Abstract
Due to their permanent and close proximity to neurons, glial cells perform essential tasks for the normal physiology of the retina. Astrocytes and Müller cells (retinal macroglia) provide physical support to neurons and supplement them with several metabolites and growth factors. Macroglia are involved in maintaining the homeostasis of extracellular ions and neurotransmitters, are essential for information processing in neural circuits, participate in retinal glucose metabolism and in removing metabolic waste products, regulate local blood flow, induce the blood-retinal barrier (BRB), play fundamental roles in local immune response, and protect neurons from oxidative damage. In response to polyetiological insults, glia cells react with a process called reactive gliosis, seeking to maintain retinal homeostasis. When malfunctioning, macroglial cells can become primary pathogenic elements. A reactive gliosis has been described in different retinal pathologies, including age-related macular degeneration (AMD), diabetes, glaucoma, retinal detachment, or retinitis pigmentosa. A better understanding of the dual, neuroprotective, or cytotoxic effect of macroglial involvement in retinal pathologies would help in treating the physiopathology of these diseases. The extensive participation of the macroglia in retinal diseases points to these cells as innovative targets for new drug therapies.
1. Introduction
The macroglial cells of the retina are the astrocytes and the Müller cells. Macroglial cells perform various essential roles for the normal physiology of the retina, maintaining a close and permanent relationship with the neurons [1]. Under normal conditions, the retinal macroglia provide trophic and metabolic support to neurons and are responsible for maintaining the homeostatic environment required for appropriate neuronal functioning. Furthermore, they are involved in the formation of the BRB and might even play a role in the correct transmission of nerve impulses [2]. Glia, as a population of immune cells residing in the retina and the optic nerve, are able to respond and become activated rapidly in the presence of any type of damage, in order to safeguard the immune privilege of nervous tissue [3]. Reactive gliosis has a direct neuroprotective effect on the retina. By contrast, chronic gliosis exacerbates disease progression, increasing vascular permeability, infiltration of toxic compounds, and even neovascularization [4].
The aforementioned data underline the importance of undertaking studies aimed at improving our understanding of the role of macroglia in the pathogenesis of retinal diseases. Such knowledge could help to develop novel neuroprotective therapies for medical treatment of these diseases.
2. Glial Cells
Glial cells have long been considered purely passive elements within the nervous system. Yet, their proximity to the neurons and blood vessels involves them in vital tasks that are essential for neuronal survival [5]. Glial cells are subdivided into macroglial cells (astrocytes and oligodendrocytes) and microglial cells. Astrocytes represent the most abundant and morphologically heterogeneous neuroglial cell, these including protoplasmic astrocytes, fibrous astrocytes, and radial glia (Bergmann glia of the cerebellum and Müller cells of the retina) [6, 7]. Oligodendroglia are responsible for myelination and metabolic support of the axon, while astrocytes are more involved as key players in neuronal circuits, information processing, and maintenance of synaptic integrity [8, 9].
3. Retinal Macroglia
Overall, in the vascular retina of many vertebrates (including mammals) two basic types of macroglial cells are found: Müller cells and astrocytes. The oligodendrocytes are seen occasionally in the retina, but only when myelinated ganglion cell axons are present in the nerve-fiber layer [1, 10–15]. Müller cells are long, radially oriented cells, which span the width of the neural retina from the outer limiting membrane (OLM), where their apical ends are located, to the inner limiting membrane (INL), where their basal end feet terminate. Müller cells ensheath all retinal neural somas and processes. Each of these cells can be considered the core of a columnar microunit of retinal neurons [16, 17]. Thus, Müller cells constitute an anatomical link between the retinal neurons and the compartments with which these need to exchange molecules (the retinal blood vessels, vitreous body, and subretinal space) [18].
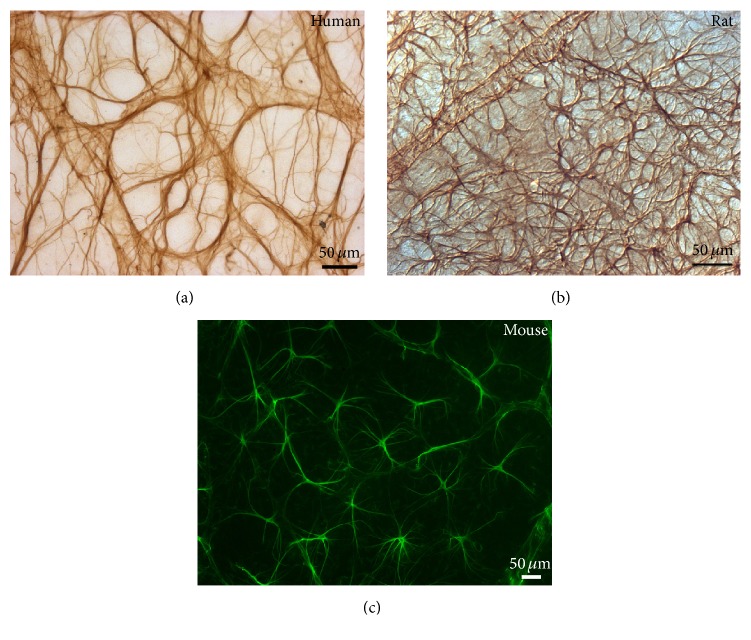
Retinal astrocytes are located mainly in the nerve-fiber layer (NFL) and ganglion cell layer (GCL) in most mammals, that is, human, rats, and mouse [1, 19, 154]. In rabbits, astrocytes are confined to the medullary nerve-fiber region, which is the only vascularized area in the rabbit retina [13]. Retinal astrocyte morphology differs between species. In humans, two types of astrocytes can be distinguished: elongated astrocytes (located in the NFL) and star-shaped astrocytes (located in the GCL) (Figure 1(a)) [12, 20]. In mice and rats the astrocytes are star-shaped (Figures 1(b) and 1(c)) [19, 20].
Figure 1.
Retinal astrocytes. Retinal whole-mount. Immunoperoxidase ((a) and (b)). Immunofluorescence (c). Astrocytes in the normal retina of a 58-year-old man. In the ganglion cell layer of the human retina, star-shaped astrocytes form a honeycomb plexus (a). In the rat (b) and mouse (c) retina star-shaped astrocytes form a plexus distributed throughout the retina. Such plexus is denser in the rat than in the mouse retina.
Macroglial cells are permanently in close relationship with neurons, performing various essential roles for the normal physiology of the retina [1, 12–14]. Thus, every aspect of the development, homeostasis, and function of the visual system involves a neuron-glia partnership. Unlike retinal ganglion cells (RGCs), astrocytes do not propagate action potentials along their processes; however, astrocytes and Müller cells do exhibit regulated increases in intracellular calcium concentrations [Ca2+]i that represent a form of astrocyte excitability [21–24]. Increases in astrocytic [Ca2+]i are of functional significance in astrocyte/astrocyte and astrocyte/neuron communication.
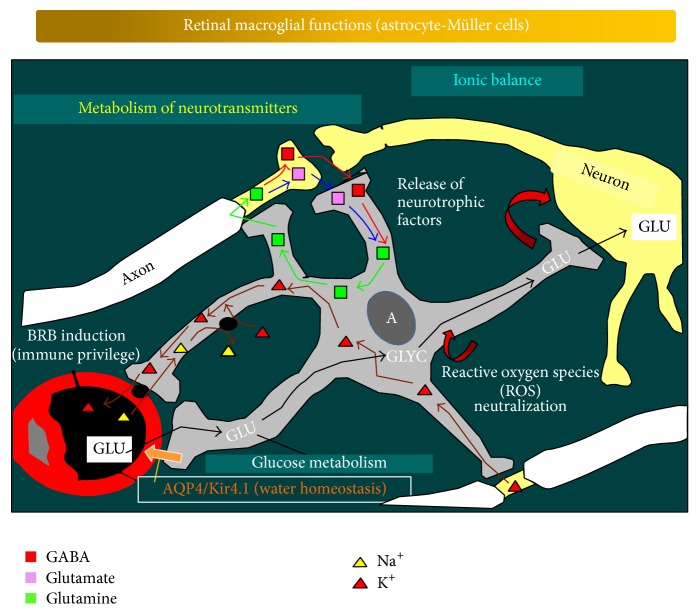
Astrocytes and Müller cells participate in the structural organization of the retina through the creation of nonoverlapping microanatomical domains that integrate into macroglial syncytia through gap junctions [12]. This organization allows long-distance communication within glial networks [25]. Macroglial cells insulate neurons, provide physical support for them, and supplement them with several metabolites and growth factors (Figure 2). These cells are also important in axon guidance and in the control of synaptogenesis [26, 27] and can adopt stem-cell properties [28, 29].
Figure 2.
Schematic drawing illustrating the functions of the retinal macroglia. Macroglial cells perform various essential roles for the normal physiology of the retina, maintaining a close and permanent relationship with the neurons. The scheme illustrates the links between the retinal macroglia, the neurons, and the blood-retinal barrier (from [154]).
Under normal conditions, astrocytes and Müller cells maintain the homeostasis of extracellular ions and other metabolites, water, and pH (Figure 2). It has been demonstrated that a complex glutamatergic-purinergic signaling cascade enables Müller cells to maintain their cell volume. Tight cell-volume regulation is a prerequisite for Müller cells to mediate transcellular ion and fluid fluxes from the extracellular space of the retina to reservoirs such as the vitreous body or blood vessels, thus in turn enabling the spatial buffering of potassium and the maintenance of retinal-fluid homeostasis [7, 30]. In addition, astrocytes and Müller cells are involved in maintaining the homeostasis of neurotransmitters such as glutamate and GABA (Figure 2) [31]. After the reuptake of neurotransmitters into astrocytes, the neurotransmitters are metabolized and transformed into precursors that can be returned to the neurons to be converted into active neurotransmitters. The astrocytes directly interact with neurons during synaptic activity in a manner that is essential for information processing in neural circuits. Such evidence has given rise to the “tripartite synapse” hypothesis [32–34]. The synapses in the CNS appear to be constituted by three elements: the perisynaptic astroglial processes, the presynaptic neuron, and the postsynaptic one [34]. In this architecture, astrocytes have a dual role. These cells in fact can sense the transmitter release as they express many neurotransmitter receptors, and, on the other hand, astrocytes can modulate the efficacy of the synapse by releasing gliotransmitters (i.e., glutamate, GABA, ATP, and D-serine), thus accurately modulating synaptic transmission [35].
Macroglial cells are also involved in retinal glucose metabolism (Figure 2), providing retinal neurons with nutrients such as lactate/pyruvate for their oxidative metabolism [18, 36, 37] and in removing metabolic waste products. These cells also produce a great quantity of cytokines and growth factors [38, 39], which may contribute to both neurotoxic and neuroprotective effects [40]. In addition, they produce laminin, fibronectin, and tropoelastin, the precursor of elastin [39]. Astrocytes and Müller cells have also been demonstrated to be more resistant to oxidative damage than neurons; this characteristic protects them against such damage. This potential is due to the fact that these cells contain high concentrations of antioxidants such as reduced glutathione and vitamins (Figure 2) [41]. Reduced glutathione is provided to neurons [42, 43] and acts as a scavenger of free radicals and reactive oxygen compounds [18]. Another way of neuroprotection is the uptake and/or detoxification of potentially harmful substances and even particles (either intrinsic or foreign). This involves the phagocytosis of debris from death neurons or pigment epithelial cells [44–48]. Consequently, depression of these cellular activities could lead to neuronal dysfunction [49].
Astrocyte and Müller cells are involved in regulating local blood flow [29] in response to changes in neuronal activity [50]. Indeed, a number of molecules, such as prostaglandins (PGE), nitric oxide (NO), and arachidonic acid (AA), which increase or decrease CNS blood-vessel diameter and blood flow, are produced by astrocytes [51, 52]. Astrocytes and Müller cells induce the properties of the barriers in the retinal capillaries, the BRB (Figure 2) [53, 54]. They release substances that stabilize the tight junctions between endothelial vascular cells [54], securing immune privilege to protect neurons from the potential damage of an inflammatory immune response (Figure 2). In addition, glial cells play fundamental roles in local immune responses and immunosurveillance [55, 56].
Finally, Müller cells act as “light guides.” Their orientation and low scattering make these cells able, like optical fibers, to conduct the light into the interior of the retina to fall on the photoreceptors with less degradation [57].
3.1. Astrocytes and Retinal Diseases
As mentioned above, the main function of astrocytes is to maintain the homeostasis of the nervous tissue and to control, protect, and support neuronal function [58]. Astroglial cells defend the CNS and therefore the retina from damage through a process called reactive gliosis. This gliosis is triggered in response to polyetiological insults [59] such as trauma, ischemic damage, neuroinflammation, or neurodegeneration. This response seeks to maintain retinal homeostasis involving both morphological and functional alteration in the glial cells [60]; however, when malfunctioning, astroglia can also constitute the primary pathogenic element [61].
Reactive astrogliosis is an evolutionarily conserved defense program, which is disease- and context-specific and involves the activation of thousands of genes [59, 61]. Thus, at least 50% of the injury-altered gene expression is injury-type specific [62].
The hallmarks of reactive astrogliosis are a burst in astrocyte number (hyperplasia/proliferation), increased number and length of astroglial processes, larger cell body size (hypertrophy), migration, and upregulation of cytoskeletal components such as glial fibrillary acidic protein (GFAP), vimentin, and nestin [45, 47, 48, 59, 61, 63]. The deletion of GFAP and vimentin genes in a genetic mice model of Alzheimer's disease (AD) in vivo resulted in a complete inhibition of astroglial activation [64]. The increased expressions of these intermediate filaments are, however, considered only as broad markers of this process, because astrogliotic metamorphosis may produce many different, yet to be fully characterized, reactive phenotypes specific to different diseases.
A reactive astrogliosis has been reported in different retinal pathologies. In AMD a large number of reactive and hypertrophic astrocytes have been found [47]. In experimental diabetic retinopathy the glial reactivity was manifested by increased GFAP immunoreactivity and content in astrocytes [65]. In the final stages of retinitis pigmentosa, when the ganglion cells disappear, the only cells left are reactive hyperplasic astrocytes [66]. In both the human glaucomatous optic nerve head and the retina of different animal models of glaucoma, greater GFAP expression has been detected [19, 67, 68]. In a mouse model of laser-induced ocular hypertension (OHT), both contralateral and OHT eyes have intensified GFAP immunoreactivity with respect to the naïve animals; the retinal macroglia of contralateral normotensive eyes exhibited morphological signs of reactivity that differed from naïve and OHT eyes. Astrocytes in contralateral eyes were more robust and had an increase in GFAP-labeled retinal area in comparison to naïve ones, although astrocytes in OHT eyes showed fewer secondary processes and a reduction in the GFAP-labeled retinal area with respect to contralateral and naïve eyes [19]. In addition, it has been noted that astrocytes proliferated at the optic nerve head and in the lateral geniculate nucleus and visual cortex in human glaucoma and animal glaucoma models [69, 70], postulating that astrocytosis is key in the remodeling of the optic nerve head during glaucomatous damage [71].
In the processes of a productive gliotic response, astrocytes undergo complex remodeling of their biochemistry and function, which generally leads to neuroprotection (Figure 3) [59]. A growing group of studies evidence a beneficial role for activated astrocytes in neuroinflammation associated with neurodegenerative diseases [72]. Activated astrocytes stimulate higher metabolic activity, increase the expression of cytoprotective factors, and restore neurotransmitter balance and ion and water concentration, among other benefits [20, 45–47, 63]. This reactive gliosis has been associated with the upregulation of enzymatic and nonenzymatic antioxidant defenses that may fortify the ability of the astrocytes to protect neurons from free radicals (Figure 3) [41]. In an experimental model of glaucoma in rats the retinal area occupied by astrocytes in eyes diminished with ocular hypertension; this trend is stronger in eyes with higher levels of intraocular pressure [20]. The authors postulate that, in RGC, death would start when astrocytes fell below a specific level, and thus a minimal amount of retina covered by astrocytes could be necessary to protect the RGC [20].
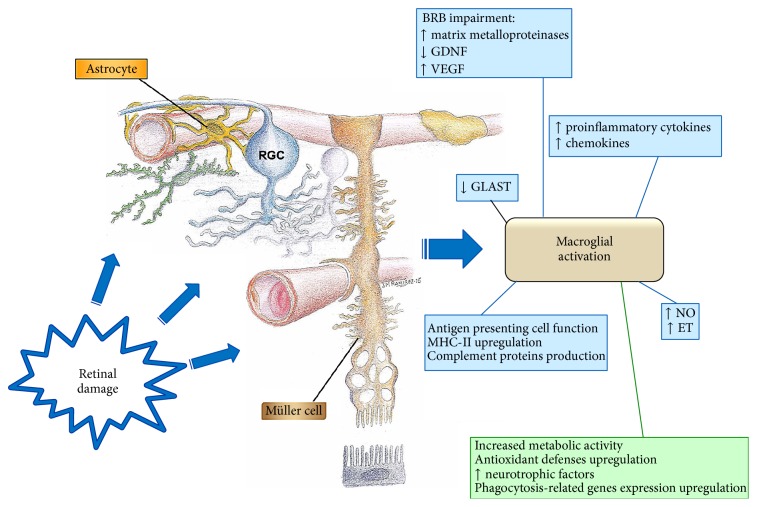
Figure 3.
Diagram summarizing the functions of activated macroglia. Under conditions of tissue stress that might represent a risk to neuronal survival, glial cells undergo reactive gliosis. The aim of acute gliosis is to protect the nervous tissue by reestablishing the extracellular medium and by supplementing neurons with factors that promote their survival (in green). An uncontrolled response (in blue), as occurs in most neurodegenerative diseases, will harm the tissue [BRB: blood-retinal barrier; ET: endothelin; GDNF: glial cell derived neurotrophic factor; GLAST: glutamate aspartate transporter; MHC: major histocompatibility complex; NO: nitric oxide; RGC: retinal ganglion cell; VEGF: vascular endothelium growth factor].
Astrocytes provide neurotrophic factors for RGC support; this is particularly important in glaucoma in which blockage in the axonal transport inherent to this pathology can impair neurotrophin delivery from the visual pathway, such as the superior colliculus. The loss of astrocytes in the lamina cribrosa during glaucomatous neurodegeneration could compromise RGC survival [73].
During the CNS injury, astrocytes become reactive and migrate to the damage site where they isolate the injured area and remove pathogens, dying cells, and cellular debris and then remodel the nerve tissue on resolving the pathology (Figure 3) [61]. In AMD, hypertrophic and reactive astrocytes have been observed to phagocytize the residues of ganglion cells that have died through necrosis or apoptosis [48]. Astrocytes in glaucoma have shown an upregulated expression of the phagocytosis-related gen Mac-2, in the laminar and orbital region of the optic nerve, suggesting that astrocytes could participate in the clearance of the RCG axonal debris [74].
As noted above, reactive gliosis includes the onset of signaling mechanisms that are primarily protective for retinal neurons but may proceed uncontrolled to augment neuronal damage [40, 75]. Chronic gliosis is typically injurious, directly and indirectly damaging neurons and the vasculature while also inhibiting tissue repair [76]. During chronic diseases such as angiogenic vascular conditions in the eye (diabetic retinopathy, retinal-vein occlusion, retinopathy of prematurity, and AMD), reactive astroglia through vascular endothelial growth factor (VEGF) production exacerbate disease progression, increasing vascular permeability and even neovascularization [4]. Also, reactive astroglia produce molecules that inhibit axon regeneration and repair, triggering neurocytotoxicity or secondary damage in nearby neurons and glial cells [59, 77, 78]. The absence of GFAP and vimentin reportedly attenuates retinal-detachment-induced reactive gliosis and subsequently limits photoreceptor degeneration [79]. Moreover, the inhibition of reactive gliosis prevents apoptotic death of retinal neurons and provides substantial neuroprotection [80].
Although the microglia cells are the main mediators in the inflammatory damage of the CNS during neurodegeneration, astrocytes behave similarly to microglia and together can act synergistically, promoting chronic neuroinflammation or fostering neuroprotection [72]. Most inflammatory mediators produced by astrocytes may act on microglial cells, thus facilitating chronic microglial activation and thereby favoring neuronal death [81]. Similarly, inflammatory mediators produced by microglia may intensify astrocyte activation [82, 83]. TNF-α promotes the synthesis and release of glutamate in microglia and glutamate uptake in astrocytes, both mechanisms augmenting neuronal loss [72, 84]. It has been shown that high extracellular levels of TNF-α exacerbate the inflammation and neurodegeneration mediated by astrocytes. However, low levels of TNF-α secreted mainly by astrocytes autocrinely stimulate the secretion of neurotrophic factors, supporting neuronal survival [85]. In an experimental model of glaucoma in rats, Lee et al. (2014) suggested that TNF-α released by activated microglia stimulated macroglial cells to produce neuroprotective factors, including nerve-growth factor, in response to a mid-hypertensive glaucomatous injury [86].
In both experimental and human glaucoma, in addition to TNF-α, astrocytes can produce and/or respond to other neurotoxic molecules such as NO, IL-6, and endothelins (ETs) which could directly damage RCG axons (Figure 3) [87–89]. ETs are potent vasoconstrictive molecules that are produced by astrocytes and act in a paracrine loop on ET receptors to trigger astrocyte activation and proliferation and to impede ocular blood circulation [90]. The expression of ET-1 receptors (ETA and ETB) has been described both in human and in experimental glaucoma [91]. In addition, the overexpression of ET-1 in the optic nerve head correlated with neural loss in an experimental model of glaucoma [92, 93].
Reactive astrocytes can secrete inflammatory cytokines, such as IL-1β, IL-6, and IL-8 (Figure 3) [94]. In diabetic retinopathy, hyperglycemia boosts astrocyte cytokine expression, activating NF-κB and intensifying oxidative stress [95]. After experimental retinal detachment, astrocytes become a major source of IL-1 production in the neural retina [96].
Apart from inflammatory cytokines reactive astrocytes can secrete chemokines, including CCL2, CCL5, CCL20, CXCL10, CXCL12, CXCL1, CXCL2, and CX3CL1 (Figure 3) [97]. These chemokines are involved in the recruitment of microglia, monocytes/macrophages, T-cells, and dendritic cells at the inflamed sites of the CNS [72]. Furthermore, the inflammatory mediators secreted by reactive astrocytes could affect the properties of the blood-brain barrier (BBB), thereby facilitating the infiltration of peripheral immune cells within the brain parenchyma during neurodegenerative diseases [98, 99]. Macroglial dysfunction in rats results in BRB breakdown of retinal vascular diseases through reduced expression of the tight-junction protein claudin-5 [100].
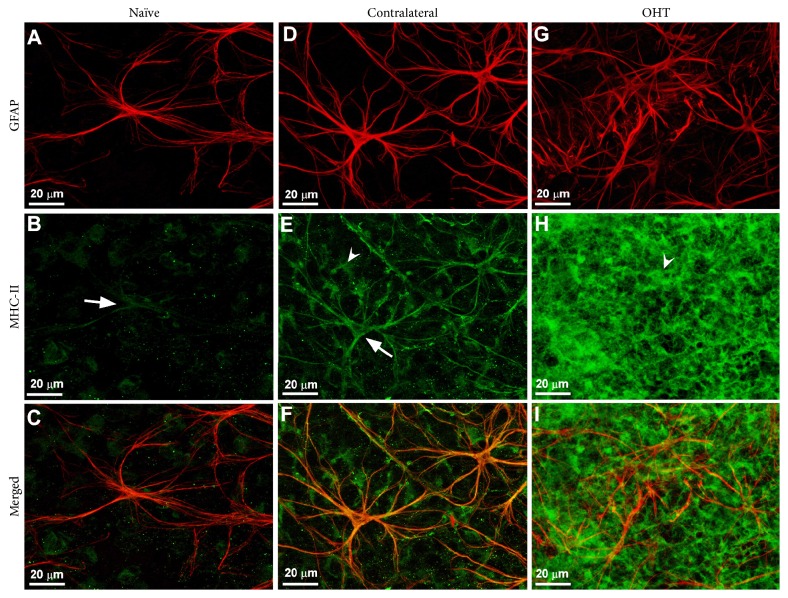
Notably, when activated, astrocytes express class II MHC and costimulatory molecules on the cell surface, thus stimulating T-cell activation in the CNS (Figure 3) [101]. In an experimental mice model of glaucoma, upregulation of MHC-II expression was found in retinal macroglia in both the hypertensive eye and the contralateral normotensive eye (Figure 4) [19].
Figure 4.
Retinal macroglia in the mouse retina. Retinal whole-mount. Double immunostaining for GFAP (red) and MHC-II (green) after 15 days of laser-induced ocular hypertension. (A)–(C): naïve eyes; (D)–(F): contralateral eyes; (G)–(I): OHT eyes. In contralateral eyes, MHC-II immunoreaction of astrocytes (arrow) and Müller cells (arrowhead) in (E) was increased with respect to naïve eyes (arrow) in (B). In OHT eyes, MHC-II immunoreaction of Müller cells (arrowhead) in (H) was notably upregulated in comparison with contralateral (E). In OHT eyes the Müller cells were GFAP+ throughout the retina and appeared as punctate structures between the astrocytes and their radiating processes (G). Fluorescence microscopy and image acquisition using the ApoTome. GFAP: glial fibrillary acidic protein; MHC: major histocompatibility complex; OHT: ocular hypertension (from Figure 10 of [19] with permission).
Astrocytes and Müller cells can also produce complement proteins (Figure 3). In glaucomatous eyes the presence of C1q in astrocytes and Müller cells lining the inner limiting membrane could be an adaptive mechanism for removing apoptotic RGC [102].
3.2. Müller Cell in Retinal Diseases
Considering their strategic location, Müller cells are in position to influence and be influenced by neuronal activity throughout the tissue [15]. Therefore, they are usually one of the first glial cells to detect retinal damage because of their radial distribution, providing a rapid response to any alteration of the retinal microenvironment [75, 103].
Müller cells are more resistant than retinal neurons to various forms of injury such as ischemia, anoxia, hypoglycemia, and elevation in the hydrostatic pressure. This resistance can be attributed to their peculiar energy metabolism and the presence of an energy reserve in the form of glycogen, their high antioxidant content, their capacity to proliferate and regenerate, and the presence of glutamate transporters and glutamine synthetase that rapidly detoxify excess glutamate, among other compounds [75, 103].
In Müller cells, gliosis is characterized by both nonspecific responses, that is, stereotypic alterations independent of the causal stimulus: the increased expression of GFAP and the activation of the extracellular signal-regulated kinases (ERKs) [18]. The upregulation of GFAP is therefore used as a common marker for reactive Müller cells and is so sensitive that it can be used as an indicator of retinal stress, retinal injury, and Müller cell activation [104]. This upregulation of intermediate filaments (GFAP, vimentin, and nestin) seems to be a crucial step for the gliotic response involved in glial scar formation, monocyte infiltration, neurite growth, neovascularization, and cell integration [18, 79].
Practically all retinal diseases are associated with the gliosis of Müller cells. In experimental diabetic retinopathy, glial reactivity is manifested by increased GFAP immunoreactivity and content in both Müller cells and astrocytes [105]. Such increment of GFAP has also been reported in the retinas of patients with nonproliferative diabetic retinopathy [106].
Müller cells are among the first to respond following intraocular pressure increase and it is thought that reactive Müller cells in glaucoma could increase the susceptibility of RGCs to stress signals and contribute to disease progression [75]. In human glaucomatous eyes and in experimental and hereditary animal models of glaucoma, more intense expression of GFAP in Müller cells has been detected in the retina [19, 67, 107–109]. In experimental glaucoma, GFAP upregulation in Müller cells is not restricted to the hypertensive eye but is also detected in the normotensive contralateral eye [19, 110, 111]. In AMD, regions of GFAP upregulation in Müller cells can be involved in drusen formation [112]. Such upregulation can occur early in the course of retinal detachment [113] or in response to degeneration of the retina in a rat model of retinitis pigmentosa [114].
Müller cell gliosis may include the dedifferentiation of the cells into pluripotent retinal progenitor/stem cells. Such dedifferentiation represents a precondition for regenerative processes in the injured retina and for glial-cell proliferation and migration [115]. It has been reported that after retinal detachment Müller cells migrate to the outer retina and undergo mitosis. Some of these displaced Müller cells stop to express Müller cell marker proteins, a feature that has been interpreted as dedifferentiation [116]. As a most important step of this dedifferentiation, the cells reduce the K+-conductance of their membrane, particularly the Kir4.1-mediated current, which is generally associated with a mislocation of the Kir4.1 channels in the Müller cell membrane [117]. This mislocation of Kir4.1 protein has been associated with a greater vulnerability of RGCs to ischemic stress [118], which will inflict a severe loss of the functions involved in normal neuron-glia interaction [18]. The alteration of Kir4.1 channels has been described in retinal tissues after retinal blue-light injury, after retinal ischemia, in ocular inflammation, and in diabetic rats [119–122].
In the retina, proliferative gliosis occurs by reentry into the cell cycle of Muller cells, accompanied by a dramatic alteration in the expression of trophic factor channels and transporters as well as the migration of these cells (Figure 5) [75, 123]. Müller cells react to retinal injury by establishing a glial scar that fills retinal breaks or holes, replacing degenerated neurons and photoreceptors [124]. Glial scars involve the expression of inhibitory molecules on the surface of reactive glial cells, which additionally inhibit regular tissue repair and neuroregeneration, harming the function and structure of retinal neurons [18, 75, 125]. A form of glial scar involves the epiretinal membranes (frequently detected in retinal detachment), AMD, and proliferative diabetic retinopathy [126]. In addition, in AMD, glial membranes constituted by astrocytes and Müller cells have been reported to be located between the vitreous humor and internal limiting membrane of the retina (Figure 5) [48].
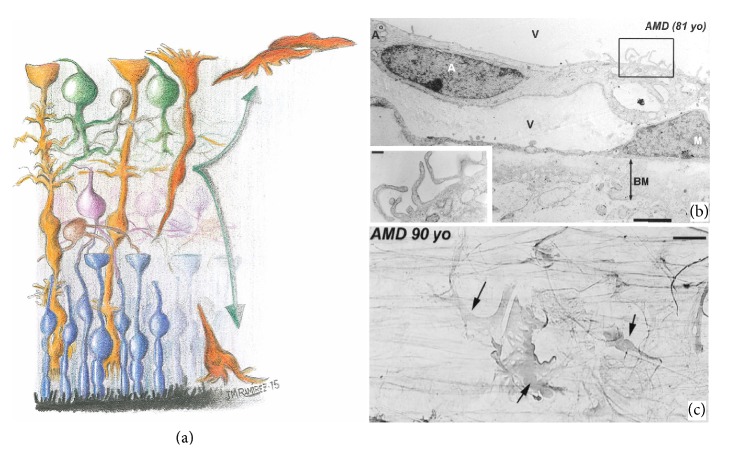
Figure 5.
Müller cell gliosis. Schematic drawing illustrating Müller cell proliferative gliosis (a). Transmission electron microscopy of a retina from an 81-year-old patient with age-related macular degeneration (b). Immunoperoxidase anti-GFAP. Retinal whole-mount from a 90-year-old patient with age-related macular degeneration (c). (a) Müller cells reenter into the cell cycle and migrate to the subretinal space and the vitreous humor where they contribute to forming the subretinal membranes and the epiretinal membranes, respectively. (b) Epiretinal astroglial membrane formed by astrocyte and Müller cells located in the vitreous humor. The Müller cells adhere to the vitreous face of the inner limiting membrane. The inset shows the astrocyte microvilli. (c) Glial membrane at the vitreoretinal interphase showing strongly GFAP+ Müller cells (arrow). A: astrocyte; BM: basement membrane; M: Müller cells; v: vitreous (schematic drawing modified from [18]; (b) and (c) from Figures 8F and 12A of [47] with permission).
Gliosis of Müller cells has both cytoprotective and cytotoxic effects on retinal neurons [127]. After retinal insults, less severe changes in Müller cells have been described as “conservative” or nonproliferative gliosis. In particular, early after injury, Müller cell gliosis is neuroprotective, due to the release of neurotrophic factors and antioxidants which favor neuronal survival and limit the extent of tissue damage [42, 75, 103, 128–130]. After axotomy, excitotoxicity, or experimental glaucoma, Müller cells increase the expression of leukemia inhibitory factor and ciliary neurotrophic factor to promote RCG survival [129, 131–133]. Both in glaucomatous donor eyes in humans and in ocular hypertension in rats, an increased concentration of hypoxia-inducible factor- (HIF-) 1α has been detected in Muller cells which induces the expression of neuroprotective factors such as VEGF or EPO [134, 135]. In diabetic retinopathy, Müller glia activation may bolster neuroprotection by releasing angiogenic and neurotrophic factors (Figure 3) in order to protect the retina from hyperglycemia-induced stress [4] and through a mechanism involving ERK1/2 activation [136].
However, the most severe insults provoke yet another level of Müller cell response described as “massive” or proliferative, in which gliosis becomes detrimental to the retinal tissue and increases neuronal death [18]. A possible trigger for the transition from “conservative” to “massive” gliosis is the breakdown of the BRB, augmenting the retinal and vitreal contents of growth factors, cytokines, and inflammatory factors, as well as an infiltration of blood-derived immune cells (Figure 3) [137]. After laser lesions in rat retina, which cause a breakdown of the BRB, the extravasated plasma protein, immunoglobulin G, may further trigger the reactive gliosis of Müller cells [138].
Furthermore hypoxia and hyperglycemia induce the overexpression of angiogenic cytokines and release of matrix metalloproteinases by Müller cells. These metalloproteinases impair the tight junctions via proteolytic degradation of occludin and claudin on retinal endothelial cells and pigment epithelial cells [139].
On the other hand, the excessive and/or prolonged expression of potential protective factors might lead to detrimental neuronal effects. An example is the expression of VEGF, which is highly induced in Müller cells following injury and which has the distinct potential of protecting retinal neurons against apoptosis [140, 141]. However, the excessive and prolonged expression of VEGF by Müller cell as occurs in diabetic retinopathy can lead to retinal inflammation, neovascularization, vascular leakage, and vascular lesion (Figure 3) [142].
Quite comparable observations have been reported concerning the release of NO by reactive Müller glia [75]. High concentrations of nitric oxide can damage neurons [143–145], while lower levels may have beneficial effects, such as the protection of neurons against glutamate excitotoxicity and the decreased retinal ischemia by its vasodilator effect [146]. With respect to glutamate, it has been demonstrated that, in glaucoma, Müller cells lose their capacity to regulate glutamate homeostasis, owing to the reduction in the biosynthesis of glutamate transporter (Figure 3) (GLAST). As a consequence, glutamate accumulates in the intercellular space, provoking neuronal death [147–149].
Another important feature in gliotic Müller cells is their intense crosstalk with cells from the immune system [126]. Molecules from inflammatory cells, platelets, and plasma may activate Müller cells, and these cells may express a wide variety of inflammation- and immune-response-related factors and enzymes such as TNF-α, IL, interferon, and ICAM-1 (Figure 3) [75]. Müller cells can mediate direct cytotoxic effects via an intensified expression of TNF-α or monocyte chemoattractant protein- (MCP-) 1 [79, 150–152]. Notably, microglial activation induces Müller responses such as an increase in Müller cell-microglia adhesive cell contacts that may guide the intraretinal mobilization of migratory microglia in a radial direction using Müller cell processes as an adhesive scaffold [153].
Under pathological conditions, for example, oxidative stress, inflammatory mediators, retinal laser photocoagulation, or increase in the intraocular pressure, Müller cells show upregulation of MHC class II molecules by acting as antigen-presenting cells (Figures 3 and 4) [19, 75]. Moreover, Müller cells are also able to produce complement proteins (Figure 3) [102].
4. Conclusions
In summary, retinal macroglial cells are fundamental for homeostasis of the retinal neurons. These cells form a defensive system of the retina through its complex program of activation termed “the reactive gliosis.” This gliosis can be neuroprotective or neurodegenerative and in the latter case may impair the course of retinal pathologies.
Acknowledgments
This work was supported by the Ophthalmological Network OFTARED (RD12-0034/0002: Prevención, Detección Precoz y Tratamiento de la Patología Ocular Prevalente Degenerativa y Crónica)‚ the Institute of Health of Carlos III of the Spanish Ministry of Economy. This work has been funded by the PN I+D+i 2008–2011, by the ISCIII-Subdirección General de Redes y Centros de Investigación Cooperativa, by the European Programme FEDER, and by the SAF2014-53779-R: Neuroinflammation in Glaucoma: Sequencing of Glial and Blood-Retinal Barrier Damage (Spanish Ministry of Economy and Competitiveness). The authors thank David Nesbitt for correcting the English version of this work.
Competing Interests
The authors declare that they have no competing interests.
References
- 1.Ramirez J. M., Triviño A., Ramirez A. I., Salazar J. J., Garcia-Sanchez J. Immunohistochemical study of human retinal astroglia. Vision Research. 1994;34(15):1935–1946. doi: 10.1016/0042-6989(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 2.Perez-Alvarez A., Araque A., Martin E. D. Confocal microscopy for astrocyte in vivo imaging: recycle and reuse in microscopy. Frontiers in Cellular Neuroscience. 2013;7, article 51 doi: 10.3389/fncel.2013.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tezel G., Wax M. B. Glaucoma. Chemical Immunology and Allergy. 2007;92:221–227. doi: 10.1159/000099273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Penn J. S., Madan A., Caldwell R. B., Bartoli M., Caldwell R. W., Hartnett M. E. Vascular endothelial growth factor in eye disease. Progress in Retinal and Eye Research. 2008;27(4):331–371. doi: 10.1016/j.preteyeres.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kandel E. R., Schwartz J. H., Jessell T. M. Neuronas y conducta. In: Kandel E. R., Schwartz J. H., Jessell T. M., editors. Principios de Neurociencia. Madrid, Spain: McGraw-Hill/Interamericana; 2001. pp. 19–35. [Google Scholar]
- 6.Kettenmann H., Verkhratsky A. Neuroglia: the 150 years after. Trends in Neurosciences. 2008;31(12):653–659. doi: 10.1016/j.tins.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Reichenbach A., Bringmann A. New functions of Müller cells. Glia. 2013;61(5):651–678. doi: 10.1002/glia.22477. [DOI] [PubMed] [Google Scholar]
- 8.Barateiro A., Brites D., Fernandes A. Oligodendrocyte development and myelination in neurodevelopment: molecular mechanisms in health and disease. Current Pharmaceutical Design. 2016;22(6):656–679. doi: 10.2174/1381612822666151204000636. [DOI] [PubMed] [Google Scholar]
- 9.Pekny M., Pekna M., Messing A., et al. Astrocytes: a central element in neurological diseases. Acta Neuropathologica. 2016;131(3):323–345. doi: 10.1007/s00401-015-1513-1. [DOI] [PubMed] [Google Scholar]
- 10.Schnitzer J. Chapter 7 Astrocytes in mammalian retina. Progress in Retinal Research. 1988;7:209–231. doi: 10.1016/0278-4327(88)90009-0. [DOI] [Google Scholar]
- 11.Triviño A., Ramírez J. M., Ramírez A. I., Salazar J. J., Garcia-Sánchez J. Retinal perivascular astroglia: an immunoperoxidase study. Vision Research. 1992;32(9):1601–1607. doi: 10.1016/0042-6989(92)90153-a. [DOI] [PubMed] [Google Scholar]
- 12.Ramírez J. M., Triviño A., Ramírez A. I., Salazar J. J., García-Sanchez J. Structural specializations of human retinal glial cells. Vision Research. 1996;36(14):2029–2036. doi: 10.1016/0042-6989(95)00322-3. [DOI] [PubMed] [Google Scholar]
- 13.Triviño A., Ramírez J. M., Ramírez A. I., Salazar J. J., García-Sanchez J. Comparative study of astrocytes in human and rabbit retinae. Vision Research. 1997;37(13):1707–1711. doi: 10.1016/S0042-6989(97)00021-7. [DOI] [PubMed] [Google Scholar]
- 14.Ramírez J. M., Triviño A., Ramírez A. I., Salazar J. J. Organization and function of astrocytes in human retina. In: Castellano B., Gonzalez B., Nieto-Sampedro M., editors. Understanding Glial Cells. Boston, Mass, USA: Kluwer Academic; 1988. pp. 47–62. [Google Scholar]
- 15.Sarthy V., Ripps H. The Retinal Müller Cell: Structure and Function. New York, NY, USA: Kluwer Academic; 2001. [Google Scholar]
- 16.Reichenbach A., Robinson S. R. Neuroglia. Oxford, UK: Oxford University Press; 1995. Ependymoglia and ependymoglia-like cells; pp. 58–84. [Google Scholar]
- 17.Reichenbach A., Robinson S. R. Phylogenetic constraints on retinal organisation and development. Progress in Retinal and Eye Research. 1995;15(1):139–171. doi: 10.1016/1350-9462(95)00008-9. [DOI] [Google Scholar]
- 18.Bringmann A., Pannicke T., Grosche J., et al. Müller cells in the healthy and diseased retina. Progress in Retinal and Eye Research. 2006;25(4):397–424. doi: 10.1016/j.preteyeres.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Gallego B. I., Salazar J. J., de Hoz R., et al. IOP induces upregulation of GFAP and MHC-II and microglia reactivity in mice retina contralateral to experimental glaucoma. Journal of Neuroinflammation. 2012;9(1, article 92) doi: 10.1186/1742-2094-9-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramírez A. I., Salazar J. J., de Hoz R., et al. Quantification of the effect of different levels of IOP in the astroglia of the rat retina ipsilateral and contralateral to experimental glaucoma. Investigative Ophthalmology & Visual Science. 2010;51(11):5690–5696. doi: 10.1167/iovs.10-5248. [DOI] [PubMed] [Google Scholar]
- 21.Charles A. C., Merrill J. E., Dirksen E. R., Sandersont M. J. Intercellular signaling in glial cells: calcium waves and oscillations in response to mechanical stimulation and glutamate. Neuron. 1991;6(6):983–992. doi: 10.1016/0896-6273(91)90238-u. [DOI] [PubMed] [Google Scholar]
- 22.Cornell-Bell A. H., Finkbeiner S. M., Cooper M. S., Smith S. J. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science. 1990;247(4941):470–473. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- 23.Nedergaard M., Ransom B., Goldman S. A. New roles for astrocytes: redefining the functional architecture of the brain. Trends in Neurosciences. 2003;26(10):523–530. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 24.Seifert G., Schilling K., Steinhäuser C. Astrocyte dysfunction in neurological disorders: a molecular perspective. Nature Reviews Neuroscience. 2006;7(3):194–206. doi: 10.1038/nrn1870. [DOI] [PubMed] [Google Scholar]
- 25.Oberheim N. A., Takano T., Han X., et al. Uniquely hominid features of adult human astrocytes. The Journal of Neuroscience. 2009;29(10):3276–3287. doi: 10.1523/jneurosci.4707-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfrieger F. W. Role of glia in synapse development. Current Opinion in Neurobiology. 2002;12(5):486–490. doi: 10.1016/S0959-4388(02)00358-6. [DOI] [PubMed] [Google Scholar]
- 27.Pfrieger F. W. Role of cholesterol in synapse formation and function. Biochimica et Biophysica Acta—Biomembranes. 2003;1610(2):271–280. doi: 10.1016/s0005-2736(03)00024-5. [DOI] [PubMed] [Google Scholar]
- 28.Chen S.-D., Wang L., Zhang X.-L. Neuroprotection in glaucoma: present and future. Chinese Medical Journal. 2013;126(8):1567–1577. doi: 10.3760/cma.j.issn.0366-6999.20123565. [DOI] [PubMed] [Google Scholar]
- 29.Verkhratsky A., Parpura V., Pekna M., Pekny M., Sofroniew M. Glia in the pathogenesis of neurodegenerative diseases. Biochemical Society Transactions. 2014;42(5):1291–1301. doi: 10.1042/BST20140107. [DOI] [PubMed] [Google Scholar]
- 30.Sanderson J., Dartt D. A., Trinkaus-Randall V., et al. Purines in the eye: recent evidence for the physiological and pathological role of purines in the RPE, retinal neurons, astrocytes, Müller cells, lens, trabecular meshwork, cornea and lacrimal gland. Experimental Eye Research. 2014;127:270–279. doi: 10.1016/j.exer.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson E. C., Morrison J. C. Friend or foe? Resolving the impact of glial responses in glaucoma. Journal of Glaucoma. 2009;18(5):341–353. doi: 10.1097/ijg.0b013e31818c6ef6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Araque A., Parpura V., Sanzgiri R. P., Haydon P. G. Tripartite synapses: glia, the unacknowledged partner. Trends in Neurosciences. 1999;22(5):208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- 33.Halassa M. M., Fellin T., Haydon P. G. The tripartite synapse: roles for gliotransmission in health and disease. Trends in Molecular Medicine. 2007;13(2):54–63. doi: 10.1016/j.molmed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 34.Perea G., Navarrete M., Araque A. Tripartite synapses: astrocytes process and control synaptic information. Trends in Neurosciences. 2009;32(8):421–431. doi: 10.1016/j.tins.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 35.Heneka M. T., Rodríguez J. J., Verkhratsky A. Neuroglia in neurodegeneration. Brain Research Reviews. 2010;63(1-2):189–211. doi: 10.1016/j.brainresrev.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 36.Tsacopoulos M., Magistretti P. J. Metabolic coupling between glia and neurons. The Journal of Neuroscience. 1996;16(3):877–885. doi: 10.1523/JNEUROSCI.16-03-00877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poitry-Yamate C. L., Poitry S., Tsacopoulos M. Lactate released by Müller glial cells is metabolized by photoreceptors from mammalian retina. Journal of Neuroscience. 1995;15(7, part 2):5179–5191. doi: 10.1523/JNEUROSCI.15-07-05179.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pena J. D. O., Taylor A. W., Ricard C. S., Vidal I., Hernandez M. R. Transforming growth factor β isoforms in human optic nerve heads. British Journal of Ophthalmology. 1999;83(2):209–218. doi: 10.1136/bjo.83.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pena J. D. O., Agapova O., Gabelt B. T., et al. Increased elastin expression in astrocytes of the lamina cribrosa in response to elevated intraocular pressure. Investigative Ophthalmology and Visual Science. 2001;42(10):2303–2314. [PubMed] [Google Scholar]
- 40.Seitz R., Ohlmann A., Tamm E. R. The role of Müller glia and microglia in glaucoma. Cell and Tissue Research. 2013;353(2):339–345. doi: 10.1007/s00441-013-1666-y. [DOI] [PubMed] [Google Scholar]
- 41.Wilson J. X. Antioxidant defense of the brain: a role for astrocytes. Canadian Journal of Physiology and Pharmacology. 1997;75(10-11):1149–1163. doi: 10.1139/cjpp-75-10-11-1149. [DOI] [PubMed] [Google Scholar]
- 42.Schütte M., Werner P. Redistribution of glutathione in the ischemic rat retina. Neuroscience Letters. 1998;246(1):53–56. doi: 10.1016/S0304-3940(98)00229-8. [DOI] [PubMed] [Google Scholar]
- 43.Francke M., Uhlmann S., Pannicke T., et al. Experimental dispase-induced retinopathy causes up-regulation of P2Y receptor-mediated calcium responses in Müller glial cells. Ophthalmic Research. 2003;35(1):30–41. doi: 10.1159/000068192. [DOI] [PubMed] [Google Scholar]
- 44.Francke M., Makarov F., Kacza J., et al. Retinal pigment epithelium melanin granules are phagocytozed by Müller glial cells in experimental retinal detachment. The Journal of Neurocytology. 2001;30(2):131–136. doi: 10.1023/a:1011987107034. [DOI] [PubMed] [Google Scholar]
- 45.Triviño A., Ramírez A. I., Salazar J. J., et al. A cholesterol-enriched diet induces ultrastructural changes in retinal and macroglial rabbit cells. Experimental Eye Research. 2006;83(2):357–366. doi: 10.1016/j.exer.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 46.Ramírez A. I., Salazar J. J., de Hoz R., et al. Macroglial and retinal changes in hypercholesterolemic rabbits after normalization of cholesterol levels. Experimental Eye Research. 2006;83(6):1423–1438. doi: 10.1016/j.exer.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 47.Triviño A., Ramírez A. I., Salazar J. J., Rojas B., de Hoz R., Ramírez J. M. Retinal changes in age-related macular degeneration. In: Ioseliane O. R., editor. Focus on Eye Research. New York, NY, USA: Nova; 2005. pp. 1–37. [Google Scholar]
- 48.Ramírez J. M., Ramírez A. I., Salazar J. J., de Hoz R., Trivio A. Changes of astrocytes in retinal ageing and age-related macular degeneration. Experimental Eye Research. 2001;73(5):601–615. doi: 10.1006/exer.2001.1061. [DOI] [PubMed] [Google Scholar]
- 49.Triviño A., de Hoz R., Rojas B., Salazar J. J., Ramírez A. I., Ramírez J. M. NPY and TH innervation in human choroidal whole-mounts. Histology and Histopathology. 2005;20(2):393–402. doi: 10.14670/HH-20.393. [DOI] [PubMed] [Google Scholar]
- 50.Koehler R. C., Roman R. J., Harder D. R. Astrocytes and the regulation of cerebral blood flow. Trends in Neurosciences. 2009;32(3):160–169. doi: 10.1016/j.tins.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 51.Gordon G. R. J., Mulligan S. J., MacVicar B. A. Astrocyte control of the cerebrovasculature. Glia. 2007;55(12):1214–1221. doi: 10.1002/glia.20543. [DOI] [PubMed] [Google Scholar]
- 52.Iadecola C., Nedergaard M. Glial regulation of the cerebral microvasculature. Nature Neuroscience. 2007;10(11):1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- 53.Abbott N. J., Rönnbäck L., Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nature Reviews Neuroscience. 2006;7(1):41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 54.Tout S., Chan-Ling T., Holländer H., Stone J. The role of Müller cells in the formation of the blood-retinal barrier. Neuroscience. 1993;55(1):291–301. doi: 10.1016/0306-4522(93)90473-s. [DOI] [PubMed] [Google Scholar]
- 55.Aloisi F. Immune function of microglia. Glia. 2001;36(2):165–179. doi: 10.1002/glia.1106. [DOI] [PubMed] [Google Scholar]
- 56.Dong Y., Benveniste E. N. Immune function of astrocytes. Glia. 2001;36(2):180–190. doi: 10.1002/glia.1107. [DOI] [PubMed] [Google Scholar]
- 57.Kimelberg H. K., Nedergaard M. Functions of astrocytes and their potential as therapeutic targets. Neurotherapeutics. 2010;7(4):338–353. doi: 10.1016/j.nurt.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pekny M., Wilhelmsson U., Pekna M. The dual role of astrocyte activation and reactive gliosis. Neuroscience Letters. 2014;565:30–38. doi: 10.1016/j.neulet.2013.12.071. [DOI] [PubMed] [Google Scholar]
- 59.Peng L., Parpura V., Verkhratsky A. Neuroglia as a central element of neurological diseases: an underappreciated target for therapeutic intervention. Current Neuropharmacology. 2014;12(4):303–307. doi: 10.2174/1570159x12999140829152550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lewis G. P., Fisher S. K. Up-regulation of glial fibrillary acidic protein in response to retinal injury: its potential role in glial remodeling and a comparison to vimentin expression. International Review of Cytology. 2003;230:263–290. doi: 10.1016/s0074-7696(03)30005-1. [DOI] [PubMed] [Google Scholar]
- 61.Pekny M., Pekna M. Astrocyte reactivity and reactive astrogliosis: costs and benefits. Physiological Reviews. 2014;94(4):1077–1098. doi: 10.1152/physrev.00041.2013. [DOI] [PubMed] [Google Scholar]
- 62.Barres B. A. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. 2008;60(3):430–440. doi: 10.1016/j.neuron.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 63.Ames A., III CNS energy metabolism as related to function. Brain Research Reviews. 2000;34(1-2):42–68. doi: 10.1016/S0165-0173(00)00038-2. [DOI] [PubMed] [Google Scholar]
- 64.Kraft A. W., Hu X., Yoon H., et al. Attenuating astrocyte activation accelerates plaque pathogenesis in APP/PS1 mice. The FASEB Journal. 2013;27(1):187–198. doi: 10.1096/fj.12-208660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lieth E., Barber A. J., Xu B., et al. Glial reactivity and impaired glutamate metabolism in short-term experimental diabetic retinopathy. Penn State Retina Research Group. Diabetes. 1998;47(5):815–820. doi: 10.2337/diabetes.47.5.815. [DOI] [PubMed] [Google Scholar]
- 66.Milam A. H., Li Z.-Y., Fariss R. N. Histopathology of the human retina in retinitis pigmentosa. Progress in Retinal and Eye Research. 1998;17(2):175–205. doi: 10.1016/S1350-9462(97)00012-8. [DOI] [PubMed] [Google Scholar]
- 67.Inman D. M., Horner P. J. Reactive nonproliferative gliosis predominates in a chronic mouse model of glaucoma. Glia. 2007;55(9):942–953. doi: 10.1002/glia.20516. [DOI] [PubMed] [Google Scholar]
- 68.Hernandez M. R., Miao H., Lukas T. Astrocytes in glaucomatous optic neuropathy. Progress in Brain Research. 2008;173:353–373. doi: 10.1016/S0079-6123(08)01125-4. [DOI] [PubMed] [Google Scholar]
- 69.Johnson E. C., Deppmeier L. M. H., Wentzien S. K. F., Hsu I., Morrison J. C. Chronology of optic nerve head and retinal responses to elevated intraocular pressure. Investigative Ophthalmology & Visual Science. 2000;41(2):431–442. [PubMed] [Google Scholar]
- 70.Lam D., Jim J., To E., Rasmussen C., Kaufman P. L., Matsubara J. Astrocyte and microglial activation in the lateral geniculate nucleus and visual cortex of glaucomatous and optic nerve transected primates. Molecular vision. 2009;15:2217–2229. [PMC free article] [PubMed] [Google Scholar]
- 71.Hernandez M. R. The optic nerve head in glaucoma: role of astrocytes in tissue remodeling. Progress in Retinal and Eye Research. 2000;19(3):297–321. doi: 10.1016/s1350-9462(99)00017-8. [DOI] [PubMed] [Google Scholar]
- 72.González H., Elgueta D., Montoya A., Pacheco R. Neuroimmune regulation of microglial activity involved in neuroinflammation and neurodegenerative diseases. Journal of Neuroimmunology. 2014;274(1-2):1–13. doi: 10.1016/j.jneuroim.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 73.Quigley H. A., McKinnon S. J., Zack D. J., et al. Retrograde axonal transport of BDNF in retinal ganglion cells is blocked by acute IOP elevation in rats. Investigative Ophthalmology & Visual Science. 2000;41(11):3460–3466. [PubMed] [Google Scholar]
- 74.Chong R. S., Martin K. R. Glial cell interactions and glaucoma. Current Opinion in Ophthalmology. 2015;26(2):73–77. doi: 10.1097/ICU.0000000000000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bringmann A., Iandiev I., Pannicke T., et al. Cellular signaling and factors involved in Müller cell gliosis: neuroprotective and detrimental effects. Progress in Retinal and Eye Research. 2009;28(6):423–451. doi: 10.1016/j.preteyeres.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 76.Coorey N. J., Shen W., Chung S. H., Zhu L., Gillies M. C. The role of glia in retinal vascular disease. Clinical & Experimental Optometry. 2012;95(3):266–281. doi: 10.1111/j.1444-0938.2012.00741.x. [DOI] [PubMed] [Google Scholar]
- 77.Robel S., Berninger B., Götz M. The stem cell potential of glia: lessons from reactive gliosis. Nature Reviews Neuroscience. 2011;12(2):88–104. doi: 10.1038/nrn2978. [DOI] [PubMed] [Google Scholar]
- 78.Burda J. E., Sofroniew M. V. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron. 2014;81(2):229–248. doi: 10.1016/j.neuron.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nakazawa T., Takeda M., Lewis G. P., et al. Attenuated glial reactions and photoreceptor degeneration after retinal detachment in mice deficient in glial fibrillary acidic protein and vimentin. Investigative Ophthalmology & Visual Science. 2007;48(6):2760–2768. doi: 10.1167/iovs.06-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ganesh B. S., Chintala S. K. Inhibition of reactive gliosis attenuates excitotoxicity-mediated death of retinal ganglion cells. PLoS ONE. 2011;6(3) doi: 10.1371/journal.pone.0018305.e18305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kettenmann H., Hanisch U.-K., Noda M., Verkhratsky A. Physiology of microglia. Physiological Reviews. 2011;91(2):461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- 82.Barcia C., Ros C., Annese V., et al. IFN-γ signaling, with the synergistic contribution of TNF-α, mediates cell specific microglial and astroglial activation in experimental models of Parkinson's disease. Cell Death & Disease. 2011;2(4, article e142) doi: 10.1038/cddis.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bezzi P., Domercq M., Brambilla L., et al. CXCR4-activated astrocyte glutamate release via TNFα: amplification by microglia triggers neurotoxicity. Nature Neuroscience. 2001;4(7):702–710. doi: 10.1038/89490. [DOI] [PubMed] [Google Scholar]
- 84.Takeuchi H., Jin S., Wang J., et al. Tumor necrosis factor-α induces neurotoxicity via glutamate release from hemichannels of activated microglia in an autocrine manner. The Journal of Biological Chemistry. 2006;281(30):21362–21368. doi: 10.1074/jbc.m600504200. [DOI] [PubMed] [Google Scholar]
- 85.Kuno R., Yoshida Y., Nitta A., et al. The role of TNF-alpha and its receptors in the production of NGF and GDNF by astrocytes. Brain Research. 2006;1116(1):12–18. doi: 10.1016/j.brainres.2006.07.120. [DOI] [PubMed] [Google Scholar]
- 86.Lee J., Shin J., Chun M., Oh S. Morphological analyses on retinal glial responses to glaucomatous injury evoked by venous cauterization. Applied Microscopy. 2014;44(1):21–29. doi: 10.9729/am.2014.44.1.21. [DOI] [Google Scholar]
- 87.Yan X., Tezel G., Wax M. B., Edward D. P. Matrix metalloproteinases and tumor necrosis factor α in glaucomatous optic nerve head. Archives of Ophthalmology. 2000;118(5):666–673. doi: 10.1001/archopht.118.5.666. [DOI] [PubMed] [Google Scholar]
- 88.Yuan L., Neufeld A. H. Tumor necrosis factor-α: a potentially neurodestructive cytokine produced by glia in the human glaucomatous optic nerve head. Glia. 2000;32(1):42–50. doi: 10.1002/1098-1136(200010)32:1<42::aid-glia40>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 89.Johnson E. C., Doser T. A., Cepurna W. O., et al. Cell proliferation and interleukin-6-type cytokine signaling are implicated by gene expression responses in early optic nerve head injury in rat glaucoma. Investigative Ophthalmology & Visual Science. 2011;52(1):504–518. doi: 10.1167/iovs.10-5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chauhan B. C. Endothelin and its potential role in glaucoma. Canadian Journal of Ophthalmology. 2008;43(3):356–360. doi: 10.1139/I08-060. [DOI] [PubMed] [Google Scholar]
- 91.Wang X., LeVatte T. L., Archibald M. L., Chauhan B. C. Increase in endothelin B receptor expression in optic nerve astrocytes in endothelin-1 induced chronic experimental optic neuropathy. Experimental Eye Research. 2009;88(3):378–385. doi: 10.1016/j.exer.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 92.Howell G. R., Macalinao D. G., Sousa G. L., et al. Molecular clustering identifies complement and endothelin induction as early events in a mouse model of glaucoma. The Journal of Clinical Investigation. 2011;121(4):1429–1444. doi: 10.1172/jci44646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Minton A. Z., Phatak N. R., Stankowska D. L., et al. Endothelin b receptors contribute to retinal ganglion cell loss in a rat model of glaucoma. PLoS ONE. 2012;7(8) doi: 10.1371/journal.pone.0043199.e43199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee H.-J., Suk J.-E., Patrick C., et al. Direct transfer of α-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. The Journal of Biological Chemistry. 2010;285(12):9262–9272. doi: 10.1074/jbc.m109.081125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shin E. S., Huang Q., Gurel Z., Sorenson C. M., Sheibani N. High glucose alters retinal astrocytes phenotype through increased production of inflammatory cytokines and oxidative stress. PLoS ONE. 2014;9(7) doi: 10.1371/journal.pone.0103148.e103148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nakazawa T., Hisatomi T., Nakazawa C., et al. Monocyte chemoattractant protein 1 mediates retinal detachment-induced photoreceptor apoptosis. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(7):2425–2430. doi: 10.1073/pnas.0608167104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Farina C., Aloisi F., Meinl E. Astrocytes are active players in cerebral innate immunity. Trends in Immunology. 2007;28(3):138–145. doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 98.Biernacki K., Prat A., Blain M., Antel J. P. Regulation of Th1 and Th2 lymphocyte migration by human adult brain endothelial cells. Journal of Neuropathology & Experimental Neurology. 2001;60(12):1127–1136. doi: 10.1093/jnen/60.12.1127. [DOI] [PubMed] [Google Scholar]
- 99.Stolp H. B., Dziegielewska K. M. Review: role of developmental inflammation and blood-brain barrier dysfunction in neurodevelopmental and neurodegenerative diseases. Neuropathology and Applied Neurobiology. 2009;35(2):132–146. doi: 10.1111/j.1365-2990.2008.01005.x. [DOI] [PubMed] [Google Scholar]
- 100.Shen W., Li S., Chung S. H., Gillies M. C. Retinal vascular changes after glial disruption in rats. Journal of Neuroscience Research. 2010;88(7):1485–1499. doi: 10.1002/jnr.22317. [DOI] [PubMed] [Google Scholar]
- 101.Nikcevich K. M., Gordon K. B., Tan L., et al. IFN-α-activated primary murine astrocytes express b7 costimulatory molecules and prime naive antigen-specific T cells. Journal of Immunology. 1997;158(2):614–621. [PubMed] [Google Scholar]
- 102.Stasi K., Nagel D., Yang X., et al. Complement component 1Q (C1Q) upregulation in retina of murine, primate, and human glaucomatous eyes. Investigative Ophthalmology & Visual Science. 2006;47(3):1024–1029. doi: 10.1167/iovs.05-0830. [DOI] [PubMed] [Google Scholar]
- 103.Bringmann A., Pannicke T., Biedermann B., et al. Role of retinal glial cells in neurotransmitter uptake and metabolism. Neurochemistry International. 2009;54(3-4):143–160. doi: 10.1016/j.neuint.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 104.Luna G., Lewis G. P., Banna C. D., Skalli O., Fisher S. K. Expression profiles of nestin and synemin in reactive astrocytes and Müller cells following retinal injury: a comparison with glial fibrillar acidic protein and vimentin. Molecular Vision. 2010;16:2511–2523. [PMC free article] [PubMed] [Google Scholar]
- 105.Lieth E., Gardner T. W., Barber A. J., Antonetti D. A. Retinal neurodegeneration: early pathology in diabetes. Clinical & Experimental Ophthalmology. 2000;28(1):3–8. doi: 10.1046/j.1442-9071.2000.00222.x. [DOI] [PubMed] [Google Scholar]
- 106.Mizutani M., Gerhardinger C., Lorenzi M. Müller cell changes in human diabetic retinopathy. Diabetes. 1998;47(3):445–449. doi: 10.2337/diabetes.47.3.445. [DOI] [PubMed] [Google Scholar]
- 107.Wang X., Tay S. S.-W., Ng Y.-K. An immunohistochemical study of neuronal and glial cell reactions in retinae of rats with experimental glaucoma. Experimental Brain Research. 2000;132(4):476–484. doi: 10.1007/s002210000360. [DOI] [PubMed] [Google Scholar]
- 108.Tezel G., Chauhan B. C., LeBlanc R. P., Wax M. B. Immunohistochemical assessment of the glial mitogen-activated protein kinase activation in glaucoma. Investigative Ophthalmology & Visual Science. 2003;44(7):3025–3033. doi: 10.1167/iovs.02-1136. [DOI] [PubMed] [Google Scholar]
- 109.Bolz S., Schuettauf F., Fries J. E., Thaler S., Reichenbach A., Pannicke T. K+ currents fail to change in reactive retinal glial cells in a mouse model of glaucoma. Graefe's Archive for Clinical and Experimental Ophthalmology. 2008;246(9):1249–1254. doi: 10.1007/s00417-008-0872-x. [DOI] [PubMed] [Google Scholar]
- 110.Kanamori A., Nakamura M., Nakanishi Y., Yamada Y., Negi A. Long-term glial reactivity in rat retinas ipsilateral and contralateral to experimental glaucoma. Experimental Eye Research. 2005;81(1):48–56. doi: 10.1016/j.exer.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 111.Zhang S., Wang H., Lu Q., et al. Detection of early neuron degeneration and accompanying glial responses in the visual pathway in a rat model of acute intraocular hypertension. Brain Research. 2009;1303:131–143. doi: 10.1016/j.brainres.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 112.Wu K. H. C., Madigan M. C., Billson F. A., Penfold P. L. Differential expression of GFAP in early v late AMD: a quantitative analysis. British Journal of Ophthalmology. 2003;87(9):1159–1166. doi: 10.1136/bjo.87.9.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Barber A. J., Antonetti D. A., Gardner T. W. Altered expression of retinal occludin and glial fibrillary acidic protein in experimental diabetes. Investigative Ophthalmology & Visual Science. 2000;41(11):3561–3568. [PubMed] [Google Scholar]
- 114.Fernandez-Sanchez L., Esquiva G., Pinilla I., Martín-Nieto J., Cuenca N. The antiapoptotic TUDCA protects against mitochondrial dysfunction, Glial cell changes and loss of the capillary network in the transgenic rat model of retinitis pigmentosa P23H. Investigative Ophthalmology & Visual Science. 2010;51(13):p. 3721. [Google Scholar]
- 115.Takeda M., Takamiya A., Jiao J.-W., et al. α-Aminoadipate induces progenitor cell properties of Müller glia in adult mice. Investigative Ophthalmology & Visual Science. 2008;49(3):1142–1150. doi: 10.1167/iovs.07-0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lewis G. P., Chapin E. A., Luna G., Linberg K. A., Fisher S. K. The fate of Muller's glia following experimental retinal detachment: nuclear migration, cell division, and subretinal glial scar formation. Molecular Vision. 2010;16:1361–1372. [PMC free article] [PubMed] [Google Scholar]
- 117.Bringmann A., Francke M., Pannicke T., et al. Role of glial K+ channels in ontogeny and gliosis: a hypothesis based upon studies on Muller cells. Glia. 2000;29(1):35–44. doi: 10.1002/(sici)1098-1136(20000101)29:1<35::aid-glia4>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 118.Dalloz C., Sarig R., Fort P., et al. Targeted inactivation of dystrophin gene product Dp71: phenotypic impact in mouse retina. Human Molecular Genetics. 2003;12(13):1543–1554. doi: 10.1093/hmg/ddg170. [DOI] [PubMed] [Google Scholar]
- 119.Pannicke T., Iandiev I., Uckermann O., et al. A potassium channel-linked mechanism of glial cell swelling in the postischemic retina. Molecular and Cellular Neuroscience. 2004;26(4):493–502. doi: 10.1016/j.mcn.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 120.Pannicke T., Uckermann O., Iandiev I., Wiedemann P., Reichenbach A., Bringmann A. Ocular inflammation alters swelling and membrane characteristics of rat Müller glial cells. Journal of Neuroimmunology. 2005;161(1-2):145–154. doi: 10.1016/j.jneuroim.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 121.Pannicke T., Iandiev I., Wurm A., et al. Diabetes alters osmotic swelling characteristics and membrane conductance of glial cells in rat retina. Diabetes. 2006;55(3):633–639. doi: 10.2337/diabetes.55.03.06.db05-1349. [DOI] [PubMed] [Google Scholar]
- 122.Iandiev I., Wurm A., Hollborn M., et al. Müller cell response to blue light injury of the rat retina. Investigative Ophthalmology & Visual Science. 2008;49(8):3559–3567. doi: 10.1167/iovs.08-1723. [DOI] [PubMed] [Google Scholar]
- 123.Dyer M. A., Cepko C. L. Control of Müller glial cell proliferation and activation following retinal injury. Nature Neuroscience. 2000;3(9):873–880. doi: 10.1038/78774. [DOI] [PubMed] [Google Scholar]
- 124.Burke J. M., Smith J. M. Retinal proliferation in response to vitreous hemoglobin or iron. Investigative Ophthalmology & Visual Science. 1981;20(5):582–592. [PubMed] [Google Scholar]
- 125.Fawcett J. W., Asher R. A. The glial scar and central nervous system repair. Brain Research Bulletin. 1999;49(6):377–391. doi: 10.1016/S0361-9230(99)00072-6. [DOI] [PubMed] [Google Scholar]
- 126.Cuenca N., Fernández-Sánchez L., Campello L., et al. Cellular responses following retinal injuries and therapeutic approaches for neurodegenerative diseases. Progress in Retinal and Eye Research. 2014;43:17–75. doi: 10.1016/j.preteyeres.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 127.Bringmann A., Reichenbach A. Role of Muller cells in retinal degenerations. Frontiers in Bioscience. 2001;6:E72–E92. doi: 10.2741/bringman. [DOI] [PubMed] [Google Scholar]
- 128.Frasson M., Picaud S., Léveillard T., et al. Glial cell line-derived neurotrophic factor induces histologic and functional protection of rod photoreceptors in the rd/rd mouse. Investigative Ophthalmology & Visual Science. 1999;40(11):2724–2734. [PubMed] [Google Scholar]
- 129.Honjo M., Tanihara H., Kido N., Inatani M., Okazaki K., Honda Y. Expression of ciliary neurotrophic factor activated by retinal Müller cells in eyes with NMDA- and kainic acid-induced neuronal death. Investigative Ophthalmology & Visual Science. 2000;41(2):552–560. [PubMed] [Google Scholar]
- 130.Oku H., Ikeda T., Honma Y., et al. Gene expression of neurotrophins and their high-affinity Trk receptors in cultured human Müller cells. Ophthalmic Research. 2002;34(1):38–42. doi: 10.1159/000048323. [DOI] [PubMed] [Google Scholar]
- 131.Sarup V., Patil K., Sharma S. C. Ciliary neurotrophic factor and its receptors are differentially expressed in the optic nerve transected adult rat retina. Brain Research. 2004;1013(2):152–158. doi: 10.1016/j.brainres.2004.03.030. [DOI] [PubMed] [Google Scholar]
- 132.Pease M. E., Zack D. J., Berlinicke C., et al. Effect of CNTF on retinal ganglion cell survival in experimental glaucoma. Investigative Ophthalmology & Visual Science. 2009;50(5):2194–2200. doi: 10.1167/iovs.08-3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kirsch M., Trautmann N., Ernst M., Hofmann H.-D. Involvement of gp130-associated cytokine signaling in Müller cell activation following optic nerve lesion. Glia. 2010;58(7):768–779. doi: 10.1002/glia.20961. [DOI] [PubMed] [Google Scholar]
- 134.Tezel G., Wax M. B. Hypoxia-inducible factor 1α in the glaucomatous retina and optic nerve head. Archives of Ophthalmology. 2004;122(9):1348–1356. doi: 10.1001/archopht.122.9.1348. [DOI] [PubMed] [Google Scholar]
- 135.Ergorul C., Ray A., Huang W., et al. Hypoxia inducible factor-1α (HIF-1α) and some HIF-1 target genes are elevated in experimental glaucoma. Journal of Molecular Neuroscience. 2010;42(2):183–191. doi: 10.1007/s12031-010-9343-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Matteucci A., Gaddini L., Villa M., et al. Neuroprotection by rat Müller glia against high glucose-induced neurodegeneration through a mechanism involving ERK1/2 activation. Experimental Eye Research. 2014;125:20–29. doi: 10.1016/j.exer.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 137.Bringmann A., Wiedemann P. Involvement of Müller glial cells in epiretinal membrane formation. Graefe's Archive for Clinical and Experimental Ophthalmology. 2009;247(7):865–883. doi: 10.1007/s00417-009-1082-x. [DOI] [PubMed] [Google Scholar]
- 138.Chu Y., Alder V. A., Humphrey M. F., Constable I. J. Localization of IgG in the normal and dystrophic rat retina after laser lesions. Australian and New Zealand Journal of Ophthalmology. 1999;27(2):117–125. doi: 10.1046/j.1440-1606.1999.00164.x. [DOI] [PubMed] [Google Scholar]
- 139.Giebel S. J., Menicucci G., McGuire P. G., Das A. Matrix metalloproteinases in early diabetic retinopathy and their role in alternation of the blood-retinal barrier. Laboratory Investigation. 2005;85(5):597–607. doi: 10.1038/labinvest.3700251. [DOI] [PubMed] [Google Scholar]
- 140.Kilic U., Kilic E., Järve A., et al. Human vascular endothelial growth factor protects axotomized retinal ganglion cells in vivo by activating ERK-1/2 and Akt pathways. The Journal of Neuroscience. 2006;26(48):12439–12446. doi: 10.1523/jneurosci.0434-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pierce I. A., Avery R. L., Foley E. D., Aiello L. P., Smith L. E. H. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(3):905–909. doi: 10.1073/pnas.92.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang J. J., Zhu M., Le Y. Z. Functions of Muller cell-derived vascular endothelial growth factor in diabetic retinopathy. World Journal of Diabetes. 2015;6(5):726–733. doi: 10.4239/wjd.v6.i5.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Roth S. Role of nitric oxide in retinal cell death. Clinical Neuroscience. 1997;4(5):216–223. [PubMed] [Google Scholar]
- 144.Koeberle P. D., Ball A. K. Nitric oxide synthase inhibition delays axonal degeneration and promotes the survival of axotomized retinal ganglion cells. Experimental Neurology. 1999;158(2):366–381. doi: 10.1006/exnr.1999.7113. [DOI] [PubMed] [Google Scholar]
- 145.Goureau O., Régnier-Ricard F., Courtois Y. Requirement for nitric oxide in retinal neuronal cell death induced by activated Muller glial cells. Journal of Neurochemistry. 1999;72(6):2506–2515. doi: 10.1046/j.1471-4159.1999.0722506.x. [DOI] [PubMed] [Google Scholar]
- 146.Kashii S., Mandai M., Kikuchi M., et al. Dual actions of nitric oxide in N-methyl-D-aspartate receptor-mediated neurotoxicity in cultured retinal neurons. Brain Research. 1996;711(1-2):93–101. doi: 10.1016/0006-8993(95)01330-x. [DOI] [PubMed] [Google Scholar]
- 147.Vorwerk C. K., Gorla M. S. R., Dreyer E. B. An experimental basis for implicating excitotoxicity in glaucomatous optic neuropathy. Survey of Ophthalmology. 1999;43(supplement 1):S142–S150. doi: 10.1016/s0039-6257(99)00017-x. [DOI] [PubMed] [Google Scholar]
- 148.Naskar R., Vorwerk C. K., Dreyer E. B. Concurrent downregulation of a glutamate transporter and receptor in glaucoma. Investigative Ophthalmology & Visual Science. 2000;41(7):1940–1944. [PubMed] [Google Scholar]
- 149.Rieck J. The pathogenesis of glaucoma in the interplay with the immune system. Investigative Ophthalmology and Visual Science. 2013;54(3):2393–2409. doi: 10.1167/iovs.12-9781. [DOI] [PubMed] [Google Scholar]
- 150.Cotinet A., Goureau O., Thillaye-Goldenberg B., Naud M. C., De Kozak Y. Differential tumor necrosis factor and nitric oxide production in retinal Muller glial cells from C3H/HeN and C3H/HeJ mice. Ocular Immunology and Inflammation. 1997;5(2):111–116. doi: 10.3109/09273949709085059. [DOI] [PubMed] [Google Scholar]
- 151.de Kozak Y., Naud M.-C., Bellot J., Faure J.-P., Hicks D. Differential tumor necrosis factor expression by resident retinal cells from experimental uveitis-susceptible and -resistant rat strains. Journal of Neuroimmunology. 1994;55(1):1–9. doi: 10.1016/0165-5728(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 152.Drescher K. M., Whittum-Hudson J. A. Modulation of immune-associated surface markers and cytokine production by murine retinal glial cells. Journal of Neuroimmunology. 1996;64(1):71–81. doi: 10.1016/0165-5728(95)00156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang M., Ma W., Zhao L., Fariss R. N., Wong W. T. Adaptive Müller cell responses to microglial activation mediate neuroprotection and coordinate inflammation in the retina. Journal of Neuroinflammation. 2011;8, article 173 doi: 10.1186/1742-2094-8-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ramírez J. M., Rojas B., Gallego B. I., et al. Cardiovascular Disease II. Hong Kong: Concept Press; 2014. Glia and blood-retinal barrier: effects of ocular hypetension; pp. 123–162. [Google Scholar]