Abstract
Primary hyperoxaluria type 1 (PH1) is caused by deficient alanine-glyoxylate aminotransferase, the human peroxisomal enzyme that detoxifies glyoxylate. Glycolate is one of the best-known substrates leading to glyoxylate production, via peroxisomal glycolate oxidase (GO). Using genetically modified mice, we herein report GO as a safe and efficient target for substrate reduction therapy (SRT) in PH1. We first generated a GO-deficient mouse (Hao1−/−) that presented high urine glycolate levels but no additional phenotype. Next, we produced double KO mice (Agxt1−/− Hao1−/−) that showed low levels of oxalate excretion compared with hyperoxaluric mice model (Agxt1−/−). Previous studies have identified some GO inhibitors, such as 4-carboxy-5-[(4-chlorophenyl)sulfanyl]-1,2,3-thiadiazole (CCPST). We herein report that CCPST inhibits GO in Agxt1−/− hepatocytes and significantly reduces their oxalate production, starting at 25 µM. We also tested the ability of orally administered CCPST to reduce oxalate excretion in Agxt1−/− mice, showing that 30–50% reduction in urine oxalate can be achieved. In summary, we present proof-of-concept evidence for SRT in PH1. These encouraging results should be followed by a medicinal chemistry programme that might yield more potent GO inhibitors and eventually could result in a pharmacological treatment for this rare and severe inborn error of metabolism.
Introduction
Primary hyperoxaluria type 1 (PH1, OMIM #259900) is a genetic disease due to a deficit of alanine-glyoxylate aminotransferase (AGT) activity in hepatocyte's peroxisomes. This enzyme metabolizes glyoxylate to glycine. The lack of AGT activity, or its mistargeting to mitochondria, allows the oxidation of glyoxylate to oxalate, which can only be excreted in the urine. High oxalate levels lead to calcium oxalate stone formation and renal parenchyma damage, which results in progressive deterioration of renal function and, eventually, end-stage renal disease. Combined renal and liver transplantation is needed in many PH1 patients to avoid the life-threatening systemic accumulation of oxalate that takes place after end-stage renal disease.1,2
Substrate reduction therapy (SRT) with small molecules is a strategy successfully used in some inborn errors of metabolism.3 Loss-of-function mutations in genes encoding key enzymes result in the harmful accumulation of substrate. SRT addresses this failure by reducing the level of the substrate to a point where residual degradative activity might be sufficient to prevent or diminish substrate accumulation to levels that can be well tolerated by the patient.
Endogenous glyoxylate production occurs mainly in the peroxisomes and mitochondria, being glycolate an important precursor of glyoxylate in humans.4 Due to the high affinity of glyoxylate reductase hydroxypyruvate reductase (GRHPR) to convert glyoxylate into glycolate, important sources of glyoxylate such as hydroxyproline are also metabolized into glycolate.5 Peroxisomal glyoxylate can result from the activity of either d-amino acid oxidase on glycine or glycolate oxidase (GO) on glycolate. GO (UNIPROT Q9UJM8), encoded by HAO1 gene, is an FMN-dependent α-hydroxyacid oxidase which transforms glycolate into glyoxylate. Peroxisomal glyoxylate is normally detoxified by AGT into pyruvate and glycine by transamination with alanine. Excess glyoxylate in peroxisomes is converted to oxalate by GO or it is transported out to the cytoplasm, where it is reduced to glycolate by GRHPR or oxidized to oxalate by lactate dehydrogenase.
The majority of PH1 alleles are missense mutations that result in severe reductions of AGT enzymatic activity in the peroxisome, with a wide range of residual activity, depending on the mutations present in both alleles. Thus, it might be beneficial for PH1 patients to reduce the production of glyoxylate by inhibiting the GO activity. Although plants and mammals have profound differences in the glyoxylate metabolism, GO is a relatively conserved protein whose structure was first elucidated in spinach.6 The potential interest of GO inhibition in agriculture prompted early investigations in small molecules capable of inhibiting GO (GO inhibitors, GOi). The structure of human GO has been recently elucidated,7,8 which facilitates the rational design of mammalian GOi.
We herein report the use of genetically modified mice to identify GO as a safe and efficient target for SRT in PH1. Indeed, GO-deficient mice, Hao1−/−, developed normally without significant phenotype, and the double knock-out (KO) animals (Agxt1−/− Hao1−/−) essentially normalized their oxalate excretion with respect to the hyperoxaluric Agxt1−/− mice. In addition, we have tested 4-carboxy-5-[(4-chlorophenyl)sulfanyl]-1,2,3-thiadiazole (CCPST), a GOi9 in hyperoxaluric Agxt1−/− mice, as a proof of concept for SRT in this orphan disease.
Results
GO-Deficient Mice Develop Normally and Show Glycolic Aciduria
We searched public databases for embryonic stem (ES) clones with putative null mutations in the mouse Hao1 gene, coding for GO. Initial attempts to generate a GO-deficient mouse model were carried out using a Hao1 gene-trapped ES clone (199G2, later renamed 199F3) from the Centre for Modeling Human Disease (University of Toronto). However, this clone, which carries a trapping vector in Hao1 intron 5 ended up producing a mouse with normal GO expression (Supplementary Figure S1).
Next, we used ES cells (129SvEvBrd, TG0109) from TIGEM (Texas Institute for Genomic Medicine) that carried a deletion of exon 3, which allowed us to generate Hao1−/− homozygous animals lacking GO expression (Figure 1). No differences were observed in growth and development of Hao1−/− mice compared with their heterozygous or wild-type littermates. The biochemical profile of 24-h urine samples collected from 6-month-old Hao1−/− and Hao1+/+ male mice (n = 6 each group) showed no significant differences, with oxalate excretion around 0.3 µmol/day (0.33 ± 0.1 versus 0.35 ± 0.11, respectively, P = 0.81). As expected, urine glycolate levels were higher in Hao1−/− mice than in the Hao1+/+ controls (11.63 ± 1.93 versus 0.83 ± 0.18 µmol/day, P = 0.005). No differences in urine sediment were detected between both genotypes. Thorough kidney histological study revealed no differences between Hao1−/− and Hao1+/+ mice, with no evidence of crystal deposition of any type. No breeding differences were observed among Hao1−/− mice compared to controls, and histological analysis of brain, lung, heart, stomach, liver, spleen, pancreas, small and large bowel, gonads, and bone revealed no differences among the various genotypes.
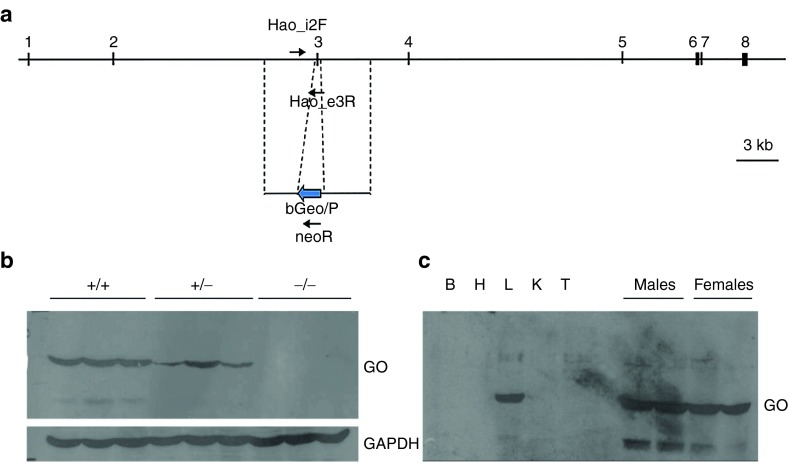
Figure 1.
Targeted mutagenesis of the mouse Hao1 locus. (a) Design of Hao1 gene exon 3 deletion by homologous recombination in ES cells. (b) Upper Western blot of 50-µg liver protein from Hao1+/+, Hao1+/−, and Hao1−/− mice probed with affinity-purified rabbit antibody raised against recombinant mouse glycolate oxidase (GO) shows lack of expression of the targeted allele and reduced levels in the heterozygous sample. Lower Reprobing of the blot with anti-glyceraldehyde-3-phosphate dehydrogenase detects even loading of the gel. (c). Left Western blot of wild-type (wt) mouse tissues (B: brain, H: heart, L: liver, K: kidney, T: testis) shows liver-specific expression of glycolate oxidase. Right No differences were found in GO expression between male and female mice. GAPDH, antiglyceraldehyde-3-phosphate dehydrogenase.
In summary, lack of GO expression in Hao1−/− mice resulted in asymptomatic elevation of urine glycolate as the only significant change, similar to what has been recently reported in a human patient with a null mutation in HAO1.10
GO Deficiency Reverses the Hyperoxaluric Phenotype in PH1 Mice
To generate a genetic mouse model for SRT of PH1, mice homozygous for GO deficiency were crossed with hyperoxaluric mice homozygous for a null mutation in the Agxt1 gene, a model for PH1.11 Double heterozygous animals were interbred to obtain double KO mice (Agxt1−/− Hao1−/− mice).
Male Agxt1−/− mice (n = 6 per group) were hyperoxaluric with respect to Agxt1+/+ controls (1.33 ± 0.21 versus 0.35 ± 0.11 µmol/24 hours, P = 0.005), while double KO mice (Agxt1−/− Hao1−/−) showed urine oxalate levels not significantly different from Agxt1+/+ controls (0.44 ± 0.12 versus 0.35 ± 0.11 µmol/24 hours, P = 0.17) (Figure 2). Conversely, urine glycolate levels were higher in Agxt1−/− Hao1−/− mice than in Agxt1−/− mice (11.70 ± 2.06 versus 0.97 ± 0.13 µmol/24 hours, P = 0.005).
Figure 2.
A 24-h urine glycolate and oxalate excretion by different mouse genotypes. Data is represented as mean ± SD (n = 6 per group). ANOVA statistical signification: ***P<0.001, NS = nonsignificative.
Sediment analysis showed calcium oxalate crystals, mostly with the tetrahedric appearance typical of calcium oxalate dihydrate in all Agxt1−/− mice at some point during a follow-up of a week. On the other hand, no calcium oxalate crystals were observed in Agxt1−/− Hao1−/− mice during the 5 days of the urine sediment study.
CCPST, a GO Inhibitor, Blunts Oxalate Production in Primary Cultures of PH1 Mouse Hepatocytes
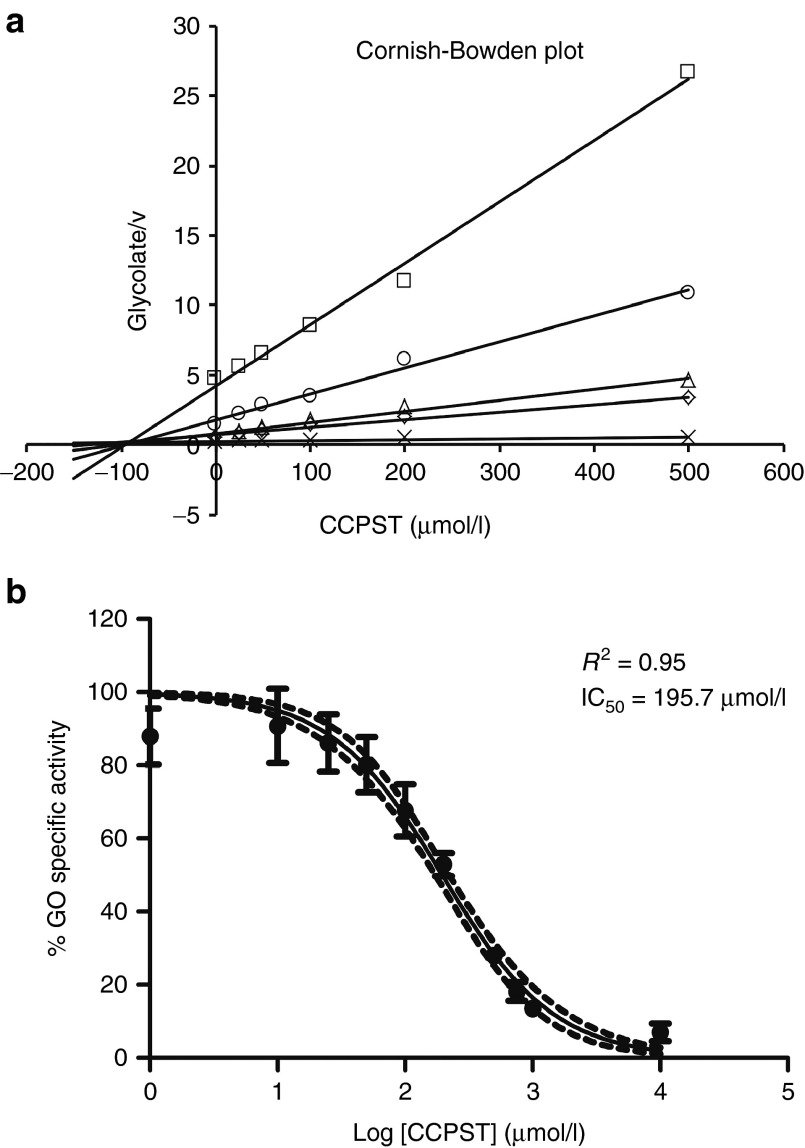
CCPST has been previously described as an inhibitor of human GO9 as well as the rat isozyme long-chain hydroxy acid oxidase.12 We have expressed and purified mouse recombinant GO (Supplementary Figure S2) to assess the inhibitory effect of CCPST on mouse GO (mGO). Various concentrations of commercial CCPST were tested on a constant amount of GO protein at each concentration of glycolate. In our assay conditions, CCPST behaves as a noncompetitive inhibitor, with a Ki value of 91.2 µM (Figure 3a). In a dose–response curve of GO enzymatic activity versus CCPST concentration for 1 µg GO, we could determine that 195.7 µM is the concentration of CCPST needed to inhibit half of the maximum enzymatic activity (log IC50 = 2.29 ± 0.026) (Figure 3b).
Figure 3.
Kinetics of the mouse glycolate oxidase (GO) inhibition by 4-carboxy-5-[(4-chlorophenyl)sulfanyl]-1,2,3-thiadiazole (CCPST). (a) Cornish-Bowden plot for the inhibition of mouse glycolate oxidase by CCPST. Increased inhibitor concentrations were tested at every glycolate (substrate) concentration and represented against glycolate/velocity (v). CCPST behaves as a noncompetitive inhibitor as all lines intersect on the x axis at the point X = −Ki = −91.2 µM. (b) Dose–response curve of mouse glycolate oxidase activity against CCPST concentration. Data are represented as mean ± SD. Discontinue lines represent 95% confidence interval; nonlinear regression analysis.
To test the efficacy of CCPST to decrease oxalate production in vitro, we cultured primary hepatocytes from hyperoxaluric Agxt1−/− mice. Less than 5-day-old cultures were used to avoid interference of primary hepatocyte dedifferentiation. To enhance the production of oxalate in these short-term cultures and make it easy to detect in standard enzymatic assays, we added 5 mM glycolate to the medium.13 Under these conditions, oxalate is detected in the media of hyperoxaluric hepatocytes (Figure 4a), in a time- and concentration-dependent manner, similar to previous descriptions on HepG2 cell line.13 We first tested the viability of cultured hepatocytes treated with increasing concentrations of CCPST up to 200 µM in the presence of glycolate. We found this compound to be nontoxic compared to control cells at least until 100 µM CCPST at each time point (Figure 4b). Using a sensitive high-performance liquid chromatography (HPLC) method to detect CCPST, we made sure that this compound was entering the hepatocytes. Indeed, the analysis of intracellular CCPST in cultures exposed to 12.5–100 µM CCPST gives a significant positive linear correlation (r = 0.952, p<0.001) with a goodness-of-fit R2 = 0.906 (F = 163.329, p<0.001) that is defined by the linear regression equation y = 0.038 + 0.007x (Figure 4c). Intracellular concentrations of CCPST were 0.44 ± 0.06, 0.74 ± 0.02, 1.37 ± 0.19, 2.26 ± 0.45, and 2.48 ± 0.29 µM at 12.5, 25, 50, 75, and 100 µM added, respectively. Thus, the intracellular concentrations were 30 times lower than concentrations of the compound in the media.
Figure 4.
In vitro response of mouse Agxt1−/− primary hepatocytes. (a) Oxalate excretion in Agxt1−/− hepatocytes treated with 5 mM glycolate compared with nontreated controls at 24, 48, and 72 hours. (b) Agxt1−/− hepatocytes viability after treatment with increased concentrations of 4-carboxy-5-[(4-chlorophenyl)sulfanyl]-1,2,3-thiadiazole (CCPST) in presence of 5 mM glycolate, measured by methyl thiazol tetrazolium reduction assay. (c) Graphic representation of the positive relationship between CCPST added to the medium and that detected in the intracellular extract. Simple linear regression analysis (r = 0.952, P < 0.001; R2 = 0.906, P < 0.001). (d) Relative amount of excreted oxalate by Agxt1−/− hepatocytes measured at 24, 48, and 72 hours post-treatment with increased concentrations of CCPST, and compared to the corresponding nontreated control. Data are represented as mean ± SD. ANOVA statistical signification: *P < 0.05, **P < 0.01, ***P < 0.001, NS = nonsignificative, relative to control at each time point.
We collected cultured media every 24 hours after treatment to measure the amount of oxalate excreted by the Agxt1−/− cells (Figure 4d). At concentrations above 25 µM CCPST, the levels of oxalate produced by Agxt1−/− hepatocytes were significantly reduced. Indeed, at 75 µM CCPST the oxalate production was 30% relative to controls. The reduction in oxalate in the culture media can be detected 24 hours after treatment and remains suppressed 72 hours post-treatment. The calculated EC50 was: 25.26 ± 1.16 µM at 24 hours, 32.94 ± 1.05 µM at 48 hours, and 33.85 ± 1.08 µM at 72 hours. Under these conditions, no significant differences were detected between 25 and 100 µM (one-way ANOVA, P > 0.05).
CCPST might have an effect on various enzymes unrelated to GO (off-target effect). To assess the relative contribution of CCPST potential off-target effects, double KO (Agxt1−/− Hao1−/−) hepatocytes were similarly treated with 12.5–50 µM CCPST, but nonsignificant further reduction in oxalate production could be detected in hepatocytes genetically lacking GO (Supplementary Figure S3).
CCPST Administration Reduces Oxalate Excretion in PH1 Mice
CCPST is not soluble in aqueous solutions at physiological pH. To explore the in vivo effects of this GO inhibitor in the Agxt1−/− mouse, we prepared complexes with β-cyclodextrin (CD), a cyclic oligosaccharide known to enhance stability and bioavailability of poorly water-soluble drugs. We solubilized CCPST in complex with CD in a 4:1 ratio at pH 7.4 and performed the same analysis in cultured hepatocytes as previously described to assure that the complex CCPST-CD also reduce oxalate excreted to the media and to confirm its lack of toxicity.
Next, Agxt1−/− mice were treated orally with CCPST-CD at daily doses of 110 mg/kg body weight during 11 days. This treatment resulted in a significant reduction of 30% in urine oxalate from the first dose to the end of the experiment. The oxalate reduction is almost 50% by the 10th and 11th doses (Figure 5a). Parallel increases in urine glycolate excretion were also observed (Figure 5b). At the end of the study, liver GO enzymatic activity was significantly reduced with respect to untreated Agxt1−/− mice (Figure 5c). The treatment was well tolerated by the mice, without clinical signs of toxicity, and necropsies performed on treated mice revealed no gross or microscopic changes (Supplementary Figure S4).
Figure 5.
In vivo effects of 4-carboxy-5-[(4-chlorophenyl)sulfanyl]-1,2,3-thiadiazole (CCPST) treatment in PH1 mice. 24-h urine oxalate (a) and glycolate (b) excretion in Agxt1−/− mice after daily oral administration of CCPST in complex with β-cyclodextrin during 11 days. Significant reduction in oxalate levels from the first dose compared to basal oxaluria (P < 0.05). At the 10th and 11th dose, the oxalate levels significantly decrease with respect to the first dose (P < 0.01). Mean ± SD of three independent assays of six male mice. Paired t-test statistical analysis. (c) Glycolate oxidase (GO) specific activity measured in perfused liver after the 11th oral dose of CCPST-β-cyclodextrin (CD) in Agxt1−/− mice. Hao1-deficient mice (Hao1−/−) was used as control of nonenzymatic GO activity. Significant reduction in GO specific activity compared to nontreated Agxt1−/− mice (P < 0.001).
Discussion
Our results indicate that glycolate oxidase (GO) is a safe and efficient target for SRT in primary hyperoxaluria type 1 (PH1) mouse model. We have generated a glycolate oxidase deficient mouse (Hao1−/−) that has no phenotype other than elevated urine glycolate levels, a situation parallel to the reported incidental finding of an individual with isolated glycolic aciduria homozygous for a null mutation in the human HAO1 gene.10 By crossing the Hao1−/− with our mouse model for PH1 (Agxt1−/−), we could demonstrate that the lack of glycolate oxidase in double knock-out mice significantly reduces the hyperoxaluric phenotype.
The idea of suppressing oxalate production by the PH1 liver with small molecules is not new. In the seventies, several candidate targets were proposed,14 and occasional chemicals were tested for their potential to reduce oxalate production by the liver of PH1 patients, without much success.15 GO, one of the enzymes upstream of the metabolic blockage present in PH1 patients (AGT deficiency) is one of such candidate targets.16
Significant advances in the understanding of GO came from the structural study of the spinach enzyme (sGOX), co-crystallized with some inhibitors originally developed as agrochemicals.6 More recently, the human GO structure was determined7,8 and the mechanism involved in the inhibition exerted by some chemicals was revealed. One of these agrochemical inhibitors, 4-carboxy-5-dodecylsulfanyl-1,2,3-triazole (CDST), has been co-crystallized with human GO and described as a potent inhibitor.8 A related compound, 4-carboxy-5-[(4-chlorophenyl)sulfanyl]-1,2,3-thiadiazole (CCPST) has been also proposed as a GO inhibitor and its interaction with the enzyme has been elucidated.9 The carboxylate groups of glyoxylate, CDST, and CCPST superimpose well in the crystal structure of human GO, interacting with the conserved active-site residues Arg167, Arg263, and Tyr26.9 The five-member rings of CDST and CCPST also fit similarly in the human GO structure, although CCPST does not carry a proton and would require the protonation of His260 upon binding to the active site. The main differences between these two inhibitors reside on the ring substituents: an aliphatic chain in CDST and a chlorophenyl ring in CCPST. Since CCPST is commercially available, we have used it to test the hypothesis that GO inhibition reduces oxalate production in a mouse model of PH1. The results herein presented provide proof of concept, in a mouse model, that GO inhibition with small molecules is a valid strategy of SRT in PH1.
Using recombinant mGO, we first performed in vitro studies to validate in our system the previously reported behavior of CCPST as an inhibitor of both human GO9 and rat long-chain hydroxy acid oxidase.12 Our results show that Km values for mGO are similar to those determined for human GO (hGO)7,8 and that CCPST inhibits mGO in a noncompetitive manner, in agreement with a previous report for hGO, but with a higher Ki = Ki′ value. This may be due to differences in the conditions on the enzymatic assays used.
Next, we tested the efficacy of CCPST in primary hepatocytes from Agxt1−/− mice. We confirmed that CCPST enters the hepatocytes in culture, by measuring the compound in cellular lysates, but the intake is limited at high concentrations and it remains in a plateau of intracellular drug and biological response in the range tested. We have found that 25 µM of CCPST added to the medium produces a significant decrease in the amount of oxalate excreted by Agxt1−/− primary hepatocytes without significant cytotoxicity, even in a medium enriched in glycolate. This concentration agrees with the EC50 at 24 hours post-treatment, and it slightly increases until 72 hours post-treatment (≈30 µM). Thus, CCPST remains inhibiting GO enzymatic activity for at least 72 h, keeping low levels of oxalate excretion.
Complexation of CCPST with β-cyclodextrin was required to solubilize this molecule in aqueous solutions at neutral pH. At a 4:1 ratio, CCPST-CD complex was stable in solution at pH 7.4 and it could be administered by gavage to Agxt1−/− mice. Oral administration of 110 mg/kg body weight reduced significantly the levels of urine oxalate by an average of 30%. After 11 daily doses, we confirmed that the GO enzymatic activity in liver lysates was blunted. We also used Agxt1−/− Hao1−/− mice to demonstrate that the decrease in oxalate excretion achieved by this high dose of CCPST is not due, to a significant degree, to off-target effect. We also noticed that these doses of CCPST were well tolerated and no lesions could be detected upon necropsy at the end of the study.
Our results provide support to the view that SRT by GO-inhibition contributes to decrease one of the main sources of glyoxylate in the liver of PH1 patients, therefore reducing oxalate renal excretion.16 In an accompanying paper, GO knock-down by siRNA administration results in significant reduction of oxalate production using our PH1 animal model (Dutta et al.,17). In addition, our results unveil CCPST as a lead compound in the search for safe and efficient small molecules that specifically inhibit glycolate oxidase. One of the limitations of our study is the high dose administered. It has been also documented that CCPST inhibits the rat long-chain hydroxy acid oxidase,12 a GO-isozyme encoded by Hao2 gene. Although not observed in the small number of animals studied, unwanted side effects could be expected at the dose used. Thus, dose-range and proper toxicity studies are needed. We are currently evaluating the pharmacokinetic profile of the complex CCPST-CD in mice. Further preclinical studies are needed, including absorption, distribution, metabolism, excretion and toxicity (ADMET) assays to gauge the potential of CCPST and related compounds in PH1 treatment. We must also keep in mind that differences in glyoxylate metabolism between mice and humans may result in large variations in the effect resulting from GO inhibition. Glycolate is known to be an important source of glyoxylate in both humans and mice, but the list of substrates that can be converted to glyoxylate by the liver metabolism in both species is not completely known, nor is the relative contribution of GO to overall oxalate production.
Materials and Methods
Development of GO-Deficient and Double-KO Mice. ES cells (129SvEvBrd, TG0109) from TIGEM reposotory were generated by homologous recombination using a targeting construct designed to delete exon 3 of the Hao1 gene. ES cells were injected into C57BL/6 blastocysts, and two chimeric males were used to generate ES-derived mice, about half of which carried the designed deletion in the Hao1 gene, as evidenced by PCR analysis performed on DNA extracted from tail tips. These heterozygous Hao1+/− mice were used both to generate an intercross colony, with mixed 129/Sv and C57BL/6 background. In order to place the mutation in a homogeneous genetic background, heterozygous Hao1+/− male mice were backcrossed with 129Sv females for nine generations, and finally intercrossed to produce homozygous Hao1-deficient mice. In parallel, Hao1+/− male mice were crossed with Agxt−/− females11 and double heterozygous animals were used to generate Agxt1−/− Hao1−/− mice. Animal studies have been approved by the University of La Laguna institutional review board (Comité ético de experimentación y bienestar animal, CEEBA-ULL).
Mice were bred and maintained in a pathogen-free facility, with free access to standard chow (Letica D04) and water. Male mice were placed in metabolic cages for single mouse (model 3600M, Tecniplast, Buguggiate, Italy) and allowed to get acclimatized to a 5 g/day powdered diet and tap water, during 3 days before the start of urine collection. Five consecutive 24-hour urine collections were performed in tubes containing 50 µl 6 N HCl. Twenty-four-hour urine samples that were <1 ml for 3-month-old mice and <1.5 ml for 6-month-old mice were typically seen in cages with signs of incomplete urine collection, and were excluded from the study. Similarly, samples with food or fecal contamination were excluded. The acidified urine samples (pH < 2) were used for oxalate measurement.
mGO Expression and Purification. The coding sequence of mouse Hao1 cDNA was inserted in the pET15b expression vector (Novagen, Madison, Wisconsin), carrying an N-terminal His-tag sequence. mGO was produced in BL21 (DE3) Escherichia coli (Novagen) in the presence of 0.1 mg/ml ampicillin in LB medium. When the OD600 of the culture was between 0.6–0.8, the expression was induced by 0.5 mM IPTG for 5 hours at 25°C. Bacteria were frozen at −80 °C until use. Cells were thawed and resuspended in Lysis buffer (50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazole, 50 µM FMN, pH 7.5), 1 mM PMSF, 0.1% Triton X-100 and 0.2 mg/ml lysozyme for 30 minutes. After sonication, cells were centrifuged at 4,800×g for 10 minutes at 4 °C. The supernatant containing the total cellular extract was loaded into a Ni-NTA agarose column (Qiagen, Germany). The column was washed with two bed volumes of Lysis buffer with 20 mM imidazole to eliminate unbound proteins. GO was eluted using the same buffer with 300 mM imidazole. Fractions containing purified GO were dialyzed against Dialysis Buffer (50 mM NaH2PO4, 300 mM NaCl, pH 7.5) at 4 °C in agitation over night, and then kept at 4 °C. Protein determination was performed using the bicinchoninic acid assay.
Western Blot. Tissues were sonicated in buffer with 50 mM TrisHCl, pH 7.4, 150-mM NaCl, 1% NP-40, 0.5% deoxycholic acid, 0.1% sodium dodecyl sulfate, 1× protease inhibitors (Complete, Roche). Protein concentration was measured using bicinchoninic acid, and equal amounts of protein (50 µg) were analyzed by immunoblotting with IgG affinity-purified from an anti-GO rabbit serum (raised against recombinant mGO), or anti-GADPH (1:5,000) (Abcam) as a control. Peroxidase-conjugated anti-rabbit IgG (Jackson IR) were used as secondary antibodies and chemiluminiscence substrate was from Pierce.
Glycolate Oxidase Enzymatic Assay. Enzymatic activity of purified mGO was determined in the presence of glycolate as substrate in 50 mM potassium phosphate buffer pH 7. The addition of sulfonated-DCIP (gift from Dr. Williams, UCL, UK) and 4-aminoantipyrine (Sigma Aldrich) in a coupled HRP reaction yields a chromogen that is measured at 515 nm.7 For kinetic parameters determination, increasing concentrations of substrate were used keeping constant the enzyme concentration (1 µg). Vmax and Km were calculated fitting the Michaelis–Menten equation:
To determine the type of inhibition of CCPST (4-carboxy-5-[(4-chlorophenyl)sulfanyl]-1,2,3-thiadiazole, Key Organics, Cornwall, UK) with glycolate oxidase and the corresponding Ki, increasing concentrations of the inhibitor at different concentrations of substrate with a constant enzyme concentration were plotted in a Cornish-Bowden plot, in which Ki was calculated as the intersection of all lines. IC50 value for CCPST was calculated using 1 µg of GO with increased concentrations of inhibitor between 1 µM and 1 mM in saturated conditions of substrate.
Hepatocytes Isolation and Culture. Hepatocytes were isolated by in situ collagenase perfusion method11 from male 129 Agxt1−/− and Agxt1−/− Hao1−/− mice liver. A total of 3.0 × 105 cells/well were cultured in six-well plates with Williams E medium supplemented with 5% fetal bovine serum, 2 mM l-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin, 2.2 mUI/ml insulin and 0.3 µg/ml hydrocortisone. After 5 hours, medium was changed to Williams E complete medium (Biochrom, Cambridge, UK) without serum and cells were treated with increased concentrations of CCPST in the presence of 5 mM glycolate. Culture medium was harvested at 24, 48, and 72 hours after treatment for oxalate quantification. For intracellular CCPST determination, 6.0 × 105 cells were seeded in 60-mm-diameter dishes following the same procedure as mentioned before. 24 h post-treatment, cells were harvested in distilled water, broken with three freeze/thaw cycles and centrifuged. Supernatants were stored at −20 °C until use.
Cell Viability and Cytotoxicity. 1.0 × 104 cells/well were seeded in 96-well plates and treated with the same concentrations of CCPST as previously described for six-well plates. At each time point, (24, 48, and 72 h), 20 µl of Cell Titer 96® Aqueous One Solution Reagent (Promega, Madison, Wisconsin) was added to the medium, incubated 2 hours at 37 °C, 5% CO2 and measured at 493 nm.
Oxalate Determination. Determination of oxalate excreted to the medium and urine oxalate was measured by the oxalate oxidase assay using a commercial kit (Trinity Biotech, Co Wicklow, Ireland), following manufacturer's instructions. GraphPad Prism 5 software was used for graphic representation of the data as mean ± SD.
Urine Glycolate Determination. Measurement of glycolate levels in urine samples was performed with the same enzymatic assay of glycolate oxidase described above, but using 2 µg of purified protein per sample.
CCPST-CD Administration and Urine Collection. Agxt1−/− mice were placed in metabolic cages for a single mouse (Tecniplast, Buguggiate, Italy) and allowed to get acclimatized for 3 days before the start of urine collection. Two consecutive 24-hour urine collections were performed for basal urine oxalate determination. Next, CCPST-CD was administered by gavage at a daily dose of 110 mg/kg body weight during 11 days. All 24-hour urine collections were obtained in tubes containing 50 µl of 6 M HCl. Samples with <1 ml urine or with food or fecal contamination were excluded from the study.
Histological Analysis. Organs were fixed in 4% buffered paraformaldehyde and embedded in paraffin. Hematoxilin/eosin staining was done in all tissues.
Reverse Phase HPLC/UV Analysis of CCPST. Deproteinized samples of total cellular extract from Agxt1−/− hepatocytes in 5% perchloric acid were separated using a HPLC analytical system Finnigan Surveyor (Thermo Fisher Scientific, San Jose, California) in combination with a 150 × 4.6 mm column packed with ACE C-18, 5 µm particle size (Advanced Chromatography Technologies Ltd, Scotland). The mobile phase consisted of deionized water pH 2.2 in phosphoric acid/acetonitrile (55:45 v/v), pumped at a flow rate of 1.0 ml/min. CCPST eluted at ≈5.5 minutes and was detected at 235 nm. Drug concentrations were determined against authentic calibrated standards and validated with in-house quality control standard.
Statistical Analysis. Descriptive data are expressed as mean ± SD and plotted in GraphPad Prism 5 software. One-way ANOVA test was used for comparison between independent groups and paired-sample t test for related groups. Tukey's range test was chosen as post-hoc analysis. Mann–Whitney U-tests were used for comparisons between two independent groups without evidence of normal distribution. All data were analyzed using SPSS v.19 statistical package. A P-value <0.05 was considered significant.
SUPPLEMENTARY MATERIAL Figure S1. Gene trapping of the mouse Hao1 locus. Figure S2. Enzymatic kinetics of mouse glycolate oxidase. Figure S3. Relative amount of excreted oxalate by Agxt1-/-Hao1-/- hepatocytes after CCPST treatment. Figure S4. Representative histological images of Agxt1-/- mice liver, kidney, lung and heart by hematoxilin-eosin staining after oral administration of CCPST-CD.
Acknowledgments
We thank Drs. Carmen Évora and Araceli Delgado for their advice, Bárbara Rodríguez, Cristina Paz and María Rosa Arnau for their technical support and help with animal care and Dr. Javier Triñanes for informatic support and Gill Rumsby and Emma Williams for the glycolate oxidase assay. This work is supported by grant SAF2011-23933 from the Spanish Ministry of Science. ES designed research; CMH and ES performed research; CMH and ES wrote the paper.
The authors declare no conflict of interest.
Supplementary Material
References
- Rumsby, G and Cochat, P (2013). Primary hyperoxaluria. N Engl J Med 369: 2163. [DOI] [PubMed] [Google Scholar]
- Salido, E, Pey, AL, Rodriguez, R and Lorenzo, V (2012). Primary hyperoxalurias: disorders of glyoxylate detoxification. Biochim Biophys Acta 1822: 1453–1464. [DOI] [PubMed] [Google Scholar]
- Smid, BE, Aerts, JM, Boot, RG, Linthorst, GE and Hollak, CE (2010). Pharmacological small molecules for the treatment of lysosomal storage disorders. Expert Opin Investig Drugs 19: 1367–1379. [DOI] [PubMed] [Google Scholar]
- Holmes, RP and Assimos, DG (1998). Glyoxylate synthesis, and its modulation and influence on oxalate synthesis. J Urol 160: 1617–1624. [PubMed] [Google Scholar]
- Knight, J, Jiang, J, Assimos, DG and Holmes, RP (2006). Hydroxyproline ingestion and urinary oxalate and glycolate excretion. Kidney Int 70: 1929–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg, K and Lindqvist, Y (1997). Three-dimensional structures of glycolate oxidase with bound active-site inhibitors. Protein Sci 6: 1009–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignaud, C, Pietrancosta, N, Williams, EL, Rumsby, G and Lederer, F (2007). Purification and characterization of recombinant human liver glycolate oxidase. Arch Biochem Biophys 465: 410–416. [DOI] [PubMed] [Google Scholar]
- Murray, MS, Holmes, RP and Lowther, WT (2008). Active site and loop 4 movements within human glycolate oxidase: implications for substrate specificity and drug design. Biochemistry 47: 2439–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourhis, JM, Vignaud, C, Pietrancosta, N, Guéritte, F, Guénard, D, Lederer, F et al. (2009). Structure of human glycolate oxidase in complex with the inhibitor 4-carboxy-5-[(4-chlorophenyl)sulfanyl]-1,2,3-thiadiazole. Acta Crystallogr Sect F Struct Biol Cryst Commun 65: 1246–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frishberg, Y, Zeharia, A, Lyakhovetsky, R, Bargal, R and Belostotsky, R (2014). Mutations in HAO1 encoding glycolate oxidase cause isolated glycolic aciduria. J Med Genet 51: 526–529. [DOI] [PubMed] [Google Scholar]
- Salido, EC, Li, XM, Lu, Y, Wang, X, Santana, A, Roy-Chowdhury, N et al. (2006). Alanine-glyoxylate aminotransferase-deficient mice, a model for primary hyperoxaluria that responds to adenoviral gene transfer. Proc Natl Acad Sci USA 103: 18249–18254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, ZW, Vignaud, C, Jaafar, A, Lévy, B, Guéritte, F, Guénard, D et al. (2012). High resolution crystal structure of rat long chain hydroxy acid oxidase in complex with the inhibitor 4-carboxy-5-[(4-chlorophenyl)sulfanyl]-1, 2, 3-thiadiazole. Implications for inhibitor specificity and drug design. Biochimie 94: 1172–1179. [DOI] [PubMed] [Google Scholar]
- Baker, PR, Cramer, SD, Kennedy, M, Assimos, DG and Holmes, RP (2004). Glycolate and glyoxylate metabolism in HepG2 cells. Am J Physiol Cell Physiol 287: C1359–C1365. [DOI] [PubMed] [Google Scholar]
- Smith, LH Jr, Bauer, RL, Craig, JC, Chan, RP and Williams, HE (1972). Inhibition of oxalate synthesis: in vitro studies using analogues of oxalate and glycolate. Biochem Med 6: 317–332. [DOI] [PubMed] [Google Scholar]
- Watts, RW, Chalmers, RA, Gibbs, DA, Lawson, AM, Purkiss, P and Spellacy, E (1979). Studies on some possible biochemical treatments of primary hyperoxaluria. Q J Med 48: 259–272. [PubMed] [Google Scholar]
- Holmes, RP (1998). Pharmacological approaches in the treatment of primary hyperoxaluria. J Nephrol 11:Suppl 1: 32–35. [PubMed] [Google Scholar]
- Dutta, C, Avitahl-Curtis, N, Pursell, N, Cohen, ML, Holmes, B, Diwanji, R et al. (2016). Inhibition of glycolate oxidase with dicer-substrate siRNA reduces calcium oxalate deposition in a mouse model of primary hyperoxaluria type I. Mol Ther, in press. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.