CD4+ and CD8+ recent thymic emigrants (RTEs) exhibit an anergic phenotype after encounter with self-antigen in the periphery. In the presence of inflammation, both CD4+ and CD8+ RTEs can be converted into competent diabetogenic effector cells.
Abstract
T cell development requires a period of postthymic maturation. Why this is the case has remained a mystery, particularly given the rigors of intrathymic developmental checkpoints, successfully traversed by only ∼5% of thymocytes. We now show that the first few weeks of T cell residence in the lymphoid periphery define a period of heightened susceptibility to tolerance induction to tissue-restricted antigens (TRAs), the outcome of which depends on the context in which recent thymic emigrants (RTEs) encounter antigen. After encounter with TRAs in the absence of inflammation, RTEs exhibited defects in proliferation, diminished cytokine production, elevated expression of anergy-associated genes, and diminished diabetogenicity. These properties were mirrored in vitro by enhanced RTE susceptibility to regulatory T cell–mediated suppression. In the presence of inflammation, RTEs and mature T cells were, in contrast, equally capable of inducing diabetes, proliferating, and producing cytokines. Thus, recirculating RTEs encounter TRAs during a transitional developmental stage that facilitates tolerance induction, but inflammation converts antigen-exposed, tolerance-prone RTEs into competent effector cells.
To ensure the generation of functional, self-tolerant T cells during thymic development, self-reactive thymocytes are negatively selected during the process of central tolerance. However, central tolerance is imperfect, and some T cells recognizing self-antigen do escape deletion and enter the lymphoid periphery.
The study of the youngest peripheral T cells, termed recent thymic emigrants (RTEs), has been facilitated by the use of RAG2p-GFP transgenic (Tg) mice in which GFP is expressed under the control of the Rag2 promoter (Yu et al., 1999). Although Rag2 expression is extinguished intrathymically, the residual GFP signal remains detectable in RTEs for ∼3 wk (Boursalian et al., 2004). GFP signal strength correlates inversely with the time since the loss of Rag2 expression (McCaughtry et al., 2007), allowing RTEs of varied ages to be distinguished from their GFP− mature naive (MN) counterparts. Using such reporter mice, we and others have shown that RTEs are phenotypically and functionally distinct from MN T cells (Priyadharshini et al., 2010; Bhaumik et al., 2013; Fink, 2013; Paiva et al., 2013; Berkley and Fink, 2014; Hogquist et al., 2015), differences also ascribed to neonatal T cells (Opiela et al., 2009; Zaghouani et al., 2009; PrabhuDas et al., 2011; Smith et al., 2014) and to human RTEs (McFarland et al., 2000; Haines et al., 2009).
Phenotypic and functional maturation of peripheral T cells requires both thymic egress and access to secondary lymphoid organs (Houston et al., 2008), but is not driven by molecules known to influence T cell homeostasis (Houston and Fink, 2009). It remains unclear what advantages are accrued by the export of T cells that interpret and respond to their immunological environment in a manner so distinct from that of their mature counterparts. RTEs have a slightly modified TCR repertoire, with longer average CDR3 lengths (Houston and Fink, 2009). Given that shorter CDR3s are associated with intrathymic tolerance (Matsutani et al., 2007), this repertoire shaping suggests that postthymic maturation might involve tolerance induction.
To test whether RTEs are tolerized to peripherally expressed self-antigen, we used RIP-mOVA Tg mice expressing a membrane-bound form of OVA under the control of the rat insulin promoter (Kurts et al., 1997a) that drives peripheral expression primarily in the pancreatic β islets and kidney proximal tubules. These mice have been used to model islet autoantigens and thereby identify the cell types involved in the islet cell destruction driving autoimmune type I diabetes. Antigen in these mice can be detected by both OVA-specific CD4 (OT-II) and CD8 (OT-I) T cells. Tolerance induction of OT-I T cells in RIP-mOVA Tg mice has been ascribed to cross presentation of OVA by dendritic cells in the pancreatic LNs (pLNs; Heath et al., 2004). Analysis of autoreactive OT-I T cells in the RIP-mOVA system indicates that control of CD4 help is critical for the maintenance of CD8 T cell tolerance induced by cross-presentation (Kurts et al., 1997a).
We show here that CD4 RTEs were more sensitive than their mature counterparts to regulatory T cell (T reg cell)–mediated suppression in vitro, and after self-antigen encounter in vivo, both CD4 and CD8 RTEs proliferated less, secreted less IL-2 and IFN-γ, and expressed elevated levels of anergy-associated genes. Correspondingly, both OT-II and OT-I RTEs were less diabetogenic than their mature counterparts after transfer into RIP-mOVA Tg hosts. However, in the presence of inflammation, RTEs proliferated to the same extent and secreted as much IL-2 as mature T cells. These results place RTEs at a crossroads between tolerance induction and effector cell differentiation, with their ultimate fate guided by the presence or absence of inflammation during antigen recognition.
RESULTS AND DISCUSSION
RTEs exhibit functional defects after in vivo exposure to peripheral self-antigen
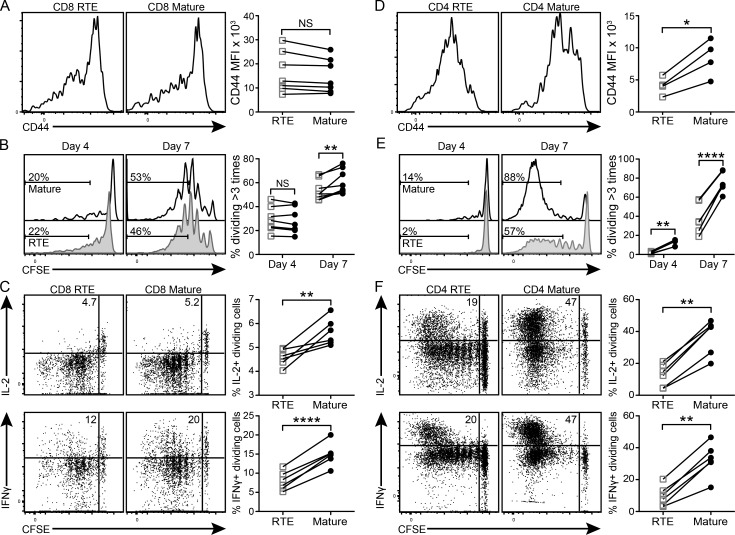
To quantify the tissue-restricted antigen (TRA) reactivity of peripheral T cells as a function of their maturation stage, an equal number of congenically marked, naive CFSE-labeled OT-I (Fig. 1, A–C) or OT-II (Fig. 1, D–F) RTEs and MN T cells were cotransferred from antigen-free donors into RIP-mOVA Tg hosts. Bulk OT-I T cells were cotransferred with OT-II RTEs and MN T cells to enhance antigen release and promote activation of donor cells in the draining pLNs. The absence of donor cell activation in either WT C57BL/6 (B6) hosts or in the nondraining LNs of RIP-mOVA Tg hosts underscores the antigen specificity of this activation (not depicted). Although there was no significant difference in the expression level of the activation marker CD44 on CD8 RTEs and mature T cells (Fig. 1 A) harvested from the pLNs of RIP-mOVA Tg hosts 4 d after transfer, CD4 RTEs were significantly less activated compared with their mature counterparts (Fig. 1 D). Correspondingly, donor CD8 RTEs only exhibited proliferative defects at day 7 after transfer (Fig. 1 B), whereas donor CD4 RTEs exhibited proliferative defects at both days 4 and 7 (Fig. 1 E).
Figure 1.
CD4 and CD8 RTEs proliferate less and secrete less IL-2 and IFN-γ than mature T cells in response to peripheral self-antigen in vivo. (A–C) 106 naive OT-I RTEs and MN T cells were cotransferred into RIP-mOVA Tg hosts. (D–F) 0.5 × 106 naive OT-II RTEs and MN T cells were cotransferred with 2 × 106 bulk naive OT-I T cells into RIP-mOVA Tg hosts. (A and D) Donor cells from the draining pLNs were analyzed 4 d later. Histograms show representative CD44 staining by RTEs and mature T cells. Data in the right panels show CD44 mean fluorescence intensity (MFI) compiled from two independent experiments (n = 4–7). (B and E) Proliferation of donor cells from the pLNs was quantified 4 and 7 d after transfer. Numbers in the histograms represent the percentages of donor RTEs (shaded) and mature T cells (open) that have divided more than three times. Data in the right panels show percentages of donor RTEs (open squares) and mature T cells (filled circles) dividing more than three times compiled from two independent experiments (n = 4–8). (C and F) 7 d after transfer, cells from recipient pLNs were restimulated in vitro for 5 h and stained for intracellular cytokines. Numbers in the top left dot plot quadrants represent the percentages of IL-2+ or IFN-γ+ dividing donor cells. Data in the right panels are compiled from two independent experiments (n = 6–8). *, P ≤ 0.05; **, P ≤ 0.01; ****, P ≤ 0.001, using a paired Student’s t test.
Next, we examined effector cytokine production by donor CD8 and CD4 RTEs and mature T cells after peripheral self-antigen encounter. 7 d after transfer into RIP-mOVA Tg hosts, divided CD8 and CD4 RTEs produced less IL-2 and IFN-γ, both proportionally (Fig. 1, C and F) and on a per cell basis, as measured by mean fluorescence intensity (not depicted). This defect in cytokine production is not simply a downstream consequence of diminished proliferation, as CD4 RTEs exhibited defects even when IL-2 and IFN-γ production were expressed as a function of cell division (not depicted). Despite the fact that CD4 RTEs are skewed away from the Th1 lineage (Hendricks and Fink, 2011), CD4 RTEs exposed to self-antigen in vivo make neither IL-4 nor IL-17 (not depicted). Thus, upon self-antigen recognition in vivo, both CD4 and CD8 RTEs exhibit functional defects similar to those described in murine (Hendricks and Fink, 2011) and human (Haines et al., 2009) RTEs stimulated in vitro.
RTEs are less diabetogenic than their mature T cell counterparts
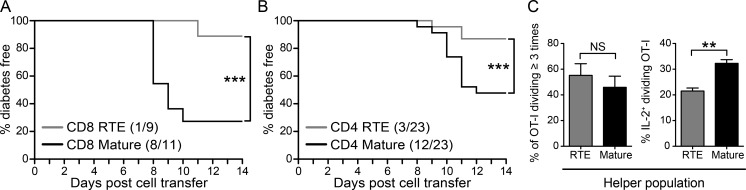
To determine whether RTE functional defects impact their autoimmune capacity, we measured diabetes induction after transfer of OT-I RTEs or MN T cells into separate RIP-mOVA Tg hosts. Although high numbers of transferred OT-I T cells alone can induce diabetes in RIP-mOVA Tg mice, help provided by cotransferred OT-II T cells (which alone fail to induce diabetes) facilitates disease initiation by a lower number of OT-I T cells (Kurts et al., 1997a). We therefore provided CD4 T cell help in the form of cotransferred bulk OT-II Rag1−/− T cells. 8 d later, recipients of OT-I MN T cells developed autoimmune diabetes; diabetes was both delayed and less prevalent in the recipients of RTEs (Fig. 2 A). Thus, CD8 RTEs are less diabetogenic than their mature counterparts.
Figure 2.
CD4 and CD8 RTEs are less efficient than mature T cells at inducing diabetes. (A) 106 naive OT-I RTEs or MN T cells were transferred with 5–8 × 105 bulk naive OT-II Rag1−/− T cells into separate RIP-mOVA Tg hosts. (B) 7 × 105 to 106 naive OT-II RTEs or MN T cells were transferred with 2–3 × 106 bulk naive OT-I T cells into separate RIP-mOVA Tg hosts. Compiled host disease incidence is shown from three to five independent experiments. Parentheses indicate the number of diabetic mice over the total number of mice per group. ***, P ≤ 0.005, using a log-rank test. (C) 106 naive OT-II RTEs or MN T cells were cotransferred with 2 × 106 bulk naive OT-I T cells into RIP-mOVA Tg hosts. Proliferation (left) and cytokine production (right) by donor OT-I T cells from recipient pLNs was quantified 7 d later. Data are presented as mean ± SEM and are representative of two independent experiments (n = 6–8). **, P ≤ 0.01, using an unpaired Student’s t test.
To determine whether the proliferative and cytokine defects of CD4 RTEs affect their capacity to facilitate CD8 T cell–mediated autoimmunity, we transferred OT-II RTEs or MN T cells plus bulk naive OT-I T cells into separate RIP-mOVA Tg hosts. Fewer of the mice that received RTE helper cells progressed to autoimmune diabetes (Fig. 2 B), indicating that the help provided by CD4 RTEs is less efficient at facilitating CD8 T cell–mediated diabetes. The impact of a helper cell source on the CD8 population is indicated by the reduction in the proportion of IL-2–producing OT-I effectors that develop in the presence of RTE helpers rather than in their proliferative capacity, which is equivalent regardless of the source of CD4 T cell help (Fig. 2 C).
RTEs are more prone to anergy than their mature counterparts
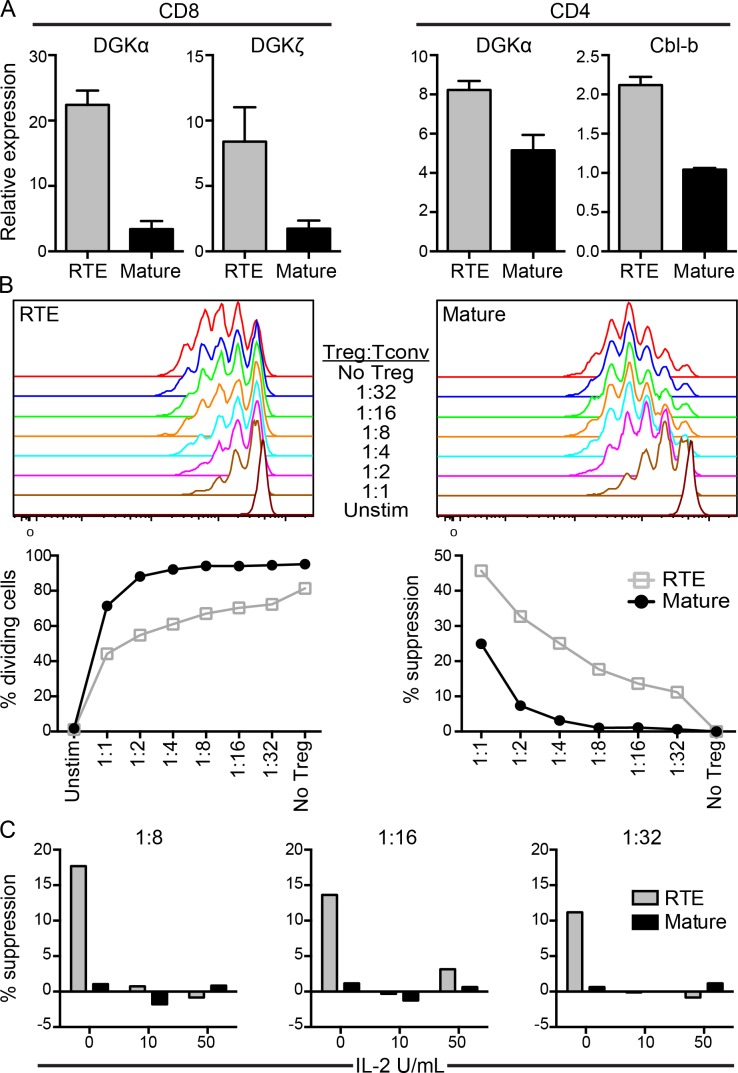
Anergy in T cells is characterized by an inability to produce IL-2, resulting in a diminished proliferative response (Parish et al., 2009; Wells, 2009). The defective proliferation and IL-2 production by RTEs in response to self-antigen as well as the fact that RTEs receive strong TCR signals but weak co-stimulation (Berkley and Fink, 2014) are all properties displayed by anergic cells (Wells, 2009). Therefore, we measured anergy-associated gene expression in OT-I and OT-II RTEs and mature T cells sorted from the pLNs of RIP-mOVA Tg hosts. Compared with their mature counterparts, antigen-exposed OT-I RTEs harvested from recipient pLNs expressed increased levels of the anergy-associated genes DGKα and ζ, whereas OT-II RTEs expressed increased levels of DGKα and Cbl-b (Fig. 3 A). OT-II RTEs harvested from the pLNs of RIP-mOVA Tg hosts up-regulated FR4 but down-regulated CD73 surface expression compared with their mature counterparts (not depicted) and thus do not fully phenocopy FR4hiCD73hi anergic mature T cells (Martinez et al., 2012).
Figure 3.
RTEs express elevated levels of anergy-associated genes after in vivo encounter with self-antigen and are more susceptible to T reg cell–mediated suppression. (A, left two graphs) 106 naive OT-I RTEs and MN T cells were cotransferred into RIP-mOVA Tg hosts. (A, right two graphs) 1–2 × 106 naive OT-II RTEs and MN T cells were cotransferred with 2 × 106 bulk naive OT-I T cells into RIP-mOVA Tg hosts. Antigen-exposed (CD44hi) donor OT-II and OT-I T cells were sorted 7 d later from pLNs and RNA was extracted for gene expression analyses. Data are presented as mean ± SD and are representative of two independent experiments. (B and C) 105 naive polyclonal CFSE-labeled CD4 RTEs or MN T cells were co-cultured in vitro with the indicated ratios of Foxp3+ T reg cells in the presence of 105 irradiated TCRβ/δ−/− splenocytes without (B) or with (C) recombinant IL-2. Cells were cultured for 72 h without (unstim) or with 50 ng/ml soluble anti-CD3 and CFSE dilution measured (top). (B) Data in the bottom left panel show the percentage of dividing cells and in the bottom right panel show the percentage of suppression of T cell proliferation by Foxp3+ T reg cells. Data are representative of three independent experiments. (C) Data show the percentage of suppression at 1:8, 1:16, and 1:32 T reg/T conv cell ratios without and with the addition of 10 or 50 U/ml recombinant IL-2, as indicated.
CD4 RTEs are more susceptible than mature T cells to T reg cell–mediated suppression
We used a standard in vitro suppression assay to determine whether the impaired proliferation and cytokine production by CD4 RTEs could also be caused by enhanced susceptibility to T reg cell–mediated suppression. Polyclonal CFSE-labeled CD4 RTEs or MN conventional T cells (T conv cells) were co-cultured with T reg cells from Foxp3-IRES-RFP Tg reporter mice (at decreasing T reg/T conv cell ratios), irradiated APCs, and soluble anti-CD3. After 72 h, both RTEs and mature T cells proliferated robustly in the absence of T reg cells, although fewer RTEs underwent division (Fig. 3 B), as expected (Boursalian et al., 2004). Furthermore, both RTE and mature T cell proliferation was suppressed in a dose-dependent manner by the addition of Foxp3+ T reg cells. Interestingly, RTE proliferation was diminished at lower T reg/T conv cell ratios, indicating their greater susceptibility to T reg cell–mediated suppression (Fig. 3 B). This inhibitory effect was not simply a consequence of reduced RTE proliferation because suppression was calculated as a function of cell division. Although the precise mechanism of T reg cell suppression is unclear, T reg cells may act as IL-2 “sinks” (Pandiyan et al., 2007), an effect that may be exacerbated for stimulated CD4 RTEs because they poorly up-regulate surface expression of the high-affinity IL-2Rα (Boursalian et al., 2004). To test this hypothesis, we added recombinant IL-2 to the in vitro suppression assay. Although CD4 RTEs were more susceptible to T reg cell–mediated suppression in the absence of exogenous IL-2 (Fig. 3 B), the addition of recombinant IL-2 inhibited the ability of T reg cells to suppress RTEs at low T reg/T conv cell ratios (Fig. 3 C). The sensitivity to suppression by T reg cells is particularly pertinent, given that CD4 T cell tolerance to TRAs is often T reg cell mediated (Legoux et al., 2015).
Inflammation converts tolerance-prone CD4 RTEs into competent effector cells
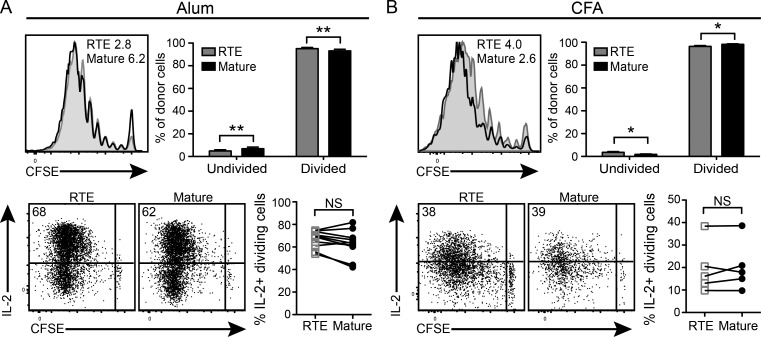
We have previously shown that, compared with mature T cells, OT-I RTEs drive increased diabetes induction in RIP-mOVA Tg hosts infected with OVA-expressing Listeria monocytogenes, perhaps a result of enhanced VLA-4 expression (Berkley and Fink, 2014), which is known to drive T cell accumulation in the prediabetic pancreas (King et al., 2012). To address the apparent contradiction between our prior and current data, we asked whether the RTE response is selectively enhanced after antigen encounter in the presence of inflammation. Adjuvants containing aluminum salts are widely used in both animal and human vaccines to induce inflammation and manipulate the T cell response to antigen (Marrack et al., 2009). With this in mind, an equal number of CFSE-labeled OT-II RTEs and MN T cells were cotransferred into recipients that were immunized subcutaneously the next day with 4-hydroxy-3-nitrophenylacetyl hapten conjugated to OVA (NP-OVA) in aluminum hydroxide (alum). In contrast to the proliferative defects observed in RTEs responding to peripheral self-antigen (Fig. 1 E), robust proliferation was observed in CD4 RTEs harvested from the draining inguinal LNs (iLNs) of mice immunized with NP-OVA in alum (Fig. 4 A). In fact, more RTEs than mature T cells divided, demonstrating that in the context of inflammation, CD4 RTEs are at least as proliferative as their mature counterparts. Furthermore, dividing RTEs and mature T cells made similar amounts of IL-2 (Fig. 4 B) and IFN-γ (not depicted). Despite alum’s reputation for mild Th2 skewing, these cells generated little to no IL-4 (not depicted). A similar recovery of effector cell function was noted in CD4 RTEs exposed to antigen in CFA (Fig. 4 B). Thus, inflammation converts antigen-exposed, tolerance-prone RTEs into competent effector cells.
Figure 4.
Antigen in the presence of inflammation converts tolerance-prone CD4 RTEs into competent effector cells. (A and B) 2 × 106 CFSE-labeled OT-II RTEs and MN T cells were cotransferred into WT B6 hosts that were immunized subcutaneously the next day with 100 µg NP-OVA in a 1:1 solution with either alum (A) or CFA (B). Donor cell proliferation and cytokine production were quantified 7 d later in the draining iLNs. Data in the top left panels represent CFSE dilution by RTEs (shaded) and mature T cells (open), and numbers denote the percentages of undivided donor cells. Data in the top right panels show the proportion of donor cells that have undergone zero (undivided) or one or more divisions (divided). Data are presented as mean ± SEM compiled from three independent experiments (n = 12). After restimulation in vitro, donor cells isolated from the draining iLNs were stained for intracellular cytokines. Numbers in the top left dot plot quadrants represent the percentages of IL-2+ dividing donor cells. Data in the bottom right panels are compiled from two independent experiments (n = 5–10). *, P < 0.05; **, P < 0.01.
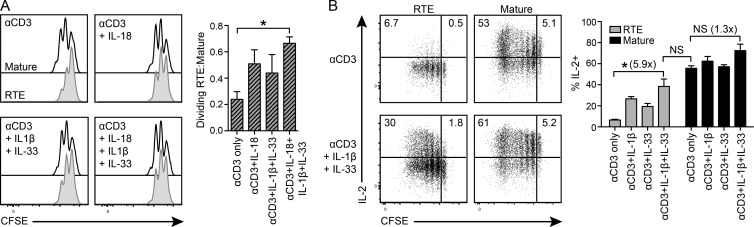
Addition of IL-1β, IL-18, and/or IL-33 disproportionately improves RTE function in vitro
To investigate how adjuvant exposure might enhance RTE effector function, we focused on alum because of its greater impact, particularly in terms of cytokine production (Fig. 4, A and B, bottom). The exposure of cells to alum in vitro activates caspase-1, resulting in the production of IL-1β, IL-18, and IL-33 (Marrack et al., 2009). Hence, we cultured polyclonal CFSE-labeled CD4 RTEs or MN T cells with irradiated APCs and soluble anti-CD3 with or without the addition of IL-1β, IL-18, and/or IL-33. After 48 h, both RTEs and mature T cells proliferated, although fewer RTEs divided two or more times (Fig. 5 A). Although the addition of exogenous IL-18 or IL-1β and IL-33 disproportionately increased the proliferation of RTEs relative to mature T cells, the addition of all three cytokines was required to drive RTE proliferation to mature T cell levels (Fig. 5 A). After 5–6 d of stimulation, ∼58% of mature CD4 T cells made IL-2, whereas only ∼7% of RTEs did. With the addition of exogenous IL-1β and IL-33, ∼32% of RTEs made IL-2, a dramatic approximately sixfold increase over anti-CD3 alone (Fig. 5 B).
Figure 5.
Addition of exogenous IL-1β, IL-18, and/or IL-33 disproportionately increases RTE proliferation and cytokine production in vitro. 105 naive polyclonal CFSE-labeled CD4 RTEs or MN T cells were cultured for 2–6 d with 2 × 105 irradiated TCRβ/δ−/− splenocytes and 50 ng/ml soluble anti-CD3 with or without 30 ng/ml IL-1β, IL-18, and/or IL-33. (A) Histograms show representative CFSE dilution by RTEs (shaded) and mature T cells (open) after 2 d of culture under the indicated conditions. Bar graph data show a ratio of the percentages of RTEs to mature T cells dividing two or more times; data are presented as mean ± SEM and are compiled from two independent experiments. (B) After 5–6 d of culture, cells were restimulated with PMA and ionomycin and stained for intracellular IL-2. Numbers in the top left and top right dot plot quadrants represent the percentage of IL-2+ divided and nondivided cells, respectively. Data in the right panel are presented as mean ± SEM and are compiled from two independent experiments. Numbers in parentheses indicate the fold change for the indicated condition relative to stimulation with anti-CD3 alone. *, P < 0.05, by unpaired Student’s t test.
Altogether, our data show that both CD4 and CD8 RTEs exposed to self-antigen in vivo exhibit defective proliferation, diminished cytokine production, and up-regulation of anergy-associated genes. OT-I T cell tolerance to peripheral self-antigen in the RIP-mOVA Tg mouse model can result from the deletion of autoreactive CD8 T cells (Kurts et al., 1997b). In our hands, OT-I RTEs are slightly underrepresented in the pLNs 7 d after transfer into RIP-mOVA Tg hosts (unpublished data), suggesting that they may also undergo deletional tolerance. However, the possibility that these differences result from differential migration into or out of the draining pLNs cannot be excluded. CD8 RTEs show reduced diabetogenic capacity, and CD4 RTEs show a reduced capacity to facilitate CD8-mediated islet cell destruction and heightened sensitivity to T reg cell suppression. However, both CD4 and CD8 RTEs can be converted into competent effector cells in the presence of inflammation, including that provided by L. monocytogenes infection or adjuvant exposure. Interestingly, the functional defects exhibited by the youngest peripheral T cells and the redirection of their immunocompetence upon exposure to antigen in the presence of inflammation mirror those of aged T cells near the end of their lifespan (Zhang et al., 2014).
Dampened immunocompetence by RTEs in the absence of inflammation may limit the production of harmful proinflammatory cytokines triggered by antigens expressed only outside the thymus. RTEs appear to be tolerized to TRAs expressed not only in the pancreas but also in the liver (Xu et al., 2016). Moreover, because of their enhanced VLA-4 expression, naive CD8 RTEs preferentially localize to the splenic white pulp (unpublished data), which may allow RTEs to access and become tolerized to TRAs not normally encountered by circulating naive T cells. Increased tissue invasiveness by RTEs in the absence of inflammation may also help explain how fetal RTEs infiltrate the organs of pregnant women, including the spleen and LNs, a process that may facilitate the onset of autoimmune disorders after pregnancy (Adams Waldorf and Nelson, 2008). Placing the inflammatory environment at the balance point between effector function and tolerance induction within RTEs may be key to ensuring that the neonate (all of whose peripheral T cells are RTEs) handles the world of antigens, both harmful and not, to its best advantage.
MATERIALS AND METHODS
Mice
WT B6, B6.SJL-PtprcaPepcb/BoyJ (B6.CD45.1), B6xB6.CD45.1, B6.129P2-Tcrbtm1MomTcrdtm1Mom/J (TCRβ/δ−/−), and RIP-mOVA Tg [B6-Tg(Ins2-TFRC/OVA)296Wehi/WehiJ] mice were purchased from The Jackson Laboratory or bred in house. RAG2p-GFP Tg mice were backcrossed in our laboratory at least 12 generations onto the B6 background to express one or both of the CD45.1 and CD45.2 congenic markers. These mice were crossed to OT-I [B6-Tg(TcraTcrb)1100Mjb/J] and OT-II [B6-Tg(TcraTcrb)425Cbn/J] mice expressing transgene-encoded TCRs that recognize OVA257–264 plus Kb or OVA323–339 plus I-Ab, respectively. B6-Foxp3tm1Flv/J (Foxp3-IRES-RFP; Wan and Flavell, 2005) and OT-II Rag1−/− mice were originally gifts from D. Campbell and S. Ziegler, respectively (Benaroya Research Institute, Seattle, WA) and were bred in house. All mice were housed under specific pathogen-free conditions and used in accordance with the University of Washington Institutional Animal Care and Use Committee guidelines.
Cell preparation, staining, and sorting
Single-cell suspensions for adoptive transfer were prepared from water-lysed splenocytes and axial, cervical, brachial, inguinal, and mesenteric LNs. T cells from RAG2p-GFP Tg TCR Tg mice were enriched using EasySep Negative Selection Mouse CD4 or CD8 T cell enrichment kits (STEMCELL Technologies), and Fc receptors were blocked with anti-CD16/32 (2.4G2; BD). Cells were stained with fluorochrome-conjugated antibodies against B220 (RA3-6B2), CD4 (RM4-5), CD8α (53-6.7), CD11b (M1/70), CD44 (IM7), CD45.1 (A20), CD45.2 (104), CD62L (MEL-14), NK1.1 (PK136), Ter119 (Ly-76), Vα2 (B20.1), and Vβ5 (MR9-4), all from BD, BioLegend, or eBioscience. After surface staining for 20 min at 4°C, a FACSAria II (BD) was used to sort cells to >98% purity as CD44loCD62LhiCD4−B220−CD11b−NK1.1−Ter119− (OT-I) or CD44loCD62LhiCD8−B220−CD11b−NK1.1−Ter119− (OT-II) cells that were either GFP+ RTEs or GFP− MN T cells. Fig. S1 shows sample sort gates and GFP curves for sort-purified populations of RTEs and MN T cells. Where indicated, cells were labeled with 2.5–5 µM CFSE for 10 min at 37°C. The GFP signal from RTEs does not interfere with the substantially brighter CFSE signal (Hendricks and Fink, 2011).
Adoptive transfers and immunizations
The indicated numbers of congenically marked, sort-purified naive TCR Tg RTEs and MN T cells were mixed 1:1, or kept separate as indicated, and transferred intravenously into adoptive hosts. Where indicated, recipients were immunized subcutaneously at the base of the tail 1 d after adoptive transfer with a total of 100 µl containing 100 µg NP-OVA (Sigma-Aldrich) in a 1:1 solution with Imject Alum (Thermo Fisher Scientific) or CFA (Sigma-Aldrich). Donor cell function was measured 7 d later in draining iLNs.
Intracellular cytokine staining
7 d after transfer or immunization, donor T cells were isolated from the draining pLNs or iLNs of adoptive hosts and restimulated in vitro using either 50 ng/ml PMA and 0.7 µM ionomycin (both from Sigma-Aldrich) for OT-II T cells or 10 nM OVA257–264 peptide for OT-I T cells, all in the presence of either brefeldin A (GolgiPlug) or monensin (GolgiStop; both from BD). Cells were surface stained and fixed using either 2% paraformaldehyde (Electron Microscopy Sciences) for OT-II T cells or Cytofix (BD) for OT-I T cells and permeabilized using Cytoperm (BD). Cells were stained intracellularly with fluorochrome-conjugated antibodies to IL-2 (JES6-5H4) and IFN-γ (XMG1.2) from either BD or eBioscience. Samples were run on an LSRII flow cytometer (BD) and analyzed with FlowJo software (Tree Star) using unstimulated controls to define gates.
Diabetes induction
5 d after transfer of the indicated numbers of OVA-specific T cells, the blood glucose of RIP-mOVA Tg hosts was monitored daily using a OneTouch Ultra2 meter (LifeScan) along with OneTouch Ultra test strips (LifeScan). Mice with blood glucose readings over 300 mg/dl for two consecutive days were considered diabetic and sacrificed.
Quantitative PCR (qPCR)
7 d after cell transfer, antigen-exposed OT-I and OT-II T cells were sort purified from pLNs, and total RNA was extracted using the RNeasy Micro kit (QIAGEN). cDNA synthesis was performed with a mix of oligo(dT) and random primers using the QuantiTect Reverse Transcription kit (QIAGEN) according to the manufacturer’s protocol. For OT-II T cells, qPCR was performed on the resulting cDNA using a ViiA 7 Real Time PCR System with TaqMan Universal Master Mix II, no uracil-N-glycosylase (both from Applied Biosystems), and with TaqMan primers/probes as indicated (Thermo Fisher Scientific). For OT-I T cells, qPCR was performed as previously described (Hendricks and Fink, 2011). Data were normalized to the expression of hypoxanthine-guanine phosphoribosyltransferase (Hprt) in each sample, and the relative expression was calculated using 2−ΔCT.
T reg cell suppression assays and in vitro cytokine stimulation
105 sort-purified naive polyclonal CFSE-labeled CD4 RTEs or MN T cells were co-cultured at the indicated ratios with sort-purified Foxp3+ T reg cells from Foxp3-IRES-RFP Tg mice and 105 TCRβ/δ−/− splenocytes irradiated with 3,000 Rad. Cells were stimulated for 72 h at 37°C in 7% CO2 with 50 ng/ml soluble anti-CD3 (BD) in complete RPMI 1640 medium containing 10% fetal bovine serum, 10 mM Hepes, 4 mM l-glutamine, and 50 µM 2-mercaptoethanol. The percentage of suppression was calculated as [(T conv cell CFSE dilution without T reg cells) − (T conv cell CFSE dilution with T reg cells)]/(T conv cell CFSE dilution without T reg cells). CFSE dilution was calculated using FlowJo software. Where indicated, cultures were supplemented with or without 10 or 50 U/ml recombinant IL-2 (originally from Cetus). For in vitro cytokine stimulation, 105 sort-purified naive polyclonal CFSE-labeled CD4 RTEs or MN T cells were cultured with 2 × 105 TCRβ/δ−/− irradiated splenocytes. Cells were stimulated for 2–6 d in complete RPMI at 37°C in 7% CO2 with 50 ng/ml soluble anti-CD3 with or without the addition of 30 ng/ml each of IL-18, IL-1β, and/or IL-33 (all from PeproTech).
Statistics
P-values were calculated using a log-rank (diabetes induction experiments) or paired (cotransfer experiments) Student’s t test, and p-values <0.05 were considered significant.
Online supplemental material
Fig. S1 shows the sort gate strategy for isolating untouched RTEs and MN T cells before cotransfer into adoptive hosts. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20151990/DC1.
Supplementary Material
ACKNOWLEDGMENTS
We thank A. Grier and Drs. D. Zehn and E.G. Houston for their help in the early stages of this work and Dr. R. Savan and S. Ozarkar for technical assistance with the qPCR analyses. We also thank our colleagues for their critical reading of the manuscript.
This work was supported by the United States National Institutes of Health (grant AI064318 to P.J. Fink) and University of Washington Training in Molecular and Cellular Biology (grant T32GM007270 to T.J. Friesen).
The authors declare no competing financial interests.
Footnotes
Abbreviations used:
- B6
- C57BL/6
- iLN
- inguinal LN
- MN
- mature naive
- pLN
- pancreatic LN
- qPCR
- quantitative PCR
- RTE
- recent thymic emigrant
- Tg
- transgenic
- TRA
- tissue-restricted antigen
References
- Adams Waldorf K.M., and Nelson J.L.. 2008. Autoimmune disease during pregnancy and the microchimerism legacy of pregnancy. Immunol. Invest. 37:631–644. 10.1080/08820130802205886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkley A.M., and Fink P.J.. 2014. Cutting edge: CD8+ recent thymic emigrants exhibit increased responses to low-affinity ligands and improved access to peripheral sites of inflammation. J. Immunol. 193:3262–3266. 10.4049/jimmunol.1401870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaumik S., Giffon T., Bolinger D., Kirkman R., Lewis D.B., Weaver C.T., and Randolph D.A.. 2013. Retinoic acid hypersensitivity promotes peripheral tolerance in recent thymic emigrants. J. Immunol. 190:2603–2613. 10.4049/jimmunol.1200852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boursalian T.E., Golob J., Soper D.M., Cooper C.J., and Fink P.J.. 2004. Continued maturation of thymic emigrants in the periphery. Nat. Immunol. 5:418–425. 10.1038/ni1049 [DOI] [PubMed] [Google Scholar]
- Fink P.J. 2013. The biology of recent thymic emigrants. Annu. Rev. Immunol. 31:31–50. 10.1146/annurev-immunol-032712-100010 [DOI] [PubMed] [Google Scholar]
- Haines C.J., Giffon T.D., Lu L.S., Lu X., Tessier-Lavigne M., Ross D.T., and Lewis D.B.. 2009. Human CD4+ T cell recent thymic emigrants are identified by protein tyrosine kinase 7 and have reduced immune function. J. Exp. Med. 206:275–285. 10.1084/jem.20080996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath W.R., Belz G.T., Behrens G.M.N., Smith C.M., Forehan S.P., Parish I.A., Davey G.M., Wilson N.S., Carbone F.R., and Villadangos J.A.. 2004. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol. Rev. 199:9–26. 10.1111/j.0105-2896.2004.00142.x [DOI] [PubMed] [Google Scholar]
- Hendricks D.W., and Fink P.J.. 2011. Recent thymic emigrants are biased against the T-helper type 1 and toward the T-helper type 2 effector lineage. Blood. 117:1239–1249. 10.1182/blood-2010-07-299263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogquist K.A., Xing Y., Hsu F.C., and Shapiro V.S.. 2015. T cell adolescence: Maturation events beyond positive selection. J. Immunol. 195:1351–1357. 10.4049/jimmunol.1501050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston E.G. Jr., and Fink P.J.. 2009. MHC drives TCR repertoire shaping, but not maturation, in recent thymic emigrants. J. Immunol. 183:7244–7249. 10.4049/jimmunol.0902313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston E.G. Jr., Nechanitzky R., and Fink P.J.. 2008. Cutting edge: Contact with secondary lymphoid organs drives postthymic T cell maturation. J. Immunol. 181:5213–5217. 10.4049/jimmunol.181.8.5213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C.G., Koehli S., Hausmann B., Schmaler M., Zehn D., and Palmer E.. 2012. T cell affinity regulates asymmetric division, effector cell differentiation, and tissue pathology. Immunity. 37:709–720. 10.1016/j.immuni.2012.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurts C., Carbone F.R., Barnden M., Blanas E., Allison J., Heath W.R., and Miller J.F.A.P.. 1997a CD4+ T cell help impairs CD8+ T cell deletion induced by cross-presentation of self-antigens and favors autoimmunity. J. Exp. Med. 186:2057–2062. 10.1084/jem.186.12.2057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurts C., Kosaka H., Carbone F.R., Miller J.F., and Heath W.R.. 1997b Class I-restricted cross-presentation of exogenous self-antigens leads to deletion of autoreactive CD8+ T cells. J. Exp. Med. 186:239–245. 10.1084/jem.186.2.239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legoux F.P., Lim J.B., Cauley A.W., Dikiy S., Ertelt J., Mariani T.J., Sparwasser T., Way S.S., and Moon J.J.. 2015. CD4+ T cell tolerance to tissue-restricted self antigens is mediated by antigen-specific regulatory T cells rather than deletion. Immunity. 43:896–908. 10.1016/j.immuni.2015.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P., McKee A.S., and Munks M.W.. 2009. Towards an understanding of the adjuvant action of aluminium. Nat. Rev. Immunol. 9:287–293. 10.1038/nri2510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez R.J., Zhang N., Thomas S.R., Nandiwada S.L., Jenkins M.K., Binstadt B.A., and Mueller D.L.. 2012. Arthritogenic self-reactive CD4+ T cells acquire an FR4hiCD73hi anergic state in the presence of Foxp3+ regulatory T cells. J. Immunol. 188:170–181. 10.4049/jimmunol.1101311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsutani T., Ohmori T., Ogata M., Soga H., Kasahara S., Yoshioka T., Suzuki R., and Itoh T.. 2007. Comparison of CDR3 length among thymocyte subpopulations: impacts of MHC and BV segment on the CDR3 shortening. Mol. Immunol. 44:2378–2387. 10.1016/j.molimm.2006.10.026 [DOI] [PubMed] [Google Scholar]
- McCaughtry T.M., Wilken M.S., and Hogquist K.A.. 2007. Thymic emigration revisited. J. Exp. Med. 204:2513–2520. 10.1084/jem.20070601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland R.D., Douek D.C., Koup R.A., and Picker L.J.. 2000. Identification of a human recent thymic emigrant phenotype. Proc. Natl. Acad. Sci. USA. 97:4215–4220. 10.1073/pnas.070061597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opiela S.J., Koru-Sengul T., and Adkins B.. 2009. Murine neonatal recent thymic emigrants are phenotypically and functionally distinct from adult recent thymic emigrants. Blood. 113:5635–5643. 10.1182/blood-2008-08-173658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paiva R.S., Lino A.C., Bergman M.L., Caramalho I., Sousa A.E., Zelenay S., and Demengeot J.. 2013. Recent thymic emigrants are the preferential precursors of regulatory T cells differentiated in the periphery. Proc. Natl. Acad. Sci. USA. 110:6494–6499. 10.1073/pnas.1221955110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandiyan P., Zheng L., Ishihara S., Reed J., and Lenardo M.J.. 2007. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat. Immunol. 8:1353–1362. 10.1038/ni1536 [DOI] [PubMed] [Google Scholar]
- Parish I.A., Rao S., Smyth G.K., Juelich T., Denyer G.S., Davey G.M., Strasser A., and Heath W.R.. 2009. The molecular signature of CD8+ T cells undergoing deletional tolerance. Blood. 113:4575–4585. 10.1182/blood-2008-10-185223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- PrabhuDas M., Adkins B., Gans H., King C., Levy O., Ramilo O., and Siegrist C.A.. 2011. Challenges in infant immunity: implications for responses to infection and vaccines. Nat. Immunol. 12:189–194. 10.1038/ni0311-189 [DOI] [PubMed] [Google Scholar]
- Priyadharshini B., Welsh R.M., Greiner D.L., Gerstein R.M., and Brehm M.A.. 2010. Maturation-dependent licensing of naive T cells for rapid TNF production. PLoS One. 5:e15038 10.1371/journal.pone.0015038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith N.L., Wissink E., Wang J., Pinello J.F., Davenport M.P., Grimson A., and Rudd B.D.. 2014. Rapid proliferation and differentiation impairs the development of memory CD8+ T cells in early life. J. Immunol. 193:177–184. 10.4049/jimmunol.1400553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y.Y., and Flavell R.A.. 2005. Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proc. Natl. Acad. Sci. USA. 102:5126–5131. 10.1073/pnas.0501701102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells A.D. 2009. New insights into the molecular basis of T cell anergy: anergy factors, avoidance sensors, and epigenetic imprinting. J. Immunol. 182:7331–7341. 10.4049/jimmunol.0803917 [DOI] [PubMed] [Google Scholar]
- Xu X., Jin R., Li M., Wang K., Zhang S., Hao J., Sun X., Zhang Y., Wu H., Zhang J., and Ge Q.. 2016. Liver sinusoidal endothelial cells induce tolerance of autoreactive CD4+ recent thymic emigrants. Sci. Rep. 6:19861 10.1038/srep19861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W., Nagaoka H., Jankovic M., Misulovin Z., Suh H., Rolink A., Melchers F., Meffre E., and Nussenzweig M.C.. 1999. Continued RAG expression in late stages of B cell development and no apparent re-induction after immunization. Nature. 400:682–687. 10.1038/23287 [DOI] [PubMed] [Google Scholar]
- Zaghouani H., Hoeman C.M., and Adkins B.. 2009. Neonatal immunity: faulty T-helpers and the shortcomings of dendritic cells. Trends Immunol. 30:585–591. 10.1016/j.it.2009.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Brahmakshatriya V., and Swain S.L.. 2014. CD4 T cell defects in the aged: causes, consequences and strategies to circumvent. Exp. Gerontol. 54:67–70. 10.1016/j.exger.2014.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.