Abstract
The co-morbidity of neuropsychiatric disorders, particularly major depressive disorder (MDD) with neurodegenerative diseases, in particular Parkinson’s disease (PD) is now well recognized. Indeed, it is suggested that depressive disorders, especially in late life, may be an indication of latent neurodegeneration. Thus, it is not unreasonable to expect that deterrents of MDD may also deter the onset and/or progression of the neurodegenerative diseases including PD. In this review, examples of neuroprotective efficacy of established as well as prospective antidepressants are provided. Conversely, mood-regulating effects of some neuroprotective drugs are also presented. Thus, in addition to currently used antidepressants, ketamine, nicotine, curcumin, and resveratrol are discussed for their dual efficacy. In addition, potential neurobiological substrates for their actions are presented. It is concluded that pharmacological developments of mood-regulating or neuroprotective drugs can have cross benefit in co-morbid conditions of neuropsychiatric and neurodegenerative disorders and that inflammatory and neurotrophic factors play important roles in both conditions.
Keywords: Major depressive disorder, Antidepressant, Neuroprotection, Neurodegenerative disorders, Neurotrophic factors, Inflammatory mediators
Introduction
Neuropsychiatric and neurodegenerative diseases are rather complex medical problems underscored by the limited and not fully effective interventions. It is now well recognized that more often than not, a number of factors, particularly genetic and environmental, act together to bring about the disorder (Roy et al. 2014; Lopizzo et al. 2015). In general, genes provide the susceptibility or vulnerability component and the environment, either internal or external, provides the knockout punch. However, if either one of the two players, genetics or environment, is severe enough it can precipitate the disease by itself. Nonetheless, these devastating diseases eventually arise because of abnormalities within the neurobiological substrates, e.g., the neurotransmitter systems, receptor functions, transduction signals, etc., that impair neuronal plasticity and function. More recently, the concept of alterations in circuit connections “connectopathy” has been introduced in both neuropsychiatric as well as neurodegenerative diseases (Martin 2012; Castrén 2013; Di Benedetto et al. 2013; Bredt et al. 2015). Thus, understanding the neurobiological underpinnings is critical in eventual treatment for either disease. Recent reports indicate a strong association between neuropsychiatric disorders (particularly, major depressive disorder = MDD) and neurological diseases (e.g., Parkinson’s disease = PD). Moreover, some common mechanisms involving neurotrophic and inflammatory factors may be responsible for both conditions. Building on above, this review starts with a brief introduction of MDD, current medications as well as promising new compounds including ketamine, nicotine, curcumin, and resveratrol for this condition. In each case, evidence of possible neuroprotective effects by the antidepressants is provided. Indeed, the duality of the effects of these compounds is a main focus of this review. Moreover, mechanism(s) of action of the compounds, vis-à-vis their interaction with neurotrophic and the inflammatory mediators, to the extent available, is discussed. It is concluded that most antidepressants and neuroprotectants may share a common mechanism of promoting neurotrophic factors while suppressing pro-inflammatory mediators and that these effects contribute to the effectiveness of an antidepressant as a neuroprotectant and vice versa.
Depression
Clinical depression, characterized by a despondent feeling, loss of interest in pleasurable activities, guilt, worthlessness, and trouble concentrating, is a serious medical illness that adversely affects a person’s level of activity. It is also associated with abnormalities in appetite and sleep. In severe cases, it can lead to suicidal ideation and actual suicide. Several types of depression are identified. Major depression is manifested by a combination of symptoms that interfere with the ability to work, sleep, eat, and enjoy once pleasurable activities. These disabling episodes of depression can occur once, twice, or several times in a lifetime. Dysthymia, a less severe type of depression, involves long-term chronic symptoms that do not disable but keep one from functioning at “full steam” or from feeling good. Manic-depressive or bipolar is not nearly as prevalent as other forms of depressive illnesses and involves cycles of depression and elation or mania.
It is estimated that 121 million people in the world live with some type of depression. In 2013, an estimated 15.7 million adults aged 18 or older in the U.S. had at least one major depressive episode in the past year. This represented 6.7 percent of all U.S. adults (NIMH 2015). Curiously, 80 % of the people who have depression are not being treated, although the number of individuals diagnosed with depression increases each year and there is a tremendous loss of productivity and increased medical expenses, estimated to be over 200 billion dollars annually (Greenberg et al. 2015). The most serious and devastating final outcome of severe depression is suicide, which amounts to approximately one million people worldwide every year (WFMH 2012).
Etiology of Depression
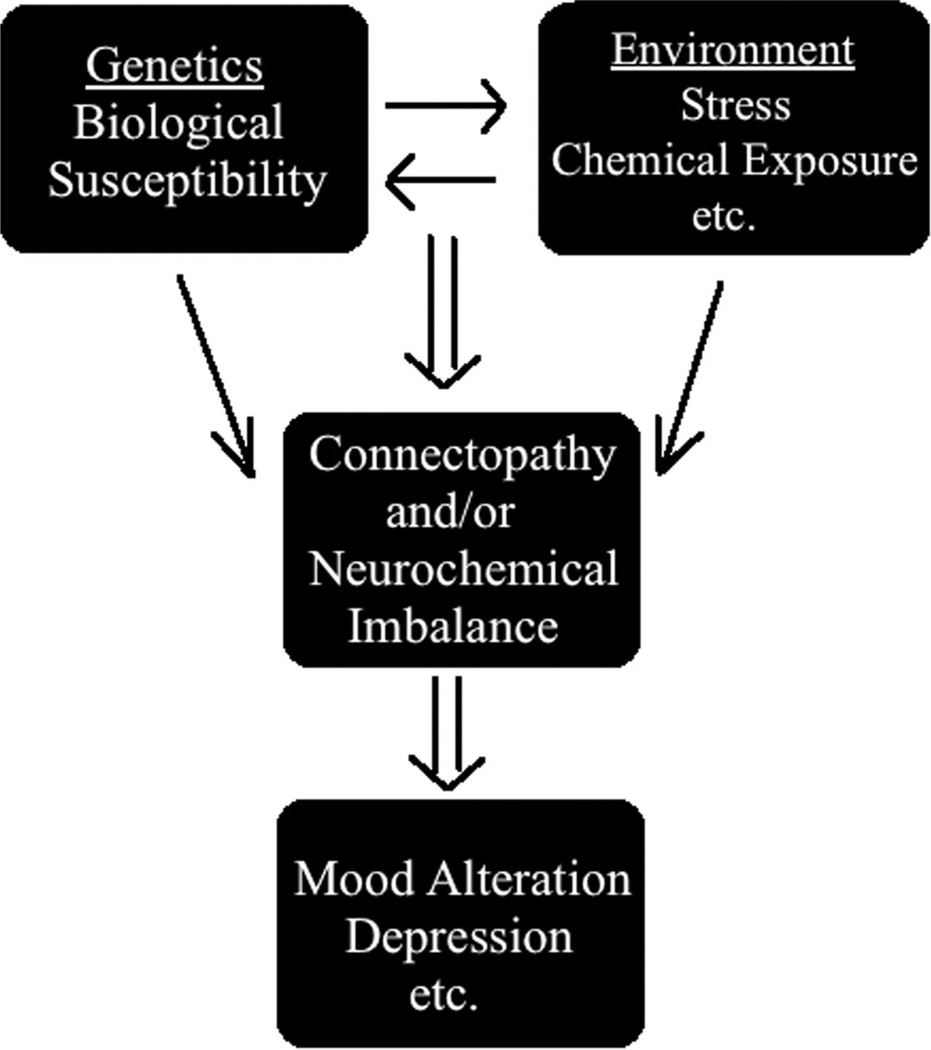
Although as noted above, genetics plays a very important role in manifestation of depression, various conditions, particularly stress, have been identified as a culprit in precipitation of this condition. Indeed, animal studies confirm that administration of chronic mild stress can result in depressive-like symptoms and that this model is commonly used to understand the neurobiological substrates of this disease and/or investigate novel therapies (Hill et al. 2012; Wiborg 2013; Lopizzo et al. 2015). As depicted in the diagram below (Fig. 1), genetic factors provide the biological vulnerability that can be directly affected by stress which can lead to connectopathy and/or neurochemical imbalance and hence manifestation of depressive-like behavior.
Fig. 1.
Interaction of genetic and environmental factors (particularly stress) can lead to abnormalities in circuit connections (connectopathy) and/or neurochemical imbalance and manifestation of depression
Role of Neurotrophins
Substantial evidence indicates that impairments in cellular plasticity underlie the pathophysiology of severe mood disorders (Manji and Duman 2001; Manji et al. 2003; Villanueva 2013; Duman 2014; Haase and Brown 2015). Neurotrophins, in particular, brain-derived neurotrophic factor (BDNF) and its receptor TrkB, the tyrosine kinase receptor for BDNF, are critically involved in regulation of synaptic plasticity and hence are strongly implicated in the pathophysiology of mood disorders (Manji and Duman 2001; Manji et al. 2003). Indeed, polymorphism in the BDNF gene has been associated with depression and some antidepressant therapies (Colle et al. 2015; Lisiecka et al. 2015). Moreover, reduced BDNF levels are observed in postmortem brain samples and in the blood of depressed patients. In addition, hippocampal volume reduction, reflective of a reduction in neurogenesis, has been detected in both animal models of depression (Zhao et al. 2008; Tizabi et al. 2010) as well as in human postmortem studies (Sheline et al. 2003; Czeh and Lucassen 2007). Interestingly, hippocampal volume reduction in the animal model of depression was also associated with a reduction in hippocampal BDNF (Hauser et al. 2011). These reductions, however, are reversible by successful antidepressant treatment (Sheline et al. 2003; Czeh and Lucassen 2007; Tizabi et al. 2010). Hence, antidepressants may, through enhanced BDNF signaling, improve the ability of critical brain circuits to optimally respond to environmental demands and help recover from depression (Castrén and Rantamäki 2008; Mendez-David et al. 2013; Duman 2014). It is noteworthy that chronic mild stress that is frequently used to induce depressive-like behavior in animal models is also associated with a reduction in neurogenesis (Sapolsky 2004; Toth et al. 2008).
Role of Inflammatory Mediators
In addition to neurotrophic factors, important roles for inflammatory mediators are evident in pathophysiology of a variety of neuropsychiatric disorders including MDD (Felger and Lotrich 2013; Postal and Appenzeller 2015; Toben and Baune 2015). This is supported by the results of numerous studies indicating elevated levels of the pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α and interleukin (IL)-1β in plasma of depressed patients (Gold and Irwin 2009; Felger and Lotrich 2013; Toben and Baune 2015). That pro-inflammatory cytokines are directly implicated in MDD has been recently reviewed (Hurley and Tizabi 2013; Anderson and Maes 2014; Müller 2014; Ogłodek et al. 2014) and hence will not be discussed in detail here. However, it is of relevance to note that the depression induced by chronic stress may also be due to an increase in the release of pro-inflammatory cytokines (Maes et al. 2009; Song and Wang 2011), which in turn can cause a reduction in neurogenesis (Song and Wang 2011; Felger and Lotrich 2013; Ogłodek et al. 2014; Stepanichev et al. 2014). Indeed, there is a reciprocal interaction between cytokines and neurotrophic factors (Spedding and Gressens 2008).
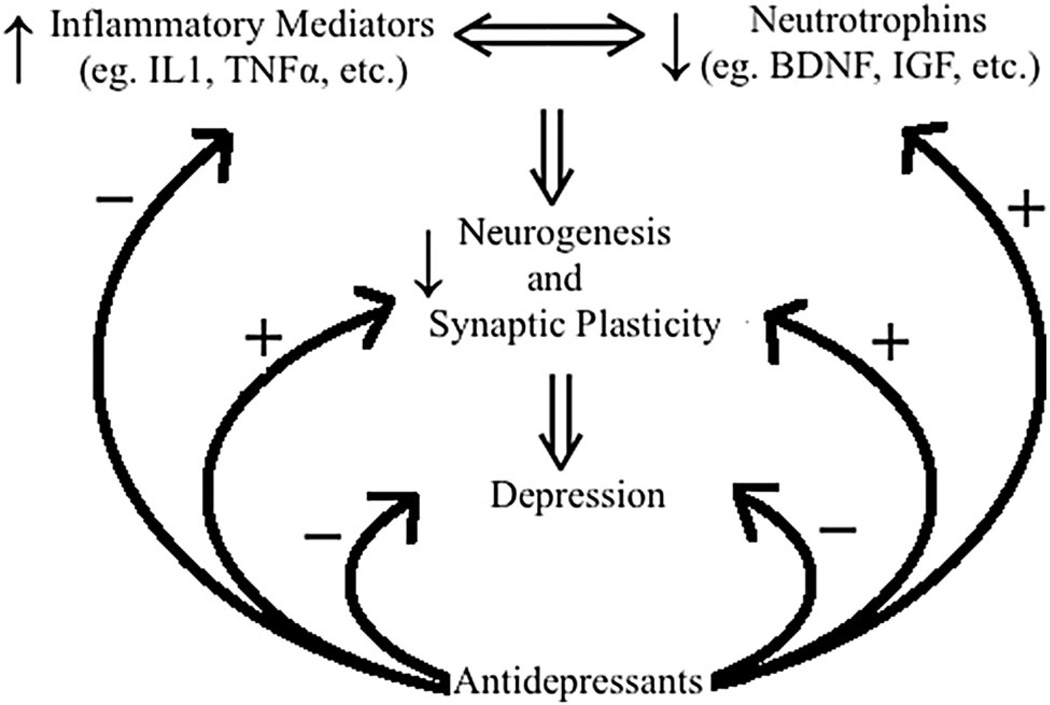
Thus, both neurotrophic factors and inflammatory mediators may play important roles in pathophysiology of depression and response to antidepressants. A summary of such interactions is depicted in Fig. 2.
Fig. 2.
Plausible relationship between pro-inflammatory mediators (e.g., cytokines) and neurotrophins (e.g., BDNF and IGF: insulin-like growth factor) in manifestation of depression and reversal by antidepressants
Current Antidepressants
The current antidepressants follow the general hypothesis of “Biogenic Amine Depletion” which suggests that low levels of serotonin, norepinephrine, or dopamine in the brain are responsible for the symptom manifestation. Hence, drugs in these categories primarily target the uptake or degradation of biogenic amines to enhance the synaptic availability of the neurotransmitters (see Table 1).
Table 1.
Various classes of currently used antidepressants
| Antidepressant class | Drugs |
|---|---|
| Monoamine oxidase inhibitors (MAOIs)a | Isocarboxazid (Marplan), Phenelzine (Nardil), Selegiline (l-Deprenyl, Emsam) |
| Tetracyclic antidepressants (TetCAs) | Mianserin (Norval), Mirtazapine (Remeron), Amitriptyline (Elavil), Clomipramine (Anafranil) |
| Tricyclic antidepressants (TCAs) | Imipramine (Tofranil), Desipramine (Norpramin), Nortriptyline (Aventyl) |
| Norepinephrine dopamine reuptake inhibitors (NDRIs) | Bupropion (Wellbutrin) |
| Serotonin norepinephrine reuptake inhibitors (SNRIs) | Desvenlafaxine (Pristiq), Duloxetine (Cymbalta) |
| Selective serotonin reuptake inhibitors (SSRIs) | Fluoxetine (Prozac), Fluvoxamine (Luvox), Citalopram (Celexa), Escitalopram (Lexapro), Paroxetine (Paxil), Sertraline (Zoloft) |
The era of antidepressants was heralded by isoniazid in 1950s, when as an antitubercular agent serendipitously showed euphoric effects in patients with tuberculosis. Probing into the mood elevating property of this drug (MAO inhibition) initiated the synthesis of generations of “biogenic based” antidepressants. Moreover, it indicated a biological basis of the illness (Lopez-Munoz et al. 2007; Ramachandraih et al. 2011)
Neuroprotection by Current Antidepressants and Involvement of Neurotrophins
A number of earlier reports indicated neurotrophic effects of antidepressants, reflected in elevated levels of brain-derived neurotrophic factor (BDNF), particularly in the hippocampus, suggesting possible neuroprotective effects as well (Nestler et al. 2002; Xu et al. 2003). These findings included all clinically relevant tri- and tetra-cyclic drugs, selective serotonin reuptake inhibitors (SSRIs), selective serotonin and norepinephrine uptake inhibitors (SSNRIs), and monoamine oxidase inhibitors (MAOIs). Indeed, Sheline and colleagues found that depression may be associated with hippocampal volume loss and that antidepressants may protect brain from damage due to depression (Sheline et al. 2003). Lately, the same group has reported on possible prophylactic use of citalopram, a SSRI in Alzheimer’s disease (AD), as this drug appears to help prevent formation of amyloid plaques in transgenic mouse model as well as in humans (Sheline et al. 2014). The potential use of other antidepressants in AD has been recently reviewed (Kim et al. 2013). In addition, several studies indicate possible utility of fluoxetine in stroke patients. However, controversy over fluoxetine’s exact mechanism of action in stroke remains. Although fluoxetine may increase neurogenesis (Corbett et al. 2015; Imoto et al. 2015; Sun et al. 2015), its beneficial effect in stroke may be due to reducing inhibitory interneuron expression in the premotor cortex (Ng et al. 2015). Nonetheless, taken together, it may be suggested that elevated levels of neurotrophins in general and BDNF in particular following antidepressants administration are at least partially responsible for the enhanced neurogenesis and hence beneficial effects in neurodegenerative diseases such as AD. As for beneficial effects of current antidepressants in stroke and their exact mechanism of action in that regard, further investigation is needed.
Neuroprotection by Current Antidepressants and Involvement of Inflammatory Mediators
Although possible interaction of currently used antidepressants and inflammatory mediators has not been adequately explored, it is of relevance to note that depression associated with low-level neuroinflammation may be at least partially due to alterations in serotonergic and noradrenergic neurotransmission (O’Sullivan et al. 2009; Müller 2014; Ménard et al. 2015). Moreover, it has been shown that norepinephrine (NE) can suppress neuroinflammation mediated by activation of microglia and astrocyte. Hence, NE uptake inhibitors’ therapeutic efficacy in depression may also be related to NE-mediated anti-inflammatory effects (O’Sullivan et al. 2009). Other investigators have also provided evidence of interaction between currently used antidepressants and inflammatory modulators. Thus, Uher et al. (2014) report that high basal levels of C-reactive protein (CRP), a marker of systemic inflammation, predict positive response to escitalopram (a serotonin reuptake inhibitor), whereas low basal levels of CRP predict a positive response to nortriptyline (a norepinephrine reuptake inhibitor). Moreover, evidence of in vitro and in vivo regulation of serotonin transporter function by pro-inflammatory mediators has been recently reviewed (Haase and Brown 2015).
Limitations with Current Antidepressants
Although the introduction of antidepressants into the medical field decades ago could be held as a scientific triumph, the fact that there is a significant delay in onset of action (it may take several weeks for the antidepressant to become effective) and that they are not effective in all patients, in addition to having undesirable side effects (e.g., anxiety, restlessness, weight gain, decreased sex drive, insomnia/sleepiness, fatigue, nausea, diarrhea/constipation, headache, etc.), indicates an urgent need to develop novel drugs with better efficacy and less side effects. In this regard, recent efforts have identified some new drugs, which will be discussed in brief here. The emphasis, however, is to show that all these possible medications also possess some neuroprotective properties.
It is also important to note that depression need not always be associated with neurodegeneration. Rather, changes in synaptic plasticity associated with decreases in neurotrophic factors and increases in inflammatory mediators are likely contributing neurobiological substrates. Fortunately, these changes are reversible, as treatment with effective antidepressants can normalize such abnormalities. On the other hand, neurodegenerative diseases that are associated with neuronal losses and can also trigger mood disorders may only be afforded a symptomatic relief, as no drug is yet available to reverse neuronal loss. Nonetheless, it would be of significant interest and relevance to evaluate any drug developed to retard neurodegenerative processes for possible mood stabilizing effects as well.
Novel Antidepressants
Ketamine
Ketamine is emerging as a very promising potent and fast-acting antidepressant, which appears to be well tolerated across patient groups, with only transient mild-to-moderate adverse effects during infusion (Abdallah et al. 2015). It is a non-competitive NMDA receptor antagonist and a derivative of phencyclidine (PCP) that blocks the NMDA receptor (Harrison and Simmonds 1985). Clinical studies have shown significant reduction of depressive symptoms within 72 h of ketamine administration (Berman et al. 2000; Zarate et al. 2006; Iadarola et al. 2015), and that a single sub-anesthetic dose of ketamine has rapid and sustained antidepressant effects in treatment-resistant patients suffering from major depressive disorder (Maeng et al. 2008; Iadarola et al. 2015; Kavalali and Monteggia 2015). Interestingly, ketamine’s antidepressant effect requires activation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, as NBQX, an AMPA receptor antagonist, can block ketamine’s effects (Maeng et al. 2008; Koike et al. 2011). Indeed, it has been shown that AMPA itself can exert an antidepressant effect in an animal model of depression (Akinfiresoye and Tizabi 2013). Moreover, clinical studies indicate that expression of AMPA receptors is altered in patients with depression (Freudenberg et al. 2015). Thus, future studies on AMPA receptor subtype involvement in depression may offer novel pharmacological interventions.
Role of Neurotrophins and Inflammatory Mediators in Ketamine’s Antidepressant Action
In a recent article examining the efficacy of ketamine as well as vagal nerve stimulation (VNS) in treatment-resistant depression, the role of BDNF and its receptor in the beneficial effects of both ketamine and VNS were highlighted (Shah et al. 2014). In addition, using conditional knockout mice, it was deducted that BDNF is required for the antidepressant-like effect of ketamine (Autry et al. 2011). Ketamine activates neurotrophin signaling through TrkB receptor (Autry et al. 2011; Duman et al. 2012; Duman 2014). Further support of neurotrophin involvement in ketamine’s effect was provided in a recent randomized clinical trial, where plasma BDNF levels were found to be significantly higher following antidepressant response to ketamine (Haile et al. 2014). Interestingly, in regard to involvement of AMPA receptors, it has been demonstrated that positive modulators of these receptors can also induce BDNF, and that some antidepressants such as tianeptine may act by restoring neuronal plasticity through this neurotrophic factor (Spedding and Gressens 2008).
As indicated above, inflammatory processes may be key contributors to depression (Hurley and Tizabi 2013; Anderson and Maes 2014; Müller 2014; Ogłodek et al. 2014). Antidepressant effects of ketamine may also be due to its suppression of pro-inflammatory cytokines such as TNF-α and IL-6 (Taniguchi et al. 2001, 2004) and promoting the activity of anti-inflammatory cytokines such as IL-10 (Simma et al. 2013). Ketamine may also suppress the expression of pro-inflammatory cytokines induced by lipopolysaccharide (LPS), an exotoxin derived from the outer membrane of Gram-negative bacteria (Helmer et al. 2003a, b; Chang et al. 2009; Yang et al. 2013a). Indeed, peripheral and central anti-inflammatory effects of ketamine are receiving substantial attention in relation to its therapeutic potential (De Kock et al. 2013; Hurley and Tizabi 2013).
Ketamine as a Neuroprotectant
Several investigations have demonstrated neuroprotective effects of ketamine both in vitro and in vivo. Indeed, a review of neuroprotective effects of ketamine against ischemic damage was published in 2010 (Hudetz and Pagel 2010). Recent reports provide further evidence of neuroprotective effects of ketamine in damage induced by oxygen glucose deprivation (Emnett et al. 2013) and against spinal ischemia (Kakinohana 2014). Interestingly, a combination of ketamine and atropine may offer neuroprotection against toxic (organophosphate)-induced status epilepticus in mice (Dhote et al. 2012). A more recent review summarizes antiepileptic as well as neuroprotective effects of ketamine (Dorandeu et al. 2013). Although the exact mechanism for neuroprotection by ketamine is not at hand, it could be speculated that its enhancement of the neurotrophic factors and reduction of inflammatory response may contribute to its neuroprotective effects. In this regard, it would be of considerable interest to determine the exact roles of signal transduction molecules, as it was demonstrated that ketamine’s protection against medial cerebral artery occlusion was associated with inhibition of neuron-specific P-CREB (phosphorylated CAMP response element-binding protein) dephosphorylation (Shu et al. 2012). It is also critical to note that ketamine’s neuroprotection is dependent on its low concentration as higher doses can result in toxicities of their own (Schifilliti et al. 2010).
Thus, ketamine is a promising antidepressant with neuroprotective properties that is at least partially mediated through activation of neurotrophic factors and suppression of pro-inflammatory mediators.
Nicotine
Nicotine as an Antidepressant
A number of preclinical (Semba et al. 1998; Djuric et al. 1999; Tizabi et al. 1999, 2000, 2009, 2010; Kalejaiye et al. 2013) as well as a limited number of clinical studies (Pomerleau and Pomerleau 1992; Salin-Pascual et al. 1995; McClernon et al. 2006; Cook et al. 2007) have verified an antidepressant effect of nicotine. Indeed, the high incidence of smoking among depressed patients has generated the “self medication” hypothesis, which posits that these individuals derive some relief of their symptoms via inhaled nicotine (Cook et al. 2007; Moreno-Coutino et al. 2007; Spring et al. 2008). This hypothesis is further supported by the findings that nicotine-withdrawal induces depression, which likely contributes to higher failure rate of smoking cessation among depressed individuals (Borrelli et al. 1996; Covey et al. 1997; Tsoh et al. 2000; Glassman et al. 2001; Edwards and Kendler 2011). Thus, sufficient evidence for applicability of nicotine or nicotinic compounds in neuropsychiatric disorders including posttraumatic stress disorder and MDD has been recently provided (Barreto et al. 2015; Picciotto et al. 2015; Rahman 2015).
Nicotine as a Neuroprotectant
In addition to its antidepressant qualities, a number of epidemiological and empirical studies also suggest neuroprotective effects for nicotine. This contention is supported by findings of an inverse relationship between Parkinson’s disease and smoking, which has been consistently demonstrated in various countries (Dorn 1959; Nefzger et al. 1968; Baumann et al. 1980; Baron 1996; Ross and Petrovitch 2001; Thacker et al. 2007). Moreover, in vitro and in vivo studies have shown that nicotine may protect against nigrostriatal damage induced by various compounds. Thus, in Parkinson’s disease cell models, nicotine protects against endogenous substances such as salsolinol and aminochrome that selectively damage dopaminergic cells (Copeland et al. 2005, 2007; Das and Tizabi 2009; Ramlochansingh et al. 2011; Munoz et al. 2012). Similarly in animal studies, including non-human primates, it has been shown that nicotine delays Parkinson’s disease-like symptoms induced by MPTP (Quik et al. 2006, 2009, 2014). Moreover, nicotine may also reduce l-Dopa-induced dyskinesia (Quik et al. 2014). A mechanism of nicotine protection against Parkinson’s disease may involve inhibition of astrocyte activation and inflammatory suppression (Liu et al. 2012). Nicotine’s modulation of neuroinflammation is further supported by a number of studies (Piao et al. 2009; Shi et al. 2009; Cui and Li 2010) and is believed to be mediated through its interaction with α7 nicotinic receptors (Cui and Li 2010). It is of relevance to note that the anti-inflammatory effects of nicotine may also be applicable to a variety of conditions including ulcerative colitis, septic kidney injury, and obesity, all of which can be precipitated or exacerbated by inflammatory processes (Lakhan and Kirchgessner 2011; Chatterjee et al. 2012).
Apart from its anti-inflammatory properties, nicotine may also modulate synaptic plasticity via its interaction with the neurotrophic mediators, particularly BDNF, which may also be responsible for its antidepressant and neuroprotective effects (Belluardo et al. 2000; Posadas et al. 2013; Yakel 2013; Barreto et al. 2015). Thus, nicotine or nicotinic agonists may offer novel intervention in depressive and/or neurodegenerative diseases, particularly PD, via their interaction with neurotrophic and/or inflammatory mediators.
Curcumin
Curcumin as an Antidepressant
Curcumin, the active ingredient in turmeric (Curcuma longa), has been shown to function as an antioxidant (Sharma 1976; Ruby et al. 1995; Sandur et al. 2007a, b), anti-inflammatory (Aggarwal and Harikumar 2009; Jurenka 2009), neuroprotectant (Singla and Dhawan 2012; Qualls et al. 2014), and antidepressant. The antidepressant effects of curcumin were originally reported in stress-induced depression models (Xu et al. 2005a,b; Li et al. 2007; Kulkarni et al. 2008; Bhutani et al. 2009), but we observed a dose-dependent antidepressant-like effect of curcumin in WKY rats, a non-induced animal model of depression (Hurley et al. 2012a). More recently, the effects of curcumin on depressive-like behavior in mice after LPS administration (Wang et al. 2014) and in unpredictable, mild stress-induced depressive-like behavior in rats (Zhang et al. 2014) were also reported. Clinically, curcumin was shown to be an effective antidepressant (Lopresti et al. 2014) as well as capable of enhancing the efficacy of currently used antidepressants (Yu et al. 2015).
Curcumin may have antidepressant activities with diverse mechanisms of action involving primarily neurotransmitters, transcription pathways, neurogenesis, the hypothalamic–pituitary–adrenal axis, and inflammatory and immune pathways, as demonstrated in various animal and human studies (reviewed in Tizabi et al. 2014; Seo et al. 2015). Hence, curcumin increases biogenic amines (e.g., dopamine, serotonin, and NE) in the cortex and hippocampus (Xu et al. 2005a, b; Kulkarni et al. 2008; Arora et al. 2011), up-regulates hippocampal BDNF (Hurley et al. 2012a; Liu et al. 2014a), reduces mRNA and protein of pro-inflammatory cytokines (e.g., TNF-α and IL-1β) as well as proteins involved in apoptotic pathways (e.g., NF-κB and caspase-3) (Abe et al. 1999; Arora et al. 2011). Thus, multiple mechanisms including interactions with the neurotrophic and inflammatory mediators may be contributing to the antidepressant-like effects of curcumin.
Curcumin as a Neuroprotectant
In regard to neuroprotection, it has been observed that societies that widely use curcumin show reduced incidence of inflammation-influenced and cognitive deficits (Chandra et al. 2001; Vas et al. 2001; Ng et al. 2006; Aggarwal et al. 2007). Indeed, anti-inflammatory effects of curcumin vis-à-vis suppression of IL-1β, tumor necrosis factor TNF-α, (Gupta et al. 2014; Yuan et al. 2015), and the nuclear factor NF-κB, considered a prototypical pro-inflammatory signaling pathway (Yang et al. Yang et al. 2014b; Yuan et al. 2015), as well as intracellular components of glial fibrillary acidic protein (GFAP), considered a marker of gliosis (Yuan et al. 2015), have been reported. Thus, curcumin may exert a neuroprotective effect against the 6-OHDA-induced rat model of PD (Yang et al. 2014aa) and may also protect axons from degeneration in the setting of local neuroinflammation (Tegenge et al. 2014). In addition, curcumin may ameliorate the adverse effects of the neurotoxin N-methyl N-nitrosourea in the cerebrum and cerebellum of mice (Singla and Dhawan 2012) and may exert a neuroprotective effect against ischemic spinal cord injury by decreasing inducible nitric oxide synthase as well as N-methyl-d-aspartate receptor expression (Zhang et al. 2013). Recently, it was demonstrated that curcumin as an anti-inflammatory and anti-oxidative agent can help myelin to repair, which can be of significant clinical relevance to multiple sclerosis (Mohajeri et al. 2015). These anti-inflammatory effects of curcumin together with its neurotrophic promoting effects (see above and Nam et al. 2014), make this compound of significant interest in both neuropsychiatric, particularly MDD, and neurodegenerative diseases, such as PD and AD (Darvesh et al. 2012; Tizabi et al. 2014).
However, two important points have to be considered in relation to therapeutic use of curcumin. The first point concerns its bioavailability as dietary curcumin exhibits poor bioavailability (Hamaguchi et al. 2010). This might be due to several factors including its insolubility in water, poor absorption, and rapid metabolism that need to be overcome for a more meaningful therapeutic intervention. In this regard, it has been suggested that addition of piperine could enhance curcumin’s absorption (Hamaguchi et al. 2010). Interestingly, a clinical study where curcumin was combined with piperine showed significant efficacy in treatment of MDD (Panahi et al. 2015). The bioavailability of curcumin may also be increased by its dissolution in oil or cooking (Yang et al. 2013b). However, structural modification and development of novel curcumin derivatives that may be administered orally or intra-nasally may offer a more realistic approach.
The other important point concerns possible combination of curcumin or its more stable derivatives with current or other medications to treat depression and/or PD. This approach may be particularly beneficial since different drugs may act at different sites and offer a better control of inflammation, neurogenesis, and other processes (e.g., oxidation) that may contribute to the pathology of these diseases. In this regard, it is of interest to note that clinically, it was demonstrated that curcumin enhances the efficacy of current antidepressants (Yu et al. 2015). In addition, as discussed in this review, a number of novel compounds including ketamine, nicotine, and resveratrol (see below) that have shown potential usefulness in major depression and PD may be combined together for a potential additive or synergistic effect (Seidl and Potashkin 2011; Mythri and Bharath 2012; Brondino et al. 2014).
Resveratrol
Resveratrol as an Antidepressant and Neuroprotectant
Resveratrol, a natural non-flavonoid polyphenol antioxidant, (3, 4’, 5-trihydroxy-trans-stilbene), is a substance extracted from red grapes in the processing of wine, but it is also found in other fruit skins. Preclinical studies have shown the antidepressant-like effect of resveratrol in various rodent models including the corticosterone-induced depression in mice (Ali et al. 2015) and the Wistar-Kyoto rat model of depression (Hurley et al. 2014). Moreover, in both cases, the antidepressant effect of resveratrol was associated with an increase in hippocampal BDNF (Hurley et al. 2014; Ali et al. 2015). Interestingly, resveratrol also prevented depression and the impaired cognition induced by chronic unpredictable mild stress in rats, and resulted in an increase in hippocampal BDNF (Ge et al. 2013; Liu et al. 2014b).
In addition to its effect on neurotrophic factors, resveratrol also possesses anti-inflammatory effects as it significantly inhibits LPS-induced microglial activation and production of TNF-α, IL-1β, and nitric oxide (another proinflammatory factor) (Zhang et al. 2012; Pallàs et al. 2013). These properties of resveratrol are likely responsible for its various beneficial effects in neurodegenerative diseases and improvement of cognitive functioning. Indeed, a number of reports have highlighted possible application of resveratrol in ischemic and traumatic CNS injury (Sinha et al. 2002; Wang et al. 2002; Lopez et al. 2015), in AD (Vingtdeux et al. 2008; Rege et al. 2014), in PD (Tredici et al. 1999; Chen et al. 2007; Lofrumento et al. 2014; Fu et al. 2015), and in improving cognitive functions (Harada et al. 2011).
Altogether, the findings confirm that resveratrol might represent a potential benefit for the treatment of inflammation-related neurological or neuropsychiatric diseases, particularly mood-related disorders. However, similar to discussion on curcumin, here also two important points have to be borne in mind. First, because naturally occurring forms of resveratrol have a very limited half-life in plasma, it is important that potent analogs with increased bioavailability be developed. Second, although the effects of resveratrol on neurotrophic and inflammatory mediators are well-established, other mechanisms (e.g., antioxidant, mitochondrial interaction, signal transduction pathways, etc.) may also be involved (Pallàs et al. 2013; Hurley et al. 2014; Lopez et al. 2015).
Concluding Remarks
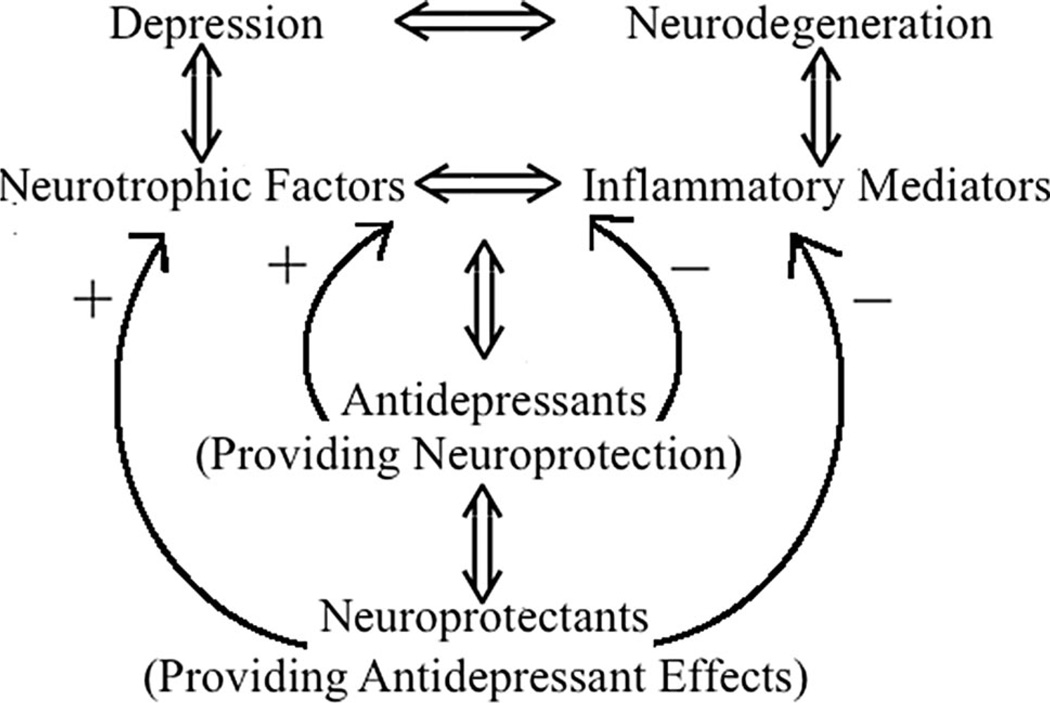
As mentioned above, to date, a true neuroprotective drug that could substantially prevent the progression of the neurodegenerative diseases is not available. The drugs discussed here have the potential of providing such neuroprotection. In this regard, one’s expectation will be to stop or retard the progression of the disease. Moreover, such a drug could be of value in normalizing mood disorders manifested alone or in conjunction with the neurodegenerative disease (see Fig. 3). Although the emphasis in this review has been on inflammatory and neurotrophic involvement, participation of other factors or systems (e.g., oxidative stress, apoptosis/necrosis, mitochondrial damage/repair, etc.) in drug’s actions cannot be overruled or ignored. Nonetheless, tapping into such an array of biological substrates, one can only hope for development of compounds that can be effective in combating the devastating neuropsychiatric/neurodegenerative diseases.
Fig. 3.
Postulated neurobiological commonality involving inflammatory mediators and neurotrophins in depression and neurodegenerative diseases and possible interaction of antidepressants and neuroprotectants with such substrates in providing relief in both conditions
Acknowledgments
This review was supported by NIH/NIAAA R03AA022479. The author wishes to thank Daniela Tizabi for proofing the article, graphic design, and compiling the references.
References
- Abdallah CG, Averill LA, Krystal JH. Ketamine as a promising prototype for a new generation of rapid-acting antidepressants. Ann N Y Acad Sci. 2015;1344:66–77. doi: 10.1111/nyas.12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe Y, Hashimoto S, Horie T. Curcumin inhibition of inflammatory cytokine production by human peripheral blood monocytes and alveolar macrophages. Pharmacol Res. 1999;39:41–47. doi: 10.1006/phrs.1998.0404. [DOI] [PubMed] [Google Scholar]
- Aggarwal BB, Harikumar KB. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int J Biochem Cell Biol. 2009;41:40–59. doi: 10.1016/j.biocel.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal BB, Sundaram C, Malani N, Ichikawa H. Curcumin: the Indian solid gold. Adv Exp Med Biol. 2007;595:1–75. doi: 10.1007/978-0-387-46401-5_1. [DOI] [PubMed] [Google Scholar]
- Ali SH, Madhana RM, Athira KV, Kasala ER, Bodduluru LN, Pitta S, Mahareddy JR, Lahkar M. Resveratrol ameliorates depressive-like behavior in repeated corticosterone-induced depression in mice. Steroids. 2015;101:37–42. doi: 10.1016/j.steroids.2015.05.010. [DOI] [PubMed] [Google Scholar]
- Akinfiresoye L, Tizabi Y. Antidepressant effects of AMPA and ketamine combination: role of hippocampal BDNF, synapsin, and mTOR. Psychopharmacol. 2013;230:291–298. doi: 10.1007/s00213-013-3153-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson G, Maes M. Oxidative/nitrosative stress and immuno-inflammatory pathways in depression: treatment implications. Curr Pharm Des. 2014;20(23):3812–3847. doi: 10.2174/13816128113196660738. [DOI] [PubMed] [Google Scholar]
- Arora V, Kuhad A, Tiwari V, Chopra K. Curcumin ameliorates reserpine-induced pain-depression dyad: behavioural, biochemical, neurochemical and molecular evidences. Psychoneuroendocrinology. 2011;36:1570–1581. doi: 10.1016/j.psyneuen.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron JA. Beneficial effects of nicotine and cigarette smoking: the real, the possible and the spurious. Br Med Bull. 1996;52:58–73. doi: 10.1093/oxfordjournals.bmb.a011533. [DOI] [PubMed] [Google Scholar]
- Barreto GE, Yarkov A, Avila-Rodriguez M, Aliev G, Echeverria V. Nicotine-derived compounds as therapeutic tools against post-traumatic stress disorder. Curr Pharm Des. 2015;21(25):3589–3595. doi: 10.2174/1381612821666150710145250. [DOI] [PubMed] [Google Scholar]
- Baumann RJ, Jameson HD, McKean HE, Haack DG, Weisberg LM. Cigarette smoking and Parkinson disease: 1. Comparison of cases with matched neighbors. Neurology. 1980;30:839–843. doi: 10.1212/wnl.30.8.839. [DOI] [PubMed] [Google Scholar]
- Belluardo N, Mudò G, Blum M, Fuxe K. Central nicotinic receptors, neurotrophic factors and neuroprotection. Behav Brain Res. 2000;113:21–34. doi: 10.1016/s0166-4328(00)00197-2. [DOI] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Bhutani MK, Bishnoi M, Kulkarni SK. Anti-depressant like effect of curcumin and its combination with piperine in unpredictable chronic stress-induced behavioral, biochemical and neurochemical changes. Pharmacol Biochem Behav. 2009;92:39–43. doi: 10.1016/j.pbb.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Borrelli B, Niaura R, Keuthen NJ, Goldstein MG, DePue JD, Murphy C, Abrams DB. Development of major depressive disorder during smoking-cessation treatment. J Clin Psychiatry. 1996;57:534–538. doi: 10.4088/jcp.v57n1106. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Furey ML, Chen G, Lovenberg T, Drevets WC, Manji HK. Translating depression biomarkers for improved targeted therapies. Neurosci Biobehav Rev. 2015;59:1–15. doi: 10.1016/j.neubiorev.2015.09.013. [DOI] [PubMed] [Google Scholar]
- Brondino N, Re S, Boldrini A, Cuccomarino A, Lanati N, Barale F, Politi P. Curcumin as a therapeutic agent in dementia: a mini systematic review of human studies. Sci World J. 2014;2014:174282. doi: 10.1155/2014/174282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrén E. Neuronal network plasticity and recovery from depression. JAMA Psychiatry. 2013;70(9):983–989. doi: 10.1001/jamapsychiatry.2013.1. [DOI] [PubMed] [Google Scholar]
- Castrén E, Rantamäki T. Neurotrophins in depression and antidepressant effects. Novartis Found Symp. 2008;289:43–52. doi: 10.1002/9780470751251.ch4. [DOI] [PubMed] [Google Scholar]
- Chandra V, Pandav R, Dodge HH, Johnston JM, Belle SH, DeKosky ST, Ganguli M. Incidence of Alzheimer’s disease in a rural community in India: the Indo-US study. Neurology. 2001;57:985–989. doi: 10.1212/wnl.57.6.985. [DOI] [PubMed] [Google Scholar]
- Chang Y, Lee JJ, Hsieh CY, Hsiao G, Chou DS, Sheu JR. Inhibitory effects of ketamine on lipopolysaccharide-induced microglial activation. Mediators Inflamm. 2009;2009:705379. doi: 10.1155/2009/705379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee PK, Yeboah MM, Dowling O, Xue X, Powell SR, Al-Abed Y, Metz CN. Nicotinic acetylcholine receptor agonists attenuate septic acute kidney injury in mice by suppressing inflammation and proteasome activity. PLoS One. 2012;7:e35361. doi: 10.1371/journal.pone.0035361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LW, Wang YQ, Wei LC, Shi M, Chan YS. Chinese herbs and herbal extracts for neuroprotection of dopaminergic neurons and potential therapeutic treatment of Parkinson’s disease. CNS Neurol Disord Drug Targets. 2007;6:273–281. doi: 10.2174/187152707781387288. [DOI] [PubMed] [Google Scholar]
- Colle R, Deflesselle E, Martin S, David DJ, Hardy P, Taranu A, Falissard B, Verstuyft C, Corruble E. BDNF/TRKB/P75NTR polymorphisms and their consequences on antidepressant efficacy in depressed patients. Pharmacogenomics. 2015;16(9):997–1013. doi: 10.2217/pgs.15.56. [DOI] [PubMed] [Google Scholar]
- Cook JW, Spring B, McChargue D. Influence of nicotine on positive affect in anhedonic smokers. Psychopharmacology. 2007;192:87–95. doi: 10.1007/s00213-006-0688-5. [DOI] [PubMed] [Google Scholar]
- Copeland RL, Jr, Leggett YA, Kanaan YM, Taylor RE, Tizabi Y. Neuroprotective effects of nicotine against salsolinol-induced cytotoxicity: implications for Parkinson’s disease. Neurotox Res. 2005;8:289–293. doi: 10.1007/BF03033982. [DOI] [PubMed] [Google Scholar]
- Copeland RL, Jr, Das JR, Kanaan YM, Taylor RE, Tizabi Y. Antiapoptotic effects of nicotine in its protection against salsolinol-induced cytotoxicity. Neurotox Res. 2007;12:61–69. doi: 10.1007/BF03033901. [DOI] [PubMed] [Google Scholar]
- Corbett AM, Sieber S, Wyatt N, Lizzi J, Flannery T, Sibbit B, Sanghvi S. Increasing neurogenesis with fluoxetine, simvastatin and ascorbic acid leads to functional recovery in ischemic stroke. Recent Pat Drug Deliv Formul. 2015;9(2):158–166. doi: 10.2174/1872211309666150122102846. [DOI] [PubMed] [Google Scholar]
- Covey LS, Glassman AH, Stetner F. Major depression following smoking cessation. Am J Psychiatry. 1997;154:263–265. doi: 10.1176/ajp.154.2.263. [DOI] [PubMed] [Google Scholar]
- Cui WY, Li MD. Nicotinic modulation of innate immune pathways via alpha7 nicotinic acetylcholine receptor. J Neuroimmune Pharmacol. 2010;5:479–488. doi: 10.1007/s11481-010-9210-2. [DOI] [PubMed] [Google Scholar]
- Czeh B, Lucassen PJ. What causes the hippocampal volume decrease in depression? Are neurogenesis, glial changes and apoptosis implicated? Eur Arch Psychiatry Clin Neurosci. 2007;257:250–260. doi: 10.1007/s00406-007-0728-0. [DOI] [PubMed] [Google Scholar]
- Darvesh AS, Carroll RT, Bishayee A, Novotny NA, Geldenhuys WJ, Van der Schyf CJ. Curcumin and neurodegenerative diseases: a perspective. Expert Opin Investig Drugs. 2012;21:1123–1140. doi: 10.1517/13543784.2012.693479. [DOI] [PubMed] [Google Scholar]
- Das JR, Tizabi Y. Additive protective effects of donepezil and nicotine against salsolinol-induced cytotoxicity in SH-SY5Y cells. Neurotox Res. 2009;16:194–204. doi: 10.1007/s12640-009-9040-2. [DOI] [PubMed] [Google Scholar]
- De Kock M, Loix S, Lavand’homme P. Ketamine and peripheral inflammation. CNS Neurosci Ther. 2013;19:403–410. doi: 10.1111/cns.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhote F, Carpentier P, Barbier L, Peinnequin A, Baille V, Pernot F, Testylier G, Beaup C, Foquin A, Dorandeu F. Combinations of ketamine and atropine are neuroprotective and reduce neuroinflammation after a toxic status epilepticus in mice. Toxicol Appl Pharmacol. 2012;259(2):195–209. doi: 10.1016/j.taap.2011.12.024. [DOI] [PubMed] [Google Scholar]
- Di Benedetto B, Rupprecht R, Czéh B. Talking to the synapse: how antidepressants can target glial cells to reshape brain circuits. Curr Drug Targets. 2013;14(11):1329–1335. doi: 10.2174/1389450111314110011. [DOI] [PubMed] [Google Scholar]
- Djuric VJ, Dunn E, Overstreet DH, Dragomir A, Steiner M. Antidepressant effect of ingested nicotine in female rats of Flinders resistant and sensitive lines. Physiol Behav. 1999;67:533–537. doi: 10.1016/s0031-9384(99)00091-8. [DOI] [PubMed] [Google Scholar]
- Dorandeu F, Dhote F, Barbier L, Baccus B, Testylier G. Treatment of status epilepticus with ketamine, are we there yet? CNS Neurosci Ther. 2013;19(6):411–427. doi: 10.1111/cns.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn HF. Tobacco consumption and mortality from cancer and other diseases. Public Health Rep. 1959;74:581–593. [PMC free article] [PubMed] [Google Scholar]
- Duman RS. Pathophysiology of depression and innovative treatments: remodeling glutamatergic synaptic connections. Dialogues Clin Neurosci. 2014;16(1):11–27. doi: 10.31887/DCNS.2014.16.1/rduman. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Li N, Liu RJ, Duric V, Aghajanian G. Signaling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacology. 2012;62:35–41. doi: 10.1016/j.neuropharm.2011.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards AC, Kendler KS. Nicotine withdrawal-induced negative affect is a function of nicotine dependence and not liability to depression or anxiety. Nicotine Tob Res. 2011;13:677–685. doi: 10.1093/ntr/ntr058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emnett CM, Eisenman LN, Taylor AM, Izumi Y, Zorumski CF, Mennerick S. Indistinguishable synaptic pharmacodynamics of the N-methyl-d-aspartate receptor channel blockers memantine and ketamine. Mol Pharmacol. 2013;84(6):935–947. doi: 10.1124/mol.113.089334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Lotrich FE. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience. 2013;246:199–229. doi: 10.1016/j.neuroscience.2013.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenberg F, Celikel T, Reif A. The role of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors in depression: central mediators of pathophysiology and antidepressant activity? Neurosci Biobehav Rev. 2015;52:193–206. doi: 10.1016/j.neubiorev.2015.03.005. [DOI] [PubMed] [Google Scholar]
- Fu W, Zhuang W, Zhou S, Wang X. Plant-derived neuroprotective agents in Parkinson’s disease. Am J Transl Res. 2015;7(7):1189–1202. [PMC free article] [PubMed] [Google Scholar]
- Ge JF, Peng L, Cheng JQ, Pan CX, Tang J, Chen FH, Li J. Antidepressant-like effect of resveratrol: involvement of antioxidant effect and peripheral regulation on HPA axis. Pharmacol Biochem Behav. 2013;114–115:64–69. doi: 10.1016/j.pbb.2013.10.028. [DOI] [PubMed] [Google Scholar]
- Glassman AH, Covey LS, Stetner F, Rivelli S. Smoking cessation and the course of major depression: a follow-up study. Lancet. 2001;357:1929–1932. doi: 10.1016/S0140-6736(00)05064-9. [DOI] [PubMed] [Google Scholar]
- Gold SM, Irwin MR. Depression and immunity: inflammation and depressive symptoms in multiple sclerosis. Immunol Allergy Clin North Am. 2009;29:309–320. doi: 10.1016/j.iac.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg PE, Fournier AA, Sisitsky T, Pike CT, Kessler RC. The economic burden of adults with major depressive disorder in the United States (2005 and 2010) J Clin Psychiatry. 2015;76(2):155–162. doi: 10.4088/JCP.14m09298. [DOI] [PubMed] [Google Scholar]
- Gupta SC, Tyagi AK, Deshmukh-Taskar P, Hinojosa M, Prasad S, Aggarwal BB. Downregulation of tumor necrosis factor and other proinflammatory biomarkers by polyphenols. Arch Biochem Biophys. 2014;559:91–99. doi: 10.1016/j.abb.2014.06.006. [DOI] [PubMed] [Google Scholar]
- Haase J, Brown E. Integrating the monoamine, neurotrophin and cytokine hypotheses of depression–a central role for the serotonin transporter? Pharmacol Ther. 2015;147:1–11. doi: 10.1016/j.pharmthera.2014.10.002. [DOI] [PubMed] [Google Scholar]
- Haile CN, Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Foulkes A, Iqbal S, Mahoney JJ, 3rd, De La Garza R, 2nd, Charney DS, Newton TF, Mathew SJ. Plasma brain derived neurotrophic factor (BDNF) and response to ketamine in treatment-resistant depression. Int J Neuropsychopharmacol. 2014;17:331–336. doi: 10.1017/S1461145713001119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaguchi T, Ono K, Yamada M. Review: curcumin and Alzheimer’s disease. CNS Neurosci Ther. 2010;16:285–297. doi: 10.1111/j.1755-5949.2010.00147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada N, Zhao J, Kurihara H, Nakagata N, Okajima K. Resveratrol improves cognitive function in mice by increasing production of insulin-like growth factor-I in the hippocampus. J Nutr Biochem. 2011;22:1150–1159. doi: 10.1016/j.jnutbio.2010.09.016. [DOI] [PubMed] [Google Scholar]
- Harrison NL, Simmonds MA. Quantitative studies on some antagonists of N-methyl d-aspartate in slices of rat cerebral cortex. Br J Pharmacol. 1985;84:381–391. doi: 10.1111/j.1476-5381.1985.tb12922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser SR, Getachew B, Taylor RE, Tizabi Y. Alcohol induced depressive-like behavior is associated with a reduction in hippocampal BDNF. Pharmacol Biochem Behav. 2011;100:253–258. doi: 10.1016/j.pbb.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmer KS, Cui Y, Chang L, Dewan A, Mercer DW. Effects of ketamine/xylazine on expression of tumor necrosis factor alpha, inducible nitric oxide synthase, and cyclo-oxygenase-2 in rat gastric mucosa during endotoxemia. Shock. 2003a;20:63–69. doi: 10.1097/01.shk.0000065766.72937.cf. [DOI] [PubMed] [Google Scholar]
- Helmer KS, Cui Y, Dewan A, Mercer DW. Ketamine/xylazine attenuates LPS-induced iNOS expression in various rat tissues. J Surg Res. 2003b;112:70–78. doi: 10.1016/s0022-4804(03)00138-0. [DOI] [PubMed] [Google Scholar]
- Hill MN, Hellemans KG, Verma P, Gorzalka BB, Weinberg J. Neurobiology of chronic mild stress: parallels to major depression. Neurosci Biobehav Rev. 2012;36(9):2085–2117. doi: 10.1016/j.neubiorev.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudetz JA, Pagel PS. Neuroprotection by ketamine: a review of the experimental and clinical evidence. J Cardiothorac Vasc Anesth. 2010;24(1):131–142. doi: 10.1053/j.jvca.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Hurley LL, Tizabi Y. Neuroinflammation neurodegeneration and depression. Neurotox Res. 2013;23(2):131–144. doi: 10.1007/s12640-012-9348-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LL, Akinfiresoye L, Tizabi Y. Behavioral and neurotrophic effects of curcumin in a putative animal model of depression. Society for Neuroscience Annual Meeting. 2012 [Google Scholar]
- Hurley LL, Akinfiresoye L, Kalejaiye O, Tizabi Y. Antidepressant effects of resveratrol in an animal model of depression. Behav Brain Res. 2014;268:1–7. doi: 10.1016/j.bbr.2014.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadarola ND, Niciu MJ, Richards EM, Vande Voort JL, Ballard ED, Lundin NB, Nugent AC, Machado-Vieira R, Zarate CA., Jr Ketamine and other N-methyl-d-aspartate receptor antagonists in the treatment of depression: a perspective review. Ther Adv Chronic Dis. 2015;6:97–114. doi: 10.1177/2040622315579059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imoto Y, Kira T, Sukeno M, Nishitani N, Nagayasu K, Nakagawa T, Kaneko S, Kobayashi K, Segi-Nishida E. Role of the 5-HT4 receptor in chronic fluoxetine treatment-induced neurogenic activity and granule cell dematuration in the dentate gyrus. Mol Brain. 2015;15(8):29. doi: 10.1186/s13041-015-0120-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurenka JS. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev. 2009;14:141–153. [PubMed] [Google Scholar]
- Kakinohana M. Protective effects of anesthetics on the spinal cord. Curr Pharm Des. 2014;20(36):5744–5750. doi: 10.2174/1381612820666140204114124. [DOI] [PubMed] [Google Scholar]
- Kalejaiye O, Bhatti BH, Taylor RE, Tizabi Y. Nicotine blocks the depressogenic effects of alcohol: implications for drinking-smoking co-morbidity. J Drug Alcohol Res. 2013;2:235709. doi: 10.4303/jdar/235709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavalali ET, Monteggia LM. How does ketamine elicit a rapid antidepressant response? Curr Opin Pharmacol. 2015;20:35–39. doi: 10.1016/j.coph.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Kim W, Kong SY. Antidepressants for neuroregeneration: from depression to Alzheimer’s disease. Arch Pharm Res. 2013;36:1279–1290. doi: 10.1007/s12272-013-0238-8. [DOI] [PubMed] [Google Scholar]
- Koike H, Iijima M, Chaki S. Involvement of AMPA receptor in both the rapid and sustained antidepressant-like effects of ketamine in animal models of depression. Behav Brain Res. 2011;224:107–111. doi: 10.1016/j.bbr.2011.05.035. [DOI] [PubMed] [Google Scholar]
- Kulkarni SK, Bhutani MK, Bishnoi M. Antidepressant activity of curcumin: involvement of serotonin and dopamine system. Psychopharmacology. 2008;201:435–442. doi: 10.1007/s00213-008-1300-y. [DOI] [PubMed] [Google Scholar]
- Lakhan SE, Kirchgessner A. Anti-inflammatory effects of nicotine in obesity and ulcerative colitis. J Transl Med. 2011;9:129. doi: 10.1186/1479-5876-9-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Wang C, Wang M, Li W, Matsumoto K, Tang Y. Antidepressant like effects of piperine in chronic mild stress treated mice and its possible mechanisms. Life Sci. 2007;80:1373–1381. doi: 10.1016/j.lfs.2006.12.027. [DOI] [PubMed] [Google Scholar]
- Lisiecka DM, O’Hanlon E, Fagan AJ, Carballedo A, Morris D, Suckling J, Frodl T. BDNF Val66Met polymorphism in patterns of neural activation in individuals with MDD and healthy controls. J Affect Disord. 2015;184:239–244. doi: 10.1016/j.jad.2015.06.002. [DOI] [PubMed] [Google Scholar]
- Liu Y, Hu J, Wu J, Zhu C, Hui Y, Han Y, Huang Z, Ellsworth K, Fan W. Alpha7 nicotinic acetylcholine receptor-mediated neuroprotection against dopaminergic neuron loss in an MPTP mouse model via inhibition of astrocyte activation. J Neuroinflammation. 2012;9:98. doi: 10.1186/1742-2094-9-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Wang Z, Gao Z, Xie K, Zhang Q, Jiang H, Pang Q. Effects of curcumin on learning and memory deficits, BDNF, and ERK protein expression in rats exposed to chronic unpredictable stress. Behav Brain Res. 2014a;271:116–121. doi: 10.1016/j.bbr.2014.05.068. [DOI] [PubMed] [Google Scholar]
- Liu D, Zhang Q, Gu J, Wang X, Xie K, Xian X, Wang J, Jiang H, Wang Z. Resveratrol prevents impaired cognition induced by chronic unpredictable mild stress in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2014b;49:21–29. doi: 10.1016/j.pnpbp.2013.10.017. [DOI] [PubMed] [Google Scholar]
- Lofrumento DD, Nicolardi G, Cianciulli A, De Nuccio F, La Pesa V, Carofiglio V, Dragone T, Calvello R, Panaro MA. Neuroprotective effects of resveratrol in an MPTP mouse model of Parkinson’s-like disease: possible role of SOCS-1 in reducing pro-inflammatory responses. Innate Immun. 2014;20(3):249–260. doi: 10.1177/1753425913488429. [DOI] [PubMed] [Google Scholar]
- Lopez MS, Dempsey RJ, Vemuganti R. Resveratrol neuroprotection in stroke and traumatic CNS injury. Neurochem Int. 2015;89:75–82. doi: 10.1016/j.neuint.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Munoz F, Alamo C, Juckel G, Assion HJ. Half a century of antidepressants: on clinical introduction of monoamine oxidase inhibitors, tricyclics and tetracyclics. Part 1: Monoamine oxidase inhibitors. J Clin Pharmacol. 2007;27:555–559. doi: 10.1097/jcp.0b013e3181bb617. [DOI] [PubMed] [Google Scholar]
- Lopizzo N, Bocchio Chiavetto L, Cattane N, Plazzotta G, Tarazi FI, Pariante CM, Riva MA, Cattaneo A. Gene-environment interaction in major depression: focus on experience-dependent biological systems. Front Psychiatry. 2015;6:68. doi: 10.3389/fpsyt.2015.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopresti AL, Maes M, Maker GL, Hood SD, Drummond PD. Curcumin for the treatment of major depression: a randomised, double-blind, placebo controlled study. J Affect Disord. 2014;167:368–375. doi: 10.1016/j.jad.2014.06.001. [DOI] [PubMed] [Google Scholar]
- Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, Manji HK. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Maes M, Yirmyia R, Noraberg J, Brene S, Hibbeln J, Perini G, Kubera M, Bob P, Lerer B, Maj M. The inflammatory & neurodegenerative (I&ND) hypothesis of depression: leads for future research and new drug developments in depression. Metab Brain Dis. 2009;24:27–53. doi: 10.1007/s11011-008-9118-1. [DOI] [PubMed] [Google Scholar]
- Manji HK, Duman RS. Impairments of neuroplasticity and cellular resilience in severe mood disorders: implications for the development of novel therapeutics. Psychopharmacol Bull. 2001;35:5–49. [PubMed] [Google Scholar]
- Manji HK, Quiroz JA, Sporn J, Payne JL, Denicoff K, Gray NA, Zarate CA, Jr, Charney DS. Enhancing neuronal plasticity and cellular resilience to develop novel, improved therapeutics for difficult-to-treat depression. Biol Psychiatry. 2003;53(8):707–742. doi: 10.1016/s0006-3223(03)00117-3. [DOI] [PubMed] [Google Scholar]
- Martin G. Network analysis and the connectopathies: current research and future approaches. Nonlinear Dynamics Psychol Life Sci. 2012;16(1):79–90. [PubMed] [Google Scholar]
- McClernon FJ, Hiott FB, Westman EC, Rose JE, Levin ED. Transdermal nicotine attenuates depression symptoms in nonsmokers: a double-blind, placebo-controlled trial. Psychopharmacology. 2006;189:125–133. doi: 10.1007/s00213-006-0516-y. [DOI] [PubMed] [Google Scholar]
- Ménard C, Hodes GE, Russo SJ. Pathogenesis of depression: insights from human and rodent studies. Neuroscience. 2015 doi: 10.1016/j.neuroscience.2015.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez-David I, Hen R, Gardier AM, David DJ. Adult hippocampal neurogenesis: an actor in the antidepressant-like action. Ann Pharm Fr. 2013;71(3):143–149. doi: 10.1016/j.pharma.2013.02.006. [DOI] [PubMed] [Google Scholar]
- Mohajeri M, Sadeghizadeh M, Najafi F, Javan M. Polymerized nano-curcumin attenuates neurological symptoms in EAE model of multiple sclerosis through down regulation of inflammatory and oxidative processes and enhancing neuroprotection and myelin repair. Neuropharmacology. 2015;99:156–167. doi: 10.1016/j.neuropharm.2015.07.013. [DOI] [PubMed] [Google Scholar]
- Moreno-Coutino A, Calderon-Ezquerro C, Drucker-Colin R. Long-term changes in sleep and depressive symptoms of smokers in abstinence. Nicotine Tob Res. 2007;9:389–396. doi: 10.1080/14622200701188901. [DOI] [PubMed] [Google Scholar]
- Müller N. Immunology of major depression. NeuroImmunoModulation. 2014;21(2–3):123–130. doi: 10.1159/000356540. [DOI] [PubMed] [Google Scholar]
- Munoz P, Huenchuguala S, Paris I, Cuevas C, Villa M, Caviedes P, Segura-Aguilar J, Tizabi Y. Protective effects of nicotine against aminochrome-induced toxicity in substantia nigra derived cells: implications for Parkinson’s disease. Neurotox Res. 2012;22:177–180. doi: 10.1007/s12640-012-9326-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mythri R, Bharath MM. Curcumin: a potential neuroprotective agent in Parkinson’s disease. Curr Pharm Des. 2012;18:91–99. doi: 10.2174/138161212798918995. [DOI] [PubMed] [Google Scholar]
- Nam SM, Choi JH, Yoo DY, Kim W, Jung HY, Kim JW, Yoo M, Lee S, Kim CJ, Yoon YS, Hwang IK. Effects of curcumin (Curcuma longa) on learning and spatial memory as well as cell proliferation and neuroblast differentiation in adult and aged mice by upregulating brain-derived neurotrophic factor and CREB signaling. J Med Food. 2014;17(6):641–649. doi: 10.1089/jmf.2013.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Mental Health. Awareness month: by the numbers. 2015 www.nimh.nih.gov/…/2015/mentalhealth-awareness-month-by-the-numbers.
- Nefzger MD, Quadfasel FA, Karl VC. A retrospective study of smoking in Parkinson’s disease. Am J Epidemiol. 1968;88:149–158. doi: 10.1093/oxfordjournals.aje.a120874. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- Ng TP, Chiam PC, Lee T, Chua HC, Lim L, Kua EH. Curry consumption and cognitive function in the elderly. Am J Epidemiol. 2006;164:898–906. doi: 10.1093/aje/kwj267. [DOI] [PubMed] [Google Scholar]
- Ng KL, Gibson EM, Hubbard R, Yang J, Caffo B, O’Brien RJ, Krakauer JW, Zeiler SR. Fluoxetine maintains a state of heightened responsiveness to motor training early after stroke in a mouse model. Stroke. 2015;46:2951–2960. doi: 10.1161/STROKEAHA.115.010471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan JB, Ryan KM, Curtin NM, Harkin A, Connor TJ. Noradrenaline reuptake inhibitors limit neuroinflammation in rat cortex following a systemic inflammatory challenge: implications for depression and neurodegeneration. Int J Neuropsychopharmacol. 2009;12:687–699. doi: 10.1017/S146114570800967X. [DOI] [PubMed] [Google Scholar]
- Ogłodek E, Szota A, Just M, Moś D, Araszkiewicz A. The role of the neuroendocrine and immune systems in the pathogenesis of depression. Pharmacol Rep. 2014;66(5):776–781. doi: 10.1016/j.pharep.2014.04.009. [DOI] [PubMed] [Google Scholar]
- Pallàs M, Porquet D, Vicente A, Sanfeliu C. Resveratrol: new avenues for a natural compound in neuroprotection. Curr Pharm Des. 2013;19(38):6726–6731. doi: 10.2174/1381612811319380005. [DOI] [PubMed] [Google Scholar]
- Panahi Y, Badeli R, Karami GR, Sahebkar A. Investigation of the efficacy of adjunctive therapy with bioavailability-boosted curcuminoids in major depressive disorder. Phytother Res. 2015;29(1):17–21. doi: 10.1002/ptr.5211. [DOI] [PubMed] [Google Scholar]
- Piao WH, Campagnolo D, Dayao C, Lukas RJ, Wu J, Shi FD. Nicotine and inflammatory neurological disorders. Acta Pharmacol Sin. 2009;30:715–722. doi: 10.1038/aps.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Lewis AS, van Schalkwyk GI, Mineur YS. Mood and anxiety regulation by nicotinic acetylcholine receptors: a potential pathway to modulate aggression and related behavioral states. Neuropharmacology. 2015;96:235–243. doi: 10.1016/j.neuropharm.2014.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerleau CS, Pomerleau OF. Euphoriant effects of nicotine in smokers. Psychopharmacology. 1992;108:460–465. doi: 10.1007/BF02247422. [DOI] [PubMed] [Google Scholar]
- Posadas I, López-Hernández B, Ceña V. Nicotinic receptors in neurodegeneration. Curr Neuropharmacol. 2013;11(3):298–314. doi: 10.2174/1570159X11311030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postal M, Appenzeller S. The importance of cytokines and autoantibodies in depression. Autoimmun Rev. 2015;14(1):30–35. doi: 10.1016/j.autrev.2014.09.001. [DOI] [PubMed] [Google Scholar]
- Qualls Z, Brown D, Ramlochansingh C, Hurley LL, Tizabi YY. Protective effects of curcumin against rotenone and salsolinol-induced toxicity: implications for Parkinson’s disease. Neurotox Res. 2014;25:81–89. doi: 10.1007/s12640-013-9433-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, Parameswaran N, McCallum SE, Bordia T, Bao S, McCormack A, Kim A, Tyndale RF, Langston JW, Di Monte DA. Chronic oral nicotine treatment protects against striatal degeneration in MPTP-treated primates. J Neurochem. 2006;98:1866–1875. doi: 10.1111/j.1471-4159.2006.04078.x. [DOI] [PubMed] [Google Scholar]
- Quik M, Huang LZ, Parameswaran N, Bordia T, Campos C, Perez XA. Multiple roles for nicotine in Parkinson’s disease. Biochem Pharmacol. 2009;78:677–685. doi: 10.1016/j.bcp.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, Zhang D, Perez XA, Bordia T. Role for the nicotinic cholinergic system in movement disorders; therapeutic implications. Pharmacol Ther. 2014;144(1):50–59. doi: 10.1016/j.pharmthera.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman S. Targeting brain nicotinic acetylcholine receptors to treat major depression and co-morbid alcohol or nicotine addiction. CNS Neurol Disord Drug Targets. 2015;14:647–653. doi: 10.2174/1871527314666150429112954. [DOI] [PubMed] [Google Scholar]
- Ramachandraih CT, Subramanyam N, Bar KJ, Baker G, Yeragani VK. Antidepressants: from MAOIs to SSRIs and more. Indian J Psychiatry. 2011;53(2):180–182. doi: 10.4103/0019-5545.82567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramlochansingh C, Taylor RE, Tizabi Y. Toxic effects of low alcohol and nicotine combinations in SH-SY5Y cells are apoptotically mediated. Neurotox Res. 2011;20:263–269. doi: 10.1007/s12640-011-9239-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rege SD, Geetha T, Griffin GD, Broderick TL, Babu JR. Neuroprotective effects of resveratrol in Alzheimer disease pathology. Front Aging Neurosci. 2014;11(6):218. doi: 10.3389/fnagi.2014.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross GW, Petrovitch H. Current evidence for neuroprotective effects of nicotine and caffeine against Parkinson’s disease. Drugs Aging. 2001;18:797–806. doi: 10.2165/00002512-200118110-00001. [DOI] [PubMed] [Google Scholar]
- Roy M, Tapadia MG, Joshi S, Koch B. Molecular and genetic basis of depression. J Genet. 2014;93(3):879–892. doi: 10.1007/s12041-014-0449-x. [DOI] [PubMed] [Google Scholar]
- Ruby AJ, Kuttan G, Babu KD, Rajasekharan KN, Kuttan R. Anti-tumour and antioxidant activity of natural curcuminoids. Cancer Lett. 1995;94:79–83. doi: 10.1016/0304-3835(95)03827-j. [DOI] [PubMed] [Google Scholar]
- Salin-Pascual RJ, de la Fuente JR, Galicia-Polo L, Drucker-Colin R. Effects of transderman nicotine on mood and sleep in nonsmoking major depressed patients. Psychopharmacology. 1995;121:476–479. doi: 10.1007/BF02246496. [DOI] [PubMed] [Google Scholar]
- Sandur SK, Ichikawa H, Pandey MK, Kunnumakkara AB, Sung B, Sethi G, Aggarwal BB. Role of pro-oxidants and antioxidants in the anti-inflammatory and apoptotic effects of curcumin (diferuloylmethane) Free Radic Biol Med. 2007a;43:568–580. doi: 10.1016/j.freeradbiomed.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandur SK, Pandey MK, Sung B, Ahn KS, Murakami A, Sethi G, Limtrakul P, Badmaev V, Aggarwal BB. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis. 2007b;28:1765–1773. doi: 10.1093/carcin/bgm123. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Is impaired neurogenesis relevant to the affective symptoms of depression? Biol Psychiatry. 2004;56:137–139. doi: 10.1016/j.biopsych.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Schifilliti D, Grasso G, Conti A, Fodale V. Anaesthetic-related neuroprotection: intravenous or inhalational agents? CNS Drugs. 2010;24:893–907. doi: 10.2165/11584760-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Seidl SE, Potashkin JA. The promise of neuroprotective agents in Parkinson’s disease. Front Neurol. 2011;2(2011):00068. doi: 10.3389/fneur.2011.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba J, Mataki C, Yamada S, Nankai M, Toru M. Antidepressant like effects of chronic nicotine on learned helplessness paradigm in rats. Biol Psychiatry. 1998;43:389–391. doi: 10.1016/s0006-3223(97)00477-0. [DOI] [PubMed] [Google Scholar]
- Seo HJ, Wang SM, Han C, Lee SJ, Patkar AA, Masand PS, Pae CU. Curcumin as a putative antidepressant. Expert Rev Neurother. 2015;15(3):269–280. doi: 10.1586/14737175.2015.1008457. [DOI] [PubMed] [Google Scholar]
- Shah A, Carreno FR, Frazer A. Therapeutic modalities for treatment resistant depression: focus on vagal nerve stimulation and ketamine. Clin Psychopharmacol Neurosci. 2014;12(2):83–93. doi: 10.9758/cpn.2014.12.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma OP. Antioxidant activity of curcumin and related compounds. Biochem Pharmacol. 1976;25:1811–1812. doi: 10.1016/0006-2952(76)90421-4. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. Am J Psychiatry. 2003;160:1516–1518. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- Sheline YI, West T, Yarasheski K, Swarm R, Jasielec MS, Fisher JR, Ficker WD, Yan P, Xiong C, Frederiksen C, Grzelak MV, Chott R, Bateman RJ, Morris JC, Mintun MA, Lee JM, Cirrito JR. An antidepressant decreases CSF Aβ production in healthy individuals and in transgenic AD mice. Sci Transl Med. 2014;6(236):236. doi: 10.1126/scitranslmed.3008169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi FD, Piao WH, Kuo YP, Campagnolo DI, Vollmer TL, Lukas RJ. Nicotinic attenuation of central nervous system inflammation and autoimmunity. J Immunol. 2009;182:1730–1739. doi: 10.4049/jimmunol.182.3.1730. [DOI] [PubMed] [Google Scholar]
- Shu L, Li T, Han S, Ji F, Pan C, Zhang B, Li J. Inhibition of neuron-specific CREB dephosphorylation is involved in propofol and ketamine-induced neuroprotection against cerebral ischemic injuries of mice. Neurochem Res. 2012;37(1):49–58. doi: 10.1007/s11064-011-0582-3. [DOI] [PubMed] [Google Scholar]
- Simma N, Bose T, Kahlfuß S, Mankiewicz J, Lowinus T, Lühder F, Schüler T, Schraven B, Heine M, Bommhardt U. NMDA-receptor antagonists block B-cell function but foster IL-10 production in BCR/CD40-activated B cells. Cell Commun Signal. 2014;12:75. doi: 10.1186/s12964-014-0075-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla N, Dhawan DK. N-methyl N-nitrosourea induced functional and structural alterations in mice brain-role of curcumin. Neurotox Res. 2012;22(2):115–126. doi: 10.1007/s12640-011-9307-2. [DOI] [PubMed] [Google Scholar]
- Sinha K, Chaudhary G, Gupta YK. Protective effect of resveratrol against oxidative stress in middle cerebral artery occlusion model of stroke in rats. Life Sci. 2002;71:655–665. doi: 10.1016/s0024-3205(02)01691-0. [DOI] [PubMed] [Google Scholar]
- Song C, Wang H. Cytokines mediated inflammation and decreased neurogenesis in animal models of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:760–768. doi: 10.1016/j.pnpbp.2010.06.020. [DOI] [PubMed] [Google Scholar]
- Spedding M, Gressens P. Neurotrophins and cytokines in neuronal plasticity. Novartis Found Symp. 2008;289:222–233. doi: 10.1002/9780470751251.ch18. [DOI] [PubMed] [Google Scholar]
- Spring B, Cook JW, Appelhans B, Maloney A, Richmond M, Vaughn J, Vanderveen J, Hedeker D. Nicotine effects on affective response in depression-prone smokers. Psychopharmacology. 2008;196:461–471. doi: 10.1007/s00213-007-0977-7. [DOI] [PubMed] [Google Scholar]
- Stepanichev M, Dygalo NN, Grigoryan G, Shishkina GT, Gulyaeva N. Rodent models of depression: neurotrophic and neuroinflammatory biomarkers. Biomed Res Int. 2014;2014:932757. doi: 10.1155/2014/932757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Sun X, Liu T, Zhao M, Zhao S, Xiao T, Jolkkonen J, Zhao C. Fluoxetine enhanced neurogenesis is not translated to functional outcome in stroke rats. Neurosci Lett. 2015;603:31–36. doi: 10.1016/j.neulet.2015.06.061. [DOI] [PubMed] [Google Scholar]
- Taniguchi T, Shibata K, Yamamoto K. Ketamine inhibits endotoxin-induced shock in rats. Anesthesiology. 2001;95:928–932. doi: 10.1097/00000542-200110000-00022. [DOI] [PubMed] [Google Scholar]
- Taniguchi T, Kanakura H, Takemoto Y, Yamamoto K. The antiinflammatory effects of ketamine in endotoxemic rats during moderate and mild hypothermia. Anesth Analg. 2004;98:1114–1120. doi: 10.1213/01.ANE.0000100740.07331.A2. [DOI] [PubMed] [Google Scholar]
- Tegenge MA, Rajbhandari L, Shrestha S, Mithal A, Hosmane S, Venkatesan A. Curcumin protects axons from degeneration in the setting of local neuroinflammation. Exp Neurol. 2014;253:102–110. doi: 10.1016/j.expneurol.2013.12.016. [DOI] [PubMed] [Google Scholar]
- Thacker EL, O’Reilly EJ, Weisskopf MG, Chen H, Schwarzschild MA, McCullough ML, Calle EE, Thun MJ, Ascherio A. Temporal relationship between cigarette smoking and risk of Parkinson disease. Neurology. 2007;68:764–768. doi: 10.1212/01.wnl.0000256374.50227.4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizabi Y, Overstreet DH, Rezvani AH, Louis VA, Clark E, Jr, Janowsky DS, Kling MA. Antidepressant effects of nicotine in an animal model of depression. Psychopharmacology. 1999;142:193–199. doi: 10.1007/s002130050879. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Rezvani AH, Russell LT, Tyler KY, Overstreet DH. Depressive characteristics of FSL rats: involvement of central nicotinic receptors. Pharmacol Biochem Behav. 2000;66:73–77. doi: 10.1016/s0091-3057(00)00236-7. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Getachew B, Rezvani AH, Hauser SR, Overstreet DH. Antidepressant-like effects of nicotine and reduced nicotinic receptor binding in the Fawn-Hooded rat, an animal model of co-morbid depression and alcoholism. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:398–402. doi: 10.1016/j.pnpbp.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizabi Y, Hauser SR, Tyler KY, Getachew B, Madani R, Sharma Y, Manaye KF. Effects of nicotine on depressive-like behavior and hippocampal volume of female WKY rats. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:62–69. doi: 10.1016/j.pnpbp.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizabi Y, Hurley LL, Qualls Z, Akinfiresoye L. Relevance of the anti-inflammatory properties of curcumin in neurodegenerative diseases and depression. Molecules. 2014;19(12):20864–20879. doi: 10.3390/molecules191220864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toben C, Baune BT. An act of balance between adaptive and maladaptive immunity in depression: a role for T lymphocytes. J Neuroimmune Pharmacol. 2015 doi: 10.1007/s11481-015-9620-2. [DOI] [PubMed] [Google Scholar]
- Toth E, Gersner R, Wilf-Yarkoni A, Raizel H, Dar DE, Richter-Levin G, Levit O, Zangen A. Age-dependent effects of chronic stress on brain plasticity and depressive behavior. J Neurochem. 2008;107:522–532. doi: 10.1111/j.1471-4159.2008.05642.x. [DOI] [PubMed] [Google Scholar]
- Tredici G, Miloso M, Nicolini G, Galbiati S, Cavaletti G, Bertelli A. Resveratrol, map kinases and neuronal cells: might wine be a neuroprotectant? Drugs Exp Clin Res. 1999;25:99–103. [PubMed] [Google Scholar]
- Tsoh JY, Humfleet GL, Munoz RF, Reus VI, Hartz DT, Hall SM. Development of major depression after treatment for smoking cessation. Am J Psychiatry. 2000;157:368–374. doi: 10.1176/appi.ajp.157.3.368. [DOI] [PubMed] [Google Scholar]
- Uher R, Tansey KE, Dew T, Maier W, Mors O, Hauser J, Dernovsek MZ, Henigsberg N, Souery D, Farmer A, McGuffin P. An inflammatory biomarker as a differential predictor of outcome of depression treatment with escitalopram and nortriptyline. Am J Psychiatry. 2014;171(12):1278–1286. doi: 10.1176/appi.ajp.2014.14010094. [DOI] [PubMed] [Google Scholar]
- Vas CJ, Pinto C, Panikker D, Noronha S, Deshpande N, Kulkarni L, Sachdeva S. Prevalence of dementia in an urban Indian population. Int Psychogeriatr. 2001;13:439–450. doi: 10.1017/s1041610201007852. [DOI] [PubMed] [Google Scholar]
- Villanueva R. Neurobiology of major depressive disorder. Neural Plast. 2013;2013:873278. doi: 10.1155/2013/873278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vingtdeux V, DresesWerringloer U, Zhao H, Davies P, Marambaud P. Therapeutic potential of resveratrol in Alzheimer’s disease. BMC Neurosci. 2008;9(Suppl 2):S6. doi: 10.1186/1471-2202-9-S2-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Huang Y, Zou J, Cao K, Xu Y, Wu JM. Effects of red wine and wine polyphenol resveratrol on platelet aggregation in vivo and in vitro. Int J Mol Med. 2002;9:77–79. [PubMed] [Google Scholar]
- Wang Z, Zhang Q, Yuan L, Wang S, Liu L, Yang X, Li G, Liu D. The effects of curcumin on depressive-like behavior in mice after lipopolysaccharide administration. Behav Brain Res. 2014;274:282–290. doi: 10.1016/j.bbr.2014.08.018. [DOI] [PubMed] [Google Scholar]
- Wiborg O. Chronic mild stress for modeling anhedonia. Cell Tissue Res. 2013;354(1):155–169. doi: 10.1007/s00441-013-1664-0. [DOI] [PubMed] [Google Scholar]
- World Federation for Mental Health. 2012 www.who.int/mental_health/…/wfmh_paper_depression_wmhd_2012.
- Xu H, Steven Richardson J, Li XM. Dose-related effects of chronic antidepressants on neuroprotective proteins BDNF, Bcl-2 and Cu/Zn-SOD in rat hippocampus. Neuropsychopharmacology. 2003;28(1):53–62. doi: 10.1038/sj.npp.1300009. [DOI] [PubMed] [Google Scholar]
- Xu Y, Ku BS, Yao HY, Lin YH, Ma X, Zhang YH, Li XJ. Antidepressant effects of curcumin in the forced swim test and olfactory bulbectomy models of depression in rats. Pharmacol Biochem Behav. 2005a;82:200–206. doi: 10.1016/j.pbb.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Xu Y, Ku BS, Yao HY, Lin YH, Ma X, Zhang YH, Li XJ. The effects of curcumin on depressive-like behaviors in mice. Eur J Pharmacol. 2005b;518:40–46. doi: 10.1016/j.ejphar.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Yakel JL. Cholinergic receptors: functional role of nicotinic ACh receptors in brain circuits and disease. Pflugers Arch. 2013;465:441–450. doi: 10.1007/s00424-012-1200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Jiang RY, Shen J, Hong T, Liu N, Ding LC, Wang DM, Chen LJ, Xu B, Zhu B. Ketamine attenuates the lipopolysaccharide-induced inflammatory response in cultured N2a cells. Mol Med Rep. 2013a;8:217–220. doi: 10.3892/mmr.2013.1465. [DOI] [PubMed] [Google Scholar]
- Yang C, Su X, Liu A, Zhang L, Yu A, Xi Y, Zhai G. Advances in clinical study of curcumin. Curr Pharm. 2013b;19:1966–1973. [PubMed] [Google Scholar]
- Yang J, Song S, Li J, Liang T. Neuroprotective effect of curcumin on hippocampal injury in 6-OHDA-induced Parkinson’s disease rat. Pathol Res Pract. 2014a;210(6):357–362. doi: 10.1016/j.prp.2014.02.005. [DOI] [PubMed] [Google Scholar]
- Yang Z, Zhao T, Zou Y, Zhang JH, Feng H. Curcumin inhibits microglia inflammation and confers neuroprotection in intracerebral hemorrhage. Immunol Lett. 2014b;160(1):89–95. doi: 10.1016/j.imlet.2014.03.005. [DOI] [PubMed] [Google Scholar]
- Yu JJ, Pei LB, Zhang Y, Wen ZY, Yang JL. Chronic supplementation of curcumin enhances the efficacy of antidepressants in major depressive disorder: a randomized, double-blind. Placebo-Controlled Pilot Study. J Clin Psychopharmacol. 2015;35(4):406–410. doi: 10.1097/JCP.0000000000000352. [DOI] [PubMed] [Google Scholar]
- Yuan J, Zou M, Xiang X, Zhu H, Chu W, Liu W, Chen F, Lin J. Curcumin improves neural function after spinal cord injury by the joint inhibition of the intracellular and extracellular components of glial scar. J Surg Res. 2015;195(1):235–245. doi: 10.1016/j.jss.2014.12.055. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Quiroz JA, De Jesus G, Denicoff KK, Luckenbaugh DA, Manji HK, Charney DS. A double-blind, placebo-controlled study of memantine in the treatment of major depression. Am J Psychiatry. 2006;163:153–155. doi: 10.1176/appi.ajp.163.1.153. [DOI] [PubMed] [Google Scholar]
- Zhang F, Wang H, Wu Q, Lu Y, Nie J, Xie X, Shi J. Resveratrol protects cortical neurons against microglia-mediated neuroinflammation. Phytother Res. 2012;27:344–349. doi: 10.1002/ptr.4734. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wei H, Lin M, Chen C, Wang C, Liu M. Curcumin protects against ischemic spinal cord injury: the pathway effect. Neural Regen Res. 2013;8(36):3391–3400. doi: 10.3969/j.issn.1673-5374.2013.36.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Luo J, Zhang M, Yao W, Ma X, Yu SY. Effects of curcumin on chronic, unpredictable, mild, stress-induced depressive-like behaviour and structural plasticity in the lateral amygdala of rats. Int J Neuropsychopharmacol. 2014;17(5):793–806. doi: 10.1017/S1461145713001661. [DOI] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]