Abstract
AABB Standards specify that ABO group-specific whole blood is the only acceptable choice for whole blood transfusions. Although universal donor group O stored whole blood (SWB) was used extensively by the military during the wars of the mid-twentieth century, its use has fallen out of favor and has never been used to great extent in the civilian trauma population. Interest in the use of whole blood has been renewed, particularly in light of its potential value in far-forward military and other austere environments. Evidence of preserved platelet function in SWB has heightened enthusiasm for a “one stop shop” resuscitation product providing volume, oxygen carrying capacity, and hemostatic effects. Experience with universal donor group O SWB is required to ascertain whether its use will be an advance in trauma care. Described here is the process of establishing a universal donor group O SWB at a civilian trauma center in the United States.
BACKGROUND
In early September 2013, a surgical colleague from the trauma, critical care, and general surgical (TCCGS) service approached the Chair of the Division of Transfusion Medicine at our facility with an inquiry as to whether Transfusion Medicine could provide ABO group-specific, leukocyte-reduced, and platelet-preserved stored whole blood (SWB) for the resuscitation support of trauma victims. This was followed shortly thereafter by a meeting of stakeholders within the Division of Transfusion Medicine (Donor Services, Component Laboratory, Quality Unit, Information Management [IM], and Billing Personnel) to discuss the logistics of developing a program that required the collection, manufacture and provision of a new blood component from our Trans-fusion Service. To help the planning process, a follow-up meeting with the surgical colleague was held to gain insight into the program from a clinical perspective. In particular, the Division of Transfusion Medicine was interested in the anticipated number of whole blood components expected to be required for this initiative.
The TCCGS service has 50-70 massive transfusion events in trauma patients plus an additional 15-25 massive transfusion events in nontrauma patients per year. Therefore, 65-95 TCCGS service massive transfusion events could be anticipated on an annual basis. The mean red blood cell (RBC) use per massive transfusion was approximately 15 units. It was estimated that 975-1425 units of RBCs are transfused as part of TCCGS service massive transfusion events annually. If 70% of RBCs are transfused after a current ABO/Rh group and type has been obtained in the transfusion recipient, the annual TCCGS demand for ABO group-specific SWB would be 683-998 units per year. If the use of SWB was restricted to only group O or group A recipients, then 587-858 SWB group-specific A or O units from male blood donors (to mitigate the risk of transfusion-related acute lung injury) would be required by the TCCGS service each year.
The ABO group-specific SWB request raised a number of concerns within the Division of Transfusion Medicine. The projected number of group A and group O SWB units to meet the annual TCCGS massive transfusion requirements was going to be considerable and it would stress the overall blood supply. The rerouting of a substantial number of male blood donors away from the donation of other blood components was likely to have a significant impact on the inventory of these other components. The inability to plan for when massive transfusions will occur, because of their unpredictable nature, was also worrisome. There were serious concerns regarding the ability to balance maintaining an adequate supply of stored group O and A whole blood units to meet TCCGS needs while simultaneously preventing an excess of SWB products outdating in the refrigerator. Furthermore, timely provision of SWB products for resuscitations was a concern. If current institutional procedures were to be followed, SWB components could not be issued for transfusion until verified, current ABO grouping results were documented. At our institution, ABO group-specific RBC containing components cannot be issued until two independent ABO grouping procedures have been performed and yield identical results (i.e., a current grouping matches historical results for previously tested patients; for patients not previously tested, two current, independent sample collections yield identical results). Getting such testing finished in a timely manner so that ABO group-specific could be issued caused concerns about unacceptable delays in getting SWB units to the bedside. An additional potential problem related to obtaining accurate ABO group results on nongroup O recipients who received uncrossmatched group O RBCs (e.g., in the helicopter) before a sample being collected for ABO group testing. Such testing could yield mixed-field ABO grouping results (due to the dual circulation of the recipient's nongroup O cells and the transfused group O cells). Mixed-field ABO grouping results trigger additional required blood group testing procedures in Transfusion Medicine, causing additional delays in the provision of ABO group-specific SWB. Because of the anticipated logistical problems with the process as well as concerns about the blood supply, it was mutually agreed on by the TCCGS and Transfusion Medicine services to abandon an approach of ABO group-specific SWB and to pursue an alternative plan utilizing universal donor blood group O SWB. Such a plan, however, would require obtaining a variance from the AABB Standards Program Unit (SPU).
AABB VARIANCE
Section 5.25 of the 28th edition of the AABB Standards for Blood Banks and Transfusion Services (BBTS) – Urgent Requirement for Blood and Blood Components contains the following: “The blood bank or transfusion service shall have a process for the provision of blood and blood components before completion of tests listed in Standards. . . when a delay in transfusion could be detrimental to the patient.”1 Section 5.25.1 of the 28th edition of the AABB Standards for BBTS specifies: “Recipients whose ABO group is not known shall receive group O RBCs.”1 Section 5.14.1 of the 28th edition of the AABB Standards for BBTS, which pertains to the Selection of Compatible Blood and Blood Components for Transfusion specifies: “Recipients shall receive ABO group-specific whole blood or ABO-compatible RBC components.”1
On January 31, 2014, the Transfusion Service at our institution submitted a variance request pertaining to Sections 5.25 (Urgent Requirement for Blood and Blood Component) and 5.14.1 (ABO Group) of the 28th edition of the AABB Standards for BBTS1:
“We are requesting a variance to the requirement that only group O red blood cell products shall be issued when a recipient's ABO and Rh group is not known; we would like the option of using group O platelet-preserved leukocyte-reduced Whole Blood for transfusion to a specific patient population.”
In correspondence dated February 11, 2014, the AABB SPU requested more information: “The SPU could not come to a definitive conclusion on whether to grant or deny your request at this time. The SPU requests you provide the following information so that a final decision regarding your request can be reached:
Does your facility have any restrictions on the use of group O platelet-preserved leukocyte-reduced whole blood for pediatric patients and small sized patients (e.g., ≤ 40 kg)?
In a subsequent April 18, 2014 correspondence to the AABB, the Transfusion Service clarified that SWB will be available as an orderable product exclusively for the TCCGS service. The use of SWB will be restricted to patients weighing 40 kg or more, and two group O SWB units will be the maximum number of units issued to patients with an unknown ABO blood group. Rh negative SWB products will be provided to women of childbearing potential (defined at our facility as less than 55 years of age), males less than 18 years of age, and in patients where such cutoff ages cannot be conclusively determined. Women 55 years of age or older and males 18 years of age or older will be provided Rh positive SWB.
On June 16, 2014, the Transfusion Service received the following correspondence pertaining to the variance request in Sections 5.25 (Urgent Requirement for Blood and Blood Component) and 5.14.1 (ABO Group) of the 28th edition of the AABB Standards for BBTS1:
“The Standards Program Unit determined that the supporting documentation provided would ensure that the Mayo Clinic's process would meet the intent of the standards.”
SWB IMPLEMENTATION PART 1
To implement the whole blood transfusion process, the TCCGS service created an Adult Practice Management Guideline. The purpose of the guideline was “To standardize the management of resuscitation with Whole Blood for hemorrhagic shock.” The guideline specifies that TCCGS verbal activation of the whole blood resuscitation protocol is required, and such activation may occur on any patient under the care of a TCCGS surgeon. The verbal whole blood protocol activation process communicated to the Transfusion Laboratory is scripted. The individual contacting the Transfusion Laboratory states “I am activating the Whole Blood Resuscitation Protocol on patient name and unique clinic number located at building/floor/patient care unit/room number.” In order for the process to continue there must be a matching read back verification of this information on the part of the Transfusion Laboratory.
The Transfusion Laboratory will maintain an inventory of four group O SWB units (two O positive and two O negative) from male donors with immediate spin immunoglobulin M (IgM) anti-A and anti-B titers less than 200. The SWB units will be stored in the Transfusion Laboratory and not in remote locations, such as the Emergency Department refrigerator, to minimize the possibility of SWB units being mistaken for RBC units and administered inadvertently to non-TCCGS patients. No more than two SWB units may be transfused to any patient unless the patient has been confirmed to be blood group O by a current ABO grouping process (an historical blood group or any other form of blood group information such as dog tags or driver's license cannot be used in lieu of a current blood group). The transfusion team nursing personnel will respond to Level I trauma activations as per standard practice. If further blood product resuscitation is required, the institutional massive blood transfusion protocol is activated.
With regard to the addition and implementation of new approaches to remote damage control resuscitation (RDCR) at our institution, the strategy that has been employed has been to first establish safety, feasibility, practicality, and competence with a specific approach or tactic within the hospital and then transition it to the field. Previous examples of this approach are group O RBC2 and group A thawed plasma transfusions,3 which are services currently provided by our air ambulance service. The same approach will be utilized for the implementation of stored group O whole blood for RDCR of trauma patients.
In conjunction with the ongoing processes of developing and implementing a whole blood transfusion protocol, a group of investigators were evaluating the hemostatic characteristics of whole blood and reconstituted whole blood (RWB). In an initial set of evaluations, in vitro hemostatic test data were obtained for RWB (a 1:1:1 volume mixture of RBCs, plasma, and platelets), warm fresh whole blood, and SWB. Testing included thromboelastography, rotational thromboelastometry, calibrated automated thrombinogram, and multiple electrode impedance platelet aggregometry. The SWB were leukocyte-reduced, platelet-preserved products stored at 1-6°C without agitation. Platelet function in SWB (which had passed through the leuko-reduction filter), as assessed by platelet aggregometry, declined rapidly beginning on day 2 of storage.4
The TCCGS Practice Committee convened October 10, 2014 to review the whole blood resuscitation protocol and, largely due to the data showing rapid loss of platelet function in leukocyte-reduced, platelet-preserved SWB, decided not to approve the protocol. It was concluded that an in vitro hemostatic study of SWB with agitation would be necessary to obtain approval.
SWB IMPLEMENTATION PART 2
Under the assumption that the whole blood resuscitation protocol would eventually be approved by the TCCGS, Transfusion Medicine continued its efforts toward the implementation of an SWB program. Because a leukocyte-reduced, platelet-preserved SWB product did not previously exist as part of the Transfusion Medicine blood component menu, it was necessary to institute it as a brand new blood product manufactured by Transfusion Medicine. Blood products must be manufactured and handled in compliance with the United States Code of Federal Regulations according to current good manufacturing practices (cGMP). The new blood bag system that would be utilized is Imuflexs® WB-SP blood bag system with integral whole blood leukoreduction filter (saving platelets) with diversion blood sampling arm anticoagulant citrate phosphate dextrose and Optisol® (AS-5) RBC preservative from TerumoBCT, Inc. (Lakewood, CO). This was manufactured to satisfy US and European requirements for leukoreduction while maintaining postfiltration platelet counts. Internal quality control (QC) procedures as part of the validation process for the new blood product confirmed acceptable leukoreduction.
To comply with cGMP and federal regulations, a multitude of blood component manufacturing details were required to be in place for Transfusion Medicine to reach the point where group O leukocyte-reduced, platelet-preserved SWB products could be collected, tested, processed, distributed, stored, and issued for transfusion. Standard operating procedures (SOPs) pertaining to the collection, testing, storage, distribution, issuing, and administration of group O SWB needed to be written or updated, validated, reviewed, communicated, and controlled (e.g., change and document controls). Training on these SOPs needed to be conducted and documented. These procedural changes would impact multiple work units including the Donor Center, Component Laboratory, Reference Laboratory, Transfusion Laboratory, Operational Support Unit, and the Department of Nursing's Transfusion Team (IVTX Team). In essence, these procedural builds needed to define who, what, why, and how the entire whole blood process would be performed so it could be completed in a consistent, reproducible manner that was fully documented. A summary of actions taken by work units in transfusion medicine to establish the SWB program is provided in Table 1.
TABLE 1.
Actions required of transfusion medicine to implement leukocyte-reduced, platelet-spared whole blood for the trauma service
| Blood donor center challenges/tasks | Implications/actions |
|---|---|
| A donor recruitment strategy targeting blood group O male donors needed development | The blood donor pool is cut by approximately 50% |
| Blood donors are deferred for 12 weeks after collection (whole blood product stored up 14 days after collection) | |
| Blood donors are diverted from donation of other blood components | |
| New blood donation visit category required | The blood donor registration SOP was updated to include a new donation visit category (new donation visit category named leukocyte-reduced WB [LW] for leukocyte-reduced whole blood) |
| New collection bag system for LW donations was being utilized | The blood collection SOP was updated to include the new collection bag system for LW donations |
| LW blood collection must come from donors who have not consumed antiplatelet medications | The Donor Center's criteria for accepting donors for LW collections must adhere to the criteria outlined for platelet collections (which differs from the acceptance criteria for “routine” whole blood donations from which platelets are not produced) |
| The computer-based blood donor questionnaire was updated to capture antiplatelet medication information for LW donations | |
| The blood donor questionnaire process was amended to include a means to reroute donors who did not qualify for LW donations to routine whole blood donations | |
| Component Laboratory challenges/tasks | Implications/actions |
| LW donations are a completely new blood component requiring manufacturing processes | SOP updates were required and made for processing (e.g., leukocyte filtration), storage, and shipping of the LW products |
| A plan for transport of whole blood units to the Transfusion Laboratory was developed | |
| A quality control (QC) plan for the new LW product needed development | A QC plan for the LW product was created so the products would satisfy cGMP acceptance criteria |
| Transfusion Laboratory challenges/tasks | Implications/actions |
| Processes for storage and issue of LW components required development | SOPs were updated to include instructions for the storage and issue of LW products |
| Electronic ordering mechanisms for LW components needed to be developed | Transfusion Laboratory worked with institutional electronic ordering personnel to create the ability to order LW products in the electronic ordering systems |
| Customer education plan for LW components was required | A customer education plan for physicians and nurses on the use and availability of the LW products was created and delivered |
| Immunohematology reference laboratory challenges/tasks | Implications/actions |
| Anti-A and anti-B isoagglutinin titer process for LW donations required development and implementation | A new policy, SOP, form, and training documents were created to describe and perform anti-A and anti-B isoagglutinin process for LW donations |
| A computer-based process for identifying and obtaining a blood sample for isoagglutinin testing was created | |
| A new test code for anti-A and anti-B titer results was created and validated in the donor computer system (anti-A and anti-B titers must be documented as <200 in the donor computer system to enable the LW products to be labeled) | |
| A new process was created in the donor computer system that prevented blood donors with unacceptable anti-A or anti-B titers from being scheduled for future LW donations | |
| IM challenges/tasks | Implications/actions |
| Numerous LW product processes required creation or updating of transfusion medicine computer systems | The computer-based donor questionnaire was amended to capture recent antiplatelet medication use in LW product donors and to defer these donors when appropriate |
| Numerous table changes were necessary and these changes were made in the donor and transfusion laboratory computer systems to incorporate the LW product process, including blood donor visit definition, blood product definition, definition of the new anti-A and anti-B titer test, and blood product labeling rules based on anti-A and anti-B titer results | |
| Computer reports were updated or created for process and quality reviews | |
| A new product label for the LW product was created in the blood labeling system | |
| Processes for shipping the LW products from the donor to the transfusion laboratory computer system were created | |
| The Circular of Information for Blood and Blood Components5 was amended to include the LW product | |
| All new and amended computer processes underwent and passed validation protocols |
There were also a number of “loose ends” and questions that needed to be answered before the leukocyte-reduced, platelet-preserved whole blood initiative for trauma patients could be initiated at our facility. The plan going into the implementation was to store the leukocyte-reduced, platelet preserved whole blood units in Transfusion Medicine for a maximum of 14 days. The question was what to do with the whole blood components once they reached their 14-day maximum storage limit. Should the units be returned to the Component Laboratory to be manufactured into an RBC component for use in the general transfusion recipient population? Based on the extensive work it would require to set up a whole blood → RBC conversion process (e.g., SOPs, process and procedure validations [including computer-based processes], new product codes, shipping procedures and processes, etc.) it was decided, at least for the initial phases of the SWB initiative, that any whole blood units not used by the TCCGS service after 14 days of storage would be discarded.
Another challenge was the question of how to document the compatibility of universal donor group O whole blood components in the Transfusion Laboratory computer system. The Transfusion Laboratory computer system is programmed to only allow ABO group-identical whole blood to be issued for transfusion recipients. The question thus became, do we alter the tables in the computer to allow for the issue of nongroup-identical universal donor group O whole blood or do we set up a human-based Transfusion Laboratory manual override process to allow these whole blood products to be issued in computer?? It was decided to proceed with the latter approach and have a manual override process for the computer.
Additional practice questions were posed by Transfusion Laboratory personnel. What if whole blood is available for use in a trauma patient, but it isn't the appropriate Rh type as outlined in the SOP? Do we give O negative SWB components to patients who should be candidates for O positive whole blood? It was decided that recipients defined as Rh positive transfusion recipients (either by Rh type triage SOP guidelines or if they are documented to be Rh positive) would be eligible for O negative SWB if such units were available. Conversely, O positive SWB units will not be issued for patients defined as candidates for the transfusion of Rh negative SWB.
Another practice question had to do with ABO group testing of the recipient: “What if current ABO group testing for the transfusion recipient has not been completed but the patient has been identified and there is a previously documented, historical blood group O typing for the patient in our Transfusion Medicine records? Can we give the additional two group O SWB units to this patient based on this historical laboratory result?” It was decided to stay consistent with Transfusion Medicine policies and SOPs and not issue group O SWB based on historical blood grouping results. A current blood group determination of group O will be required to issue an additional two group O SWB units.
Finally, Transfusion Laboratory personnel asked: “What should be done if the identity of a patient to receive SWB is obtained and a review of the patient's transfusion history reveals documentation of previously identified clinically significant RBC antibodies? Should the group O SWB units still be issued?” It was decided that when a history of clinically significant RBC alloantibodies is known, group O SWB will not be issued.
SWB IMPLEMENTATION PROCESS PART 3
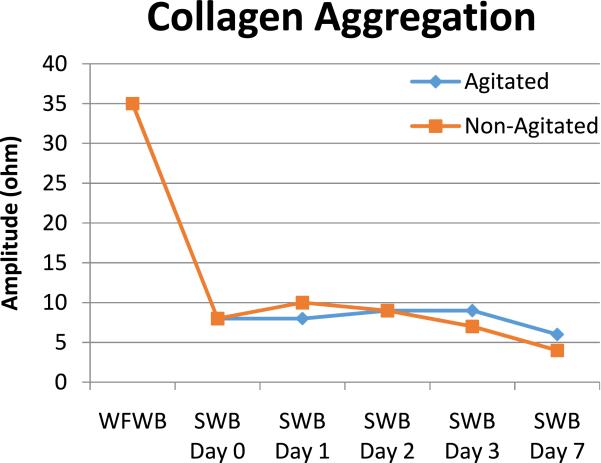
Due to the suboptimal platelet function results obtained in the first set of hemostatic studies of stored leukocyte-reduced platelet-preserved whole blood, it was decided to repeat the studies using whole blood that had been agitated during storage. The hypothesis was that the agitation of stored, refrigerated leukocyte-reduced whole blood components would result in improved platelet function. In the study, whole blood units were collected and leukocyte-reduced per manufacturer's instructions and our internal SOP. The whole blood units were then divided in half (i.e., two leukocyte-reduced, “twin daughter units” were produced) for storage comparison studies. After division, one of the daughter units was stored in the refrigerator (1-6°C) without agitation while the second of the daughter units was stored in the refrigerator with constant agitation in the same manner as platelets on a platelet rotator (a horizontal orbital rotator at 90 rpm). The results of the second study, as can be seen in Fig. 1, showed rapid loss of platelet function following leukocyte filtration when assessed with multichannel platelet function studies in both the nonagitated or agitated SWB components (Furthermore, agitation of the SWB components resulted in visibly evident hemolysis of RBCs). After the results of these studies became known to the TCCGS surgical group, it was decided that the use of leukocyte-reduced, platelet-preserved SWB in trauma patients needed to be reconsidered. Interestingly, the loss of platelet function in SWB is inconsistent with the findings of others,6,7 but to the best of our knowledge, we are the only group to evaluate platelet function with multichannel platelet aggregation studies on the SWB product following the passage of the platelets through the leukoreduction filter.
Fig. 1.
Multichannel platelet aggregation study of warm fresh whole blood (WFWB) and postfiltration leukocyte-reduced agitated and nonagitated stored whole blood (SWB) at Days 0, 1, 2, 3, and 7 using collagen as the platelet agonist.
Because of concerns regarding the preservation of platelet function in the leukocyte-reduced SWB components, combined with a strong desire to continue to evaluate the potential benefits of SWB use in trauma patients, the Chair of the Trauma Service at our institution proposed a shift in approach. Because stored nonfiltered whole blood has been shown to retain platelet function for 14 days (or longer),6 it was felt that the rapid loss of platelet function seen with the leukocyte-reduced SWB components seemed to be related to the leukocyte filtration process. Therefore, the shift in approach was to utilize stored group O whole blood components in exactly the same manner and utilizing most of the same processes that were going to be used for the leukocyte-reduced, platelet-preserved product, however, standard whole blood components would be collected (using the bags normally used for routine whole blood collections) and these components would be stored in the refrigerator unmanipulated (this product will be named Trauma Whole Blood [T-WB]). The blood bag system that will be utilized for T-WB is the Leukotrap® WB with SAVE System from Haemonetics, Inc. (Braintree, MA). These T-WB products, therefore, would be platelet-rich and non-leukocyte-reduced. The T-WB products will be trans-fused to trauma patients in an unmanipulated state through standard blood administration filters according to procedures utilized for the transfusion of trauma patients. T-WB should have preserved platelet function, but the lack of leukoreduction added circumstances that needed to be considered. First of all, T-WB is not a cytomegalovirus (CMV)-safe product because it is not leukocyte-reduced. The question was posed to the trauma team whether that was a concern and should we provide T-WB only from CMV-negative group O male blood donors? Due to the significant impact on the number of donors satisfying all of those requirements (particularly group O negative donors), the trauma team decided it was okay to bypass the requirement that these products be CMV negative.
What about the risk of transfusion-associated graft-versus-host disease (TA-GVHD) associated with the trans-fusion of a non-leukocyte-reduced blood component? Leukocyte-filtration does not totally prevent TA-GVHD, but the use of non-leukocyte-reduced blood components could potentially increase the risk of this serious, often fatal, transfusion-associated adverse event. The question was posed as to whether T-WB should be irradiated to prevent TA-GVHD. The decision was to proceed without irradiation because of the logistical challenges of providing irradiated products in a timely manner during an emergency. The closest blood irradiator to our trauma center is approximately 1 mile away in a separate building. Although these buildings are connected by a pneumatic tube system, the time delays of providing “on-demand” irradiated products associated with tubing products from the site of the irradiator to the trauma center and the lack of 100% reliability of the tube system to deliver products to the appropriate destination, did not support the adoption of such an approach. As an alternative, T-WB units could be irradiated at the time of storage. It was felt that the risk of increased potassium related to storage of irradiated RBCs outweighed the risk of TA-GVHD so this approach was rejected as well.
DISCUSSION
It is fair to say that the implementation of universal donor, group O SWB for the TCCGS service has been a long and challenging process at our institution. Some readers of this paper might even ask: “Why was this route of implementation chosen at your facility? Given that group O universal donor whole blood is an “experimental therapy” was it really necessary for the transfusion services to implement so many changes when this “research” could have been performed under the auspices of the facility's Institutional Review Board?” In response to such questions it is our view that the use of whole blood is not an “experimental therapy.” To illustrate this point, a recent survey of transfusion policies at US and Canadian children's hospitals documented the use of fresh whole blood at 6 (15%) of 40 responding hospitals.8 Furthermore, whole blood as a product for transfusion is approved by the US Food and Drug Administration and the AABB has described the appropriate use of whole blood in its Standards.1 The goal of the TCCGS service (due to knowledge gained through the efforts of groups such as the Trauma, Hemostasis, and Oxygenation Research Network, the TCCGS service was already convinced of superior efficacy) was to transition to the use of whole blood for their patient population as a long-term practice change rather than as a short-term research project. Since whole blood is an FDA-approved product, no consent (individual patient or community) is required to implement such a practice change. A long-term practice change using universal donor group O SWB thus posed two major obstacles to overcome to allow implementation at our facility. The first obstacle was the variance process with the AABB SPU to receive permission to use group O universal donor SWB. As documented above, this “give and take” took numerous months and did not resolve until approval was received for the use of non-leukocyte-reduced group O universal donor SWB in the Fall of 2015. The second obstacle related to the process required of a Blood Donor Center to collect and manufacture a brand new blood component. The cGMP process for blood products is rigorous, time-consuming, and requires the coordinated effort of many work units and individuals as described above. These two obstacles constituted the major factors influencing on the long time frame for implementation of SWB at our institution. Would other institutions be able to implement SWB faster than us? The answer is probably yes. First of all, we have obtained the AABB variance so a precedent has now been established for universal donor group O SWB transfusions. Second, hospital-based trans-fusion services that receive their blood components from an outside blood supplier will not have to go through many of the implementation steps outlined above as long as their blood supplier (who will have the predominance of the cGMP responsibilities) has the SWB product of interest available to distribute to the transfusion service. Finally, we lost a lot of time trying to implement the “new” leukocyte-reduced, platelet-spared SWB product. If we had started this initiative with the non-leukocyte-reduced whole blood products that were already being collected at our Blood Donor Center, this project would have been less complicated and would have taken much less time.
Another question has arisen pertaining to the anti-A and anti-B isoagglutinin titer process used to qualify group O blood donors for SWB donation. The question raised concerns that this seems to be a cumbersome process so are there systems available to perform these tests in routine donor testing platforms? The answer to this question is that it is a cumbersome process and it is not currently amenable to being added to routine donor testing platforms. What makes the process a little bit more “user friendly” for our facility is that the testing is performed “in-house” and it follows a work flow that is similar to the one established for testing group O apheresis platelet donations. If the SWB program significantly expands at our institution in the future, we will investigate more automated solutions to the isoagglutinin process.
As we proceed with the implementation of group O, universal donor T-WB at our facility two key questions await more definitive answers:
Is non-leukocyte-reduced universal group O SWB safe?
Is 0053WB the preferable transfusion option for the resuscitation (e.g., RDCR) of trauma patients?
With regard to question number 1, the approach soon to be implemented at our facility most closely resembles US Military practices during World War II, the Korean War, and the Vietnam War.
During World War II, the initial approach taken by the US Army Medical Service was to transfuse group O whole blood regardless of the recipient's blood group and without regard to the blood donor's isoagglutinin titers. In a study of 265 patients who received ABO-incompatible group O whole blood during this period of World War II, three transfusion reactions with the manifestations of fever, hemoglobinemia, and hyperbilirubinemia were identified (incidence 1.1%), but no other serous clinical signs or symptoms were seen in association with these transfusion episodes.9 The group O whole blood units implicated in the three transfusion reactions all had anti-A/anti-B IgM isoagglutinin titers in excess of 500. The transfusion practice during World War II did not change until after an April 1944 report of a severe hemolytic trans-fusion reaction in a group A recipient who received 75 mL of a group O whole blood unit with an IgM anti-A titer of 8000. Subsequently, the US Army adopted a policy declaring that all group O whole blood units with an IgM anti-A/anti-B titer higher than 250 be labeled as “high-titer” and these high-titer units could only be transfused to group O recipients.10
During the Korean War only group O whole blood components were shipped to the war zone. These group O whole blood units were labeled as low-titer (IgM titer less than 256) and high-titer.11 High-titer whole blood units were transfused to group O recipients and low-titer units were transfused to all nongroup O recipients.12 Nearly 400,000 units of blood were transfused during the Korean War with no reported reactions implicating the use of group O whole blood. The use of universal donor group O whole blood, directed to recipients on the basis of high-titer and low-titer anti-A/anti-B results, was concluded to be a safe practice.11
In early 1965, during the Vietnam War, a decision was made to only ship universal donor, low-titer, group O whole blood to the war zone. However, as blood requirements increased, the policy was changed to allow for the shipment of nongroup O whole blood to Vietnam. The initial shipment of group A whole blood was delivered to Vietnam in December 1965 and additional whole blood units with random blood group distributions were delivered starting in January 1966. Exclusive use of low-titer, group O whole blood continued to be the practice utilized by forward medical personnel and forward surgical hospitals where pretransfusion testing and compatibility testing could not be performed.13 Between September 1967 and February 1969, 230,323 whole blood units (all ABO groups included) were transfused in Vietnam. During this time period, 24 hemolytic transfusion reactions were documented. Only one of these transfusion reactions, however, was caused by ABO isoagglutinins in a transfused group O whole blood unit. This reaction occurred in a far-forward setting when a high-titer unit (IgM and IgG titers of 256 and 32,768, respectively) was transfused as universal blood by mistake.10,14,15 This patient experienced oliguria and hemolysis for 2 days and then recovered.10,14 The experience in Vietnam served to reinforce the concept that the transfusion of universal donor low-titer group O whole blood was a safe practice.
It appears, therefore, that the use of universal donor, low-titer, and group O SWB is a safe transfusion practice.
With regard to question number 2, information pertaining to outcomes in trauma patients receiving SWB is limited. In the military, despite extensive use of SWB in World War II, the Korean War, and the Vietnam War, and evidence of its safety, there are few data on the impact of such transfusions on patient outcomes. In the civilian setting, a recent pilot study evaluated modified whole blood versus blood component therapy in severely injured patients.16 The modified whole blood product used in the study was leukocyte-reduced and stored in the refrigerator (4°C). On arrival to the trauma center, patients were randomized to receive either modified whole blood or blood component therapy consisting of RBCs and plasma provided at a 1:1 ratio. Patients in both groups received a platelet transfusion for every six units of modified whole blood or six units of RBCs/plasma. Subjects randomized to the modified whole blood arm received significantly lower blood transfusion volumes at 24 hours. It is difficult to definitively draw a conclusion of a clear benefit of the cold, modified SWB versus blood component therapy from this pilot study.
It is apparent that the question of whether SWB is the preferable transfusion option for the resuscitation of trauma patients has not been answered and the only way to answer this question is through studies and the accumulation of experience with the product in this setting.
CONCLUSION
After all the planning, changes of plans, and preparations, our facility is “rolling out” group O non-leukocyte-reduced SWB (i.e., T-WB) for our TCCGS service in October, 2015. Going into the endeavor, there is confidence in the safety of the product, particularly with the use being restricted to two group O T-WB units in non-group O recipients. The question of whether T-WB will result in improved outcomes in trauma patients remains to be determined. This question can only be answered if trauma centers are willing to evaluate SWB in their patient population.
ABBREVIATIONS
- cGMP
current good manufacturing practices
- IM
information management
- QC
quality control
- RDCR
remote damage control resuscitation
- RWB
reconstituted whole blood
- SOPs
standard operating procedures
- SPU
Standards Program Unit
- SWB
stored whole blood
- TA-GVHD
transfusion-associated graft-versus-host disease
- TCCGS
trauma, critical care, and general surgical
- WFWB
warm fresh whole blood
Footnotes
CONFLICT OF INTEREST
The authors report no conflicts of interest.
REFERENCES
- 1.AABB. Standards for blood banks and transfusion services. 28th ed. AABB; Bethesda (MD): 2012. [Google Scholar]
- 2.Berns KS, Zietlow SP. Blood usage in rotor-wing transport. Air Med J. 1998;17:105–8. doi: 10.1016/s1067-991x(98)90104-3. [DOI] [PubMed] [Google Scholar]
- 3.Kim BD, Zielinski MD, Jenkins DH, et al. The effects of prehospital plasma on patients with injury: a prehospital plasma resuscitation. J Trauma Acute Care Surg. 2012;73:S49–53. doi: 10.1097/TA.0b013e31826060ff. [DOI] [PubMed] [Google Scholar]
- 4.Polites SF, Park MS, Stubbs JR, et al. Whole blood platelet function degrades quickly after storage: in vitro comparison of fresh whole blood, stored whole blood, and reconstituted whole blood.. 46th World Congress of Surgery Abstract Book; Oral Presentation at 46th World Congress of Surgery Meeting; 2015. 2015. Abstract # 177.05. [Google Scholar]
- 5.AABB, American Red Cross, America's Blood Centers, and the Armed Forces Blood Program 2013. AABB Press; 2013. Circular of Information for the Use of Human Blood and Blood Components. [Google Scholar]
- 6.Pidcoke HF, McFaul SJ, Ramasubramanian AK, et al. Primary hemostatic capacity of whole blood: a comprehensive analysis of pathogen reduction and refrigeration effects over time. Transfusion. 2013;53:137S–49S. doi: 10.1111/trf.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strandenes G, Austlin I, Apelseth TO, et al. Coagulation function of stored whole blood is preserved for 14 days in austere conditions: a ROTEM feasibility study during a Norwegian antipiracy mission and comparison to equal ratio reconstituted blood. J Trauma Acute Care Surg. 2015;78:S31–8. doi: 10.1097/TA.0000000000000628. [DOI] [PubMed] [Google Scholar]
- 8.Spinella PC, Dressler A, Tucci M, et al. Survey of transfusion policies at US and Canadian children's hospitals in 2008 and 2009. Transfusion. 2010;50:2328–35. doi: 10.1111/j.1537-2995.2010.02708.x. [DOI] [PubMed] [Google Scholar]
- 9.Ebert RV, Emerson CP., Jr A clinical study of transfusion reactions: the hemolytic effect of group-O blood and pooled plasma containing incompatible isoagglutinins. J Clin Invest. 1946;25:627–38. doi: 10.1172/JCI101745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnes A., Jr Status of the use of universal donor blood trans-fusion. CRC Crit Care Rev Clin Lab Sci. 1973;4:147–60. doi: 10.3109/10408367309151555. [DOI] [PubMed] [Google Scholar]
- 11.Kendrick D. Blood Program in World War II. [2015 Sept 29];Chapter XX: the blood, plasma, and related programs in the Korean War. 1964 Available from: http://history.amedd.army.mil/booksdocs/wwii/blood/chapter20.htm.
- 12. [2015 Sept 29];International Trauma Care: Blood Use Issues. 2005 Available from: http://www.itaccs.com/traumacare/archive/05_01Winter_2005/blood-use.pdf.
- 13.Neel MG. Vietnam studies: medical support of the US Army in Vietnam 1965-1970. Department of the Army; 1991. [2015 Sept 29]. Available from: http://history.amedd.army.mil/booksdocs/vietnam/medicalsupport/default.html. [Google Scholar]
- 14.Barnes A. Transfusion of universal donor and uncross-matched blood. Bibl Haematol. 1980;46:132–42. doi: 10.1159/000430554. [DOI] [PubMed] [Google Scholar]
- 15.Ballas M, Nordstrom E. Severe hemolysis following the trans-fusion of one unit of O negative blood. USARV Med Bull. 1968;40:50–1. [Google Scholar]
- 16.Cotton BA, Podbielski J, Camp E, et al. A randomized controlled pilot trial of modified whole blood versus component therapy in severely injured patients requiring large volume transfusions. Ann Surg. 2013;258:527–32. doi: 10.1097/SLA.0b013e3182a4ffa0. [DOI] [PubMed] [Google Scholar]