Abstract
Deficiency of the antiaging gene Klotho (KL) induces renal damage and hypertension through unknown mechanisms. In this study, we assessed whether KL regulates expression of CYP11B2, a key rate–limiting enzyme in aldosterone synthesis, in adrenal glands. We found that haplodeficiency of KL(+/−) in mice increased the plasma level of aldosterone by 16 weeks of age, which coincided with spontaneous and persistent elevation of BP. Blockade of aldosterone actions by eplerenone reversed KL deficiency–induced hypertension and attenuated the kidney damage. Protein expression of CYP11B2 was upregulated in adrenal cortex of KL(+/−) mice. KL and CYP11B2 proteins colocalized in adrenal zona glomerulosa cells. Silencing of KL upregulated and overexpression of KL downregulated CYP11B2 expression in human adrenocortical cells. Notably, silencing of KL decreased expression of SF-1, a negative transcription factor of CYP11B2, but increased phosphorylation of ATF2, a positive transcription factor of CYP11B2, which may contribute to upregulation of CYP11B2 expression. Therefore, these results show that KL regulates adrenal CYP11B2 expression. KL deficiency–induced spontaneous hypertension and kidney damage may be partially attributed to the upregulation of CYP11B2 expression and aldosterone synthesis.
Keywords: BP, aldosterone, hypertension, immune complexes, kidney dysfunction, macrophages
Klotho (KL) was identified as an antiaging gene.1 Mutation of this gene resulted in extensive aging phenotypes in mice resembling human aging, including short lifespan and arteriosclerosis.1 Overexpression of KL gene slowed down the aging process and extended lifespan by 20%–30% in mice.1–3 Therefore, KL plays an important role in aging.
The KL gene generates two different transcripts because of alternative RNA splicing at exon 34 (i.e., full–length [130 kD] and short–form [68 kD] KL).5,6 KL protein is primarily expressed in kidney tubule cells and regulates phosphate reabsorption.1,4 However, KL and its function in adrenal glands have never been reported. Adrenal zona glomerulosa cells are the primary source of the circulating aldosterone. CYP11B2 in zona glomerulosa cells is a specific and rate–limiting enzyme for aldosterone synthesis. The relationship of KL and CYP11B2 in adrenals has not been investigated. Aldosterone is a major hormone that regulates Na and fluid balance. Overproduction of aldosterone could impair kidney function and increase BP.7,8 In this study, we investigated if haplodeficiency of KL(+/−) gene affects adrenal CYP11B2 expression and aldosterone synthesis.
It is well documented that the prevalence of hypertension increases with age.9 The prevalence of hypertension is doubled in the elderly versus the young population.9 On the basis of the Seventh Report of the Joint National Committee,10 more than two thirds of individuals after age 65 years old suffer from hypertension; >90% of subjects who are nonhypertensive at 65 years old or older would eventually develop hypertension in their remaining life.11 Therefore, hypertension is an aging-related disorder.12 Unfortunately, the etiology and mechanism of hypertension remain poorly understood. In humans, the serum level of antiaging KL protein declines with age after age 40 years old.13 Our recent studies showed that in vivo KL gene delivery prevented the progression of hypertension in spontaneous hypertensive rats.14 RNAi silencing of brain KL potentiated cold-induced elevation of BP.15 We recently reported that KL deficiency causes hypertension and kidney damage.16 However, the mechanism of KL deficiency–induced hypertension is not fully understood. We hypothesize that haplodeficiency of KL upregulates adrenal CYP11B2 expression and aldosterone synthesis, which contribute to KL deficiency–induced hypertension and kidney damage.
Results
Haplodeficiency of KL Increased Plasma Levels of Aldosterone and Caused Spontaneous Hypertension
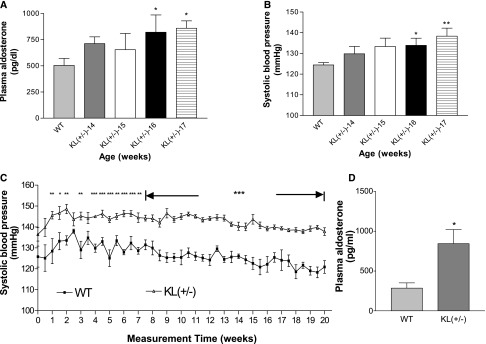
We found that plasma levels of aldosterone were elevated in KL(+/−) mice versus wild-type (WT) mice (Figure 1A). Notably, plasma levels of aldosterone started to increase in KL(+/−) mice by 16 weeks of age (Figure 1A), which concurred with elevation of BP (Figure 1B). Systolic BP was normal (at the WT levels) before 15 weeks of age. BP started to elevate significantly in KL(+/−) mice by 16 weeks of age (Figure 1B). Thus, the increase in BP is age dependent. Systolic BP remained elevated in middle–aged KL(+/−) mice (8–13 months) (Figure 1C). Therefore, KL deficiency caused spontaneous and persistent hypertension.
Figure 1.
Haplodeficiency of KL increased systolic BP and plasma aldosterone levels in KL(+/−) mice. (A) The time course of changes in plasma aldosterone levels in young KL(+/−) and WT mice from ages 14 to 17 weeks old (n=5 mice per group). (B) The time course of changes in systolic BP of young KL(+/−) and WT mice from ages of 14 to 17 weeks (n=5 mice per group). (C) The time course of systolic BP of middle–aged KL(+/−) and age–matched WT mice from ages of 8 to 13 months old (n=14 mice per group). Systolic BP was continuously measured two times per week for 5 months by a computerized VPR tail–cuff method. (D) Plasma aldosterone levels in KL(+/−) and WT mice at age 15 months (n=14 mice per group). Data are means±SEMs. *P<0.05 versus WT; **P<0.01 versus WT; ***P<0.001 versus WT.
Plasma levels of aldosterone remained elevated in KL(+/−) mice by 15 months of age (middle aged) (Figure 1D) when hypertension persisted (Figure 1C). Therefore, we further assessed if increased aldosterone levels are involved KL deficiency–induced hypertension.
Haplodeficiency of KL Increased Adrenal CYP11B2 Expression
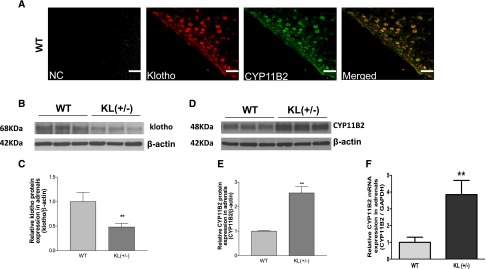
To further explore how KL deficiency increases plasma levels of aldosterone, we assessed the immunofluorescence staining of KL and CYP11B2, an aldosterone synthase, in adrenal zona glomerulosa. It is noticed that both KL (Figure 2A, red) and CYP11B2 (Figure 2A, green) were expressed in zona glomerulosa cells. Although the merged photo (Figure 2A, yellow) shows potential overlap of KL and CYP11B2, additional studies are required to determine whether there is colocalization of these proteins.
Figure 2.
Haplodeficiency of KL increased adrenal CYP11B2 expression in KL(+/−) mice. (A) Representative photomicrographs of dual-immunofluorescence staining of KL (red) and CYP11B2 (green) and confocal analysis of colocalization (yellow) in adrenal zona glomerulosa of WT mice. (B) Representative Western blots of KL protein expression in adrenal glands. (C) Quantitative analysis of KL protein expression. (D) Representative Western blots of CYP11B2 protein expression in adrenal glands. (E) Quantitative analysis of CYP11B2 protein expression. (F) Quantitative real–time RT-PCR analysis of CYP11B2 mRNA expression. These measurements were done when animals were euthanized at age 15 months old. Western blots results were normalized to β-actin and then expressed as fold changes to the WT group. Data are means±SEMs (n=5 mice per group). NC, negative control (without first antibody). Scale bars, 20 µm for black bars; 50 µm for white bars. **P<0.01 versus WT. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Western blot analysis showed that short–form KL protein (approximately 68 KD) was expressed in adrenals and decreased by approximately 50% in KL(+/−) mice (Figure 2, B and C). In contrast, CYP11B2 protein and mRNA expression were increased significantly in adrenals of KL(+/−) mice (Figure 2, D–F). These results showed that KL deficiency may upregulate CYP11B2 expression in adrenals. It is likely that the increase in plasma aldosterone was attributed to the upregulation of CYP11B2.
Blockade of Aldosterone Receptors Attenuated Hypertension in KL(+/−) Mice
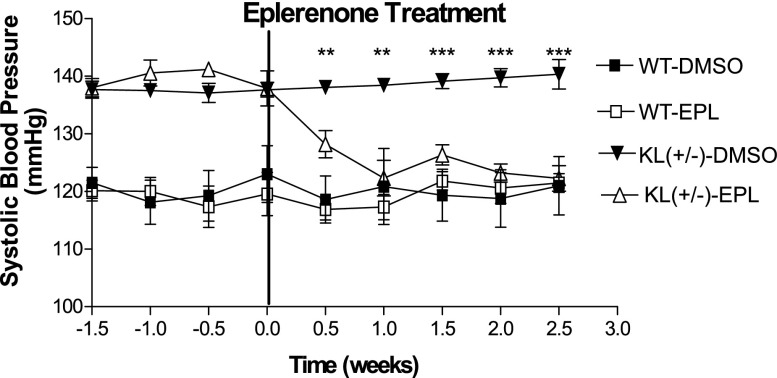
Next, we investigated if the upregulation of plasma aldosterone is involved in KL deficiency–induced hypertension using a specific aldosterone receptor antagonist (eplerenone) (Supplemental Figure 1). Blockade of aldosterone receptors decreased high BP of KL(+/−) mice to the control levels within 1 week of treatment (Figure 3), suggesting that upregulation of plasma levels of aldosterone is involved in KL deficiency–induced hypertension. Eplerenone did not alter BP significantly in WT mice (Figure 3). Body weights were not affected by treatments (data not shown).
Figure 3.
Blockade of aldosterone receptor attenuated the elevation of systolic BP in KL(+/−) mice. After BP was stabilized, two groups of each strain [KL(+/−) and WT; 13 months] were treated with eplerenone (6 mg/kg per day intraperitoneally) and DMSO, respectively, for 3 weeks. BP was measured two times per week. Data are means±SEMs (n=7 mice per group). **P<0.01 versus WT-DMSO; ***P<0.001 versus WT-DMSO. EPL, eplerenone.
Blockade of Aldosterone Receptors Abolished Upregulation of the SGK1-Na, Cl Cotransporter Signaling in Kidneys in KL(+/−) Mice
There was no significant difference in mineralocorticoid receptor (MR) protein expression in kidneys of KL(+/−)-DMSO group and WT-DMSO group (Supplemental Figure 2, A and B). Eplerenone treatment had no effect on MR expression in kidneys in either KL(+/−) or WT mice (Supplemental Figure 2, A and B). Protein expressions of SGK1, Na, Cl cotransporter (NCC), and ATP synthase-β, the sodium reabsorption–related signaling molecules, were increased in the KL(+/−)-DMSO group versus the WT-DMSO group (Supplemental Figure 2, C–H). The upregulation of these signaling proteins in kidneys in KL(+/−) mice was likely caused by increased aldosterone levels, because it can be eliminated by eplerenone (Supplemental Figure 2, C–H). The results also indicate that the increased aldosterone actions were effectively blocked by eplerenone.
Blockade of Aldosterone Receptors Attenuated Renal Structural Damage in KL(+/−) Mice
The periodic acid–Schiff (PAS) staining showed greater glomerular mesangial matrix expansion in KL(+/−)-DMSO mice versus WT-DMSO mice. Blockade of aldosterone receptors significantly attenuated glomerular remodeling in KL(+/−) mice (Supplemental Figure 3, A and B). The number of collapsed glomeruli was increased significantly in KL(+/−)-DMSO mice versus WT-DMSO mice. Eplerenone significantly reduced the glomerular damage in KL(+/−) mice, although it did not eliminate the damage completely (Supplemental Figure 3C). KL deficiency also caused renal tubular damage, including dilation, atrophy, and cast formation, in KL(+/−)-DMSO mice (Supplemental Figure 3A). Eplerenone partially attenuated tubular damages. Tubular cast formation was attenuated significantly, although it was not reversed by eplerenone (Supplemental Figure 2, A and D).
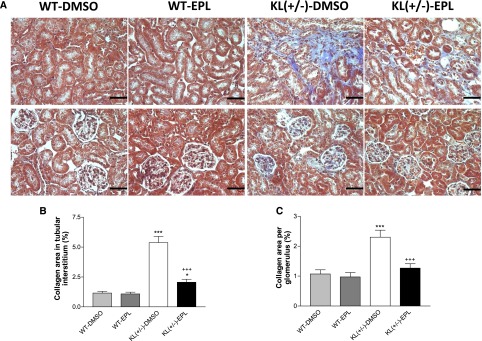
The Masson trichrome staining showed that collagen deposition was increased in renal tubular interstitia and glomeruli in KL(+/−)-DMSO mice versus WT-DMSO mice (Figure 4), indicating that one–half KL deficiency caused kidney remodeling. Blockade of aldosterone receptors significantly alleviated kidney remodeling (Figure 4), although it did not completely reverse tubulointerstitial fibrosis (Figure 4, A and B).
Figure 4.
Blockade of aldosterone receptors attenuated renal structural damage in KL(+/−) mice. (A) Representative photomicrographs of Masson trichrome–stained kidney sections. Tubulointerstitial and glomerular fibrosis (blue staining) were increased in KL(+/−) mice versus in WT mice. Eplerenone alleviated renal remodeling. (B) Semiquantitative analysis of collagen staining in tubular interstitial space. (C) Semiquantitative analysis of collagen staining in glomeruli. Staining was carried out when animals were euthanized at 3 weeks after treatment with eplerenone. Data are means±SEMs (n=5 mice per group). Scale bars, 50 μm. *P<0.05 versus WT-DMSO; ***P<0.001 versus WT-DMSO; +++P<0.001 versus KL(+/−)-DMSO. EPL, eplerenone.
Blockade of Aldosterone Receptors Improved Renal Function in KL(+/−) Mice
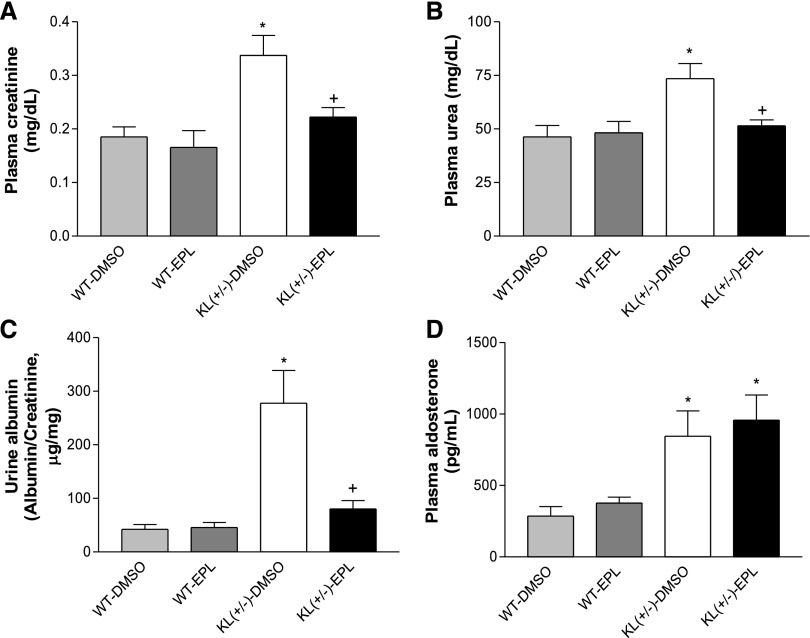
Renal function was significantly impaired in KL(+/−)-DMSO mice versus the age–matched WT-DMSO mice as evidenced by increased levels of plasma creatinine, plasma urea, and urine albumin in the KL(+/−)-DMSO group (Figure 5, A–C). Blockade of aldosterone receptors by eplerenone almost abolished KL deficiency–induced impairment in renal function (Figure 5, A–C). Eplerenone did not affect renal function in WT mice.
Figure 5.
Blockade of aldosterone receptors ameliorated renal function in KL(+/−) mice. (A) Plasma creatinine concentration. (B) Plasma urea concentration. (C) Urine albumin normalized to creatinine. (D) Plasma aldosterone concentration. These parameters were measured when animals were euthanized at 3 weeks after treatment with eplerenone. Data are means±SEMs (n=7 mice per group). *P<0.05 versus WT-DMSO; +P<0.05 versus KL(+/−)-DMSO. EPL, eplerenone.
Plasma aldosterone levels of the KL(+/−) groups were higher than those of the WT groups (Figure 5D). Eplerenone did not affect plasma aldosterone levels in either WT or KL(+/−) mice.
Blockade of Aldosterone Receptors Abolished the Upregulation of Proinflammatory Cytokine Expression in Kidneys of KL(+/−) Mice
Protein expression of several proinflammatory cytokines and chemokines, including TNF-α, MCP-1, IL-6, and osteopontin, in kidneys was increased significantly in the KL(+/−)-DMSO group versus the WT-DMSO group (Supplemental Figure 4). Eplerenone completely abolished the upregulation of these proinflammatory factors in kidneys in KL(+/−) mice. Protein expression of these proinflammatory factors was low in WT groups and was not affected by eplerenone (Supplemental Figure 4).
Blockade of Aldosterone Receptors Inhibited T Cell and Macrophage Infiltration in Kidneys of KL(+/−) Mice
The immunostaining analysis showed that infiltration of T cells (CD4 and CD8) and macrophages (CD68) was increased in kidneys of KL(+/−) mice (Supplemental Figure 5). Thus, KL deficiency resulted in kidney inflammation. Eplerenone treatment abolished T cell infiltration and partially inhibited macrophage infiltration in KL(+/−) mice, suggesting that KL deficiency–induced kidney inflammation is largely caused by upregulation of aldosterone levels.
KL Regulated CYP11B2 Expression in Human NCI H295R Adrenocortical Cells
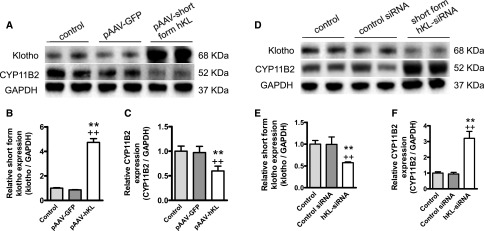
To determine if KL directly regulates CYP11B2 protein expression, we assessed the effects of overexpression and silencing of KL on CYP11B2 protein expression. Western blot analysis indicated that only short-form KL (approximately 68 kD) was expressed in human NCI H295R adrenocortical cells (Figure 6A). The full-length KL was not detectable. Overexpression of KL effectively increased KL protein expression (Figure 6, A and B), which resulted in a significant decrease in CYP11B2 protein expression (Figure 6, A and C). However, silencing of short-form KL effectively decreased KL protein expression (Figure 6, D and E), which resulted in a significant increase in CYP11B2 protein expression (Figure 6, D and F). Therefore, the results provided the first evidence that KL negatively regulated CYP11B2 protein expression.
Figure 6.
KL regulates CYP11B2 protein expression in human NCI H295R adrenocortical cells. (A–C) Overexpression of human KL decreased CYP11B2 protein expression in NCI H295R cells. Cells were transfected with pAAV-hKL, pAAV-GFP, or vehicle (transfection agent alone) for 48 hours. (A) Representative Western blots of KL and CYP11B2 protein expression in NCI H295R cells. (B and C) Quantitative analysis of KL and CYP11B2 expression in NCI H295R cells. (D–F) Suppression of human KL protein expression increased CYP11B2 protein expression in NCI H295R cells. Cells were transfected with hKL-siRNA, control siRNA, or vehicle (transfection agent alone) for 48 hours. (D) Representative Western blots of KL and CYP11B2 protein expression in NCI H295R cells. (E and F) Quantitative analysis of KL and CYP11B2 expression in NCI H295R cells. Western blots results were normalized to β-actin and then expressed as fold changes to control group. Data are means±SEMs (n=4 independent experiments). *P<0.05 versus control; **P<0.01 versus control; ++P<0.01 versus pAAV-GFP or control siRNA. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Silencing of KL Altered Protein Expression of Transcription Factors for CYP11B2 Expression in Human NCI H295R Adrenocortical Cells
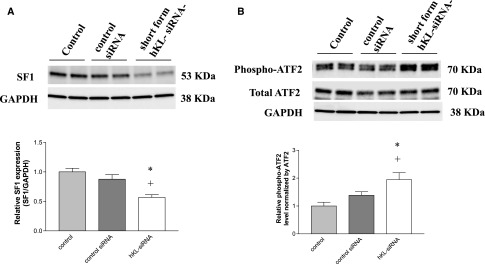
To further explore the potential molecular mechanism by which KL deficiency possibly upregulates CYP11B2 protein expression, we assessed expression of several transcription factors (steroidogenic factor-1 [SF-1], activator transcription factor 2 [ATF2], CREB, and Jun B) for CYP11B2 expression in H295R adrenocortical cells transfected with KL small interfering RNA (siRNA) for 48 hours. Silencing of KL downregulated expression of SF-1 (Figure 7A), a negative transcription factor for CYP11B2 expression. However, silencing of KL upregulated expression of phosphorylated ATF2 (Figure 7B), an active form of ATF2 that is a positive transcription factor for CYP11B2 expression. Expression of total ATF2 was not significantly affected by silencing of KL. CREB or Jun B was not affected by overexpression or silencing of KL (data not shown). Therefore, KL regulates CYP11B2 expression likely through SF-1 and ATF2 transcription factors.
Figure 7.
Silencing of KL altered protein expression of transcription factors for CYP11B2 expression in human NCI H295R adrenocortical cells. (A) Western blot analysis of protein expression of SF-1 in adrenocortical cells transfected with hKL-siRNA, control siRNA, or vehicle (transfection agent alone) for 48 hours. The results were normalized to GAPDH and then expressed as fold changes of the control group. (B) Western blot analysis of protein expression of phosphorylated ATF2 and total ATF2 in adrenocortical cells transfected with hKL-siRNA, control siRNA, or vehicle (transfection agent alone) for 48 hours. The results were normalized to GAPDH and then calculated as the ratio of phosphorylated ATF2 to total ATF2. Data are means±SEMs (n=4 independent experiments). *P<0.05 versus control; +P<0.05 versus control siRNA. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Discussion
Plasma aldosterone levels were significantly elevated in KL(+/−) mice, which coincided with elevation of BP (Figure 1). Interestingly, blockade of aldosterone actions by a specific aldosterone receptor antagonist eplerenone abolished high BP in KL(+/−) mice (Figure 3). Thus, upregulation of aldosterone levels is sufficient to explain KL deficiency–induced hypertension.
It is unexpected that KL deficiency upregulated CYP11B2 expression in adrenal cortex (Figure 2), because the relationship of KL and CYP11B2 has never been reported. It was believed that KL was primarily expressed in kidneys.4 This study shows, for the first time, that short-form KL (approximately 68 kD) is also expressed in adrenal zona glomerulosa cells (Figure 2A). Full-length KL (130 kD) is not apparent in adrenal glands, although it is a major form of KL in kidneys. CYP11B2 is a key rate–limiting enzyme in aldosterone synthesis17 that is uniquely expressed in zona glomerulosa cells of the adrenal gland. Interestingly, KL deficiency (Figure 2, B and C) was associated with upregulation of CYP11B2 protein and mRNA expression in adrenals (Figure 2, D–F). These results suggest that upregulation of CYP11B2 may be responsible for the increase in plasma aldosterone levels seen in KL(+/−) mice (Figure 1). Although hyperaldosteronism was also found in homozygous KL mutant mice [KL(−/−)],18 the underlying mechanism was not investigated. In this study, we show, for the first time, that haplodeficiency of KL upregulated expression of CYP11B2, a rate-limiting enzyme in aldosterone synthesis, which may be responsible for the KL deficiency–induced hypertension in aldosterone levels. It is new and interesting that KL regulates CYP11B2 expression in adrenals. The KL deficiency–induced increase in aldosterone is not caused by overactivity of the renin-angiotensin system, because plasma levels of renin and angiotensin II were not altered in KL(+/−) mice (Supplemental Figure 7). The elevation of aldosterone may be an atypical primary aldosteronism that does not completely reproduce primary aldosteronism (e.g., low renin).
In aldosterone-producing cells, we found that overexpression of KL downregulated CYP11B2 protein expression, whereas silencing of KL upregulated CYP11B2 protein expression (Figure 6). To the best of our knowledge, this is the first study showing that KL directly regulates expression of CYP11B2, a key enzyme for aldosterone synthesis. This finding is of physiologic significance, because it advances the current understanding of the regulation of CYP11B2 expression and aldosterone generation.
SF-1 inhibits aldosterone synthase (CYP11B2) and decreases aldosterone production,19 indicating that SF-1 is a negative transcription factor for CYP11B2 expression. Therefore, a decrease in SF-1 by silencing of KL (Figure 7A) may contribute to the upregulation of CYP11B2 expression (Figure 6, D–F). However, ATF2 serves as a positive transcription factor that increases CYP11B2 expression.20 Activation of ATF2 requires phosphorylation. Silencing of KL increased phosphorylation of ATF2 (Figure 7B), which also could contribute to upregulation of CYP11B2 expression (Figure 6, D–F). Therefore, KL deficiency–induced upregulation of CYP11B2 expression may be mediated, in part, by regulation of SF-1 and ATF2 transcription factors.
Aldosterone regulates volume homeostasis and BP by enhancing sodium reabsorption in renal distal nephrons. Our study revealed that the expressions of SGK1 and NCC were upregulated in KL(+/−) mice (Supplemental Figure 2, C–F). KL deficiency–induced upregulation of SGK1 and NCC is likely caused by elevation of aldosterone levels, which can be abolished by eplerenone. NCC is a key sodium transporter located in the distal convoluted tubule, which has abundance and activity that are modulated by the With-no-lysine kinase (WNK) family of serine/threonine kinases.21 It was reported that WNK4 could reduce cell surface NCC expression by disrupting NCC trafficking to the apical membrane.22 Furthermore, SGK1, an aldosterone–stimulated signaling molecule,23 binds to and phosphorylates WNK4, which subsequently reverses the inhibitory effect of WNK4 on NCC trafficking, leading to an increase in net effect of NCC.24 Our study also showed that expression of β-subunit of ATP synthase (Supplemental Figure 2, G and H), an enzyme providing ATP for active transport of sodium, was upregulated in KL(+/−) mice. Therefore, the elevation of BP induced by KL deficiency may be partially attributed to activation of the aldosterone-SGK1-NCC signaling pathway in kidneys. KL deficiency may impair vascular function,25,26 which may facilitate the elevation of BP. However, elevation of aldosterone levels may promote hypertension through its actions on endothelial function,27 vascular compliance,28 and central nervous system,29 which go beyond the scope of this study.
It seems that KL is essential to the maintenance of normal BP, because KL gene deficiency [KL(+/−)] causes spontaneous and persistent elevation of BP (Figure 1). It is interesting that KL plays an important role in the regulation of BP. KL(+/−) mice had one-half KL versus WT mice (Figure 2, Supplemental Figure 6). In humans, the KL level declines gradually with age after age 40 years old.4,13 At age 70 years old, the KL level is about one half of what it was at age 40 years old.13 Coincidently, the prevalence of hypertension increases with age.9,10 The prevalence of essential hypertension (EH) is more than doubled in the elderly than in the young population.10 Thus, EH is an aging-related disorder.9,11 It was reported that KL gene polymorphism is associated with human EH.30 Therefore, this study introduces a new concept that KL deficiency may be an important pathologic factor and a potential interventional target for EH. Our recent studies showed that in vivo expression of KL gene prevented the progression of spontaneous hypertension in spontaneous hypertensive rats.14 Saito et al.31 also reported that in vivo KL gene delivery reduced elevated BP in Otsuka Long–Evans Tokushima Fatty rats.
In this study, BP was measured using a computerized volume-pressure recording (VPR) tail–cuff method, a noninvasive and high–throughput measurement technique. It facilitates long-term monitoring of BP in unanesthetized animals. This method has been confirmed to be in good agreement with the radiotelemetry measurement and is recommended by the American Heart Association.32,33 The repeatable measurements of BP over a 5-month period are a strong guarantee for the reliability of the BP data.
Glomerulosclerosis (mesangial matrix expansion, increased collagen deposition, and glomerular collapse) and tubular damage (dilation, atrophy, and cast formation) were found in KL(+/−) mice (Figure 4, Supplemental Figure 3). Obvious fibrosis was also observed in tubular interstitium in KL(+/−) mice, suggesting that one–half KL deficiency causes kidney remodeling. In parallel with the structural damage, the renal function was markedly impaired in KL(+/−) mice as evidenced by the increases in plasma urea, plasma creatinine, and urine albumin (Figure 5). Although recent clinical studies implied that KL deficiency may be involved in the progression of kidney diseases,34,35 the underlying mechanisms have not been elucidated.
It is surprising that blockade of aldosterone receptors almost abolished KL deficiency–induced kidney damage, although the morphologic recovery was slower than the functional rehabilitation (Figures 4 and 5, Supplemental Figure 3). This finding suggests that the renal damage may also be largely caused by elevation of plasma aldosterone levels rather than the direct effects of KL deficiency in kidneys. Notably, proinflammatory cytokines and chemokines (TNF-α, MCP-1, IL-6, and osteopontin) were upregulated and inflammatory cell infiltration (T cells and macrophages) was increased in kidneys of KL(+/−) mice (Supplemental Figures 4 and 5). Eplerenone significantly attenuated the activation of the inflammatory process in KL(+/−) mice, suggesting that renal inflammation was largely owing to the increase in plasma aldosterone. Recent studies showed that aldosterone/MR increased TNF-α expression and subsequently, activated the NF-κB pathway by upregulating SGK1, leading to increased expression of several NF-κB–targeted proinflammatory chemokine and cytokine genes, such as MCP-1 and IL-6.36,37 The upregulation of proinflammatory factors and the consequent infiltration of leukocytes, such as macrophages and T cells, result in epithelial cellular apoptosis, mesangial cellular proliferation and matrix expansion, interstitial fibrosis, activation of the coagulation cascade, and further recruitment of leukocytes, which eventually lead to a loss of renal function.38,39 Our recent study showed that high salt–induced kidney damage in KL(+/−) mice may involve activation of the MCP-1/CCR2 pathway and infiltration of T cells and macrophages.16 Although we cannot exclude the contribution of hypertension to renal impairment in KL(+/−) mice, consistent results of other studies without the confounding interference of hypertension7,40,41 suggest that the activated inflammatory process could play an independent and important role in renal damage.
We recently reported that KL gene deficiency causes salt-sensitive hypertension by inflammation.16 This study further shows that upregulation of aldosterone levels is responsible for the inflammation seen in KL(+/−) mice, because blockade of aldosterone receptors abolishes leukocyte infiltration and cytokine releases in kidneys (Supplemental Figures 4 and 5). Although FGF23 was reported to increase the membrane abundance of NCC, tubular uptake of sodium, and BP in WT mice, high serum levels of FGF231,26 failed to increase Na reabsorption and BP in KL(−/−) mice.42 Therefore, FGF23 does not regulate Na uptake and BP in KL-deficient (−/−) mice, because the function of FGF23 is dependent on KL.42 Our study showed that increased aldosterone levels may be responsible for KL deficiency–induced hypertension, which can be abolished by an aldosterone blocker, eplerenone, in KL(+/−) mice (Figure 3). The relationship of aldosterone and FGF23 in the regulation of NCC and Na reabsorption in renal distal tubules in KL(+/−) mice remains to be determined.
Concise Methods
Animal Study Protocols
This study was carried out according to the guidelines of the National Institutes of Health on the care and use of laboratory animals. The project was approved by the Institutional Animal Care and Use Committee. Heterozygous KL mutant mice [KL(+/−)] with more than nine generations in 129Sv background were provided by Makoto Kuro-o.1 Heterozygous mutant mice were used, because they have one–half KL expression levels versus WT mice, which mimic the decline in KL levels associated with human ageing.13 In humans, at age 70 years old, the KL level is about one half of what it was at age 40 years old.13 Homozygous KL(−/−) mice show early and extensive aging phenotypes and die before the age of 8 weeks old. Homozygous KL(−/−) mice suffer from severe hyperphosphatemia, which may impair adrenal glands.26 As a result, KL homozygous mice were not used.26 The WT littermate 129Sv mice were used as controls.
In total, 20 KL(+/−) mice and 20 WT mice were used to monitor the time course of changes in BP and plasma aldosterone. BP was measured two times per week. At the ages of 14, 15, 16, and 17 weeks old, five KL(+/−) mice and five WT mice were euthanized (halothane) for measuring plasma levels of aldosterone using an ELISA kit. For confocal analysis of colocalization of KL and CYP11B2, some adrenal glands of WT mice were embedded in O.C.T., frozen at −80°C, and sectioned at 10 μm using a cryostat.
For the interventional study, one group of KL(+/−) mice and one group of WT mice (n=14) were used. The time course schema is provided in Supplemental Figure 1. BP was measured two times per week when the mice were from 8 to 13 months of age. After BP was stabilized, each strain group was divided into two subgroups when the mice were 15 months of age. One subgroup of each strain received eplerenone (6 mg/kg per day intraperitoneally), whereas the other received an equal volume of DMSO and served as a control. BP and body weight were measured two times per week, and urine was collected once a week for assessing renal function (urea, creatinine, and albumin) during the treatment. After 3 weeks of the treatments, animals were euthanized (halothane). Blood was collected for measuring plasma levels of aldosterone, urea, and creatinine. Animals were then perfused transcardially using heparinized saline. One kidney and one adrenal gland were collected and embedded in paraffin for histologic and immunohistochemical analysis. The other kidney and adrenal gland were saved in −80°C for molecular assays.
Measurements of BP
BP was measured by a computerized VPR tail–cuff method with slight warming (28°C) but not heating of the tail using a CODA 6 BP Monitoring System (Kent Scientific). This method has been validated by using a telemetry system.32,33 Animals were gently handled and trained for the VPR tail–cuff measurement to minimize handling stress. No signs of stress were observed during BP measurements. The operator was also strictly trained for the measurement procedure. At least 20 stable cycles were obtained for data analysis for each measurement. The VPR tail–cuff procedure can reliably monitor BP and is a common method for monitoring BP in our laboratory.14,43–45
Measurements of Plasma Aldosterone
Plasma aldosterone levels were measured using an aldosterone ELISA kit (ADI-901–173; Enzo Life Sciences, Inc., Farmingdate, NY) according to the manufacturer’s instruction.
Morphologic and Immunohistochemical Investigations
Paraffin-embedded kidney and adrenal gland were cut at 5-μm intervals. A series of tissue sections (at least three to five sections) of each mouse (five mice per group) was processed for staining.
Immunohistochemical Staining
Staining was performed as we described before.14,46,47 Briefly, the sections were incubated overnight (4°C) with primary antibodies against CD4 (GK1.5; 1:100; Santa Cruz Biotechnology, Santa Cruz, CA), CD8-α (H-160; 1:100; Santa Cruz Biotechnology), and CD68 (KP1; 1:100; Abcam, Inc., Cambridge, MA). Subsequently, the sections were incubated with secondary antibody, including donkey anti–goat, goat anti–mouse, goat anti–rabbit, and chicken anti–rat IgG-HRP (1:1000–2000; Santa Cruz Biotechnology) for 1 hour. The sections stained without the primary antibody served as negative controls. The sections were examined and photographed using a Nikon Eclipse Ti-U Microscope coupled with a digital color camera. The semiquantitative analysis was done using the Image J software (NIH Freeware, Bethesda, MD) as described in our recent studies.15,43,46 The numbers of CD4-, CD8-, and CD68-positive cells infiltrated in kidneys were counted in three random fields per section.
PAS Staining
PAS staining in kidneys was performed using a PAS Staining Reagents System (Sigma-Aldrich, St. Louis, MO) according to the manufacturer’s instruction. Images of 10 glomeruli for each section were randomly collected at an equal exposure condition and magnified under a Nikon Eclipse Ti-U Microscope. Mesangial matrix area was defined by the PAS–positive and nuclei–free area in the mesangium as described previously.48 The glomerular area was defined by tracing along the borders of the capillary loop. Relative mesangial area (defined as the fraction of mesangial matrix area over glomerular area) was obtained using NIS-Elements BR 3.0 software (Nikon, Melville, NY). A collapsed glomerulus was defined as an atrophic and heavy–staining glomerulus without normal structure under the microscope. The average percentage of collapsed glomeruli in all glomeruli was calculated (three random fields per section). Tubular cast formation was defined as the red deposition of proteinaceous material in renal tubules. The semiquantitative analysis of relative cast area (percentage of cast area over the total area in a section) was measured using NIS-Elements BR 3.0 software (three random fields per section).
Masson Trichrome Staining
Trichrome staining in kidneys was performed for detecting renal fibrosis. The blue staining represented collagen deposition. Blue–stained collagen area in the total tubular interstitium area and each glomerular area (defined by tracing along the borders of the capillary loop) were measured using NIS-Elements BR 3.0 software (three random fields per section).
Measurements of Renal Function
Plasma urea levels were detected using a Quantichrom Urea Assay Kit (DIUR-500; BioAssay Systems, Hayward, CA) according to the manufacturer’s instruction. Plasma creatinine levels were detected using a Quantichrom Creatinine Assay Kit (DICT-500; BioAssay Systems). Urinary albumin concentration was measured with a mouse–specific microalbuminuria ELISA kit (Albuwell M; Exocell, Philadelphia, PA). Urinary albumin excretion was normalized to urine creatinine.
Western Blotting Analyses
Western blotting analysis was performed as we described previously.14,30,46,49,50 Briefly, the membranes were blocked in 2%–3% BSA or 2.5%–5% milk in TBST for 2 hours and then incubated overnight (4°C) with primary antibodies against KL (15 μg/ml; AF1819; in mouse adrenal glands of mice; R&D Systems), CYP11B2 (1:200; MAB602151; in mouse adrenal glands; EMD Millipore), MR (1:250; ab2774; Abcam, Inc.), SGK1 (1:100; sc-28338; Santa Cruz Biotechnology), NCC (1:1000; ab3553; EMD Millipore), ATP synthase-β (1:15,000; 612518; BD Transduction Laboratories Inc., Mississauga, ON, Canada), TNF-α (1 μg/ml; ab6671; Abcam, Inc.), MCP-1 (1:500; 2029; Cell Signaling Technology, Danvers, MA), IL-6 (dilution 1:350; MABF41; EMD Millipore), osteopontin (1:1000; ab91665; Abcam, Inc.), and β-actin (1:7500; ab6267; Abcam, Inc.). Goat anti-mouse (1:2000; sc-2005; Santa Cruz Biotechnology) or goat anti-rabbit with HRP (1:10,000; sc-2004; Santa Cruz Biotechnology) was used as a secondary antibody and incubated for 1 hour at room temperature. Proteins were visualized by ECL, exposed to an x-ray film, and developed with an x-ray processor (SRA-101A; Canon). Relative protein expression was normalized to the expression of β-actin.
In human NCI H295R adrenocortical cells, protein expression of KL (1:200; AF1819; R&D Systems) and CYP11B2 (1:1000) was measured after overexpression and silencing of KL gene. The CYP11B2 mAb was produced and validated in the laboratory of Celso Gomez-Sanchez (University of Mississippi Medical Center).52,53
Quantitative Real–Time RT-PCR Analyses
Adrenal CYP11B2 mRNA expression was quantified using quantitative real–time RT-PCR as described in our previous studies.15,50,54
Cell Culture
Human NCI H295R adrenocortical aldosterone–producing cells (CRL-2128; ATCC, Manassas, VA) were cultured in DMEM:F-12 medium (ATCC) supplemented with 2.5% Nu-Serum (BD Biosciences, San Jose, CA) and the additives (insulin, transferrin, selenium, BSA, and linoleic acid) in the form of ITS+Premix (BD Biosciences) at 37°C and 5% CO2.
Confocal Immunofluorescence Microscopy
Both adrenal O.C.T. sections of WT mice were fixed with 4% paraformaldehyde for 15 minutes at room temperature. Tissues were permeabilized using 0.1% Triton X-100 (in PBS) for 5 minutes before incubation with primary antibody. Goat anti–mouse KL (15 μg/ml; AF1819; R&D Systems) and mouse anti–rat CYP11B2 (1:100; MAB6021; EMD Millipore)51 antibodies were used for revealing the localization of KL and CYP11B2 in mouse adrenal glands, respectively. Alexa Fluor 568 donkey anti–goat IgG (H+L) antibody (1:200; Life Technologies, Grand Island, NY) and Alexa Fluor 488 donkey anti–mouse IgG (H+L) antibody (1:650; Life Technologies) were supplied as secondary antibodies.
Recombinant Plasmid DNA Transfection
pAAV-hKL-short form (pAAV-IRES-hrGFP expression vector + human secreted KL cDNA) was constructed and purified as described previously.14 NCI H295R cells cultured in six-well plates were transfected with recombinant plasmid DNA (pAAV-hKL-short form or pAAV-GFP) at the concentration of 0.87 g/ml using Optifect Transfection Reagent (Life Technologies) according to the manufacturer’s protocol followed by 48 hours of incubation in DMEM:F-12 medium with 2.5% Nu-Serum and ITS+Premix at 37°C in a CO2 incubator.
siRNA Transfection
Human KL siRNA was purchased from Life Technologies (catalog no. AM16704). The sequences are sense 5′-GGU CAA GUA CUG GAU CAC Ctt-3′ and antisense 5′-GGU GAU CCA GUA CUU GAC Ctg-3′. The scrambled siRNA was purchased as a control siRNA (catalog no. AM4611; Life Technologies). NCI H295R cells cultured in six-well plates were transfected with siRNAs (KL siRNA and control siRNA; 50 nM) using Lipofectamine RNAiMAX Transfection Reagent (Life Technologies) according to the manufacturer’s instruction followed by 48 hours of incubation in DMEM:F-12 medium with 2.5% Nu-Serum and ITS+Premix at 37°C in a CO2 incubator.
Statistical Analyses
BP was analyzed by a repeated measures one–way ANOVA. All other data were analyzed using a one-way ANOVA or an unpaired t test. Data are expressed as means±SEMs. A value of P<0.05 was considered statistically significant.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Drs. Celso Gomez-Sanchez and Elise Gomez-Sanchez at the University of Mississippi Medical Center for providing validated CYP11B2 antibodies.
This work was supported by National Institutes of Health (NIH) Grants R01 DK093403, HL105302, HL102074, HL116863, HL118558, HL122166, and AG049780. This publication was made possible by NIH Grant 9P20GM104934-06 from the Center of Biomedical Research Excellence Program of the National Institute of General Medical Sciences.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015010093/-/DCSupplemental.
References
- 1.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI: Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390: 45–51, 1997 [DOI] [PubMed] [Google Scholar]
- 2.Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M: Suppression of aging in mice by the hormone Klotho. Science 309: 1829–1833, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Masuda H, Chikuda H, Suga T, Kawaguchi H, Kuro-o M: Regulation of multiple ageing-like phenotypes by inducible klotho gene expression in klotho mutant mice. Mech Ageing Dev 126: 1274–1283, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Sun Z: Current understanding of klotho. Ageing Res Rev 8: 43–51, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsumura Y, Aizawa H, Shiraki-Iida T, Nagai R, Kuro-o M, Nabeshima Y: Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem Biophys Res Commun 242: 626–630, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Shiraki-Iida T, Aizawa H, Matsumura Y, Sekine S, Iida A, Anazawa H, Nagai R, Kuro-o M, Nabeshima Y: Structure of the mouse klotho gene and its two transcripts encoding membrane and secreted protein. FEBS Lett 424: 6–10, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Siragy HM, Xue C: Local renal aldosterone production induces inflammation and matrix formation in kidneys of diabetic rats. Exp Physiol 93: 817–824, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stewart PM: Mineralocorticoid hypertension. Lancet 353: 1341–1347, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Ong KL, Cheung BM, Man YB, Lau CP, Lam KS: Prevalence, awareness, treatment, and control of hypertension among United States adults 1999-2004. Hypertension 49: 69–75, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr., Jones DW, Materson BJ, Oparil S, Wright JT Jr., Roccella EJ Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute National High Blood Pressure Education Program Coordinating Committee : Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 42: 1206–1252, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK: The progression from hypertension to congestive heart failure. JAMA 275: 1557–1562, 1996 [PubMed] [Google Scholar]
- 12.Sun Z: Aging, arterial stiffness, and hypertension. Hypertension 65: 252–256, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao NM, Zhang YM, Zheng Q, Gu J: Klotho is a serum factor related to human aging. Chin Med J (Engl) 117: 742–747, 2004 [PubMed] [Google Scholar]
- 14.Wang Y, Sun Z: Klotho gene delivery prevents the progression of spontaneous hypertension and renal damage. Hypertension 54: 810–817, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, Sun Z: RNAi silencing of brain klotho potentiates cold-induced elevation of blood pressure via the endothelin pathway. Physiol Genomics 41: 120–126, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou X, Chen K, Lei H, Sun Z: Klotho gene deficiency causes salt-sensitive hypertension via monocyte chemotactic protein-1/CC chemokine receptor 2-mediated inflammation. J Am Soc Nephrol 26: 121–132, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Curnow KM, Tusie-Luna MT, Pascoe L, Natarajan R, Gu JL, Nadler JL, White PC: The product of the CYP11B2 gene is required for aldosterone biosynthesis in the human adrenal cortex. Mol Endocrinol 5: 1513–1522, 1991 [DOI] [PubMed] [Google Scholar]
- 18.Fischer SS, Kempe DS, Leibrock CB, Rexhepaj R, Siraskar B, Boini KM, Ackermann TF, Föller M, Hocher B, Rosenblatt KP, Kuro-O M, Lang F: Hyperaldosteronism in Klotho-deficient mice. Am J Physiol Renal Physiol 299: F1171–F1177, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ye P, Nakamura Y, Lalli E, Rainey WE: Differential effects of high and low steroidogenic factor-1 expression on CYP11B2 expression and aldosterone production in adrenocortical cells. Endocrinology 150: 1303–1309, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nogueira EF, Rainey WE: Regulation of aldosterone synthase by activator transcription factor/cAMP response element-binding protein family members. Endocrinology 151: 1060–1070, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veríssimo F, Jordan P: WNK kinases, a novel protein kinase subfamily in multi-cellular organisms. Oncogene 20: 5562–5569, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Kahle KT, Wilson FH, Lifton RP: Regulation of diverse ion transport pathways by WNK4 kinase: A novel molecular switch. Trends Endocrinol Metab 16: 98–103, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Pearce D, Kleyman TR: Salt, sodium channels, and SGK1. J Clin Invest 117: 592–595, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rozansky DJ, Cornwall T, Subramanya AR, Rogers S, Yang YF, David LL, Zhu X, Yang CL, Ellison DH: Aldosterone mediates activation of the thiazide-sensitive Na-Cl cotransporter through an SGK1 and WNK4 signaling pathway. J Clin Invest 119: 2601–2612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Six I, Okazaki H, Gross P, Cagnard J, Boudot C, Maizel J, Drueke TB, Massy ZA: Direct, acute effects of Klotho and FGF23 on vascular smooth muscle and endothelium. PLoS One 9: e93423, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu Y, Sun Z: Molecular basis of Klotho: From gene to function in aging. Endocr Rev 36: 174–193, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rocha R, Funder JW: The pathophysiology of aldosterone in the cardiovascular system. Ann N Y Acad Sci 970: 89–100, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Blacher J, Amah G, Girerd X, Kheder A, Ben Mais H, London GM, Safar ME: Association between increased plasma levels of aldosterone and decreased systemic arterial compliance in subjects with essential hypertension. Am J Hypertens 10: 1326–1334, 1997 [DOI] [PubMed] [Google Scholar]
- 29.Gómez-Sánchez EP, Zhou M, Gomez-Sanchez CE: Mineralocorticoids, salt and high blood pressure. Steroids 61: 184–188, 1996 [DOI] [PubMed] [Google Scholar]
- 30.Wang HL, Xu Q, Wang Z, Zhang YH, Si LY, Li XJ, Yang QH, Xiao H: A potential regulatory single nucleotide polymorphism in the promoter of the Klotho gene may be associated with essential hypertension in the Chinese Han population. Clin Chim Acta 411: 386–390, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Saito Y, Nakamura T, Ohyama Y, Suzuki T, Iida A, Shiraki-Iida T, Kuro-o M, Nabeshima Y, Kurabayashi M, Nagai R: In vivo klotho gene delivery protects against endothelial dysfunction in multiple risk factor syndrome. Biochem Biophys Res Commun 276: 767–772, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Feng M, Whitesall S, Zhang Y, Beibel M, D’Alecy L, DiPetrillo K: Validation of volume-pressure recording tail-cuff blood pressure measurements. Am J Hypertens 21: 1288–1291, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Whitesall SE, Hoff JB, Vollmer AP, D’Alecy LG: Comparison of simultaneous measurement of mouse systolic arterial blood pressure by radiotelemetry and tail-cuff methods. Am J Physiol Heart Circ Physiol 286: H2408–H2415, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Kuro-o M: Klotho in health and disease. Curr Opin Nephrol Hypertens 21: 362–368, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Hu MC, Kuro-o M, Moe OW: The emerging role of Klotho in clinical nephrology. Nephrol Dial Transplant 27: 2650–2657, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanz-Rosa D, Cediel E, de las Heras N, Miana M, Balfagón G, Lahera V, Cachofeiro V: Participation of aldosterone in the vascular inflammatory response of spontaneously hypertensive rats: Role of the NFkappaB/IkappaB system. J Hypertens 23: 1167–1172, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Leroy V, De Seigneux S, Agassiz V, Hasler U, Rafestin-Oblin ME, Vinciguerra M, Martin PY, Féraille E: Aldosterone activates NF-kappaB in the collecting duct. J Am Soc Nephrol 20: 131–144, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Furuichi K, Kaneko S, Wada T: Chemokine/chemokine receptor-mediated inflammation regulates pathologic changes from acute kidney injury to chronic kidney disease. Clin Exp Nephrol 13: 9–14, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Duffield JS: Macrophages and immunologic inflammation of the kidney. Semin Nephrol 30: 234–254, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blasi ER, Rocha R, Rudolph AE, Blomme EA, Polly ML, McMahon EG: Aldosterone/salt induces renal inflammation and fibrosis in hypertensive rats. Kidney Int 63: 1791–1800, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Irita J, Okura T, Jotoku M, Nagao T, Enomoto D, Kurata M, Desilva VR, Miyoshi K, Matsui Y, Uede T, Denhardt DT, Rittiling SR, Higaki J: Osteopontin deficiency protects against aldosterone-induced inflammation, oxidative stress, and interstitial fibrosis in the kidney. Am J Physiol Renal Physiol 301: F833–F844, 2011 [DOI] [PubMed] [Google Scholar]
- 42.Andrukhova O, Slavic S, Smorodchenko A, Zeitz U, Shalhoub V, Lanske B, Pohl EE, Erben RG: FGF23 regulates renal sodium handling and blood pressure. EMBO Mol Med 6: 744–759, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crosswhite P, Sun Z: Ribonucleic acid interference knockdown of interleukin 6 attenuates cold-induced hypertension. Hypertension 55: 1484–1491, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun Z, Cade R, Zhang Z, Alouidor J, Van H: Angiotensinogen gene knockout delays and attenuates cold-induced hypertension. Hypertension 41: 322–327, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Sun Z, Bello-Roufai M, Wang X: RNAi inhibition of mineralocorticoid receptors prevents the development of cold-induced hypertension. Am J Physiol Heart Circ Physiol 294: H1880–H1887, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Crosswhite P, Sun Z: Inhibition of phosphodiesterase-1 attenuates cold-induced pulmonary hypertension. Hypertension 61: 585–592, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin Y, Kuro-o M, Sun Z: Genetic deficiency of anti-aging gene klotho exacerbates early nephropathy in STZ-induced diabetes in male mice. Endocrinology 154: 3855–3863, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zuo Z, Lei H, Wang X, Wang Y, Sonntag W, Sun Z: Aging-related kidney damage is associated with a decrease in klotho expression and an increase in superoxide production. Age (Dordr) 33: 261–274, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang X, Skelley L, Wang B, Mejia A, Sapozhnikov V, Sun Z: AAV-based RNAi silencing of NADPH oxidase gp91(phox) attenuates cold-induced cardiovascular dysfunction. Hum Gene Ther 23: 1016–1026, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin Y, Sun Z: In vivo pancreatic β-cell-specific expression of antiaging gene Klotho: A novel approach for preserving β-cells in type 2 diabetes. Diabetes 64: 1444–1458, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bailey SD, Xie C, Do R, Montpetit A, Diaz R, Mohan V, Keavney B, Yusuf S, Gerstein HC, Engert JC, Anand S DREAM investigators : Variation at the NFATC2 locus increases the risk of thiazolidinedione-induced edema in the Diabetes REduction Assessment with ramipril and rosiglitazone Medication (DREAM) study. Diabetes Care 33: 2250–2253, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gomez-Sanchez CE, Qi X, Velarde-Miranda C, Plonczynski MW, Parker CR, Rainey W, Satoh F, Maekawa T, Nakamura Y, Sasano H, Gomez-Sanchez EP: Development of monoclonal antibodies against human CYP11B1 and CYP11B2. Mol Cell Endocrinol 383: 111–117, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heitzmann D, Derand R, Jungbauer S, Bandulik S, Sterner C, Schweda F, El Wakil A, Lalli E, Guy N, Mengual R, Reichold M, Tegtmeier I, Bendahhou S, Gomez-Sanchez CE, Aller MI, Wisden W, Weber A, Lesage F, Warth R, Barhanin J: Invalidation of TASK1 potassium channels disrupts adrenal gland zonation and mineralocorticoid homeostasis. EMBO J 27: 179–187, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Crosswhite P, Chen K, Sun Z: AAV delivery of tumor necrosis factor-α short hairpin RNA attenuates cold-induced pulmonary hypertension and pulmonary arterial remodeling. Hypertension 64: 1141–1150, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.