Abstract
Accurate memory retrieval from partial or degraded input requires the reactivation of memory traces, a hippocampal mechanism termed pattern completion. Age-related changes in hippocampal integrity have been hypothesized to shift the balance of memory processes in favor of the retrieval of already stored information (pattern completion), to the detriment of encoding new events (pattern separation). Using a novel behavioral paradigm, we investigated the impact of cognitive aging (1) on recognition performance across different levels of stimulus completeness, and (2) on potential response biases. Participants were required to identify previously learned scenes among new ones. Additionally, all stimuli were presented in gradually masked versions to alter stimulus completeness. Both young and older adults performed increasingly poorly as the scenes became less complete, and this decline in performance was more pronounced in elderly participants indicative of a pattern completion deficit. Intriguingly, when novel scenes were shown, only the older adults showed an increased tendency to identify these as familiar scenes. In line with theoretical models, we argue that this reflects an age-related bias towards pattern completion.
Keywords: pattern completion bias, recognition memory deficit, cognitive aging, memory retrieval, hippocampus
1. Introduction
All too often we find ourselves faced with the problem of recognizing something familiar even though its appearance may have changed; for example, finding our way across a park with all the trees having lost their leaves, or recognizing a person wearing a different haircut. Pattern completion is essential for the successful retrieval of memories from such degraded or partial cues. This process has been defined as a hippocampal computation during which the original memory trace is restored (completed) via reactivation (Marr, 1971; McClelland, McNaughton, & O’Reilly, 1995). However, behavioral evidence for such computations in episodic memory processing in humans is rare. One line of evidence comes from studies using continuous object recognition tasks to assess pattern separation – a concurrent process which differentiates new input from stored representations (for review, see Yassa & Stark, 2011). Typically, stimuli used in these paradigms are similar lures, and participants’ ability to correctly reject them as similar and not identify them as old is interpreted as behavioral pattern separation (Stark, Yassa, Lacy, & Stark, 2013). The identification of pattern completion processes is usually a by-product of this assessment; that is, the failure to correctly reject a lure as similar and judging it as old (false alarms) is interpreted as behavioral pattern completion (Ally, Hussey, Ko, & Molitor, 2013). However, as of yet, it is unclear how exactly pattern separation and completion contribute to behavior, and whether they are distinct processes that work concurrently or in competition, or whether they represent two ends of a unified process (for review, see Hunsaker & Kesner, 2013).
Because the structural integrity of the hippocampus is particularly sensitive to the aging process, it has been suggested that the aged brain should show a bias toward pattern completion (Wilson, Gallagher, Eichenbaum, & Tanila, 2006). Behavior concomitant with these age-related changes in hippocampal processing has been assessed with a similar focus on pattern separation, only indirectly showing a shift towards pattern completion (Toner, Pirogovsky, Kirwan, & Gilbert, 2009; Yassa, Mattfeld, Stark, & Stark, 2011). However, a more recent study has raised objections to these conceptualizations by showing that both measures (lure correct rejections and false alarms) likely entail both pattern separation and completion, suggesting that more process-pure behavioral measurements need to be developed (Molitor, Ko, Hussey, & Ally, 2014). In that study, eye-tracking data revealed that performance differences were driven by differential encoding rather than retrieval, hence lure correct rejections and false alarms should rather be interpreted as successful and unsuccessful pattern separation during encoding as opposed to pattern completion biases during retrieval.
In the present study, we devised a behavioral paradigm more suitable to assess pattern completion, and to test the hypothesis that older adults would show a bias towards this process. We developed a recognition task that required participants to learn simple line-drawn scenes and later identify them amongst new scenes. During recognition, we manipulated stimulus completeness by gradually reducing scene information similar to Gollin figures (Gollin, 1960). The resulting partial input was intended to trigger the pattern completion process, a manipulation suggested by Hunsaker and Kesner (2013). With this paradigm, we could (1) assess the recognition ability across different levels of stimulus completeness, and (2) calculate a response bias score by comparing the performance for learned versus new stimuli, while simultaneously characterizing age effects.
2. Materials and Methods
2.1. Subjects
All participants were recruited by the German Center for Neurodegenerative Diseases (DZNE), Magdeburg. After screening for mild cognitive impairment using the Montreal Cognitive Assessment (MoCA; Nasreddine et al., 2005), we excluded 4 older participants, because they scored lower than 23 (Luis, Keegan, & Mullan, 2009). Thirty young (20-35 years old; 15 males) and 30 older adults (62-78 years old; 15 males) were included in the study. Informed consent was obtained in writing before the experiment, and the study received approval from the Ethics Committee of the University of Magdeburg. All participants received monetary compensation of 6.50€/h.
2.2. Materials
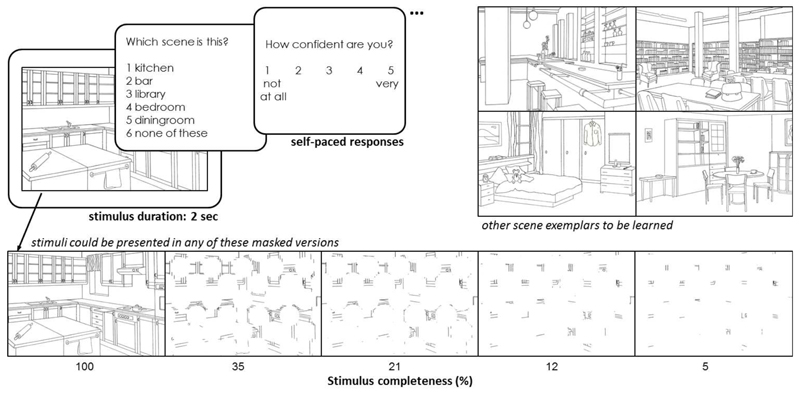
The experimental stimuli comprised 15 black and white line-drawn images (Hollingworth & Henderson, 1998) depicting simple indoor scenes (e.g. kitchen, bar, library, etc.). Stimulus completeness was manipulated for 10 of the 15 line-drawn images by masking them with a grid (5×6) of white circles. Four different completeness levels (35%, 21%, 12%, and 5%; percentages reflect the amount of the image visible through the mask) were created by gradually increasing the circle by a factor of 1.2 after each iteration (the size of this manipulation was determined by careful piloting of the paradigm). The original stimulus (100%), therefore, became progressively more occluded by the mask and appeared less complete (see Fig. 1, bottom panel). All stimuli were presented on a 15” computer screen.
Fig. 1. Experimental design of the test phase.
Each stimulus was presented for 2 seconds each, followed by 2 self-paced forced choice tasks - stimulus identification and confidence rating. In a previous study phase, participants learned the 5 depicted stimuli (kitchen, bar, library, bedroom, diningroom; from Hollingworth & Henderson, 1998). Those were then mixed with 5 novel items and all 10 were randomly presented in complete or masked form as shown in the bottom panel; percentages reflect the amount of the image visible through the mask.
2.3. Procedure
Prior to the test phase of the experiment (the results of which are outlined in this paper), participants learned 5 different scene exemplars. Each exemplar was presented for 2 seconds in the center of the screen, on a gray background; a verbal label of the image (e.g. ‘dining room’) preceded each scene for 1 second. All items were presented 3 times in a random order throughout the learning phase. To ensure that participants remembered the 5 scene exemplars, these items were presented again, intermixed with 5 new scene foils. Each stimulus was presented for 2 seconds, after which participants were required to indicate whether they had seen it before; if so, they had to select the corresponding description from among 3 semantically similar options (e.g. ‘kitchen’, ‘canteen’, ‘cafeteria’). Participants were allowed to proceed with the experiment only after correctly identifying each learned scene on 3 consecutive trials.
In the test phase of the experiment (see Fig. 1), the 5 original scene exemplars were again presented intermixed with 5 novel scene items; all stimuli were presented unmasked (100%) and in the 4 incomplete versions (35%, 21%, 12%, and 5%), resulting in 50 test items. Each item was shown 4 times in a random order with a duration of 2 seconds. On each trial, participants had to indicate which of the 5 learned scenes was presented or whether it was a new scene (i.e. ‘bar’, ‘library’, ‘dining room’, ‘bedroom’, ‘kitchen’, ‘none of these’). Responses were self-paced. Performance was scored as correct only when participants identified the one appropriate response (i.e. the exact stimulus name for learned stimuli, and 'none of these' for new stimuli), resulting in a chance level of 1/6 for each trial. Additionally, participants had to rate their confidence in this decision on a scale from 1 (‘not at all confident’) to 5 (‘very confident’).
2.4. Bias measure
Test performance for both learned and new items can rely upon pattern completion. The identification of learned items from partial cues (35%, 21%, 12%, and 5%) provides a demonstration of this process. Similarly, to identify new items, participants might employ a recall-to-reject strategy, whereby they retrieve a learned stimulus to compare it to the current sensory input before deciding on whether it is in fact new or not; this strategy, therefore, also relies upon pattern completion. It should be noted, that at the same time pattern separation is likely required to compare and orthogonalize the new item to the retrieved one.
First, we obtained performance scores for the learned stimuli (i.e. correctly selecting the exact stimulus name as a response), which served as an index of the individual recognition ability. We could then assess by how much performance for the new stimuli (i.e. correctly selecting ‘none of these’ as a response) deviated from this value, to test whether there was behavioral evidence for a response bias in older adults. Therefore, the difference in accuracy scores for learned minus new stimuli was calculated separately for each participant and for each level of stimulus completeness. Positive-going values were obtained if a participant’s performance for new stimuli was worse than for learned stimuli. This pattern of performance is indicative of a higher tendency to select one of the five learned options when presented with a new stimulus, which is interpreted here as a bias to complete towards a familiar pattern.
3. Results
3.1. Accuracy
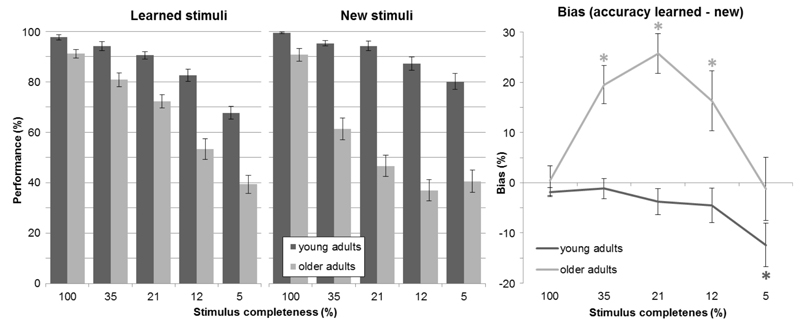
First, we assessed recognition ability by computing accuracy scores separately for learned and new stimuli (see Fig. 2, left panel). A three-way mixed analysis of variance (ANOVA) with a between-subjects factor of age (young, old), and two within-subjects factors (stimulus completeness: 100%, 35%, 21%, 12%, 5%; stimulus type: learned, new) revealed that young participants performed better than older participants (main effect of age: F(1,58) = 128.342, p < 0.001). For both groups, performance was modulated by the degree of stimulus completeness (main effect of stimulus completeness: F(4,232) = 256.981, p < 0.001), i.e. reduced stimulus completeness resulted in less accurate performance. This decrease was more pronounced in the elderly, as was revealed by a two-way interaction (age × stimulus completeness: F(4,232) = 46.104, p < 0.001). Interestingly, performance was differentially affected between age groups relative to whether they saw a learned or a new stimulus (age × stimulus type × stimulus completeness: F(4,232) = 5.54, p < 0.001). In fact, even though the performance per stimulus type (learned, new) was not different overall (main effect of stimulus type: F(1,58) = 3.517, p = 0.066), the two-way interaction of age and stimulus type showed that only older adults performed worse for new stimuli as compared to learned stimuli (see Fig. 2; age × stimulus type: F(1,58) = 18.227, p < 0.001). Post-hoc independent t-tests revealed age group differences in performance for all levels of stimulus completeness for both learned and new stimuli (after Holm-Bonferroni multiple comparisons correction; all p < 0.001; level 100%: tlearned(58) = 3.397, tnew(58) = 3.416, level 35%: tlearned(50.248) = 4.059, tnew(58) = 7.695, level 21%: tlearned(45.464) = 6.125, tnew(58) = 10.348, level 12%: tlearned(58) = 6.227, tnew(58) = 10.195, level 5%: tlearned(58) = 6.45, tnew(58) = 7.38). Altogether, these findings show that older adults’ recognition ability was impaired across all levels of stimulus completeness in relation to young adults, and even more so for new stimuli as compared to learned ones.
Fig. 2. Performance and bias measures.
Left, performance for both age groups, separately for learned and new stimuli for the 5 different levels of stimulus completeness (mean ± SE); right, bias measure (see section 2.4. for a detailed explanation) - difference in accuracy scores for learned minus new stimuli calculated separately for each participant (mean ± SE); positive values indicate a bias toward pattern completion, significant differences from 0 are indicated with * separately for each age group as indicated by color.
3.2. Response bias
Because we were interested in the identification of response biases, we looked at the distribution of response errors for learned items only. If false familiar responses (false alarms) occur more often than false ‘new’ responses (misses), this could potentially reveal a pattern completion bias. Numerically, older adults had higher false alarm rates than misses, while the reverse was true for young participants (descriptive statistics can be viewed in Table 1). However, the proportion of errors was too small for a detailed analysis.
Table 1. False alarm rates for learned stimuli.
| stimulus completeness | false alarms - mean (SE) | |
| young adults | older adults | |
| 100% | 0.22 (0.16) | 0.35 (0.09) |
| 35% | 0.24 (0.10) | 0.67 (0.07) |
| 21% | 0.39 (0.65) | 0.65 (0.05) |
| 12% | 0.63 (0.07) | 0.53 (0.04) |
| 5% | 0.44 (0.05) | 0.54 (0.05) |
False alarms and misses add up to 1, so that values higher than 0.5 indicate more false alarms, and values lower than 0.5 indicate more misses; values do not comprise the data of all participants since not all of them made errors for each completeness level.
To investigate a potential bias in more detail, accuracy scores for learned stimuli were treated as indices of the individual recognition ability, and we then assessed how much the performance for new stimuli deviated from this index. Therefore, we calculated individual bias scores by subtracting the accuracy scores for new stimuli from the learned stimuli for each participant separately (see section 2.4. for details). The resulting bias measures were submitted to a mixed ANOVA (age × stimulus completeness). Older adults had higher scores than young adults, i.e. a positive bias (see Fig. 2, right; main effect of age: F(1,58) = 18.227, p < 0.001). The bias scores were influenced by stimulus completeness (main effect of stimulus completeness: F(4,232) = 11.09, p < 0.001), and the two-way interaction with age was also significant (age × stimulus completeness: F(4,232) = 5.54, p < 0.001). To explore this interaction in more detail, we performed five planned post-hoc comparisons. Independent t-tests demonstrated significant between-group differences only for the middle three completeness levels after Holm-Bonferroni multiple comparisons correction (level 35%: t(58) = -4.82, p < 0.001, level 21%: t(58) = -6.216, p < 0.001; level 12%: t(58) = -3.016, p = 0.004).
To test the levels of stimulus completeness at which this score establishes a bias, group average bias scores for both the young and older adults were tested against 0 with five one-sample t-tests. Only the older adults showed a positive bias for the middle three completeness levels, indicative of a pattern completion bias (after Holm-Bonferroni multiple comparisons corrections; level 35%: t(29) = 5.131, p < 0.001, level 21%: t(29) = 6.466, p < 0.001; level 12%: t(29) = 2.717, p = 0.011), while younger participants showed the opposite, negative bias with the least complete stimuli (level 5%: t(29) = -2.868, p = 0.008). There was no evidence of a bias for the complete versions of the stimuli (level 100%: tyoung(29) = -2.009, pyoung = 0.054; told(29) = -0.145, pold = 0.886). This was to be expected as participants were allowed to continue to this part of the experiment only if they had demonstrated accurate memory for the stimuli and should therefore be able to discriminate learned from new stimuli equally well.
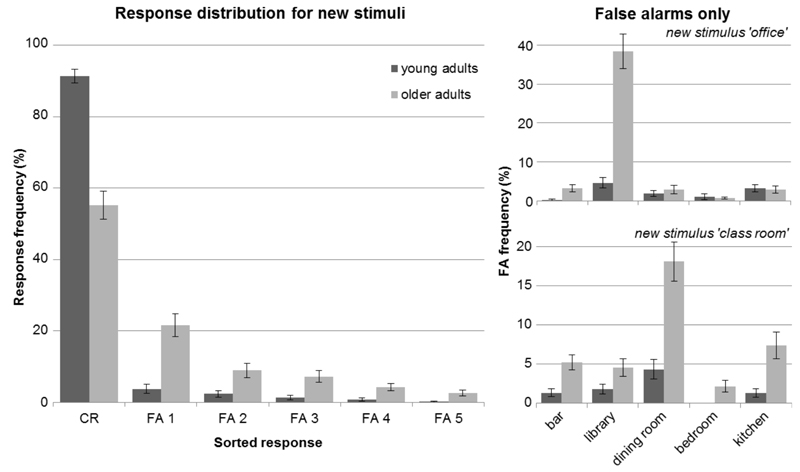
An alternative explanation for the lower performance of older adults for new stimuli could be that they guess more. Therefore, we looked at the distribution of errors for each specific new stimulus. If participants were simply guessing, then each false response choice (i.e. the learned stimuli’s labels ‘bar’, ‘library’, ‘dining room’, ‘bedroom’, ‘kitchen’) should occur equally frequent. Fig. 3 (right panel) shows the false alarm distribution for 2 exemplary stimuli. In fact, especially older adults chose one answer a lot more often than any of the other responses (i.e. ‘library’ was the most frequent false choice for stimulus ‘office’, and ‘dining room’ was the most frequent false choice for stimulus ‘class room’). This indicates that the participants were not randomly guessing. Instead, they chose the 1 of the 5 learned stimuli that was presumably perceived as most similar to the new one. To show that this error pattern was not simply driven by those 2 examples, we used the group-average frequencies of all false choice options, and sorted them from most (FA 1) to least (FA 5) chosen option for each new stimulus. Subsequently, we averaged across all new stimuli and obtained a generalized response distribution differentiating false alarms. The left panel of Fig. 3 shows that older adults indeed chose one particular false response option most often (FA 1) and did not simply guess more, which was confirmed by a χ²-test of goodness-of-fit on the 5 false alarm options (χ² = 716.949, df = 4, p < 0.001). Additionally, for each specific new stimulus, we tested the most frequent false alarm against the average of the other false alarms to show that there was one dominant option per stimulus (stimulus ‘office’: χ² = 185.719, df = 1, p < 0.001; stimulus ‘class room’: χ² = 44.899, df = 1, p < 0.001; stimulus ‘restaurant’: χ² = 44.024, df = 1, p < 0.001; stimulus ‘locker room’: χ² = 15.791, df = 1, p < 0.001; stimulus ‘living room’: χ² = 9.391, df = 1, p = 0.002). This indicates that older adults completed towards the stimulus perceived as most similar.
Fig. 3. Response distribution for new stimuli.
Left, responses are depicted over the 6 possible choice options (i.e. 'none of these' as correct rejections - CR, and the false alarms sorted according to frequency – FA 1-5; mean ± SE) showing that older adults chose one particular false response option most often (FA 1) rather than guess more overall, which would lead to similar frequencies for all 5 response options. Right, distributions of false alarms are depicted for 2 exemplary stimuli per actual false response option (i.e. label of the learned stimuli; mean ± SE).
3.3. Reaction times
Reaction times followed the profile of performance values as assessed by a three-way mixed ANOVA (age × stimulus completeness × stimulus type). Older adults were generally slower than young adults (main effect of age: F(1,58) = 25.333, p < 0.001), both groups became slower with decreasing stimulus completeness (main effect of stimulus completeness: F(4,232) = 37.01, p < 0.001), and older adults slowed down more with decreasing information (age × stimulus completeness: F(4,232) = 4.748, p = 0.001). Overall, there was no difference between learned and new stimuli (main effect of stimulus type: F(1,58) = 0.008, p = 0.93), but interestingly younger adults were faster for new stimuli while older adults were faster for learned stimuli (age × stimulus type: F(1,58) = 11.867, p = 0.001; age × stimulus type × stimulus completeness: F(4,232) = 3.123, p = 0.016).
3.4. Confidence ratings
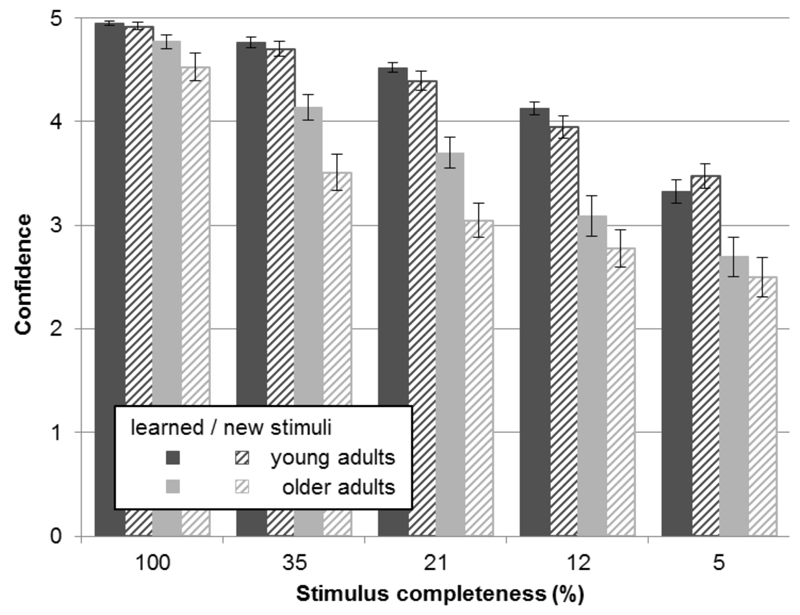
The confidence ratings further support the present findings (see Fig. 4). A three-way mixed ANOVA (age × stimulus completeness × stimulus type) revealed that older participants were generally less confident than young participants (main effect of age: F(1,58) = 33.499, p < 0.001). All participants were more confident in their responses when less of the image was masked (main effect of stimulus completeness: F(4,232) = 205.047, p < 0.001), and were also more confident when responding to learned, relative to new, items (main effect of stimulus type: F(1,58) = 26.931, p < 0.001). Mirroring task performance, relative to young adults, older participants were less confident in their responses (interaction of age × stimulus completeness: F(4,232) = 11.887, p < 0.001). Significant interactions (age × stimulus type: F(1,58) = 16.733, p < 0.001; age × stimulus type × stimulus completeness: F(4,232) = 3.58, p = 0.007) indicate that the age groups’ confidence was differentiallly affected depending on whether the stimuli were learned or new. As can be seen in Fig. 4, older adults were less confident in the identification of new stimuli, whereas young adults were equally confident for learned and new stimuli. This further supports the finding that older adults’ performance is adversely affected by unknown stimuli as compared to learned stimuli.
Fig. 4. Confidence ratings.
Scores for both age groups, separately for learned and new stimuli for the 5 different levels of stimulus completeness (mean ± SE). Ratings ranged from 1 (‘not at all confident’) to 5 (‘very confident’). Scores are depicted in dark gray for young adults, and in light gray for older adults; dashed lines indicate new stimuli, solid bars represent learned stimuli.
4. Discussion
We used a novel recognition memory paradigm to assess pattern completion and the impact of cognitive aging on this process. In contrast to previous studies investigating episodic memory processing we have shifted the focus from pattern separation to pattern completion. In the experiment, participants were asked to identify complete or partially masked stimuli, half of which they had learned previously. For both age groups, recognition accuracy was reduced with decreasing stimulus completeness. This effect, however, was more pronounced in older adults, suggesting that pattern completion may be adversely affected by aging. Older adults also showed a response bias toward familiar stimuli, as evidenced by the profile of errors for new items (i.e., a tendency to incorrectly select a familiar item as a response). This behavior may be the result of an underlying pattern completion bias as suggested by theoretical models of aging (Wilson et al., 2006).
The current paradigm is based on the original computational definition of pattern completion as a recollective process that restores a complete memory trace from partial or degraded input (Marr, 1971). Hunsaker and Kesner (2013, p. 40) have suggested that presenting subsets of an original cue could engage pattern completion more independently than degraded versions of it. We have incorporated this idea by manipulating stimulus completeness, in contrast to previous studies that have used altered versions of familiar stimuli (for review, see Yassa & Stark, 2011; Stokes, Kyle, & Ekstrom, 2014). Assuming that our manipulation was successful, the reported recognition memory deficits for previously experienced stimuli in older adults would indicate that pattern completion becomes deficient with age. Paleja and Spaniol (2013) reported similar findings in a task requiring participants to relocate a familiar target in a virtual environment. Older adults performed significantly worse than young adults when fewer cues were available at test, compared to study. This difference, however, was apparent only when no extra-maze cues were available and it remains open whether this could be explained by higher exploration rates of the young participants in comparison to older adults.
In a more general view of age-related memory changes, it is often reported that recognition memory is not as impaired as for example free recall is (Danckert & Craik, 2013; Luo & Craik, 2008). In contrast, our data demonstrate that recognition memory is impaired in the older age-group in a paradigm like ours and gets significantly more impaired when the retrieval stimuli become less complete. These results can provide a link by illustrating age-related impairments on the spectrum from environmentally aided (recognition) to more self-initiated (recall) processing (for theoretical accounts, see Craik, 1983, and Luo & Craik, 2008). Our finding that older adults were already impaired in the complete conditions (100%) may be explained by the fact that, unlike in many recognition paradigms in the literature, correct responses here demanded exact identification/naming of the stimulus as opposed to old/new/similar or remember/know judgments. We therefore suggest that previous assumptions about recognition memory in aging would need to be revisited in scenarios where the stimuli prompting retrieval are not exactly the same as during learning, or where tasks demand precise identification.
The second major finding of our study – the positive response bias in older adults – shows that this group tended to increasingly choose familiar responses even though they were presented with new stimuli. Thus, new partial information seemed to trigger the recognition of learned items although it was not part of the original cues. We interpret this as a bias toward pattern completion. Our findings would imply that with age even though the process of pattern completion seems to be deficient during actual memory retrieval, it is increasingly initiated despite the new (partial) information not corresponding to a stored memory.
Given its extensive excitatory recurrent connections, region CA3 within the hippocampus has been identified as a likely candidate to execute the auto-associative processing essential for pattern completion (for review, see Hunsaker & Kesner, 2013). This theory has very recently received direct empirical evidence from rodent data (Neunuebel & Knierim, 2014) showing that CA3 effectively performs pattern completion on the sensory input it receives from entorhinal cortex and the dentate gyrus. It should be noted that CA3 is not exclusively involved in pattern completion, but has also been found to contribute to other processing like rapid encoding, short-term memory or recall (for review, see Rolls & Kesner, 2006). Importantly, aging appears to selectively affect parts of the hippocampal circuit (Smith et al., 2000), because the perforant path degenerates - hence sensory input to CA3 is diminished – while CA3’s auto-associative network remains relatively intact. As a result, even when less information is fed forward to CA3, that is, only a subset of neurons is activated, neighboring CA3 cells can still be co-activated and memory traces be restored. Additionally, there is evidence from the rodent literature showing hyperactivity of CA3 cells in older rats (Wilson, Ikonen, Gallagher, Eichenbaum, & Tanila, 2005), and an age-related decline in cholinergic modulation which reduces inhibition in CA3 (Hasselmo, Schnell, & Barkai, 1995). Theoretical models of hippocampal function therefore predict that older adults should show a bias toward pattern completion (Wilson et al., 2006). Simultaneously, less sensory input to the dentate gyrus is suggested to result in the encoding of less distinct (or pattern separated) memory traces. The conjunction of those degenerative aspects could lead to an increased tendency to reactivate stored memory traces rather than encode new input. Our findings further support this notion, since older adults demonstrated a bias toward familiar stimuli, whereas young adults did not.
As mentioned earlier, the focus of many human studies has been directed towards pattern separation. Yassa and colleagues (2011) have reported an age-related shift from pattern separation to completion, which correlated with the integrity of the perforant path and the dentate gyrus/CA3 complex. In that study, participants saw pictures of objects with gradually decreasing mnemonic similarity among old and new items, and young adults readily exhibited separation-like BOLD responses for all the similar items while older adults only shifted from completion-like to separation-like activity with a bigger stimulus change. These findings were interpreted as a reduction in pattern separation processes in older adults, based also on behavioral discrimination deficits (i.e. incorrectly identifying a similar item as old). Their use of very similar stimuli might prompt this interpretation; however, we would like to point out that the reported shift might not necessarily stem from impaired pattern separation only. Our results could provide an additional explanation, highlighting the importance of pattern completion. Our stimulus selection did not require strong pattern separation as discussed earlier, because the images here were very different. Behavioral discrimination deficits could therefore also result from a bigger impact of pattern completion processes as compared to pattern separation. However, we cannot rule out the contribution of other processes in our task. Especially during the identification of new stimuli, pattern separation is potentially involved to compare the new item to the retrieved one. The observed response bias in older adults may therefore partly be explained by a failure to separately encode new stimuli, i.e. orthogonalize the new item to the retrieved one.
Our experiment was designed to specifically tap into retrieval processes by introducing a learning criterion during the study phase to ensure equal encoding, yet, it is possible that young and older adults exhibited differential learning. Given that there is no bias for complete stimuli, however, the observed group differences are likely to result from impaired retrieval processes rather than differential encoding. Along the same lines, we believe one can rule out a perceptual deficit of older adults as a primary cause of the reported bias. Any perceptual influence should follow the linear profile of the completeness manipulation, independent of learned or new stimuli, that is, less visibility should lead to weaker perception. This assumption cannot explain why older adults made more errors identifying new stimuli in the middle completeness levels as compared to the lowest level. Nevertheless, in future studies, it may be worth employing a methodology such as eye-tracking to control for potential encoding or perceptual differences similarly to Molitor and colleagues (2014).
An alternative explanation for a pattern completion bias in older adults is an inability to detect novelty. It has been suggested that the hippocampus acts as a match-mismatch detector that evaluates current sensory input in relation to stored representations to identify novelty (Kumaran & Maguire, 2007; 2009). In light of this mechanism, the reported pattern completion bias of older adults could be interpreted as a failure or impairment of novelty detection. Indeed, our results show that older adults are worse at identifying something new. However, Kumaran and Maguire (2007) have argued that hippocampal mismatch signals occur only when new input is very similar to stored memories, and interferes with predictions derived from previous experience. The observed linear performance decline with decreasing stimulus completeness suggests a mechanism signaling the degree of familiarity rather than a pure match-mismatch model as reasoned by Kumaran and Maguire (2009).
Finally, there is a wide literature documenting increased false alarm rates in older adults (for review, see Schacter, Koutstaal, & Norman, 1997), that is, an age-related increase in judging new items as old. Several reasons have been described to account for this change, including more liberal response criteria, decreasing overall sensitivity, increasing reliance on gist memory, or decreasing item-specific memory. Our data are not consistent with global shifts in sensitivity or response criteria, due to the reported bias curve; i.e. if there was a global criterion shift, false alarm rates should be uniformly distributed across all levels of stimulus completeness, but instead their probability varied. We want to point out that our data are different to some extent, because we did not find an increase in false alarms for complete stimuli, which are the standard material in most previous studies. Related to this, an fMRI study has suggested frontal regions as the origin of higher false alarm rates in older adults as opposed to medial temporal regions, pointing to impaired monitoring (Duarte, Graham, & Henson, 2010). However, the univariate analyses employed by the authors might not be sensitive enough to detect activity differences in medial temporal regions, because the average signal intensity in a voxel does not inform about subtle signal variations across voxels and conditions likely to occur during processes like pattern completion.
One major limitation of our study is that we cannot unravel the underlying neural processes and identify the involvement of different hippocampal subfields with a behavioral experiment like this. It may well be that pattern separation in the dentate gyrus is also impaired during encoding or even retrieval, while there are pattern completion deficits in CA3, and that the conjunction of these alleged processes produced the observed behavior. It remains a challenge for future neuroimaging research to disentangle these different processes, the involved brain areas and the resulting behavioral responses.
5. Conclusion
To summarize, we have used a novel recognition memory paradigm designed to target pattern completion processes by manipulating stimulus completeness. On the one hand, we demonstrated age-related recognition memory deficits for learned items, suggesting an underlying deficit in pattern completion. On the other hand, we showed a bias in older adults toward familiar responses during the identification of new items, strongly suggesting increased initiation of pattern completion processes even though the trigger stimuli should not have a corresponding memory trace. These findings are in line with predictions derived from theoretical models based on the literature about hippocampal circuitry. Our results provide more detailed insights into recognition memory deficits reported in older adults, and they serve as a starting point for further investigations to shed light on the underlying neural processes.
Highlights.
We use a new recognition paradigm to behaviorally test pattern completion in aging.
We examine how manipulating stimulus completeness influences recognition memory.
Less information adversely affects recognition accuracy especially in older adults.
Older adults show a pattern completion bias when limited information is available.
In old age, retrieving stored information is favored over encoding new events.
Acknowledgements
This research was funded by the European Research Council (Starting Investigator Grant AGESPACE 335090). The funding source had no direct involvement in the study. We thank Christin Ruß and Franziska Schulze for their help with data collection, and Jonathan Shine for valuable comments on the manuscript.
Footnotes
The authors declare no competing financial interests.
References
- 1.Ally BA, Hussey EP, Ko PC, Molitor RJ. Pattern separation and pattern completion in Alzheimer’s disease: Evidence of rapid forgetting in amnestic mild cognitive impairment. Hippocampus. 2013;23(12):1246–58. doi: 10.1002/hipo.22162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Craik FIM. On the transfer of information from temporary to permanent memory. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 1983;359:341–359. [Google Scholar]
- 3.Danckert SL, Craik FIM. Does aging affect recall more than recognition memory? Psychology and Aging. 2013;28(4):902–9. doi: 10.1037/a0033263. [DOI] [PubMed] [Google Scholar]
- 4.Duarte A, Graham KS, Henson RN. Age-related changes in neural activity associated with familiarity, recollection and false recognition. Neurobiology of Aging. 2010;31:1814–1830. doi: 10.1016/j.neurobiolaging.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 5.Gollin ES. Developmental studies of visual recognition of incomplete objects. Perceptual and Motor Skills. 1960;11:289–298. doi: 10.2466/pms.1962.15.3.583. [DOI] [PubMed] [Google Scholar]
- 6.Hasselmo ME, Schnell E, Barkai E. Dynamics of Learning and Recall at Excitatory Recurrent Synapses and Cholinergic Modulation in Rat Hippocampal Region CA3. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 1995;15(7):5249–5262. doi: 10.1523/JNEUROSCI.15-07-05249.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunsaker MR, Kesner RP. The operation of pattern separation and pattern completion processes associated with different attributes or domains of memory. Neuroscience and Biobehavioral Reviews. 2013;37(1):36–58. doi: 10.1016/j.neubiorev.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 8.Kumaran D, Maguire EA. Match mismatch processes underlie human hippocampal responses to associative novelty. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2007;27(32):8517–24. doi: 10.1523/JNEUROSCI.1677-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumaran D, Maguire EA. Novelty signals: a window into hippocampal information processing. Trends in Cognitive Sciences. 2009;13(2):47–54. doi: 10.1016/j.tics.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Luis CA, Keegan AP, Mullan M. Cross validation of the Montreal Cognitive Assessment in community dwelling older adults residing in the Southeastern US. International Journal of Geriatric Psychiatry. 2009;24:197–201. doi: 10.1002/gps.2101. (April 2008) [DOI] [PubMed] [Google Scholar]
- 11.Luo L, Craik FIM. Aging and memory: a cognitive approach. Canadian Journal of Psychiatry Revue Canadienne de Psychiatrie. 2008;53(6):346–53. doi: 10.1177/070674370805300603. [DOI] [PubMed] [Google Scholar]
- 12.Marr D. Simple Memory: A Theory for Archicortex. Philosophical Transactions of the Royal Society of London Series B Biological Sciences. 1971;262(841):23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- 13.McClelland JL, McNaughton BL, O’Reilly RC. Why There Are Complementary Leaming Systems in the Hippocampus and Neocortex: Insights From the Successes and Failures of Connectionist Models of Learning and Memory. Psychological Review. 1995;102(3):419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- 14.Molitor RJ, Ko PC, Hussey EP, Ally Ba. Memory-related eye movements challenge behavioral measures of pattern completion and pattern separation. Hippocampus. 2014;24(6):666–72. doi: 10.1002/hipo.22256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neunuebel JP, Knierim JJ. CA3 Retrieves Coherent Representations from Degraded Input: Direct Evidence for CA3 Pattern Completion and Dentate Gyrus Pattern Separation. Neuron. 2014;81(2):416–427. doi: 10.1016/j.neuron.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paleja M, Spaniol J. Spatial pattern completion deficits in older adults. Frontiers in Aging Neuroscience. 2013;5:3. doi: 10.3389/fnagi.2013.00003. (February) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rolls ET, Kesner RP. A computational theory of hippocampal function, and empirical tests of the theory. Progress in Neurobiology. 2006;79:1–48. doi: 10.1016/j.pneurobio.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Schacter DL, Koutstaal W, Norman KA. False memories and aging. Trends in Cognitive Sciences. 1997;1:229–236. doi: 10.1016/S1364-6613(97)01068-1. [DOI] [PubMed] [Google Scholar]
- 19.Smith TD, Adams MM, Gallagher M, Morrison JH, Rapp PR. Circuit-specific alterations in hippocampal synaptophysin immunoreactivity predict spatial learning impairment in aged rats. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2000;20(17):6587–93. doi: 10.1523/JNEUROSCI.20-17-06587.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stark SM, Yassa MA, Lacy JW, Stark CEL. A task to assess behavioral pattern separation (BPS) in humans: Data from healthy aging and mild cognitive impairment. Neuropsychologia. 2013;51(12):2442–9. doi: 10.1016/j.neuropsychologia.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stokes J, Kyle C, Ekstrom AD. Complementary Roles of Human Hippocampal Subfields in Differentiation and Integration of Spatial Context. Journal of Cognitive Neuroscience. 2014:1–14. doi: 10.1162/jocn. (Sep 30) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toner CK, Pirogovsky E, Kirwan CB, Gilbert PE. Visual object pattern separation deficits in nondemented older adults. Learning & Memory (Cold Spring Harbor, N.Y.) 2009;16(5):338–42. doi: 10.1101/lm.1315109. [DOI] [PubMed] [Google Scholar]
- 23.Wilson IA, Gallagher M, Eichenbaum H, Tanila H. Neurocognitive aging: prior memories hinder new hippocampal encoding. Trends in Neurosciences. 2006;29(12):662–70. doi: 10.1016/j.tins.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson IA, Ikonen S, Gallagher M, Eichenbaum H, Tanila H. Age-associated alterations of hippocampal place cells are subregion specific. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2005;25(29):6877–86. doi: 10.1523/JNEUROSCI.1744-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yassa MA, Mattfeld AT, Stark SM, Stark CEL. Age-related memory deficits linked to circuit-specific disruptions in the hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(21):8873–8. doi: 10.1073/pnas.1101567108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yassa MA, Stark CEL. Pattern separation in the hippocampus. Trends in Neurosciences. 2011;34(10):515–525. doi: 10.1016/j.tins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]