Abstract
Vitamin D plays a significant role in our health, including cancer incidence and mortality. Vitamin D receptor (VDR) single-nucleotide polymorphisms (SNPs) may affect its activity, influencing the risk of cancer. Several studies have investigated VDR SNPs, but the association with the risk of cancer is controversial. Here, we present a meta-analysis to assess the association of TaqI, ApaI, and Cdx2 SNPs with the risk of cancer. A systematic literature search was performed following a predefined protocol and using validated search strategies. This meta-analysis shows the summary odd ratio (SOR) overall, by cancer sites and by ethnicity. Up to January 2014, we identified 73 independent studies with 35 525 cases and 38 675 controls. The meta-analysis of Cdx2 gg versus GG showed a significant 12% increased risk for all cancers [SOR=1.12; 95% confidence interval (CI): 1.00–1.25]. The other SNPs analyzed did not show an overall significant association with the risk of cancer: SOR=0.98 (95% CI: 0.90–1.07) and 1.06 (95% CI: 0.95–1.19) for TaqI tt versus TT and ApaI aa versus AA, respectively. TaqI shows a significant 43% increased risk for colorectal cancer (SOR=1.43; 95% CI: 1.30–1.58 for tt vs. TT). Strong frequency variations are present among different ethnic groups. This meta-analysis showed an overall increased risk of cancer associated with Cdx2 SNP and a specific higher risk of colorectal cancer associated with the TaqI polymorphism. The VDR genotype might become more relevant when clustered in a specific haplotype, associated with other SNPs of genes involved in vitamin D metabolism, or for specific tumors and/or patient characteristics.
Keywords: ApaI, cancer, Cdx2, polymorphisms, TaqI, vitamin D, vitamin D receptor
Introduction
Vitamin D comes from two sources: endogenous, which is produced in the skin on exposure to sunlight, and exogenous, to a minor extent, which is ingested in food. Vitamin D is transported by vitamin D-binding protein (GC) and hydroxylated in the liver into 25-hydroxyvitamin D [25(OH)D], the more stable circulating metabolite. 25(OH)D is further hydroxylated into 1,25-dihydroxyvitamin D [1,25(OH)2D] in the kidney. This is the biologically active metabolite that binds to nuclear vitamin D receptors (VDR). VDR is expressed in bone, intestine, and in many other tissues and cells including cancer cells.
So far, vitamin D has mainly been studied for its role in the maintenance of calcium and phosphate homeostasis, and bone health. However, it is also involved in a wide range of other health issues, cardiovascular diseases, metabolic disorders, allergy, and cancer (Deeb et al., 2007; Liu et al., 2008; Minambres et al., 2012). Numerous in-vitro studies have indicated that 1,25(OH)2D can inhibit cell proliferation and promote cell differentiation in tumor tissue, suggesting that vitamin D may be protective against cancer (Deeb et al., 2007). Concomitantly, epidemiological studies have shown an inverse relationship between the incidence of cancer, mortality, and plasma levels of vitamin D (Autier and Gandini, 2007; Gandini et al., 2011).
Vitamin D activity is mediated by its receptor (VDR). The VDR is a type II nuclear receptor that interacts with the promoters of vitamin-D-responsive genes. VDR is found bound to DNA in the presence of corepressors; when 1,25(OH)2D binds to the VDR, it triggers a series of conformational changes including the release of corepressors and the recruitment of coactivators (Strugnell and DeLuca, 1997). VDR is differentially expressed in many types of cancer including breast, cervix, ovary, and many others (Friedrich et al., 2003). Its expression, together with the enzyme involved in vitamin D hydroxylation, suggests a paracrine/autocrine vitamin D metabolism at cancer sites.
Several VDR single-nucleotide polymorphisms (SNPs) have been identified that may deregulate vitamin D activity, interfering with its role in the risk of cancer (Uitterlinden et al., 2004; Kostner et al., 2009). Our previous meta-analysis (Gnagnarella et al., 2014; Raimondi et al., 2014) suggested that the most studied SNPs, FokI (rs2228570) and BsmI (rs1544410), can determine risk factors for cancer. More recently, other SNPs have been investigated: TaqI (rs731236), ApaI (rs7975232), and Cdx2 (rs11568820). TaqI and ApaI polymorphisms, located near the 3′-UTR of the VDR gene, do not alter the protein’s amino acid sequence, and it remains difficult to explain how these variants might influence VDR function. However, even if they do not have a direct action, they can be in linkage with other gene polymorphisms and act as markers (Kostner et al., 2009) of other sequences in the VDR gene that regulate transcription, translation, or RNA processing (Durrin et al., 1999; Whitfield et al., 2001). Cdx2, located in the 5′ region of the VDR, has been suggested to modulate promoter activity, and the Cdx2 g allele showed 30% less transcriptional activity compared with the a allele (Arai et al., 2001). To clarify the possible role of TaqI, ApaI, and Cdx2 VDR polymorphisms in the risk of cancer, we carried out a comprehensive literature search and meta-analysis of published studies. We calculated risk estimates for each specific SNP for any cancer and for specific organs, and we examined extensively estimate inconsistencies, variability, and between-study heterogeneity.
Methods
A systematic literature search and quantitative analysis were planned, carried out, and reported following MOOSE guidelines on the meta-analysis of observational studies (Stroup et al., 2000).
Published reports were obtained from the following databases using validated search strategies: PUBMED, Ovid Medline, EMBASE, and ISI Web of Knowledge up to January 2014. We used the MeSH index terms ‘VDR’, ‘Vitamin D receptor’, or ‘TaqI’, ‘ApaI,’ and ‘Cdx2’ in combination with ‘cancer’ or ‘tumor’. We also performed manual searches of references cited in the retrieved articles and preceding reviews on the topic. Ecological studies, case reports, reviews, and editorials were not considered eligible. We screened titles, looked at abstracts and, if the abstract content was relevant, full copies of articles were retrieved and read by at least two coauthors.
We selected studies reporting the minimum information on relative risks necessary to carry out an adequate meta-analysis:
Sufficient information to estimate the relative risk and 95% confidence intervals (95% CI) for the association between TaqI, ApaI, or Cdx2 polymorphisms and cancer [odds ratios (OR), relative risks or crude data and corresponding SEs, variance, CIs, or P-value of the significance of the estimates].
Studies had to be independent and not duplicate results published in another article. When some articles studied the same population, results from the publication using the largest sample of patients were used.
A standardized data-collection protocol was used to gather the relevant data from each selected article. When data were reported by ethnicity or by cancer sites, the estimates were extracted separately for the two factors.
We excluded studies evaluating the risk of colorectal adenoma and benign prostatic hyperplasia as our endpoint was the risk of cancer and we included studies with disease-free controls.
When possible, we considered fully adjusted estimates of the association between VDR polymorphisms and cancer, both for heterozygous and minor allele homozygous patients compared with wild-type patients.
Data extraction was carried out by one coauthor in a predefined database and then revised by a second coauthor. For each study selected for this meta-analysis, we extracted information on authors, journal and year of publication, country, ethnicity of the study population, source of controls (hospital or population), number of cases and controls, risk estimates, and the corresponding CI along with variables adjusted for in the analysis.
Statistical analysis
The summary odds ratios (SORs) for heterozygous carriers and homozygous mutant carriers compared with wild-type patients were calculated. As cancer is a relatively rare disease, we ignored the distinction between the various estimates of relative risk (i.e. OR, rate ratio, risk ratio) and all measures were interpreted as relative risk. Every measure of association, and corresponding CIs, was transformed into log relative risks, and the corresponding variance was calculated using the formula proposed by Greenland (1987). When no estimates were given, crude estimates were calculated from tabular data. We used Woolf’s formula to evaluate the SE of the log relative risk.
The SOR was estimated by pooling the study-specific estimates with random-effects models as described by Van Houwelingen et al. (2002), with summary effect size obtained from maximum likelihood estimation. CIs were computed assuming an underlying t-distribution.
The measure of heterogeneity I2 has been considered to compare heterogeneities for different numbers of pooled studies. It can be interpreted as the percentage of total variation across several studies that is attributable to heterogeneity: larger values of I2 indicate greater heterogeneity. A threshold of I2 below 50% is generally considered an acceptable level of variability (Higgins and Thompson, 2002).
We presented SORs overall and separately for each cancer site (for which at least three papers were found unless differentially indicated), and stratified by ethnicity (White and other than White); moreover, we produced forest plots including the single studies and the SOR.
To assess the influence of possible sources of bias, we considered the STROBE checklist proposed for observational epidemiologic studies (Von Elm et al., 2008). According to the STROBE checklist, using metaregression, we evaluated between-study heterogeneity assessing the influence of different study features, such as the study population and study design. We also examined changes in results after exclusion of specific studies to evaluate the stability of the pooled estimates. Metaregressions and subgroup analyses were carried out to quantify between-study heterogeneity (Greenland, 1987). Heterogeneity was investigated by examining possible factors that could influence the estimates: ethnicity, source of SNP determination (blood vs. tissue), type of controls, race, adjustment for confounding factors, etc.
Furthermore, deviations from the Hardy–Weinberg (H–W) equilibrium for frequency of VDR genotypes of TaqI, ApaI, and Cdx2 polymorphisms in controls were assessed using the χ2-test.
Publication bias was evaluated graphically with a funnel plot and we carried out the Macaskill test (Macaskill et al., 2001), which is more powerful than the Egger test when fewer than 20 estimates are included in the analysis.
All the statistical analyses were carried out using SAS software (version 9.2; SAS Institute Inc., Cary, North Carolina, USA).
Results
In this meta-analysis, we investigated the associations between the VDR gene polymorphisms TaqI, ApaI, and Cdx2 and the risk of cancer. Seventy-three independent studies were identified. Some of them reported different estimates within the same manuscript (Table 1). Information on allele frequencies for each SNP, deviation from H–W equilibrium, and information on adjusting variables are presented in Supplementary Table 1. In Table 2, the SORs are reported, overall, by cancer site (prostate, breast, colorectal, skin, and ovary, and all the remaining organs grouped in ‘other sites’) and by ethnic groups, separately for TaqI, ApaI, and Cdx2. Estimates of between-study heterogeneity are also reported.
Table 1.
Characteristics of the studies included in the meta-analysys on the association between VDR TaqI, ApaI, and Cdx2 polymorphism and different types of cancer
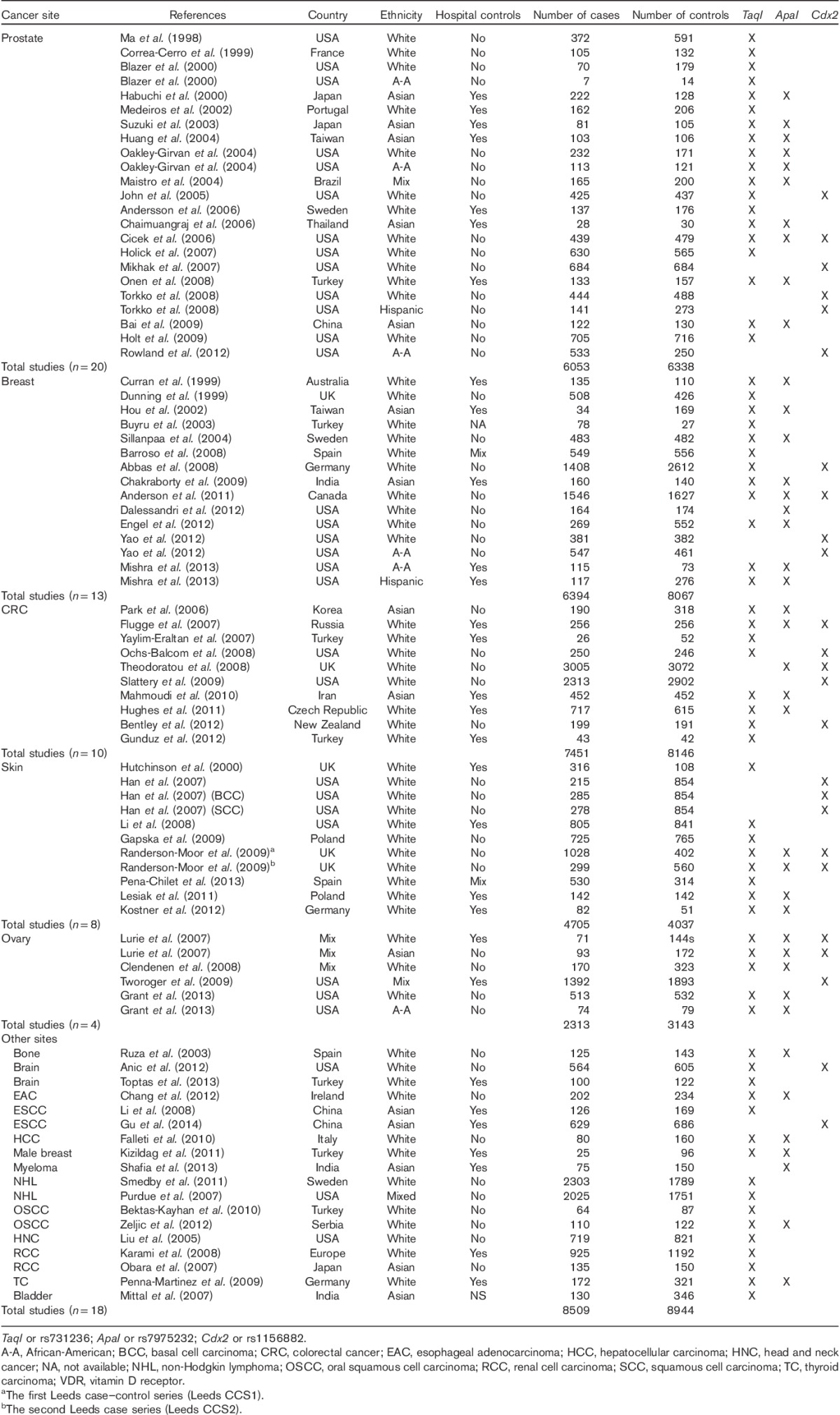
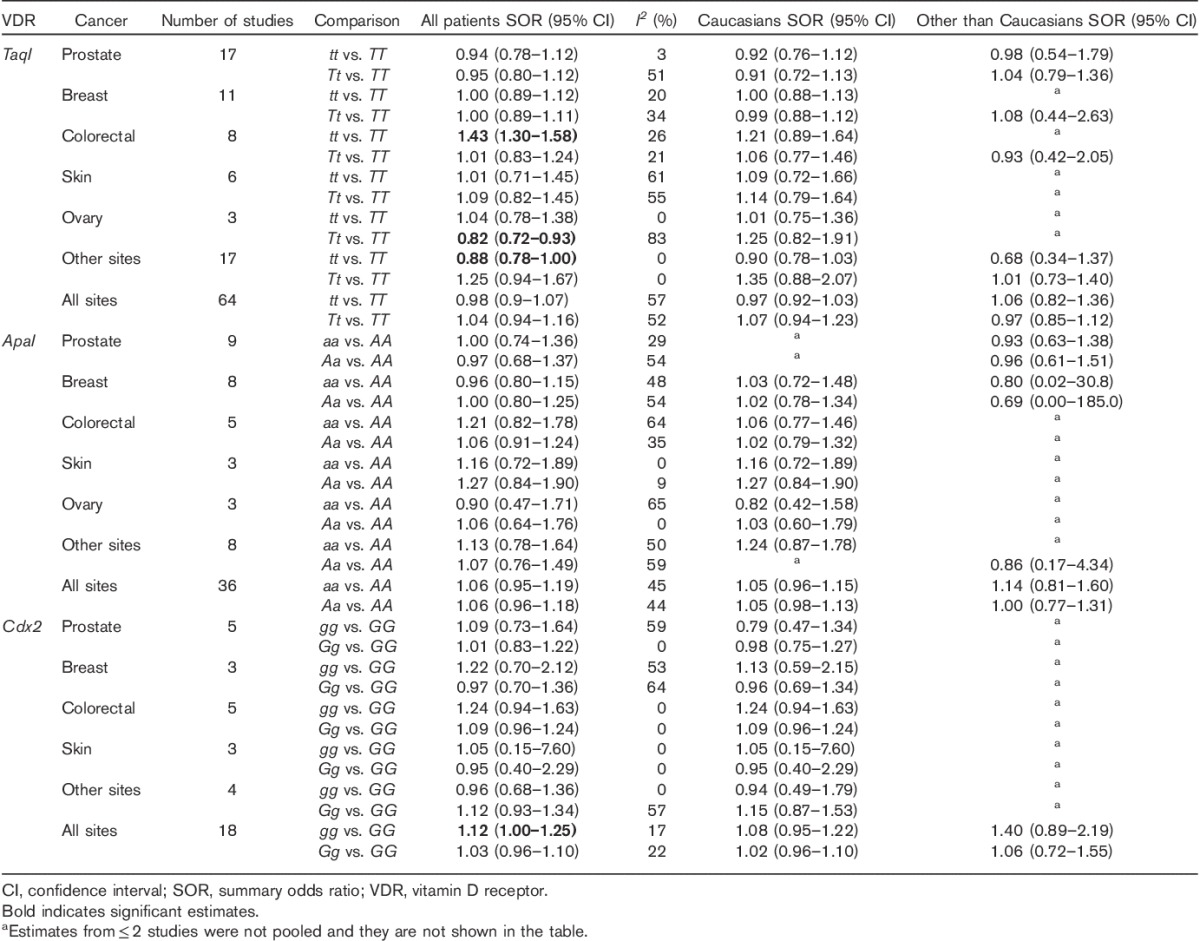
Table 2.
Overall summary odds ratios for the association of VDR TaqI, ApaI, and Cdx2 polymorphism with different types of cancer and ethnicity
TaqI
The role of TaqI polymorphism in the risk of cancer was investigated in 64 studies (Table 1). A total of 24 439 cases and 26 406 controls were included. Seventeen studies published results on the associations with prostate cancer, 11 with breast cancer, eight with colorectal, six with skin cancer, three with ovarian cancer, and 17 with other cancer sites. Overall, no significant association with the risk of cancer was observed for all cancer sites SOR=0.98 (95% CI: 0.9–1.07) and 1.04 (95% CI: 0.94–1.16) for tt and Tt versus the TT genotype, respectively (Table 2 and Fig. 1 and Supplementary Figure 1), and no major differences have been observed as stratified by ethnicity (White vs. other than White). The TaqI tt genotype has shown an increased risk for colorectal cancer, SOR 1.43 (95% CI: 1.30–1.58); the data lose significance in Caucasians [SOR=1.21 (95% CI: 0.89–1.64)]. An opposite trend was found in ovarian cancer, with an 18% risk reduction for the Tt genotype [SOR=0.82 (95% CI: 0.72–0.93], but with a large heterogeneity between study estimates (I2=83%), probably related to the inclusion in this analysis of different ethnic groups. Indeed, the calculated SOR for Caucasians was in the opposite direction, suggesting a possible risk reduction only for patients other than Caucasians (data not shown). A similar risk reduction was also observed for other cancer groups [SOR 0.88 (95% CI: 0.78–1.00].
Fig. 1.
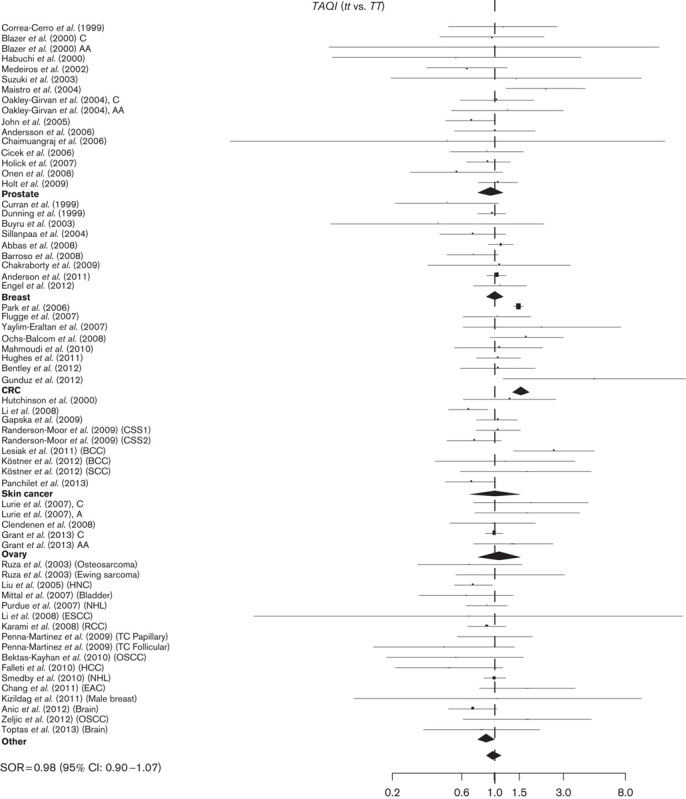
Study-specific SORs with 95% confidence intervals forest plot for the association between the development of cancer and TaqI tt versus TT genotype by cancer sites and overall. CRC, colorectal cancer; SORs, summary odds ratios.
The range of allele frequencies is relatively broad among the controls, the allele frequency ranging from 4 to 48%. Interestingly, in Asian populations, the t allele appears to be quite rare (Supplementary Table 1), and several studies from an Asian cohort do not have homozygote tt carriers, but only patients with the tT genotype. In five studies, a significant departure from H–W equilibrium was observed (Supplementary Table 1).
ApaI
The role of ApaI polymorphism in the risk of cancer has been investigated for a total of 12 542 cases and 13 574 controls (Table 1). The allele frequencies range from 23 to 70% for the a allele (Supplementary Table 1). In seven studies, a significant departure from H–W equilibrium was observed (Supplementary Table 1).
No significant association with the risk of cancer has been observed for any cancer site: SORs were 1.06 (95% CI: 0.95–1.19) and 1.06 (95% CI: 0.96–1.18) for aa and Aa versus the AA genotype, respectively (Table 2, Fig. 2 and Supplementary Figure 2).
Fig. 2.
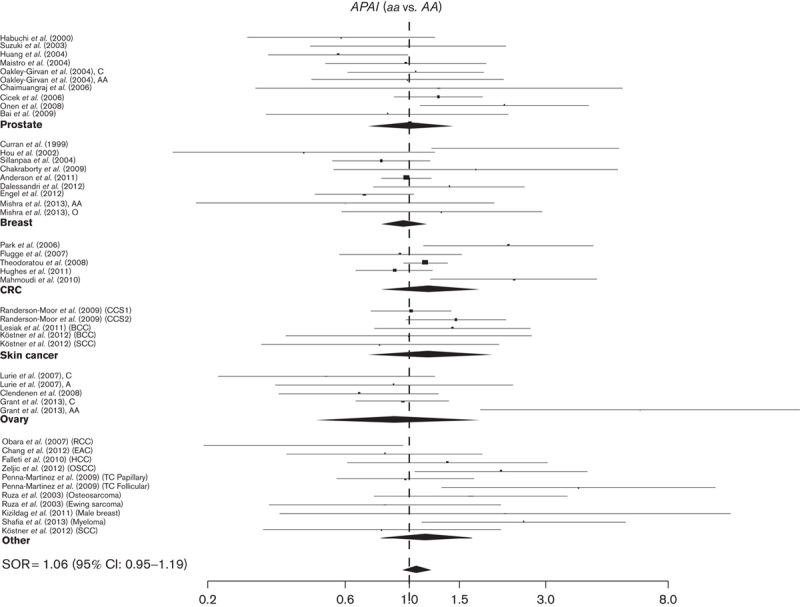
Study-specific SORs with 95% confidence intervals for the association between the development of cancer and ApaI aa versus AA genotype by cancer sites and overall. CRC, colorectal cancer; SORs, summary odd ratios.
Cdx2
A total of 25 studies (17 425 cases and 21 384 controls) were analyzed for the association between the Cdx2 polymorphism and the risk of cancer. Cdx2 showed a modest but significant association with all cancer sites: SOR was 1.12 (95% CI: 1.00–1.25) and 1.03 (95% CI: 0.96–1.10) for gg and Gg versus the GG genotype, respectively, with acceptable between-study heterogeneity (I2≤22%). Even if they do not reach statistical significance similar to the TaqI polymorphism, the non-Caucasians might predominantly contribute to the cancer risk association SOR 1.40 (95% CI: 0.89–2.19) (Table 2, Fig. 3 and Supplementary Figure 3).
Fig. 3.
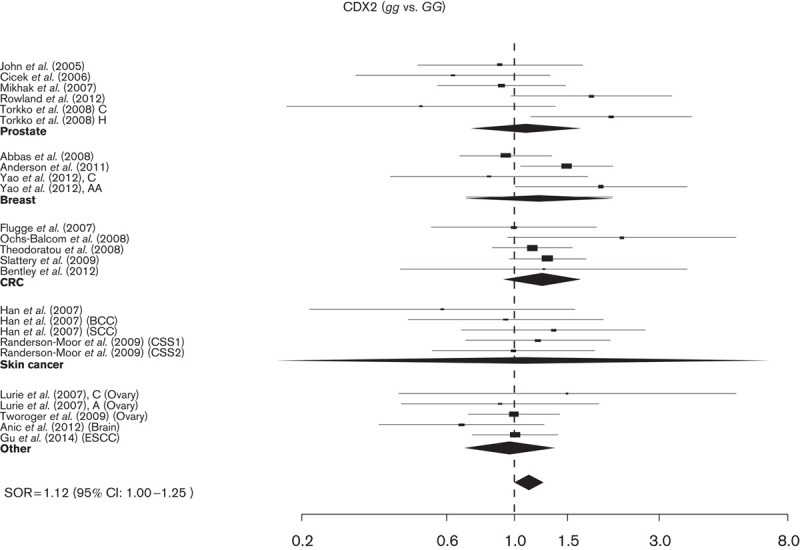
Study-specific SORs with 95% confidence intervals for the association between the development of cancer and Cdx2 gg versus GG genotype by cancer sites and overall. CRC, colorectal cancer; SORs, summary odds ratios.
Overall, the allele frequency ranged from 19 to 79% for the g allele (Supplementary Table 1). In Caucasians and Asian populations, it ranged from 20 to 45%, whereas in African-Americans g ranged from 73 to 79%. In three studies, a significant departure from H–W equilibrium was observed (Supplementary Table 1).
No evidence of publication bias was found for any of the investigated VDR polymorphisms and cancer sites.
Discussion
The role of VDR polymorphisms in the risk of cancer is controversial. To better define the possible clinical relevance, we carried out a comprehensive meta-analysis of TaqI, ApaI, and Cdx2 VDR polymorphisms. We found that the Cdx2 gg genotype was associated with a 12% increased risk of cancer overall. In-vitro reported gene assays found that the Cdx2 protein binds more efficiently to the a than to the g allele, and the g allele has been shown to drive transcription less efficiently (Crofts et al., 1998). The g allele has also been associated with lower bone mineral density in candidate gene studies (Arai et al., 2001) and in genome-wide association studies (Styrkarsdottir et al., 2008). The g allele frequency is particularly high in African Americans and it is possible that the association found in our meta-analysis was driven by non-White populations. Overall TaqI and ApaI variant genotypes did not show a significant association with the risk of cancer. For specific cancer sites, colorectal cancer showed a 43% increased risk with the tt TaqI genotype. Polymorphism frequencies have a very broad range, with a very low TaqI t allele frequency among Asians; the frequencies are consistent with the one reported in the literature (Uitterlinden et al., 2004).
Some VDR meta-analyses have been published (Touvier et al., 2011; Bai et al., 2012; Guo et al., 2012; Huang et al., 2013; Liu et al., 2013; Song and Lee, 2013; Wang et al., 2013; Xu et al., 2014), but differently from others, here we analyzed all cancer sites with a panel of different SNPs and we investigated sources of heterogeneity, including ethnicity, which seems to explain much of the between-study variation. The Huang group showed an overall 16% increased risk, increasing to 31% in African-American patients for Cdx2 SNP (variant homozygous condition versus the heterozygote plus the wild-type genotype) for any cancer. These data are similar to those we have reported here. BsmI was found to be associated with an increased risk of cancer by Xu et al. (2014). In his meta-analysis, FokI, TaqI, and ApaI did not show an overall cancer risk association, but only for specific cancer sites. The meta-analysis on colorectal cancer by Touvier et al. (2011) and a second one by Bai et al. (2012) reached similar conclusions: the BsmI polymorphism was found to be associated with a reduced risk of cancer, whereas FokI TaqI, ApaI, and Cdx2 did not show an association with the risk of colorectal cancer. In our meta-analysis, the TaqI tt genotype was associated with a 43% increased risk. Our analysis included eight studies, whereas the Bai et al. (2012) publication analyzed nine studies, including three studies on adenoma risk, which might weaken the data. The study by Touvier et al. (2011) had a smaller sample size. The TaqI polymorphism has been suggested to be associated with the risk of prostate cancer in Asian populations (Guo et al., 2012).
A meta-analysis on ovarian cancer (Song and Lee, 2013) consistently showed an increased risk associated with FokI and ApaI SNP. The FokI data are consistent with those reported by (Liu et al. (2013), even if ApaI was not associated with the risk of ovarian cancer. Again, conflicting results have been reported for ApaI and breast cancer by the Wang group (Wang et al., 2013) that suggested an association in Asian populations, whereas Luo et al. (2014) did not report this association. Cdx2 might be associated with the risk of breast cancer in African-Americans (Zhou et al., 2013), consistent with the data reported by Huang et al. (2013).
These data reinforce the hypothesis that VDR SNPs, and overall vitamin D metabolism, are correlated to the risk of cancer, and might be more relevant in specific ethnic groups. Moreover, single studies have suggested a correlation with vitamin D plasma level and tumor characteristics. The TaqI polymorphism, as reported by Ma et al. (1998), showed a reduction in the risk of prostate cancer only in patients with lower circulating vitamin D. Low vitamin D level and Cdx2 SNP were also associated with poorly differentiated prostate cancer (Mikhak et al., 2007). In colon cancer, vitamin D plasma level and BMI may interact with VDR SNPs (Yaylim-Eraltan et al., 2007; Ochs-Balcom et al., 2008; Gunduz et al., 2012). In breast cancer, the estrogen receptor status and vitamin D level may interact with VDR polymorphisms (Swami et al., 2000; Engel et al., 2012; Yao et al., 2012).
To strengthen the role of the VDR polymorphisms, some authors have defined haplotypes, analyzing more SNPs simultaneously, but this approach is difficult in a meta-analytic context, because of the inconsistent analysis throughout the different studies and generally low statistical power for haplotype analyses (McCullough et al., 2007; Abbas et al., 2008; Engel et al., 2012).
Most likely, to identify a clinically relevant VDR phenotype, it is necessary to include in the analysis the 25(OH)D plasma levels and other genes involved in vitamin D activity, such as the binding protein (GC) and the anabolic and catabolic enzymes (CYP27A1, CYP27B1, CYP24A1, and CYP2R1) (Lauridsen et al., 2005; Deeb et al., 2007). The multifunctional plasma protein GC is the major transporter of vitamin D metabolites in the circulation. Plasma 1,25(OH)2D and 25(OH)D levels are related to the GC genotype (Lauridsen et al., 2005). GC SNPs may have a dual effect and may be associated with lower or higher vitamin D, and also the response to vitamin D supplementation may be modified (Muindi et al., 2013). In a recent genome-wide association study, the combination of GC (rs2282679), DHCR7 (rs12785878), and CYP2R1 (rs10741657) SNPs conferred an approximately two-fold increase in the risk of vitamin D deficiency (Wang et al., 2010). In addition, for specific GC SNP, an association with the risk of cancer for melanoma and hepatocellular carcinoma has also been shown; for colon cancer GC SNP may correlate with the prognosis, whereas the association with the risk of cancer has not been confirmed (Hiraki et al., 2013; Lange et al., 2013; Pena-Chilet et al., 2013; Szkandera et al., 2013). One more factor is the variability within the tumor tissue of the complex vitamin D metabolisms’ differential exposure of VDR and other enzymes to promote vitamin D anabolisms or catabolism within the tumor itself (Anderson et al., 2006; Thill et al., 2010).
In conclusion, our study represents an updated and comprehensive meta-analysis on the role of TaqI, ApaI, and Cdx2 VDR polymorphisms and the risk of cancer at any site including 23 cancer types and provided a complete picture of the role of VDR polymorphisms in the risk of cancer. Among the SNPs included in this meta-analysis, the Cdx2 polymorphism has shown a general trend toward an increased risk of cancer. TaqI has been found to be associated with an increased risk for colorectal cancer, whereas overall, ApaI is not associated significantly with the risk of cancer. Limitations are because of the low number of studies available for some cancer sites or for some ethnic groups.
The VDR genotype might become more clinically relevant clustered in a specific haplotype, considering the CG-binding protein or for specific tumors and/or patient characteristics.
Acknowledgements
This work was supported by grants from the Fondazione Umberto Veronesi.
The authors thank William Russel-Edu for help with the literature.
Conflicts of interest
There are no conflicts of interest.
Footnotes
All supplementary digital content is available directly from the corresponding author.
References
- Abbas S, Nieters A, Linseisen J, Slanger T, Kropp S, Mutschelknauss EJ, et al. (2008). Vitamin D receptor gene polymorphisms and haplotypes and postmenopausal breast cancer risk. Breast Cancer Res 10:R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson LN, Cotterchio M, Cole DE, Knight JA. (2011). Vitamin D-related genetic variants, interactions with vitamin D exposure, and breast cancer risk among Caucasian women in Ontario. Cancer Epidemiol Biomarkers Prev 20:1708–1717. [DOI] [PubMed] [Google Scholar]
- Anderson MG, Nakane M, Ruan X, Kroeger PE, Wu-Wong JR. (2006). Expression of VDR and CYP24A1 mRNA in human tumors. Cancer Chemother Pharmacol 57:234–240. [DOI] [PubMed] [Google Scholar]
- Andersson P, Varenhorst E, Soderkvist P. (2006). Androgen receptor and vitamin D receptor gene polymorphisms and prostate cancer risk. Eur J Cancer 42:2833–2837. [DOI] [PubMed] [Google Scholar]
- Anic GM, Thompson RC, Nabors LB, Olson JJ, Browning JE, Madden MH, et al. (2012). An exploratory analysis of common genetic variants in the vitamin D pathway including genome-wide associated variants in relation to glioma risk and outcome. Cancer Causes Control 23:1443–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai H, Miyamoto KI, Yoshida M, Yamamoto H, Taketani Y, Morita K, et al. (2001). The polymorphism in the caudal-related homeodomain protein Cdx-2 binding element in the human vitamin D receptor gene. J Bone Miner Res 16:1256–1264. [DOI] [PubMed] [Google Scholar]
- Autier P, Gandini S. (2007). Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med 167:1730–1737. [DOI] [PubMed] [Google Scholar]
- Bai Y, Yu Y, Yu B, Ge J, Ji J, Lu H, et al. (2009). Association of vitamin D receptor polymorphisms with the risk of prostate cancer in the Han population of Southern China. BMC Med Genet 10:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai YH, Lu H, Hong D, Lin CC, Yu Z, Chen BC. (2012). Vitamin D receptor gene polymorphisms and colorectal cancer risk: a systematic meta-analysis. World J Gastroenterol 18:1672–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barroso E, Fernandez LP, Milne RL, Pita G, Sendagorta E, Floristan U, et al. (2008). Genetic analysis of the vitamin D receptor gene in two epithelial cancers: melanoma and breast cancer case–control studies. BMC Cancer 8:385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bektas-Kayhan K, Unur M, Yaylim-Eraltan I, Ergen HA, Toptas B, Hafiz G, et al. (2010). Association of vitamin D receptor Taq I polymorphism and susceptibility to oral squamous cell carcinoma. In Vivo 24:755–759. [PubMed] [Google Scholar]
- Bentley RW, Keown DA, Gearry RB, Cameron VA, Keenan J, Roberts RL, Day AS (2012). Vitamin D receptor polymorphisms in colorectal cancer in New Zealand: an association study. N Z Med J 125:47–51. [PubMed] [Google Scholar]
- Blazer DG, III, Umbach DM, Bostick RM, Taylor JA. (2000). Vitamin D receptor polymorphisms and prostate cancer. Mol Carcinog 27:18–23. [DOI] [PubMed] [Google Scholar]
- Buyru N, Tezol A, Yosunkaya-Fenerci E, Dalay N. (2003). Vitamin D receptor gene polymorphisms in breast cancer. Exp Mol Med 35:550–555. [DOI] [PubMed] [Google Scholar]
- Chaimuangraj S, Thammachoti R, Ongphiphadhanakul B, Thammavit W. (2006). Lack of association of VDR polymorphisms with Thai prostate cancer as compared with benign prostate hyperplasia and controls. Asian Pac J Cancer Prev 7:136–139. [PubMed] [Google Scholar]
- Chakraborty A, Mishra AK, Soni A, Regina T, Mohil R, Bhatnagar D, et al. (2009). Vitamin D receptor gene polymorphism(s) and breast cancer risk in north Indians. Cancer Detect Prev 32:386–394. [DOI] [PubMed] [Google Scholar]
- Chang CK, Mulholland HG, Cantwell MM, Anderson LA, Johnston BT, McKnight AJ, et al. (2012). Vitamin D receptor gene variants and esophageal adenocarcinoma risk: a population-based case-control study. J Gastrointest Cancer 43:512–517. [DOI] [PubMed] [Google Scholar]
- Cicek MS, Liu X, Schumacher FR, Casey G, Witte JS. (2006). Vitamin D receptor genotypes/haplotypes and prostate cancer risk. Cancer Epidemiol Biomarkers Prev 15:2549–2552. [DOI] [PubMed] [Google Scholar]
- Clendenen TV, Arslan AA, Koenig KL, Enquist K, Wirgin I, Agren A, et al. (2008). Vitamin D receptor polymorphisms and risk of epithelial ovarian cancer. Cancer Lett 260:209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa-Cerro L, Berthon P, Haussler J, Bochum S, Drelon E, Mangin P, et al. (1999). Vitamin D receptor polymorphisms as markers in prostate cancer. Hum Genet 105:281–287. [DOI] [PubMed] [Google Scholar]
- Crofts LA, Hancock MS, Morrison NA, Eisman JA. (1998). Multiple promoters direct the tissue-specific expression of novel N-terminal variant human vitamin D receptor gene transcripts. Proc Natl Acad Sci USA 95:10529–10534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran JE, Vaughan T, Lea RA, Weinstein SR, Morrison NA, Griffiths LR. (1999). Association of A vitamin D receptor polymorphism with sporadic breast cancer development. Int J Cancer 83:723–726. [DOI] [PubMed] [Google Scholar]
- Dalessandri KM, Miike R, Wiencke JK, Farren G, Pugh TW, Manjeshwar S, et al. (2012). Vitamin D receptor polymorphisms and breast cancer risk in a high-incidence population: a pilot study. J Am Coll Surg 215:652–657. [DOI] [PubMed] [Google Scholar]
- Deeb KK, Trump DL, Johnson CS. (2007). Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer 7:684–700. [DOI] [PubMed] [Google Scholar]
- Dunning AM, McBride S, Gregory J, Durocher F, Foster NA, Healey CS, et al. (1999). No association between androgen or vitamin D receptor gene polymorphisms and risk of breast cancer. Carcinogenesis 20:2131–2135. [DOI] [PubMed] [Google Scholar]
- Durrin LK, Haile RW, Ingles SA, Coetzee GA. (1999). Vitamin D receptor 3′-untranslated region polymorphisms: lack of effect on mRNA stability. Biochim Biophys Acta 1453:311–320. [DOI] [PubMed] [Google Scholar]
- Engel LS, Orlow I, Sima CS, Satagopan J, Mujumdar U, Roy P, et al. (2012). Vitamin D receptor gene haplotypes and polymorphisms and risk of breast cancer: a nested case–control study. Cancer Epidemiol Biomarkers Prev 21:1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falleti E, Bitetto D, Fabris C, Cussigh A, Fontanini E, Fornasiere E, et al. (2010). Vitamin D receptor gene polymorphisms and hepatocellular carcinoma in alcoholic cirrhosis. World J Gastroenterol 16:3016–3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flugge J, Krusekopf S, Goldammer M, Osswald E, Terhalle W, Malzahn U, et al. (2007). Vitamin D receptor haplotypes protect against development of colorectal cancer. Eur J Clin Pharmacol 63:997–1005. [DOI] [PubMed] [Google Scholar]
- Friedrich M, Rafi L, Mitschele T, Tilgen W, Schmidt W, Reichrath J. (2003). Analysis of the vitamin D system in cervical carcinomas, breast cancer and ovarian cancer. Recent Results Cancer Res 164:239–246. [DOI] [PubMed] [Google Scholar]
- Gandini S, Boniol M, Haukka J, Byrnes G, Cox B, Sneyd MJ, et al. (2011). Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int J Cancer 128:1414–1424. [DOI] [PubMed] [Google Scholar]
- Gapska P, Scott RJ, Serrano-Fernandez P, Mirecka A, Rassoud I, Gorski B, et al. (2009). Vitamin D receptor variants and the malignant melanoma risk: a population-based study. Cancer Epidemiol 33:103–107. [DOI] [PubMed] [Google Scholar]
- Gnagnarella P, Pasquali E, Serrano D, Raimondi S, Disalvatore D, Gandini S. (2014). Vitamin D receptor polymorphism FokI and cancer risk: a comprehensive meta-analysis. Carcinogenesis 35:1913–1919. [DOI] [PubMed] [Google Scholar]
- Grant DJ, Hoyo C, Akushevich L, Iversen ES, Whitaker R, Marks J, et al. (2013). Vitamin D receptor (VDR) polymorphisms and risk of ovarian cancer in Caucasian and African American women. Gynecol Oncol 129:173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenland S. (1987). Quantitative methods in the review of epidemiologic literature. Epidemiol Rev 9:1–30. [DOI] [PubMed] [Google Scholar]
- Gu H, Wang X, Zheng L, Tang W, Dong C, Wang L, et al. (2014). Vitamin D receptor gene polymorphisms and esophageal cancer risk in a Chinese population: a negative study. Med Oncol 31:827. [DOI] [PubMed] [Google Scholar]
- Gunduz M, Cacina C, Toptas B, Yaylim-Eraltan I, Tekand Y, Isbir T. (2012). Association of vitamin D receptor gene polymorphisms with colon cancer. Genet Test Mol Biomarkers 16:1058–1061. [DOI] [PubMed] [Google Scholar]
- Guo YJ, Shi ZM, Liu JD, Lei N, Chen QH, Tang Y. (2012). Meta-analysis of the relation between the VDR gene TaqI polymorphism and genetic susceptibility to prostate cancer in Asian populations. Asian Pac J Cancer Prev 13:4441–4444. [DOI] [PubMed] [Google Scholar]
- Habuchi T, Suzuki T, Sasaki R, Wang L, Sato K, Satoh S, et al. (2000). Association of vitamin D receptor gene polymorphism with prostate cancer and benign prostatic hyperplasia in a Japanese population. Cancer Res 60:305–308. [PubMed] [Google Scholar]
- Han J, Colditz GA, Hunter DJ. (2007). Polymorphisms in the MTHFR and VDR genes and skin cancer risk. Carcinogenesis 28:390–397. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG. (2002). Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558. [DOI] [PubMed] [Google Scholar]
- Hiraki LT, Qu C, Hutter CM, Baron J, Berndt SI, Bezieau S, et al. (2013). Genetic predictors of circulating 25-hydroxyvitamin D and risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev 22:2037–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick CN, Stanford JL, Kwon EM, Ostrander EA, Nejentsev S, Peters U. (2007). Comprehensive association analysis of the vitamin D pathway genes, VDR, CYP27B1, and CYP24A1, in prostate cancer. Cancer Epidemiol Biomarkers Prev 16:1990–1999. [DOI] [PubMed] [Google Scholar]
- Holt SK, Kwon EM, Peters U, Ostrander EA, Stanford JL. (2009). Vitamin D pathway gene variants and prostate cancer risk. Cancer Epidemiol Biomarkers Prev 18:1929–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou MF, Tien YC, Lin GT, Chen CJ, Liu CS, Lin SY, et al. (2002). Association of vitamin D receptor gene polymorphism with sporadic breast cancer in Taiwanese patients. Breast Cancer Res Treat 74:1–7. [DOI] [PubMed] [Google Scholar]
- Huang J, Huang J, Ma Y, Wang H, Yang J, Xiong T, et al. (2013). The Cdx-2 polymorphism in the VDR gene is associated with increased risk of cancer: a meta-analysis. Mol Biol Rep 40:4219–4225. [DOI] [PubMed] [Google Scholar]
- Huang SP, Chou YH, Wayne Chang WS, Wu MT, Chen YY, Yu CC, et al. (2004). Association between vitamin D receptor polymorphisms and prostate cancer risk in a Taiwanese population. Cancer Lett 207:69–77. [DOI] [PubMed] [Google Scholar]
- Hughes DJ, Hlavata I, Soucek P, Pardini B, Naccarati A, Vodickova L, et al. (2011). Variation in the vitamin D receptor gene is not associated with risk of colorectal cancer in the Czech Republic. J Gastrointest Cancer 42:149–154. [DOI] [PubMed] [Google Scholar]
- Hutchinson PE, Osborne JE, Lear JT, Smith AG, Bowers PW, Morris PN, et al. (2000). Vitamin D receptor polymorphisms are associated with altered prognosis in patients with malignant melanoma. Clin Cancer Res 6:498–504. [PubMed] [Google Scholar]
- John EM, Schwartz GG, Koo J, Van Den Berg D, Ingles SA. (2005). Sun exposure, vitamin D receptor gene polymorphisms, and risk of advanced prostate cancer. Cancer Res 65:5470–5479. [DOI] [PubMed] [Google Scholar]
- Karami S, Brennan P, Hung RJ, Boffetta P, Toro J, Wilson RT, et al. (2008). Vitamin D receptor polymorphisms and renal cancer risk in Central and Eastern Europe. J Toxicol Environ Health A 71:367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizildag S, Gulsu E, Bagci O, Yuksel E, Canda T. (2011). Vitamin D receptor gene polymorphisms and male breast cancer risk in Turkish population. J BUON 16:640–645. [PubMed] [Google Scholar]
- Kostner K, Denzer N, Muller CS, Klein R, Tilgen W, Reichrath J. (2009). The relevance of vitamin D receptor (VDR) gene polymorphisms for cancer: a review of the literature. Anticancer Res 29:3511–3536. [PubMed] [Google Scholar]
- Kostner K, Denzer N, Koreng M, Reichrath S, Graber S, Klein R, et al. (2012). Association of genetic variants of the vitamin D receptor (VDR) with cutaneous squamous cell carcinomas (SCC) and basal cell carcinomas (BCC): a pilot study in a German population. Anticancer Res 32:327–333. [PubMed] [Google Scholar]
- Lange CM, Miki D, Ochi H, Nischalke HD, Bojunga J, Bibert S, et al. (2013). Genetic analyses reveal a role for vitamin D insufficiency in HCV-associated hepatocellular carcinoma development. PLoS One 8:e64053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauridsen AL, Vestergaard P, Hermann AP, Brot C, Heickendorff L, Mosekilde L, et al. (2005). Plasma concentrations of 25-hydroxy-vitamin D and 1,25-dihydroxy-vitamin D are related to the phenotype of Gc (vitamin D-binding protein): a cross-sectional study on 595 early postmenopausal women. Calcif Tissue Int 77:15–22. [DOI] [PubMed] [Google Scholar]
- Lesiak A, Norval M, Wodz-Naskiewicz K, Pawliczak R, Rogowski-Tylman M, Sysa-Jedrzejowska A, et al. (2011). An enhanced risk of basal cell carcinoma is associated with particular polymorphisms in the VDR and MTHFR genes. Exp Dermatol 20:800–804. [DOI] [PubMed] [Google Scholar]
- Li C, Liu Z, Wang LE, Gershenwald JE, Lee JE, Prieto VG, et al. (2008). Haplotype and genotypes of the VDR gene and cutaneous melanoma risk in non-Hispanic whites in Texas: a case–control study. Int J Cancer 122:2077–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Nguyen L, Chun RF, Lagishetty V, Ren S, Wu S, et al. (2008). Altered endocrine and autocrine metabolism of vitamin D in a mouse model of gastrointestinal inflammation. Endocrinology 149:4799–4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Li C, Chen P, Li X, Li M, Guo H, et al. (2013). Polymorphisms in the vitamin D receptor (VDR) and the risk of ovarian cancer: a meta-analysis. PLoS One 8:e66716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Calderon JI, Zhang Z, Sturgis EM, Spitz MR, Wei Q. (2005). Polymorphisms of vitamin D receptor gene protect against the risk of head and neck cancer. Pharmacogenet Genomics 15:159–165. [DOI] [PubMed] [Google Scholar]
- Luo S, Guo L, Li Y, Wang S. (2014). Vitamin D receptor gene ApaI polymorphism and breast cancer susceptibility: a meta-analysis. Tumour Biol 35:785–790. [DOI] [PubMed] [Google Scholar]
- Lurie G, Wilkens LR, Thompson PJ, McDuffie KE, Carney ME, Terada KY, et al. (2007). Vitamin D receptor gene polymorphisms and epithelial ovarian cancer risk. Cancer Epidemiol Biomarkers Prev 16:2566–2571. [DOI] [PubMed] [Google Scholar]
- Ma J, Stampfer MJ, Gann PH, Hough HL, Giovannucci E, Kelsey KT, et al. (1998). Vitamin D receptor polymorphisms, circulating vitamin D metabolites, and risk of prostate cancer in United States physicians. Cancer Epidemiol Biomarkers Prev 7:385–390. [PubMed] [Google Scholar]
- Macaskill P, Walter SD, Irwig L. (2001). A comparison of methods to detect publication bias in meta-analysis. Stat Med 20:641–654. [DOI] [PubMed] [Google Scholar]
- Mahmoudi T, Mohebbi SR, Pourhoseingholi MA, Fatemi SR, Zali MR. (2010). Vitamin D receptor gene ApaI polymorphism is associated with susceptibility to colorectal cancer. Dig Dis Sci 55:2008–2013. [DOI] [PubMed] [Google Scholar]
- Maistro S, Snitcovsky I, Sarkis AS, da Silva IA, Brentani MM. (2004). Vitamin D receptor polymorphisms and prostate cancer risk in Brazilian men. Int J Biol Markers 19:245–249. [DOI] [PubMed] [Google Scholar]
- McCullough ML, Stevens VL, Diver WR, Feigelson HS, Rodriguez C, Bostick RM, et al. (2007). Vitamin D pathway gene polymorphisms, diet, and risk of postmenopausal breast cancer: a nested case–control study. Breast Cancer Res 9:R9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros R, Morais A, Vasconcelos A, Costa S, Pinto D, Oliveira J, et al. (2002). The role of vitamin D receptor gene polymorphisms in the susceptibility to prostate cancer of a southern European population. J Hum Genet 47:413–418. [DOI] [PubMed] [Google Scholar]
- Mikhak B, Hunter DJ, Spiegelman D, Platz EA, Hollis BW, Giovannucci E. (2007). Vitamin D receptor (VDR) gene polymorphisms and haplotypes, interactions with plasma 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D, and prostate cancer risk. Prostate 67:911–923. [DOI] [PubMed] [Google Scholar]
- Minambres I, Sanchez-Hernandez J, Sanchez-Quesada JL, Rodriguez J, de Leiva A, Perez A. (2012). The association of hypovitaminosis D with the metabolic syndrome is independent of the degree of obesity. ISRN Endocrinol 2012691803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra DK, Wu Y, Sarkissyan M, Sarkissyan S, Chen Z, Shang X, et al. (2013). Vitamin D receptor gene polymorphisms and prognosis of breast cancer among African-American and Hispanic women. PLoS One 8:e57967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal RD, Manchanda PK, Bhat S, Bid HK. (2007). Association of vitamin-D receptor (Fok-I) gene polymorphism with bladder cancer in an Indian population. BJU Int 99:933–937. [DOI] [PubMed] [Google Scholar]
- Muindi JR, Adjei AA, Wu ZR, Olson I, Huang H, Groman A, et al. (2013). Serum vitamin D metabolites in colorectal cancer patients receiving cholecalciferol supplementation: correlation with polymorphisms in the vitamin D genes. Horm Cancer 4:242–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley-Girvan I, Feldman D, Eccleshall TR, Gallagher RP, Wu AH, Kolonel LN, et al. (2004). Risk of early-onset prostate cancer in relation to germ line polymorphisms of the vitamin D receptor. Cancer Epidemiol Biomarkers Prev 13:1325–1330. [PubMed] [Google Scholar]
- Obara W, Suzuki Y, Kato K, Tanji S, Konda R, Fujioka T. (2007). Vitamin D receptor gene polymorphisms are associated with increased risk and progression of renal cell carcinoma in a Japanese population. Int J Urol 14:483–487. [DOI] [PubMed] [Google Scholar]
- Ochs-Balcom HM, Cicek MS, Thompson CL, Tucker TC, Elston RC, Plummer J, et al. (2008). Association of vitamin D receptor gene variants, adiposity and colon cancer. Carcinogenesis 29:1788–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onen IH, Ekmekci A, Eroglu M, Konac E, Yesil S, Biri H. (2008). Association of genetic polymorphisms in vitamin D receptor gene and susceptibility to sporadic prostate cancer. Exp Biol Med (Maywood) 233:1608–1614. [DOI] [PubMed] [Google Scholar]
- Park K, Woo M, Nam J, Kim JC. (2006). Start codon polymorphisms in the vitamin D receptor and colorectal cancer risk. Cancer Lett 237:199–206. [DOI] [PubMed] [Google Scholar]
- Pena-Chilet M, Ibarrola-Villava M, Martin-Gonzalez M, Feito M, Gomez-Fernandez C, Planelles D, et al. (2013). rs12512631 on the group specific complement (vitamin D-binding protein GC) implicated in melanoma susceptibility. PLoS One 8:e59607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penna-Martinez M, Ramos-Lopez E, Stern J, Hinsch N, Hansmann ML, Selkinski I, et al. (2009). Vitamin D receptor polymorphisms in differentiated thyroid carcinoma. Thyroid 19:623–628. [DOI] [PubMed] [Google Scholar]
- Purdue MP, Hartge P, Davis S, Cerhan JR, Colt JS, Cozen W, et al. (2007). Sun exposure, vitamin D receptor gene polymorphisms and risk of non-Hodgkin lymphoma. Cancer Causes Control 18:989–999. [DOI] [PubMed] [Google Scholar]
- Raimondi S, Pasquali E, Gnagnarella P, Serrano D, Disalvatore D, Johansson HA, Gandini S. (2014). BsmI polymorphism of vitamin D receptor gene and cancer risk: a comprehensive meta-analysis. Mutat Res 769:17–34. [DOI] [PubMed] [Google Scholar]
- Randerson-Moor JA, Taylor JC, Elliott F, Chang YM, Beswick S, Kukalizch K, et al. (2009). Vitamin D receptor gene polymorphisms, serum 25-hydroxyvitamin D levels, and melanoma: UK case-control comparisons and a meta-analysis of published VDR data. Eur J Cancer 45:3271–3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland GW, Schwartz GG, John EM, Ingles SA. (2012). Calcium intake and prostate cancer among African Americans: effect modification by vitamin D receptor calcium absorption genotype. J Bone Miner Res 27:187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruza E, Sotillo E, Sierrasesumaga L, Azcona C, Patino-Garcia A. (2003). Analysis of polymorphisms of the vitamin D receptor, estrogen receptor, and collagen I alpha1 genes and their relationship with height in children with bone cancer. J Pediatr Hematol Oncol 25:780–786. [DOI] [PubMed] [Google Scholar]
- Shafia S, Qasim I, Aziz SA, Bhat IA, Nisar S, Shah ZA. (2013). Role of vitamin D receptor (VDR) polymorphisms in susceptibility to multiple myeloma in ethnic Kashmiri population. Blood Cells Mol Dis 51:56–60. [DOI] [PubMed] [Google Scholar]
- Sillanpaa P, Hirvonen A, Kataja V, Eskelinen M, Kosma VM, Uusitupa M, et al. (2004). Vitamin D receptor gene polymorphism as an important modifier of positive family history related breast cancer risk. Pharmacogenetics 14:239–245. [DOI] [PubMed] [Google Scholar]
- Slattery ML, Wolff RK, Curtin K, Fitzpatrick F, Herrick J, Potter JD, et al. (2009). Colon tumor mutations and epigenetic changes associated with genetic polymorphism: insight into disease pathways. Mutat Res 660:12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smedby KE, Eloranta S, Duvefelt K, Melbye M, Humphreys K, Hjalgrim H, et al. (2011). Vitamin D receptor genotypes, ultraviolet radiation exposure, and risk of non-Hodgkin lymphoma. Am J Epidemiol 173:48–54. [DOI] [PubMed] [Google Scholar]
- Song GG, Lee YH. (2013). Vitamin D receptor FokI, BsmI, ApaI, and TaqI polymorphisms and susceptibility to ovarian cancer: a meta-analysis. Immunol Invest 42:661–672. [DOI] [PubMed] [Google Scholar]
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. (2000). Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283:2008–2012. [DOI] [PubMed] [Google Scholar]
- Strugnell SA, DeLuca HF. (1997). The vitamin D receptor – structure and transcriptional activation. Proc Soc Exp Biol Med 215:223–228. [DOI] [PubMed] [Google Scholar]
- Styrkarsdottir U, Halldorsson BV, Gretarsdottir S, Gudbjartsson DF, Walters GB, Ingvarsson T, et al. (2008). Multiple genetic loci for bone mineral density and fractures. N Engl J Med 358:2355–2365. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Matsui H, Ohtake N, Nakata S, Takei T, Koike H, et al. (2003). Vitamin D receptor gene polymorphism in familial prostate cancer in a Japanese population. Int J Urol 10:261–266. [DOI] [PubMed] [Google Scholar]
- Swami S, Krishnan AV, Feldman D. (2000). 1alpha, 25-Dihydroxyvitamin D3 down-regulates estrogen receptor abundance and suppresses estrogen actions in MCF-7 human breast cancer cells. Clin Cancer Res 6:3371–3379. [PubMed] [Google Scholar]
- Szkandera J, Absenger G, Pichler M, Stotz M, Langsenlehner T, Samonigg H, et al. (2013). Association of common gene variants in vitamin D modulating genes and colon cancer recurrence. J Cancer Res Clin Oncol 139:1457–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodoratou E, Farrington SM, Tenesa A, McNeill G, Cetnarskyj R, Barnetson RA, et al. (2008). Modification of the inverse association between dietary vitamin D intake and colorectal cancer risk by a FokI variant supports a chemoprotective action of vitamin D intake mediated through VDR binding. Int J Cancer 123:2170–2179. [DOI] [PubMed] [Google Scholar]
- Thill M, Fischer D, Kelling K, Hoellen F, Dittmer C, Hornemann A, et al. (2010). Expression of vitamin D receptor (VDR), cyclooxygenase-2 (COX-2) and 15-hydroxyprostaglandin dehydrogenase (15-PGDH) in benign and malignant ovarian tissue and 25-hydroxycholecalciferol (25(OH2)D3) and prostaglandin E2 (PGE2) serum level in ovarian cancer patients. J Steroid Biochem Mol Biol 121:387–390. [DOI] [PubMed] [Google Scholar]
- Toptas B, Kafadar AM, Cacina C, Turan S, Yurdum LM, Yigitbasi N, et al. (2013). The vitamin D receptor (VDR) gene polymorphisms in Turkish brain cancer patients. Biomed Res Int 2013:295791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torkko KC, van Bokhoven A, Mai P, Beuten J, Balic I, Byers TE, et al. (2008). VDR and SRD5A2 polymorphisms combine to increase risk for prostate cancer in both non-Hispanic White and Hispanic White men. Clin Cancer Res 14:3223–3229. [DOI] [PubMed] [Google Scholar]
- Touvier M, Chan DS, Lau R, Aune D, Vieira R, Greenwood DC, et al. (2011). Meta-analyses of vitamin D intake, 25-hydroxyvitamin D status, vitamin D receptor polymorphisms, and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev 20:1003–1016. [DOI] [PubMed] [Google Scholar]
- Tworoger SS, Gates MA, Lee IM, Buring JE, Titus-Ernstoff L, Cramer D, et al. (2009). Polymorphisms in the vitamin D receptor and risk of ovarian cancer in four studies. Cancer Res 69:1885–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uitterlinden AG, Fang Y, van Meurs JB, Pols HA, van Leeuwen JP. (2004). Genetics and biology of vitamin D receptor polymorphisms. Gene 338:143–156. [DOI] [PubMed] [Google Scholar]
- Van Houwelingen HC, Arends LR, Stijnen T. (2002). Advanced methods in meta-analysis: multivariate approach and meta-regression. Stat Med 21:589–624. [DOI] [PubMed] [Google Scholar]
- Von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. (2008). The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 61:344–349. [DOI] [PubMed] [Google Scholar]
- Wang J, He Q, Shao YG, Ji M, Bao W. (2013). Associations between vitamin D receptor polymorphisms and breast cancer risk. Tumour Biol 34:3823–3830. [DOI] [PubMed] [Google Scholar]
- Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, et al. (2010). Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet 376:180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield GK, Remus LS, Jurutka PW, Zitzer H, Oza AK, Dang HT, et al. (2001). Functionally relevant polymorphisms in the human nuclear vitamin D receptor gene. Mol Cell Endocrinol 177:145–159. [DOI] [PubMed] [Google Scholar]
- Xu Y, He B, Pan Y, Deng Q, Sun H, Li R, et al. (2014). Systematic review and meta-analysis on vitamin D receptor polymorphisms and cancer risk. Tumour Biol 35:4153–4169. [DOI] [PubMed] [Google Scholar]
- Yao S, Zirpoli G, Bovbjerg DH, Jandorf L, Hong CC, Zhao H, et al. (2012). Variants in the vitamin D pathway, serum levels of vitamin D, and estrogen receptor negative breast cancer among African-American women: a case–control study. Breast Cancer Res 14:R58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaylim-Eraltan I, Arzu Ergen H, Arikan S, Okay E, Ozturk O, Bayrak S, et al. (2007). Investigation of the VDR gene polymorphisms association with susceptibility to colorectal cancer. Cell Biochem Funct 25:731–737. [DOI] [PubMed] [Google Scholar]
- Zeljic K, Supic G, Stamenkovic Radak M, Jovic N, Kozomara R, Magic Z. (2012). Vitamin D receptor, CYP27B1 and CYP24A1 genes polymorphisms association with oral cancer risk and survival. J Oral Pathol Med 41:779–787. [DOI] [PubMed] [Google Scholar]
- Zhou ZC, Wang J, Cai ZH, Zhang QH, Cai ZX, Wu JH. (2013). Association between vitamin D receptor gene Cdx2 polymorphism and breast cancer susceptibility. Tumour Biol 34:3437–3441. [DOI] [PubMed] [Google Scholar]


