Abstract
It has been reported that salicylic acid (SA) induces both immediate spike and long lasting phases of oxidative burst represented by the generation of reactive oxygen species (ROS) such as superoxide anion radical (O2•−). In general, in the earlier phase of oxidative burst, apoplastic peroxidase are likely involved and in the late phase of the oxidative burst, NADPH oxidase is likely involved. Key signaling events connecting the 2 phases of oxidative burst are calcium channel activation and protein phosphorylation events. To date, the known earliest signaling event in response to exogenously added SA is the cell wall peroxidase-catalyzed generation of O2•− in a hydrogen peroxide (H2O2)-dependent manner. However, this model is incomplete since the source of the initially required H2O2 could not be explained. Based on the recently proposed role for H2O2-independent mechanism for ROS production catalyzed by plant peroxidases (Kimura et al., 2014, Frontiers in Plant Science), we hereby propose a novel model for plant peroxidase-catalyzed oxidative burst fueled by SA.
Keywords: alkalization, auxin, Compound III, oxidative burst, peroxidase, superoxide
Introduction
It has been reported that salicylic acid (SA) induces both immediate and long lasting phases of oxidative burst represented by generation of reactive oxygen species (ROS), chiefly superoxide anion radical (O2•−) and hydrogen peroxide (H2O2).1-3 Early studies have indicated that SA is an active signal accompanying oxidative burst by which development of systemic acquired resistance (SAR) against a wide range of pathogens is induced, as reviewed in several articles by our group.2-6 In 1990s, it has been proposed that SA signaling paths leading to SAR require the accumulation of ROS derived from H2O2,7 based on the observation that SA binds and inhibits 2 types of H2O2-detoxifying enzymes, namely catalase8,9 and ascorbate peroxidase.10 In addition to these passive modes of ROS production by SA, more active modes of SA action involving extracellular peroxidase and NADPH oxidase that directly generates ROS in the presence of SA was reported later.1,11,12 Interestingly, multiple roles for ROS confirmed to date include (i) activation SAR associated with systemic propagation of the oxidative burst, (ii) induction of intracellular signaling pathway such as the further synthesis and release of SA and activation of mitogen-activated protein kinase (MAPK) cascade, (iii) strengthening of cell wall through oxidative cross-linking of glycoproteins, and (iv) direct microbicidal actions.2,7
In general, in the earlier phase of oxidative burst, apoplastic peroxidase are likely involved and in the late phase of the oxidative burst, NADPH oxidase activity is likely involved.13 The key signaling events connecting 2 phases of oxidative burst are calcium channel activation and protein phosphorylation events.6,11,13,14 Real-time detections of both SA-induced ROS generation (especially of O2•−) and SA-induced increase in cytosolic calcium concentration were first performed by our group by using Cypridina luciferin analog (CLA)-treated and aequorin-expressing model plant cells (tobacco BY-2 cells).11 CLA is an O2•−-specific chemiluminescent probe and aequorin is a calcium responsive luminescence protein. Treatment of tobacco BY-2 cells with SA (sub-mM) resulted in rapid and transient generation of O2•− and in turn, O2•− stimulated the influx of extracellular Ca2+ into the cytosolic space in the tobacco cells in a ROS scavenger-sensitive manner. Here, we need to emphasize that SA-induced extracellular O2•− generation was shown to be catalyzed by apoplastic free and cell wall-bound peroxidases.11,15
To date, known earliest signaling event in response to exogenously added SA is the cell wall peroxidase-catalyzed generation of O2•− in a H2O2-dependent manner.11 Interestingly, peroxidase-mediated O2•− generation and secondarily induced calcium signaling are important events in the early signaling phase of SA-induced rapid stomatal closure in Vicia faba16 and Arabidopsis thaliana.17
Previously proposed formulae for SA-dependent generation of O2•− in plants11,15 and model enzyme system using horseradish peroxidase (HRP),18,19 suggest that the byproducts of peroxidase-catalyzed oxidation of SA is involved in generation of O2•− as follows:
| (1) |
| (2) |
| (3) |
| (4) |
where POX N, CI, and CII stands for native ferric form, Compound I and Compound II of plant peroxidase, respectively. SA• and SA+ are free radical species and the 2-electron oxidized intermediate products derived from substrate SA, respectively. The likely structures of SA• and SA+ were proposed by Gozzo.20 The formal oxidation states of the heme within the peroxidase enzyme are indicated by numbers in the small brackets. As above, SA is an e− donating substrate while H2O2 is viewed as the e− acceptor. Then, phenoxy radical species derived from SA (shown as SA•) released thereafter may react with molecular oxygen to form O2•−. Since O2•− is readily transformed into H2O2 in biological systems, a single cycle of SA-oxidizing peroxidase reactions initiated by single unit of H2O2 results in yield of 2 units of O2•− which is equivalent to 2 units of H2O2, and therefore, by this way, ROS could be amplified.1,11 Electron spin resonance spectroscopic analysis has shown that production of SA• occurs in SA-treated plant cells and reaction mixture of purified HRP.15 Since this mechanism requires the initial input of low level of H2O2 as one of starters, supplementation of low dose H2O2 reportedly enhances the SA-induced production of O2•− in cell suspension culture11 and model enzyme system.15
However, above model is incomplete since the source of the initially required H2O2 could not be explained. Here, we propose a novel model for plant peroxidase-catalyzed oxidative burst fueled by SA.
H2O2-independent ROS production
When plants are threaten by pathogens or recognized the molecules derived from microorganisms, extracellular space alkalization is often induced, under which pH-dependent extracellular oxidative burst involving peroxidase reportedly proceeds, especially at the site of microbial challenge. However, direct stimulus involved in activation of peroxidase-catalyzed oxidative burst has not been fully understood. We have recently studied a likely role for free ferrous ion (Fe2+) in reduction of ferric native enzyme of HRP (with heme at FeIII) into ferrous enzyme intermediate (FeII) which H2O2-independently produces O2•− via mechanism involving Compound III (FeIII-O2•−), especially under alkaline condition, thus, possibly contributing to the plant mechanism combatting against the microbial invasion.21 This H2O2-independent cycle of enzyme is now referred to as oxygenase-like cycle as molecular oxygen participates and binds to the enzyme. In addition to Fe2+, indole-3-acetic acid (IAA) is an active inducer of O2•− production in H2O2-independent manner1,22-24 possibly involving molecular oxygen25 to form Compound III.26
Based on the views that formation of enzyme-substrate complexes such as [POX-IAA-O2] equivalent to Compound III results in release of O2•−,24 medical application of HRP-labeled antibodies and IAA has been proposed as a novel O2•−-generating system for cancer cell-targeted and controlled cell death induction, by designing the HRP-conjugated immuno-labeling of cancer-related molecules or expression of recombinant HRP in mammalian cells.27-30
Furthermore, we have previously proposed our view that nitric oxide (NO) is also one of candidate chemicals for reducing native plant peroxidase into ferrous intermediate to initiate the oxygenase-like cycle of plant peroxidases.2 As summarized in Figure 1A, after completion of the oxygenase-like cycle, O2•− which could be converted to H2O2 via disproportionation can be released, suggesting that H2O2-dependent cycle of peroxidase reaction can be concomitantly achieved depending on the types and combination of the substrates or chemicals added to the system (Fig. 1B).
Figure 1.

Mechanism of peroxidase-catalyzed ROS production. (A) Hourglass model which summarizes the inter-conversions among active and inactive forms of peroxidases involved in ROS generation. This model emphasizes that 2 distinct cycles are initiated by conversion of native peroxidase with e− acceptor via conventional peroxidase cycle or with e− donor via oxygenase-like cycle. (B) Graph theoretical summary of redox state shifts in plant peroxidase upon treatment with various substrates or chemicals. Each of small graphs is consisted with 5 vertices corresponding to the enzyme intermediates at different redox states, and directed edges (maximally 6 edges allowed). The formal oxidation states of the heme within the enzyme are indicated by numbers in the circles (vertices). Directed edges (arrows) indicate the transitions of the redox states. Red and blue edges represent the steps with and without direct and/or concomitant release of O2•−. Final states of the enzyme following various chemical treatments are highlighted with red circle. Graphs in (B) were made based on the documented knowledge (Kawano, 2003a, 2003b, 2013; Kawano et al., 1998, 2001, 2002a, 2002b, 2004; Kawano and Bouteau, 2013a; Kawano and Muto, 2000; Kimura et al., 2014; Takayama et al., 2012). (C) Expanded model for SA-induced oxidative burst, based on the relay of H2O2-independnet and H2O2-dependent peroxidase actions. Series of reactions were numbered from (1) to (8).
Algebraic and graphical handling of enzyme behaviors
Graphs presented in Figure 1B are summary of redox state shifts in plant peroxidase upon treatment with various substrates or chemicals. These theoretical graph models are based on our previous bio-computational approach for algebraically expressing the cyclic behavior (redox cycling) of HRP among native enzyme and its 2 electron-oxidized and single electron-oxidized intermediates (Compounds I and II) as a cyclic additive group Z3 = {C0, C2, C1} = {C0, 1C2, 2C2} = {0, 2, 1}, and a cyclic multiplicative group Z3* = {C1, C2} = {C1, C21} = {1, 2}, with C2 as the common generator (generalized inputs of e-donating and e-accepting substrates); by viewing that the system is simply consisted of “states” and “transitions.”31
Each of small graphs in Figure 1B is consisted of 5 vertices and directed edges corresponding to the enzyme intermediates and their transitions, respectively. Red and blue edges represent the steps with and without (direct and/or concomitant) release of O2•−, respectively. Final state of the enzyme following various chemical treatments are highlighted with red-colored circles. Chemicals listed are H2O2, typical peroxidase substrates (AH) such as phenolics, Na ditionate (an active reductant with deoxygenation effect), nitric oxide (NO), Fe2+, IAA, and SA.
Additions of Fe2+ to HRP21 and NO to soybean peroxidase (SBP),32 reportedly result in arrest of the enzymes at Compound II suggesting that these enzymes enter the conventional peroxidase cycle after oxygenation cycle. Upon addition of peroxidase substrates such as phenolics or amines, the peroxidase cycle is completed and native enzyme was shown to be regenerated.32 In case of IAA, multiple roles are played by IAA, primarily as an enzyme-reducing agent converting native enzyme to ferrous enzyme, and secondarily as a conventional peroxidase substrate being oxidized by compound I and II.24 Thus, both reaction cycles are completed eventually leaving native enzyme. By analogy to the roles of NO, Fe2+ and IAA, modes of SA actions leading to release of O2•− catalyzed by plant peroxidase must be revised.
Proposed model
Among three potential inducers of O2•− (IAA, SA, Fe2+), only Fe2+ induced an intense and long-lasting peak of CLA-CL in both HRP21 and SBP (unpublished results). These data might be reflecting the facts that both IAA and SA present at excess level behave as suicide substrates by targeting Compound III (Fig. 1A).19,33
SA has affinity for various forms of heme proteins such as native form of catalase8,9 and ascorbate peroxidase,15 Compound II of HRP18 and SBP,32 and Compound III of HRP.19 We can expect that SA may interact with native form of plant peroxidases. In fact, such preliminary data on the SA-dependent stimulation of native HRP21 and SBP32 leading to generation of O2•− have been shown in our previous articles. However, when we have reported such data, it was early for us to clarify our working hypothesis on the mode of SA action in the H2O2-independent peroxidase action leading to generation of ROS.
After publication of recent work by Kimura et al.,21 we examined and confirmed that SA actually induces the generation of O2•− in the presence of model enzymes such as HRP and SBP, even in the absence of H2O2 supplementation. Here, typical data obtained with SBP are shown (Fig. 2). The data presented here simply but clearly supported our view that plant peroxidases catalyze the SA-dependent O2•− generation in H2O2-independent manner. Furthermore, comparison of the actions of SA and IAA at neutral and alkaline condition (Fig. 2) suggests that there is possibility that SA but not IAA contributes to the alkaline-responsive oxidative burst in plant defense mechanisms associated with microbe-associated molecular patterns, by involving the extracellular peroxidases as proposed by the group of Bolwell.34-37
Figure 2.
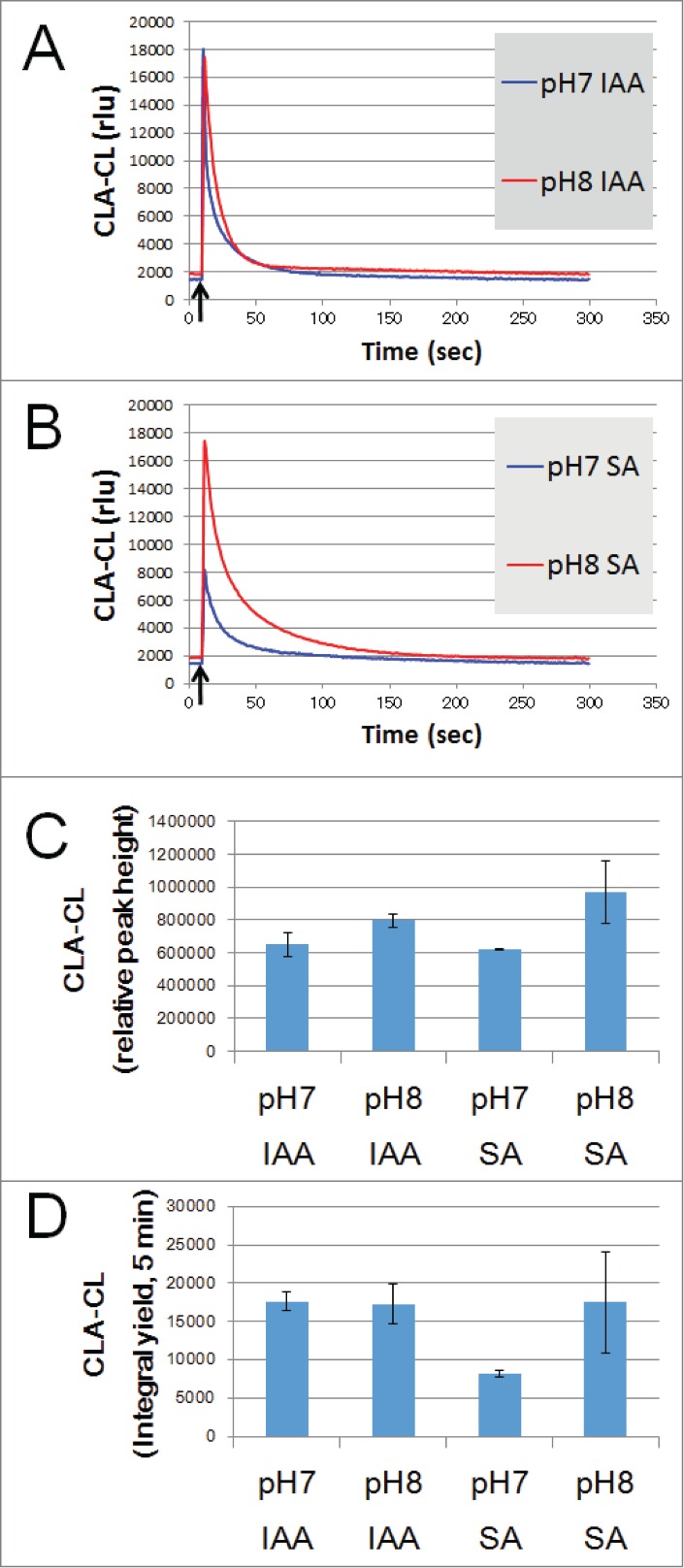
Effects of SA and IAA on O2•− generation in SBP reaction mixture. SA or IAA or water was added to SBP reaction mixture at pH 7.0 and 8.0. Temporal changes in H2O2-independent O2•−-dependent CLA-CL upon addition of IAA (A) or SA (B) are compared. Arrows indicate the timing of chemical additions. Peak height (C) and integral yields (D) of CLA-CL induced by IAA and SA measured at pH 7.0 and 8.0 are compared. Bars, SD (n = 3). Conditions: total volume, 0.2 ml; K-phosphate, 25 mM (pH 7.0 or 8.0); SBP, 1.5 μM; CLA, 10 μM, reducing agents (IAA, SA), 100 μM.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
TK was supported by a grant of Regional Innovation Strategy Support Program implemented by Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan.
References
- 1. Kawano T. Roles of the reactive oxygen species-generating peroxidase reactions in plant defense and growth induction. Plant Cell Rep 2003; 21:829-37; PMID:12789499 [DOI] [PubMed] [Google Scholar]
- 2. Kawano T, Furuichi T, Muto S. Controlled free salicylic acid levels and corresponding signaling mechanisms in plants. Plant Biotechnol 2004; 21:319-35; http://dx.doi.org/ 10.5511/plantbiotechnology.21.319 [DOI] [Google Scholar]
- 3. Kawano T, Hiramatsu T, Bouteau F. Signaling role of salicylic acid in abiotic stress responses in plants. Salicylic Acid. Eds., Hayat S, Ahmad A, Alyemeni MN. Netherland, Dordrecht: Springer; 2013; 249-75. [Google Scholar]
- 4. Kawano T, Furuichi T. Salicylic acid as a defense-related plant hormone: Roles of oxidative and calcium signaling paths in salicylic acid biology. Salicylic Acid - A Plant Hormone. Eds, Hayat S, Ahmad A. Dordrecht, Netherland: Springer; 2007:277-321. [Google Scholar]
- 5. Kawano T, Bouteau F. Salicylic acid-induced local and long-distance signaling models in plants. Long-Distance Systemic Signaling and Communication in Plants. Eds, Baluska F. Berlin Heidelberg: Springer-Verlag; 2013:23-52. [Google Scholar]
- 6. Kawano T, Bouteau F. Crosstalk between intracellular and extracellular salicylic acid signaling events leading to long-distance spread of signals. Plant Cell Rep 2013; 32:1125-38; PMID:23689257; http://dx.doi.org/ 10.1007/s00299-013-1451-0 [DOI] [PubMed] [Google Scholar]
- 7. Chen Z, Silva H, Klessig DF. Active oxygen species in the induction of plant systemic acquired resistance induced by salicylic acid. Science 1993; 262:1883-6; PMID:8266079; http://dx.doi.org/ 10.1126/science.8266079 [DOI] [PubMed] [Google Scholar]
- 8. Chen Z, Ricigliano JR, Klessig DF. Purification and characterization of soluble salicylic acid binding protein from tobacco. Proc Natl Acad Sci USA 1993; 90:9533-7; PMID:8415736; http://dx.doi.org/ 10.1073/pnas.90.20.9533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Durner J, Klessig DF. Salicylic acid is a modulator of tobacco and mammalian catalases. J Biol Chem 1996; 272:28492-501; PMID:8910477; http://dx.doi.org/ 10.1074/jbc.271.45.28492 [DOI] [PubMed] [Google Scholar]
- 10. Durner J, Klessig DF. Inhibition of ascorbate peroxidase by salicylic acid and 2,6-dichloroisonicotinic acid, two inducers of plant defense responses. Proc Natl Acad Sci USA 1995; 92:11312-6; PMID:7479986; http://dx.doi.org/ 10.1073/pnas.92.24.11312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kawano T, Sahashi N, Takahashi K, Uozumi N, Muto S. Salicylic acid induces extracellular superoxide generation followed by an increase in cytosolic calcium ion in tobacco suspension culture: The earliest events in salicylic acid signal transduction. Plant Cell Physiol 1998; 39:721-30; http://dx.doi.org/ 10.1093/oxfordjournals.pcp.a029426 [DOI] [Google Scholar]
- 12. Kunihiro S, Hiramatsu T, Kawano T. Involvement of salicylic acid signal transduction in aluminum-responsive oxidative burst in Arabidopsis thaliana cell suspension culture. Plant Sig Behav 2011; 6:611-6; PMID:21447999; http://dx.doi.org/ 10.4161/psb.6.5.14895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yoshioka H, Bouteau F, Kawano T. Discovery of oxidative burst in the field of plant immunity: looking back at the early pioneering works and towards the future development. Plant Signal Behav 2008; 3:153-5; PMID:19513209; http://dx.doi.org/ 10.4161/psb.3.3.5537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kurusu T, Yagala T, Miyao A, Hirochika H, Kuchitsu K. Identification of a putative voltage-gated Ca2+ channel as a key regulator of elicitor-induced hypersensitive cell death and mitogen-activated protein kinase activation in rice. Plant J 2005; 42:798-809; PMID:15941394; http://dx.doi.org/ 10.1111/j.1365-313X.2005.02415.x [DOI] [PubMed] [Google Scholar]
- 15. Kawano T, Muto S. Mechanism of peroxidase actions for salicylic acid-induced generation of active oxygen species and an increase in cytosolic calcium in tobacco suspension culture. J Exper Bot 2000; 51:685-93; PMID:10938860; http://dx.doi.org/ 10.1093/jexbot/51.345.685 [DOI] [PubMed] [Google Scholar]
- 16. Mori IC, Pinontoan R, Kawano T, Muto S. Involvement of superoxide generation in salicylic acid-induced stomatal closure in Vicia faba. Plant Cell Physiol 2001; 42, 1383-8; PMID:11773531; http://dx.doi.org/ 10.1093/pcp/pce176 [DOI] [PubMed] [Google Scholar]
- 17. Khokon MAR, Okuma E, Hossain MA, Munemasa S, Uraji M, Nakamura Y, Mori IC, Murata Y. Involvement of extracellular oxidative burst in salicylic acid-induced stomatal closure in Arabidopsis. Plant Cell Environ 2011; 34:434-43; PMID:21062318; http://dx.doi.org/ 10.1111/j.1365-3040.2010.02253.x [DOI] [PubMed] [Google Scholar]
- 18. Kawano T, Muto S, Adachi M, Hosoya H, Lapeyrie F. Spectroscopic evidence in support of horseradish peroxidase Compound II-catalyzed oxidation of salicylic acid but not of phenylethylamine. Biosci Biotechnol Biochem 2002; 66:651-4; PMID:12005064; http://dx.doi.org/ 10.1271/bbb.66.651 [DOI] [PubMed] [Google Scholar]
- 19. Kawano T, Muto S, Adachi M, Hosoya H, Lapeyrie F. Spectroscopic evidence that salicylic acid converts a temporal inactive form of horseradish peroxidase (Compound III) to the irreversibly inactivated verdohemoprotein (P-670). Biosci Biotechnol Biochem 2002; 66:646-50; PMID:12005063; http://dx.doi.org/ 10.1271/bbb.66.646 [DOI] [PubMed] [Google Scholar]
- 20. Gozzo F. Systemic acquired resistance in crop protection: from nature to a chemical approach. J Agric Food Chem 2003; 51:4487-503; PMID:14705870; http://dx.doi.org/ 10.1021/jf030025s [DOI] [PubMed] [Google Scholar]
- 21. Kimura M, Umemoto Y, Kawano T. Hydrogen peroxide-independent generation of superoxide by plant peroxidase: Hypotheses and supportive data employing ferrous ion as a model stimulus. Frontiers in Plant Science 2014; 5:article 285; PMID:25071789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gazaryan IG, Lagrimini LM, Ashby GA, Thorneley NF. Mechanism of indole-3-acetic acid oxidation by plant peroxidases: anaerobic stopped-flow spectrophotometric studies on horseradish and tobacco peroxidases. Biochem J 1996; 313:841-7; PMID:8611164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gazarian IG, Lagrimini LM. Anaerobic stopped-flow studies of indole-3-acetic acid oxidation by dioxygen catalyzed by horseradish peroxidase C and anionic tobacco peroxidase at neutral pH: catalase effect. Biophys Chem 1998; 72:231-7; PMID:17029711; http://dx.doi.org/ 10.1016/S0301-4622(98)00098-2 [DOI] [PubMed] [Google Scholar]
- 24. Kawano T, Kawano N, Hosoya H, Lapeyrie F. Fungal auxin antagonist hypaphorine competitively inhibits indole-3-acetic acid-dependent superoxide generation by horseradish peroxidase. Biochem Biophys Res Commun 2001; 288:546-51; PMID:11676477; http://dx.doi.org/ 10.1006/bbrc.2001.5800 [DOI] [PubMed] [Google Scholar]
- 25. Savitsky PA, Gazaryan IG, Tishkov VI, Lagrimini LM, RuzGas T, Gorton L. Oxidation of indole-3-acetic acid by dioxygen catalyzed by plant peroxidases: specificity for the enzyme structure. Biochem J 1999; 340:579-83; PMID:10359640; http://dx.doi.org/ 10.1042/0264-6021:3400579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Smith AM, Morrison WL, Milham PJ. Oxidation of indole-3-acetic acid by peroxidase: involvement of reduced peroxidase and compound III with superoxide as a product. Biochemistry 1982; 21:4414-9; PMID:6289882; http://dx.doi.org/ 10.1021/bi00261a034 [DOI] [PubMed] [Google Scholar]
- 27. Folkes LK, Wardman P. Oxidative activation of indole-3-acetic to cytotoxic species apotential new role for plant auxins in cancer therapy. Biochem Pharmacol 2001; 61:129-36; PMID:11163327; http://dx.doi.org/ 10.1016/S0006-2952(00)00498-6 [DOI] [PubMed] [Google Scholar]
- 28. Folkes LK, Greco O, Dachs GU, Stratfoed MRL, Wardman P. 5-Fluoroindole-3-acetic acid:a prodrug activated by a peroxidase with potential for use in targeted cancer therapy. Biochem Pharmacol 2002; 63:265-72; PMID:11841802; http://dx.doi.org/ 10.1016/S0006-2952(01)00868-1 [DOI] [PubMed] [Google Scholar]
- 29. Kawano T. Possible use of indole-3-acetic acid and its antagonist tryptophan betaine in controlled killing of horseradish peroxidase-labeled human cells. Med Hypoth 2003; 60:664-6; PMID:12710900; http://dx.doi.org/ 10.1016/S0306-9877(03)00012-4 [DOI] [PubMed] [Google Scholar]
- 30. Dai M, Liu J, Chen D-E, Rao D-E, Tang Z-J, Ho W-Z, Dong C-Y. Tumor-targeted gene therapy using Adv-AFP-HRPC/IAA prodrug system suppresses growth of hepatoma xenografted in mice. Cancer Gene Therapy 2012; 19:77-83; PMID:21959967; http://dx.doi.org/ 10.1038/cgt.2011.65 [DOI] [PubMed] [Google Scholar]
- 31. Kawano T. Biomolecule-assisted natural computing approaches for simple polynomial algebra over fields. ICIC Express Lett 2013; 7:2023-8 [Google Scholar]
- 32. Takayama A, Kadono T, Kawano T. Heme redox cycling in soybean peroxidase: hypothetical model and supportive data. Sens Mater 2012; 24:87-97 [Google Scholar]
- 33. Kawano T, Kawano N, Lapeyrie F. A fungal auxin antagonist, hypaphorine prevents the indole-3-acetic acid-dependent irreversible inactivation of horseradish peroxidase: inhibition of Compound III-mediated formation of P-670. Biochem Biophys Res Commun 2002; 294, 553-9; PMID:12056802; http://dx.doi.org/ 10.1016/S0006-291X(02)00513-2 [DOI] [PubMed] [Google Scholar]
- 34. Bolwell GP, Davies DR, Gerrish C, Auh CK, Murphy TM. Comparative biochemistry of the oxidative burst produced by rose and French bean cells reveals two distinct mechanisms. Plant Physiol 1998; 116, 1370-85; PMID:9536055; http://dx.doi.org/ 10.1104/pp.116.4.1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bolwell GP, Bindschedler LV, Blee KA, Butt VS, Davies DR, Gardner SL, Gerrish C, Minibayeva F. The apoplastic oxidative burst in response to biotic stress in plants: a three-component system. J Exper Bot 2002; 53:1367-76; PMID:11997382; http://dx.doi.org/ 10.1093/jexbot/53.372.1367 [DOI] [PubMed] [Google Scholar]
- 36. O'Brien JA, Daudi A, Finch P, Butt VS, Whitelegge JP, Souda P, Ausubel FM, Bolwell GP. A peroxidase-dependent apoplastic oxidative burst in cultured Arabidopsis cells functions in MAMP-elicited defense. Plant Physiol 2012; 158:2013-27; PMID:22319074; http://dx.doi.org/ 10.1104/pp.111.190140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. O'Brien JA, Daudi A, Butt VS, Bolwell GP. Reactive oxygen species and their role in plant defence and cell wall metabolism. Planta 2012; 236:765-79; PMID:22767200; http://dx.doi.org/ 10.1007/s00425-012-1696-9 [DOI] [PubMed] [Google Scholar]


