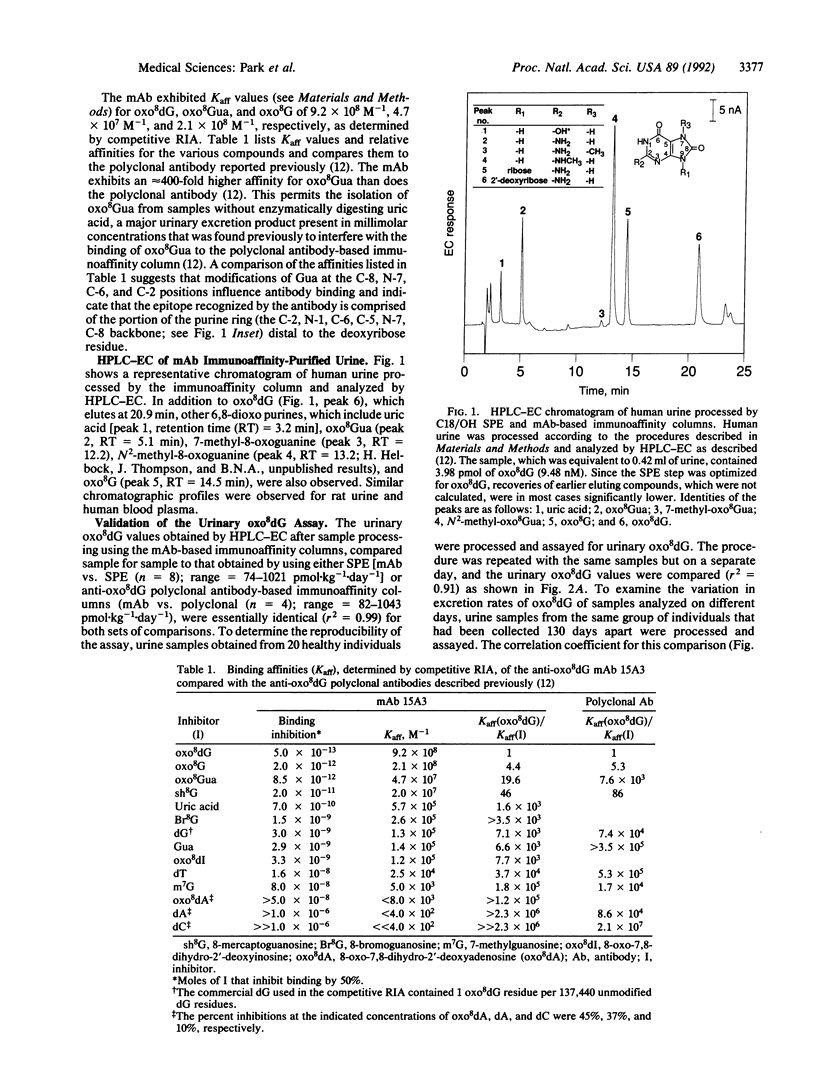
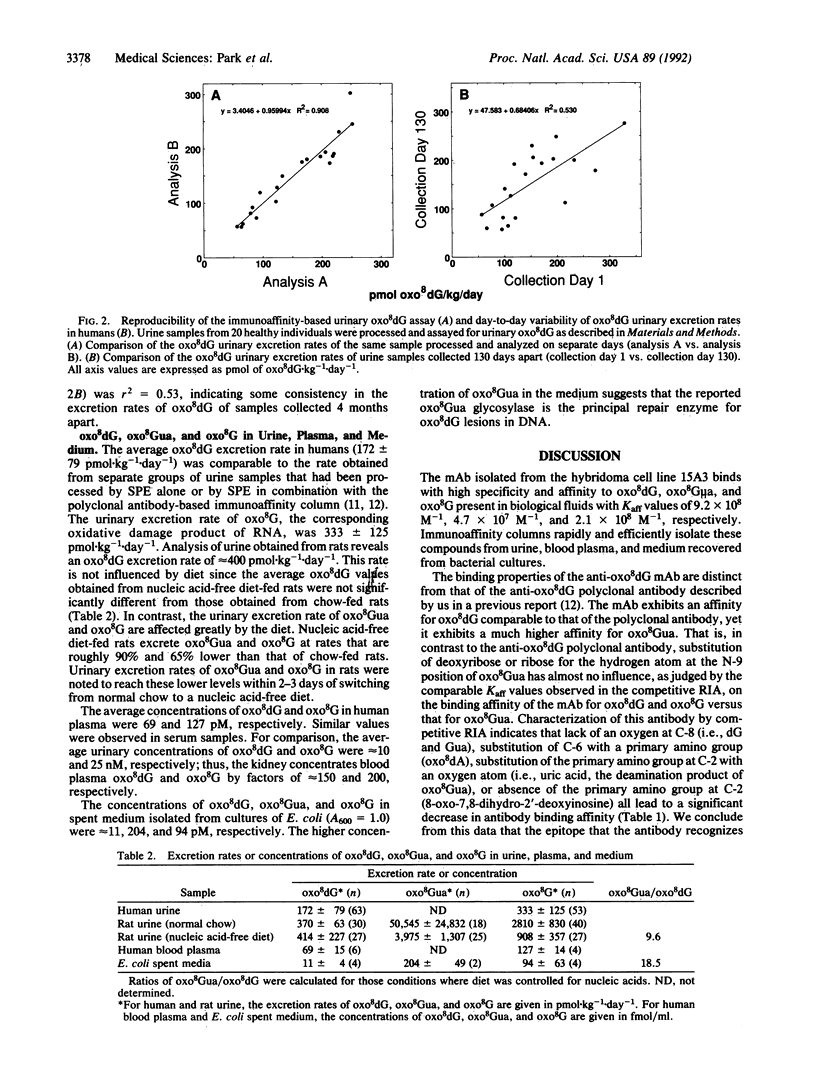
Abstract
An immunoaffinity column is described that facilitates the analysis of oxidative damage products of DNA and RNA in urine, blood plasma, and medium isolated from cultures of Escherichia coli. In intact animals, lesions (adducts) excised from DNA are transported from the cell through the circulation and excreted in urine. In bacteria, DNA adducts are excreted directly into the medium. In either case, the adducts can be assayed as a measure of oxidative damage to DNA. A monoclonal antibody that recognizes 8-oxo-7,8-dihydro-2'-deoxyguanosine (oxo8dG;8-hydroxy-2'-deoxyguanosine), a bio-marker of oxidative damage to DNA, has been isolated, and its substrate binding properties have been characterized. The relative binding affinities of this monoclonal antibody for oxo8dG, unmodified nucleosides, or derivatives of Gua made it suitable for the preparation of immunoaffinity columns that greatly facilitate the isolation of oxo8dG, 8-oxo-7,8-dihydroguanine, and 8-oxo-7,8-dihydroguanosine from various biological fluids. Quantitative analysis of these adducts in urine of rats fed a nucleic acid-free diet and in the medium from cultures of E. coli suggests that oxo8-7,8-dihydroguanine is the principal repair product from oxo8-dG in DNA of both eukaryotes and prokaryotes. The results support our previous estimate of about 10(5) oxidative lesions to DNA being formed and excised in an average rat cell per day.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman R., Saul R. L., Ames B. N. Oxidative damage to DNA: relation to species metabolic rate and life span. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2706–2708. doi: 10.1073/pnas.85.8.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames B. N. Endogenous oxidative DNA damage, aging, and cancer. Free Radic Res Commun. 1989;7(3-6):121–128. doi: 10.3109/10715768909087933. [DOI] [PubMed] [Google Scholar]
- Boiteux S., O'Connor T. R., Lederer F., Gouyette A., Laval J. Homogeneous Escherichia coli FPG protein. A DNA glycosylase which excises imidazole ring-opened purines and nicks DNA at apurinic/apyrimidinic sites. J Biol Chem. 1990 Mar 5;265(7):3916–3922. [PubMed] [Google Scholar]
- Cathcart R., Schwiers E., Saul R. L., Ames B. N. Thymine glycol and thymidine glycol in human and rat urine: a possible assay for oxidative DNA damage. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5633–5637. doi: 10.1073/pnas.81.18.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung M. H., Kasai H., Jones D. S., Inoue H., Ishikawa H., Ohtsuka E., Nishimura S. An endonuclease activity of Escherichia coli that specifically removes 8-hydroxyguanine residues from DNA. Mutat Res. 1991 Jan;254(1):1–12. doi: 10.1016/0921-8777(91)90035-n. [DOI] [PubMed] [Google Scholar]
- Degan P., Shigenaga M. K., Park E. M., Alperin P. E., Ames B. N. Immunoaffinity isolation of urinary 8-hydroxy-2'-deoxyguanosine and 8-hydroxyguanine and quantitation of 8-hydroxy-2'-deoxyguanosine in DNA by polyclonal antibodies. Carcinogenesis. 1991 May;12(5):865–871. doi: 10.1093/carcin/12.5.865. [DOI] [PubMed] [Google Scholar]
- Dizdaroglu M. Formation of an 8-hydroxyguanine moiety in deoxyribonucleic acid on gamma-irradiation in aqueous solution. Biochemistry. 1985 Jul 30;24(16):4476–4481. doi: 10.1021/bi00337a032. [DOI] [PubMed] [Google Scholar]
- Fiala E. S., Conaway C. C., Mathis J. E. Oxidative DNA and RNA damage in the livers of Sprague-Dawley rats treated with the hepatocarcinogen 2-nitropropane. Cancer Res. 1989 Oct 15;49(20):5518–5522. [PubMed] [Google Scholar]
- Floyd R. A. The role of 8-hydroxyguanine in carcinogenesis. Carcinogenesis. 1990 Sep;11(9):1447–1450. doi: 10.1093/carcin/11.9.1447. [DOI] [PubMed] [Google Scholar]
- Floyd R. A., Watson J. J., Wong P. K., Altmiller D. H., Rickard R. C. Hydroxyl free radical adduct of deoxyguanosine: sensitive detection and mechanisms of formation. Free Radic Res Commun. 1986;1(3):163–172. doi: 10.3109/10715768609083148. [DOI] [PubMed] [Google Scholar]
- Fraga C. G., Motchnik P. A., Shigenaga M. K., Helbock H. J., Jacob R. A., Ames B. N. Ascorbic acid protects against endogenous oxidative DNA damage in human sperm. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11003–11006. doi: 10.1073/pnas.88.24.11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraga C. G., Shigenaga M. K., Park J. W., Degan P., Ames B. N. Oxidative damage to DNA during aging: 8-hydroxy-2'-deoxyguanosine in rat organ DNA and urine. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4533–4537. doi: 10.1073/pnas.87.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei B., England L., Ames B. N. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6377–6381. doi: 10.1073/pnas.86.16.6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei B., Kim M. C., Ames B. N. Ubiquinol-10 is an effective lipid-soluble antioxidant at physiological concentrations. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4879–4883. doi: 10.1073/pnas.87.12.4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei B., Stocker R., Ames B. N. Antioxidant defenses and lipid peroxidation in human blood plasma. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9748–9752. doi: 10.1073/pnas.85.24.9748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H., Nishimura S., Kurokawa Y., Hayashi Y. Oral administration of the renal carcinogen, potassium bromate, specifically produces 8-hydroxydeoxyguanosine in rat target organ DNA. Carcinogenesis. 1987 Dec;8(12):1959–1961. doi: 10.1093/carcin/8.12.1959. [DOI] [PubMed] [Google Scholar]
- Michaels M. L., Pham L., Cruz C., Miller J. H. MutM, a protein that prevents G.C----T.A transversions, is formamidopyrimidine-DNA glycosylase. Nucleic Acids Res. 1991 Jul 11;19(13):3629–3632. doi: 10.1093/nar/19.13.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R. Calculation of average antibody affinity in anti-hapten sera from data obtained by competitive radioimmunoassay. J Immunol Methods. 1980;34(4):345–352. doi: 10.1016/0022-1759(80)90107-6. [DOI] [PubMed] [Google Scholar]
- Müller R., Rajewsky M. F. Immunological quantification by high-affinity antibodies of O6-ethyldeoxyguanosine in DNA exposed to N-ethyl-N-nitrosourea. Cancer Res. 1980 Mar;40(3):887–896. [PubMed] [Google Scholar]
- Prevost V., Shuker D. E., Bartsch H., Pastorelli R., Stillwell W. G., Trudel L. J., Tannenbaum S. R. The determination of urinary 3-methyladenine by immunoaffinity chromatography-monoclonal antibody-based ELISA: use in human biomonitoring studies. Carcinogenesis. 1990 Oct;11(10):1747–1751. doi: 10.1093/carcin/11.10.1747. [DOI] [PubMed] [Google Scholar]
- Shibutani S., Takeshita M., Grollman A. P. Insertion of specific bases during DNA synthesis past the oxidation-damaged base 8-oxodG. Nature. 1991 Jan 31;349(6308):431–434. doi: 10.1038/349431a0. [DOI] [PubMed] [Google Scholar]
- Shigenaga M. K., Gimeno C. J., Ames B. N. Urinary 8-hydroxy-2'-deoxyguanosine as a biological marker of in vivo oxidative DNA damage. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9697–9701. doi: 10.1073/pnas.86.24.9697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker R., Yamamoto Y., McDonagh A. F., Glazer A. N., Ames B. N. Bilirubin is an antioxidant of possible physiological importance. Science. 1987 Feb 27;235(4792):1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- Tchou J., Kasai H., Shibutani S., Chung M. H., Laval J., Grollman A. P., Nishimura S. 8-oxoguanine (8-hydroxyguanine) DNA glycosylase and its substrate specificity. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4690–4694. doi: 10.1073/pnas.88.11.4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood M. L., Dizdaroglu M., Gajewski E., Essigmann J. M. Mechanistic studies of ionizing radiation and oxidative mutagenesis: genetic effects of a single 8-hydroxyguanine (7-hydro-8-oxoguanine) residue inserted at a unique site in a viral genome. Biochemistry. 1990 Jul 31;29(30):7024–7032. doi: 10.1021/bi00482a011. [DOI] [PubMed] [Google Scholar]


