Abstract
Purpose
The primary purpose of this study was to compare maternal plasma inflammation between physically active and inactive obese women during late pregnancy. The secondary purpose was to examine the relationships between maternal plasma inflammation and lipid metabolism and maternal and neonatal metabolic health in these women.
Methods
A cross-sectional, observational study design was performed in 16 obese-inactive ((OBI) age: 25.0 ± 4.8 years, pre-pregnancy BMI: 36.3 ± 4.3kg/m2, body fat percentage in late gestation: 37.7 ± 3.5%) and 16 obese-active ((OBA) age: 28.9 ± 4.8 years, pre-pregnancy BMI: 34.0±3.7kg/m2, body fat in late gestation: 36.6 ± 3.8%) women during the third trimester of pregnancy. Maternal plasma inflammation (C -reactive protein (CRP)) and insulin resistance (Homeostatic Model Assessment-Insulin Resistance (HOMA-IR)) were measured at rest. Plasma lipid concentration and metabolism (lipid oxidation and lipolysis) were measured at rest, during a 30-minute bout of low-intensity (40% VO2peak) exercise, and during a resting recovery period using indirect calorimetry. Umbilical cord blood was collected for measurement of neonatal plasma insulin resistance, inflammation, and lipid concentration. Neonatal body composition was measured via air displacement plethysmography.
Results
Maternal plasma CRP concentration was significantly higher in OBI compared to OBA women (9.1 ± 4.0 mg/L versus 6.3 ±2.5mg/L, p=0.02). Maternal plasma CRP concentration was significantly associated with maternal lipolysis (r=0.43, p=0.02), baseline lipid oxidation rate (r=0.39, p=0.03), and baseline plasma free fatty acid concentration (r=0.36, p=0.04).
Conclusions
Maternal physical activity may reduce inflammation during pregnancy in obese women. Maternal lipid metabolism is related to systemic inflammation.
Keywords: Pregnancy, inflammation, C-reactive protein, lipid metabolism, obesity, exercise
Introduction
Maternal obesity (pre-pregnancy BMI ≥30kg/m2) prevalence is at a historic high with nearly one in three women entering pregnancy obese (King 2006). Pre-pregnancy obesity contributes to maternal inflammation, insulin resistance, and altered lipid metabolism (Herrera 2002; Ramsay et al. 2002), as well as neonatal adiposity and insulin resistance; all of which can have serious long-term health implications for women and their offspring (Borengasser et al. 2011; Heerwagen et al. 2010; Catalano et al. 2009b; Jarvie et al. 2010). In particular, maternal inflammation may play a significant role in the development of maternal insulin resistance and hypertension- two of the most common health issues diagnosed in obese pregnant women (Borzychowski et al. 2006; Schmatz et al. 2010; Ozgu-Erdinc et al. 2014).
Maternal inflammation is elevated in normal-weight pregnant women (Watts et al. 1991). In obese pregnant women, maternal inflammation is further elevated (Schmatz et al. 2010), and might also negatively contribute to maternal long-term health as it is predictive of future cardiovascular disease risk in non-gravid adults (Lagrand et al. 1999). Interestingly, maternal inflammatory changes during pregnancy are believed to extend into the placenta, suggesting that the fetus of a woman with excessive inflammation is exposed to an inflammatory environment during development (Challier et al. 2008). This exposure might predispose neonates to have a higher risk for the development of metabolic disease in adulthood (Barker 2004; Segovia et al. 2014)
Physical inactivity is recognized as an independent risk factor for obesity, insulin resistance, and type 2 diabetes in non-gravid adults (Blair and Brodney 1999; Blair 2009). The physiological and hormonal changes associated with pregnancy magnify this risk during and after pregnancy by causing an increase in adiposity and insulin resistance (Artal 2015). In pregnant women of normal body weight, physical activity reduces inflammation (Hawkins et al. 2014; Wang et al. 2014), as well as improves maternal insulin sensitivity (Hopkins and Artal 2013). In obese pregnant women, physical activity may decrease insulin resistance (van Poppel et al. 2014). In addition, neonates of normal-weight physically active women have lower adiposity compared to neonates born to inactive women (Clapp and Capeless 1990; Hayes et al. 2014). However, the role of a physically active lifestyle on maternal metabolic health, particularly systemic inflammation, in at-risk obese pregnant women and their neonates is poorly understood. To our knowledge, the impact of physical activity on maternal systemic inflammation and lipid metabolism, and neonatal adiposity, insulin resistance, and inflammation has not been studied in exclusively obese pregnant women.
The primary purpose of this study was to compare maternal plasma inflammation between physically active and inactive obese women during pregnancy. The secondary purpose was to examine the relationships between maternal plasma inflammation and lipid metabolism and maternal and neonatal metabolic health in these women. We hypothesized that during late pregnancy, physically active obese women will have lower plasma inflammation, lipid oxidation rate, lipolysis, and insulin resistance compared to inactive obese women. We also hypothesized that neonates of physically active obese women will have lower adiposity, inflammation, and insulin resistance than neonates of inactive obese women.
Methods
Subjects
Thirty-two women participated in the study (16 obese-inactive (OBA), 16 obese-active (OBA)). Four-hundred women receiving prenatal care at the Women’s Health Center or Women’s Health Clinic at Barnes Jewish Hospital/Washington University between August 2013 and November 2014 were screened for inclusion. Sixty women who met all criteria with ongoing pregnancies were approached for participation late in their second trimester, and 40 agreed to participate. Eight were excluded due to delivering prior to scheduled study visits (n=2), non-compliance with study visits (n=4), or loss to follow-up once entered into the study (n=2). Inclusion criteria included women ages 18–44 years with a confirmed singleton viable pregnancy and no identified fetal abnormalities (as determined by routine anatomy ultrasound at 18–22 weeks) and pre-pregnancy BMI between 30 and 45 kg/m2. Physical activity information was gathered from standard-of-care health history questionnaires distributed by the clinic (questionnaires asked if women exercised regularly (yes/no). If they answered yes, they were given an open-ended question asking for type, frequency, and duration of activity). This form was used to identify potentially active women based on what they reported, and then once identified and confirmed via face-to-face conversation, they were recruited and potentially enrolled. All women in the active group reported exercising ≥150 minutes per week. Physical activity levels were then objectively confirmed via accelerometry. Patients were excluded for any of the following reasons: 1) multiple gestation pregnancy, 2) inability to provide voluntary informed consent, 3) self-reported use of illegal drugs (cocaine, methamphetamine, opiates), 4) current smoker who did not consent to cessation, 5) current usage of daily medications by class: corticosteroids, beta-blockers, or anti-psychotics (known to alter insulin resistance and metabolic profiles), 6) diagnosis of gestational diabetes in current pregnancy, 7) history of gestational diabetes, pre-pregnancy diabetes or prior macrosomic (>4500g) infant (each elevates the risk for gestational diabetes in the current pregnancy, or undiagnosed gestational diabetes), 8) history of heart disease, or 9) any other condition that would preclude exercise. Approval for this study was granted by the Institutional Review Board at Washington University (IRB ID: 201306109, NCT: NCT02039414). All women gave informed consent prior to participating in the study.
Study Procedures
All study procedures were performed at the Washington University School of Medicine Institute for Clinical and Translational Science’s Clinical Research Unit (CRU). All pregnant women participated in two maternal visits between 32 and 37 weeks of gestation.
Maternal Visit 1
Body composition was measured using skinfold anthropometry in order to determine maternal percent body fat. Body fat percentage was determined by pressing folds of the skin at seven sites with a caliper (Harpenden Skinfolds Caliper, Baty International, United Kingdom), recording skin fold thickness, and entering the data into a standardized equation that accounts for age as previously described (Jackson et al. 1980), a technique that has been used during pregnancy in prior studies (Taggart et al. 1967; Kannieappan et al. 2013). Cardiorespiratory fitness levels were assessed using the YMCA submaximal multistage cycle ergometer test as previously described (Beekley et al. 2004) using a Lode Corvial Recumbent cycle ergometer (Lode B.V., The Netherlands). Results were used to predict participant’s VO2peak via the VO2-Heart Rate extraction method, which is considered more accurate for pregnant women than the Astrand-Ryhming test (Sady et al. 1988; Thorell and Kristiansson 2012; Thorell et al. 2015). A three-lead electrocardiogram was applied to monitor heart rate throughout the duration of the exercise test. Subjects also completed the National Institutes of Health’s validated Dietary History Questionnaire II to determine potential differences in diet (Subar et al. 2001).
Maternal physical activity levels were objectively assessed during the week following visit one using the ActiGraph GT3X+ accelerometer (ActiGraph LLC, Pensacola, FL). The GTX3+ was placed on the non-dominant wrist with non-removable wristbands. The wristbands were cut off by the study team when they returned for their second visit to ensure the accelerometers were worn for the entire data collection period. Wrist-worn tri-axel accelerometers can be used as a valid measure of physical activity energy expenditure in pregnant women (van Hees et al. 2011). In addition, previous data on acceptability suggest participants do not object to wearing wrist-worn devices 24 hours/day, and we believe wrist-worn devices with hospital-type wristbands would give us the best compliance and 24 hours/day wear data (van Hees et al. 2011). Data was collected for seven consecutive days at 30 Hz. The accelerometer output was sampled by a 12-bit analog-to-digital converter. The percentage of time spent sedentary as well as the amount of time spent participating in different categories of physical activity ranging from light and lifestyle to moderate were calculated using algorithms corresponding to the following activity counts: sedentary: 0 – 99 counts/min, light: 100 – 759 counts/min, lifestyle: 760 – 1951 counts/min, moderate: 1952–5724 counts/min (Freedson et al. 1998).
Maternal Visit 2
Approximately one week after Visit 1, participants were admitted to the CRU the morning after an overnight fast. The night prior, subjects were provided with written instructions from the CRU dietician for consuming a balanced meal with approximately 50% carbohydrate, 30% fat, and 20% protein. Upon admission to the CRU, height, weight, and vital signs were obtained. A catheter (IV) was placed in a hand vein and heated to 55 °C by using a thermostatically controlled box in order to obtain arterialized blood samples as previously described (Zello et al. 1990). Participants kept their hand in the box throughout the entire study visit. Participants rested in supine for 30 minutes prior to a baseline 15-minute resting indirect calorimetry measurement using the TrueOne Canopy Option and TrueOne Metabolic Cart (TrueOne 2400, Parvomedics, Sandy, UT). Lipid oxidation rate was calculated by measurement of oxygen consumption and carbon dioxide production as previously described (Frayn 1983). After a baseline blood draw, participants exercised at approximately 40% of their predicted VO2peak (based on the YMCA submaximal cycle test) for 30 minutes. Blood was drawn at 10, 20, and 30 minute time points during submaximal exercise. Indirect calorimetry was also performed at 10, 20, and 30 minutes (for 2 minutes at a time) during submaximal exercise using an exercise mouthpiece and nose clip for calculation of lipid oxidation rate during low-intensity exercise. Lipid metabolism was examined during exercise because exercise upregulates adipose tissue breakdown (lipolysis) and lipid oxidation (Horowitz and Klein 2000). In addition, we believe the exercise paradigm mimicked daily activity levels (~3–5 METS, e.g. household chores, caring for other children, walking), thus, representing metabolism during and after normal daily activities. After exercise termination, participants returned to supine and blood was drawn 10, 30, and 60 minutes-post cessation of exercise (i.e. recovery). Resting indirect calorimetry using the canopy option was performed a second time for 15 minutes midway through the post-exercise recovery period. Visit 2 study procedures are shown in Figure 1.
Figure 1.
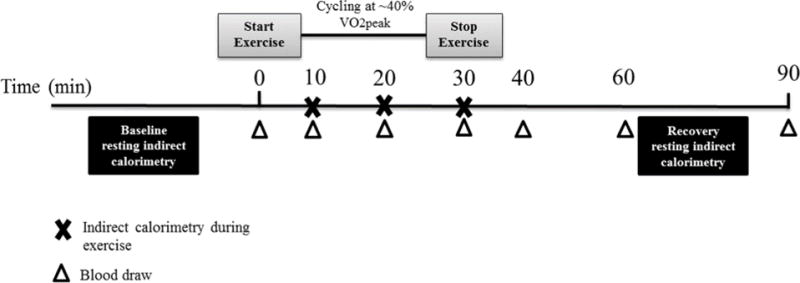
On visit 2, resting indirect calorimetry will be administered before and after a 30-minute bout of low-intensity exercise on a recumbent bicycle. Indirect calorimetry will also occur during the exercise bout at the time points indicated. Blood will be drawn before exercise, three times during exercise, and three times post-exercise.
Sample Analyses and Calculations
All blood samples were immediately placed on ice and plasma was separated by centrifugation within 30 minutes of collection. Plasma samples were stored at −80°C until final analyses were performed. Blood samples for glucose were collected in heparinized tubes and analyzed immediately with an automated glucose analyzer (Yellow Springs Instruments Co, Yellow Springs, OH). Plasma insulin concentration was measured by electrochemiluminescence technology (Elecsys 2010, Roche Diagnostics, Indianapolis, IN). Insulin and glucose levels were used to calculate the homeostatic model assessment-insulin resistance (HOMA-IR), an index of insulin resistance that reflects fasting glucose concentration measured at the fasting insulin concentration (Matthews et al. 1985; Cohen et al. 2006). High-sensitivity C-reactive protein (CRP) was measured by immunoturbidimetric assay (Roach Diagnostics, Indianapolis, IN). Blood samples used to determine plasma free fatty acids were collected in tubes containing EDTA. Plasma free fatty acid concentrations were determined by enzymatic colorimetric assay (Wako Pure Chemical Industries, Osaka, Japan). All blood sample analyses were run in duplicate by trained experts in the Core Laboratory for Clinical Studies as part of the Washington University Institute of Clinical and Translational Sciences. The inter-assay coefficients of variation in the Core Laboratory are ≤1.2% for glucose, ≤3.2% for CRP, ≤2.7% for free fatty acids, and ≤4.3% for insulin. Lipolysis was calculated by the area under the curve (Pruessner et al. 2003; Allirot et al. 2014) for free fatty acids from free fatty acid concentrations from baseline and throughout the exercise and recovery periods.
Neonatal Measurements
Upon admission to labor and delivery, maternal weight was measured and gestational weight gain was calculated using pregnancy weight at initiation for prenatal care (or self-report if >10 weeks gestation at first prenatal appointment) and subtracting that number from their weight upon admission to labor and delivery. At parturition, neonatal birth weight was obtained. In addition, 44 mL of umbilical cord blood was collected, centrifuged within 30 minutes of delivery, and stored at −80°C until further analyses were performed. Umbilical cord blood was used to determine neonatal HOMA-IR, free fatty acid concentration, and inflammation (CRP).
Within 48 hours of delivery, neonatal anthropometrics were measured in the CRU. Neonatal length (Pediatric Length Board, Ellard Instrumentation LTD, Monroe, WA) and head circumference (Gulick II Tape Measure, model 67020, Country Technology Inc., Gays Mills, WI) were measured. Body composition (fat and lean mass) was measured by skin fold thickness (Harpenden calipers, Baty International, West Sussex, UK) at four different sites (triceps, subscapular, ilium, and thigh) and by air displacement plethysmography (Pea Pod, Life Measurement, Inc., Concord, CA). Air displacement plethysmography is a superior, validated, measure of neonatal body composition(Ma et al. 2004; Ellis et al. 2007), but skinfold measurements were performed to better access any unusual distributions of body fat as well as provide the study team with baseline data that could be used for long-term follow-up for the neonates when they outgrow the peapod. All neonates were full-term (≥37+0 weeks) at the time of delivery except for one in the OBI group. Anthropometric measurements for this neonate were not included in the study results.
Statistical Analysis
The sample size (N=32) was sufficient to detect a difference in maternal CRP with a two-tailed alpha of 0.05 and a power of 0.85 (beta=0.15), based on CRP data from a cohort of obese, non-gravid women as part of a 16-week exercise intervention trial (Arikawa et al. 2011).
Normality of the distribution for each variable was tested using Kolmogorov-Smirnov tests. Student’s independent t-tests for normally distributed variables and Mann-Whitney U tests for non-normally distributed variables were used to compare metabolic outcomes between OBA and OBI women. Two-way repeated-measures ANOVAs (group × time) were used with Tukey post hoc analyses when comparing baseline, exercise, and recovery conditions. Groups were not perfectly matched therefore age, race, parity, and income were used as covariates when comparing CRP values between OBI and OBA women to ensure differences between groups were not due to any of these factors. Because fat consumption trended towards significance between OBI and OBA women, percent of calories from fat was used as a covariate when comparing lipid oxidation, lipolysis, and free fatty acids between groups. Pearson product-moment correlation coefficients for normally distributed variables or Spearman’s rank-order correlation coefficient for non-normally distributed variables were used to assess the degree of the relationship between variables. Partial correlations were used to adjust for potential confounders. All tests were two-sided with a p-value <0.05 denoting statistical significance. Data were collected and managed using Research Electronic Data Capture (REDCap), hosted at Washington University School of Medicine (Harris et al. 2009). All data analyses were conducted using IBM SPSS Statistics, Version 22 (Armonk, New York).
Results
Maternal demographic variables
OBA women were older than OBI women, but all other demographics were similar between the groups (Table 1). No current smokers agreed to cessation in order to participate, thus, no current smokers were included in the study. Dietary composition was similar between groups; however, there was a trend for OBA women to consume a higher percentage of dietary fats (Table 2). Dietary fats were used as a covariate in subsequent analysis involving lipid metabolism because dietary fat consumption significantly influences lipid metabolism (Lawrence 2013).
Table 1.
Maternal baseline demographic and metabolic characteristics between OBI and OBA pregnant women
| OBI (n=16) | OBA (n=16) | p-value | |
|---|---|---|---|
|
| |||
| Age (y)* | 25.0 ± 4.8 | 28.9 ± 4.8 | 0.03 |
|
| |||
| Pre-pregnancy BMI (kg/m2) | 36.3 ± 4.3 | 34.0 ± 3.7 | 0.09 |
|
| |||
| Body fat percentage (%) | 37.7 ± 3.5 | 36.6 ± 3.8 | 0.43 |
|
| |||
| Gestation age at visit 2 (wks) | 34+7 ± 1.4 | 34+4 ± 1.3 | 0.58 |
|
| |||
| Gestational weight gain (kg) | 10.3 ± 9.2 | 9.5 ± 6.8 | 0.78 |
|
| |||
| Heart rate (bpm) | 90.0 ± 10.8 | 88.5 ± 12.3 | 0.73 |
|
| |||
| Systolic blood pressure (mmHg) | 112.0 ± 10.9 | 111.6 ± 8.8 | 0.96 |
|
| |||
| Diastolic blood pressure (mmHg) | 71.5 ± 4.3 | 69.3 ± 7.9 | 0.33 |
|
| |||
| Glucose (mg/dL) | 80.3 ± 7.9 | 80.2 ± 8.3 | 0.96 |
|
| |||
| Insulin (uU/mL) | 18.7 ± 8.4 | 15.6 ± 9.1 | 0.24 |
|
| |||
| HOMA-IR | 3.8 ± 1.9 | 3.2 ± 2.3 | 0.24 |
|
| |||
| C-reactive protein (mg/L)* | 9.1 ± 4.0 | 6.3 ± 2.5 | 0.02 |
|
| |||
| Total cholesterol (mg/dL) | 207.8 ± 35.9 | 212.6 ± 46.1 | 0.74 |
|
| |||
| HDL (mg/dL) | 65.1 ± 12.3 | 68.5 ± 14.1 | 0.47 |
|
| |||
| LDL (mg/dL) | 110.6 ± 34.0 | 112.9 ± 39.9 | 0.87 |
|
| |||
| Triglycerides (mg/dL) | 160.0 ± 60.9 | 155.9 ± 59.9 | 0.85 |
|
| |||
| Free fatty acids (meq/L) | 0.49 ± 0.15 | 0.45 ± 0.14 | 0.46 |
|
| |||
| Lipolysis (meq·min/L)* | 42.1 ± 11.0 | 36.9 ± 7.2 | 0.03 |
|
| |||
| Resting energy expenditure (kcal/day) | 2167 ± 291 | 2099 ± 338 | 0.55 |
|
| |||
| p-value (χ2-test) | |||
|
| |||
| Parity | 0.29 | ||
| Nulliparous | 10 (63%) | 7 (44%) | |
| Multiparous | 6 (37%) | 9 (56%) | |
|
| |||
| Income | 0.28 | ||
| Low income | 8 (50%) | 5 (31%) | |
| Mod/high income | 8 (50%) | 11 (69%) | |
|
| |||
| Race | 0.34 | ||
| Caucasian | 5 (31%) | 9 (56%) | |
| African-American | 10 (63%) | 6 (38%) | |
| Other | 1 (6%) | 1 (6%) | |
Data presented as mean ± SD for continuous variables and number of participants and percent for discrete variables,
p<0.05
Table 2.
Average daily dietary composition between OBI and OBA pregnant women
| OBI (n=15) | OBA (n=16) | p-value (t-test) | |
|---|---|---|---|
| Energy intake (kcal) | 2058.7 ± 845 | 2369.0 ± 1252 | 0.43 |
| Fat (g) | 69.1 ± 31.2 | 94.0 ± 62.9 | 0.18 |
| Fat (% of kcal/day) | 30.6 ± 7.1 | 34.9 ± 5.0 | 0.06 |
| Carbohydrate (g) | 295.4 ± 137 | 295.0 ± 122 | 0.99 |
| Carbohydrate (% of kcal/day) | 56.9 ± 10.6 | 51.4 ± 7.9 | 0.11 |
| Protein (g) | 73.3 ± 35.9 | 98.5 ± 68.1 | 0.21 |
| Protein (% of kcal/day) | 14.3 ± 3.5 | 16.1 ± 3.2 | 0.15 |
All data presented as mean ± SD,
p<0.05
Based on accelerometer data, OBA women spent significantly less time sedentary, and more time in light, lifestyle, and moderate physical activities than the OBI women (p≤0.001). Compliance was 100% as all women wore the accelerometer without removal for the entire seven days. OBA women also had higher predicted fitness levels compared to OBI women (33.7± 6.4 ml/kg/min vs. 24.7± 3.5 ml/kg/min, p<0.001). All OBA reported their primary mode of activity being walking (n=16), while secondary activities reported were yoga (n=2), weight-lifting (n=2), elliptical machine (n=2), biking (n=1), and swimming (n=1).
Maternal metabolic characteristics
Maternal plasma CRP concentration was significantly lower in OBA women compared to OBI women (6.3 ± 2.5 mg/L versus 9.1 ± 4.0 mg/L, p=0.02) (Figure 2). When adjusting for differences in age, race, parity, and income between groups, CRP remained significantly lower in OBA women compared to OBI women (p=0.002). When adjusting for pre-pregnancy BMI, CRP was not statistically significant between groups (p=0.077). However, when adjusting for maternal body fat percentage at 32–37 weeks gestation, CRP remained significant between OBA and OBI women (p=0.035). Maternal HOMA-IR was similar between OBA and OBI women. All other maternal metabolic characteristics are shown in Table 1.
Figure 2.
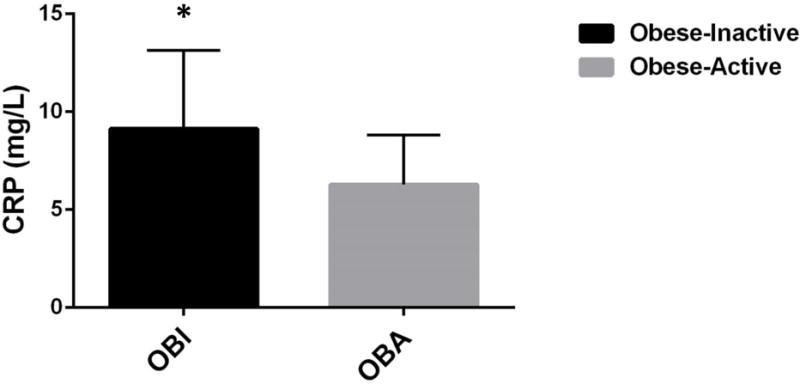
Maternal CRP was significantly higher in the OBI group compared to the OBA group. *p<0.05
OBA women had lower lipolysis (36.9 ± 7.2 meq·min/L vs. 42.1 ± 11.0 meq·min/L, p=0.03) and had lower free fatty acids during the exercise bout (30 minute time point) (0.36 ± 0.10 meq/L vs. 0.42 ± 0.13 meq/L, p=0.03) compared to OBI women. Maternal lipid metabolism between OBI and OBA groups is presented in Figure 3.
Figure 3.
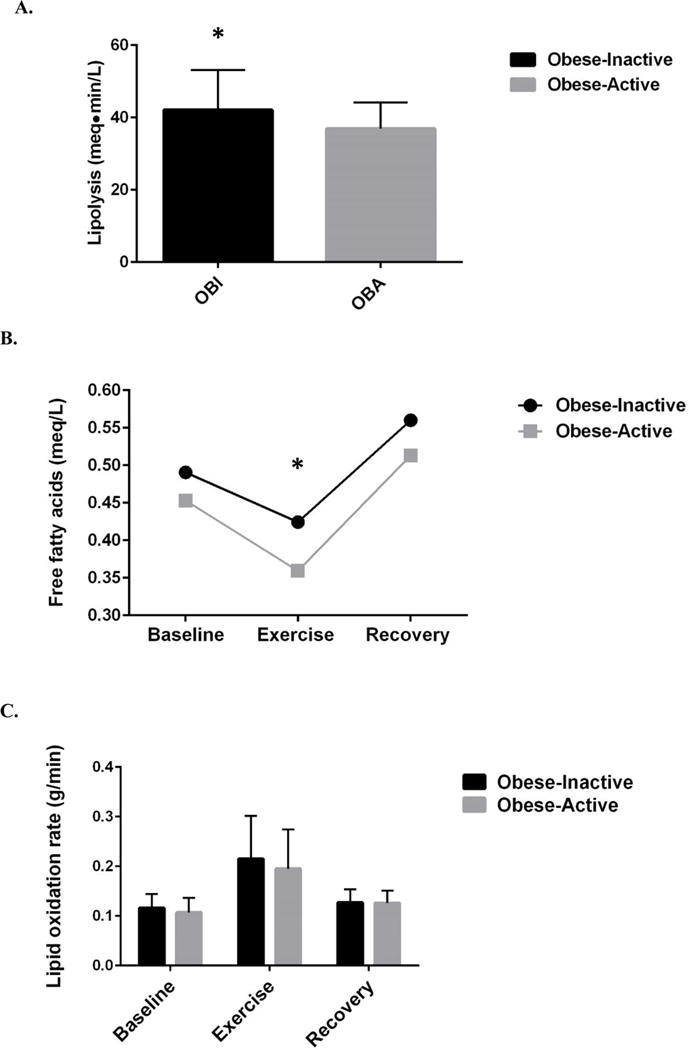
A. Maternal lipolysis was significantly higher in OBI women compared to OBA. B. Maternal plasma circulating free fatty acids were higher in OBA women during exercise compared to OBI women. C. There were no significant differences between maternal lipid oxidation rates between OBI and OBA women. *p<0.05
Maternal lipolysis was significantly correlated to maternal plasma CRP concentration (r=0.43, p=0.02). Maternal resting free fatty acid concentration was also correlated with maternal plasma CRP concentration (r=0.36, p=0.04). Similarly, maternal baseline (i.e. resting/before exercise) lipid oxidation rate and maternal plasma CRP concentration were positively correlated (r=0.39, p=0.03). When the relationship between lipid oxidation and inflammation was analyzed in the OBA and OBI groups individually, there was a trend for a positive correlation between lipid oxidation and CRP in OBI women (r=0.50 p=0.06), but not in OBA women (r= −0.12, p=0.67) (Figure 4).
Figure 4.
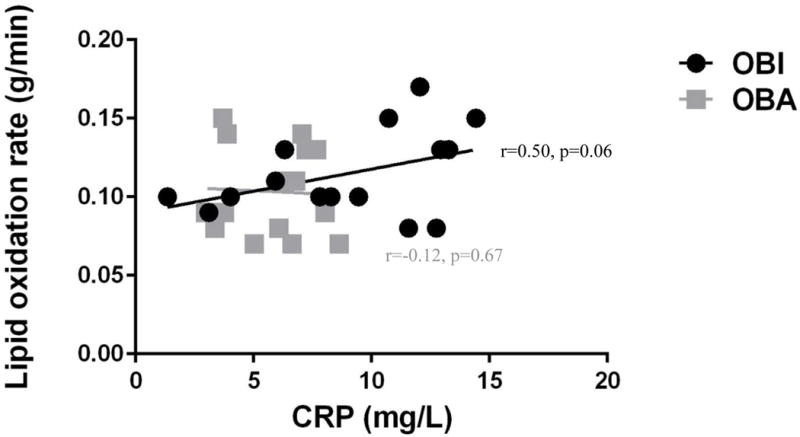
Maternal lipid oxidation rate was positively correlated to maternal CRP across all participants (r=0.39. p=0.03), but this relationship appears to be driven by the OBI group (r=0.50, p=0.06), as this relationship did not exist in OBA women (r= −0.12, p=0.67).
Neonatal Results
Neonatal anthropometric and metabolic outcomes were similar between groups (Table 3). Neonatal anthropometrics were not obtained for one neonate who was lost to follow-up. Cord blood could not be obtained for two OBI and four OBA women/neonates. There were no significant relationships between maternal and neonatal adiposity (r= −0.26, p=0.16), maternal and neonatal inflammation (r=0.19, p=0.37), or maternal and neonatal insulin resistance (r=0.20, p=0.35).
Table 3.
Neonatal outcomes for neonates of OBI and OBA pregnant women
| Anthropometric outcomes | OBI (n= 15) | OBA (n=15) | p-value |
|---|---|---|---|
|
| |||
| Birth weight (g) | 3344 ± 428 | 3230 ± 515 | 0.80 |
|
| |||
| Length (cm) | 49.9 ± 2.0 | 49.6 ± 2.3 | 0.75 |
|
| |||
| Head circumference (cm) | 34.4 ± 1.2 | 34.3 ± 1.3 | 0.72 |
|
| |||
| Fat mass via PeaPod (%) | 11.2 ± 3.6 | 12.4 ± 2.9 | 0.35 |
|
| |||
| Skinfolds | |||
| Triceps | 5.1 ± 1.1 | 4.7 ± 0.9 | 0.35 |
| Subscapular | 4.4 ± 0.9 | 4.3 ± 0.6 | 0.72 |
| Ilium | 5.2 ± 1.4 | 4.7 ± 1.2 | 0.35 |
| Thigh | 6.9 ± 1.9 | 6.4 ± 1.3 | 0.49 |
|
| |||
| OBI (n=16) | OBA (n=15) | ||
|
| |||
| Gestational age at delivery (wks) | 39+5 ± 1.4 | 39+0 ± 1.3 | 0.25 |
|
| |||
| Mode of delivery | 0.72 | ||
| Vaginal | 9 (56%) | 10 (67%) | |
| Cesarean | 7 (44%) | 5 (33%) | |
|
| |||
| Gender | 0.72 | ||
| Male | 10 (63%) | 8 (53%) | |
| Female | 6 (37%) | 7 (47%) | |
|
| |||
| Cord blood values | OBI (n=14) | OBA (n=12) | |
|
| |||
| Glucose (mg/dL) | 80.7 ± 12.8 | 88.3 ± 20.7 | 0.31 |
|
| |||
| Insulin (uU/mL) | 7.5 ± 4.9 | 9.0 ± 7.2 | 0.57 |
|
| |||
| HOMA-IR | 1.6 ± 1.2 | 1.9 ± 1.6 | 0.55 |
|
| |||
| Free fatty acids (meq/L) | 0.16 ±0.06 | 0.16 ± 0.07 | 0.89 |
|
| |||
| C-reactive protein (mg/L) | 0.24 ± 0.21 | 0.16 ± 0.14 | 0.32 |
Data are presented as mean ±SD for continuous variables or number of participants and percent for discrete variables,
p<0.05
Discussion
Maternal outcomes
The primary finding of this study was that during late pregnancy, maternal systemic inflammation, measured by C-reactive protein, was lower in physically active obese women compared to inactive obese women. In non-pregnant populations, CRP values ≥3.0 mg/L have been associated with increased cardiovascular risk (Pearson et al. 2003). It appears both groups have elevated cardiovascular disease risk; however, physical activity during pregnancy might modulate this risk in obese women. Our finding is consistent with Hawkins et al. who found that physical activity is associated with lower CRP in normal-weight pregnant women (Hawkins et al. 2014). Our study extends this work by demonstrating the benefits of physical activity on inflammation also apply to obese pregnant women during late pregnancy- a population at high risk for excessive inflammation during pregnancy and its downstream sequela. This finding is clinically significant as higher inflammation might contribute to the increased acute and chronic risk for the development of metabolic complications (e.g. insulin resistance, gestational diabetes, hypertension, metabolic syndrome, cardiovascular disease) (Catalano et al. 2009a; Kosus et al. 2014; Ozgu-Erdinc et al. 2014) as well as additional risks for maternal infection, preterm delivery, and severe preeclampsia (Schmatz et al. 2010). Additionally, higher inflammation in inactive obese women during pregnancy will likely persist into postpartum (Christian and Porter 2014); thus, potentially contributing to a higher long-term diabetes and cardiovascular disease risk (Lagrand et al. 1999).
Of note, maternal CRP was not different when adjusting for maternal pre-pregnancy BMI. Thus, pre-pregnancy BMI could play a role in determining late-pregnancy maternal CRP values irrespective of physical activity levels. However, when adjusting for maternal body fat percentage at 32–37 weeks gestation, maternal CRP remained statistically higher in obese inactive women. Adjusting for maternal body fat percentage instead of BMI is warranted as skinfolds provide a more accurate measure of body fat; BMI does not distinguish fat and lean body mass (Nooyens et al. 2007). In addition, maternal body fat percentage and maternal CRP were measured at the same time (32–37 weeks gestation); thus, we believe that accounting for maternal body fat percentage more accurately accounts for any differences in maternal body composition between groups.
In non-gravid obese adults, excessive plasma free fatty acids and lipid oxidation are believed to initiate an inflammatory response (McIntyre and Hazen 2010). In the current study, maternal lipid oxidation and lipolysis were associated with maternal inflammation, suggesting that this relationship between lipid metabolism and inflammation also exists in obese pregnant women. We suspect maternal physical activity during pregnancy might modulate lipid metabolism as physically active obese women had lower plasma free fatty acid concentrations during exercise and lower lipolysis across the entire study period. It is plausible that the difference in plasma free fatty acids between groups was only detected during exercise due to exercise being a condition in which lipid metabolism is upregulated (Horowitz and Klein 2000). Therefore, the exercise bout elucidated possible differences in lipid metabolism between groups that might not be seen during rest. In addition, differences in lipid metabolism during exercise (i.e. free fatty acids and lipolysis) might be clinically meaningful as this metabolic environment may represent a pregnant woman’s metabolism at intermittent times throughout the day when she is participating in daily activities such as housework, caring for other children, grocery shopping, or completing work-related tasks.
Our findings are consistent with data in non-gravid adults that suggests endurance exercise training increases efficiency of lipid metabolism including decreasing plasma free fatty acid turnover and oxidation during submaximal exercise (Martin 1996). Our study suggests maternal lipid metabolism might be moderately improved in obese women who participate in physical activity, and that this improvement may contribute to lower inflammation. Our finding is clinically important as lipid metabolism may be a modifiable upstream target that contributes to systemic inflammation in obese pregnant women (McIntyre and Hazen 2010).
Upon further examination, we noted that the relationship between lipid oxidation and inflammation was primarily driven by the obese inactive group. Lipid oxidation and CRP trended towards a positive correlation in inactive obese women, but this relationship did not exist in the physically active obese group. This suggests that although lipid oxidation rates were not different between inactive and active groups, the nearly positive correlation between lipid oxidation and inflammation in obese inactive pregnant women may be due to inactive women also having higher incomplete lipid oxidation. Incomplete lipid oxidation may then be contributing to downstream inflammation, which is supported by the increased C-reactive protein in the obese inactive group. Bell et al. suggests that in obesity, lipid oxidation may be incomplete and inefficient, leading to oxidative stress (Bell et al. 2010; McIntyre and Hazen 2010)- a known contributor to inflammation (Fernandez-Sanchez et al. 2011). It is well-established that exercise improves efficiency of lipid metabolism as well as reduces oxidative stress (Martin 1996; Fisher-Wellman et al. 2009). Therefore, it is plausible that the reason for the lack-of association between lipid oxidation and inflammation in the obese active group was due to obese active women having improved efficiency of lipid metabolism resulting in complete oxidation of lipids, lower oxidative stress, and lower systemic inflammation. Further support of this theory is the connection between lipid metabolism and oxidative stress; oxidative stress is a known by-product of inefficient lipid metabolism (Bell et al. 2010). In addition, inflammation is believed to be a manifestation of oxidative stress, which is increased in obese individuals (Fernandez-Sanchez et al. 2011). Unfortunately, intermediate by-products of lipid metabolism were not measured, and we did not assess whether or not lipid oxidation was complete; therefore, this is speculative.
Neonatal outcomes
Contrary to our hypothesis, there were no differences in neonatal metabolic outcomes between obese inactive and obese active women. Our study suggests that although neonates of obese active women were not leaner, exercise during pregnancy in this at-risk population does not appear to have a harmful impact on neonatal birth weight. These data are clinically important as the neonatal risks associated with obese pregnant women participating in exercise regimes are largely unknown. However, our neonatal outcomes should be interpreted carefully as our small sample size makes it difficult to draw any conclusions as we were likely underpowered to look at these outcomes.
Maternal inflammation and insulin resistance were not related to any neonatal metabolic outcomes (adiposity, insulin resistance, or inflammation). These findings suggest that maternal metabolic health may not have an acute impact on neonatal metabolic health. However, metabolic abnormalities may be programmed but not apparent or measurable until later in life (Almond and Currie 2011). One of the primary ideas behind the “fetal origins hypothesis” is the programming of poor metabolic health can remain latent for many years in the offspring (Barker et al. 2002; Almond and Currie 2011). Several studies have noted a long-term impact of maternal obesity or physical activity on neonatal adiposity and inflammation (Leibowitz et al. 2012; Mourtakos et al. 2015). Thus, long-term follow-up of neonates born to obese inactive and obese active women are needed to truly determine the impact of physical activity on long-term offspring health in obese pregnant women.
Limitations
Despite the current study’s novel findings, this study had several notable limitations. First, this study was powered based on the primary outcome of maternal plasma C-reactive protein concentration, thus, examination of other variables were very likely to be underpowered. Second, our cross-sectional, observational study design did not allow us to explore cause and effect relationships. Also, skinfold measurements, as used to determine maternal body fat percentage, have not been validated in pregnant women. Similarly, the YMCA submaximal cycle test protocol, as used to determine maternal fitness level, has not been validated in pregnancy. Another notable limitation is that we measured neonatal body composition between 24–48 hours postpartum when neonatal weight is known to slightly decrease (Flaherman et al. 2015; Miller et al. 2015), thus, this may have influenced our body composition measurements. However, we used birth weight as our primary measure of neonatal weight, which would not yet be influenced by neonatal postpartum weight loss. We also waited at least 24 hours to obtain the body composition measurements, thus, changes within the first 24 hours were less likely to influence our results.
Conclusions
We found that inflammation is lower in physically active obese pregnant women when compared to inactive obese pregnant women. The reduction in inflammation among physically active obese women might be related to improvements in lipid metabolism as measures of lipid metabolism and inflammation were related. Maternal physical activity did not influence neonatal metabolic outcomes, but long-term follow-up is needed. Future studies should investigate the effect of physical activity on maternal inflammation via randomized clinical trials, as well as the possible role of incomplete lipid oxidation and oxidative stress on maternal and neonatal metabolic outcomes. In addition, the long-term impact of maternal physical activity on neonatal outcomes should be investigated.
Acknowledgments
The nursing staff at the Washington University Institute for Clinical and Translational Sciences Clinical Research Unit as well as Barnes-Jewish Hospital’s Labor and Delivery floor should be acknowledged for their hard work and altruism. This work was funded by Clinical and Translational Science Award (CTSA) program of the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH) under Award TL1 TR000449 and the Movement Science Program as part of Washington University School of Medicine’s Program in Physical Therapy.
Footnotes
Contributions: RAT and WTC researched data, wrote/edited manuscript. EAS and AGC researched data and reviewed/edited the manuscript.
The authors declare no conflicts of interest.
References
- Allirot X, Seyssel K, Saulais L, Roth H, Charrie A, Drai J, et al. Effects of a breakfast spread out over time on the food intake at lunch and the hormonal responses in obese men. Physiol Behav. 2014;127:37–44. doi: 10.1016/j.physbeh.2014.01.004. [DOI] [PubMed] [Google Scholar]
- Almond D, Currie J. Killing Me Softly: The Fetal Origins Hypothesis. J Econ Perspect. 2011;25(3):153–172. doi: 10.1257/jep.25.3.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arikawa AY, Thomas W, Schmitz KH, Kurzer MS. Sixteen weeks of exercise reduces C-reactive protein levels in young women. Med Sci Sports Exerc. 2011;43(6):1002–1009. doi: 10.1249/MSS.0b013e3182059eda. [DOI] [PubMed] [Google Scholar]
- Artal R. The role of exercise in reducing the risks of gestational diabetes mellitus in obese women. Best Pract Res Clin Obstet Gynaecol. 2015;29(1):123–132. doi: 10.1016/j.bpobgyn.2014.05.013. [DOI] [PubMed] [Google Scholar]
- Barker DJ. The developmental origins of adult disease. J Am Coll Nutr. 2004;23(6 Suppl):588S–595S. doi: 10.1080/07315724.2004.10719428. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Eriksson JG, Forsen T, Osmond C. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol. 2002;31(6):1235–1239. doi: 10.1093/ije/31.6.1235. [DOI] [PubMed] [Google Scholar]
- Beekley MD, Brechue WF, deHoyos DV, Garzarella L, Werber-Zion G, Pollock ML. Cross-validation of the YMCA submaximal cycle ergometer test to predict VO2max. Res Q Exerc Sport. 2004;75(3):337–342. doi: 10.1080/02701367.2004.10609165. [DOI] [PubMed] [Google Scholar]
- Bell JA, Reed MA, Consitt LA, Martin OJ, Haynie KR, Hulver, et al. Lipid partitioning, incomplete fatty acid oxidation, and insulin signal transduction in primary human muscle cells: effects of severe obesity, fatty acid incubation, and fatty acid translocase/CD36 overexpression. J Clin Endocrinol Metab. 2010;95(7):3400–3410. doi: 10.1210/jc.2009-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair SN. Physical inactivity: the biggest public health problem of the 21st century. Br J Sports Med. 2009;43(1):1–2. [PubMed] [Google Scholar]
- Blair SN, Brodney S. Effects of physical inactivity and obesity on morbidity and mortality: current evidence and research issues. Med Sci Sports Exerc. 1999;31(11 Suppl):S646–662. doi: 10.1097/00005768-199911001-00025. [DOI] [PubMed] [Google Scholar]
- Borengasser SJ, Lau F, Kang P, Blackburn ML, Ronis MJ, Badger TM, et al. Maternal obesity during gestation impairs fatty acid oxidation and mitochondrial SIRT3 expression in rat offspring at weaning. PLoS One. 2011;6(8):e24068. doi: 10.1371/journal.pone.0024068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borzychowski AM, Sargent IL, Redman CW. Inflammation and pre-eclampsia. Semin Fetal Neonatal Med. 2006;11(5):309–316. doi: 10.1016/j.siny.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Catalano PM, Farrell K, Thomas A, Huston-Presley L, Mencin P, de Mouzon SH, et al. Perinatal risk factors for childhood obesity and metabolic dysregulation. Am J Clin Nutr. 2009;90(5):1303–1313. doi: 10.3945/ajcn.2008.27416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalano PM, Presley L, Minium J, Hauguel-de Mouzon S. Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care. 2009;32(6):1076–1080. doi: 10.2337/dc08-2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challier JC, Basu S, Bintein T, Minium J, Hotmire K, Catalano PM, et al. Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta. 2008;29(3):274–281. doi: 10.1016/j.placenta.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian LM, Porter K. Longitudinal changes in serum proinflammatory markers across pregnancy and postpartum: effects of maternal body mass index. Cytokine. 2014;70(2):134–140. doi: 10.1016/j.cyto.2014.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp JF, 3rd, Capeless EL. Neonatal morphometrics after endurance exercise during pregnancy. Am J Obstet Gynecol. 1990;163(6 Pt 1):1805–1811. doi: 10.1016/0002-9378(90)90754-u. [DOI] [PubMed] [Google Scholar]
- Cohen O, Epstein GS, Weisz B, Homko CJ, Sivan E. Longitudinal assessment of insulin sensitivity in pregnancy. Validation of the homeostasis model assessment. Clin Endocrinol (Oxf) 2006;64(6):640–644. doi: 10.1111/j.1365-2265.2006.02519.x. [DOI] [PubMed] [Google Scholar]
- Ellis KJ, Yao M, Shypailo RJ, Urlando A, Wong WW, Heird WC. Body-composition assessment in infancy: air-displacement plethysmography compared with a reference 4-compartment model. Am J Clin Nutr. 2007;85(1):90–95. doi: 10.1093/ajcn/85.1.90. [DOI] [PubMed] [Google Scholar]
- Fernandez-Sanchez A, Madrigal-Santillan E, Bautista M, Esquivel-Soto J, Morales-Gonzalez A, Esquivel-Chirino C, et al. Inflammation, oxidative stress, and obesity. Int J Mol Sci. 2011;12(5):3117–3132. doi: 10.3390/ijms12053117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher-Wellman K, Bell HK, Bloomer RJ. Oxidative stress and antioxidant defense mechanisms linked to exercise during cardiopulmonary and metabolic disorders. Oxid Med Cell Longev. 2009;2(1):43–51. doi: 10.4161/oxim.2.1.7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherman VJ, Schaefer EW, Kuzniewicz MW, Li SX, Walsh EM, Paul IM. Early weight loss nomograms for exclusively breastfed newborns. Pediatrics. 2015;135(1):e16–23. doi: 10.1542/peds.2014-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol Respir Environ Exerc Physiol. 1983;55(2):628–634. doi: 10.1152/jappl.1983.55.2.628. [DOI] [PubMed] [Google Scholar]
- Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc. 1998;30(5):777–781. doi: 10.1097/00005768-199805000-00021. [DOI] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins M, Pekow P, Chasan-Taber L. Physical activity, sedentary behavior, and C-reactive protein in pregnancy. Med Sci Sports Exerc. 2014;46(2):284–292. doi: 10.1249/MSS.0b013e3182a44767. [DOI] [PubMed] [Google Scholar]
- Hayes L, Bell R, Robson S, Poston L, Consortium U. Association between physical activity in obese pregnant women and pregnancy outcomes: the UPBEAT pilot study. Ann Nutr Metab. 2014;64(3–4):239–246. doi: 10.1159/000365027. [DOI] [PubMed] [Google Scholar]
- Heerwagen MJ, Miller MR, Barbour LA, Friedman JE. Maternal obesity and fetal metabolic programming: a fertile epigenetic soil. Am J Physiol Regul Integr Comp Physiol. 2010;299(3):R711–722. doi: 10.1152/ajpregu.00310.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera E. Lipid metabolism in pregnancy and its consequences in the fetus and newborn. Endocrine. 2002;19(1):43–55. doi: 10.1385/ENDO:19:1:43. [DOI] [PubMed] [Google Scholar]
- Hopkins SA, Artal R. The role of exercise in reducing the risks of gestational diabetes mellitus. Womens Health (Lond Engl) 2013;9(6):569–581. doi: 10.2217/whe.13.52. [DOI] [PubMed] [Google Scholar]
- Horowitz JF, Klein S. Lipid metabolism during endurance exercise. Am J Clin Nutr. 2000;72(2 Suppl):558S–563S. doi: 10.1093/ajcn/72.2.558S. [DOI] [PubMed] [Google Scholar]
- Jackson AS, Pollock ML, Ward A. Generalized equations for predicting body density of women. Med Sci Sports Exerc. 1980;12(3):175–181. [PubMed] [Google Scholar]
- Jarvie E, Hauguel-de-Mouzon S, Nelson SM, Sattar N, Catalano PM, Freeman DJ. Lipotoxicity in obese pregnancy and its potential role in adverse pregnancy outcome and obesity in the offspring. Clin Sci (Lond) 2010;119(3):123–129. doi: 10.1042/CS20090640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannieappan LM, Deussen AR, Grivell RM, Yelland L, Dodd JM. Developing a tool for obtaining maternal skinfold thickness measurements and assessing inter-observer variability among pregnant women who are overweight and obese. BMC Pregnancy Childbirth. 2013;13:42. doi: 10.1186/1471-2393-13-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JC. Maternal obesity, metabolism, and pregnancy outcomes. Annu Rev Nutr. 2006;26:271–291. doi: 10.1146/annurev.nutr.24.012003.132249. [DOI] [PubMed] [Google Scholar]
- Kosus N, Kosus A, Turhan N. Relation between abdominal subcutaneous fat tissue thickness and inflammatory markers during pregnancy. Arch Med Sci. 2014;10(4):739–745. doi: 10.5114/aoms.2014.44865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrand WK, Visser CA, Hermens WT, Niessen HW, Verheugt FW, Wolbink GJ, et al. C-reactive protein as a cardiovascular risk factor: more than an epiphenomenon? Circulation. 1999;100(1):96–102. doi: 10.1161/01.cir.100.1.96. [DOI] [PubMed] [Google Scholar]
- Lawrence GD. Dietary fats and health: dietary recommendations in the context of scientific evidence. Adv Nutr. 2013;4(3):294–302. doi: 10.3945/an.113.003657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz KL, Moore RH, Ahima RS, Stunkard AJ, Stallings VA, Berkowitz RI, et al. Maternal obesity associated with inflammation in their children. World J Pediatr. 2012;8(1):76–79. doi: 10.1007/s12519-011-0292-6. [DOI] [PubMed] [Google Scholar]
- Ma G, Yao M, Liu Y, Lin A, Zou H, Urlando A, et al. Validation of a new pediatric air-displacement plethysmograph for assessing body composition in infants. Am J Clin Nutr. 2004;79(4):653–660. doi: 10.1093/ajcn/79.4.653. [DOI] [PubMed] [Google Scholar]
- Martin WH., 3rd Effects of acute and chronic exercise on fat metabolism. Exerc Sport Sci Rev. 1996;24:203–231. [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- McIntyre TM, Hazen SL. Lipid oxidation and cardiovascular disease: introduction to a review series. Circ Res. 2010;107(10):1167–1169. doi: 10.1161/CIRCRESAHA.110.224618. [DOI] [PubMed] [Google Scholar]
- Miller JR, Flaherman VJ, Schaefer EW, Kuzniewicz MW, Li SX, Walsh EM, et al. Early weight loss nomograms for formula fed newborns. Hosp Pediatr. 2015;5(5):263–268. doi: 10.1542/hpeds.2014-0143. [DOI] [PubMed] [Google Scholar]
- Mourtakos SP, Tambalis KD, Panagiotakos DB, Antonogeorgos G, Arnaoutis G, Karteroliotis A, et al. Maternal lifestyle characteristics during pregnancy, and the risk of obesity in the offspring: a study of 5,125 children. BMC Pregnancy Childbirth. 2015;15:66. doi: 10.1186/s12884-015-0498-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nooyens AC, Koppes LL, Visscher TL, Twisk JW, Kemper HC, Schuit AJ, et al. Adolescent skinfold thickness is a better predictor of high body fatness in adults than is body mass index: the Amsterdam Growth and Health Longitudinal Study. Am J Clin Nutr. 2007;85(6):1533–1539. doi: 10.1093/ajcn/85.6.1533. [DOI] [PubMed] [Google Scholar]
- Ozgu-Erdinc AS, Yilmaz S, Yeral MI, Seckin KD, Erkaya S, Danisman AN. Prediction of gestational diabetes mellitus in the first trimester: comparison of C-reactive protein, fasting plasma glucose, insulin and insulin sensitivity indices. J Matern Fetal Neonatal Med. 2014:1–6. doi: 10.3109/14767058.2014.973397. [DOI] [PubMed] [Google Scholar]
- Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M, et al. Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28(7):916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Ramsay JE, Ferrell WR, Crawford L, Wallace AM, Greer IA, Sattar N. Maternal obesity is associated with dysregulation of metabolic, vascular, and inflammatory pathways. J Clin Endocrinol Metab. 2002;87(9):4231–4237. doi: 10.1210/jc.2002-020311. [DOI] [PubMed] [Google Scholar]
- Sady SP, Carpenter MW, Sady MA, Haydon B, Hoegsberg B, Cullinane EM, et al. Prediction of VO2max during cycle exercise in pregnant women. J Appl Physiol. 1988;65(2):657–661. doi: 10.1152/jappl.1988.65.2.657. [DOI] [PubMed] [Google Scholar]
- Schmatz M, Madan J, Marino T, Davis J. Maternal obesity: the interplay between inflammation, mother and fetus. J Perinatol. 2010;30(7):441–446. doi: 10.1038/jp.2009.182. [DOI] [PubMed] [Google Scholar]
- Segovia SA, Vickers MH, Gray C, Reynolds CM. Maternal obesity, inflammation, and developmental programming. Biomed Res Int. 2014;2014:418975. doi: 10.1155/2014/418975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, et al. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires : the Eating at America’s Table Study. Am J Epidemiol. 2001;154(12):1089–1099. doi: 10.1093/aje/154.12.1089. [DOI] [PubMed] [Google Scholar]
- Taggart NR, Holliday RM, Billewicz WZ, Hytten FE, Thomson AM. Changes in skinfolds during pregnancy. Br J Nutr. 1967;21(2):439–451. doi: 10.1079/bjn19670045. [DOI] [PubMed] [Google Scholar]
- Thorell E, Goldsmith L, Weiss G, Kristiansson P. Physical fitness, serum relaxin and duration of gestation. BMC Pregnancy Childbirth. 2015;15:168. doi: 10.1186/s12884-015-0607-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorell E, Kristiansson P. Pregnancy related back pain, is it related to aerobic fitness? A longitudinal cohort study. BMC Pregnancy Childbirth. 2012;12:30. doi: 10.1186/1471-2393-12-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hees VT, Renstrom F, Wright A, Gradmark A, Catt M, Chen KY, et al. Estimation of daily energy expenditure in pregnant and non-pregnant women using a wrist-worn tri-axial accelerometer. PLoS One. 2011;6(7):e22922. doi: 10.1371/journal.pone.0022922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Poppel MN, Peinhaupt M, Eekhoff ME, Heinemann A, Oostdam N, Wouters MG, et al. Physical activity in overweight and obese pregnant women is associated with higher levels of proinflammatory cytokines and with reduced insulin response through interleukin-6. Diabetes Care. 2014;37(4):1132–1139. doi: 10.2337/dc13-2140. [DOI] [PubMed] [Google Scholar]
- Wang Y, Cupul-Uicab LA, Rogan WJ, Eggesbo M, Travlos G, Wilson R, et al. Recreational Exercise Before and During Pregnancy in Relation to Plasma C-Reactive Protein Concentrations in Pregnant Women. J Phys Act Health. 2015;12(6):770–775. doi: 10.1123/jpah.2013-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts DH, Krohn MA, Wener MH, Eschenbach DA. C-reactive protein in normal pregnancy. Obstet Gynecol. 1991;77(2):176–180. doi: 10.1097/00006250-199102000-00002. [DOI] [PubMed] [Google Scholar]
- Zello GA, Smith JM, Pencharz PB, Ball RO. Development of a heating device for sampling arterialized venous blood from a hand vein. Ann Clin Biochem. 1990;27(Pt 4):366–372. doi: 10.1177/000456329002700414. [DOI] [PubMed] [Google Scholar]


