Abstract
Inositol 1,4,5‐trisphosphate receptors (IP3Rs) are expressed in nearly all animal cells, where they mediate the release of Ca2+ from intracellular stores. The complex spatial and temporal organization of the ensuing intracellular Ca2+ signals allows selective regulation of diverse physiological responses. Interactions of IP3Rs with other proteins contribute to the specificity and speed of Ca2+ signalling pathways, and to their capacity to integrate information from other signalling pathways. In this review, we provide a comprehensive survey of the proteins proposed to interact with IP3Rs and the functional effects that these interactions produce. Interacting proteins can determine the activity of IP3Rs, facilitate their regulation by multiple signalling pathways and direct the Ca2+ that they release to specific targets. We suggest that IP3Rs function as signalling hubs through which diverse inputs are processed and then emerge as cytosolic Ca2+ signals.
Abbreviations
- AC
adenylyl cyclase
- B2R
type 2 bradykinin receptor
- cAMP
cyclic adenosine monophosphate
- CREB
cAMP response element‐binding protein
- EB3
end‐binding protein 3
- ER
endoplasmic reticulum
- GPCR
G protein‐coupled receptor
- IBC
IP3‐binding core
- IP3
inositol 1,4,5‐trisphosphate
- IP3R
IP3 receptor
- IRBIT
IP3R‐binding protein released with IP3
- M1R
type 1 muscarinic acetylcholine receptor
- PKA
protein kinase A
- PLC
phospholipase C
- SD
suppressor domain
- TMD
transmembrane domain
Introduction
Ca2+ signals within cells are spatially and temporally intricate, allowing them to elicit a multitude of specific downstream effects (Berridge, 2009). Inositol 1,4,5‐trisphosphate receptors (IP3Rs), the most widely expressed class of intracellular Ca2+ channel, release Ca2+ from intracellular stores in response to binding of IP3 and Ca2+ (Foskett et al. 2007; Taylor & Tovey, 2010). Dual regulation of IP3Rs by two essential stimuli, IP3 and Ca2+, is important because it endows IP3Rs with a capacity to propagate Ca2+ signals regeneratively by Ca2+‐induced Ca2+ release, as Ca2+ released by an active IP3R ignites the activity of adjacent IP3Rs that have bound IP3 (Smith & Parker, 2009). This in turn plays a key role in defining the spatial organization of IP3‐evoked Ca2+ signals.
Activation of IP3Rs is initiated by binding of IP3 within a clam‐like structure, the IP3‐binding core (IBC) (Bosanac et al. 2002), located near the N‐terminus of each IP3R subunit. Binding of IP3 causes a conformational change that rearranges the association of the IBC with the N‐terminal suppressor domain (SD). These changes are proposed to disrupt interactions between the N‐terminal regions of the four subunits of the IP3R, leading to opening of the channel. The latter is formed by transmembrane domains (TMDs) towards the C‐terminus of each IP3R subunit (Seo et al. 2012) (Fig. 1). It is not yet clear where binding of Ca2+ to the IP3R lies within the sequence of events linking binding of IP3 to channel gating. One possibility is that the conformational changes evoked by binding of IP3 expose a site to which Ca2+ must bind before the channel can open (Marchant & Taylor, 1997; Foskett et al. 2007). However, neither the structural identity of this stimulatory Ca2+‐binding site, nor that of the inhibitory site through which higher concentrations of Ca2+ inhibit IP3Rs have been resolved. The inhibitory site may reside on an accessory protein associated with IP3Rs.
Figure 1. Association of proteins with IP3Rs .
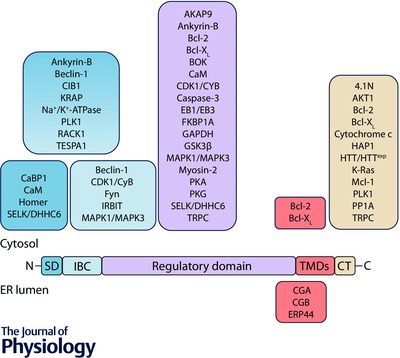
Key functional domains of a single IP3R subunit are shown: the suppressor domain (SD), IP3‐binding core (IBC), cytosolic regulatory domain, transmembrane domains (TMDs) and the cytosolic C‐terminus (CT). The sites to which proteins are proposed to bind are shown. Many additional proteins are thought to associate with IP3Rs, but the binding sites have not been identified. Abbreviations and references are provided in Tables 1, 2, 3, 4.
IP3Rs are present in almost all animal cells and some protozoa (Prole & Taylor, 2011), but there are no homologous proteins in plants (Wheeler & Brownlee, 2008) or fungi (Prole & Taylor, 2012). The genomes of vertebrates encode three subtypes of IP3R subunit (IP3R1–3), which can form homo‐tetrameric or hetero‐tetrameric channels (Joseph et al. 1995) with differing properties and distributions (Foskett et al. 2007; Mikoshiba, 2007). In mammalian cells, IP3Rs have been reported to release Ca2+ mainly from the endoplasmic reticulum (ER) (Streb et al. 1984; Volpe et al. 1985), but the Golgi apparatus (Pinton et al. 1998) and secretory vesicles (Yoo, 2011) also respond to IP3. Although IP3 initiates Ca2+ signals by stimulating Ca2+ release from intracellular stores, the signals are sustained by Ca2+ entry across the plasma membrane. That too is indirectly regulated by IP3, because store‐operated Ca2+ entry is stimulated by loss of Ca2+ from the ER (Parekh & Putney, 2005; Lewis, 2012). Ca2+ signals initiated by IP3Rs evoke a wide variety of cellular events, ranging from embryological development (Kume et al. 1997; Uchida et al. 2010) to cellular metabolism (Cardenas et al. 2010), gluconeogenesis (Wang et al. 2012), exocrine secretion (Futatsugi et al. 2005) and neuronal function (Matsumoto et al. 1996).
Specificity within Ca2+ signalling pathways, or indeed any signalling pathway (Scott & Pawson, 2009; Scott et al. 2013), is achieved, in part, by the formation of macromolecular signalling complexes. Within the signalling pathways that involve phospholipase C (PLC), these complexes regulate the activity of IP3Rs, their distribution, and their association with both the plasma membrane receptors that evoke IP3 formation and the downstream targets of the Ca2+ released by IP3Rs (Konieczny et al. 2012). The interactions of IP3Rs with other proteins have been reviewed previously (Choe & Ehrlich, 2006; Foskett et al. 2007; Mikoshiba, 2007; Vanderheyden et al. 2009 a), but continued progress and the advent of high‐throughput proteomics methods (Havugimana et al. 2012; Rolland et al. 2014) suggest that an update is timely.
Searches of proteomic databases and published literature reveal a large number of proteins that form complexes with IP3Rs (Tables 1, 2, 3, 4). For some of these proteins, the regions within IP3Rs that are important for the interaction have been mapped (Fig. 1). At the outset, it is worth sounding some notes of caution regarding the reported interactions. Firstly, it is often difficult to establish that two proteins interact directly, rather than via intermediate proteins. Many of these complexes may, therefore, be formed by direct or indirect interactions of IP3Rs with other proteins. For example, association of protein phosphatase 1 with IP3Rs may be mediated in part by IRBIT (IP3R‐binding protein released with IP3), which binds directly to both proteins (Ando et al. 2014). Secondly, the interactions and their effects may depend on the cellular context, including such factors as the subtype of IP3R, the physiological status of the IP3R (e.g. phosphorylation), the cell type and the expression levels of the interacting proteins and IP3Rs. Thirdly, interactions that occur in cellular lysates may be precluded within intact cells. For example, the interaction of two proteins may be prevented by their physical separation within the cell or by mutually exclusive binding of other proteins or ligands. IRBIT, for example, binds to IP3R subunits only when they have no IP3 bound. Lastly, some forms of experimental evidence are more discriminating than others, and it will be necessary to verify the putative interactions indicated by methods such as yeast two‐hybrid screening and mass spectrometry.
Table 1.
Proteins that form complexes with IP3Rs and enhance their activity
| Protein | References |
|---|---|
| Effective delivery of messengers | |
| Adenylyl cyclase 6 (AC6) | Tovey et al. 2008 |
| Bradykinin receptor B2 (B2R) | Delmas et al. 2002; Jin et al. 2013 |
| Epidermal growth factor receptor (EGFR) | Hur et al. 2005 |
| Erythropoietin receptor (EPO‐R) | Tong et al. 2004 |
| Glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) | Patterson et al. 2005 |
| Metabotropic glutamate receptor 1 (mGluR1;GRM1) | Tu et al. 1998 |
| Phospholipase C‐β1 (PLCβ1) | Shin et al. 2000 |
| Phospholipase C‐β4 (PLCβ4) | Nakamura et al. 2004 |
| Phospholipase C‐γ1 (PLCγ1) | Tong et al. 2004; Yuan et al. 2005 |
| Protease‐activated receptor 2 (PAR‐2) | Jin et al. 2013 |
| Sensitization to IP3/Ca2 + | |
| Bcl‐2 (B‐cell lymphoma 2)a | Chen et al. 2004; Eckenrode et al. 2010; Monaco et al. 2012; Chang et al. 2014 |
| Bcl‐XL (B‐cell lymphoma extra large) | White et al. 2005; Eckenrode et al. 2010; Monaco et al. 2012 |
| Chromogranin A (CGA) | Yoo & Lewis, 1998; Thrower et al. 2002 |
| Chromogranin B (CGB; secretogranin‐1) | Yoo & Lewis, 2000; Thrower et al. 2003 |
| Cyclin‐A | Soghoian et al. 2005 |
| Cyclin‐B1 (CYB) | Malathi et al. 2003; Malathi et al. 2005 |
| Cyclin‐dependent kinase 1 (CDK1) | Malathi et al. 2003; Malathi et al. 2005 |
| Cytochrome c 1 | Boehning et al. 2004 |
| Fyn (tyrosine‐protein kinase) | Jayaraman et al. 1996; Cui et al. 2004 |
| Glucosidase 2 subunit β (80K‐H) | Kawaai et al. 2009 |
| Glycogen synthase kinase‐3β (GSK3β) | Gomez et al. 2016 |
| Huntingtin‐associated protein 1 (HAP‐1) | Tang et al. 2003 b |
| Huntingtin (HTT) (with poly‐Q expansion, HTTexp)b | Tang et al. 2003 b |
| Lyn (tyrosine‐protein kinase) | Yokoyama et al. 2002 |
| Mcl‐1 (myeloid cell leukemia‐1) | Eckenrode et al. 2010 |
| mTOR (mammalian target of rapamycin) | Fregeau et al. 2011 |
| Neuronal Ca2+ sensor 1 (NCS‐1) | Schlecker et al. 2006 |
| Polo‐like kinase 1 (PLK1) | Ito et al. 2008; Vanderheyden et al. 2009 b |
| Presenilin‐1/Presenilin‐2 (PS‐1/PS‐2) | Cheung et al. 2008 |
| Protein kinase A (PKA; cAMP‐dependent protein kinase) | Ferris et al. 1991; Bruce et al. 2002 |
| Receptor of activated protein kinase C1 (RACK1) | Patterson et al. 2004 |
| Rho‐associated protein kinase (ROCK) | Singleton & Bourguignon, 2002 |
| TRISK 32 (cardiac triadin TRISK 32 isoform) | Olah et al. 2011 |
| Direct activation of IP3Rs | |
| Ca2+‐binding protein 1 (CaBP1)c | Yang et al. 2002; Li et al. 2013 |
| CIB1 (Ca2+ and integrin‐binding protein 1; calmyrin)c | White et al. 2006 |
| Gβγ complex | Shin et al. 2000; Zeng et al. 2003 |
| Other | |
| DARPP‐32 (protein phosphatase 1 regulatory subunit 1B) | Chang et al. 2014 |
| DHHC6 | Fredericks et al. 2014 |
| EB3 (end‐binding protein 3) | Geyer et al. 2015 |
| GRP‐78 (78 kDa glucose‐regulated protein; BiP) | Higo et al. 2010 |
| Phosphatidylinositol trisphosphate 3‐phosphatase (PTEN) | Bononi et al. 2013 |
| Selenoprotein K (SELK) | Fredericks et al. 2014 |
Data for Tables 1, 2, 3, 4 were derived from manual searches of the literature, reviews (Choe & Ehrlich, 2006; Foskett et al. 2007; Mikoshiba, 2007; Vanderheyden et al. 2009 a) and databases, including BioGRID (Chatr‐Aryamontri et al. 2015) and IntAct (Orchard et al. 2013). The nomenclature of proteins shown is consistent with the human homologues, although some data are derived from interactions of IP3Rs and proteins from other species. aSome studies report sensitization of IP3Rs by Bcl‐2, while others report inhibition. bHTTexp, but not wild‐type HTT, sensitizes IP3Rs to IP3/Ca2+. cCaBP1 and CIB1 are also reported to inhibit IP3Rs (see Table 2); direct activation seems to occur only transiently, and is controversial.
Table 2.
Proteins that form complexes with IP3Rs and inhibit their activity
| Protein | References |
|---|---|
| Proteins that bind reversibly and disrupt activation by IP3 and/or Ca2+ | |
| Ankyrin‐R (ANK1) | Bourguignon et al. 1993; Joseph & Samanta, 1993 |
| Bcl‐2 (B‐cell lymphoma 2)a | Chen et al. 2004; Monaco et al. 2012; Chang et al. 2014 |
| Ca2+‐binding protein 1 (CaBP1)b | Yang et al. 2002; Li et al. 2013 |
| Calmodulin (CaM) | Maeda et al. 1991; Yamada et al. 1995 |
| Carbonic anhydrase‐related protein (CARP; CA8) | Hirota et al. 2003 |
| Caspase‐3 | Hirota et al. 1999 |
| CIB1 (Ca2+ and integrin‐binding protein 1; calmyrin)b | White et al. 2006 |
| DANGER (IP3R‐interacting protein) | van Rossum et al. 2006 |
| ERp44 (endoplasmic reticulum resident protein 44) | Higo et al. 2005 |
| FKBP1A (FK506‐binding protein 1A; FKBP12) | Cameron et al. 1995 b |
| GIT1/GIT2 (ARF GTPase‐activating protein 1/2) | Zhang et al. 2009 |
| IRBIT (IP3‐binding protein released with IP3) | Ando et al. 2003 |
| K‐Ras | Sung et al. 2013 |
| MRVI1 (IRAG; IP3R‐associated cGMP kinase substrate) | Schlossman et al. 2000 |
| Nuclear protein localization protein 4 homologue (NPL4) | Alzayady et al. 2005 |
| Polycystin‐1 (PC1; TRPP1) | Li et al. 2005 |
| Proteins that post‐translationally modify IP3Rs | |
| AKT1 (RAC‐α serine/threonine protein kinase; PKB) | Khan et al. 2006; Szado et al. 2008 |
| Ca2+/calmodulin‐dependent protein kinase II (CaMKII) | Ferris et al. 1991; Bare et al. 2005 |
| Calpain | Μagnusson et al. 1993; Wojcikiewicz & Oberdorf, 1996 |
| E3 ubiquitin ligase AMFR (GP78)c | Pearce et al. 2007 |
| E3 ubiquitin ligase RNF170c | Lu et al. 2011 |
| Erlin‐1/Erlin‐2 (SPFH domain‐containing protein 1/2)c | Pearce et al. 2007; Pearce et al. 2009 |
| MAPK1/MAPK3 (mitogen‐activated protein kinase 1/3) | Bai et al. 2006 |
| Protein phosphatase 1A (PP1A) | Tang et al. 2003 a; Chang et al. 2014 |
| Transglutaminase‐2 (TGM2) | Hamada et al. 2014 |
| Transitional endoplasmic reticulum ATPase (p97)c | Alzayady et al. 2005 |
| Ubiquitinc | Bokkala & Joseph, 1997; Oberdorf et al. 1999 |
| Ubiquitin‐conjugating enzyme E2 7 (UBC7)c | Webster et al. 2003 |
| Ubiquitin conjugation factor E4A (UFD2)c | Alzayady et al. 2005 |
| Ubiquitin fusion degradation 1 protein (UFD1)c | Alzayady et al. 2005 |
Table 3.
Proteins that form complexes with IP3Rs and act as downstream effectors
| Protein | References |
|---|---|
| Anoctamin‐1 (ANO1, Ca2+‐activated Cl− channel) | Jin et al. 2013 |
| Calcineurin (CN; protein phosphatase 2B) | Cameron et al. 1995 a; Chang et al. 2014 |
| CASK (Ca2+/calmodulin‐dependent serine protein kinase) | Maximov et al. 2003 |
| CRTC2 (CREB‐regulated transcription coactivator 2) | Wang et al. 2012 |
| IRBIT (IP3‐binding protein released with IP3)a | Ando et al. 2003 |
| KCa1.1 (BKCa; large conductance Ca2+‐activated K+ channel) | Zhao et al. 2010; Mound et al. 2013 |
| Na+/Ca2+ exchanger 1 (NCX1) | Lencesova et al. 2004; Mohler et al. 2005 |
| Orai‐1 (Ca2+ release‐activated Ca2+ channel 1) | Woodard et al. 2010; Lur et al. 2011 |
| Plasma membrane Ca2+ ATPase (PMCA) | Shin et al. 2000; Huang et al. 2006 |
| Protein kinase C (PKC) | Ferris et al. 1991; Rex et al. 2010 |
| SERCA 2B/3 (sarco/endoplasmic reticulum Ca2+‐ATPase) | Redondo et al. 2008 |
| STIM1 (stromal interaction molecule 1) | Santoso et al. 2011 |
| TRPC1‐7 (transient receptor potential canonical channels) | Boulay et al. 1999; Mery et al. 2001; Tang et al. 2001; Yuan et al. 2003; Tong et al. 2004 |
| VDAC1 (voltage‐dependent anion channel 1) | Szabadkai et al. 2006 |
IRBIT also inhibits IP3Rs by occluding the IP3‐binding site (Table 2).
Table 4.
Other proteins that form complexes with IP3Rs
| Protein | References |
|---|---|
| Cytoskeletal, scaffolding and adaptor proteins | |
| 14‐3‐3 protein zeta/delta (PKC inhibitor protein 1) | Angrand et al. 2006 |
| α‐Actin | Sugiyama et al. 2000 |
| Ankyrin‐B (ANK2) | Hayashi & Su, 2001; Mohler et al. 2004; Kline et al. 2008 |
| AKAP9 (A‐kinase anchor protein 9; Yotiao) | Tu et al. 2004 |
| BANK1 (B‐cell scaffold protein with ankyrin repeats) | Yokoyama et al. 2002 |
| Caveolin‐1 | Murata et al. 2007; Sundivakkam et al. 2009; Jin et al. 2013 |
| Coiled‐coil domain‐containing protein 8 | Hanson et al. 2014 |
| Homer 1/2/3 | Tu et al. 1998 |
| EB1 / EB3 (end‐binding protein 1/3)a | Geyer et al. 2015 |
| KRAP (K‐Ras‐induced actin‐interacting protein) | Fujimoto et al. 2011 |
| LAT (linker of activated T‐cells) | deSouza et al. 2007 |
| Myosin‐2A | Walker et al. 2002; Hours & Mery, 2010 |
| Obscurin‐like protein 1 | Hanson et al. 2014 |
| Protein 4.1N (band 4.1‐like protein 1) | Maximov et al. 2003 |
| SEC8 (exocyst complex component) | Shin et al. 2000 |
| SNAP‐29 (synaptosomal‐associated protein 29) | Huttlin et al. 2013 |
| α‐Spectrin/β‐spectrin (α/β‐fodrin) | Lencesova et al. 2004 |
| Syntaxin 1B | Tanaka et al. 2011 |
| Talin | Sugiyama et al. 2000 |
| Vimentin | Dingli et al. 2012 |
| Vinculin | Sugiyama et al. 2000 |
| Other proteins | |
| Anaplastic lymphoma kinase (ALK) | Crockett et al. 2004 |
| ARHGAP1 (Rho GTPase‐activating protein 1) | Nagaraja & Kandpal, 2004 |
| γ‐BBH (γ‐butyrobetaine dioxygenase) | Huttlin et al. 2013 |
| Beclin‐1 | Vicencio et al. 2009 |
| BOK (Bcl‐2‐related ovarian killer protein) | Schulman et al. 2013 |
| Calnexin | Joseph et al. 1999 |
| CD44 antigen (heparin sulphate proteoglycan) | Singleton & Bourguignon, 2004 |
| CEMIP (cell migration‐inducing and hyaluronan‐binding protein) | Tiwari et al. 2013 |
| Cyclophilin D (peptidyl‐prolyl cis‐trans isomerase F) | Paillard et al. 2013 |
| FAM19A4 (chemokine‐like protein TAFA‐4) | Huttlin et al. 2013 |
| F‐box and leucine‐rich repeat protein 14 | Huttlin et al. 2013 |
| FGL2 (fibrinogen‐like 2) | Huttlin et al. 2013 |
| FERM domain‐containing 1 | Huttlin et al. 2013 |
| GluRδ2 (ionotropic glutamate receptor δ2) | Nakamura et al. 2004 |
| Golgi anti‐apoptotic protein (GAAP; Lifeguard 4; TMBIM4) | de Mattia et al. 2009 |
| GRP‐75 (glucose‐regulated protein 75; stress‐70 protein) | Szabadkai et al. 2006 |
| Heat shock protein 90 (HSP90) | Nguyen et al. 2009 |
| Junctate | Treves et al. 2004 |
| Lethal(3)malignant brain tumor‐like protein 2 | Huttlin et al. 2013 |
| Lymphoid‐restricted membrane protein (LRMP; JAW1) | Shindo et al. 2010 |
| Na+/K+‐transporting ATPase | Mohler et al. 2005; Yuan et al. 2005 |
| Neuronal acetylcholine receptor α3 | Huttlin et al. 2013 |
| PASK (PAS domain‐containing protein kinase) | Schlafli et al. 2011 |
| Phospholamban | Koller et al. 2003 |
| Polycystin‐2 (PC2; TRPP2) | Li et al. 2005 |
| Protein kinase G1 (PKG1; cGMP‐dependent protein kinase 1) | Schlossman et al. 2000 |
| PTPα (protein tyrosine phosphatase‐α) | Wang et al. 2009 |
| Rab29 (Ras‐related protein Rab7L1) | Huttlin et al. 2013 |
| Rac1 (Ras‐related C3 botulinum toxin substrate 1; TC25) | Natsvlishvili et al. 2015 |
| RhoA | Mehta et al. 2003 |
| Sigma 1 receptor (σ1R) | Hayashi & Su, 2001; Natsvlishvili et al. 2015 |
| Sirtuin‐7 | Tsai et al. 2012 |
| c‐Src (proto‐oncogene tyrosine‐protein kinase Src) | Jayaraman et al. 1996; Wang et al. 2009 |
| STARD13 (StAR‐related lipid transfer protein 13; RhoGAP) | Nagaraja & Kandpal, 2004 |
| Syndecan‐1 (SYND1; CD138) | Maximov et al. 2003 |
| TESPA1 (thymocyte‐expressed positive selection‐associated protein 1) | Matsuzaki et al. 2012 |
Although we focus on the ability of IP3Rs to release Ca2+ from intracellular stores, IP3Rs have additional roles. For example, binding of IP3 is proposed to release IRBIT from the IP3‐binding site, freeing IRBIT to regulate additional targets that include ion channels, transporters and the enzyme ribonucleotide reductase (Ando et al. 2014; Arnaoutov & Dasso, 2014). IP3Rs may also regulate associated proteins independently of their ability to release Ca2+. For example, a direct interaction between IP3Rs and TRPC (transient receptor potential canonical) channels is proposed to stimulate opening of the latter (Zhang et al. 2001). Hence, when reviewing the effects of proteins associated with IP3Rs, we should look beyond the effects of IP3 on cytosolic Ca2+ signals, to consider also consequences within the ER lumen, effects on Ca2+ entry, and effects unrelated to Ca2+ signalling. That scope is too ambitious for this short review. Instead we provide a comprehensive summary of proteins suggested to interact with IP3Rs (Tables 1, 2, 3, 4, within which we provide most references) and then explore a few selected examples to illustrate some general features.
Signalling complexes containing IP3Rs span entire signalling pathways
The sheer number of proteins reported to form complexes with IP3Rs is striking and so too is their diversity, in terms of both cellular geography and function (Tables 1, 2, 3, 4). IP3Rs form complexes with many of the proteins that link extracellular stimuli to formation of IP3, including G protein‐coupled receptors (GPCRs), the epidermal growth factor receptor (EGFR), the erythropoietin receptor, the Gβγ complexes of G proteins, and some forms of PLC. IP3Rs also associate with other signalling proteins linked to PLC signalling, including protein kinase C (PKC), RACK1 (receptor of activated PKC) and the phosphoinositide phosphatase PTEN. The interactions extend also to proteins from other signalling pathways, including adenylyl cyclase (AC), the small G protein K‐Ras, and the protein kinases AKT1 (RAC‐α serine/threonine protein kinase), mTOR (mammalian target of rapamycin), c‐Src and MAPK1/MAPK3 (mitogen‐activated protein kinase 1/3) (Tables 1, 2, 3, 4 and Fig. 1). Proteins that respond to the Ca2+ released by IP3Rs also form complexes with IP3Rs. These include ion channels, exchangers and pumps within the plasma membrane. It is clear that IP3Rs reside within macromolecular complexes that both span entire signalling pathways from cell‐surface receptors to the effectors that respond to Ca2+, and include proteins that integrate signals from other signalling pathways.
The advantages of these signalling complexes are clear. They allow information to be directed selectively from specific extracellular stimuli to specific intracellular targets through conserved signalling pathways. Furthermore, associated proteins can integrate signals from different signalling pathways and so modulate traffic through the complex. Hence, protein complexes confer both specificity and plasticity. A third advantage is speed. Signalling pathways must be able to turn on and off quickly. Fast activation benefits from high concentrations of reactants and fast on‐rates (k 1) for association of messengers with their targets. Rapid de‐activation requires rapid destruction or dissipation of the messenger and a fast dissociation rate (k −1). By facilitating delivery of messengers at high local concentrations to their targets (e.g. IP3 to IP3Rs), signalling complexes contribute to both rapid activation and de‐activation, the latter because diffusion of messengers away from the site of delivery may be sufficient to allow their concentration to fall below that required for activation as soon as synthesis of the messenger ceases. Secondly, targets can have fast off‐rates (k −1) with a corresponding loss of affinity (equilibrium association constant, K A = k 1/k −1) that does not compromise their capacity to respond to high local concentrations of messenger. We suggest, then, that assembly of proteins around IP3Rs contributes to fast and specific signalling, while providing opportunities for signal integration and plasticity.
For convenience, we consider the proteins that associate with IP3Rs under four somewhat arbitrary (and overlapping) headings: proteins that enhance or inhibit the activity of IP3Rs (Tables 1 and 2); proteins that respond to Ca2+ released by IP3Rs (Table 3); and proteins with more general roles, including those associated with movement of IP3Rs (Table 4).
Proteins that enhance the function of IP3Rs
Usually, IP3Rs open only when they have bound both IP3 and Ca2+ (Foskett et al. 2007; Taylor & Tovey, 2010). Unsurprisingly, therefore, most of the proteins that associate with IP3Rs and enhance their activity do so either by allowing more effective delivery of IP3 and/or Ca2+ to IP3Rs, or by enhancing the responsiveness of IP3Rs to IP3 and/or Ca2+ (Table 1).
The association of IP3Rs with GPCRs, EGFR and erythropoietin receptors, with the βγ subunits of G proteins, with some isoforms of PLC, and with scaffold proteins, like Homer 1 that tethers IP3Rs to metabotropic glutamate receptors and PLC (Tu et al. 1998), suggest mechanisms by which receptors may effectively deliver IP3 to specific IP3Rs. This targeted delivery of IP3 provides two advantages: it allows rapid responses and it may allow spatially organized Ca2+ signals to retain an ‘imprint’ of the stimulus that evoked them. Bradykinin B2 receptors (B2Rs) are a well‐defined example. In sympathetic neurons, both muscarinic M1 receptors (M1Rs) and B2Rs activate PLC, but only activation of B2Rs evokes Ca2+ release through IP3Rs (Delmas et al. 2002). This selectivity arises because B2Rs, but not M1Rs, form complexes with IP3Rs. Rapid generation of IP3 in response to activation of B2Rs thereby generates relatively high concentrations of IP3 in the vicinity of IP3Rs, which are not achieved by the more distant M1Rs. In this case, selective coupling between plasma membrane receptors and IP3Rs may allow sympathetic neurons to generate different intracellular responses to pro‐inflammatory and cholinergic inputs.
Rather than enhancing the delivery of IP3 to IP3Rs, many other proteins sensitize IP3Rs to prevailing concentrations of IP3 and/or Ca2+ (Table 1). An example, which may play an important role in human disease, is the sensitization of IP3Rs by mutant forms of presenilins (Cheung et al. 2008). Mutations in presenilin‐1 (PS1) and presenilin‐2 (PS2) are major causes of familial Alzheimer's disease. Although both wild‐type and mutant presenilins associate with IP3Rs, only the disease‐causing mutant forms of PS1 and PS2 enhance the activity of IP3Rs in response to IP3 and Ca2+. The mechanism involved may be a change in the modal gating of IP3Rs (Cheung et al. 2010). This increased activity of IP3Rs results in enhanced release of Ca2+, which may lead to aberrant processing of β‐amyloid (Cheung et al. 2008), constitutive activation of cyclic AMP response element binding protein (CREB)‐mediated transcription (Muller et al. 2011), synaptic dysfunction and neuronal degeneration (Mattson, 2010).
Although activation of IP3Rs normally requires binding of IP3 and Ca2+, a few proteins have been reported to cause reversible activation of IP3Rs directly, without the coincident presence of IP3 and Ca2+ (Table 1). These include Gβγ (Zeng et al. 2003), CIB1 (White et al. 2006) and, more controversially, CaBP1 (Yang et al. 2002). The initial report on the actions of CaBP1 described an activation of Xenopus IP3Rs in the absence of IP3 in vitro. However, subsequent studies have demonstrated that CaBP1 inhibits Ca2+ release via mammalian and Xenopus IP3Rs by stabilizing an inactive state of the IP3R (Haynes et al. 2004; Nadif Kasri et al. 2004; White et al. 2006; Li et al. 2013). Similarly, CIB1 was reported to activate IP3Rs in Xenopus oocytes and Sf9 insect cells in the absence of IP3, but it too inhibits Ca2+ release via mammalian IP3Rs (White et al. 2006). Uniquely, an irreversible activation of IP3Rs appears to occur after proteolytic cleavage by caspase‐3 (Assefa et al. 2004; Nakayama et al. 2004), a process that may play a prominent role in apoptosis.
Proteins that inhibit the function of IP3Rs
Many proteins that interact with IP3Rs inhibit their function (Table 2). These interactions may enable rapid feedback regulation of Ca2+ release and provide long‐term attenuation of IP3R activity by promoting degradation or irreversible inhibition of IP3Rs. These mechanisms contribute to the tight regulation of IP3R activity needed to achieve spatial and temporal organization of Ca2+ signals (Konieczny et al. 2012). They also provide protection from the damaging consequences of excessive increases in cytosolic free Ca2+ concentration (Orrenius et al. 2015) and disturbance of the other essential roles of the ER while it fulfils its role in Ca2+ signalling (Berridge, 2002). Proteins that inhibit IP3Rs in a Ca2+‐dependent manner, like calmodulin, CaBP1, calcineurin, CaMKII and the unidentified protein(s) that may mediate the universal inhibition of IP3Rs by Ca2+, are prime candidates for mediating this negative feedback. Proteins that inhibit IP3Rs fall into two broad categories: those that bind reversibly to interfere with binding of IP3 and/or Ca2+ or their links to gating; and those that cause post‐translational modifications of the IP3R (Table 2).
IRBIT inhibits all three IP3R subtypes by competing with IP3 for binding to the IBC (Ando et al. 2003). IRBIT binds only when it is phosphorylated at several sites, probably because the phosphorylated residues mimic the essential phosphate groups of IP3 (Fig. 2 A). Residue S68 is the ‘master’ phosphorylation site. When it is phosphorylated by a Ca2+‐dependent kinase, perhaps a Ca2+/calmodulin‐dependent protein kinase (CaMK), it allows casein kinase I‐mediated phosphorylation of the two residues (S71 and S74, residue numbering relates to mouse IP3R1) that are critical for binding of IRBIT to IP3Rs (and its other targets) (Ando et al. 2014). Dephosphorylation of S68 is catalysed by protein phosphatase 1 (PP1), which also associates with IRBIT. The competition between phospho‐IRBIT and IP3 for occupancy of the IBC through which IP3 initiates activation of IP3Rs allows IRBIT to tune the sensitivity of IP3Rs to IP3. Hence, inhibiting expression of IRBIT, or expression of a dominant negative form (IRBIT‐S68A), allows Ca2+ release at lower concentrations of IP3 (Ando et al. 2014). This tuning of IP3R sensitivity has been demonstrated in sympathetic neurons where, as discussed earlier, M1Rs do not associate with IP3Rs and do not normally generate sufficient IP3 to activate more distant IP3Rs (Delmas et al. 2002). However, expression of the dominant negative IRBIT allows M1Rs to evoke Ca2+ release through IP3Rs (Zaika et al. 2011). Although the details are not fully resolved, the interplay between Ca2+ and the activation of IRBIT is intriguing because it suggests potential feedback loops that might control the sensitivity of IP3Rs to IP3 (Ando et al. 2014). The phosphorylation (of S68) that initiates activation of IRBIT is Ca2+ sensitive, deactivation of IRBIT by proteolytic cleavage within its N‐terminal may be mediated by Ca2+‐sensitive calpain, and IRBIT itself inhibits Ca2+/calmodulin‐dependent protein kinase IIα (CaMKIIα) (Kawaai et al. 2015) (Fig. 2 B).
Figure 2. IRBIT controls the sensitivity of IP3Rs .
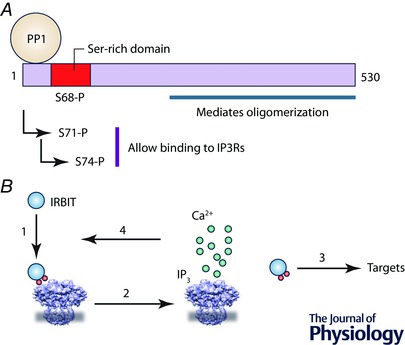
A, the N‐terminal region of IRBIT includes a serine‐rich domain. Phosphorylation of S68, the ‘master’ phosphorylation site, allows sequential phosphorylation of the two residues, S71 and S74, that must be phosphorylated for IRBIT to bind to IP3Rs. Protein phosphatase 1 (PP1) bound to IRBIT dephosphorylates S68. B, phosphorylation of IRBIT (1) allows it to bind to the IBC and so compete with IP3 for binding to the IP3R. Phospho‐IRBIT thereby sets the sensitivity of the IP3R to IP3. IP3 binding to the IBC (2) prevents IRBIT binding and initiates activation of the IP3R. The displaced phospho‐IRBIT can regulate many additional targets, including ion channels and transporters (3). The Ca2+ released by active IP3Rs may control the phosphorylation state of IRBIT, and thereby complete a feedback loop that regulates IP3R sensitivity (4).
Post‐translational modification of IP3Rs by associated proteins may be reversible (e.g. phosphorylation) (Betzenhauser & Yule, 2010) or irreversible (e.g. proteolysis and some covalent modifications). An example of the latter is the Ca2+‐dependent enzyme transglutaminase type 2 (TGM2). By covalently modifying a glutamine residue within the C‐terminal tail of IP3R1, TGM2 causes irreversible cross‐linking of adjacent IP3R subunits via a lysine residue and the modified glutamine. This prevents the conformational changes required for activation of IP3Rs, and so inhibits IP3‐evoked Ca2+ release (Hamada et al. 2014). The Ca2+ sensitivity of TGM2 may allow it to contribute to feedback control of Ca2+ release and to disruption of IP3R function when dysregulation of Ca2+ signalling occurs in pathological conditions such as Huntington's disease (Hamada et al. 2014). Activation of IP3Rs and the ensuing release of Ca2+ also trigger ubiquitination and proteasomal degradation of IP3Rs (Pearce et al. 2009) and their cleavage by calpains (Μagnusson et al. 1993; Wojcikiewicz & Oberdorf, 1996). Hence, proteins that associate with IP3Rs provide mechanisms that allow both acute and long‐term feedback regulation of IP3R activity.
Downstream effectors
IP3Rs also form complexes with proteins that are downstream effectors of IP3R activation; most of these respond to the Ca2+ released by IP3Rs (Table 3). Many of these proteins are cytosolic, but others reside within membranes that allow IP3Rs within the ER to communicate with other intracellular organelles or the plasma membrane. The importance of this communication between organelles, mediated by junctional complexes between them, is increasingly recognized (Lam & Galione, 2013).
Hepatic gluconeogenesis, which is likely to play an important role in diabetes and obesity, is stimulated by glucagon released by the pancreas during fasting, and inhibited by insulin released when the plasma glucose concentration increases. A complex containing IP3Rs, the Ca2+‐regulated protein phosphatase calcineurin, the transcriptional co‐activator of CREB‐regulated transcription CRTC2 (CREB‐coactivator C2), PKA and AKT1 coordinates gluconeogenesis (Wang et al. 2012) (Fig. 3). De‐phosphorylated CRTC2 binds to nuclear CREB and up‐regulates genes that promote gluconeogenesis. This is repressed by SIK2, a kinase that phosphorylates CRTC2. IP3‐evoked Ca2+ release activates calcineurin, which de‐phosphorylates CRTC2. Glucagon receptors stimulate production of both cAMP and IP3 (Wakelam et al. 1986; Wang et al. 2012). The cAMP activates PKA, which phosphorylates, and thereby inhibits, SIK2; and it phosphorylates IP3Rs, sensitizing them to activation by IP3 and Ca2+. IP3Rs are also directly sensitized by cAMP (Tovey et al. 2008). Increased release of Ca2+ via IP3Rs activates calcineurin, which dephosphorylates CRTC2 (Vanderheyden et al. 2009 a; Wang et al. 2012). Hence glucagon both inhibits the kinase (SIK2) and stimulates the phosphatase (calcineurin) that control phosphorylation of CRTC2. Glucagon also reduces binding of CRTC2 to IP3Rs (Wang et al. 2012), further enhancing the nuclear translocation of dephosphorylated CRTC2. The signals evoked by insulin receptors also feed into this IP3R complex. Insulin stimulates phosphatidylinositol 3‐kinase (PI3K) and thereby AKT1. The latter phosphorylates IP3Rs and attenuates their activity. Hence insulin, by inhibiting IP3Rs, opposes the actions of glucagon by restraining the activation of calcineurin and so maintains CRTC2 in its inactive phosphorylated state (Wang et al. 2012). This example illustrates some of the intricate interactions that the assembly of proteins around IP3Rs can allow: signals from a GPCR and a receptor tyrosine kinase converge at IP3Rs, which then integrate the inputs and transduce them into a regulation of gene expression (Fig. 3).
Figure 3. A signalling complex assembled around IP3Rs controls gluconeogenesis .

Glucagon and insulin exert opposing effects on hepatic gluconeogenesis. Their signalling pathways converge to a protein complex assembled around IP3Rs, the activity of which controls phosphorylation of the transcription factor CRTC2. Dephosphorylated CRTC2 translocates to the nucleus, where it associates with CREB and stimulates transcription of genes required for gluconeogenesis. SIK2 phosphorylates CRTC2, while calcineurin dephosphorylates it. Glucagon, via a GPCR, stimulates both PLC and AC. The IP3 produced by PLC stimulates IP3Rs. The cAMP generated by AC stimulates PKA and that promotes dephosphorylation of CRTC2 by phosphorylating both SIK2 (inhibiting its activity) and IP3Rs, sensitizing the latter to IP3. The larger Ca2+ signal then activates calcineurin. Insulin causes activation of AKT1, which phosphorylates IP3Rs and inhibits their activity; it thereby opposes the effects of glucagon and attenuates calcineurin activity. Phosphorylation is indicated by red circles, black arrows denote stimulation and the red arrow denotes inhibition. Abbreviations and further details in the text and tables.
Proteins that determine the distribution of IP3Rs
The subcellular distribution of IP3Rs is an important influence on their behaviour, not least because it defines the sites at which they will release Ca2+, and whether they will be exposed to effective concentrations of the stimuli that activate them, IP3 and Ca2+. Assembly of IP3Rs with components of the PLC signalling pathway (see above) can ensure targeted delivery of IP3, but Ca2+ is most often provided by neighbouring IP3Rs. An important interaction, therefore, is that between IP3Rs themselves, because their proximity to neighbours dictates whether Ca2+ released by an active IP3R can ignite the activity of other IP3Rs. Considerable evidence suggests that clustering of IP3Rs within the plane of the ER membrane is dynamically regulated by IP3 and/or Ca2+ (Tateishi et al. 2005; Rahman et al. 2009; and see references in Geyer et al. 2015), although the role of this process in shaping Ca2+ signals remains controversial (Smith et al. 2014). We have suggested that IP3‐evoked clustering of IP3Rs may contribute to the coordinated openings of IP3Rs that underlie the small Ca2+ signals (‘Ca2+ puffs’) evoked by low stimulus intensities, by both bringing IP3Rs together and retuning their Ca2+ sensitivity (Rahman et al. 2009). Head‐to‐head interactions of IP3Rs have also been observed in electron micrographs of purified IP3Rs (Hamada et al. 2003), between opposing ER membranes within cells (Takei et al. 1994) and between the isolated N‐terminal domains of IP3Rs (Chavda et al. 2013). The functional significance of these interactions has not been established.
A recent study of the Ca2+ signals evoked by thrombin‐mediated stimulation of the protease‐activated receptor PAR‐1 in endothelial cells provides evidence that microtubules may guide IP3Rs into the clusters within which Ca2+ release can most effectively recruit neighbouring IP3Rs (Geyer et al. 2015). In lung microvascular endothelial cells, thrombin, which activates PAR‐1 by cleaving its N‐terminal, stimulates PLC and thereby evokes Ca2+ release through IP3Rs. The resulting increase in cytosolic Ca2+ concentration contributes to disassembly of the adherens junctions that maintain the integrity of the endothelium (Komarova & Malik, 2010). These effects are attenuated when the interaction between type 3 IP3Rs (IP3R3) and end‐binding protein 3 (EB3) are disrupted. EB3 belongs to a family of proteins that bind to the plus‐end of growing microtubules and recruit other proteins, often via an S/TxIP motif (where x denotes any residue) (Honnappa et al. 2009). Mutation of the TxIP motif within the regulatory domain of IP3R3 prevents its binding to EB3, attenuates thrombin‐evoked Ca2+ signals, and reduces both the basal clustering of IP3R3 and the enhanced clustering evoked by thrombin. Hence, in endothelial cells, the association of IP3R3 with EB3 and microtubules is required for both clustering of IP3Rs and effective Ca2+ signalling. This suggests that clustering allows IP3Rs to deliver Ca2+ more effectively to other IP3Rs and so allows the amplification provided by Ca2+‐induced Ca2+ release (Fig. 4). We conclude that association of IP3Rs with other proteins, components of the PLC signalling pathway or EB3, contributes to effective delivery of the two essential regulators of IP3Rs, IP3 and Ca2+, respectively.
Figure 4. EB3 is required for effective signalling by IP3Rs in endothelial cells .
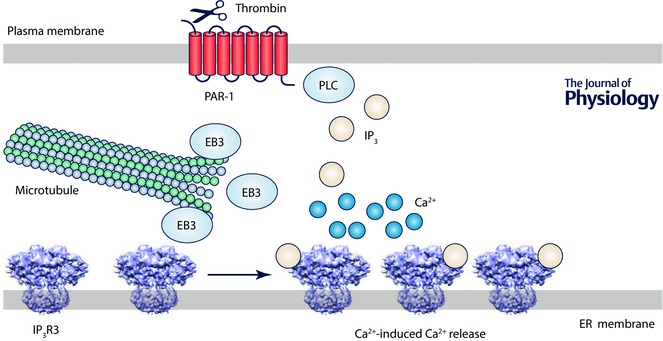
In endothelial cells, EB3 binds to a TxIP motif within the regulatory domain of IP3R3, allowing IP3Rs to associate with the plus‐end of microtubules. Disrupting this interaction prevents clustering of IP3Rs and attenuates the Ca2+ signals evoked by thrombin, which cleaves within the N‐terminus of PAR‐1 and allows it to stimulate PLC. The evidence (Geyer et al. 2015) suggests that the EB3‐mediated interaction of IP3R3 with microtubules is essential for the clustering of IP3Rs that allows the Ca2+ released by one IP3R to be amplified by recruitment of neighbouring IP3Rs.
Conclusions
IP3Rs and the Ca2+ they release are called upon to specifically regulate many physiological processes (Berridge, 2009), while neither perturbing the other essential roles of the ER (Berridge, 2002) nor subjecting the cell to the deleterious consequences of excessive increases in cytosolic Ca2+ concentration (Orrenius et al. 2015). These demands impose a need for complex regulation of IP3Rs, much of which is achieved by assembling proteins around IP3Rs to form signalling complexes (Konieczny et al. 2012). These complexes allow signals to be directed through conserved signalling pathways and endow the pathways with speed, integrative capacity and plasticity. The very large size of IP3Rs relative to most other ion channels might be viewed as an evolutionary adaptation to meet this need for them to function as signalling hubs.
Advances in genomics, proteomics, antibody technologies and bioinformatics have transformed analyses of protein–protein interactions. It is now possible to interrogate these interactions on a whole‐proteome scale (Havugimana et al. 2012; Rolland et al. 2014). Bioinformatic methods can predict protein–protein interactions (Baughman et al. 2011; Kotlyar et al. 2015) and even the regions of the proteins that are involved (Gavenonis et al. 2014). These powerful technologies, and the opportunities they provide to design new therapies (Wells & McClendon, 2007), cannot displace the need for direct confirmation of the interactions and their functional significance. Together, these approaches pave the way to defining the properties and functional importance of IP3R signalling hubs in normal physiology and disease.
Additional information
Competing interests
None declared.
Author contributions
All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was supported by the Biotechnology and Biological Sciences Research Council (L0000075) and the Wellcome Trust (101844).
Biographies
David Prole studied Natural Sciences at the University of Cambridge before exploring the structure and function of K+ channels during his PhD with Neil Marrion at the University of Bristol and HCN pacemaker channels during postdoctoral training with Gary Yellen at Harvard Medical School. After moving back to the University of Cambridge to work with Colin Taylor he held a Meres Research Associateship from St John's College and now explores the roles of ion channels in cell signalling.

Colin Taylor began his career as an insect physiologist with Mike Berridge, before moving into phosphoinositide and Ca2+ signalling during a postdoc with Jim Putney in Virginia. He is presently Professor of Cellular Pharmacology and a Wellcome Trust Senior Investigator in the Department of Pharmacology in Cambridge, where he continues to explore the workings of IP3 receptors.
This review was presented at the symposium “Molecular and Cellular Mechanisms in Health and Disease”, which took place at the Gordon Research Conference on Calcium Signalling ‐ Molecular and Cellular Mechanisms in Health and Disease in Maine, USA, 7–12 June, 2015.
References
- Alzayady KJ, Panning MM, Kelley GG & Wojcikiewicz RJ (2005). Involvement of the p97‐Ufd1‐Npl4 complex in the regulated endoplasmic reticulum‐associated degradation of inositol 1,4,5‐trisphosphate receptors. J Biol Chem 280, 34530–34537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando H, Kawaai K & Mikoshiba K (2014). IRBIT: A regulator of ion channels and ion transporters. Biochim Biophys Acta 1843, 2195–2204. [DOI] [PubMed] [Google Scholar]
- Ando H, Mizutani A, Matsu‐ura T & Mikoshiba K (2003). IRBIT, a novel inositol 1,4,5‐trisphosphate (IP3) receptor‐binding protein, is released from the IP3 receptor upon IP3 binding to the receptor. J Biol Chem 278, 10602–10612. [DOI] [PubMed] [Google Scholar]
- Angrand PO, Segura I, Volkel P, Ghidelli S, Terry R, Brajenovic M, Vintersten K, Klein R, Superti‐Furga G, Drewes G, Kuster B, Bouwmeester T & Acker‐Palmer A (2006). Transgenic mouse proteomics identifies new 14‐3‐3‐associated proteins involved in cytoskeletal rearrangements and cell signaling. Mol Cell Proteomics 5, 2211–2227. [DOI] [PubMed] [Google Scholar]
- Arnaoutov A & Dasso M (2014). Enzyme regulation. IRBIT is a novel regulator of ribonucleotide reductase in higher eukaryotes. Science 345, 1512–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assefa Z, Bultynck G, Szlufcik K, Nadif Kasri N, Vermassen E, Goris J, Missiaen L, Callewaert G, Parys JB & De Smedt H (2004). Caspase‐3‐induced truncation of type 1 inositol trisphosphate receptor accelerates apoptotic cell death and induces inositol trisphosphate‐independent calcium release during apoptosis. J Biol Chem 279, 43227–43236. [DOI] [PubMed] [Google Scholar]
- Bai GR, Yang LH, Huang XY & Sun FZ (2006). Inositol 1,4,5‐trisphosphate receptor type 1 phosphorylation and regulation by extracellular signal‐regulated kinase. Biochem Biophys Res Commun 348, 1319–1327. [DOI] [PubMed] [Google Scholar]
- Bare DJ, Kettlun CS, Liang M, Bers DM & Mignery GA (2005). Cardiac type‐2 inositol 1,4,5‐trisphosphate receptor: interaction and modulation by CaMKII. J Biol Chem 280, 15912–15920. [DOI] [PubMed] [Google Scholar]
- Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher‐Timme CA, Sancak Y, Bao XR, Strittmatter L, Goldberger O, Bogorad RL, Koteliansky V & Mootha VK (2011). Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 476, 341–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ (2002). The endoplasmic reticulum: a multifunctional signaling organelle. Cell Calcium 32, 235–249. [DOI] [PubMed] [Google Scholar]
- Berridge MJ (2009). Inositol trisphosphate and calcium signalling mechanisms. Biochim Biophys Acta 1793, 933–940. [DOI] [PubMed] [Google Scholar]
- Betzenhauser MJ & Yule DI (2010). Regulation of inositol 1,4,5‐trisphosphate receptors by phosphorylation and adenine nucleotides. Curr Top Membr 66, 273–298. [DOI] [PubMed] [Google Scholar]
- Boehning D, Patterson RL & Snyder SH (2004). Apoptosis and calcium: new roles for cytochrome c and inositol 1,4,5‐trisphosphate. Cell Cycle 3, 252–254. [PubMed] [Google Scholar]
- Bokkala S & Joseph SK (1997). Angiotensin II‐induced down‐regulation of inositol trisphosphate receptors in WB rat liver epithelial cells. Evidence for involvement of the proteasome pathway. J Biol Chem 272, 12454–12461. [DOI] [PubMed] [Google Scholar]
- Bononi A, Bonora M, Marchi S, Missiroli S, Poletti F, Giorgi C, Pandolfi PP & Pinton P (2013). Identification of PTEN at the ER and MAMs and its regulation of Ca2+ signaling and apoptosis in a protein phosphatase‐dependent manner. Cell Death Differ 20, 1631–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosanac I, Alattia J‐R, Mal TK, Chan J, Talarico S, Tong FK, Tong KI, Yoshikawa F, Furuichi T, Iwai M, Michikawa T, Mikoshiba K & Ikura M (2002). Structure of the inositol 1,4,5‐trisphosphate receptor binding core in complex with its ligand. Nature 420, 696–700. [DOI] [PubMed] [Google Scholar]
- Boulay G, Brown DM, Qin N, Jiang M, Dietrich A, Zhu MX, Chen Z, Birnbaumer M, Mikoshiba K & Birnbaumer L (1999). Modulation of Ca2+ entry by polypeptides of the inositol 1,4,5‐trisphosphate receptor (IP3R) that bind transient receptor potential (TRP): evidence for roles of TRP and IP3R in store depletion‐activated Ca2+ entry. Proc Natl Acad Sci USA 96, 14955–14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon LYW, Jin H, Iida N, Brandt NR & Zhang SH (1993). The involvement of ankyrin in the regulation of inositol 1,4,5‐trisphosphate receptor‐mediated internal Ca2+ release from the Ca2+ storage vesicles of mouse T‐lymphoma cells. J Biol Chem 268, 7290–7297. [PubMed] [Google Scholar]
- Bruce JIE, Shuttleworth TJ, Giovannucci DR & Yule DI (2002). Phosphorylation of inositol 1,4,5‐trisphosphate receptors in parotid acinar cells. A mechanism for the synergistic effects of cAMP on Ca2+ signaling. J Biol Chem 277, 1340–1348. [DOI] [PubMed] [Google Scholar]
- Cameron AM, Steiner JP, Roskams AJ, Ali SM, Ronnett GV & Snyder SH (1995. a). Calcineurin associated with the inositol 1,4,5‐trisphosphate receptor‐FKBP12 complex modulates Ca2+ flux. Cell 83, 463–472. [DOI] [PubMed] [Google Scholar]
- Cameron AM, Steiner JP, Sabatini DM, Kaplin AI, Walensky LD & Snyder SH (1995. b). Immunophilin FK506 binding protein associated with inositol 1,4,5‐trisphosphate receptor modulates calcium flux. Proc Natl Acad Sci USA 92, 1784–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas C, Miller RA, Smith I, Bui T, Molgo J, Muller M, Vais H, Cheung KH, Yang J, Parker I, Thompson CB, Birnbaum MJ, Hallows KR & Foskett JK (2010). Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell 142, 270–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang MJ, Zhong F, Lavik AR, Parys JB, Berridge MJ & Distelhorst CW (2014). Feedback regulation mediated by Bcl‐2 and DARPP‐32 regulates inositol 1,4,5‐trisphosphate receptor phosphorylation and promotes cell survival. Proc Natl Acad Sci USA 111, 1186–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatr‐Aryamontri A, Breitkreutz BJ, Oughtred R, Boucher L, Heinicke S, Chen D, Stark C, Breitkreutz A, Kolas N, O'Donnell L, Reguly T, Nixon J, Ramage L, Winter A, Sellam A, Chang C, Hirschman J, Theesfeld C, Rust J, Livstone MS, Dolinski K & Tyers M (2015). The BioGRID interaction database: 2015 update. Nucleic Acids Res 43, D470–D478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavda AP, Prole DL & Taylor CW (2013). A bead aggregation assay for detection of low‐affinity protein‐protein interactions reveals interactions between N‐terminal domains of inositol 1,4,5‐trisphosphate receptors. PLoS One 8, e60609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Valencia I, Zhong F, McColl KS, Roderick HL, Bootman MD, Berridge MJ, Conway SJ, Holmes AB, Mignery GA, Velez P & Distelhorst CW (2004). Bcl‐2 functionally interacts with inositol 1,4,5‐trisphosphate receptors to regulate calcium release from the ER in response to inositol 1,4,5‐trisphosphate. J Cell Biol 166, 193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung KH, Mei L, Mak DO, Hayashi I, Iwatsubo T, Kang DE & Foskett JK (2010). Gain‐of‐function enhancement of IP3 receptor modal gating by familial Alzheimer's disease‐linked presenilin mutants in human cells and mouse neurons. Sci Signal 3, ra22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung KH, Shineman D, Muller M, Cardenas C, Mei L, Yang J, Tomita T, Iwatsubo T, Lee VM & Foskett JK (2008). Mechanism of Ca2+ disruption in Alzheimer's disease by presenilin regulation of InsP3 receptor channel gating. Neuron 58, 871–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe C & Ehrlich BE (2006). The inositol 1,4,5‐trisphosphate receptor (IP3R) and its regulators: sometimes good and sometimes bad teamwork. Science STKE 2006, re15. [DOI] [PubMed] [Google Scholar]
- Crockett DK, Lin Z, Elenitoba‐Johnson KS & Lim MS (2004). Identification of NPM‐ALK interacting proteins by tandem mass spectrometry. Oncogene 23, 2617–2629. [DOI] [PubMed] [Google Scholar]
- Cui J, Matkovich SJ, deSouza N, Li S, Rosemblit N & Marks AR (2004). Regulation of the type 1 inositol 1,4,5‐trisphosphate receptor by phosphorylation at tyrosine 353. J Biol Chem 279, 16311–16316. [DOI] [PubMed] [Google Scholar]
- Delmas P, Wanaverbecq N, Abogadie FC, Mistry M & Brown DA (2002). Signaling microdomains define the specificity of receptor‐mediated InsP3 pathways in neurons. Neuron 34, 209–220. [DOI] [PubMed] [Google Scholar]
- de Mattia F, Gubser C, van Dommelen MM, Visch HJ, Distelmaier F, Postigo A, Luyten T, Parys JB, de Smedt H, Smith GL, Willems PH & van Kuppeveld FJ (2009). Human Golgi antiapoptotic protein modulates intracellular calcium fluxes. Mol Biol Cell 20, 3638–3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deSouza N, Cui J, Dura M, McDonald TV & Marks AR (2007). A function for tyrosine phosphorylation of type 1 inositol 1,4,5‐trisphosphate receptor in lymphocyte activation. J Cell Biol 179, 923–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingli F, Parys JB, Loew D, Saule S & Mery L (2012). Vimentin and the K‐Ras‐induced actin‐binding protein control inositol‐(1,4,5)‐trisphosphate receptor redistribution during MDCK cell differentiation. J Cell Sci 125, 5428–5440. [DOI] [PubMed] [Google Scholar]
- Eckenrode EF, Yang J, Velmurugan GV, Foskett JK & White C (2010). Apoptosis protection by Mcl‐1 and Bcl‐2 modulation of inositol 1,4,5‐trisphosphate receptor‐dependent Ca2+ signaling. J Biol Chem 285, 13678–13684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris CD, Huganir RL, Bredt DS, Cameron AM & Snyder SH (1991). Inositol trisphosphate receptor: phosphorylation by protein kinase C and calcium calmodulin‐dependent protein kinases in reconstituted lipid vesicles. Proc Natl Acad Sci USA 88, 2232–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foskett JK, White C, Cheung KH & Mak DO (2007). Inositol trisphosphate receptor Ca2+ release channels. Physiol Rev 87, 593–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredericks GJ, Hoffmann FW, Rose AH, Osterheld HJ, Hess FM, Mercier F & Hoffmann PR (2014). Stable expression and function of the inositol 1,4,5‐triphosphate receptor requires palmitoylation by a DHHC6/selenoprotein K complex. Proc Natl Acad Sci USA 111, 16478–16483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregeau MO, Regimbald‐Dumas Y & Guillemette G (2011). Positive regulation of inositol 1,4,5‐trisphosphate‐induced Ca2+ release by mammalian target of rapamycin (mTOR) in RINm5F cells. J Cell Biochem 112, 723–733. [DOI] [PubMed] [Google Scholar]
- Fujimoto T, Machida T, Tanaka Y, Tsunoda T, Doi K, Ota T, Okamura T, Kuroki M & Shirasawa S (2011). KRAS‐induced actin‐interacting protein is required for the proper localization of inositol 1,4,5‐trisphosphate receptor in the epithelial cells. Biochem Biophys Res Commun 407, 438–443. [DOI] [PubMed] [Google Scholar]
- Futatsugi A, Nakamura T, Yamada MK, Ebisui E, Nakamura K, Uchida K, Kitaguchi T, Takahashi‐Iwanaga H, Noda T, Aruga J & Mikoshiba K (2005). IP3 receptor types 2 and 3 mediate exocrine secretion underlying energy metabolism. Science 309, 2232–2234. [DOI] [PubMed] [Google Scholar]
- Gavenonis J, Sheneman BA, Siegert TR, Eshelman MR & Kritzer JA (2014). Comprehensive analysis of loops at protein‐protein interfaces for macrocycle design. Nat Chem Biol 10, 716–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer M, Huang F, Sun Y, Vogel SM, Malik AB, Taylor CW & Komarova YA (2015). Microtubule‐associated protein EB3 regulates IP3 receptor clustering and Ca2+ signaling in endothelial cells. Cell Reports 12, 79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez L, Thiebaut PA, Paillard M, Ducreux S, Abrial M, Crola Da Silva C, Durand A, Alam MR, Van Coppenolle F, Sheu SS & Ovize M (2016). The SR/ER‐mitochondria calcium crosstalk is regulated by GSK3β during reperfusion injury. Cell Death Differ 23, 313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada K, Terauchi A & Mikoshiba K (2003). Three‐dimensional rearrangements with inositol 1,4,5‐trisphosphate receptor by calcium. J Biol Chem 278, 52881–52889. [DOI] [PubMed] [Google Scholar]
- Hamada K, Terauchi A, Nakamura K, Higo T, Nukina N, Matsumoto N, Hisatsune C, Nakamura T & Mikoshiba K (2014). Aberrant calcium signaling by transglutaminase‐mediated posttranslational modification of inositol 1,4,5‐trisphosphate receptors. Proc Natl Acad Sci USA 111, E3966–E3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson D, Stevens A, Murray PG, Black GC & Clayton PE (2014). Identifying biological pathways that underlie primordial short stature using network analysis. J Mol Endocrinol 52, 333–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havugimana PC, Hart GT, Nepusz T, Yang H, Turinsky AL, Li Z, Wang PI, Boutz DR, Fong V, Phanse S, Babu M, Craig SA, Hu P, Wan C, Vlasblom J, Dar VU, Bezginov A, Clark GW, Wu GC, Wodak SJ, Tillier ER, Paccanaro A, Marcotte EM & Emili A (2012). A census of human soluble protein complexes. Cell 150, 1068–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T & Su T‐P (2001). Regulating ankyrin dynamics: roles of sigma‐1 receptors. Proc Natl Acad Sci USA 98, 491–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes LP, Tepikin AV & Burgoyne RD (2004). Calcium‐binding protein 1 is an inhibitor of agonist‐evoked, inositol 1,4,5‐trisphosphate‐mediated calcium signaling. J Biol Chem 279, 547–555. [DOI] [PubMed] [Google Scholar]
- Higo T, Hamada K, Hisatsune C, Nukina N, Hashikawa T, Hattori M, Nakamura T & Mikoshiba K (2010). Mechanism of ER stress‐induced brain damage by IP3 receptor. Neuron 68, 865–878. [DOI] [PubMed] [Google Scholar]
- Higo T, Hattori M, Nakamura T, Natsume T, Michikawa T & Mikoshiba K (2005). Subtype‐specific and ER lumenal environment‐dependent regulation of inositol 1,4,5‐trisphosphate receptor type 1 by ERp44. Cell 120, 85–98. [DOI] [PubMed] [Google Scholar]
- Hirota J, Ando H, Hamada K & Mikoshiba K (2003). Carbonic anhydrase‐related protein is a novel binding protein for inositol 1,4,5‐trisphosphate receptor type 1. Biochem J 372, 435–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota J, Furuichi T & Mikoshiba K (1999). Inositol 1,4,5‐trisphosphate receptor type 1 is a substrate for caspase‐3 and is cleaved during apoptosis in a caspase‐3‐dependent manner. J Biol Chem 274, 34433–34437. [DOI] [PubMed] [Google Scholar]
- Honnappa S, Gouveia SM, Weisbrich A, Damberger FF, Bhavesh NS, Jawhari H, Grigoriev I, van Rijssel FJ, Buey RM, Lawera A, Jelesarov I, Winkler FK, Wuthrich K, Akhmanova A & Steinmetz MO (2009). An EB1‐binding motif acts as a microtubule tip localization signal. Cell 138, 366–376. [DOI] [PubMed] [Google Scholar]
- Hours MC & Mery L (2010). The N‐terminal domain of the type 1 Ins(1,4,5)P3 receptor stably expressed in MDCK cells interacts with myosin IIA and alters epithelial cell morphology. J Cell Sci 123, 1449–1459. [DOI] [PubMed] [Google Scholar]
- Huang G, Yao J, Zeng W, Mizuno Y, Kamm KE, Stull JT, Harding HP, Ron D & Muallem S (2006). ER stress disrupts Ca2+‐signaling complexes and Ca2+ regulation in secretory and muscle cells from PERK‐knockout mice. J Cell Sci 119, 153–161. [DOI] [PubMed] [Google Scholar]
- Hur E‐M, Park Y‐S, Huh YH, Yoo SH, Woo K‐C, Choi B‐H & Kim K‐T (2005). Junctional membrane inositol 1,4,5‐trisphosphate receptor complex coordinates sensitization of the silent EGF‐induced Ca2+ signaling. J Cell Biol 169, 657–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttlin EL, Ting L, Bruckner R, Paulo JA, Gygi MP, Rad R, Kolippakkam D, Szpyt J, Zarraga G, Tam S, Gebreab F, Colby G, Pontano‐Vaites L, Obar RA, Guarani‐Pereira V, Harris T, Artavanis‐Tsakonas S, Sowa ME, Harper JW & Gygi SP (2013). High‐throughput proteomic mapping of human interaction networks via affinity‐purification mass spectrometry (pre publication). BioGRID http://thebiogrid.org/166968/publication/high‐throughput‐proteomic‐mapping‐of‐human‐interaction‐networks‐via‐affinity‐purification‐mass‐spectrometry.html [Google Scholar]
- Ito J, Yoon SY, Lee B, Vanderheyden V, Vermassen E, Wojcikiewicz R, Alfandari D, De Smedt H, Parys JB & Fissore RA (2008). Inositol 1,4,5‐trisphosphate receptor 1, a widespread Ca2+ channel, is a novel substrate of polo‐like kinase 1 in eggs. Dev Biol 320, 402–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman T, Ondrias K, Ondriasová E & Marks AR (1996). Regulation of the inositol 1,4,5‐trisphosphate receptor by tyrosine phosphorylation. Science 272, 1492–1494. [DOI] [PubMed] [Google Scholar]
- Jin X, Shah S, Liu Y, Zhang H, Lees M, Fu Z, Lippiat JD, Beech DJ, Sivaprasadarao A, Baldwin SA & Gamper N (2013). Activation of the Cl− channel ANO1 by localized calcium signals in nociceptive sensory neurons requires coupling with the IP3 receptor. Sci Signal 6, ra73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph SK, Boehning D, Bokkala S, Watkins R & Widjaja J (1999). Biosynthesis of inositol trisphosphate receptors: selective association with the molecular chaperone calnexin. Biochem J 342, 153–161. [PMC free article] [PubMed] [Google Scholar]
- Joseph SK, Lin C, Pierson S, Thomas AP & Maranto AR (1995). Heteroligomers of type‐I and type‐III inositol trisphosphate receptors in WB rat liver epithelial cells. J Biol Chem 270, 23310–23315. [DOI] [PubMed] [Google Scholar]
- Joseph SK & Samanta S (1993). Detergent solubility of the inositol trisphosphate receptor in rat brain membranes. Evidence for association of the receptor with ankyrin. J Biol Chem 268, 6477–6486. [PubMed] [Google Scholar]
- Kawaai K, Hisatsune C, Kuroda Y, Mizutani A, Tashiro T & Mikoshiba K (2009). 80K‐H interacts with inositol 1,4,5‐trisphosphate (IP3) receptors and regulates IP3‐induced calcium release activity. J Biol Chem 284, 372–380. [DOI] [PubMed] [Google Scholar]
- Kawaai K, Mizutani A, Shoji H, Ogawa N, Ebisui E, Kuroda Y, Wakana S, Miyakawa T, Hisatsune C & Mikoshiba K (2015). IRBIT regulates CaMKIIα activity and contributes to catecholamine homeostasis through tyrosine hydroxylase phosphorylation. Proc Natl Acad Sci USA 112, 5515–5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MT, Wagner L 2nd, Yule DI, Bhanumathy C & Joseph SK (2006). Akt kinase phosphorylation of inositol 1,4,5‐trisphosphate receptors. J Biol Chem 281, 3731–3737. [DOI] [PubMed] [Google Scholar]
- Kline CF, Cunha SR, Lowe JS, Hund TJ & Mohler PJ (2008). Revisiting ankyrin‐InsP3 receptor interactions: ankyrin‐B associates with the cytoplasmic N‐terminus of the InsP3 receptor. J Cell Biochem 104, 1244–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller A, Schlossmann J, Ashman K, Uttenweiler‐Joseph S, Ruth P & Hofmann F (2003). Association of phospholamban with a cGMP kinase signaling complex. Biochem Biophys Res Commun 300, 155–160. [DOI] [PubMed] [Google Scholar]
- Komarova Y & Malik AB (2010). Regulation of endothelial permeability via paracellular and transcellular transport pathways. Annu Rev Physiol 72, 463–493. [DOI] [PubMed] [Google Scholar]
- Konieczny V, Keebler MV & Taylor CW (2012). Spatial organization of intracellular Ca2+ signals. Semin Cell Dev Biol 23, 172–180. [DOI] [PubMed] [Google Scholar]
- Kotlyar M, Pastrello C, Pivetta F, Lo Sardo A, Cumbaa C, Li H, Naranian T, Niu Y, Ding Z, Vafaee F, Broackes‐Carter F, Petschnigg J, Mills GB, Jurisicova A, Stagljar I, Maestro R & Jurisica I (2015). In silico prediction of physical protein interactions and characterization of interactome orphans. Nat Methods 12, 79–84. [DOI] [PubMed] [Google Scholar]
- Kume S, Muto A, Inoue T, Suga K, Okano H & Mikoshiba K (1997). Role of inositol 1,4,5‐trisphosphate receptor in ventral signaling in Xenopus embryos. Science 278, 1940–1943. [DOI] [PubMed] [Google Scholar]
- Lam A & Galione A (2013). The endoplasmic reticulum and junctional membrane communication during calcium signaling. Biochim Biophys Acta 1833, 2542–2559. [DOI] [PubMed] [Google Scholar]
- Lencesova L, O'Neill A, Resneck WG, Bloch RJ & Blaustein MP (2004). Plasma membrane‐cytoskeleton‐endoplasmic reticulum complexes in neurons and astrocytes. J Biol Chem 279, 2885–2893. [DOI] [PubMed] [Google Scholar]
- Lewis RS (2012). Store‐operated calcium channels: new perspectives on mechanism and function. Cold Spring Harb Persp Biol 3, a003970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Enomoto M, Rossi AM, Seo M‐D, Rahman T, Stathopulos PB, Taylor CW, Ikura M & Ames JB (2013). CaBP1, a neuronal Ca2+ sensor protein, inhibits inositol trisphosphate receptors by clamping inter‐subunit interactions. Proc Natl Acad Sci USA 110, 8507–8512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wright JM, Qian F, Germino GG & Guggino WB (2005). Polycystin 2 interacts with type I inositol 1,4,5‐trisphosphate receptor to modulate intracellular Ca2+ signaling. J Biol Chem 280, 41298–41306. [DOI] [PubMed] [Google Scholar]
- Lu JP, Wang Y, Sliter DA, Pearce MM & Wojcikiewicz RJ (2011). RNF170 protein, an endoplasmic reticulum membrane ubiquitin ligase, mediates inositol 1,4,5‐trisphosphate receptor ubiquitination and degradation. J Biol Chem 286, 24426–24433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lur G, Sherwood MW, Ebisui E, Haynes L, Feske S, Sutton R, Burgoyne RD, Mikoshiba K, Petersen OH & Tepikin AV (2011). InsP3 receptors and Orai channels in pancreatic acinar cells: co‐localization and its consequences. Biochem J 436, 231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda N, Kawasaki T, Nakade S, Yokota N, Taguchi T, Kasai M & Mikoshiba K (1991). Structural and functional characterization of inositol 1,4,5‐trisphosphate receptor channel from mouse cerebellum. J Biol Chem 266, 1109–1116. [PubMed] [Google Scholar]
- Magnusson A, Haug LS, Walaas I & Ostvold AC (1993). Calcium‐induced degradation of the inositol (1,4,5)‐trisphosphate receptor/Ca2+ channel. FEBS Lett 323, 229–232. [DOI] [PubMed] [Google Scholar]
- Malathi K, Kohyama S, Ho M, Soghoian D, Li X, Silane M, Berenstein A & Jayaraman T (2003). Inositol 1,4,5‐trisphosphate receptor (type 1) phosphorylation and modulation by Cdc2. J Cell Biochem 90, 1186–1196. [DOI] [PubMed] [Google Scholar]
- Malathi K, Li X, Krizanova O, Ondrias K, Sperber K, Ablamunits V & Jayaraman T (2005). Cdc2/cyclin B1 interacts with and modulates inositol 1,4,5‐trisphosphate receptor (type 1) functions. J Immunol 175, 6205–6210. [DOI] [PubMed] [Google Scholar]
- Marchant JS & Taylor CW (1997). Cooperative activation of IP3 receptors by sequential binding of IP3 and Ca2+ safeguards against spontaneous activity. Curr Biol 7, 510–518. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Nakagawa T, Inoue T, Nagata E, Tanaka K, Takano H, Minowa O, Kuno J, Sakakibara S, Yamada M, Yoneshima H, Miyawaki A, Fukuuchi Y, Furuichi T, Okano H, Mikoshiba K & Noda T (1996). Ataxia and epileptic seizures in mice lacking type 1 inositol 1,4,5‐trisphosphate receptor. Nature 379, 168–171. [DOI] [PubMed] [Google Scholar]
- Matsuzaki H, Fujimoto T, Ota T, Ogawa M, Tsunoda T, Doi K, Hamabashiri M, Tanaka M & Shirasawa S (2012). Tespa1 is a novel inositol 1,4,5‐trisphosphate receptor binding protein in T and B lymphocytes. FEBS Open Bio 2, 255–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP (2010). ER calcium and Alzheimer's disease: in a state of flux. Sci Signal 3, pe10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maximov A, Tang TS & Bezprozvanny I (2003). Association of the type 1 inositol (1,4,5)‐trisphosphate receptor with 4.1N protein in neurons. Mol Cell Neurosci 22, 271–283. [DOI] [PubMed] [Google Scholar]
- Mehta D, Ahmmed GU, Paria BC, Holinstat M, Voyno‐Yasenetskaya T, Tiruppathi C, Minshall RD & Malik AB (2003). RhoA interaction with inositol 1,4,5‐trisphosphate receptor and transient receptor potential channel‐1 regulates Ca2+ entry. Role in signaling increased endothelial permeability. J Biol Chem 278, 33492–33500. [DOI] [PubMed] [Google Scholar]
- Mery L, Magnino F, Schmidt K, Krause KH & Dufour JF (2001). Alternative splice variants of hTrp4 differentially interact with the C‐terminal portion of the inositol 1,4,5‐trisphosphate receptors. FEBS J 487, 377–383. [DOI] [PubMed] [Google Scholar]
- Mikoshiba K (2007). IP3 receptor/Ca2+ channel: from discovery to new signaling concepts. J Neurochem 102, 1426–1446. [DOI] [PubMed] [Google Scholar]
- Mohler PJ, Davis JQ & Bennett V (2005). Ankyrin‐B coordinates the Na/K ATPase, Na/Ca exchanger, and InsP3 receptor in a cardiac T‐tubule/SR microdomain. PLoS Biol 3, e423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler PJ, Davis JQ, Davis LH, Hoffman JA, Michaely P & Bennett V (2004). Inositol 1,4,5‐trisphosphate receptor localization and stability in neonatal cardiomyocytes requires interaction with ankyrin‐B. J Biol Chem 279, 12980–12987. [DOI] [PubMed] [Google Scholar]
- Monaco G, Beckers M, Ivanova H, Missiaen L, Parys JB, De Smedt H & Bultynck G (2012). Profiling of the Bcl‐2/Bcl‐X(L)‐binding sites on type 1 IP3 receptor. Biochem Biophys Res Commun 428, 31–35. [DOI] [PubMed] [Google Scholar]
- Mound A, Rodat‐Despoix L, Bougarn S, Ouadid‐Ahidouch H & Matifat F (2013). Molecular interaction and functional coupling between type 3 inositol 1,4,5‐trisphosphate receptor and BKCa channel stimulate breast cancer cell proliferation. Eur J Cancer 49, 3738–3751. [DOI] [PubMed] [Google Scholar]
- Muller M, Cardenas C, Mei L, Cheung KH & Foskett JK (2011). Constitutive cAMP response element binding protein (CREB) activation by Alzheimer's disease presenilin‐driven inositol trisphosphate receptor (InsP3R) Ca2+ signaling. Proc Natl Acad Sci USA 108, 13293–13298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata T, Lin MI, Stan RV, Bauer PM, Yu J & Sessa WC (2007). Genetic evidence supporting caveolae microdomain regulation of calcium entry in endothelial cells. J Biol Chem 282, 16631–16643. [DOI] [PubMed] [Google Scholar]
- Nadif Kasri N, Holmes AM, Bultynck G, Parys JB, Bootman MD, Rietdorf K, Missiaen L, McDonald F, De Smedt H, Conway SJ, Holmes AB, Berridge MJ & Roderick HL (2004). Regulation of InsP3 receptor activity by neuronal Ca2+‐binding proteins. EMBO J 23, 312–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraja GM & Kandpal RP (2004). Chromosome 13q12 encoded Rho GTPase activating protein suppresses growth of breast carcinoma cells, and yeast two‐hybrid screen shows its interaction with several proteins. Biochem Biophys Res Commun 313, 654–665. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Sato K, Fukaya M, Araishi K, Aiba A, Kano M & Watanabe M (2004). Signaling complex formation of phospholipase Cβ4 with metabotropic glutamate receptor type 1α and 1,4,5‐trisphosphate receptor at the perisynapse and endoplasmic reticulum in the mouse brain. Eur J Neurosci 20, 2929–2944. [DOI] [PubMed] [Google Scholar]
- Nakayama T, Hattori M, Uchida K, Nakamura T, Tateishi Y, Bannai H, Iwai M, Michikawa T, Inoue T & Mikoshiba K (2004). The regulatory domain of the inositol 1,4,5‐trisphosphate receptor is necessary to maintain the channel domain closed: possible physiological significance of specific cleavage by caspase. Biochem J 377, 299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natsvlishvili N, Goguadze N, Zhuravliova E & Mikeladze D (2015). Sigma‐1 receptor directly interacts with Rac1‐GTPase in the brain mitochondria. BMC Biochem 16, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen N, Francoeur N, Chartrand V, Klarskov K, Guillemette G & Boulay G (2009). Insulin promotes the association of heat shock protein 90 with the inositol 1,4,5‐trisphosphate receptor to dampen its Ca2+ release activity. Endocrinology 150, 2190–2196. [DOI] [PubMed] [Google Scholar]
- Oberdorf J, Webster JM, Zhu CC, Luo SG & Wojcikiewicz RJ (1999). Down‐regulation of types I, II and III inositol 1,4,5‐trisphosphate receptors is mediated by the ubiquitin/proteasome pathway. Biochem J 339, 453–461. [PMC free article] [PubMed] [Google Scholar]
- Olah T, Fodor J, Oddoux S, Ruzsnavszky O, Marty I & Csernoch L (2011). Trisk 32 regulates IP3 receptors in rat skeletal myoblasts. Pflugers Arch 462, 599–610. [DOI] [PubMed] [Google Scholar]
- Orchard S, Ammari M, Aranda B, Breuza L, Briganti L, Broackes‐Carter F, Campbell NH, Chavali G, Chen C, del‐Toro N, Duesbury M, Dumousseau M, Galeota E, Hinz U, Iannuccelli M, Jagannathan S, Jimenez R, Khadake J, Lagreid A, Licata L, Lovering RC, Meldal B, Melidoni AN, Milagros M, Peluso D, Perfetto L, Porras P, Raghunath A, Ricard‐Blum S, Roechert B, Stutz A, Tognolli M, van Roey K, Cesareni G & Hermjakob H (2013). The MIntAct project–IntAct as a common curation platform for 11 molecular interaction databases. Nucleic Acids Res 42, D358–D363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrenius S, Gogvadze V & Zhivotovsky B (2015). Calcium and mitochondria in the regulation of cell death. Biochem Biophys Res Commun 460, 72–81. [DOI] [PubMed] [Google Scholar]
- Paillard M, Tubbs E, Thiebaut PA, Gomez L, Fauconnier J, Da Silva CC, Teixeira G, Mewton N, Belaidi E, Durand A, Abrial M, Lacampagne A, Rieusset J & Ovize M (2013). Depressing mitochondria‐reticulum interactions protects cardiomyocytes from lethal hypoxia‐reoxygenation injury. Circulation 128, 1555–1565. [DOI] [PubMed] [Google Scholar]
- Parekh AB & Putney JW (2005). Store‐operated calcium channels. Physiol Rev 85, 757–810. [DOI] [PubMed] [Google Scholar]
- Patterson RL, van Rossum DB, Barrow RK & Snyder SH (2004). RACK1 binds to inositol 1,4,5‐trisphosphate receptors and mediates Ca2+ release. Proc Natl Acad Sci USA 101, 2328–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson RL, van Rossum DB, Kaplin AI, Barrow RK & Snyder SH (2005). Inositol 1,4,5‐trisphosphate receptor/GAPDH complex augments Ca2+ release via locally derived NADH. Proc Natl Acad Sci USA 102, 1357–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce MM, Wang Y, Kelley GG & Wojcikiewicz RJ (2007). SPFH2 mediates the endoplasmic reticulum‐associated degradation of inositol 1,4,5‐trisphosphate receptors and other substrates in mammalian cells. J Biol Chem 282, 20104–20115. [DOI] [PubMed] [Google Scholar]
- Pearce MM, Wormer DB, Wilkens S & Wojcikiewicz RJ (2009). An endoplasmic reticulum (ER) membrane complex composed of SPFH1 and SPFH2 mediates the ER‐associated degradation of inositol 1,4,5‐trisphosphate receptors. J Biol Chem 284, 10433–10445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinton P, Pozzan T & Rizzuto R (1998). The Golgi apparatus is an inositol 1,4,5‐trisphosphate‐sensitive Ca2+ store, with functional properties distinct from those of the endoplasmic reticulum. EMBO J 17, 5298–5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prole DL & Taylor CW (2011). Identification of intracellular and plasma membrane calcium channel homologues in pathogenic parasites. PLoS One 6, e26218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prole DL & Taylor CW (2012). Identification and analysis of cation channel homologues in human pathogenic fungi. PLoS One 7, e42404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman TU, Skupin A, Falcke M & Taylor CW (2009). Clustering of IP3 receptors by IP3 retunes their regulation by IP3 and Ca2+ . Nature 458, 655–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redondo PC, Jardin I, Lopez JJ, Salido GM & Rosado JA (2008). Intracellular Ca2+ store depletion induces the formation of macromolecular complexes involving hTRPC1, hTRPC6, the type II IP3 receptor and SERCA3 in human platelets. Biochim Biophys Acta 1783, 1163–1176. [DOI] [PubMed] [Google Scholar]
- Rex EB, Rankin ML, Yang Y, Lu Q, Gerfen CR, Jose PA & Sibley DR (2010). Identification of RanBP 9/10 as interacting partners for protein kinase C (PKC) γ/δ and the D1 dopamine receptor: regulation of PKC‐mediated receptor phosphorylation. Mol Pharmacol 78, 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland T, Tasan M, Charloteaux B, Pevzner SJ, Zhong Q, Sahni N, Yi S, Lemmens I, Fontanillo C, Mosca R, Kamburov A, Ghiassian SD, Yang X, Ghamsari L, Balcha D, Begg BE, Braun P, Brehme M, Broly MP, Carvunis AR, Convery‐Zupan D, Corominas R, Coulombe‐Huntington J, Dann E, Dreze M, Dricot A, Fan C, Franzosa E, Gebreab F, Gutierrez BJ, Hardy MF, Jin M, Kang S, Kiros R, Lin GN, Luck K, MacWilliams A, Menche J, Murray RR, Palagi A, Poulin MM, Rambout X, Rasla J, Reichert P, Romero V, Ruyssinck E, Sahalie JM, Scholz A, Shah AA, Sharma A, Shen Y, Spirohn K, Tam S, Tejeda AO, Trigg SA, Twizere JC, Vega K, Walsh J, Cusick ME, Xia Y, Barabasi AL, Iakoucheva LM, Aloy P, De Las Rivas J, Tavernier J, Calderwood MA, Hill DE, Hao T, Roth FP & Vidal M (2014). A proteome‐scale map of the human interactome network. Cell 159, 1212–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoso NG, Cebotaru L & Guggino WB (2011). Polycystin‐1, 2, and STIM1 interact with IP3R to modulate ER Ca release through the PI3K/Akt pathway. Cell Physiol Biochem 27, 715–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlafli P, Troger J, Eckhardt K, Borter E, Spielmann P & Wenger RH (2011). Substrate preference and phosphatidylinositol monophosphate inhibition of the catalytic domain of the Per‐Arnt‐Sim domain kinase PASKIN. FEBS J 278, 1757–1768. [DOI] [PubMed] [Google Scholar]
- Schlecker C, Boehmerle W, Jeromin A, DeGray B, Varshney A, Sharma Y, Szigeti‐Buck K & Ehrlich BE (2006). Neuronal calcium sensor‐1 enhancement of InsP3 receptor activity is inhibited by therapeutic levels of lithium. J Clin Invest 116, 1668–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlossmann J, Mendola A, Ashman K, Zong X, Huber A, Neubauer G, Ang G‐X, Allescher H‐D, Korth M, Wilm M, Hofmann F & Ruth P (2000). Regulation of intracellular calcium by a signalling complex of IRAG, IP3 receptor and cGMP kinase Ib. Nature 404, 197–201. [DOI] [PubMed] [Google Scholar]
- Schulman JJ, Wright FA, Kaufmann T & Wojcikiewicz RJ (2013). The Bcl‐2 family member Bok binds to the coupling domain of inositol 1,4,5‐trisphosphate receptors and protects them from proteolytic cleavage. J Biol Chem 288, 25340–25349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JD, Dessauer CW & Tasken K (2013). Creating order from chaos: cellular regulation by kinase anchoring. Annu Rev Pharmacol Toxicol 53, 187–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JD & Pawson T (2009). Cell signaling in space and time: where proteins come together and when they're apart. Science 326, 1220–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo M‐D, Velamakanni S, Ishiyama N, Stathopulos PB, Rossi AM, Khan SA, Dale P, Li C, Ames JB, Ikura M & Taylor CW (2012). Structural and functional conservation of key domains in InsP3 and ryanodine receptors. Nature 483, 108–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin DM, Zhao X‐S, Zeng W, Mozhayeva M & Muallem S (2000). The mammalian Sec 6/8 complex interacts with Ca2+ signaling complexes and regulates their activity. J Cell Biol 150, 1101–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindo Y, Kim MR, Miura H, Yuuki T, Kanda T, Hino A & Kusakabe Y (2010). Lrmp/Jaw1 is expressed in sweet, bitter, and umami receptor‐expressing cells. Chem Senses 35, 171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton PA & Bourguignon LY (2002). CD44v10 interaction with Rho‐kinase (ROK) activates inositol 1,4,5‐triphosphate (IP3) receptor‐mediated Ca2+ signaling during hyaluronan (HA)‐induced endothelial cell migration. Cell Motil Cytoskeleton 53, 293–316. [DOI] [PubMed] [Google Scholar]
- Singleton PA & Bourguignon LY (2004). CD44 interaction with ankyrin and IP3 receptor in lipid rafts promotes hyaluronan‐mediated Ca2+ signaling leading to nitric oxide production and endothelial cell adhesion and proliferation. Exp Cell Res 295, 102–118. [DOI] [PubMed] [Google Scholar]
- Smith IF & Parker I (2009). Imaging the quantal substructure of single IP3R channel activity during Ca2+ puffs in intact mammalian cells. Proc Natl Acad Sci USA 106, 6404–6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith IF, Swaminathan D, Dickinson GD & Parker I (2014). Single‐molecule tracking of inositol trisphosphate receptors reveals different motilities and distributions. Biophys J 107, 834–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soghoian D, Jayaraman V, Silane M, Berenstein A & Jayaraman T (2005). Inositol 1,4,5‐trisphosphate receptor phosphorylation in breast cancer. Tumour Biol 26, 207–212. [DOI] [PubMed] [Google Scholar]
- Streb H, Bayerdörffer E, Haase W, Irvine RF & Schulz I (1984). Effect of inositol‐1,4,5‐trisphosphate on isolated subcellular fractions of rat pancreas. J Membr Biol 81, 241–253. [DOI] [PubMed] [Google Scholar]
- Sugiyama T, Matsuda Y & Mikoshiba K (2000). Inositol 1,4,5‐trisphosphate receptor associated with focal contact cytoskeletal proteins. FEBS Lett 466, 29–34. [DOI] [PubMed] [Google Scholar]
- Sundivakkam PC, Kwiatek AM, Sharma TT, Minshall RD, Malik AB & Tiruppathi C (2009). Caveolin‐1 scaffold domain interacts with TRPC1 and IP3R3 to regulate Ca2+ store release‐induced Ca2+ entry in endothelial cells. Am J Physiol Cell Physiol 296, C403–C413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung PJ, Tsai FD, Vais H, Court H, Yang J, Fehrenbacher N, Foskett JK & Philips MR (2013). Phosphorylated K‐Ras limits cell survival by blocking Bcl‐xL sensitization of inositol trisphosphate receptors. Proc Natl Acad Sci USA 110, 20593–20598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabadkai G, Bianchi K, Varnai P, De Stefani D, Wieckowski MR, Cavagna D, Nagy AI, Balla T & Rizzuto R (2006). Chaperone‐mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J Cell Biol 175, 901–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szado T, Vanderheyden V, Parys JB, De Smedt H, Rietdorf K, Kotelevets L, Chastre E, Khan F, Landegren U, Soderberg O, Bootman MD & Roderick HL (2008). Phosphorylation of inositol 1,4,5‐trisphosphate receptors by protein kinase B/Akt inhibits Ca2+ release and apoptosis. Proc Natl Acad Sci USA 105, 2427–2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei K, Mignery GA, Mugnaini E, Südhof TC & De Camilli P (1994). Inositol 1,4,5‐trisphosphate receptor causes formation of ER cisternal stacks in transfected fibroblasts and in cerebellar Purkinje cells. Neuron 12, 327–342. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Kabayama H, Enomoto M, Saito N & Mikoshiba K (2011). Inositol 1, 4, 5‐trisphosphate receptor interacts with the SNARE domain of syntaxin 1B. J Physiol Sci 61, 221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Lin Y, Zhang Z, Tikunova S, Birnbaumer L & Zhu MX (2001). Identification of common binding sites for calmodulin and inositol 1,4,5‐trisphosphate receptors on the carboxyl termini of trp channels. J Biol Chem 276, 21303–21310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang TS, Tu H, Chan EY, Maximov A, Wang Z, Wellington CL, Hayden MR & Bezprozvanny I (2003. b). Huntingtin and huntingtin‐associated protein 1 influence neuronal calcium signaling mediated by inositol‐(1,4,5) triphosphate receptor type 1. Neuron 39, 227–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang T‐S, Tu H, Wang Z & Bezprozvanny I (2003. a). Modulation of type 1 inositol (1,4,5)‐trisphosphate receptor function by protein kinase A and protein phosphatase 1α. J Neurosci 23, 403–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateishi Y, Hattori M, Nakayama T, Iwai M, Bannai H, Nakamura T, Michikawa T, Inoue T & Mikoshiba K (2005). Cluster formation of inositol 1,4,5‐trisphosphate receptor requires its transition to open state. J Biol Chem 280, 6816–6822. [DOI] [PubMed] [Google Scholar]
- Taylor CW & Tovey SC (2010). IP3 receptors: toward understanding their activation. Cold Spring Harb Persp Biol 2, a004010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrower EC, Choe CU, So SH, Jeon SH, Ehrlich BE & Yoo SH (2003). A functional interaction between chromogranin B and inositol 1,4,5‐trisphosphate receptor/Ca2+ channel. J Biol Chem 278, 49699–49708. [DOI] [PubMed] [Google Scholar]
- Thrower EC, Park HY, So SH, Yoo SH & Ehrlich BE (2002). Activation of inositol 1,4,5‐trisphosphate receptor by the calcium storage protein chromogranin A. J Biol Chem 277, 15801–15806. [DOI] [PubMed] [Google Scholar]
- Tiwari A, Schneider M, Fiorino A, Haider R, Okoniewski MJ, Roschitzki B, Uzozie A, Menigatti M, Jiricny J & Marra G (2013). Early insights into the function of KIAA1199, a markedly overexpressed protein in human colorectal tumors. PLoS One 8, e69473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Q, Chu X, Cheung JY, Conrad K, Stahl R, Barber DL, Mignery G & Miller BA (2004). Erythropoietin‐modulated calcium influx through TRPC2 is mediated by phospholipase Cγ and IP3R. Am J Physiol Cell Physiol 287, C1667–C1678. [DOI] [PubMed] [Google Scholar]
- Tovey SC, Dedos SG, Taylor EJA, Church JE & Taylor CW (2008). Selective coupling of type 6 adenylyl cyclase with type 2 IP3 receptors mediates a direct sensitization of IP3 receptors by cAMP. J Cell Biol 183, 297–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treves S, Franzini‐Armstrong C, Moccagatta L, Arnoult C, Grasso C, Schrum A, Ducreux S, Zhu MX, Mikoshiba K, Girard T, Smida‐Rezgui S, Ronjat M & Zorzato F (2004). Junctate is a key element in calcium entry induced by activation of InsP3 receptors and/or calcium store depletion. J Cell Biol 166, 537–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai YC, Greco TM, Boonmee A, Miteva Y & Cristea IM (2012). Functional proteomics establishes the interaction of SIRT7 with chromatin remodeling complexes and expands its role in regulation of RNA polymerase I transcription. Mol Cell Proteomics 11, 60–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu H, Tang T‐S, Wang Z & Bezprozvanny I (2004). Association of type 1 inositol 1,4,5‐trisphosphate receptor with AKAP9 (Yotiao) and protein kinase A. J Biol Chem 279, 19375–19382. [DOI] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Yuan JP, Lanahan AA, Leoffert K, Li M, Linden DJ & Worley PF (1998). Homer binds a novel proline‐rich motif and links group 1 metabotropic glutamate receptors with IP3 receptors. Neuron 21, 717–726. [DOI] [PubMed] [Google Scholar]
- Uchida K, Aramaki M, Nakazawa M, Yamagishi C, Makino S, Fukuda K, Nakamura T, Takahashi T, Mikoshiba K & Yamagishi H (2010). Gene knock‐outs of inositol 1,4,5‐trisphosphate receptors types 1 and 2 result in perturbation of cardiogenesis. PLoS One 5, e12500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderheyden V, Devogelaere B, Missiaen L, De Smedt H, Bultynck G & Parys JB (2009. a). Regulation of inositol 1,4,5‐trisphosphate‐induced Ca2+ release by reversible phosphorylation and dephosphorylation. Biochim Biophys Acta 1793, 959–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderheyden V, Wakai T, Bultynck G, De Smedt H, Parys JB & Fissore RA (2009. b). Regulation of inositol 1,4,5‐trisphosphate receptor type 1 function during oocyte maturation by MPM‐2 phosphorylation. Cell Calcium 46, 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rossum DB, Patterson RL, Cheung KH, Barrow RK, Syrovatkina V, Gessell GS, Burkholder SG, Watkins DN, Foskett JK & Snyder SH (2006). DANGER: A novel regulatory protein of IP3‐receptor activity. J Biol Chem 281, 37111–37116. [DOI] [PubMed] [Google Scholar]
- Vicencio JM, Ortiz C, Criollo A, Jones AW, Kepp O, Galluzzi L, Joza N, Vitale I, Morselli E, Tailler M, Castedo M, Maiuri MC, Molgo J, Szabadkai G, Lavandero S & Kroemer G (2009). The inositol 1,4,5‐trisphosphate receptor regulates autophagy through its interaction with Beclin 1. Cell Death Differ 16, 1006–1017. [DOI] [PubMed] [Google Scholar]
- Volpe P, Salviati G, Di Virgilio F & Pozzan T (1985). Inositol 1,4,5‐trisphosphate induces calcium release from sarcoplasmic reticulum of skeletal muscle. Nature 316, 347–349. [DOI] [PubMed] [Google Scholar]
- Wakelam MJO, Murphy GJ, Hruby VJ & Houslay MD (1986). Activation of two signal‐transduction cascades in hepatocytes by glucagon. Nature 323, 68–71. [DOI] [PubMed] [Google Scholar]
- Walker DS, Ly S, Lockwood KC & Baylis HA (2002). A direct interaction between IP3 receptors and myosin II regulates IP3 signaling in C. elegans . Curr Biol 12, 951–956. [DOI] [PubMed] [Google Scholar]
- Wang Q, Rajshankar D, Branch DR, Siminovitch KA, Herrera Abreu MT, Downey GP & McCulloch CA (2009). Protein‐tyrosine phosphatase‐alpha and Src functionally link focal adhesions to the endoplasmic reticulum to mediate interleukin‐1‐induced Ca2+ signaling. J Biol Chem 284, 20763–20772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Li G, Goode J, Paz JC, Ouyang K, Screaton R, Fischer WH, Chen J, Tabas I & Montminy M (2012). Inositol‐1,4,5‐trisphosphate receptor regulates hepatic gluconeogenesis in fasting and diabetes. Nature 485, 128–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster JM, Tiwari S, Weissman AM & Wojcikiewicz RJH (2003). Inositol 1,4,5‐trisphosphate receptor ubiquitination is mediated by mammalian Ubc7, a component of the endoplasmic reticulum‐associated degradation pathway, and is inhibited by chelation of intracellular Zn2+ . J Biol Chem 278, 38238–38246. [DOI] [PubMed] [Google Scholar]
- Wells JA & McClendon CL (2007). Reaching for high‐hanging fruit in drug discovery at protein‐protein interfaces. Nature 450, 1001–1009. [DOI] [PubMed] [Google Scholar]
- Wheeler GL & Brownlee C (2008). Ca2+ signalling in plants and green algae – changing channels. Trends Plant Sci 13, 506–514. [DOI] [PubMed] [Google Scholar]
- White C, Li C, Yang J, Petrenko NB, Madesh M, Thompson CB & Foskett JK (2005). The endoplasmic reticulum gateway to apoptosis by Bcl‐XL modulation of InsP3R. Nat Cell Biol 7, 1022–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White C, Yang J, Monteiro MJ & Foskett JK (2006). CIB1, a ubiquitously expressed Ca2+‐binding protein ligand of the InsP3 receptor Ca2+ release channel. J Biol Chem 281, 20825–20833. [DOI] [PubMed] [Google Scholar]
- Wojcikiewicz RJH & Oberdorf JA (1996). Degradation of inositol 1,4,5‐trisphosphate receptors during cell stimulation is a specific process mediated by a cysteine protease activity. J Biol Chem 271, 16652–16655. [DOI] [PubMed] [Google Scholar]
- Woodard GE, Lopez JJ, Jardin I, Salido GM & Rosado JA (2010). TRPC3 regulates agonist‐stimulated Ca2+ mobilization by mediating the interaction between type I inositol 1,4,5‐trisphosphate receptor, RACK1, and Orai1. J Biol Chem 285, 8045–8053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Miyawaki A, Saito K, Yamamoto‐Hino M, Ryo Y, Furuichi T & Mikoshiba K (1995). The calmodulin‐binding domain in the mouse type 1 inositol 1,4,5‐trisphosphate receptor. Biochem J 308, 83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, McBride S, Mak D‐O, Vardi, N , Palczewski K, Haeseleer F & Foskett JK (2002). Identification of a family of calcium sensors as protein ligands of the inositol trisphosphate receptor Ca2+ release channels. Proc Natl Acad Sci USA 99, 7711–7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama K, Su I, Tezuka T, Yasuda T, Mikoshiba K, Tarakhovsky A & Yamamoto T (2002). BANK regulates BCR‐induced calcium mobilization by promoting tyrosine phosphorylation of IP3 receptor. EMBO J 21, 83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SH (2011). Role of secretory granules in inositol 1,4,5‐trisphosphate‐dependent Ca2+ signaling: From phytoplankton to mammals. Cell Calcium 50, 175–183. [DOI] [PubMed] [Google Scholar]
- Yoo SH & Lewis MS (1998). Interaction between an intraluminal loop peptide of the inositol 1,4,5‐trisphosphate receptor and the near N‐teminal peptide of chromogranin A. FEBS Lett 427, 55–58. [DOI] [PubMed] [Google Scholar]
- Yoo SH & Lewis MS (2000). Interaction of chromogranin B and the near N‐terminal region of chromogranin B with an intraluminal loop peptide of the inositol 1,4,5‐trisphosphate receptor. J Biol Chem 275, 30293–30300. [DOI] [PubMed] [Google Scholar]
- Yuan JP, Kiselyov K, Shin DM, Shcheynikov N, Kang SH, Dehoff MH, Schwarz MK, Seeburg PH, Muallem S & Worley PF (2003). Homer binds TRPC family channels and is required for gating of TRPC1 by IP3 receptors. Cell 114, 777–789. [DOI] [PubMed] [Google Scholar]
- Yuan Z, Cai T, Tian J, Ivanov AV, Giovannucci DR & Xie Z (2005). Na/K‐ATPase tethers phospholipase C and IP3 receptor into a calcium‐regulatory complex. Mol Biol Cell 16, 4034–4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaika O, Zhang J & Shapiro MS (2011). Combined phosphoinositide and Ca2+ signals mediating receptor specificity toward neuronal Ca2+ channels. J Biol Chem 286, 830–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng W, Mak DD, Li Q, Shin DM, Foskett JK & Muallem S (2003). A new mode of Ca2+ signaling by G protein‐coupled receptors: gating of IP3 receptor Ca2+ release channels by Gβγ. Curr Biol 13, 872–876. [DOI] [PubMed] [Google Scholar]
- Zhang S, Hisatsune C, Matsu‐Ura T & Mikoshiba K (2009). G‐protein‐coupled receptor kinase‐interacting proteins inhibit apoptosis by inositol 1,4,5‐triphosphate receptor‐mediated Ca2+ signal regulation. J Biol Chem 284, 29158–29169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Tang J, Tikunova S, Johnson JD, Chen Z, Qin N, Dietrich A, Stefani E, Birnbaumer L & Zhu MX (2001). Activation of Trp3 by inositol 1,4,5‐trisphosphate receptors through displacement of inhibitory calmodulin from a common binding domain. Proc Natl Acad Sci USA 98, 3168–3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G, Neeb ZP, Leo MD, Pachuau J, Adebiyi A, Ouyang K, Chen J & Jaggar JH (2010). Type 1 IP3 receptors activate BKCa channels via local molecular coupling in arterial smooth muscle cells. J Gen Physiol 136, 283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]


