Abstract
The development and strategic application of effective anticancer therapies have turned out to be one of the most critical approaches of managing human cancers. Nevertheless, drug resistance is the major obstacle for clinical management of these diseases especially ovarian cancer. In the past years, substantial studies have been carried out with the aim of exploring alternative therapeutic approaches to enhance efficacy of current chemotherapeutic regimes and reduce the side effects caused in order to produce significant advantages in overall survival and to improve patients' quality of life. Targeting cancer cell metabolism by the application of AMP-activated protein kinase (AMPK)-activating agents is believed to be one of the most plausible attempts. AMPK activators such as 5-aminoimidazole-4-carboxamide 1-β-d-ribofuranoside, A23187, metformin, and bitter melon extract not only prevent cancer progression and metastasis but can also be applied as a supplement to enhance the efficacy of cisplatin-based chemotherapy in human cancers such as ovarian cancer. However, because of the undesirable outcomes along with the frequent toxic side effects of most pharmaceutical AMPK activators that have been utilized in clinical trials, attentions of current studies have been aimed at the identification of replaceable reagents from nutraceuticals or traditional medicines. However, the underlying molecular mechanisms of many nutraceuticals in anticancer still remain obscure. Therefore, better understanding of the functional characterization and regulatory mechanism of natural AMPK activators would help pharmaceutical development in opening an area to intervene ovarian cancer and other human cancers.
Keywords: AMPK, cell metabolism, ovarian cancer, chemoresistance, nutraceuticals
Introduction: Ovarian Carcinoma at a Glance
Ovarian cancer is the gynecological malignance that evolves from tissues of the ovary. About 90% of all ovarian neoplasms are derived from the coelomic surface epithelium cells of the ovary and categorized as the epithelial ovarian carcinoma. The remaining 10% is either the malignant germ cell tumors developed from the germ cells or the mesenchyme tumors arose from the sex cords and stroma of the ovary [1–6]. Ovarian cancer indeed is very subtle as it is relatively asymptomatic. In contradistinction to other gynecologic disorders, ovarian cancer surprisingly is the most lethal gynecological malignance accounting for more deaths than the sum of both endometrial and cervical carcinomas [7]. The reason for this high mortality is probably due to the lack of specific screening tools and early detection methods for early-stage disease. Ovarian cancer, therefore, has been notoriously renowned as ‘silent killer’ and most patients (∼65%) are poorly diagnosed until late stages (III or IV) while the cancer has already metastasized beyond the confines of the ovary [4,7–12].
Ovarian cancer is found generally in postmenopausal women in 50s and 60s; however, it is comparatively rare in women of reproductive age [13]. Although 70%–80% of patients in the beginning respond well to treatment, but recurrence due to chemotherapeutic resistance always tends to occur in most patients (∼60%) within 5 years, and when this does, it is usually fatal [1,3,4,9,14]. In recent years, multimodality approaches with aggressive cytoreductive surgery and platinum-based chemotherapy, most frequently the combination of paclitaxel and carboplatin, have been applied as the mainstay of regimen in patients with advanced ovarian cancer [1,4,9,14–17]. Nevertheless, conventional therapy is still not very satisfactory since it pays little attention to the aspects of tumor biology [4]. Understanding the biological mechanisms underlying recurrence of ovarian cancer and addressing chemoresistance is, therefore, of the utmost significance for better treatment and outcome of the disease.
Chemoresistance Is the Major Hurdle in Successful Treatment and Prognosis in Ovarian Carcinoma
Ovarian carcinoma has long been a surgically treated malignance [12]. The standard initial management of primary ovarian cancer among most women involves specific surgical staging procedures, optimal surgical debulking with improved patient outcomes typically defined as reduction of residual tumor deposits of <1 cm in size, and eventually followed by administration of six cycles of intravenous platinum-based chemotherapy with carboplatin and paclitaxel [1,12,18].
The survival advantage of cytoreduction, in particular for patients with late-stage disease, has been reported retrospectively in studies since 1934. With reference to a meta-analysis of 53 studies involving 6885 patients with late-stage malignance (Stage III or IV), Bristow and colleagues had demonstrated that each 10% increase in surgical debulking was correlated with a 5.5% improvement in median survival [19]. Therefore, after surgery and subsequent adjuvant chemotherapy in women with ovarian carcinoma, it seems that the duration of survival has somewhat extended. Nonetheless, the overall 5-year survival rate for patients is still as low as 45% at present. The prognosis of this disease is poor because nearly 80% of patients presented at advanced stage have already metastasized. Despite the majority of these patients are responsive to the first-line treatment, most of them acquire resistance to conventional chemotherapeutics and experience more aggressive tumor recurrence at a median of 15 months from diagnosis [18–20]. Even if second-line therapies have recently been suggested to increase survival and quality of life, they are just palliative. Thus, there is a compelling need to further advance our knowledge in ovarian tumor biology and explore novel therapeutic interventions for this disease.
Current Complementary Therapeutic Approaches
In view of the limitations of current cancer therapies like chemotherapy, radiotherapy, and surgery in treating ovarian carcinoma, many alternative therapeutic approaches have been evolved in recent years with the aim of improving prognosis and overall survival rates. Such kinds of alternative therapies sometimes act as a complementary character and therefore are given in addition to the primary treatments. Among multiple complementary treatments, targeted cancer therapies, immunotherapy, and nutraceutical medication are somewhat believed to lessen the side effects and show benefits in a proportion of cancer patients.
Many of the targeted cancer therapies nowadays are pharmaceuticals that halt the growth and metastasis of cancer through blocking or intervening with specific protein molecules involved in tumor growth and progression. By blocking the essential cellular signal transductions critical for tumor growth, targeted cancer therapies either, on one hand, directly cause cancer cell death by inducing apoptosis or, on the other hand, activate the immune system to distinguish and then eliminate the malignant cells. Identification of ‘good targets’, which should be proven to play a key role in malignant cells’ growth and survival, is therefore with the utmost importance for the effectiveness of targeted cancer therapy development.
Once a target has been recognized, targeted cancer therapies can be subsequently developed. In fact, most of the targeted cancer therapies using today are either small-molecule inhibitors or monoclonal antibodies. For the small-molecule inhibitors, they are able to target cancer cells specifically. On the contrary, the monoclonal antibodies usually cannot infiltrate the plasma membrane of intact cells and are hence directed against their targets, which are normally located outside cells or on the surface of the cancer cells. Targeted cancer therapies are more specific than conventional therapies and are less likely to exert side effects to normal cells. Nevertheless, targeted cancer therapies are comparable in higher cost and they usually can only address one target at a time. In cancer treatment, fixing one molecular or cellular target may be effective for some cancer patients; however, it is understood that multiple molecular and cellular abnormalities should actually be involved in many cases, and thus, other patients may need treatments that address more than one target.
Apart from targeted cancer therapies, immunotherapy is regarded as biological therapy, which makes use of the host's immune system to fight against cancer with little side effects. Almost a century ago, Paul Ehrlich hypothesized the theory of immune surveillance that cancer cells are rapidly eradicated by the immune system on a daily basis [21]. At that time, his hypothesis could not be proven due to lack of appropriate models and in vitro systems. Today, it is understood that malignant tumors are immunogenic in certain cancer sites, including ovarian cancer [21]. Accumulating evidence associating with the linkage between antitumor immunity and carcinoma has also been found in ovarian cancer [22–25]. Understanding how the immune system is stimulated and responded in ovarian carcinoma is consequently a prerequisite for scheming clinically meaningful immunologic approaches against this malignance.
The hypothesis of immunotherapy as a potential strategy for the treatment of ovarian cancer is indeed based on the several points of evidence. First of all, many tumor-associated antigens such as HER2/neu [26,27], MUC1 [28], OA3 [29], membrane folate receptor [30], TAG-72 [31], mesothelin [32], NY-ESO-1 [33], and sialyl-Tn [34] are commonly expressed in ovarian cancer and can serve as targets for cellular immune responses. Secondly, the presence of tumor-infiltrating lymphocytes was strongly and positively correlated with patient survival [22]. Thirdly, it has been reported that peptide/MHC complexes that can be recognized by CD8+ T lymphocytes are always expressed in ovarian cancer [21]. Finally and most importantly, the dynamic interaction between immune response of the host and cancer implies that the equilibrium between the two parties can be prone to favor the host immunity, with the ever enhancing arsenals of the immunological nature [21]. Collectively, it has been recommended that immunotherapy can be an innovative and useful complementary therapy for ovarian cancer.
When employing immunotherapy for cancer treatment, the involvement of biological response modifiers (BRMs) is very important. BRMs can alter the interaction between the cancer cells and the body's immune defenses that direct body's immune system to specifically tackle the malignancies. BRMs, therefore, act as the key executioner, which include a wide variety of biological compounds such as antibodies, cytokines, and other biological substances of the immune system. Many BRMs have already been developed as a standard part of medication for certain kinds of carcinomas, whereas some of them are still undergoing clinical trials. Besides being used in addition to the conventional treatments, BRMs can be applied singly or in combination with each other.
Cancer vaccines, on the other hand, are another contemporary immunotherapy presently under intense investigation. They are available for both cancer patients and healthy individuals because two different groups of cancer vaccines have been developed so far, including the therapeutic vaccines for treatment of existing cancers and the prophylactic vaccines for prevention of cancer development. In brief, therapeutic vaccines are usually given to patients after cancer is diagnosed with the aim of terminating the growth of existing tumors, precluding recurrence of cancer, and eliminating cancer cells not fully killed by previous conventional treatments. In contrast, prophylactic vaccines are administrated in healthy people prior to the development of diseases. Such kinds of vaccines are intended to activate the body's immune system to fight against viruses, which may induce cancer. It is hoped that the development of certain kinds of cancers can be prevented by targeting these cancer-inducing viruses.
Early cancer vaccine clinical trials involved mainly patients with melanoma. Therapeutic vaccines are now being investigated in the treatment of numerous carcinomas, including ovarian cancer. Additionally, studies on prophylactic vaccines are also continued in an attempt to prevent cervical and liver cancers worldwide. Besides these, researchers are finding ways to combine cancer vaccines with other BRMs.
Although different modalities of immunotherapy have already been developed and entered the clinic, most of them have demonstrated limited efficacy. The genomic instability plus intrinsic heterogeneity of the tumor, together with immune suppression triggered by both the tumor and its microenvironment, remain the key obstacles to the success of the immunotherapy [35]. Similar to other kinds of cancer treatments, multiple side effects may also arise during immunotherapy. Usually rashes or swellings may occur at the site where the BRMs are given. Several types of BRMs, involving cytokines like interferons and interleukins, may bring along some flu-like symptoms such as fever, chills, nausea, fatigue, vomiting, and loss of appetite. In serious cases, blood pressure of the patients may also be influenced. In view of these negative drawbacks, patients should better consult their physicians and think twice to strike a right balance between the pros and cons of immunotherapy before choosing it in their medication.
Last but not least, nutraceutical medication by either folk medicine or food supplements has been claimed to possess physiological benefits and provide protection against chronic diseases, including cancer [36]. Traditional medicine has a very long history and it involves the use of herbal medicines, animal parts, and minerals. Among these three, herbal medicines are the most commonly used, which include herbs, herbal materials, herbal preparations, and finished herbal products. The application of herbal medicines predates written human history, and a number of the earliest written records from China, Egypt, and Sumeria deal with this topic. Although herbal medicines are generally acknowledged to be safe and effective, they are not always appreciated by national authorities and by international agencies. However, considerable benefits are believed to be possible when the traditional herbal medicines are subject to scientific methods of validation of conventional use and quality control. In fact, many pharmacological classes of drugs also include a natural product prototype. Artimesinin, atropine, digoxin, morphine, quinine, physostigmine, pilocarpine, reserpine, taxol, vincristine, and vinblastine are some of the examples of what medicinal plants have given us in the past. Contemporary medicine is now, therefore, beginning to accept the use of botanicals once they are scientifically validated. Garlic, ginkgo, ginseng, and in particular bitter melon are some instances of botanicals that are gaining more and more popularities in modern cancer medications. Yet, the number of plants that have not been examined for content of biologically active components is vast. It is estimated that only 3%–5% of terrestrial botanicals have been reasonably well studied, and thus, there is great potential on research using medicinal plants as cancer therapy in the near future[37,38].
AMP-activated Protein Kinase in Human Cancer and its Significance in Cancer Metabolism
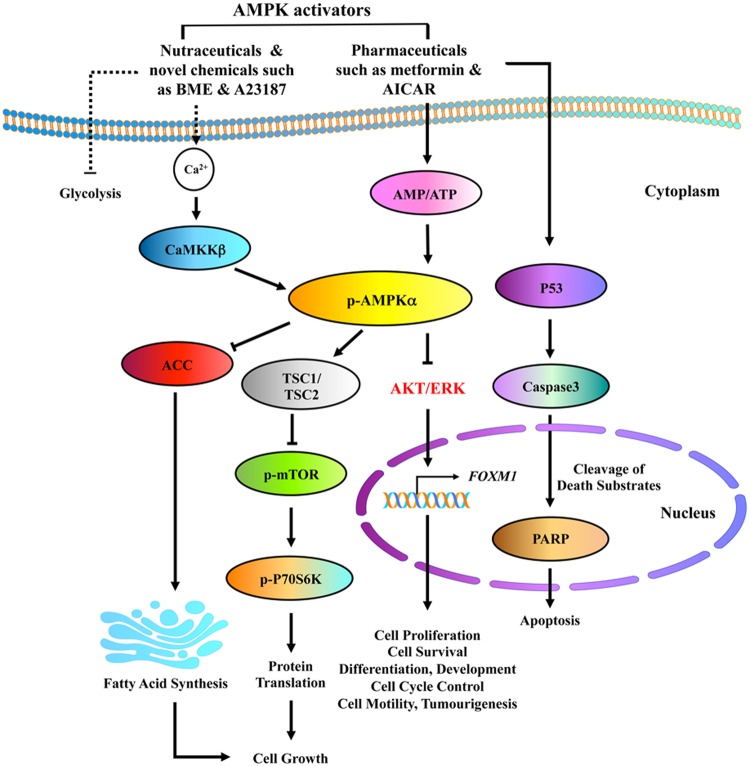
In the 1920s, Warburg was the first to suggest the idea that cancer is in part a metabolic disorder. Today, emerging evidence confirmed that targeting cancer cell metabolism is a promising therapeutic approach in human cancers. AMP-activated protein kinase (AMPK) is a known cellular metabolic sensor that plays a significant role in the control of energy homeostasis in response to external stresses [39–42]. Recent studies have revealed that activation of AMPK by pharmaceuticals or natural compounds, e.g. bitter melon extract (BME), is able to block the malignant cell growth in numerous human cancers [42–46]. Indeed, previous studies reported by our team showed that pharmaceutical AMPK activators such as 5-aminoimidazole-4-carboxamide 1-β-d-ribofuranoside (AICAR) (ATP-dependent) and A23187 (ATP-independent) could be used to suppress cervical cancer cell growth harboring with or without LKB1, an upstream kinase of AMPK [44]. Our group also proposed mechanistic evidence showing that metformin, AICAR, and A23187 suppress cell growth of cervical cancer through reducing AKT/FOXO3a/FOXM1 signaling [47] and DVL3, a positive effector of Wnt/β-catenin signaling cascade, has been shown to be activated constitutively in cervical cancer development [48]. More importantly, our latest study of the molecular mechanism revealed that BME acts as a natural AMPK activator through CaMKKβ signaling in an AMP-independent manner, which in turn represses both mTOR/p70S6K and AKT/ERK/FOXM1 signals in ovarian cancer cells (Fig. 1). Yet, it is still possible that there are other molecular mechanisms altered by these AMPK activators in suppressing the growth of cancer cells. The understanding of these uncharted mechanisms will assist in exploring better therapeutic regimes when using these activators to treat malignance, in particular, gynecological cancers.
Figure 1.
Proposed mechanisms summarizing pharmaceutical and natural AMPK activators mediated by cell growth inhibition and apoptosis in ovarian cancer
Structure and properties of AMPK
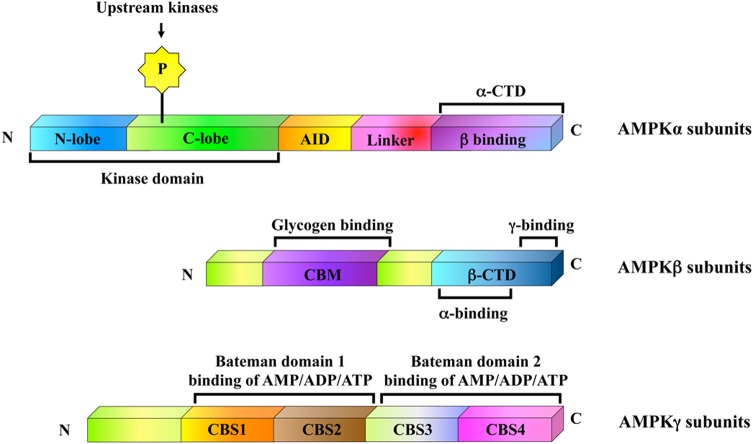
AMPK was first identified in 1987 [49,50]. It is a universal heterotrimeric serine/threonine protein kinase composed of a catalytic subunit α and two regulatory subunits β and γ. Each subunit has different isoforms such as α1, α2, β1, β2, γ1, γ2, and γ3 encoded by distinct genes, which enables the yielding of 12 possible heterotrimeric combinations (Fig. 2) [39,51,52]. The predominant variants in the majority of cells are α1, β1, and γ1, while cardiac and skeletal muscle cells also express α2, β2, γ2, and γ3 [53]. The α, β, and γ subunits of AMPK have their distinctive structural components. This property not only allows their differential roles in the regulation of AMPK activity but also facilitates the physiological functions of AMPK in mammalian cells. Encoded by the gene PRKAA1, AMPKα1 is a 63-kDa protein with 548-amino-acid residues, whereas AMPKα2 is a 552-amino-acid protein with the same molecular weight as α1 variant encoded by the gene PRKAA2. Both variants possess a highly conserved catalytic domain at the N terminus (residues 1–312) that contains the activating phosphorylation site (Thr172) immediately followed by an autoinhibitory domain and a C terminus that possesses a divergent C terminal regulatory domain (residues 313–548 and residues 313–552 for α1 and α2 subunits, respectively) implicated in the interaction with the β and γ subunits [39,52,54]. Interestingly, both AMPK α1 and α2 catalytic subunits are found to have a differential subcellular localization pattern in eukaryotic cells. For instance, α1 subunit appears to be largely enriched in the cytoplasm and excluded from the nucleus, but α2 subunit is localized in both the nucleus and cytoplasm [53]. With regard to tissue distribution, α1 subunit is found to be universally expressed; however, α2 subunit is highly expressed in specific tissues such as skeletal and cardiac muscle, and is not present in blood cells or endothelial cell lineages [51]. Similarly, AMPKα2 was found to be widely repressed in human breast cancer tissues, whereas AMPKα1 subunit was not [55]. Functional study characterized that re-expression of AMPKα2 in the estrogen-dependent breast cancer MCF-7 cells displayed lesser proliferation and underwent apoptosis more readily than control cells both in vitro and in vivo [55], implicating the tumor suppressor-like contribution of AMPKα2 in human carcinogenesis.
Figure 2.
Schematic diagram of domain structures of mammalian AMPK is a highly conserved heterotrimeric complex composed of catalytic α, regulatory β and γ subunits in a 1 : 1 : 1 ratio The C terminal domain (CTD) of β subunit forms the core of the heterotrimers, interacting with the α-CTD and the N terminus of the γ subunit prior to CBS1. AID stands for the autoinhibitory domain, CBM stands for the carbohydrate-binding module, and CBS1 to CBS4 stand for the CBS repeats in the γ subunit [52].
On the other hand, two isoforms of AMPKβ have been identified so far, which are β1 and β2. Encoded by the gene PRKAB1, AMPKβ1 is a 38-kDa protein with 270-amino-acid residues, whereas AMPKβ2 is a 271-amino-acid protein with a molecular weight of 34 kDa and encoded by the gene PRKAB2. The AMPKβ family members are highly similar, and they are 71% identical with only slight difference at the N terminus [51,56]. Via the α and γ subunits’ binding domain at the C terminus, both AMPKβ1 and AMPK β2 act as scaffold/docking subunits and interact at the same efficiency with AMPKα and AMPKγ subunits, so as to facilitate the formation of stable AMPK heterotrimeric complex [39,54,57]. In addition, the central carbohydrate-binding module (CBM), also called the internal glycogen-binding domain of AMPKβ subunit, has high affinity for glycogen particles and may bring about abnormal glycogen containing inclusions when the AMPK heterotrimers are overexpressed. However, the functional importance of such binding remains uncertain because the truncated β isoforms without an intact glycogen-binding domain still form active and functional heterotrimeric complex with AMPKα and AMPKγ, indicating that glycogen-binding domain is not necessary for the enzymatic activity of AMPK [51,54]. Moreover, AMPKβ subunit has multiple regulatory phosphorylation sites such as Ser24/25 (only in AMPKβ1), Ser108, and Ser182. With the participation of AMPKα1, autophosphorylation at Ser24, Ser25, and Ser108 is successfully accomplished; while through the action of the upstream AMPK kinase, phosphorylation at Ser182 is effectively achieved. Although none of the mutations in these reported AMPKβ1 phosphorylation sites influence the rate of phosphorylation at Thr172 on AMPKα1 subunit, phosphorylation at Ser24, Ser25, and Ser182 residues is critical for the nuclear exclusion of AMPKβ1 subunit, and phosphorylation at Ser108 is necessary for the regulation of AMPK catalytic activity. Consistent with this finding, AMPKβ subunits appear to be confined in both the nuclear and the nonnuclear cell fractions [39,54]. Our recent study reported that AMPKβ1 is consistent with the amount of AMPK heterotrimeric complexes and the AMPK activity in advanced ovarian cancers [58]. Both functional and mechanistic studies showed that the reduced AMPKβ1 expression leads to decreased AMPK activity, but enhanced oncogenic capacities of ovarian cancer cells via modulating the AKT/ERK and JNK signaling pathways [59]. These findings underscore the significance of AMPKβ subunit in carcinogenesis by means of its competence to modulate AMPK activity and other oncogenic pathways throughout the progression of ovarian cancer.
Apart from α and β subunits, there are three kinds of AMPKγ isoforms that have been identified in mammalian cells. AMPKγ1 is a 37-kDa protein with 331-amino-acid residues, AMPKγ2 is a 569-amino-acid protein with a molecular weight of 63 kDa, and AMPKγ3 is a protein of 492-amino-acid residues (55 kDa). They are encoded by three alternate genes PRKAG1–3, respectively. They differ at the N-terminal region followed by four highly conserved cystathione β-synthase (CBS) repeats. In pairs, the CBS tandem repeats form two functional structures known as Bateman domains 1 and 2 that are involved in binding of adenosine-containing ligands such as AMP, ADP, and ATP. Among the three γ isoforms, γ1 is ubiquitously expressed, while γ2 and γ3 appear to be most abundant in muscle with less preferential nuclear localization than γ1 variant [39,54,60,61].
Regulation of AMPK
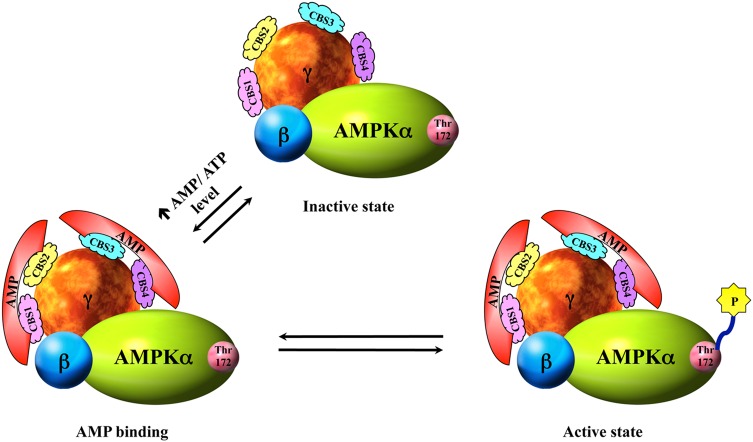
AMPK is a well-known cellular and whole-body energy balancing sensor that is implicated in the regulation of multiple cellular metabolic pathways [42,44,62–64]. It is believed that in the inactivated AMPK, the kinase catalytic domain of the α subunit interacts with its autoinhibitory region located adjacent to the C-terminal regulatory domain. However, AMPK is activated in response to depletion of intracellular ATP levels by environmental, physiological, or pathological stress factors such as exercise, heat shock, hypoxia, ischemia, nutrient deprivation, redox imbalance, and changes in cellular pH, which all induce a concomitant elevation of cellular AMP : ATP ratio. The accumulation of cellular AMP due to the rise in the ratio of AMP : ATP not only favors the allosteric regulation of AMPK by the binding of AMP to the Bateman domains of AMPK γ subunit but also prevents association of the autoinhibitory segment of the α subunit with its kinase domain. As a result, a conformational change in the AMPK heterotrimer is induced to expose the active site Thr172 on the α subunit. An increase in the activity of AMPK is eventually facilitated by subsequent phosphorylation at the exposed active site Thr172 [39–41,65,66] (Fig. 3). Contrary to the effect of AMP, studies have shown that high concentration of ATP antagonizes the binding of AMP to the γ subunit and leads to a decrease in AMPK activity [39].
Figure 3.
Allosteric regulation of AMPK In order to have an optimal AMPK activity, a functional AMPK complex is a must. When AMPK is in the inactive state, the catalytic domain of α subunit is suppressed by interactions with the autoinhibitory domain on the same subunit. However, in the presence of elevated AMP : ATP ratio upon stimuli, AMP molecules bind to the two Bateman domains on the γ subunit and induce a conformational change in the AMPK complex which thereafter exposes the kinase active site Thr172 of the α subunit. Ultimately, AMPK kinase is activated by phosphorylation on the active site Thr172 [66].
In addition to the AMP allosteric regulation, increase in the cellular AMP : ATP ratio can also induce phosphorylation of AMPK by upstream AMPK kinase, and such activation causes at least a 50- to 100-fold increase in the AMPK activity [49,64]. For example, in the presence of high concentration of AMP, dephosphorylation of AMPKα catalytic subunit by protein phosphatases is prevented and at the same time net activating phosphorylation of Thr172 residue on AMPKα is potentiated by its upstream kinase, LKB1, which is a tumor suppressor gene responsible for the inherited disease called Peutz-Jeghers Syndrome [40,41,43,44,62,65,67]. However, a low basal activity of AMPK and phosphorylation of Thr172 residue on the α catalytic domain can still be detected in cells lacking functional LKB1 (e.g. HeLa cells), suggesting that kinases other than LKB1 might be involved in upstream activation of AMPK [68–70].
Recently, it has been revealed that elevation of intracellular calcium concentration in cells lacking LKB1 but expressing calcium/calmodulin-dependent protein kinase kinase α and β (CaMKKα and β) can activate AMPK activity by phosphorylating AMPKα at residue Thr172, independent of changes in concentration of AMP [39,41,68,69]. Subsequent experimentations by means of pharmacological inhibitors and short interfering RNAs directed against the CaMKKs confirmed that CaMKKs, in particular the β isoform, are accountable for the activation of AMPK by phosphorylation of AMPKα at Thr172 under these circumstances [68,71]. These results imply that there are two converging pathways of AMPK regulation. One is directed by LKB1, which is regulated by alterations in AMP concentration upon cellular stresses, whereas the other one is directed by CaMKKs, which is dependent on changes in intracellular calcium level [54].
Besides the action of LKB1 and CaMKKs, transforming growth factor-β-activated kinase-1 (TAK1), also known as MAPKK kinase-7 (MAP3K7), has been proposed to be another upstream kinase of AMPK [72–74]. TAK1 is a serine/threonine protein kinase, which is activated by numerous cytokines, for example, transforming growth factor β and tumor necrosis factor α in mammalian cells, and it is frequently associated with human cancer progression including ovarian cancer [75]. Activation of endogenous TAK1 has been demonstrated in experiments using chemical AMPK activators such as AICAR and metformin, which directly affect or imitate changes in cellular AMP : ATP ratio. Subsequent experiments revealed that TAK1 is capable of phosphorylating AMPKα at Thr172 residue as LKB1 and CaMKKs do. In addition, coexpression of TAK1 with its binding partner TAK-1-binding protein 1 in HeLa cells or treatment of HeLa cells with cytokines induced phosphorylation of AMPKα at residue Thr172 [39,54,73]. However, the physiological relevance of some of these findings remains uncertain, and therefore, more investigations are required to find out the significance of this upstream kinase in cellular physiology.
Importance of LKB1/AMPK signaling and carcinogenesis
The discovery of LKB1, which is a well-known upstream kinase of AMPK and also an important tumor suppressor, provided the first evidence of an association between AMPK and cancer [76]. In cells with functional LKB1/AMPK signaling pathway, energy depletion generally causes an inhibition of cell growth or induces a p53-dependent cell cycle arrest until levels of ATP are restored [43,70,77]. Whereas in cells lacking intact LKB1 signaling, retardation of cell proliferation would not happen even during the time of reduced energy production, and this facilitates dysregulated cellular proliferation which is a hallmark of the malignant phenotype [43]. Nevertheless, when energy depletion was serious, cancer cells without LKB1 would undergo apoptosis rather than growth arrest. These phenomena were supported by a recent study showing that cells lacking intact tuberous sclerosis complex (TSC), a downstream target of AMPK, increased their cellular size upon glucose deprivation and died while glucose was completely removed. On the contrary, cells bearing wild-type TSC decreased in size under low-glucose conditions but continued to survive upon glucose withdrawal [78].
Activation of AMPK suppresses the growth of malignant cells
Although functional LKB1/AMPK signaling pathway in ordinary tissues may protect against cancer development, in transformed cells, activation of AMPK has been reported to be considerably cytotoxic to different kinds of human carcinoma cell lines such as breast [79,80], glial, liver [81], lung [82], stomach [83], prostate [79,84], and ovary [85]. Moreover, tumor growth in both rat C6 glioma allograft [79] and MDA-MB-231 human breast cancer xenografts [80] was significantly reduced after AICAR treatment. The detailed mechanism whereby activation of AMPK induces cell growth arrest or apoptosis in malignant cells is very complicated and its understanding is still not comprehensive enough. Yet, a scenario is currently emerging that intervention of cancer cell metabolism by activation of AMPK may be mainly mediated via the oncogene AKT.
In general, two common metabolic phenotypes including aerobic glycolysis [86] and lipogenesis [87] are always expressed in human cancers. In normal human tissues, the integration of aerobic glycolysis as well as enhanced de novo fatty acid synthesis is not often, but it is extensively unique to malignant cells instead. Activation of the oncogene AKT is perhaps in large part accounts for this metabolic profile. In the energy-surfeit state, the levels of ATP are usually far more than necessary so that both the LKB1 and AMPK are in their inactive state. However, AKT is constitutively activated in energy-surplus cancer cells, and it drives glycolysis by translocating hexokinase from the cytoplasm to the place close to the mitochondria [43,88]. On the other hand, phosphorylated AKT strengthens mTOR signaling through the inactivation of TSC1/TSC2 [43]. As a result of the increased mTOR activity, transcription of glycolytic enzymes [89,90], protein translation via phosphorylation of S6K and 4EBP1, and cell proliferation [78] are all subsequently enhanced.
In addition to augmented glycolysis, the upsurge of fatty acid synthesis is promoted by the AKT-activated phosphorylation on ATP citrate lyase [91], while nucleic acid synthesis is increased as a secondary consequence of enhanced flux through glycolysis [43]. Taken together, activation of AKT triggers a series of anabolic processes including aerobic glycolysis and increased syntheses of fatty acid, protein, and nucleic acid, but with reduced fatty acid oxidation.
When cancer continues to grow and proliferate, regions of the tumor may perhaps extend beyond the capacity of its vasculature to supply adequate nutrients. Accordingly, cellular energy homeostasis is dysregulated, and as a result, AMPK is phosphorylated and activated by upstream kinase LKB1. It is understood that activation of AMPK promotes catabolism and inhibits anabolism in order to conserve cellular energy [53,92]. To curb anabolism during the period of energy deprivation, catabolic AKT signaling is interfered by the activated AMPK through a two-prong approach involving direct inhibition [79] on one hand, and activation of the TSC on the other hand, which in turn suppresses the activity of mTOR [78]. On the whole, a net reduction of glycolysis occurs by restricting the hexokinase activity and the transcription of glycolytic enzymes. Such decline in glycolysis and the decline in the carbon flux result in less citrate available for fatty acid synthesis and less carbon available for nucleic acid synthesis. In addition, fatty acid synthesis is even further inhibited by the AMPK-suppressive phosphorylation on acetyl-CoA carboxylase (ACC). Besides the inhibition of fatty acid synthesis and nucleic acid synthesis, new protein synthesis is also reduced due to the resultant inhibition of mTOR signaling. Importantly, p53 is also activated by AMPK, which leads to growth arrest so as to save energy and avoid the onset of cell division with insufficient energy capacity [43].
With reference to this scenario, physiological activation of AMPK is likely to be antiapoptotic, protecting the integrities of cancer cells during energy depletion. When there is an increase in nutrient supply again, the AMP : ATP ratio decreases, and so does the activity of AMPK. Whereas AKT is believed to be reactivated under this energy-surplus condition, and macromolecular synthesis as well as tumor cell growth can be resumed with relief of the cell cycle blockage. Nevertheless, prompt induction of AMPK by pharmaceuticals seems to attenuate cancer cell growth more when it is compared with mere physiological AMPK activation. For example, pharmaceutical activation of AMPK could give rise to a rapid decrease in fatty acid synthesis with intensified levels of citrate and malonyl-CoA. It is known that high concentrations of cellular citrate can repress glycolysis by inhibiting phosphofructo-kinase-1, while high level of malonyl-CoA suppresses oxidation of fatty acid. The combination of repressed fatty acid synthesis and repressed glycolysis severely compromise the cellular AMP : ATP ratio and the normal cellular metabolism resulting in cell fatality [43]. Determined by the metabolic stage and cell cycle position of the cancer cells, any rapid intervention in the synthesis of nucleic acid, protein, and fatty acid through pharmacological AMPK activation is believed to provide the coup de grâce for cancer.
AMPK activation—a two-edged sword?
Although mounting evidence has documented that enforced activation of AMPK could not only induce a cytostatic effect in cancer cells but also inhibit tumor growth and metastasis, emerging findings have reported that physiological activation of AMPK in malignant cells might promote their survival especially at the initial stages of tumor development [93,94]. It is well known that most malignant cells are subject to both limited nutrient and oxygen supplies on account of the insufficient vascularization in early stage of tumor formation [95]. Tolerance to nutrient deprivation and angiogenesis may, therefore, be critical in those malignancies. To this end, activation of AMPK could provide a window-of-opportunity to keep the cancer cells alive by mediating a metabolic adaptation among them. Using an established model of glioblastoma, it was shown that AMPK is highly activated at the early stages of tumorigenesis [94,96], and LKB1/AMPK signaling cascade is essential for glioma cell survival and spheroid migration under low-glucose conditions [94,97]. Consistently, it has been demonstrated in another study that silencing of AMPK severely impaired the anchorage-independent growth manner and the in vivo tumor formation ability on human pancreatic carcinoma [95]. The significance of AMPK activation for cell growth and survival of cancer has also been revealed in lung carcinoma [98]. Re-expression of LKB1 on LKB1-deficient human lung adenocarcinoma (A549) cells protected the cancer cells against cell death triggered by glucose deprivation via suppression of fatty acid synthesis by AMPK and subsequent sparing of NADPH, which could be exploited to safeguard against oxidative stress induced by glucose starvation [93,98]. Owning to the emergence of such a tumor-promoting paradox in AMPK, it is believed that a better understanding of AMPK activation and its role in various pathological conditions could enable the development of strategies to use it as a therapeutic target.
Biochemical activation of AMPK
AMPK is emerging as a potentially attractive therapeutic target for the treatment of cancer. Understanding of the physiological role of AMPK in cells is, therefore, of the utmost importance, and the approaches can be greatly enhanced by the application of different pharmacological AMPK activators. In fact, the majority of these activators are novel chemicals [99,100] or drugs in clinical use in conventional medicines, while others are some natural compounds found in traditional medicines, food, and drink derivatives [51,101–103]. The mechanisms of most activators can be briefly classified into two categories—those that stimulate AMPK through the major upstream activating kinase LKB1 by increasing the cellular AMP : ATP ratio and those that activate AMPK through alternative pathways, independent of the AMP : ATP ratio [54] (Tables 1 and 2).
Table 1.
Differential behaviors of AMPK-activating compounds in stimulating AMPK
| Compounds | Mediation of AMPK activation | Reference |
|---|---|---|
| Activators interfere cellular energy homeostasis | ||
| AICAR | AMP mimic that induces allosteric activation and promoting phosphorylation through upstream kinase LKB1 | [39,51,54,104] |
| Metformin | Biguanide derivative that is an inhibitor of complex I of the mitochondrial respiratory chain | [105,106] |
| 2-DG | Non-hydrolysable d-glucose that impedes glycolysis and results in depletion of intracellular ATP | [51,78,107,108] |
| Mitochondrial toxins (DNP) | Possessing a remarkable increase in AMP : ATP ratio by defeating the proton gradient across the inner mitochondrial membrane | [39] |
| Oxidative stress | Hydrogen peroxide reduces ATP production and concomitantly rise in the level of AMP | [109,110] |
| Compound 14 | Accumulation of endogenous ZMP through suppressing the homodimerization of aminoimidazole carboxamide ribonucleotide transformylase/inosine monophosphate cyclohydrolase (ATIC) | [99] |
| R419 | Similar to metformin that modulates mitochondrial function via inhibiting respiratory complex I | [100] |
| Activators without interrupting cellular energy homeostasis | ||
| A-769662 | Thienopyridone induces allosteric activation and interacts with uncharacterized site(s) of AMPK instead of displacing AMP from the Bateman domains | [39,51,111] |
| A23187 | Calcium ionophores that target CaMKKβ, the upstream kinase of AMPK | [68] |
The manners of AMPK activation can be categorized into two groups—most of them interfere cellular energy homeostasis by altering the AMP : ATP ratio and the others trigger initiation of AMPK without affecting cellular energy homeostasis.
Table 2.
Natural AMPK activators
| Compounds | Mediation of AMPK activation | Reference |
|---|---|---|
| Nutraceuticals and traditional medicines—novel natural AMPK activators | ||
| Resveratrol (3,5,4′-trihydroxystilbene) | A molecule produced by a variety of plants especially grapes. It inhibits mitochondrial F1 ATPase resulting in increase of AMP : ATP ratio | [101,112] |
| Red ginseng extract (RGE) | Activates the AMPK-ACC pathway in cells via activation of LKB1 | [113] |
| Curcumin | A phytochemical isolated from the rhizome of turmeric. It possesses change in cellular AMP : ATP ratio, resulting in activation of AMPK and its downstream mTOR and STAT-3 signaling | [114] |
| Puerarin | Activates AMPK signaling through CaMKII | [102] |
| α-Lipoic acid | Activation of AMPK is through the CaMKKβ-mediated phosphorylation of Thr172 | [101,103] |
| BME | BME and its active ingredients activate AMPK via CaMKKβ signaling in an AMP-independent manner | [115] |
Many natural activators of AMPK are nutraceuticals, traditional medicines, or unexplored secondary plant metabolites that not only inhibit mitochondria but also mediate activation of AMPK by other novel manners.
Activators of AMPK that interfere cellular energy homeostasis
AICAR
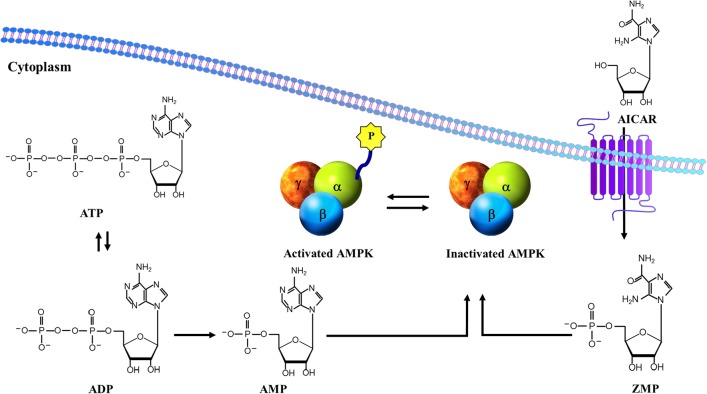
AICAR is one of the best characterized activators of AMPK. It is a cell permeable adenosine analog actively transported into cells by adenosine transporters and phosphorylated by the enzyme adenosine kinase into the equivalent ribotide, 5-aminoimidazole-4-carboxamide 1-β-d-ribofuranosyl monophosphate (ZMP) (Fig. 4). ZMP is an AMP mimic that activates AMPK by binding to the Bateman domains of the AMPKγ subunit and inducing allosteric activation, as well as by promoting its phosphorylation via upstream kinases [39,51,54,104]. Although ZMP, as an activator of AMPK, is only ∼2% as potent as AMP, activation of AMPK can be detected in cells just after 30–60 min exposure to AICAR because ZMP can accumulate to millimolar concentrations inside the cells [51,116]. Besides in vitro experiments, AICAR is also effective in vivo that has been applied to examine physiological processes associated with AMPK in animal models [51,117].
Figure 4.
Structural similarity between AMPK-activating compounds AICAR, ZMP, and AMP
The critical mediator of AICAR-induced AMPK activation in cells is believed to be LKB1, because experiments performed utilizing LKB1 wild-type and LKB1-deficient murine embryonic fibroblasts showed that AICAR-stimulated AMPK phosphorylation was significantly attenuated in the absence of LKB1 [104,118]. Furthermore, AMPK activation by AICAR also suppresses the mTOR signaling cascade both in vitro [119] and in vivo [120]. Collectively, these findings provided the preliminary link between AICAR and cancer treatment. In addition, the capability of AICAR to inhibit cancer cell proliferation has been assessed both in vitro and in vivo [79]. Research conducted in a panel of cancer cell lines revealed that AICAR-induced activation of AMPK suppressed cell proliferation dose dependently and arrested cell cycle progression in S phase [79,104]. In addition, after activating AMPK with AICAR in C6 glioma cell xenografts, tumor growth in rats from the experimental group decreased by >50% when compared with untreated control [79].
Although preclinical studies have demonstrated that AICAR plays an important role in AMPK activation, its use is still very limited. One may expect that the rise in the level of ZMP when AICAR is continuously metabolized by the adenosine kinase would lead to more activation of AMPK, but in fact the opposite result occurs. The main reason is that a triply phosphorylated form (ZTP) may also accumulate and serve as an antagonist of ATP, hence inhibiting AMPK in the long term. Additionally, some AMP sensitive enzymes like fructose-1,6-bisphosphatase and glycogen phosphorylase can be influenced by the AMP-mimetic effect of ZMP. In terms of clinical use, AICAR likewise is a lukewarm participant. On one hand, the pharmacokinetic of AICAR is very poor, and on the other hand, its toxicity in patients is rather high. Patients with AICAR orally or intravenously administrated during clinical trials revealed that the bioavailability of AICAR is <5% and its half-life is just ∼2 h [121]. More importantly, patients treated with AICAR have adverse side effects that they exhibit significant increases in the production of lactic and uric acid [121–123]. Although AICAR is an important research tool for investigating the effects of AMPK activation and mTOR inhibition on tumorigenesis, it is obviously not suitable for clinical use yet. In any case, off target effects do seem likely to occur as aforementioned, and therefore, alternative means for activating AMPK should always be included to confirm that the observed result is AMPK dependent.
Metformin
In fact, the most clinically developed activator of AMPK is metformin (N,N-dimethylimidodicarbonimidic diamide), which is a first-line prescribed drug commonly utilized for the treatment of Type II diabetes [62,117,124]. The very high prevalence of Type II diabetes mellitus is reaching epidemic proportions and it is estimated that >1% of the world population (i.e. ∼100 million people) have been prescribed for this drug [51,62]. Metformin is a biguanide derivative originally derived from guanidine and galegine, which can be naturally obtained in the plant Galega officinalis also known as Goat's Rue, French lilac, and Italian fitch [125,126]. As early as in the 18th century, Goat's Rue was used as an herbal medicine to cure symptoms like thirst and intense urination [51,125]. In the 1920s, metformin was chemically synthesized and demonstrated to be useful in reducing blood glucose in animals [127]. Unfortunately, further investigations of the biguanide drugs have been put on hold since the successful development of insulin as the main therapy for diabetes. Until the late 1950s, by the time the discrimination between Type I and Type II diabetes had been made, metformin and its sister drugs buformin and phenformin were eventually introduced into clinical application [125,126]. Nevertheless, because of a rare but life-threatening side effect of frequent lactic acidosis, the use of phenformin was discontinued in the US market in 1978. Whereas impressive outcomes of metformin were always reported after 20 years of use in Europe especially in the UK Prospective Diabetic Study, it was even approved for use in US in 1995 [51,125,128].
Although biguanide drugs have been in clinical practice for decades and their major effect is known to exert a significant decrease in the rate of hepatic glucose production in Type II diabetic patients [129], their precise mode of action still remains obscure until recent studies [124,130,131] demonstrated that metformin activated AMPK both in vitro and in vivo. Activation appears soon after treatment for 30–60 min and causes a phosphorylation of both AMPKα1 and α2 subunits at the catalytic site Thr172 in a time-dependent manner [54,124]. Although metformin has been demonstrated to activate AMPK, the site(s) of action plus the biochemical mechanism by which metformin stimulates AMPK remains controversial. Earlier studies [105,106] have indicated that metformin is an inhibitor of Complex I of the mitochondrial respiratory chain, which may activate AMPK by inhibiting the generation of cellular ATP energy and hence increasing the AMP : ATP ratio inside the living cells. Intriguingly, a number of latest investigations [124,126,132] argued against this notion because in these studies, metformin was found to stimulate AMPK without affecting the AMP : ATP ratio. For example, Zou et al. [124] have demonstrated that reactive nitrogen species generated within mitochondria by metformin lead to activation of AMPK through the c-Src/PI3 kinase pathway without a noticeable change in the intracellular AMP or ATP contents. Consequently, up to this time, it is sorry to tell that none of these investigations have fully elucidated the mechanism by which metformin activates AMPK.
Recently, increasing evidence has suggested that metformin lowers the risk of cancer via the activation of AMPK [62,133]. In unison with this association, it has been reported that diabetic patients who received metformin treatment in general got a lower incidence of cancer than those who had other medications [70,134]. Further confirmation on this correlation has also been done in different retrospective studies. For instance, diabetic patients with breast cancer who had already taken metformin apparently showed a better response to chemotherapy than nondiabetics and diabetics without metformin treatment [135]. In fact, activation of AMPK by metformin per se may be required, but not adequate, to suppress tumor growth. Research conducted employing a panel of breast carcinoma cell lines revealed that treatment with metformin remarkably repressed cancer cell proliferation, which was associated with inhibition of S6K and phosphorylation of S6 by mTOR in these cells [136]. Such effects of metformin were AMPK dependent, since AMPK knockdown mediated by siRNA approach in breast cancer cells prevented metformin-induced inhibition of the mTOR signaling and rescued cells from metformin-induced growth inhibition [136]. In addition, cap-dependent translation in breast cancer cells was also inhibited by metformin through an AMPK- and mTOR-dependent mechanism [137]. Taken together, these findings indicate that metformin suppress cancer cell growth by inhibiting protein synthesis.
Indeed, in vivo study has also been performed to assess the efficacy of metformin as an anticancer drug in mouse model expressing moderately reduced LKB1 and deficient PTEN (LKB1fl/+PTEN+/−) [138]. It is understood that both LKB1 and PTEN are constituents of signaling pathways that regulate mTOR, and therefore, tissues from these mice are usually characterized by hyperactivity of the mTOR signaling. Results from such experiment showed that administration of metformin to LKB1fl/+PTEN+/− mice did stimulate AMPK in various kinds of tissues; however, tumorigenesis was only modestly inhibited. In general, it just delayed the onset of tumors by 1 month, but did nothing in the tumor incidence or morphology. It is really a wonder that the cancer cells can ultimately run away from the cytostatic effects of metformin. One possible explanation is that additional mutation has been acquired among the tumor cells to prevent or reduce continued activation of AMPK. In some respects, loss of LKB1 may be involved because it has been estimated to appear in up to 20% of cervical carcinomas [139] and 30% of non-small cell lung carcinomas [70,140,141]. Further investigations are, therefore, required to better determine which molecular contexts would be predictive in response to biguanide derivatives.
Thus far, the majority of cancer research has been focused on the signaling effects of biguanide derivatives and AMPK activation, whereas little investigations have been directed to the massive metabolic alterations, which are correlated with AMPK activation. By virtue of the metabolic regulatory role of AMPK, both adipogenesis and lipolysis should be properly regulated, making AMPK as a potential candidate to modulate the adipocytes generating microenvironmental milieu for the malignant cells [142–144]. Given that ovarian cancer cells exploit omental adipocytes as their primary repository to derive energy for proliferation and metastasis [142,145], while biguanide AMPK activators such as metformin have been reported to restrain adipogenesis and the stimulant effects on ovarian cancer cells driven by adipocytes at a time [142], implying a conceivable therapeutic option of applying biguanide derivatives for ovarian carcinoma.
2-Deoxy-d-glucose
2-Deoxy-d-glucose (2-DG) is another major representative of AMPK activator. 2-DG actually is a non-hydrolyzable d-glucose analog that is actively taken up into living cells by glucose transporters and subsequently catalyzed by hexokinase into 2-deoxglucose-6-phosphate [51]. This catalysis impedes glycolysis and as a result stimulates AMPK in part by depleting intracellular level of ATP but increasing AMP : ATP ratio in cells [51,78,107,108]. It is believed that 2-DG stimulates AMPK and represses mTOR via LKB1-dependent mechanism since these consequences of 2-DG are significantly reduced in LKB1-deficient murine embryonic fibroblasts and in LKB1 mutant cancer cells [107,146]. The moderate level of AMPK activation detected in LKB1 mutant cancer cells (i.e. HeLa and A549) with regard to 2-DG is probably mediated by CaMKKβ, as pretreatment of LKB1 mutant cancer cells with the CaMKK-specific inhibitor, STO-609, attenuates 2-DG-induced activation of AMPK [147]. Unlike AICAR, 2-DG may possess higher clinical potential since oral administration of 2-DG is excellently tolerant among cancer patients and can also generate plasma concentrations as high as 5 mM. Moreover, medication with 2-DG may lead to a higher therapeutic index in patients with cancer because cancer cells that have increased glycolytic activity preferentially take up 2-DG. Phase I/II clinical trials in the treatment of solid tumors with the use of 2-DG are recently being undertaken [108].
Mitochondrial toxins
Besides 2-DG, it has been demonstrated that high concentration of mitochondrial toxins such as 2,4-dinitrophenol (DNP) (0.5 mM) can stimulate AMPK in a number of cell types, especially in adipocytes and skeletal muscle cells through a mechanism possessing a remarkable increase in AMP : ATP ratio and phosphorylation on Thr172 of the AMPKα subunit [132,148,149]. From the 1930s, DNP was extensively used as a dieting aid in the treatment of nutritional disorders [150], but it is considered too harmful for that application nowadays. DNP actually is a cell permeable and benzene-based chemical compound, which generally behaves as a proton ionophore in biological membranes and particularly defeats the proton gradient across the inner mitochondrial membrane. This gives rise to a less effective ATP energy production because the proton motive force that living cells use to generate most of their ATP is collapsed, and in effect some of the cellular energy that is normally generated from respiration is lost as heat production [39].
Oxidative stress
In addition to the elevation of AMPK activity with respect to DNP treatment, oxidative stress induced by hydrogen peroxide was also found to stimulate AMPK. The mechanism involves both a significant reduction in ATP and a concomitant rise in AMP [109,110]. Activation is again tightly associated with the increased phosphorylation of AMPKα1 at Thr172 [110].
Activators of AMPK independent of AMP : ATP ratio
A-769662
In order to have a better understanding about the mechanisms of action of AMPK and the regulatory functions of AMPK in cellular metabolism, scientists have kept on pursuing the discovery and development of more potent activators of AMPK. By a high-throughput screening in a chemical library having >700,000 chemical compounds, Abbott Laboratories have currently identified a thienopyridone compound known as A-769662 that functions as a reversible small-molecule activator of AMPK both in cell-free assay and in intact cells like primary rat hepatocytes [39,51,138,151].
In cell-free assay, A-769662 not only directly activated partially purified AMPK from rat hepatocytes with half-maximal effect (EC50) of 0.8 μM, a concentration much lower than that needed for AMP (EC50 of 112 μM), but it also significantly generated a higher activation (4.1 ± 0.5 versus 3.1 ± 0.5 folds) than AMP [111,151]. Whereas in animal experiments, both wild-type and LKB1fl/+PTEN+/− mice orally administered with A-769662 over 2 weeks had remarkably enhanced levels of AMPK activity and phosphorylation in their livers, spleens, and intestines [138]. On the other hand, when A-769662 was administrated intraperitoneally, it possessed metabolic effects similar to other common AMPK activators that it not only enhanced fatty acid oxidation, inhibited de novo fatty acid synthesis, and decreased weight gain in normal rats, but it also reduced plasma glucose and triglycerides levels and the hepatic triglycerides among obese mice [151]. Alternatively, A-769662 seems likewise to have the potential as an anticancer agent as it apparently improved latency of tumor and reduced incidence of tumor in LKB1fl/+PTEN+/− mice [104,138].
The mechanism by which A-769662 stimulates AMPK was unclear, until recent studies demonstrated that it allosterically activates AMPK without altering the cellular AMP : ATP ratio and protects against dephosphorylation of AMPKα subunit at residue Thr172 by inhibiting the activity of the protein phosphatases. Although allosteric activation is involved, a scintillation proximity assay carried out by Göransson and colleagues revealed that A-769662 elicits its effects by interacting with some currently uncharacterized site(s) instead of displacing AMP from the γ subunit Bateman domains [39,51,111]. In addition, it was quite interesting to find out that A-769662 did not stimulate AMPK heterotrimers having β2 subunit or heterotrimers in which the β1 subunit had an S108A mutation, while both of them yet stay highly sensitive to activation by AMP [51,152,153]. Despite recent progress, many unknowns in the mode of action of A-769662 in stimulating AMPK remain to be explicated. Nevertheless, the fact that A-769662 directly interacts with and activates AMPK is very encouraging, and it may be useful for researchers attempting to further elucidate the role of AMPK in the regulation of cellular metabolism.
A23187
As aforementioned, frequent mutations and deletions of LKB1 found in some cancer cells limit the application of various well-known AMPK activators such as AICAR and 2-DG as efficient therapeutic drugs. Alternative class of pharmacological AMPK activator, the Ca2+ ionophores including A23187 and ionomycin, which target Ca2+/CaMKKβ may help activating AMPK in those LKB1-deficient cells [68].
Since CaMKKβ is a Ca2+/calmodulin-activated AMPK upstream kinase, a sudden increase in concentration of cytosolic calcium ions by Ca2+ ionophore would probably induce phosphorylation and activation of AMPK. In favor of this idea, cells lacking functional LKB1 such as HeLa cells, which therefore cannot respond to the phosphorylation of Thr172 of the AMPKα subunit, showed a maximal activation of AMPK upon Ca2+ ionophore A23187 treatment at 10 μM and a half-maximal effect at 1–2 μM without affecting the cellular AMP: ATP ratio. The effect of either concentration was fully blocked by STO-609, an inhibitor of CaMKKα and CaMKKβ. Similar effects also occur when another Ca2+ ionophore, ionomycin, was used [68,154]. Collectively, these findings suggested that CaMKKs, in particular β isoform, might be responsible for the A23187-stimulated activation of AMPK. Consistent with this study, it had been demonstrated by our group that A23187 was able to inhibit cervical cancer cell growth through activation of CaMKKβ [44]. Using cervical cancer cell models, it was found that LKB1-deficient cell line HeLa responded less to the antiproliferative effect exerted by AICAR treatment (P< 0.001) when compared with LKB1-expressing cervical cancer cell lines CaSki and C41. Conversely, under the treatment of A23187 (P< 0.001), the antiproliferative effect was increased significantly in HeLa cells, but not in CaSki and C41 cells. Moreover, cotreatment of AICAR and A23187 was able to further enhance the inhibitory effect on cell growth of HeLa, CaSki, and C41 cells. Notably, both AICAR and A23187 exerted inhibitory effect on cell growth of cervical cancer cells by suppressing AMPK/mTOR signaling pathway. These findings suggested that A23187 could be a potential alternative therapeutic drug for antiproliferation in LKB-deficient cancer cells. More importantly, AMPK can be activated by multiple means and may become a potential target for gynecological cancer therapy.
Nutraceuticals and traditional medicines
Among the numerous pharmacological AMPK activators available today, there are very few that deserve more attention of the public than many currently described natural plant products with AMPK-activating activity in intact cells. Actually, many of these natural plant products are found in foods and beverages consumed in our diets. Since most of them are supposed to be beneficial for human health, they are often being touted as nutraceuticals in the markets. Aside from this, some xenobiotic compounds, which are components of traditional herbal medicines, are also reported to have the ability in activating AMPK. These plant products include salicylate conventionally purified from willow bark [155,156], berberine extracted from Chinese Goldthread [157], resveratrol found in grapes and red wine [112,133], epigallocatechin-3-gallate found in green tea [158,159], hispidulin taken from Snow Lotus [160], the aflavins obtained from black tea [161], genistein derived from soybean [159], capsaicin that exists in Chili peppers [159], caffeic acid phenethyl ester present in honeybee propolis [162], curcumin present in turmeric Curcuma longa [114], garlic oil present in Allium sativum [163], extracts of Korean red ginseng (RGE) [113], and last but not least the extracts from bitter melon (BME) [164].
Although in most cases the mechanism of action of these natural substances in activating AMPK is still not completely understood, pilot studies have already been started in recent times to examine the effects of these dietary compounds and their potential application as a cotherapy together with standard therapeutics in malignancies especially ovarian cancer. For example, berberine has been reported to significantly sensitize cisplatin-resistant ovarian cancer cells to cisplatin treatment through miR-21/PDCD4 axis [165]. On the other hand, Opipari et al. presented the first evidence that resveratrol promotes cell death via autophagy in five ovarian cancer cell lines, which implied resveratrol as a promising regime in treating apoptosis-resistant cancer cells [166]. In addition, quercetin has antitumorigenic effects linked to its capacity for targeting key tumorigenic pathways such as mTOR/elF4E-BP1/P70S6K signaling in ovarian cancer [167]. All the evidence demonstrates that many phytochemical compounds present in diets not only are inherently lesser toxicity but may also give novel therapeutic strategies in the treatment of ovarian cancer.
BME as a capable natural activator of AMPK in cancer treatment
Besides the above encouraging contributions by others in recognizing novel natural AMPK activators on cancer treatment, our group has recently reported that BME significantly inhibits tumorigenicity and overcomes cisplatin resistance in ovarian cancer cells through targeting AMPK signaling cascade [115]. Over the years, BME has already drawn global attention due to its increasing and valuable medicinal values. Apart from its amazing hypoglycemia action, previous findings from independent laboratories have revealed that constituents of bitter melon can have analgesic, antioxidant, anticancer, antiviral, antimutagenic, abortifacient, cytotoxic, and immunomodulating properties [168–170]. Lots of BME dietary supplement products are currently available and marketed in numerous health food stores worldwide. Nowadays, the antitumor activity of BME has even become one of the most attractive research areas, since the anticancer property of the extract against a wide variety of human cancers such as breast carcinoma, melanoma, myeloma tumor, skin tumor, prostatic carcinoma, colorectal carcinoma, epidermoid carcinoma, hepatoblastoma, brain glioblastoma, bladder cancer, cervical carcinoma, and ovarian carcinoma has been gradually discovered [115,168,169,171–173]. BME is potent in its antitumor activity against different cancer cells probably due to the presence of some of its active components including triterpenoids like cucurbitacin B (cucB) and kuguacin J, flavonoids like rutin and naringin, and phenolic acids.
Triterpenoids from a wide range of botanicals have already been confirmed to possess antiproliferative [174–176] and anti-invasive [177,178] features. More than 50 triterpenoids have been extracted from bitter melon; however, their biological functions have yet to be investigated in detail. Recently, it has been reported that cucB, a triterpenoid from Cucurbitaceae vegetables, exists in the seeds of bitter melon as well, which can induce cell cycle arrest and apoptosis in human colon adenocarcinoma cancer cells [176]. Another triterpenoid, kuguacin J, which accounts for only ∼1.6% of bitter melon leave extract, has been shown to significantly inhibit cancer and/or carcinogenesis by causing cell cycle arrest at G1 phase and inducing apoptosis in preinitiated or initiated tumor cells. Whereas in more advanced tumors, kuguacin J not only has the ability to sensitize chemoresistant cancer cells to anticancer drug-induced cell death, but it also successfully blocks tumor progression and metastasis, implying that natural compounds from BME might bear the scientific potential in the development of useful chemopreventive and chemotherapeutic agents. Except for triterpenoids, flavonoid rutin has also been reported to effectively inhibit the growth of both leukemia and ovarian carcinoma, with anti-invasive effects on melanoma [179–183].
The fact that BME appears to bear both the hypoglycemic and anticarcinogenic responses is reminiscent of the pharmaceutical AMPK activator metformin, which is originally an insulin-sensitizing and antihyperglycemic agent used in the treatment of Type II diabetes mellitus [184]. Recently, the antidiabetic efficacy and the anticancer effect of metformin have been traced to its capability to stimulate AMPK [85,130,131]. It is rational to hypothesize that BME may contain at least one natural activator of AMPK and such hypothesis is in unison with some of the findings between the biological effects of bitter melon and metformin [185]. For example, bitter melon has been reported to have the ability to reduce both weight gain and body fat accumulation in rats fed with diets containing high fat content [186]. Similar effects have been reported in metformin for its tendency to promote body weight loss instead of weight gain [184,187,188]. Moreover, elevated levels of serum cholesterol and triglycerides in hyperlipidemic, diabetic, or insulin-resistant rodents were shown to be suppressed by BME [189–192], which is consistent with the clinical hyperlipidemic impact of metformin [193–196]. On the other hand, BME was demonstrated to possess an antipromotional effect on cancer induction in rodent models of either spontaneous or carcinogen-induced carcinoma [170,197,198]. Coincidently, metformin's fellow biguanide, phenformin, has also been revealed to exert similar effects by downregulating IGFI activity [185,199]. With reference to these concomitant similarities between BME and metformin, the presence of a nutraceutical activator of AMPK in BME would be of great likelihood. Aforementioned, our recent report in fact has confirmed the activity of BME to suppress proliferation, migration, and invasion of ovarian cancer cells [115]. Notably, BME showed no obvious toxicity in normal ovarian epithelial cells (HOSEs) or nude mice, but enhanced the cytotoxicity of cisplatin in ovarian cancer cells, both in vitro and in vivo. Importantly, our mechanistic studies are the first to show that BME differs from other xenobiotic AMPK activators in that it activates AMPK in an AMP-independent manner through CaMKKβ signaling. Such BME-mediated AMPK activation significantly inhibits ovarian cancer cell growth by repressing both mTOR/P70S6K and AKT/ERK/FOXM1 cascades [115]. This is definitely of practical importance, as modulating cellular metabolism by well-tolerated AMPK activators will have substantial therapeutic value especially for cancer treatment.
Conclusion
Recent studies have suggested that targeting cancer cell metabolism is an alternative therapeutic approach in cancer treatment. AMPK is the pivotal energy sensor governing normal and cancer cell metabolism. Pharmaceutical AMPK activators are able to repress cervical cancer cell growth through targeting DVL3 in Wnt/β-catenin signaling and FOXM1 in AKT/FOXO3a/FOXM1 signaling cascade. The reduction of FOXM1 by AMPK/AKT/ERK signaling axis is attributed to aforementioned AMPK-mediated ovarian cancer cell growth retardation. AMPK activated by either pharmaceutical or natural AMPK activators could reduce FOXM1 expression through suppressing the AKT/ERK signaling pathway, which thereby impairs ovarian cancer cell growth. Additionally, AMPK activation in response to natural AMPK activators such as BME has been shown to attenuate mTOR and its corresponding P70S6K activities, validating that BME has the ability to inhibit cell growth in ovarian cancer cells via suppressing mTOR-mediated protein translation process. This result is consistent with other findings using pharmaceutical AMPK activators in suppression of cancer cell growth through activation of AMPK, blocking of mTOR signaling and its downstream regulators p70S6 kinase, and 4E-BP1 activity. Therefore, nutraceuticals and traditional medicines in particular BME act as natural AMPK activators governing AMPK activity through an AMP-independent mechanism. These natural AMPK activators may directly activate AMPK via a mechanism closely related to A769662 or behave as Ca2+ ionophore A23187 to stimulate CaMKKβ or function as osmotic stress and quercetin to have more than one of these mechanisms in AMPK activation. Hence, it is worth to input more endeavors onto this topic to test these recommended hypotheses so as to have a better understanding of the multifaceted mechanism of AMPK activation and it is hoped that discussions herein would shed light on the application of natural AMPK activators in human ovarian cancer treatment.
Funding
This study was supported by a grant from the National Natural Science Foundation of China (NSFC)—General Program (No. 81472721).
References
- 1.Al Rawahi T, Lopes AD, Bristow RE, Bryant A, Elattar A, Chattopadhyay S, Galaal K. Surgical cytoreduction for recurrent epithelial ovarian cancer. Cochrane Database Syst Rev 2013, 2: CD008765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.del Carmen MG, Birrer M, Schorge JO. Clear cell carcinoma of the ovary: a review of the literature. Gynecol Oncol 2012, 126: 481–490. [DOI] [PubMed] [Google Scholar]
- 3.Wang V, Li C, Lin M, Welch W, Bell D, Wong YF, Berkowitz R et al. Ovarian cancer is a heterogeneous disease. Cancer Genet Cytogenet 2005, 161: 170–173. [DOI] [PubMed] [Google Scholar]
- 4.Laios A, O'Toole SA, Flavin R, Martin C, Ring M, Gleeson N, D'Arcy T et al. An integrative model for recurrence in ovarian cancer. Mol Cancer 2008, 7: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sankaranarayanan R, Ferlay J. Worldwide burden of gynaecological cancer: the size of the problem. Best Pract Res Clin Obstet Gynaecol 2006, 20: 207–225. [DOI] [PubMed] [Google Scholar]
- 6.Kaku T, Ogawa S, Kawano Y, Ohishi Y, Kobayashi H, Hirakawa T, Nakano H. Histological classification of ovarian cancer. Med Electron Microsc 2003, 36: 9–17. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen L, Cardenas-Goicoechea SJ, Gordon P, Curtin C, Momeni M, Chuang L, Fishman D. Biomarkers for early detection of ovarian cancer. Womens Health (Lond Engl) 2013, 9: 171–187. [DOI] [PubMed] [Google Scholar]
- 8.Gui T, Shen K. The epidermal growth factor receptor as a therapeutic target in epithelial ovarian cancer. Cancer Epidemiol 2012, 36: 490–496. [DOI] [PubMed] [Google Scholar]
- 9.Kikkawa F, Nawa A, Ino K, Shibata K, Kajiyama H, Nomura S. Advances in treatment of epithelial ovarian cancer. Nagoya J Med Sci 2006, 68: 19–26. [PubMed] [Google Scholar]
- 10.Shanmughapriya S, Nachiappan V, Natarajaseenivasan K. BRCA1 and BRCA2 mutations in the ovarian cancer population across race and ethnicity: special reference to Asia. Oncology 2013, 84: 226–232. [DOI] [PubMed] [Google Scholar]
- 11.Gentry-Maharaj A, Menon U. Screening for ovarian cancer in the general population. Best Pract Res Clin Obstet Gynaecol 2012, 26: 243–256. [DOI] [PubMed] [Google Scholar]
- 12.Stewart SL, Rim SH, Richards TB. Gynecologic oncologists and ovarian cancer treatment: avenues for improved survival. J Womens Health (Larchmt) 2011, 20: 1257–1260. [DOI] [PubMed] [Google Scholar]
- 13.Morimura Y, Hoshi K, Watanabe F, Honda T, Yamada H, Sato A. Ovarian epithelial borderline tumor and carcinoma in young women. Tohoku J Exp Med 1996, 180: 319–326. [DOI] [PubMed] [Google Scholar]
- 14.Parmar MK, Ledermann JA, Colombo N, du Bois A, Delaloye JF, Kristensen GB, Wheeler S et al. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet 2003, 361: 2099–2106. [DOI] [PubMed] [Google Scholar]
- 15.van der Burg ME. Advanced ovarian cancer. Curr Treat Options Oncol 2001, 2: 109–118. [DOI] [PubMed] [Google Scholar]
- 16.Dizon DS, Hensley ML, Poynor EA, Sabbatini P, Aghajanian C, Hummer A, Venkatraman E et al. Retrospective analysis of carboplatin and paclitaxel as initial second-line therapy for recurrent epithelial ovarian carcinoma: application toward a dynamic disease state model of ovarian cancer. J Clin Oncol 2002, 20: 1238–1247. [DOI] [PubMed] [Google Scholar]
- 17.Rose PG, Fusco N, Fluellen L, Rodriguez M. Second-line therapy with paclitaxel and carboplatin for recurrent disease following first-line therapy with paclitaxel and platinum in ovarian or peritoneal carcinoma. J Clin Oncol 1998, 16: 1494–1497. [DOI] [PubMed] [Google Scholar]
- 18.Hennessy BT, Coleman RL, Markman M. Ovarian cancer. Lancet 2009, 374: 1371–1382. [DOI] [PubMed] [Google Scholar]
- 19.Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol 2002, 20: 1248–1259. [DOI] [PubMed] [Google Scholar]
- 20.Elit L, Hirte H. Palliative systemic therapy for women with recurrent epithelial ovarian cancer: current options. Onco Targets Ther 2013, 6: 107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu B, Nash J, Runowicz C, Swede H, Stevens R, Li Z. Ovarian cancer immunotherapy: opportunities, progresses and challenges. J Hematol Oncol 2010, 3: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 2003, 348: 203–213. [DOI] [PubMed] [Google Scholar]
- 23.Tomsova M, Melichar B, Sedlakova I, Steiner I. Prognostic significance of CD3+ tumor-infiltrating lymphocytes in ovarian carcinoma. Gynecol Oncol 2008, 108: 415–420. [DOI] [PubMed] [Google Scholar]
- 24.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA 2005, 102: 18538–18543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 2004, 10: 942–949. [DOI] [PubMed] [Google Scholar]
- 26.Disis ML, Gooley TA, Rinn K, Davis D, Piepkorn M, Cheever MA, Knutson KL et al. Generation of T-cell immunity to the HER-2/neu protein after active immunization with HER-2/neu peptide-based vaccines. J Clin Oncol 2002, 20: 2624–2632. [DOI] [PubMed] [Google Scholar]
- 27.Disis ML, Goodell V, Schiffman K, Knutson KL. Humoral epitope-spreading following immunization with a HER-2/neu peptide based vaccine in cancer patients. J Clin Immunol 2004, 24: 571–578. [DOI] [PubMed] [Google Scholar]
- 28.Vlad AM, Kettel JC, Alajez NM, Carlos CA, Finn OJ. MUC1 immunobiology: from discovery to clinical applications. Adv Immunol 2004, 82: 249–293. [DOI] [PubMed] [Google Scholar]
- 29.Kenemans P. CA 125 and OA 3 as target antigens for immunodiagnosis and immunotherapy in ovarian cancer. Eur J Obstet Gynecol Reprod Biol 1990, 36: 221–228. [DOI] [PubMed] [Google Scholar]
- 30.Coliva A, Zacchetti A, Luison E, Tomassetti A, Bongarzone I, Seregni E, Bombardieri E et al. 90Y labeling of monoclonal antibody MOv18 and preclinical validation for radioimmunotherapy of human ovarian carcinomas. Cancer Immunol Immunother 2005, 54: 1200–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenblum MG, Verschraegen CF, Murray JL, Kudelka AP, Gano J, Cheung L, Kavanagh JJ. Phase I study of 90Y-labeled B72.3 intraperitoneal administration in patients with ovarian cancer: effect of dose and EDTA coadministration on pharmacokinetics and toxicity. Clin Cancer Res 1999, 5: 953–961. [PubMed] [Google Scholar]
- 32.Chang K, Pastan I. Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc Natl Acad Sci USA 1996, 93: 136–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Odunsi K, Jungbluth AA, Stockert E, Qian F, Gnjatic S, Tammela J, Intengan M et al. NY-ESO-1 and LAGE-1 cancer-testis antigens are potential targets for immunotherapy in epithelial ovarian cancer. Cancer Res 2003, 63: 6076–6083. [PubMed] [Google Scholar]
- 34.Sandmaier BM, Oparin DV, Holmberg LA, Reddish MA, MacLean GD, Longenecker BM. Evidence of a cellular immune response against sialyl-Tn in breast and ovarian cancer patients after high-dose chemotherapy, stem cell rescue, and immunization with Theratope STn-KLH cancer vaccine. J Immunother 1999, 22: 54–66. [DOI] [PubMed] [Google Scholar]
- 35.Zigler M, Shir A, Levitzki A. Targeted cancer immunotherapy. Curr Opin Pharmacol 2013, 13: 504–510. [DOI] [PubMed] [Google Scholar]
- 36.Das L, Bhaumik E, Raychaudhuri U, Chakraborty R. Role of nutraceuticals in human health. J Food Sci Technol 2012, 49: 173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gilani AH. Role of medicinal plants in modern medicine. Malaysian J Sci 2005, 24: 1–5. [Google Scholar]
- 38.Houghton PJ. The role of plants in traditional medicine and current therapy. J Altern Complement Med 1995, 1: 131–143. [DOI] [PubMed] [Google Scholar]
- 39.Witczak CA, Sharoff CG, Goodyear LJ. AMP-activated protein kinase in skeletal muscle: from structure and localization to its role as a master regulator of cellular metabolism. Cell Mol Life Sci 2008, 65: 3737–3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carling D. AMP-activated protein kinase: balancing the scales. Biochimie 2005, 87: 87–91. [DOI] [PubMed] [Google Scholar]
- 41.Hardie DG. AMPK: a key regulator of energy balance in the single cell and the whole organism. Int J Obes (Lond) 2008, 32(Suppl. 4): S7–S12. [DOI] [PubMed] [Google Scholar]
- 42.Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer 2009, 9: 563–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuhajda FP. AMP-activated protein kinase and human cancer: cancer metabolism revisited. Int J Obes (Lond) 2008, 32(Suppl. 4): S36–S41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu SY, Chan DW, Liu VW, Ngan HY. Inhibition of cervical cancer cell growth through activation of upstream kinases of AMP-activated protein kinase. Tumour Biol 2009, 30: 80–85. [DOI] [PubMed] [Google Scholar]
- 45.Fay JR, Steele V, Crowell JA. Energy homeostasis and cancer prevention: the AMP-activated protein kinase. Cancer Prev Res (Phila) 2009, 2: 301–309. [DOI] [PubMed] [Google Scholar]
- 46.Li W, Saud SM, Young MR, Chen G, Hua B. Targeting AMPK for cancer prevention and treatment. Oncotarget 2015, 6: 7365–7378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yung MM, Chan DW, Liu VW, Yao KM, Ngan HY. Activation of AMPK inhibits cervical cancer cell growth through AKT/FOXO3a/FOXM1 signaling cascade. BMC Cancer 2013, 13: 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kwan HT, Chan DW, Cai PC, Mak CS, Yung MM, Leung TH, Wong OG et al. AMPK activators suppress cervical cancer cell growth through inhibition of DVL3 mediated Wnt/beta-catenin signaling activity. PLoS One 2013, 8: e53597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hardie DG, Hawley SA. AMP-activated protein kinase: the energy charge hypothesis revisited. Bioessays 2001, 23: 1112–1119. [DOI] [PubMed] [Google Scholar]
- 50.Carling D, Zammit VA, Hardie DG. A common bicyclic protein kinase cascade inactivates the regulatory enzymes of fatty acid and cholesterol biosynthesis. FEBS Lett 1987, 223: 217–222. [DOI] [PubMed] [Google Scholar]
- 51.Hardie DG, Ross FA, Hawley SA. AMP-activated protein kinase: a target for drugs both ancient and modern. Chem Biol 2012, 19: 1222–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol 2012, 13: 251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hardie DG. Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology 2003, 144: 5179–5183. [DOI] [PubMed] [Google Scholar]
- 54.Sanz P. AMP-activated protein kinase: structure and regulation. Curr Protein Pept Sci 2008, 9: 478–492. [DOI] [PubMed] [Google Scholar]
- 55.Fox MM, Phoenix KN, Kopsiaftis SG, Claffey KP. AMP-activated protein kinase alpha 2 isoform suppression in primary breast cancer alters AMPK growth control and apoptotic signaling. Genes Cancer 2013, 4: 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thornton C, Snowden MA, Carling D. Identification of a novel AMP-activated protein kinase beta subunit isoform that is highly expressed in skeletal muscle. J Biol Chem 1998, 273: 12443–12450. [DOI] [PubMed] [Google Scholar]
- 57.Gimeno-Alcaniz JV, Sanz P. Glucose and type 2A protein phosphatase regulate the interaction between catalytic and regulatory subunits of AMP-activated protein kinase. J Mol Biol 2003, 333: 201–209. [DOI] [PubMed] [Google Scholar]
- 58.Li C, Liu VW, Chiu PM, Chan DW, Ngan HY. Over-expressions of AMPK subunits in ovarian carcinomas with significant clinical implications. BMC Cancer 2012, 12: 357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li C, Liu VW, Chiu PM, Yao KM, Ngan HY, Chan DW. Reduced expression of AMPK-beta1 during tumor progression enhances the oncogenic capacity of advanced ovarian cancer. Mol Cancer 2014, 13: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bateman A. The structure of a domain common to archaebacteria and the homocystinuria disease protein. Trends Biochem Sci 1997, 22: 12–13. [DOI] [PubMed] [Google Scholar]
- 61.Scott JW, Hawley SA, Green KA, Anis M, Stewart G, Scullion GA, Norman DG et al. CBS domains form energy-sensing modules whose binding of adenosine ligands is disrupted by disease mutations. J Clin Invest 2004, 113: 274–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boyle JG, Salt IP, McKay GA. Metformin action on AMP-activated protein kinase: a translational research approach to understanding a potential new therapeutic target. Diabet Med 2010, 27: 1097–1106. [DOI] [PubMed] [Google Scholar]
- 63.Liang J, Shao SH, Xu ZX, Hennessy B, Ding Z, Larrea M, Kondo S et al. The energy sensing LKB1-AMPK pathway regulates p27(kip1) phosphorylation mediating the decision to enter autophagy or apoptosis. Nat Cell Biol 2007, 9: 218–224. [DOI] [PubMed] [Google Scholar]
- 64.Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J Biol Chem 2002, 277: 23977–23980. [DOI] [PubMed] [Google Scholar]
- 65.Greer EL, Oskoui PR, Banko MR, Maniar JM, Gygi MP, Gygi SP, Brunet A. The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J Biol Chem 2007, 282: 30107–30119. [DOI] [PubMed] [Google Scholar]
- 66.Ahn YJ, Kim H, Lim H, Lee M, Kang Y, Moon S, Kim HS et al. AMP-activated protein kinase: implications on ischemic diseases. BMB Rep 2012, 45: 489–495. [DOI] [PubMed] [Google Scholar]
- 67.Jones RG, Plas DR, Kubek S, Buzzai M, Mu J, Xu Y, Birnbaum MJ et al. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell 2005, 18: 283–293. [DOI] [PubMed] [Google Scholar]
- 68.Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG et al. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab 2005, 2: 9–19. [DOI] [PubMed] [Google Scholar]
- 69.Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, Carlson M et al. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab 2005, 2: 21–33. [DOI] [PubMed] [Google Scholar]
- 70.Hardie DG. Adenosine monophosphate-activated protein kinase: a central regulator of metabolism with roles in diabetes, cancer, and viral infection. Cold Spring Harb Symp Quant Biol 2011, 76: 155–164. [DOI] [PubMed] [Google Scholar]
- 71.Hardie DG, Sakamoto K. AMPK: a key sensor of fuel and energy status in skeletal muscle. Physiology (Bethesda) 2006, 21: 48–60. [DOI] [PubMed] [Google Scholar]
- 72.Bright NJ, Thornton C, Carling D. The regulation and function of mammalian AMPK-related kinases. Acta Physiol (Oxf) 2009, 196: 15–26. [DOI] [PubMed] [Google Scholar]
- 73.Momcilovic M, Hong SP, Carlson M. Mammalian TAK1 activates Snf1 protein kinase in yeast and phosphorylates AMP-activated protein kinase in vitro. J Biol Chem 2006, 281: 25336–25343. [DOI] [PubMed] [Google Scholar]
- 74.Xie M, Zhang D, Dyck JR, Li Y, Zhang H, Morishima M, Mann DL et al. A pivotal role for endogenous TGF-beta-activated kinase-1 in the LKB1/AMP-activated protein kinase energy-sensor pathway. Proc Natl Acad Sci USA 2006, 103: 17378–17383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cai PC, Shi L, Liu VW, Tang HW, Liu IJ, Leung TH, Chan KK et al. Elevated TAK1 augments tumor growth and metastatic capacities of ovarian cancer cells through activation of NF-kappaB signaling. Oncotarget 2014, 5: 7549–7562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP, Alessi DR et al. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol 2003, 2: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Imamura K, Ogura T, Kishimoto A, Kaminishi M, Esumi H. Cell cycle regulation via p53 phosphorylation by a 5′-AMP activated protein kinase activator, 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside, in a human hepatocellular carcinoma cell line. Biochem Biophys Res Commun 2001, 287: 562–567. [DOI] [PubMed] [Google Scholar]
- 78.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell 2003, 115: 577–590. [DOI] [PubMed] [Google Scholar]
- 79.Rattan R, Giri S, Singh AK, Singh I. 5-Aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside inhibits cancer cell proliferation in vitro and in vivo via AMP-activated protein kinase. J Biol Chem 2005, 280: 39582–39593. [DOI] [PubMed] [Google Scholar]
- 80.Swinnen JV, Beckers A, Brusselmans K, Organe S, Segers J, Timmermans L, Vanderhoydonc F et al. Mimicry of a cellular low energy status blocks tumor cell anabolism and suppresses the malignant phenotype. Cancer Res 2005, 65: 2441–2448. [DOI] [PubMed] [Google Scholar]
- 81.Meisse D, Van de Casteele M, Beauloye C, Hainault I, Kefas BA, Rider MH, Foufelle F et al. Sustained activation of AMP-activated protein kinase induces c-Jun N-terminal kinase activation and apoptosis in liver cells. FEBS Lett 2002, 526: 38–42. [DOI] [PubMed] [Google Scholar]
- 82.Li J, Jiang P, Robinson M, Lawrence TS, Sun Y. AMPK-beta1 subunit is a p53-independent stress responsive protein that inhibits tumor cell growth upon forced expression. Carcinogenesis 2003, 24: 827–834. [DOI] [PubMed] [Google Scholar]
- 83.Saitoh M, Nagai K, Nakagawa K, Yamamura T, Yamamoto S, Nishizaki T. Adenosine induces apoptosis in the human gastric cancer cells via an intrinsic pathway relevant to activation of AMP-activated protein kinase. Biochem Pharmacol 2004, 67: 2005–2011. [DOI] [PubMed] [Google Scholar]
- 84.Xiang X, Saha AK, Wen R, Ruderman NB, Luo Z. AMP-activated protein kinase activators can inhibit the growth of prostate cancer cells by multiple mechanisms. Biochem Biophys Res Commun 2004, 321: 161–167. [DOI] [PubMed] [Google Scholar]
- 85.Li C, Liu VW, Chan DW, Yao KM, Ngan HY. LY294002 and metformin cooperatively enhance the inhibition of growth and the induction of apoptosis of ovarian cancer cells. Int J Gynecol Cancer 2012, 22: 15–22. [DOI] [PubMed] [Google Scholar]
- 86.Elstrom RL, Bauer DE, Buzzai M, Karnauskas R, Harris MH, Plas DR, Zhuang H et al. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res 2004, 64: 3892–3899. [DOI] [PubMed] [Google Scholar]
- 87.Kuhajda FP. Fatty acid synthase and cancer: new application of an old pathway. Cancer Res 2006, 66: 5977–5980. [DOI] [PubMed] [Google Scholar]
- 88.Majewski N, Nogueira V, Bhaskar P, Coy PE, Skeen JE, Gottlob K, Chandel NS et al. Hexokinase-mitochondria interaction mediated by Akt is required to inhibit apoptosis in the presence or absence of Bax and Bak. Mol Cell 2004, 16: 819–830. [DOI] [PubMed] [Google Scholar]
- 89.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev 2004, 18: 1926–1945. [DOI] [PubMed] [Google Scholar]
- 90.Kim JW, Zeller KI, Wang Y, Jegga AG, Aronow BJ, O'Donnell KA, Dang CV. Evaluation of myc E-box phylogenetic footprints in glycolytic genes by chromatin immunoprecipitation assays. Mol Cell Biol 2004, 24: 5923–5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bauer DE, Hatzivassiliou G, Zhao F, Andreadis C, Thompson CB. ATP citrate lyase is an important component of cell growth and transformation. Oncogene 2005, 24: 6314–6322. [DOI] [PubMed] [Google Scholar]
- 92.Carling D. The AMP-activated protein kinase cascade—a unifying system for energy control. Trends Biochem Sci 2004, 29: 18–24. [DOI] [PubMed] [Google Scholar]
- 93.Hardie DG, Alessi DR. LKB1 and AMPK and the cancer-metabolism link—ten years after. BMC Biol 2013, 11: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jeon SM, Hay N. The dark face of AMPK as an essential tumor promoter. Cell Logist 2012, 2: 197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kato K, Ogura T, Kishimoto A, Minegishi Y, Nakajima N, Miyazaki M, Esumi H. Critical roles of AMP-activated protein kinase in constitutive tolerance of cancer cells to nutrient deprivation and tumor formation. Oncogene 2002, 21: 6082–6090. [DOI] [PubMed] [Google Scholar]
- 96.Jang T, Calaoagan JM, Kwon E, Samuelsson S, Recht L, Laderoute KR. 5′-AMP-activated protein kinase activity is elevated early during primary brain tumor development in the rat. Int J Cancer 2011, 128: 2230–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Godlewski J, Nowicki MO, Bronisz A, Nuovo G, Palatini J, De Lay M, Van Brocklyn J et al. MicroRNA-451 regulates LKB1/AMPK signaling and allows adaptation to metabolic stress in glioma cells. Mol Cell 2010, 37: 620–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jeon SM, Chandel NS, Hay N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature 2012, 485: 661–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Asby DJ, Cuda F, Beyaert M, Houghton FD, Cagampang FR, Tavassoli A. AMPK activation via modulation of de novo purine biosynthesis with an inhibitor of ATIC homodimerization. Chem Biol 2015, 22: 838–848. [DOI] [PubMed] [Google Scholar]
- 100.Jenkins Y, Sun TQ, Markovtsov V, Foretz M, Li W, Nguyen H, Li Y et al. AMPK activation through mitochondrial regulation results in increased substrate oxidation and improved metabolic parameters in models of diabetes. PLoS One 2013, 8: e81870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Coughlan KA, Valentine RJ, Ruderman NB, Saha AK. AMPK activation: a therapeutic target for type 2 diabetes? Diabetes Metab Syndr Obes 2014, 7: 241–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hwang YP, Kim HG, Hien TT, Jeong MH, Jeong TC, Jeong HG. Puerarin activates endothelial nitric oxide synthase through estrogen receptor-dependent PI3-kinase and calcium-dependent AMP-activated protein kinase. Toxicol Appl Pharmacol 2011, 257: 48–58. [DOI] [PubMed] [Google Scholar]
- 103.Shen QW, Zhu MJ, Tong J, Ren J, Du M. Ca2+/calmodulin-dependent protein kinase kinase is involved in AMP-activated protein kinase activation by alpha-lipoic acid in C2C12 myotubes. Am J Physiol Cell Physiol 2007, 293: C1395–C1403. [DOI] [PubMed] [Google Scholar]
- 104.Memmott RM, Dennis PA. Akt-dependent and -independent mechanisms of mTOR regulation in cancer. Cell Signal 2009, 21: 656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.El-Mir MY, Nogueira V, Fontaine E, Averet N, Rigoulet M, Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem 2000, 275: 223–228. [DOI] [PubMed] [Google Scholar]
- 106.Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J 2000, 348(Pt 3): 607–614. [PMC free article] [PubMed] [Google Scholar]
- 107.Corradetti MN, Inoki K, Bardeesy N, DePinho RA, Guan KL. Regulation of the TSC pathway by LKB1: evidence of a molecular link between tuberous sclerosis complex and Peutz-Jeghers syndrome. Genes Dev 2004, 18: 1533–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mohanti BK, Rath GK, Anantha N, Kannan V, Das BS, Chandramouli BA, Banerjee AK et al. Improving cancer radiotherapy with 2-deoxy-D-glucose: phase I/II clinical trials on human cerebral gliomas. Int J Radiat Oncol Biol Phys 1996, 35: 103–111. [DOI] [PubMed] [Google Scholar]
- 109.Daniel T, Carling D. Expression and regulation of the AMP-activated protein kinase-SNF1 (sucrose non-fermenting 1) kinase complexes in yeast and mammalian cells: studies using chimaeric catalytic subunits. Biochem J 2002, 365: 629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Choi SL, Kim SJ, Lee KT, Kim J, Mu J, Birnbaum MJ, Soo Kim S et al. The regulation of AMP-activated protein kinase by H(2)O(2). Biochem Biophys Res Commun 2001, 287: 92–97. [DOI] [PubMed] [Google Scholar]
- 111.Goransson O, McBride A, Hawley SA, Ross FA, Shpiro N, Foretz M, Viollet B et al. Mechanism of action of A-769662, a valuable tool for activation of AMP-activated protein kinase. J Biol Chem 2007, 282: 32549–32560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444: 337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dong GZ, Jang EJ, Kang SH, Cho IJ, Park SD, Kim SC, Kim YW. Red ginseng abrogates oxidative stress via mitochondria protection mediated by LKB1-AMPK pathway. BMC Complement Altern Med 2013, 13: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lim HW, Lim HY, Wong KP. Uncoupling of oxidative phosphorylation by curcumin: implication of its cellular mechanism of action. Biochem Biophys Res Commun 2009, 389: 187–192. [DOI] [PubMed] [Google Scholar]
- 115.Yung MM, Ross FA, Hardie DG, Leung TH, Zhan J, Ngan HY, Chan DW. Bitter melon (Momordica charantia) extract inhibits tumorigenicity and overcomes cisplatin-resistance in ovarian cancer cells through targeting AMPK signaling cascade. Integr Cancer Ther 2015. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 116.Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-Aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem 1995, 229: 558–565. [DOI] [PubMed] [Google Scholar]
- 117.Jorgensen SB, Viollet B, Andreelli F, Frosig C, Birk JB, Schjerling P, Vaulont S et al. Knockout of the alpha2 but not alpha1 5′-AMP-activated protein kinase isoform abolishes 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranosidebut not contraction-induced glucose uptake in skeletal muscle. J Biol Chem 2004, 279: 1070–1079. [DOI] [PubMed] [Google Scholar]
- 118.Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci USA 2004, 101: 3329–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shaw RJ, Bardeesy N, Manning BD, Lopez L, Kosmatka M, DePinho RA, Cantley LC. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell 2004, 6: 91–99. [DOI] [PubMed] [Google Scholar]
- 120.Lee MJ, Feliers D, Mariappan MM, Sataranatarajan K, Mahimainathan L, Musi N, Foretz M et al. A role for AMP-activated protein kinase in diabetes-induced renal hypertrophy. Am J Physiol Renal Physiol 2007, 292: F617–F627. [DOI] [PubMed] [Google Scholar]
- 121.Dixon R, Gourzis J, McDermott D, Fujitaki J, Dewland P, Gruber H. AICA-riboside: safety, tolerance, and pharmacokinetics of a novel adenosine-regulating agent. J Clin Pharmacol 1991, 31: 342–347. [DOI] [PubMed] [Google Scholar]
- 122.Holmes BF, Kurth-Kraczek EJ, Winder WW. Chronic activation of 5′-AMP-activated protein kinase increases GLUT-4, hexokinase, and glycogen in muscle. J Appl Physiol 1999, 87: 1990–1995. [DOI] [PubMed] [Google Scholar]
- 123.Leung JM, Stanley T III, Mathew J, Curling P, Barash P, Salmenpera M, Reves JG et al. An initial multicenter, randomized controlled trial on the safety and efficacy of acadesine in patients undergoing coronary artery bypass graft surgery. SPI Research Group. Anesth Analg 1994, 78: 420–434. [DOI] [PubMed] [Google Scholar]
- 124.Zou MH, Kirkpatrick SS, Davis BJ, Nelson JS, Wiles WGT, Schlattner U, Neumann D et al. Activation of the AMP-activated protein kinase by the anti-diabetic drug metformin in vivo. Role of mitochondrial reactive nitrogen species. J Biol Chem 2004, 279: 43940–43951. [DOI] [PubMed] [Google Scholar]
- 125.Witters LA. The blooming of the French lilac. J Clin Invest 2001, 108: 1105–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hawley SA, Gadalla AE, Olsen GS, Hardie DG. The antidiabetic drug metformin activates the AMP-activated protein kinase cascade via an adenine nucleotide-independent mechanism. Diabetes 2002, 51: 2420–2425. [DOI] [PubMed] [Google Scholar]
- 127.Hesse G, Taubmann G. Die wirkung des biguanids und seiner derivate aud den zuckerstoffwechsel. Arch Exp Path Pharm 1929, 142: 290–308. [Google Scholar]
- 128.Xie Z, Dong Y, Scholz R, Neumann D, Zou MH. Phosphorylation of LKB1 at serine 428 by protein kinase C-zeta is required for metformin-enhanced activation of the AMP-activated protein kinase in endothelial cells. Circulation 2008, 117: 952–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hundal RS, Krssak M, Dufour S, Laurent D, Lebon V, Chandramouli V, Inzucchi SE et al. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes 2000, 49: 2063–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Musi N, Hirshman MF, Nygren J, Svanfeldt M, Bavenholm P, Rooyackers O, Zhou G et al. Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes 2002, 51: 2074–2081. [DOI] [PubMed] [Google Scholar]
- 131.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 2001, 108: 1167–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fryer LG, Parbu-Patel A, Carling D. The anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J Biol Chem 2002, 277: 25226–25232. [DOI] [PubMed] [Google Scholar]
- 133.Vakana E, Platanias LC. AMPK in BCR-ABL expressing leukemias. Regulatory effects and therapeutic implications. Oncotarget 2011, 2: 1322–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ 2005, 330: 1304–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jiralerspong S, Palla SL, Giordano SH, Meric-Bernstam F, Liedtke C, Barnett CM, Hsu L et al. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J Clin Oncol 2009, 27: 3297–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zakikhani M, Dowling R, Fantus IG, Sonenberg N, Pollak M. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res 2006, 66: 10269–10273. [DOI] [PubMed] [Google Scholar]
- 137.Dowling RJ, Zakikhani M, Fantus IG, Pollak M, Sonenberg N. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res 2007, 67: 10804–10812. [DOI] [PubMed] [Google Scholar]
- 138.Huang X, Wullschleger S, Shpiro N, McGuire VA, Sakamoto K, Woods YL, McBurnie W et al. Important role of the LKB1-AMPK pathway in suppressing tumorigenesis in PTEN-deficient mice. Biochem J 2008, 412: 211–221. [DOI] [PubMed] [Google Scholar]
- 139.Wingo SN, Gallardo TD, Akbay EA, Liang MC, Contreras CM, Boren T, Shimamura T et al. Somatic LKB1 mutations promote cervical cancer progression. PLoS One 2009, 4: e5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sanchez-Cespedes M, Parrella P, Esteller M, Nomoto S, Trink B, Engles JM, Westra WH et al. Inactivation of LKB1/STK11 is a common event in adenocarcinomas of the lung. Cancer Res 2002, 62: 3659–3662. [PubMed] [Google Scholar]
- 141.Ji H, Ramsey MR, Hayes DN, Fan C, McNamara K, Kozlowski P, Torrice C et al. LKB1 modulates lung cancer differentiation and metastasis. Nature 2007, 448: 807–810. [DOI] [PubMed] [Google Scholar]
- 142.Tebbe C, Chhina J, Dar SA, Sarigiannis K, Giri S, Munkarah AR, Rattan R. Metformin limits the adipocyte tumor-promoting effect on ovarian cancer. Oncotarget 2014, 5: 4746–4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Daval M, Foufelle F, Ferre P. Functions of AMP-activated protein kinase in adipose tissue. J Physiol 2006, 574: 55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gaidhu MP, Bikopoulos G, Ceddia RB. Chronic AICAR-induced AMP-kinase activation regulates adipocyte lipolysis in a time-dependent and fat depot-specific manner in rats. Am J Physiol Cell Physiol 2012, 303: C1192–C1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, Zillhardt MR, Romero IL et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med 2011, 17: 1498–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhong D, Guo L, de Aguirre I, Liu X, Lamb N, Sun SY, Gal AA et al. LKB1 mutation in large cell carcinoma of the lung. Lung Cancer 2006, 53: 285–294. [DOI] [PubMed] [Google Scholar]
- 147.Hurley RL, Anderson KA, Franzone JM, Kemp BE, Means AR, Witters LA. The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J Biol Chem 2005, 280: 29060–29066. [DOI] [PubMed] [Google Scholar]
- 148.Hayashi T, Hirshman MF, Fujii N, Habinowski SA, Witters LA, Goodyear LJ. Metabolic stress and altered glucose transport: activation of AMP-activated protein kinase as a unifying coupling mechanism. Diabetes 2000, 49: 527–531. [DOI] [PubMed] [Google Scholar]
- 149.Almeida A, Cidad P, Delgado-Esteban M, Fernandez E, Garcia-Nogales P, Bolanos JP. Inhibition of mitochondrial respiration by nitric oxide: its role in glucose metabolism and neuroprotection. J Neurosci Res 2005, 79: 166–171. [DOI] [PubMed] [Google Scholar]
- 150.Tainter ML, Cutting WC, Stockton AB. Use of dinitrophenol in nutritional disorders: a critical survey of clinical results. Am J Public Health Nations Health 1934, 24: 1045–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cool B, Zinker B, Chiou W, Kifle L, Cao N, Perham M, Dickinson R et al. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab 2006, 3: 403–416. [DOI] [PubMed] [Google Scholar]
- 152.Scott JW, van Denderen BJ, Jorgensen SB, Honeyman JE, Steinberg GR, Oakhill JS, Iseli TJ et al. Thienopyridone drugs are selective activators of AMP-activated protein kinase beta1-containing complexes. Chem Biol 2008, 15: 1220–1230. [DOI] [PubMed] [Google Scholar]
- 153.Sanders MJ, Ali ZS, Hegarty BD, Heath R, Snowden MA, Carling D. Defining the mechanism of activation of AMP-activated protein kinase by the small molecule A-769662, a member of the thienopyridone family. J Biol Chem 2007, 282: 32539–32548. [DOI] [PubMed] [Google Scholar]
- 154.Hawley SA, Ross FA, Chevtzoff C, Green KA, Evans A, Fogarty S, Towler MC et al. Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab 2010, 11: 554–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Stone E. An account of the success of the bark of the willow in the cure of agues. Phil Trans 1763, 53: 195–200. [Google Scholar]
- 156.Hawley SA, Fullerton MD, Ross FA, Schertzer JD, Chevtzoff C, Walker KJ, Peggie MW et al. The ancient drug salicylate directly activates AMP-activated protein kinase. Science 2012, 336: 918–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Turner N, Li JY, Gosby A, To SW, Cheng Z, Miyoshi H, Taketo MM et al. Berberine and its more biologically available derivative, dihydroberberine, inhibit mitochondrial respiratory complex I: a mechanism for the action of berberine to activate AMP-activated protein kinase and improve insulin action. Diabetes 2008, 57: 1414–1418. [DOI] [PubMed] [Google Scholar]
- 158.Collins QF, Liu HY, Pi J, Liu Z, Quon MJ, Cao W. Epigallocatechin-3-gallate (EGCG), a green tea polyphenol, suppresses hepatic gluconeogenesis through 5′-AMP-activated protein kinase. J Biol Chem 2007, 282: 30143–30149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hwang JT, Park IJ, Shin JI, Lee YK, Lee SK, Baik HW, Ha J et al. Genistein, EGCG, and capsaicin inhibit adipocyte differentiation process via activating AMP-activated protein kinase. Biochem Biophys Res Commun 2005, 338: 694–699. [DOI] [PubMed] [Google Scholar]
- 160.Lin YC, Hung CM, Tsai JC, Lee JC, Chen YL, Wei CW, Kao JY et al. Hispidulin potently inhibits human glioblastoma multiforme cells through activation of AMP-activated protein kinase (AMPK). J Agric Food Chem 2010, 58: 9511–9517. [DOI] [PubMed] [Google Scholar]
- 161.Lin CL, Huang HC, Lin JK. Theaflavins attenuate hepatic lipid accumulation through activating AMPK in human HepG2 cells. J Lipid Res 2007, 48: 2334–2343. [DOI] [PubMed] [Google Scholar]
- 162.Lee ES, Uhm KO, Lee YM, Han M, Lee M, Park JM, Suh PG et al. CAPE (caffeic acid phenethyl ester) stimulates glucose uptake through AMPK (AMP-activated protein kinase) activation in skeletal muscle cells. Biochem Biophys Res Commun 2007, 361: 854–858. [DOI] [PubMed] [Google Scholar]
- 163.Ki SH, Choi JH, Kim CW, Kim SG. Combined metadoxine and garlic oil treatment efficaciously abrogates alcoholic steatosis and CYP2E1 induction in rat liver with restoration of AMPK activity. Chem Biol Interact 2007, 169: 80–90. [DOI] [PubMed] [Google Scholar]
- 164.Kaur M, Deep G, Jain AK, Raina K, Agarwal C, Wempe MF, Agarwal R. Bitter melon juice activates cellular energy sensor AMP-activated protein kinase causing apoptotic death of human pancreatic carcinoma cells. Carcinogenesis 2013, 34: 1585–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Liu S, Fang Y, Shen H, Xu W, Li H. Berberine sensitizes ovarian cancer cells to cisplatin through miR-21/PDCD4 axis. Acta Biochim Biophys Sin 2013, 45: 756–762. [DOI] [PubMed] [Google Scholar]
- 166.Opipari AW Jr, Tan L, Boitano AE, Sorenson DR, Aurora A, Liu JR. Resveratrol-induced autophagocytosis in ovarian cancer cells. Cancer Res 2004, 64: 696–703. [DOI] [PubMed] [Google Scholar]
- 167.Hasima N, Ozpolat B. Regulation of autophagy by polyphenolic compounds as a potential therapeutic strategy for cancer. Cell Death Dis 2014, 5: e1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ray RB, Raychoudhuri A, Steele R, Nerurkar P. Bitter melon (Momordica charantia) extract inhibits breast cancer cell proliferation by modulating cell cycle regulatory genes and promotes apoptosis. Cancer Res 2010, 70: 1925–1931. [DOI] [PubMed] [Google Scholar]
- 169.Pitchakarn P, Suzuki S, Ogawa K, Pompimon W, Takahashi S, Asamoto M, Limtrakul P et al. Induction of G1 arrest and apoptosis in androgen-dependent human prostate cancer by Kuguacin J, a triterpenoid from Momordica charantia leaf. Cancer Lett 2011, 306: 142–150. [DOI] [PubMed] [Google Scholar]
- 170.Ganguly C, De S, Das S. Prevention of carcinogen-induced mouse skin papilloma by whole fruit aqueous extract of Momordica charantia. Eur J Cancer Prev 2000, 9: 283–288. [DOI] [PubMed] [Google Scholar]
- 171.Pitchakarn P, Ohnuma S, Pintha K, Pompimon W, Ambudkar SV, Limtrakul P. Kuguacin J isolated from Momordica charantia leaves inhibits P-glycoprotein (ABCB1)-mediated multidrug resistance. J Nutr Biochem 2011, 23: 76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Fan JM, Luo J, Xu J, Zhu S, Zhang Q, Gao DF, Xu YB et al. Effects of recombinant MAP30 on cell proliferation and apoptosis of human colorectal carcinoma LoVo cells. Mol Biotechnol 2008, 39: 79–86. [DOI] [PubMed] [Google Scholar]
- 173.Lee-Huang S, Huang PL, Chen HC, Bourinbaiar A, Huang HI, Kung HF. Anti-HIV and anti-tumor activities of recombinant MAP30 from bitter melon. Gene 1995, 161: 151–156. [DOI] [PubMed] [Google Scholar]
- 174.Lavhale MS, Kumar S, Mishra SH, Sitasawad SL. A novel triterpenoid isolated from the root bark of Ailanthus excelsa Roxb (Tree of Heaven), AECHL-1 as a potential anti-cancer agent. PLoS One 2009, 4: e5365. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 175.Sun C, Zhang M, Shan X, Zhou X, Yang J, Wang Y, Li-Ling J et al. Inhibitory effect of cucurbitacin E on pancreatic cancer cells growth via STAT3 signaling. J Cancer Res Clin Oncol 2010, 136: 603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yasuda S, Yogosawa S, Izutani Y, Nakamura Y, Watanabe H, Sakai T. Cucurbitacin B induces G2 arrest and apoptosis via a reactive oxygen species-dependent mechanism in human colon adenocarcinoma SW480 cells. Mol Nutr Food Res 2010, 54: 559–565. [DOI] [PubMed] [Google Scholar]
- 177.Yanamandra N, Berhow MA, Konduri S, Dinh DH, Olivero WC, Nicolson GL, Rao JS. Triterpenoids from glycine max decrease invasiveness and induce caspase-mediated cell death in human SNB19 glioma cells. Clin Exp Metastasis 2003, 20: 375–383. [DOI] [PubMed] [Google Scholar]
- 178.Weng CJ, Chau CF, Chen KD, Chen DH, Yen GC. The anti-invasive effect of lucidenic acids isolated from a new Ganoderma lucidum strain. Mol Nutr Food Res 2007, 51: 1472–1477. [DOI] [PubMed] [Google Scholar]
- 179.Zhang M, Hettiarachchy NS, Horax R, Chen P, Over KF. Effect of maturity stages and drying methods on the retention of selected nutrients and phytochemicals in bitter melon (Momordica charantia) leaf. J Food Sci 2009, 74: C441–C448. [DOI] [PubMed] [Google Scholar]
- 180.Lin JP, Yang JS, Lu CC, Chiang JH, Wu CL, Lin JJ, Lin HL et al. Rutin inhibits the proliferation of murine leukemia WEHI-3 cells in vivo and promotes immune response in vivo. Leuk Res 2009, 33: 823–828. [DOI] [PubMed] [Google Scholar]
- 181.Luo H, Jiang BH, King SM, Chen YC. Inhibition of cell growth and VEGF expression in ovarian cancer cells by flavonoids. Nutr Cancer 2008, 60: 800–809. [DOI] [PubMed] [Google Scholar]
- 182.Martinez Conesa C, Vicente Ortega V, Yanez Gascon MJ, Alcaraz Banos M, Canteras Jordana M, Benavente-Garcia O, Castillo J. Treatment of metastatic melanoma B16F10 by the flavonoids tangeretin, rutin, and diosmin. J Agric Food Chem 2005, 53: 6791–6797. [DOI] [PubMed] [Google Scholar]
- 183.Limtrakul P, Pitchakarn P, Suzuki S, Kuguacin J. A Triterpenoid from Momordica charantia Linn: A Comprehensive Review of Anticarcinogenic Properties. Carcinogenesis, Dr Kathryn Tonissen (Ed) 2013: 275–296. [Google Scholar]
- 184.Kay JP, Alemzadeh R, Langley G, D'Angelo L, Smith P, Holshouser S. Beneficial effects of metformin in normoglycemic morbidly obese adolescents. Metabolism 2001, 50: 1457–1461. [DOI] [PubMed] [Google Scholar]
- 185.McCarty MF. Does bitter melon contain an activator of AMP-activated kinase? Med Hypotheses 2004, 63: 340–343. [DOI] [PubMed] [Google Scholar]
- 186.Chen Q, Chan LL, Li ET. Bitter melon (Momordica charantia) reduces adiposity, lowers serum insulin and normalizes glucose tolerance in rats fed a high fat diet. J Nutr 2003, 133: 1088–1093. [DOI] [PubMed] [Google Scholar]
- 187.Stumvoll M, Nurjhan N, Perriello G, Dailey G, Gerich JE. Metabolic effects of metformin in non-insulin-dependent diabetes mellitus. N Engl J Med 1995, 333: 550–554. [DOI] [PubMed] [Google Scholar]
- 188.Fontbonne A, Charles MA, Juhan-Vague I, Bard JM, Andre P, Isnard F, Cohen JM et al. The effect of metformin on the metabolic abnormalities associated with upper-body fat distribution. BIGPRO Study Group. Diabetes Care 1996, 19: 920–926. [DOI] [PubMed] [Google Scholar]
- 189.Rao BK, Kesavulu MM, Giri R, Appa Rao C. Antidiabetic and hypolipidemic effects of Momordica cymbalaria Hook. fruit powder in alloxan-diabetic rats. J Ethnopharmacol 1999, 67: 103–109. [DOI] [PubMed] [Google Scholar]
- 190.Ahmed I, Lakhani MS, Gillett M, John A, Raza H. Hypotriglyceridemic and hypocholesterolemic effects of anti-diabetic Momordica charantia (karela) fruit extract in streptozotocin-induced diabetic rats. Diabetes Res Clin Pract 2001, 51: 155–161. [DOI] [PubMed] [Google Scholar]
- 191.Platel K, Shurpalekar KS, Srinivasan K. Influence of bitter gourd (Momordica charantia) on growth and blood constituents in albino rats. Nahrung 1993, 37: 156–160. [DOI] [PubMed] [Google Scholar]
- 192.Singh N, Tyagi SD, Agarwal SC. Effects of long term feeding of acetone extract of Momordica charantia (whole fruit powder) on alloxan diabetic albino rats. Indian J Physiol Pharmacol 1989, 33: 97–100. [PubMed] [Google Scholar]
- 193.Fanghanel G, Sanchez-Reyes L, Trujillo C, Sotres D, Espinosa-Campos J. Metformin's effects on glucose and lipid metabolism in patients with secondary failure to sulfonylureas. Diabetes Care 1996, 19: 1185–1189. [DOI] [PubMed] [Google Scholar]
- 194.Guthrie R. Treatment of non-insulin-dependent diabetes mellitus with metformin. J Am Board Fam Pract 1997, 10: 213–221. [PubMed] [Google Scholar]
- 195.Palumbo PJ. Metformin: effects on cardiovascular risk factors in patients with non-insulin-dependent diabetes mellitus. J Diabetes Complications 1998, 12: 110–119. [DOI] [PubMed] [Google Scholar]
- 196.Charles MA, Eschwege E, Grandmottet P, Isnard F, Cohen JM, Bensoussan JL, Berche H et al. Treatment with metformin of non-diabetic men with hypertension, hypertriglyceridaemia and central fat distribution: the BIGPRO 1.2 trial. Diabetes Metab Res Rev 2000, 16: 2–7. [DOI] [PubMed] [Google Scholar]
- 197.Chiampanichayakul S, Kataoka K, Arimochi H, Thumvijit S, Kuwahara T, Nakayama H, Vinitketkumnuen U et al. Inhibitory effects of bitter melon (Momordica charantia Linn.) on bacterial mutagenesis and aberrant crypt focus formation in the rat colon. J Med Invest 2001, 48: 88–96. [PubMed] [Google Scholar]
- 198.Nagasawa H, Watanabe K, Inatomi H. Effects of bitter melon (Momordica charantia l.) or ginger rhizome (Zingiber offifinale rosc) on spontaneous mammary tumorigenesis in SHN mice. Am J Chin Med 2002, 30: 195–205. [DOI] [PubMed] [Google Scholar]
- 199.Dilman VM, Berstein LM, Zabezhinski MA, Alexandrov VA, Bobrov JF, Pliss GB. Inhibition of DMBA-induced carcinogenesis by phenformin in the mammary gland of rats. Arch Geschwulstforsch 1978, 48: 1–8. [PubMed] [Google Scholar]