Abstract
The earliest symptom of glomerular injury in patients with sickle cell disease (SCD) is microalbuminuria. The effect of hydroxyurea (HU) on urine albumin-to-creatinine ratio (ACR) is unclear and should be determined, because increasing numbers of patients with SCD take this drug to improve red blood cell function. In this cohort study of 58 SS-homozygous adults with SCD who initiated HU therapy, we evaluated ACR changes and relationships of these changes with demographic, clinical, and biologic parameters at HU initiation (baseline) and 6 months later (follow-up). Between baseline and follow-up, ACR declined significantly for the entire population (3.0–1.7 mg/mmol; P<0.01), but this was primarily driven by the ACR reduction in the microalbuminuria subgroup (8.1–2.3 mg/mmol; P=0.03; n=23). According to bivariate analyses on 39 patients who did not receive a blood transfusion during the study period, the baseline to follow-up ACR decline was strongly associated with decreases in levels of hemolysis markers, percentage of dense red blood cells, and systolic BP. Bivariate analysis also revealed a close association between the ACR decrease and high baseline levels of hemolysis markers and percentage of dense red blood cells. These results show that urine ACR decreased significantly after 6 months of HU and confirm a close relationship between ACR and hemolysis evolution in patients with SCD.
Keywords: albuminuria, sickle cell disease, hydroxyurea
Sickle cell nephropathy (SCN), a major mortality risk factor among patients with sickle cell disease (SCD), is now a well described renal entity with specific risk factors and clinical manifestations.1–3 Glomerular involvement, one of the most prominent renal manifestations observed in these patients, is characterized by an early increase of the GFR associated with micro- or macroalbuminuria, subsequently leading to progressive GFR decline and chronic renal failure.4–6 Reported results showed an increased frequency of ESRD in the SCD population.7,8 The albuminuria level is currently considered a relevant biomarker to detect glomerular damage in patients with SCD.1 The results of two large studies showed that albuminuria was abnormal in 20.7% and 68% of juvenile and adult patients with SCD, respectively.5,6 As observed during diabetic nephropathy, proteinuria begins as microalbuminuria and progresses to overt macroalbuminuria and nephrotic syndrome, which reflect the worsening of glomerular damage.5,6 A broad spectrum of glomerular diseases has been described in patients with SCD, with elevated albuminuria, isolated glomerular enlargement, and FSGS being the most frequent lesions.9 The underlying pathophysiologic mechanisms involved in SCD-associated glomerulopathy remain poorly understood. Endothelial dysfunction related to chronic hemolysis and the relative renal hypoxia caused by vaso–occlusive sickle red blood cells (RBCs) are probably two key factors contributing to SCN development.10,11
The optimal therapeutic management of adults with SCD and proteinuria associated or not with CKD remains challenging and undetermined.12 Usual renal–protective measures, including renin-angiotensin system inhibitors (angiotensin–converting enzyme [ACE] inhibitor and angiotensin II receptor blocker [ARB]), probably slow renal disease progression in patients with SCD, which is observed in diabetic or nondiabetic proteinuric kidney diseases. Nevertheless, to date, no randomized, prospective clinical trials have clearly shown this hypothesis. Falk et al.13 first showed, in 10 patients, that 2 weeks of enalapril was associated with a 57% proteinuria-level decrease that rebounded after treatment withdrawal. Those preliminary results were confirmed by a 6-month controlled study on 22 patients14 and suggested that using an ACE inhibitor might be appropriate to reduce proteinuria. However, in SCD, the long–term ACE inhibitor effect and its tolerance remain unknown. Hydroxyurea (HU) is one of the cornerstones of SCD therapeutic management.15,16 It was previously shown that HU lowered the frequencies of vaso-occlusive crises (VOCs), acute chest syndrome, and need for RBC transfusions and finally, improved patient survival.17,18 Despite those obviously beneficial effects, the potential effect of HU on renal function parameters is still controversial, with contradictory results.19,20
In this cohort study, we sought to determine the potential effects of HU on albuminuria in a cohort of 58 adults with SCD who had not simultaneously received renal–protective renin-angiotensin system inhibitors.
Results
Patients and Hematologic Parameters
Between January of 2011 and December of 2012, among 118 cohort study patients, 61 patients with SCD and SCD homozygous hemoglobinopathy (SS-SCD) fulfilled inclusion criteria; 58 patients were included (24 men and 34 women; mean age, 35±8.67 years old), because 3 of them did not have urine albumin-to-creatinine ratio (ACR) evaluation at follow-up. The main indications of HU prescription for 58 patients included one episode of acute chest syndrome (n=26) and at least three VOCs during the preceding year (n=23); four patients had poorly tolerated low hemoglobin (Hb) levels, and the last five took HU for nonconventional indications (i.e., pulmonary hypertension [n=2], cerebral vasculopathy [n=2], and priapism [n=1]). At HU onset, only four (6.9%) patients were taking antihypertensive agents: calcium-channel blockers (n=2) or a β-blocker (n=2). The median month 6 HU dose was 15 (12–18) mg/kg per day. During the study period, no patients were excluded for HU intolerance. Demographic and biologic data at HU onset (henceforth referred to as baseline) and month 6 (henceforth referred to as follow-up) are summarized in Table 1. Body surface area and body mass index increased significantly during 6 months of observation (P=0.002 for both), whereas systolic and diastolic BPs remained unchanged. For the entire population, 81 VOCs requiring hospitalization occurred during the 6 months before starting HU versus 16 VOCs during the 6 months thereafter (P<0.001). As expected, HU was associated with significantly increased percentage of fetal hemoglobin (HbF), mean corpuscular volume, and mean corpuscular hemoglobin (MCH) levels and significantly decreased white blood cell and platelet counts. Biologic hemolysis parameter values were improved: decreased lactate dehydrogenase (LDH), aspartate aminotransferase (AST), total bilirubin levels, and reticulocyte counts. We also confirmed the percentage of dense red blood cells (%DRBCs) decline in patients with SCD on HU.21 We examined the eGFR change under HU. For the entire population, eGFR did not decrease significantly between baseline and follow-up (P=0.15) (Table 1). The hyperfiltration frequency for these patients was 25.8% (35.3% for women and 16.6% for men).
Table 1.
Comparisons of demographic and biologic parameters between HU onset (baseline) and after 6 months of treatment (follow-up; n=58)
| Parameter (n) | Baseline | Follow-Up | P Value |
|---|---|---|---|
| eGFR (ml/min per 1.73 m2) | 124.5 [112–132] | 120.5 [112–129] | 0.15 |
| Albuminuria (mg/L) | 22 [10–56] | 14 [6–53] | 0.02 |
| ACR (mg/mmol) | 3 [1.3–10.5] | 1.7 [1–7] | <0.01 |
| ACR subgroups | |||
| Normal, <3 mg/mmol (29) | 1.3 [0.9–2.4] | 1.3 [0.4–1.9] | 0.76 |
| Microalbuminuria (23) | 8.1 [4.9–17.8] | 2.3 [1.1–7.1] | 0.03 |
| Macroalbuminuria (6) | 72 [37–119] | 36 [22–75] | 0.15 |
| Body mass index | 21.2 [19.2–22.9] | 21.7 [19.6–23.2] | 0.002 |
| Body surface area (m2) | 1.75 [1.65–1.83] | 1.75 [1.65–1.86] | 0.002 |
| Systolic BP (mmHg) | 114 [103–122] | 117 [109–127] | 0.78 |
| Diastolic BP (mmHg) | 70 [64–75] | 70 [60–71] | 0.48 |
| VOCs during the last 6 mo | 81 | 16 | <0.001 |
| White blood cells (×109/L) | 9.1 [8–11.7] | 6.8 [5.2–8.5] | <0.001 |
| Platelets (×109/L) | 366 [271–424] | 272 [210–366] | <0.01 |
| Hb (g/dl) | 8.9 [8–10] | 9.9 [8.9–10.7] | <0.001 |
| Reticulocytes (×109/L) | 262[178–294] | 121 [90–181] | <0.001 |
| Mean corpuscular volume (fl) | 89[83–94] | 106 [96–115] | <0.001 |
| MCHC (g/dl) | 33.5[33–35] | 33 [33–34] | 0.24 |
| MCH (pg) | 30 [28–32] | 35 [33–39] | <0.001 |
| HbF (%) | 5.7 [2.5–8.4] | 14.4 [8.8–23.3] | <0.001 |
| %DRBCs>1.11 | 10.5 [5–20.5] | 6 [2–10.5] | <0.001 |
| Serum creatinine (μmol/L) | 52 [44–64] | 54 [45–65] | 0.31 |
| Urine creatinine (mmol/L) | 7.2 [4.8–8.6] | 6.3 [4.6–9.4] | 0.86 |
| BUN (mmol/L) | 3.1 [2.3–3.8] | 3 [2.4–3.9] | 0.64 |
| LDH (IU/ml) | 408 [316–582] | 344 [261–500] | 0.003 |
| AST (IU/L) | 51 [27–58] | 35 [27–53] | 0.04 |
| Alanine aminotransferase (IU/L) | 25 [15–36] | 25 [16–37] | 0.79 |
| Total bilirubin (µmol/L) | 37 [26–60] | 28 [16–44] | <0.001 |
Values are expressed as medians [interquartile ranges], except for VOCs during the last 6 months, which are expressed as absolute numbers.
HU Effect on Urine ACR
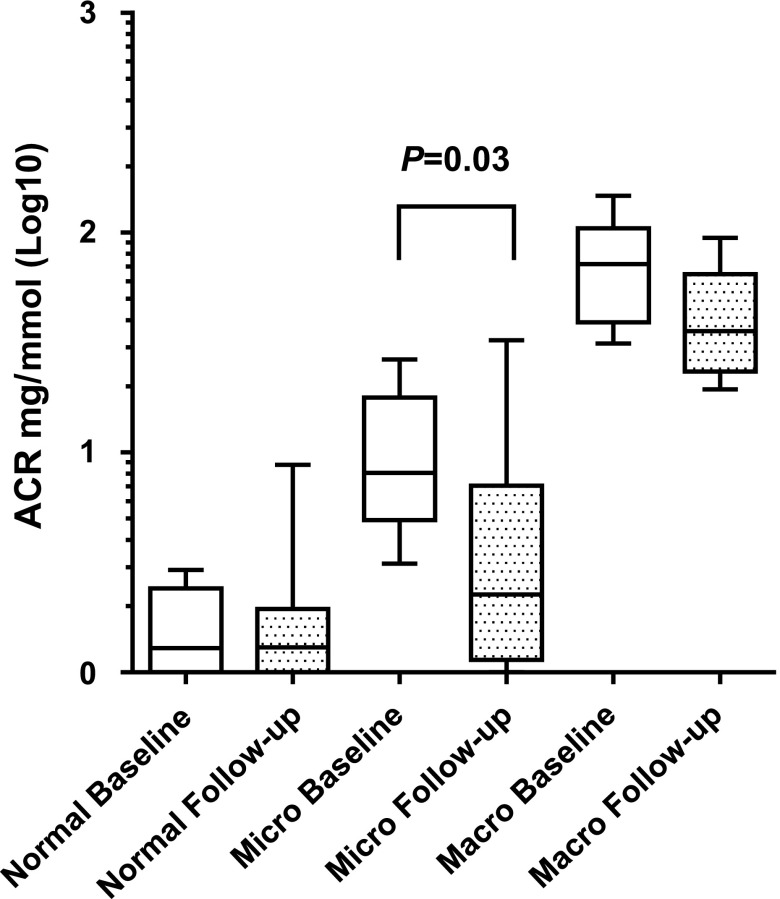
Our analysis of the urine ACR evolution for 58 patients with SCD taking HU showed that it was significantly lower at HU follow-up, with the median ACR declining from 3 (1.3–10.5) mg/mmol at baseline to 1.7 (1–7) mg/mmol at follow-up (P<0.01). This diminution, observed as of month 3 (1.725 [1–9.3] mg/mmol; P<0.01), led us to investigate whether the positive HU effect on albuminuria might differ according to the baseline level (Figure 1). For 29 of 58 (50%) patients with normal baseline ACRs, HU did not lower their ratios; by contrast, patients with abnormal ACR at baseline had significantly lower ACRs at follow-up (n=29; median, 9.34 [6.1–24.8] versus 3.69 [1.3–24.1] mg/mmol, respectively; P=0.01). After additional stratification according to baseline micro- and macroalbuminuria, the positive HU effect on ACR was only evident in 23 of 58 (40%) patients with baseline microalbuminuria: their ACRs declined 72% between baseline and follow-up, and compared with baseline, the median follow-up ACR had returned to within the normal range (2.3 [1.1–7.1] mg/mmol) (Table1). For 6 of 58 (10.3%) patients with SCD and macroalbuminuria, we observed a nearly 50% reduction of the median ACR that did not reach statistical significance (P=0.15). During 6 months, three patients with normal albuminuria (10.3%) progressed to microalbuminuria, and one patient with microalbuminuria (4.3%) progressed to macroalbuminuria.
Figure 1.
Comparisons of ACRs between HU initiation (baseline) and follow-up (month 6; n=58) revealed that HU effect on ACR is primarily driven by the ACR reduction in the microalbuminuria subgroup.
Biologic Parameter Variations Associated with ACR Change in Patients with SCD
We analyzed the data of 39 patients not transfused during the period of study to identify demographic, clinical, and biologic parameters that might be associated with lower ACR at HU follow-up. Bivariate analyses of baseline to follow-up ACR change and laboratory parameter variations are given in Table 2. Between baseline and follow-up, decreases of %DRBCs, mean corpuscular hemoglobin concentration (MCHC), hemolysis markers (bilirubin, LDH, and AST), and systolic BP were correlated with an ACR decline.
Table 2.
Correlation between clinical or biologic parameter variation and ACR change (n=39)
| Parameter | Bivariate Analysis | |
|---|---|---|
| r | P Value | |
| Body mass index | 0.11 | 0.50 |
| Body surface area | 0.08 | 0.60 |
| Systolic BP | 0.51 | 0.02 |
| Diastolic BP | 0.17 | 0.40 |
| VOC variationa | 0.17 | 0.30 |
| White blood cells | 0.29 | 0.08 |
| Mean corpuscular volume | −0.04 | 0.70 |
| MCHC | 0.33 | 0.05 |
| MCH | 0.04 | 0.80 |
| Hb | 0.13 | 0.40 |
| HbF (%) | −0.11 | 0.50 |
| %DRBCs | 0.39 | 0.02 |
| Reticulocytes | 0.13 | 0.40 |
| Platelets | 0.01 | 0.90 |
| eGFR | 0.26 | 0.11 |
| BUN | 0.17 | 0.30 |
| Total bilirubin | 0.31 | 0.05 |
| Alanine aminotransferase | 0.24 | 0.20 |
| AST | 0.38 | 0.02 |
| LDH | 0.43 | <0.01 |
VOC variation: comparison of the numbers of VOCs occurring during the 6 months before starting HU and the 6 months thereafter.
Baseline Parameters Associated with ACR Change under HU
We next conducted bivariate analyses of baseline demographic, clinical, and biologic parameters according to ACR change to determine whether they might be predictive of improved ACR under HU at follow-up (Table 3). Higher baseline MCH, MCHC, %DRBC, and hemolysis marker (bilirubin and LDH) levels were predictive of a significant ACR decrease.
Table 3.
Bivariate analyses of baseline parameters associated with ACR change under HU (n=39)
| Parameter | Bivariate Analysis | |
|---|---|---|
| r | P Value | |
| Age | 0.23 | 0.15 |
| Body mass index | −0.13 | 0.40 |
| Body surface area | 0.06 | 0.70 |
| Systolic BP | 0.4 | 0.06 |
| Diastolic BP | 0.2 | 0.30 |
| VOCs during the last 6 mo | 0.06 | 0.70 |
| White blood cells | 0.29 | 0.08 |
| Mean corpuscular volume | −0.28 | 0.09 |
| MCHC | −0.36 | 0.02 |
| MCH | −0.32 | 0.05 |
| Hb | 0.17 | 0.30 |
| HbF (%) | −0.13 | 0.40 |
| %DRBCs | −0.45 | <0.01 |
| Reticulocytes | −0.25 | 0.13 |
| Platelets | −0.06 | 0.70 |
| eGFR | −0.15 | 0.40 |
| BUN | −0.16 | 0.30 |
| Total bilirubin | −0.37 | 0.02 |
| Alanine aminotransferase | −0.05 | 0.70 |
| AST | −0.24 | 0.13 |
| LDH | −0.37 | 0.02 |
Discussion
Microalbuminuria, associated or not with hyperfiltration, is the earliest renal symptom reflecting glomerular injury in adult and juvenile patients with SCD.4,5 However, the long-term outcome and the natural history of kidney disease in patients with SCD and albuminuria remain largely unknown. It is likely, as classically observed in patients with other proteinuric kidney diseases, including diabetic nephropathy, that, in the absence of specific treatment, albuminuria rises progressively over time. In a retrospective study on 38 patients with albuminuria until the age of 21 years old, 10.5% of them had progressive renal disease after approximately 20 months of follow-up.22 A more recent study showed that, after a mean follow-up of 5 years, the CKD frequency increased to 41.8%, and multivariate analysis retained the importance of baseline albuminuria levels and each 1-mmHg systolic BP increase as two prominent risk factors for subsequent CKD development.23 At present, optimal therapeutic management to prevent albuminuria progression and the progressive eGFR decline in patients with SCD and glomerular involvement remains to be determined.12 Renin-angiotensin system inhibitors and/or HU, the standard of care to prevent VOCs in patients with SCD, should probably be considered to prevent kidney disease progression in this population.12 While awaiting the results of ongoing and future studies evaluating the potential contributions of promising biomarkers of tubular damage (kidney injury molecule -1, neutrophil gelatinase-associated lipocalin, and N-acetyl-β-D glucosaminidase) for early SCN detection, we focused herein on the effect of HU on the ACR value, which is currently considered the most relevant biomarker of early renal injury in patients with SCD.
Still, definitive proof of the potential beneficial HU effect on albuminuria is lacking. In this study, we sought to determine the effect of HU at follow-up on albuminuria (normal and micro- and macroalbuminuria) in 58 adults with SS-SCD. Because nonsteroidal anti–inflammatory drugs may be used to control VOC-related pain, it is noticeable that, in our institution, this therapeutic approach is not part of our standard protocol for VOC treatment, because our previous prospective, controlled trial results showed limited ketoprofen effect on VOCs requiring hospitalization24 and because of the direct effect of those drugs on eGFR in patients with SCD.25 Our results showed that patients with SCD taking HU without concomitant use of renin-angiotensin system inhibitors had significantly reduced urine ACRs at follow-up. By considering patients according to baseline ACR value subgroups, HU significantly affected patients with microalbuminuria. In adults with SS-SCD, the frequencies of micro- and macroalbuminuria were 42% and 26%, respectively.6 Our patients’ microalbuminuric rates were similar to those reported by Guasch et al.6 The approximately 10% lower macroalbuminuria frequency found in our study is probably explained by our inclusion criteria, which excluded patients treated with ACE inhibitors or ARBs. Although these observations were not significant for patients who were macroproteinuric, we cannot definitively exclude that HU might prevent the increase of their albuminuria, because only six patients were concerned. However, our findings suggest a potential benefit, even in this group. The HU effect on ACR has not been extensively investigated in SCD. The retrospective study by Lebensburger et al.26 on children with SCD showed a lower microalbuminuria frequency for HU-treated patients than those not receiving this drug (13% and 24%, respectively), suggesting a potentially beneficial preventive HU effect against glomerular injury. Aygun et al.19 did not find that microalbuminuria significantly changed in 23 children taking HU for 3 years. A recent publication on adults with SCD showed lower albuminuria in patients after ≥3 months on HU compared with patients not taking HU.27 Our results showed that 6 months of HU was associated with significantly decreased urine ACRs, particularly in adult patients with SCD and microalbuminuria. This finding suggests the potential benefit of prescribing HU as soon as microalbuminuria appears.
Our results strongly suggest that 6 months of HU significantly attenuated the microalbuminuria observed during early stages of SCN. A close relationship between hyperfiltration and the subsequent risk of developing microalbuminuria was recently advanced.28 Increased renal blood flow and glomerular filtration are well documented in patients with SCD.29 As observed in diabetic nephropathy, compelling evidence suggests that hyperfiltration probably plays a crucial role in the progressive renal dysfunction seen in older patients with SCD.30–32 Moreover, hyperfiltration is a well known risk factor contributing to secondary FSGS, the most frequent glomerular lesion in patients with SCD.9,33 One limitation of our study is that the definition of hyperfiltration on the basis of eGFR evaluation may be limited by the enhanced tubular secretion of creatinine observed in patients with SCD. Despite an apparent moderate decrease over the study period, no statistically significant evolution of eGFR was found at follow-up. However, given our relatively small sample size, it is possible that our study was underpowered to detect that difference as statistically significant.
The pathophysiologic mechanism involved in this beneficial HU effect remains uncertain. Compelling evidence from clinical studies on adults and children with SCD showed strong associations between biologic hemolysis parameters and increased albuminuria, thereby suggesting that chronic hemolysis–related endothelial dysfunction is probably a key factor in SCN development.4,10,34–36 In accordance with those observations, Saraf et al.37 recently observed, in two large SCD cohorts, that hemoglobinuria was associated with increasing albuminuria and CKD progression. We found that ACR reduction was closely correlated to decreased hemolysis marker (LDH, total bilirubin, and AST) levels between baseline and follow-up, suggesting that the diminished hemolysis rate might be one mechanism by which HU achieves significantly lower albuminuria. We recently showed that the %DRBCs was correlated to the risk of leg ulcers, priapism, renal dysfunction, and hemolysis.21 Notably, 6 months of HU was associated with a 34% DRBC decline, independent of the increased HbF level.21 In that study, bivariate analyses showed that a high baseline %DRBCs >1.11 and the %DRBC decrease at follow-up were significantly correlated with improved urine ACRs. Other than hemolysis, DRBCs have deleterious hemorrheologic properties that could explain the patients’ improved renal laboratory parameters after their decline. A direct action of HU on podocyte dysfunction, widely observed in patients with FSGS, was postulated but remains hypothetical.27 Definitive conclusions concerning the exact mechanism involved in the albuminuria decline under HU remain uncertain. It might result from decreased glomerular filtered load of albumin (related to reduced GFR) or could indicate improved glomerular permeability to macromolecules. HU might also improve renal laboratory parameters by preventing VOCs that are associated with subclinical renal tubular injury.11 Consistent with that hypothesis, we observed significantly more VOCs during the 6 months preceding HU onset than the 6 months thereafter. However, this clinical improvement was not statistically associated with the ACR change.
In conclusion, the results of this cohort study showed a beneficial effect of short-term HU (6 months) on the ACRs of patients with SCD, suggesting that, in the future, its use in SCD could include a renal protection indication. This finding emphasizes the need to start kidney-protective therapy at the early stages of glomerular injury (microalbuminuria) to reduce the risk of progressive renal function deterioration. The observations of previous nonprospective studies suggested that combining HU and an ACE inhibitor might be considered for patients with persistent significant proteinuria taking only the latter.20,38 Optimal preventive management of SCD with microalbuminuria and/or hyperfiltration requires additional larger prospective, randomized, controlled trials to clearly show the positive effect of HU (possibly in association with an ACE inhibitor) to reduce albuminuria level and hence, delay CKD progression.
Concise Methods
Patient Population and Methods
This monocenter cohort study, conducted in the Adult Sickle-Cell Referral Center of Henri Mondor Hospital, was approved by our local Ethics Committee. In our practice, as of HU initiation, all patients are systematically evaluated for efficacy and tolerance, including renal laboratory parameter evaluations every 3 months during the first 6 months and then, every 6 months. All adult patients with SS-SCD (18 years old or older) from our cohort study on HU who underwent eGFR and ACR evaluation at HU onset (baseline) and month 6 (follow-up) were included. All participants gave their signed informed consent to participate in the cohort study in accordance with the Declaration of Helsinki. Eligibility criteria to start HU consisted of at least one episode of acute chest syndrome and/or at least three VOCs during the preceding year and/or poorly tolerated low Hb (<7 g/dl). VOC was defined as an episode of pain or tenderness requiring opioids and not attributable to other causes.
Demographic, clinical, and laboratory data were prospectively assessed for each patient at baseline and follow-up. We did not include patients who were pregnant, had stage 3 CKD defined as eGFR≤60 ml/min per 1.73 m2, or were taking an ACE inhibitor or ARB at HU onset or requiring such antihypertensive medication during the study. Patients who required blood transfusion and/or had ≥10% HbA were excluded from the correlation analysis because of potential laboratory parameter modifications post-transfusion. Patients requiring HU withdrawal during the study period were excluded. For all patients, demographic and clinical data, including sex, age, weight, body mass index, body surface area, systolic and diastolic BPs, and HU dose (milligrams per kilogram per day), were recorded. We compared the numbers of VOCs during 6 months before starting HU and during the study period (VOC variation in Table 2).
Complete blood cell and reticulocyte (expressed as absolute reticulocytes ×109/L) counts were determined with a Coulter LH 750 Counter (Beckman Coulter). Measured parameters included mean corpuscular volume, MCH, MCHC, and Hb levels. HbS and HbF values were determined by cation–exchange high–performance liquid chromatography using a Variant Hb Analyzer (Variant Hemoglobin Testing System; Bio-Rad). RBC density and %DRBCs, defined as having a density >1.11, were determined as previously described.21 Biochemical analyses included determination of LDH, total bilirubin, creatinine, BUN, urine creatinine levels, and liver enzymes (AST and alanine aminotransferase) levels. The urine ACR was expressed as milligrams per millimole creatinine. ACRs of ≤3, 3–30, and >30 mg/mmol creatinine defined normal, microalbuminuria, and macroalbuminuria, respectively.39 ACR determination at baseline and months 3 and 6 (follow-up) was on the basis of a single measurement. We used the Chronic Kidney Disease Epidemiology Collaboration formula without adjustment for ethnicity, which was shown to be the best method to calculate eGFR for patients with SCD: GFR (milliliters per minute per 1.73 m2) =141× min(Scr/k,1)α × max(Scr/k,1)−1.209 ×0.993Age×1.018 (for women), where Scr is serum creatinine, k is 0.7 for women and 0.9 for men, α is −0.329 for women and −0.411 for men, min indicates the minimum of Scr/k or 1, and max indicates the maximum of Scr/k or 1.40 Renal hyperfiltration was defined as eGFR>130 ml/min per 1.73 m2 for women or >140 ml/min per 1.73 m2 for men.4 HU was initially started at a daily dose of 15 mg/kg and adjusted after 1 and 3 months according to clinical benefit and hematologic tolerance, and then, it was maintained stable unless clinical efficacy required additional modification.
Statistical Analyses
Descriptive results are presented as medians (interquartile ranges) for continuous variables or n (%) for categorical parameters. Univariate analyses used Mann–Whitney or Wilcoxon signed–rank tests for comparisons of continuous biologic parameters between baseline and follow-up as appropriate. Spearman correlation coefficients were computed to assess the association between ACR change and collected biologic parameters, considering first their variation between baseline and follow-up and then, their punctual baseline estimates. A two-tailed P<0.05 defined significance. All statistical analyses were computed with using Graphpad or Statview software.
Disclosures
None.
Acknowledgments
We thank Jugurtha Berkenou and Christine Fauroux for data management, Christian Godart and Jean Riou for percentage of dense red blood cells determinations, and Janet Jacobson for editorial assistance.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Ataga KI, Derebail VK, Archer DR: The glomerulopathy of sickle cell disease. Am J Hematol 89: 907–914, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharpe CC, Thein SL: Sickle cell nephropathy - a practical approach. Br J Haematol 155: 287–297, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Platt OS, Brambilla DJ, Rosse WF, Milner PF, Castro O, Steinberg MH, Klug PP: Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med 330: 1639–1644, 1994 [DOI] [PubMed] [Google Scholar]
- 4.Haymann JP, Stankovic K, Levy P, Avellino V, Tharaux PL, Letavernier E, Grateau G, Baud L, Girot R, Lionnet F: Glomerular hyperfiltration in adult sickle cell anemia: A frequent hemolysis associated feature. Clin J Am Soc Nephrol 5: 756–761, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McPherson Yee M, Jabbar SF, Osunkwo I, Clement L, Lane PA, Eckman JR, Guasch A: Chronic kidney disease and albuminuria in children with sickle cell disease. Clin J Am Soc Nephrol 6: 2628–2633, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guasch A, Navarrete J, Nass K, Zayas CF: Glomerular involvement in adults with sickle cell hemoglobinopathies: Prevalence and clinical correlates of progressive renal failure. J Am Soc Nephrol 17: 2228–2235, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Powars DR, Chan LS, Hiti A, Ramicone E, Johnson C: Outcome of sickle cell anemia: A 4-decade observational study of 1056 patients. Medicine (Baltimore) 84: 363–376, 2005 [DOI] [PubMed] [Google Scholar]
- 8.McClellan AC, Luthi JC, Lynch JR, Soucie JM, Kulkarni R, Guasch A, Huff ED, Gilbertson D, McClellan WM, DeBaun MR: High one year mortality in adults with sickle cell disease and end-stage renal disease. Br J Haematol 159: 360–367, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maigne G, Ferlicot S, Galacteros F, Belenfant X, Ulinski T, Niaudet P, Ronco P, Godeau B, Durrbach A, Sahali S, Lang P, Lambotte O, Audard V: Glomerular lesions in patients with sickle cell disease. Medicine (Baltimore) 89: 18–27, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Nath KA, Katusic ZS: Vasculature and kidney complications in sickle cell disease. J Am Soc Nephrol 23: 781–784, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Audard V, Moutereau S, Vandemelebrouck G, Habibi A, Khellaf M, Grimbert P, Levy Y, Loric S, Renaud B, Lang P, Godeau B, Galactéros F, Bartolucci P: First evidence of subclinical renal tubular injury during sickle-cell crisis. Orphanet J Rare Dis 9: 67, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharpe CC, Thein SL: How I treat renal complications in sickle cell disease. Blood 123: 3720–3726, 2014 [DOI] [PubMed] [Google Scholar]
- 13.Falk RJ, Scheinman J, Phillips G, Orringer E, Johnson A, Jennette JC: Prevalence and pathologic features of sickle cell nephropathy and response to inhibition of angiotensin-converting enzyme. N Engl J Med 326: 910–915, 1992 [DOI] [PubMed] [Google Scholar]
- 14.Foucan L, Bourhis V, Bangou J, Mérault L, Etienne-Julan M, Salmi RL: A randomized trial of captopril for microalbuminuria in normotensive adults with sickle cell anemia. Am J Med 104: 339–342, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Bartolucci P, Galactéros F: Clinical management of adult sickle-cell disease. Curr Opin Hematol 19: 149–155, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Platt OS: Hydroxyurea for the treatment of sickle cell anemia. N Engl J Med 358: 1362–1369, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Charache S, Terrin ML, Moore RD, Dover GJ, Barton FB, Eckert SV, McMahon RP, Bonds DR Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia : Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. N Engl J Med 332: 1317–1322, 1995 [DOI] [PubMed] [Google Scholar]
- 18.Steinberg MH, Barton F, Castro O, Pegelow CH, Ballas SK, Kutlar A, Orringer E, Bellevue R, Olivieri N, Eckman J, Varma M, Ramirez G, Adler B, Smith W, Carlos T, Ataga K, DeCastro L, Bigelow C, Saunthararajah Y, Telfer M, Vichinsky E, Claster S, Shurin S, Bridges K, Waclawiw M, Bonds D, Terrin M: Effect of hydroxyurea on mortality and morbidity in adult sickle cell anemia: Risks and benefits up to 9 years of treatment. JAMA 289: 1645–1651, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Aygun B, Mortier NA, Smeltzer MP, Shulkin BL, Hankins JS, Ware RE: Hydroxyurea treatment decreases glomerular hyperfiltration in children with sickle cell anemia. Am J Hematol 88: 116–119, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alvarez O, Miller ST, Wang WC, Luo Z, McCarville MB, Schwartz GJ, Thompson B, Howard T, Iyer RV, Rana SR, Rogers ZR, Sarnaik SA, Thornburg CD, Ware RE BABY HUG Investigators : Effect of hydroxyurea treatment on renal function parameters: Results from the multi-center placebo-controlled BABY HUG clinical trial for infants with sickle cell anemia. Pediatr Blood Cancer 59: 668–674, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartolucci P, Brugnara C, Teixeira-Pinto A, Pissard S, Moradkhani K, Jouault H, Galacteros F: Erythrocyte density in sickle cell syndromes is associated with specific clinical manifestations and hemolysis. Blood 120: 3136–3141, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Alvarez O, Lopez-Mitnik G, Zilleruelo G: Short-term follow-up of patients with sickle cell disease and albuminuria. Pediatr Blood Cancer 50: 1236–1239, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Gosmanova EO, Zaidi S, Wan JY, Adams-Graves PE: Prevalence and progression of chronic kidney disease in adult patients with sickle cell disease. J Investig Med 62: 804–807, 2014 [DOI] [PubMed] [Google Scholar]
- 24.Bartolucci P, El Murr T, Roudot-Thoraval F, Habibi A, Santin A, Renaud B, Noël V, Michel M, Bachir D, Galactéros F, Godeau B: A randomized, controlled clinical trial of ketoprofen for sickle-cell disease vaso-occlusive crises in adults. Blood 114: 3742–3747, 2009 [DOI] [PubMed] [Google Scholar]
- 25.de Jong PE, de Jong-Van Den Berg TW, Sewrajsingh GS, Schouten H, Donker AJ, Statius van Eps LW: The influence of indomethacin on renal haemodynamics in sickle cell anaemia. Clin Sci (Lond) 59: 245–250, 1980 [DOI] [PubMed] [Google Scholar]
- 26.Lebensburger J, Johnson SM, Askenazi DJ, Rozario NL, Howard TH, Hilliard LM: Protective role of hemoglobin and fetal hemoglobin in early kidney disease for children with sickle cell anemia. Am J Hematol 86: 430–432, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Laurin LP, Nachman PH, Desai PC, Ataga KI, Derebail VK: Hydroxyurea is associated with lower prevalence of albuminuria in adults with sickle cell disease. Nephrol Dial Transplant 29: 1211–1218, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vazquez B, Shah B, Zhang X, Lash JP, Gordeuk VR, Saraf SL: Hyperfiltration is associated with the development of microalbuminuria in patients with sickle cell anemia. Am J Hematol 89: 1156–1157, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Becker AM: Sickle cell nephropathy: Challenging the conventional wisdom. Pediatr Nephrol 26: 2099–2109, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Wesson DE: The initiation and progression of sickle cell nephropathy. Kidney Int 61: 2277–2286, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Helal I, Fick-Brosnahan GM, Reed-Gitomer B, Schrier RW: Glomerular hyperfiltration: Definitions, mechanisms and clinical implications. Nat Rev Nephrol 8: 293–300, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Serjeant GR, Serjeant BE, Mason KP, Hambleton IR, Fisher C, Higgs DR: The changing face of homozygous sickle cell disease: 102 patients over 60 years. Int J Lab Hematol 31: 585–596, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Hostetter TH: Hyperfiltration and glomerulosclerosis. Semin Nephrol 23: 194–199, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Day TG, Drasar ER, Fulford T, Sharpe CC, Thein SL: Association between hemolysis and albuminuria in adults with sickle cell anemia. Haematologica 97: 201–205, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gurkan S, Scarponi KJ, Hotchkiss H, Savage B, Drachtman R: Lactate dehydrogenase as a predictor of kidney involvement in patients with sickle cell anemia. Pediatr Nephrol 25: 2123–2127, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Kato GJ, Gladwin MT, Steinberg MH: Deconstructing sickle cell disease: Reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Rev 21: 37–47, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saraf SL, Zhang X, Kanias T, Lash JP, Molokie RE, Oza B, Lai C, Rowe JH, Gowhari M, Hassan J, Desimone J, Machado RF, Gladwin MT, Little JA, Gordeuk VR: Haemoglobinuria is associated with chronic kidney disease and its progression in patients with sickle cell anaemia. Br J Haematol 164: 729–739, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fitzhugh CD, Wigfall DR, Ware RE: Enalapril and hydroxyurea therapy for children with sickle nephropathy. Pediatr Blood Cancer 45: 982–985, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Maier-Redelsperger M, Lévy P, Lionnet F, Stankovic K, Haymann JP, Lefèvre G, Avellino V, Perol JP, Girot R, Elion J: Strong association between a new marker of hemolysis and glomerulopathy in sickle cell anemia. Blood Cells Mol Dis 45: 289–292, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Arlet JB, Ribeil JA, Chatellier G, Eladari D, De Seigneux S, Souberbielle JC, Friedlander G, de Montalembert M, Pouchot J, Prié D, Courbebaisse M: Determination of the best method to estimate glomerular filtration rate from serum creatinine in adult patients with sickle cell disease: A prospective observational cohort study. BMC Nephrol 13: 83, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]