Abstract
Calcium signalling within normal and cancer cells regulates many important cellular functions such as migration, proliferation, differentiation and cytokine secretion. Store operated Ca2+ entry (SOCE) via the Ca2+ release activated Ca2+ (CRAC) channels, which are composed of the plasma membrane based Orai channels and the endoplasmic reticulum stromal interaction molecules (STIMs), is a major Ca2+ entry route in many cell types. Orai and STIM have been implicated in the growth and metastasis of multiple cancers; however, while their involvement in cancer is presently indisputable, how Orai–STIM‐controlled Ca2+ signals affect malignant transformation, tumour growth and invasion is not fully understood. Here, we review recent studies linking Orai–STIM Ca2+ channels with cancer, with a particular focus on melanoma. We highlight and examine key molecular players and the signalling pathways regulated by Orai and STIM in normal and malignant cells, we expose discrepancies, and we reflect on the potential of Orai–STIMs as anticancer drug targets. Finally, we discuss the functional implications of future discoveries in the field of Ca2+ signalling.
Abbreviations
- Brn2
POU domain
- class 3
transcription factor 2
- CRAC
calcium release activated calcium
- ER
endoplasmic reticulum
- ERK
extracellular‐signal‐regulated kinases
- JARID1B
histone demethylase Jumonji AT‐rich interactive domain 1B
- MITF
microphthalmia‐associated transcription factor
- PKB
protein kinase B
- PKC
protein kinase C
- SOCE
store operated calcium entry
- STIM
stromal interaction molecule
- Tg
thapsigargin
- TRP
transient receptor potential
- Wnt5a
wingless‐type MMTV integration site family, member 5A
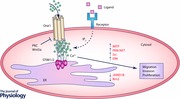
Store operated Ca2+ entry and Ca2+ release activated Ca2+ channels
Store operated Ca2+ entry (SOCE), also referred to as capacitive Ca2+ entry, is a mechanism by which Ca2+ enters cells from the extracellular space (Putney, 1986, 1990; Parekh & Putney, 2005). SOCE is initiated by the engagement of surface receptors which cause depletion of the endoplasmic reticulum (ER) Ca2+ stores and subsequent opening of Ca2+ selective ion channels at the plasma membrane. While the phenomenon of SOCE was discovered almost 30 years ago, the molecular identity of the plasma membrane channels defining this process took longer to reveal. The first major breakthrough in identifying and characterizing these channels arose in 1992, when Hoth et al. recorded a small Ca2+ current in mast cells that was induced by the depletion of ER stores (Hoth & Penner, 1992, 1993). The current was named Ca2+ release activated Ca2+ (CRAC) current (I CRAC). Studies that followed contributed in further characterizing the biophysical properties of the channel responsible for I CRAC (Fasolato et al. 1993; Hoth, 1995; Zweifach & Lewis, 1995; Lepple‐Wienhues & Cahalan, 1996; Parekh et al. 1997; Gilabert & Parekh, 2000; Hoth et al. 2000; Mignen & Shuttleworth, 2000; Prakriya & Lewis, 2001; Voets et al. 2001; Ashot Kozak et al. 2002; Glitsch et al. 2002; Bautista & Lewis, 2004; Zitt et al. 2004). However, the molecular identity of CRAC channels remained elusive until 2005 and 2006 when an ER based Ca2+ binding protein named stromal interaction molecule (STIM) and plasma membrane proteins termed Orai were identified as major components of the channel (Liou et al. 2005; Roos et al. 2005; Zhang et al. 2005; Feske et al. 2006; Vig et al. 2006; Yeromin et al. 2006). Presently, three isoforms of Orai (Orai1–3) and two of STIM (STIM1–2) are accepted as major molecular components of the CRAC channel. In the following years, many studies further characterized the properties of Orai–STIM channels (Luik et al. 2006; Soboloff et al. 2006 b; Brandman et al. 2007; Ong et al. 2007; Parvez et al. 2008; Fahrner et al. 2009; Lur et al. 2009; Park et al. 2009; Wang et al. 2010; Bogeski et al. 2010 b; Darbellay et al. 2011; Singaravelu et al. 2011; Hou et al. 2012; Kar et al. 2012; Ng et al. 2012; Zhang et al. 2014; Kar & Parekh, 2015; Miederer et al. 2015). With the molecular components of CRAC channels known, the next task was to understand the function and regulation of Orai and STIM. Not surprisingly, it was soon shown that Orai and STIM are essential in many organs and tissues; they are determinants of immune responses, the cardiovascular system, skin, muscle and blood for example. For the interested reader, we recommend the following review papers (Smyth et al. 2006; Soboloff et al. 2006 a; Hogan & Rao, 2007; Frischauf et al. 2008; Oh‐hora & Rao, 2008; Cahalan, 2009; Cahalan & Chandy, 2009; Di Capite & Parekh, 2009; Dirksen, 2009; Feske, 2009; Shuttleworth, 2009; Vig & Kinet, 2009; Feske, 2010; Hogan et al. 2010; Lee et al. 2010; Parekh, 2010, 2011; Qu et al. 2011; Beech, 2012).
Within the skin, Orai–STIM channels were identified in keratinocytes where they are involved in migration and differentiation as well as in pathological conditions such as atopic dermatitis (Jans et al. 2013; Numaga‐Tomita & Putney, 2013; Vandenberghe et al. 2013; Wilson et al. 2013; Brun et al. 2014). The functional importance of Orai and STIM in melanocytes and in melanoma is increasingly gaining recognition.
Calcium channels in melanocytes and melanoma
Melanocytes are cells that originate from the neural crest. They reside in the epidermal basement layer of the skin or in the eyes where they produce melanin pigment that protects against UV irradiation. Melanocyte malignant transformation leads to melanoma, which is the deadliest form of skin cancer. Once spread into distant organs, metastatic melanoma significantly reduces patient survival (Balch et al. 2009; Garbe et al. 2011). Unfortunately, melanoma incidence is steadily rising and it has been estimated that in the USA 73,870 new cases will be diagnosed in 2015 (4.5% of all US cancers). Around 10,000 patients will succumb to the disease within the same time period (http://seer.cancer.gov/statfacts/html/melan.html).
The recent advances in the treatment of melanoma, achieved by targeted therapies mostly aiming at the mitogen‐activated protein kinase (MAPK) pathway (e.g. vemurafenib, cobimetinib, dabrafenib, trametinib) or immunotherapeutic agents (e.g. the monoclonal antibodies against CTLA‐4 (ipilimumab) and PD‐1 (nivolumab, pembrolizumab)), significantly improved the prognosis for many melanoma patients (Schadendorf et al. 2015). Nevertheless, not all patients respond to treatments and resistance often occurs; therefore, it is still highly important to understand how and why melanocytes transform into melanoma and spread to distant organs. Besides the genetic mutations such as BRAF and NRAS commonly found in cutaneous melanomas, which are clearly linked with the malignant transformation of melanocytes, it has been suggested that the extracellular environment plays an important role in tumour cell growth and metastasis (Villanueva & Herlyn, 2008; Schadendorf et al. 2015). The Ca2+ gradient within the skin epidermis is a perfect example for such an environmental cancer modulation. For example, keratinocytes use the Ca2+ gradient to control their stage of differentiation by expressing the Ca2+ sensing receptor on their surface (Bikle et al. 1996, 2012; Hofer & Brown, 2003). Melanocytes, as all other cells in an organism, also need Ca2+ ions as regulators of important cellular functions. For this purpose cells possess a complex molecular machinery which allows them to process Ca2+ ions with strict spatial and temporal control. This Ca2+‐handling molecular machinery includes Ca2+ channels, pumps, transporters and chelators (Clapham, 2007).
The role of Ca2+ in melanoma was established in the late 70s and early 80s when researchers established a link between melanoma growth and migration and inhibitors of voltage gated Ca2+ channels (CaV) (Parsons et al. 1983). Of note, CaV inhibitors have been shown to also block other Ca2+ channels (Bogeski et al. 2010 a). Probably the first detailed study on Ca2+ channels, their expression, and electrophysiological characterization in melanoma cells was conducted in 1997 by Allen and colleagues (Allen et al. 1997). In this study, the authors identified and characterized two types of Ca2+ channels in four melanoma cell types; the CaV channels and the so‐called Ca2+ release activated Ca2+ (CRAC) channels, whose molecular identity was elusive at the time. Since then, a number of studies examined the functional importance of different Ca2+ channels and pumps in melanocytes and melanoma (Guo et al. 2012; Stanisz, 2013; Bellono & Oancea, 2014; Saul et al. 2014). In the late nineties, a third major group of Ca2+ channels known as transient receptor potential (TRP) channels was shown to play a role in melanocytes and melanoma (Duncan et al. 1998). TRP channels belong to a superfamily of close to 30 channels which are responsible for transporting not only Ca2+ but also Mg2+ and Na+ (Montell, 2005; Pedersen et al. 2005). In melanocytes, the melastatin TRP (TRPM) subfamily is represented by four members, including TRPM1, TRPM2, TRPM7 and TRPM8 (Guo et al. 2012). In fact, TRPM1 was originally cloned from melanocytes and was later shown to serve as a tumour suppressor in melanoma (Duncan et al. 1998, 2001; Deeds et al. 2000). TRPM1 is now established as an ion channel required for melanin synthesis and as a tumour marker since its expression levels inversely correlate with melanoma stage and prognosis (Miller et al. 2004; Erickson et al. 2009; Oancea et al. 2009; Oancea & Wicks, 2011; Bellono & Oancea, 2014). Other TRPM family members as well as TRPA1, TRPV1 and TRPML3 channels have been identified and demonstrated to control melanocyte and/or melanoma function (Atoyan et al. 2009; Choi et al. 2009; Oehler et al. 2012; Pucci et al. 2012; Bellono et al. 2013). Since this review focuses mostly on the Orai–STIM Ca2+ channels, we will not go into further details regarding the CaV and the TRP channels and suggest several publications which describe the role of ion channels in melanocytes and melanoma in more detail (Devi et al. 2009; Oancea et al. 2009; Lu et al. 2010; Oancea & Wicks, 2011; Guo et al. 2012; Saul et al. 2014; Ho & Lee, 2015).
Orai and STIM in cancer: a brief overview
Orai and STIM proteins, and thereby the CRAC channels, are important determinants of Ca2+ homeostasis and have been reported to be regulated in different tissues and cells, but also in several cancers (Yang et al. 2009; Feng et al. 2010; Flourakis et al. 2010; Chen et al. 2011; Hou et al. 2011; McAndrew et al. 2011; Aytes et al. 2012; Holzmann et al. 2013; Motiani et al. 2013 a; Dragoni et al. 2014; Kim et al. 2014; Kondratska et al. 2014; Stanisz et al. 2014; Holzmann et al. 2015; Hooper et al. 2015; Wang et al. 2015; Wong & Chang, 2015; Xu et al. 2015). The most extensively examined proteins in this regard are the predominant isoforms Orai1 and STIM1. Less is known about the role of the other CRAC channel components Orai2, Orai3 and STIM2 (Aytes et al. 2012; Faouzi et al. 2013; Motiani et al. 2013 b; Sobradillo et al. 2014; Stanisz et al. 2014). According to current published reports, Orai1 and STIM1 are upregulated in most cancers, suggesting an increased SOCE as a result. In some studies, this was proven experimentally, but in others this important parameter was, unfortunately, not quantified. Having in mind that the relative expression between Orai and STIM determines SOCE amplitude (Hoover & Lewis, 2011) rather than the absolute levels (e.g. upregulation of Orai1 alone would lead to decreased SOCE) such an experimental examination of not only SOCE but also of resting Ca2+ levels is absolutely critical. This information is essential in our understanding of the role of Orai and STIM in cancer development, but also for cell growth and metastasis. Interestingly, functionally relevant single nucleotide polymorphisms in Orai and STIM have also been reported in some cancers (Chang et al. 2014).
It is well known that Ca2+ is needed for proliferation and migration of various cell types (Clapham, 2007). Hence, changes in Ca2+ homeostasis can undoubtedly affect cancer cell biology. However, it is still unclear if alterations in Ca2+ homeostasis can contribute to malignant transformation and/or change the metastatic potential. For more on this issue, please refer to a recent review by Hoth (2016). A more in‐depth look into the role of Orai and STIM in different cancers will bring researchers one step further in their quest for using Orai–STIM Ca2+ channels as anticancer drug targets. In this regard the melanocyte–melanoma system, which we describe below, provides important hints.
Orai and STIM in melanocytes and melanoma
The first evidence regarding the expression and functional role of Orai and STIM in mouse melanoma cells was reported in 2010 (Feldman et al. 2010). The authors showed that Orai1 and STIM1 are expressed in B16BL6 mouse cells where they control SOCE. Moreover, SOCE was shown to be essential for the activation of protein kinase B (PKB/AKT), which in turn is able to control proliferation and apoptosis in malignant cells. The authors demonstrate that pharmacological inhibition of either PKB/AKT or SOCE, as well as silencing of STIM1, caused a reduction in cell growth and increased cell death. Hence, they proposed that SOCE might be a potential target for melanoma. Interestingly, the authors could detect a much higher SOCE in malignant B16BL6 cells compared to their non‐malignant counterparts; however, they could not assign the difference to altered Orai1 and STIM1 expression because both cell types had comparable protein levels. Since the plasma membrane Ca2+‐ATPase (PMCA) was also refuted as a cause for the different SOCE amplitudes, Feldman and colleagues tested mitochondrial Ca2+ buffering as a possible regulation mechanism and found that, indeed, mitochondrial Ca2+ uptake after SOCE was much higher in the malignant cells. This allowed the Orai1 channels to remain open for a longer time period and thereby efficiently activate PKB/AKT. A study published by the same group 2 years later expanded on these observations and suggested that lipid rafts are essential for proper SOCE function and activation of PKB/AKT in malignant B16BL6 cells (Fedida‐Metula et al. 2012).
Although these first studies were valuable they were performed in mouse cell lines, which are physiologically different from human skin cells (Herlyn & Fukunaga‐Kalabis, 2010). Hence, we examined Orai and STIM in primary human melanocytes and showed that all three Orai isoforms as well as the two STIMs are expressed in these cells (Stanisz et al. 2012). At the mRNA level, Orai1 and STIM2 were the predominant isoforms expressed and this was an interesting finding given that cells of neural origin usually utilize Orai2 and STIM2 as the major channel components. Why would melanocytes, which also originate from the neural crest, switch from Orai2 to Orai1 remains to be answered. It should be noted that Orai and STIM expression levels did not depend on the skin type since we did not observe any significant difference across a variety of human donors displaying distinct skin types and pigmentation (Stanisz et al. 2012). To analyse the functional role of Orai and STIM in this study, we used endothelin‐1, which is a hormone released upon UV radiation and known to activate SOCE. Measurements of melanin content and tyrosinase activity suggested that Orai–STIM channels control melanin synthesis and are thereby involved in adaptive tanning. This hypothesis, while needing confirmation, indicates a potential use for modulators of Orai and STIM in skin pigmentation control.
Given the important role of Orai and STIM in healthy melanocytes, we next examined the function of these channels in human melanoma (Stanisz et al. 2014). Our expression analyses showed no significant differences between melanoma cells and primary melanocytes or between different melanoma cell lines. Moreover, SOCE was also not overtly different between healthy and malignant cells, which did not fit with the findings of Feldman et al. (2010). This, however, does not prove that SOCE is not involved in malignant transformation of melanocytes or has no additional role in melanoma. In fact, further experiments showed that the melanoma phenotype is dependent on Orai‐ and STIM‐mediated SOCE. Inhibition of the channel by pharmacological inhibitors, or by silencing Orai1 and/or STIM2, caused melanoma cells to grow faster, but reduced their invading potential. This correlated with the upregulation of proteins such as microphthalmia‐associated transcription factor (MITF) that indicate a proliferative melanoma cell phenotype and the downregulation of slow‐cycling cell markers such as JARID1B and Brn2 (Hoek & Goding, 2010; Roesch et al. 2013; Stanisz et al. 2014). Immunostaining of human melanoma patient samples confirmed the expression of Orai1 and STIM2 in vivo. Higher expression levels near the melanoma invasive front further strengthened the hypothesis that Orai1 and STIM2 are involved in tumour invasion.
Another study on Orai and STIM in melanoma, published by Umemura and colleagues, showed that Orai1 and STIM1 are expressed in both melanoma and melanocytes (Umemura et al. 2014). However, none of the other three CRAC channel components were detected in melanoma cells or in melanocytes. Moreover, the authors observed high SOCE in metastatic melanomas, with much lower SOCE in primary melanomas and healthy melanocytes, an observation that was not in full agreement with the work by Feldman et al. or our own observations. Umemura et al. next investigated the effects of Orai–STIM channels on melanoma proliferation and invasion by using gene silencing and pharmacological inhibitors. Reduction of SOCE strongly suppressed cell migration in vitro and the formation of tumour colonies in vivo. However, the effects on cellular proliferation were mild; for example, silencing of Orai1 did not cause any change in cell growth in two out of three examined melanoma cell lines. Moreover, an inhibitor of SOCE (YM58483) slightly suppressed cell growth but mostly at concentrations higher than that required to block the channels, indicating possible off‐target effects. Interestingly, the authors identified the extracellular‐signal‐regulated kinases (ERK) signalling pathway to be regulated by SOCE and to affect phenotypic changes (Umemura et al. 2014). This suggests that there is a link between SOCE and MAPK signalling, a key pathway involved in melanoma pathobiology.
Recently, another study focusing on Orai and STIM in melanoma suggested that SOCE is involved in invadopodia formation and cancer invasion (Sun et al. 2014). Sun et al. came to this conclusion by counting invadopodia and measuring extracellular matrix degradation in melanoma cells treated with Ca2+ chelators (BAPTA‐AM and EGTA), pharmacological inhibitors of Orai1 (2‐APB and SKF96365), and by silencing Orai1 and STIM1 expression. The authors did not perform expression analyses on the other three CRAC channel components, namely Orai2, Orai3 and STIM2, and thereby did not consider the possibility that STIM2 might be more functionally important than STIM1 as suggested by our study (Stanisz et al. 2014). In another set of experiments, the authors tested the effects of SOCE on invadopodia formation by overexpressing STIM1 but not Orai1. To activate SOCE, the authors starved the cells and reintroduced 10% fetal calf serum (FCS) in the measuring bath. They examined the possible link between SOCE and invadopodia formation by screening for kinase expression and identified a master regulator of invasive cell phenotypes, the SRC kinase, to be regulated by cytosolic Ca2+ and hence, by Orai1 and STIM1. Moreover, they showed that inhibition of SOCE reduced the localization of membrane type 1 metalloprotease (MT1‐MMP) to the plasma membrane and thereby explained how Orai1 and STIM1 can promote invasion. Next, Sun and colleagues questioned the role of STIM1 in melanoma metastasis. For this purpose, the authors used 1205Lu melanoma cells, which are highly metastatic and form lung metastases in immunosuppressed mice. The cells were first stably transfected with STIM1 shRNA and then they were tested for proliferation changes. Contrary to previously published findings, the authors did not detect any difference in proliferation between control and STIM1 silenced cells. Next, the authors asked if the specific pattern of serum induced Ca2+ oscillations were necessary for invadopodia formation; this was tested by stimulating the cells with the sarco(endo)plasmic reticulum Ca2+‐ATPase (SERCA) inhibitor thapsigargin (Tg) or ionomycin which both induce sustained Ca2+ entry. Quantification of invadopodia formation showed that Tg and ionomycin caused a reduction in invadopodia formation in contrast to the serum‐induced activation of SOCE. Although this is an intriguing observation, the authors did not further analyse the mechanism behind the opposite effects of drug versus serum‐induced SOCE in melanoma invasion.
The most recent paper on Orai and STIM in melanoma was published by Hooper et al.; the authors examined and compared the role of CRAC channels in melanoma cells previously characterized as invasive and non‐invasive, mostly based on Wnt5a expression levels (Hooper et al. 2015). SOCE was induced either via receptor stimulation (histamine) or by Tg or ionomycin treatment. Interestingly, the authors found that the invasive Wnt5a expressing cells had a very small SOCE when compared to the non‐invasive melanomas. This finding is not in a full agreement with the observations of some of the above‐mentioned studies which reported that invasive melanoma has much higher SOCE than primary melanoma cell lines or melanocyte cell lines. Expression analysis, however, showed no overt differences in the abundance of Orai1 and STIM1, or of the other three CRAC channel components Orai2, Orai3 and STIM2 at the protein as well as the mRNA level. Of note, at the mRNA level it appears that Orai2 and Orai3 are more highly expressed than Orai1 in almost all examined melanoma cells. This interesting observation was not further examined. Remarkably, even the transient overexpression of Orai1 and STIM1 could not reinstate SOCE in the invasive cells. Hence, the authors went on to explore the molecular mechanism(s) responsible for the inhibition of the Orai–STIM driven Ca2+ entry. Because the cells examined were characterized by the expression pattern of Wnt5a, they were able to demonstrate that by manipulating Wnt5a levels, they could also affect SOCE. In invasive cells, silencing of Wnt5a led to SOCE recovery while in non‐invasive cells, overexpression of Wnt5a reduced the Ca2+ entry. In addition, based on the fact that Orai1 is inhibited by phosphorylation and that some melanomas have high protein kinase C (PKC) activity, the authors examined the role of this kinase in their cellular model. Their results demonstrate that high Wnt5a levels correlate with high PKC activity, which phosphorylates and inhibits the channel. Accordingly, by using PKC inhibitors or by mutating the phosphorylation sites in Orai1, the authors were able to recover SOCE in the highly invasive melanoma cells (Hooper et al. 2015).
Concluding remarks and future perspectives
The Orai–STIM research field in melanocytes and melanoma is still in its infancy, yet findings already point to their important role in normal skin function and malignancy. As shown in Table 1, all studies examining the role of Orai and STIM in melanocytes and melanoma demonstrate the important functional role of these channels. These studies show that SOCE regulates proliferation, migration and invasion as well as melanin synthesis in melanocytes and melanoma. Moreover, they identify several key signalling pathways involved in disease which are controlled by Ca2+ and hence, by Orai and STIM. However, the findings are far from being in a full agreement and need careful dissection and confirmation in the future. The most obvious reasons for the discrepant findings might be the dynamic nature of the calcium signalling system and its dependency on endogenous and exogenous parameters such as the heterogeneity of melanoma cell lines, varying culturing conditions, and different experimental techniques. While additional work is required in order to fully explain how Orai and STIM regulate melanocyte and melanoma biology, it is clear that their functional role in these cells is important and further knowledge in this area is very much needed.
Table 1.
Summary of the major findings regarding Orai and STIM functional effects in melanocytes and melanoma
| Effects of | Signalling | ||||||
|---|---|---|---|---|---|---|---|
| CRAC | Effects of | Orai–STIM on | pathways/ | ||||
| components | Orai–STIM on | migration and | proteins | Other functional | |||
| Cells examined | identified | SOCE/I CRAC | proliferation | invasion | involved | effects | |
| Feldman et al. 2010 | Malignant and non‐malignant B16BL6 mouse cells | Orai1 and STIM1 | SOCE is detectable and is higher in the malignant cells | ND | ND | PKB/AKT | Inhibition of mitochondrial Ca2+ buffering sensitizes melanoma cells to apoptosis |
| Stanisz et al. 2012 | Primary human melanocytes | Orai1–3, STIM1–2(Orai1 and STIM2 highest expression) | SOCE and I CRAC are detectable | Promote | ND | ND | Orai1 and STIM2 promote melanin synthesis |
| Stanisz et al. 2014 | Human melanoma | Orai1–3, STIM1–2 (Orai1 and STIM2 highest expression) | SOCE is detectable (no differences between melanocytes and different melanoma cell lines) | Suppress | Promote | MITFJARID1BBrn2 | Orai1 and STIM2 are highly expressed in invasive tumour fronts in human melanoma patient samples |
| Umemura et al. 2014 | Human melanoma | Orai1, STIM1 (other Orai isoforms and STIM2 not detected) | SOCE is increased in invasive melanoma cell lines vs. primary melanoma cells and melanocytes | Promote (minor effect) | Promote | ERK | Orai1‐ and STIM1‐silencing resulted in fewer lung metastases |
| Melanocyte cell line | Low Orai1 expression | ||||||
| Sun et al. 2014 | Human melanoma | Orai1, STIM1 | SOCE is detectable10% FCS induces Ca2+ oscillations while Tg sustained Ca2+ entry | No effect | Promote (oscillatory SOCE)Suppress (sustained SOCE) | Src | Promotion of plasma membrane localization of MT1‐MMP and invadopodium maturation |
| Hooper et al. 2015 | Human melanoma | Orai1‐3, STIM1‐2 (Orai2, ‐3 higher expression than Orai1; STIM1 higher expression than STIM2) | SOCE and I CRAC are detectable but are much lower in highly invasive melanoma cells | ND | Suppress | Wnt5a, PKC | Wnt5a regulates SOCE amplitude and melanoma invasiveness |
ND, not determined. MT1‐MMP, membrane type 1 metalloprotease; Src, proto‐oncogene tyrosine‐protein kinase.
Targeting these channels for the purpose of pigmentation or disease control may be challenging; however, this can be overcome with additional studies focusing on function, dynamics, signalling, mutations and observations with current therapies. Given the recent success of immunotherapies in melanoma patients, the melanoma field could benefit from studies that will examine the role of Orai–STIM channels in the immune‐cancer interplay. Finally, Orai and STIM are only one of the channels involved in melanocyte/melanoma Ca2+ homeostasis and they showcase the intricate yet important role of Ca2+ in melanocyte physiology and pathology. Our knowledge of these channels and calcium‐related processes is integral to both understanding normal skin biology and for curbing disease.
Additional information
Competing interests
None declared.
Funding
This work is supported by the Medical Faculty of Saarland (HOMFOR excellent research grant to I.B. and HOMFOR research grant to H.S.) and by the Deutsche Forschungsgemeinschaft (DFG) (SFB1027 project C4 and BO3643/3‐1 research grant to I.B.; R3577/3‐1 to A.R.). A.V. and M.H. acknowledge the NIH grant R01 CA047159.
Acknowledgements
H.S. and I.B. thank Professor Markus Hoth for his continuous support.
Biographies
Hedwig Stanisz graduated with an MD from Homburg Medical School, University of Saarland, Germany in 2008. Subsequently, she started her dermatology training at the Department of Dermatology at the University Clinics in Homburg, Germany, achieving board certification in Dermatology in 2013. In her doctoral work, which she defended in 2012, she examined the functional role of MCR1 receptor polymorphisms in primary human melanocytes and fibroblasts. Her current research interests are in understanding melanocyte biology with an emphasis on melanogenesis and mitogenesis, but also in examining the role of calcium metabolism in melanomagenesis and melanoma pathobiology.

Adina Vultur received her Ph.D. from the Department of Pathology at Queen's University, Canada in 2005 with a governor general's gold medal award. With an NSERC fellowship, she obtained postdoctoral training at City of Hope, CA, USA, with Dr. Richard Jove, focusing on Src and Stat3 signalling in breast cancer. She then joined Meenhard Herlyn's laboratory at the Wistar Institute, PA, USA, in 2007, where she is now a staff scientist. Dr. Vultur's current goals are to identify novel targets and treatment strategies against malignant melanoma. Her investigations also focus on signal transduction and the role of the microenvironment in tumor heterogeneity, treatment resistance, and metastasis.
This review was presented at the symposium “Ca2+ Signaling in Inflammatory and Immunological Disease”, which took place at the Gordon Research Conference on Calcium Signalling – Molecular and Cellular Mechanisms in Health and Disease in Maine, USA, 7–12 June, 2015.
References
- Allen DH, Lepple‐Wienhues A & Cahalan MD (1997). Ion channel phenotype of melanoma cell lines. J Membr Biol 155, 27–34. [DOI] [PubMed] [Google Scholar]
- Ashot Kozak J, Kerschbaum HH & Cahalan MD (2002). Distinct properties of CRAC and MIC channels in RBL cells. J Gen Physiol 120, 221–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atoyan R, Shander D & Botchkareva NV (2009). Non‐neuronal expression of transient receptor potential type A1 (TRPA1) in human skin. J Invest Dermatol 129, 2312–2315. [DOI] [PubMed] [Google Scholar]
- Aytes A, Molleví DG, Martinez‐Iniesta M, Nadal M, Vidal A, Morales A, Salazar R, Capellà G & Villanueva A (2012). Stromal interaction molecule 2 (STIM2) is frequently overexpressed in colorectal tumors and confers a tumor cell growth suppressor phenotype. Mol Carcinog 51, 746–753. [DOI] [PubMed] [Google Scholar]
- Balch CM, Gershenwald JE, Soong S‐j, Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG, Ding S, Eggermont AM, Flaherty KT, Gimotty PA, Kirkwood JM, McMasters KM, Mihm MC, Morton DL, Ross MI, Sober AJ & Sondak VK (2009). Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 27, 6199–6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista DM & Lewis RS (2004). Modulation of plasma membrane calcium‐ATPase activity by local calcium microdomains near CRAC channels in human T cells. J Physiol 556, 805–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech DJ (2012). Orai1 calcium channels in the vasculature. Pflugers Arch 463, 635–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellono NW, Kammel LG, Zimmerman AL & Oancea E (2013). UV light phototransduction activates transient receptor potential A1 ion channels in human melanocytes. Proc Natl Acad Sci USA 110, 2383–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellono NW & Oancea EV (2014). Ion transport in pigmentation. Arch Biochem Biophys 563, 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle DD, Ratnam A, Mauro T, Harris J & Pillai S (1996). Changes in calcium responsiveness and handling during keratinocyte differentiation: Potential role of the calcium receptor. J Clin Invest 97, 1085–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle DD, Xie Z & Tu CL (2012). Calcium regulation of keratinocyte differentiation. Expert Rev Endocrinol Metab 7, 461–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogeski I, Al‐Ansary D, Qu B, Niemeyer BA, Hoth M & Peinelt C (2010. a). Pharmacology of ORAI channels as a tool to understand their physiological functions. Expert Rev Clin Pharmacol 3, 291–303. [DOI] [PubMed] [Google Scholar]
- Bogeski I, Kummerow C, Al‐Ansary D, Schwarz EC, Koehler R, Kozai D, Takahashi N, Peinelt C, Griesemer D, Bozem M, Mori Y, Hoth M & Niemeyer BA (2010. b). Differential redox regulation of ORAI ion channels: a mechanism to tune cellular calcium signaling. Sci Signal 3, ra24. [DOI] [PubMed] [Google Scholar]
- Brandman O, Liou J, Park WS & Meyer T (2007). STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell 131, 1327–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun C, Demeaux A, Guaddachi F, Jean‐Louis F, Oddos T, Bagot M, Bensussan A, Jauliac S & Michel L (2014). T‐plastin expression downstream to the calcineurin/NFAT pathway is involved in keratinocyte migration. PLoS One 9, e104700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan MD (2009). STIMulating store‐operated Ca2+ entry. Nat Cell Biol 11, 669–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan MD & Chandy KG (2009). The functional network of ion channels in T lymphocytes. Immunol Rev 231, 59–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang WC, Fang YY, Chang HW, Chuang LY, Lin YD, Hou MF & Yang CH (2014). Identifying association model for single‐nucleotide polymorphisms of ORAI1 gene for breast cancer. Cancer Cell Int 14, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y‐F, Chiu W‐T, Chen Y‐T, Lin P‐Y, Huang H‐J, Chou C‐Y, Chang H‐C, Tang M‐J & Shen M‐R (2011). Calcium store sensor stromal‐interaction molecule 1‐dependent signaling plays an important role in cervical cancer growth, migration, and angiogenesis. Proc Natl Acad Sci USA 108, 15225–15230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi TY, Park SY, Jo JY, Kang G, Park JB, Kim JG, Hong SG, Kim CD, Lee JH & Yoon TJ (2009). Endogenous expression of TRPV1 channel in cultured human melanocytes. J Dermatol Sci 56, 128–130. [DOI] [PubMed] [Google Scholar]
- Clapham DE (2007). Calcium signaling. Cell 131, 1047–1058. [DOI] [PubMed] [Google Scholar]
- Darbellay B, Arnaudeau S, Bader CR, Konig S & Bernheim L (2011). STIM1L is a new actin‐binding splice variant involved in fast repetitive Ca2+ release. J Cell Biol 194, 335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeds J, Cronin F & Duncan LM (2000). Patterns of melastatin mRNA expression in melanocytic tumors. Hum Pathol 31, 1346–1356. [PubMed] [Google Scholar]
- Devi S, Kedlaya R, Maddodi N, Bhat KMR, Weber CS, Valdivia H & Setaluri V (2009). Calcium homeostasis in human melanocytes: Role of transient receptor potential melastatin 1 (TRPM1) and its regulation by ultraviolet light. Am J Physiol Cell Physiol 297, C679–C687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Capite J & Parekh AB (2009). CRAC channels and Ca2+ signaling in mast cells. Immunol Rev 231, 45–58. [DOI] [PubMed] [Google Scholar]
- Dirksen RT (2009). Checking your SOCCs and feet: The molecular mechanisms of Ca2+ entry in skeletal muscle. J Physiol 587, 3139–3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragoni S, Turin I, Laforenza U, Potenza DM, Bottino C, Glasnov TN, Prestia M, Ferulli F, Saitta A, Mosca A, Guerra G, Rosti V, Luinetti O, Ganini C, Porta C, Pedrazzoli P, Tanzi F, Montagna D & Moccia F (2014). Store‐operated Ca2+ entry does not control proliferation in primary cultures of human metastatic renal cellular carcinoma. BioMed Res Int 2014, 739494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan LM, Deeds J, Cronin FE, Donovan M, Sober AJ, Kauffman M & McCarthy JJ (2001). Melastatin expression and prognosis in cutaneous malignant melanoma. J Clin Oncol 19, 568–576. [DOI] [PubMed] [Google Scholar]
- Duncan LM, Deeds J, Hunter J, Shao J, Holmgren LM, Woolf EA, Tepper RI & Shyjan AW (1998). Down‐regulation of the novel gene melastatin correlates with potential for melanoma metastasis. Cancer Res 58, 1515–1520. [PubMed] [Google Scholar]
- Erickson LA, Letts GA, Shah SM, Shackelton JB & Duncan LM (2009). TRPM1 (Melastatin‐1/MLSN1) mRNA expression in Spitz nevi and nodular melanomas. Mod Pathol 22, 969–976. [DOI] [PubMed] [Google Scholar]
- Fahrner M, Muik M, Derler I, Schindl R, Fritsch R, Frischauf I & Romanin C (2009). Mechanistic view on domains mediating STIM1‐Orai coupling. Immunol Rev 231, 99–112. [DOI] [PubMed] [Google Scholar]
- Faouzi M, Kischel P, Hague F, Ahidouch A, Benzerdjeb N, Sevestre H, Penner R & Ouadid‐Ahidouch H (2013). ORAI3 silencing alters cell proliferation and cell cycle progression via c‐myc pathway in breast cancer cells. Biochim Biophys Acta 1833, 752–760. [DOI] [PubMed] [Google Scholar]
- Fasolato C, Hoth M & Penner R (1993). A GTP‐dependent step in the activation mechanism of capacitative calcium influx. J Biol Chem 268, 20737–20740. [PubMed] [Google Scholar]
- Fedida‐Metula S, Feldman B, Koshelev V, Levin‐Gromiko U, Voronov E & Fishman D (2012). Lipid rafts couple store‐operated Ca2+ entry to constitutive activation of PKB/Akt in a Ca2+/calmodulin‐, Src‐ and PP2A‐mediated pathway and promote melanoma tumor growth. Carcinogenesis 33, 740–750. [DOI] [PubMed] [Google Scholar]
- Feldman B, Fedida‐Metula S, Nita J, Sekler I & Fishman D (2010). Coupling of mitochondria to store‐operated Ca2+‐signaling sustains constitutive activation of protein kinase B/Akt and augments survival of malignant melanoma cells. Cell Calcium 47, 525–537. [DOI] [PubMed] [Google Scholar]
- Feng M, Grice DM, Faddy HM, Nguyen N, Leitch S, Wang Y, Muend S, Kenny PA, Sukumar S, Roberts‐Thomson SJ, Monteith GR & Rao R (2010). Store‐independent activation of Orai1 by SPCA2 in mammary tumors. Cell 143, 84–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feske S (2009). ORAI1 and STIM1 deficiency in human and mice: roles of store‐operated Ca2+ entry in the immune system and beyond. Immunol Rev 231, 189–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feske S (2010). CRAC channelopathies. Pflugers Arch 460, 417–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M & Rao A (2006). A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 441, 179–185. [DOI] [PubMed] [Google Scholar]
- Flourakis M, Lehen'kyi V, Beck B, Raphael M, Vandenberghe M, Abeele FV, Roudbaraki M, Lepage G, Mauroy B, Romanin C, Shuba Y, Skryma R & Prevarskaya N (2010). Orai1 contributes to the establishment of an apoptosis‐resistant phenotype in prostate cancer cells. Cell Death Dis 1, e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischauf I, Schindl R, Derler I, Bergsmann J, Fahrner M & Romanin C (2008). The STIM/Orai coupling machinery. Channels 2, 261–268. [DOI] [PubMed] [Google Scholar]
- Garbe C, Eigentler TK, Keilholz U, Hauschild A & Kirkwood JM (2011). Systematic review of medical treatment in melanoma: current status and future prospects. Oncologist 16, 5–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilabert JA & Parekh AB (2000). Respiring mitochondria determine the pattern of activation and inactivation of the store‐operated Ca2+ current ICRAC . EMBO J 19, 6401–6407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glitsch MD, Bakowski D & Parekh AB (2002). Store‐operated Ca2+ entry depends on mitochondrial Ca2+ uptake. EMBO J 21, 6744–6754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Carlson JA & Slominski A (2012). Role of TRPM in melanocytes and melanoma. Exp Dermatol 21, 650–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlyn M & Fukunaga‐Kalabis M (2010). What is a good model for melanoma. J Invest Dermatol 130, 911–912. [DOI] [PubMed] [Google Scholar]
- Ho JC & Lee CH (2015). TRP channels in skin: From physiological implications to clinical significances. Biophysics (Japan) 11, 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek KS & Goding CR (2010). Cancer stem cells versus phenotype‐switching in melanoma. Pigment Cell Melanoma Res 23, 746–759. [DOI] [PubMed] [Google Scholar]
- Hofer AM & Brown EM (2003). Extracellular calcium sensing and signalling. Nat Rev Mol Cell Biol 4, 530–538. [DOI] [PubMed] [Google Scholar]
- Hogan PG, Lewis RS & Rao A (2010). Molecular basis of calcium signaling in lymphocytes: STIM and ORAI. Annu Rev Immunol 28, 491–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan PG & Rao A (2007). Dissecting ICRAC, a store‐operated calcium current. Trends Biochem Sci 32, 235–245. [DOI] [PubMed] [Google Scholar]
- Holzmann C, Kilch T, Kappel S, Armbruster A, Jung V, Stockle M, Bogeski I, Schwarz EC & Peinelt C (2013). ICRAC controls the rapid androgen response in human primary prostate epithelial cells and is altered in prostate cancer. Oncotarget 4, 2096–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzmann C, Kilch T, Kappel S, Dörr K, Jung V, Stöckle M, Bogeski I & Peinelt C (2015). Differential redox regulation of Ca2+ signaling and viability in normal and malignant prostate cells. Biophys J 109, 1410–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper R, Zhang X, Webster M, Go C, Kedra J, Marchbank K, Gill DL, Weeraratna AT, Trebak M & Soboloff J (2015). Novel protein kinase C‐mediated control of Orai1 function in invasive melanoma. Mol Cell Biol 35, 2790–2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover PJ & Lewis RS (2011). Stoichiometric requirements for trapping and gating of Ca2+ release‐activated Ca2+ (CRAC) channels by stromal interaction molecule 1 (STIM1). Proc Natl Acad Sci USA 108, 13299–13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoth M (1995). Calcium and barium permeation through calcium release‐activated calcium (CRAC) channels. Pflugers Arch 430, 315–322. [DOI] [PubMed] [Google Scholar]
- Hoth M (2016). CRAC channels, calcium, and cancer in light of the driver and passenger concept. Biochim Biophys Acta 1863, 1408–1417. [DOI] [PubMed] [Google Scholar]
- Hoth M, Button DC & Lewis RS (2000). Mitochondrial control of calcium‐channel gating: A mechanism for sustained signaling and transcriptional activation in T lymphocytes. Proc Natl Acad Sci USA 97, 10607–10612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoth M & Penner R (1992). Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature 355, 353–356. [DOI] [PubMed] [Google Scholar]
- Hoth M & Penner R (1993). Calcium release‐activated calcium current in rat mast cells. J Physiol 465, 359–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou M‐F, Kuo H‐C, Li J‐H, Wang Y‐S, Chang C‐C, Chen K‐C, Chen W‐C, Chiu C‐C, Yang S & Chang W‐C (2011). Orai1/CRACM1 overexpression suppresses cell proliferation via attenuation of the store‐operated calcium influx‐mediated signalling pathway in A549 lung cancer cells. Biochim Biophys Acta 1810, 1278–1284. [DOI] [PubMed] [Google Scholar]
- Hou X, Pedi L, Diver MM & Long SB (2012). Crystal structure of the calcium release‐activated calcium channel Orai. Science 338, 1308–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jans R, Mottram L, Johnson DL, Brown AM, Sikkink S, Ross K & Reynolds NJ (2013). Lysophosphatidic acid promotes cell migration through STIM1‐and Orai1‐mediated Ca2+ i mobilization and NFAT2 activation. J Invest Dermatol 133, 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar P, Bakowski D, Di Capite J, Nelson C & Parekh AB (2012). Different agonists recruit different stromal interaction molecule proteins to support cytoplasmic Ca2+ oscillations and gene expression. Proc Natl Acad Sci USA 109, 6969–6974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar P & Parekh AB (2015). Distinct spatial Ca2+ signatures selectively activate different NFAT transcription factor isoforms. Mol Cell 58, 232–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Lkhagvadorj S, Lee MR, Hwang KH, Chung HC, Jung JH, Cha SK & Eom M (2014). Orai1 and STIM1 are critical for cell migration and proliferation of clear cell renal cell carcinoma. Biochem Biophys Res Commun 448, 76–82. [DOI] [PubMed] [Google Scholar]
- Kondratska K, Kondratskyi A, Yassine M, Lemonnier L, Lepage G, Morabito A, Skryma R & Prevarskaya N (2014). Orai1 and STIM1 mediate SOCE and contribute to apoptotic resistance of pancreatic adenocarcinoma. Biochim Biophys Acta 1843, 2263–2269. [DOI] [PubMed] [Google Scholar]
- Lee KP, Yuan JP, Hong JH, So I, Worley PF & Muallem S (2010). An endoplasmic reticulum/plasma membrane junction: STIM1/Orai1/TRPCs. FEBS Lett 584, 2022–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepple‐Wienhues A & Cahalan MD (1996). Conductance and permeation of monovalent cations through depletion‐activated Ca2+ channels (ICRAC) in Jurkat T cells. Biophys J 71, 787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE Jr & Meyer T (2005). STIM is a Ca2+ sensor essential for Ca2+‐store‐depletion‐triggered Ca2+ influx. Curr Biol 15, 1235–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S, Slominski A, Yang SE, Sheehan C, Ross J & Carlson JA (2010). The correlation of TRPM1 (Melastatin) mRNA expression with microphthalmia‐associated transcription factor (MITF) and other melanogenesis‐related proteins in normal and pathological skin, hair follicles and melanocytic nevi. J Cutan Pathol 37, 26–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luik RM, Wu MM, Buchanan J & Lewis RS (2006). The elementary unit of store‐operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER‐plasma membrane junctions. J Cell Biol 174, 815–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lur G, Haynes LP, Prior IA, Gerasimenko OV, Feske S, Petersen OH, Burgoyne RD & Tepikin AV (2009). Ribosome‐free terminals of rough ER allow formation of STIM1 puncta and segregation of STIM1 from IP3 receptors. Curr Biol 19, 1648–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAndrew D, Grice DM, Peters AA, Davis FM, Stewart T, Rice M, Smart CE, Brown MA, Kenny PA, Roberts‐Thomson SJ & Monteith GR (2011). ORAI1‐mediated calcium influx in lactation and in breast cancer. Mol Cancer Ther 10, 448–460. [DOI] [PubMed] [Google Scholar]
- Miederer AM, Alansary D, Schwär G, Lee PH, Jung M, Helms V & Niemeyer BA (2015). A STIM2 splice variant negatively regulates store‐operated calcium entry. Nat Commun 6, 6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignen O & Shuttleworth TJ (2000). I ARC, a novel arachidonate‐regulated, noncapacitative Ca2+ entry channel. J Biol Chem 275, 9114–9119. [DOI] [PubMed] [Google Scholar]
- Miller AJ, Du J, Rowan S, Hershey CL, Widlund HR & Fisher DE (2004). Transcriptional regulation of the melanoma prognostic marker melastatin (TRPM1) by MITF in melanocytes and melanoma. Cancer Res 64, 509–516. [DOI] [PubMed] [Google Scholar]
- Montell C (2005). The TRP superfamily of cation channels. Sci STKE 2005, re3. [DOI] [PubMed] [Google Scholar]
- Motiani RK, Hyzinski‐García MC, Zhang X, Henkel MM, Abdullaev IF, Kuo YH, Matrougui K, Mongin AA & Trebak M (2013. a). STIM1 and Orai1 mediate CRAC channel activity and are essential for human glioblastoma invasion. Pflugers Arch 465, 1249–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motiani RK, Zhang X, Harmon KE, Keller RS, Matrougui K, Bennett JA & Trebak M (2013. b). Orai3 is an estrogen receptor α‐regulated Ca2+ channel that promotes tumorigenesis. FASEB J 27, 63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng SW, Bakowski D, Nelson C, Mehta R, Almeyda R, Bates G & Parekh AB (2012). Cysteinyl leukotriene type I receptor desensitization sustains Ca2+‐dependent gene expression. Nature 482, 111–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numaga‐Tomita T & Putney JW (2013). Role of STIM1‐and Orai1‐mediated Ca2+ entry in Ca2+‐induced epidermal keratinocyte differentiation. J Cell Sci 126, 605–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oancea E, Vriens J, Brauchi S, Jun J, Splawski I & Clapham DE (2009). TRPM1 forms ion channels associated with melanin content in melanocytes. Sci Signal 2, ra21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oancea E & Wicks NL (2011). TRPM1: new trends for an old TRP. Adv Exp Med Biol 704, 135–145. [DOI] [PubMed] [Google Scholar]
- Oehler B, Scholze A, Schaefer M & Hill K (2012). TRPA1 is functionally expressed in melanoma cells but is not critical for impaired proliferation caused by allyl isothiocyanate or cinnamaldehyde. Naunyn Schmiedebergs Arch Pharmacol 385, 555–563. [DOI] [PubMed] [Google Scholar]
- Oh‐hora M & Rao A (2008). Calcium signaling in lymphocytes. Curr Opin Immunol 20, 250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong HL, Cheng KT, Liu X, Bandyopadhyay BC, Paria BC, Soboloff J, Pani B, Gwack Y, Srikanth S, Singh BB, Gill DL & Ambudkar IS (2007). Dynamic assembly of TRPC1‐STIM1‐Orai1 ternary complex is involved in store‐operated calcium influx. Evidence for similarities in store‐operated and calcium release‐activated calcium channel components. J Biol Chem 282, 9105–9116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh AB (2010). Store‐operated CRAC channels: Function in health and disease. Nat Rev Drug Discov 9, 399–410. [DOI] [PubMed] [Google Scholar]
- Parekh AB (2011). Decoding cytosolic Ca2+ oscillations. Trends Biochem Sci 36, 78–87. [DOI] [PubMed] [Google Scholar]
- Parekh AB, Fleig A & Penner R (1997). The store‐operated calcium current ICRAC: Nonlinear activation by InsP3 and dissociation from calcium release. Cell 89, 973–980. [DOI] [PubMed] [Google Scholar]
- Parekh AB & Putney JW Jr (2005). Store‐operated calcium channels. Physiol Rev 85, 757–810. [DOI] [PubMed] [Google Scholar]
- Park CY, Hoover PJ, Mullins FM, Bachhawat P, Covington ED, Raunser S, Walz T, Garcia KC, Dolmetsch RE & Lewis RS (2009). STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell 136, 876–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons PG, Musk P, Goss PD & Leah J (1983). Effects of calcium depletion on human cells in vitro and the anomalous behavior of the human melanoma cell line MM170. Cancer Res 43, 2081–2087. [PubMed] [Google Scholar]
- Parvez S, Beck A, Peinelt C, Soboloff J, Lis A, Monteilh‐Zoller M, Gill DL, Fleig A & Penner R (2008). STIM2 protein mediates distinct store‐dependent and store‐independent modes of CRAC channel activation. FASEB J 22, 752–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen SF, Owsianik G & Nilius B (2005). TRP channels: An overview. Cell Calcium 38, 233–252. [DOI] [PubMed] [Google Scholar]
- Prakriya M & Lewis RS (2001). Potentiation and inhibition of Ca2+ release‐activated Ca2+ channels by 2‐aminoethyldiphenyl borate (2‐APB) occurs independently of IP3 receptors. J Physiol 536, 3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucci M, Pasquariello N, Battista N, Di Tommaso M, Rapino C, Fezza F, Zuccolo M, Jourdain R, Agrò AF, Breton L & Maccarrone M (2012). Endocannabinoids stimulate human melanogenesis via type‐1 cannabinoid receptor. J Biol Chem 287, 15466–15478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney JW Jr (1986). A model for receptor‐regulated calcium entry. Cell Calcium 7, 1–12. [DOI] [PubMed] [Google Scholar]
- Putney JW Jr (1990). Capacitative calcium entry revisited. Cell Calcium 11, 611–624. [DOI] [PubMed] [Google Scholar]
- Qu B, Al‐Ansary D, Kummerow C, Hoth M & Schwarz EC (2011). ORAI‐mediated calcium influx in T cell proliferation, apoptosis and tolerance. Cell Calcium 50, 261–269. [DOI] [PubMed] [Google Scholar]
- Roesch A, Vultur A, Bogeski I, Wang H, Zimmermann KM, Speicher D, Korbel C, Laschke MW, Gimotty PA, Philipp SE, Krause E, Patzold S, Villanueva J, Krepler C, Fukunaga‐Kalabis M, Hoth M, Bastian BC, Vogt T & Herlyn M (2013). Overcoming intrinsic multidrug resistance in melanoma by blocking the mitochondrial respiratory chain of slow‐cycling JARID1Bhigh cells. Cancer Cell 23, 811–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Veliçelebi G & Stauderman KA (2005). STIM1, an essential and conserved component of store‐operated Ca2+ channel function. J Cell Biol 169, 435–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saul S, Stanisz H, Backes CS, Schwarz EC & Hoth M (2014). How ORAI and TRP channels interfere with each other: Interaction models and examples from the immune system and the skin. Eur J Pharmacol 739, 49–59. [DOI] [PubMed] [Google Scholar]
- Schadendorf D, Fisher DE, Garbe C, Gershenwald JE, Grob J‐J, Halpern A, Herlyn M, Marchetti MA, McArthur G, Ribas A, Roesch A & Hauschild A (2015). Melanoma. Nat Rev Dis Primers, 15003 (DOI: 10.1038/nrdp.2015.3). [DOI] [PubMed] [Google Scholar]
- Shuttleworth TJ (2009). Arachidonic acid, ARC channels, and Orai proteins. Cell Calcium 45, 602–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singaravelu K, Nelson C, Bakowski D, De Brito OM, Ng SW, Di Capite J, Powell T, Scorrano L & Parekh AB (2011). Mitofusin 2 regulates STIM1 migration from the Ca2+ store to the plasma membrane in cells with depolarized mitochondria. J Biol Chem 286, 12189–12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth JT, DeHaven WI, Jones BF, Mercer JC, Trebak M, Vazquez G & Putney JW Jr (2006). Emerging perspectives in store‐operated Ca2+ entry: Roles of Orai, Stim and TRP. Biochim Biophys Acta 1763, 1147–1160. [DOI] [PubMed] [Google Scholar]
- Soboloff J, Spassova MA, Dziadek MA & Gill DL (2006. a). Calcium signals mediated by STIM and Orai proteins – A new paradigm in inter‐organelle communication. Biochim Biophys Acta 1763, 1161–1168. [DOI] [PubMed] [Google Scholar]
- Soboloff J, Spassova MA, Tang XD, Hewavitharana T, Xu W & Gill DL (2006. b). Orai1 and STIM reconstitute store‐operated calcium channel function. J Biol Chem 281, 20661–20665. [DOI] [PubMed] [Google Scholar]
- Sobradillo D, Hernández‐Morales M, Ubierna D, Moyer MP, Núñez L & Villalobos C (2014). A reciprocal shift in transient receptor potential channel 1 (TRPC1) and stromal interaction molecule 2 (STIM2) contributes to Ca2+ remodeling and cancer hallmarks in colorectal carcinoma cells. J Biol Chem 289, 28765–28782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanisz H (2013). Calcium signalling in inflammatory skin disease In Dermatitis: Causes, Symptoms and Treatment Options, ed. Müller CSL pp. 120–130. Nova Science, New York. [Google Scholar]
- Stanisz H, Saul S, Müller CS, Kappl R, Niemeyer BA, Vogt T, Hoth M, Roesch A & Bogeski I (2014). Inverse regulation of melanoma growth and migration by Orai1/STIM2‐dependent calcium entry. Pigment Cell Melanoma Res 27, 442–453. [DOI] [PubMed] [Google Scholar]
- Stanisz H, Stark A, Kilch T, Schwarz EC, Müller CSL, Peinelt C, Hoth M, Niemeyer BA, Vogt T & Bogeski I (2012). ORAI1 Ca2+ channels control endothelin‐1‐induced mitogenesis and melanogenesis in primary human melanocytes. J Invest Dermatol 132, 1443–1451. [DOI] [PubMed] [Google Scholar]
- Sun J, Lu F, He H, Shen J, Messina J, Mathew R, Wang D, Sarnaik AA, Chang WC, Kim M, Cheng H & Yang S (2014). STIM1‐ and Orai1‐mediated Ca2+ oscillation orchestrates invadopodium formation and melanoma invasion. J Cell Biol 207, 535–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemura M, Baljinnyam E, Feske S, De Lorenzo MS, Xie LH, Feng X, Oda K, Makino A, Fujita T, Yokoyama U, Iwatsubo M, Chen S, Goydos JS, Ishikawa Y & Iwatsubo K (2014). Store‐operated Ca2+ entry (SOCE) regulates melanoma proliferation and cell migration. PLoS One 9, e89292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe M, Raphaël M, Lehen'kyi V, Gordienko D, Hastie R, Oddos T, Rao A, Hogan PG, Skryma R & Prevarskaya N (2013). ORAI1 calcium channel orchestrates skin homeostasis. Proc Natl Acad Sci USA 110, E4839–E4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vig M & Kinet JP (2009). Calcium signaling in immune cells. Nat Immunol 10, 21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan‐Huberson M, Kraft S, Turner H, Fleig A, Penner R & Kinet JP (2006). CRACM1 is a plasma membrane protein essential for store‐operated Ca2+ entry. Science 312, 1220–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva J & Herlyn M (2008). Melanoma and the tumor microenvironment. Curr Oncol Rep 10, 439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voets T, Prenen J, Fleig A, Vennekens R, Watanabe H, Hoenderop JGJ, Bindels RJM, Droogmans G, Penner R & Nilius B (2001). CaT1 and the calcium release‐activated calcium channel manifest distinct pore properties. J Biol Chem 276, 47767–47770. [DOI] [PubMed] [Google Scholar]
- Wang JY, Sun J, Huang MY, Wang YS, Hou MF, Sun Y, He H, Krishna N, Chiu SJ, Lin S, Yang S & Chang WC (2015). STIM1 overexpression promotes colorectal cancer progression, cell motility and COX‐2 expression. Oncogene 34, 4358–4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Deng X, Mancarella S, Hendron E, Eguchi S, Soboloff J, Tang XD & Gill DL (2010). The calcium store sensor, STIM1, reciprocally controls Orai and CaV1.2 channels. Science 330, 105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SR, Thé L, Batia LM, Beattie K, Katibah GE, McClain SP, Pellegrino M, Estandian DM & Bautista DM (2013). The epithelial cell‐derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell 155, 285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong HSC & Chang WC (2015). Correlation of clinical features and genetic profiles of stromal interaction molecule 1 (STIM1) in colorectal cancers. Oncotarget 6, 42169–42182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Zhang S, Niu H, Ye Y, Hu F, Chen S, Li X, Luo X, Jiang S, Liu Y, Chen Y, Li J, Xiang R & Li N (2015). STIM1 accelerates cell senescence in a remodeled microenvironment but enhances the epithelial‐to‐mesenchymal transition in prostate cancer. Sci Rep 5, 11754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Zhang JJ & Huang X‐Y (2009). Orai1 and STIM1 are critical for breast tumor cell migration and metastasis. Cancer Cell 15, 124–134. [DOI] [PubMed] [Google Scholar]
- Yeromin AV, Zhang SL, Jiang W, Yu Y, Safrina O & Cahalan MD (2006). Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature 443, 226–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, Stauderman KA & Cahalan MD (2005). STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature 437, 902–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhang W, González‐Cobos JC, Jardin I, Romanin C, Matrougui K & Trebak M (2014). Complex role of STIM1 in the activation of store‐independent Orai1/3 channels. J Gen Physiol 143, 345–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitt C, Strauss B, Schwarz EC, Spaeth N, Rast G, Hatzelmann A & Hoth M (2004). Potent inhibition of Ca2+ release‐activated Ca2+ channels and T‐lymphocyte activation by the pyrazole derivative BTP2. J Biol Chem 279, 12427–12437. [DOI] [PubMed] [Google Scholar]
- Zweifach A & Lewis RS (1995). Rapid inactivation of depletion‐activated calcium current (ICRAC) due to local calcium feedback. J Gen Physiol 105, 209–226. [DOI] [PMC free article] [PubMed] [Google Scholar]


