Abstract
In six control subjects pars compacta nerve cells in the ventrolateral substantia nigra had a lower melanin content than nerve cells in the dorsomedial region. This coincides with a natural anatomical division into ventral and dorsal tiers, which represent functionally distinct populations. In six cases of Parkinson's disease (PD) the ventral tier showed very few surviving nerve cells compared with preservation of cells in the dorsal tier. In 13 subjects without PD, but with nigral Lewy bodies and cell loss, the degenerative process started in the ventral tier, and spread to the dorsal tier. This pattern of selective degeneration of nigrostriatal neurons is not seen in ageing or after acute administration of MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine).
Full text
PDF
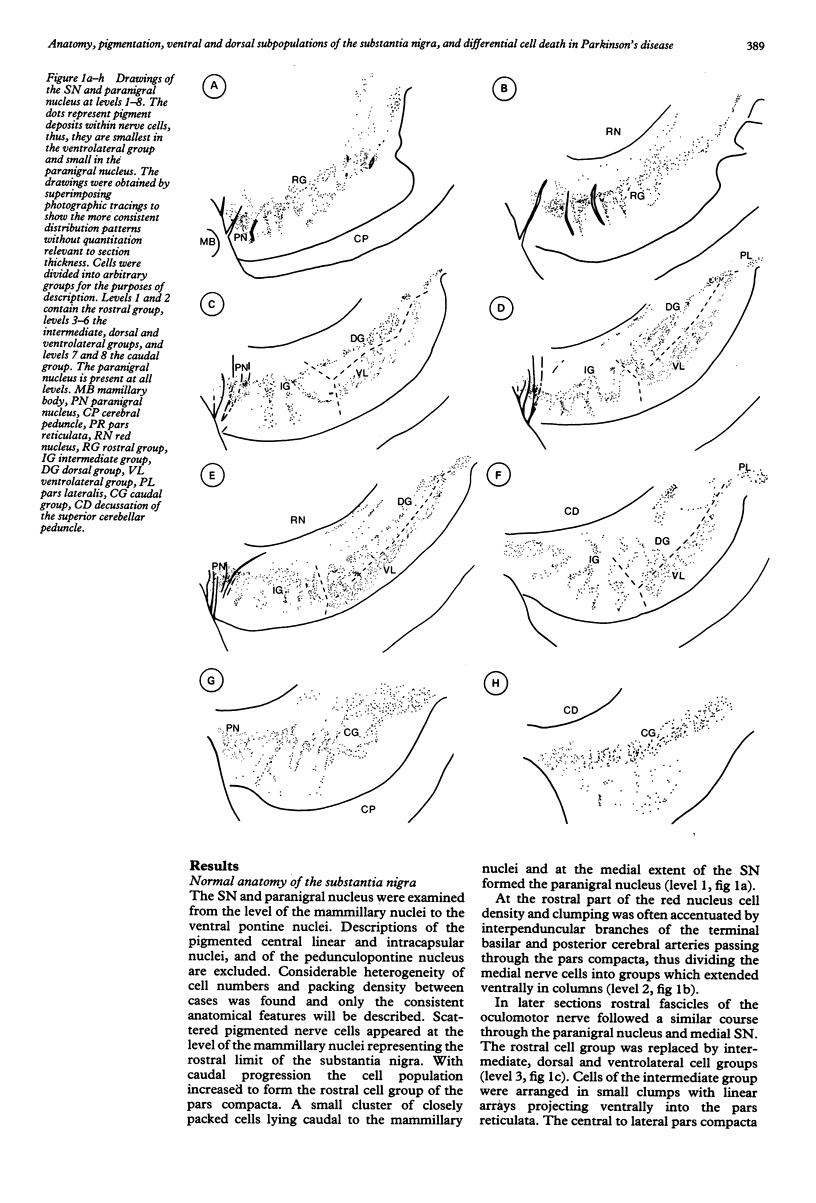


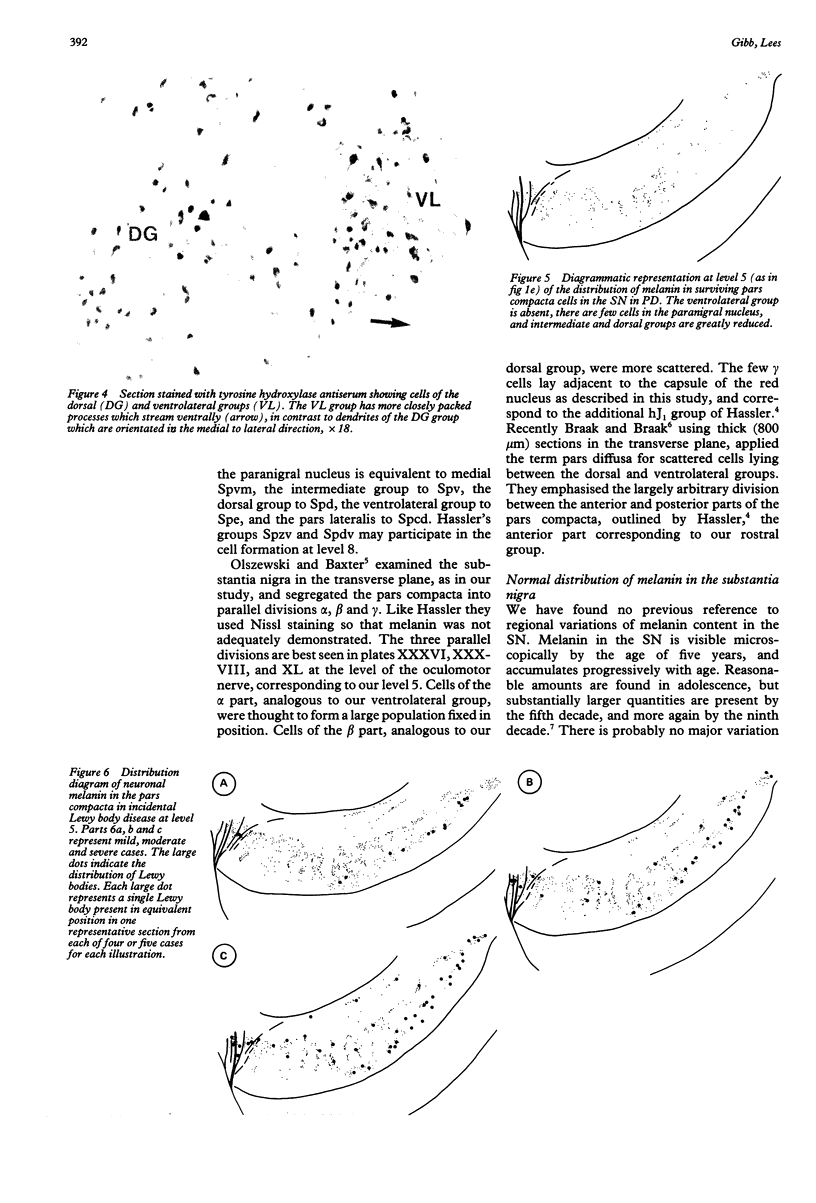



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADAMS R. D., VANBOGAERT L., VANDEREECKEN H. STRIATO-NIGRAL DEGENERATION. J Neuropathol Exp Neurol. 1964 Oct;23:584–608. [PubMed] [Google Scholar]
- Bernheimer H., Birkmayer W., Hornykiewicz O., Jellinger K., Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci. 1973 Dec;20(4):415–455. doi: 10.1016/0022-510x(73)90175-5. [DOI] [PubMed] [Google Scholar]
- Braak H., Braak E. Nuclear configuration and neuronal types of the nucleus niger in the brain of the human adult. Hum Neurobiol. 1986;5(2):71–82. [PubMed] [Google Scholar]
- Bédard P., Larochelle L., Parent A., Poirier L. J. The nigrostriatal pathway: a correlative study based on neuroanatomical and neurochemical criteria in the cat and the monkey. Exp Neurol. 1969 Nov;25(3):365–377. doi: 10.1016/0014-4886(69)90131-9. [DOI] [PubMed] [Google Scholar]
- Carpenter M. B., Peter P. Nigrostriatal and nigrothalamic fibers in the rhesus monkey. J Comp Neurol. 1972 Jan;144(1):93–115. doi: 10.1002/cne.901440105. [DOI] [PubMed] [Google Scholar]
- D'Amato R. J., Alexander G. M., Schwartzman R. J., Kitt C. A., Price D. L., Snyder S. H. Evidence for neuromelanin involvement in MPTP-induced neurotoxicity. 1987 May 28-Jun 3Nature. 327(6120):324–326. doi: 10.1038/327324a0. [DOI] [PubMed] [Google Scholar]
- D'Amato R. J., Lipman Z. P., Snyder S. H. Selectivity of the parkinsonian neurotoxin MPTP: toxic metabolite MPP+ binds to neuromelanin. Science. 1986 Feb 28;231(4741):987–989. doi: 10.1126/science.3080808. [DOI] [PubMed] [Google Scholar]
- Fallon J. H., Moore R. Y. Catecholamine innervation of the basal forebrain. IV. Topography of the dopamine projection to the basal forebrain and neostriatum. J Comp Neurol. 1978 Aug 1;180(3):545–580. doi: 10.1002/cne.901800310. [DOI] [PubMed] [Google Scholar]
- Fearnley J. M., Lees A. J. Striatonigral degeneration. A clinicopathological study. Brain. 1990 Dec;113(Pt 6):1823–1842. doi: 10.1093/brain/113.6.1823. [DOI] [PubMed] [Google Scholar]
- GREENFIELD J. G., BOSANQUET F. D. The brain-stem lesions in Parkinsonism. J Neurol Neurosurg Psychiatry. 1953 Nov;16(4):213–226. doi: 10.1136/jnnp.16.4.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen C. R., Baimbridge K. G., Thibault J. The neostriatal mosaic: III. Biochemical and developmental dissociation of patch-matrix mesostriatal systems. J Neurosci. 1987 Dec;7(12):3935–3944. doi: 10.1523/JNEUROSCI.07-12-03935.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen C. R., Herkenham M., Thibault J. The neostriatal mosaic: II. Patch- and matrix-directed mesostriatal dopaminergic and non-dopaminergic systems. J Neurosci. 1987 Dec;7(12):3915–3934. doi: 10.1523/JNEUROSCI.07-12-03915.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen C. R. The neostriatal mosaic. I. Compartmental organization of projections from the striatum to the substantia nigra in the rat. J Comp Neurol. 1985 Jun 22;236(4):454–476. doi: 10.1002/cne.902360404. [DOI] [PubMed] [Google Scholar]
- Gibb W. R., Lees A. J. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry. 1988 Jun;51(6):745–752. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel A. M., Moratalla R. Dopamine uptake sites in the striatum are distributed differentially in striosome and matrix compartments. Proc Natl Acad Sci U S A. 1989 Nov;86(22):9020–9024. doi: 10.1073/pnas.86.22.9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M., Edley S. M., Stuart J. Cell clusters in the nucleus accumbens of the rat, and the mosaic relationship of opiate receptors, acetylcholinesterase and subcortical afferent terminations. Neuroscience. 1984 Mar;11(3):561–593. doi: 10.1016/0306-4522(84)90045-9. [DOI] [PubMed] [Google Scholar]
- Hirsch E., Graybiel A. M., Agid Y. A. Melanized dopaminergic neurons are differentially susceptible to degeneration in Parkinson's disease. Nature. 1988 Jul 28;334(6180):345–348. doi: 10.1038/334345a0. [DOI] [PubMed] [Google Scholar]
- Jimenez-Castellanos J., Graybiel A. M. Subdivisions of the dopamine-containing A8-A9-A10 complex identified by their differential mesostriatal innervation of striosomes and extrastriosomal matrix. Neuroscience. 1987 Oct;23(1):223–242. doi: 10.1016/0306-4522(87)90285-5. [DOI] [PubMed] [Google Scholar]
- Kish S. J., Shannak K., Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson's disease. Pathophysiologic and clinical implications. N Engl J Med. 1988 Apr 7;318(14):876–880. doi: 10.1056/NEJM198804073181402. [DOI] [PubMed] [Google Scholar]
- Langer L. F., Graybiel A. M. Distinct nigrostriatal projection systems innervate striosomes and matrix in the primate striatum. Brain Res. 1989 Oct 2;498(2):344–350. doi: 10.1016/0006-8993(89)91114-1. [DOI] [PubMed] [Google Scholar]
- Mann D. M., Yates P. O. Lipoprotein pigments--their relationship to ageing in the human nervous system. II. The melanin content of pigmented nerve cells. Brain. 1974 Sep;97(3):489–498. doi: 10.1093/brain/97.1.489. [DOI] [PubMed] [Google Scholar]
- Mann D. M., Yates P. O. Pathological basis for neurotransmitter changes in Parkinson's disease. Neuropathol Appl Neurobiol. 1983 Jan-Feb;9(1):3–19. doi: 10.1111/j.1365-2990.1983.tb00320.x. [DOI] [PubMed] [Google Scholar]
- Mann D. M., Yates P. O. Possible role of neuromelanin in the pathogenesis of Parkinson's disease. Mech Ageing Dev. 1983 Feb;21(2):193–203. doi: 10.1016/0047-6374(83)90074-x. [DOI] [PubMed] [Google Scholar]
- McGeer P. L., McGeer E. G., Suzuki J. S. Aging and extrapyramidal function. Arch Neurol. 1977 Jan;34(1):33–35. doi: 10.1001/archneur.1977.00500130053010. [DOI] [PubMed] [Google Scholar]
- Pifl C., Schingnitz G., Hornykiewicz O. The neurotoxin MPTP does not reproduce in the rhesus monkey the interregional pattern of striatal dopamine loss typical of human idiopathic Parkinson's disease. Neurosci Lett. 1988 Oct 5;92(2):228–233. doi: 10.1016/0304-3940(88)90066-3. [DOI] [PubMed] [Google Scholar]
- Ruberg M., Javoy-Agid F., Hirsch E., Scatton B., LHeureux R., Hauw J. J., Duyckaerts C., Gray F., Morel-Maroger A., Rascol A. Dopaminergic and cholinergic lesions in progressive supranuclear palsy. Ann Neurol. 1985 Nov;18(5):523–529. doi: 10.1002/ana.410180503. [DOI] [PubMed] [Google Scholar]
- Scherman D., Desnos C., Darchen F., Pollak P., Javoy-Agid F., Agid Y. Striatal dopamine deficiency in Parkinson's disease: role of aging. Ann Neurol. 1989 Oct;26(4):551–557. doi: 10.1002/ana.410260409. [DOI] [PubMed] [Google Scholar]
- Smith Y., Parent A. Differential connections of caudate nucleus and putamen in the squirrel monkey (Saimiri sciureus). Neuroscience. 1986 Jun;18(2):347–371. doi: 10.1016/0306-4522(86)90159-4. [DOI] [PubMed] [Google Scholar]
- Snyder S. H., D'Amato R. J. MPTP: a neurotoxin relevant to the pathophysiology of Parkinson's disease. The 1985 George C. Cotzias lecture. Neurology. 1986 Feb;36(2):250–258. doi: 10.1212/wnl.36.2.250. [DOI] [PubMed] [Google Scholar]
- Spence A. M., Gilles F. H. Underpigmentation of the substantia nigra in chronic disease in children. Neurology. 1971 Apr;21(4):386–390. doi: 10.1212/wnl.21.4.386. [DOI] [PubMed] [Google Scholar]
- Spokes E. G., Bannister R., Oppenheimer D. R. Multiple system atrophy with autonomic failure: clinical, histological and neurochemical observations on four cases. J Neurol Sci. 1979 Sep;43(1):59–82. doi: 10.1016/0022-510x(79)90073-x. [DOI] [PubMed] [Google Scholar]
- Szabo J. Organization of the ascending striatal afferents in monkeys. J Comp Neurol. 1980 Jan 15;189(2):307–321. doi: 10.1002/cne.901890207. [DOI] [PubMed] [Google Scholar]
- Turner B. H., Wilson J. S., McKenzie J. C., Richtand N. MPTP produces a pattern of nigrostriatal degeneration which coincides with the mosaic organization of the caudate nucleus. Brain Res. 1988 Nov 8;473(1):60–64. doi: 10.1016/0006-8993(88)90315-0. [DOI] [PubMed] [Google Scholar]