abstract
Volatile organic compounds (VOC) play an important role in protecting plants from insect and pathogen attack. In this study, we investigated the leaf volatile profiles of 14 citrus varieties. The VOC in citrus leaves were extracted with n-hexane and analyzed using gas chromatography-mass spectrometry (GC-MS). Overall, 4six volatile compounds were identified in the n-hexane extract from citrus leaves. Most of the detected compounds belonged to 3 main groups (monoterpenes, sesquiterpenes, and aliphatic aldehydes). Principle component analysis was used to examine the relative distribution of the studied varieties to each other. Interestingly, volatile profiles of varieties that are tolerant to Candidatus Liberibacter asiaticus (CLas) were different from those of the susceptible ones. Tolerant and moderately-tolerant cultivars contained relatively higher amounts of volatiles than susceptible varieties. In addition, tolerant varieties were also higher in specific compounds which are known for their antimicrobial activities. These compounds include Aldehydes (undecanal, neral, geranial, and citronellal) and some monoterpenes such as linalool, d-limonene, myrcene, α- and β- phellandrene. In addition, some sesquiterpene compounds including t-caryophellene, γ-elemene, β-elemene, germacrene D, and geranyl acetate were higher in tolerant and moderately tolerant cultivars. Severinia buxifolia which is known for its tolerance to CLas and many other pathogens contained higher levels of santalenes and coumarins. Our results indicated that citrus leaf volatiles might play a role in citrus tolerance to CLas. The results of this study may help in understanding of the mechanism of citrus tolerance against CLas.
Keywords: Aliphatic aldehydes, chemotaxonomy, Candidatus Liberibacter asiaticus, citrus, gas chromatography, huanglongbing, monoterpene, sesquiterpene, volatile organic compounds
Introduction
Citrus is one of the world's major fruit crops with an annual global production of more than 100 million metric tons.1 Citrus fruits are rich in carbohydrates and biological components that are important to human health such as dietary fiber, vitamins, minerals, carotenoids, and flavonoids.1 Unfortunately, nowadays Huanglongbing (HLB) is threatening the global citrus industry. The HLB-associated bacterium, Candidatus Liberibacter asiaticus (CLas) is an uncultivable gram-negative bacterium, phloem-restricted, and it is transmitted by the Asian citrus psyllid (ACP), Diaphorina citri.2
ACP transmits CLas while feeding on citrus phloem sap. Citrus and citrus-relatives are the main host plants for the ACP.3 Field observation indicated that sweet orange and Murraya paniculata (L.) Jack (orange jasmine) were the most preferred host for ACP, whereas Poncirus trifoliata was an occasional host.3 Tsai & Liu tested the preference of ACP to 4 hosts: M. paniculata, Citrus aurantium L. (sour orange), Citrus jambhiri Lushington (rough lemon), and Citrus × paradisi Macfadyen (grapefruit).4 Tsai & Liu showed that grapefruit was the best host among the tested plants.4 Richardson and Hall also showed that accessions of Poncirus trifoliata and x Citroncirus spp. (hybrids of P. trifoliata and Citrus spp) were more resistant to ACP than Citrus macrophylla Wester (Alemow).5 Richardson and Hall concluded that P. trifoliata may have antixenosis and antibiosis resistance to ACP.5 Similarly, the presence of the antifeedant compounds (limonoids) in Severinia buxifolia may also make it less attractive to insects.6
Field observation and green house controlled studies also showed that some citrus varieties are more tolerant to CLas pathogen than others.7,8 Based on intensity of symptoms and plant growth, the tested citrus genotypes were divided into 3 groups (sensitive, moderately tolerant, and tolerant).7 The sensitive genotypes showed severe leaf chlorosis, reduced growth, and death. The moderately tolerant plants showed mild symptoms and the tolerant varieties showed little or no symptoms. Based on severity of symptoms caused by CLas, Sagaram and Burns also classified inoculated plants into 3 different groups: mild, moderate, and severe.8
Although field and lab studies showed that some citrus varieties were more tolerant to CLas pathogen than others, so far we do not know what plant characteristics makes some citrus varieties more sensitive to CLas than others. Answering this question is necessary for the understanding of the mechanisms of pathogenicity of CLas and developing effective disease management strategies.7 Some of CLas-tolerant cultivars like P. trifoliata are also known for their tolerance to citrus tristeza virus (CTV).9 However, the mechanism of their resistance to CTV is still unknown.9 Because many antibacterial compounds were found in fruits and seeds of P. trifoliata, it is suggested that the phloem of CLas-tolerant cultivars may contain similar antimicrobial compounds.10,11,12
Volatile compounds, otherwise known as essential oils, are produced in oil glands in the peels, leaves, and other parts of the citrus plant.13 It has been shown that biotic and abiotic stress can stimulate plant volatiles.14 Upon infection, plants produce volatiles that possess an antimicrobial activity to inhibit pathogens' mobility within tissues.13,15 Volatile compounds (citral, citronellal, and linalool) released from wounded rough lemon leaves significantly inhibited hyphal and spore germination growth of Alternaria alternata.16 In addition, terpene down-regulation in orange fruits induced fruits' tolerance against Penicillium digitatum and Xanthomonas citri subsp. citri.17 Fruits from the transgenic plants were also less attractive to citrus pest medfly (Ceratitis capitata).17 Alteration of green leaf volatile biosynthesis in Arabidopsis improved its resistance against both herbivores and pathogens.18 The increase in the expression of hydroperoxide lyase (HPL) in Arabidopsis was attributed to the significant increase Z-3-hexenal.18
Our previous research showed that the levels undecanal, citronellal, β-phellandrene and d-limonene were induced in CLas-infected Valencia plants.19 On the other hand, the levels of δ-3-carene, neral, geranial, α-phellandrene, and α-terpinolene slightly decreased. In addition, 17 VOCs were induced in Valencia plants infested with ACP. Fourteen out of the 17 detected compounds were monoterpenes and the rest were sesquiterpenes. These finding indicated that citrus plant volatiles might play an important role against insects and pathogens attack.
In this study, we investigated the leaf volatile composition of 14 different citrus varieties in order to examine the relative distribution of these varieties to each other and to check whether this distribution is related to their resistance to CLas.
Material and methods
Plants materials and plants growing conditions
The fourteen citrus varieties used in this study are listed in Table 1. Some of these varieties were previously determined to be tolerant or moderately-tolerant to CLas whereas others are sensitive.7 Plants (12 months old, 0.75 to 1 m height) were kept in a greenhouse with the temperature controlled at 28–32°C. Plants were watered twice a week and fertilized once a week using 20-10-20 fertilizer (Allentown, PA).
Table 1.
Citrus plants used in this study.
| Citrus variety | Symptoms severity* |
|---|---|
| Valencia sweet orange (C. sinensis (L.) Osbeck) | Sensitive |
| Hamlin sweet orange (C. sinensis (L.) Osbeck) | Sensitive |
| Madam Vinous sweet orange (C. sinensis (L.) Osbeck) | Sensitive |
| Duncan grapefruit (C. paradisi MacFadyen) | Sensitive |
| Ruby red grapefruit (C. paradisi MacFadyen) | Sensitive |
| Sour orange (C. aurantium L.) | Moderately tolerant |
| Volkamer lemon (C. limonia Osbeck ‘Volkameriana’) | Moderately tolerant |
| Alemow (C. macrophylla Wester) | Moderately tolerant |
| Palestine Sweet lime (C. aurantifolia (Christm.) | Moderately tolerant |
| Mexican lime (C. aurantifolia (Christm.) | Moderately tolerant |
| Carrizo citrange (X Citroncirus webberi) | Tolerant |
| Severinia buxifolia (Poiret) Ten. | Tolerant |
| Poncirus trifoliata (L.) Raf. | Tolerant |
| Citrus latipes | Tolerant |
Folimonova et al. (2009)
Sample collection and volatile organic compound extraction from citrus leaves
Three leaves were collected from each tree from 3 different locations (top, middle, and bottom) and at least 5 trees were sampled from each variety. Collected leaves from each tree were pooled together and ground to a fine powder with liquid nitrogen. A100 mg of the powder was transferred to a 1.5 ml micro-centrifuge tube. A 0.5-ml aliquot of n-hexane was added and the sample was vortexed for 30 sec. Samples were left on ice for 10 min and the vortexing step was repeated 2 more times. At the end of the extraction, samples were removed from the ice and centrifuged at 12,000 rpm for 1 min. A 0.2-ml aliquot of the organic layer was spiked with the internal standard (trans, trans-2,4-nonadienal) at a final concentration of 200 ppm.19
GC-MS analysis
Citrus leaf volatiles were analyzed using a Clarus 500 GC-MS system (Perkin Elmer) fitted with an HP-5MS column (crosslinked 5% Ph Me siloxane, 30 m × 0.25 mm × 0.025 µm film thickness). The flow rate for the helium carrier gas was 0.7 ml/min. The following GC temperature program was used: initial oven temperature was 50°C held for 3 min, then increased to 200°C at a rate of 5°C/min, increased further to 250°C at 10°C/min, and finally held at 250°C for 2 min. The injector and the detector temperatures were 250°C and 180°C, respectively. A 1 μl of the spiked sample extract was injected in splitless mode into the GC-MS which was running in the full scan mode (40–450 m/z). A1 μl of the non-spiked sample extract was also injected into the GC-MS to make sure no compounds elute at the same retention time of the internal standard (LRI: 1243). Each sample was injected twice into the GC-MS and the average of the 2 injections was used in the statistical analysis.
Peak identification and quantification
GC-MS chromatograms were analyzed using TurboMass software version 5.4.2 (Perkin Elmer). Peak identifications were achieved using NIST (Natl. Inst. of Standards and Technology) and Wiley 9th edition (John Wiley and Sons, Inc..,) mass spectra database libraries and linear retention index (LRI). The LRI values were calculated using a calibration curve generated by injecting a mixture of alkanes (C8–C18) under the previously described conditions. The identity of some compound was also confirmed by comparing their LRI and mass spectra with those of authentic standards. To fairly compare the relative amount (abundance) of each compound in different varieties, the peak area of each compound was normalized by dividing it by the peak area of the internal standard and multiplying it by 100 to make a larger number. The percentages were calculated by dividing the peak area of each compound by the total area and multiplying it by 100.
Statistical analyses
Statistical analyses were performed using JMP version 9.0 (SAS Institute Inc.,). Data were normally distributed. The statistical analysis between the different varieties was performed using analysis of variance (ANOVA), followed by Post hoc pairwise comparisons using the Tukey honestly significant difference test (HSD). Differences between varieties were considered significant if P values were lower than 0.05. Principle component analysis (PCA) and cluster analysis (CA) were performed using normalized data (Abundance) of individual volatiles and main groups as well as the percentages of individual volatiles and main groups. Additionally, the biplot were generated using the singular value decomposition (SVD). Hierarchical cluster analysis (HCA), based on Ward's method (with 95% confidence) between different varieties, was also used to construct the similarity dendrograms.
Result
Volatiles content as chemotaxonomy for citrus varieties
Forty-six different compounds were detected and identified in the n-hexane extract of citrus leaves. The abundances of these compounds after normalizing to the internal standard are shown in Table 2 and the percentages (proportions) are shown in Table 3. Most of the detected compounds belonged to 3 main groups (monoterpenes, sesquiterpenes, and aliphatic aldehydes). In addition, few coumarin compounds were detected in S. buxifolia.
Table 2.
Abundance (1 abundance unit is equal to 10 µg g−1 fresh weight) of volatile organic compounds in different citrus varieties.
| Compound | LRI HP-5MS | Hamlin sweet orange | Madam vinou sweet orange | Carrizo citrange | Valencia sweet orange | Ruby redgrapefruit | Duncan grapefruit | Volkamer lemon | Mexican lime | Palestine sweet lime | Sour orange | Poncirus trifoliata | Citrus latipes | Alemow | Severinia buxifolia |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (E)-2-hexenal | 836 | 1.2 ± 0.8a | nd | 2.2 ± 2.2a | 7.0 ± 12.8a | 2.7 ± 2.5a | nd | 10.3 ± 3.5a | 7.3 ± 8.7a | 9.3 ± 3.9a | 0.2 ± 0.3a | 8.8 ± 7.02a | 0.9 ± 1.6a | 2.1 ± 3.0a | 0.4 ± 0.4a |
| α-thujene | 911 | 0.2 ± 0.1a | nd | nd | nd | 0.4 ± 0.2a | nd | nd | nd | nd | nd | nd | nd | 0.9 ± 1.1a | nd |
| α-pinene | 921 | 2.4 ± 1.0b | 2.6 ± 1.5b | nd | 3.1 ± 0.8b | 1.9 ± 0.4b | 0.5 ± 0.2b | 0.7 ± 0.2b | nd | 1.0 ± 0.2 | 0.1 ± 0.1b | nd | 15.3 ± 15.0a | 2.6 ± 2.9b | nd |
| sabinene | 967 | 72.2 ± 10.9a | 45.5 ± 26.1ab | nd | 76.3 ± 21.4a | 27.4 ± 8.0bc | 18.2 ± 9.9bc | 18.8 ± 5.4bc | nd | nd | 0.3 ± 0.1c | nd | 8.1 ± 7.3c | 0.4 ± 0.5c | nd |
| β-pinene | 974 | 2.00 ± 0.6a | 1.4 ± 1.4a | nd | 2.5 ± 0.8a | 1.6 ± 0.2a | 1.1 ± 0.5a | nd | nd | nd | 2.0 ± 0.4a | nd | nd | 1.6 ± 2.0a | nd |
| β -myrcene | 983 | 6.1 ± 1.6ab | 11.3 ± 5.2ab | 16.6 ± 6.3ab | 10.5 ± 4.0ab | 7.0 ± 1.4ab | 1.3 ± 0.9b | 3.5 ± 0.7b | 0.7 ± 0.5b | 6.3 ± 1.2ab | 11.8 ± 4.9ab | 11.8 ± 10.2ab | 21.4 ± 11.6a | 3.9 ± 3.1b | nd |
| α-phelandrene | 1005 | 0.5 ± 0.2b | nd | nd | 1.0 ± 0.2b | nd | nd | nd | nd | nd | nd | nd | 60.6 ± 27.7a | nd | nd |
| 3-carene | 1008 | 15.2 ± 3.0bc | 30.2 ± 14.1ab | 30.1 ± 9.6abc | 37.7 ± 10.0a | nd | nd | nd | nd | nd | 0.2 ± 0.1c | nd | nd | 0.1 ± 0.1c | nd |
| d-limonene | 1033 | 5.2 ± 2.1b | 461.6 ± 168ab | 820.4 ± 452.6a | 16.4 ± 8.8 | 11.1 ± 1.0b | 6.6 ± 5.3b | 171.6 ± 44.5ab | 51.8 ± 25.9ab | 392.0 ± 42.4ab | nd | 0.5 ± 0.3b | 196.4 ± 25.1ab | 170.6 ± 65.3ab | 22.5 ± 10.6b |
| β-phellandrene | 1042 | nd | 5.1 ± 2.3b | nd | 0.4 ± 0.3b | nd | nd | nd | nd | nd | nd | nd | 210.9 ± 22.6a | 1.8 ± 0.8b | nd |
| ocimene | 1050 | 8.4 ± 2.2b | 15.5 ± 9.8ab | 55.4 ± 2.4a | 20.9 ± 8.7ab | 6.7 ± 1.1b | 1.3 ± 1.2b | 12.3 ± 2.9ab | 3.3 ± 2.1b | 8.5 ± 1.3b | 4.4 ± 1.2b | 1.1 ± 0.9b | 6.5 ± 3.4b | 5.1 ± 1.6b | nd |
| α-terpinene | 1066 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 20.0 ± 14.6 | nd |
| t-sabinene H2O | 1084 | 1.4 ± 0.5ab | 2.4 ± 2.2a | nd | 0.8 ± 0.6ab | 1.3 ± 0.0ab | 1.5 ± 1.3ab | 0.3 ± 0.1ab | nd | nd | nd | nd | nd | 0.1 ± 0.1b | nd |
| δ-terpinolene | 1098 | 1.9 ± 0.5b | 3.0 ± 2.0b | 2.8 ± 0.3b | 4.3 ± 1.6b | nd | nd | nd | nd | nd | nd | nd | 42.8 ± 21.4a | 0.6 ± 0.4b | nd |
| linalool | 1114 | 21.7 ± 5.7c | 37.1 ± 26.6c | 3.5 ± 0.0c | 30.2 ± 12.1c | 12.1 ± 0.4c | 17.4 ± 18.3c | 11.0 ± 3.1c | 0.7 ± 0.6c | 106.3 ± 9.3ab | 91.6 ± 15.2b | nd | 149.6 ± 58.4a | 8.1 ± 2.9c | 2.5 ± 1.2c |
| citronellal | 1172 | 1.0 ± 0.4d | 31.0 ± 9.7bcd | 9.0 ± 1.1d | 33.5 ± 10.0bcd | 50.2 ± 3.0bc | 13.6 ± 5.8cd | 64.8 ± 16.7b | 2.9 ± 1.3d | 272.6 ± 41.9a | 0.1 ± 0.1d | nd | 17.5 ± 10.4cd | 70.2 ± 24.7b | nd |
| (E,Z)-2,4-nonadienal | 1222 | 6.1 ± 2.8ab | 3.2 ± 1.0b | nd | 5.0 ± 1.7ab | 10.5 ± 3.5a | 4.1 ± 0.6ab | 5.4 ± 1.0ab | 2.7 ± 0.7b | 4.5 ± 1.9ab | 4.7 ± 0.5ab | 2.8 ± 1.0b | 9.9 ± 5.1a | 4.4 ± 2.3ab | 4.1 ± 3.6ab |
| fenchol | 1224 | 0.3 ± 0.3b | 2.6 ± 0.9ab | nd | 1.2 ± 0.8b | nd | nd | nd | nd | 2.4 ± 1.8ab | nd | nd | 4.2 ± 3.2a | 0.2 ± 0.1b | nd |
| undecanal | 1230 | 0.2 ± 0.1b | 1.7 ± 2.2b | nd | 0.5 ± 0.1b | 0.5 ± 0.3b | 0.1 ± 0.0b | 1.0 ± 0.4b | 0.2 ± 0.2b | 0.5 ± 0.1b | 0.4 ± 0.3b | nd | 374.2 ± 88.0a | 0.7 ± 0.1b | 3.3 ± 1.0b |
| neral | 1268 | 5.2 ± 1.3d | 80.4 ± 33.5bc | nd | 22.5 ± 14.7cd | 6.1 ± 2.7d | 5.6 ± 3.3 | nd | 80.1 ± 30.0bc | 2.2 ± 0.3d | 0.1 ± 0.2d | nd | 447.1 ± 63.4a | 91.2 ± 44.1b | nd |
| geranial | 1299 | 7.0 ± 1.8c | 96.0 ± 35.7ab | nd | 28.6 ± 20.6bc | 3.2 ± 1.2c | 10.7 ± 5.9c | nd | 159.0 ± 77.0a | nd | nd | nd | nd | 131.5 ± 70.1a | nd |
| δ-elemene | 1365 | nd | nd | 29.0 ± 12.0b | nd | nd | nd | 2.3 ± 0.6b | 4.5 ± 2.7b | nd | nd | 3.7 ± 3.6b | 152.1 ± 60.5a | 1.6 ± 0.7b | 22.2 ± 19.4b |
| citronellyl acetate | 1375 | nd | nd | nd | nd | nd | nd | 0.7 ± 0.1b | 1.7 ± 2.3b | 3.6 ± 0.4a | nd | nd | nd | 0.3 ± 0.1b | nd |
| neryl acetate | 1385 | nd | nd | nd | nd | nd | nd | 2.6 ± 0.2ab | 8.7 ± 8.8a | 0.5 ± 0.2b | 3.1 ± 0.5ab | nd | 1.0 ± 0.6b | 1.3 ± 0.6b | 0.3 ± 0.3b |
| geranyl acetate | 1404 | nd | nd | 48.7 ± 36.2abc | nd | nd | nd | 0.4 ± 0.1c | 62.6 ± 53.2ab | 1.8 ± 0.3c | 4.0 ± 0.8c | nd | 68.8 ± 27.2a | 5.0 ± 1.7bc | nd |
| β-elemene | 1420 | 20.7 ± 1.8ab | 2.6 ± 0.7b | 226.9 ± 152.9a | 39.2 ± 19.0ab | 12.5 ± 1.3ab | 2.1 ± 1.3ab | 0.3 ± 0.1b | 9.3 ± 0.6ab | nd | 0.2 ± 0.1b | 9.2 ± 11.9ab | 2.2 ± 0.7 | 1.0 ± 0.4ab | 2.6 ± 1.8 |
| α-santalene | 1450 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 458.6 ± 333.1 |
| t-caryophyllene | 1453 | 5.5 ± 0.9a | 5.3 ± 2.4a | 472.3 ± 305.8a | 11.3 ± 2.8a | 8.8 ± 2.3a | 0.9 ± 0.5a | 13.5 ± 2.9a | 42.3 ± 15.4a | 12.6 ± 1.8a | 7.3 ± 1.5a | 69.4 ± 56.5a | 40.0 ± 9.4a | 9.2 ± 6.7a | 251.6 ± 199.2a |
| γ-elemen | 1458 | nd | nd | 228.6 ± 140.5a | nd | nd | nd | 0.3 ± 0.0b | 4.6 ± 2.1b | nd | nd | 22.1 ± 18.5ab | nd | 0.8 ± 0.4b | nd |
| t-α-bergamotene | 1460 | nd | nd | 6.7 ± 3.3b | nd | nd | nd | 2.0 ± 0.5b | 2.7 ± 1.1b | 4.0 ± 0.6b | nd | 0.2 ± 0.2b | nd | 3.8 ± 3.1b | 14.6 ± 11.4a |
| t-β-farnesene | 1474 | 2.5 ± 0.4ab | 0.7 ± 0.4b | 47.2 ± 31.1a | 3.2 ± 2.4ab | 3.6 ± 1.1ab | 0.9 ± 0.5b | nd | 0.5 ± 0.7b | nd | 0.2 ± 0.1b | nd | nd | 0.1 ± 0.1b | nd |
| epi-β-santalene | 1475 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 45.9 ± 35.2 |
| β-santalene | 1487 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 69.3 ± 50.5 |
| α-humulene | 1487 | 1.1 ± 0.2ab | 0.2 ± 0.1b | 28.2 ± 17.7a | 2.8 ± 1.2ab | nd | nd | 0.4 ± 0.1ab | 7.0 ± 9.2ab | 0.6 ± 0.2ab | 0.3 ± 0.0ab | 2.2 ± 1.9ab | 3.4 ± 0.3ab | 1.4 ± 0.6ab | nd |
| germacrene D | 1510 | 0.8 ± 0.2b | nd | 147.9 ± 99.2a | nd | nd | nd | 0.4 ± 0.1b | 3.1 ± 1.7ab | 0.5 ± 0.1b | nd | 13.9 ± 11.5ab | 11.8 ± 0.4ab | 2.2 ± 1.0ab | 0.5 ± 0.4b |
| α-farnesene | 1521 | nd | nd | nd | 1.0 ± 0.8b | nd | nd | nd | 22.6 ± 7.3a | nd | 1.7 ± 0.3b | nd | nd | nd | nd |
| bicyclogermacrene | 1524 | nd | nd | 8.4 ± 3.5b | nd | nd | nd | 0.5 ± 0.1b | 0.5 ± 0.5b | nd | 1.7 ± 0.3b | 0.5 ± 0.3b | 83.3 ± 32.4a | 3.9 ± 2.1 | 91.3 ± 63.9a |
| β-bisabolene | 1528 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 4.2 ± 2.0a | 6.2 ± 4.9a |
| sesquiterpenol 1 | 1598 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 7.0 ± 1.0 | nd | nd |
| coumarin 1 | 1624 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 2.7 ± 0.9 |
| sesquiterpenol 2 | 1669 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 195.1 ± 168.9 |
| sesquiterpenol 3 | 1679 | 2.3 ± 0.3b | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 38.7 ± 30.3a |
| β-sinensal | 1691 | 0.9 ± 0.4b | 2.3 ± 0.9b | 5.2 ± 3.1ab | 6.4 ± 6.6ab | nd | nd | 3.1 ± 0.7b | 11.2 ± 0.6a | 5.4 ± 0.6ab | nd | nd | nd | nd | nd |
| coumarin 2 | 1703 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 25.0 ± 24.2 |
| seselin | 1898 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 69.4 ± 54.3 |
| coumarin 3 | 1911 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 17.4 ± 17.7 |
Means in the same line with the same letters are not significantly different from each other (Tukey test, p < 0.05). nd: not detected and tr: < 0.01.
Bolded compound were identified by LRI and mass spectra of authentic standards; non-bolded compounds were tentatively identified by LRI and mass spectrum.
Coumarin 1: 7-Methoxy-8-(2-formyl-2-methylpropyl) coumarin.
Coumarin 2: 7-Methoxy-8-(2-oxo-3-methylbutyl) coumarin.
Coumarin 3: coumarin-6-ol, 3,4-dihydro-7,8-dimethoxy-4,4,5-trimethyl-
Table 3.
Percentages of volatile ornagic compounds in different citrus varieties.
| Compound | LRI HP-5MS | Hamlin sweet orange | Madam vinous sweet orange | Carrizo citrange | Valencia sweet orange | Ruby red grapefruit | Duncan grapefruit | Volkamer lemon | Mexican lime | Palestine sweet lime | Sour orange | Poncirus trifoliata | Citrus latipes | Alemow | Severinia buxifolia |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (E)-2-hexenal | 836 | 0.6 ± 0.3ab | nd | 0.6 ± 0.6ab | 2.3 ± 4.4ab | 1.6 ± 1.4ab | nd | 3.2 ± 0.9ab | 2.1 ± 2.9ab | 1.1 ± 0.3ab | 0.1 ± 0.2ab | 6.8 ± 6.7a | 0.1 ± 0.1ab | 0.3 ± 0.4ab | 0.1 ± 0.0ab |
| α-thujene | 911 | 0.1 ± 0.0b | 0.1 ± 0.13b | nd | nd | 0.3 ± 0.1a | nd | nd | nd | nd | nd | nd | nd | 0.1 ± 0.1ab | nd |
| α-pinene | 921 | 1.2 ± 0.3a | 0.3 ± 0.1cde | nd | 0.8 ± 0.1abc | 1.1 ± 0.3ab | 0.5 ± 0.1bcde | 0.2 ± 0.1de | nd | 0.1 ± 0.0de | 0.1 ± 0.1e | nd | 0.7 ± 0.6abcd | 0.4 ± 0.4cde | nd |
| sabinene | 967 | 37.9 ± 2.5a | 5.1 ± 1.1cd | nd | 20.8 ± 4.3b | 15.1 ± 12.4bc | 20.6 ± 2.2b | 5.7 ± 0.4cd | nd | nd | 0.2 ± 0.1d | nd | 0.4 ± 0.3d | 0.1 ± 0.1d | nd |
| β -pinene | 974 | 1.0 ± 0.1bc | 0.2 ± 0.1d | nd | 0.7 ± 0.1c | 1.0 ± 0.2bc | 1.3 ± 0.0ab | nd | nd | nd | 1.5 ± 0.4a | nd | nd | 0.3 ± 0.2d | nd |
| β -myrcene | 983 | 3.1 ± 0.3bc | 1.3 ± 0.1cde | 0.9 ± 0.2cde | 2.8 ± 0.5bcd | 4.3 ± 1.1b | 1.4 ± 0.4bcde | 1.1 ± 0.1cde | 0.1 ± 0.1de | 0.8 ± 0.2cde | 8.5 ± 2.3a | 7.4 ± 2.7a | 1.1 ± 0.4cde | 0.7 ± 0.3cde | nd |
| α-phellandrene | 1005 | 0.2 ± 0.0b | nd | nd | 0.3 ± 0.0b | nd | nd | nd | nd | nd | nd | nd | 3.1 ± 0.9a | nd | nd |
| 3-carene | 1008 | 8.0 ± 0.5b | 3.5 ± 0.3c | 1.8 ± 0.6d | 10.2 ± 0.9a | nd | nd | nd | tr | nd | 0.2 ± 0.1e | nd | nd | 3.5 ± 0.3d | nd |
| d-limonene | 1033 | 2.6 ± 0.6ef | 55.3 ± 2.4a | 40.0 ± 3.1cd | 4.3 ± 1.7ef | 6.9 ± 2.4ef | 8.4 ± 7.8ef | 52.5 ± 0.8ab | 10.8 ± 4.7e | 47.0 ± 1.8bc | nd | 0.3 ± 0.2f | 10.5 ± 1.3e | 31.4 ± 1.9d | 2.7 ± 2.7ef |
| β-phellandrene | 1042 | nd | 0.6 ± 0.1b | nd | 0.1 ± 0.1b | nd | nd | nd | nd | nd | nd | nd | 11.4 ± 2.6a | 0.3 ± 0.1b | nd |
| ocimene | 1050 | 4.4 ± 0.5ab | 1.8 ± 0.5bcdef | 3.3 ± 2.2abcde | 5.7 ± 1.9a | 4.1 ± 0.8abc | 1.7 ± 1.7bcdef | 3.8 ± 0.3abcd | 0.7 ± 0.4def | 1.0 ± 0.1cdef | 3.4 ± 1.3abcde | 0.8 ± 0.5def | 0.3 ± 0.1ef | 1.0 ± 0.2cdef | nd |
| α-terpinene | 1066 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 3.4 ± 1.4a | nd |
| t-sabinene H2O | 1084 | 0.7 ± 0.1bc | 0.3 ± 0.1bc | nd | 0.2 ± 0.2bc | 0.8 ± 0.1b | 1.6 ± 1.0a | 0.1 ± 0.0bc | nd | nd | nd | nd | nd | 0.1 ± 0.0c | nd |
| δ-terpinolene | 1098 | 1.0 ± 0.1b | 0.3 ± 0.1c | 0.2 ± 0.1c | 1.2 ± 0.2b | nd | nd | nd | nd | nd | nd | nd | 2.2 ± 0.7a | 0.1 ± 0.0c | nd |
| linalool | 1114 | 11.2 ± 0.7bcd | 4.1 ± 1.4cd | 0.3 ± 0.2d | 8.1 ± 1.6bcd | 7.4 ± 2.0bcd | 18.1 ± 14.3b | 3.3 ± 0.1cd | 0.1 ± 0.1d | 12.8 ± 0.5bc | 68.3 ± 1.4a | nd | 7.6 ± 1.8bcd | 1.5 ± 0.1cd | 0.3 ± 0.3d |
| citronellal | 1172 | 0.5 ± 0.1e | 3.8 ± 0.5de | 0.7 ± 0.4 | 9.1 ± 2.0cd | 30.3 ± 4.3a | 16.1 ± 5.2b | 19.8 ± 0.3b | 0.6 ± 0.2e | 32.6 ± 2.2a | 0.1 ± 0.0e | nd | 0.9 ± 0.4e | 13.9 ± 5.2bc | nd |
| (E,Z)-2,4-nonadienal | 1222 | 3.2 ± 1.3abc | 0.5 ± 0.3bc | nd | 1.5 ± 0.7bc | 6.2 ± 1.8a | 5.6 ± 2.6a | 1.8 ± 0.7bc | 0.6 ± 0.2bc | 0.5 ± 0.2bc | 3.5 ± 0.5ab | 3.1 ± 2.2abc | 0.5 ± 0.2bc | 1.1 ± 1.1bc | 0.3 ± 0.7bc |
| fenchol | 1224 | 0.2 ± 0.2abc | 0.3 ± 0.0a | nd | 0.3 ± 0.2ab | nd | nd | nd | nd | 0.3 ± 0.2abc | nd | nd | 0.2 ± 0.2abc | 0.0 ± 0.0bc | nd |
| undecanal | 1230 | 0.1 ± 0.0b | 0.2 ± 0.2b | nd | 0.1 ± 0.0b | 0.3 ± 0.3b | 0.1 ± 0.0b | 0.3 ± 0.1b | 0.1 ± 0.0b | 0.1 ± 0.0b | 0.3 ± 0.2b | nd | 19.6 ± 0.8a | 0.1 ± 0.1b | 0.3 ± 0.3b |
| neral | 1268 | 2.7 ± 0.2cd | 9.7 ± 2.1bc | nd | 5.9 ± 2.9cd | 3.7 ± 1.8cd | 8.2 ± 5.9bcd | nd | 16.6 ± 4.5ab | 0.3 ± 0.0d | 0.1 ± 0.2d | nd | 24.6 ± 8.3a | 16.1 ± 2.8ab | nd |
| geranial | 1299 | 3.6 ± 0.2de | 11.6 ± 1.6c | nd | 7.4 ± 4.3cd | 1.9 ± 0.7de | 12.1 ± 1.4c | nd | 31.5 ± 4.4a | nd | nd | nd | nd | 22.8 ± 4.1b | nd |
| δ-elemene | 1365 | nd | nd | 1.4 ± 0.4b | nd | nd | nd | 0.7 ± 0.0b | 0.9 ± 0.2b | nd | nd | 2.2 ± 0.6ab | 5.7 ± 5.0a | 0.3 ± 0.0b | 1.6 ± 0.4b |
| citronellyl acetate | 1375 | nd | nd | nd | nd | nd | nd | 0.2 ± 0.0ab | 0.3 ± 0.3ab | 0.4 ± 0.0a | nd | nd | nd | 0.1 ± 0.0b | nd |
| neryl acetate | 1385 | nd | nd | nd | nd | nd | nd | 0.8 ± 0.1bc | 1.6 ± 1.1ab | 0.1 ± 0.0c | 2.3 ± 0.0a | nd | 0.1 ± 0.0c | 0.2 ± 0.0c | 0.1 ± 0.0c |
| geranyl acetate | 1404 | nd | nd | 1.8 ± 0.6b | nd | nd | nd | 0.1 ± 0.0b | 11.7 ± 6.3a | 0.2 ± 0.0 | 3.0 ± 0.2b | nd | 3.5 ± 0.8b | 0.9 ± 0.1b | nd |
| β-elemene | 1420 | 10.92 ± 1.1ab | 0.3 ± 0.1e | 9.0 ± 1.7abc | 11.4 ± 6.4a | 7.5 ± abcd | 2.4 ± 0.4cde | 0.1 ± 0.0e | 2.1 ± 0.7de | nd | 0.1 ± 0.0e | 4.6 ± 3.2bcde | 0.11 ± 0.0e | 0.2 ± 0.0e | 0.2 ± 0.0e |
| α-santalene | 1450 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 34.6 ± 1.0 |
| t-caryophyllene | 1453 | 2.87 ± 0.1cd | 0.6 ± 0.1d | 20.5 ± 2.1b | 3.1 ± 0.8cd | 5.3 ± 1.3cd | 1.0 ± 0.1d | 4.2 ± 0.2cd | 8.6 ± 1.0c | 1.5 ± 0.2d | 5.4 ± 0.6cd | 48.5 ± 8.2a | 2.1 ± 0.4 | 1.8 ± 1.2cd | 17.3 ± 1.4b |
| γ-elemen | 1458 | nd | nd | 9.6 ± 1.1b | nd | nd | nd | 0.1 ± 0.0c | 0.9 ± 0.1c | nd | nd | 14.9 ± 0.8a | nd | 0.1 ± 0.0c | nd |
| t-α-bergamotene | 1460 | nd | nd | 0.3 ± 0.1cd | nd | nd | nd | 0.6 ± 0.0bc | 0.5 ± 0.1bc | 0.5 ± 0.0bcd | nd | 0.1 ± 0.0d | nd | 0.7 ± 0.5ab | 1.0 ± 0.1a |
| t-β-farnesene | 1474 | 1.3 ± 0.0abc | 0.1 ± 0.0d | 2.0 ± 0.2ab | 0.9 ± 0.7cd | 2.2 ± 1.0a | 1.0 ± 0.1bcd | nd | 0.1 ± 0.1d | nd | 0.1 ± 0.1d | nd | nd | 0.1 ± 0.0d | nd |
| epi-β-santalene | 1475 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 3.2 ± 0.2 |
| β-santalene | 1487 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 5.1 ± 0.1 |
| α-humulene | 1487 | 0.6 ± 0.0abcd | 0.1 ± 0.0d | 1.22 ± 0.1ab | 0.8 ± 0.4abcd | nd | nd | 0.1 ± 0.0cd | 1.2 ± 1.3abc | 0.1 ± 0.0d | 0.2 ± 0.0bcd | 1.4 ± 0.2a | 0.2 ± 0.0bcd | 0.3 ± 0.1bcd | nd |
| germacrene D | 1510 | 0.4 ± 0.0c | nd | 5.88 ± 1.1b | nd | nd | nd | 0.1 ± 0.0c | 0.8 ± 0.7c | 0.1 ± 0.0c | nd | 9.5 ± 1.0a | 0.6 ± 0.1c | 0.4 ± 0.0c | 0.1 ± 0.0c |
| α-farnesene | 1521 | nd | nd | nd | 0.3 ± 0.2b | nd | nd | nd | 5.5 ± 3.9a | nd | 1.3 ± 0.1b | nd | nd | nd | nd |
| bicyclogermacrene | 1524 | nd | nd | 0.39 ± 0.1cd | nd | nd | nd | 0.2 ± 0.0d | 0.1 ± 0.2d | nd | 1.3 ± 0.1c | 0.4 ± 0.1cd | 4.3 ± 1.2b | 0.7 ± 0.2cd | 6.6 ± 0.4a |
| β-bisabolene | 1528 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 0.8 ± 0.1a | 0.4 ± 0.0b |
| sesquiterpenol 1 | 1598 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 0.4 ± 0.1 | nd | nd |
| coumarin 1 | 1624 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 0.2 ± 0.2 |
| sesquiterpenol 2 | 1669 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 13.7 ± 2.7 |
| sesquiterpenol 3 | 1679 | 1.2 ± 0.1b | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 3.5 ± 0.9a |
| β-sinensal | 1691 | 0.5 ± 0.1b | 0.3 ± 0.2b | 0.2 ± 0.0b | 1.9 ± 2.0ab | nd | nd | 1.0 ± 0.0ab | 2.5 ± 1.0a | 0.7 ± 0.1ab | nd | nd | nd | nd | nd |
| coumarin 2 | 1703 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 4.8 ± 2.6 |
| seselin | 1898 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 4.7 ± 0.4 |
| coumarin 3 | 1911 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 0.9 ± 0.4 |
Means in the same line with the same letters are not significantly different from each other (Tukey test, p < 0.05). nd: not detected and tr: < 0.01.
Bolded compound were identified by LRI and mass spectra of authentic standards; non-bolded compounds were tentatively identified by LRI and mass spectrum.
Coumarin 1: 7-Methoxy-8-(2-formyl-2-methylpropyl) coumarin
Coumarin 2: 7-Methoxy-8-(2-oxo-3-methylbutyl) coumarin
Coumarin 3: coumarin-6-ol, 3,4-dihydro-7,8-dimethoxy-4,4,5-trimethyl-
Data presented in Table 2 show that, d-limonene was the most abundant in all varieties. Carrizo citrange had the highest levels of d-limonene (820.4±452.6), followed by Madam vinous sweet orange and Palestine sweet lime (461.6±168 and 392.0±42.4, respectively). Interestingly, the volatile profiles of some citrus varieties that are closely related were similar to each other such as Valencia and Hamlin sweet oranges.
Several compounds were detected only in Severinia buxifolia including α-santalene (458.6 ± 333.1), β-santalene (69.3 ± 50.5), epi-β-santalene (45.9 ± 35.2), coumarin 1 (2.7 ± 0.9), coumarin 2 (25.0 ± 24.2), coumarin 3 (17.4 ± 17.7), seselin (69.4 ± 54.3), and sesquiterpenol 2 (195.1 ± 168.9). Furthermore, Citrus latipes was the highest in neral (447.1 ± 63.4), and undecanal (374.2 ± 88.0), β-phelandrene (210.9 ± 22.6), δ-elemene (152.1 ± 60.5), linalool (149.6 ± 58.4), δ-terpinolene (42.8 ± 21.4) (Table 2).
The results showed that this method could be used to differentiate among citrus varieties. For example, the volatile profile of Palestine sweet lime can be distinguished from that of sour orange by d-limonene. The percentage of d-limonene in Palestine lime was 47.0% while it was absent in sour orange (Table 3). In the same manner, (Z)-citral can also differentiate between Mexican lime (31.5%) and sour orange (0.0%) and Palestine lime (0.0%). Although the volatile profile of the Valencia and Hamlin sweet oranges were very close to each other, the percentage of citronellal in Valencia (9.1%) was higher than that in Hamlin (0.5%) (Table 3). The volatile profile of Ruby red and Duncan grapefruits were also similar. However, the percentages of some volatiles were different between these 2 cultivars. For example, (Z)-citral (12.1%) in Duncan grapefruit was higher than that in Ruby red grapefruit (1.9%) (Table 3).
Principle component and clustering analysis revealed the differences between citrus varieties
The principle component analysis (PCA) using the abundance (Table 2) of individual volatiles is shown in Fig. 1A and the associated biplot is shown in Fig. 1B. Most of the cultivars clustered in one side of the scatter plot except Citrus latipes, and S. buxifolia (Fig. 1A). Palestine sweet lime and P. trifoliata were also slightly separated (Fig. 1A). As shown in Fig. 1A, PC1 and PC2 accounted for 69% of the variation. The biplot obtained via PCA is presented in Fig. 1B. Across the 46 detected volatile compounds, α-santalene, sesquiterepenol 2, seselin, t-caryophellene, d-limonene, undecanal, neral, geranial, citronellal, and linalool were the compounds with the highest absolute scores values in both PC1 and PC2.
Figure 1.
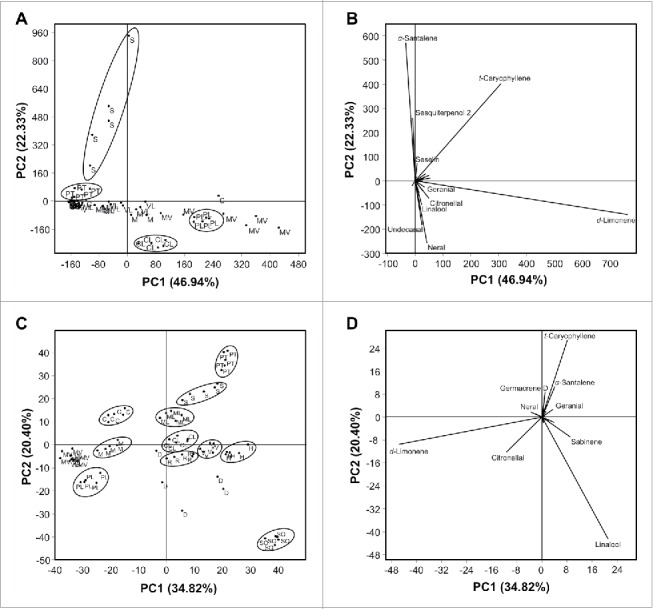
Principal components analysis (PCA) and its associated biplot showing the distribution of different citrus varieties using the abundances (Table 2) and percentages (Table 3) of volatiles in the hexane extract. (n = 5). (A) PCA-scatterplot using the abundances of all volatiles and its PCA-biplot (B), (C) PCA-scatterplot using the percentages of all volatiles and its PCA-biplot (D). Abbreviations: Valencia sweet orange (V), Hamlin sweet orange (H), Madam Vinous sweet orange (MV), Duncan grapefruit (D), Ruby red grapefruit (R), Sour orange (SO), Volkamer lemon (VL), Alemow (M), Palestine sweet lime (PL), Mexican lime (ML), Carrizo citrange (C), Severinia buxifolia (S), Poncirus trifoliata (PT), and Citrus latipes (CL). Some of the volatiles compounds names have been deleted from the biplots (B&D) for better presentation.
The PCA generated using the percentages (Table 3) of the individual volatile compound is shown in Fig. 1C and the associated biplot is shown in Fig. 1D. As shown in Fig. 1C, P. trifoliata, Carrizo citrange, Palestine sweet lime, sour orange, and S. buxifolia were totally separated from the rest of the other cultivars. PC1 and PC2 explained 55.2% of the variation (Fig. 1C). The compounds with the highest loading scores in both PC1 and PC2 were α-santalene, t-caryophellene, d-limonene, neral, geranial, citronellal, sabinene, germacrene and linalool (Fig. 1D)
The PCA was also used to examine the relative distribution of the studied cultivars using the abundance and percentage of volatile compounds categorized into their main groups. As shown in Fig. 2A, Citrus latipes, S. buxifolia, P. trifoliata, and Palestine sweet lime were clearly separated from the rest of the other varieties. PC1 and PC2 accounted for about 91% of the variation (Fig. 2A). The biplot in Fig. 2B presents data of 9 main volatile groups of citrus leaves. Across the 9 analyzed groups, monoterpene hydrocarbon group was positively associated with C. latipes, sesquiterpene hydrocarbons were correlated with S. buxifolia, the sesquiterpene alcohol group was associated with P. trifoliata and monoterpene aldehydes were positively correlated with Palestine sweet lime (Fig. 2B).
Figure 2.
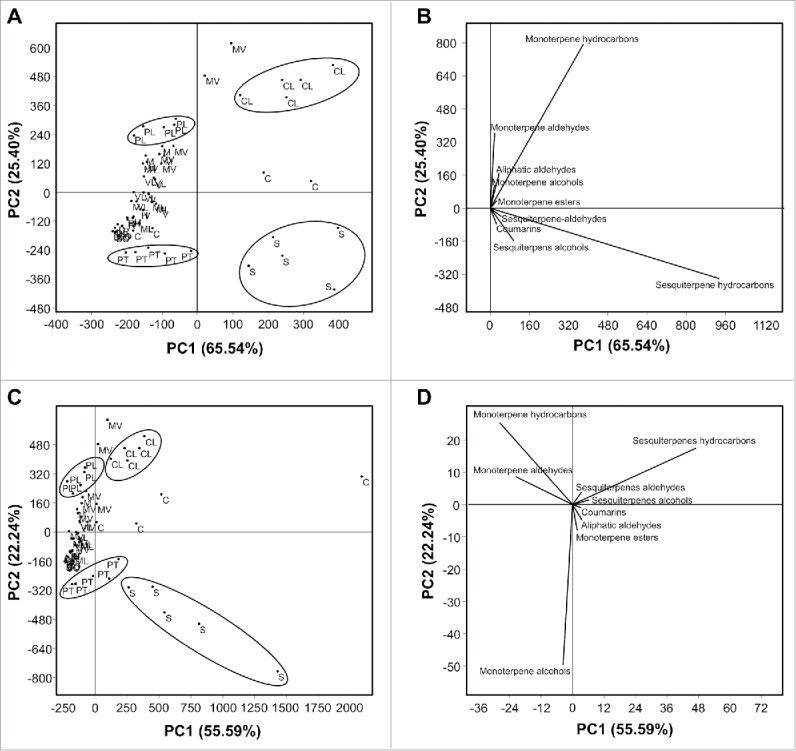
Principal components analysis (PCA) and its associated biplot showing the distribution of different citrus varieties using the abundances and percentages of the main volatile groups in the hexane extract (n = 5). (A) PCA-scatterplot using the abundances of the main volatile groups and its PCA-biplot (B), (C) PCA-scatterplot using the percentages of the main volatile groups and its PCA-biplot (D). Abbreviations: Valencia sweet orange (V), Hamlin sweet orange (H), Madam Vinous sweet orange (MV), Duncan grapefruit (D), Ruby red grapefruit (R), Sour orange (SO), Volkamer lemon (VL), Alemow (M), Palestine sweet lime (PL), Mexican lime (ML), Carrizo citrange (C), Severinia buxifolia (S), Poncirus trifoliata (PT), and Citrus latipes (CL). Some of the volatiles compounds names have been deleted from the biplots (B&D) for better presentation.
The PCA constructed using the percentages of volatile compounds categorized into the main groups also showed that C. latipes, S. buxifolia, P. trifoliata, and Palestine sweet lime were different from the rest of the other varieties (Fig. 2C). As shown in Fig. 2C, PC1 and PC2 accounted for about 78% of the variation. The groups with the highest loading values in both PC1 and PC2 were sesquiterpene hydrocarbons, monoterpene aldehydes, monoterpene alcohols, and monoterpene hydrocarbons.
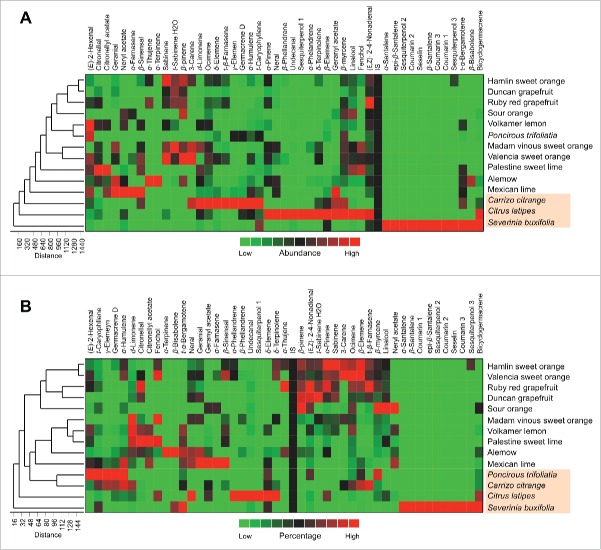
For further characterization of the citrus leaves volatile, the 2-way hierarchical cluster analysis (HCA), followed by the heat-map using the abundance (Table 2) of individual volatiles were performed. The total HCA dendogram showed that the volatile profile of citrus leaf of S. buxifolia, C. latipes, and C. citrange were close to each other (Fig. 3A). In addition, the HCA dendogram showed that the moderately tolerant varieties (Mexican lime, Alemow, and Palestine sweet lime) were closer to the previous varieties than the rest of other varieties (Fig. 3A).
Figure 3.
Hierarchical cluster analysis (HCA) and heat-map using the abundances (A) and percentages (B) of all volatiles of different citrus varieties in the hexane extract (n = 5). Row represents variety and column compound abundance (A ) or percentage in (B). Cells are colored in based on abundances (A) or percentages (B). Red represents high abundances (A) or high percentages (B).
Likewise, the HCA analysis using the percentages (Table 3) of the detected single volatiles also showed that S. buxifolia was closely related to C. latipes and C. citrange was closely related to P. trifoliata (Fig. 3B). Furthermore, the HCA dendogram showed that Mexican lime, Alemow, Palestine sweet lime, and Volkamer lemon (moderately tolerant varieties) were also close to the previous varieties (tolerant varieties). On the other hand, the sensitive varieties (Hamlin sweet orange, Valencia sweet orange, Ruby red grapefruit, and Duncan grapefruit) clustered in the top of dendogram (Fig. 3B).
The HCA results generated using the abundance of the volatile compounds categorized into the main groups also showed that S. buxifolia, C. latipes, and C. citrange close to each others; clustered close to each others at the bottom of dendrogram (Fig. 4A). In addition, HCA obtained using the percentages of the main groups showed that C. latipes, P. trifoliata and C. citrange were close to each other; clustered at the bottom half of the dendrogram (Fig. 4B). Collectively, the PCA and HCA results showed that the citrus leaf volatile profiles of CLas-sensitive varieties were different from those of tolerant varieties.
Figure 4.
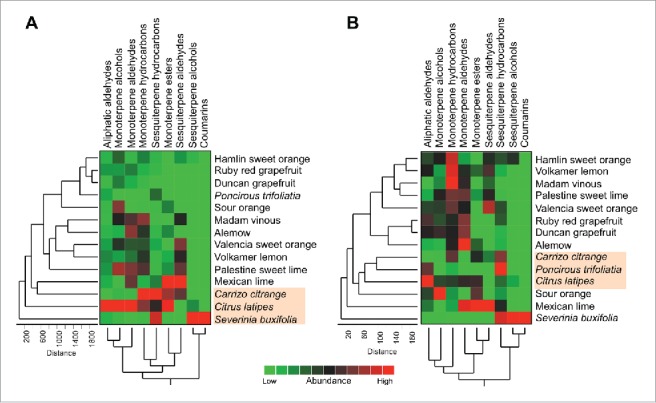
Two-way hierarchical cluster analysis (HCA) and heat-map using the abundances (A) and percentages (B) of the main volatile groups of different citrus varieties in the hexane extract (n = 5). Row represents variety and column compound abundance (A) or percentage (B). Cells are colored in based on abundances (A) or percentages (B). Red represents high abundances (A) or high percentages (B).
Analysis of variance confirmed the multivariate analysis results and the heat map visualized them
Because the PCA and CA showed that the volatile profiles of some of the citrus varieties were different from the rest of the other varieties, the volatile composition of these varieties was examined in more detail using Tukey's test (Table 2 and 3). In addition, the abundance and the percentages of individual volatiles as well as the abundance and the percentages of the main volatile groups were visualized using hierarchically clustered heat maps (Fig. 3 and 4). C. citrange was the highest variety in total volatiles and sesquiterpenes (Fig. 4A and B; Fig. 5A and B). In addition, it was the second highest variety in total monoterpenes (Fig. 5C). With respect to single compounds, C. citrange was the highest cultivar in d-limonene, t-caryophyllene, γ-elemene, β-elemene, and germacrene D (Table 2; Fig. 3A). The percentage of these compounds in Carrizo citrange was also high (Fig. 3B). The actual percentages of these compounds are shown in (Table 3) and they were as follows: d-limonene: 40; t-caryophyllene: 20.6; γ-elemene: 9.6: β-elemene: 9.0; and germacrene D: 5.9%..
Figure 5.
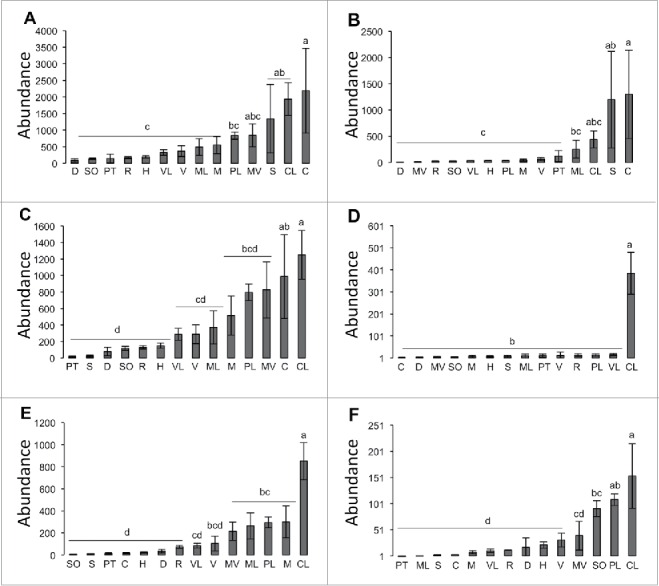
Abundance (1 abundance unit is equal to 10 µg g−1 fresh weight) of total volatiles (A), total sesquiterpenes (B), total monoterpenes (C), total aliphatic aldehydes (D), total aldehydes (E), and total monoterpene alcohols (F), in different citrus varieties in the hexane extract (n = 5). Different letters indicate statistically significantly differences among the studied varieties, while “ns” or the same letter means no differences between varieties. Abbreviations: Valencia sweet orange (V), Hamlin sweet orange (H), Madam Vinous sweet orange (MV), Duncan grapefruit (D), Ruby red grapefruit (R), Sour orange (SO), Volkamer lemon (VL), Alemow (M), Palestine sweet lime (PL), Mexican lime (ML), Carrizo citrange (C), Severinia buxifolia (S), Poncirus trifoliata (PT), and Citrus latipes (CL).
C. latipes was the highest variety in total monoterpenes (Fig. 4A; Fig. 5C), aliphatic aldehydes (Fig. 4A; Fig. 5D), total aldehydes (Fig. 4A; Fig. 5E), and monoterpene alcohols (Fig. 4A; Fig. 5F). In addition, C. latipes was the second highest variety in total volatiles (Fig. 5A) and the third in total sesquiterpenes (Fig. 5B). With respect to single volatiles, C. latipes was the highest cultivar in neral, undecanal, linalool, α- and β-phellandrene and the fourth in d-limonene (Table 2). Neral composed about (24.6%) of the total volatile of C. latipes followed by undecanal (19.5%), β-phellandrene, (11.4%), d-limonene (10.5%), linalool (7.6%) and δ-elemene (5.7%) (Table 3). The heat maps also showed that the abundances and the percentage of these compounds were high in C. latipes (Fig. 3A and B).
S. buxifolia was the third in total volatiles (Fig. 5A) and the second in total sesquiterpenes (Fig. 5B). In addition, S. buxifolia was the only variety that contained high levels of santalene compounds (about 42%), coumarins (10.6%) and sesquiterpene alcohols (17.2%) (Table 3). This variety was the second highest cultivar in t-caryophyllene (Table 2) and this compound accounted for 17.3% of its total volatiles (Table 3). The previous groups in S. buxifolia appeared in red color (Fig. 4A and B) indicating that they were found at high levels.
P. trifoliata was the fifth cultivar in total sesquiterpenes (Fig. 5B). Specifically, P. trifoliata was the second cultivar in germacrene D, γ-elemene, and β-elemene (Table 2). In addition it was the third highest cultivar in t-caryophyllene and myrcene (Table 2). The sesquiterpene (t-caryophyllene) composed about 50% of P. trifoliata total volatiles (Table 3). γ-elemene, germacrene D, myrcene, and β-elemene, were also abundant and their percentages were (14.9%), (9.5%), (7.4%), and (4.5%), respectively (Table 3). The previous compounds in P. trifoliata appeared in black in the heat maps (Fig. 4A and B) which means they were in moderate amounts.
Mexican lime was the fourth highest variety in total sesquiterpenes (Fig. 5B) and total aldehydes (Fig. 5E) and the sixth in total monoterpenes (Fig. 5C). With respect to single volatiles, Mexican lime was the highest variety in geranial, the second in neral andgaranyl acetate, and the fourth in t-caryophllene (Table 2). The monoterpene aldehydes (geranial and neral) accounted for 31.5 and 16.6% of the total volatiles in Mexican lime, respectively (Table 3).The heat maps also shows that Mexican lime was high in these 2 aldehyde compounds (Fig. 3A and B) as well as in total monoterpene aldehydes (Fig. 4A and B) The most predominant volatiles after geranial and neral were geranyl acetate (11.7%), d-limonene (10.8%), t-caryophyllene (8.6%), α-farnesene (5.5%), β-sinensal (2.5%), 2-hexenal (2.1%), and β-elemene (Table 3).
Palestine lime was the second highest in monoterpene alcohols (Fig. 5F), third in aliphatic and total aldehydes (Fig. 5D and E), fourth in total monoterpenes (Fig. 5C), and the fifth in total volatiles (Fig. 5A). With respect to single volatiles, Palestine lime was the highest variety in citronellal, second in linalool, and the third in d-limonene (Table 2). The d-limonene, citronellal, and linalool were the main volatiles in Palestine lime and they composed 47.0, 32.6, and 12.77 % of its total volatiles, respectively (Table 3). The heat map also showed that citronellal (Fig. 3A and B) and total monoterpene aldehydes (Fig. 4A and B) were also high in Palestine lime.
Sour orange was the third highest variety in monoterpene alcohols (Fig. 5F). Regarding single volatiles, sour orange was the third in linalool and β-myrcene (Table 2). Linalool composed 68.3% of the total volatile followed by β-myrcene (8.5%), and t-caryophyllene (5.4%) (Table 3).
Volkamer lemon was the second highest cultivar in aliphatic aldehydes (Fig. 5D) and it contained moderate amounts of the other volatile groups (Fig. 5A-F). With respect to single volatiles, Volkamer lemon was the second highest variety in citronellal (Table 2). Citronellal also appeared in red color in the heat maps (Fig 3A and B) indicating that its level was high in Volkamer lemon. As a percentage, d-limonene composed 52.5%, citronellal 19.8%, sabinene 5.7%, t-caryophyllene 4.5%, and ocimene 3.8% from the total volatiles of Volkamer lemon (Table 3).
Alemow was the second highest variety in total aldehydes (Fig. 5E), fifth highest cultivar in total monoterpenes (Fig. 5C), and it also contained moderate amount of the other volatile groups (Fig. 4A-F). Regarding single volatiles, Alemow was the second highest cultivar in neral, geranial, and citronellal (Table 2). The heat maps (Fig. 3A and B) also showed that Alemow was high in these 2 aldehyde compounds. As percentages, the d-limonene was the main volatile (31.37%), followed by geranial (22.8%), neral (16.0%), citronellal (13.9%), and ocimene (3.4%) (Table 3).
Discussion
Citrus leaf volatiles from different citrus varieties were extracted with n-hexane and directly analyzed using GC-MS.19 This method was found to be fast and reliable and requires only a small amount of plant tissue.19 In addition, because the extraction is performed at low temperature, this decreases the decomposition of the volatile compounds which may occur during distillation.19 Using this method, we were able to study the effect of CLas and ACP attack on the volatile profile of citrus.19Analysis of citrus leaf volatiles by GC-MS is widely used in citrus genetics and breeding studies. In the current study, this method was used to investigate the volatile constituents of leaves from different citrus varieties. Our results showed that this method could be used for chemotaxonomy. It distinguished between closely related cultivars using quantitative and qualitative differences for specific volatiles.
Our findings showed that the total leaf volatile profile varies from one variety to another. In agreement with our results, previous research also showed that total leaf volatile profile varies among citrus species. For example, the total leaf volatiles in Mexican lime was higher than that of lemon (apireno Cantinella), citron (Corsican), sweet orange (Shamouti), and star grapefruit.20,21 In this study, we found that the total amount of volatiles in Mexican lime was higher than 7 of the studied varieties. C. citrange, C. latipes, and S. buxifolia were the highest in total volatiles. Interestingly, these 3 cultivars are known for their tolerance to CLas-infection.7 Our previous results showed that the total abundance of volatile compounds was doubled upon CLas infection.19 the current and previous findings together indicated that the level of volatiles in citrus may play an important role in citrus resistance against plant pathogens.
In addition to the differences in total volatile between cultivars, our results also showed that there were differences in the abundance of the main volatile group among the different varieties. Total monoterpene aldehydes in Mexican lime were higher than those of other cultivars (Corsican, Shamouti, apireno Cantinella, and star grapefruit).21 In agreement with the previous result, the current results showed that Mexican lime was rich in aldehydes. 21
Interestingly, the PCA results showed that volatile profiles of CLas tolerant varieties were different from those of CLas-susceptible varieties which indicated that these volatiles could be implicated in citrus resistance to CLas. Upon infection with plant pathogens, plants can induce production of volatiles organic compounds possessing powerful antimicrobial activity to inhibit the movement of the pathogens within plant tissues.13,15 Our previous research showed that the levels of d-limonene, β-phellandrene, citronellal, and undecanal were induced in CLas-infected Valencia plants.19 On the other hand, the levels of δ-3-carene, neral, geranial, α-phellandrene, and α-terpinolene slightly decreased. The change in the level of these compounds indicated that these compounds may play a role in citrus response to CLas infection. Our current study showed that all of the compounds that were induced in CLas-infected plants were high in one or more of the CLas-tolerant cultivars.19 Surprisingly, neral and geranial which were reduced in CLas-infected plant were also high in some of the tolerant cultivars.19 These compounds could be either depleted as a result of CLas infection or they were reduced because their precursors were consumed by other synthesis pathways, especially those that were upregulated.
Our results showed that most CLas-tolerant varieties were high in aldehyde compounds such as undecanal, neral, geranial, and citronellal. These aldehydes may inhibit the movement of CLas inside the citrus phloem tissues. Aldehydes such as formaldehyde glutaraldehydes, pelargon aldehyde, decanal, and benzaldehyde are known for their antimicrobial activity.15,22 The conjugation of the double bond with the aldehyde group in neral, geranial, and citronellal increases their electronegativity.22 As a result of their high electronegativity, these compounds may interfere with biological processes involving electron transport, and may react with nucleic acids.16 Geranial and neral showed moderate antibacterial activity against several pathogens while citronellal was the only active against some microorganisms. 22 However, these aldehydes were shown to significantly inhibit the spore germination and hyphal growth of Alternaria alternata.16 The alcohol form of citronellal (Citronellol) also had an antibacterial activity against Staphylococcus aureus.23 An increase in the production of β-citronellol and nerol in peel of citrus fruits improved fruit peel resistance against pathogens and insect attack.17 In addition, an increase in green leaf volatiles (Z-3-hexenal) production in transgenic Arabidopsis plant improved its resistance to white butterfly larvae attack and gray mold infection.18
Some monoterpenes such as d-limonene, linalool, myrcene, and α- and β-phellandrene were high in some of the tolerant varieties. This also indicates that these compounds may possess an antimicrobial activity to CLas. The antimicrobial effect of monoterpenes was attributed to the disruption of the lipid layer of the cell membrane.24 This disruption increases cell permeability and can result in leakage of intracellular components.24Linalool was found to have a wide spectrum of activity against many pathogens.22 Linalool has a strong antibacterial effect against periodontopathic and cariogenic bacteria.25 In addition, it has been found that linalool has a fungicidal effect against Alternaria alternata.16 The antimicrobial activity of d-limonene and α-phellandrene have been also reported.15,22
Our results also showed that some sesquiterpenes such as t-caryophyellene, γ-elemene, β-elemene, germacrene D, geranyl acetate were also high in some of the CLas-tolerant cultivars. This result indicated that these compounds may have an antimicrobial effects against CLas. It has also been reported that t-caryophyllene and geranyl acetate were active against many microorganisms.22 Geranyl acetate showed a higher antibacterial activity compared to geraniol.22 The essential oil of Stachys cretica subsp smyrnaea (t-β-caryophyllene: 51.0%; germacrene-D: 32.8%; α-humulene: 3.1%; δ-cadinene: 2.1%; δ-elemene: 2.1%) and pure t-β-caryophyllene exhibited antimicrobial activity against Pseudomonas aeruginosa and Bacillus subtilis.26 Germacrene-D is also known to have strong antibacterial and antifungal activities.27 In addition, β-elemene has shown promising anti-cancer effects against a broad spectrum of tumors.28
It is noteworthy to mention that the volatile profile of the CLas-tolerant cultivar Severinia buxifolia was very different from the rest of the tested cultivars; it contained unique type of chemicals (coumarins, santalenes, and sesquiterpenols) and it was also high in t-caryophyllene. Besides its tolerance to CLas, S. buxifolia is also known for its resistance to nematodes.7,9 Many coumarins and limonoids including were reported in S. buxifolia essential oil.6 The α-santalene and t-β-santalol were also reported in its essential oil.6 These compounds may contribute to S. buxifolia's resistance to insects and pathogens. Limonoids may act as an insecticide, insect antifeedant, and insect growth regulators.29 Limonoids are known for their antibacterial, antifungal, antimalarial, and antiviral properties.29 α- and β-santalols, their mixtures, and their derivatives demonstrated antiviral and antibacterial activity against many viral and bacterial diseases.30
Although the PCA and CA showed that the leaf volatile profiles of CLas-tolernat varieties were different from those of CLas-sensitive varieties, the separation was not clear in few cases. The lack of total separation was expected because the multivariate analysis was only based on the volatile metabolites and not on the total metabolomic profile. Consequently, future studies should focus on the non-volatile metabolites and their possible roles in citrus resistance against CLas. In conclusion, our results showed that the leaf volatile profiles of CLas-tolerant varieties were different from those of sensitive varieties. This finding may indicate a possible role of these volatiles in citrus resistance against CLas. The results of this study may lead to production of transgenic plants with modified volatile profiles that are more tolerant to citrus greening disease.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgment
We thank our lab members for critical reading of the manuscript. This study was supported with a grant for NK received Citrus initiative program, IFAS, University of Florida.
References
- 1.Liu Y, Heying E, Tanumihardjo SA. History, global distribution, and nutritional importance of citrus fruits. Compr Rev Food Sci F 2012; 11(6):530-45; http://dx.doi.org/ 10.1111/j.1541-4337.2012.00201.x [DOI] [Google Scholar]
- 2.Jagoueix S, Bove JM, Garnier M. The phloem-limited bacterium of greening disease of citrus is a member of the alpha subdivision of the Proteobacteria. Int J Syst Bacteriol 1994; 44:379-86; PMID:7520729; http://dx.doi.org/ 10.1099/00207713-44-3-379 [DOI] [PubMed] [Google Scholar]
- 3.Halbert SE, Manjunath KL. Asian citrus psyllids (Sternorrhyncha: Psyllidae) greening disease of citrus: a literature review and assessment of risk in Florida. Fla Entomol 2004; 87(3):330-53; PMID: 7520729; http://dx.doi.org/11142304 10.1653/0015-4040(2004)087%5b0330:ACPSPA%5d2.0.CO;2 [DOI] [Google Scholar]
- 4.Tsai JH, Liu YH. Biology of Diaphorina citri (Homoptera: Psyllidae) on four host plants. J Econ Entomol 2000; 93(6):1721-5; PMID:11142304; http://dx.doi.org/ 10.1603/0022-0493-93.6.1721 [DOI] [PubMed] [Google Scholar]
- 5.Richardson ML, Hall DG. Resistance of Poncirus and citrus x Poncirus germplasm to the Asian citrus psyllid. Crop Sci 2013; 53(1):183-8; http://dx.doi.org/ 10.2135/cropsci2012.02.0091 [DOI] [Google Scholar]
- 6.Scora RW, Ahmed M. The leaf oils of Severinia buxifolia (Poir.) Tenore. J of Essent Oil Res 1994; 6(4):363-7; http://dx.doi.org/ 10.1080/10412905.1994.9698400 [DOI] [Google Scholar]
- 7.Folimonova SY, Robertson C J, Garnsey SM, Gowda S, Dawson WO. Examination of the responses of different genotypes of citrus to huanglongbing (citrus greening) under different conditions. Phytopathology 2009; 99(12):1346-54; PMID:19900000; http://dx.doi.org/ 10.1094/PHYTO-99-12-1346 [DOI] [PubMed] [Google Scholar]
- 8.Sagaram M, Burns JK. Leaf chlorophyll fluorescence parameters and huanglongbing. J Am Soc Hortic Sci 2009; 134(2):194-201 [Google Scholar]
- 9.Albiach-Marti MR, Grosser JW, Gowda S, Mawassi M, Satyanarayana T, Garnsey SM, Dawson WO. Citrus tristeza virus replicates and forms infectious virions in protoplasts of resistant citrus relatives. Mol Breeding 2004; 14(2):117-28; http://dx.doi.org/ 10.1023/B:MOLB.0000038000.51218.a7 [DOI] [Google Scholar]
- 10.Kim DH, Bae EA, Han MJ. Anti-Helicobacter pylori activity of the metabolites of poncirin from Poncirus trifoliata by human intestinal bacteria. Biol Pharm Bull 1999; 22(4):422-4; PMID:10328566; http://dx.doi.org/ 10.1248/bpb.22.422 [DOI] [PubMed] [Google Scholar]
- 11.Rahman A, Al-Reza SM, Yoon JI, Kang SC. In vitro inhibition of foodborne pathogens by volatile oil and organic extracts of Poncirus trifoliata Rafin. seeds. J Food Sci Agric 2009; 89(5):876-81; http://dx.doi.org/ 10.1002/jsfa.3527 [DOI] [Google Scholar]
- 12.Albrecht U, Bowman KD. Tolerance of the trifoliate citrus hybrid US-897 (citrus reticulata Blanco x Poncirus trifoliata L. Raf.) to huanglongbing. Hort Sci 2011; 46(1):16-22 [Google Scholar]
- 13.Maffei ME. Sites of synthesis, biochemistry, and functional role of plant volatiles. J Bot 2010; 76:612-31; http://dx.doi.org/ 10.1016/j.sajb.2010.03.003 [DOI] [Google Scholar]
- 14.Arimura G, Matsui K, Takabayashi J. Chemical and molecular ecology of herbivore-induced plant volatiles: proximate factors and their ultimate functions. Plant Cell Physiol 2009; 50:911-23; PMID:19246460; http://dx.doi.org/ 10.1093/pcp/pcp030 [DOI] [PubMed] [Google Scholar]
- 15.Gao Y, Jin YJ, Li HD, Chen HJ. Volatile organic compounds and their roles in bacteriostasis in five conifer species. J Integr Plant Biol 2005; 47:499-507; http://dx.doi.org/ 10.1111/j.1744-7909.2005.00081.x [DOI] [Google Scholar]
- 16.Yamasaki Y, Kunoh H, Yamamoto H, Akimitsu K. Biological roles of monoterpene volatiles derived from rough lemon (Citrus jambhiri Lush) in citrus defense. J Gen Plant Pathol 2007; 73:168-79; http://dx.doi.org/ 10.1007/s10327-007-0013-0 [DOI] [Google Scholar]
- 17.Rodríguez A, San Andres V, Cervera M, Redondo A, Alquezar B, Shimada T, Gadea J, Jesus Rodrigo M, Zacarias L, Palou L, Lopez M M, Castanera P, Pena L. Terpene down-regulation in orange reveals the role of fruit aromas in mediating interactions with insect herbivores and pathogens. Plant Physiol 2011; 156(2):793-802; PMID:21525333; http://dx.doi.org/17075049 10.1104/pp.111.176545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shiojiri K, Kishimoto K, Ozawa R, Kugimiya S, Urashimo S, Arimura G, Horiuchi J, Nishioka T, Matsui K, Takabayashi J. Changing green leaf volatile biosynthesis in plants: An approach for improving plant resistance against both herbivores and pathogens. Proc Natl Acad Sci U S A 2006; 103(45):16672-6; PMID:17075049; http://dx.doi.org/ 10.1073/pnas.0607780103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hijaz F, El-Shesheny I, Killiny N., Herbivory by the insect Diaphorina citri induces greater change in citrus plant volatile profile than does infection by the bacterium, Candidatus Liberibacter asiaticus. Plant Signal and Behav 2013; 8(10): e25677-e25677; PMID:23857364; http://dx.doi.org/13129309 10.4161/psb.25677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gancel AL, Ollitrault P, Froelicher Y, Tomi F, Jacquemond C, Luro F, Brillouet JM. Leaf volatile compounds of seven citrus somatic tetraploid hybrids sharing willow leaf mandarin (Citrus deliciosa Ten.) as their common parent. J Agric Food Chem 2003; 51(20):6006-13; PMID:13129309; http://dx.doi.org/ 10.1021/jf0345090 [DOI] [PubMed] [Google Scholar]
- 21.Gancel AL, Ollitrault P, Froelicher Y, Tomi F, Jacquemond C, Luro F, Brillouet JM. Leaf volatile compounds of six citrus somatic allotetraploid hybrids originating from various combinations of lime, lemon, citron, sweet orange, and grapefruit. J Agric Food Chem 2005; 53(6):2224-30; PMID:15769160; http://dx.doi.org/ 10.1021/jf048315b [DOI] [PubMed] [Google Scholar]
- 22.Dorman HJD, Deans SG. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J Appl Microbiol 2000; 88(2):308-16; PMID:10736000; http://dx.doi.org/ 10.1046/j.1365-2672.2000.00969.x [DOI] [PubMed] [Google Scholar]
- 23.Griffin SG, Wyllie SG, Markham JL, Leach DN. The role of structure and molecular properties of terpenoids in determining their antimicrobial activity. Flavour Frag J 1999; 14(5):322-32; http://dx.doi.org/ 10.1002/(SICI)1099-1026(199909/10)14:5%3c322::AID-FFJ837%3e3.0.CO;2-4 [DOI] [Google Scholar]
- 24.Trombetta D, Castelli F, Sarpietro MG, Venuti V, Cristani M, Daniele C, Saija A, Mazzanti G, Bisignano G. Mechanisms of antibacterial action of three monoterpenes. Antimicrob Agents Ch 2005; 49(6):2474-8; PMID:15917549; http://dx.doi.org/ 10.1128/AAC.49.6.2474-2478.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park SN, Lim YK, Freire MO, Cho E, Jin DC, Kook JK. Antimicrobial effect of linalool and α-terpineol against periodontopathic and cariogenic bacteria. Anaerobe 2012; 18(3):369-72; PMID:22537719; http://dx.doi.org/ 10.1016/j.anaerobe.2012.04.001 [DOI] [PubMed] [Google Scholar]
- 26.Oeztuerk M, Duru ME, Aydogmus-Oeztuerk F, Harmandar M, Mahhch M, Kolak U, Ulubelen A. GC-MS analysis and antimicrobial activity of essential oil of Stachys cretica subsp smyrnaea. Nat Prod Commun 2009; 4(1):109-14; PMID:19370886 [PubMed] [Google Scholar]
- 27.Sahin F, Gulluc M, Daferera D, Sokmen A, Sokmen M, Polissiou M, Agar G, Ozer H. Biological activities of the essential oils and methanol extract of Origanum vulgare ssp. vulgare in the eastern Anatolia region of Turkey. Food Control 2004; 15:549-57; http://dx.doi.org/ 10.1016/j.foodcont.2003.08.009 [DOI] [Google Scholar]
- 28.Liu J, Zhang Y, Qu J, Xu L, Hou K, Zhang J, Qu X, Liu Y. Beta-Elemene-induced autophagy protects human gastric cancer cells from undergoing apoptosis. BMC Cancer 2011; 11:183; PMID:21595977; http://dx.doi.org/ 10.1186/1471-2407-11-183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roy A, Saraf S. Limonoids: Overview of significant bioactive triterpenes distributed in plants kingdom. Biol Pharm Bull 2006; 29(2):191-201; PMID:16462017; http://dx.doi.org/ 10.1248/bpb.29.191 [DOI] [PubMed] [Google Scholar]
- 30.Xu HX, Zeng FQ, Wan M, Sim KY. Anti- HIV triterpenes acids from Geum japonicum. J of Nat Prod 1996; 59(7):643-45; http://dx.doi.org/ 10.1021/np960165e [DOI] [PubMed] [Google Scholar]