Abstract
The mechanisms of kidney aging are not yet clear. Studies have shown that immunological inflammation is related to kidney aging. Inflammasomes are important components of innate immune system in the body. However, the function of inflammasomes and their underlying mechanisms in renal aging remain unclear. In this study, for the first time, we systematically investigated the role of the inflammasomes and the inflammatory responses activated by inflammasomes during kidney aging. We found that during kidney aging, the expression levels of the molecules associated with the activation of inflammasomes, including toll-like receptor-4 and interleukin-1 receptor (IL-1R), were significantly increased; their downstream signaling pathway molecule interleukin-1 receptor-associated kinase-4 (IRAK4) was markedly activated (Phospho-IRAK4 was obviously increased); the nuclear factor-κB (NF-κB) signaling pathway was activated (the activated NF-κB pathway molecules Phospho-IKKβ, Phospho-IκBα, and Phospho-NF-κBp65 were significantly elevated); the levels of the inflammasome components NOD-like receptor P3 (NLRP3), NLRC4, and pro-caspase-1 were prominently upregulated; and the proinflammatory cytokines IL-1β and IL-18 were notably increased in the kidneys of 24-month-old (elderly group) rats. These results showed that inflammasomes are markedly activated during the renal aging process and might induce inflamm-aging by promoting the maturation and secretion of the proinflammatory cytokines IL-1β and IL-18.
Keywords: Kidneys, Inflamm-aging, Inflammasome, Innate immune system, Inflammation
Despite the many theories of aging that have been proposed, such as the oxidative stress theory, the mechanism of aging remains less than clear (1,2). Recent studies have shown that immunological inflammation may be related to aging. During aging, the adaptive immunity response significantly declines (immunosenescence), and the innate immunity response is markedly activated, which leads to the aging type of low-level, chronic inflammatory phenotype (inflamm-aging) (3,4). Kidneys exhibit high energy metabolism and are thus prone to aging. Kidney function in the elderly decreases with increasing age (5). However, the mechanisms of renal aging also remain unclear.
The inflammasome is a high molecular weight, multiprotein complex composed of intracellular sensor molecules such as pattern recognition receptors (PRRs), the adaptor protein ASC (apoptosis-associated speck-like protein containing a CARD), and effector protein pro-caspase-1 (precursor of cysteinyl aspartate specific proteinase-1). PRRs include NLRP1 (NOD-, LRR- and pyrin domain-containing-1), NLRP3 (NOD-, LRR- and pyrin domain-containing-3, also known as NALP3 or cryopyrin), NLRC4 (NOD-, LRR- and CARD domain-containing-4, also known as IPAF), and AIM2 (absent in melanoma 2), which can separately assemble with ASC and pro-caspase-1. The inflammasome is an important component of the innate immune system. In particular, NLRP1 and NLRC4 can also directly interact with pro-caspase-1 to assemble the inflammasome. The inflammasome can sense numerous intracytoplasmic microbial metabolites and metabolic stress molecules and is thus called “the sensor of innate immunity.” Formation of inflammasome is triggered by a range of foreign molecules that emerge during infections, which are designated pathogen-associated molecular patterns, as well as molecules native to the body that are altered during tissue damage or metabolic imbalances, which are designated danger-associated molecular patterns (DAMPs). Once inflammasomes have formed, they can recruit and activate caspase-1(cysteinyl aspartate specific proteinase-1). Activated caspase-1 can cleave proinflammatory cytokines, such as pro-interleukin-1β (pro-IL-1β) and pro-IL-18, producing corresponding mature cytokines IL-1β and IL-18 to be released into the extracellular plasma, which triggers immune response. However, overactivation of inflammasomes can induce serious inflammatory reactions and eventually cause the occurrence and development of various inflammatory diseases such as type 2 diabetes (6), Alzheimer’s disease (7), atherosclerosis (8), and gouty arthritis (9).
It is currently not clear how pathogen-associated molecular pattern and DAMP molecules, such as bacterial peptidoglycan, bacterial toxins, oxidized mitochondrial DNA, and adenosine triphosphate, promote the priming and activation of inflammasomes. Studies have shown that certain ligands can activate the nuclear factor-κB (NF-kB) signal transduction pathway by binding to PRRs, such as toll-like receptor-4 (TLR4) in the cell membrane, hence promoting the upregulated expression of intracellular PRRs such as NLRP1, NLRP3, NLRC4, AIM2, and proinflammatory cytokines such as pro-IL-1β and pro-IL-18 (10,11). A few molecules, such as amyloid-β, can induce both NLRP3 priming through TLR activation and NLRP3 inflammasome activation (12). Proinflammatory cytokines such as IL-1, binding to cytokine receptors such as IL-1 receptor (IL-1R), can activate the transcription of NLRP3. Currently, the role of the inflammasome system in kidney aging is unclear.
In this study, for the first time, we systematically observed the changes in the expression of TLR4 and IL-1R and their downstream signaling pathway molecule Phospho-IRAK4 (interleukin-1 receptor-associated kinase-4); NF-κB signaling pathway molecules Phospho-IKKβ (IκB kinase complex β subunit), Phospho-IκBα(Phospho-inhibitor α of NF-κB), and Phospho-NF-κBp65; inflammasome components NLRP1, NLRP3, NLRC4, AIM2, and pro-caspase-1; and proinflammatory cytokines IL-1β and IL-18 during the renal aging process to clarify the role of the inflammasome and its mechanisms in kidney aging. We found that inflammasomes are activated in the ageing kidneys, which results in the activation of caspase-1. Activated caspase-1 can cleave the precursors of the proinflammatory cytokines IL-1β and IL-18, and mature IL-1β and IL-18 are produced and secreted into the extracellular milieu to induce immune and inflammatory responses, which eventually lead to senescence of the kidney tissues.
Materials and methods
Experimental Animals and Specimen Handling
Clean-grade inbred male F344 rats aged 2 months (weighing 180–190g) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. They were divided into two groups: the 3-month-old group (youth group) (n = 20) and the 24-month-old group (elderly group) (n = 20). Three rats were kept in each cage, and they were housed in an environment at 22±1°C with 40% humidity and a 12-hour photoperiod. They had free access to food and water. The rats were reared for 3 or 24 months, depending on the group, and their body weights were then measured. The rats were anesthetized with an intraperitoneal injection of sodium pentobarbital (40mg/kg), and all efforts were made to minimize suffering. Blood samples were taken, and their kidneys were removed. After rinsing in ice-cold normal saline to remove residual blood, the kidneys were weighed. The renal tissue on the upper pole of the kidneys was placed into 10% neutral formalin for fixation and used for immunohistochemistry and renal pathological examination. The lower pole of the kidneys was quickly placed in OCT compound and used for immunofluorescence staining. The remaining kidney tissue was cut into small pieces and placed into liquid nitrogen for Western blotting and real-time–quantitative polymerase chain reaction (RT-qPCR) analysis. This study was conducted in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the Chinese PLA General Hospital. The protocol was approved by the Committee on the Ethics of Animal Experiments of the Chinese PLA General Hospital (Permit Number: X6-27).
Biochemical Analyses
The blood samples were centrifuged at 3,000rpm for 10 minutes, and the sera were collected and sent to the Department of Biochemistry in our hospital to measure blood urea nitrogen, serum creatinine, glucose (GLU), triglyceride, and total cholesterol. Blood urea nitrogen and triglyceride were measured by colorimetric methods. Enzymatic methods were used to detect serum creatinine and total cholesterol, and the hexokinase method was used to detect blood sugar. Urine samples were collected and sent to the Department of Clinical Laboratory in our hospital to detect the urinary protein/creatinine ratio, with urinary protein measured by the coomassie brilliant blue method and urine creatinine measured by the sarcosine oxidase method. Urinary protein concentration was determined using a protein concentration kit based on coomassie brilliant blue. The procedural details are as follows. (i) Three sterile Eppendorf tubes were designated as a blank tube, which was supplemented with 0.05mL of ddH2O; a standard tube, which was supplemented with 0.05mL of standard protein solution; and a sample tube, which was supplemented with 0.05mL of a urine sample. (ii) A volume of 3mL of coomassie brilliant blue reagent was added to each tube. (iii) Samples were fully mixed and left to stand for 5 minutes. (iv) Absorbance at 595nm was measured for all of the tubes using a UV spectrometer. (v) Urine protein concentrations were calculated using the equation: Concentration = (Detected optical density [OD] − blank OD) / (Standard OD − Blank OD) × Standard substance concentration. Standard substance concentration: 563mg/L. Urine creatinine level was determined using a creatinine kit based on sarcosine oxidase. The procedural details are as follows. (i) Three sterile Eppendorf tubes were designated as a blank tube, which was supplemented with 6 µL of ddH2O; a standard tube, which was supplemented with 6 µL of a standard specimen; and a sample tube, which was supplemented with 6 µL of a urine sample. (ii) A volume of 180 µL of enzyme solution A was added to each tube. (iii) The samples were fully mixed and incubated at 37°C for 5 minutes. (iv) Absorbance at 546nm was measured for all of the tubes using the UV spectrometer, and the values were defined as A1. (v) Following the method of absorbance measurement, a blank tube, which was supplemented with 6 µL of ddH2O; a standard tube, wd and incubated at 37°C for 5 minutes. (vi) Absorbance at 546nm was again measured for all of the tubes and defined as A2. (vii) Urine creatinine concentration was calculated using the equation: Concentration = [(Detected A2-K × Detected A1) − (Blank A2-K × Blank A1)] / [(Standard A2-K × Standard A1) − (Blank A2-K × Blank A1)] × Standard substance concentration. Standard substance concentration: 50 µmol/L; Dilution factor K: 0.76.
Periodic Acid–Schiff Staining
Kidneys from the rats were excised, fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned at 4mm for histological staining with periodic acid–Schiff followed by examination under the microscope. Periodic acid–Schiff staining methods are as follows. (i) Thin-section slides were oxidized in 1% periodic acid for 10–15 minutes and then rinsed with water. (ii) The slides were stained with Schiff’s reagent for 10–30 minutes and then rinsed with water. (iii) The slides were then incubated with hematoxylin for 1–2 minutes to stain the nucleus and then rinsed with water. (iv) The slides were immersed in 1% HCl in ethanol for several seconds and then rinsed with water. (v) The slides were stained blue in ammonia water and then rinsed with running water; microscopy revealed that the nucleus was stained blue, whereas the basement membrane was stained red. (vi) The slides were subsequently subjected to serial alcohol dehydration and xylene clearing, and the coverslips were sealed with gum.
Western Blot Analysis
The frozen kidney tissues were lysed with radio immunoprecipitation assay lysis buffer (50mM Tris-Cl [pH 7.6], 150mM NaCl, 1% NP-40, 0.1% sodium dodecyl sulfate, 0.5% deoxycholic acid, 50mM sodium fluoride (ser/thr phosphatase inhibitor), 0.2mM sodium orthovanadate (tyr phosphatase inhibitor), 1 µg/mL leupeptin, 1 µg/mL aprotinin, and 0.5mM phenylmethylsulfonyl fluoride) and were centrifuged at 12,000g at 4°C for 30 minutes to obtain the cellular proteins in the supernatant. Equal amounts of proteins from each sample were resolved by 6%–15% sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to NC membranes, blocked with 5% skim milk for 1 hour at room temperature, and probed with the following primary antibodies at 4°C overnight: NLRP3 (Millipore, Billerica, MA); TLR4, Phospho-IKKβ, NLRP1, NLRC4, IL-1β, and IL-18 (Abcam, Cambridge, UK); Phospho-IRAK4, Phospho-IκBα, and Phospho-NF-κB p65 (Cell Signaling Technology, Danvers, MA); and IL-1R, AIM2, caspase-1, and β-actin (Santa Cruz Biotechnology, Dallas, TX). The blots were subsequently incubated with horseradish peroxidase–conjugated anti-mouse or anti-rabbit IgG (Santa Cruz Biotechnology) at 1:1,000–1:5,000. Immunoreactive bands were visualized using enhanced chemiluminescence, and densitometry was performed using Quantity One software (Bio-Rad Laboratories, Hercules, CA).
Total RNA Extraction and Reverse Transcription
Total RNA was isolated from renal tissues using TRIzol (Invitrogen, Carlsbad, CA) following the manufacturer’s instructions. A UV spectrophotometer was used to measure the concentrations of total RNA. Reverse transcription was performed using a TIANScript RT kit (Tiangen Biotech, Beijing, China).
Real-Time–Quantitative Polymerase Chain Reaction
Amplification was performed in a 7500 real-time PCR System (Applied Biosystems, Foster, CA). The reaction contained 50ng of complementary deoxyribonucleic acid, 0.2 µM primers, and 10 µL of 2 × SYBR green buffer (TaKaRa, Dalian, Liaoning, China) in a final volume of 20 µL. The primers (Table 2) were designed using the software package Primer Express 2.0 (Applied Biosystems) based on GenBank nucleotide sequences. PCR was performed using the following cycling conditions: 95°C for 30 seconds followed by 40 cycles of denaturation at 95°C for 15 seconds and extension at 60°C for 30 seconds. All samples were run in triplicate. The relative abundance of the target messenger ribonucleic acid was determined using the comparative cycle threshold method (52).
Table 2.
Real-Time–Quantitative Polymerase Chain Reaction Primer Sequences and the Lengths of the Products
| Name | GenBank No. | Primers | Length of Products (bp) |
|---|---|---|---|
| NLRP3 | NM_017008.4 | F:CAGAAGCTGGGGTTGGTGAA | 125 |
| R:CCCATGTCTCCAAGGGCATT | |||
| IL-1β | NM_031512.2 | F:CAGCTTTCGACAGTGAGGAGA | 139 |
| R:TTGTCGAGATGCTGCTGTGA | |||
| IL-18 | NM_019165.1 | F:ACCGCAGTAATACGGAGCAT | 109 |
| R:TCTGGGATTCGTTGGCTGTT | |||
| GAPDH | NM_017008.4 | F:TGCACCACCAACTGCTTAG | 176 |
| R:GATGCAGGGATGATGTTC |
Notes: F = forward; GAPDH = glyceraldehyde-3-phosphate dehydrogenase; IL-18 = interleukin-18; IL-1β = interleukin-1β; NLRP3 = NLR family, pyrin domain containing 3; R = reverse.
Immunohistochemical Staining
Kidneys were fixed in 10% formaldehyde at 4°C overnight and then processed for paraffin embedding, following standard procedures. Sections were prepared at 3 µm. For immunohistochemical analysis, tissue sections were subjected to antigen retrieval by microwaving or autoclaving for 10 or 15 minutes in 10mM sodium citrate buffer (pH 6.0). Endogenous peroxidase was blocked by incubation with 3% hydrogen peroxide for 10 minutes. Sections were washed in phosphate-buffered saline and incubated with 1.5% normal goat serum for 20 minutes, followed by incubation with a 1:4,000 dilution of rabbit polyclonal anti-NLRP3 antibody (Millipore) overnight at 4°C. Sections were washed three times with PBS and incubated with biotin-conjugated goat anti-rabbit IgG (Invitrogen) for 30 minutes at room temperature. Sections were again washed with PBS and incubated with streptavidin-conjugated peroxidase (Invitrogen) for 30 minutes at room temperature. Sections were washed for a final time in PBS and incubated with diaminobenzidine (Invitrogen) followed by examination under a microscope.
Immunofluorescent Dual Staining
Renal tissues were embedded in Tissue-Tek O.C.T complex (Sakura Finetek Europe BV, Zoeterwoude, the Netherlands). For immunofluorescent analysis, sections were prepared at 4 µm. After drying at room temperature for 10 minutes, sections were fixed in 4% paraformaldehyde at 4°C for 30 minutes. Sections were washed in PBS three times (5min each) and incubated with 5% bovine serum albumin for 40 minutes at room temperature, followed by synchronous incubation with goat polyclonal anti-NLRC4 antibody (Santa Cruz Biotechnology) at 1:10 and mouse monoclonal anti-CD31 antibody (Abcam) at 1:50; or goat polyclonal anti-caspase-1 antibody (Santa Cruz Biotechnology) at 1:10 and mouse monoclonal anti-CD31 antibody at 1:50 overnight at 4°C. Sections were washed three times with PBS and incubated sequentially with Cy3-conjugated donkey anti-goat IgG (red) or FITC-conjugated goat anti-mouse IgG (green) (Jackson ImmunoResearch Laboratories, West Grove, PA) at room temperature for 1 hour. Following washing in PBS three times (5 minutes each), 4, 6-diamidino-2-phenylindole counterstain was performed, sections were mounted with glycerin, and the results were analyzed under an Olympus laser scanning confocal microscope.
Statistical Methods
All data were analyzed using the SPSS 20.0 (SPSS, Chicago, IL) software package. Comparisons between the data of various groups were performed using analyses of variance, with p values < .05 indicating significant differences.
Results
Changes in Renal Function in the Aging Rats
First, the body weights and kidney weights of rats from young and old groups were measured. The results showed that compared with the rats in the 3-month-old group (young), the rats in the 24-month-old group (old) showed significant increases in body weight and kidney weight. We then examined the changes in the renal and metabolic function parameters of the rats in the 3-month-old and 24-month-old groups. We found that compared with the rats in the 3-month-old group, rats in the 24-month-old group showed significant increases in triglyceride, blood urea nitrogen, and the urinary protein/creatinine ratio, whereas serum creatinine, glucose, and total cholesterol levels did not change significantly (Table 1).
Table 1.
Changes in the Kidney Function and Metabolic Function in the Aging Rats
| Parameters | At 3 Months | At 24 Months |
|---|---|---|
| Body weight (g) | 236.3±12.28 | 617.8±57.34* |
| Kidney weight (g) | 1.67±0.68 | 5.01±1.0* |
| Blood urea nitrogen (mmol/L) | 5.73±0.47 | 6.86±1.10* |
| Serum creatinine (μmol/L) | 27.15±2.60 | 25.8±2.04 |
| Serum glucose (mmol/L) | 7.09±1.02 | 6.09±1.31 |
| Serum triglyceride (mmol/L) | 1.20±0.32 | 3.22±0.82* |
| Serum cholesterol (mmol/L) | 1.55±0.16 | 3.11±0.36 |
| Urine protein/creatinine ratio (mg/mmol) | 146.01±22.72 | 256±49.39* |
Compared with the 3-month-old group.
*Indicates p < .05.
Pathological Changes in the Aged Rat Kidney Tissues
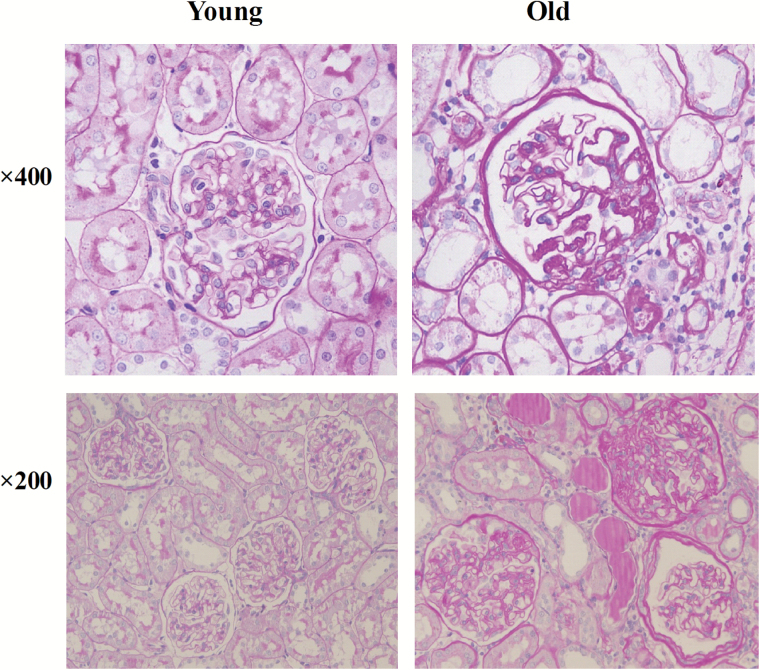
Renal histological changes in young (3-month-old) and old (24-month-old) male rats were evaluated by periodic acid–Schiff staining. Compared with young renal tissues, old renal tissues showed marked pathological features of aging, including focal segmental glomerulosclerosis, atrophy of renal tubules, and interstitial fibrosis, along with some inflammatory cell infiltration (Figure 1).
Figure 1.
Periodic acid–Schiff staining results in the renal tissues of young (3 months) and old (24 months) rats.
The Expression of Priming Signal Transduction Molecules that Mediate Inflammasome Activation in the Aging Rat Kidney Tissues
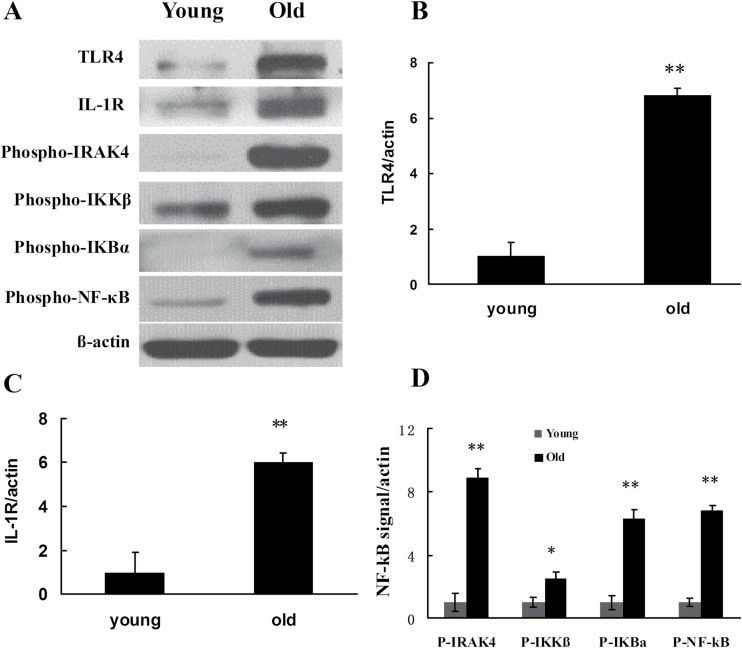
Although the exact molecular mechanisms of inflammasome assembly are incompletely understood, it is well established that inflammasome activation requires priming signals. Various exogenous and endogenous ligand molecules, after binding with TLR4 and IL-1R, can regulate the expression of intracellular PRRs such as NLRP1, NLRP3, NLRC4, and AIM2 and proinflammatory cytokines such as pro-IL-1β and pro-IL-18 by activating the NF-kB signal transduction pathway (13). In this study, Western blotting was used to detect the activation and expression of TLR4, IL-1R, and some key molecules in the NF-κB signal transduction pathway. These results revealed significant upregulated expression of TLR4 and IL-1R in the group of 24-month-old rats. A further study showed that, compared with the 3-month-old young rats, the levels of Phospho-IRAK4, Phospho-IKKβ, Phospho-IKBα, and Phospho-NF-kBp65 (all are activated forms) were significantly higher (Figure 2) in the old rat kidney tissue.
Figure 2.
Changes in the levels of TLR4, IL-1R, and NF-kB signal pathway molecules in the renal tissues of young and old rats. (A) Western blotting was used to detect the levels of expression or activation of proteins in each group of rats. (B–D) Quantitative gray scale analysis of protein levels. β-actin: internal reference. Compared with the young group, *indicates p < .05, **indicates p < .01. IL-1R = interleukin-1 receptor; NF-kB = nuclear factor-κB; P-IRAK4 = Phospho-IRAK4; P-IKKβ = Phospho-IKKβ; P-IKBα = Phospho-IKBα; P-NF-kB = Phospho-NF-kBp65; TLR4 = toll-like receptor 4.
The Expression Changes of Inflammasome Subunits in Aging Rat Kidney Tissues
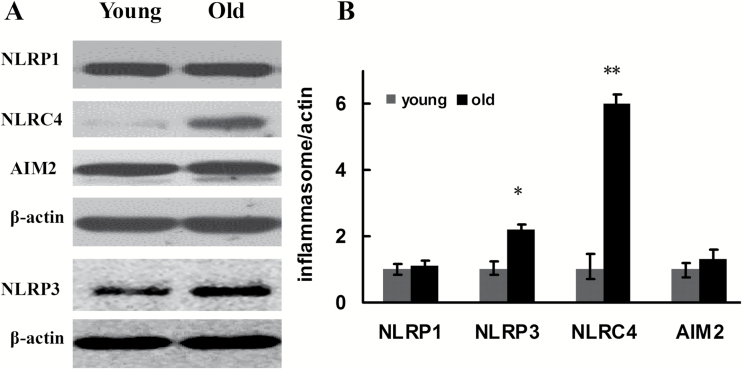
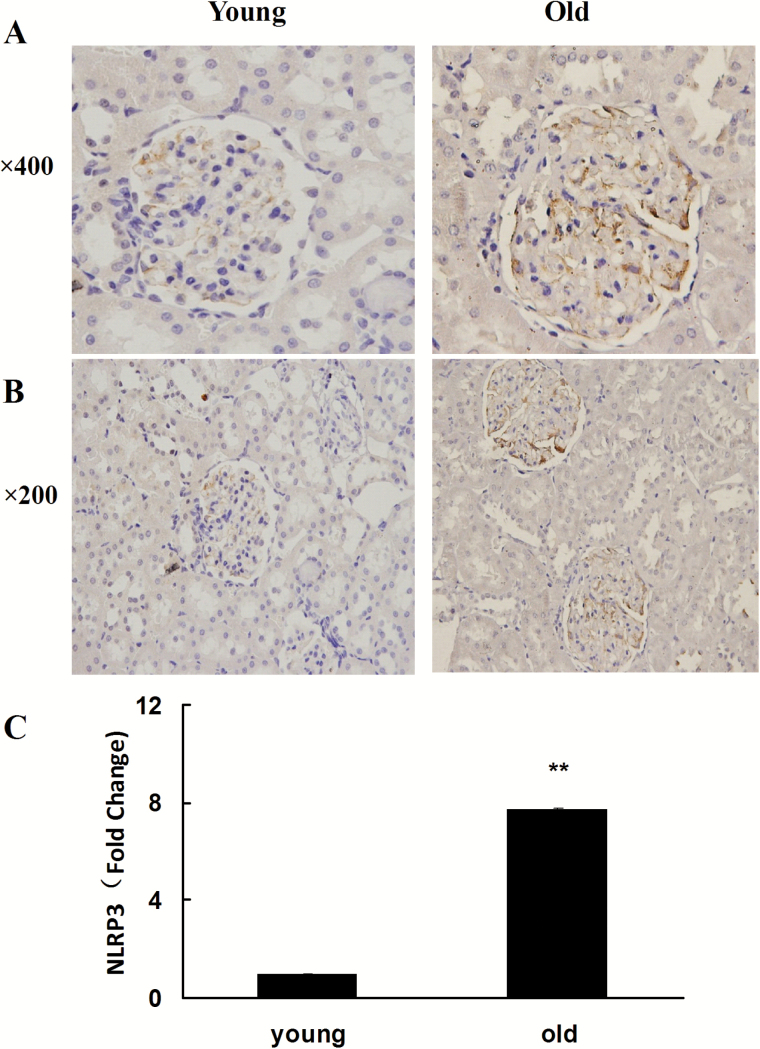
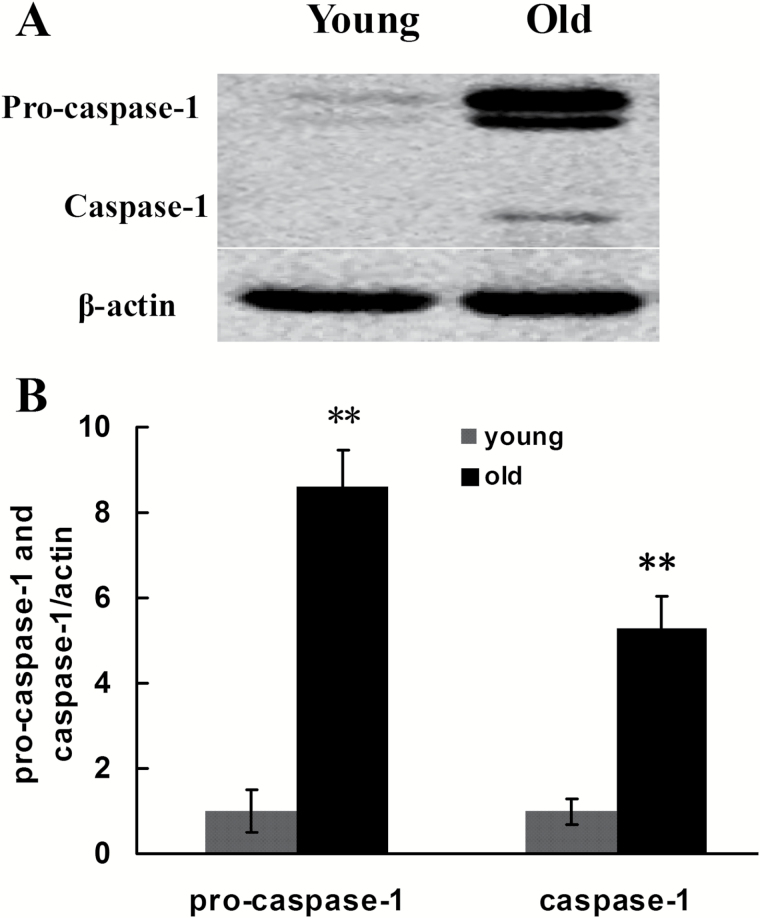
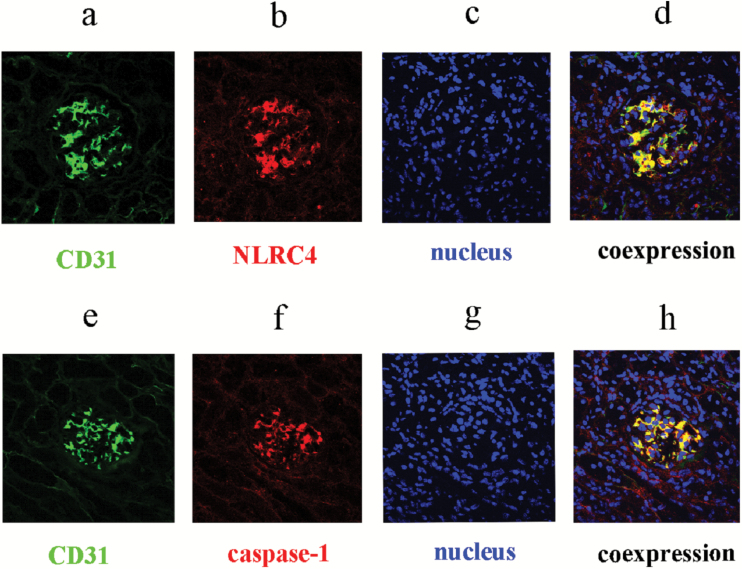
Previous studies showed that various pathogen-associated molecular patterns and DAMPs, by binding with intracellular PRRs such as NLRP1, NLRP3, NLRC4, and AIM2, promoted the assembly and activation of four types of inflammasomes and further activated pro-caspase-1. Specifically, NLRP3 or AIM2 interacted with adaptor proteins ASC and pro-caspase-1, whereas NLRP1 or NLRC4 directly interacted with pro-caspase-1, assembling into high molecular weight inflammasomes. In this case, the inflammasomes can transform the inactive pro-caspase-1 into activated caspase-1 with proteolytic function. In this study, we performed Western blotting to determine the expression of inflammasome subunits including NLRP1, NLRP3, NLRC4, AIM2, and pro-caspase-1 in the aging rat kidney tissues. The results showed that, compared with the young group, the expression of NLRP3 and NLRC4 increased significantly in the aging kidney tissue, whereas the expression of NLRP1 and AIM2 demonstrated no significant difference between the two groups (Figure 3). The NLRP3 inflammasome is a special inflammasome that can be activated by various types of endogenous or exogenous ligands. To further confirm the expression of NLRP3 in the aging rat kidney tissues, immunohistochemistry and RT-qPCR were utilized to detect its expression and intracellular location. As shown in Figure 4, compared with the young group, the expression of NLRP3 significantly increased in the old rat kidney tissues and was primarily distributed in the renal glomeruli. Compared with the young group, both pro-caspase-1 and its activated form, caspase-1, showed significantly increased levels in the renal tissues of old rats (Figure 5). To further define the intracellular location of inflammasome molecules NLRC4 and caspase-1, we performed immunofluorescent dual staining using the antibodies against NLRC4 or caspase-1 with the antibody against CD31 (marker of endothelial cells) in the aging renal tissues. The results showed that NLRC4 and caspase-1 expressed predominately in glomeruli; and dual staining results demonstrated that NLRC4 or caspase-1 (red) colocalized completely with marker for endothelial cells (CD31, green), indicating that both cytokines located in the endothelial cells of glomeruli (Figure 6D and H, yellow).
Figure 3.
Changes in the expression of intracytoplasm pattern recognition receptors in inflammasomes in the renal tissues of young and old rats. (A) Western blotting was used to detect the expression levels of proteins in each group of rats. (B) Quantitative gray scale analysis of protein levels. β-actin: internal reference. Compared with the young group, *indicates p < .05, **indicates p < .01.
Figure 4.
Analysis of NLRP3 expression level in the renal tissues of young and old rats. (A) Immunohistochemical result of NLRP3 expression. ×400; (B) ×200; (C) Real-time–quantitative polymerase chain reaction detection results of NLRP3 expression. Compared with the young group, the fold change in NLRP3 in the old group = 2 − ΔΔCT. Compared with the young group, **indicates p < .01. NLRP3 = NOD-like receptor P3.
Figure 5.
Changes in the expression of pro-caspase-1 and caspase-1 in the renal tissues of young and old rats. (A) Western blotting was used to detect the expression levels of proteins in each group of rats. (B) Quantitative gray scale analysis of protein levels. β-actin: internal reference. Compared with the young group, **indicates p < .05.
Figure 6.
The expression and location of inflammasome molecules in aging renal tissues. (A), (E) Markers for endothelial cells (CD31, green); (B) NLRC4 (red), (F) caspase-1(red); (C), (G) 4,6-diamidino-2-phenylindole counterstain (blue); (D) NLRC4 colocalized with marker for endothelial cells (yellow); (H) caspase-1 colocalized with marker for endothelial cells (yellow). NLRP3 = NOD-like receptor P3.
The Expression of Inflammasome-Activated Downstream Proinflammatory Factors IL-1β and Il-18 in Aging Rat Kidney Tissues
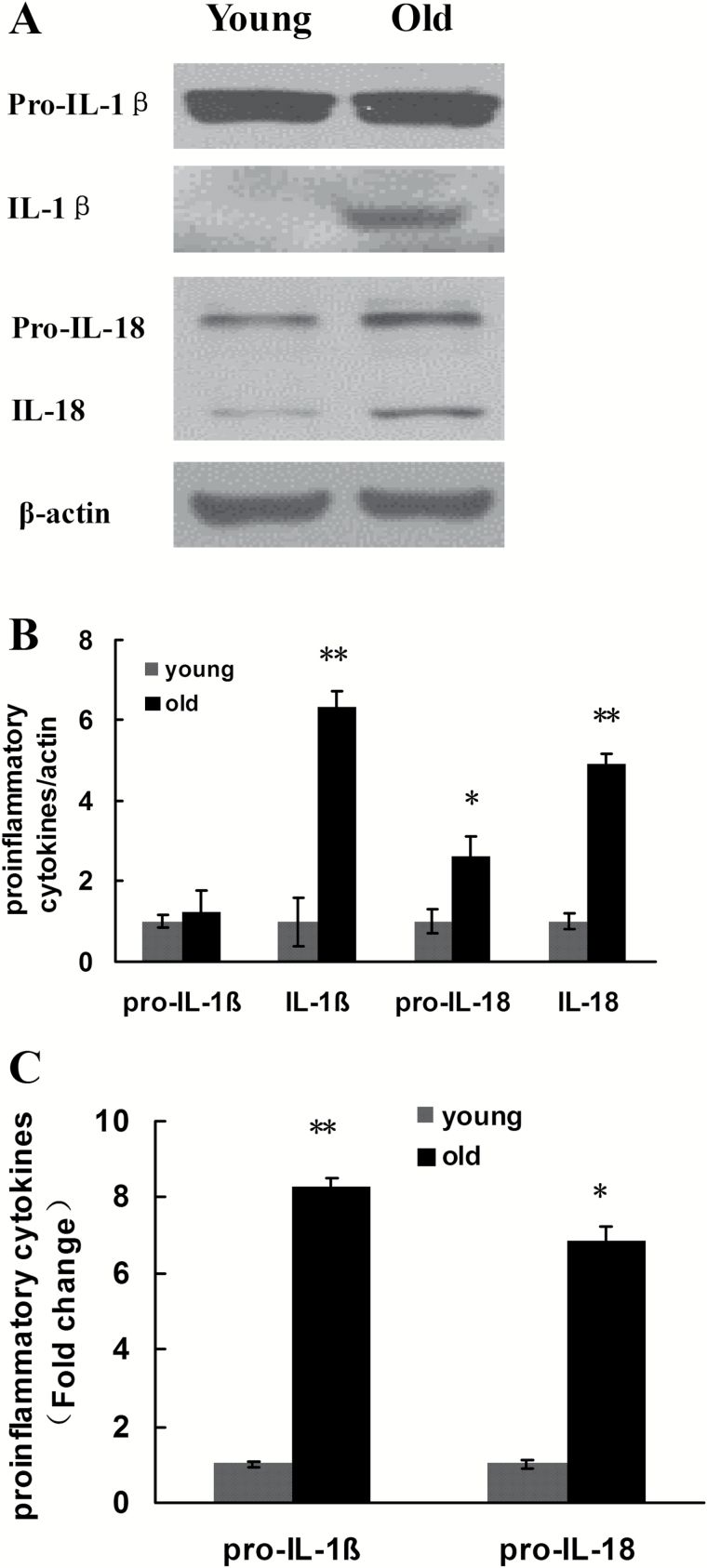
The activated caspase-1 can promote the maturation and secretion of pro-IL-1β and pro-IL-18, thus exerting an important effect during the process of innate immune response. To test whether kidney aging was correlated with activated IL-1β and IL-18, the expression of the precursors and mature bodies of IL-1β and IL-18 in the aging rat kidney tissues was determined by Western blotting. The results demonstrated that the downstream cytokine IL-1β precursor did not differ significantly in the old rat kidney tissue compared with that in the young group, but the activated IL-1β exhibited significantly higher expression in the aged group. Intriguingly, another important cytokine in this process, pro-IL-18 and its active form IL-18, exhibited significantly higher expression in the aged group than in the young group (Figure 7A and B). The RT-qPCR showed a similar result: compared with the 3-month-old group, the expression of pro-IL-1β and pro-IL-18 increased significantly in the 24-month-old group (Figure 7C).
Figure 7.
Changes in the expression of pro-IL-1β, IL-1β, pro-IL-18, and IL-18 in the renal tissues of young and old rats. (A) Western blotting was used to detect the expression levels of proteins in each group of rats. (B) Quantitative gray scale analysis of protein levels. β-actin: internal reference. Compared with the young group, *indicates p < .05, **indicates p < .01. (C) Real-time–quantitative polymerase chain reaction detection results of pro-IL-1β and pro-IL-18. Compared with the young group, the fold changes in pro-IL-1β and pro-IL-18 in the old group = 2 − ΔΔCT. Compared with the young group, *indicates p < .05, **p < .01. IL-1βinterleukin-1β.
Discussion
Age-associated renal anatomic and functional phenotypes occur during kidney senescence. The histology of the senescent kidneys is characterized by diffuse glomerular sclerosis, with tubulointerstitial fibrosis. The aging glomeruli, mainly in the superficial cortex, are subjected to progressive expansion of mesangium, obliteration of the glomerular loops and capillary tuft collapse. Podocytes show hypertrophy, foot process fusion, and detachment from the glomerular basement membrane. These alterations represent progressive glomerulosclerosis (14–16). Along with glomerular changes, several tubulointerstitial alterations have been noted in the aging kidneys. Interstitial fibrosis which is due to excessive collagen deposition in the extracellular matrix with infiltration of inflammatory cells, tubular dilation, and thickening of the basement membranes are all hallmarks of the aging kidneys. Furthermore, a decrease in size of the proximal tubular epithelial cells and length of the proximal convoluting tubules has been observed in the senescent kidneys (15,17). Alterations in renal function occur along with anatomical changes, including decreased glomerular filtration rate, decreased renal plasma flow, decreased functional reserve, impaired urine dilution/concentration ability, impaired Na+/K+ handling, and impaired oxidants handling (18). Recent findings suggest significant effects of replicative and environmental stress-induced senescences in kidney aging. In particular, ataxia telangiectasia mutated (ATM)/p53/p21 and Ras/p38/p16 pathways have been shown to co-contribute to the senescence, which is caused by intrinsic and extrinsic stimuli (19,20). Telomere shortening and deprotection defined as intrinsic stimuli trigger replicative senescence (16). As an extrinsic stimuli, oxidative injury is believed to play a major role in the process of cellular aging. Oxidative stress and generation of free radicals increase with aging (21,22). Inhibition of autophagy, as happens with aging, can accelerate the aging process (16). During aging, adaptive immunity significantly declines, whereas innate immunity is activated. The master regulator of innate immunity is NF-κB signal pathway system. NF-κB is a transcription factor in the recognition signaling and host responses to immune attacks. As the host defense receptor system, PRRs including TLRs and NLRs can recognize pathogen-associated molecular patterns and DAMPs. Signaling of TLRs and NLRs are linked to NF-κB system via different adapter proteins and protein kinases to enhance the inflammatory responses (3).
The innate immune system enables immune response and inflammation to eliminate infections and repairs injured tissues. Inflammasomes play an important role in mediating innate immune and adaptive immune responses. NLRP1, NLRP3, and NLRC4 in NLR family and AIM2 in ALR family can assemble into corresponding inflammasomes. AIM2, as an intracellular sensor, can be activated by various microorganisms such as Francisella tularensis, the Listeria monocytogenes cytomegalovirus, and the vaccinia virus (23). In addition, AIM2 is also related to the occurrence and development of certain autoimmune diseases such as systemic lupus erythematosus (24).
Among all currently discovered inflammasomes, the NLRP3 inflammasome has the largest number of corresponding ligands. Its ligands comprise not only various bacteria, viruses, yeasts, parasites, and their toxins but also DAMPs, such as adenosine triphosphate (25), reactive oxygen species (26), saturated fatty acids (27), uric acid crystals (9), and cholesterol crystals (8). In recent years, NLRP3 inflammasome has attracted increasing attention for its effects in age-related metabolism and degenerative diseases such as type 2 diabetes mellitus, atherosclerosis, gout, and Alzheimer’s disease. Previous studies have confirmed that the secretion of IL-1β through NLRP3 leads to the occurrence and development of type II diabetes by mediating the apoptosis of β-cells in the islet and insulin resistance (28–30). One study has reported that cholesterol crystal-induced NLRP3 inflammasome activation and IL-1β secretion could cause atherosclerosis (31,32). The precipitation of monosodium urate and calcium pyrophosphate dihydrate crystals at joints and surrounding tissues could promote the assembly and activation of NLRP3 inflammasome and induce arthrolithiasis attack (33). A recent in vivo experiment suggests that the amyloid-β-activated NLRP3/caspase-1 axis in microglia plays an important role in the pathological process of Alzheimer’s disease (30,34). The NLRP3 inflammasome could be activated not only during the pathological conditions but also during the natural aging process in the hippocampus, thymus gland and adipose tissues (35).
Studies discovered that NLRP3 inflammasome was closely related to the occurrence of kidney diseases, such as renal tubulointerstitial disease (36). A recent experiment supports that the activation of NLRP3 inflammasome in non-bone marrow–derived cells such as glomerular capillary endothelial cells and podocytes plays an important role in the pathogenesis of diabetic nephropathy (37). The kidney is an organ with an active energy metabolism and is prone to aging. Do inflammasomes exert any influence on the kidney aging process?
Our results have verified that expression of the key signal transduction molecules activating NLRP3 showed a significant increase in aging rat kidney tissues. Activation of NLRP3 inflammasome needs two signal transduction pathways. One signal pathway was through an NF-κB–dependent priming signal and the other was through an inflammasome formation signal. Firstly, after binding to their respective ligands, TLR4/IL-1R recruited interleukin-1 receptor-associated kinase-4 (IRAK4) to join the receptor complexes and further activate IkB kinases (IKKs). IKKs (especially the catalytic subunit IKKβ) can phosphorylate IκB, the inhibitor of NF-κB, and further promote its ubiquitination, resulting in the release of IκB from the IκB/NF-κB complex. In this way, NF-κB is activated and is transferred into the nucleus to participate in the transcription and expression of inflammasomes and proinflammatory cytokines such as IL-1β and IL-18 precursor (38,39). For the first time, we discovered that the above discussed signal transduction molecules, including TLR4, IL-1R, Phospho-IRAK4, Phospho-IKKβ, Phospho-IκBα, and Phospho-NF-κBp65, all showed significant increases in aging rat kidney tissues. Furthermore, the second signal transduction pathway involving the NLRP3-caspase-1-IL-1β/IL-18 axis plays an important role in mediating the immune response. PRRs such as NLRP3 can be activated by various exogenous or endogenous stimulating signals, triggering itself oligomerization and the recruitment of ASC and pro-caspase-1 to form a multiprotein complex, the “NLRP3 inflammasome.” This complex can further activate pro-caspase-1 (IL-1β invertase), and the activated caspase-1 can promote the cleavage, maturation, and secretion of proinflammatory cytokines such as IL-1β and IL-18, which results in inflammatory reactions. In this study, we discovered that the average expression levels of NLRP3, pro-caspase-1, and caspase-1 all significantly increased in aging rat kidney tissues. In addition to NLRP3, we also measured the expression of NLRP1, NLRC4, and AIM2 in aging rat kidney tissues. The result showed that NLRC4 was also activated. A previous study showed that the pathogenic microorganism Salmonella enterica serovar Typhimurium can simultaneously activate both NLRP3 and NLRC4, and only one type of inflammasome was observed in the infected macrophages (40).
The “Inflamm-aging theory” posits that “aging either physiologically or pathologically can be driven by the proinflammatory cytokines and substances produced by the innate immune system” (41,42). Previous studies suggest that the IL-1 family plays an important role in the occurrence and development of age-related degenerative diseases (43). A recent study showed that, in the noninfected state, the expression of age-related IL-1β was significantly upregulated in the adipose tissues (35). A recent study showed that senescence at a cellular level is directly linked to an IL-dependent inflammatory network (44). Kidney resident cells including endothelial cells, mesenchymal cells, and epithelial cells are actively involved in inflammatory responses by producing cytokines, chemokines, and growth factors. During kidney aging process, upregulated chemokines in resident cells may initiate the inflammation via the recruitment of inflammatory cells. Kidney resident cells also could increase the proliferation, differentiation, and survival of inflammatory cells via the production of cytokines, such as IL-1 and IL-6, and growth factors. Cytokines released from inflammatory cells could further stimulate the expression of proinflammatory molecules by resident cells, the interaction between kidney resident cells and inflammatory cells may form a vicious cycle that leads to chronic inflammation (15,45). Both IL-1β and IL-18 are important members of the same structural family (IL-1 super family) (46,47). Previous studies demonstrated that proinflammatory cytokines IL-1β and IL-18 could enhance apoptosis of mesenchymal cells and podocytes, extracellular matrix deposition, consequently leading to glomerulosclerosis during kidney aging process, indicating that IL-1β and IL-18 participate in inflammatory processes that increase during the kidney aging process (15,45). In this study, we discovered that, as the key effect factors downstream of inflammasomes, proinflammatory cytokines including IL-1β and IL-18 all showed significantly increased expression levels in aging kidney tissues. These results suggest that both the cytokines are crucial factors in the aging process of the kidneys.
By 2050, 2 billion of the estimated 9 billion people in the world will be older than 60 years. With the proportion of older people among the global population being now higher than at any time in history and still expanding, maintaining health into old age (or healthspan) has become a new and urgent frontier for modern medicine (48). In recent years, studies have suggested a correlation between immune inflammation, aging, and age-related diseases (49). It has also been discovered that the serum levels of TNF-α, IL-6, and C-reactive protein were significantly elevated in the aged population (45). There is evidence that these higher levels of proinflammatory cytokines and acute-phase proteins are linked to increased mortality (50). However, it is not clear by what mechanism inflammation is involved in the organ aging process. This study suggests that the inflammasome is closely related to kidney aging, which provides a new direction for studying the kidney aging mechanism and devising novel therapeutic strategies (51).
Funding
This work was supported by a grant (No. 2011CBA01003 to X.-Y.B.) from the National Basic Research Program of China, a grant (No. 2011CB964904 to X.-Y.B.) from the National Key Scientific Program of China, and grants (No. 30870920, 30270505, and 30070288 to X.-Y.B.) from the National Natural Science Foundation of China.
Acknowledgments
The English in this document has been checked by at least two professional editors, both native speakers of English. For a certificate, please see: http://www.textcheck.com/certificate/qmxdNA.
References
- 1. Baylis C. Changes in renal hemodynamics and structure in the aging kidney; sexual dimorphism and the nitric oxide system. Exp Gerontol. 2005;40:271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cai W, He JC, Zhu L, et al. Oral glycotoxins determine the effects of calorie restriction on oxidant stress, age-related diseases, and lifespan. Am J Pathol. 2008;173:327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Salminen A, Huuskonen J, Ojala J, Kauppinen A, Kaarniranta K, Suuronen T. Activation of innate immunity system during aging: NF-kB signaling is the molecular culprit of inflamm-aging. Ageing Res Rev. 2008;7:83–105. [DOI] [PubMed] [Google Scholar]
- 4. Arranz L, Caamaño JH, Lord JM, De la Fuente M. Preserved immune functions and controlled leukocyte oxidative stress in naturally long-lived mice: possible role of nuclear factor kappa B. J Gerontol A Biol Sci Med Sci. 2010;65:941–950. doi:10.1093/gerona/glq101 [DOI] [PubMed] [Google Scholar]
- 5. Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. [DOI] [PubMed] [Google Scholar]
- 6. Vandanmagsar B, Youm YH, Ravussin A, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–188. doi:10.1038/nm.2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu L, Chan C. The role of inflammasome in Alzheimer’s disease. Ageing Res Rev. 2014;15:6–15. doi:10.1016/j.arr.2013.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Samstad EO, Niyonzima N, Nymo S, et al. Cholesterol crystals induce complement-dependent inflammasome activation and cytokine release. J Immunol. 2014;192:2837–2845. doi:10.4049/jimmunol.1302484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. [DOI] [PubMed] [Google Scholar]
- 10. Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi:10.1016/j.cell.2010.01.040 [DOI] [PubMed] [Google Scholar]
- 11. Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157:1013–1022. doi:10.1016/j.cell.2014.04.007 [DOI] [PubMed] [Google Scholar]
- 12. Halle A, Hornung V, Petzold GC, et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9:857–865. doi:10.1038/ni.1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bauernfeind FG, Horvath G, Stutz A, et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–791. doi:10.4049/jimmunol.0901363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wiggins JE. Aging in the glomerulus. J Gerontol A Biol Sci Med Sci. 2012;67:1358–1364. doi:10.1093/gerona/gls157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bolignano D, Mattace-Raso F, Sijbrands EJ, Zoccali C. The aging kidney revisited: a systematic review. Ageing Res Rev. 2014;14:65–80. doi:10.1016/j.arr.2014.02.003 [DOI] [PubMed] [Google Scholar]
- 16. Zhou XJ, Saxena R, Liu Z, Vaziri ND, Silva FG. Renal senescence in 2008: progress and challenges. Int Urol Nephrol. 2008;40:823–839. doi:10.1007/s11255-008-9405-0 [DOI] [PubMed] [Google Scholar]
- 17. Buemi M, Nostro L, Aloisi C, Cosentini V, Criseo M, Frisina N. Kidney aging: from phenotype to genetics. Rejuvenation Res. 2005;8:101–109. [DOI] [PubMed] [Google Scholar]
- 18. Sands JM. Urine concentrating and diluting ability during aging. J Gerontol A Biol Sci Med Sci. 2012;67:1352–1357. doi:10.1093/gerona/gls128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Melk A, Schmidt BM, Takeuchi O, Sawitzki B, Rayner DC, Halloran PF. Expression of p16INK4a and other cell cycle regulator and senescence associated genes in aging human kidney. Kidney Int. 2004;65:510–520. [DOI] [PubMed] [Google Scholar]
- 20. Famulski KS, Halloran PF. Molecular events in kidney ageing. Curr Opin Nephrol Hypertens. 2005;14:243–248. [DOI] [PubMed] [Google Scholar]
- 21. Bhayadia R, Schmidt BM, Melk A, Homme M. Senescence-induced oxidative stress causes endothelial dysfunction. J Gerontol A Biol Sci Med Sci. 2015. May 3. pii: glv008. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22. Percy CJ, Power D, Gobe GC. Renal ageing: changes in the cellular mechanism of energy metabolism and oxidant handling. Nephrology (Carlton). 2008;13:147–152. doi:10.1111/j.1440-1797.2008.00924.x [DOI] [PubMed] [Google Scholar]
- 23. Rathinam VA, Jiang Z, Waggoner SN, et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol. 2010;11:395–402. doi:10.1038/ni.1864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Panchanathan R, Duan X, Shen H, et al. Aim2 deficiency stimulates the expression of IFN-inducible Ifi202, a lupus susceptibility murine gene within the Nba2 autoimmune susceptibility locus. J Immunol. 2010;185:7385–7393. doi:10.4049/jimmunol.1002468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mariathasan S, Weiss DS, Newton K, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. [DOI] [PubMed] [Google Scholar]
- 26. Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–286. doi:10.1038/nature10759 [DOI] [PubMed] [Google Scholar]
- 27. L’homme L, Esser N, Riva L, et al. Unsaturated fatty acids prevent activation of NLRP3 inflammasome in human monocytes/macrophages. J Lipid Res. 2013;54:2998–3008. doi:10.1194/jlr.M037861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fève B, Bastard JP. The role of interleukins in insulin resistance and type 2 diabetes mellitus. Nat Rev Endocrinol. 2009;5:305–311. doi:10.1038/nrendo.2009.62 [DOI] [PubMed] [Google Scholar]
- 29. Schroder K, Zhou R, Tschopp J. The NLRP3 inflammasome: a sensor for metabolic danger? Science. 2010;327:296–300. doi:10.1126/science.1184003 [DOI] [PubMed] [Google Scholar]
- 30. Choi AJ, Ryter SW. Inflammasomes: molecular regulation and implications for metabolic and cognitive diseases. Mol Cells. 2014;37:441–448. doi:10.14348/molcells.2014.0104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Duewell P, Kono H, Rayner KJ, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi:10.1038/nature08938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grebe A, Latz E. Cholesterol crystals and inflammation. Curr Rheumatol Rep. 2013;15:313. doi:10.1007/s11926-012-0313-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Martinon F. Mechanisms of uric acid crystal-mediated autoinflammation. Immunol Rev. 2010;233:218–232. doi:10.1111/j.0105-2896.2009.00860.x [DOI] [PubMed] [Google Scholar]
- 34. Heneka MT, Kummer MP, Stutz A, et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493:674–678. doi:10.1038/nature11729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Youm YH, Grant RW, McCabe LR, et al. Canonical Nlrp3 inflammasome links systemic low-grade inflammation to functional decline in aging. Cell Metab. 2013;18:519–532. doi:10.1016/j.cmet.2013.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Andersen K, Eltrich N, Lichtnekert J, Anders HJ, Vielhauer V. The NLRP3/ASC inflammasome promotes T-cell-dependent immune complex glomerulonephritis by canonical and noncanonical mechanisms. Kidney Int. 2014;86:965–978. doi:10.1038/ki.2014.161 [DOI] [PubMed] [Google Scholar]
- 37. Shahzad K, Bock F, Dong W, et al. Nlrp3-inflammasome activation in non-myeloid-derived cells aggravates diabetic nephropathy. Kidney Int. 2014. doi:10.1038/ki.2014.271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Janssens S, Beyaert R. Functional diversity and regulation of different interleukin-1 receptor-associated kinase (IRAK) family members. Mol Cell. 2003;11:293–302. [DOI] [PubMed] [Google Scholar]
- 39. Ungvari Z, Bailey-Downs L, Gautam T, et al. Age-associated vascular oxidative stress, Nrf2 dysfunction, and NF-{kappa}B activation in the nonhuman primate Macaca mulatta. J Gerontol A Biol Sci Med Sci. 2011;66:866–875. doi:10.1093/gerona/glr092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Man SM, Hopkins LJ, Nugent E, et al. Inflammasome activation causes dual recruitment of NLRC4 and NLRP3 to the same macromolecular complex. Proc Natl Acad Sci USA. 2014;111:7403–7408. doi:10.1073/pnas.1402911111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Goto M. Inflammaging (inflammation + aging): a driving force for human aging based on an evolutionarily antagonistic pleiotropy theory? Biosci Trends. 2008;2:218–230. [PubMed] [Google Scholar]
- 42. Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69(suppl 1):S4–S9. doi:10.1093/gerona/glu057 [DOI] [PubMed] [Google Scholar]
- 43. McGillicuddy FC, Harford KA, Reynolds CM, et al. Lack of interleukin-1 receptor I (IL-1RI) protects mice from high-fat diet-induced adipose tissue inflammation coincident with improved glucose homeostasis. Diabetes. 2011;60:1688–1698. doi:10.2337/db10-1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tsirpanlis G. Cellular senescence and inflammation: a noteworthy link. Blood Purif. 2009;28:12–14. doi:10.1159/000210032 [DOI] [PubMed] [Google Scholar]
- 45. Mei C, Zheng F. Chronic inflammation potentiates kidney aging. Semin Nephrol. 2009;29:555–568. doi:10.1016/j.semnephrol.2009.07.002 [DOI] [PubMed] [Google Scholar]
- 46. Dinarello CA. Interleukin 1 and interleukin 18 as mediators of inflammation and the aging process. Am J Clin Nutr. 2006;83:447S–455S. [DOI] [PubMed] [Google Scholar]
- 47. Miles EA, Rees D, Banerjee T, et al. Age-related increases in circulating inflammatory markers in men are independent of BMI, blood pressure and blood lipid concentrations. Atherosclerosis. 2008;196:298–305. [DOI] [PubMed] [Google Scholar]
- 48. Burch JB, Augustine AD, Frieden LA, et al. Advances in geroscience: impact on healthspan and chronic disease. J Gerontol A Biol Sci Med Sci. 2014;69(suppl 1):S1–S3. doi:10.1093/gerona/glu041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Craft S, Foster TC, Landfield PW, Maier SF, Resnick SM, Yaffe K. Session III: Mechanisms of age-related cognitive change and targets for intervention: inflammatory, oxidative, and metabolic processes. J Gerontol A Biol Sci Med Sci. 2012;67:754–759. doi:10.1093/gerona/ gls112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Swain SL, Nikolich-Zugich J. Key research opportunities in immune system aging. J Gerontol A Biol Sci Med Sci. 2009;64:183–186. doi:10.1093/gerona/gln068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Le Couteur DG, McLachlan AJ, Quinn RJ, Simpson SJ, de Cabo R. Aging biology and novel targets for drug discovery. J Gerontol A Biol Sci Med Sci. 2012;67:168–174. doi:10.1093/gerona/glr095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lee EJ, Schmittgen TD. Comparison of RNA assay methods used to normalize cDNA for quantitative real-time PCR. Anal Biochem. 2006;357:299–301. [DOI] [PubMed] [Google Scholar]