Abstract
Campylobacteriosis is the most common cause of bacterial gastroenteritis worldwide. Campylobacter species involved in this infection usually include the thermotolerant species Campylobacter jejuni. The major reservoir for C. jejuni leading to human infections is commercial broiler chickens. Poultry flocks are frequently colonized by C. jejuni without any apparent symptoms. Risk assessment analyses have identified the handling and consumption of poultry meat as one of the most important sources of human campylobacteriosis, so elimination of Campylobacter in the poultry reservoir is a crucial step in the control of this foodborne infection. To date, the use of probiotics has demonstrated promising results to reduce Campylobacter colonization. This review provides recent insights into methods used for probiotic screening to reduce the prevalence and colonization of Campylobacter at the farm level. Different eukaryotic epithelial cell lines are employed to screen probiotics with an anti-Campylobacter activity and yield useful information about the inhibition mechanism involved. These in vitro virulence models involve only human intestinal or cervical cell lines whereas the use of avian cell lines could be a preliminary step to investigate mechanisms of C. jejuni colonization in poultry in the presence of probiotics. In addition, in vivo trials to evaluate the effect of probiotics on Campylobacter colonization are conducted, taking into account the complexity introduced by the host, the feed, and the microbiota. However, the heterogeneity of the protocols used and the short time duration of the experiments lead to results that are difficult to compare and draw conclusions at the slaughter-age of broilers. Nevertheless, the combined approach using complementary in vitro and in vivo tools (cell cultures and animal experiments) leads to a better characterization of probiotic strains and could be employed to assess reduced Campylobacter spp. colonization in chickens if some parameters are optimized.
Keywords: Campylobacter, poultry, probiotics, screening, in vitro virulence, in vivo colonization
Introduction
Food safety is of fundamental importance to the consumer, the food industry and the economy. The incidence of foodborne diseases is still increasing in the European Union (EU) (Hugas et al., 2009; EFSA, 2015), mainly caused by the presence and/or the growth of pathogenic bacteria in food. Campylobacter and Salmonella are among the leading causes of bacterial foodborne illness and are therefore considered as major public health concern (Scallan et al., 2011). In many countries, the number of human campylobacteriosis cases has considerably increased to exceed the number of Salmonella infections in humans by 2–3-fold (EFSA, 2010). The disease is characterized by watery or bloody diarrhea, abdominal cramps and nausea (Blaser et al., 2008). Post-infection complications include peripheral neuropathies, Guillain-Barré and Miller Fisher syndromes, and functional bowel diseases, such as irritable bowel syndrome (Moore et al., 2005). Hospitalization occurs in 10% of cases (Bessell et al., 2010) and 0.2% end in death (Adak et al., 2005). In 2013, with 214,779 confirmed cases corresponding to a notification rate of 64.8 cases per 100,000 inhabitants, campylobacteriosis was the most frequently reported zoonotic disease in humans in the EU (EFSA, 2015). There are several species of Campylobacter (C. jejuni, C. coli, C. lari, and C. upsaliensis) capable of causing human illness. However, C. jejuni is the one most frequently involved in zoonotic infections (Hugas et al., 2009). It is believed to be responsible for 400–500 million cases of gastroenteritis worldwide per year (Olson et al., 2008). Campylobacter cases are often associated with very large costs, i.e., medical expenses, lost wages, legal costs, and other indirect expenses. Only sporadic data are available on the overall costs of Campylobacter infections but campylobacteriosis and its sequelae in the EU are calculated to cost 0.35 million disability-adjusted life-years per year, totaling 2.4 billion per year (EFSA, 2014). Annual costs for the US were calculated to range between 1.2 and 4 billion $ (Batz et al., 2012; Eberle and Kiess, 2012). Batz et al. (2014) estimated 16 QALY (quality-adjusted life years) lost per 1000 campylobacteriosis cases; with more than 828,500 cases annually reported, global estimation is around 13,256 QALY losses in the US per year. More recently, Scharff (2015) gives a QALY analysis for all foodborne pathogens including Campylobacter.
Campylobacter is a commensal organism routinely found in cattle, sheep, swine, and avian species, the latter being the most common host. Numerous studies have already emphasized the importance of poultry as a reservoir of Campylobacter (Herman et al., 2003; Hermans et al., 2012; Sasaki et al., 2013) and epidemiological evidence indicates poultry and poultry products are a significant source of human infection (Mor-Mur and Yuste, 2010; EFSA, 2011). In particular, broiler meat is considered the main foodborne source of Campylobacter human infection (Nadeau et al., 2003; Nielsen et al., 2006; Silva et al., 2011; EFSA, 2014). Recently, a national prospective case-control study of factors associated with Campylobacter infection confirmed that consumption of poultry remains an important exposure for campylobacteriosis in Norway (MacDonald et al., 2015). Good hygiene and biosecurity practices have been implemented to avoid, or at least, reduce contamination (Gibbens et al., 2001) but are considered as not sufficient (Hermans et al., 2011). Considering this information, it is imperative to find a way to minimize Campylobacter presence at the farm level in order to reduce the risk of transmission throughout the processing stages. Reducing the proportion of Campylobacter-infected poultry flocks and/or reducing the number of Campylobacter in live poultry will considerably lower the risk to consumers (Keener et al., 2004; Westrell et al., 2009). Furthermore, prevention of disease in humans and a reduction in the pathogen reservoir in farm animals, without the need for antibiotics, are of both ecological and financial benefit to society.
Regarding the emergence of antibiotic resistance in livestock breeding (Schwarz and Chaslus-Dancla, 2001), poultry farmers turned to new solutions to maintain animal welfare without affecting performance parameters. Over the past years, researchers are considering the use of probiotics as feed additives in poultry nutrition (Kabir, 2009). Probiotics are usually defined as “live microorganisms which, when administered in adequate amounts, confer a health benefit on the host” (Hill et al., 2014). In 2002, the United Nations FAO/WHO Working Group generated new guidelines for the development and evaluation of probiotics found in foods (Reid, 2005). They are acceptable and cost-effective alternatives to antibiotics.
This review provides recent insights into the technological and scientific advances to reduce the prevalence and colonization of Campylobacter at the farm level with an emphasis on the screening of probiotics.
Campylobacter in poultry
The prevalence of Campylobacter spp. in broiler chicken batches varies considerably between EU countries; in 2008, it ranged from 2 to 100% (average of 71%) (EFSA, 2010). In France, Campylobacter is present at all stages of the food chain with a very high prevalence of infection: 70–100% of broiler chicken batches on their arrival at the slaughterhouse (Hue et al., 2010); 72–77% of individual cecal portage during rearing and on arrival at the slaughterhouse; 88% of carcasses and 76% of products at the retail level (Chemaly et al., 2012; Guyard-Nicodème et al., 2015). These results for France are broadly comparable to some high-prevalence countries in the EU. Epidemiological studies have identified potential risk factors associated with Campylobacter colonization of flocks (Refrégier-Petton et al., 2001; Bull et al., 2006; Allain et al., 2014; Robyn et al., 2015), including season (Huneau-Salaün et al., 2007), drinking water quality (Ellis-Iversen et al., 2009), or lack of hygienic barriers (Huneau-Salaün et al., 2007).
Colonization of broiler flocks with Campylobacter species typically occurs between 2 and 3 weeks of age (Newell et al., 2011). The infection is mostly asymptomatic although chickens can harbor very high levels of Campylobacter in the gut, from 5 to 9 log10 CFU/g of cecal content (Saleha, 2002; Hansson et al., 2010). Once in a flock, Campylobacter is rapidly transmitted between birds by the fecal-oral route (Wassenaar, 2011) and Campylobacter-positive birds often remain colonized until slaughter (Newell et al., 2011). During transport of birds (Hansson et al., 2005) and carcass dressing, the surface of broiler carcasses and the plant environment are contaminated by fecal material from the gastrointestinal tract (Herman et al., 2003; Rasschaert et al., 2006; Rosenquist et al., 2006; Reich et al., 2008). Contamination of carcasses with Campylobacter occurs mainly during defeathering, evisceration and chilling operations (Sánchez et al., 2002; Stern and Robach, 2003; Takahashi et al., 2006). The bacteria can thus survive during poultry processing through to human consumption, causing subsequent illness as demonstrated by a Danish prospective case-control study (MacDonald et al., 2015). Reducing the cecal Campylobacter load in poultry during primary production is expected to decrease significantly the contamination levels of the carcasses of colonized animals after processing, and to reduce the incidence of human campylobacteriosis (Lin, 2009; Hermans et al., 2012).
Campylobacter control at farm level
A possible way to reduce Campylobacter contamination in poultry is by actions at the primary production level. To date, three general strategies have been proposed to control Campylobacter in poultry at the farm level: (i) a reduction in environmental exposure (Van de Giessen et al., 1998), (ii) an increase in the poultry host's resistance to reduce Campylobacter carriage in the gut (Neal-McKinney et al., 2014), and (iii) the use of antimicrobial alternatives to reduce and even eliminate Campylobacter from colonized chickens (Ghareeb et al., 2012).
Preventive strategy consists in the application of generic control measures that have an impact on transmission routes of pathogens; and therefore may reduce Campylobacter level in poultry. This includes in particular biosecurity, good husbandry as well as hygiene measures. Biosecurity practices at the farm have been reviewed by Newell et al. (2011) and include disinfecting poultry houses, boot dips (Galanis, 2007), fly screens (Hald et al., 2007), disinfecting equipment and vehicles, and treating the flock water supply (Wassenaar, 2011). Nevertheless, contamination is only reduced at the farm level while Campylobacter remains widespread in the outside environment, for example in other animal reservoirs (Devane et al., 2005). Once the flock was infected by Campylobacter, biosecurity measures became useless. Therefore, additional actions are necessary to fight this foodborne pathogen (Hermans et al., 2011; Robyn et al., 2015), such as vaccination, bacteriocin treatment, or probiotics.
Strategies in progress
Complementary practices currently being investigated (Table 1) include vaccination (De Zoete et al., 2007; Meunier et al., 2016b), bacteriocins (Svetoch and Stern, 2010; Messaoudi et al., 2012a), bacteriophages (Monk et al., 2010), prebiotics (Gaggìa et al., 2010), and probiotics (Kergourlay et al., 2012; Messaoudi et al., 2012b, 2013). To date, there are still no effective and consistent immune interventions, primarily due to the lack of understanding of the protective immunity, the antigenic variability of different Campylobacter strains, and the inability of current vaccination to induce a strong and persistent mucosal immune response in chickens (Meunier et al., 2016a). Studies using bacteriophages showed that they were partly and temporally effective in reducing Campylobacter in broilers. This could be explained by the fact that Campylobacter develop resistance to bacteriophages (Janež and Loc-Carrillo, 2013) and that these may be strain-specific and only effective against certain Campylobacter strains (Loc-Carrillo et al., 2005).
Table 1.
Strategies in progress to control Campylobacter at the farm level.
| Strategy | Principle | Advantage | Drawback |
|---|---|---|---|
| Vaccination | Improvement of the immune response against Campylobacter | Easy to use | Antigenic variability of Campylobacter strains |
| Bacteriophage therapy | Use of specific bacterial virus to kill Campylobacter | Rapid action | Selection of resistant Campylobacter strains Production cost Diversity of Campylobacter strains |
| Bacteriocin treatment | Use of bacteria-produced antimicrobial compounds against Campylobacter | Easy to use | Production cost Variable sensitivity of Campylobacter strains |
| Prebiotics | Incorporation of feed additives to improve beneficial avian gut microbiota | Easy to use Production cost | Dependence on the avian gut microbiota |
| Probiotics | Administration of beneficial microorganisms with anti-Campylobacter activity | Easy to produce and to use Production cost Mix of multiple species Different ways of inhibiting Campylobacter | Variable sensitivity of Campylobacter strains |
Interestingly, prebiotics and bacteriocins can be used together to probiotics to potentially increase the anti-Campylobacter activity. Prebiotics are non-digestible ingredients, such as fructo-oligosaccharides (Patterson and Burkholder, 2003), which enhance the growth of gut commensal bacteria that have probiotic properties, i.e., Bifidobacterium (Bf.) and Lactobacillus (Lb.) (Roberfroid, 1998), while bacteriocins are ribosomally-synthesized antimicrobial peptides produced by bacteria. Few studies have been conducted to evaluate the efficacy of prebiotics in reducing Campylobacter colonization in poultry. The addition of mannanoligosaccharide to the feed of naturally-infected birds and xylanase to artificially-infected broilers resulted in a statistically significant decrease of 0.3 log in cecal C. jejuni counts (Baurhoo et al., 2009). Concerning bacteriocins, for example, Messaoudi et al. (2012a) showed that the viable population of C. jejuni NCTC 11168 pure cultures decreased by 2 log when growth was performed in the presence of salivaricin SMXD51. Administration of enterocin E-760-treated feed significantly reduced the colonization of young broiler chicks experimentally challenged and colonized with two strains of C. jejuni by more than 8 log CFU (Line et al., 2008). Another in vivo study on chickens infected with C. jejuni and Salmonella enteritidis, demonstrated that treatment with L-1077, the bacteriocin produced by Lb. salivarius NRRL B-50053, reduced by more than 4 log the number of bacteria per gram of cecal content (Svetoch et al., 2011). Majority of the bacterial antimicrobial peptides active against C. jejuni were isolated from Bacillus and Paenibacillus spp., and from the lactic acid bacteria (Lohans et al., 2015). Svetoch and Stern (2010) have reviewed bacteriocin applications to reduce the cecal Campylobacter counts in broiler chickens of colonized flocks. This strategy is of limited relevance for the moment because purity and yields of bacteriocins, after purification, are low. This could be due, in part, to their low molecular weight and to the design of the purification processes employed so far (Carolissen-Mackay et al., 1997). In addition, hydrophobic peptides are often only produced in small amounts (Berjeaud and Cenatiempo, 2004). But efforts are underway and current strategies to enhance yield of bacteriocins were recently described by Zacharof (2015).
Prohibition of antibiotics in poultry feed in Europe and the problems inherent in developing new vaccines make probiotics a promising prophylactic alternative to control C. jejuni in broiler chickens during rearing at the farm level (Table 1). They could act in multiple ways, at the same time, against pathogens in contrast to other more specific strategies (vaccination or bacteriophages) (Figure 1). In fact, probiotics are already used in the poultry industry for preventing or reducing the occurrence of Salmonella infection in poultry and for enhancing the growth performance of broiler chickens (Tellez et al., 2013). Their impact on poultry nutrition is of great importance for the proper utilization of nutrients.
Figure 1.
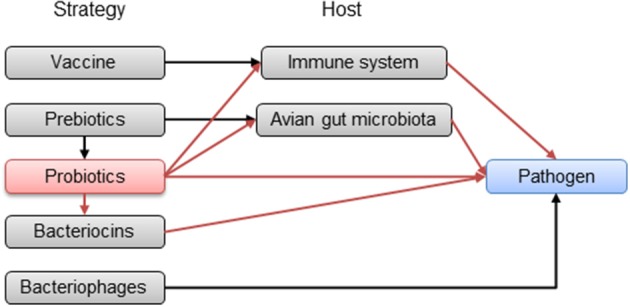
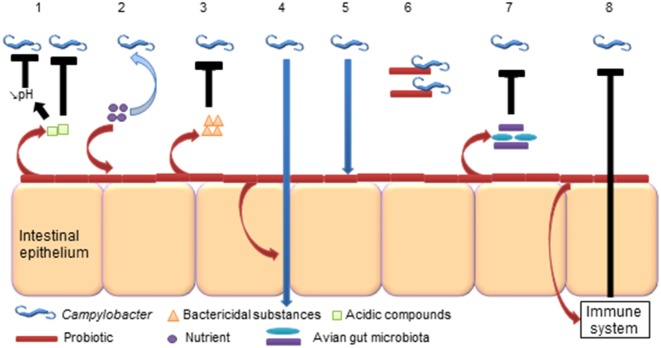
Potential pathways of the strategies in progress to reduce avian gut pathogens in poultry. Red arrows represent probiotic pathways.
Probiotics: Attractive and natural antimicrobial agents
Being living microorganisms, probiotics can stimulate gut microbiota which contributes to keep the host healthy (Fuller, 1989; Sanders, 2011). Based on in vitro assays, these modifications include stimulation of the immune system (Smits et al., 2005), acidification of the environment (Ogawa et al., 2001), secretion of active metabolites against pathogens, such as bacteriocins (Marciňáková et al., 2004) or hydrogen peroxide (Batdorj et al., 2007), and competition with the pathogens for nutrients or sites for adherence on the mucous membrane or the host epithelial cells (Bernet et al., 1994). These abilities can be useful to control pathogen infection and probiotic treatment has been linked with beneficial effects against gastrointestinal pathogens using animal models. For example, a mixture of Lactobacillus spp. strains reduced gastric inflammation and bacterial colonization in Helicobacter pylori-infected mice (Johnson-Henry et al., 2004). A five-strain probiotic combination (two strains of Lb. murinus and one strain each of Lb. salivarius, Lb. pentosus, and Pediococcus pentosaceous) reduced pathogen shedding and alleviated disease signs in pigs challenged with S. enterica serovar Typhimurium (Casey et al., 2007). Pascual et al. (1999) showed that a treatment with Lb. salivarius CTC2197 prevented S. enterica serovar Enteritidis colonization in chickens. In addition, some probiotic strains as feed supplements can also prevent gastrointestinal infection in broiler chickens (Tellez et al., 2001).
As probiotics inhibit foodborne pathogens such as Salmonella (Nurmi and Rantala, 1973), often designated as competitive exclusion, they could potentially have an effect on Campylobacter (Figure 2). Indeed, probiotic bacteria successfully excluded C. jejuni from mice (Sorokulova et al., 1997; Wagner et al., 2009). Regarding chickens, potential probiotic mechanisms associated with the inhibition of Campylobacter have been reviewed and detailed by Mohan (2015).
Figure 2.
Potential probiotic abilities to reduce Campylobacter in the avian gut. (1) Probiotics produce acidic compounds (lactic acid), which could inhibit Campylobacter and reduce the gut luminal pH that could affect Campylobacter (Neal-McKinney et al., 2012). (2) Probiotics compete for nutrients with Campylobacter (Aho et al., 1992). (3) Probiotics produce bactericidal substances (bacteriocins, H2O2) that could kill Campylobacter (Messaoudi et al., 2012a). (4) Probiotics strengthen tight junctions of intestinal epithelium and prevent Campylobacter translocation (Messaoudi et al., 2012b). (5) Probiotics colonize intestinal epithelium and prevent adhesion and invasion of Campylobacter (Wine et al., 2009). (6) Probiotics bind Campylobacter (Nishiyama et al., 2014). (7) Probiotics alter the avian gut microbiota, which could affect Campylobacter colonization (Sanders, 2011). (8) Probiotics modulate the immune system, which acts against Campylobacter (Brisbin et al., 2011).
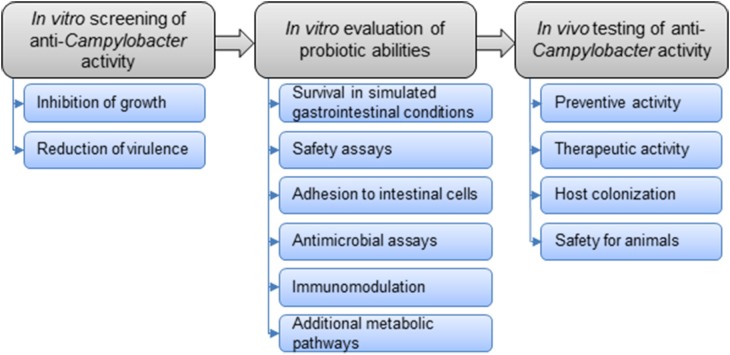
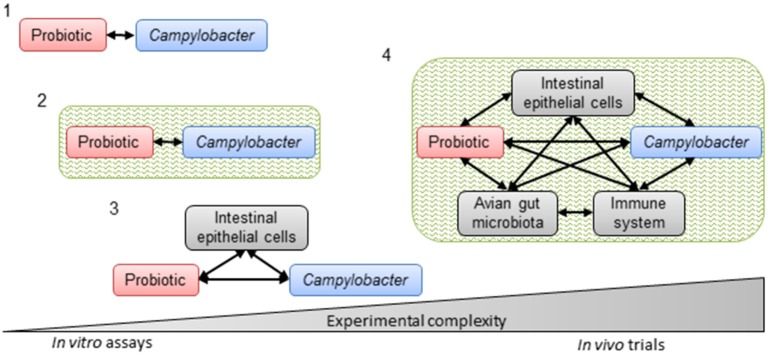
The general strategy for the selection of probiotic strains requires a set of experiments to identify the most promising candidates (Figure 3). In vitro studies include aggregation, co-aggregation, cell surface hydrophobicity and adhesion activities on epithelial cells. Additionally, growth with bile acids (chicken bile) and tolerance to acidic pH are checked. In addition to in vitro assays, in vivo experiments on chickens are carried out to highlight the impact of probiotics on foodborne pathogen colonization and/or the effect on growth performances in animals. This strategy includes simplified in vitro assays for probiotic screening, followed by more complex in vivo trials to confirm the anti-Campylobacter activity (Figure 4).
Figure 3.
Overall strategy to select potential probiotics to control Campylobacter in chickens.
Figure 4.
Progressive complexity of methods used to select probiotics with in vitro and in vivo anti-Campylobacter activity. Simplified in vitro assays to assess direct interactions between the probiotic and Campylobacter (1) without (co-culture and agar plate diffusion) or (2) with an intestinal environment (co-culture in batch) or (3) interactions between the probiotic, Campylobacter and intestinal epithelial cells (adhesion and invasion assays). Complex in vivo trials (4) with their potential interactions to corroborate in vitro assays. Black arrows represent potential interactions. Green represents the intestinal environment.
In vitro screening for anti-Campylobacter probiotics
In vitro studies are preliminary screening tools for the selection of potential probiotic cultures, and the first stage for further application in poultry production. Based on tests that confirm some antimicrobial properties, several potential anti-Campylobacter bacteria have been isolated (Table 2). A whole set of experiments can be carried out to identify the mechanism involved in the anti-Campylobacter activity. The ability to inhibit the pathogen's growth can be evaluated by co-culture experiments as well as by antimicrobial assays with cell-free culture supernatant, while interference with the adhesion to/invasion of intestinal cells can be studied by adhesion and invasion inhibition assays.
Table 2.
In vitro experiments related to the probiotic impact on Campylobacter.
| Study | Probiotic agents | C. jejuni strain (origin) | Test | Epithelial cells or mucus | Temperature | Time of incubation | Observed effects (results) | Mechanism involved |
|---|---|---|---|---|---|---|---|---|
| PATHOGEN + PROBIOTIC | ||||||||
| Fooks and Gibson, 2003 |
Lb. plantarum 0407 + oligofructose Bf. bifidum Bb12 + oligofructose + xylo-oligosaccharides |
CIP 70.2 (bovine) | Co-culture in batch and continuous culture anaerobic fermentation systems | – | 37°C | 24 h | Growth inhibition (8 log reduction) | Lactic and acetic acid production |
| Fernández et al., 2003 |
Lb. acidophilus UO 001 Lb. gasseri UO 002 |
Clinical isolate (human) | Agar plate diffusion | – | 37°C | 48 h | Inhibition zone (NI) | Lactic acid production |
| Chaveerach et al., 2004 | Lactobacillus spp. P93 | C2146 (chicken) C186 (chicken) C350 (chicken) C591 (chicken) C690 (chicken) C144 (chicken) | Co-culture and agar plate diffusion | – | 37°C | 72 h | Growth inhibition (4–6 log reduction) Inhibition zone (9–15 mm) | Organic acid and bacteriocin production |
| Messaoudi et al., 2011 |
Lb. salivarius SMXD51 Lb. salivarius MMS122 Lb. salivarius MMS151 |
NCTC 11168 (human) 81–176 (human) |
Agar plate diffusion | – | 37°C | 24 h | Inhibition zone (NI) | Bacteriocin production |
| Dubois Dauphin et al., 2011 |
E. faecium THT Lb. pentosus CWBI B78 |
LMG 6446 (human) CWBI B1444 (NI) |
Co-culture and agar plate diffusion | – | 37°C | 100 h | Inhibition zone (10–15 mm) | Lactic and acetic acid production |
| Robyn et al., 2012 | E. faecalis MB 5259 | MB 4185 (chicken) | Co-culture in batch and agar plate diffusion | – | 37°C | 48 h | Growth inhibition (0.5–1 log reduction) Inhibition zone (NI) |
NI |
| Mundi et al., 2013 |
Lb. acidophilus La-5 Bf. longum NCC2705 |
81–176 (human) | Campylobacter culture with neutralized cell-free supernatants from probiotics | – | 42°C | 2 h | Virulence gene down-regulation (3–7-fold reduction for ciaB and flaA genes) |
Biologically active molecules production |
| Menconi et al., 2014 |
Pediococcus parvulus Lb. salivarius |
NI | Agar plate diffusion | – | 37°C | 24 h | Inhibition zone (NI) | NI |
| Bratz et al., 2015 |
Lb. fermentum ATCC 14931 Lb. johnsonii BFE 663 Lb. paracasei IMT 22353 |
NCTC 11168 (human) CIP 70.2 (bovine) |
Agar plate diffusion | – | 37°C | 24 h | Inhibition zone (NI) | Organic acid production |
| PATHOGEN + PROBIOTIC + MUCUS | ||||||||
| Ganan et al., 2013 |
Propionibacterium freudenreichii DSM 7067 Lb. rhamnosus ATCC 53103 Lactococcus lactis N8 Broilact® (facultative anaerobic bacteria) |
NCTC 11168 (human) 118 (human) |
Adhesion assay (exclusion testa, competition testb) | Chicken intestinal mucus | 37°C | 1 h | Adhesion reduction (8–23%) | Competition for adhesion site |
| Tareb et al., 2013 |
Lb. rhamnosus CNCM-I-3698 Lb. farciminis CNCM-I-3699 |
CIP 70.2 (bovine) | Adhesion assay (exclusion testa, competition testb) | Mucin | 37°C | 1 h | Adhesion reduction (17–70%) | Co-aggregation |
| PATHOGEN + PROBIOTIC + EPITHELIAL CELLS | ||||||||
| Wine et al., 2009 |
Lb. helveticus R0052 Lb. rhamnosus R0011 Lb. rhamnosus ATCC 53103 |
NCTC 11168 (human) 81–176 (human) |
Invasion assay (exclusion testa, competition testb) | T84 INT-407 | 37°C | 1 or 4 h (probiotics) 4 h (Campylobacter) | Invasion reduction (35–55%) | Competition for adhesion site |
| Alemka et al., 2010 |
Lb. rhamnosus R0011 Lb. helveticus R0052 Lb. salivarius AH102 Bf. longum AH1205 Lacidofil® (Lb. rhamnosus R0011 + Lb. helveticus R0052) Mixture (Lb. rhamnosus + Lb. helveticus + Lb. salivarius) |
81–176 (human) | Invasion assay (exclusion testa) | HT29-MTXE12 HT29 | 37°C | 4 or 15 h (probiotics) 24 h (Campylobacter) | Invasion reduction (1–1.5 log) and translocation reduction (3–4 log) | NI |
| Campana et al., 2012 | Lb. acidophilus ATCC 4356 | Hom 107 (human) ISS 9 (human) ISS 3 (human) Hom 13 (human) 241 (human) ISS 1 (human) Hom 88 (human) Hom 14 (human) Hom 7 (human) |
Adhesion and invasion assays (exclusion testa, competition testb, displacement testc) | Caco-2 | 37°C | 1 or 4 h (probiotics) 4 h (Campylobacter) | Adhesion reduction (10–50%) and invasion reduction (10–50%) | Competition for adhesion site Bacteriocin production |
| Wang et al., 2014 |
Lb. plantarum N8 Lb. plantarum N9 Lb. plantarum ZL5 Lb. casei ZL4 |
NCTC 11168 (human) ATCC 33291 (human) ATCC BAA-1153 (human) |
Adhesion and invasion assays (exclusion testa, competition testb, displacement testc) | HT29 | 37°C | 1 or 4 h (probiotics) 4 h (Campylobacter) | Adhesion reduction (40–70%) and invasion reduction (30–60%) | Organic acid and bacteriocin production |
Lb, Lactobacillus; Bf, Bifidobacterium; E, Enterococcus; NI, not indicated.
Probiotics were incubated before Campylobacter to assess a preventive effect.
Probiotics and Campylobacter were incubated at the same time to assess a therapeutic effect.
Campylobacter were incubated before probiotics to assess a therapeutic effect.
Lacidofil® is produced by Xymogen (Orlando, FL, USA); Broilact® is produced by Nimrod Veterinary Products, (Gloucester, UK).
Probiotic identification
Identification of probiotic strain at species level is still important as the GRAS (Generally Recognized As Safe) and QPS (Qualified Presumption of Safety) status defined in USA and Europe, respectively, are both based on the species name. Traditional methods for bacterial identification and phenotypical characterization, such as API system, BIOLOG or culture-based techniques can be used to identify probiotics strains (Herbel et al., 2013; Bagheripoor-Fallah et al., 2015; Galanis et al., 2015; Cherdyntseva et al., 2016). For instance, the main phenotypic methods for Lactobacillus probiotic identification were discussed in Herbel et al. (2013). However, these conventional microbiological tests may have limitations in discriminating large numbers of isolates with similar physiological characteristics (Herbel et al., 2013; Bagheripoor-Fallah et al., 2015; Yadav and Shukla, 2015). In addition, culture-based techniques provide strains able to replicate under experimental conditions, indeed selective media exist only for a limited subset of potential strains of interest (Davis, 2014).
Several DNA-based techniques have been developed to overcome this obstacle (Bagheripoor-Fallah et al., 2015; Yadav and Shukla, 2015), such as the pulsed field gel electrophoresis (PFGE) mainly used for probiotic strain differentiation and discrimination (Tynkkynen et al., 1999; Gosiewski and Brzychczy-Wloch, 2015). However, it cannot be applied for direct detection of a particular strain, in a single reaction (Tynkkynen et al., 1999). Moreover, it is laborious, time-consuming and, thus, inappropriate for large scale screening experiments from environmental samples, especially when microbial groups, other than those needed to be identified, are at higher population levels. In addition, the PCR methodology (mostly on 16S and 23S ribosomal RNA) coupled to sequencing is commonly employed for efficient identification of lactic acid bacteria (Allegretti et al., 2014; Yadav and Shukla, 2015; Cherdyntseva et al., 2016). It is easy to implement, fast, cost efficient, and requires a small amount of template DNA. However, when the design of specific primers is not feasible, the random amplified polymorphic DNA (RAPD) technique may be applied. RAPD is a PCR-based assay that uses short arbitrary primers that anneal to multiple random target sequences to generate the needed polymorphism (Galanis et al., 2015). A recent article published by Yadav and Shukla (2015) reviewed molecular and analytical techniques to identify and screen probiotics. Among the methods discussed, quantitative analysis by real-time PCR (RT-PCR or qPCR) and fluorescent based-methods (fluorescent in situ hybridization and fluorescent activated cell sorting) enables the discrimination of different species and to quantify the amount of bacteria used in a sample (Herbel et al., 2013; Yadav and Shukla, 2015). These last years, development of new techniques to improve bacterial strain identification and characterization is facilitated by the next-generation sequencing (NGS) technologies (Herbel et al., 2013). These techniques would allow identification of non-cultivable strains and also analyze of metabolites produced by probiotics by metabolomics. In addition, whole genome sequencing (WGS) offers an insight regarding evolutionary background and diversity of lactic acid bacteria belonging to one species (Herbel et al., 2013). For example, comparative genome analysis of published Lb. salivarius sequences led to the identification several genes known to be important for gastrointestinal survival, adherence to cells, and bacteriocin production in Lb. salivarius SMXD51 (Kergourlay et al., 2012).
Growth inhibition assays
This first step of screening consists of monitoring Campylobacter growth in the presence of the probiotic in a co-culture assay or its supernatant in an agar plate diffusion assay (Table 2). This method is easy, applicable to a large number of test strains and, in addition, does not require expensive laboratory equipment. Using this approach, Lb. acidophilus and Lb. gasseri have been shown to inhibit strongly C. jejuni by lactic acid production (Fernández et al., 2003). Similarly, the ability of Lactobacillus spp. isolated from chickens to inhibit the growth of C. jejuni has been demonstrated by Chaveerach et al. (2004). These results suggest that the inhibitory effect of Lactobacillus strains on Campylobacter growth is a combination of organic acid and bacteriocin production (Chaveerach et al., 2004). These findings have been supported by Dubois Dauphin et al. (2011) who observed the antimicrobial effect of E. faecium THT due to lactic acid production and Lb. pentosus CWBI B78 due to lactic and acetic acid production. Messaoudi et al. (2011) identified three Lb. salivarius strains, i.e., SMXD51, MMS122, and MMS151, from chicken ceca with antagonism against C. jejuni strains NCTC 11168 and 81–176 due to the production of bacteriocins. Recently, Lb. fermentum ATCC 1493, Lb. johnsonii BFE 663 and Lb. paracasei IMT 22353 showed antimicrobial activity against C. jejuni NCTC 11168 and C. jejuni CIP 70.2 (Bratz et al., 2015). It turned out that the anti-Campylobacter activity of the Lactobacillus strains was pH-dependent, i.e., pH < 4.3.
In vitro fermentation experiments under controlled temperature, pH and atmosphere were carried out to elucidate further the ability of probiotics to inhibit Campylobacter growth under conditions simulating those in broiler ceca. Chang and Chen (2000) demonstrated an antagonistic effect on C. jejuni by four lactobacilli, including Lb. acidophilus, Lb. fermentum, Lb. crispatus, and Lb. brevis, in a complete simulated digestive tract model. Similarly, Robyn et al. (2012) showed the in vitro anti-Campylobacter activity of E. faecalis MB 5259. Even though the model mimics the broiler cecal environment, i.e., pH and bile salts, and anaerobic incubation, a major limitation of this approach is the lack of epithelial cells and avian gut microbiota that compose the intestine.
Adhesion and invasion inhibition assays
The inhibition assays described in Section Growth Inhibition Assays are not solely suitable to confirm the anti-Campylobacter effect of probiotics because these experiments do not take into account the complexity of interactions occurred in vivo, whose interaction with the epithelial intestinal cells. Thus, a better characterization of the mechanisms of action of probiotic strains on Campylobacter is required. Another screening step is to test the ability of the probiotic strain to inhibit or modulate Campylobacter infection in epithelial intestinal cells. Probiotic and pathogen are incubated with intestinal monolayer cells and then all the pathogens that adhere to and invade eukaryotic cells are enumerated in order to determine the adhesion and invasion indexes. In addition, the number of probiotic cells that adhered to the monolayer could be also counted to assess the adhesion ability of the probiotic strain. The possible impact of the probiotic on the structure and integrity of the eukaryotic cells could be also evaluated to provide useful information on the mode of action of the probiotic.
Although the purpose of the in vitro experiments presented in the Table 1 is to highlight the anti-Campylobacter activity of probiotics for further application at the farm level, particularly in poultry farms, no in vitro experiments including avian intestinal cell lines have been carried out as, to our knowledge, these cell lines are not yet commercialized. For example, Van Deun et al. (2008) used ceca from commercial brown laying hens at the age of 12–20 weeks to isolate primary epithelial cells from crypts according to a modified protocol of Booth et al. (1994), which requires specialized expertise (Booth et al., 1994; Van Deun et al., 2008). It is worth noting that the LMH cell line (Kawaguchi et al., 1987) is the only chicken epithelial cell line currently available to researchers from the ATCC culture collection (Larson et al., 2008). LMH is a primary hepatocellular carcinoma epithelial cell line and has been used previously as an in vitro model to investigate mechanisms of C. jejuni colonization in poultry (Smith et al., 2005; Byrne et al., 2007). Although the LMH chicken epithelial cells are derived from the liver, the results obtained with this cell line in vitro were correlated with in vivo findings (Konkel et al., 2007). In addition, Smith et al. (2005) and Byrne et al. (2007) reported that C. jejuni isolates invade chicken primary cells and human cells at comparable levels. In contrast to these results, Larson et al. (2008) found that C. jejuni invades chicken LMH epithelial cells in significantly lower numbers (0.6–1.7 log differences) than it invades human INT 407 epithelial cells, although the bacterial adhesion assays showed that C. jejuni adhere to LMH cells and INT 407 cells in comparable numbers. The chicken LMH cell line has also been used to evaluate the adhesion of Lactobacillus cultures to epithelial cells (Spivey et al., 2014). Thus, LMH epithelial cells may represent an alternative cell line for the investigation of probiotic functionality and mechanistic studies, but efforts should be made to develop a stable avian intestinal cell line.
On the contrary, the in vitro human cell lines are well established and have been used for many years to investigate specific aspects of small intestinal function. They could reflect the interaction between the pathogen and the probiotic bacteria. They are useful for the evaluation of the immunomodulation activity of probiotic strains, by assaying cytokine production (Ashraf and Shah, 2014; Vitaliti et al., 2014; Frei et al., 2015). Moreover, as shown previously, several steps of C. jejuni pathogeny, including adhesion, invasion and translocation, could be assessed using these cell lines (Haddad et al., 2010a,b). Thereby, this model may help to clarify whether probiotic strains prevent or reduce damage to epithelial integrity caused by a pathogenic challenge. Although this model does not completely reflect the in vivo setting, it does provide a valuable opportunity to study the interactions between the enteric pathogen, potentially beneficial microorganisms, and host epithelial cells.
The different experiments involving epithelial cells are described and summarized in Table 2. Most studies showed slight reductions in adhesion and invasion ranging from 8 to 70%. For example, a 55% reduction in the invasion of human intestinal epithelial cells by C. jejuni was observed after treatment with Lb. helveticus R0052, which suggested that competitive exclusion could contribute to protection by adherent probiotics (Wine et al., 2009). One important point highlighted by these authors is the strain specificity of the described effects. Their results demonstrated that Lb. helveticus R0052 is more effective than either Lb. rhamnosus R0011 or Lb. rhamnosus GG in interfering with C. jejuni invasion of intestinal epithelial cells. This observation highlights the complexity of the interactions between microorganisms and mammalian cells.
Similarly, probiotics attenuated C. jejuni association with and internalization within HT29-MTXE12 cells, and translocation of the bacteria to the basolateral medium of transwells (Alemka et al., 2010). The studies mentioned above emphasized probiotics as a preventive/protective measure to limit Campylobacter infection. Interestingly, HT29-MTXE12 cells are a cell line that provides an opportunity to study the role of mucus in vitro, and the relationship of mucus-associated factors with the anti-Campylobacter activity of probiotics, as Campylobacter inhabits the mucus layer in the avian host (Van Deun et al., 2008). Simplified models with mucin (Tareb et al., 2013) or chicken intestinal mucus (Ganan et al., 2013) showed that probiotics were able to reduce the binding of Campylobacter spp. when the probiotics colonized the mucus before the pathogen (Table 2).
Campana et al. (2012) also observed the inhibitory properties of Lb. acidophilus ATCC 4356 on Caco-2 cell adhesion to/invasion of C. jejuni. More recently, Wang et al. (2014) isolated four adhesive Lactobacillus strains able to exert significant antagonistic activity against C. jejuni in vitro and to promote effective inhibition of the adhesion to and invasion of HT29 cells by C. jejuni. Their bactericidal capacity is probably related to the low pH and the production of metabolites, such as lactic acid and antibiotic-like substances. These last two works emphasized the beneficial effects of probiotics not only as a preventive/protective measure but also as a therapeutic one.
Several limitations of these kinds of experiment need to be reported. C. jejuni isolates from humans, chickens or pigs are capable of adhering to and invading human, avian and porcine cell lines (Biswas et al., 2000; Gripp et al., 2011) with different efficiencies (Poly et al., 2007; Larson et al., 2008; Wine et al., 2008). Moreover, the capabilities between strains vary significantly (Newell et al., 1985; Fauchere et al., 1986; Biswas et al., 2000; Fearnley et al., 2008; Zheng et al., 2008). It is also well-known that Campylobacter spp. exhibit high genetic and phenotypic variability and flexibility (Gripp et al., 2011; Rodrigues et al., 2015; Bronnec et al., 2016), and, as a consequence, are not equally virulent and probably not equally sensitive to probiotic actions (Wine et al., 2009).
Another limitation is the growth conditions, which are very beneficial for the bacteria but do not reflect a realistic intestinal environment. Similarly, in almost all the studies described in Table 2, experiments and strain cultures were carried out at 37°C, which is not in accordance with the temperature of chicken, i.e., 42°C. This difference could have an impact on the in vitro anti-Campylobacter activity. For example, it has been documented that bacteriocin production can be sensitive to environmental changes and parameters including temperature, pH and growth medium (Cintas et al., 2000; Diep et al., 2000; Qi et al., 2001). Therefore, the models used are not optimized to characterize completely C. jejuni virulence or colonization, and thus the effect of probiotics on the infection biology of this pathogen.
Despite these limitations, the techniques mentioned (Table 2) are relevant for initially screening probiotic strains with anti-Campylobacter activity and speculating on the mechanisms involved. However, improved adhesion and invasion inhibition assays would use an avian intestinal cell line that secretes mucus, an incubation temperature of 42°C (temperature of chicken), field Campylobacter strains (isolated from chicken), and a reference strain. This strain of choice should exhibit efficient adherence and invasion characteristics but also robustly infect animal models (Ahmed et al., 2002; Seal et al., 2007; Hiett et al., 2008). These strains, such as NCTC 11168, 81–176, RM1221 and 81116, are the most commonly used isolates in laboratories and have been successfully used for in vitro and in vivo infection studies. Such reference strains will ensure a critical comparison of the impact of probiotics between the different studies performed. In addition, complete genome sequences of these reference strains are available and could allow further investigations of the interactions between pathogens and probiotics at the genomic level (Parkhill et al., 2000; Fouts et al., 2005; Gundogdu et al., 2007; Pearson et al., 2007).
Nevertheless, the results obtained need to be confirmed by in vivo experiments because in vitro experiments do not take into account major parameters (Figure 4), such as avian gut microbiota, immune response and feed, which could interact with the probiotic and its anti-Campylobacter activity.
Anti-Campylobacter activity of probiotics in broilers
Many early reports showed that the administration of probiotics, especially Lactobacillus and Bifidobacterium, improved growth performances in animals such as broilers by increasing the utilization of nutrients (Jin et al., 1998). An overview of the effects of probiotics was given by Oelschlaeger (2010). Among them, the exclusion of pathogens (Tsai et al., 2005) seems to be a valid approach to counteract foodborne pathogen contamination. Published in vivo studies, summarized in Table 3, have pointed out a possible role of probiotics in preventing the shedding of C. jejuni at the level of primary production.
Table 3.
In vivo studies using probiotics to reduce Campylobacter colonization in broilers.
| Study | Probiotic administration | C. jejuni contamination | C. jejuni enumeration | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain | Dose | Route | Period | Type | Chicken age or period | Strain (origin) | Dose | Organ | Chicken age | Results* | |
| PROBIOTIC ALONE | |||||||||||
| Netherwood et al., 1999 | E. faecium NCIMB 11508 transformed with plasmid pVACMC1 containing the Ruminococcus flavefaciens b-1,4-glucanase gene | 1.106 CFU/chick | Per os | From day of hatching to day 28 | Natural | – | – | – | Crops, duodena, ceca | 14, 28, 30, 33, and 35 days | No reduction |
| Fritts et al., 2000 | Calsporin® (B. subtilis C-3102) | NI | Diet | From day of hatching to day 42 | Natural | – | – | – | Processed carcasses | 42 days | 0.2 log reduction |
| Line et al., 2008 |
Lb. salivarius NRRL B-30514 Paenibacillus polymyxa NRRL B-30509 |
2.108 CFU/chick | Per os | From day of hatching to day 4 twice daily | Artificial | From day of hatching to day 10 | NI | 1.103, 1.104 or 1.105 CFU/chick | Ceca | 7 days | No reduction |
| Santini et al., 2010 | Bf. longum PCB133 | 1.108 CFU/chick | Per os | From day of hatching to day 14 | Natural | – | – | – | Feces | 15 days | 1 log reduction |
| Neal-McKinney et al., 2012 | Lb. crispatus JCM5810 | 1.108 CFU/chick | Per os | 4 days | Artificial | 14 days | F38011 (human) | 1.108 CFU/chick | Ceca | 21 days | 2 log reduction |
| Robyn et al., 2013 | E. faecalis MB5259 | 1.104 or 1.108 CFU/chick | Per os | NI | Artificial | 15 days | MB 4185 (chicken) | 2.104 CFU/chick | Ceca | 21 days | No reduction |
| Nishiyama et al., 2014 | Lb. gasseri SBT2055 | 1.108 CFU/chick | Per os | From day of hatching to day 15 | Artificial | 1 day | 81–176 (human) | 1.106 CFU/chick | Ceca | 15 days | 2 log reduction |
| Arsi et al., 2015a | Bacillus spp. | NI | Intracloacal or per os | Day of hatching | Artificial | 7 days | Four-strain mixture | 1.106 CFU/chick | Ceca | 14 days | 1–3 log reduction (intracloacal) No reduction (per os) |
| Arsi et al., 2015b | Bacillus spp. Lb. salivarius subsp. salivarius Lb. salivarius subsp. salicinius | 2.106 CFU/chick | Per os | Day of hatching | Artificial | 7 days | Four-strain mixture | 1.106 CFU/chick | Ceca | 14 days | 1–3 log reduction |
| Nishiyama et al., 2015 | Lb. gasseri SBT2055 | 1.108 CFU/chick | Per os | From day of hatching to day 15 | Artificial | 1 day | 81–176 (human) | 1.106 CFU/chick | Ceca | 15 days | 1–2 log reduction |
| Gracia et al., 2016 | B. subtilis DSM17299 | 0.05% (w/w) | Diet | From day of hatching to day 42 | Artificial | 14 days | Isolate from ST45 complex (chicken) | 1.104 CFU/chick | Ceca | 21, 35 and 42 days | No reduction |
| Guyard-Nicodème et al., 2016 | Calsporin® Ecobiol® (B. amyloliquefaciens) | 0.01% (w/w) 0.1% (w/w) | Diet | From day of hatching to day 42 | Artificial | 11 days | C97ANSES640 (chicken) | 1.104 CFU/chick | Ceca | 14, 35 and 42 days | 1.7 log reduction at 42 days (Calsporin®) No reduction (Ecobiol®) |
| PROBIOTIC MIXTURE | |||||||||||
| Aho et al., 1992 | K-bacteria (microaerophilic adaptive-mucus bacteria) + Broilact® (facultative anaerobic bacteria) | NI | Water | From day of hatching to day 38 | Artificial | 4 days | T23/42 (chicken) | 1.104 CFU/chick | Ceca | 38 days | 1.5–2 log reduction |
| Schoeni and Wong, 1994 | Citrobacter diversus 22 + Klebsiella pneumonia 23 + Escherichia coli 25 + mannose | 1.108 CFU/chick | Per os | Days 1 and 3 | Artificial | 1 day | 108 (chicken) | 1.108 CFU/chick | Ceca | 7 days | 62% reduction in the colonization rate |
| Morishita et al., 1997 | Avian PAC Soluble®; (Lb. acidophilus + Streptococcus faecium) | 400 mg/L | Water | From day of hatching to day 3 | Artificial | 1 day | C101 (chicken) | 1.104 CFU/chick | Cloacal swabs | 39 days | 70% reduction in prevalence |
| Willis and Reid, 2008 | Starter diet (Lb. acidophilus + Lb. casei + Bf. thermophilus + E. faecium) | 1.108 CFU/kg of feed | Diet | From day of hatching to day 42 | Natural | – | – | – | Cloacal swabs | 42 days | 10% reduction in prevalence |
| Baffoni et al., 2012 | Microencapsulated Bf. longum PCB133 + oligosaccharides | 1.109 CFU/chick + 3% of galactooligosaccharide (w/w) | Diet | From day of hatching to day 14 | Natural | – | – | – | Feces | 15 days | 0.5 log reduction |
| Ghareeb et al., 2012 | PoultryStar sol® (E. faecium + P. acidilactici + Bf. animalis + Lb. salivarius + Lb. reuteri) | 2 or 20 mg | Water | From day of hatching to day 14 | Artificial | 1 day | 3015/2010 (chicken) | 1.104 CFU/chick | Ceca | 15 days | 3.7–5.5 log reduction |
| Aguiar et al., 2013 | Three B. subtilis sp. mixture | 2.106 CFU/chick | Per os | Day of hatching | Artificial | 7 days | Four-strain mixture (chicken) | 1.105 CFU/chick | Ceca | 14 days | 1–4 log reduction |
| Cean et al., 2015 | Lb. paracasei J.R + Lb. rhamnosus 15b + Lb. lactis Y + Lb. lactis FOa | NI | Water | From day of hatching to day 42 | Natural | – | – | – | Duodena, ceca, feces | 42 days | 5 log reduction (duodena and ceca) |
| Guyard-Nicodème et al., 2016 | PoultryStar ME® | 0.1% (w/w) | Diet | From day of hatching to day 42 | Artificial | 11 days | C97ANSES640 (chicken) | 1.104 CFU/chick | Ceca | 14, 35 and 42 days | 0.5 log reduction at 14 days and 1.9 log reduction at 35 days |
Only results with statistical reduction are presented.
E, Enterococcus; B, Bacillus; Lb, Lactobacillus; Bf, Bifidobacterium; P, Pediococcus; NI, not indicated; Calsporin® is produced by Calpis (Tokyo, Japan); Broilact® is produced by Nimrod Veterinary Products (Gloucester, UK); PoultryStar sol® and PoultryStar ME® are produced by BIOMIN (Holding GmbH, Getzersdorf, Austria); Avian PAC Soluble® is produced by Pacific Agri-Sales (Visalia, CA, USA); Ecobiol® is produced by Norel Animal Nutrition (Madrid, Spain).
Probiotics used alone
Several studies suggest that a therapeutic treatment could be useful in suppressing C. jejuni colonization of chicks at early growth stages (Table 3). Birds fed diets including Bacillus subtilis C-3102 had significantly reduced numbers of Campylobacter (0.2 log) than birds fed with the control diet (Fritts et al., 2000). Neal-McKinney et al. (2012) found that the number of C. jejuni was reduced by almost two orders of magnitude in commercial broiler chickens fed with Lb. crispatus showing a potential role of the probiotic as a preventive/protective measure. After investigating possible mechanisms for this reduction, including production of bacteriocins, stimulation of antibody production, alteration of the cecal microbiome, and production of lactic acid, the authors concluded that only the production of lactic acid was supported by their data (Neal-McKinney et al., 2012). Recently, Nishiyama et al. (2014) demonstrated the ability of Lb. gasseri SBT2055 to inhibit the adhesion and invasion of C. jejuni in vitro and C. jejuni colonization of chicks in vivo. Their data suggested a pivotal role for APF1 in mediating the interaction of LG2055 with human intestinal cells and in inhibiting C. jejuni colonization of the gastrointestinal tract (Nishiyama et al., 2015). Recently, Arsi et al. (2015a) collected bacterial isolates (Bacillus spp.) with anti-Campylobacter activity in vitro and evaluated their efficacy in vivo after oral or intracloacal inoculation into chicks. They demonstrated that, when dosed orally, only one isolate had a 1 log reduction in cecal Campylobacter counts, whereas when administered intracloacally, six isolates produced a 1–3 log reduction in cecal Campylobacter counts in 14-day-old chickens (Arsi et al., 2015a). Their results highlight the fact that if probiotics are protected during transit through the upper gastrointestinal tract and are thus available in the lower intestinal tract, they could reduce Campylobacter colonization in broiler chickens. This is also the first study to show an anti-Campylobacter effect of a single probiotic on a four-strain mixture of Campylobacter.
Contrary to these results, treatments with viable probiotic bacterial cultures (Lb. salivarius NRRL B-30514 or Paenibacillus polymyxa NRRL B-30509) were ineffective in reducing C. jejuni in chickens, using both prophylactic or therapeutic administration (Line et al., 2008), while treatment with bacteriocins from these corresponding bacteria substantially reduced C. jejuni colonization in live chickens (Svetoch et al., 2005). Finally, this anti-Campylobacter activity of P. polymyxa was not due to a bacteriocin and was reassigned to the lipopeptide tridecaptin A1 (Lohans et al., 2014). Another study showed that in vitro activity of Bf. longum PCB 133 against C. jejuni was confirmed in in vivo trials while Lb. plantarum PCS 20 failed to show any efficacy (Santini et al., 2010). Netherwood et al. (1999) also showed no evidence of a beneficial effect on the shedding of Campylobacter by chickens treated with the probiotic E. faecium NCIMB 11508. This result was corroborated by Robyn et al. (2013) with another Enterococcus strain. No evidence for inhibition was identified after challenging the probiotic E. faecalis MB 5259 with Campylobacter in broilers, although an in vitro inhibitory influence of the E. faecalis strain on C. jejuni had previously been shown in a system mimicking the broiler cecal environment (Robyn et al., 2013).
As a general remark, studies using probiotics individually have demonstrated heterogeneous results (Table 3). The presence of the complex avian gut microbiota, which could interact with the anti-Campylobacter activity, might partly explain this difference between in vitro and in vivo results. Thus, an effective alternative can be combinations of probiotic strains, which individually show anti-Campylobacter activities such as aggregation, competition for site adhesion, bacteriocin and acid production.
Probiotic mixture
In their search for a competitive flora against Campylobacter, Aho et al. (1992) isolated two K-bacteria, i.e., two strains of Campylobacter-like organisms, from the ceca of an adult hen. They found that these mucin-adapted microaerophilic bacteria combined with Broilact® (Nimrod Veterinary Products, Gloucester, United Kingdom) a commercial mix of facultative anaerobic bacteria, delayed the onset of Campylobacter colonization by 1.5 weeks, and maintained a low level of colonization of 1.5–2 log10 CFU/g in broiler chickens (Aho et al., 1992). These bacteria, as a preventive/protective measure, may compete with Campylobacter for the same ecological niche in the intestinal ecosystem. Nevertheless, the problem with using undefined bacterial mixtures was that the antagonistic activities of the supplied bacteria were not well understood and presented the potential risk of introducing avian or human pathogens into the food chain (Stavric, 1992). This study appears to be in line with Schoeni and Wong (1994) who found that a defined mixture of Citrobacter diversus, Klebsiella pneumoniae, and Escherichia coli reduced the colonization of Campylobacter by 62% in chicken. Morishita et al. (1997) orally administered a mixture of Lb. acidophilus and Streptococcus faecium (isolated from chicken gut) to chickens in their drinking water for the first 3 days of life. Six hours after the first treatment, they orally challenged them with C. jejuni. Chickens receiving the treatment were significantly less colonized with C. jejuni (70% reduction) than those in the control group. Similarly, Willis and Reid (2008) showed a lower level of C. jejuni in broiler chickens fed with a standard diet supplemented with a probiotic mixture containing Lb. acidophilus, Lb. casei, Bf. thermophilus, and E. faecium (108 CFU/g).
Ghareeb et al. (2012) infected 1-day-old broiler chicks, which then received 2 or 20 mg/chick per day of a commercialized probiotic via their drinking water for 15 days. The protective administration of the multispecies probiotic product, containing avian-derived Enterococcus, Pediococcus, Lactobacillus, and Bifidobacterium microorganisms, to broiler chickens reduced the cecal colonization by 3.8–5.5 log of C. jejuni at both 8 and 15 days post-challenge and may have changed their gut microbiota in a way that is beneficial to the health of consumers by reducing the number of Campylobacter (Ghareeb et al., 2012). Cean et al. (2015) went a step further and investigated the presence of the pathogen in the feces, duodenal and cecal content and the duodenal and cecal mucosa after a 42-day treatment with a combination of Lb. paracasei J.R., Lb. rhamnosus 15b, Lb. lactis Y, and Lb. lactis FOa. A significant reduction in the pathogen load from 0.5 to 5 log in both intestinal content and mucus colonization was observed (Cean et al., 2015). The highest effect of the mixture was observed in the duodenal content while the reduction in Campylobacter loads in the cecal content was the lowest. This observation highlights that probiotic activity may depend on the part of gastrointestinal tract considered and suggests that probiotic concentrations may be lower in the ceca than in the duodenum and/or Campylobacter may be better protected in the ceca. In addition, these probiotics were effective even when introduced in broiler feed 7 days before slaughter, thus as a therapeutic measure.
Recently, Baffoni et al. (2012) evaluated the therapeutic ability of a synbiotic mixture of Bf. longum PCB133 and prebiotic oligosaccharides to reduce the presence of C. jejuni in broiler chicken gut. In their in vivo experiment, C. jejuni quantification showed a 0.5 log decrease while total bifidobacteria were significantly increased after 2 weeks of treatment compared with the control group. On one hand, they speculated that the increased number of bifidobacteria, determined by prebiotic oligosaccharide intake, helps modulate the expression of Campylobacter genes involved in adhesion, as reported by Ding et al. (2005). On the other hand, the probiotic strain PCB133 exerts an anti-Campylobacter effect through antibacterial metabolite production, mainly acidic products, as well evidenced in the literature for other probiotic strains (Marianelli et al., 2010). These results illustrate the concept of synbiotics, which is a synergistic combination of probiotics and prebiotics (Roberfroid, 1998).
By targeting motility properties of bacteria in the development of probiotic cultures, Aguiar et al. (2013) selected three B. subtilis sp. with enhanced motility. The mixture, administered on the day of hatching, was able to reduce C. jejuni colonization in chicken challenged with a mixture of four different wild-type strains of C. jejuni. These motility-selected bacteria may have the marked ability to reach the same gastrointestinal niche in poultry, i.e., cross the protective avian intestinal mucus and reach cecal crypts, and then competitively reduce C. jejuni. Their findings support the theory that the motility enhancement of potential probiotic bacteria may provide a strategy for reduction of C. jejuni in chickens. This is also the first study to show an anti-Campylobacter effect of a probiotic mixture administrated once (day of hatching) on a four-strain mixture of Campylobacter.
Critical parameters of in vivo trials
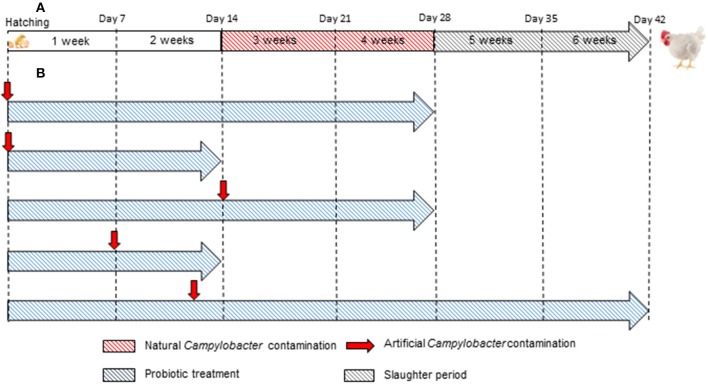
Figure 5 presents a comparison of commercial broiler chicken production and in vivo studies with an emphasis on the duration and timing of Campylobacter contamination.
Figure 5.
Comparison between (A) commercial broiler chicken production and (B) in vivo studies designed to evaluate the efficacy of probiotics to reduce the colonization of C. jejuni. In almost all in vivo studies, the duration and/or artificial Campylobacter contamination are not in accordance with the duration and natural Campylobacter contamination in commercial broiler chicken production.
Most commercial broilers reach slaughter-weight between 5 and 7 weeks of age, although slower growing races reach slaughter-weight at approximately 14 weeks of age. As indicated in Table 3 and illustrated in Figure 5, almost all in vivo studies have only lasted from 2 to 4 weeks. Thus, it may be very difficult to conclude about the positive or negative effectiveness of the tested probiotics at the slaughter-age of broilers. On one hand, studies may not take into account the resilience of Campylobacter to the presence of probiotic in the intestinal environment. The pathogen might implement strategies to overcome the anti-Campylobacter activity, as they do against bacteriophages (Hammerl et al., 2014) and then, despite a decrease at the beginning, the pathogen could adapt and grow once more. On the other hand, a delay could be necessary for the probiotic to express the genes required for the anti-Campylobacter activity, which could explain why some studies do not show positive results.
In addition, infection by Campylobacter is rarely detected in chicks that are less than a week old; flocks usually become infected when the birds are 2–3 weeks of age (Neill et al., 1984; Jacobs-Reitsma et al., 1995; Berndtson et al., 1996). In the majority of the studies conducted to date, researchers inoculated with C. jejuni in the very first days of life (Figure 5 and Table 3).
Different host factors can justify the variations in the results of probiotic use in poultry (Otutumi et al., 2012). Chicken lineage could potentially influence probiotic treatments. Recent studies showed that behavior of C. jejuni in the broiler chicken may differ considerably to that in chicken breeds used in experimental studies (Humphrey et al., 2015). Modern rapidly growing chicken breeds used in intensive production systems exhibited a strong inflammatory response to C. jejuni infection that can lead to diarrhea (Humphrey et al., 2014). As demonstrated with Campylobacter, probiotics could have a different efficacy depending on the chicken breeds. The immunologic status of the animals is different between different chicken breeds (Korver, 2012) and therefore is also an inherent characteristic that could modulate the probiotic action. Interactions of probiotics with different chicken breeds need to be considered. Age of the chicken when the probiotic is administrated could also affect the activity of the probiotic strain. Indeed, Mohan et al. (1996) have found that beneficial effects of probiotics on zootechnical parameters were seen during the initial growth phase, suggesting that during this stage of life the intestinal microbiota is still in an unstable condition, and the microorganisms given orally probably find a niche where they can occupy (Fuller, 1995). Therefore, the existence of an intestinal microbiota at the time of administration and the health of the host must be considered when a probiotic is supplemented for the suppression of pathogenic bacteria (Siriken et al., 2003). Antimicrobial and antiparasitic treatments received by the animals before or during the probiotic administration could also influence the survival of the probiotic strain (Jin et al., 1997).
With artificially colonized chicks, the origin of the pathogen strain is very important as the ability to colonize chickens is dependent on the original source of the isolate (Pielsticker et al., 2012). In some studies, only human isolates of C. jejuni (81–176, F38011) were used and might not be relevant for chicken colonization trials. As we suggest for in vitro assays (Sections Growth Inhibition Assays and Adhesion and Invasion Inhibition Assays), it could be interesting to include in the trials reference strains to compare results between different studies and field strains to be closer to the field.
When natural contamination occurred, it raised a particularly important point about Campylobacter: they exhibit high genetic and phenotypic variability (Gripp et al., 2011). As a consequence, they are not equally able to colonize chickens (Chaloner et al., 2014) and probably not equally sensitive to probiotic actions (Wine et al., 2009). Therefore, it could be important to characterize these C. jejuni strains. In addition, research on Campylobacter control has focused on C. jejuni and the probiotic strains used in the in vivo trials showed an in vitro anti-Campylobacter activity against C. jejuni. However, broilers can also be contaminated by C. coli (Rivoal et al., 2005; Hue et al., 2011) and when natural contamination occurred, C. jejuni could not be distinguished from C. coli as the enumeration was done by microbiological methods. It cannot be excluded that C. coli was responsible for the contamination and therefore the in vivo anti-Campylobacter activity was low or absent.
Samples used to enumerate Campylobacter may have an impact on the results and their interpretation. Feces and cloacal swabs are very useful for performing longitudinal studies with repeated measures on one animal. Nevertheless, cloacal swabs can only be used for detection and give information on the prevalence of Campylobacter while Campylobacter concentrations are also important when comparing an effect of a treatment. Feces could induce a bias in the results because bacterial diversity and community composition in fecal samples differ from cecal content (Pauwels et al., 2015). Bahrndorff et al. (2015) recently evaluated the colonization of individual broiler chickens by C. jejuni over time. They pointed out large differences between broiler chickens in the number of C. jejuni in cecal and fecal samples at 4, 7, and 12 days post-infection (Bahrndorff et al., 2015). These differences could be due to the fact that this foodborne pathogen requires a microaerophilic atmosphere (Macé et al., 2015) and this condition is not optimal in the fecal samples. Cecal drops could be a valuable alternative to feces and cloacal swabs in longitudinal studies (Pauwels et al., 2015).
The form and route of probiotic administration are two critical points for a future industrial application. Fresh cultures that are individually inoculated are clearly not possible at the farm level, even if the probiotics are highly active and efficient. It will be important for probiotic producers to use production processes and modified preservation and administration strategies to guarantee the delivery of active strains to the poultry. As several papers have shown, the industrial processing of a probiotic preparation has a fundamental impact on its functionality in the host (Bron et al., 2012; Van Bokhorst-Van de Veen et al., 2012). Viability, the presence or absence of pili, the cell wall condition, the matrix or the growth stage of the probiotic seem to have an important influence on its performance and its interaction with the host (Papadimitriou et al., 2015). Defining the mechanism of action of a probiotic might therefore also include some critical parameters of the production process. Their activity and survival during storage must also be assessed (FAO/WHO, 2001). Little information on these aspects are available for probiotics with an anti-Campylobacter. However, several studies focusing on probiotics for poultry mentioned that moisture and cell conditions have an impact on survival of probiotics during long-term storage. Freeze-drying and freezing with cryoprotective agents seemed to be suitable conditions to store probiotic strains (Pascual et al., 1999). In addition, Khoramnia et al. (2011) have shown that cryoprotectants significantly increase storage life of freeze-died lactobacilli probiotics, intended for poultry, during several months at refrigerated temperature. The probiotic could be administrated to poultry by different routes, including to animal feed. However, inclusion to the commercial feed mixture can affect probiotic survival by the temperatures used during the feed mixture storage and in the chicken incubator rooms (Pascual et al., 1999). Microencapsulation of probiotics appeared to be a promising alternative to improve their viability and survival against adverse conditions during processing, storage and gastrointestinal passage (Baffoni et al., 2012; Dianawati et al., 2015). To our knowledge, few probiotics, in the form of commercial feed additives, have exhibited a strong anti-Campylobacter activity (>2 log reduction) (Ghareeb et al., 2012; Guyard-Nicodème et al., 2016).
Even if the purpose of these studies is to reduce Campylobacter loads in poultry, it is important to keep in mind that the final destination of the broilers is the retail market. The administration of large amounts of bacteria could not only reduce Campylobacter but also impair the homeostasis of the avian gut microbiota. Indeed, this ecosystem is crucial for the fermentation of undigested carbohydrates (Józefiak et al., 2004). Therefore, it is necessary to examine the probiotic impact on performance parameters including average daily feed intake, body weight gain and feed conversion ratio. These parameters have not always been assessed because of the lack of a group treated with the probiotic and unchallenged with C. jejuni in the experimental design. In addition to zootechnical parameters, it might be interesting to monitor immune and inflammatory responses during animal experiments (Awad et al., 2014a,b; Humphrey et al., 2014).
Impact on consumers
When considering the results of the different studies summarized in Table 3, an issue that needs to be addressed is the biological meaning of Campylobacter reduction in broilers. For example, the questions could be whether a statistically significant 0.5 log reduction in C. jejuni has important effects on the risk for consumers and what is the minimal reduction in order to conclude that a probiotic is efficient. Quantitative microbial risk assessment analyses of human campylobacteriosis associated with thermotolerant Campylobacter spp. in broiler chickens have been performed. In Denmark, a reduction in Campylobacter counts on chicken carcasses by 2 log predicted a 30-fold reduction in the incidence of campylobacteriosis in humans (Rosenquist et al., 2003). Another study conducted in Belgium demonstrated that the incidence would be reduced by 48, 85, and 96% when a 1 log, 2 log or 3 log reduction, respectively, of Campylobacter contamination on carcasses was achieved (Messens et al., 2007). Based on a quantitative microbiological risk assessment on Campylobacter in broilers at EU level, Romero-Barrios et al. (2013) estimated that the potential risk reduction would range from 48 to 100% for reductions of 1–6 log in Campylobacter in the intestines. According to these assessments, the minimum reduction in cecal Campylobacter loads that needs to be achieved to ensure a substantial reduction in human campylobacteriosis is at least 1 log10 CFU/g (Nauta et al., 2016).
An added value might be to extend the work to assess the impact of the probiotic strains on the prevalence or the level of Campylobacter on processed birds, i.e., carcasses. This could provide an overview of a part of the poultry chain production from the farm to the slaughterhouse.
Conclusion
To conclude, research has shown that probiotics have potential for limiting Campylobacter colonization in broiler chickens. The oral administration of probiotic bacteria is advantageous, as they are easy to administer, i.e., in feed or drinking water, inexpensive to produce, and may persist in the animal. In vitro studies can indicate a possible anti-Campylobacter activity and yield useful information about the inhibition mechanism involved. Nevertheless, given the limitations of individual methods, no in vitro assay alone seems ideal to affirm a potential anti-Campylobacter activity. Therefore, studies must combine in vitro and in vivo methods to take into account the complexity introduced by the host, the feed, and the microbiota. This recommended combined approach may use multiple complementary tools (cell cultures, animal experiments) and address different points (molecular and overall interactions). In vivo studies using defined bacterial strains and various mixtures have shown promising results in reducing the colonization of Campylobacter spp. in chicken.
This review highlights, in particular, the intensive use of Lactobacillus spp., i.e., acidophilus, casei, crispatus, gasseri, helveticus, pentosus, plantarum, rhamnosus, and salivarius, which exhibit relevant in vitro and in vivo anti-Campylobacter activities. In the future, it may be important to investigate different and varied bacterial species.
Finally, a valuable perspective would be to look at strain combinations enhanced by prebiotics. This strategy could be relevant for additives to poultry feed for the reduction of food-borne campylobacteriosis in humans. There is still a long way to go because processing could influence the in vivo anti-Campylobacter activity.
Author contributions
MS analyzed data from the literature and drafted the manuscript. NH, MG, and DX conceived the review, participated in its organization and helped to draft the manuscript. SM, MC, and JC revising the manuscript critically for important intellectual content. All authors read and approved the final manuscript.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The work was supported by the CAMPYLOW project founded by Région Pays de la Loire and Bretagne. We are grateful to Stephan Rouverand and the PAO (Pôle Agronomique Ouest) for the assistance in the project conception. We thank our collaborators Cécile Guillon-Kroon (TERRENA, Ancenis, France), Florence Quéré (Nutréa, Languidic, France), Jean-Christophe Bodin and Nicolas Destombes (Jefo, Carquefou, France).
References
- Adak G. K., Meakins S. M., Yip H., Lopman B. A., O'Brien S. J. (2005). Disease risks from foods, England and Wales, 1996–2000. Emerg. Infect. Dis. 11, 365–372. 10.3201/eid1103.040191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguiar V. F., Donoghue A. M., Arsi K., Reyes-Herrera I., Metcalf J. H., de los Santos F. S., et al. (2013). Targeting motility properties of bacteria in the development of probiotic cultures against Campylobacter jejuni in broiler chickens. Foodborne Pathog. Dis. 10, 435–441. 10.1089/fpd.2012.1302 [DOI] [PubMed] [Google Scholar]
- Ahmed I. H., Manning G., Wassenaar T. M., Cawthraw S., Newell D. G. (2002). Identification of genetic differences between two Campylobacter jejuni strains with different colonization potentials. Microbiology 148, 1203–1212. 10.1099/00221287-148-4-1203 [DOI] [PubMed] [Google Scholar]
- Aho M., Nuotio L., Nurmi E., Kiiskinen T. (1992). Competitive exclusion of campylobacters from poultry with K-bacteria and Broilact®. Int. J. Food Microbiol. 15, 265–275. 10.1016/0168-1605(92)90057-A [DOI] [PubMed] [Google Scholar]
- Alemka A., Clyne M., Shanahan F., Tompkins T., Corcionivoschi N., Bourke B. (2010). Probiotic colonization of the adherent mucus layer of HT29MTXE12 cells attenuates Campylobacter jejuni virulence properties. Infect. Immun. 78, 2812–2822. 10.1128/IAI.01249-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allain V., Chemaly M., Laisney M. J., Rouxel S., Quesne S., Le Bouquin S. (2014). Prevalence of and risk factors for Campylobacter colonisation in broiler flocks at the end of the rearing period in France. Br. Poult. Sci. 55, 452–459. 10.1080/00071668.2014.941788 [DOI] [PubMed] [Google Scholar]
- Allegretti L., Revolledo L., Astolfi-Ferreira C. S., Chacón J. L, Martins, L. M., Seixas G. H. F., et al. (2014). Isolation and molecular identification of lactic acid bacteria and Bifidobacterium spp. from faeces of the blue-fronted Amazon parrot in Brazil. Benef. Microbes 5, 497–503. 10.3920/BM2013.0082 [DOI] [PubMed] [Google Scholar]
- Arsi K., Donoghue A. M., Woo-Ming A., Blore P. J., Donoghue D. J. (2015b). The efficacy of selected probiotic and prebiotic combinations in reducing Campylobacter colonization in broiler chickens. J. Appl. Poult. Res. 24, 327–334. 10.3382/japr/pfv032 [DOI] [Google Scholar]
- Arsi K., Donoghue A., Woo-Ming A., Blore P. J., Donoghue D. J. (2015a). Intracloacal Inoculation, an effective screening method for determining the efficacy of probiotic bacterial isolates against Campylobacter colonization in broiler chickens. J. Food Prot. 78, 209–213. 10.4315/0362-028X.JFP-14-326 [DOI] [PubMed] [Google Scholar]
- Ashraf R., Shah N. P. (2014). Immune system stimulation by probiotic microorganisms. Crit. Rev. Food Sci. Nutr. 54, 938–956. 10.1080/10408398.2011.619671 [DOI] [PubMed] [Google Scholar]
- Awad W. A., Aschenbach J. R., Ghareeb K., Khayal B., Hess C., Hess M. (2014a). Campylobacter jejuni influences the expression of nutrient transporter genes in the intestine of chickens. Vet. Microbiol. 172, 195–201. 10.1016/j.vetmic.2014.04.001 [DOI] [PubMed] [Google Scholar]
- Awad W. A., Molnár A., Aschenbach J. R., Ghareeb K., Khayal B., Hess C., et al. (2014b). Campylobacter infection in chickens modulates the intestinal epithelial barrier function. Innate Immun. 21, 151–160. 10.1177/1753425914521648 [DOI] [PubMed] [Google Scholar]
- Baffoni L., Gaggìa F., Di Gioia D., Santini C., Mogna L., Biavati B. (2012). A Bifidobacterium-based synbiotic product to reduce the transmission of C. jejuni along the poultry food chain. Int. J. Food Microbiol. 157, 156–161. 10.1016/j.ijfoodmicro.2012.04.024 [DOI] [PubMed] [Google Scholar]
- Bagheripoor-Fallah N., Mortazavian A., Hosseini H., Khoshgozaran-Abras S., Rad A. H. (2015). Comparison of molecular techniques with other methods for identification and enumeration of probiotics in fermented milk products. Crit. Rev. Food Sci. Nutr. 55, 396–413. 10.1080/10408398.2012.656771 [DOI] [PubMed] [Google Scholar]
- Bahrndorff S., Garcia A. B., Vigre H., Nauta M., Heegaard P. M., Madsen M., et al. (2015). Intestinal colonization of broiler chickens by Campylobacter spp. in an experimental infection study. Epidemiol. Infect. 143, 2381–2389. 10.1017/S0950268814003239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batdorj B., Trinetta V., Dalgalarrondo M., Prévost H., Dousset X., Ivanova I., et al. (2007). Isolation, taxonomic identification and hydrogen peroxide production by Lactobacillus delbrueckii subsp. lactis T31, isolated from Mongolian yoghurt: inhibitory activity on food-borne pathogens. J. Appl. Microbiol. 103, 584–593. 10.1111/j.1365-2672.2007.03279.x [DOI] [PubMed] [Google Scholar]
- Batz M. B., Hoffmann S., Morris J. G., Jr. (2012). Ranking the disease burden of 14 pathogens in food sources in the United States using attribution data from outbreak investigations and expert elicitation. J. Food Prot. 75, 1278–1291. 10.4315/0362-028X.JFP-11-418 [DOI] [PubMed] [Google Scholar]
- Batz M., Hoffmann S., Morris J. G., Jr. (2014). Disease-outcome trees, EQ-5D scores, and estimated annual losses of quality-adjusted life years (QALYs) for 14 foodborne pathogens in the United States. Foodborne Pathog. Dis. 11, 395–402. 10.1089/fpd.2013.1658 [DOI] [PubMed] [Google Scholar]
- Baurhoo B., Ferket P. R., Zhao X. (2009). Effects of diets containing different concentrations of mannanoligosaccharide or antibiotics on growth performance, intestinal development, cecal and litter microbial populations, and carcass parameters of broilers. Poult. Sci. 88, 2262–2272. 10.3382/ps.2008-00562 [DOI] [PubMed] [Google Scholar]
- Berjeaud J.-M., Cenatiempo Y. (2004). Purification of antilisterial bacteriocins, in Public Health Microbiology, eds Spencer J. F. T., de Spencer A. L. R. (Totowa, NJ: Springer; ), 225–233. [DOI] [PubMed] [Google Scholar]
- Berndtson E., Danielsson-Tham M. L., Engvall A. (1996). Campylobacter incidence on a chicken farm and the spread of Campylobacter during the slaughter process. Int. J. Food Microbiol. 32, 35–47. 10.1016/0168-1605(96)01102-6 [DOI] [PubMed] [Google Scholar]
- Bernet M. F., Brassart D., Neeser J., Servin A. (1994). Lactobacillus acidophilus LA 1 binds to cultured human intestinal cell lines and inhibits cell attachment and cell invasion by enterovirulent bacteria. Gut 35, 483–489. 10.1136/gut.35.4.483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessell P. R., Matthews L., Smith-Palmer A., Rotariu O., Strachan N. J., Forbes K. J., et al. (2010). Geographic determinants of reported human Campylobacter infections in Scotland. BMC Public Health 10:423. 10.1186/1471-2458-10-423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas D., Itoh K., Sasakawa C. (2000). Uptake pathways of clinical and healthy animal isolates of Campylobacter jejuni into INT-407 cells. FEMS Immunol. Med. Microbiol. 29, 203–211. 10.1111/j.1574-695X.2000.tb01524.x [DOI] [PubMed] [Google Scholar]
- Blaser M. J., Engberg J., Nachamkin I., Szymanski C. (2008). Clinical Aspects of Campylobacter jejuni and Campylobacter coli Infections. Washington, DC: ASM Press. [Google Scholar]
- Booth C., Patel S., Bennion G. R., Potten C. S. (1994). The isolation and culture of adult mouse colonic epithelium. Epithelial Cell Biol. 4, 76–86. [PubMed] [Google Scholar]
- Bratz K., Gölz G., Janczyk P., Nöckler K., Alter T. (2015). Analysis of in vitro and in vivo effects of probiotics against Campylobacter spp. Berl. Münc. Tierärztl. Wochenschr. 128, 155–162. Available online at: http://vetline.de/open-access/158/3216/ [PubMed] [Google Scholar]
- Brisbin J. T., Gong J., Orouji S., Esufali J., Mallick A. I., Parvizi P., et al. (2011). Oral treatment of chickens with lactobacilli influences elicitation of immune responses. Clin. Vaccine Immunol. 18, 1447–1455. 10.1128/CVI.05100-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bron P. A., Wels M., Bongers R. S., van Bokhorst-van de Veen H., Wiersma A., Overmars L., et al. (2012). Transcriptomes reveal genetic signatures underlying physiological variations imposed by different fermentation conditions in Lactobacillus plantarum. PLoS ONE 7:e38720. 10.1371/journal.pone.0038720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronnec V., Haddad N., Cruveiller S., Hernould M., Tresse O., Zagorec M. (2016). Draft genome sequence of Campylobacter jejuni Bf, an atypical strain able to grow under aerobiosis. Genome Announc. 4:e00120-16. 10.1128/genomeA.00120-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull S. A., Allen V. M., Domingue G., Jørgensen F., Frost J. A., Ure R., et al. (2006). Sources of Campylobacter spp. colonizing housed broiler flocks during rearing. Appl. Environ. Microbiol. 72, 645–652. 10.1128/AEM.72.1.645-652.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne C. M., Clyne M., Bourke B. (2007). Campylobacter jejuni adhere to and invade chicken intestinal epithelial cells in vitro. Microbiology 153, 561–569. 10.1099/mic.0.2006/000711-0 [DOI] [PubMed] [Google Scholar]
- Campana R., Federici S., Ciandrini E., Baffone W. (2012). Antagonistic activity of Lactobacillus acidophilus ATCC 4356 on the growth and adhesion/invasion characteristics of human Campylobacter jejuni. Curr. Microbiol. 64, 371–378. 10.1007/s00284-012-0080-0 [DOI] [PubMed] [Google Scholar]
- Carolissen-Mackay V., Arendse G., Hastings J. W. (1997). Purification of bacteriocins of lactic acid bacteria: problems and pointers. Int. J. Food Microbiol. 34, 1–16. 10.1016/S0168-1605(96)01167-1 [DOI] [PubMed] [Google Scholar]
- Casey P. G., Gardiner G. E., Casey G., Bradshaw B., Lawlor P. G., Lynch P. B., et al. (2007). A five-strain probiotic combination reduces pathogen shedding and alleviates disease signs in pigs challenged with Salmonella enterica serovar Typhimurium. Appl. Environ. Microbiol. 73, 1858–1863. 10.1128/AEM.01840-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cean A., Stef L., Simiz E., Julean C., Dumitrescu G., Vasile A., et al. (2015). Effect of human isolated probiotic bacteria on preventing Campylobacter jejuni colonization of poultry. Foodborne Pathog. Dis. 12, 122–130. 10.1089/fpd.2014.1849 [DOI] [PubMed] [Google Scholar]
- Chaloner G., Wigley P., Humphrey S., Kemmett K., Lacharme-Lora L., Humphrey T., et al. (2014). Dynamics of dual infection with Campylobacter jejuni strains in chickens reveals distinct strain-to-strain variation in infection ecology. Appl. Environ. Microbiol. 80, 6366–6372. 10.1128/AEM.01901-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang M. H., Chen T. C. (2000). Reduction of Campylobacter jejuni in a simulated chicken digestive tract by lactobacilli cultures. J. Food Prot. 63, 1594–1597. [DOI] [PubMed] [Google Scholar]
- Chaveerach P., Lipman L. J., Van Knapen F. (2004). Antagonistic activities of several bacteria on in vitro growth of 10 strains of Campylobacter jejuni/coli. Int. J. Food Microbiol. 90, 43–50. 10.1016/S0168-1605(03)00170-3 [DOI] [PubMed] [Google Scholar]
- Chemaly M., Magras C., Madec J.-Y., Santolini J., Denis M. (2012). Campylobacter dans les filières de production animale. Bull. Epidémiol. Santé Anim. Aliment. 50, 19–22. Available online at: https://pro.anses.fr/bulletin-epidemiologique/Documents/BEP-mg-BE50-art5.pdf [Google Scholar]
- Cherdyntseva T. A., Kotova I. B., Netrusov A. I. (2016). The isolation, identification and analyses of lactobacillus genus bacteria with probiotic potential, in Advances in Microbiology, Infectious Diseases and Public Health, Vol. 1, ed Donelli G. (Springer International Publishing; ), 103–111. [DOI] [PubMed] [Google Scholar]
- Cintas L. M., Casaus P., Herranz C., Håvarstein L. S., Holo H., Hernández P. E., et al. (2000). Biochemical and genetic evidence that Enterococcus faecium L50 produces enterocins L50A and L50B, thesec-dependent enterocin P, and a novel bacteriocin secreted without an N-terminal extension termed enterocin Q. J. Bacteriol. 182, 6806–6814. 10.1128/JB.182.23.6806-6814.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C. (2014). Enumeration of probiotic strains: review of culture-dependent and alternative techniques to quantify viable bacteria. J. Microbiol. Methods 103, 9–17. 10.1016/j.mimet.2014.04.012 [DOI] [PubMed] [Google Scholar]
- Devane M. L., Nicol C., Ball A., Klena J. D., Scholes P., Hudson J. A., et al. (2005). The occurrence of Campylobacter subtypes in environmental reservoirs and potential transmission routes. J. Appl. Microbiol. 98, 980–990. 10.1111/j.1365-2672.2005.02541.x [DOI] [PubMed] [Google Scholar]
- De Zoete M. R., Van Putten J. P., Wagenaar J. A. (2007). Vaccination of chickens against Campylobacter. Vaccine 25, 5548–5557. 10.1016/j.vaccine.2006.12.002 [DOI] [PubMed] [Google Scholar]
- Dianawati D., Mishra V., Shah N. P. (2015). Survival of microencapsulated probiotic bacteria after processing and during storage: a review. Crit. Rev. Food Sci. Nutr. 10.1080/10408398.2013.798779. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Diep D. B., Axelsson L., Grefsli C., Nes I. F. (2000). The synthesis of the bacteriocin sakacin A is a temperature-sensitive process regulated by a pheromone peptide through a three-component regulatory system. Microbiology 146, 2155–2160. 10.1099/00221287-146-9-2155 [DOI] [PubMed] [Google Scholar]
- Ding W., Wang H., Griffiths M. W. (2005). Probiotics down-regulate flaA σ28 promoter in Campylobacter jejuni. J. Food Prot. 68, 2295–2300. [DOI] [PubMed] [Google Scholar]
- Dubois Dauphin R., Didderen I., Marcq C., Thewis A., Thonart P. (2011). In vitro antagonistic activity evaluation of lactic acid bacteria (LAB) combined with cellulase enzyme against Campylobacter jejuni growth in co-culture. J. Microbiol. Biotechnol. 21, 62–70. 10.4014/jmb.1007.07006 [DOI] [PubMed] [Google Scholar]
- Eberle K., Kiess A. (2012). Phenotypic and genotypic methods for typing Campylobacter jejuni and Campylobacter coli in poultry. Poult. Sci. 91, 255–264. 10.3382/ps.2011-01414 [DOI] [PubMed] [Google Scholar]
- EFSA (2010). Analysis of the baseline survey on the prevalence of Campylobacter in broiler batches and of Campylobacter and Salmonella on broiler carcasses, in the EU, 2008—Part A: Campylobacter and Salmonella prevalence estimates. EFSA J. 8, 1503–1550. 10.2903/j.efsa.2011.2017 [DOI] [Google Scholar]
- EFSA (2011). Panel on Biological Hazards (BIOHAZ); scientific opinion on Campylobacter in broiler meat production: control options and performance objectives and/or targets at different stages of the food chain. EFSA J. 9, 141. 10.2903/j.efsa.2011.210523486439 [DOI] [Google Scholar]
- EFSA (2014). EU Summary Report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2012. EFSA J. 12, 3547 10.2903/j.efsa.2014.3547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (2015). EU summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2013. EFSA J. 13, 3991 10.2903/j.efsa.2015.3991 [DOI] [Google Scholar]
- Ellis-Iversen J., Jorgensen F., Bull S., Powell L., Cook A. J., Humphrey T. J. (2009). Risk factors for Campylobacter colonisation during rearing of broiler flocks in Great Britain. Prev. Vet. Med. 89, 178–184. 10.1016/j.prevetmed.2009.02.004 [DOI] [PubMed] [Google Scholar]
- FAO/WHO (2001). Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria. Córdoba: Joint FAO/WHO Expert Consultation. [Google Scholar]
- Fauchere J., Rosenau A., Veron M., Moyen E., Richard S., Pfister A. (1986). Association with HeLa cells of Campylobacter jejuni and Campylobacter coli isolated from human feces. Infect. Immun. 54, 283–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearnley C., Manning G., Bagnall M., Javed M. A., Wassenaar T. M., Newell D. G. (2008). Identification of hyperinvasive Campylobacter jejuni strains isolated from poultry and human clinical sources. J. Med. Microbiol. 57, 570–580. 10.1099/jmm.0.47803-0 [DOI] [PubMed] [Google Scholar]
- Fernández M., Boris S., Barbés C. (2003). Probiotic properties of human lactobacilli strains to be used in the gastrointestinal tract. J. Appl. Microbiol. 94, 449–455. 10.1046/j.1365-2672.2003.01850.x [DOI] [PubMed] [Google Scholar]
- Fooks L. J., Gibson G. R. (2003). Mixed culture fermentation studies on the effects of synbiotics on the human intestinal pathogens Campylobacter jejuni and Escherichia coli. Anaerobe 9, 231–242. 10.1016/S1075-9964(03)00043-X [DOI] [PubMed] [Google Scholar]
- Fouts D. E., Mongodin E. F., Mandrell R. E., Miller W. G., Rasko D. A., Ravel J., et al. (2005). Major structural differences and novel potential virulence mechanisms from the genomes of multiple Campylobacter species. PLoS Biol. 3:e15. 10.1371/journal.pbio.0030015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei R., Akdis M., O'Mahony L. (2015). Prebiotics, probiotics, synbiotics, and the immune system: experimental data and clinical evidence. Curr. Opin. Gastroenterol. 31, 153–158. 10.1097/MOG.0000000000000151 [DOI] [PubMed] [Google Scholar]
- Fritts C. A., Kersey J. H., Motl M. A., Kroger E. C., Yan F., Si J., et al. (2000). Bacillus subtilis C-3102 (Calsporin) improves live performance and microbiological status of broiler chickens. J. Appl. Poult. Res. 9, 149–155. 10.1093/japr/9.2.149 [DOI] [Google Scholar]
- Fuller R. (1989). Probiotics in man and animals. J. Appl. Bacteriol. 66, 365–378. 10.1111/j.1365-2672.1989.tb05105.x [DOI] [PubMed] [Google Scholar]
- Fuller R. (1995). Probiotics: their development and use, in Old Herborn University Seminar Monograph 8, eds Van der Waaji D., Heidt P. J., Rusch V. C. (Reading, UK: Institute for Microbiology and Biochemistry; ), 1–8. [Google Scholar]
- Gaggìa F., Mattarelli P., Biavati B. (2010). Probiotics and prebiotics in animal feeding for safe food production. Int. J. Food Microbiol. 141, S15–S28. 10.1016/j.ijfoodmicro.2010.02.031 [DOI] [PubMed] [Google Scholar]
- Galanis A., Kourkoutas Y., Tassou C. C., Chorianopoulos N. (2015). Detection and identification of probiotic Lactobacillus plantarum strains by multiplex PCR using RAPD-derived primers. Int. J. Mol. Sci. 16, 25141–25153. 10.3390/ijms161025141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanis E. (2007). Campylobacter and bacterial gastroenteritis. Can. Med. Assoc. J. 177, 570–571. 10.1503/cmaj.070660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganan M., Martinez-Rodriguez A. J., Carrascosa A. V., Vesterlund S., Salminen S., Satokari R. (2013). Interaction of Campylobacter spp. and human probiotics in chicken intestinal mucus. Zoonoses Public Health 60, 141–148. 10.1111/j.1863-2378.2012.01510.x [DOI] [PubMed] [Google Scholar]
- Ghareeb K., Awad W. A., Mohnl M., Porta R., Biarnés M., Böhm J., et al. (2012). Evaluating the efficacy of an avian-specific probiotic to reduce the colonization of Campylobacter jejuni in broiler chickens. Poult. Sci. 91, 1825–1832. 10.3382/ps.2012-02168 [DOI] [PubMed] [Google Scholar]
- Gibbens J. C., Pascoe S. J., Evans S. J, Davies, R. H., Sayers A. R. (2001). A trial of biosecurity as a means to control Campylobacter infection of broiler chickens. Prev. Vet. Med. 48, 85–99. 10.1016/S0167-5877(00)00189-6 [DOI] [PubMed] [Google Scholar]
- Gosiewski T., Brzychczy-Wloch M. (2015). The use of PFGE method in genotyping of selected bacteria species of the Lactobacillus genus. Methods Mol. Biol. 1301, 225–240. 10.1007/978-1-4939-2599-5_18 [DOI] [PubMed] [Google Scholar]
- Gracia M., Millán C., Sánchez J., Guyard-Nicodème M., Mayot J., Carre Y., et al. (2016). Efficacy of feed additives against Campylobacter in live broilers during the entire rearing period: PartB. Poult. Sci. 95, 886–892. 10.3382/ps/pev346 [DOI] [PubMed] [Google Scholar]
- Gripp E., Hlahla D., Didelot X., Kops F., Maurischat S., Tedin K., et al. (2011). Closely related Campylobacter jejuni strains from different sources reveal a generalist rather than a specialist lifestyle. BMC Genomics 12:584. 10.1186/1471-2164-12-584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundogdu O., Bentley S. D., Holden M. T., Parkhill J., Dorrell N., Wren B. W. (2007). Re-annotation and re-analysis of the Campylobacter jejuni NCTC11168 genome sequence. BMC Genomics 8:162. 10.1186/1471-2164-8-162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyard-Nicodème M., Keita A., Quesne S., Amelot M., Poezevara T., Le Berre B., et al. (2016). Efficacy of feed additives against Campylobacter in live broilers during the entire rearing period. Poult. Sci. 95, 298–305. 10.3382/ps/pev303. [DOI] [PubMed] [Google Scholar]
- Guyard-Nicodème M., Rivoal K., Houard E., Rose V., Quesne S., Mourand G., et al. (2015). Prevalence and characterization of Campylobacter jejuni from chicken meat sold in French retail outlets. Int. J. Food Microbiol. 203, 8–14. 10.1016/j.ijfoodmicro.2015.02.013 [DOI] [PubMed] [Google Scholar]
- Haddad N., Maillart G., Garénaux A., Jugiau F., Federighi M., Cappelier J.-M. (2010a). Adhesion ability of Campylobacter jejuni to Ht-29 cells increases with the augmentation of oxidant agent concentration. Curr. Microbiol. 61, 500–505. 10.1007/s00284-010-9644-z [DOI] [PubMed] [Google Scholar]
- Haddad N., Marce C., Magras C., Cappelier J. M. (2010b). An overview of methods used to clarify pathogenesis mechanisms of Campylobacter jejuni. J. Food Prot. 73, 786–802. [DOI] [PubMed] [Google Scholar]
- Hald B., Sommer H. M., Skovgård H. (2007). Use of fly screens to reduce Campylobacter spp. introduction in broiler houses. Emerg. Infect. Dis. 13, 1951. 10.3201/eid1312.070488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerl J. A., Jäckel C., Alter T., Janzcyk P., Stingl K., Knüver M. T., et al. (2014). Reduction of Campylobacter jejuni in broiler chicken by successive application of group II and group III phages. PLoS ONE 9:e114785. 10.1371/journal.pone.0114785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson I., Ederoth M., Andersson L., Vågsholm I., Olsson Engvall E. (2005). Transmission of Campylobacter spp. to chickens during transport to slaughter. J. Appl. Microbiol. 99, 1149–1157. 10.1111/j.1365-2672.2005.02689.x [DOI] [PubMed] [Google Scholar]
- Hansson I., Pudas N., Harbom B., Engvall E. O. (2010). Within-flock variations of Campylobacter loads in caeca and on carcasses from broilers. Int. J. Food Microbiol. 141, 51–55. 10.1016/j.ijfoodmicro.2010.04.019 [DOI] [PubMed] [Google Scholar]
- Herbel S. R., Vahjen W., Wieler L. H., Guenther S. (2013). Timely approaches to identify probiotic species of the genus Lactobacillus. Gut Pathog. 5:27. 10.1186/1757-4749-5-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman L., Heyndrickx M., Grijspeerdt K., Vandekerchove D., Rollier I., De Zutter L. (2003). Routes for Campylobacter contamination of poultry meat: epidemiological study from hatchery to slaughterhouse. Epidemiol. Infect. 131, 1169–1180. 10.1017/S0950268803001183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans D., Pasmans F., Messens W., Martel A., Van Immerseel F., Rasschaert G., et al. (2012). Poultry as a host for the zoonotic pathogen Campylobacter jejuni. Vector-Borne Zoonotic Dis. 12, 89–98. 10.1089/vbz.2011.0676 [DOI] [PubMed] [Google Scholar]
- Hermans D., Van Deun K., Messens W., Martel A., Van Immerseel F., Haesebrouck F., et al. (2011). Campylobacter control in poultry by current intervention measures ineffective: urgent need for intensified fundamental research. Vet. Microbiol. 152, 219–228. 10.1016/j.vetmic.2011.03.010 [DOI] [PubMed] [Google Scholar]
- Hiett K. L., Stintzi A., Andacht T. M., Kuntz R. L., Seal B. S. (2008). Genomic differences between Campylobacter jejuni isolates identify surface membrane and flagellar function gene products potentially important for colonizing the chicken intestine. Funct. Integr. Genomics 8, 407–420. 10.1007/s10142-008-0087-6 [DOI] [PubMed] [Google Scholar]
- Hill C., Guarner F., Reid G., Gibson G. R., Merenstein D. J., Pot B., et al. (2014). Expert consensus document: the international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 11, 506–514. 10.1038/nrgastro.2014.66 [DOI] [PubMed] [Google Scholar]
- Hue O., Allain V., Laisney M. J., Le Bouquin S., Lalande F., Petetin I., et al. (2011). Campylobacter contamination of broiler caeca and carcasses at the slaughterhouse and correlation with Salmonella contamination. Food Microbiol. 28, 862–868. 10.1016/j.fm.2010.11.003 [DOI] [PubMed] [Google Scholar]
- Hue O., Le Bouquin S., Laisney M. J., Allain V., Lalande F., Petetin I., et al. (2010). Prevalence of and risk factors for Campylobacter spp. contamination of broiler chicken carcasses at the slaughterhouse. Food Microbiol. 27, 992–999. 10.1016/j.fm.2010.06.004 [DOI] [PubMed] [Google Scholar]
- Hugas M., Tsigarida E., Robinson T., Calistri P. (2009). The EFSA scientific panel on biological hazards first mandate: may 2003–may 2006. insight into foodborne zoonoses. Trends Food Sci. Technol. 20, 188–193. 10.1016/j.tifs.2009.03.001 [DOI] [Google Scholar]
- Humphrey S., Chaloner G., Kemmett K., Davidson N., Williams N., Kipar A., et al. (2014). Campylobacter jejuni is not merely a commensal in commercial broiler chickens and affects bird welfare. mBio 5:e01364-14. 10.1128/mBio.01364-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey S., Lacharme-Lora L., Chaloner G., Gibbs K., Humphrey T., Williams N., et al. (2015). Heterogeneity in the infection biology of Campylobacter jejuni isolates in three infection models reveals an invasive and virulent phenotype in a st21 isolate from poultry. PLoS ONE 10:e0141182. 10.1371/journal.pone.0141182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huneau-Salaün A., Denis M., Balaine L., Salvat G. (2007). Risk factors for Campylobacter spp. colonization in French free-range broiler-chicken flocks at the end of the indoor rearing period. Prev. Vet. Med. 80, 34–48. 10.1016/j.prevetmed.2007.02.001 [DOI] [PubMed] [Google Scholar]
- Jacobs-Reitsma W. F., Van de Giessen A. W., Bolder N. M., Mulder R. W. (1995). Epidemiology of Campylobacter spp. at two Dutch broiler farms. Epidemiol. Infect. 114, 413–421. 10.1017/S0950268800052122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janež N., Loc-Carrillo C. (2013). Use of phages to control Campylobacter spp. J. Microbiol. Methods 95, 68–75. 10.1016/j.mimet.2013.06.024 [DOI] [PubMed] [Google Scholar]
- Jin L. Z., Ho Y. W., Abdullah N., Jalaludin S. (1997). Probiotics in poultry: modes of action. World's Poultry Sci. J. 53, 351–368. 10.1079/WPS19970028 [DOI] [Google Scholar]
- Jin L. Z., Ho Y. W., Abdullah N., Jalaludin S. (1998). Growth performance, intestinal microbial populations, and serum cholesterol of broilers fed diets containing Lactobacillus cultures. Poult. Sci. 77, 1259–1265. 10.1093/ps/77.9.1259 [DOI] [PubMed] [Google Scholar]
- Johnson-Henry K. C., Mitchell D. J., Avitzur Y., Galindo-Mata E., Jones N. L., Sherman P. M. (2004). Probiotics reduce bacterial colonization and gastric inflammation in H. pylori-infected mice. Dig. Dis. Sci. 49, 1095–1102. 10.1023/B:DDAS.0000037794.02040.c2 [DOI] [PubMed] [Google Scholar]
- Józefiak D., Rutkowski A., Martin S. (2004). Carbohydrate fermentation in the avian ceca: a review. Anim. Feed Sci. Technol. 113, 1–15. 10.1016/j.anifeedsci.2003.09.007 [DOI] [Google Scholar]
- Kabir S. M. (2009). The role of probiotics in the poultry industry. Int. J. Mol. Sci. 10, 3531–3546. 10.3390/ijms10083531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi T., Nomura K., Hirayama Y., Kitagawa T. (1987). Establishment and characterization of a chicken hepatocellular carcinoma cell line, LMH. Cancer Res. 47, 4460–4464. [PubMed] [Google Scholar]
- Keener K., Bashor M., Curtis P., Sheldon B., Kathariou S. (2004). Comprehensive review of Campylobacter and poultry processing. Compr. Rev. Food Sci. Food Saf. 3, 105–116. 10.1111/j.1541-4337.2004.tb00060.x [DOI] [PubMed] [Google Scholar]
- Kergourlay G., Messaoudi S., Dousset X., Prévost H. (2012). Genome sequence of Lactobacillus salivarius SMXD51, a potential probiotic strain isolated from chicken cecum, showing anti-campylobacter activity. J. Bacteriol. 194, 3008–3009. 10.1128/JB.00344-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoramnia A., Abdullah N., Liew S. L., Sieo C. C., Ramasamy K., Ho Y. W. (2011). Enhancement of viability of a probiotic Lactobacillus strain for poultry during freeze-drying and storage using the response surface methodology. Anim. Sci. J. 82, 127–135. 10.1111/j.1740-0929.2010.00804.x [DOI] [PubMed] [Google Scholar]
- Konkel M. E., Christensen J. E., Dhillon A. S., Lane A. B., Hare-Sanford R., Schaberg D. M., et al. (2007). Campylobacter jejuni strains compete for colonization in broiler chicks. Appl. Environ. Microbiol. 73, 2297–2305. 10.1128/AEM.02193-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korver D. (2012). Implications of changing immune function through nutrition in poultry. Anim. Feed Sci. Technol. 173, 54–64. 10.1016/j.anifeedsci.2011.12.019 [DOI] [Google Scholar]
- Larson C. L., Shah D. H., Dhillon A. S., Call D. R., Ahn S., Haldorson G. J., et al. (2008). Campylobacter jejuni invade chicken LMH cells inefficiently and stimulate differential expression of the chicken CXCLi1 and CXCLi2 cytokines. Microbiology 154, 3835–3847. 10.1099/mic.0.2008/021279-0 [DOI] [PubMed] [Google Scholar]
- Lin J. (2009). Novel Approaches for Campylobacter control in poultry. Foodborne Pathog. Dis. 6, 755–765. 10.1089/fpd.2008.0247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Line J. E., Svetoch E. A., Eruslanov B. V., Perelygin V. V., Mitsevich E. V., Mitsevich I. P., et al. (2008). Isolation and purification of enterocin E-760 with broad antimicrobial activity against gram-positive and gram-negative bacteria. Antimicrob. Agents Chem. 52, 1094–1100. 10.1128/AAC.01569-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loc-Carrillo C., Atterbury R. J., El-Shibiny A., Connerton P. L., Dillon E., Scott A., et al. (2005). Bacteriophage therapy to reduce Campylobacter jejuni colonization of broiler chickens. Appl. Environ. Microbiol. 71, 6554–6563. 10.1128/AEM.71.11.6554-6563.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohans C. T., van Belkum M. J., Cochrane S. A., Huang Z., Sit C. S., McMullen L. M., et al. (2014). Biochemical, structural, and genetic characterization of tridecaptin A1, an antagonist of Campylobacter jejuni. ChemBioChem 15, 243–249. 10.1002/cbic.201300595 [DOI] [PubMed] [Google Scholar]
- Lohans C. T., van Belkum M. J., Li J., Vederas J. C. (2015). Characterization of bacterial antimicrobial peptides active against Campylobacter jejuni. Can. J. Chem. 93, 381–388. 10.1139/cjc-2014-0411 [DOI] [Google Scholar]
- Macé S., Haddad N., Zagorec M., Tresse O. (2015). Influence of measurement and control of microaerobic gaseous atmospheres in methods for Campylobacter growth studies. Food Microbiol. 52, 169–176. 10.1016/j.fm.2015.07.014 [DOI] [PubMed] [Google Scholar]
- MacDonald E., White R., Mexia R., Bruun T., Kapperud G., Lange H., et al. (2015). Risk factors for sporadic domestically acquired Campylobacter infections in Norway 2010–2011: A national prospective case-control study. PLoS ONE 10:e0139636. 10.1371/journal.pone.0139636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciňáková M., Simonová M., Lauková A. (2004). Probiotic properties of Enterococcus faecium EF9296 strain isolated from silage. Acta Veterinaria Brno 73, 513–519. 10.2754/avb200473040513 [DOI] [Google Scholar]
- Marianelli C., Cifani N., Pasquali P. (2010). Evaluation of antimicrobial activity of probiotic bacteria against Salmonella enterica subsp. enterica serovar typhimurium 1344 in a common medium under different environmental conditions. Res. Microbiol. 161, 673–680. 10.1016/j.resmic.2010.06.007 [DOI] [PubMed] [Google Scholar]
- Menconi A., Kallapura G., Latorre J. D., Morgan M. J., Pumford N. R., Hargis B. M., et al. (2014). Identification and characterization of lactic acid bacteria in a commercial probiotic culture. Biosci. Microbiota Food Health 33, 25–30. 10.12938/bmfh.33.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messaoudi S., Kergourlay G., Dalgalarrondo M., Choiset Y., Ferchichi M., Prévost H., et al. (2012a). Purification and characterization of a new bacteriocin active against Campylobacter produced by Lactobacillus salivarius SMXD51. Food Microbiol. 32, 129–134. 10.1016/j.fm.2012.05.002 [DOI] [PubMed] [Google Scholar]
- Messaoudi S., Kergourlay G., Rossero A., Ferchichi M., Prévost H., Drider D., et al. (2011). Identification of lactobacilli residing in chicken ceca with antagonism against Campylobacter. Int. Microbiol. 14, 103–110. 10.2436/20.1501.01.140 [DOI] [PubMed] [Google Scholar]
- Messaoudi S., Madi A., Prévost H., Feuilloley M., Manai M., Dousset X., et al. (2012b). In vitro evaluation of the probiotic potential of Lactobacillus salivarius SMXD51. Anaerobe 18, 584–589. 10.1016/j.anaerobe.2012.10.004 [DOI] [PubMed] [Google Scholar]
- Messaoudi S., Manai M., Kergourlay G., Prévost H., Connil N., Chobert J. M., et al. (2013). Lactobacillus salivarius: Bacteriocin and probiotic activity. Food Microbiol. 36, 296–304. 10.1016/j.fm.2013.05.010 [DOI] [PubMed] [Google Scholar]
- Messens W., Hartnett E., Gellynck X., Viaene J., Halet D., Herman L., et al. (2007). Quantitative risk assessment of human campylobacteriosis through the consumption of chicken meat in Belgium, in XVIII European Symposium on the Quality of Poultry Meat and The XII European Symposium on the Quality of Eggs and Egg products (Prague: ). [Google Scholar]
- Meunier M., Chemaly M., Dory D. (2016a). DNA vaccination of poultry: the current status in 2015. Vaccine 34, 202–211. 10.1016/j.vaccine.2015.11.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier M., Guyard-Nicodème M., Dory D., Chemaly M. (2016b). Control strategies against Campylobacter at the poultry production level: biosecurity measures, feed additives and vaccination. J. Appl. Microbiol. 120, 1139–1173. 10.1111/jam.12986 [DOI] [PubMed] [Google Scholar]
- Mohan B., Kadirvel R., Natarajan A., Bhaskaran M. (1996). Effect of probiotic supplementation on growth, nitrogen utilisation and serum cholesterol in broilers. Br. Poult. Sci. 37, 395–401. 10.1080/00071669608417870 [DOI] [PubMed] [Google Scholar]
- Mohan V. (2015). The role of probiotics in the inhibition of Campylobacter jejuni colonization and virulence attenuation. Eur. J. Clin. Microbiol. Infect. Dis 34, 1503–1513. 10.1007/s10096-015-2392-z [DOI] [PubMed] [Google Scholar]
- Monk A. B., Rees C. D., Barrow P., Hagens S., Harper D. R. (2010). Bacteriophage applications: where are we now? Lett. Appl. Microbiol. 51, 363–369. 10.1111/j.1472-765X.2010.02916.x [DOI] [PubMed] [Google Scholar]
- Moore J. E., Corcoran D., Dooley J. S., Fanning S., Lucey B., Matsuda M., et al. (2005). Campylobacter. Vet. Res. 36, 351–382. 10.1051/vetres:2005012 [DOI] [PubMed] [Google Scholar]
- Morishita T. Y., Aye P. P., Harr B. S., Cobb C. W., Clifford J. R. (1997). Evaluation of an avian-specific probiotic to reduce the colonization and shedding of Campylobacter jejuni in broilers. Avian Dis. 41, 850–855. 10.2307/1592338 [DOI] [PubMed] [Google Scholar]
- Mor-Mur M., Yuste J. (2010). Emerging bacterial pathogens in meat and poultry: an overview. Food Bioprocess Technol. 3, 24–35. 10.1007/s11947-009-0189-8 [DOI] [Google Scholar]
- Mundi A., Delcenserie V., Amiri-Jami M., Moorhead S., Griffiths M. W. (2013). Cell-free preparations of Lactobacillus acidophilus strain La-5 and Bifidobacterium longum strain NCC2705 affect virulence gene expression in Campylobacter jejuni. J. Food Prot. 76, 1740–1746. 10.4315/0362-028X.JFP-13-084 [DOI] [PubMed] [Google Scholar]
- Nadeau É., Messier S., Quessy S. (2003). Comparison of Campylobacter isolates from poultry and humans: association between in vitro virulence properties, biotypes, and pulsed-field gel electrophoresis clusters. Appl. Environ. Microbiol. 69, 6316–6320. 10.1128/AEM.69.10.6316-6320.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauta M., Johannessen G., Adame L. L., Williams N., Rosenquist H. (2016). The effect of reducing numbers of Campylobacter in broiler intestines on human health risk. Microb. Risk Anal. I 10.1016/j.mran.2016.02.001 [DOI] [Google Scholar]
- Neal-McKinney J. M., Lu X., Duong T., Larson C. L., Call D. R., Shah D. H., et al. (2012). Production of organic acids by probiotic lactobacilli can be used to reduce pathogen load in poultry. PLoS ONE 7:e43928. 10.1371/journal.pone.0043928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal-McKinney J. M., Samuelson D. R., Eucker T. P., Nissen M. S., Crespo R., Konkel M. E. (2014). Reducing Campylobacter jejuni colonization of poultry via vaccination. PLoS ONE 9:e114254. 10.1371/journal.pone.0114254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill S. D., Campbell J. A., Greene J. A. (1984). Campylobacter species in broiler chickens. Avian Pathol. 13, 777–785. 10.1080/03079458408418574 [DOI] [PubMed] [Google Scholar]
- Netherwood T., Gilbert H. J., Parker D. S., O'Donnell A. G. (1999). Probiotics shown to change bacterial community structure in the avian gastrointestinal tract. Appl. Environ. Microbiol. 65, 5134–5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell D. G., Elvers K. T., Dopfer D., Hansson I., Jones P., James S., et al. (2011). Biosecurity-based interventions and strategies to reduce Campylobacter spp. on poultry farms. Appl. Environ. Microbiol. 77, 8605–8614. 10.1128/AEM.01090-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell D. G., McBride H., Saunders F., Dehele Y., Pearson A. D. (1985). The virulence of clinical and environmental isolates of Campylobacter jejuni. J. Hygiene 94, 45–54. 10.1017/S0022172400061118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen E. M., Fussing V., Engberg J., Nielsen N. L., Neimann J. (2006). Most Campylobacter subtypes from sporadic infections can be found in retail poultry products and food animals. Epidemiol. Infect. 134, 758–767. 10.1017/S0950268805005509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama K., Nakazato A., Ueno S., Seto Y., Kakuda T., Takai S., et al. (2015). Cell surface-associated aggregation-promoting factor from Lactobacillus gasseri SBT2055 facilitates host colonization and competitive exclusion of Campylobacter jejuni. Mol. Microbiol. 98, 712–726. 10.1111/mmi.13153 [DOI] [PubMed] [Google Scholar]
- Nishiyama K., Seto Y., Yoshioka K., Kakuda T., Takai S., Yamamoto Y., et al. (2014). Lactobacillus gasseri SBT2055 reduces infection by and colonization of Campylobacter jejuni. PLoS ONE 9:e108827. 10.1371/journal.pone.0108827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurmi E., Rantala M. (1973). New aspects of Salmonella infection in broiler production. Nature 241, 210–211. 10.1038/241210a0 [DOI] [PubMed] [Google Scholar]
- Oelschlaeger T. A. (2010). Mechanisms of probiotic actions–a review. Int. J. Med. Microbiol. 300, 57–62. 10.1016/j.ijmm.2009.08.005 [DOI] [PubMed] [Google Scholar]
- Ogawa M., Shimizu K., Nomoto K., Tanaka R., Hamabata T., Yamasaki S., et al. (2001). Inhibition of in vitro growth of Shiga toxin-producing Escherichia coli O157: H7 by probiotic Lactobacillus strains due to production of lactic acid. Int. J. Food Microbiol. 68, 135–140. 10.1016/S0168-1605(01)00465-2 [DOI] [PubMed] [Google Scholar]
- Olson C. K., Ethelberg S., Pelt W. V., Tauxe R. V., Nachamkin I., Szymanski C., et al. (2008). Epidemiology of Campylobacter jejuni infections in industrialized nations, in Campylobacter, eds Nachamkin I., Szymanski C. M., Blaser M. J. (Washington, DC: ASM Press; ), 163–189. 10.1128/9781555815554.ch9 [DOI] [Google Scholar]
- Otutumi L. K., De Moraes Garcia E. R., Góis M. B., Loddi M. M. (2012). Variations on the efficacy of probiotics in poultry, in Probiotic in Animals, ed Rigobelo E. C. (Rijeka: InTech; ), 203–230. [Google Scholar]
- Papadimitriou K., Zoumpopoulou G., Foligné B., Alexandraki V., Kazou M., Pot B., et al. (2015). Discovering probiotic microorganisms: in vitro, in vivo, genetic and omics approaches. Front. Microbiol. 6:58. 10.3389/fmicb.2015.00058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhill J., Wren B. W., Mungall K., Ketley J. M., Churcher C., Basham D., et al. (2000). The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature 403, 665–668. 10.1038/35001088 [DOI] [PubMed] [Google Scholar]
- Pascual M., Hugas M., Badiola J. I., Monfort J. M., Garriga M. (1999). Lactobacillus salivarius CTC2197 prevents Salmonella enteritidis colonization in chickens. Appl. Environ. Microbiol. 65, 4981–4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson J. A., Burkholder K. M. (2003). Application of prebiotics and probiotics in poultry production. Poult. Sci. 82, 627–631. 10.1093/ps/82.4.627 [DOI] [PubMed] [Google Scholar]
- Pauwels J., Taminiau B., Janssens G., De Beenhouwer M., Delhalle L., Daube G., et al. (2015). Cecal drop reflects the chickens' cecal microbiome, fecal drop does not. J. Microbiol. Methods 117, 164–170. 10.1016/j.mimet.2015.08.006 [DOI] [PubMed] [Google Scholar]
- Pearson B. M., Gaskin D. J., Segers R. P., Wells J. M., Nuijten P. J., van Vliet A. H. (2007). The complete genome sequence of Campylobacter jejuni strain 81116 (NCTC11828). J. Bacteriol. 189, 8402–8403. 10.1128/JB.01404-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pielsticker C., Glünder G., Rautenschlein S. (2012). Colonization properties of Campylobacter jejuni in chickens. Eur. J. Microbiol. Immunol. 2, 61–65. 10.1556/EuJMI.2.2012.1.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poly F., Read T., Tribble D. R., Baqar S., Lorenzo M., Guerry P. (2007). Genome sequence of a clinical isolate of Campylobacter jejuni from Thailand. Infect. Immun. 75, 3425–3433. 10.1128/IAI.00050-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi F., Chen P., Caufield P. W. (2001). The group I strain of Streptococcus mutans, UA140, produces both the lantibiotic mutacin I and a nonlantibiotic bacteriocin, mutacin IV. Appl. Environ. Microbiol. 67, 15–21. 10.1128/AEM.67.1.15-21.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasschaert G., Houf K., Van Hende J., De Zutter L. (2006). Campylobacter contamination during poultry slaughter in Belgium. J. Food Prot. 69, 27–33. [DOI] [PubMed] [Google Scholar]
- Refrégier-Petton J., Rose N., Denis M., Salvat G. (2001). Risk factors for Campylobacter spp. contamination in French broiler-chicken flocks at the end of the rearing period. Prev. Vet. Med. 50, 89–100. 10.1016/S0167-5877(01)00220-3 [DOI] [PubMed] [Google Scholar]
- Reich F., Atanassova V., Haunhorst E., Klein G. (2008). The effects of Campylobacter numbers in caeca on the contamination of broiler carcasses with Campylobacter. Int. J. Food Microbiol. 127, 116–120. 10.1016/j.ijfoodmicro.2008.06.018 [DOI] [PubMed] [Google Scholar]
- Reid G. (2005). The importance of guidelines in the development and application of probiotics. Curr. Pharm. Des. 11, 11–16. 10.2174/1381612053382395 [DOI] [PubMed] [Google Scholar]
- Rivoal K., Ragimbeau C., Salvat G., Colin P., Ermel G. (2005). Genomic diversity of Campylobacter coli and Campylobacter jejuni isolates recovered from free-range broiler farms and comparison with isolates of various origins. Appl. Environ. Microbiol. 71, 6216–6227. 10.1128/AEM.71.10.6216-6227.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberfroid M. (1998). Prebiotics and synbiotics: concepts and nutritional properties. Br. J. Nutr. 80, S197–202. [PubMed] [Google Scholar]
- Robyn J., Rasschaert G., Hermans D., Pasmans F., Heyndrickx M. (2013). In vivo broiler experiments to assess anti-Campylobacter jejuni activity of a live Enterococcus faecalis strain. Poult. Sci. 92, 265–271. 10.3382/ps.2012-02712 [DOI] [PubMed] [Google Scholar]
- Robyn J., Rasschaert G., Messens W., Pasmans F., Heyndrickx M. (2012). Screening for lactic acid bacteria capable of inhibiting Campylobacter jejuni in in vitro simulations of the broiler chicken caecal environment. Benef. Microbes 3, 299–308. 10.3920/BM2012.0021 [DOI] [PubMed] [Google Scholar]
- Robyn J., Rasschaert G., Pasmans F., Heyndrickx M. (2015). Thermotolerant Campylobacter during broiler rearing: risk factors and intervention. Compr. Rev. Food Sci. Food Saf. 14, 81–105. 10.1111/1541-4337.12124 [DOI] [PubMed] [Google Scholar]
- Rodrigues R. C., Pocheron A. L., Hernould M., Haddad N., Tresse O., Cappelier J. M. (2015). Description of Campylobacter jejuni Bf, an atypical aero-tolerant strain. Gut Pathog. 7, 1. 10.1186/s13099-015-0077-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Barrios P., Hempen M., Messens W., Stella P., Hugas M. (2013). Quantitative microbiological risk assessment (QMRA) of food-borne zoonoses at the European level. Food Control 29, 343–349. 10.1016/j.foodcont.2012.05.043 [DOI] [Google Scholar]
- Rosenquist H., Nielsen N. L., Sommer H. M., Nørrung B., Christensen B. B. (2003). Quantitative risk assessment of human campylobacteriosis associated with thermophilic Campylobacter species in chickens. Int. J. Food Microbiol. 83, 87–103. 10.1016/S0168-1605(02)00317-3 [DOI] [PubMed] [Google Scholar]
- Rosenquist H., Sommer H. M., Nielsen N. L., Christensen B. B. (2006). The effect of slaughter operations on the contamination of chicken carcasses with thermotolerant Campylobacter. Int. J. Food Microbiol. 108, 226–232. 10.1016/j.ijfoodmicro.2005.12.007 [DOI] [PubMed] [Google Scholar]
- Saleha A. (2002). Isolation and characterization of Campylobacter jejuni from broiler chickens in Malaysia. Int. J. Poult. Sci. 1, 94–97. 10.3923/ijps.2002.94.97 [DOI] [Google Scholar]
- Sánchez M. X., Fluckey W. M., Brashears M. M., McKee S. R. (2002). Microbial profile and antibiotic susceptibility of Campylobacter spp. and Salmonella spp. in broilers processed in air-chilled and immersion-chilled environments. J. Food Prot. 65, 948–956. [DOI] [PubMed] [Google Scholar]
- Sanders M. E. (2011). Impact of probiotics on colonizing microbiota of the gut. J. Clin. Gastroenterol. 45, S115–S119. 10.1097/MCG.0b013e318227414a [DOI] [PubMed] [Google Scholar]
- Santini C., Baffoni L., Gaggia F., Granata M., Gasbarri R., Di Gioia D., et al. (2010). Characterization of probiotic strains: an application as feed additives in poultry against Campylobacter jejuni. Int. J. Food Microbiol. 141, S98–S108. 10.1016/j.ijfoodmicro.2010.03.039 [DOI] [PubMed] [Google Scholar]
- Sasaki Y., Maruyama N., Zou B., Haruna M., Kusukawa M., Murakami M., et al. (2013). Campylobacter cross-contamination of chicken products at an abattoir. Zoonoses Public Health 60, 134–140. 10.1111/j.1863-2378.2012.01509.x [DOI] [PubMed] [Google Scholar]
- Scallan E., Hoekstra R. M., Angulo F. J., Tauxe R. V., Widdowson M. A., Roy S. L., et al. (2011). Foodborne illness acquired in the United States-major pathogens. Emerg. Infect. Dis. 17, 7–15. 10.3201/eid1701.p11101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharff R. L. (2015). State estimates for the annual cost of foodborne illness. J. Food Prot. 78, 1064–1071. 10.4315/0362-028X.zJFP-14-505 [DOI] [PubMed] [Google Scholar]
- Schoeni J. L., Wong A. (1994). Inhibition of Campylobacter jejuni colonization in chicks by defined competitive exclusion bacteria. Appl. Environ. Microbiol. 60, 1191–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz S., Chaslus-Dancla E. (2001). Use of antimicrobials in veterinary medicine and mechanisms of resistance. Vet. Res. 32, 201–225. 10.1051/vetres:2001120 [DOI] [PubMed] [Google Scholar]
- Seal B. S., Hiett K. L., Kuntz R. L., Woolsey R., Schegg K. M., Ard M., et al. (2007). Proteomic analyses of a robust versus a poor chicken gastrointestinal colonizing isolate of Campylobacter jejuni. J. Proteome Res. 6, 4582–4591. 10.1021/pr070356a [DOI] [PubMed] [Google Scholar]
- Silva J., Leite D., Fernandes M., Mena C., Gibbs P. A., Teixeira P. (2011). Campylobacter spp. as a foodborne pathogen: a review. Front. Microbiol 2:200 10.3389/fmicb.2011.00200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siriken B., Bayram I., Önol A. G. (2003). Effects of probiotics: alone and in a mixture of Biosacc® plus zinc bacitracin on the caecal microflora of Japanese quail. Res. Vet. Sci. 75, 9–14. 10.1016/S0034-5288(03)00036-5 [DOI] [PubMed] [Google Scholar]
- Smith C. K., Kaiser P., Rothwell L., Humphrey T., Barrow P. A., Jones M. A. (2005). Campylobacter jejuni-induced cytokine responses in avian cells. Infect. Immun. 73, 2094–2100. 10.1128/IAI.73.4.2094-2100.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits H. H., Engering A., Van Der Kleij D., De Jong E. C., Schipper K., Van Capel T. M., et al. (2005). Selective probiotic bacteria induce IL-10–producing regulatory T cells in vitro by modulating dendritic cell function through dendritic cell–specific intercellular adhesion molecule 3–grabbing nonintegrin. J. Allergy Clin. Immunol. 115, 1260–1267. 10.1016/j.jaci.2005.03.036 [DOI] [PubMed] [Google Scholar]
- Sorokulova I. B., Kirik D. L., Pinchuk I. V. (1997). Probiotics against Campylobacter pathogens. J. Travel Med. 4, 167–170. 10.1111/j.1708-8305.1997.tb00813.x [DOI] [PubMed] [Google Scholar]
- Spivey M. A., Dunn-Horrocks S. L., Duong T. (2014). Epithelial cell adhesion and gastrointestinal colonization of Lactobacillus in poultry. Poult. Sci. 93, 2910–2919. 10.3382/ps.2014-04076 [DOI] [PubMed] [Google Scholar]
- Stavric S. (1992). Defined cultures and prospects. Int. J. Food Microbiol. 15, 245–263. 10.1016/0168-1605(92)90056-9 [DOI] [PubMed] [Google Scholar]
- Stern N. J., Robach M. C. (2003). Enumeration of Campylobacter spp. in broiler feces and in corresponding processed carcasses. J. Food Prot. 66, 1557–1563. [DOI] [PubMed] [Google Scholar]
- Svetoch E. A., Eruslanov B. V., Levchuk V. P., Perelygin V. V., Mitsevich E. V., Mitsevich I. P., et al. (2011). Isolation of Lactobacillus salivarius 1077 (NRRL B-50053) and characterization of its bacteriocin and spectra of antimicrobial activity. Appl. Environ. Microbiol. 77, 2749–2754. 10.1128/AEM.02481-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svetoch E. A., Stern N. J., Eruslanov B. V., Kovalev Y. N., Volodina L. I., Perelygin V. V., et al. (2005). Isolation of Bacillus circulans and Paenibacillus polymyxa strains inhibitory to Campylobacter jejuni and characterization of associated bacteriocins. J. Food Prot. 68, 11–17. [DOI] [PubMed] [Google Scholar]
- Svetoch E. A., Stern N. J. (2010). Bacteriocins to control Campylobacter spp. in poultry—a review. Poult. Sci. 89, 1763–1768. 10.3382/ps.2010-00659 [DOI] [PubMed] [Google Scholar]
- Takahashi R., Shahada F., Chuma T., Okamoto K. (2006). Analysis of Campylobacter spp. contamination in broilers from the farm to the final meat cuts by using restriction fragment length polymorphism of the polymerase chain reaction products. Int. J. Food Microbiol. 110, 240–245. 10.1016/j.ijfoodmicro.2006.04.043 [DOI] [PubMed] [Google Scholar]
- Tareb R., Bernardeau M., Gueguen M., Vernoux J. P. (2013). In vitro characterization of aggregation and adhesion properties of viable and heat-killed forms of two probiotic Lactobacillus strains and interaction with foodborne zoonotic bacteria, especially Campylobacter jejuni. J. Med. Microbiol. 62, 637–649. 10.1099/jmm.0.049965-0 [DOI] [PubMed] [Google Scholar]
- Tellez G., Petrone V. M., Escorcia M., Morishita T. Y., Cobb C. W., Villaseñor L. (2001). Evaluation of avian-specific probiotic and Salmonella Enteritidis-, Salmonella Typhimurium-, and Salmonella Heidelberg-specific antibodies on cecal colonization and organ invasion of Salmonella Enteritidis in broilers. J. Food Prot. 64, 287–291. [DOI] [PubMed] [Google Scholar]
- Tellez G., Rodríguez-Fragoso L., Kuttappan V., Kallapura G., Velasco X., Menconi A., et al. (2013). Probiotics for human and poultry use in the control of gastrointestinal disease: a review of real-world experiences. Altern. Integr. Med. 2:4 10.4172/2327-5162.1000118 [DOI] [Google Scholar]
- Tsai C. C., Hsih H. Y., Chiu H. H., Lai Y. Y., Liu J. H., Yu B., et al. (2005). Antagonistic activity against Salmonella infection in vitro and in vivo for two Lactobacillus strains from swine and poultry. Int. J. Food Microbiol. 102, 185–194. 10.1016/j.ijfoodmicro.2004.12.014 [DOI] [PubMed] [Google Scholar]
- Tynkkynen S., Satokari R., Saarela M., Mattila-Sandholm T., Saxelin M. (1999). Comparison of ribotyping, randomly amplified polymorphic DNA analysis, and pulsed-field gel electrophoresis in typing of Lactobacillus rhamnosus and L. casei strains. Appl. Environ. Microbiol. 65, 3908–3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bokhorst-Van de Veen H., Lee I. C., Marco M. L., Wels M., Bron P. A., Kleerebezem M. (2012). Modulation of Lactobacillus plantarum gastrointestinal robustness by fermentation conditions enables identification of bacterial robustness markers. PLoS ONE 7:e39053. 10.1371/journal.pone.0039053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Giessen A. W., Tilburg J. J., Ritmeester W. S., Van Der Plas J. (1998). Reduction of Campylobacter infections in broiler flocks by application of hygiene measures. Epidemiol. Infect. 121, 57–66. 10.1017/S0950268898008899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Deun K., Pasmans F., Ducatelle R., Flahou B., Vissenberg K., Martel A., et al. (2008). Colonization strategy of Campylobacter jejuni results in persistent infection of the chicken gut. Vet. Microbiol. 130, 285–297. 10.1016/j.vetmic.2007.11.027 [DOI] [PubMed] [Google Scholar]
- Vitaliti G., Pavone P., Guglielmo F., Spataro G., Falsaperla R. (2014). The immunomodulatory effect of probiotics beyond atopy: an update. J. Asthma 51, 320–332. 10.3109/02770903.2013.862259 [DOI] [PubMed] [Google Scholar]
- Wagner R. D., Johnson S. J., Kurniasih Rubin D. (2009). Probiotic bacteria are antagonistic to Salmonella enterica and Campylobacter jejuni and influence host lymphocyte responses in human microbiota-associated immunodeficient and immunocompetent mice. Mol. Nutr. Food Res. 53, 377–388. 10.1002/mnfr.200800101 [DOI] [PubMed] [Google Scholar]
- Wang G., Zhao Y., Tian F., Jin X., Chen H., Liu X., et al. (2014). Screening of adhesive lactobacilli with antagonistic activity against Campylobacter jejuni. Food Control 44, 49–57. 10.1016/j.foodcont.2014.03.042 [DOI] [Google Scholar]
- Wassenaar T. (2011). Following an imaginary Campylobacter population from farm to fork and beyond: a bacterial perspective. Lett. Appl. Microbiol. 53, 253–263. 10.1111/j.1472-765X.2011.03121.x [DOI] [PubMed] [Google Scholar]
- Westrell T., Ciampa N., Boelaert F., Helwigh B., Korsgaard H., Chriél M., et al. (2009). Zoonotic infections in Europe in 2007: a summary of the EFSA-ECDC annual report. Euro surveill. 14, 785–794. Avaiable online at: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19100 [PubMed] [Google Scholar]
- Willis W. L, Reid, L. (2008). Investigating the effects of dietary probiotic feeding regimens on broiler chicken production and Campylobacter jejuni presence. Poult. Sci. 87, 606–611. 10.3382/ps.2006-00458 [DOI] [PubMed] [Google Scholar]
- Wine E., Chan V. L., Sherman P. M. (2008). Campylobacter jejuni mediated disruption of polarized epithelial monolayers is cell-type specific, time dependent, and correlates with bacterial invasion. Pediatr. Res. 64, 599–604. 10.1203/PDR.0b013e31818702b9 [DOI] [PubMed] [Google Scholar]
- Wine E., Gareau M. G., Johnson-Henry K., Sherman P. M. (2009). Strain-specific probiotic (Lactobacillus helveticus) inhibition of Campylobacter jejuni invasion of human intestinal epithelial cells. FEMS Microbiol. Lett. 300, 146–152. 10.1111/j.1574-6968.2009.01781.x [DOI] [PubMed] [Google Scholar]
- Yadav R., Shukla P. (2015). An overview of advanced technologies for selection of probiotics and their expediency: a review. Crit. Rev. Food Sci. Nutr. 10.1080/10408398.2015.1108957. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Zacharof M.-P. (2015). Strategies for advantageous antimicrobial activity by bacteriocins from lactic acid bacteria: higher yield, enhanced activity and successful application in foods, in Advances in Food Biotechnology, ed Rai V. R. (Chichester, UK: John Wiley and Sons Ltd.), 511–526. [Google Scholar]
- Zheng J., Meng J., Zhao S., Singh R., Song W. (2008). Campylobacter-induced interleukin-8 secretion in polarized human intestinal epithelial cells requires Campylobacter-secreted cytolethal distending toxin-and Toll-like receptor-mediated activation of NF-κB. Infect. Immun. 76, 4498–4508. 10.1128/IAI.01317-07 [DOI] [PMC free article] [PubMed] [Google Scholar]