we focus on early embryonic development, when stripes of genetic expression appear and define the location of insect segments (“segmentation”). We use an evolutionary algorithm to reconstruct and predict dynamics of genes controlling stripes in the last common ancestor of fly and mosquito. We predict a new and different combinatorial logic of stripe formation in mosquito compared to fly, which is fully consistent with development of intermediate species such as moth-fly (Clogmia). Our simulations further suggest that the dynamics of gene expression in this last common ancestor were similar to other insects, such as wasps (Nasonia). Our method illustrates how computational methods inspired by machine learning and non-linear physics can be used to infer gene dynamics in species that disappeared millions of years ago.
Introduction
Molecular phylogenies based on protein coding genes have greatly enhanced evolutionary theory, and in favorable cases even allow a reconstruction of the last common ancestral gene or even full evolutionary pathways [1]. However regulatory sequence evolves more rapidly than coding sequence and the functional binding sites can move around without impacting the function of a ∼1kb functional regulatory module [2, 3]. Thus one is often in the situation where gene homologies are obvious, yet there is no visible sequence homology in the regulatory regions. At the phenotypic level, gene expression domains can be easily mapped by in-situ hybridization yet a molecular understanding is limited outside of model organisms. There is considerable need for a computational tool that can take sparse phenotypic information, e.g., broadly defined space-time gene expression, and construct the simplest phylogenetic relationships consistent with data, thereby highlighting interesting events for molecular follow up.
Drosophila segmentation is a paradigmatic example of dynamic developmental network. Positional information propagates from maternal gradients such as bicoid (bcd) and caudal (cad) to gap genes such as hunchback, giant, knirps and Kruppel (respectively hb, gt, kni, Kr), and then to the striped expression of primary pair-rule genes such as even-skipped (eve), hairy (h), runt (run), and partially fushi-tarazu (ftz) [4, 5]. The pair-rule genes in turn control the segment polarity genes that are broadly conserved across the arthropods [6]. Mutagenesis and bioinformatics studies have revealed the main DNA motifs controlling the expression of gap and pair-rule genes [7] while systematic quantitative imaging has led to phenomenological models for segmentation dynamics [8, 9].
Recent evo-devo studies have started to map the segmentation hierarchy in other dipterans (Anopheles [10], Clogmia [11], Megaselia [12]). Almost all information comes from localizing the relevant mRNA by in-situ hybridization, and knocking down (KD) various transcripts with RNA interference. Information in each of these three species is still very sparse: while we know the position of the gap genes and the single pair-rule gene eve, there is only few information on the phasing of the other pair-rule genes relative to eve. Whether they are positioned by the gap genes or other so-called primary pair-rule genes is not known in those Dipterans. There are no defined gene regulatory modules in these species, so all information about gap gene regulation is inferred from their position and shifts in putative targets under KD.
In spite of this sparse information, some interesting questions can be posed. The anterior gap gene pattern appears invariant in all species as do the eve stripes, though there are only six in Clogmia before gastrulation vs 7 in Drosophila and up to 8 in Anopheles. There is more variability in the posterior. The relative positions of the posterior domains of hb and gt are inverted in Anopheles with respect to Drosophila, while in Clogmia, neither of these gap genes are expressed posteriorly before gastrulation. It is reasonable to assume that the primary pair-rule stripes are positioned by gap gene repression, so the evolutionary interchange of the posterior hb and giant domains poses problems for individual eve stripe regulatory modules. For instance, eve 5 in Drosophila is repressed posteriorly by gt so if the posterior gt domain is removed, eve 5 extends broadly posteriorly in Drosophila [13]. So how can gt domain be much more posterior in Anopheles, and virtually nonexistent in Clogmia? Similarly the two nested modules eve3+7 and 4+6 are both defined by kni repression from the interior and hb repression from the exterior [14], which seems less plausible in Anopheles based on the relative positions of the eve stripes and gap genes.
How is computational modeling best harnessed to the task of inferring the evolutionary path between fly and mosquito with such sparse information about one endpoint and intermediates? One very general lesson from the machine learning field is to avoid overfitting [15] [16]. More parameters make less predictive, “hairball” models [17] that can always be complexified rather than falsified. The temptation in the present instance is to import into the evolutionary simulation all the molecular details we have accumulated about Drosophila. A realistic model for the AP patterning in Drosophila with multiple factors, short range repression and cooperativity, was formulated in [18], and applied to the evolution of new enhancers in [3]. When guided by strong selection for the correct domain of expression [3], new modules can evolve on the time scale of 107 years [19]. The key point made in these and related papers is that de novo evolution of enhancers is fast because their genotype to phenotype map can be optimized by point mutations and hill climbing. These papers also observe that under the quick and sloppy logic of evolution, the excess of binding sites or the prevalence of generic activators and position specific inhibitors can all be understood as the most quickly realized solutions to the fitness optimization problem.
We do not see the creation of new modules in response to strong selection as necessary for the transition from fly and mosquito back to their last common ancestor (LCA). Rather via the logic of evolutionary bricolage [20], organic evolution and thus computation, should seek the most quickly evolved repurposing of existing components that connects the two defined endpoints subject to the constraint of viability for all intermediates. We will show that gap and pair-rule regulation in fly can be continuously adjusted to accommodate the observed changes in the posterior gap gene expression patterns. Given the range of times we have to cover, the high rate of churn in regulatory sequence among the Drosophlids [19](with little effect on phenotype), and the changes in regulatory factors such as the absence of bicoid in Anopheles, it is thus most practical and informative to simulate the phenotype and ignore the molecular level.
Phenotypic models have been informative in other areas [21, 22] and in the present context fit quantitative genetic data as to how expression domains shift when upstream factors are altered. Similar approaches are found in [23], and [8]. We then use an evolutionary computation that initializes the network model with Drosophila parameters, and mutates and selects with a ‘fitness’ that directs the model towards Anopheles. Putting aside the specific molecular information we have for Drosophila makes our approach applicable to a wider range of problems.
Invariably we find, eve stripe 5 disappears and either (or both) the eve 4+6 or 3+7 modules add a third posterior stripe to compensate. Thus the posterior eve stripes are not homologous in Drosophila and Anopheles. When we consider regulation of the other primary pair rule genes in fly, we conclude that the most plausible common long-germ ancestor of fly and mosquito employed a dynamic patterning system based on a forward shift in the eve pattern as observed in Clogmia and Drosophila [24] to impose phase relationships on the remaining pair rule genes. Thus there should be no homology in the posterior gap gene regulation of run, h, or ftz between fly and mosquito.
We emphasize that no computation, no matter how complex, will ever prove one evolutionary scenario over another. Computation is at best a heuristic tool to uncover interesting hypothesis that one could not guess, and buttress those hypothesis by their fidelity with a quantitative phenotypic model for regulation. The computation is like a screen for all solutions to an evolutionary problem given defined rules. To the extent the ingredients of the phenotypic model are plausible and transparent, and the predictions intuitive, they may stimulate experiments.
Materials and Methods
Evolutionary algorithm
The main lesson of two decades of quantitative analysis of Drosophila segmentation is that positional information of pair-rule stripes is essentially defined by gap-gene repression (see e.g. review in [5]). Gap genes themselves are positioned by a mixture of cross-repression [9, 25] and activation provided by maternal gradients. We will build our genetic or phenotypic model for Drosophila by defining an interaction kernel for each gap gene and pair-rule regulatory module. The kernel takes the numerical values of the inputs and outputs the expression. The general functional form is given in the S1 Text, and the specific inputs shown in the next subsection.
The evolutionary algorithm that will produce the Anopheles network is allowed to change only the numerical parameters within the kernel functions. Thus the parameters that define the maternal to gap regulation, interactions among the gap genes, and their regulation of the pair rule genes all change. The algorithm does not create new kernels nor add new inputs to existing kernels, but it is important to include from the start all potential regulatory inputs that might play a role during evolution, even if their effect is minor in Drosophila. The output of a kernel is allowed to become 0 signifying its elimination.
This conservative choice for the allowed ‘mutations’, was motivated above, and justified here. Firstly we show that the desired conversion from fly to mosquito can be realized without adding new kernels, and merely modifying existing ones. Binding sites turn over rapidly in modules so parameter evolution in existing kernels should be fast, while creating kernels in the absence of directional selection in anticipation of a future need is more speculative, and arguably slower. Secondly the anterior (roughly eve stripe 4 and forward) gap gene pattern in Anopheles and the intermediate species is largely invariant, while several of the pair-rule gene modules control both an anterior and a posterior stripe. Since we will impose that the anterior regulation is invariant, it was most logical to keep the inputs to these two stripe kernels invariant also. Once the allowed mutations are defined, the algorithm proceeds by rounds of mutation-selection. A population of networks is initialized to the Drosophila parameters, each network is mutated and retained if it is more fit than its parent. The most fit half of the population is duplicated and forms the next generation. Details on the code can be found in [26, 27], and our code is available upon request.
The function (negative fitness) that we want to minimize for each network is a sum of terms measuring (1) deviation of the posterior hb and gt profiles from the Anopheles pattern (2) deviation of the anterior eve profile from Drosophila. In addition there must be at least 7 eve stripes. From [10] we know hb moves forward in mosquito, while posterior gt is weak and probably plays no role in patterning so we assume it’s absent. Note we constrain the expression profiles, so evolution has to find a way to alter the posterior hb kernel to move its expression forward and match Anopheles. When we include a second pair rule gene, ftz, to define the 14 stripe segment polarity pattern, we insist its stripes alternate with those of eve. Nothing about intermediate species such as Clogmia is assumed. Once the evolutionary path to Anopheles is understood, and with it the regulation, the homology of the 6 Clogmia stripes becomes obvious without any further computation as we explain below.
We do not impose that the eve stripes be of equal width, though in a number of instances we checked that local parameter optimization can readily satisfy this constraint, see e.g. S4 Video. Normal Drosophila segmentation is known to be extremely precise, [28, 29]. However considerable change in eve expression in the blastula is not incompatible with adult viability. An early example was induced by variable bcd dosage [30]. Later examples include loss of parasegments 7 and 11 [31], and even abdominal segment A5 [32, 33], with further details left for the discussion. Some variation in phenotype is essential for evolution. Since one can only claim heuristic value for our evolutionary computations, trying to better define the fitness costs of quantitatively imperfect patterns adds more uncertainty than it resolves and encumbers a simple story.
Idealized Drosophila network
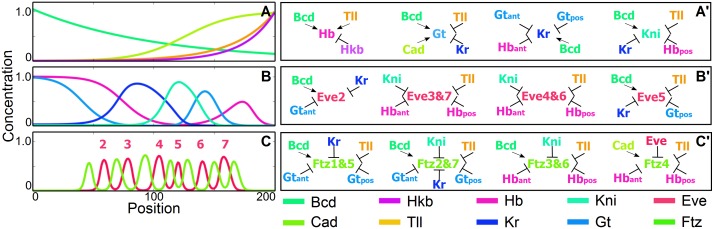
The starting point of our simulations is an idealized Drosophila shown in Fig 1. S1 Text details our assumptions, we summarize their main features below.
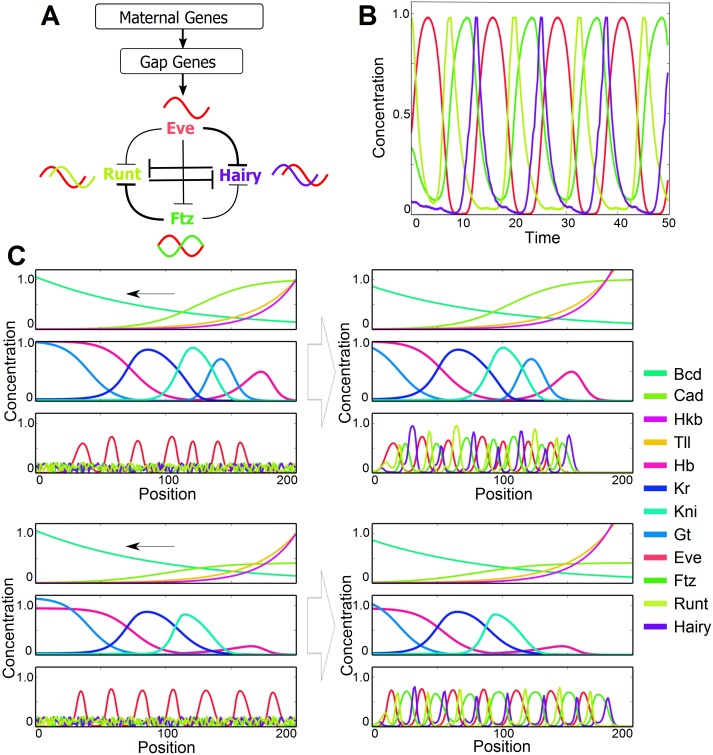
Fig 1. Simplified model of Drosophila network.
A-B: simulated maternal and gap gene profiles. C: simulated pair-rule gene profiles. A’-C’ Summary of interactions used to generate these profiles. Equations and references for the interactions are given in the S1 Text. A generic spatially uniform activator is assumed where needed.
There are maternal input gradients, bcd anterior and cad posterior, repressed by bcd. bcd is frozen throughout the simulation. While there is no bcd in mosquito, we assume some other gene such as otd takes its place [34]. In addition we have fixed profiles of tailless (tll) and huckebein (hkb) in the posterior. Those gradients supply positional information to the gap genes, hb, gt, kni, Kr, which are the only ones we need to follow during the evolution. At the phenotypic level we consider, gap domains look very similar in Drosophila and Anopheles, the main difference being the posterior exchanges between hb and gt.
Our description of the kernels defining gap territories incorporates regulatory interactions inferred from genetics, that are presumably conserved in evolution given the observed similarities of the gap patterns (see details in S1 Text). Repression comes from more than the immediately adjacent gap genes, since when these are mutated, expression typically does not extend to the anterior or posterior pole of the embryo. We omit other potential interactions because they do not impact the conserved qualitative gap pattern and would further require detailed molecular data to be fit in a species-specific manner [9, 35]. The reader will observe that all gap gene expression patterns along the computed pathways from fly to mosquito remain fixed in size, suggesting we are not omitting any essential interactions as gt and hb interchange.
For eve we include only stripes 2 to 7. (We do not simulate eve stripe 1 because we focus on the posterior regulation, and its regulation is decoupled from the other stripes.) and thus have four eve modules to consider eve 2 [36], eve 3+7, eve 4+6 [14, 37] and eve 5 [13]. There is good genetic evidence, reinforced by bioinformatic studies [5, 7], that their position is largely defined by gap gene repression. We include more than the minimal interactions required to fit the wild type eve and gap gene patterns in the posterior since hb and gt domains interchange as we evolve to mosquito, and mutagenesis experiments in fly suggest stripe regulation by more than the closest gap genes. For instance, in a hb mutant background, neither eve 6 nor eve stripe 7 expand much in the posterior [13] and in a gt mutant, eve 5 stripe only extends posterior to eve 7 stripe [13]. Thus there must be additional repression from the posterior that we assume comes from tll. We allow a uniform activator for stripes 3–7 and 4–6 (supplied by DSTAT [7, 37] or Zelda [38, 39]), but in our framework no positional information is given by activators.
There are a similar set of ftz modules defined by gap gene repression in Fig 1. ftz 4 represents a special case in that there is no stripe specific element and it appears that ftz stripe 4 is only expressed as part of the 7 stripe ‘zebra’ element [5]. Thus ftz has partially the character of a secondary pair rule gene that takes input from other primary genes, a fact that will be important in the following.
Results
Gap gene Anopheles pattern has been described by Goltsev et al. [10]. As explained before, the main difference between Drosophila and Anopheles gap patterns is in the relative positioning of hb and gt. Specifically hb moves forward and the gt domain becomes so posterior in Anopheles that it is unlikely to set stripe boundaries.
This interchange of localization in the course of evolution poses a problem for eve 5 whose posterior boundary is regulated by gt in Drosophila which is implausible in Anopheles, and also in Clogmia where posterior gt is absent (and therefore in the LCA of these three insects). So it is very plausible that eve regulation has changed between these insects. Similarly, the relation between eve stripes and gap genes in the posterior is rather different: for instance, Anopheles eve 6/7 are symmetrical on either side of posterior hb (Fig. 6 in [10]), while they are both anterior to Drosophila hb. Finally, Anopheles even has a weak (and late) extra 8th eve stripe compared to Drosophila.
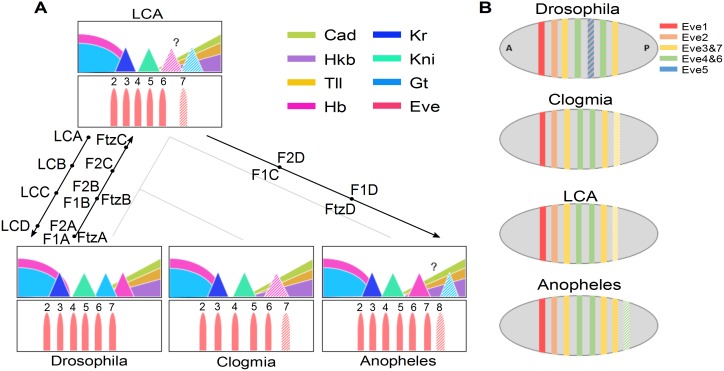
In the following we use computation to evolve evolutionary pathways between Drosophila and Anopheles and infer a LCA. (As with simple models of molecular evolution, our mutation rates are the same forward or backward in time.) All solutions described here were found several times and for varying parameters of the viability functions or the initial network itself. S1 and S2 Videos summarize the evolutionary pathways. Predicted evolutionary pathways are displayed on a simplified insect evolutionary tree in Fig 2A.
Fig 2. Summary of our predictions.
A Predicted evolutionary pathways from different simulations detailed in Fig 3 (label F1), Fig 4 (label F2), Fig 5 (label Ftz), Fig 7 (label LC). The times shown for the intermediates are only schematic. Gap and eve patterns in three insect species and the inferred last common ancestor (LCA) are indicated. B Summary of homology between Eve modules in different species predicted by our evolutionary simulations.
Variation in eve stripes and subsequent loss of eve 5
Simulations begin from the Drosophila network in Fig 1 and target the Anopheles gap pattern as an end point. The number of eve stripes (including 1) must be at least 7, and there is no restriction on their relative size or position.
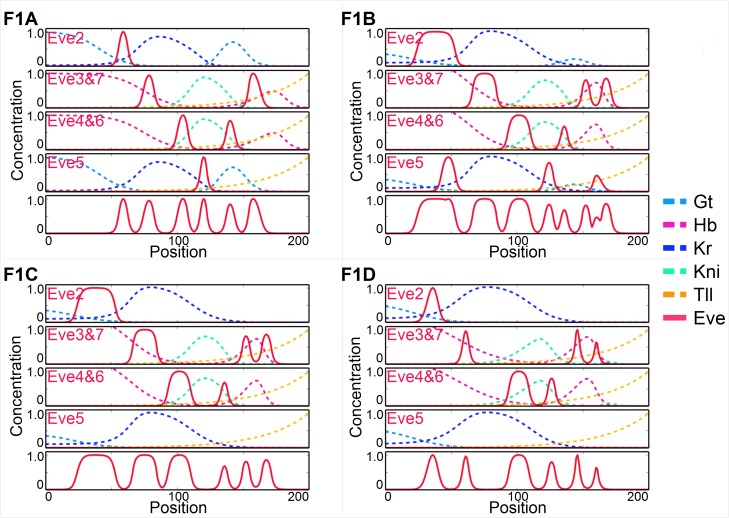
A typical example of such simulation is provided on Fig 3, with intermediate steps pictured on the phylogeny in Fig 2A. As the posterior hb domain moves forward it splits eve stripe 7. The repression from hb that defined the posterior boundaries of eve 6–7 gradually shifts to tll. Stripe 5 transiently fragments into two additional domains, Fig 3B, neither of which emerges as an distinct stripe. But once eve 7 splits in two, the stripe 5 element can disappear while respecting our constraint of at least 7 stripes. After it disappears posterior gt, is superfluous.
Fig 3. Simulated evolutionary pathway (label F1 on Fig 2A) from Drosophila to Anopheles, with salient changes discussed in the main text.
For each transcriptional eve module only the gap genes that regulate it are shown with the same color scheme as Figs 1 and 2A. eve stripe 1 is not shown and the maximum expression of each module is normalized to 1 except when it dips beneath a threshold equivalent to its loss.
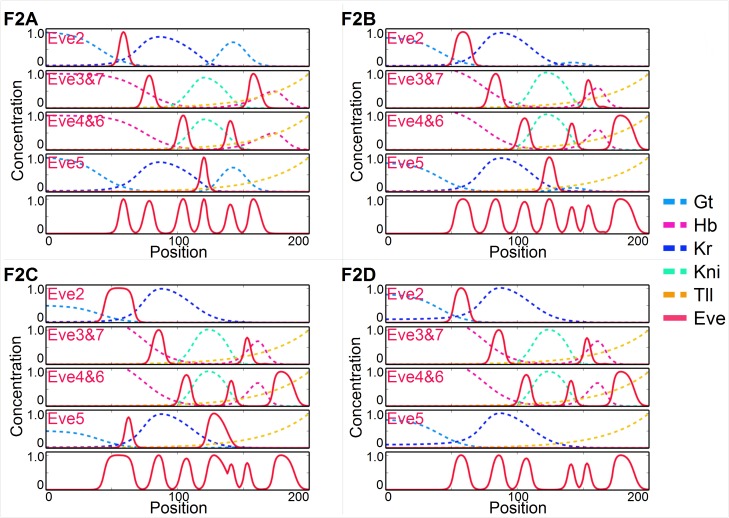
A variation on this pathway is presented on Fig 4. This time the evolution of the posterior hb domain anterior, splits eve 6 to create a new eve 8. Once a new eve stripe appears in the posterior (Fig 4B), posterior gt first disappears so that eve 5 expands posteriorly, fusing with eve 6 (thus effectively disappearing, (Fig 4C). Thus the eve 5 stripe module is no longer needed and disappears, leading to a final configuration similar to Fig 3D.
Fig 4. Simulated evolutionary pathway (label F2 on Fig 2A) from Drosophila to Anopheles, following the conventions of Fig 3.
In C the posterior eve 5 stripe is counted as merged with stripe 6.
The evolutionary scenario with creation of a new eve stripe in the posterior and subsequent removal of eve 5 and gt posterior is highly reproducible in our simulations for a variety of conditions that implement the same evolutionary pressures. Thus stripes 4+6 and 3+7 in Drosophila become stripes 4+5 and 3+6 in Anopheles. Furthermore one of these Drosophila modules controls 3 stripes in Anopheles. On Fig 3, Drosophila eve3+7 gives rise to stripes 3,6,7, while on fig 4, Drosophila eve4+6 gives rise to stripes 4,5,7. In both cases, one eve stripe is split by hb to give two stripes in the posterior that are symmetrically positioned around the hb domain. The 8 stripes in Anopheles would be most easily explained if both modules grow another stripe with Drosophila eve 4+6 (resp. 3+7) becoming 4,5,8 (resp. 3,6,7) in mosquito.
Our simulations also predict that intermediate dipterans must have retained this logic where eve 4+5 (resp. 3+6) are homologous to the eve 4+6 (resp. 3+7) module in Drosophila. Both modules are repressed by kni and hb, so in particular stripes 4–5 and 3–6 should be laid symmetrically with respect to kni in those intermediate dipterans. This is a prediction of our computation, that exploits the known gap gene regulation, but was in no way imposed. Strikingly, eve stripes 4+5 and 3+6 in both Clogmia and Anopheles are indeed laid rather symmetrically with respect to kni contrary to the situation in Drosophila. Furthermore Clogmia has only 6 eve stripes prior to gastrulation and consistent with our model, lack the posterior hb domain that generated the two additional posterior stripes in Anopheles.
Keeping segmentation logic by including ftz
We could not find examples of viable mutant flies missing 2 consecutive segments (corresponding to one full pair-rule period). This suggests that, even if in some mutants (e.g. the hopscotch mutant [32]), when one eve stripe disappears, the embryo needs to keep some polarity information required for the definition of parasegments.
Since eve 5 overlaps and defines A4p and A5a, proper parasegment definition means that the polarity of A4a and A5p must be maintained (and subsume cells that were in A4p and A5a). A natural hypothesis is then to assume another pair rule gene, out of phase with eve, must persist when eve stripe 5 disappears to provide input to the segment polarity system. We chose to add ftz to our model. We recognize that the proximate input to the segment polarity genes is not directly from eve and ftz but we have to insist that the model respect the minimal information logically required for the segment polarity pattern. Thus when an eve stripe disappears, the neighboring ftz stripes merge and only one parasegment disappears. (We will consider below how the constraints on evolution imposed by the other primary pair-rule genes in Drosophila [5] can be satisfied, if we insist that the relative phase among the pair rules genes is maintained.)
In a first round of simulations where we model ftz as a primary pair-rule gene (and postulate a pure ftz stripe 4 module delimited by hb on its anterior side and gt posteriorly), the evolutionary pathway observed in the previous section dies. There are several reasons for this: first gt controls both eve 5 and the putative ftz 4, so it is very difficult to have it disappear given this dual role while keeping the ftz/eve alternation that we impose. Second, if a new eve stripe appears in the posterior as before, it has no reason to be coupled properly to a corresponding alternating ftz stripe. Essentially, if ftz is primary, simulations fail to evolve new eve posterior stripes without breaking the alternation of ftz/eve.
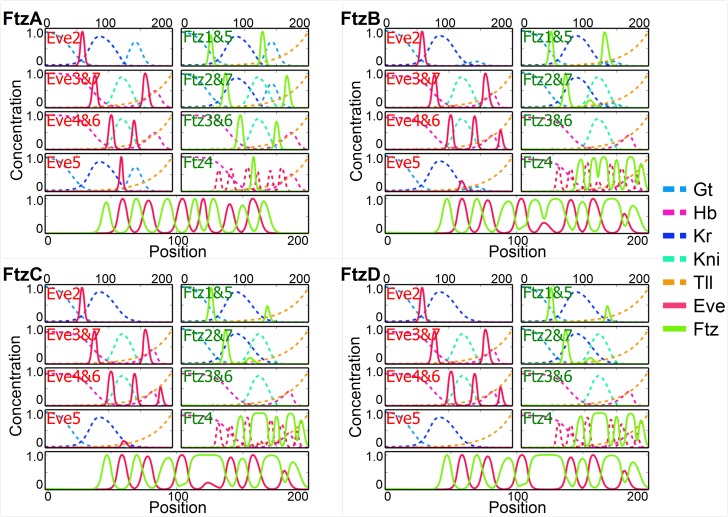
It is thus interesting in this context that ftz stipe 4 appears only together with the 7 stripe zebra element [5]. Thus if we allow repression of ftz 4-zebra by eve and not gt, it becomes slaved to eve in the posterior and functions as a secondary pair rule gene. In the simulation, Fig 5, ftz stripes 5,6,7 that are positioned by gap genes, gradually disappear in favor of the zebra element. The modules that controlled pairs of stripes 1+5, 2+7 and 3+6 now control only the anterior member and can evolve to interdigitate with the eve stripes. With the posterior ftz stripes controlled by eve repression, the pattern can evolve to the Anopheles configuration as before while preserving eve and ftz alternation throughout, Fig 5. When eve 5 disappears ftz 4 and ftz 5 merge (since eve repression is keeping them distinct) Fig 5B and 5C, thus preserving the eve, ftz alternation.
Fig 5. Simulated evolutionary pathway (label ftz on Fig 2A) from Drosophila to Anopheles, including ftz.
Conventions of Fig 3 for eve and ftz stripes are used.
Is the ancestral pair-rule patterning dynamically generated?
The fact that our evolutionary simulations fail when ftz is purely primary and succeed when ftz is more secondary suggests that it will be the same for other primary pair-rule genes such as h and runt. We nevertheless need to ask how the relative phase of the primary pair rule could be conserved in the evolutionary scenarios presented here. We propose, by means of a quantitative model, that pair-rule regulation in the posterior of the LCA is more dynamic than conventionally assumed in Drosophila. This will imply that there is no homology between the posterior regulation of the pair-rule genes by gap genes, other than for eve itself.
Specifically both in Drosophila [40] and Clogmia [24]eve stripes move from posterior to anterior prior to gastrulation. Our idea is that suitable combinations of strong and weak repression among run, h, ftz, and eve can read this phase information and stabilize the pair-rule pattern we observe in Drosophila, without direct gap gene input. The model is related to the pair-rule gene oscillator that patterns the posterior of short germ insects, as previously suggested in [41]. (However the model is not capable of intrinsic oscillations since eve is driven by gap genes and not by other pair rule genes, though it is easy to envisage how intrinsic pair rule feedback on eve could be gradually replaced by extrinsic gap regulation during the short to long germ band transition.) Viewed within a single cell, the forward displacement of eve appears as one complete temporal cycle, thus a gene regulatory network derived from a delayed negative feed back oscillator among the pair rule genes can use the same interactions to produce stable phases in space. In certain respects our conjectured LCA resembles Nasonia [42] where the segments posterior to A5 are patterned dynamically as we reconsider in more detail in the Discussion.
To implement our model, we control the maternal gradients to move the gap-genes forward and they drag eve along with them. The maternal gradients are adjusted to induce a forward shift of precisely one period in the eve pattern, Fig 6. Then for both the Drosophila and Anopheles gap gene patterns, the interactions shown in Fig 6A will direct an arbitrary expression pattern for the pair-rule genes other than eve to stably assume the relative phases we know for Drosophila, (or any other one by adjusting the strengths of repressions, see S3 and S4 Videos). For demonstration purposes only, we applied our model to the entire anterior-posterior axis, though we expect as in Fig 5 that the anterior gap gene regulation can persist.
Fig 6. A model for the LCA that imparts stable phase relations among the pair-rule genes and remains consistent with the evolutionary pathway from fly to mosquito.
(A) Schematic of the model showing gap input to eve only. The intensity of repression among the remaining genes (chosen arbitrarily as the primary pair-rule genes in fly) is shown by the line intensity and defines their relative phase. (B) Behavior of the model in A in response to imposed temporal oscillations of Eve, showing phase relationships between different pair-rule genes. Viewed within a cell, one cycle of temporal oscillations would result from the forward shift of the entire Eve pattern by one period. (C,D) If we implement a forward shift of eve by one stripe (left to right panels), by suitably scaling the maternal gradients, then an arbitrary initial arrangement of the three remaining genes is reset to the proper phasing for fly. We show the gap gene configuration for fly in (C) and for mosquito in (D). S3 and S4 Videos show the evolution from the left to right panels respectively for panels (C) and (D). For simplicity only, the model is applied across the entire embryo, though in reality the anterior gap gene input to the primary pair rule genes can remain invariant.
More detailed models of the gap gene network reproduce directly the anterior shift that we put in by hand [9, 35]. However they take as input the dynamic maternal gradients (in particular cad), and further observe that bcd itself is dynamical, which is consistent with what we assumed. A difference is that their models, along with [23], aim to reproduce precise developmental dynamics, while we have sacrificed this level of detail and prefer to reveal parsimonious phenotypic mechanisms that have a greater claim to validity over the large evolutionary distances we cover.
From LCA to Drosophila: Creation of eve 5
In our simulated evolution from Drosophila to Anopheles, eve 5 always disappeared. Thus an important consistency check is to show how eve 5 can appear when evolving from a LCA as appears in Figs 3 and 5C, to Drosophila. The solution was already suggested in Fig 4C, when a weak eve 2 stripe emerged from the stripe 5 module. Indeed these two stripes share the Kr and gt repressors.
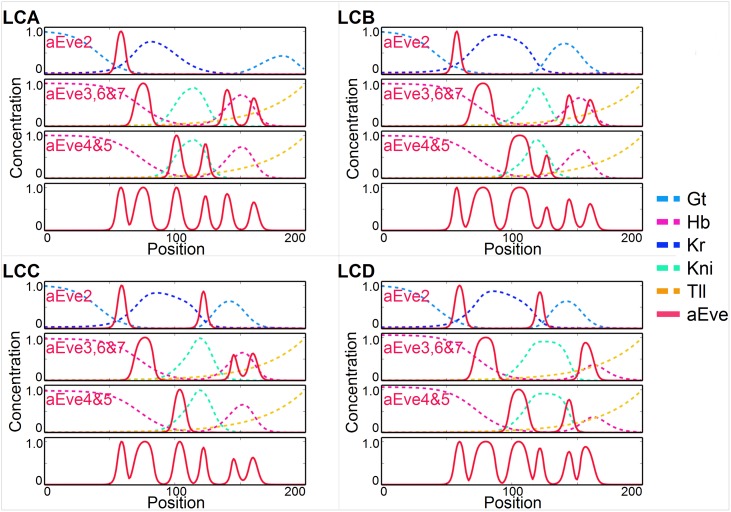
In Fig 7, we indeed see that when the posterior gt domain moves anterior the LCA-eve 2 module develops a second stripe, (and we imagine a distinct stripe 5 later evolves by drift). The tripartite 3,6,7 stripe loses its last component as hb moves posterior, and a reasonable Drosophila pattern is restored. Our LCA will generate a Clogmia like pattern if we remove posterior hb, illustrated on ??. There also is one eve stripe less than our presumptive LCA and Drosophila, because hb is not here to split ancestral eve 3–6 module in two in the posterior. Both these features qualitatively correspond to the observed pre-gastrulation Clogmia pattern, with only 6 stripes (vs 7 in Drosophila).
Fig 7. Simulated evolutionary pathway (label LC on Fig 4) from a presumptive LCA back to Drosophila.
4 steps of evolution are shown (A-D), details are given in the main text. Gap genes profiles are shown as well as activity of different transcriptional eve modules. eve1 is not simulated.
Discussion
We have used computational evolution and the observed gap gene patterns in Drosophila and Anopheles to suggest how the gap and pair-rule network evolved between these species and their LCA as well as the relation to Clogmia. Our strongest conclusions are that Anopheles eve stripes 3,6,7 (resp. stripes 4,5,8) are derived Drosophila eve modules 3+7 (resp. 4+6) by the elimination of Drosophila eve stripe 5, and the forward shift and renumbering of stripes 6,7, Fig 2B. Stripes 7 and 8 in Anopheles are the reflection of stripes 6 and 5 respectively in the repositioned hb domain. The Clogmia pattern follows Anopheles, except for the elimination of stripes 7,8 which are generated by the posterior hb domain, which is absent in Clogmia. Drosophila eve 5 arises from stripe 2 of the LCA.
Our model is formulated entirely within a phenotypic or genetic description of the regulatory network, yet generated surprising, but after the fact, plausible predictions. The homologies between the eve stripe 3+6 and 4+5 modules in Anopheles or Clogmia and Drosophila could have been guessed from their symmetries around the kni domain, but we are not aware of a reference to that effect. But it could not be guessed that the continuous transition from Drosophila to Anopheles could be accomplished merely by adjusting the parameters within the regulatory kernels defined by Drosophila. This is surely the most parsimonious route between fly and mosquito, and perhaps the most rapidly evolved since it only requires mutating binding sites in existing gene regulatory modules which we know to be rapid [19, 43]. The complex structure of Drosophila regulation, such as duplicate enhancers, only becomes implicated in the evolutionary transition if they were found to persist in intermediate species. We can never preclude more complex scenarios, such as new modules, but their rate of evolution is uncertain in the absence of positive selection. Thus our computation is a useful heuristic tool to show that the desired transition can be accomplished by reparametrizing existing kernels without creating new ones, via evolutionary bricolage [20].
The most immediate tests of our predictions require identifying pair-rule gene regulatory modules in Anopheles and in fact none has been found in that species or Clogmia to our knowledge. The most expeditious route to their discovery, with modern technology (e.g. [44]) would be CHIP-seq with antibodies against the gap genes. Putative binding sites could be refined computationally and then clusters of them could be matched numerically against the regulatory regions of relevant genes, [45, 46]. In Drosophila, computationally defined clusters of binding sites were very successful in reconstructing gap and pair-rule regulation and this approach could in principle be applied to other species. Our first prediction is the existence of eve modules in Clogmia with purely kni hb and tll binding sites and suggestive of their expression as stripes 3+6 and 4+5, and the analogous prediction of putative 3-stripe modules in Anopheles. A more dramatic confirmation of theory would be observing the expression of these modules in Drosophila. This requires sufficient homology between the gap gene proteins, which is not a given, since computational screens of these other genomes with the Drosophila binding site weight matrices has yielded nothing. However the conservation of enhancer function between Drosophila and Tribolium, in several cases gives one hope [47, 48].
More speculatively, our model requires a LCA where the posterior pair-rule genes other than eve derive their phasing from the anterior shift of eve observed in Drosophila and Clogmia [24, 40]. The connection between an anterior shift of the posterior pair-rule genes and a segmentation clock was made in the discussion of a paper that revealed that mechanism in Tribolium, [41], but is speculative. Our model concerns only long-germ dipterians.
Recent work on Nasonia, a long-germ band Hymenoptera, provides a very informative bridge between short and long germ band insects and the state we impute to our LCA. Hymenoptera is an out-group for the order Diptera considered here [42, 49]. Nasonia gap/eve pattern is qualitatively very similar to Drosophila in the anterior part of the embryo [50, 51], precisely until abdominal segment A5 (corresponding to ftz 5 in fly) [42] which is the position where some strong variability between species in gap/eve is observed in our simulations. Posterior to this segment, Nasonia pair-rule pattern presents all the characteristic of an insect segmentation clock, with eve on the top of the hierarchy controlling waves of expression of odd [42]. (Note eve has the segmental period in the posterior, as also seen in the centipede Strigamia [52].) Other pair-rule genes necessary to set proper segment polarity appear downstream of this clock system [42].
We chose ftz as the second pair-rule gene in the simulations to define the 14 parasegments since it regulates engrailed and has its zebra regulatory element that allowed the simulation to position the posterior ftz stripes by repression from eve. odd might seem a more logical choice to define the 14 parasegments, since it is part of the posterior oscillator in short germ insects, but the simulation would encounter the same difficulty as found for ftz, namely it is impossible to find paths for posterior hb and gt that are compatible with the known gap gene regulatory kernels in Drosophila and preserve the pair-rule gene alternation.
If we combine the phylogenic evidence from Nasonia with our inability to evolve multiple pair-rule genes with purely gap gene regulation, then an alternate conceptual distinction between primary and secondary pair-rule genes naturally arises, along the lines already suggested in [53] using data from Strigamia (see also data from Glomeris [54]). Primary pair-rule genes are those involved in the posterior segmentation clock, the secondary genes take input from the primary and control the segment polarity layer. Delayed negative feedback is a natural way to build an oscillator with a stable period. If the segmentation clock operates by phased sequential repression among the primary pair-rule genes then the same repression could operate in space, anterior to the oscillating growth zone, to fix the relative position of these same genes with the same relative phases. (A related conversion of a temporal signal to a static one was derived in a prior study on the evolution of Hox patterning during the short to long germ transition [55].) This is a prediction that could be tested in Tribolium [56]. We have assumed that in the ancestral short to long germ transition (or fly to mosquito), it is eve that first acquires gap gene input and breaks the negative feedback oscillator, based on circumstantial evidence, but this is not a logical necessity of the model.
If we are correct that the LCA used the anterior shift of eve to set the relative phase of the other pair-rule genes, then the posterior regulation of these genes by the gap genes would be recent and derived, and it should not be the basis for classifying primary vs secondary. Thus we would not expect any homology between the posterior gap gene input to the pair-rule genes other than eve in fly and mosquito. This proposal is difficult to test since convergent evolution is a real possibility here, since any module will use the gap genes that are appropriately positioned for its regulation.
Most evo-devo studies involve close enough species that there is no question that intermediates are viable. However the LCA of fly and mosquito was more than 200 million years ago [57] and we are proposing an evolutionary chain of events in the blastula and presuming viable adults exist along the way! The best evidence we can offer, is the hopscotch mutants [32] (a component of the Jak-Stat pathway). A maternal hypomorph rescued by a wildtype male, loses A5, yet gives rise to fertile flies of both sexes. So in this mutant, no essential part of the anatomy is lost with A5. The gap gene expression is unaffected, but stripe run 5 is absent, and eve 3/5 are suppressed, eve 5 more so than eve 3 [33]. The embryo tolerates other abdominal segment loss, e.g., reduced expression of eve 4/6 results in loss of two abdominal segments but viable adults [31]. If we consider the Hox genes as the basic mediators of segment identity then based on expression, abdominal segments 2–7 are identical [58], but more subtle differences in Hox regulation remain [59]
Our modeling differs from earlier work that focused more on the developmental dynamics of gap gene expression in Drosophila, such as [8, 9]. As noted in [9], fitting dynamic data will be difficult to scale up for more complex pathways, and these authors did not consider the pair rule genes. Thus it might prove challenging to study significant evolutionary changes with such detailed models. Our coarse-grained description, relying on minimal interaction (in a spirit similar to [23]) allows us to model long evolutionary time-scales, moving from microevolution to mesoevolution [60].
Our approach illustrates the interest of phenotypic models for evolutionary systems biology. It gives a quantitative framework to make qualitative predictions (such as “one stripe appears in this region while one stripe disappears in another region”) using semi-quantitative phenotypic data directly obtained from experiment (here, gap gene positioning and constraints on stripe number/alternation). The ability to generate novel predictions directly from currently available measurements is thus of interest for a broad swath of biological modeling [21, 27].
Supporting Information
(AVI)
(AVI)
The gap and pair-rule genes downstream shift forward by one eve stripe and in the process generate the known phasing among the primary pair-rule genes.
(AVI)
We took as initial pattern a network similar to our evolved Anopheles network and adjusted parameters to have stripes or equal size. Then, similar to Drosophila, after adding hairy and runt to the network of Anopheles a shift of the maternal gap genes towards the anterior is introduced then stopped. The pair-rule genes again acquire a defined phasing, starting from arbitrary initial conditions.
(AVI)
(PDF)
In the last common ancestor profile with hb, Fig 5B, the ancestral eve 6 and 7 are found symmetrically positioned on each side of the hb posterior peak. In Fig 5A, the deletion of this concentration of hb combines these stripes and the subsequent extension of kni condenses the form into a single eve stripe, obtaining a profile qualitatively similar to Clogmia.
(PNG)
(ZIP)
Acknowledgments
We thank Erik Clark for help with the literature on AP patterning mutants and Michael Akam and Eric Weischaus for discussions.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Simons Investigator mathematical modelling of biological systems, https://www.simonsfoundation.org to PF, the National Science Foundation grant PHY-1502151 http://www.nsf.gov/ to EDS, the Fonds de Recherche du Québec Nature et Technologies, Programe Nouveaux Chercheurs http://www.frqnt.gouv.qc.ca/accueil to PF, and the Natural Science and Engineering Research Council of Canada, Undergraduate Summer Research Award http://www.nserc-crsng.gc.ca/index_eng.asp to PT. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Ugalde JA, Chang BSW, Matz MV. Evolution of coral pigments recreated. Science. 2004. September;305(5689):1433–1433. 10.1126/science.1099597 [DOI] [PubMed] [Google Scholar]
- 2. Kim J, He X, Sinha S. Evolution of Regulatory Sequences in 12 Drosophila Species. PLoS Genetics. 2009. January;5(1):e1000330 10.1371/journal.pgen.1000330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Duque T, Sinha S. What does it take to evolve an enhancer? A simulation-based study of factors influencing the emergence of combinatorial regulation. Genome Biology and Evolution. 2015. June;7(6):1415–1431. 10.1093/gbe/evv080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wolpert L. Principles of Development. Oxford University Press; 2006. [Google Scholar]
- 5. Schroeder MD, Greer C, Gaul U. How to make stripes: deciphering the transition from non-periodic to periodic patterns in Drosophila segmentation. Development (Cambridge, England). 2011. July;138(14):3067–3078. 10.1242/dev.062141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peel AD, Chipman AD, Akam M. Arthropod segmentation: beyond the Drosophila paradigm. Nat Rev Genet. 2005. December;6(12):905–916. 10.1038/nrg1724 [DOI] [PubMed] [Google Scholar]
- 7. Schroeder MD, Pearce M, Fak J, Fan H, Unnerstall U, Emberly E, et al. Transcriptional control in the segmentation gene network of Drosophila. PLoS Biology. 2004. September;2(9):E271 10.1371/journal.pbio.0020271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jaeger J, Surkova S, Blagov M, Janssens H, Kosman D, Kozlov KN, et al. Dynamic control of positional information in the early Drosophila embryo. Nature. 2004. July;430(6997):368–371. 10.1038/nature02678 [DOI] [PubMed] [Google Scholar]
- 9. Crombach A, Wotton KR, Cicin-Sain D, Ashyraliyev M, Jaeger J. Efficient reverse-engineering of a developmental gene regulatory network. PLoS Comput Biol. 2012. July;8(7):e1002589 10.1371/journal.pcbi.1002589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goltsev Y, Hsiong W, Lanzaro G, Levine M. Different combinations of gap repressors for common stripes in Anopheles and Drosophila embryos. Developmental Biology. 2004;275(2):435–446. 10.1016/j.ydbio.2004.08.021 [DOI] [PubMed] [Google Scholar]
- 11. García-Solache M, Jaeger J, Akam M. A systematic analysis of the gap gene system in the moth midge Clogmia albipunctata. Developmental Biology. 2010. August;344(1):306–318. 10.1016/j.ydbio.2010.04.019 [DOI] [PubMed] [Google Scholar]
- 12. Wotton KR, Jiménez-Guri E, Crombach A, Janssens H, Alcaine-Colet A, Lemke S, et al. Quantitative system drift compensates for altered maternal inputs to the gap gene network of the scuttle fly Megaselia abdita. eLife. 2015. January;4:–. 10.7554/eLife.04785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fujioka M, Emi-Sarker Y, Yusibova GL, Goto T, Jaynes JB. Analysis of an even-skipped rescue transgene reveals both composite and discrete neuronal and early blastoderm enhancers, and multi-stripe positioning by gap gene repressor gradients. Development (Cambridge, England). 1999. June;126(11):2527–2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Clyde DE, Corado MSG, Wu X, Paré A, Papatsenko D, Small S. A self-organizing system of repressor gradients establishes segmental complexity in Drosophila. Nature. 2003. December;426(6968):849–853. 10.1038/nature02189 [DOI] [PubMed] [Google Scholar]
- 15. Daniels BC, Nemenman I. Automated adaptive inference of phenomenological dynamical models. Nature communications. 2015;6:8133 10.1038/ncomms9133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duvenaud D, Lloyd JR, Grosse R, Tenenbaum JB, Ghahramani Z. Structure Discovery in Nonparametric Regression through Compositional Kernel Search. 2013 Feb;.
- 17. Lander AD. The edges of understanding. BMC biology. 2010;8:40 10.1186/1741-7007-8-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. He X, Samee MAH, Blatti C, Sinha S. Thermodynamics-Based Models of Transcriptional Regulation by Enhancers: The Roles of Synergistic Activation, Cooperative Binding and Short-Range Repression. PLoS Comput Biol. 2010. September;6(9):e1000935 10.1371/journal.pcbi.1000935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arnold CD, Gerlach D, Spies D, Matts JA, Sytnikova YA, Pagani M, et al. Quantitative genome-wide enhancer activity maps for five Drosophila species show functional enhancer conservation and turnover during cis-regulatory evolution. Nature Genetics. 2014. June;46(7):685–692. 10.1038/ng.3009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jacob F. Evolution and tinkering. Science. 1977. June;196(4295):1161–1166. 10.1126/science.860134 [DOI] [PubMed] [Google Scholar]
- 21. François P, Siggia ED. Phenotypic models of evolution and development: geometry as destiny. Current Opinion in Genetics & Development. 2012. December;22(6):627–633. 10.1016/j.gde.2012.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. François P, Voisinne G, Siggia ED, Altan-Bonnet G, Vergassola M. Phenotypic model for early T-cell activation displaying sensitivity, specificity, and antagonism. Proc Natl Acad Sci U S A. 2013. February;p. 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Papatsenko D, Levine M. The Drosophila gap gene network is composed of two parallel toggle switches. PLoS ONE. 2011;6(7):e21145 10.1371/journal.pone.0021145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Janssens H, Siggens K, Cicin-Sain D, Jiménez-Guri E, Musy M, Akam M, et al. A quantitative atlas of Even-skipped and Hunchback expression in Clogmia albipunctata (Diptera: Psychodidae) blastoderm embryos. EvoDevo. 2014;5(1):1–13. 10.1186/2041-9139-5-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Krotov D, Dubuis JO, Gregor T, Bialek W. Morphogenesis at criticality. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(10):3683–3688. 10.1073/pnas.1324186111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. François P. Evolution in silico: from network structure to bifurcation theory. Advances in experimental medicine and biology. 2012;751:157–182. [DOI] [PubMed] [Google Scholar]
- 27. François P. Evolving phenotypic networks in silico. Seminars in cell & developmental biology. 2014. November;35:90–97. 10.1016/j.semcdb.2014.06.012 [DOI] [PubMed] [Google Scholar]
- 28. Gregor T, Tank DW, Wieschaus EF, Bialek W. Probing the limits to positional information. Cell. 2007;130(1):153–164. 10.1016/j.cell.2007.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dubuis JO, Tkacik G, Wieschaus EF, Gregor T, Bialek W. Positional information, in bits. PNAS. 2013. October;110(41):16301–16308. 10.1073/pnas.1315642110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Struhl G, Johnston P, Lawrence PA. Control of Drosophila body pattern by the hunchback morphogen gradient. Cell. 1992. April;69(2):237–249. 10.1016/0092-8674(92)90405-2 [DOI] [PubMed] [Google Scholar]
- 31. Fujioka M, Yusibova GL, Patel NH, Brown SJ, Jaynes JB. The repressor activity of Even-skipped is highly conserved, and is sufficient to activate engrailed and to regulate both the spacing and stability of parasegment boundaries. Development (Cambridge, England). 2002. October;129(19):4411–4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Perrimon N, Mahowald AP. l(1)hopscotch, a larval-pupal zygotic lethal with a specific maternal effect on segmentation in Drosophila. Developmental Biology. 1986. November;118(1):28–41. 10.1016/0012-1606(86)90070-9 [DOI] [PubMed] [Google Scholar]
- 33. Binari R, Perrimon N. Stripe-specific regulation of pair-rule genes by hopscotch, a putative Jak family tyrosine kinase in Drosophila. Genes & Development. 1994. February;8(3):300–312. 10.1101/gad.8.3.300 [DOI] [PubMed] [Google Scholar]
- 34. Lynch J, Desplan C. Evolution of Development: Beyond Bicoid. Current Biology. 2003. July;13(14):R557–R559. 10.1016/S0960-9822(03)00472-X [DOI] [PubMed] [Google Scholar]
- 35. Crombach A, Wotton KR, Jiménez-Guri E, Jaeger J. Gap gene regulatory dynamics evolve along a genotype network. Molecular biology and evolution. 2016. January;p. msw013 10.1093/molbev/msw013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stanojevic D, Small S, Levine M. Regulation of a segmentation stripe by overlapping activators and repressors in the Drosophila embryo. Science. 1991. November;254(5036):1385–1387. 10.1126/science.1683715 [DOI] [PubMed] [Google Scholar]
- 37. Struffi P, Corado M, Kaplan L, Yu D, Rushlow C, Small S. Combinatorial activation and concentration-dependent repression of the Drosophila even skipped stripe 3+7 enhancer. Development (Cambridge, England). 2011. October;138(19):4291–4299. 10.1242/dev.065987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Harrison MM, Li XY, Kaplan T, Botchan MR, Eisen MB. Zelda binding in the early Drosophila melanogaster embryo marks regions subsequently activated at the maternal-to-zygotic transition. PLoS Genetics. 2011. October;7(10):e1002266 10.1371/journal.pgen.1002266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nien CY, Liang HL, Butcher S, Sun Y, Fu S, Gocha T, et al. Temporal coordination of gene networks by Zelda in the early Drosophila embryo. PLoS Genetics. 2011. October;7(10):e1002339 10.1371/journal.pgen.1002339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Keränen SVE, Fowlkes CC, Luengo Hendriks CL, Sudar D, Knowles DW, Malik J, et al. Three-dimensional morphology and gene expression in the Drosophila blastoderm at cellular resolution II: dynamics. Genome Biology. 2006;7(12):R124 10.1186/gb-2006-7-12-r124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. El-Sherif E, Averof M, Brown SJ. A segmentation clock operating in blastoderm and germband stages of Tribolium development. Development (Cambridge, England). 2012;139(23):4341–4346. 10.1242/dev.085126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rosenberg MI, Brent AE, Payre F, Desplan C, Pan D. Dual mode of embryonic development is highlighted by expression and function of Nasonia pair-rule genes. eLife. 2014. March;3:1120 10.7554/eLife.01440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kazemian M, Suryamohan K, Chen JY, Zhang Y, Samee MAH, Halfon MS, et al. Evidence for Deep Regulatory Similarities in Early Developmental Programs across Highly Diverged Insects. Genome Biology and Evolution. 2014. September;6(9):2301–2320. 10.1093/gbe/evu184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. He Q, Johnston J, Zeitlinger J. ChIP-nexus enables improved detection of in vivo transcription factor binding footprints. Nature biotechnology. 2015. April;33(4):395–401. 10.1038/nbt.3121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zinzen RP, Girardot C, Gagneur J, Braun M, Furlong EEM. Combinatorial binding predicts spatio-temporal cis-regulatory activity. Nature. 2009. November;462(7269):65–70. 10.1038/nature08531 [DOI] [PubMed] [Google Scholar]
- 46. Sinha S, van Nimwegen E, Siggia ED. A probabilistic method to detect regulatory modules. Bioinformatics (Oxford, England). 2003;19 Suppl 1:i292–301. 10.1093/bioinformatics/btg1040 [DOI] [PubMed] [Google Scholar]
- 47. Wolff C, Schröder R, Schulz C, Tautz D, Klingler M. Regulation of the Tribolium homologues of caudal and hunchback in Drosophila: evidence for maternal gradient systems in a short germ embryo. Development (Cambridge, England). 1998. September;125(18):3645–3654. [DOI] [PubMed] [Google Scholar]
- 48. Cande J, Goltsev Y, Levine MS. Conservation of enhancer location in divergent insects. Proc Natl Acad Sci U S A. 2009. August;106(34):14414–14419. 10.1073/pnas.0905754106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lynch JA, El-Sherif E, Brown SJ. Comparisons of the embryonic development of Drosophila, Nasonia, and Tribolium. Wiley Interdisciplinary Reviews-Developmental Biology. 2012;1(1):16–39. 10.1002/wdev.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Olesnicky EC, Brent AE, Tonnes L, Walker M, Pultz MA, Leaf D, et al. A caudal mRNA gradient controls posterior development in the wasp Nasonia. Development (Cambridge, England). 2006. October;133(20):3973–3982. 10.1242/dev.02576 [DOI] [PubMed] [Google Scholar]
- 51. Lynch JA, Brent AE, Leaf DS, Pultz MA, Desplan C. Localized maternal orthodenticle patterns anterior and posterior in the long germ wasp Nasonia. Nature. 2006;439(7077):728–732. 10.1038/nature04445 [DOI] [PubMed] [Google Scholar]
- 52. Brena C, Akam M. An analysis of segmentation dynamics throughout embryogenesis in the centipede Strigamia maritima. BMC biology. 2013. January;11:112–112. 10.1186/1741-7007-11-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Green J, Akam M. Evolution of the pair rule gene network: Insights from a centipede. Developmental Biology. 2013. October;382(1):235–245. 10.1016/j.ydbio.2013.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Janssen R, Budd GE, Prpic NM, Damen WG. Expression of myriapod pair rule gene orthologs. EvoDevo. 2011;2(1):5 10.1186/2041-9139-2-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. François P, Siggia ED. Predicting embryonic patterning using mutual entropy fitness and in silico evolution. Development (Cambridge, England). 2010. June;137(14):2385–2395. 10.1242/dev.048033 [DOI] [PubMed] [Google Scholar]
- 56. Choe CP, Miller SC, Brown SJ. A pair-rule gene circuit defines segments sequentially in the short-germ insect Tribolium castaneum. Proceedings of the National Academy of Sciences of the United States of America. 2006. April;103(17):6560–6564. 10.1073/pnas.0510440103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Grimaldi D, Engel MS. Evolution of the Insects. Cambridge University Press; 2005. [Google Scholar]
- 58. Akam M. Hox Genes and the Evolution of Diverse Body Plans. Philosophical Transactions of the Royal Society B: Biological Sciences. 1995. September;349(1329):313–319. 10.1098/rstb.1995.0119 [DOI] [PubMed] [Google Scholar]
- 59. Bowman SK, Deaton AM, Domingues H, Wang PI, Sadreyev RI, Kingston RE, et al. H3K27 modifications define segmental regulatory domains in the Drosophila bithorax complex. eLife. 2014;3:e02833 10.7554/eLife.02833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Abouheif E. Parallelism as the pattern and process of mesoevolution. Evolution & Development. 2008;10(1):3–5. 10.1111/j.1525-142X.2007.00208.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(AVI)
(AVI)
The gap and pair-rule genes downstream shift forward by one eve stripe and in the process generate the known phasing among the primary pair-rule genes.
(AVI)
We took as initial pattern a network similar to our evolved Anopheles network and adjusted parameters to have stripes or equal size. Then, similar to Drosophila, after adding hairy and runt to the network of Anopheles a shift of the maternal gap genes towards the anterior is introduced then stopped. The pair-rule genes again acquire a defined phasing, starting from arbitrary initial conditions.
(AVI)
(PDF)
In the last common ancestor profile with hb, Fig 5B, the ancestral eve 6 and 7 are found symmetrically positioned on each side of the hb posterior peak. In Fig 5A, the deletion of this concentration of hb combines these stripes and the subsequent extension of kni condenses the form into a single eve stripe, obtaining a profile qualitatively similar to Clogmia.
(PNG)
(ZIP)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.