Abstract
Recent clinical studies have suggested a role for immune/inflammatory responses in the pathophysiology of psychosis. However, a mechanistic understanding of this process and its application for drug discovery is underdeveloped. Here we assessed our recently developed cuprizone short-term exposure (CSE) mouse model across behavioral domains targeting neurocognitive and neuroaffective systems. We propose that the CSE model may be useful for understanding the mechanism associating inflammation and psychosis, with applications for drug discovery in that context.
Keywords: Cuprizone, cuprizone short-term exposure (CSE), psychosis, behavior, inflammation, hippocampus, Research Domain Criteria (RDoC), dimensional approach
Recent studies have suggested a role for immune/inflammatory responses in the pathophysiology of psychosis, including schizophrenia (Hayes et al., 2014; Miller et al., 2011). A popular model for studying schizophrenia is maternal immune activation (MIA) which uses immunological insults such as the viral mimic polyriboinosinic-polyribocytidilic acid [poly(I:C)] or the bacterial endotoxin lipopolysaccharide (LPS), to induce postnatal alterations in the immune/inflammatory response, neurocircuitry, and behavior of the adult offspring (Borrell et al., 2002; Reul et al., 1994; Zuckerman et al., 2003). We sought to develop a higher throughput model in which inflammatory processes underlie neurochemical and behavioral changes relevant to psychosis.
We recently reported that systemic exposure to cuprizone for one week could elevate expression of interleukin-6 (IL-6) and gliosis markers [glial fibrillary acidic protein (GFAP) and ionized calcium-binding adaptor molecule 1 (Iba1)] in the hippocampus, frontal cortex, and striatum, leading to behavioral abnormalities, including hypersensitivity to psychostimulants and deficits in some memory tasks (Tezuka et al., 2013). In contrast to prolonged cuprizone exposure (4–8 weeks), which elicits robust demyelination (Matsushima and Morell, 2001; Xu et al., 2011), one-week exposure to the chemical does not cause overt white matter pathology (Tezuka et al., 2013). Based on the findings we proposed that cuprizone short-term exposure (CSE) may be a promising method to study inflammation-associated psychosis. Since a systematic assessment of behaviors in the CSE model was not included in the original study, here we present a more comprehensive assessment, integrating new data with those recently reported (Tezuka et al., 2013), to assess multiple behavioral domains associated with psychiatric disorders. The results have been organized into eight broad dimensions of neurobehavioral function: motor system, memory, executive function, social cognition, anxiety, depression, psychosis, and information processing.
Eight-week-old C57BL/6J male mice were randomly assigned to two groups. For one-week, mice were fed either a diet containing 0.2% cuprizone (Sigma- Aldrich), or a control diet consisting of standard chow obtained from Harlan Teklad. Unless otherwise stated, experiments were performed on separate cohorts on the 7th day of diet exposure (exposure day 7; ED 7). All experimental procedures followed the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals, and the Johns Hopkins University Animal Care and Use Guidelines. Data were assessed for normal distribution using the Shapiro-Wilk test, and homogeneity of variance using the F-test. Outliers were identified using a Grubbs test with α = 0.01. Where assumptions were met, parametric statistical comparisons were performed using a two-tailed unpaired Student's t-test, except for the rotarod motor learning, reversal learning test, fear conditioning extinction, social interaction, and amphetamine-induced hyperactivity, which were analyzed with two-way analysis of variance (ANOVA) or two-way repeated measures ANOVA. An α value of p < 0.05 was considered statistically significant.
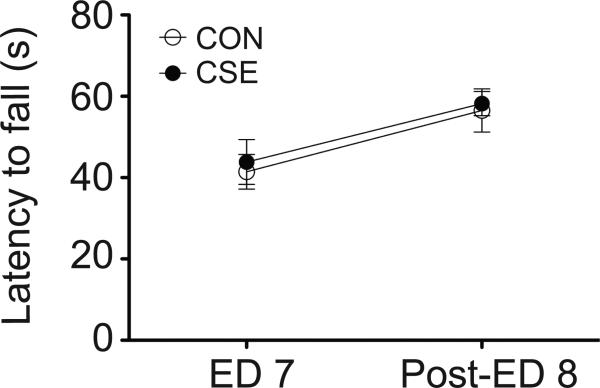
Our previous study reported that CSE mice show no difference in baseline locomotor activity (Tezuka et al., 2013). Because longer-term exposure to cuprizone (5 weeks) results in poor motor coordination (Ye et al., 2013), we first conducted an assessment of motor function using the rotarod test to ensure that motor coordination and motor learning are not confounding other behavioral tasks. There was no significant difference in baseline motor coordination or motor learning between control (CON) mice and CSE mice (Fig.1). Briefly, mice were placed on a rotarod (Rotamex-5; Columbus Instruments) that accelerated from 4 to 40 rpm over 120 s and the latency to fall onto a platform below was recorded. Motor learning was assessed with four trials per day for two consecutive days (ED 7 and post-exposure day 8).
Figure 1. Motor function in cuprizone short-term exposure (CSE) mice.
CSE mice demonstrated normal motor coordination and motor learning on the rotarod. Assessments were made on two consecutive days [exposure day (ED) 7 and post-ED 8]. Data are presented as mean ± S.E.M. (n=8 for each group). CON, control
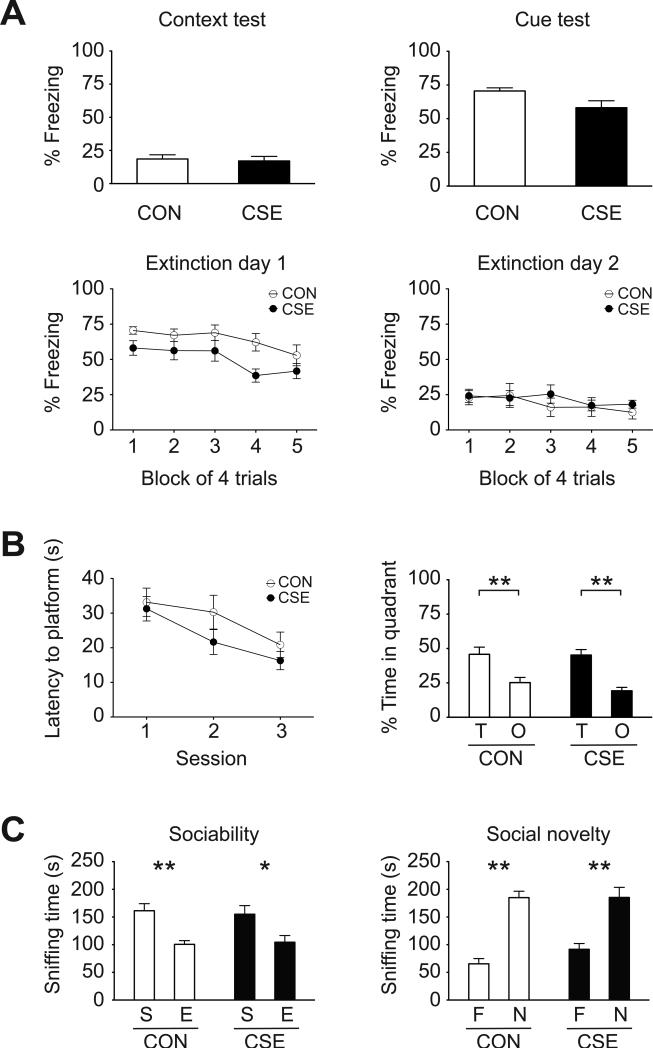
In our previous study CSE mice displayed decreased preference for the novel object in the novel object recognition test and decreased spontaneous alternation in the Y-maze test, indicating deficits for some types of memory dependent on the medial temporal lobe (Tezuka et al., 2013). To test additional forms of learning and memory, in the current study we used a fear-conditioning procedure to assess associative learning and memory (Tovote et al., 2015). CON and CSE mice showed similar levels of baseline freezing (data not shown). There was no significant difference in freezing between CON mice and CSE mice during tests for the acquisition of learned fear to either the context or associative cue used during training, indicating that retrieval of fear memories is intact in CSE mice (Fig. 2A, top). We also examined extinction of associative learning by testing extinction to the context and cue but found no significant difference in rate of extinction between the groups (Fig. 2A, bottom). Fear conditioning and extinction were conducted as follows. On ED 5, mice were habituated to the training chamber for 10 min. On ED 6, mice were returned to the training chamber and after 120 s, presented with a 20 s tone that co-terminates with a 2 s foot-shock (0.5 mA). The tone-shock pairing presentation was repeated 3 times, with an intertone interval of 10 min. On ED 7, mice were returned to the training chamber and allowed to move freely for 5 min (no presentation of tone or shock). Mice were then placed in a novel context. Following a 120 s acclimation period, the tone is presented for 20 s. The tone presentation is repeated 20 times with an intertone interval of 180 s. Data was analyzed in five blocks of four tone presentations each (the first block on extinction day 1 corresponds to the cued test). On day post-exposure 8, the procedure of ED 7 was repeated.
Figure 2. Memory, executive function, and social cognition in CSE mice.
(A) Fear conditioning: there were no significant differences between CON and CSE mice in freezing behavior during the context and cue tests of fear retrieval (top), or freezing during extinction of fear (bottom; Block 1 of first extinction day corresponds to the cued fear retrieval test, above). (B) Reversal learning: CSE mice exhibited normal spatial reversal learning in the Morris water maze based on latency to the hidden platform and time in target quadrant during the probe trial. **p<0.01, as analyzed by 2-way ANOVA with post hoc Bonferroni test (quadrant occupancy F1,42 = 33.11, p<0.0001). (C) Three-chamber social interaction test: both CON and CSE mice preferred sniffing the enclosure with the stranger mouse during the sociability phase and preferred the unfamiliar stranger in the social novelty phase. *p<0.05, **p<0.01, as analyzed by 2-way ANOVA with post hoc Bonferroni test (sociability phase; preference F1,28 = 20.63, p<0.0001, social novelty phase; preference F1,28 = 68.09, p<0.0001). Data are presented as mean ± S.E.M. (CON n=7, CSE n=8 for fear conditioning, CON n=12, CSE n=11 for reversal learning, n=8 for each group for social interaction test). T, target; O, opposite; S, stranger; E, empty; F, familiar; and N, novel.
Behavioral/cognitive flexibility, a neurocognitive function that depends heavily on prefrontal cortical function, was assessed by reversal learning in the water maze. The water maze reversal learning task was designed to test the effect of cuprizone on memory performance and reversal learning. There was no significant difference between CON and CSE mice in latency to reach the hidden platform during the reversal training trials or percent time spent in the target quadrant during the probe trial following reversal training (Fig. 2B). These data indicate that CSE mice do not show deficits in behavioral flexibility in this assessment. The test was conducted according to a published protocol (Smith et al., 2015) with some modifications. Briefly, beginning at age 7 weeks, all mice were trained in an initial water maze environment (maze 1) to locate a hidden escape platform and at the end of training all received a probe trial test. Training and testing then ceased and the mice were evenly split into exposure groups (cuprizone or control) such that the resulting groups showed equal performance in maze 1. Following three days of cuprizone or control diet exposure (but still no training), on ED 4 mice started training in a completely new water maze with different cues (maze 2). Reversal training (where the hidden platform was moved to the opposite quadrant) was started in maze 2 on ED 7 and continued on post-exposure day 8. Mice had four training trials per session over three sessions and a probe trial was conducted on post-exposure day 8 at the conclusion of session 3. A visual platform test showed that visual acuity was intact in CSE mice (data not shown).
To assess social cognition, we conducted a test of sociability and preference for social novelty using a three-chambered apparatus. This apparatus is frequently used to evaluate social preference in rodents (Silverman et al., 2010). The CSE mice showed no deficits in social interaction preferring the stranger mouse to the empty cage (Fig. 2C, left). In the social novelty phase, both CON and CSE mice preferred the new stranger (Fig. 2C, right). These data indicate that CSE mice show normal sociability and preference for social novelty. The test was conducted according to a published protocol (Johnson et al., 2013).
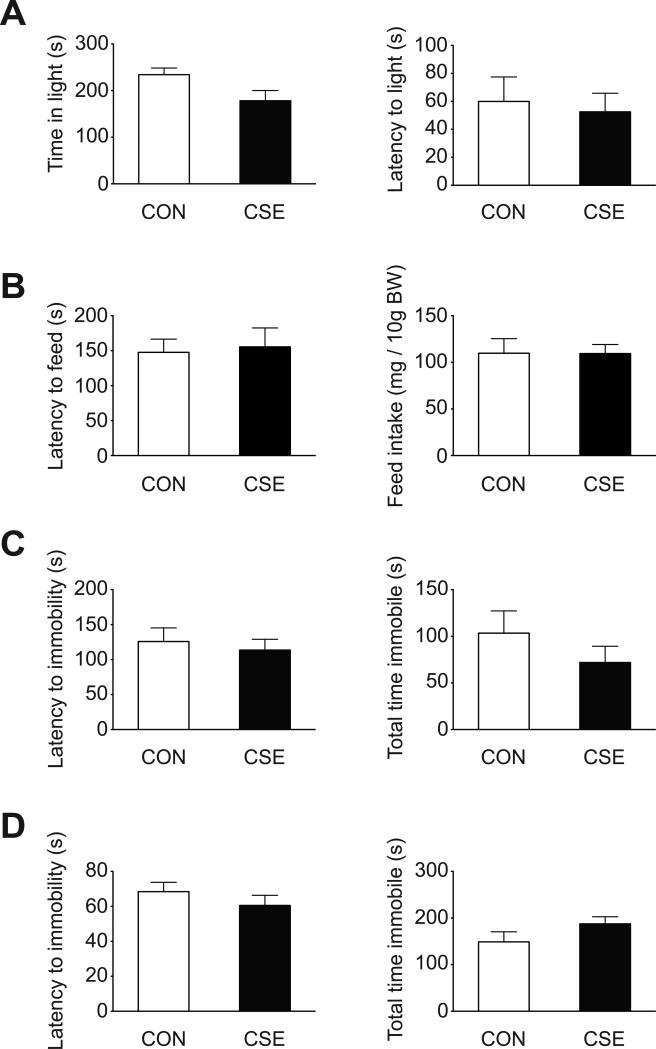
Anxiety-like behavior was assessed using the light/dark and novelty-suppressed feeding tests. The light/dark box is a common test of anxiety that relies on the conflict between a rodent's desire to explore a novel environment, and its desire to avoid exposure (Adhikari, 2014). CSE mice spent less time in the light compartment compared with CON mice (Fig. 3A, left, p=0.05). However, latency to enter the light compartment did not differ between groups (Fig. 3A, right). The light/dark box test was conducted according to a published protocol (Smith et al., 2015) with the minor modification of 10 min sessions. The novelty-suppressed feeding test is also an anxiety test with a strong hedonic and motivation component (Bodnoff et al., 1988). There was no significant difference between CON and CSE mice in latency to feed, or food consumption in the home cage (Fig. 3B). The test was conducted as previously described (Kondo et al., 2015).
Figure 3. Anxiety and depression-like behaviors in CSE mice.
(A) Light/dark box test: CSE mice spent less time in the light compartment (p=0.05). There was no significant difference between groups in latency to enter the light compartment. (B) Novelty suppressed feeding test: there were no significant differences between CON mice and CSE mice in latency to feed or food consumed. (C) Forced swim test: there were no significant differences between CON mice and CSE mice in latency to immobility or total time immobile. (D) Tail suspension test: there were no significant differences between CON mice and CSE mice in latency to immobility or total time immobile. Data are presented as mean ± S.E.M. (n=8 for each group for light/dark box test, forced swim test, and tail suspension test, CON n=7, CSE n=8 for novelty suppressed feeding test). BW, body weight
To assess depression-associated phenotypes, we used the forced swim test (FST) and tail suspension test (TST), two representative tests of behavioral despair. CON and CSE mice did not differ in latency to immobility or total time immobile during the FST and TST (Fig. 3C, D), suggesting that CSE mice do not have a depression-like phenotype. Both tests were carried out as previously described (Hikida et al., 2007; Holmes et al., 2002). The FST used Plexiglas cylinder tanks measuring 19 cm in height, 13 cm in diameter and the water level was 16 cm. In both the FST and TST tests mice were scored for immobility over a 6-min uninterrupted test session. These two tests were carried out in the same cohort of mice in morning (AM) and afternoon (PM) sessions wherein during the AM, half of the CON mice and half of the CSE mice were tested on the FST while the other half of each group was tested on the TST. Four hours later during the PM the mice that had been tested on FST in the AM were tested on TST and those that were tested on TST in the AM were tested on FST in the PM.
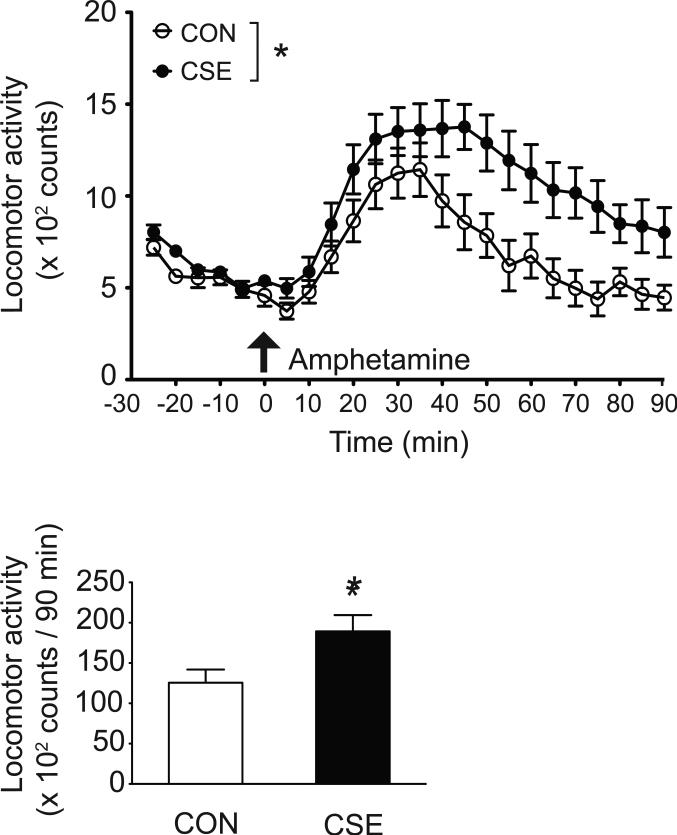
Hypersensitivity to psychostimulants is seen in some patients with psychosis (Laruelle et al., 1996), and may represent a biological feature associated with psychosis. We previously reported hypersensitivity to methamphetamine and phencyclidine in CSE mice (Tezuka et al., 2013). Here we tested the response to amphetamine: mice received one subcutaneous injection of amphetamine (1.5 mg/kg) after 30 min of habituation to the open field. A significantly greater augmentation of locomotion was observed in CSE mice following amphetamine injection (Fig. 4).
Figure 4. Hypersensitivity to amphetamine in CSE mice.
Amphetamine (1.5 mg/kg, s.c.)-induced hyperactivity is augmented in CSE mice (top). *p<0.05, as analyzed by 2-way repeated measures ANOVA (treatment F1,22 = 6.00, p<0.05, time F17,374 = 26.42, p<0.0001, and treatment × time interaction F17,374 = 2.26, p< 0.01). Total locomotor activity assessed after injection of amphetamine (90 min) (bottom). *p<0.05, as analyzed by Student's t-test (t=2.45). Data are presented as mean ± S.E.M. (n=12 for each group).
Our previous study reported no significant changes in information processing (sensory motor gating) based on the acoustic startle response and prepulse inhibition (Tezuka et al., 2013). Taken together, CSE mice display hypersensitivity to psychostimulants, and deficits in a limited subset of memory paradigms. In contrast, we detected no deficits in a range of other behavioral assessments for motor function, executive function, social cognition, affective behavior, and information processing (Table 1).
Table 1.
Comprehensive behavioral characterization of Cuprizone short-exposure mice.
| Dimensions | Index | Test | Results | RDoC Domains/Constructs and sub-constructs |
|---|---|---|---|---|
| Motor systems | Motor coordination/learning | Rotarod | Normal, Fig.1 | Unclassifiable |
| Memory | Recognition memory | NORT | ↓, [1] | Cognitive Systems/Declarative Memory |
| Spontaneous alternation | Y-maze | ↓, [1] | Cognitive Systems/Perception | |
| Context/cue-induced fear Fear extinction | FC | Normal, Fig.2A | Negative Valence Systems/Responses to acute threat (Fear) | |
| Executive function | Behavioral flexibility | RL | Normal, Fig.2B | Cognitive Systems/Cognitive Control |
| Social cognition | Sociability/social novelty | SIT | Normal, Fig.2C | Systems for Social Processes/Affiliation and Attachment |
| Anxiety | Neophobia | LDT | ↑ (p=0.05), Fig.3A | Negative Valence Systems/Responses to potential harm (Anxiety) |
| Hyponeophagia | NSFT | Normal, Fig.3B | Negative Valence Systems/Responses to potential harm (Anxiety) | |
| Depression | Behavioral despair | FST | Normal, Fig.3C | Negative Valence Systems/Responses to sustained threat |
| Behavioral despair | TST | Normal, Fig.3D | Negative Valence Systems/Responses to sustained threat | |
| Psychosis | Sensitivity to psychostimulants | OF | ↑, Fig.4 and [1] | Arousal and Regulatory Systems/Arousal |
| Information processing | Sensorimotor gating | PPI | Normal, [1] | Cognitive Systems/Perception |
[1], Tezuka et al., 2013
NORT, Novel object recognition test; FC, Fear conditioning; RL, Reversal learning; SIT, Social interaction test; LDT, Light/dark box test; NSFT, Novelty suppressed feeding test; FST, Forced swim test; TST, Tail suspension test; OF, Open field; PPI, Prepulse inhibition
The National Institutes of Mental Health recently proposed a framework called the Research Domain Criteria (RDoC), to assist in the scientific assessment of mental disturbances (Cuthbert, 2014). This framework was designed to integrate pathophysiology based constructs, such as those of genes and molecules, cells, circuits and overall physiology, associated with neurobehavioral systems, according to the basic functional dimensions that underlie human behaviors and neural systems. RDoC is an ambitious and intriguing approach to understanding mental disturbances, which may translate into improved outcome prediction and treatment response. We generally appreciate the introduction of dimensional approaches beyond the classic categorical frameworks seen in the Diagnostic and Statistical Manual of Mental Disorders (DSM). Thus, we have integrated our present and past studies on the CSE model, organizing them into multiple constructs in an attempt to compare with the RDoC framework (Table 1).
Through multiple behavioral experiments we examined the behavioral deficits of the CSE model from a dimensional approach and conclude that limited memory deficits and psychosis-associated phenotypes are preferentially emphasized in the model. In this aspect, this model is different from other inflammation-associated models for mental illness (e.g., MIA model), in which multiple domains of behavioral deficits are observed (Meyer, 2014). As this is a chemical-induced model in early adulthood, experiments are higher throughput compared to using genetically engineered mice or the MIA model. Furthermore, CSE could be combined with genetic models or other environmental models to examine gene-environment interactions or environment-environment interactions, respectively. Although cuprizone itself is not relevant to psychosis pathogenesis, it is an effective driver of inflammatory responses (e.g., elevation of IL-6 in the hippocampus) that may underlie psychosis. It is important to demonstrate how the inflammatory processes cause psychosis at the mechanistic level in the next study.
Psychosis is seen in many neuropsychiatric diseases, including schizophrenia and mood disorders, and also in other conditions such as frontotemporal dementia, Alzheimer's disease, epilepsy, and autoimmune disorders (Arciniegas et al., 2001; Najjar et al., 2013; Shinagawa et al., 2014). Although many antipsychotic drugs are available, control of psychotic symptoms are not perfectly optimized yet, with many treatment resistant cases (Miyamoto et al., 2014). Therefore, through characterization of the CSE model, we highlight the importance of developing higher throughput mouse models for psychosis to further our understanding of the mechanisms and to aid in drug discovery.
Highlights.
- Cuprizone short-term exposure (CSE) models brain inflammation-associated psychosis.
- Comprehensive behavior assessment of CSE model is done using a dimensional approach.
- The CSE model may be useful to further our understanding of psychosis mechanisms.
Acknowledgements
We thank Ms. Yukiko Lema for organizing the figures. This work was supported by the National Institute of Mental Health MH-084018, MH-094268 Silvio O. Conte center, MH-069853 (A.S., A.K., and M.G.), MH-085226 (A.S.), MH-088753 (A.S.), MH-092443 (A.S.), MH-105660 (A.S.), MH-091230 (A.K.), AT-008547 (A.K.), as well as foundation grants from Stanley (A.S.), S-R (A.S.), RUSK (A.S.), NARSAD (A.S. and A.K.), and MSCRF (A.S.). This study was also supported in part by Mitsubishi Tanabe Pharma Corporation.
Abbreviations
- CON
control
- CSE
cuprizone short-term exposure
- DSM
Diagnostic and Statistical Manual of Mental Disorders
- ED
exposure day
- FST
forced swim test
- GFAP
glial fibrillary acidic protein
- Iba1
ionized calcium-binding adaptor molecule 1
- IL-6
interleukin-6
- LPS
lipopolysaccharide
- MIA
maternal immune activation
- poly(I:C)
polyriboinosinic-polyribocytidilic acid
- RDoC
Research Domain Criteria
- TST
tail suspension test
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adhikari A. Distributed circuits underlying anxiety. Front Behav Neurosci. 2014;8:112. doi: 10.3389/fnbeh.2014.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arciniegas DB, Topkoff JL, Held K, Frey L. Psychosis Due to Neurologic Conditions. Curr Treat Options Neurol. 2001;3:347–366. doi: 10.1007/s11940-001-0039-0. [DOI] [PubMed] [Google Scholar]
- Bodnoff SR, Suranyi-Cadotte B, Aitken DH, Quirion R, Meaney MJ. The effects of chronic antidepressant treatment in an animal model of anxiety. Psychopharmacology (Berl) 1988;95:298–302. doi: 10.1007/BF00181937. [DOI] [PubMed] [Google Scholar]
- Borrell J, Vela JM, Arevalo-Martin A, Molina-Holgado E, Guaza C. Prenatal immune challenge disrupts sensorimotor gating in adult rats. Implications for the etiopathogenesis of schizophrenia. Neuropsychopharmacology. 2002;26:204–215. doi: 10.1016/S0893-133X(01)00360-8. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN. The RDoC framework: facilitating transition from ICD/DSM to dimensional approaches that integrate neuroscience and psychopathology. World Psychiatry. 2014;13:28–35. doi: 10.1002/wps.20087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes LN, Severance EG, Leek JT, Gressitt KL, Rohleder C, Coughlin JM, Leweke FM, Yolken RH, Sawa A. Inflammatory molecular signature associated with infectious agents in psychosis. Schizophr Bull. 2014;40:963–972. doi: 10.1093/schbul/sbu052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikida T, Jaaro-Peled H, Seshadri S, Oishi K, Hookway C, Kong S, Wu D, Xue R, Andrade M, Tankou S, Mori S, Gallagher M, Ishizuka K, Pletnikov M, Kida S, Sawa A. Dominant-negative DISC1 transgenic mice display schizophrenia-associated phenotypes detected by measures translatable to humans. Proc Natl Acad Sci U S A. 2007;104:14501–14506. doi: 10.1073/pnas.0704774104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Yang RJ, Murphy DL, Crawley JN. Evaluation of antidepressant-related behavioral responses in mice lacking the serotonin transporter. Neuropsychopharmacology. 2002;27:914–923. doi: 10.1016/S0893-133X(02)00374-3. [DOI] [PubMed] [Google Scholar]
- Johnson AW, Jaaro-Peled H, Shahani N, Sedlak TW, Zoubovsky S, Burruss D, Emiliani F, Sawa A, Gallagher M. Cognitive and motivational deficits together with prefrontal oxidative stress in a mouse model for neuropsychiatric illness. Proc Natl Acad Sci U S A. 2013;110:12462–12467. doi: 10.1073/pnas.1307925110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo MA, Gray LJ, Pelka GJ, Leang SK, Christodoulou J, Tam PP, Hannan AJ. Affective dysfunction in a mouse model of Rett syndrome: Therapeutic effects of environmental stimulation and physical activity. Dev Neurobiol. 2015 doi: 10.1002/dneu.22308. doi: 10.1002/dneu.22308. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A, van Dyck CH, Gil R, D'Souza CD, Erdos J, McCance E, Rosenblatt W, Fingado C, Zoghbi SS, Baldwin RM, Seibyl JP, Krystal JH, Charney DS, Innis RB. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc Natl Acad Sci U S A. 1996;93:9235–9240. doi: 10.1073/pnas.93.17.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima GK, Morell P. The neurotoxicant, cuprizone, as a model to study demyelination and remyelination in the central nervous system. Brain Pathol. 2001;11:107–116. doi: 10.1111/j.1750-3639.2001.tb00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U. Prenatal poly(i:C) exposure and other developmental immune activation models in rodent systems. Biol Psychiatry. 2014;75:307–315. doi: 10.1016/j.biopsych.2013.07.011. [DOI] [PubMed] [Google Scholar]
- Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70:663–671. doi: 10.1016/j.biopsych.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto S, Jarskog LF, Fleischhacker WW. New therapeutic approaches for treatment-resistant schizophrenia: a look to the future. J Psychiatr Res. 2014;58:1–6. doi: 10.1016/j.jpsychires.2014.07.001. [DOI] [PubMed] [Google Scholar]
- Najjar S, Pearlman DM, Alper K, Najjar A, Devinsky O. Neuroinflammation and psychiatric illness. J Neuroinflammation. 2013;10:43. doi: 10.1186/1742-2094-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reul JM, Stec I, Wiegers GJ, Labeur MS, Linthorst AC, Arzt E, Holsboer F. Prenatal immune challenge alters the hypothalamic-pituitary-adrenocortical axis in adult rats. J Clin Invest. 1994;93:2600–2607. doi: 10.1172/JCI117272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinagawa S, Nakajima S, Plitman E, Graff-Guerrero A, Mimura M, Nakayama K, Miller BL. Psychosis in frontotemporal dementia. J Alzheimers Dis. 2014;42:485–499. doi: 10.3233/JAD-140312. [DOI] [PubMed] [Google Scholar]
- Silverman JL, Yang M, Lord C, Crawley JN. Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci. 2010;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D, Aherrera A, Lopez A, Neptune E, Winickoff JP, Klein JD, Chen G, Lazarus P, Collaco JM, McGrath-Morrow SA. Adult Behavior in Male Mice Exposed to E-Cigarette Nicotine Vapors during Late Prenatal and Early Postnatal Life. PLoS One. 2015;10:e0137953. doi: 10.1371/journal.pone.0137953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezuka T, Tamura M, Kondo MA, Sakaue M, Okada K, Takemoto K, Fukunari A, Miwa K, Ohzeki H, Kano S, Yasumatsu H, Sawa A, Kajii Y. Cuprizone short-term exposure: astrocytic IL-6 activation and behavioral changes relevant to psychosis. Neurobiol Dis. 2013;59:63–68. doi: 10.1016/j.nbd.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovote P, Fadok JP, Luthi A. Neuronal circuits for fear and anxiety. Nat Rev Neurosci. 2015;16:317–331. doi: 10.1038/nrn3945. [DOI] [PubMed] [Google Scholar]
- Xu H, Yang HJ, Rose GM, Li XM. Recovery of behavioral changes and compromised white matter in C57BL/6 mice exposed to cuprizone: effects of antipsychotic drugs. Front Behav Neurosci. 2011;5:31. doi: 10.3389/fnbeh.2011.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye JN, Chen XS, Su L, Liu YL, Cai QY, Zhan XL, Xu Y, Zhao SF, Yao ZX. Progesterone alleviates neural behavioral deficits and demyelination with reduced degeneration of oligodendroglial cells in cuprizone-induced mice. PLoS One. 2013;8:e54590. doi: 10.1371/journal.pone.0054590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman L, Rehavi M, Nachman R, Weiner I. Immune activation during pregnancy in rats leads to a postpubertal emergence of disrupted latent inhibition, dopaminergic hyperfunction, and altered limbic morphology in the offspring: a novel neurodevelopmental model of schizophrenia. Neuropsychopharmacology. 2003;28:1778–1789. doi: 10.1038/sj.npp.1300248. [DOI] [PubMed] [Google Scholar]