Abstract
AIM
To explore whether ectopic expression of human melanopsin can effectively and safely restore visual function in rd1 mice.
METHODS
Hematoxylin-eosin staining of retinal sections from rd1 mice was used to detect the thickness of the outer nuclear layer to determine the timing of surgery. We constructed a human melanopsin-AAV2/8 viral vector and injected it into the subretinal space of rd1 mice. The Phoenix Micron IV system was used to exclude the aborted injections, and immunohistochemistry was used to validate the ectopic expression of human melanopsin. Furthermore, visual electrophysiology and behavioral tests were used to detect visual function 30 and 45d after the injection. The structure of the retina was compared between the human melanopsin-injected group and phosphate buffer saline (PBS)-injected group.
RESULTS
Retinas of rd1 mice lost almost all of their photoreceptors on postnatal day 28 (P28). We therefore injected the human melanopsin-adeno-associated virus (AAV) 2/8 viral vector into P30 rd1 mice. After excluding aborted injections, we used immunohistochemistry of the whole mount retina to confirm the ectopic expression of human melanopsin by co-expression of human melanopsin and YFP that was carried by a viral vector. At 30d post-injection, visual electrophysiology and the behavioral test significantly improved. However, restoration of vision disappeared 45d after human melanopsin injection. Notably, human melanopsin-injected mice did not show any structural differences in their retinas compared with PBS-injected mice.
CONCLUSION
Ectopic expression of human melanopsin effectively and safely restores visual function in rd1 mice.
Keywords: human melanopsin, retinal degenerative diseases, visual restoration
INTRODUCTION
Retinal degenerative diseases (RDDs) are the leading cause of vision loss and blindness. With the development of medical technology, an increasing number of mutant genes that cause inherited retinal diseases have been identified, and most of these genes are related to photo transduction pathways[1]–[3]. As a rapid retinal degeneration model, rd1 mice have a mutation in the phosphodiesterase type 6 (PDE6)-β subunit that causes a complete loss of photoreceptors by postnatal day 30[4]. This animal model has similar gene mutations and phenotypes as some human RDDs[5] and is therefore commonly used as a model to test potential treatments of RDDs. More recently, experimental efforts have explored new drugs[6]–[7], cell therapies[8]–[9] and light-sensitive proteins[10]–[12]. However, drugs can only provide functional benefits in the early stage of RDDs, and the efficacy of cell therapy is dependent on remnant photoreceptor cells. By comparison, light-sensitive proteins show a great potential in the treatment of late RDDs due to their independence on photoreceptor cells. Channelrhodopsin (ChR) and melanopsin, two types of light-sensitive proteins, have raised much attention for restoration of vision. However, ChR originates from Chlamydomonas reinhardtii and is less sensitive to light compared with melanopsin[13].
Melanopsin, which exists in the retinal ganglion cells of mammalian retina, is a G-protein coupled receptor that couples to the canonical transient receptor potential channels via Gq-type G protein activation[14]. As a result, melanopsin can absorb photons by itself and melanopsin-containing photosensitive ganglion cells have been directly linked to brain functions[15]. It is plausible that melanopsin can restore visual function in the advanced stages of RDDs. Some researchers have demonstrated that melanopsin plays important roles in the circadian rhythm[16], depression[17] and pupil light reflex[18]. In addition, we have found that ectopic expression of mouse melanopsin can restore visual function in rd1 mice[11]. This work suggests that melanopsin may be a candidate therapeutic method for advanced stage RDDs. However, human melanopsin is a prerequisite to advance clinical applications of melanopsin. Therefore, in this study, we constructed a human melanopsin-adeno-associated virus (AAV) 2/8 vector (AAV-hMel-YFP) to transfect rd1 mice retinas.
MATERIALS AND METHODS
Animals
Twenty-five healthy SPF-grade rd1 mice and three C57 normal control mice of both sexes were provided by the Experimental Animal Center of Southwest Hospital, Third Military Medical University. Three C57 mice were used for hematoxylin-eosin staining, ten randomly selected rd1 mice were used for the flash electroretinogram (FERG) and flash visually evoked potentials (FVEP) experiments and fifteen randomly selected rd1 mice were used for the behavioral tests. The mice were reared in a light-controlled room that has a fixed lighting schedule (8:00 to 20:00). Light was generated by two fluorescent lamps that created 60 lx of intensity at the animal level. The room humidity was controlled at 50% to 60%, while the temperature was held at 22°C-25°C. All experimental protocols were performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research[19].
Anesthesia
Animals were anesthetized via an intraperitoneal injection of 4% chloral hydrate at a dose of 1 mL/kg. Oxybuprocaine eye drops (0.4%) were used for superficial anesthesia (Santen Pharmaceutical Co., Ltd., Osaka, Japan).
Construct of Viral Vectors
Full-length human melanopsin was cloned into an AAV2/8 vector under the transcriptional control of a mCMV promoter. Because of the mCMV promoter, viral vectors easily transfected all retinal neurons in the retina of rd1 mice. The constructs were packaged at GENECHEM biological company's virus production core in Shanghai, China. The packaged viruses were concentrated and purified in phosphate buffer saline (PBS) with a titer of 3.5×10[12] AAV-hMel-YFP genome copies per mL.
Subretinal Injection of Adeno-associated Virus
After administration of anesthesia, rd1 mice were moved to an animal operating table under a microscope. Then, 1 µL of a viral suspension was delivered to the subretinal space using a Hamilton micro-injector. To minimize individual differences in the FERG and FVEP testing, AAV-hMel-YFP was injected into the right eye, and the left eye was used as a PBS-injected control (n=10). For behavioral testing, ten rd1 mice received an AAV-hMel-YFP injection in both eyes and the control group (n=5) received PBS injections. After 30d of subretinal injections, we used phoenix Micron IV system (Phoenix company, USA) to observe the retinas of the injected mice to exclude mice with retinal puncture or cataracts due to aborted injections.
Immunohistochemistry
Frozen sections were air-dried, washed in PBS for 5min, and then stained with hematoxylin and eosin. For fluorescence immunohistochemistry, whole-mount retinas were blocked in 3% bovine serum albumin (BSA) and 10% normal goat serum in 0.5% Triton X 100 (Sigma-Aldrich, USA) for 1h at room temperature (25°C). Then, the whole retinas were incubated with a rabbit polyclonal antibody against human melanopsin (1:500, Abcam, England) overnight at 4°C. The following day, after washing in PBS for 45min (3×15min), the retinas were incubated for 4h in Cy3-conjugated goat anti-rabbit IgG (1:1000, life technologies, USA). Finally, the nuclei were stained with 4,6-diamidino-2-phenylindole (DAPI; Invitrogen, USA) for 4h. Confocal images were acquired using Zeiss LSM 510 microscope (Carl Zeiss Co. Ltd., Oberkohen, Germany).
Flash Electroretinogram Recording
For FERG recordings, rd1 mice were dark adapted for nearly 12h and prepared for recording under dim red light. After administration of anesthesia, the pupils were dilated with tropicamide and phenylephrine. FERG responses were recorded from both eyes simultaneously with gold wire loops. Saline (0.9%) was frequently applied on the cornea to prevent its dehydration and to allow for electrical contact with the recording electrode. Two of the needle electrodes were inserted under the skin of the angulus oculi temporalis and served as the reference electrodes. Another electrode was placed in the tail and served as the ground electrode. Data were acquired by the phoenix Micron IV system (Phoenix company, USA). Dark-adapted intensity responses were 0 Log(cd·s/m2). To avoid any adapting effect from the previous flash, the flash interval was set between 60-120s, depending on stimulus intensity. Data were exported and processed by Igor.
Flash Visually Evoked Potentials Recording
The mice were reared in a normal, light-controlled room (8:00 to 20:00). After administration of anesthesia, the electrical activity was recorded by silver wire needle electrodes, which were placed in the visual cortex region, with the reference electrodes placed under the skin on the chin and the ground electrode was placed in the tail. The data acquisition was provided performed by the Reti-scan system (Roland, Germany). The stimulus intensity was -0.02 log(cd·s/m2), frequency was 1 Hz and the flash duration was <5ms. The bandpass of the filter was between 0.01 Hz and 300 Hz. Data were exported and processed by Igor.
Open Field Test
The open field test was performed according to previous studies[11],[20]. The open field test box was 45×30×40 cm. It was divided into a white open field and a dark zone with a door (10×10 cm) between the two areas. The mice were adapted in the dark room for 2min, and then, the door was opened for behavioral observations. The amount of time spent in the dark zone and white open field was recorded. The open field test was performed under 100 lx light intensity and recorded using a video camera to enable subsequent evaluation. The total time of the test was 300s.
Statistical Analysis
Data analysis was carried out using the SPSS 13.0 statistical package (SPSS, Chicage, IL, USA). Data are expressed as the mean±standard deviation (SD) and were analyzed using the independent-samples t-test to compare the treated and control groups in both the electrophysiology recording and behavioral testing. A P value of less than 0.05 was considered statistically significant.
RESULTS
Ectopic Expression of Human Melanopsin Protein in Retina of rd1 Mice
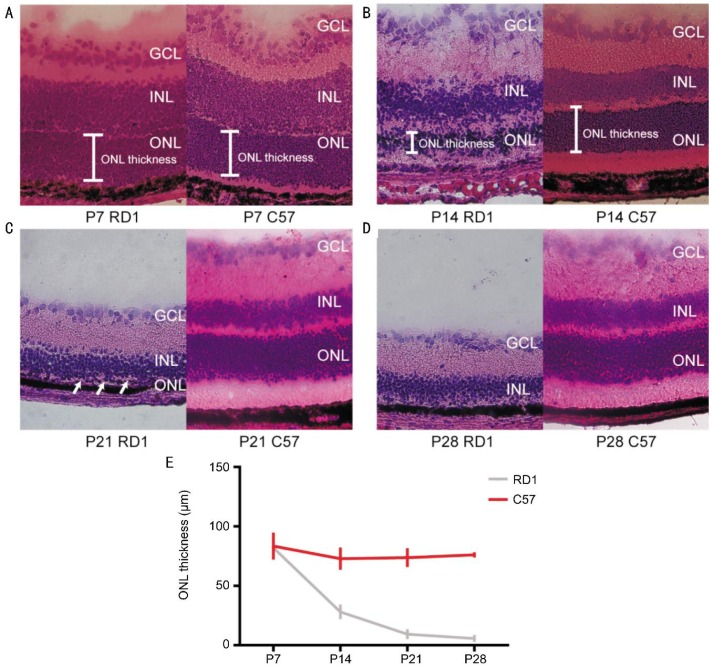
Using hematoxylin-eosin staining, we confirmed that the retinas of rd1 mice lost almost all of the photoreceptors on postnatal day 28 (P28) (Figure 1). To study whether human melanopsin protein could restore visual function in advanced retinal degeneration, we used AAV to ectopically express human melanopsin in the retina of P30 rd1 mice.
Figure 1. Hematoxylin-eosin staining of retinal sections from C57 and rd1 mice.
A-D: Representative hematoxylin-eosin staining of retinal sections from C57 and rd1 mice on postnatal days 7, 14, 21 and 28. Arrows indicate monolayer of ONL. E: Quantification of ONL thickness in C57 and rd1 mice. ONL thickness in rd1 mice gradually decreased. ONL: Outer nuclear layer; INL: Inner nuclear layer; GCL: Ganglion cell layer.
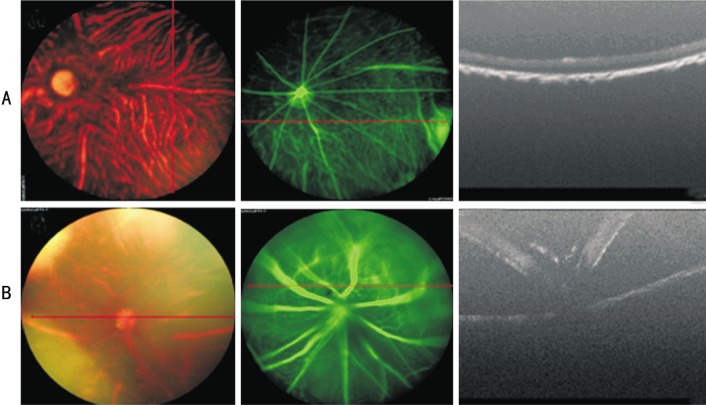
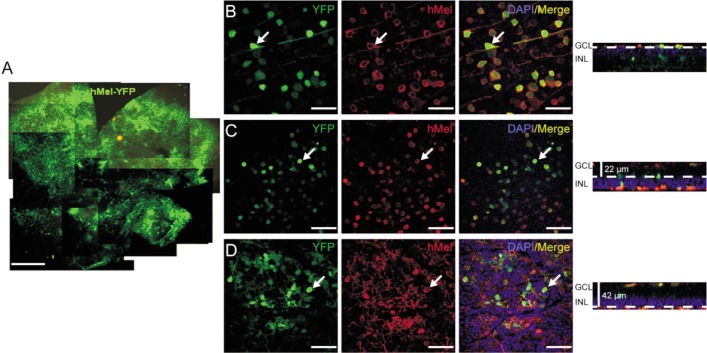
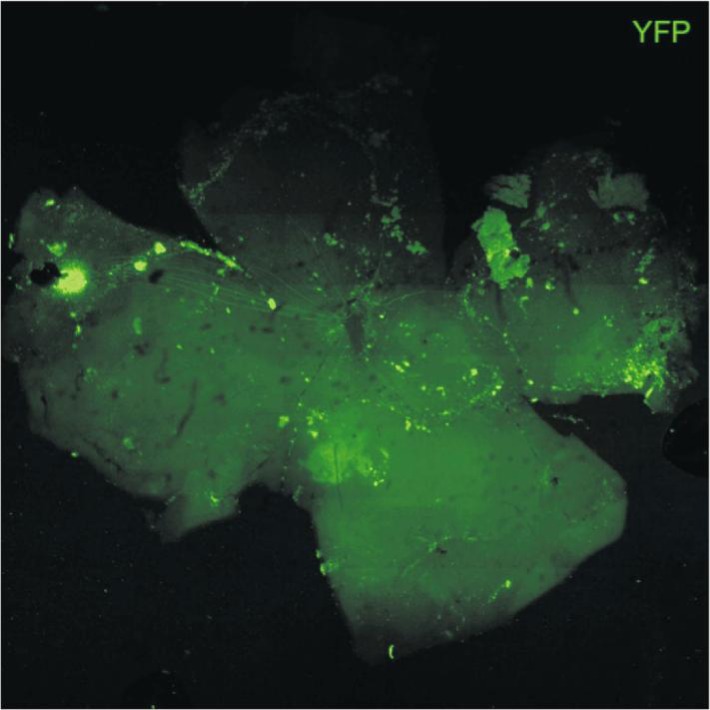
First, we excluded mice with cataracts or retinal puncture due to aborted injections (Figure 2). Subsequently, immunohistochemistry of the whole mount retina was used to investigate the ectopic expression of human melanopsin. Thirty days post-injection, more than 80% of the retina displayed hMel-YFP fluorescence (Figure 3A). Furthermore, we scanned the mount retina at a higher magnification. In three random fields, co-expression of hMel and YFP fluorescence was observed in the ganglion cell layer (GCL) (Figure 3B), the inner plexiform layer (22 µm depth to GCL) (Figure 3C) and the inner nuclear layer (42 µm depth to GCL) (Figure 3D). However, 45d post-injection, the expression of human melanopsin significantly decreased (Figure 4).
Figure 2. Screening the hMel subretinal injected mice by phoenix Micron IV system.
A: Successfully injected mice showing normal fundus, fundus fluorescein angiography (FFA) and optical coherence tomography (OCT); B: Aborted injections caused diffusion of fluorescence in FFA and retinal puncture in OCT.
Figure 3. Ectopic expression of human melanopsin protein in the retina of rd1 mice 30d post-injection.
A: YFP expression of the whole mount retina. Scale bar: 500 µm. B-D: Co-expression of YFP and human melanopsin in the whole mount retina from superficial ganglion cell layer (B) to deeper layers (C, D). Arrows indicate colocalization of YFP and hMel. Scale bar: 50 µm.
Figure 4. Ectopic expression of melanopsin protein in the retina of rd1 mice 45d post-injection.
Assessment of Visual Function After Ectopic Expression of Human Melanopsin Protein in the Retina of rd1 Mice
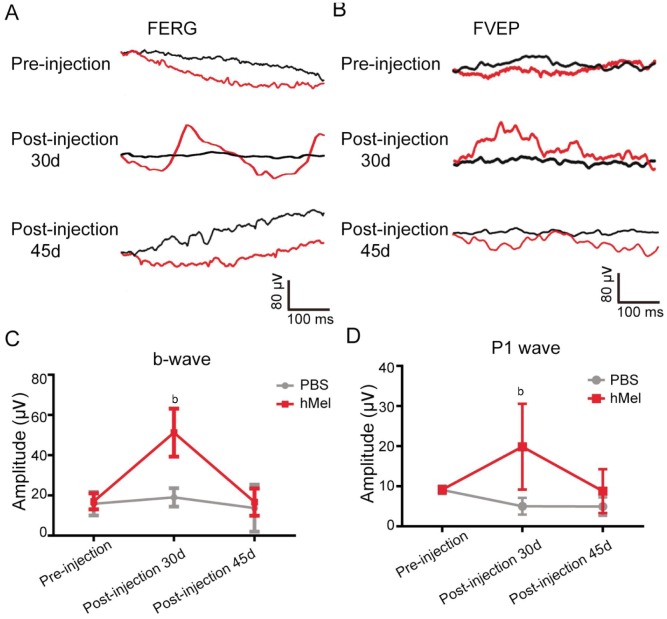
Visual function was assessed using FERG, FVEP and behavioral tests. Previous studies have indicated that the b-wave and P1 wave amplitude of rd1 mice were abolished quickly after postnatal P30 due to a complete loss of photoreceptors[21]. However, the b-wave of FERG and P1 wave of FVEP in P60 rd1 mice were well restored 30d post-injection, with the injected eye demonstrating significantly higher amplitude compared to the control eye (Figure 5). Forty-five days post-injection, the amplitude of b-wave and P1 wave in post-injection 45d rd1 mice decreased to control levels (Figure 5).
Figure 5. The FERG and FVEP test of subretinal hMel injections in rd1 mice.
A, B: Representative traces of FERG and FVEP before the injection, 30d post-injection and 45d post-injection. The black line represents the left eye (PBS-injected eye), red line indicates the right eye (hMel-injected eye). C: The mean amplitude of b-wave before the injection, 30d post-injection and 45d post-injection (mean±SD, n=4). bP<0.01. Thirty days post injection, the mean amplitude of the b-wave in the hMel-injected eye was significantly higher than that in the PBS-injected eye (P=0.008) (mean±SD, n=4). D: Thirty days post-injection, the mean amplitude of the P1-wave in the hMel-injected eye was significantly higher than that in the PBS-injected eye (P=0.008) (mean±SD, n=6). However, there was no significant difference in the P1-wave amplitude pre-injection (P=0.916) and 45d post-injection (P=0.203).
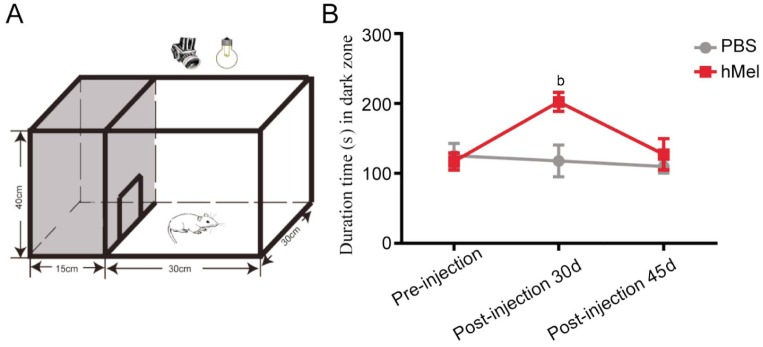
To further assess visual function, we used an open field test, which is a behavioral test of visual function. Many researchers have demonstrated that normal mice avoid open, brightly lit spaces and that this innate tendency depends on their ability to distinguish light from dark[11],[20]. Mice were placed in the apparatus shown in Figure 6A. We showed that hMel-injected mice spent more time in the dark zone compared with PBS-injected mice 30d post-injection (P=0.000). However, 45d post-injection, there was no significant difference in the amount of time spent in the dark zone between hMel-injected mice and PBS-injected mice (P=0.126).
Figure 6. Behavioral test of rd1 mice with subretinal hMel injections.
A: Schematic diagram of the open field test equipment. The testing chamber was divided into a white open field and a dark zone. Mice could move freely through a door (10×10 cm) between the white and dark zones; B: The amount of time spent in the dark zone by hMel-injected and PBS-injected mice pre-injection, 30d post-injection and 45d post-injection (mean±SD, n=8). bP<0.01.
Safety Assessment of Ectopic Expression of Human Melanopsin Protein in rd1 Mice Retina
To validate the safety of a human melanopsin transfection, we examined the morphology of the retinas and the optic nerve from hMel-injected mice. Importantly, no structural differences were observed between hMel-injected mice and PBS-injected mice using hematoxylin-eosin staining. In addition, the critical organs, such as the heart, liver, spleen, lung and kidney, all showed normal morphology, just as in the control mice (data not shown).
DISCUSSION
In this study, we demonstrated that ectopic expression of human melanopsin in the degenerated retina of rd1 mice transiently restored visual function.
To study the late stages of retinal degeneration, we used P30 rd1 mice based on the results that almost all of the photoreceptors were absent in P28 rd1 mice. We then injected human melanopsin into the subretinal space of P30 rd1 mice and confirmed the ectopic expression of human melanopsin. Consistent with our previous work[11], we found that the AAV-hMel-YFP virus not only transduced retinal ganglion cells but also transduced other retinal neurons. Furthermore, we used FERG and FVEP to evaluate the effect of hMel treatment because FERG and FVEP are the most effective standard methods used for evaluation of visual function[21]–[23]. FERG is a method that reflects the whole retinal function, including many neuronal types in the retina, such as photoreceptor cells, bipolar cells and amacrine cells[21]–[22]. FVEP reflects the function of visual pathways that connect ganglion cells to the visual cortex[23]. To minimize surgery damage due to aborted injections, we used the Phoenix Micron IV system to exclude mice with cataracts or retinal puncture. We showed that the amplitude of the b-wave in FERG and the P1 wave in FVEP were significantly higher in the hMel-treated mice 30d post-injection compared with PBS-treated mice. Furthermore, we showed that the hMel-treated rd1 mice showed better behavioral aversion to light compared with PBS-treated mice 30d after the injection. This finding suggests that hMel-treated rd1 mice had a restored visual function on postnatal day 60. However, transplantation of retinal pigment epithelial (RPE) cells, neural stem cells (NSCs), and mesenchymal stromal cells (MSCs) can only provide photoreceptor preservation for 21d in P14 rd1 mice[24]. In a previous study, we intravitreally injected mouse melanopsin in P80 rd1 mice and used a behavioral test to confirm that it could restore visual function 4wk post-injection[11]. Furthermore, to advance the clinical application of melanopsin, we used FERG, FVEP and behavioral tests to validate that ectopic expression of human melanopsin could also restore visual function in RDDs at the late stage. Although human melanopsin has been found to be effective in control of wakefulness[25], this is the first time that human melanopsin has been shown to restore visual function in late retinal degeneration.
However, we found that the restoration of visual function disappeared 45d after hMel injection, suggesting that ectopic expression of human melanopsin alone could only transiently restore visual function in late retinal degeneration. We inferred that the short-lived rescue effects were due to the loss of hMel expression, which might result from the species differences between human and mouse. When applied to human retinas, hMel might restore visual function for a longer period. Nevertheless, further studies are needed, such as the use of a better vector for ectopic expression of hMel, to accomplish the long-term restoration of visual function. Notably, ectopic expression of human melanopsin did not induce any serious immune reaction or toxicity.
In conclusion, human melanopsin is a candidate for clinical treatment of retinal degeneration patients. However, the molecular mechanism of the visual restoration by human melanopsin is not clear, and further studies are needed to elucidate the mechanism of visual restoration by human melanopsin.
Acknowledgments
Liu MM contributed to conception and design, data collection and analysis and writing of the manuscript. Dai JM and Liu WY contributed to data collection and analysis. Zhao CJ and Lin B contributed to experimental design, data analysis and interpretation and revised the manuscript. Yin ZQ contributed to conception and design of the study, data analysis and revised the manuscript.
Foundations: Supported by the Chongqing International Cooperation Key Projects (No. CSTC2013GJHZ10004); National Basic Research Program of China (973 Program, No. 2013CB967002).
Conflicts of Interest: Liu MM, None; Dai JM, None; Liu WY, None; Zhao CJ, None; Lin B, None; Yin ZQ, None.
REFERENCES
- 1.Fishman GA, Jacobson SG, Alexander KR, Cideciyan AV, Birch DG, Weleber RG, Hood DC. Outcome measures and their application in clinical trials for retinal degenerative diseases: outline, review, and perspective. Retina. 2005;25(6):772–777. doi: 10.1097/00006982-200509000-00014. [DOI] [PubMed] [Google Scholar]
- 2.Veleri S, Lazar CH, Chang B, Sieving PA, Banin E, Swaroop A. Biology and therapy of inherited retinal degenerative disease: insights from mouse models. Dis Model Mech. 2015;8(2):109–129. doi: 10.1242/dmm.017913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yi J, Li S, Jia X, Xiao X, Wang P, Guo X, Zhang Q. Evaluation of the ELOVL4, PRPH2 and ABCA4 genes in patients with Stargardt macular degeneration. Mol Med Rep. 2012;6(5):1045–1049. doi: 10.3892/mmr.2012.1063. [DOI] [PubMed] [Google Scholar]
- 4.Bowes C, Li T, Danciger M, Baxter LC, Baxter LC, Applebury ML, Farber DB. Retinal degeneration in the rd mouse is caused by a defect in the beta subunit of rod cGMP-phosphodiesterase. Nature. 1990;347(6294):677–680. doi: 10.1038/347677a0. [DOI] [PubMed] [Google Scholar]
- 5.McLaughlin ME, Sandberg MA, Berson EL, Dryja TP. Recessive mutations in the gene encoding the beta-subunit of rod phosphodiesterase in patients with retinitis pigmentosa. Nat Genet. 1993;4(2):130–134. doi: 10.1038/ng0693-130. [DOI] [PubMed] [Google Scholar]
- 6.Zarbin MA, Arlow T, Ritch R. Regenerative nanomedicine for vision restoration. Mayo Clin Proc. 2013;88(12):148–190. doi: 10.1016/j.mayocp.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 7.Zhu Q, Su G, Nie L, Wang C, He Y, Liu X. Salvia miltiorrhiza extracts protect against retinal injury in a rat glaucoma model. Exp Ther Med. 2014;7(6):1513–1515. doi: 10.3892/etm.2014.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ballios BG, Cooke MJ, van der Kooy D, Shoichet MS. A hydrogel-based stem cell delivery system to treat retinal degenerative diseases. Biomaterials. 2010;31(9):2555–2564. doi: 10.1016/j.biomaterials.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Kamao H, Mandai M, Okamoto S, Sakai N, Suga A, Sugita S, Kiryu J, Takahashi M. Characterization of human induced pluripotent stem cell-derived retinal pigment epithelium cell sheets aiming for clinical application. Stem Cell Rep. 2014;2(2):205–218. doi: 10.1016/j.stemcr.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomita H, Sugano E, Yawo H, Ishizuka T, Isago H, Narikawa S, Kügler S, Tamai M. Restoration of visual response in aged dystrophic RCS rats using AAV-mediated channelopsin-2 gene transfer. Invest Ophthalmol Vis Sci. 2007;48(8):3821–3826. doi: 10.1167/iovs.06-1501. [DOI] [PubMed] [Google Scholar]
- 11.Lin B, Koizumi A, Tanaka N, Panda S, Masland RH. Restoration of visual function in retinal degeneration mice by ectopic expression of melanopsin. Proc Natl Acad Sci USA. 2008;105(41):16009–16014. doi: 10.1073/pnas.0806114105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qiu X, Kumbalasiri T, Carlson SM, Wong KY, Krishna V, Provencio I, Berson DM. Induction of photosensitivity by heterologous expression of melanopsin. Nature. 2005;433(7027):745–749. doi: 10.1038/nature03345. [DOI] [PubMed] [Google Scholar]
- 13.Sexton T, Buhr E, Van Gelder RN. Melanopsin and mechanisms of non-visual ocular photoreception. J Biol Chem. 2012;87(3):1649–1656. doi: 10.1074/jbc.R111.301226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt TM, Chen SK, Hattar S. Intrinsically photosensitive retinal ganglion cells: many subtypes, diverse functions. Trends Neurosci. 2011;34(11):572–580. doi: 10.1016/j.tins.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koizumi A, Tanaka KF, Yamanaka A. The manipulation of neural and cellular activities by ectopic expression of melanopsin. Neurosci Res. 2013;75(1):3–5. doi: 10.1016/j.neures.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295(5557):1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 17.Roecklein KA, Wong PM, Miller MA, Donofry SD, Kamarck ML, Brainard GC. Melanopsin, photosensitive ganglion cells, and seasonal affective disorder. Neurosci Biobehav Rev. 2013;37(3):229–239. doi: 10.1016/j.neubiorev.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou W, Lou Y, Pan B, Huang J. Reliability of field chromatic pupillometry for assessing the function of melanopsin-containing retinal ganglion cells. Invest Ophthalmol Vis Sci. 2015;56(4):2519. doi: 10.1167/iovs.15-16672. [DOI] [PubMed] [Google Scholar]
- 19.The Ministry of Science and Technology of the People's Republic of China Guidance Suggestions for the Care and Use of Laboratory Animals. 09, 2012.
- 20.Go RE, Hwang KA, Kim SH, Lee MY, Kim CW, Jeon SY, Kim YB, Choi KC. Effects of anti-obesity drugs, phentermine and mahuang, on the behavioral patterns in Sprague-Dawley rat model. Lab Anim Res. 2014;30(2):73–78. doi: 10.5625/lar.2014.30.2.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinilla I, Lund RD, Sauvé Y. Contribution of rod and cone pathways to the dark-adapted electroretinogram (ERG) b-wave following retinal degeneration in RCS rats. Vision Res. 2004;44(21):2467–2474. doi: 10.1016/j.visres.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 22.Kakiuchi D, Uehara T, Shiotani M, Nakano-Ito K, Suganuma A, Aoki T, Tsukidate K, Sawada K. Oscillatory potentials in electroretinogram as an early marker of visual abnormalities in vitamin A deficiency. Mol Med Rep. 2015;11(2):995–1003. doi: 10.3892/mmr.2014.2852. [DOI] [PubMed] [Google Scholar]
- 23.Bullock TH, Hofmann MH, New JG, Nahm FK. Dynamic properties of visual evoked potentials in the tectum of cartilaginous and bony fishes, with neuroethological implications. J Exp Zool Suppl. 1990;5:142–155. doi: 10.1002/jez.1402560519. [DOI] [PubMed] [Google Scholar]
- 24.Sun J, Mandai M, Kamao H, Hashiguchi T, Shikamura M, Kawamata S, Sugita S, Takahashi M. Protective effects of human iPS-derived retinal pigmented epithelial cells in comparison with human mesenchymal stromal cells and human neural stem cells on the degenerating retina in rd1 mice. Stem Cells. 2015;33(5):1543–1553. doi: 10.1002/stem.1960. [DOI] [PubMed] [Google Scholar]
- 25.Tsunematsu T, Tanaka KF, Yamanaka A, Koizumi A. Ectopic expression of melanopsin in orexin/hypocretin neurons enables control of wakefulness of mice in vivo by blue light. Neurosci Res. 2013;75(1):23–28. doi: 10.1016/j.neures.2012.07.005. [DOI] [PubMed] [Google Scholar]