Abstract
Ultraviolet radiation (UVR) induces immunosuppression and is a major factor for development of skin cancer. Numerous efforts have been made to determine mechanisms for UVR-induced immunosuppression and to develop strategies for prevention and treatment of UVR-induced cancers. In the current study, we use IL-17 receptor (IL-17R) deficient mice to examine whether IL-17 mediated responses have a role in UVB (290–320)-induced immunosuppression of contact hypersensitivity responses. Results demonstrate that IL-17 mediated responses are required for UVB-induced immunosuppression of contact hypersensitivity responses. The systemic immune suppression and development of regulatory T cells are inhibited in UVB-treated IL-17R deficient mice compared to wild-type animals. The deficiency in IL-17R inhibits the infiltration and development of a tolerogenic myeloid cell population in UVB-treated skin, which expresses CD11b and Gr-1 and produces reactive oxygen species. We speculate that the development of the tolerogenic myeloid cells is dependent on IL-17-induced chemokines and inflammatory mediators in UVB-treated skin. The inhibition of the tolerogenic myeloid cells may be attributed to the suppression of regulatory T cells in UVR-treated IL-17R−/− mice. The findings may be exploited to new strategies for prevention and treatment of UVR-induced skin diseases and cancers.
INTRODUCTION
It has been known that ultraviolet radiation (UVR) in the UVB range (290–320) induces immunosuppression and the immunosuppression induced by UVR exposure is a major risk factor for skin cancer (1–8). Immunosuppression induced by UVR is confirmed in several animal models, including contact hypersensitivity (CHS) (9,10). CHS response to allergens is the most used model for study in UVR-induced immune suppression in mice and humans (9,11). CHS response is inhibited in skin cancer patients whereas a suppression of CHS responses is associated with increased skin carcinogenesis in UVR-treated animals (2,8,11,12). UVR-induced immune suppression is triggered by UVR-induced DNA damage in the skin and is mediated by the alteration in antigen presenting cell function, UVR-induced cytokines and soluble mediators, and ultimately the development of regulatory T cells (6,7,13,14). UVR-induced regulatory T cells have important roles in UVR-induced immune suppression and skin carcinogenesis (3,15) and are able to transfer antigen-specific immune suppression to normal animals which are not treated with UVR (6,15–17). UVR-induced CD4+ regulatory T cells include IL-10 producing Treg (CD4/CD25/Foxp-3) and IL-4-producing Th2 and NKT cells (6,7,18).
Studies in humans and animals demonstrate that UVR induces infiltrations of CD11b+ myeloid cells in the skin, which are able to produce immune suppressive cytokine IL-10 or IL-4 (19–21). Depletion of CD11b+/CD15+ cells inhibits the ability of UVR-treated human skin cells to induce Th2 cells (20) whereas depletion of epidermal CD11b+ cells diminishes the ability of UVR-treated mouse skin cells to induce regulatory T cells and suppress CHS responses in mice (22,23). In vivo treatment of mice with an anti-CD11b antibody reduces the number of CD11b+ cells in the UVR-treated skin and inhibits UVR-induced tolerance (24). Interestingly, patients with polymorphic light eruption (PLE) are hyper-responsive to UVR exposure, which is associated with a reduced infiltration of CD11b+ myeloid cells in the skin (25,26). Moreover, PLE patients are resistant to UVR-induced suppression of CHS responses (26–28). Mechanisms for the infiltration and development of CD11b+ tolerogenic myeloid cells in UVR-exposed skin remain to be fully elucidated. Langerhans cells (LC) were originally considered to be primary cells responsible for UVR-induced immune suppression. Recent studies in mouse models with depletion of LC show that LC may (29) or may not be required (30). Interestingly, blood monocytes which express Gr-1 and CD11b molecules are able to infiltrate, proliferate and differentiate into LC in UVR-treated skin (31). The infiltration of these monocytes in the skin is dependent on the chemokine receptor CCR2 (32). Collectively, CD11b+ myeloid cells in UVR-treated skin are likely heterogeneous and tolerogenic myeloid cells remain to be fully characterized.
IL-17 is an inflammatory cytokine produced by immune cells as well as nonimmune cells such as keratinocytes (33–35). The receptor for IL-17 (IL-17R) is ubiquitously expressed (36,37). On the one hand, IL-17 is important for protective responses against infectious agents and environmental hazards. On the other, it is involved in autoimmune diseases (38) and cancers (39). A prominent activity of IL-17 is to induce the infiltration of myeloid cells in inflammatory tissues (33,34,37). Little is known about the role of IL-17 in UVR-induced immune suppression. A study shows high levels of IL-17 in the serum and Th17 cell-related signal molecules RORγt, Stat3 and IL-6 in UVR-treated skin and skin tumors. It suggests that IL-17 may be related with UVR-induced skin cancers (40). Studies from our laboratory and others have demonstrated that IL-17 is required for cutaneous inflammation and promotes chemical carcinogen-induced skin cancers in mice (41,42). One mechanism for IL-17-mediated tumor promotion is to enhance the development and infiltration of myeloid derived suppressor cells (43). A role of IL-17 in UVR-induced immune suppression has not been investigated. The current studies will examine whether UVR-induced immunosuppression of CHS responses is altered in mice which are deficient in the receptor for IL-17 and whether IL-17 mediated effects are required for the development of regulatory T cells which are able to transfer UVR-induced tolerance in normal mice.
MATERIALS AND METHODS
Mice and reagents
IL-17R−/− mice on C57BL/6 background were provided by Amgen and were bred in our laboratory (44). The gene phenotype was routinely confirmed. Wild-type C57Bl/6 mice were purchased from Jacksons Laboratory. Both wild-type and IL-17R−/− mice used in this study were 6–8 weeks old. All animal procedures were performed according to NIH guidelines and the protocols were approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham.
UVR-induced immune suppression of contact hypersensitivity responses
Contact hypersensitivity responses to 2,4-dinitro-1-fluorobenzene (DNFB) are examined as described in our previous studies (45,46). To examine a role of IL-17 in UVR-induced immunosuppression, wildype and IL-17R−/− mice (both on C57Bl/6) were irradiated with UVB (290–320) at 200 mJ cm−2 on shaved back skin once a day for four consecutive days. This protocol has been selected based on our preliminary studies and literatures for the mouse strain (47,48). The mice were sensitized with DNFB on the UVB-treated back skin 24 h after the last UVB exposure. Five days later, the mice were challenged with DNFB on the right ear skin and the ear thickness was read prior to and 24 h after the challenge. To examine whether the UVB irradiation induces tolerance, the mice were re-sensitized with DNFB 7 days after the first challenge on shaved abdominal skin which was not exposed to UVB. Five days after the re-sensitization, the mice were challenged on the left ear skin and ear thickness was read.
Analysis of UVR-induced leukocyte infiltrations in the skin
To examine whether the deficiency in IL-17R affects UVB-induced inflammation in the skin, mice were irradiated with UVB on shaved back skin four times on four consecutive days as described above. UVB-treated skin tissues were harvested 24 h after the last UVR and digested with collagenase IV (1 mg mL−1) at 37° C for 1 h. Cell suspensions were stained with antibodies and results were analyzed by flow cytometry (46).
To examine the production of reactive oxygen species (ROS), UVR-treated skin cell suspensions were stained with an oxidation-sensitive dye DCFDA and antibodies. The level of ROS in specific subsets of myeloid cells was analyzed by flow cytometry (49).
Analysis of UVR-induced inflammatory mediators in the skin
Levels of mRNA for inflammatory mediators were quantified by real-time RT-PCR (44). Mice were treated with UVB as described above and the UVB-treated skin tissues were harvested 24 h after the last UVB exposure. The skin tissues were homogenized in TRIzol and total RNA was isolated according to the manufacturer’s instructions (GibcoBRL). Real time RT-PCR was performed with SYBRO Green Supermix Kit in an ABL Quantumstudio 6 system according to the manufacturer’s instructions (Applied System). The expression level of cytokines was normalized to the house-keeping gene GAPDH in each sample. The sequences for primers were: CXCL1: forward, 5′-GCTGGGATTCACCTCAAGAA C-3′, reverse, 5′ -TGGGGACACCTTTTAGCATC-3′, Cox-2: forward, 5′-A TCTACCCTCCTCACATCCC-3′, reverse, 5′-TAGTTGCTCATCACCCC ACTC-3′, IL-6: forward, 5′-CCTCTCTGCAAGAGACTTCC-3′, reverse, 5′-GCACAACTCTTTTCTCATTTCC-3′, CCL2: forward, 5′-AACTCCC ATCCCAATCACC-3′, reverse, 5′-CCTCCATCAACCACTTTTCC-3′, GApDH: forward, 5′-AATGGTGAAGGTCGGTGTGAAC-3′, reverse, 5′-GAAGATGGTGATGGGCTTCC-3′.
The concentration of PGE2 in the UVB-treated skin tissues was measured by using a prostaglandin E2 EIA kit according to the manufacturer’s instructions (Cayman Chemical, Ann Arbor, MI). Briefly, skin samples were homogenized in the buffer provided in the kit and centrifuged at 11200 g for 15 min. The concentration of PGE2 in supernatants was determined and normalized to the protein concentration in supernatants (41).
Examination of UVR-induced systemic immune suppression
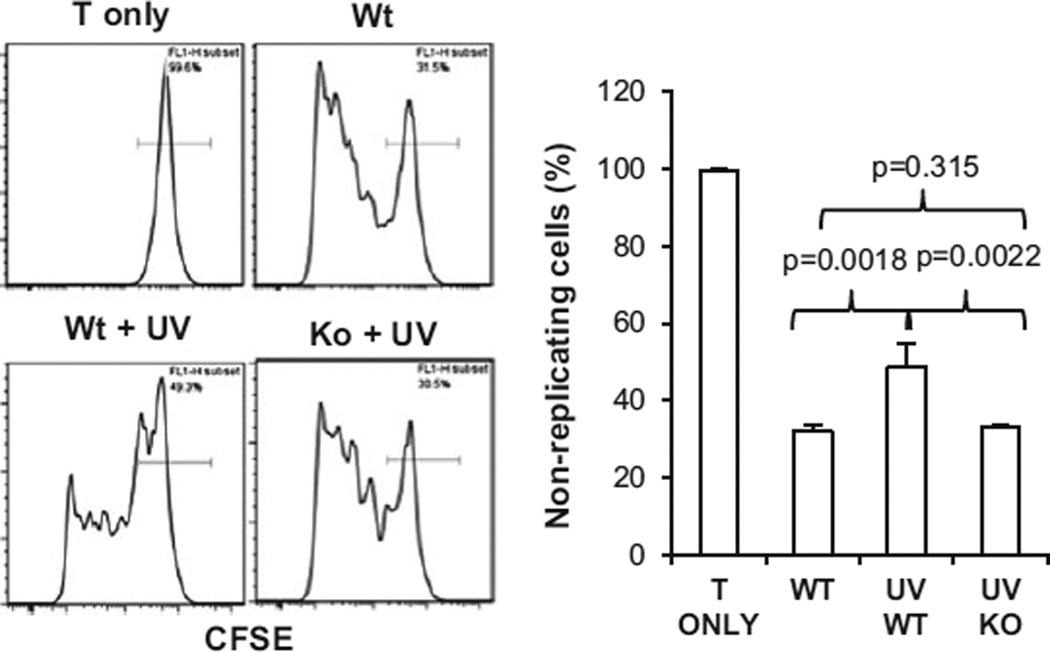
To examine whether IL-17 affected the development of UVR-induced systemic immune suppression, mice were treated with UVB as described above and spleens of the mice were harvested 24 h after the last UVB treatment. Spleen cell suspensions were prepared. CD4+ transgenic T cells specific for ovalbumin were isolated from OT-II mice and labeled with a fluorescence dye CFSE. These CD4+ T cells (2 × 106 cells mL−1) were incubated with the spleen cells from UVR-treated mice (1 × 107 cells mL−1) in the presence of an MHC class II-specific peptide (OVA323–339, ISQAVHAAHAEINEAGR, AnaSpec, Fremont, CA)(50). Four days later, the cells were harvested and CFSE+ cells were analyzed by flow cytometry. Replicating cells will have a lower level of CFSE than those without replication (Fig. 2). T cells which were incubated without the peptide served as controls. The percentage of nonreplicating cells is calculated for statistical analysis.
Figure 2.
Ultraviolet radiation-induced immune suppression is diminished in IL-17R−/− mice. Wild-type and IL-17R−/− mice were irradiated with UVB (200 mJ cm−2) on shaved back skin once a day for four consecutive days. The mice were sacrificed 24 h later and spleens were harvested. Spleen cell suspensions were prepared and cultured with CFSE labeled OT-II cells in the presence of a MHC class II specific peptide for 4 days. CFSE+ cells were gated and analyzed. Histograms show that replicating cells express lower levels of CFSE than the nonreplicating cells. Graph shows the percentage of nonreplicating cells in each group. Data show mean ± SE (3 mice/group) of one of three independent experiments.
Transfer of UVR-induced tolerance by CD4+ T cells
To examine UVR-induced regulatory T cells, CD4+ T cells were purified from the draining lymph nodes of wild-type and IL-17R−/− mice, which were treated with UVB and sensitized with DNFB. MACS beads coupled with anti-CD4 antibodies were used for CD4+ T-cell purification according to the manufacturer’s instruction (Miltenyi Biotec Inc.) Control CD4+ T cells were purified from wild-type and IL-17R−/− mice which were sensitized with DNFB but not treated with UVB. The CD4+ T cells from control and UVB-treated mice were transferred intravenously to naïve wild-type mice which were not treated with UVR (5 × 106/mouse). The recipient mice were sensitized with DNFB 24 h after the CD4+ T cell transfer and then challenged 5 days after the sensitization. Ear swelling was read 24 h after the challenge.
Statistical analysis
All data are presented as means ± SEM. The two tailed Student’s t-test was applied for statistical analysis with P < 0.05 being considered statistically significant.
RESULTS AND DISCUSSION
A deficiency in IL-17R inhibits UVB-induced tolerance of CHS responses
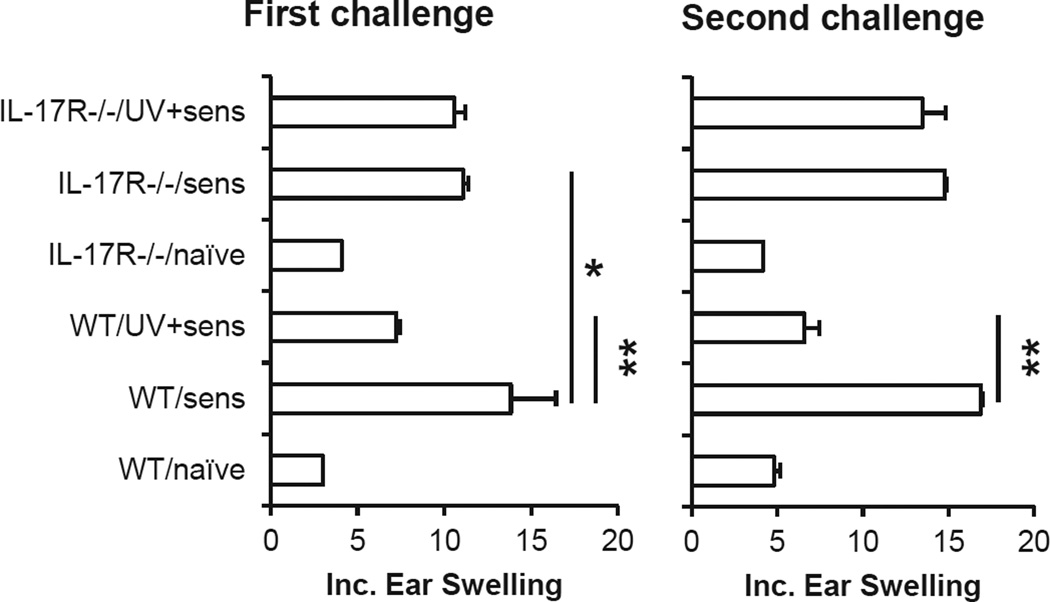
Literature shows that UVR will induce the suppression of CHS responses in mice (9–11). Our results showed that CHS responses were significantly inhibited in UVB-treated wild-type mice following the first challenge compared to non-UVR-treated control mice (Fig. 1). Moreover, the CHS response in UVR-treated wild-type mice was also significantly inhibited after re-sensitization with DNFB on the abdominal skin which was not exposed to UVB. It indicates that UVR not only suppresses the development but also induces tolerance of CHS responses in the mice. The results are in accordance with literature (22). In contrast to the results in wild-type- mice, there was no significant difference in CHS responses between UVB-treated and non-UVR control IL-17R−/− mice. Further results showed that UVR did not have an effect on CHS responses in UVB-treated IL-17R−/− mice which were re-sensitized on the abdominal skin which was not exposed to UVR, either. The results suggest that IL-17R−/− mice are resistant to UVR-induced immune suppression and tolerance. It is to note that the CHS response was lower in IL-17R−/− than in wild-type mice, which were not irradiated with UVB. This is in accordance with our previous report that a deficiency in IL-17R inhibits CHS responses (44,46). However, CHS response is mediated not only by IL-17 but also by INF-γ (44). Therefore, the deficiency in IL-17R does not abrogate CHS responses.
Figure 1.
Ultraviolet radiation-induced immune tolerance is diminished in IL-17R−/− mice. Wild-type and IL-17R−/− mice were irradiated with UVB (200 mJ cm−2) on shaved back skin once a day for four consecutive days. The mice were sensitized once with DNFB on the UVR-treated skin area 24 h after the last UVR. The mice were then challenged on the right ears 5 days after the sensitization. The mice were resensitized with DNFB on shaved abdominal skin 7 days after the first challenge and were rechallenged on left ears 5 days after the resensitization. Data show mean ± SE (5 mice/group) of one of three independent experiments, *P < 0.05, **P < 0.01.
Literature shows that IL-23, a stimulator for IL-17 production, inhibits UVR-induced tolerance (47). However, the effect is attributed to IL-23-mediated repair of UVR-induced DNA damage (47). We have found that the deficiency in IL-17R does not have a significant effect on UVR-induced DNA damage in the skin (not shown).
Little is known about a role of IL-17-mediated responses in UVR-induced immune suppression in the skin. A study shows high levels of IL-17 in the serum and Th17 cell-related signal molecules RORγt, Stat3 and IL-6 in UVR-treated skin and skin tumors. It suggests that IL-17 may be related with UVR-induced skin cancers (40). The suppression of UVR-induced immunosuppression and tolerance of CHS responses in IL-17R−/− mice suggests that the increase in IL-17 production in the UVR-treated skin may be a mechanism for UVR-induced immune suppression which is known to have a key role in the development of skin tumors (1,2,4,6–8,51).
IL-17R deficiency diminishes UVB-induced systemic immune suppression in spleen
Ultraviolet radiation induces not only local immune suppression in the skin and the draining lymph node but also systemic tolerance in the immune system (2). Although our results show that UVR-induced immune suppression of CHS responses is inhibited in IL-17R−/− mice, it remains to be determined whether IL-17-mediated responses are required for UVR-induced systemic immune suppression. Our results showed that spleen cells from UVR-treated wild-type mice induced a lower level of T-cell activation (higher percentage of nonreplicating cells) than those from control mice which were not treated with UVB (Fig. 2). In contrast, spleen cells from UVR-treated IL-17R−/− mice induced a similar level of T-cell proliferation to those from control mice which were not treated with UVB. We have examined the suppressive effect of spleen cells from UVR-treated animals at various times up to 6 days after the UVB treatment. Similar results were observed (not shown), implicating that the systemic immune suppression in UVB-treated wild-type mice is long lasting, and that UVB-induced systemic immune suppression is inhibited in IL-17R−/− mice. The results provide evidence that the deficiency in IL-17R inhibits UVR-induced systemic immune suppression.
IL-17R deficiency inhibits the development of UVB-induced regulatory CD4+ T cells
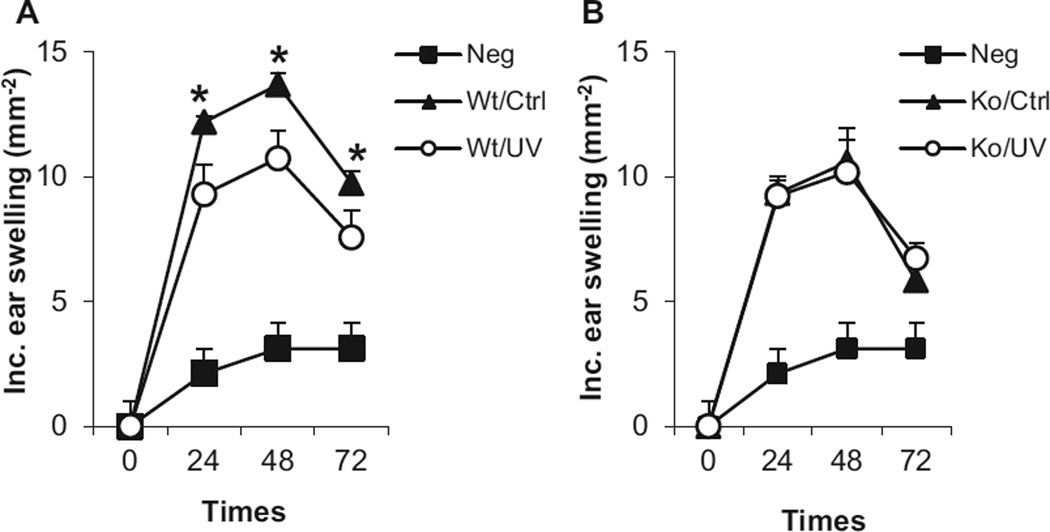
Ultraviolet radiation-induced regulatory T cells have important roles in UVR-induced immune suppression and skin carcinogenesis (1,3,15). They are able to transfer antigen-specific immune suppression to normal animals which are not treated with UVR (6,15–17). Our results show that in accordance to literature (15,17), transfer of CD4+ T cells from UVB-treated wild-type mic inhibited CHS responses in the recipient wild-type mice which were not treated with UVB (Fig. 3A). However, the transfer of CD4+ T cells from UVB-treated IL-17R−/− mice did not have a significant effect on CHS responses in wild-type recipient mice (Fig. 3B). It implicates that UVR-induced development of regulatory T cells is inhibited in IL-17R−/− mice. The results not only provide a mechanism for the suppression of UVR-induced tolerance of CHS responses but also indicate a clue to the inhibition of systemic immune suppression in IL-17R−/− mice.
Figure 3.
Interleukin-17 regulates the development of UVR-induced CD4+ regulatory T cells. Wild-type and IL-17R−/− mice were irradiated with UVB (200 mJ cm−2) on shaved back skin once a day for four consecutive days (Wt/UV and Ko/UV). The mice were sensitized once with DNFB on the UVR-treated skin area 24 h after the last UVR. Control mice which were not treated with UVR (Wt/Ctrl and Ko/Ctrl) were also sensitized with DNFB. Spleen and the draining lymph node cells from all sensitized mice were harvested 5 days later. CD4+ T cells were purified and transferred i.v. into naïve wild-type mice (5 × 106 cells/mouse), which were not treated with UVR. The recipient mice were sensitized with DNFB 24 h later and challenged 5 days after the sensitization. Data show mean ± SE (5 mice/group) of one of two independent experiments, *P < 0.05.
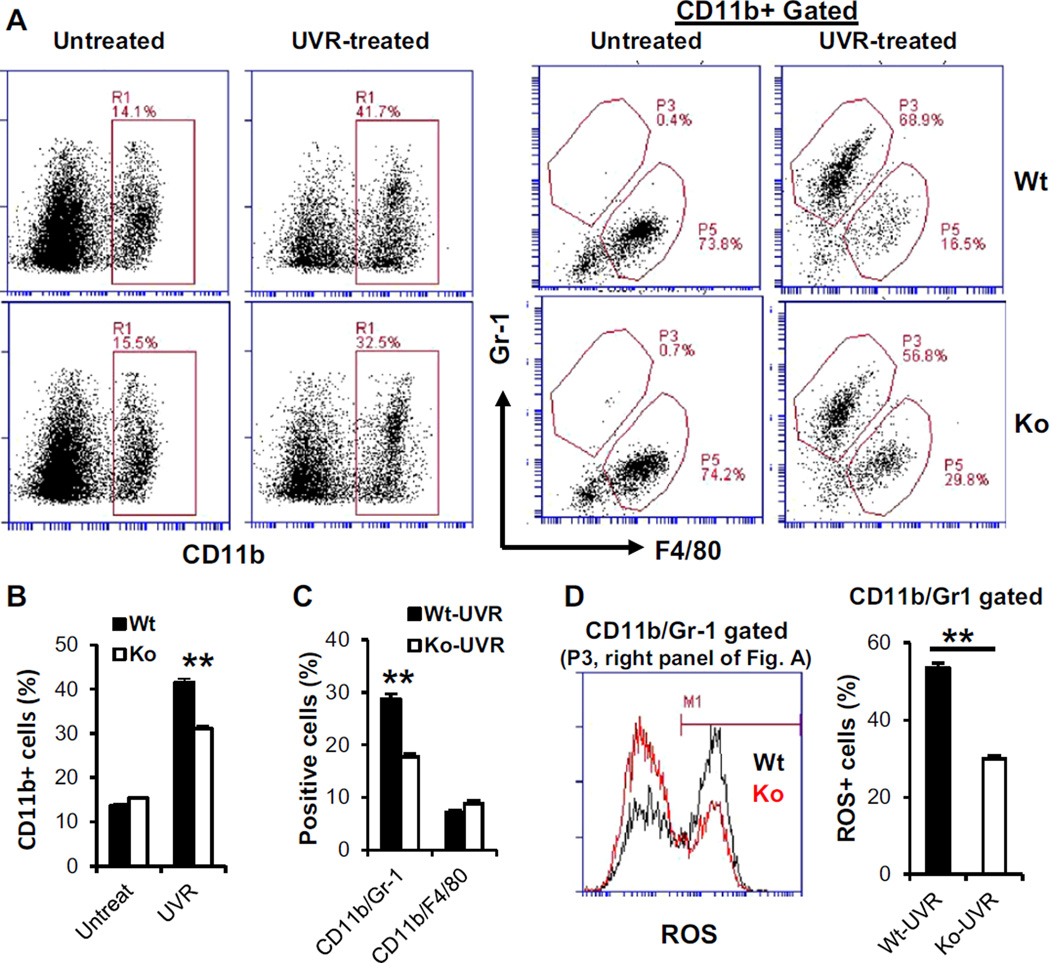
A deficiency in IL-17R reduces UVB-induced tolerogenic myeloid cells in the skin
UVR-induced DNA damage is a trigger for UVR-induced inflammation in the skin and immune suppression (6,13,52,53). An important feature is that UVR exposure induces infiltrations of myeloid cells in the skin (9,54). These myeloid cells are tolerogenic, which induce the development of regulatory T cells and play an important role in UVR-induced immune tolerance (6,9,19,22). IL-17 has a prominent effect on the infiltration of myeloid cells in inflammatory tissues (34,37,55). A role of IL-17 in the development of tolerogenic myeloid cells in UVB-treated skin has not yet been investigated. Our experiments showed that the deficiency in IL-17R did not have a significant effect on CD11b+ cells in the skin of untreated naïve mice (Fig. 4A). However, the level of CD11b+ cells was significantly lower in UVB-treated skin of IL-17R−/− mice than in that of wild-type mice (Fig. 4A,B). Further analysis of gated CD11b+ cells showed that a CD11b+/Gr-1+ /F4/80- cell population, which was hardly detectable in normal skin, was greatly increased in UVB-treated skin (Fig. 4A). Remarkably, this subset was selectively reduced in UVB-treated skin of IL-17R−/− compared to that of wild-type mice. In contrast, the other subset which was CD11b+/Gr-1-/F4/80+ and the dominant population in normal skin, did not show a significant difference between UVB-treated wild-type and IL-1R−/− mice (Fig. 4A,C). To further analyze the CD11b+/Gr-1+ cells which were induced in the UVB-treated skin and were significantly reduced in IL-17R−/− mice, the production of reactive oxygen species (ROS) by the cell population was examined. Results showed that the gated CD11b+/Gr-1+ cells from UVB-treated skin of IL-17R−/− mice had a significant fewer number of ROS positive cells than those from UVB-treated skin of wild-type mice (Fig. 4D). Literature shows that infiltrating CD11b+ myeloid cells are required for UVR-induced immune tolerance in mice (22,24). Our findings demonstrate that infiltrating CD11b+ cells in UVB-treated skin are composed of at least two subpopulations, CD11b+/Gr-1+/F4/80- and CD11b+/Gr-1-/F4/80+, respectively. IL-17 has a selective effect on the number and ROS production of CD11b+/Gr-1+/F4/80- subset in UVR-treated skin. Recent studies indicate that CD11b+/Gr-1+ is a specific marker for myeloid-derived suppressor cells (MDSC) which are able to induce Treg cells, inhibit effector T cells and suppress immune responses (56–58). Studies from our lab have shown that IL-17 promotes the development of MDSC in tumor-bearing mice (41,43). Furthermore, animal and human studies indicate that ROS have a pivotal role in MDSC-mediated suppression of T cells (49,57,59). Importantly, the production of ROS has been implicated as a mechanism for UVB-induced suppression of immune responses (60). The data in the current study indicate that IL-17-mediated effects on the development and function of suppressive myeloid cells in UVB-treated skin may be a novel mechanism for UVR-induced tolerance. We have shown previously that UVB exposure induces depletion of antioxidant enzymes and enhances ROS generation in the skin (61). It is possible that IL-17 regulates the activity of specific antioxidant enzymes. Further studies are required to determine whether IL-17 has a role in this process.
Figure 4.
Interleukin-17 regulates myeloid cells in UVR-exposed skin. Wild-type and IL-17R−/− mice were irradiated with UVB (200 mJ/cm−2) on shaved back skin once a day for four consecutive days. Skin tissues were harvested 24 h after the last UVR and digested with collagenase. (A) Skin cells were stained and analyzed by flow cytometry. (B,C) Percentage of positive cells from total skin cells. (D) Positive cells with reactive oxygen species (ROS) in the gated CD11b/Gr-1 population. Data show mean ± SE (3–4 mice/group), one of three independent experiments **P < 0.01.
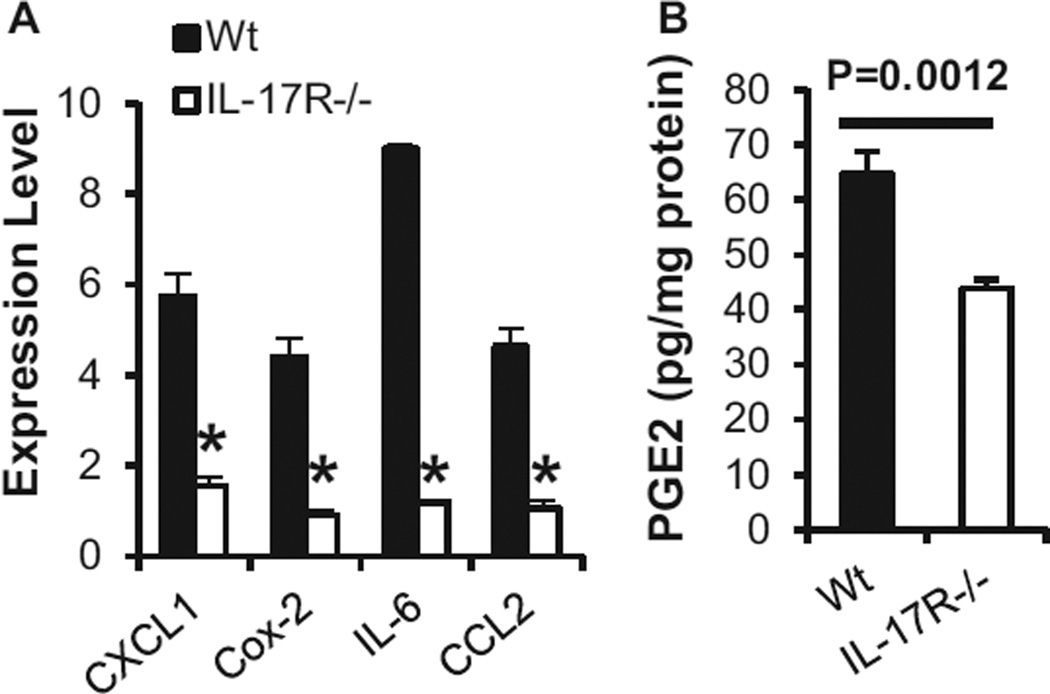
Ultraviolet radiation-induced production of inflammatory mediators and infiltration of myeloid cells create an environment for the migration and development of tolerogenic myeloid cells in the skin (6,7,19,53,62). Mechanisms underlying the effects remain to be fully defined. Our data showed that the deficiency in IL-17R reduced levels of IL-6 and Cox-2/PGE2 and chemokines CCL2 and CXCL1 in UVB-treated skin (Fig. 5A,B). PGE2 and IL-6 have been reported to have effects on the development of tumor MDSC (56,57,63). UVR-induced Cox-2/PGE2 has important roles in UVR-induced immunosuppression (64) and appears to be an initial mediator for the production of immunosuppressive cytokines IL-4 and IL-10 (7,19,53,62,65). Chemokines CXCL1 and CCL2 have a role in the infiltration of myeloid cells in UVR-treated skin (31,32,66). A deficiency in CCR2, the receptor for CXCL1 and CCL2, inhibits the infiltration of MDSC into tumor tissues (67). Our data show that CD11b+/Gr-1+ myeloid cells are hardly detectable in normal skin and are induced in UVB-exposed skin (Fig. 4), implicating that they are newly infiltrated and developed myeloid cells in the UVB-treated skin. The fact that a reduced number of UVB-induced myeloid cells is associated with a reduction of CCL2 in UVB-irradiated skin of IL-17R−/− mice suggests that the induction of the chemokine by IL-17 is a mechanism for the infiltration of the myeloid cells. Taken together, these results indicate that IL-17 has a critical role in UVB-induced suppressive environments for the infiltration and development of tolerogenic myeloid cells in the skin. Previous studies show that depletion of CD11b+ cells from UVB-treated skin cells abrogates their ability to induce tolerance and that treatment of UVB-treated mice with an anti-CD11b antibody reduces the number of CD11b+ cells in the UVB-treated skin and diminishes UVR-induced tolerance (22,24). Moreover, polymorphic light eruption (PLE), a UVR-induced skin disease with hyper-responses to UVR, is associated with a decreased infiltration of CD11b+ myeloid cells in the skin (25,26,68). Interestingly, UVR causes less immunosuppression in patients with PLE (27). Certainly, more studies are required for further determining mechanisms for IL-17-mediated regulation of tolerogenic myeloid cells in UVB-treated skin and UVR-induced immune tolerance.
Figure 5.
Interleukin-17 regulates UVR-induced chemokines and Cox-2/PGE2. Wild-type and IL-17R−/− mice were irradiated with UVB (200 mJ cm−2) on shaved back skin once a day for four consecutive days. Skin tissues were harvested 24 h after the last UVR. (A) Levels of mRNA in ear skin tissues were quantified by real-time RT-PCR (4/group, *P < 0.05). (B) Concentrations of PGE2 in ear skin tissues were measured by ELISA (6/group). Data show mean ± SE of one of two independent experiments.
In summary, the current study demonstrates that IL-17-mediated responses are required for UVR-induced immune tolerance. The systemic immune suppression and the development of regulatory T cells are inhibited in UVB-treated IL-17R−/− mice. The deficiency in IL-17 responses inhibits the infiltration, development and function of a tolerogenic myeloid cell population in the UVB-treated skin, which expresses CD11b and Gr-1 and produces ROS. We speculate that the development of tolerogenic myeloid cells is dependent on IL-17-induced chemokines and inflammatory mediators in UVB-treated skin. The reduction of suppressive myeloid cells in UVB-treated skin is attributed to the inhibition of regulatory T cell development and systemic immune suppression in UVB-treated IL-17R−/− mice. The findings in this study may be exploited to new strategies for prevention and treatment of UVR-induced skin diseases and cancers.
Acknowledgments
This study was supported by R01CA140197, P30 AR050948, P30 CA013148, and by VA Merit Review18-103-02.
Footnotes
This paper is part of the Special Issue commemorating the 65th birthday of Dr. Craig A. Elmets.
REFERENCES
- 1.Elmets CA, Athar M. Milestones in photocarcinogenesis. J Invest Dermatol. 2013;133:E13–E17. doi: 10.1038/skinbio.2013.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher MS, Kripke ML. Systemic alteration induced in mice by ultraviolet light irradiation and its relationship to ultraviolet carcinogenesis. Proc. Natl Acad. Sci. USA. 1977;74:1688–1692. doi: 10.1073/pnas.74.4.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Granstein RD, Parrish JA, McAuliffe DJ, Waltenbaugh C, Greene MI. Immunologic inhibition of ultraviolet radiation-induced tumor suppressor cell activity. Science. 1984;224:615–617. doi: 10.1126/science.6231725. [DOI] [PubMed] [Google Scholar]
- 4.Hart PH, Gorman S, Finlay-Jones JJ. Modulation of the immune system by UV radiation: more than just the effects of vitamin D? Nat. Rev. Immunol. 2011;11:584–596. doi: 10.1038/nri3045. [DOI] [PubMed] [Google Scholar]
- 5.Kripke ML. Immunological unresponsiveness induced by ultraviolet radiation. Immunol. Rev. 1984;80:87–102. doi: 10.1111/j.1600-065x.1984.tb00496.x. [DOI] [PubMed] [Google Scholar]
- 6.Schwarz T, Beissert S. Milestones in photoimmunology. J Invest Dermatol. 2013;133:E7–E10. doi: 10.1038/skinbio.2013.177. [DOI] [PubMed] [Google Scholar]
- 7.Ullrich SE, Byrne SN. The immunologic revolution: photoimmunology. J Invest Dermatol. 2012;132:896–905. doi: 10.1038/jid.2011.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Urbach F. Ultraviolet radiation and skin cancer of humans. J Photochem and Photobiol B. Biology. 1997;40:3–7. doi: 10.1016/s1011-1344(97)00029-8. [DOI] [PubMed] [Google Scholar]
- 9.Cooper K, Oberhelman DL, Hamilton TA, Baadsgaard O, Terhune M, LeVee G, Anderson T, Koren H. UV exposure reduces immunization rates and promotes tolerance to epicutaneous antigens in humans: relationship to dose, CD1a-DR+ epidermal macrophage induction, and Langerhans cell depletion. Proc. Natl Acad. Sci. USA. 1992;89:8497–8501. doi: 10.1073/pnas.89.18.8497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Becklund BR, Severson KS, Vang SV, DeLuca HF. UV radiation suppresses experimental autoimmune encephalomyelitis independent of vitamin D production. Proc. Natl Acad. Sci. USA. 2010;107:6418–6423. doi: 10.1073/pnas.1001119107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwarz T. The dark and the sunny sides of UVR-induced immunosuppression: photoimmunology revisited. J Invest Dermatol. 2009;130:49–54. doi: 10.1038/jid.2009.217. [DOI] [PubMed] [Google Scholar]
- 12.Yoshikawa T, Rae V, Bruins-Slot W, van den Berg J-W, Taylor JR, Streilein JW. Susceptibility to effects of UVB radiation on induction of contact hypersensitivity as a risk factor for skin cancer in humans. J Invest Dermatol. 1990;95:530–536. doi: 10.1111/1523-1747.ep12504877. [DOI] [PubMed] [Google Scholar]
- 13.Kripke ML, Cox PA, Alas LG, Yarosh DB. Pyrimidine dimers in DNA initiate systemic immunosuppression in UV- irradiated mice. Proc. Natl Acad. Sci. USA. 1992;89:7516–7520. doi: 10.1073/pnas.89.16.7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shreedhar VK, Pride MW, Sun Y, Kripke ML, Strickland FM. Origin and characteristics of ultraviolet-B radiation- induced suppressor T lymphocytes. J Immunol. 1998;161:1327–1335. [PubMed] [Google Scholar]
- 15.Fisher MS, Kripke ML. Suppressor T lymphocytes control the development of primary skin cancers in ultraviolet-irradiated mice. Science. 1982;216:1133–1134. doi: 10.1126/science.6210958. [DOI] [PubMed] [Google Scholar]
- 16.Elmets CA, Bergstresser PR, Tigelaar RE, Wood PJ, Streilein JW. Analysis of the mechanism of unresponsiveness produced by haptens painted on skin exposed to low dose ultraviolet radiation. J Exp. Med. 1983;158:781–794. doi: 10.1084/jem.158.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fisher MS, Kripke ML. Further studies on the tumor-specific suppressor cells induced by ultraviolet radiation. J Immunol. 1978;121:1139–1144. [PubMed] [Google Scholar]
- 18.Elmets CA, Cala CM, Xu H. Photoimmunology. Dermatol. Clin. 2014;32:277–290. doi: 10.1016/j.det.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang K, Hammerberg C, Meunier L, Cooper KD. CD11b+ macrophages that infiltrate human epidermis after in vivo ultraviolet exposure potently produce IL-10 and represent the major secretory source of epidermal IL-10 protein. J Immunol. 1994;153:5256–5264. [PubMed] [Google Scholar]
- 20.Teunissen MB, Piskin MG, Nuzzo SD, Sylva-Steenland RMR, deRie MA, Bos JD. Ultraviolet B radiation induces a transient appearance of IL-4+ neutrophils, which support the development of Th2 responses. J Immunol. 2002;168:3732–3739. doi: 10.4049/jimmunol.168.8.3732. [DOI] [PubMed] [Google Scholar]
- 21.Yoshida Y, Kang K, Berger M, Chen G, Gilliam AC, Moser A, Wu L, Hammerberg C, Cooper KD. Monocyte induction of IL-10 and down-regulation of IL-12 by iC3b deposited in ultraviolet-exposed human skin. J Immunol. 1998;161:5873–5879. [PubMed] [Google Scholar]
- 22.Hammerberg C, Duraiswamy N, Cooper KD. Active induction of unresponsiveness (tolerance) to DNFB by in vivo ultraviolet-exposed epidermal cells is dependent upon infiltrating class II MHC+ CD11bbright monocytic/macrophagic cells. J Immunol. 1994;153:4915–4924. [PubMed] [Google Scholar]
- 23.Hammerberg C, Katiyar SK, Carroll MC, Cooper KD. Activated complement component 3 (C3) is required for ultraviolet induction of immunosuppression and antigenic tolerance. J Exp. Med. 1998;187:1133–1138. doi: 10.1084/jem.187.7.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hammerberg C, Duraiswamy N, Cooper KD. Reversal of immunosuppression inducible through ultraviolet-exposed skin by in vivo anti-CD11b treatment. J Immunol. 1996;157:5254–5261. [PubMed] [Google Scholar]
- 25.Kolgen W, Van Weelden H, Den Hengst S, Guikers KLH, Kiekens RCH, Knol EF, Bruijnzeel-Koomen CAFM, van Vloten WA, de Gruijl FR. CD11b+ cells and ultraviolet-B-resistant CD1a+ cells in skin of patients with polymorphous light eruption1. J Invest Dermatol. 1999;113:4–10. doi: 10.1046/j.1523-1747.1999.00625.x. [DOI] [PubMed] [Google Scholar]
- 26.Wolf P, Byrne SN, Gruber-Wackernagel A. New insights into the mechanisms of polymorphic light eruption: resistance to ultraviolet radiation-induced immune suppression as an aetiological factor. Exp. Dermatol. 2009;18:350–356. doi: 10.1111/j.1600-0625.2009.00859.x. [DOI] [PubMed] [Google Scholar]
- 27.Palmer RA, Friedmann PS. Ultraviolet radiation causes less immunosuppression in patients with polymorphic light eruption than in controls. J Invest Dermatol. 2004;122:291–294. doi: 10.1046/j.0022-202X.2004.22213.x. [DOI] [PubMed] [Google Scholar]
- 28.Van de Pas CB, Kelly DA, Seed PT, Young AR, Hawk JLM, Walker SL. Ultraviolet-radiation-induced erythema and suppression of contact hypersensitivity responses in patients with polymorphic light eruption. J Invest Dermatol. 2004;122:295–299. doi: 10.1046/j.0022-202X.2004.22201.x. [DOI] [PubMed] [Google Scholar]
- 29.Schwarz A, Noordegraaf M, Maeda A, Torii K, Clausen BE, Schwarz T. Langerhans cells are required for UVR-induced immunosuppression. J Invest Dermatol. 2010;130:1419–1427. doi: 10.1038/jid.2009.429. [DOI] [PubMed] [Google Scholar]
- 30.Wang L, Jameson SC, Hogquist KA. Epidermal langerhans cells are not required for UV-induced immunosuppression. J Immunol. 2009;183:5548–5553. doi: 10.4049/jimmunol.0900235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ginhoux F, Tacke F, Angeli V, Bogunovic M, Loubeau M, Dai X-M, Stanley ER, Randolph GJ, Merad M. Langerhans cells arise from monocytes in vivo. Nat. Immunol. 2006;7:265. doi: 10.1038/ni1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merad M, Manz MG, Karsunky H, Wagers A, Peters W, Charo I, Weissman IL, Cyster JG, Engleman EG. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat. Immunol. 2002;3:1135. doi: 10.1038/ni852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin-17 family members. Immunity. 2011;34:149–162. doi: 10.1016/j.immuni.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 34.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 35.McGeachy MJ, Cua DJ. Th17 cell differentiation: the long and winding road. Immunity. 2008;28:445–453. doi: 10.1016/j.immuni.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 36.O’Connor W, Zenewicz LA, Flavell RA. The dual nature of TH17 cells: shifting the focus to function. Nat. Immunol. 2010;11:471–476. doi: 10.1038/ni.1882. [DOI] [PubMed] [Google Scholar]
- 37.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Ann Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 38.Baeten DLP, Kuchroo VK. How cytokine networks fuel inflammation: interleukin-17 and a tale of two autoimmune diseases. Nat. Med. 2013;19:824–825. doi: 10.1038/nm.3268. [DOI] [PubMed] [Google Scholar]
- 39.Zou W, Restifo NP. TH17 cells in tumour immunity and immunotherapy. Nat. Rev. Immunol. 2010;10:248–256. doi: 10.1038/nri2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nasti TH, Iqbal O, Tamimi IA, Geise JT, Katiyar SK, Yusuf N. Differential roles of T-cell subsets in regulation of ultraviolet radiation induced cutaneous photocarcinogenesis. Photochem. Photobiol. 2011;87:387–398. doi: 10.1111/j.1751-1097.2010.00859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He D, Li H, Yusuf N, Elmets CA, Athar M, Katiyar SK, Xu H. IL-17 mediated inflammation promotes tumor growth and progression in the skin. PLoS ONE. 2012;7:e32126. doi: 10.1371/journal.pone.0032126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang L, Yi T, Zhang W, Pardoll DM, Yu H. IL-17 enhances tumor development in carcinogen-induced skin cancer. Cancer Res. 2010;70:10112–10120. doi: 10.1158/0008-5472.CAN-10-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He D, Li H, Yusuf N, Elmets CA, Li J, Mountz JD, Xu H. IL-17 promotes tumor development through the induction of tumor promoting microenvironments at tumor sites and myeloid-derived suppressor cells. J Immunol. 2010;184:2281–2288. doi: 10.4049/jimmunol.0902574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.He D, Wu L, Kim HK, Li H, Elmets CA, Xu H. IL-17 and IFN-{gamma} mediate the elicitation of contact hypersensitivity responses by different mechanisms and both are required for optimal responses. J Immunol. 2009;183:1463–1470. doi: 10.4049/jimmunol.0804108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu H, Dilulio NA, Fairchild RL. T cell populations primed by hapten sensitization in contact sensitivity are distinguished by polarized patterns of cytokine production: Interferon γ-producing (Tc1) effector CD8+ T cells and interleukin 4/IL-10-producing (Th2) negative regulatory CD4+ T cells. J Exp. Med. 1996;183:1001–1012. doi: 10.1084/jem.183.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.He D, Wu L, Kim HK, Li H, Elmets CA, Xu H. CD8+ IL-17-producing T cells are important in effector functions for the elicitation of contact hypersensitivity responses. J Immunol. 2006;177:6852–6858. doi: 10.4049/jimmunol.177.10.6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Majewski S, Jantschitsch C, Maeda A, Schwarz T, Schwarz A. IL-23 antagonizes UVR-induced immunosuppression through two mechanisms: reduction of UVR-induced DNA damage and inhibition of UVR-induced regulatory T cells. J Invest Dermatol. 2009;130:554–562. doi: 10.1038/jid.2009.274. [DOI] [PubMed] [Google Scholar]
- 48.Schwarz A, Maeda A, Kernebeck K, van Steeg H, Beissert S, Schwarz T. Prevention of UV radiation–induced immunosuppression by IL-12 is dependent on DNA repair. J Exp. Med. 2005;201:173–179. doi: 10.1084/jem.20041212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Corzo CA, Cotter MJ, Cheng P, Cheng F, Kusmartsev S, Sotomayor E, Padhya T, McCaffrey TV, McCaffrey JC, Gabrilovich DI. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J Immunol. 2009;182:5693–5701. doi: 10.4049/jimmunol.0900092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oran AE, Robinson HL. DNA vaccines, combining form of antigen and method of delivery to raise a spectrum of IFN-γ and IL-4-producing CD4+ and CD8+ T Cells. J Immunol. 2003;171:1999–2005. doi: 10.4049/jimmunol.171.4.1999. [DOI] [PubMed] [Google Scholar]
- 51.Granstein RD, Matsui MS. UV radiation-induced immunosuppression and skin cancer. Cutis. 2004;74:4–9. [PubMed] [Google Scholar]
- 52.Applegate LA, Ley RD, Alcalay J, Kripke ML. Identification of the molecular target for the suppression of contact hypersensitivity by ultraviolet radiation. J Exp. Med. 1989;170:1117–1131. doi: 10.1084/jem.170.4.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shreedhar V, Giese T, Sung VW, Ullrich SE. A cytokine cascade including prostaglandin E2, IL-4, and IL-10 is responsible for UV-induced systemic immune suppression. J Immunol. 1998;160:3783–3789. [PubMed] [Google Scholar]
- 54.Sluyter R, Halliday GM. Infiltration by inflammatory cells required for solar-simulated ultraviolet radiation enhancement of skin tumor growth. Cancer Immunol. Immunother. 2001;50:151–156. doi: 10.1007/PL00006685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat. Rev. Immunol. 2009;9:556–567. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goh C, Narayanan S, Hahn YS. Myeloid-derived suppressor cells: the dark knight or the joker in viral infections? Immunol. Rev. 2013;255:210–221. doi: 10.1111/imr.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lindau D, Gielen P, Kroesen M, Wesseling P, Adema GJ. The immunosuppressive tumour network: myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology. 2013;138:105–115. doi: 10.1111/imm.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee K, Won HY, Bae MA, Hong J-H, Hwang ES. Spontaneous and aging-dependent development of arthritis in NADPH oxidase 2 deficiency through altered differentiation of CD11b+ and Th/Treg cells. Proc. Natl Acad. Sci. USA. 2011;108:9548–9553. doi: 10.1073/pnas.1012645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rana S, Rogers LJ, Halliday GM. Systemic low-dose UVB inhibits CD8 T cells and skin inflammation by alternative and novel mechanisms. Am J Path. 2011;178:2783–2791. doi: 10.1016/j.ajpath.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vayalil PK, Elmets CA, Katiyar SK. Treatment of green tea polyphenols in hydrophilic cream prevents UVB-induced oxidation of lipids and proteins, depletion of antioxidant enzymes and phosphorylation of MAPK proteins in SKH-1 hairless mouse skin. Carcinogenesis. 2003;24:927–936. doi: 10.1093/carcin/bgg025. [DOI] [PubMed] [Google Scholar]
- 62.Rivas JM, Ullrich SE. Systemic suppression of delayed-type hypersensitivity by supernatants from UV-irradiated keratinocytes. An essential role for keratinocyte-derived IL-10. J Immunol. 1992;149:3865–3871. [PubMed] [Google Scholar]
- 63.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.An KP, Athar M, Tang X, Katiyar SK, Russo J, Beech J, Aszterbaum M, Kopelovich L, Epstein EH, Jr, Mukhtar H, Bickers DR. Cyclooxygenase-2 expression in murine and human nonmelanoma skin cancers: implications for therapeutic approaches. Photochem. Photobiol. 2002;76:73–80. doi: 10.1562/0031-8655(2002)076<0073:ceimah>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 65.Soontrapa K, Honda T, Sakata D, Yao C, Hirata T, Hori S, Matsuoka T, Kita Y, Shimizu T, Kabashima K, Narumiya S. Prostaglandin E2–prostoglandin E receptor subtype 4 (EP4) signaling mediates UV irradiation-induced systemic immunosuppression. Proc. Natl Acad. Sci. USA. 2011;108:6668–6673. doi: 10.1073/pnas.1018625108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dawes JM, Calvo M, Perkins JR, Paterson KJ, Kiesewetter H, Hobbs C, Kaan TKY, Orengo C, Bennett DLH, McMahon SB. CXCL5 mediates UVB irradiation–induced pain. Science Trans Med. 2011;3:90ra60. doi: 10.1126/scitranslmed.3002193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lesokhin AM, Hohl TM, Kitano S, Cortez C, Hirschhorn-Cymerman D, Avogadri F, Rizzuto GA, Lazarus JJ, Pamer EG, Houghton AN, Merghoub T, Wolchok JD. Monocytic CCR2+ myeloid-derived suppressor cells promote immune escape by limiting activated CD8 T-cell infiltration into the tumor microenvironment. Cancer Res. 2012;72:876–886. doi: 10.1158/0008-5472.CAN-11-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rhodes LE. Polymorphic light eruption: does a neutrophil defect contribute to the pathogenesis? J Invest Dermatol. 2004;123:xiii–xv. doi: 10.1111/j.0022-202X.2004.22733.x. [DOI] [PubMed] [Google Scholar]