Abstract
Arsenic trioxide (ATO) is highly effective for treating acute promyelocytic leukemia. It also holds the promise for treating solid tumors, including gastric carcinoma. However, the molecular mechanism of the effectiveness of ATO to solid tumor is still poorly understood. In this study, we chosed gastric carcinoma as an example and tried to reveal the antitumor mechanism through metabolomics. Gastric carcinoma cell line SGC7901 was treated with ATO for 6, 12, and 24 h. The global metabolite profiles were monitored by metabolomics analysis using gas chromatography (GC)/mass spectrometry (MS) and liquid chromatography/MS/MS. A total of 281 certified metabolites were reliably detected. Bioinformatics analysis showed that glycerophospholipid synthesis, one-carbon synthesis, and glutathione synthesis were affected dramatically. Other cellular functions/pathways that had been affected included inflammatory response, nicotinamide adenine dinucleotide (NAD+), and polyamine biosynthesis pathway. The metabolomics data from this study, in combination with previous transcriptomics and proteomics data, could serve as valuable resources for the understanding of the specific antitumor mechanism of ATO treatment.
Keywords: arsenic trioxide, gastric carcinoma, metabolomics
Introduction
According to the incidence rate, gastric cancer ranks the fifth among all the cancers, and it is estimated that there are 952,000 new cases (7% of total cancer incidence) and 723,000 deaths (9% of total cancer mortality) in 2012 worldwide. Among them, ∼75% of the new cases occurred in Asia [1], and the mortality-to-incidence ratio in >70% countries is over 0.8 [2]. Gastric cancer is difficult to be cured and has a poor overall prognosis with a high recurrence rate. Radical surgical resection remains the only curative treatment; however, it is only effective for early stage gastric cancer patients.
Arsenic trioxide (ATO) has been applied for treating a variety of diseases for thousands of years and shows extreme effectiveness for acute promyelocytic leukemia (APL) [3]. Besides APL, ATO showed an antitumor activity for a wide range of other tumors, including chronic myelocytic leukemia [4], hepatocellular carcinoma [5], lung cancer [6], and gastric carcinoma [7,8]. Although previous studies showed that ATO could induce apoptosis in gastric cancer cell lines AGS and MKN-28 by upregulating P53 and activation of caspase-3, or induce apoptosis in gastric cancer cell line SGC7901 by upregulating FAS [9,10], the mode of action (MOA) of ATO's antitumor activity for gastric cancer is still elusive. Therefore, to facilitate the application of ATO for therapeutic intervention of gastric cancer, systematical understanding of the MOA is needed. To meet this requirement, systematical studies have already been performed on the levels of genomics [11], transcriptomics [12], and proteomics [13]. However, metabolomics data are still lacking.
Metabolomics study can provide data of global biochemical events by analyzing thousands of metabolites in cells, tissue, or body fluid, and leads to enhanced knowledge of diseases and drug mechanisms [14]. The metabolism of cancer is quite different from that of the healthy physiological state, and numerous studies have demonstrated that aberrant metabolism plays crucial roles in tumor genesis and tumor development [15–17]. For example, in cancer cells, glucose is commonly consumed for glycolysis even under aerobic condition, and this phenomenon is also called Warburg effect. A better understanding of these metabolic changes will prompt new approaches toward cancer therapy [18].
Comprehensive metabolomics approaches that simultaneously detect changes in a variety of metabolites in cancer cells under ATO is critical for the identification of potential metabolic ‘Achilles’ heels’ of the solid tumor. In this study, we applied metabolomics profiling to investigate the dynamics of metabolic responses to ATO treatment in human gastric cancer cell line SGC7901.
Materials and Methods
Cell culture
SGC7901 cell line was grown in RPMI-1640 medium (Invitrogen, Grand Island, USA) supplemented with 10% fetal bovine serum (Invitrogen), 100 U/ml penicillin, and 100 μg/ml streptomycin (Corning, Manassas, USA) in humidified air at 37°C with 5% CO2.
Sample preparation
ATO (2 μM) was added into SGC7901 cell line to treat the cells for 6, 12, and 24 h. The sample preparation step was carried out employing the automated MicroLab STAR system. The resulting extract was split into two fractions: one for liquid chromatography (LC) analysis, and the other for gas chromatography (GC) analysis. The organic solvent of samples was removed by employing TurboVap® (Zymark, Hopkinton, USA). Samples were then frozen and dried under vacuum condition individually, then prepared for either LC/mass spectrometry (MS)/MS or GC/MS analysis.
LC/MS/MS and GC/MS analyses
The LC/MS/MS portion of the platform was based on a ACQUITYUPLC (Waters, Milford, USA)-LTQ XL (Thermo, Waltham, USA). The sample extract was divided into two aliquots, dried, and then reconstituted in acidic or basic LC-compatible solvent, and each of them contained 11 or more injection standards at fixed concentrations. One aliquot was analyzed using acidic positive-ion optimized conditions and the other using negative-ion optimized conditions. Two independent injections were performed using independent columns. The extracts under acidic conditions were reconstituted using water and methanol both containing 0.1% formic acid with gradient elution, while the basic extracts were dissolved in 6.5 mM ammonium bicarbonate.
For GC (Thermo), the samples were redried under vacuum desiccation for a minimum of 24 h prior to be derivatized under dried nitrogen using bistrimethyl-silyl-trifluoroacetamide. The GC column was 5% phenyl and the temperature ramp was from 40 to 300°C in a 16-min period. The Thermo-Finnigan Trace DSQ fast-scanning single-quadrupole mass spectrometer using electron impact ionization was employed to analyze samples.
Data extraction and quality assurance
The raw data extracted from the MS were loaded into a related database. Peaks were identified after data were examined on the database with appropriate quality control limits using Shanghai Jiao Tong University-Metabolon's proprietary peak integration software, and component parts were stored in a designed data structure.
Heatmap analysis
The heatmap was performed using Cluster 3.0 (Stanford University, Palo Alto, USA) by a three cluster and 100 runs k-means clustering methods, and visualized using Java Treeview [19] (http://jtreeview.sourceforge.net).
Statistical analysis
For pair-wise comparisons, Welch's t-tests and/or Wilcoxon's rank sum tests were performed. For other statistical designs, analysis of variance procedure was performed. For classification, random forest analyzes were used. Statistical analyses were performed with the program ‘R’ (http://cran.r-project.org/).
Results
Overview of the dynamic changes at the three time points
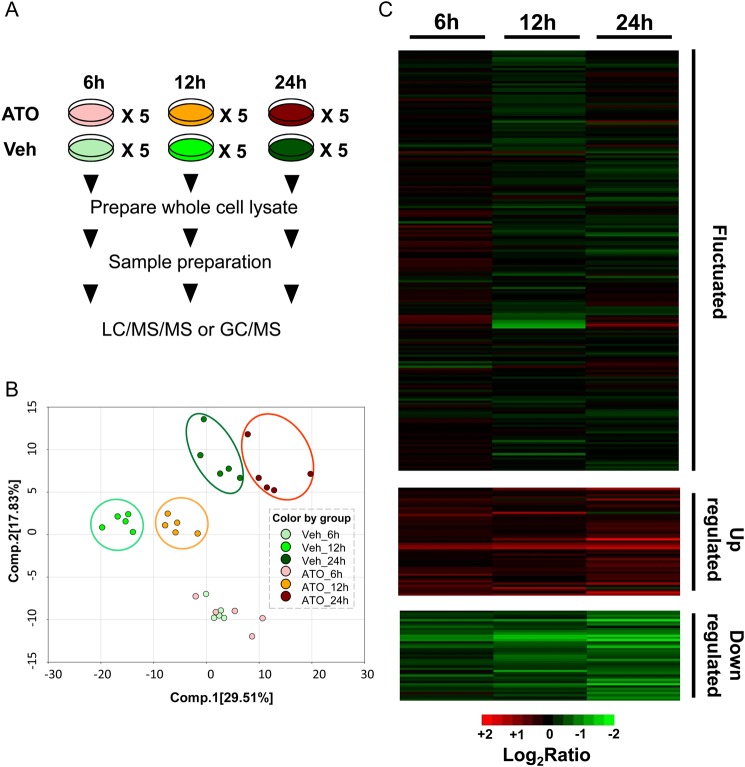
The goal of this study is to characterize metabolic adaptation and MOA associated with ATO treatment in the gastric carcinoma cell line SGC7901. To achieve this goal, the gastric carcinoma cell line SGC7901 was chosen as an example, and a standard extraction workflow was performed on the cytosolic lysate in samples of both vehicle control (dimethyl sulfoxide treatment) groups and ATO groups at 6, 12, and 24 h (Fig. 1A). Totally, 30 cell lysate samples were collected and stored immediately at −80°C. At the time of analysis, samples were extracted and prepared using standard solvent extraction method. The extracted samples were split into two equal parts and analyzed on GC/MS and LC/MS/MS platforms. Several technical replicate samples created from a homogeneous pool containing a small amount of all study samples were included as controls.
Figure 1.
Dynamic changes at three time points (A) Workflow of ATO treatment and vehicle control at three time points, five replicates for each. (B) PCA of all quantified metabolites from cells treated with/without ATO at three time points. The score of two components with time and ATO treatment is 29.51% and 17.83%, suggesting distinct separation among the three time points and distinct separations of treated vs. vehicle control groups at 12 and 24 h (color circles). (C) The k-means cluster analysis of all metabolites quantified in each experiment. Log2(ATO/Veh) refers to the ratio of the abundance of a given metabolite between the cells with ATO treatment and vehicle. Veh, vehicle control group.
Principal component analysis (PCA) (Fig. 1B) showed that there was no statistical difference between ATO-treated group and vehicle group at 6 h, indicating that the majority of cellular metabolomics response to ATO treatment is not fast. However, PCA demonstrated distinct separations of ATO-treated groups from vehicle groups at 12 and 24 h, suggesting fundamental difference of cellular behavior resulted from ATO treatment. In total, 281 quantified metabolites were identified (Supplementary Table S1). According to the metabolomics changes among these three time points, three sets of metabolites were revealed after clustering analysis, i.e. upregulated, downregulated, or fluctuated as shown in the heatmap (Fig. 1C and Supplementary Table S2).
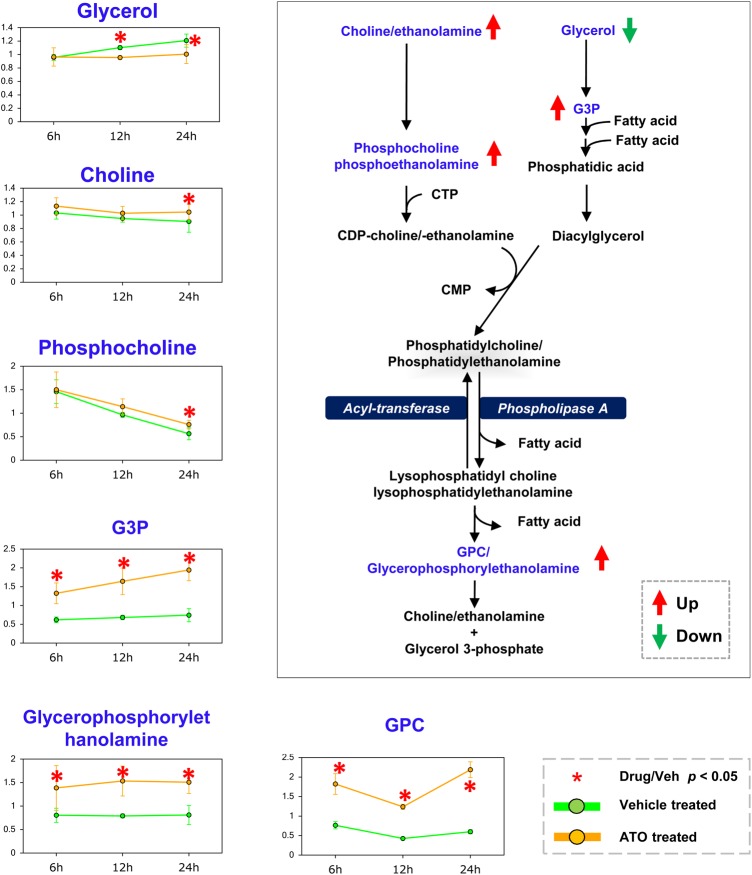
Upregulated glycerophospholipid metabolism pathway
ATO treatment induced many changes in the levels of biomolecules involved in glycerophospholipid metabolism (Fig. 2), which plays a pivotal role in various cellular functions. Recent studies showed that inhibition of glycerophospholipid is through lysophosphatidic acid acyltransferase-β, the key enzyme involved in glycerophospholipid synthesis [20], and may be a potential therapy for osteosarcoma. These changes in glycerophospholipid were accompanied by elevated levels of biomolecules involved in phospholipid metabolism in the ATO-treated groups, relative to the vehicle control groups across all three time points, i.e. glycerophosphorylethanolamine, glycerol-3-phosphate (G3P), and glycerophosphorylcholine (GPC).
Figure 2.
The changes of glycerophospholipid metabolism at three time points ATO treatment caused many changes in the levels of biomolecules involved in glycerophospholipid metabolism. Glycerophospholipids identified in this study are highlighted in blue. Up- and downregulation of metabolites are shown with red and green arrows, respectively. *P < 0.05. G3P, glycerol-3-phosphate; GPC, glycerophosphorylcholine; CMP, cytidine monophosphate; CDP, cytidine diphosphate; CTP, cytidine triphosphate.
Endometrial differential 3 (EDI3) cleaves GPC to form G3P and choline, thus decreasing the ratio of GPC to phosphocholine (PC). When EDI3 was inhibited, lower migration capacity of breast cancer was observed [21]. Our data are consistent with this observation. An elevated level of GPC but not PC or choline was observed in the ATO-treated group when compared with the control group. These results indicate that ATO may inhibit EDI3's activity in gastric cancer directly or indirectly.
In addition, the levels of choline, PC, and many lysolipids were significantly higher, while the level of glycerol was significantly lower in ATO-treated group than that in the control group at the time point of 24 h. These observations were consistent with an earlier active membrane remodeling and a later increase in membrane breakdown in response to ATO treatment [22].
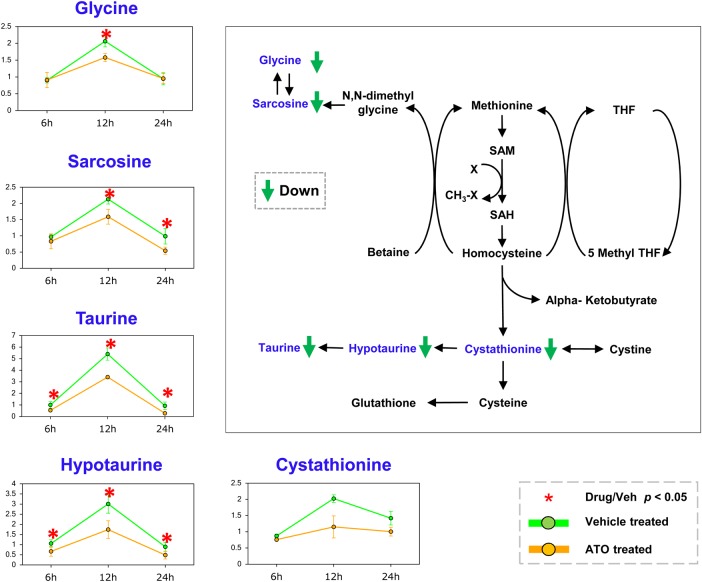
Downregulated one-carbon metabolism, fatty acid β-oxidation, and polyamine metabolism pathway
One-carbon metabolism is involved in the folate and methionine cycles [23]. Genetic and functional evidence show that high activation of one-carbon metabolism pathway is a driver of oncogenesis and correlated with cellular epigenetic status. In methionine metabolism pathway (Fig. 3), sarcosine was identified as both a biomarker and a promoter for prostate cancer progression [24]. The reduced level of sarcosine in response to ATO treatment at 12 and 24 h would be potentially consistent with the antimetastatic activity of ATO [24]. These metabolomics changes upon ATO treatment indicate changes in cellular methylation process. Alterations in acetylation and methylation have been shown to affect the epigenetic programming of cells, and the changes of one-carbon metabolism may imply changes in these regulatory pathways. The observation here may potentially suggest an antimetastatic effect of ATO.
Figure 3.
The changes of one-carbon metabolism pathway at three time points Diversion of cysteine to taurine and 5-methyl THF were followed by decreases at ATO treatment with an increase in transmethylation, transsulfuration, and methionine salvage. Metabolites with changed level are highlighted in blue, coupled with line chart nearby. The down trends are shown with green arrow, respectively. *P< 0.05. SAM, S-adenosyl methionine; SAH, S-adenosyl-l-homocysteine; THF, tetrahydrofuran.
Fatty acids are essential energy source, which are catabolized by the fatty acid β-oxidation (FAO) [25]. In the FAO pathway, activated fatty acids are transported across the mitochondrial membrane by the carnitine shuttle, and undergo a series of shortening of their carbon chain in mitochondria, which are catalyzed by acyl-CoA dehydrogenases and other enzymes, generating NADH, FADH2, and acetyl-CoA (Supplementary Fig. S1). The FAO pathway is beneficial for cancer survival and resistance to metabolic stress [18]. In this study, the levels of carnitine-related fatty acids were lower upon ATO treatment in comparison with the vehicle control. Meanwhile, 3-hydroxybutyrate (a ketone body) (at 6 and 12 h) and free carnitine (at 12 and 24 h) showed significantly reduced levels. These results suggest that ATO inhibits the activities of mitochondrial acyl-CoA dehydrogenases, i.e. very long-chain acyl-CoA dehydrogenase, long-chain acyl-CoA dehydrogenase, and medium-chain acyl-CoA dehydrogenase, thus causes mitochondrial dysfunction [26].
As another downregulated pathway, polyamine biosynthesis affects many processes in carcinogenesis. Elevated polyamine levels correlate with increased cell proliferation, decreased apoptosis, and affected tumor invasion and metastasis [27].
The polyamine levels displayed a consistent decreasing over the course of ATO treatment, suggesting a progressive decline of cellular growth and proliferation (Supplementary Fig. S2). The polyamine biosynthetic pathway produces putrescine from arginine through either ornithine or agmatine. Putrescine is converted into spermidine with 5-methylthioadenosine as a by-product. The levels of putrescine, spermidine, and N-acetylputrescine were all decreased significantly in ATO-treated groups in comparison with those of the vehicle controls. This decline was most prominent when the gastric cancer cells were treated with ATO for 12 h, with the levels of putrescine and N-acetylputrescine progressively declined over time. In addition, other bimolecules involved in polyamine metabolism (N-acetylornithine, 4-acetamidobutanoate, creatine, and creatinine) also showed significant decreases at 12 h. These results suggest an overall inhibition of polyamine metabolism in response to ATO treatment.
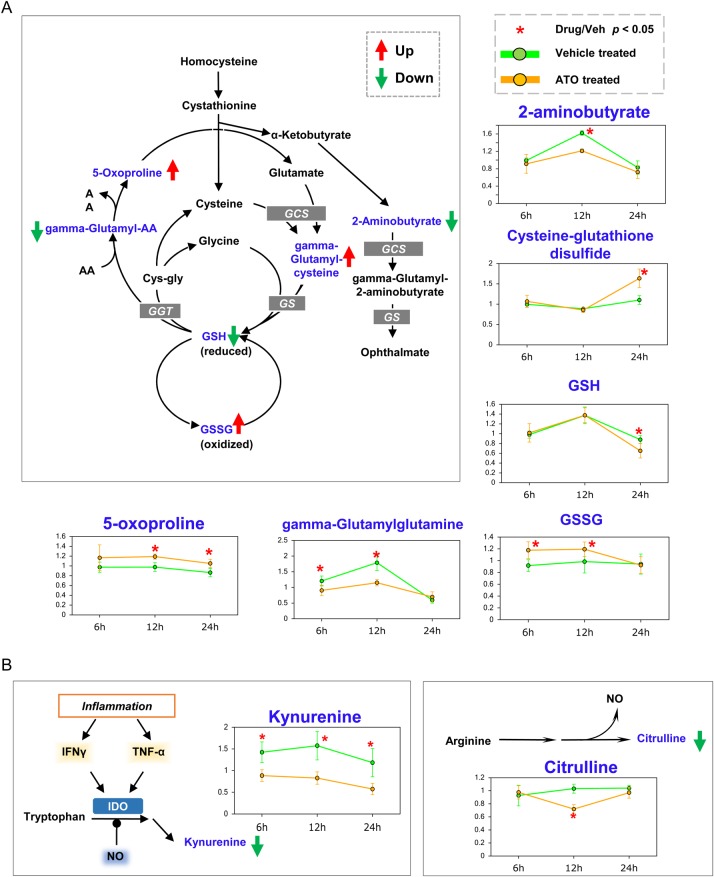
Fluctuated glutathione biosynthesis, inflammatory response, and NAD+ pathway
Glutathione (GSH) is formed from glutamic acid, cysteine, and glycine, which is catalyzed by GSH synthetase (GS). GSH plays a central role in the detoxification and the maintenance of the intracellular redox balance and proteins' essential thiol status [28]. The level of GSH is related to cell cycle progression, especially in cancer cells. In many types of tumors, the level of GSH is elevated for self-cellular proliferation and resistance to chemotherapy [29,30]. In this study, ATO treatment resulted in the decrease of GSH, and the corresponding elevated level of oxidized GSH (GSSG) (Fig. 4A). These results indicate that ATO may inhibit the activity of GS for producing GSH, thus causing the elevation of the cellular oxidative stress level with a high level of reactive oxygen species (ROS). The excessive increase of cellular ROS level induces tumor cell cycle arrest or cell death.
Figure 4.
The changes of GSH biosynthesis and inflammation response at three time points (A) ATO treatment caused many changes in the levels of metabolites involved in GSH metabolism pathway. (B) The inflammation pathway. Kynurenine and citrulline levels were decreased with ATO treatment. Metabolites with changed level are highlighted in blue, coupled with line chart nearby. Up and down trends are shown with red and green arrows, respectively. *P< 0.05.
The increased ROS level causes cell inflammatory response with the secretion of cytokines (Fig. 4B), e.g. interferon γ and tumor necrosis factor α [31]. These cytokines regulate indoleamine 2,3-dioxygenase, which is responsible for the conversion of tryptophan into kynurenine. Interestingly, ATO treatment caused a decrease in kynurenine level.
NAD+ plays an important role in many cellular processes, such as redox reactions where NAD+ participates in hydride transfer and nonredox reactions where NAD+ acts as a donor of ADP-ribose units. NAD+ biosynthesis consists of three pathways: de novo pathway using tryptophan as an endogenous precursor, import pathway using an exogenous niacin, and salvage pathway where the breakdown product nicotinamide is recycled back to NAD+ [32].
ATO treatment caused a significant decrease in the level of kynurenine in comparison with that of the control group across all the three time points. This is consistent with a possible reduced de novo biosynthesis of NAD+ besides implication in the inflammatory pathway that was discussed earlier. The changes in the NAD+ biosynthesis pathway (Supplementary Fig. S3), especially the prominent accumulation of nicotinate, nicotinateribonucleoside, and NAD+ at 24 h of treatment, suggest the inhibition of glutamine-dependent NAD+ synthetase (NADSYN1). At 6 h, there was a significant accumulation of nicotinamide riboside and nicotinamide mononucleotide (NMN) in the ATO-treated group, suggesting the inhibition of NMN adenylytransferase (NMNAT). NMNAT is a rate-limiting enzyme that catalyzes the biosynthesis of NAD from adenosine triphosphate and NMN, and is essential for cell survival under conditions of oxidative stress and DNA damage [33]. These results suggest that NAD+ biosynthesis inhibitor and ATO may have a synergistic effect.
Discussion
ATO holds the potential for treating solid tumors. In this study, we took gastric cancer as an example and tried to systematically investigate the MOA of ATO's antitumor activity through dynamic metabolomics profiling of gastric cancer cell line SGC7901 upon ATO treatment. The results clearly showed that a variety of metabolomics pathways have been disturbed, e.g. glycerophospholipid, one-carbon metabolism, FAO, NAD+ biosynthesis, and polyamine metabolism. Although in some cases, 12 h treatment has given a more dramatic change than 24 h treatment. The reason maybe that the biological systems are very robust; one plausible explanation is that when a key metabolic enzyme/pathway was inhibited by ATO, a complementary pathway may be triggered through a feedback loop. So we can anticipate a multilayer and deeper understanding of the MOA of ATO's antitumor activity when the metabolomics data are combined with other systematic data, such as genomics, transcriptomics, and proteomics.
Several studies have shown that ATO perturbation in solid tumor may have different mechanisms from that of hematological carcinoma [34–37]. According to our recent study [38], ATO binds to >300 proteins. Usually ATO causes activity loss of the proteins that it binds with, but it will not cause direct change of the targeting proteins at either mRNA level or protein level. Thus, it may be not necessary to check the mRNA level and protein level of a specific target, such as EDI3 at current stage. But, as demonstrated by a variety of other studies, cell lines of solid tumors were usually treated with 1–2 μM ATO for 12–24 h, and significant growth inhibition and apoptosis were observed in many of these cell lines, such as MGC803 [39], MCF-7, HeLa, and HIC [40]. According to our recent study [38], significant growth inhibition was also observed in SGC7901 cells after 12–24 h of ATO treatment at a dose of 2 μM. Our study employing the human protein microarray showed that ATO-binding proteins are involved in a variety of cellular signaling pathway, such as apoptosis, protein kinase, and acetylation/deacetylation pathway. Interestingly, ATO-binding proteins were highly enriched in glycolysis pathway, particularly, overexpression of tumor-specific glycolysis rate-limiting enzyme hexokinase-2 could dramatically rescue ATO-treated cells from apoptosis [38].
The metabolomics data are consistent with some of the previous ATO-related -omics studies. Ge et al. [41] monitored the global changes in protein expression in a multiple myeloma cell line that was perturbed with arsenic. The results showed that significant variations occurred in carbohydrate metabolism and nucleotide metabolism. Zhang and colleagues [12] treated leukemia cell line NB4 with ATO, and discovered that the apoptosis regulators and stress response-related genes were modulated. These results are consistent with the oxides stress-induced metabolic changes that were observed in our metabolomics profiling. It would be nice to verify the finding by other fast and handy technologies such as cDNA microarray and/or RNA-seq on mRNA level, and western blotting, enzyme-linked immunosorbent assay, or protein microarray on protein level. As such, we could then correlate the metabolomics data with transcriptomics and proteomics. This multiple level investigation would definitely help us to understand MOA of ATO at a more systematical and comprehensive level [38].
When the metabolomics data are integrated with other ATO-related -omics data, we could anticipate a more comprehensive understanding of the underlying mechanisms of ATO's antitumor activity, which may guide us to develop combinatorial therapy with other compounds/strategies for more effective treatment of a variety of tumors. For example, immune checkpoint therapy [42,43], which targets regulatory pathway in T cells and enhances antitumor immune response, may be a good choice to be combined with ATO treatment.
Taken together, the present study represents the first metabolomic study of solid tumor cell line, SGC7901 of gastric cancer, upon ATO treatment, and the dynamics of 281 well-defined metabolites were successfully documented. This study provides the first framework on metabolomics for understanding the molecular basis of ATO perturbation. The metabolomic data will serve as a valuable resource for future clinical application of ATO, either alone or in combination with other antitumor agents.
Supplementary Data
Funding
This work was supported in part by the grants from the National High Technology Research and Development Program of China (Nos. 2012AA020103 and 2012AA020203), the National Natural Science Foundation of China (Nos. 31370813 and 31370750), and the Medicine-engineering Collaboration Grant of Shanghai Jiao Tong University (No. YG2012MS43) and Key Project Specialized for Infectious Diseases of the Chinese Ministry of Health Grant (No. 2013ZX10003006).
Supplementary Material
Acknowledgements
We are grateful to Sheng Quan (Shanghai Jiaotong University) and SJTU-Metabolon Joint Metabolomics Laboratory for their expert research assistance, and Prof. Bingya Liu (Shanghai Jiaotong University) for providing the SGC7901 cell line.
References
- 1.Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014, 513: 202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shen L, Shan YS, Hu HM, Price TJ, Sirohi B, Yeh KH, Yang YH et al. . Management of gastric cancer in Asia: resource-stratified guidelines. Lancet Oncol 2013, 14: e535–e547. [DOI] [PubMed] [Google Scholar]
- 3.Zhang XW, Yan XJ, Zhou ZR, Yang FF, Wu ZY, Sun HB, Liang WX et al. . Arsenic trioxide controls the fate of the PML-RARalpha oncoprotein by directly binding PML. Science 2010, 328: 240–243. [DOI] [PubMed] [Google Scholar]
- 4.Mao JH, Sun XY, Liu JX, Zhang QY, Liu P, Huang QH, Li KK et al. . As4S4 targets RING-type E3 ligase c-CBL to induce degradation of BCR-ABL in chronic myelogenous leukemia. Proc Natl Acad Sci USA 2010, 107: 21683–21688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li JJ, Tang Q, Li Y, Hu BR, Ming ZY, Fu Q, Qian JQ et al. . Role of oxidative stress in the apoptosis of hepatocellular carcinoma induced by combination of arsenic trioxide and ascorbic acid. Acta Pharmacol Sin 2006, 27: 1078–1084. [DOI] [PubMed] [Google Scholar]
- 6.Walker AM, Stevens JJ, Ndebele K, Tchounwou PB. Arsenic trioxide modulates DNA synthesis and apoptosis in lung carcinoma cells. Int J Environ Res Public Health 2010, 7: 1996–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang XH, Chun-Yu-Wong B, Yuen ST, Jiang SH, Cho CH, Lai KC, Lin M et al. . Arsenic trioxide induces apoptosis in human gastric cancer cells through up-regulation of P53 and activation of caspase-3. Int J Cancer 2001, 91: 173–179. [DOI] [PubMed] [Google Scholar]
- 8.Sun XP, Zhang X, He C, Qiao H, Jiang X, Jiang H, Sun X. ABT-737 synergizes with arsenic trioxide to induce apoptosis of gastric carcinoma cells in vitro and in vivo. J Int Med Res 2012, 40: 1251–1264. [DOI] [PubMed] [Google Scholar]
- 9.Wu DD, Xiao YF, Geng Y, Hou J. Antitumor effect and mechanisms of arsenic trioxide on subcutaneously implanted human gastric cancer in nude mice. Cancer Genet Cytogen 2010, 198: 90–96. [DOI] [PubMed] [Google Scholar]
- 10.Jiang X-H, Chun-Yu Wong B, Yuen S-T, Jiang S-H, Cho C-H, Lai K-C, Lin MCM et al. . Arsenic trioxide induces apoptosis in human gastric cancer cells through up-regulation of P53 and activation of caspase-3. Int J Cancer 2001, 91: 173–179. [DOI] [PubMed] [Google Scholar]
- 11.Liu TX, Zhang JW, Tao J, Zhang RB, Zhang QH, Zhao CJ, Tong JH et al. . Gene expression networks underlying retinoic acid-induced differentiation of acute promyelocytic leukemia cells. Blood 2000, 96: 1496–1504. [PubMed] [Google Scholar]
- 12.Zheng PZ, Wang KK, Zhang QY, Huang QH, Du YZ, Zhang QH, Xiao DK et al. . Systems analysis of transcriptome and proteome in retinoic acid/arsenic trioxide-induced cell differentiation/apoptosis of promyelocytic leukemia. Proc Natl Acad Sci USA 2005, 102: 7653–7658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wan J, Wang J, Cheng H, Yu Y, Xing G, Oiu Z, Qian X et al. . Proteomic analysis of apoptosis initiation induced by all-trans retinoic acid in human acute promyelocytic leukemia cells. Electrophoresis 2001, 22: 3026–3037. [DOI] [PubMed] [Google Scholar]
- 14.Kaddurah-Daouk R, Kristal BS, Weinshilboum RM. Metabolomics: a global biochemical approach to drug response and disease. Annu Rev Pharmacol 2008, 48: 653–683. [DOI] [PubMed] [Google Scholar]
- 15.Schulze A, Harris AL. How cancer metabolism is tuned for proliferation and vulnerable to disruption. Nature 2012, 491: 364–373. [DOI] [PubMed] [Google Scholar]
- 16.Dang CV. Links between metabolism and cancer. Gene Dev 2012, 26: 877–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffin JL, Shockcor JP. Metabolic profiles of cancer cells. Nat Rev Cancer 2004, 4: 551–561. [DOI] [PubMed] [Google Scholar]
- 18.Currie E, Schulze A, Zechner R, Walther TC, Farese RV Jr. Cellular fatty acid metabolism and cancer. Cell Metab 2013, 18: 153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saldanha AJ. Java Treeview-extensible visualization of microarray data. Bioinformatics 2004, 20: 3246–3248. [DOI] [PubMed] [Google Scholar]
- 20.Springett GM, Bonham L, Hummer A, Linkov I, Misra D, Ma C, Pezzoni G et al. . Lysophosphatidic acid acyltransferase-β is a prognostic marker and therapeutic target in gynecologic malignancies. Cancer Res 2005, 65: 9415–9425. [DOI] [PubMed] [Google Scholar]
- 21.Stewart JD, Marchan R, Lesjak MS, Lambert J, Hergenroeder R, Ellis JK, Lau CH et al. . Choline-releasing glycerophosphodiesterase EDI3 drives tumor cell migration and metastasis. Proc Natl Acad Sci USA 2012, 109: 8155–8160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hishikawa D, Hashidate T, Shimizu T, Shindou H. Diversity and function of membrane glycerophospholipids generated by the remodeling pathway in mammalian cells. J Lipid Res 2014, 55: 799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Locasale JW. Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat Rev Cancer 2013, 13: 572–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B et al. . Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature 2009, 457: 910–914. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 25.Carracedo A, Cantley LC, Pandolfi PP. Cancer metabolism: fatty acid oxidation in the limelight. Nat Rev Cancer 2013, 13: 227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vankoningsloo S, Piens M, Lecocq C, Gilson A, De Pauw A, Renard P, Demazy C et al. . Mitochondrial dysfunction induces triglyceride accumulation in 3T3-L1 cells: role of fatty acid beta-oxidation and glucose. J Lipid Res 2005, 46: 1133–1149. [DOI] [PubMed] [Google Scholar]
- 27.Gerner EW, Meyskens FL Jr. Polyamines and cancer: old molecules, new understanding. Nat Rev Cancer 2004, 4: 781–792. [DOI] [PubMed] [Google Scholar]
- 28.Sies H. Glutathione and its role in cellular functions. Free Radic Biol Med 1999, 27: 916–921. [DOI] [PubMed] [Google Scholar]
- 29.Estrela JM, Ortega A, Obrador E. Glutathione in cancer biology and therapy. Crit Rev Clin Lab Sci 2006, 43: 143–181. [DOI] [PubMed] [Google Scholar]
- 30.Traverso N, Ricciarelli R, Nitti M, Marengo B, Furfaro AL, Pronzato MA, Marinari UM et al. . Role of glutathione in cancer progression and chemoresistance. Oxid Med Cell Longev 2013, 2013: 972913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 2007, 39: 44–84. [DOI] [PubMed] [Google Scholar]
- 32.Khan JA, Forouhar F, Tao X, Tong L. Nicotinamide adenine dinucleotide metabolism as an attractive target for drug discovery. Expert Opin Ther Targets 2007, 11: 695–705. [DOI] [PubMed] [Google Scholar]
- 33.Jayaram HN, Kusumanchi P, Yalowitz JA. NMNAT expression and its relation to NAD metabolism. Curr Med Chem 2011, 18: 1962–1972. [DOI] [PubMed] [Google Scholar]
- 34.Wang GZ, Zhang W, Fang ZT, Zhang W, Yang MJ, Yang GW, Li S et al. . Arsenic trioxide: marked suppression of tumor metastasis potential by inhibiting the transcription factor Twist in vivo and in vitro. J Cancer Res Clin 2014, 140: 1125–1136. [DOI] [PubMed] [Google Scholar]
- 35.Zhou W, Cheng L, Shi Y, Ke SQ, Huang Z, Fang X, Chu CW et al. . Arsenic trioxide disrupts glioma stem cells via promoting PML degradation to inhibit tumor growth. Oncotarget 2015, 6: 37300–37315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kerl K, Moreno N, Holsten T, Ahlfeld J, Mertins J, Hotfilder M, Kool M et al. . Arsenic trioxide inhibits tumor cell growth in malignant rhabdoid tumors in vitro and in vivo by targeting overexpressed Gli1. Int J Cancer 2014, 135: 989–995. [DOI] [PubMed] [Google Scholar]
- 37.Ma ZB, Xu HY, Jiang M, Yang YL, Liu LX, Li YH. Arsenic trioxide induces apoptosis of human gastrointestinal cancer cells. World J Gastroenterol 2014, 20: 5505–5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang HN, Yang L, Ling JY, Czajkowsky DM, Wang JF, Zhang XW, Zhou YM et al. . Systematic identification of arsenic-binding proteins reveals that hexokinase-2 is inhibited by arsenic. Proc Natl Acad Sci USA 2015, 112: 15084–15089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y, Qu X, Qu J, Zhang Y, Liu J, Teng Y, Hu X et al. . Arsenic trioxide induces apoptosis and G2/M phase arrest by inducing Cbl to inhibit PI3K/Akt signaling and thereby regulate p53 activation. Cancer Lett 2009, 284: 208–215. [DOI] [PubMed] [Google Scholar]
- 40.Zhang TC, Cao EH, Li JF, Ma W, Qin JF. Induction of apoptosis and inhibition of human gastric cancer MGC-803 cell growth by arsenic trioxide. Eur J Cancer 1999, 35: 1258–1263. [DOI] [PubMed] [Google Scholar]
- 41.Ge F, Lu XP, Zeng HL, He QY, Xiong S, Jin L, He QY. Proteomic and functional analyses reveal a dual molecular mechanism underlying arsenic-induced apoptosis in human multiple myeloma cells. J Proteome Res 2009, 8: 3006–3019. [DOI] [PubMed] [Google Scholar]
- 42.Sharma P, Wagner K, Wolchok JD, Allison JP. Novel cancer immunotherapy agents with survival benefit: recent successes and next steps. Nat Rev Cancer 2011, 11: 805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Binder DC, Fu YX, Weichselbaum RR. Radiotherapy and immune checkpoint blockade: potential interactions and future directions. Trends Mol Med 2015, 21: 463–465. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.