Abstract
Objectives
To compare the prevalence of cognitive, neurological, and behavioral outcomes at 10 years of age in 428 girls and 446 boys who were born extremely preterm (EP).
Study design
889 of 966 eligible children previously enrolled in the multi-center Extremely Low Gestational Age Newborns (ELGAN) Study from 2002–2004 were evaluated at 10 years of age. Children underwent a neuropsychological battery and testing for autism spectrum disorder (ASD), and parents reported on their child’s behavior, development, and seizures.
Results
28% of boys and 21% of girls exhibited moderate to severe impairment on summary measures of cognitive abilities. Boys had a higher prevalence of impairment than girls in nearly all measures of cognition, were more than twice as likely to have microcephaly (15% in boys, 8% in girls), and require more often assistive devices to ambulate (6% in boys, 4% in girls). In contrast, boys and girls had comparable risk for a history of seizure (identified in 10% of the cohort) or epilepsy (identified in 7% of the cohort). The boy-to-girl ratio of ASD (9% in boys, 5% in girls) was lower than expected compared with the overall US autism population.
Conclusion
In this contemporary cohort of children born extremely premature and evaluated at school age, boys had higher prevalence of cognitive, neurological, and behavioral deficits than girls. The ratio of boys to girls among those with ASD deserves further study as does the perinatal environmental- genetic interactions that might contribute to male preponderance of deficits in this high-risk sample.
Compared with term-born peers, children born extremely premature (EP) are at greatly increased risk for cognitive, behavioral, and neurological disabilities.(1–4) Among EP children, follow-up studies in early childhood report higher rates of neurological and early cognitive impairment in boys compared with girls.(5–14). Some of this increase in risk may be attributable to higher rates of neonatal morbidities among boys.(15)
Although many studies provide evidence for a male disadvantage on early neurodevelopmental assessments among children born EP, only a few large, epidemiological studies of children born EP after the early 1990s, in the era of routine surfactant and antenatal steroid use, have assessed cognitive outcomes in later childhood (7–12 years old) or adolescence. In a United Kingdom cohort of 11-year-old EP children, boys had lower scores than girls on assessments of cognition and reading achievement.(16, 17) In addition, boys were more likely than girls to have cerebral palsy, special education needs, and autism spectrum disorders (ASD). Neither a study of 219 extremely low birth weight children (< 1000 grams) born in the United States between 1992 and 1995 in which 8-year-old children had a mean IQ score 10 points lower than those of term-born controls(18), nor a study of 154 children born EP in Germany in which one-quarter of the children had intellectual disability at age 8 years, reported important sex-related differences in outcome. (19) Moreover, a recent meta-analysis of studies published through 2014 reported that the greater severity of neurocognitive deficits among boys in early childhood appears to ameliorate after age 5.(20) Our epidemiological study examined the school-age cognitive, behavioral, and neurological outcomes of EP boys compared with those of EP girls to verify this trend. This comparison is especially important in light of recently-reported reductions in the prevalence of cognitive and neurological disability in those born EP.(9) Here we compare the prevalence of dysfunctions among 428 EP girls and 446 EP boys born from 2002 to 2004 on a wide spectrum of cognitive, neurological, and behavioral outcomes, including intelligence quotient (IQ), executive function (EF), language ability, epilepsy, motor impairment, microcephaly, and ASD.
Methods
The Extremely Low Gestational Age Newborns (ELGAN) Study is a national, multicenter, observational study of the risk of structural and functional neurological disorders in extremely preterm infants.(21) During the years 2002–2004, women delivering before 28 weeks gestation were asked to enroll in the study. 1249 mothers of 1506 infants consented to participate. Of 1200 ELGAN Study survivors, 1102 (92%) were evaluated at age 2 years. At 10 years of age, 966 surviving children, on whom blood specimens were collected in the first weeks of life for assessment of inflammation-related proteins, were recruited. The families of 889 (92%) of these children returned for follow up and 874 children were assessed on cognitive, neurological, and behavioral outcomes. The institutional review boards of all participating institutions approved enrollment and consent procedures for this follow-up study.
Families willing to participate were scheduled for one visit, usually at the institution of birth. The cognitive evaluations were administered by certified child psychologists in a 3- to 4-hour session that included breaks. All psychologist examiners underwent a 1-day in-person training and verification of competency for administering the neurocognitive test battery. Further, all evaluators for ASD participated in research-level training in the administration and scoring of the Autism Diagnostic Interview - Revised (ADI-R) and Autism Diagnostic Observation Schedule-2 (ADOS) and established inter-rater reliability with the study autism expert (R.M.J.). Parent and child measures were selected to provide the most comprehensive assessment possible in one 3–4 hour testing session, with the exception of children who screened positive for ASD, who returned for a second visit.
The goal was to assess children’s abilities in a number of key domains, some of which were assessable by direct examination of the child, and others were provided by the parent (and teacher). The direct assessments reported in this manuscript evaluated general cognitive ability, language ability, EF, aspects of ASD, and presence of seizure or epilepsy.
General cognitive ability (or IQ) was assessed with the School-Age Differential Ability Scales–II (DAS-II[22]) Verbal and Nonverbal Reasoning scales.
Expressive and receptive language skills were evaluated with the Oral and Written Language Scales (OWLS [23]), which assesses semantic, morphological, syntactic, and pragmatic production and comprehension of elaborated sentences.
Attention and executive function were assessed with the DAS-II and the NEuroPSYchological Assessment (NEPSY-II).(24) DAS-II Recall of Digits Backward and Recall of Sequential Order measured verbal working memory. NEPSY-II Auditory Attention and Auditory Response Set measured sustained auditory attention, set switching and inhibition. NEPSY-II Inhibition-Inhibition and Inhibition-Switching measured simple inhibition and inhibition in the context of set shifting, respectively. NEPSY-II Animal Sorting measured visual concept formation and mental flexibility.
Using latent profile analysis (LPA) to find EP children with similar distinctive profiles on measures of cognitive and executive functioning, we identified four subgroups corresponding to functioning that was normal (34% of cohort), low-normal (41%), moderately impaired (17%), and severely impaired (8%).
Identification of seizures involved a two-stage process.(25) First, a “yes” answer to any of 11 broad questions for history of possible seizures prompted the expert pediatric epileptologist to conduct a structured interview followed by an open-ended interview, and then to determine whether a reported event was a seizure. A second epileptologist independently reviewed interview responses and similarly rated the event type. When the two physicians’ disagreed on the presence of seizures, which occurred in 3% of the children whose parent was interviewed, a third epileptologist reviewed the interview responses and made the final determination as to whether or not a seizure had occurred. For these analyses, we defined epilepsy as having 2 or more seizures unassociated with fever or other provocation.
After undergoing initial training, a research assistant rated each child on the Gross Motor Function Classification System (GMFCS). A level of 0 indicates normal motor function without limitation. A level of 1 or 2 indicates that the child can walk independently with limitations, but does not require assistive mobility devices, whereas children with a level of ≥ 3 require such devices.(26)
The head circumference was measured as the largest possible occipital- frontal circumference. Measurements were rounded to the closest 0.1 centimeter. All head circumferences are presented as Z-scores.(27)
All children were screened by parent report with the Social Communication Questionnaire (SCQ) for risk of ASD. Children determined to be at risk on the SCQ, were assessed with the Autism Diagnostic Interview – Revised (ADI-R), an in-depth parent interview. Children meeting ADI-R criteria for ASD were administered the Autism Diagnostic Observation Schedule-2 (ADOS-2). Children meeting standardized research criteria for ASD on both the ADI-R and ADOS-2 were classified as having ASD.
Statistical Analyses
To compare the distribution of neurocognitive test scores among boys and girls born EP with the expected distribution for the normal population, test scores were re-expressed as Z-scores using normative means and standard deviations (SD). For a normally-distributed population, 2.3% of children would be expected to have Z-scores at or below −2, and 13.6% to have Z-scores greater than −2 and less than or equal to −1. Adjusted odds ratios (OR) and 95% confidence intervals (CI) comparing boys to girls on the likelihood of having a test score more than 2 SD below the normative mean were calculated using multivariable logistic regression (multivariable multinomial logistic regression for the LPA classification) adjusting for variables that are associated both with outcome and preterm birth: maternal age, race, education, insurance status, and child gestational age, and birth weight Z-score. Parents of 48 children out of the 315 who screened positive for possible seizures did not complete the seizure interviews with the epileptologist. Inverse probability weighting was used to account for these missing data in estimating seizure prevalence.
Results
Boys were less likely than girls to be growth restricted, and more likely than girls to be born at earlier gestational age (Table I). At age 2 years, boys were more likely than girls to have a Mental Development Index or a Psychomotor Development Index (Bayley Scales of Infant Development-II) more than 3 SD below the normative mean (<55).(1)
Table 1. Sample characteristics by sex (column percents).
| Sex | Row N |
|||
|---|---|---|---|---|
| Girls (n=428) |
Boys (n=446) |
|||
| Maternal characteristics | ||||
| Racial identity | White | 62 | 64 | 543 |
| Black | 26 | 26 | 226 | |
| Other | 12 | 10 | 95 | |
| Hispanic | Yes | 12 | 8 | 86 |
| Age, years | < 21 | 15 | 11 | 113 |
| 21–35 | 65 | 69 | 586 | |
| > 35 | 20 | 20 | 175 | |
| Education, years | ≤ 12 (high school) | 42 | 40 | 347 |
| > 12, < 16 | 25 | 22 | 198 | |
| ≥ 16 (≥ college) | 33 | 38 | 304 | |
| Single marital status | Yes | 41 | 39 | 348 |
| Public insurance | Yes | 38 | 32 | 301 |
| Newborn characteristics | ||||
| Gestational age, weeks | 23–24 | 18 | 23 | 180 |
| 25–26 | 46 | 45 | 396 | |
| 27 | 36 | 32 | 298 | |
| Birth weight, grams | ≤ 750 | 40 | 34 | 323 |
| 751–1000 | 43 | 44 | 379 | |
| > 1000 | 17 | 22 | 172 | |
| Birth weight Z-score | < −2 | 8 | 4 | 51 |
| ≥ −2, < −1 | 18 | 9 | 116 | |
| ≥ −1 | 74 | 87 | 707 | |
Distribution of verbal and nonverbal IQ (DAS-II) and NEPSY-II
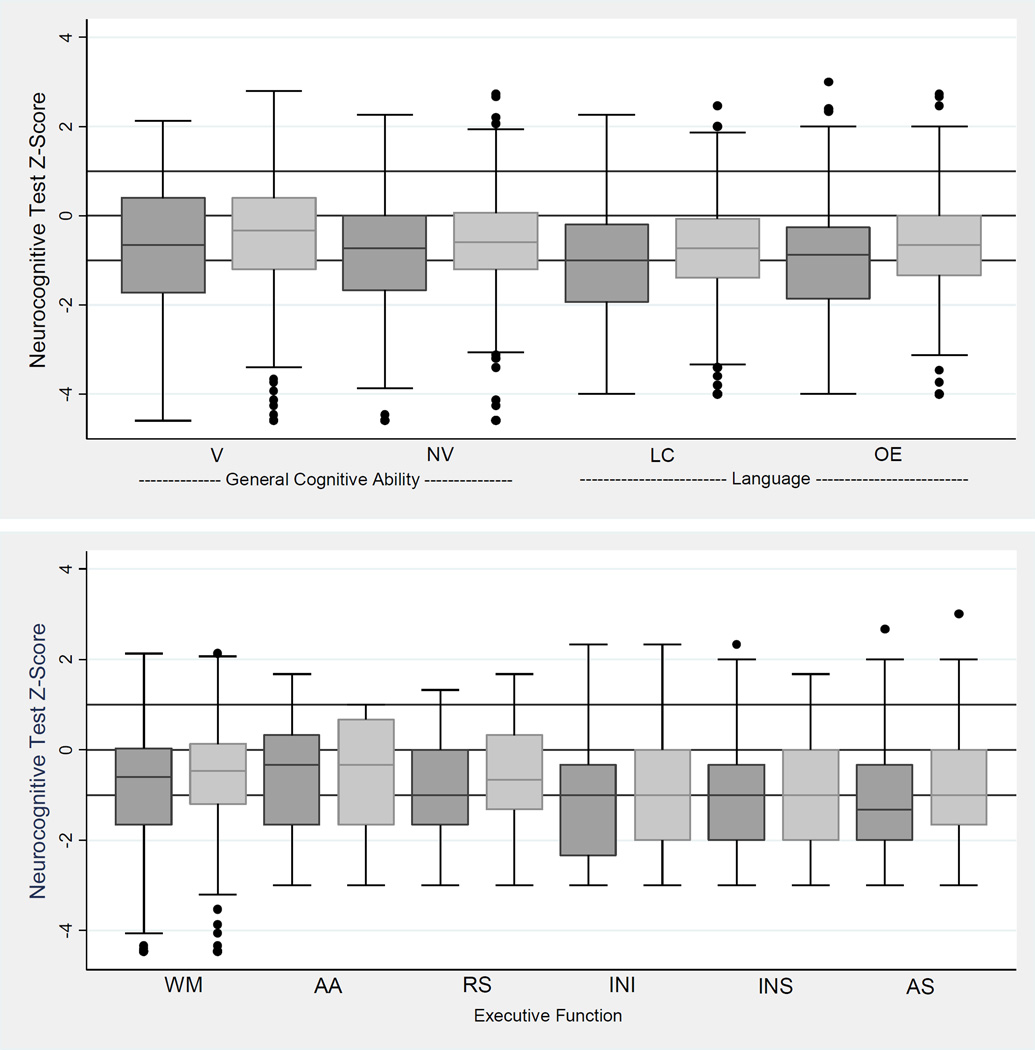
For both girls and boys, the distributions of DAS-II and NEPSY-II scores were shifted to lower values compared with the expected normal distribution (horizontal lines at 0, 1, and −1 on the vertical scale indicate values at the mean and 1 SD above and below the population mean, respectively) (Figure 1). Boys had lower scores than girls across all neurocognitive tests, including DAS verbal and nonverbal IQ, and language, working memory, and executive function measures.
Figure 1.
Box-and-whisker plots of General Cognitive Ability scores (DAS-II), Language scores (OWLS) and Executive Function scores (DAS-II and NEPSY-II). All test Z-scores are adjusted to population norms. Key: darker gray is boys and lighter gray is girls. The central line in the box indicates the median (50th centile), while the top of the box indicates the 75th centile and the bottom of the box indicates the 25th centile. If ELGANs had the expected normal distribution of term-born children, the middle of the box would be at Z=0 and the upper and lower ends of the box would be at Z=1 and Z= −1, respectively. V=Verbal Reasoning (DAS-II), NV=Nonverbal Reasoning (DAS-II), LC=Listening Comprehension (OWLS), OE=Oral Expression (OWLS), WM=Working Memory (DAS-II), AA=Auditory Attention (NEPSY-II), RS=Auditory Response Set (NEPSY-II), INI=Inhibition Inhibition (NEPSY-II), INS=Inhibition Switching (NEPSY-II), AS=Animal Sorting (NEPSY-II). Maximum N=874.
Distribution of DAS and other neuropsychological measures by sex
Z-scores (ie, the number of standard deviations above or below the normative mean) of −2 or more are expected 2.3% of the time in the normal population. The distribution of almost every assessment is shifted toward lower scores, with scores 2 or more SD below the normal population mean occurring in 12 to 36% of the sample, in contrast to the expected 2.3%, and scores 1 to 2 SD below the mean occurring in 14 to 31%, in contrast to the expected 13.6%. On most neurocognitive tests, boys had lower scores than girls. The most prominent sex differences were found in the proportion of children who had abilities more than 2 SD below the mean on DAS-II Verbal Reasoning Ability (12% of girls v. 22% of boys) and OWLS Listening Comprehension (13% of girls v. 24% of boys) (Table 2).
Table 2. Row percents of neurocognitive test Z-scores by sex.
Neurocognitive assessments are on the left. Adjusted ORs compare boys to girls on risk of a score 2 or more SD below the expected mean.
| Z-score (row percent) | aOR* (95% CI) of a Z-score ≤ −2 for boys |
|||||
|---|---|---|---|---|---|---|
| ≤ −2 | > −2, ≤ −1 | > −1, ≤ 1 | > 1 | |||
| Expected % | 2.3% | 13.6% | 68.2% | 15.9% | ||
| General Cognitive Ability | ||||||
| DAS-II Verbal Reasoning Ability (V) | Girls | 12 | 19 | 62 | 7 | |
| Boys | 22 | 19 | 51 | 8 | 2.3 (1.5, 3.4) | |
| DAS-II Nonverbal Reasoning Ability (NV) | Girls | 12 | 23 | 60 | 6 | |
| Boys | 18 | 26 | 51 | 4 | 1.6 (1.1, 2.4) | |
| Language | ||||||
| OWLS Listening Comprehension | Girls | 13 | 28 | 55 | 4 | |
| Boys | 24 | 26 | 45 | 4 | 2.4 (1.6, 3.5) | |
| OWLS Oral Expression Expression | Girls | 15 | 22 | 56 | 7 | |
| Boys | 24 | 22 | 48 | 6 | 2.2 (1.5, 3.2) | |
| Executive Function | ||||||
| DAS-II Working Memory | Girls | 12 | 14 | 72 | 2 | |
| Boys | 18 | 15 | 65 | 3 | 1.5 (1.02, 2.2) | |
| NEPSY-II Auditory Attention | Girls | 20 | 21 | 58 | 0 | |
| Boys | 25 | 20 | 54 | 0 | 1.3 (0.9, 1.8) | |
| NEPSY-II Auditory Response Set | Girls | 16 | 26 | 54 | 4 | |
| Boys | 24 | 30 | 43 | 4 | 1.9 (1.3, 2.7) | |
| NEPSY-II Inhibition Inhibition | Girls | 31 | 25 | 41 | 3 | |
| Boys | 36 | 22 | 38 | 4 | 1.3 (0.9, 1.7) | |
| NEPSY-II Inhibition Switching | Girls | 27 | 29 | 38 | 5 | |
| Boys | 28 | 29 | 36 | 8 | 1.1 (0.8, 1.5) | |
| NEPSY-II Animal Sorting | Girls | 24 | 30 | 42 | 4 | |
| Boys | 33 | 31 | 35 | 2 | 1.7 (1.3, 2.4) | |
Adjusted for mother's age, race, education, and insurance status, and child's gestational age and birth weight Z-score. Bolded results are significant at p <0.05.
Summary IQ-executive function classification, neurobehavioral and neurological outcomes
With LPA classification as the outcome, a multivariable multinomial logistic regression analysis that adjusted for mother's age, race, education, insurance status, and child's gestational age and birth weight Z-score found boys were twice as likely as girls to be classified as severely impaired (OR: 2.1; 95% CI:1.2, 3.8), and 1.8 times more likely than girls to be classified as moderately impaired (95% CI:1.1, 2.7) (Table 3).
Table 3. Differences between boys and girls on latent profiles of cognitive and executive function, autism and seizures.
The column percents show the prevalence in the outcome strata listed on the left for girls and for boys, while the adjusted Odds Ratios (95% confidence intervals) on the right compare boys’ risk to girls’ risk for each stratum.
| Outcome | Column percent | Row N | aOR* (95% CI) for boys |
||
|---|---|---|---|---|---|
| Girls | Boys | ||||
| LPA profile | Normal | 36 | 33 | 300 | 1.0 |
| Low normal | 44 | 39 | 360 | 1.1 (0.9, 1.5) | |
| Moderately impaired | 15 | 18 | 145 | 1.8 (1.1, 2.7) | |
| Severely impaired | 6 | 10 | 69 | 2.1 (1.2, 3.8) | |
| ASD | 5 | 9 | 61 | 2.0 (1.1, 3.6) | |
| Seizures | Any | 11 | 12 | 105 | 1.1 (0.7, 1.7) |
| Epilepsy | 7 | 8 | 66 | 1.3 (0.7, 2.2) | |
| Microcephaly | at 10 years | 8 | 15 | 104 | 2.4 (1.5, 4.0) |
| Motor impairment (GMFCS) | 0 | 77 | 65 | 624 | 1.0 |
| 1–2 | 19 | 28 | 217 | 1.6 (1.2, 2.3) | |
| 3–5 | 4 | 6 | 43 | 2.1 (1.1, 4.2) | |
| Column Maximum N | 428 | 446 | 874 | ||
Adjusted for mother's age, race, education, and insurance status and child's gestational age and birth weight Z-score. Bolded results are significant at p<0.05.
Boys were more than twice as likely as girls to meet diagnostic criteria for ASD (OR: 2.0; 95% CI 1.1, 3.6), identified in 7% of the cohort. Also, boys had a 50% greater risk than girls for mild limitations in gross motor function (OR: 1.6; 95% CI 1.2, 2.3), identified in nearly one quarter of the cohort. Furthermore, boys were two times more likely than girls to be unable to ambulate independently without assistive devices (OR: 2.1; 95% CI 1.1, 4.0), seen in 5% of the cohort. Also, boys were more than twice as likely as girls to have microcephaly at age 10 (OR: 2.4; 95% CI 1.5, 4.0), identified in 11.9% of the cohort. The greater number of boys with microcephaly and gross motor impairment remained even when children identified to have cerebral palsy at age 2 years were excluded (data not shown). On the other hand, boys and girls had the same rates of seizures and epilepsy, identified in 10% and 7% of the cohort, respectively.
Discussion
In this report of cognitive, behavioral, and neurological outcomes of school-age children born EP, we found that a quarter of the children (28% of boys and 21% of girls) exhibited moderate to severe impairment on summary measures of cognitive abilities. Impairment was more prevalent among boys for nearly all measures of cognition,(28–30) even after adjustment for socio-economic indicators, gestational age, and birth weight Z-score. Furthermore, boys were more than twice as likely to have microcephaly, and to be unable to ambulate without assistive devices. However, boys were not at increased risk for developing a seizure or epilepsy before age 10. The approximately 2:1 boy-girl ratio among those with ASD in our cohort is lower than has been described in unselected samples from mainly term-born populations.(31)
Our data confirm the few other recent large, epidemiological studies that children born EP have an excessive burden of cognitive deficits in later childhood (7–12 years old),(16, 18, 19) and only the Epicure Study found a sex discrepancy with 49% of boys and 30% of girls having moderate to severe cognitive impairment.(16) We confirm that the excessive burden of cognitive deficits reported in boys in early childhood (5–10, 14, 18, 32) also is evident in later childhood. The excess of neurocognitive deficit seen in boys at age 10, evident across nearly all individual and summary neurocognitive test scores, does not support the finding in a study of children born at less than 32 weeks gestation that the cognitive disparity between boys and girls in early childhood is ameliorated after 5 years of age.(20) On the other hand, within the narrow frame of 24 to 28 weeks, our multivariate analyses affirm findings of others that gestational age does not account fully for the outcome differences seen between boys and girls.(5)
One explanation for the observed sex differences is that preterm birth may disrupt hormonal signals such as the conversion of testosterone to estradiol,(33) and that higher circulating concentrations of testosterone in males may accentuate the deleterious consequences of early insults to brain, as reflected in measures of both neuropathology and behavior.(34) Another possibility is that EP boys and girls differ in their rates of brain maturation,(35) as has been observed among children born at term, resulting in differences in vulnerability or resilience to insults. For example, genetic control of critical neurotransmitter development, such as dopamine, appears to differ in boys and girls, and may predispose to greater disease risk in boys.(36) Boys and girls also appear to differ in their response to stimuli that lead to excessive neuronal loss related to dysregulated apoptosis.(37, 38)
Three lines of evidence support the validity of our finding that boys have modestly increased risks in neurodevelopmental morbidities. First, a number of published studies describe similar modest increase in risk of morbidities among boys.(5–10, 14, 18) Second, the higher risks among boys persisted after adjustment for confounders, including gestational age, which is an effective proxy for unmeasured indicators of vulnerability among individuals born extremely premature.(39) Finally, sex differences were found across several neurological domains, decreasing the likelihood that our findings are attributable to chance.
We found that compared with girls, boys are more likely to be microcephalic and to have motor impairment, a finding that we identified also in this cohort at age 2 years even after excluding children with cerebral palsy from the analyses (Data not shown). Although cerebral palsy occurs more often in boys(40), and microcephaly and gross motor abilities are correlates of cerebral palsy, we are not aware of previous reports that identify microcephaly or gross motor function abnormalities that affect boys more than girls in the absence of cerebral palsy. Our observations indicate that the neurological disadvantages boys experience encompass broad structural (ultrasound, microcephaly) and functional (cognition and motor) domains.
On the other hand, the risk of postnatal seizures and epilepsy in boys and girls is comparable in our cohort. This observation is surprising given that compared with girls, boys are more likely to have motor impairment, ASD, and cognitive disabilities, all of which are associated with an elevated risk of having seizures and epilepsy. The vulnerability of premature brains to ultimately develop epilepsy is complex and may involve age-related interplay among the influences of predisposing brain injury, sex hormone status and cellular excitability.(41) Although boys may be at greater risk of structural brain injury, the cellular mechanisms that underlie seizures or epilepsy may be less sex-specific, and may involve inflammatory, genetic, and epigenetic mechanisms.(41, 42) The similar male/female prevalence rates of seizures and epilepsy in our ELGAN cohort, though unexpected, afford an opportunity to explore the relationship between prematurity and neuronal excitability.
Compared with the general population, the rate of ASD in children born EP is approximately 20 times higher in girls and about 9 times higher in boys. Overall in our cohort, boys had a 2-fold increased risk of ASD compared with girls, which stands in contrast to the 4-fold increased risk for autism among boys in the general population (43, 44). We consider three explanations for a lower male-female ratio among children born EP. First, emerging data suggest that the autism phenotype in girls, with a larger proportion of higher functioning girls, may be less likely to be diagnosed in community-based epidemiological studies in early years of life.(31) This could explain the observation in some studies that the ratio of boys to girls is about 4 at younger ages, but rises closer to 2 in late childhood and beyond.(45–47)
Second, the decreased sex ratio in children born EP may reflect differences in risk factors for ASD compared with the general ASD population, including the possibility that factors, which protect girls born at term, are not present among those born EP. Third, the impact of environmental-inflammatory-gene interactions, associated with EP, might not be sex-specific.(48–52)
As a final point, the autism identified in children born EP may differ from that found in the general population because of the higher rates of sensorimotor and intellectual disability.(53) The prevalence of intellectual disability or sensorimotor impairment in our cohort, however, did not differ substantially from rates seen among autistic children in the general population (manuscript in preparation). Consequently, the autism we have identified in EP children using “gold-standard” instruments appears very similar to autism in the general population.
The strengths of this study include the large number of children who were born at an extremely low gestational age and followed until age 10 years, the relatively low rate of loss to follow-up, and the range of neurological and neurocognitive dysfunctions identified using well-validated tools for assessment. Additionally, the cohort represents a broad, unbiased sampling of children living in several regions of the country with diverse socioeconomic, racial, and ethnic characteristics, which make the findings generalizable to most children born EP. One limitation is that we did not evaluate term-born control children, but rather compared the distribution of cognitive outcomes in our cohort with standardized population-based normative means and standard deviations. Although population norms may change over time (Flynn effect)(54), such drift is most often towards higher scores on standardized tests of cognition. Thus, the magnitude of differences seen in our cohort is unlikely explained by modest drift in normative test performance. Finally, as with all observational studies, we are limited in our ability to infer causation or pathways from associations. Regardless, our findings have implications for research on the relationship of perinatal environmental-genetic interactions and cognitive and behavioral outcome.
Acknowledgments
We acknowledge the contributions of the subjects and their families.
Supported by the National Institute of Neurological Disorders and Stroke (5U01NS040069-05 and 2R01NS040069-09). Center grant award from the National Institute of Child Health and Human Development (5P30HD018655-28).
Abbreviations used
- EP
Extremely premature
- IQ
Intelligent quotient
- ASD
Autism spectrum disorder
- ELGAN
Extremely low gestational age newborn
- ADI-R
Autism Diagnostic Interview – Revised
- ADOS
Autism Diagnostic Observation Schedule-2
- EF
Executive function
- DAS-II
Differential Ability Scales–II
- OWLS
Oral and Written Language Scales
- NEPSY-II
NEuroPSYchological Assessment-II
- LPA
Latent profile analysis
- GMFCS
Gross Motor Function Classification System
- SCQ
Social Communication Questionnaire
- SD
Standard deviation
Appendix
Additional ELGAN Study Group Investigators include: Boston Children’s Hospital, Boston, MA: Janice Ware, PhD Taryn Coster, BA Brandi Hanson, PsyD Rachel Wilson, PhD Kirsten McGhee, PhD Patricia Lee, PhD Aimee Asgarian, PhD Anjali Sadhwani, PhD Tufts Medical Center, Boston, MA: Ellen Perrin, MD Emily Neger, MA Kathryn Mattern, BA Jenifer Walkowiak, PhD Susan Barron, PhD Baystate Medical Center Bhavesh Shah, MD Rachana Singh, MD, MS Anne Smith, PhD Deborah Klein, BSN, RN Susan McQuiston, PhD University of Massachusetts Medical School, Worcester, MA: Lauren Venuti, BA Beth Powers, RN Ann Foley, Ed M Brian Dessureau, PhD Molly Wood, PhD Jill Damon-Minow, PsyD Yale University School of Medicine, New Haven, CT: Richard Ehrenkranz, MD Jennifer Benjamin, MD Elaine Romano, APRN Kathy Tsatsanis, PhD Katarzyna Chawarska, PhD Sophy Kim, PhD Susan Dieterich, PhD Karen Bearrs, PhD Wake Forest University Baptist Medical Center, Winston-Salem, NC: Nancy Peters, RN Patricia Brown, BSN Emily Ansusinha, BA Ellen Waldrep, PhD Jackie Friedman, PhD Gail Hounshell. PhD Debbie Allred, PhD University Health Systems of Eastern Carolina, Greenville, NC: Stephen C. Engelke, MD Nancy Darden-Saad, BS, RN, CCRC Gary Stainback, PhD North Carolina Children’s Hospital, Chapel Hill, NC: Diane Warner, MD, MPH Janice Wereszczak, MSN, PNP Janice Bernhardt, MS, RN Joni McKeeman, PhD Echo Meyer, PhD Helen DeVos Children’s Hospital, Grand Rapids, MI: Steve Pastyrnak, PHD Julie Rathbun, BSW, BSN, RN Sarah Nota, BS Teri Crumb, BSN, RN, CCRC Sparrow Hospital, Lansing, MI: Madeleine Lenski, MPH Deborah Weiland, MSN Megan Lloyd, MA, EdS University of Chicago Medical Center, Chicago, IL: Scott Hunter, PhD Michael Msall, MD Rugile Ramoskaite, BA Suzanne Wiggins, MA Krissy Washington, MA Ryan Martin, MA Barbara Prendergast, BSN, RN Megan Scott, PhD William Beaumont Hospital, Royal Oak, MI: Judith Klarr, MD Beth Kring, RN Jennifer DeRidder, RN Kelly Vogt, PhD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
Citations
- 1.O'Shea TM, Allred EN, Kuban KC, Dammann O, Paneth N, Fichorova R, et al. Elevated concentrations of inflammation-related proteins in postnatal blood predict severe developmental delay at 2 years of age in extremely preterm infants. J Pediatr. 2012;160:395–401. e4. doi: 10.1016/j.jpeds.2011.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leviton A, Kuban KC, Allred EN, Fichorova RN, O'Shea TM, Paneth N, et al. Early postnatal blood concentrations of inflammation-related proteins and microcephaly two years later in infants born before the 28th post-menstrual week. Early Hum Dev. 2011;87:325–330. doi: 10.1016/j.earlhumdev.2011.01.043. [DOI] [PubMed] [Google Scholar]
- 3.Kuban KCK, O'Shea TM, Alled EN, Paneth N, Hirtz D, Fichorova RN, Leviton A. Systemic inflammation and cerebral palsy risk in extremely preterm infants. Journal of Child Neurology. 2014;29:1692–1698. doi: 10.1177/0883073813513335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Shea TM, Joseph RM, Kuban KC, Allred EN, Ware J, Coster T, et al. Elevated blood levels of inflammation-related proteins are associated with an attention problem at age 24 mo in extremely preterm infants. Pediatr Res. 2014;75:781–787. doi: 10.1038/pr.2014.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peacock JL, Marston L, Marlow N, Calvert SA, Greenough A. Neonatal and infant outcome in boys and girls born very prematurely. Pediatr Res. 2012;71:305–310. doi: 10.1038/pr.2011.50. [DOI] [PubMed] [Google Scholar]
- 6.Skiold B, Alexandrou G, Padilla N, Blennow M, Vollmer B, Aden U. Sex differences in outcome and associations with neonatal brain morphology in extremely preterm children. J Pediatr. 2014;164:1012–1018. doi: 10.1016/j.jpeds.2013.12.051. [DOI] [PubMed] [Google Scholar]
- 7.Kent AL, Wright IM, Abdel-Latif ME, New South W Australian Capital Territory Neonatal Intensive Care Units Audit G. Mortality and adverse neurologic outcomes are greater in preterm male infants. Pediatrics. 2012;129:124–131. doi: 10.1542/peds.2011-1578. [DOI] [PubMed] [Google Scholar]
- 8.Johnson S, Fawke J, Hennessy E, Rowell V, Thomas S, Wolke D, et al. Neurodevelopmental disability through 11 years of age in children born before 26 weeks of gestation. Pediatrics. 2009;124:e249–e257. doi: 10.1542/peds.2008-3743. [DOI] [PubMed] [Google Scholar]
- 9.Moore T, Hennessy EM, Myles J, Johnson SJ, Draper ES, Costeloe KL, et al. Neurological and developmental outcome in extremely preterm children born in England in 1995 and 2006: the EPICure studies. BMJ. 2012;345:e7961. doi: 10.1136/bmj.e7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frondas-Chauty A, Simon L, Branger B, Gascoin G, Flamant C, Ancel PY, et al. Early growth and neurodevelopmental outcome in very preterm infants: impact of gender. Arch Dis Child Fetal Neonatal Ed. 2014;99:F366–F372. doi: 10.1136/archdischild-2013-305464. [DOI] [PubMed] [Google Scholar]
- 11.Farooqi A, Hagglof B, Sedin G, Serenius F. Impact at age 11 years of major neonatal morbidities in children born extremely preterm. Pediatrics. 2011;127:e1247–e1257. doi: 10.1542/peds.2010-0806. [DOI] [PubMed] [Google Scholar]
- 12.Mansson J, Fellman V, Stjernqvist K, Group ES. Extremely preterm birth affects boys more and socio-economic and neonatal variables pose sex-specific risks. Acta Paediatr. 2015;104:514–521. doi: 10.1111/apa.12937. [DOI] [PubMed] [Google Scholar]
- 13.Wood NS, Marlow N, Costeloe K, Gibson AT, Wilkinson AR. Neurologic and developmental disability after extremely preterm birth. EPICure Study Group. N Engl J Med. 2000;343:378–384. doi: 10.1056/NEJM200008103430601. [DOI] [PubMed] [Google Scholar]
- 14.Hintz SR, Kendrick DE, Vohr BR, Poole WK, Higgins RD. Changes in neurodevelopmental outcomes at 18 to 22 months' corrected age among infants of less than 25 weeks' gestational age born in 1993–1999. Pediatrics. 2005;115:1645–1651. doi: 10.1542/peds.2004-2215. [DOI] [PubMed] [Google Scholar]
- 15.Stevenson DK, Verter J, Fanaroff AA, Oh W, Ehrenkranz RA, Shankaran S, et al. Sex differences in outcomes of very low birthweight infants: the newborn male disadvantage. Arch Dis Child Fetal Neonatal Ed. 2000;83:F182–F185. doi: 10.1136/fn.83.3.F182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson S, Hennessy E, Smith R, Trikic R, Wolke D, Marlow N. Academic attainment and special educational needs in extremely preterm children at 11 years of age: the EPICure study. Arch Dis Child Fetal Neonatal Ed. 2009;94:F283–F289. doi: 10.1136/adc.2008.152793. [DOI] [PubMed] [Google Scholar]
- 17.Johnson S, Hollis C, Kochhar P, Hennessy E, Wolke D, Marlow N. Psychiatric disorders in extremely preterm children: longitudinal finding at age 11 years in the EPICure study. J Am Acad Child Adolesc Psychiatry. 2010;49:453–463. e1. [PubMed] [Google Scholar]
- 18.Taylor HG, Klein N, Drotar D, Schluchter M, Hack M. Consequences and Risks of <1000-g Birth Weight for Neuropsychological Skills, Achievement, and Adaptive Functioning. Developmental and Behavioral Pediatrics. 2006;27:459–469. doi: 10.1097/00004703-200612000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Stahlmann N, Rapp M, Herting E, Thyen U. Outcome of extremely premature infants at early school age: health-related quality of life and neurosensory, cognitive, and behavioral outcomes in a population-based sample in northern Germany. Neuropediatrics. 2009;40:112–119. doi: 10.1055/s-0029-1243166. [DOI] [PubMed] [Google Scholar]
- 20.Linsell L, Malouf R, Morris J, Kurinczuk JJ, Marlow N. Prognostic Factors for Poor Cognitive Development in Children Born Very Preterm or With Very Low Birth Weight: A Systematic Review. JAMA Pediatr. 2015:1–11. doi: 10.1001/jamapediatrics.2015.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Shea TM, Allred EN, Dammann O, Hirtz D, Kuban KC, Paneth N, et al. The ELGAN study of the brain and related disorders in extremely low gestational age newborns. Early Hum Dev. 2009;85:719–725. doi: 10.1016/j.earlhumdev.2009.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elliott CD. Differential ability scales. second. San Antonio, TX: The Psychological Corporation; 2007. [Google Scholar]
- 23.Carrow-Woolfolk E. Oral and Written Language Skills: Written Expression Scale Manual. Circle Pines, MN: American Guidance Service; 1996. [Google Scholar]
- 24.Korkman M, Kirk U, Kemp S. NEPSY-second edition (NEPSY-II) San Antonio, Texas: The Psychological Corporation; 2007. [Google Scholar]
- 25.Douglass LM, Kuban K, Tarquinio D, Schraga L, Jonas R, Heeren T, et al. A Novel Parent Questionnaire for the Detection of Seizures in Children. Pediatr Neurol. 2016;54:64–69. doi: 10.1016/j.pediatrneurol.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 26.Palisano RJ, Rosenbaum P, Bartlett D, Livingston MH. Content validity of the expanded and revised Gross Motor Function Classification System. Dev Med Child Neurol. 2008;50:744–750. doi: 10.1111/j.1469-8749.2008.03089.x. [DOI] [PubMed] [Google Scholar]
- 27.Roche AF, Mukherjee D, Guo SM, Moore WM. Head circumference reference data: birth to 18 years. Pediatrics. 1987;79:706–712. [PubMed] [Google Scholar]
- 28.Barabasi AL. The origin of bursts and heavy tails in human dynamics. Nature. 2005;435:207–211. doi: 10.1038/nature03459. [DOI] [PubMed] [Google Scholar]
- 29.Eguiluz VM, Zimmermann MG. Transmission of information and herd Behavior: an application to financial markets. Phys Rev Lett. 2000;85:5659–5662. doi: 10.1103/PhysRevLett.85.5659. [DOI] [PubMed] [Google Scholar]
- 30.Viswanathan GM, Buldyrev SV, Havlin S, da Luz MG, Raposo EP, Stanley HE. Optimizing the success of random searches. Nature. 1999;401:911–914. doi: 10.1038/44831. [DOI] [PubMed] [Google Scholar]
- 31.Lai MC, Lombardo MV, Auyeung B, Chakrabarti B, Baron-Cohen S. Sex/gender differences and autism: setting the scene for future research. J Am Acad Child Adolesc Psychiatry. 2015;54:11–24. doi: 10.1016/j.jaac.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Streimish IG, Ehrenkranz RA, Allred EN, O'Shea TM, Kuban KC, Paneth N, et al. Birth weight- and fetal weight-growth restriction: impact on neurodevelopment. Early Hum Dev. 2012;88:765–771. doi: 10.1016/j.earlhumdev.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crider A, Thakkar R, Ahmed AO, Pillai A. Dysregulation of estrogen receptor beta (ERbeta), aromatase (CYP19A1), and ER co-activators in the middle frontal gyrus of autism spectrum disorder subjects. Mol Autism. 2014;5:46. doi: 10.1186/2040-2392-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hill CA, Threlkeld SW, Fitch RH. Early testosterone modulated sex differences in behavioral outcome following neonatal hypoxia ischemia in rats. Int J Dev Neurosci. 2011;29:381–388. doi: 10.1016/j.ijdevneu.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Bellis MD, Keshavan MS, Beers SR, Hall J, Frustaci K, Masalehdan A, et al. Sex differences in brain maturation during childhood and adolescence. Cereb Cortex. 2001;11:552–557. doi: 10.1093/cercor/11.6.552. [DOI] [PubMed] [Google Scholar]
- 36.Loke H, Harley V, Lee J. Biological factors underlying sex differences in neurological disorders. Int J Biochem Cell Biol. 2015;65:139–150. doi: 10.1016/j.biocel.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 37.Zhu C, Xu F, Wang X, Shibata M, Uchiyama Y, Blomgren K, et al. Different apoptotic mechanisms are activated in male and female brains after neonatal hypoxia-ischaemia. J Neurochem. 2006;96:1016–1027. doi: 10.1111/j.1471-4159.2005.03639.x. [DOI] [PubMed] [Google Scholar]
- 38.Johnston MV, Ishida A, Ishida WN, Matsushita HB, Nishimura A, Tsuji M. Plasticity and injury in the developing brain. Brain Dev. 2009;31:1–10. doi: 10.1016/j.braindev.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leviton A, Blair E, Dammann O, Allred EN. The wealth of information conveyed by gestational age. J Pediatr. 2005;146:123–127. doi: 10.1016/j.jpeds.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 40.Chounti A, Hagglund G, Wagner P, Westbom L. Sex differences in cerebral palsy incidence and functional ability: a total population study. Acta Paediatr. 2013;102:712–717. doi: 10.1111/apa.12240. [DOI] [PubMed] [Google Scholar]
- 41.Scharfman HE, MacLusky NJ. Sex differences in the neurobiology of epilepsy: a preclinical perspective. Neurobiol Dis. 2014;72(Pt B):180–192. doi: 10.1016/j.nbd.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akman O, Moshe SL, Galanopoulou AS. Sex-specific consequences of early life seizures. Neurobiol Dis. 2014;72(Pt B):153–166. doi: 10.1016/j.nbd.2014.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Developmental Disabilities Monitoring Network Surveillance Year Principal I, Centers for Disease C, Prevention. Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveill Summ. 2014;63:1–21. [PubMed] [Google Scholar]
- 44.Frazier TW, Georgiades S, Bishop SL, Hardan AY. Behavioral and cognitive characteristics of females and males with autism in the Simons Simplex Collection. J Am Acad Child Adolesc Psychiatry. 2014;53:329–340. e1–e3. doi: 10.1016/j.jaac.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sandin S, Lichtenstein P, Kuja-Halkola R, Larsson H, Hultman CM, Reichenberg A. The familial risk of autism. JAMA. 2014;311:1770–1777. doi: 10.1001/jama.2014.4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zwaigenbaum L, Bryson SE, Szatmari P, Brian J, Smith IM, Roberts W, et al. Sex differences in children with autism spectrum disorder identified within a high-risk infant cohort. J Autism Dev Disord. 2012;42:2585–2596. doi: 10.1007/s10803-012-1515-y. [DOI] [PubMed] [Google Scholar]
- 47.Hiller RM, Young RL, Weber N. Sex differences in pre-diagnosis concerns for children later diagnosed with autism spectrum disorder. Autism. 2016;20:75–84. doi: 10.1177/1362361314568899. [DOI] [PubMed] [Google Scholar]
- 48.Mazina V, Gerdts J, Trinh S, Ankenman K, Ward T, Dennis MY, et al. Epigenetics of Autism-related Impairment: Copy Number Variation and Maternal Infection. J Dev Behav Pediatr. 2015;36:61–67. doi: 10.1097/DBP.0000000000000126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Himes KP, Simhan HN. Risk of recurrent preterm birth and placental pathology. Obstet Gynecol. 2008;112:121–126. doi: 10.1097/AOG.0b013e318179f024. [DOI] [PubMed] [Google Scholar]
- 50.Wei SQ, Fraser W, Luo ZC. Inflammatory cytokines and spontaneous preterm birth in asymptomatic women: a systematic review. Obstet Gynecol. 2010;116:393–401. doi: 10.1097/AOG.0b013e3181e6dbc0. [DOI] [PubMed] [Google Scholar]
- 51.Bastek JA, Brown AG, Anton L, Srinivas SK, D'Addio A, Elovitz MA. Biomarkers of inflammation and placental dysfunction are associated with subsequent preterm birth. J Matern Fetal Neonatal Med. 2011;24:600–605. doi: 10.3109/14767058.2010.511340. [DOI] [PubMed] [Google Scholar]
- 52.Leviton A, Allred EN, Yamamoto H, Fichorova RN, Investigators ES. Relationships among the concentrations of 25 inflammation-associated proteins during the first postnatal weeks in the blood of infants born before the 28th week of gestation. Cytokine. 2012;57:182–190. doi: 10.1016/j.cyto.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnson S, Hollis C, Kochhar P, Hennessy E, Wolke D, Marlow N. Autism Spectrum Disorders in Extremely Preterm Children. J Pediatr. 2010;156:525–531. doi: 10.1016/j.jpeds.2009.10.041. [DOI] [PubMed] [Google Scholar]
- 54.Pietschnig J, Voracek M. One Century of Global IQ Gains: A Formal Meta-Analysis of the Flynn Effect (1909–2013) Perspect Psychol Sci. 2015;10:282–306. doi: 10.1177/1745691615577701. [DOI] [PubMed] [Google Scholar]